Abstract
Defensins are widely distributed and broad-spectrum antimicrobial peptides with activities against bacteria, fungi, and enveloped viruses. Defensins have been isolated from granules of neutrophils from humans, rabbits, rats, and guinea pigs. They have also been found in lung macrophages as well as in Paneth cells of the human, rabbit, and mouse small intestine. The human β-defensin-2 was recently isolated from human skin. In this study, we detected the expression of mRNA for the defensin cryptdin in BALB/c mouse skin by means of reverse transcriptase PCR amplification. Expression was also detected in dispase-separated epidermis and cultured keratinocytes, but expression was not detected in fibroblasts. The expression of cryptdin mRNA was found to begin on embryonic day 17.5. As determined with specific primers, the cDNA sequence cloned from the skin was found to be identical to that previously reported for cryptdin-5. cDNA derived from cultured keratinocytes demonstrated the sequences of the cryptdin-6 and cryptdin-1 isoforms. In situ hybridization analysis showed that the mRNA of cryptdin was expressed in the suprabasal keratinocytes of the skin in embryonic and neonatal days and then shifted to the hair bulbs in the skin of adult mice.
Antimicrobial polypeptides are widely distributed within animal tissues and cells that frequently encounter microorganisms (4). Some antimicrobial peptides are stabilized by disulfide bonds, which may increase their resistance to proteolysis or loss of conformation. Antimicrobial peptides that contain six cysteines have been classified as defensins (7). They are cationic, 3- to 4-kDa peptides characterized by nine highly conserved amino acids, including six invariant cysteine residues in a unique disulfide motif (21). They are divided into the α-defensins, the β-defensins, and the insect defensins. The three defensin groups differ from each other in the spacing and connectivity of their six cysteine residues (7). These peptides exhibit broad-range activities against gram-negative and gram-positive bacteria, many fungi, and some enveloped viruses (11, 13, 17), including the human immunodeficiency virus (16).
Mammalian defensins were first identified in phagocytic leukocytes from humans, rabbits, guinea pigs, and rats (12). Subsequently, they were found in certain epithelial cells (3) and human and murine Paneth cells of the small intestine (10, 18). The defensin cryptdin is a Paneth cell corticostatin and defensin found in the mouse small bowel. The mRNA of cryptdin is one of many highly abundant mRNAs of low molecular weight that appear during postnatal intestinal development (18).
Some antimicrobial peptides have been isolated from animal and human skin, e.g., LL-37 (5), a class of peptides known as magainins (24), and dermaseptin (15). Recently, β-defensin-2 was isolated from human skin (9). However, defensins have not yet been reported in the skin of rodents. In this study, we detected mRNA expression of the defensin cryptdin in the skin of BALB/c mice using reverse transcriptase (RT) PCR (RT-PCR) amplification and subsequent sequence analysis. We also examined the developmental expression pattern of cryptdin mRNA in the embryonic mouse skin and performed in situ hybridization analysis to localize cryptdin.
MATERIALS AND METHODS
Preparation of tissues and cell cultures.
The strain of BALB/c mice used in this study was purchased from the mouse colony of Okayama University. Skin samples were taken from adult (6-week-old) female mice, neonatal mice, and mouse embryos on embryonic days 15.5 (E15.5 mice) and 17.5 (E17.5 mice). The intestines of adult mice were used as positive controls. For in situ hybridization, the skin samples from E17.5, neonatal, and adult mice and the intestines of adult mice were fixed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline at 4°C for 5 h. The fixed tissues were dehydrated through a series of ethanol washes, immersed in xylene, and embedded in paraffin wax.
Primary cultures of keratinocytes and fibroblasts were obtained from the back skin of neonatal mice. Skin samples were minced and incubated in phosphate-buffered saline containing 1000 pU of dispase (Goudou Shusei, Tokyo, Japan) per ml at 4°C for 24 h. The specimen was separated into the epidermis and the dermis, and the epidermis was trypsinized (0.25% trypsin, 0.02% EDTA) at 37°C for 20 min. Keratinocytes were cultured in Keratinocyte Basal Medium (Biowhittaker, Walkersville, Md.) containing 30 μg of bovine pituitary extract per ml, 0.1 ng of epidermal growth factor per ml, 0.25 μg of hydrocortisone per μl, 5 ng of insulin per ml, 50 μg of gentamicin per ml, and 50 ng of amphotericin B per ml. Fibroblasts were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (GIBCO, Grand Island, N.Y.). Cells were maintained at 37°C in a humidified atmosphere of 5% CO2. Keratinocytes that were passaged three times and fibroblasts that were passaged twice were used.
