Highlights
-
•
The spectral characteristics of resting-state BOLD signals carry prognostic significance.
-
•
Spectral flattening in glioblastoma patients is a negative prognostic indicator.
-
•
Spectral flattening is associated with clinical comorbidities.
-
•
Spectral flattening is paradoxically associated with MGMT methylation status.
Keywords: Glioblastoma, Resting-state fMRI, Intrinsic brain activity, Power-law exponent, Fractional amplitude of low-frequency fluctuations (fALFF), Clinical comorbidities, Prognosis
Abstract
Glioblastoma, a highly aggressive form of brain tumor, is a brain-wide disease. We evaluated the impact of tumor burden on whole brain resting-state functional magnetic resonance imaging (rs-fMRI) activity. Specifically, we analyzed rs-fMRI signals in the temporal frequency domain in terms of the power-law exponent and fractional amplitude of low-frequency fluctuations (fALFF). We contrasted 189 patients with newly-diagnosed glioblastoma versus 189 age-matched healthy reference participants from an external dataset. The patient and reference datasets were matched for age and head motion. The principal finding was markedly flatter spectra and reduced grey matter fALFF in the patients as compared to the reference dataset. We posit that the whole-brain spectral change is attributable to global dysregulation of excitatory and inhibitory balance and metabolic demand in the tumor-bearing brain. Additionally, we observed that clinical comorbidities, in particular, seizures, and MGMT promoter methylation, were associated with flatter spectra. Notably, the degree of change in spectra was predictive of overall survival. Our findings suggest that frequency domain analysis of rs-fMRI activity provides prognostic information in glioblastoma patients and offers a means of noninvasively studying the effects of glioblastoma on the whole brain.
1. Introduction
Glioblastomas exhibit markedly altered metabolism that sustains and expands the tumor microenvironment (Agnihotri and Zadeh, 2016, Al-Zhoughbi et al., 2014, Dong and Cui, 2019, Vander Heiden et al., 2009). Accordingly, cerebrospinal fluid (CSF) metabolomic studies demonstrate significant differences between tumor patients and healthy controls (Locasale et al., 2012, Nakamizo et al., 2013). Importantly, glioma cells and glioma stem-like cells efficiently employ metabolic strategies (Vander Heiden et al., 2009, Warburg, 1956) that favor biosynthesis and cellular proliferation, and induce changes beyond the tumor microenvironment. The products of this altered metabolism promote neovascularization and a tolerogenic environment (Agnihotri and Zadeh, 2016, Al-Zhoughbi et al., 2014, Mantovani et al., 2008). Newly synthesized blood and lymph vessels facilitate the exchange of metabolites and signaling molecules between the tumor and the parenchymal macroenvironment: oxygen, glucose, and other nutrients are delivered to the tumor (Weis and Cheresh, 2011), and soluble factors are released from the tumor into the bloodstream (Al-Zhoughbi et al., 2014). These findings raise the question of whether the global effects of glioblastoma can be quantified and, if so, whether such quantifications carry prognostic significance.
Functional magnetic resonance imaging using resting-state (rs-fMRI) enables brain-wide monitoring of regional neural activity at millimeter scale resolution via the blood-oxygen-level-dependent (BOLD) signal, which spontaneously fluctuates over tens of seconds (infra-slow activity). Most studies that use rs-fMRI to study gliomas have focused on functional connectivity (FC), which measures the Pearson temporal correlation between BOLD signals extracted from region of interest pairs (e.g.,(Gordon et al., 2017, Seitzman et al., 2020)). These studies have demonstrated brain-wide changes in resting-state FC extending beyond the anatomical limits of the tumor (Daniel et al., 2021, Park et al., 2022, Ghinda et al., 2018, Fox and King, 2018, Stoecklein et al., 2020) as well as globally attenuated FC strength (Fox and King, 2018).
Here, we utilize a complementary analytic approach focused on the spectral properties of spontaneous BOLD signal fluctuations. Specifically, we evaluate the power-law exponent, , which quantitates the relative prevalence of slow vs. fast activity (He et al., 2010, He, 2011, He, 2014, Bullmore et al., 2001); and the fractional amplitude of low-frequency fluctuations (fALFF) (Zou et al., 2008), which evaluates relative power in selected frequency bands. fALFF and power-law estimation both enable quantification of regional neural activity over the whole brain, hence, are well suited to investigation of the global effects of glioblastomas. We evaluate brain-wide spectral properties in a group of patients with glioblastoma (N = 189) vs. a group of age-matched reference participants (N = 189). We find that glioblastomas induce brain-wide shifts in both measures and that these metrics correlate with epigenetic features as well as comorbid conditions. Finally, we demonstrate that these spectral measures are predictive of overall survival.
2. Materials and methods
2.1. Datasets
2.1.1. Reference dataset
The reference dataset comprised 189 healthy individuals obtained from the OASIS3 dataset (LaMontagne et al., 2019), which includes 1098 participants. For this work, we included participants that met the following inclusion criteria: Clinical Dementia Rating (CDR) score of zero at every assessment session, MRI acquired with Siemens TIM Trio 3 T scanner; presence of at least two rs-fMRI runs plus T1-weighted (T1w) and T2-weighted (T2w) structural images used for anatomic registration; participants with root mean square head motion (in mm) as determined by the realignment procedure < 1.15 mm (see Appendix A for details). Of all participants that met the inclusion criteria, we selected 189 individuals to statistically match the head motion profile to the patient group (Table C.1). The resting state fMRI data included two 6-minute runs (328 frames) acquired while participants were asked to remain still with their eyes open (voxel size (3 mm)3 isotropic; echo time = 27 ms; repetition time = 2.2 or 2.5 s).
2.1.2. Washington University School of Medicine glioblastoma dataset
The clinical dataset comprised 189 glioblastoma patients aged 21 – 86 (average 61 years), which was retrospectively identified in the Washington University School of Medicine (WUSM) neurosurgery brain tumor database. Inclusion criteria included: a new diagnosis of primary glioblastoma; age above 18 years; MRI at WUSM including fMRI for pre-surgical planning; adequate tumor segmentation; IDH1 wild-type; and root mean square head motion < 1.15 mm (Table C.1). Exclusion criteria included: prior brain surgery and inability to have an MRI scan. The Washington University Institutional Review Board oversees all aspects of the study. All analyses were conducted retrospectively using preoperative data.
Patients were scanned with either a 3 T Trio or Skyra scanner (Siemens, Erlangen, Germany) using a standard clinical pre-surgical tumor protocol. Anatomical imaging included a T1w magnetization prepared rapid acquisition (MP-RAGE) and a T2w fast spin echo, both with a voxel size of (1 mm)3 and a FLAIR image used for tumor segmentation. rs-fMRI was acquired using a BOLD-sensitized EPI sequence (voxel size (3 mm)3 isotropic; echo time = 27 ms; repetition time = 2.2 – 2.9 s; field of view = 256 mm; flip angle = 90°). Two rs-fMRI runs were obtained in each patient (320 frames); each run included 160 frames.
