Abstract
Purpose
Many studies have reported declines in semen quality mainly focused on total sperm counts (TSC) and sperm concentration (SC), ignoring the importance of progressive motile sperm (PR), total motile sperm (TM), and normal morphological sperm (NM). Therefore, we performed a comprehensive meta-analysis to explore the trend in semen quality of young men.
Methods
We searched 3 English databases and 4 Chinese databases from January 1980 to August 2022. Random-effect meta-analyses and weighted linear regression models were conducted to perform the trend in semen quality.
Results
Finally, 162 eligible studies including 264,665 men from 28 countries were got between 1978 and 2021. Significant decreases were observed in TSC (− 3.06 million/year, 95% CI − 3.28 to − 2.84), SC (− 0.47 million/ml/year, 95% CI − 0.51 to − 0.43), and PR (− 0.15%/year, 95% CI − 0.20 to − 0.09), and there was an upward trend in TM (0.28%/year, 95% CI 0.24 to 0.32). The results of meta-regression analyses indicated that age, continent, income, WHO criteria, and abstinence time significantly impacted on TSC, SC, PR, and TM. Positive regression coefficients were observed in some categories suggesting that outcomes might not be declining and even increasing in these subgroups.
Conclusions
Downward trends in semen quality among global young men were observed in our study, including TSC, SC, and PR. But TM did not appear to be trending down or even to be leveling off. More studies are needed to focus on the causes of the declines.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02859-z.
Keywords: Global trends, Semen quality, Total sperm counts, Sperm concentration, Progressive motile sperm, Total motile sperm
Introduction
Infertility is becoming a major health problem, causing serious economic burdens for people. It is reported that infertility has affected 8–12% of couples worldwide, and male factors are contributing to approximately 50% of couples [1]. A review explored the potential associations between infertility and health, suggesting that semen quality and male infertility may be fundamental biomarkers of overall health and also be a predictor of hospitalization and mortality [2]. Recently, a global burden of disease study indicated that the annual age-standardized prevalence rate of infertility of males and females increased by 0.29% and 0.37%, respectively, from 1990 to 2017 [3].
Spermatogenesis is a complex and precisely orchestrated process. Sperm quality, including sperm concentration (SC), total sperm count (TSC), semen volume, sperm motility, and sperm morphology, can reflect the functional status of the reproductive system and fecundity in males [4, 5]. Nowadays, sperm quality analysis is the first step to identify male factor infertility [6], and sperm quality may be considered reliable factors for measuring the male role in assisted reproductive technology of infertile couples [7].
In 1992, Carlsen et al. [8] reviewed 61 papers published from 1938 to 1991 and found a significant decline in SC over the past 50 years, which aroused a wide debate more than two decades. Some authors hold the opinion that worldwide sperm counts are not declining [9]. Ramírez et al. [10] retrospectively analyzed the semen quality of 23,130 patients from 2001 to 2020 in Argentina, finding no impairment in seminal quality over time. Jørgensen et al. [11] showed the sperm concentration and sperm count of 4867 men in Copenhagen, Denmark, increased significantly over 15 years. However, many studies have confirmed the decline in TSC [4, 12, 13]. Lv et al. [4] reviewed 111 studies including 32,7373 Chinese healthy men and found a temporal decrease existed in SC and TSC among Chinese men from 1981 to 2019. Similar results were also found in the European [13] and African populations [14]. Recently, Levine et al. [15] updated their research “Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis” published in 2017, by adding the sperm count among unselected men from South/Central America-Asia-Africa. The results further suggested that this worldwide decline is continuing in the twenty-first century at an accelerated pace.
Studies about sperm quality are mainly focused on SC and TSC, because of stable methods and criteria for assessment over time [12]. Sperm motility and morphology are critical parameters of sperm quality and are closely related to clinical pregnancy [16, 17]. Given this background, in this study, we performed a meta-regression analysis of SC, TSC, and sperm motility from reports beginning in the 1980s worldwide. We performed multiple subgroup analyses, including age, region, and income level, to explore the sperm quality in healthy men globally over time.
Materials and methods
Study design
This study was performed and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 [18]. We had registered in PROSPERO (CRD 42022352085).