RT-PCR.
Total RNA from tissue samples and cultured cells was extracted with the TRIzol Reagent (Life Technologies, Gaithersburg, Md.), according to the manufacturer’s instructions. RNA was reverse transcribed with Ready to go You prime First-Strand Beads (Pharmacia Biotech, Grand Island, N.Y.), according to the manufacturer’s instructions. Briefly, total RNA (5 μg) from each sample was reverse transcribed with 5 μg of oligo(dT) primer.
Amplification by PCR was conducted in a 30-μl reaction volume containing 10× PCR Buffer II, 0.5 μM each primer, 1.7 mM MgCl2 solution, each deoxynucleoside triphosphate at a concentration of 0.2 mM, and 1.5 U of Taq polymerase (AmpliTaq Gold; Perkin-Elmer Corporation, Foster City, Calif.). One microliter of each cDNA mixture was used as a template. The PCR mixture was overlaid with mineral oil. Amplification of cryptdin was conducted in a thermocycler (PC-700; ASTEC, Fukuoka, Japan) with the following profiles: 95°C for 9 min (1 cycle) and 94°C for 1 min, 60°C for 1 min, and 72°C for 2 min (35 cycles). Amplification of β-actin was conducted with the following profiles: 95°C for 9 min (1 cycle) and 94°C for 1 min, 50°C for 1 min, and 72°C for 2 min (35 cycles). The products of PCR were analyzed by gel electrophoresis on 2% agarose gels. Amplification by PCR of cryptdin from cDNA was accomplished with primers Defcrp130 (AAGAGACTAAAACTGAGGAGCAGC) and Defcrm380 (GGTGATCATCAGACCCCAGCATCAGT) (2). The primer Defcrp130 corresponds to nucleotides 80 to 103 in cryptdin-1 cDNA. It is an exact match with cryptdin-1 and -4 cDNAs and has a single mismatch at primer nucleotide 11 (A→T) with the cryptdin-5 mRNA sequence. The primer Defcrm380 hybridizes to the sequence 3′ of the termination codon on the sense strand and primes all known cryptdin mRNAs. Amplification by PCR of mouse β-actin from cDNA included a commercial amplimer set (CLONTECH Laboratories, Palo Alto, Calif.) both to confirm the integrity of the cDNA transcripts and to check for the presence of contaminating genomic DNA.
Clone construction and selection.
Cryptdin PCR products were ligated directly into PCR-Script Amp SK(+) cloning vectors with a PCR-Script Amp SK(+) cloning kit (Stratagene, La Jolla, Calif.) according to the manufacturer’s instructions. Constructs were transformed into Escherichia coli XL1-Blue MRF′ Kan supercompetent cells (Stratagene). Transformants were selected on laked blood agar containing ampicillin (50 μg/ml).
DNA sequencing.
Double-stranded plasmid DNA bearing an appropriate insert was isolated for further use as a sequencing template. Cycle sequencing was accomplished with an ABI Prism Dye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, Warrington, Great Britain), in accordance with the manufacturer’s instructions. M13 universal and reverse primers were used for sequencing. Sequence homologies were determined by using the BLAST server of the National Center for Biotechnology Information.
In situ hybridization. (i) Preparation of DIG-labeled RNA probes.
Digoxigenin (DIG)-11-UTP-labeled single-stranded RNA probes were prepared with the DIG RNA Labeling Kit (Boehringer Mannheim, Mannheim, Germany), according to the manufacturer’s instructions. Briefly, plasmid DNA bearing cryptdin was linearized with appropriate restriction enzymes, and DIG-labeled sense and antisense probes were obtained by in vitro transcription with T3 and T7 RNA polymerases (Stratagene) together with 10× transcription buffer, a DIG-RNA labeling mixture, and an excess of RNase inhibitor (Stratagene). After digestion of the original linearized template cDNA with DNase and several ethanol precipitation steps, the probes were dissolved in water and used for hybridization.