2.2. Demographics, clinical characteristics, clinical comorbidities
Participant demographics and dataset information are listed in Table C.1. Clinical characteristics, including the extent of resection, tumor volume, Karnofsky Performance Status (KPS), MGMT methylation status, EGFR amplification status, TERT mutation status, and PTEN mutation status, are tabulated in Table 1. Survival time was calculated as the difference between the patient’s first clinical visit and the date of death. Comorbidities included in the analysis are stroke and seizure (either had a history of or presented with); history of deep vein thrombosis (DVT) or pulmonary embolism (PE); chronic kidney disease (CKD); diabetes; hypertension; tobacco use; hyperlipidemia; cardiac comorbidity (including previous myocardial infarction, arrhythmia, valvular dysfunction, and congestive heart failure) and BMI > 30 (see Appendix E and Table E.1. for details).
Table 1.
Clinical characteristics of glioblastoma patients.
| CE volume (cm3) | 42.5 ± 34.2 |
| FLAIR volume (cm3) | 119.0 ± 67.8 |
| Extent of resection | GTR = 60, NTR = 28, STR = 47, Biopsy = 3, LITT = 4, Missing = 4, None = 43 |
| MGMT promoter methylation | Methylated = 69, Non-methylated = 113, Missing = 7 |
| EGFR amplification | Positive = 69, Negative = 108, Missing = 12 |
| PTEN mutation | Mutant = 97, Wildtype = 78, Missing = 14 |
| TERT promoter mutation | Mutant = 48, Wildtype = 116, Missing = 25 |
| KPS | < 70 = 27, ≥ 70 = 154, Missing = 8 |
| Stupp Protocol | Received Stupp protocol = 121, Radiotherapy, no chemo = 39, None = 18, Missing = 11 |
CE = contrast-enhanced; FLAIR = fluid attenuated inversion recovery; GTR = gross total resection; NTR = near total resection; STR = sub-total resection; LITT = laser interstitial thermal therapy; KPS = Karnofsky performance score.
2.3. Spatial normalization and fMRI preprocessing
Initial fMRI preprocessing followed conventional practice (Shulman et al., 2010). Briefly, this included compensation for slice-dependent time shifts, elimination of systematic odd–even slice intensity differences due to the interleaved acquisition, and rigid body correction of head movement within and across runs (Power et al., 2012). The preprocessed fMRI data were then resampled in register with the structural data in (3 mm)3 atlas space using a composition of the initial affine transform and a warping map (computed using the ANTs registration with cost function masking) connecting the fMRI volumes with the T1w structural image. Details of the steps regarding Advanced Normalization Tools (ANTs) registration and cost function masking (i.e., tumor masking) are outlined in (Park et al., 2022) and in Appendix B. Motion correction was included in the final resampling to generate volumetric timeseries in (3 mm)3 atlas space. Additional preprocessing included voxel-wise removal of linear trends over each fMRI run, temporal low-pass filtering retaining frequencies below 0.15 Hz and regression of nuisance waveforms. Regressors were derived from the 6 head motion timeseries, timeseries extracted from regions in CSF, and the signal evaluated over the whole brain. Finally, spatial smoothing was applied (6 mm full width at half maximum (FWHM) Gaussian blur in each direction).
2.4. Head motion
To minimize the impact of subject head motion at both the individual-level analysis and group-level comparison, we evaluated root mean square (RMS) head motion (in mm) as determined by the realignment procedure (see Appendix A for details). The exclusion threshold of head motion was determined as an RMS head motion value > 3 mm scaled median absolute deviation (MAD) from median of RMS head motion values evaluated over all patients. Participants with RMS head motion > 1.15 mm were excluded from reference and patient groups. The finally obtained head motion distributions were comparable across the reference and patient groups (Figs. A1,2).
2.5. Power spectrum calculation
The time series extracted from each voxel was transformed to the frequency domain using Welch's averaged modified periodogram method (pwelch function in Matlab). Each run consists of 164 and 160 frames for the reference and patient datasets, respectively. The first three frames were ignored to allow for steady state magnetization. A rectangular window of 75 frames with a 50% overlap between segments was used to compute the power spectral density (PSD) estimate in each run (150 frames for both datasets) and power spectra were averaged across runs. Since the lowest detectable frequency is determined by the window size, the lowest detectable frequencies are different across patients (0.023 – 0.03 Hz) based on varying sampling rates (TR = 2.2 s – 2.9 s; SR = 0.345 – 0.455 Hz). Accordingly, we obtained the power spectra within the frequency range of 0.025 – 0.105 Hz for subsequent analyses.
2.6. Fractional ALFF (fALFF) and power-law exponent calculation
Fractional ALFF (fALFF) at each frequency (0.025 to 0.105 Hz at a 0.01 Hz interval) was computed as the ratio of the power of the specific frequency to that of the frequency range, 0.03 – 0.15 Hz. Power-law scaling behavior is indicative of scale invariance: if , then the ratio of measured at two different frequencies, and , depends on the ratio of the two defined frequencies. Several studies have shown that fMRI signal exhibits a scale-free activity similar to that seen in electrophysiologic and cellular studies, such as local field potentials (LFP), electroencephalography (EEG), action potential rates, and the dynamics of neurotransmitter release (Buzsaki, 2006, Freeman and Zhai, 2009, Monto et al., 2008, Lowen et al., 1997, Ward Dynamical Cognitive Science, 2001). Specifically, He and colleagues have shown that the power-law function using the < 0.1 Hz frequency region better fits the fMRI power spectrum than exponential and log-normal functions (He, 2011). Accordingly, the power-law exponent, , within the frequency range of interest (0.025 – 0.105 Hz) was computed in subcortical regions (amygdala, hippocampus, thalamus, ventral and dorsal striatum), gray matter cortical regions, and white matter (regions shown in Figs. F2-4). We obtained the power-law exponent by fitting a power-law function to the 0.025–0.105 Hz frequency region of the power spectral density.
2.7. Statistical analyses
The difference in spectral properties between patients with glioblastoma and reference subjects was examined using a two-sided Wilcoxon rank sum test (significance level of p < 0.05). A two-sample t-test (significance level p < 0.05) was used to compare power-law exponents between two groups based on their mutational status, and we excluded patients with missing data for this analysis (Fig. F5, Table 1). For the gene that showed a significant difference between the two groups, we identified a cross-over frequency that demonstrated a statistically significant difference in spectra. Finally, we used a two-sided Wilcoxon rank sum test to evaluate the mutation status-dependent difference in fALFF at two frequency bands.
To explore the association between clinical comorbidities and the power-law exponent, patients were ranked based on their power-law exponents averaged across cortical voxels. The top and bottom 20% were then selected for each group (N = 38 in each group). We used Fisher’s exact test (MATLAB function fishertest) to evaluate the significance of the association between the power-law exponent and the presence of comorbidities. Due to the exploratory nature of this analysis and the relatively small sample size (N = 38) in each group, we chose not to apply a multiple comparison adjustment. This decision prioritized identifying potentially relevant associations for future investigations. Additionally, a linear regression model (MATLAB function fitlm) was used to evaluate the association between the number of clinical comorbidities and power-law exponents.