Eligibility criteria
Studies meeting the following criteria were included: (i) the target population was unselected fertility status healthy men aged 18–45 years or a subgroup that includes this age range; (ii) the study period was from 1980 (when the first edition of the WHO laboratory manual for examination of human semen was published) to the present; (iii) outcomes included total sperm counts (TSC), sperm concentration or sperm density (SC), progressive motile sperm (PR), total motile sperm (TM), and normal morphological sperm (NM); (iv) observational study; and (v) English or Chinese language publications. The exclusion criteria were (i) patients suffered from disorders probably affecting semen quality, such as dysplastic disorders, sexually transmitted diseases, infertility or subfertility, asthenospermia, or oligospermia; (ii) studies interested that some exposures might affect semen quality, such as pollution, occupational exposure, drugs, smoking, and alcohol abuse; (iii) semen did not be collected or analyzed following the WHO laboratory manual; (iv) duplicate publications that ranged from publishing the same study in different journals; and (v) publications were not full text or non-peer reviewed, such as conference abstract.
Search strategy and study selection
We searched PubMed, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) (via Ovid), China National Knowledge Infrastructure (CNKI), Chinese Scientific Journals database (VIP), WanFang database, and Chinese Biomedical Literature database (CBM) from January 1980 to August 2022 and compare the references of similar studies to supplement the studies that may be included. The search strategies are available in Supplementary Table SI.
Parsifal, an online collaborative tool, and EndNote were used to screen records based on the title and abstract. Three reviewers independently completed the process and compared the screening results, and any differences were resolved by discussion. Each excluded study was marked with the reason for exclusion The same approach was used in the full-text screening. During the data extraction, ineligible studies were also excluded by carefully reading the full text of each article.
Data extraction
Data extraction was performed by two reviewers using Excel independently, including basic information (publication year, country, start date and end date, study design), population information (recruitment source and process, sample size, fertility status, age, abstinence), semen collection and analysis methods, and statistics (mean, standard deviation, minimum, maximum, median, and percentiles) on outcomes (TSC, SC, PR, TM, NM). Meanwhile, the subgroup data were also extracted.
If an article did not report the study date, the year of publication or submission was extracted to replace it. When outcome statistics lacked standard deviations (SDs), data from the article with similar sample size and mean were “borrowed” [19]. For the missing means and SDs, we estimated the mean and SDs from the sample size, median, range, and/or interquartile range reported in the text [20, 21]. GetData Graph Digitizer 2.22 was used to extract the data in the statistical plots.
Quality assessment
Two reviewers independently assessed the quality of the included studies by the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools) developed by the National Heart, Blood, and Blood Institute of the National Institutes of Health. Any differences were discussed and reassessed to reach a final consensus.
Statistical analysis
First, the eligible studies were divided into subgroups based on their start years and end years (not publication years). If the start years and end years were not reported, the received years or publication years were used to replace them. When the outcomes were reported for a span of 5 years or less, the midpoint year of that span was used to represent this period (e.g., if a study reported outcomes for 2017–2019, then these outcomes were classified as 2018). We conducted random-effect meta-analyses that the means of each outcome were pooled in the subgroups year by year.
Second, weighted linear regression models were used to perform the trend in semen quality. The pooled means of each outcome were used as the dependent variables (TSC, SC, PR, TM; but NM not included due to subjectivity and lack of uniform criteria), and the years were used as the independent variables. The weight arguments were set to the inverse of the squared residuals [22]. Meta-regression analysis with random-effect model was performed. The means for each outcome (TSC, SC, PR, TM) were included separately as dependent variables and modeled as function of the years, with the potential covariates on semen quality including age (18–20 years, 18–25 years, 18–30 years, 18–35 years, 18–40 years, 18–45 years), geographical position (Africa, Asia, Europe, North America, Oceania, South America), income based on World Bank country classifications in 2022 (high income, upper middle income, lower middle income, low income) [23], all editions of the WHO Laboratory Manual for the Examination and Processing of Human Semen (first edition, second edition, third edition, fourth edition, fifth edition, multiple editions), and abstinence time (2–3 days, 2–4 days, 2–5 days, 2–6 days, 2–7 days). The restricted maximum likelihood method was used to estimate regression coefficients and the heterogeneity variance between studies. Any multicollinear covariates were removed. We performed regression diagnostics, and the variance inflation factor (VIF) was used to judge the severity of multicollinearity. The Knapp-Hartung method was used to determine the significance of the regression coefficients (P ≤ 0.05 was considered statistically significant) and calculate the 95% confidence interval (95% CI) of the pooled effects.