(ii) Hybridization procedure.
In situ hybridization was performed by the methods previously reported by Senboshi et al. (23). Deparaffinized sections were treated with 1 μg of proteinase K (Boehringer Mannheim) per ml, acetylated in acetic anhydride solution, and then dehydrated. Hybridization with freshly denatured sense or antisense RNA probes was performed in humidified chambers at 50°C for 15 h. Sections were washed after hybridization at 50°C under highly stringent conditions. Prior to immunodetection of the in situ hybridization signal, the sections were incubated in blocking solution (DIG Nucleic Acid Detection Kit; Boehringer Mannheim). Incubation with polyclonal sheep anti-DIG Fab fragments conjugated to alkaline phosphatase (Boehringer Mannheim) was performed for 30 min in humidified chambers at room temperature. The sections were stained by incubation in nitroblue tetrazolium and β-chloroindolyl phosphate solution (Boehringer Mannheim) in darkness at room temperature. The time required for color development was dependent on the probes used.
RESULTS
PCR amplification of cryptdin.
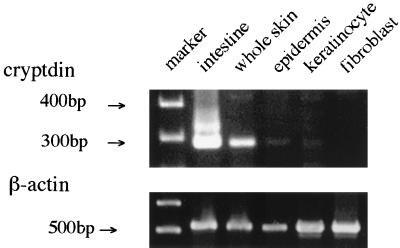
The amplification products of murine small intestine, whole skin, epidermis, and keratinocyte demonstrated the same single band of 272 bp (Fig. 1). However, the cDNA produced from cultured fibroblasts did not yield the band, indicating that mesenchymal cells such as fibroblasts may not be involved in cryptdin mRNA production. An amplification product of 500 bp was also synthesized from all cDNA templates with the β-actin primer set (Fig. 1).
FIG. 1.
PCR amplification of cryptdin. RNA samples isolated from whole skin, dispase-separated epidermis, cultured keratinocytes, and cultured fibroblasts were reverse transcribed and amplified with the cryptdin primer set and the β-actin primer set as described in Materials and Methods. PCR products were analyzed by electrophoresis.
Cryptdin gene expression in skin development.
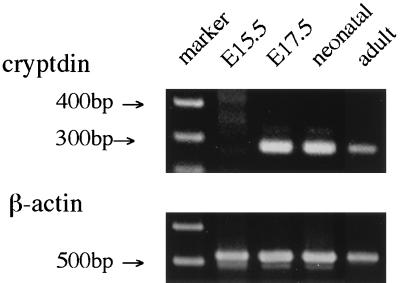
The band corresponding to β-actin was detected from all samples by PCR amplification with specific primers. Cryptdin gene expression was detected in the skin of E17.5, neonatal, and adult mice (Fig. 2). Each of these samples produced a clear, single, 272-bp band. Those bands from samples from E17.5 and neonatal mice were more intense than those from samples from adult mice. No band was detected in the skin of E15.5 mice.
FIG. 2.
Cryptdin gene expression in skin development. RNA samples extracted from the skin of E15.5, E17.5, neonatal, and adult mice were reverse transcribed and amplified with the cryptdin primer set and the β-actin primer set as described in Materials and Methods. PCR products were analyzed by electrophoresis.
Sequence analysis.