Survival analysis was performed by first median-splitting patients into two groups (N = 95 vs. 94) based on their overall survival (time of diagnosis to death). The average power spectra for each group were computed, and cross-over frequency points (0.045 and 0.085 Hz) were identified. Using these frequency points, a voxel-wise % difference in each frequency-band specific fALFF (0.025–0.045 Hz, 0.045–0.085 Hz, 0.085–0.105 Hz) was computed by averaging fALFF maps for all patients in each group.
Next, patients were median-split into two groups based on the averaged fALFF values in the gray matter, and the log-rank test was used to compare Kaplan-Meier survival curves (Creed et al., 2020). Additionally, univariate regression analysis was used to evaluate the effects of variables (age, sex, tumor volume, KPS, MGMT methylation status, surgical status, and fALFF in gray matter) on survival using Cox proportional hazards regression (MATLAB function coxphfit). Variables with significant p-values in the univariate analysis were included in a multivariate model using Cox regression proportional hazard model. Bonferroni corrected p-values indicate statistical significance. All statistical analyses were performed in Matlab 2019a.
3. Results
3.1. Demographics
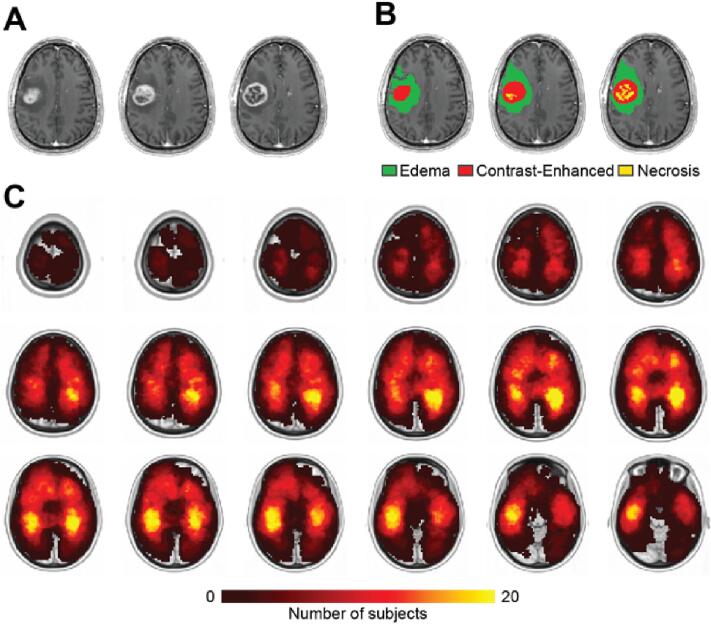
189 patients with glioblastoma (Age = 61.1 ± 11.3; M:F ratio = 119:80) and 189 reference subjects (Age = 68.0 ± 10.8 M:F ratio = 80:109) were included in this study (Table C.1). Head movement during scanning was used as an index of fMRI data quality and was matched across two groups (Figs. F1,2). Glioblastomas were segmented on the basis of contrast-enhanced T1w and FLAIR images (see Appendix B). Fig. 1 shows a tumor distribution heatmap compiled in the patient group. Tumor volume, the extent of resection, genetic status (MGMT, EGFR, PTEN, and TERT), Karnofsky Performance Scale (KPS), and the follow-up administration of Stupp protocol post-surgery are included in Table 1.
Fig. 1.
A. Postcontrast T1w image of an exemplar glioblastoma patient. B. Example of segmentation. Vasogenic edema (potentially including infiltrative non-enhancing tumor core) (green), necrotic/non-enhancing tumor core (yellow), and enhancing tumor core (red) are visualized. C. Tumor frequency heatmap shows the distribution of contrast-enhanced tumor volume in all 189 subjects. The intensity of the color scale represents the number of patients, ranging from 0 to 20. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Brain-wide power-law exponent change in patients with glioblastoma
The power-law exponent, , averaged over the whole brain was clearly different in the patient group as compared to the reference group (Fig. 2A). Fig. 2B shows power spectra averaged over cortical gray matter, subcortical gray matter, and white matter (region masks illustrated in Figs. F2-4). Gray matter (cortical + subcortical, region masks illustrated in Figs. F3, 4) median values ranged from 0.6 to 1.2 and 0.44 to 0.86 in reference subjects and glioblastoma patients, respectively (Fig. 2C). In cortical networks, was greatest in the visual network and least in the cingulo-opercular network. Group differences in were significant across both gray matter (cortical/subcortical) and white matter (Fig. 2B, C; Wilcoxon rank-sum test, p < 1e-4 for all regions). We observed no clear evidence of focal or resting state network (RSN) specific aberrancy in tumor patients compared to the reference group, based on our averaged data (Fig. 2C, Fig. F1). The RSNs we evaluated included cingulo-opercular network, salience network, somatomotor network, among others (see Fig. 2C for a comprehensive list). Our observations were based on the averaged data across these networks. This result is consistent with our prior finding that functional connectivity in glioblastoma patients is globally weaker but remains generally preserved (Park et al., 2022).
Fig. 2.
Brain-wide power-law exponent change in patients with glioblastoma. A. Averaged voxel-wise obtained in the OASIS subjects and glioblastoma patient dataset. Red hues indicate a relatively steeper slope, i.e., more power at low frequencies; blue hues indicate a relatively flatter slope. OASIS subjects are referred to as Reference. B. fMRI signal power spectra (mean ± standard deviation) were computed for the gray matter cortical (left), gray matter sub-cortical (middle), and white matter (right) for each subject in each group (black = reference dataset; red = patient dataset) and averaged across the subjects. Spectra computed at all three regions were significantly different between the two groups. ** (p < 0.0001 based on Wilcoxon rank-sum test between computed spectral slopes ()). C. Averaged across 5 subcortical regions (amygdala, hippocampus, thalamus, dorsal, and ventral striatum) and 8 cortical regions in the reference and glioblastoma patient dataset. Gray matter cortical and subcortical regions defined in (Xue et al., 2014). Abbreviations: amygdala (Amyg), hippocampus (Hp), thalamus (Thal), dorsal striatum (DS), ventral striatum (VS), cingulo-opercular network (CON), salience network (Sal), somatomotor network (SMN), ventral attention network (VAN), dorsal attention network (DAN), default mode network (DMN), fronto-parietal network (FPN), visual network (VIS), gray matter (GM), white matter (WM). ** (p < 0.0001). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.3. fALFF changes parametric in selected spectral bands
Fig. 3 shows whole-brain fALFF in spectral bands subdivided at 0.01 Hz intervals. fALFF evaluated over the entire spectral range between 0.025 Hz and 0.105 Hz was significantly lower in patients compared to the reference cohort in both gray and white matter (Fig. 3A). Fig. 3B shows that this effect was largely driven by the lowest frequencies. Restricting fALFF to frequencies centered around 0.06 Hz yielded no group differences in either gray or white matter (Fig. 3C). In contrast, fALFF evaluated at frequencies > 0.075 Hz was greater in patients in both gray and white matter and this effect was more pronounced in white matter (Fig. 3D). These results suggest that spontaneous BOLD fluctuations in glioblastoma patients include more activity conventionally regarded as “noise” (discussed further below).
Fig. 3.
Frequency-specific fALFF in the reference vs. glioblastoma patient groups. fALFF in selected frequency bands was averaged across subjects in each group. The shaded boxes indicate spectral bands contributing to the fALFF measure. A. fALFF topography computed within the 0.025 – 0.105 Hz band. B. Group-averaged fALFF topography in selected bands in gray vs. white matter. Abbreviations: reference dataset (REF), patient dataset (PAT). ** indicates a significant group difference (p < 0.0001).
3.4. Changes in the spectral content are associated with the MGMT promoter methylation status
Previous work has demonstrated a close association between epigenetic markers and tumor progression (Dong and Cui, 2019, Etchegaray and Mostoslavsky, 2016). To examine such associations in our data, we evaluated in patients categorized according to the methylation status of MGMT as well as three other somatic mutations commonly seen in glioblastoma, (EGFR, PTEN, and TERT; Table 1). differed significantly only in relation to MGMT mutation status. Positive methylation (N = 63 vs. non-methylated, N = 113) status was associated with flatter spectra (decrease in (p = 0.017, two-sample t-test), Fig. F5). To further investigate this difference, we carried out analyses at the voxel level, contrasting patients according to MGMT mutational status. Averaged voxel-wise was subtly smaller in patients with methylated MGMT (Fig. 4A). Moreover, the average power spectrum was generally flatter in the methylated group (Fig. 4B). Spectral band analyses parallel to those in Fig. 3 showed that patients with methylated MGMT exhibited decreased fALFF at lower-frequencies and increased fALFF at higher-frequencies (Fig. 4C). The direction of these MGMT-dependent findings suggests that methylation exacerbates glioblastoma-associated spectral changes. This effect is potentially anomalous as methylation is associated with increased survival in treated patients.
Fig. 4.
Higher frequency fALFF is associated with MGMT promoter methylation. A. Averaged voxel-wise power-law exponent obtained in the unmethylated and methylated MGMT groups. B. fMRI signal power spectra were averaged across the subjects in each group (mean ± standard error of mean). Frequency-specific fALFF maps are divided based on where the two groups cross over at the corresponding frequency bands. C. Lower- and higher-fALFF maps of glioblastoma patients based on the tumor’s MGMT promoter methylation status. Warm hues, compared to cool hues, indicate an increased contribution of the frequency band to the power spectra. (** p < 0.005).
3.5. Associations of power-law exponent with comorbid medical conditions
Brain function is not independent of other organs and systems in the body. Accordingly, we hypothesized that clinical comorbidities would also be associated with spectral changes in spontaneous BOLD fluctuations. To test this hypothesis, we ranked patients according to averaged over cortical gray matter. Fig. 5 shows results comparing patients in the quintile with flattest spectra vs. the quintile with steepest spectra (N = 38 in each group). Comorbid conditions were more prevalent in patients with the flattest spectra, i.e., smaller (Fig. 5A). Using Fisher’s exact test, we found that seizure was significantly associated with power-law exponent (p < 0.05). We also observed a statistically significant negative correlation between the number of comorbid conditions and cortical gray matter (Fig. 5B, p < 0.005). This result suggests that clinical comorbidities (other than tumor) are associated with flattened resting state BOLD spectra in a manner similar to glioblastoma.
Fig. 5.
change is associated with comorbid medical conditions. A. Patients were ranked based on their averaged power-law exponent in the cortical gray matter voxels. Next, the top 20% of patients with smaller and greater were selected. In each group, we computed the percentage of patients with the corresponding comorbid conditions shown on the left of the bar. There was generally a greater percentage of patients with smaller (white–gray bars) with comorbid conditions than those with greater (pink-red bars). Seizure was significantly associated with the power-law exponent (** p < 0.05), as determined by Fisher’s exact test. B. For each patient, we compared their averaged gray-matter cortical to their number of comorbidities shown on panel A. There was a significant negative correlation between the number of comorbidities patients presented and their averaged (p < 0.005). Abbreviations: deep vein thrombosis (DVT), pulmonary embolism (PE), chronic kidney disease (CKD), hyperlipidemia (HLD), hypertension (HTN), presented with at the time of evaluation (Pw), history (Hx). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.6. Frequency-specific fALFF in the gray matter is associated with prognosis
Significant differences in the spectral properties of the patient vs. reference cohorts raise the possibility that these effects are biomarkers of the brain’s functional status and, hence, prognosis. Our findings thus far show that low frequency fALFF is decreased while higher frequency fALFF is increased in glioblastoma patients. Similar findings were observed in association with clinical comorbidities in the patient group. Based on these observations, we hypothesized that the spectral properties of spontaneous BOLD fluctuations carry prognostic significance. To examine this hypothesis, we divided the patients into two subgroups based on overall survival of greater than vs. <14 months (median survival of our dataset; N = 95 vs. 94). Power spectra averaged over the cortical gray matter were evaluated in each group (Fig. 6A). Fig. 6B shows significant fALFF differences in the two groups broken down by spectral band (two-sided Wilcoxon rank-sum test, p < 0.0001). The plotted quantity is () / (). Fig. 6C shows voxel-wise fALFF in the two groups evaluated over three spectral bands. In parallel, we computed Kaplan-Meier survival curves based on cortical gray matter fALFF evaluated in the same spectral bands. Patients were divided into two subgroups based on their respective cortical gray matter fALFF values for this analysis. Significant differences in median survival were found for the lowest (0.025–0.045 Hz) and highest (0.085–0.105 Hz) frequency bands (first and third panels in Fig. 6D; log-rank test, p = 0.038 and p = 0.017, respectively). Univariate and multivariate Cox regression results are shown in Table 2. Variables that were significant (p < 0.05) in the univariate analysis were included in the multivariate analysis. Frequency-specific fALFF was a significant prognostic factor (HR = 1.45 and 1.49, respectively) even after accounting for age, tumor volume, MGMT methylation status, KPS, and surgical status.
Fig. 6.
fALFF at the lowest and highest frequency bands is associated with prognosis. A. Patients were median split into two groups based on their overall survival (OS), and fMRI signal power spectra were averaged across the subjects in each group (mean ± standard error of mean). Patients with greater OS had greater gray matter fALFF in the lowest frequency band (0.025–0.45 Hz), comparable in the middle frequency band (0.045–0.085 Hz), and a smaller fALFF in the highest frequency band (0.085–0.105 Hz). B. fALFF maps in each frequency band (0.025–0.045; 0.045–0.085; 0.085–0.105 Hz) were averaged in each group, and the voxel-wise % difference between the two maps () were computed. C. Averaged fALFF maps in three frequency bands in each group based on their OS. Warm hues, compared to cool hues, indicate an increased relative power in the corresponding frequency band. D. Kaplan-Meier survival analysis for comparing OS of glioblastoma patients with low and high fALFF (based on C).
Table 2.