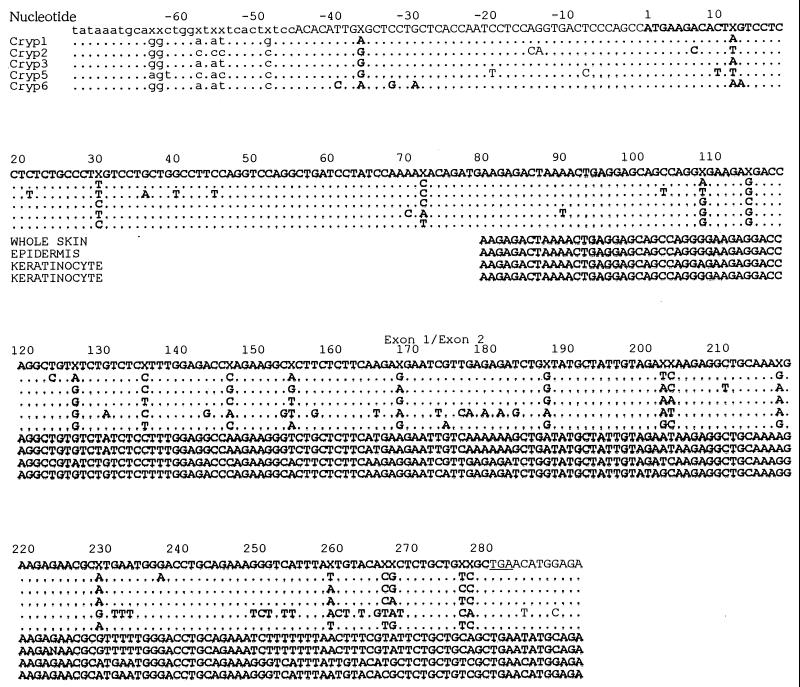
The primer pair used amplifies approximately half of exon 1 (nucleotides 80 to 172) and all of exon 2 of cryptdin cDNA. We performed sequence analysis in these regions of cryptdin cDNA with PCR-Script Amp SK(+)-cloned PCR products obtained from samples from the small intestine, whole skin, epidermis, and keratinocytes (Fig. 3). The sequence of cDNA derived from whole skin demonstrated nearly perfect identity with the previously reported cryptdin-5 sequence except for a single mismatch at cryptdin nucleotide 90 (T→A; primer nucleotide 11). The DNA sequence amplified from the epidermis was also aligned with the sequence of cryptdin-5. The cDNA sequences amplified from cultured keratinocytes perfectly corresponded to exon 1 of cryptdin-6 and showed mismatches only at cryptdin nucleotides 183 (T→G) and 200 (G→T). The transition at position 183 resulted in a missense mutation that changed a tyrosine codon to one for aspartic acid, and the transition at position 200 resulted in a missense mutation that changed an arginine codon to one for isoleucine. Another clone derived from keratinocytes demonstrated the sequence of a cryptdin-1-like peptide, cryptdin-11. Exon 1 of the clone correlated with that of cryptdin-1 completely, and only one substitution was found at nucleotide 237 (A→G) in codon 79, resulting in a silent mutation.
FIG. 3.
Murine cDNA sequences obtained from whole skin, dispase-separated epidermis, and cultured keratinocytes were compared with established cDNA sequences from the consensus cryptdin gene and the cryptdin-1, -2, -3, -5, and -6 isoforms. Nucleotides are numbered from the first residue of the initiating methionine codon. An X in the consensus sequence indicates a position at which at least two sequences contain nucleotide substitutions. Coding sequences are indicated with uppercase letters, while untranslated sequences are indicated with lowercase letters. The junction between exon 1 and exon 2 is also indicated.
Developmental appearance of cryptdin mRNA in murine skin.
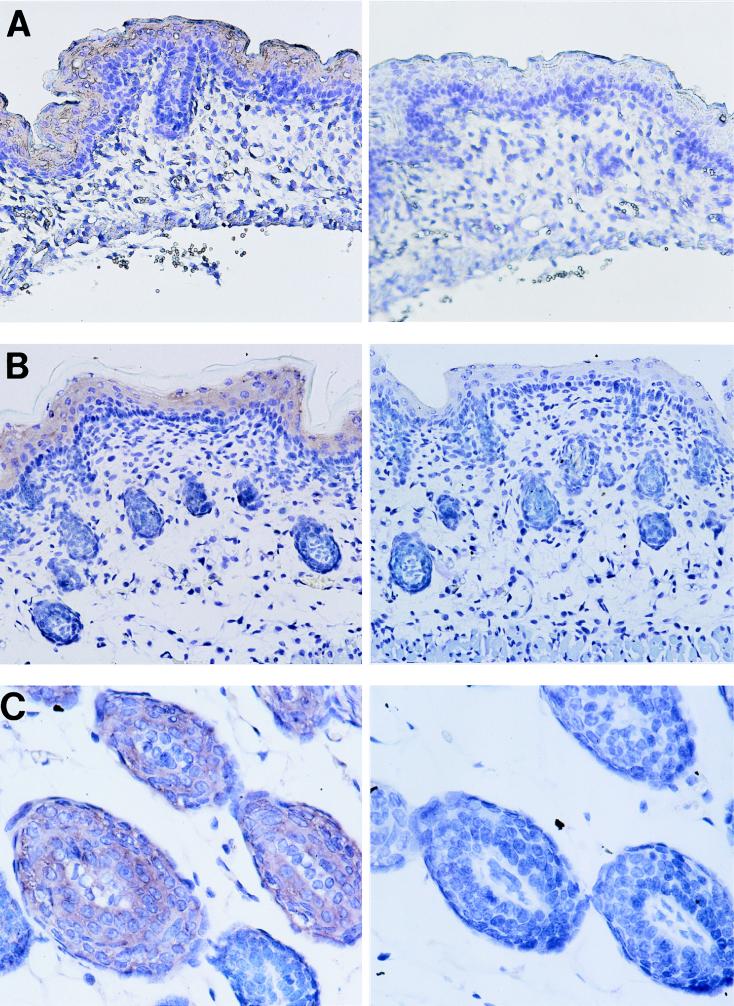
The expression of cryptdin mRNA was clearly detected in suprabasal keratinocytes of the epidermis of the skin of E17.5 and neonatal mice when a DIG-labeled antisense cRNA probe was used (left-hand panels of Fig. 4A and B, respectively). No signal was detected in the dermis. In the skin of adult mice, the epidermis did not have a detectable signal for cryptdin, but a signal was localized to keratinocytes of the hair bulbs (left-hand panel of Fig. 4C). No signal was observed in the skin when the sense probe was used (Fig. 4A, B, and C, right-hand panels).
FIG. 4.
Developmental appearance of cryptdin mRNA in murine skin. The expression of cryptdin mRNA was determined in the skin of E17.5 (A), neonatal (B), and adult (C) mice. Antisense probe was used for samples in the left-hand panels. Sense probe was used for samples in the right-hand panels. DIG-labeled probe was detected with sheep antibody to DIG.
DISCUSSION
Understanding of the mechanism by which the body naturally defends itself against bacterial invasion through the skin may lead to a new approach to the control of bacterial infections. In this context, defensins and similar antimicrobial peptides have become the objects of research. Recently, defensins have been detected in Paneth cells of human and murine intestines, in tracheal epithelial cells, as well as in phagocytes, where it is presumed that they are involved in the inhibition of bacterial colonization in the mucosa. The report of human β-defensin-2 expression in human keratinocytes by Harder et al. (9) in 1997 has stimulated interest in the function of the defensin system in the skin. Further investigation of this system requires experimental models, but such models have yet to be reported. The present study investigated antimicrobial peptide expression in mouse skin by using murine Paneth cell defensin (cryptdin)-specific primers.
Amplification by RT-PCR with a cryptdin-specific primer set demonstrated the expression of cryptdin mRNA in whole skin, epidermis, and cultured keratinocytes but detected no cryptdin message in cultured fibroblasts. Our data suggest that epithelial cells are the primary source of murine skin cryptdin.
Cryptdin message expression during the development of murine skin was also examined. The intensity of the band identified with cryptdin increased drastically from embryonic day 17.5. This suggests that the expression of cryptdin in the skin may not require contact with microorganisms. Ouellette et al. (18) reported that enteric cryptdin mRNAs were equally abundant in germ-free adult mice and naturally reared adult mice. During embryonic development, murine skin differentiates rapidly: on embryonic day 14, an intermediate layer forms above the basal layer; on embryonic days 15 and 16, keratohyaline granules appear in the uppermost layer, known as the granular layer, and hair bulbs begin to form. A cornified layer forms above the granular layer on embryonic days 16 to 18. Finally, on neonatal day 2, the epidermis is fully developed and consists of four histologically distinct cell layers: the basal, spinous, granular, and cornified layers (14). The expression of cryptdin appears to correlate with the maturity and the differentiation of the skin. In an experiment with athymic nude mice, enteric defensin mRNA was found to be abundant, indicating that cellular immunity has no influence on cryptdin expression (18). The neonatal immune system is functionally immature; shortly after birth, the counts of mature T cells in the spleen are 1,000-fold lower than those in the spleens of adults (20). During the neonatal period, cryptdin may compensate for this immature immune system by acting as a biochemical shield.