Univariate and multivariate survival analysis.
| Characteristic |
Univariate Cox |
Multivariate Cox |
||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age at initial dx | 1.03 (1.01, 1.04) | <1e-3 | 1.03 (1.01, 1.04) | <1e-3 |
| CE volume (cm3) | 1.01 (1.00, 1.01) | 0.003 | 1.01 (1.00, 1.01) | <1e-3 |
| Sex | 1.16 (0.86, 1.56) | 0.327 | ||
| MGMT methylation | 0.68 (0.51, 0.92) | 0.013 | 0.38 (0.27, 0.53) | <1e-7 |
| KPS (>70) | 0.33 (0.23, 0.49) | <1e-7 | 0.45 (0.29, 0.69) | <1e-3 |
| Surgical Status (GTR/NTR/STR vs. LITT/Biopsy/None) | 0.52 (0.38, 0.72) | <1e-4 | 0.49 (0.35, 0.69) | <1e-4 |
| Cortical gray matter fALFF (0.025–0.045 Hz) = High | 1.36 (1.01, 1.81) | 0.038 | 1.45 (1.01, 1.94) | 0.014 |
| Cortical gray matter fALFF (0.085–0.105 Hz) = Low | 1.43 (1.06, 1.91) | 0.017 | 1.49 (1.11, 2.01) | 0.009 |
4. Discussion
Our principal finding is that glial tumors induce widely distributed changes in the spectral content of spontaneous BOLD fMRI fluctuations. These changes manifest as flatter power spectra (lower value of the power-law exponent, ) and reduced gray matter fALFF relative to a reference cohort. Importantly, similar spectral findings were observed in association with clinical comorbidities, especially seizures, as well as MGMT promoter methylation status. Moreover, flattened spectra, relatively depressed lower-band fALFF and elevated higher-band fALFF were associated with shortened survival, i.e., unfavorable prognosis. As far as we are aware, this is the first study to focus on the spectral properties of whole-brain spontaneous BOLD fMRI fluctuations in glioblastoma patients and to evaluate spectral properties in relation to clinical comorbidities, genetic markers, and prognosis.
Our findings are consistent with the perspective that glioblastoma is a brain-wide disease. Previous studies have demonstrated that tumor cells often infiltrate the entire brain and brainstem (Sahm et al., 2012, Drumm et al., 2020). Cerebrospinal fluid metabolomics differ between tumor patients and healthy controls (Locasale et al., 2012, Nakamizo et al., 2013, Ballester et al., 2018, Kalinina et al., 2016). The global nature of rs-fMRI abnormalities in glioblastoma patients is also reflected in the resting state fMRI literature. Multiple studies have reported altered functional connectivity (FC) extending beyond the anatomical limits of the tumor (Daniel et al., 2021, Park et al., 2022, Ghinda et al., 2018, Fox and King, 2018, Stoecklein et al., 2020). FC is used to map the representation of function within the brain and is calculated as the Pearson temporal correlation between pairs of time series extracted from regions of interest, e.g., (Schaefer et al., 2018, Seitzman et al., 2020). Here, we evaluated BOLD fMRI fluctuations using a complementary approach, specifically, voxelwise analysis of resting state data in the temporal frequency domain. We find no evidence of lateralization. Direct comparison of spectral properties in ipsi- vs. contra-lesional hemispheres (excluding the tumor and the corresponding homotopic region) revealed no significant differences (Fig. D in Appendix).
Regarding the significance of power spectrum flattening, it is well established that BOLD fMRI signals of neural origin normally exhibit a “1/f-like” spectral characteristic (He, 2011, Tagliazucchi et al., 2013). Power at faster frequencies is attenuated by the kinetics of neurovascular coupling (Boynton et al., 1996). Thus, in the reference cohort, power at low and high frequencies is concentrated in gray and white matter, respectively (Fig. 3) (Zuo et al., 2010). In other words, spontaneous BOLD fluctuations of neural origin are represented primarily at lower temporal frequencies. In practice, electronic noise and artifact (principally generated by head motion) contaminate BOLD fMRI signals at all frequencies (Liu, 2016). Accordingly, measured power becomes increasingly dominated by variance of non-neural origin at frequencies > ∼0.1 Hz (He, 2011). It is for this reason that functional connectivity studies conventionally limit the analysis to frequencies below ∼ 0.1 Hz (Ciric et al., 2017). These considerations imply that flattening of the BOLD fMRI power spectrum reflects a relative loss of BOLD fluctuations of neural origin, hence, represents a potential biomarker of impaired brain integrity. This perspective is supported by the observation that comorbidities in the present patient sample were associated with smaller , i.e., flatter spectra. Similar considerations explain why attenuation of global FC strength is the most commonly reported finding in rs-fMRI studies of glioblastoma (Fox and King, 2018).
The pathophysiology responsible for flattening BOLD power spectra is as yet uncertain. One possibility is excess glutamate, the principal excitatory neurotransmitter in the brain (Zhou and Danbolt, 2014). Glioma cells release excess glutamate into the CSF and extracellular fluid (Pereira et al., 2017, de Groot and Sontheimer, 2011, Ye and Sontheimer, 1999). Excess glutamate may shift the excitatory/inhibitory (E/I) balance throughout the brain towards hyperexcitability (McCormick and Contreras, 2001). Such a shift in E/I balance would be expected to disrupt neural processes giving rise to normal spontaneous BOLD activity (Deco et al., 2014) and depress power in low frequency end of the power spectrum. [footnote: Conversely, administering midazolam, a GABAA receptor agonist, decreases the global E/I ratio (Kohno et al., 2000, Olkkola et al., 2008, Hong and Rajendram, 2022), enhancing power in low frequency end of the power spectrum (J. Kiviniemi et al., 2005).] Glutamate-induced hyperexcitability may also contribute to the high incidence (30–50%) of seizures in glioblastoma patients (Ruda et al., 2010). In our data, seizures were significantly more prevalent in patients with smaller s (Fig. 5A). An alternative mechanism leading to flatter spectra is a shift of intrinsic time scales toward faster frequencies as a correlate on increased neural excitability. This mechanism occurs as a lateralized finding in temporal lobe epilepsy (Xie et al., 2023). Here, we posit that increased excitability occurs globally in brains with glioblastomas.
Additionally, prior studies have shown that power-law exponent of fMRI signals correlates with a cerebral metabolic rate for glucose (CMRGlu) (He, 2011). Simultaneous fMRI/FDG-PET studies have also demonstrated regionally specific correlations between fALFF and CMRGlu (Nugent et al., 2015, Aiello et al., 2015, Deng et al., 2022). This relationship is particularly relevant to our current work, as it aligns with earlier findings linking increased glioma metabolic activity (measured using FET-PET) to disrupted whole-brain FC (Stoecklein et al., 2020). Accordingly, it is conceivable that alterations in the whole-brain fALFF in the presence of glioblastoma may reflect changes in the underlying metabolic demand of the tumor-bearing brain. Furthermore, BOLD fMRI fluctuations are closely linked to cerebral blood flow (CBF), which may be disrupted by invasive glioma cells that impair astrocyte function (Watkins et al., 2014). Notably, changes in CBF are also associated with E/I balance dysregulation, increased neural excitability, and increased metabolic demand (see above). These glioma-associated mechanisms likely contribute to altered BOLD fMRI fluctuations.
Reduced gray matter fALFF, a principal present finding (Fig. 3A), has been previously related to cognitive performance measures in nominally healthy individuals (Garrett et al., 2013, Grady and Garrett, 2014), as well patients with Alzheimer’s disease (Strain et al., 2022). Other evidence demonstrates a tight link between impaired cognitive status and shortened survival in glioblastoma patients (Johnson and Wefel, 2013). Thus, there appears to exist a triple association between reduced gray matter fALFF (Fig. 3), impaired cognitive status, and shortened survival (Fig. 6) in patients with glioblastoma.
Perhaps the most salient feature in the present results is the prognostic value of spectral changes in glioblastoma patients. We previously reported that functional connectivity changes have prognostic value in glioblastoma patients (Daniel et al., 2021, Daniel et al., 2021). The present finding is distinct, based on spectral as opposed to connectivity analysis, and is an empirical finding about which we cannot be etiologically more specific. However, it is consistent with the observation that a variety of comorbid conditions are associated with flattened BOLD power spectra (Fig. 5).
MGMT methylation status was also associated with flattened power spectra, particularly in white matter and the brain stem (Fig. 4C). According to the present considerations, this should be a negative prognostic indicator, in agreement with previous work showing that epigenetic regulations are associated with tumor progression (Dong and Cui, 2019, Johnson et al., 2015). In practice, however, hypermethylation of the MGMT gene is associated with better prognosis in treated patients as it enhances sensitivity to alkylating agents (Hegi et al., 2005). This presents a seemingly paradoxical relationship between MGMT methylation status, BOLD spectral changes, and prognosis, necessitating further investigation. The key is distinguishing between three different associations: 1) MGMT and fALFF, 2) MGMT and prognosis, 3) fALFF and prognosis. These are distinct phenomena, reflecting different contexts and variables. Spectral flattening is associated with multiple comorbidities (Fig. 5), thus appears to be a non-specific marker of compromised brain integrity. Spectral flattening also is associated with MGMT methylation, which seems anomalous or discrepant as MGMT methylation is a positive prognostic indicator. We suggest a parsimonious resolution of the apparent discrepancy as follows. The key point is that MGMT methylation is associated with improved survival in treated patients as it enhances sensitivity to alkylating agents and radiation therapy. Thus, it is hypothetically possible that MGMT methylation in isolation may actually be a marker compromised brain integrity, as implied by the results shown in Fig. 5 and potentially reduced survival in untreated patients. In principle, this account could be empirically tested. In practice, it cannot be tested as very few glioma patients remain untreated. In our dataset, only 3 of 189 patients did not receive either chemotherapy or radiation therapy.
Fig. F6 (in Appendix) clarifies the relation between MGMT methylation status and fALFF in low vs. high-frequency bands. Regardless of MGMT methylation status, predominance of low frequency power in the spectrum is a positive prognostic indicator. In addition, the survival advantage of MGMT methylation is evident on comparison of the red survival curves in the left panels of the figure. Crucially, all patients represented in the figure have been treated. Potentially, treatments such as radiation therapy and chemotherapy may “normalize” fALFF, implying potential restorative effects on brain physiology that could lead to improved prognosis. Future work is required to better understand changes in BOLD spectral characteristics in diverse contexts.
We mention several limitations and future directions. Our reference subject sample was curated from an external dataset. However, we carefully matched reference subjects to the glioblastoma patients with respect to age, prevalence of head motion, and fMRI echo time (details provided in Appendix A, C and Table C.1.). Hence, it is unlikely that our results are biased by those factors. However, the duration of rs-fMRI runs was only 6 min, which is standard in clinical practice (Van Dijk et al., 2010). Thus, the lowest frequency at which BOLD spectral content could be analyzed was 0.025 Hz (see Section 2.5.). Longer duration runs, e.g., as in (Gordon et al., 2017), would enable extending the analysis to lower frequencies to enhance the estimation of spontaneous BOLD fluctuations of neural origin, thereby providing further insight into the manner in which glioblastoma impacts the brain. With regard to tumor mutation status, we examined MGMT, EGFR, TERT, and PTEN. Other genes subject to epigenetic regulations (Dong and Cui, 2019) may provide further insights into the influence of mutations on the spectral content of resting state BOLD data.
Our findings suggest that frequency domain analysis of spontaneous whole-brain activity provides prognostic information and offers a noninvasive means of studying the tumor-bearing brain. However, as our study population consists of newly diagnosed glioblastoma patients, further research is necessary to determine if our findings also apply to patients with recurrent glioblastoma or at different stages of the disease. Moreover, the generalizability of our findings to real-world clinical practice may depend on factors such as institution-specific MRI protocols, preprocessing pipelines, and different patient characteristics in a larger population. Therefore, our observed associations require further validation in independent cohorts. Finally, we here speculate that glioblastoma alters E/I balance, metabolic demand, and CBF throughout the brain. These hypotheses could be tested in animal models using invasive methodologies (Xue et al., 2014).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Parts of this work were funded by the Intellectual and Developmental Disabilities Research Center at Washington University award Number P50 HD103525.
Funding
Cognitive, Computational, and Systems Neuroscience (CCSN) Fellowship, McDonnell Center for Systems Neuroscience, Washington University School of Medicine (KYP).
National Institutes of Health grant R01CA203861 (KYP, AZS, PHL, JJL, JSS, ECL).
The McDonnell Center for Systems Neuroscience (TX).
National Institutes of Health grant P41EB018783 (ECL).
National Institutes of Health grant U24NS109103 (ECL).
National Institutes of Health grant R01EB026439 (ECL).