Sequence analysis showed that all clones derived from the whole skin and epidermis demonstrated nearly perfect identity with cryptdin-5. This suggests that the skin of BALB/c mice primarily expresses the cryptdin-5 isoform in vivo. However, cDNA derived from cultured keratinocytes corresponded to cryptdin-6 and cryptdin-11. The sequence of cryptdin-11 that we isolated had one mismatch at position 237 in the exon 2 coding the mature peptide. Thus, it appears that keratinocytes may produce some forms of cryptdin. Darmoul et al. (1) reported that intestinal mRNAs coding for cryptdin-1-like isoforms were chiefly detected in adult mice but that cryptdin-6 was the most abundant enteric defensin mRNA in the newborn. The expression of cryptdin isoforms may be influenced by the degree of development and differentiation. More than 20 cryptdin isoform mRNAs have been found in the full-length mouse small intestine, and 17 of these cryptdin-coding mRNAs were cloned from a single jejunum crypt (19). Further examination may reveal that other forms of cryptdin are expressed by keratinocytes.
Whether the expression of cryptdin-5 is species specific or organ specific remains unclear. Compared to myeloid defensins, intestinal defensins have variably extended NH2 termini that contain from three amino acids (as in cryptdin-4) to six amino acids (as in cryptdin-5) preceding the first cysteine (22). Because such variations in defensin amino termini have been shown to correlate with relative antimicrobial potency in vitro (8), the structure of cryptdin-5 may be appropriate for the containment of bacterial colonization and invasion of the skin. When the activities of cryptdins 1 through 6 against E. coli ML35 were compared by a plate diffusion assay, cryptdin-4 and cryptdin-5 were substantially more active than the other four intestinal cryptdins (19).
In situ hybridization analysis showed that cryptdin transcripts were expressed within the keratinocytes of the suprabasal layer from the embryonic to the neonatal days. Fulton et al. (6) showed that human β-defensin-1 transcripts localize within the suprabasal keratinocytes of the skin. This pattern of cryptdin distribution appears to be efficient for protection against pathogens from the outside of skin with few hairs. However, in this study, we showed that in the skin of adult mice, the mRNA of cryptdin localized to the keratinocytes of hair bulbs. Because the body surface of most animals is covered with hair, this localization pattern may be favorable. The change in the distribution pattern of cryptdin in the small intestine during development was reported by Darmoul et al. (1). Unlike in adult mice, where only Paneth cells are immunopositive for cryptdin, cryptdin-containing cells were distributed throughout the developing intestinal epithelium of the newborn and not in association with rudimentary crypts (1). These facts suggest that cryptdin localization in the skin may change during development and differentiation.
In this study, we detected cryptdin-5 mRNA expression in mouse skin, we determined that the expression of cryptdin mRNA began between embryonic days 15.5 and 17.5, and we discovered that cryptdin transcripts were initially expressed within the keratinocytes of the suprabasal layer from the embryonic to the neonatal days and then shifted to the hair bulbs. We speculate that cryptdin-5 is a more efficient protector than any other isoform of cryptdin in limiting bacterial colonization and invasion of the skin.
ACKNOWLEDGMENT
This work was supported by Grant-in-Aid for Scientific Research 09877159 (to H.K. and T.O.) from the Ministry of Education, Science and Culture of Japan.