Competing interests
KYP, JJL, JSS, PHL, AZS, report the following conflict of interest. Licensing of Intellectual Property: Sora Neuroscience. ECL reports the following conflicts of interest. Stock ownership: Neurolutions, General Sensing, Osteovantage, Pear Therapeutics, Face to Face Biometrics, Immunovalent, Caeli Vascular, Acera, Sora Neuroscience, Inner Cosmos, Kinetrix, NeuroDev. Petal Surgical. Consultant: Monteris Medical, E15, Acera, Alcyone, Intellectual Ventures, Medtronic, Neurolutions, Osteovantage, Pear Therapeutics, Sante Ventures, Microbot. Licensing of Intellectual Property: Neurolutions, Osteovantage, Caeli Vascular, Sora Neuroscience. Washington University owns equity in Neurolutions. The other authors report they have no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103476.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Agnihotri S., Zadeh G. Metabolic reprogramming in glioblastoma: the influence of cancer metabolism on epigenetics and unanswered questions. Neuro Oncol. 2016;18(2):160–172. doi: 10.1093/neuonc/nov125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello M., Salvatore E., Cachia A., Pappatà S., Cavaliere C., Prinster A., Nicolai E., Salvatore M., Baron J.-C., Quarantelli M. Relationship between simultaneously acquired resting-state regional cerebral glucose metabolism and functional MRI: a PET/MR hybrid scanner study. Neuroimage. 2015;113:111–121. doi: 10.1016/j.neuroimage.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Al-Zhoughbi W., Huang J., Paramasivan G.S., Till H., Pichler M., Guertl-Lackner B., Hoefler G. Tumor macroenvironment and metabolism. Semin. Oncol. 2014;41(2):281–295. doi: 10.1053/j.seminoncol.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester L.Y., Lu G., Zorofchian S., Vantaku V., Putluri V., Yan Y., Arevalo O., Zhu P., Riascos R.F., Sreekumar A., Esquenazi Y., Putluri N., Zhu J.-J. Analysis of cerebrospinal fluid metabolites in patients with primary or metastatic central nervous system tumors. Acta Neuropathol. Commun. 2018;6(1) doi: 10.1186/s40478-018-0588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton G.M., Engel S.A., Glover G.H., Heeger D.J. Linear systems analysis of functional magnetic resonance imaging in human V1. J. Neurosci. 1996;16(13):4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E.d., Long C., Suckling J., Fadili J., Calvert G., Zelaya F., Carpenter T.A., Brammer M. Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum. Brain Mapp. 2001;12(2):61–78. doi: 10.1002/1097-0193(200102)12:2<61::AID-HBM1004>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Oxford University Press; 2006. Rhythms of the Brain. [Google Scholar]
- Ciric R., Wolf D.H., Power J.D., Roalf D.R., Baum G.L., Ruparel K., Shinohara R.T., Elliott M.A., Eickhoff S.B., Davatzikos C., Gur R.C., Gur R.E., Bassett D.S., Satterthwaite T.D. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017;154:174–187. doi: 10.1016/j.neuroimage.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed J.H., Gerke T.A., Berglund A.E. MatSurv: survival analysis and visualization in MATLAB. J. Open Source Software. 2020;5(46):1830. [Google Scholar]
- Daniel A.G.S., Park K.Y., Roland J.L., Dierker D., Gross J., Humphries J.B., Hacker C.D., Snyder A.Z., Shimony J.S., Leuthardt E.C. Functional connectivity within glioblastoma impacts overall survival. Neuro Oncol. 2021;23(3):412–421. doi: 10.1093/neuonc/noaa189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel A.G.S., Hacker C.D., Lee J.J., Dierker D., Humphries J.B., Shimony J.S., Leuthardt E.C. Homotopic functional connectivity disruptions in glioma patients are associated with tumor malignancy and overall survival. Neurooncol. Adv. 2021;3(1) doi: 10.1093/noajnl/vdab176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot J., Sontheimer H. Glutamate and the biology of gliomas. Glia. 2011;59(8):1181–1189. doi: 10.1002/glia.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G., Ponce-Alvarez A., Hagmann P., Romani G.L., Mantini D., Corbetta M. How local excitation-inhibition ratio impacts the whole brain dynamics. J. Neurosci. 2014;34(23):7886–7898. doi: 10.1523/JNEUROSCI.5068-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S., Franklin C.G., O'Boyle M., et al. Hemodynamic and metabolic correspondence of resting-state voxel-based physiological metrics in healthy adults. Neuroimage. 2022;250 doi: 10.1016/j.neuroimage.2022.118923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z., Cui H. Epigenetic modulation of metabolism in glioblastoma. Semin. Cancer Biol. 2019;57:45–51. doi: 10.1016/j.semcancer.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Drumm M.R., Dixit K.S., Grimm S., Kumthekar P., Lukas R.V., Raizer J.J., Stupp R., Chheda M.G., Kam K.-L., McCord M., Sachdev S., Kruser T., Steffens A., Javier R., McCortney K., Horbinski C. Extensive brainstem infiltration, not mass effect, is a common feature of end-stage cerebral glioblastomas. Neuro Oncol. 2020;22(4):470–479. doi: 10.1093/neuonc/noz216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchegaray J.-P., Mostoslavsky R. Interplay between metabolism and epigenetics: a nuclear adaptation to environmental changes. Mol. Cell. 2016;62(5):695–711. doi: 10.1016/j.molcel.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.E., King T.Z. Functional connectivity in adult brain tumor patients: a systematic review. Brain Connect. 2018;8(7):381–397. doi: 10.1089/brain.2018.0623. [DOI] [PubMed] [Google Scholar]
- Freeman W.J., Zhai J. Simulated power spectral density (PSD) of background electrocorticogram (ECoG) Cogn. Neurodyn. 2009;3(1):97–103. doi: 10.1007/s11571-008-9064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett D.D., Kovacevic N., McIntosh A.R., Grady C.L. The modulation of BOLD variability between cognitive states varies by age and processing speed. Cereb Cortex. 2013;23(3):684–693. doi: 10.1093/cercor/bhs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinda D.C., Wu J.S., Duncan N.W., Northoff G. How much is enough-Can resting state fMRI provide a demarcation for neurosurgical resection in glioma? Neurosci. Biobehav. Rev. 2018;84:245–261. doi: 10.1016/j.neubiorev.2017.11.019. [DOI] [PubMed] [Google Scholar]
- Gordon E.M., Laumann T.O., Gilmore A.W., Newbold D.J., Greene D.J., Berg J.J., Ortega M., Hoyt-Drazen C., Gratton C., Sun H., Hampton J.M., Coalson R.S., Nguyen A.L., McDermott K.B., Shimony J.S., Snyder A.Z., Schlaggar B.L., Petersen S.E., Nelson S.M., Dosenbach N.U.F. Precision functional mapping of individual human brains. Neuron. 2017;95(4):791–807.e7. doi: 10.1016/j.neuron.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C.L., Garrett D.D. Understanding variability in the BOLD signal and why it matters for aging. Brain Imaging Behav. 2014;8(2):274–283. doi: 10.1007/s11682-013-9253-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J. Scale-free properties of the functional magnetic resonance imaging signal during rest and task. J. Neurosci. 2011;31(39):13786–13795. doi: 10.1523/JNEUROSCI.2111-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J. Scale-free brain activity: past, present, and future. Trends Cogn. Sci. 2014;18(9):480–487. doi: 10.1016/j.tics.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.J., Zempel J.M., Snyder A.Z., Raichle M.E. The temporal structures and functional significance of scale-free brain activity. Neuron. 2010;66(3):353–369. doi: 10.1016/j.neuron.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegi M.E., Diserens A.-C., Gorlia T., Hamou M.-F., de Tribolet N., Weller M., Kros J.M., Hainfellner J.A., Mason W., Mariani L., Bromberg J.E.C., Hau P., Mirimanoff R.O., Cairncross J.G., Janzer R.C., Stupp R. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- Hong J.C. In: Treatments, Mechanisms, and Adverse Reactions of Anesthetics and Analgesics. Rajendram R., editor. Academic Press; 2022. Chapter 14 – midazolam: mechanism and perioperative applications; pp. 131–138. [Google Scholar]
- Johnson C., Warmoes M.O., Shen X., Locasale J.W. Epigenetics and cancer metabolism. Cancer Lett. 2015;356(2, Part A):309–314. doi: 10.1016/j.canlet.2013.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.R., Wefel J.S. Relationship between cognitive function and prognosis in glioblastoma. CNS Oncol. 2013;2(2):195–201. doi: 10.2217/cns.13.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinina, J., J. Ahn, N.S. Devi, et al., Selective detection of the d-enantiomer of 2-hydroxyglutarate in the csf of glioma patients with mutated isocitrate dehydrogenase. Clin. Cancer Res., 2016. 22(24): p. 6256–6265. [DOI] [PMC free article] [PubMed]
- Kiviniemi V.J., Haanpää H., Kantola J.-H., Jauhiainen J., Vainionpää V., Alahuhta S., Tervonen O. Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Magn. Reson. Imaging. 2005;23(4):531–537. doi: 10.1016/j.mri.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Kohno T., Kumamoto E., Baba H., Ataka T., Okamoto M., Shimoji K., Yoshimura M. Actions of midazolam on GABAergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Anesthesiology. 2000;92(2):507. doi: 10.1097/00000542-200002000-00034. [DOI] [PubMed] [Google Scholar]
- LaMontagne, P.J., T.L.S. Benzinger, J.C. Morris, et al., OASIS-3: Longitudinal Neuroimaging, Clinical, and Cognitive Dataset for Normal Aging and Alzheimer Disease. medRxiv, 2019.
- Liu T.T. Noise contributions to the fMRI signal: an overview. Neuroimage. 2016;143:141–151. doi: 10.1016/j.neuroimage.2016.09.008. [DOI] [PubMed] [Google Scholar]
- Locasale, J.W., T. Melman, S. Song, et al., Metabolomics of human cerebrospinal fluid identifies signatures of malignant glioma. Mol. Cell. Proteomics: MCP, 2012. 11(6): p. M111.014688. [DOI] [PMC free article] [PubMed]
- Lowen S.B., Cash S.S., Poo M.-M., Teich M.C. Quantal neurotransmitter secretion rate exhibits fractal behavior. J. Neurosci. 1997;17(15):5666–5677. doi: 10.1523/JNEUROSCI.17-15-05666.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- McCormick D.A., Contreras D. On the cellular and network bases of epileptic seizures. Annu. Rev. Physiol. 2001;63(1):815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- Monto S., Palva S., Voipio J., Palva J.M. Very slow EEG fluctuations predict the dynamics of stimulus detection and oscillation amplitudes in humans. J. Neurosci. 2008;28(33):8268–8272. doi: 10.1523/JNEUROSCI.1910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamizo S., Sasayama T., Shinohara M., Irino Y., Nishiumi S., Nishihara M., Tanaka H., Tanaka K., Mizukawa K., Itoh T., Taniguchi M., Hosoda K., Yoshida M., Kohmura E. GC/MS-based metabolomic analysis of cerebrospinal fluid (CSF) from glioma patients. J. Neurooncol. 2013;113(1):65–74. doi: 10.1007/s11060-013-1090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent A.C., Martinez A., D'Alfonso A., Zarate C.A., Theodore W.H. The relationship between glucose metabolism, resting-state fMRI BOLD signal, and GABAA-binding potential: a preliminary study in healthy subjects and those with temporal lobe epilepsy. J. Cereb. Blood Flow Metab. 2015;35(4):583–591. doi: 10.1038/jcbfm.2014.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkola K.T., Ahonen J. In: Modern Anesthetics. Schüttler J., Schwilden H., editors. Springer; Berlin, Heidelberg: 2008. Midazolam and other benzodiazepines; pp. 335–360. [Google Scholar]
- Park, K.Y., J.S. Shimony, S. Chakrabarty, et al., Optimal Atlas Registration and Resting State Functional Architecture in Patients with Glioblastoma. 2022.
- Pereira M.S.L., Klamt F., Thomé C.C., Worm P.V., de Oliveira D.L. Metabotropic glutamate receptors as a new therapeutic target for malignant gliomas. Oncotarget. 2017;8(13):22279–22298. doi: 10.18632/oncotarget.15299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruda R., Trevisan E., Soffietti R. Epilepsy and brain tumors. Curr Opin Oncol. 2010;22(6):611–620. doi: 10.1097/CCO.0b013e32833de99d. [DOI] [PubMed] [Google Scholar]
- Sahm F., Capper D., Jeibmann A., et al. Addressing diffuse glioma as a systemic brain disease with single-cell analysis. Arch. Neurol. 2012;69(4):523–526. doi: 10.1001/archneurol.2011.2910. [DOI] [PubMed] [Google Scholar]
- Schaefer A., Kong R.u., Gordon E.M., Laumann T.O., Zuo X.-N., Holmes A.J., Eickhoff S.B., Yeo B.T.T. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 2018;28(9):3095–3114. doi: 10.1093/cercor/bhx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitzman B.A., Gratton C., Marek S., et al. A set of functionally-defined brain regions with improved representation of the subcortex and cerebellum. Neuroimage. 2020;206 doi: 10.1016/j.neuroimage.2019.116290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G.L., Pope D.L.W., Astafiev S.V., McAvoy M.P., Snyder A.Z., Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J. Neurosci. 2010;30(10):3640–3651. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoecklein V.M., Stoecklein S., Galiè F., Ren J., Schmutzer M., Unterrainer M., Albert N.L., Kreth F.-W., Thon N., Liebig T., Ertl-Wagner B., Tonn J.-C., Liu H. Resting-state fMRI detects alterations in whole brain connectivity related to tumor biology in glioma patients. Neuro Oncol. 2020;22(9):1388–1398. doi: 10.1093/neuonc/noaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain J.F., Brier M.R., Tanenbaum A., et al. Covariance-based vs. correlation-based functional connectivity dissociates healthy aging from Alzheimer disease. Neuroimage. 2022;261 doi: 10.1016/j.neuroimage.2022.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E., von Wegner F., Morzelewski A., Brodbeck V., Jahnke K., Laufs H. Breakdown of long-range temporal dependence in default mode and attention networks during deep sleep. Proc. Natl. Acad. Sci. U.S.A. 2013;110(38):15419–15424. doi: 10.1073/pnas.1312848110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Hedden T., Venkataraman A., et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Ward, L.M., Dynamical Cognitive Science. 2002: MIT Press.
- Watkins S., Robel S., Kimbrough I.F., et al. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun. 2014;5:4196. doi: 10.1038/ncomms5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S.M., Cheresh D.A. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat. Med. 2011;17(11):1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- Xie K., Royer J., Lariviere S., et al. Atypical intrinsic neural timescales in temporal lobe epilepsy. Epielpsia. 2023;64(4):998–1011. doi: 10.1111/epi.17541. [DOI] [PubMed] [Google Scholar]
- Xue M., Atallah B.V., Scanziani M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature. 2014;511(7511):596–600. doi: 10.1038/nature13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z.C., Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59(17):4383–4391. [PubMed] [Google Scholar]
- Zhou Y., Danbolt N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transm. 2014;121(8):799–817. doi: 10.1007/s00702-014-1180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q.-H., Zhu C.-Z., Yang Y., Zuo X.-N., Long X.-Y., Cao Q.-J., Wang Y.-F., Zang Y.-F. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods. 2008;172(1):137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.-N., Di Martino A., Kelly C., Shehzad Z.E., Gee D.G., Klein D.F., Castellanos F.X., Biswal B.B., Milham M.P. The oscillating brain: complex and reliable. Neuroimage. 2010;49(2):1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.