REFERENCES
- 1.Darmoul D, Brown D, Selsted M E, Ouellette A J. Cryptdin gene expression in developing mouse small intestine. Am J Physiol. 1997;272:G197–G206. doi: 10.1152/ajpgi.1997.272.1.G197. [DOI] [PubMed] [Google Scholar]
- 2.Darmoul D, Huttner K M, Frederick D M, Ouelette A J. Positional specificity of defensin gene expression reveals Paneth cell heterogeneity in mouse small intestine. Am J Physiol. 1996;271:G68–G74. doi: 10.1152/ajpgi.1996.271.1.G68. [DOI] [PubMed] [Google Scholar]
- 3.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy W L, Bevins C L. Tracheal antimicrobial peptide, a novel cysteine-rich peptide from mammalian tracheal mucosa: isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenhauer P B, Harwig S S, Lehrer R I. Cryptdins: antimicrobial defensins of the murine small intestine. Infect Immun. 1992;60:3556–3565. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson G H. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 6.Fulton C, Anderson G M, Zasloff M, Bull R, Quinn A J. Expression of natural peptide antibiotics in human skin. Lancet. 1997;350:1750–1751. doi: 10.1016/S0140-6736(05)63574-X. [DOI] [PubMed] [Google Scholar]
- 7.Ganz T, Lehrer R I. Defensins. Curr Opin Immunol. 1994;6:584–589. doi: 10.1016/0952-7915(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 8.Ganz T, Selsted M E, Szklarek D, Harwig S S, Daher K, Bainton D F, Lehrer R I. Defensins: natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harder J, Bartels J, Christophers E, Schroder J M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 10.Jones D E, Bevins C L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992;267:23216–23225. [PubMed] [Google Scholar]
- 11.Kohashi O, Ono T, Ohki K, Soejima T, Moriya T, Umeda A, Meno Y, Amako K, Funakosi S, Masuda M. Bactericidal activities of rat defensins and synthetic rabbit defensins on staphylococci, Klebsiella pneumoniae (Chedid, 277, and 8N3), Pseudomonas aeruginosa (mucoid and nonmucoid strains), Salmonella typhimurium (Ra, Rc, Rd, and Re of LPS Mutants) and Escherichia coli. Microb Immunol. 1992;36:369–380. doi: 10.1111/j.1348-0421.1992.tb02036.x. [DOI] [PubMed] [Google Scholar]
- 12.Lehrer R I, Ganz T, Selsted M E. Defensins: endogenous antibiotic peptides of animal cells. Cell. 1991;64:229–230. doi: 10.1016/0092-8674(91)90632-9. [DOI] [PubMed] [Google Scholar]
- 13.Lehrer R I, Lichtenstein A K, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 14.Makino E, Namba M, Arata J. Are anionic sites involved in changes in cell layer susceptibility to exfoliative toxin during epidermal development? Eur J Dermatol. 1996;6:191–195. [Google Scholar]
- 15.Mor A, Nguyen V H, Delfour A, Migliore-Samour D, Nicolas P. Isolation, amino acid sequence and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry. 1991;30:8824–3017. doi: 10.1021/bi00100a014. [DOI] [PubMed] [Google Scholar]
- 16.Nakashima H, Yamamoto N, Masuda M, Fujii N. Defensins inhibit HIV replication in vitro. AIDS. 1993;7:1129. doi: 10.1097/00002030-199308000-00019. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 17.Ogata K, Linzer B A, Zuberi R I, Ganz T, Lehrer R I, Catanzaro A. Activity of defensins from human neutrophilic granulocytes against Mycobacterium avium and Mycobacterium intracellulare. Infect Immun. 1992;60:4720–4725. doi: 10.1128/iai.60.11.4720-4725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouellette A J, Greco R M, James M, Frederick D, Naftilan J, Fallon J T. Developmental regulation of cryptdin, a corticostatin/defensin precursor mRNA in mouse small intestinal crypt epithelium. J Cell Biol. 1989;108:1687–1695. doi: 10.1083/jcb.108.5.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouellette A J, Hsieh M M, Nosek M T, Cano-Gauci D F, Huttner K M, Buick R N, Selsted M E. Mouse Paneth cell defensins: primary structures and antimicrobial activities of numerous cryptdin isoforms. Infect Immun. 1994;62:5040–5047. doi: 10.1128/iai.62.11.5040-5047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridge J P, Fuchs E J, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 21.Selsted M E, Harwig S S. Determination of the disulfide array in the human defensin HNP-2: a covalently cyclized peptide. J Biol Chem. 1989;264:4003–4007. [PubMed] [Google Scholar]
- 22.Selsted M E, Miller S I, Henschen A H, Ouellette A J. Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol. 1992;118:929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senboshi Y, Oono T, Arata J. Prolidase gene expression in scar tissue using in situ hybridization. J Dermatol Sci. 1996;12:163–171. doi: 10.1016/0923-1811(95)00505-6. [DOI] [PubMed] [Google Scholar]
- 24.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA. 1987;84:5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]