Third, when ten or more data points were included, subgroup analyses were performed according to age, continent, income, and abstinence time (the different editions of the WHO manual were not included because of its interaction with year) in TSC, SC, PR, and TM. Sensitivity analyses were performed to verify the robustness of the regression model, where only cross-sectional studies were included, removing studies of sperm donors, removing studies not reporting start years and end years, removing years before 1999, removing studies with poor quality, and removing studies with sample size < 100.
Meta-analysis and meta-regression were conducted using Comprehensive Meta Analysis 3.3. Weighted linear regression models were developed by SPSS 28.0, and scatter plots were drawn by GraphPad Prism 9.0.
Results
Study selection and summary of characteristics
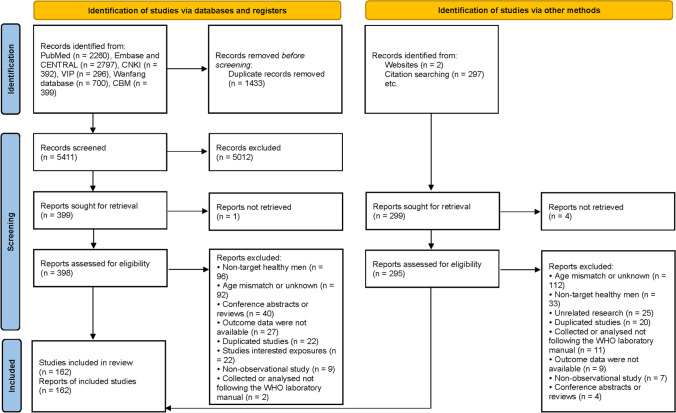
The PRISMA 2020 flow diagram details the process of study selection (Fig. 1). We got 6844 records from databases and 299 records from websites and references of similar studies. Finally, 162 studies meet the eligibility criteria, and they were all included in analysis. The basic characteristics of all eligible studies are in Supplementary Table SII. The studies included data on 264,665 men from 28 countries between 1978 and 2021. The recruited population came from the healthy general population (65/162, 40.12%), unselected donors (36/162, 22.22%), college students (29/162, 17.90%), premarital examination or pregnancy checkup (18/162, 11.11%), eligible donors (9/162, 5.56%), soldiers (2/162, 1.23%), and multiple recruitment populations (3/162, 1.85%). One hundred three (63.58%) studies reported the outcomes of TSC, 159 (98.15%) studies were SC, 114 (70.37%) studies were PR, 84 (51.85%) studies were TM, and 99 (61.11%) studies were NM.
Fig. 1.
PRISMA 2020 flow diagram of the study selection process
Quality of eligible studies
Summary of the quality of each item presented as percentages across all eligible studies is shown in Supplementary Figure SI. There was considerable certainty relating to clearly specified and defined research question and population in the eligible studies. Almost all studies did not provide the sample size justification, power description, or variance and effect estimates. For the questions about exposure measures, some studies were not applicable because they were cross-sectional studies. Finally, 58 (35.80%) studies were good quality, 99 (61.11%) studies were fair quality, and 5 (3.09%) were poor quality.
Global trends in semen quality
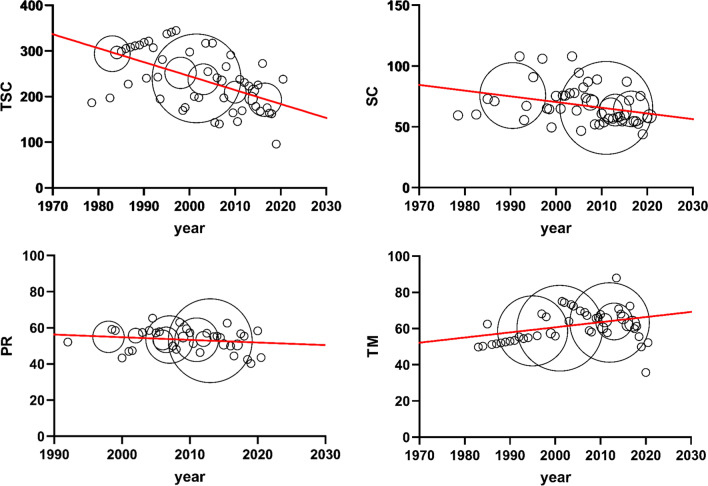
In the weighted linear regression model, significant decreases were observed in TSC (regression coefficient = − 3.06 million/year, 95% CI − 3.28 to − 2.84), SC (regression coefficient = − 0.47 million/ml/year, 95% CI − 0.51 to − 0.43), and PR (regression coefficient = − 0.15%/year, 95% CI − 0.20 to − 0.09), and there was an upward trend in TM (regression coefficient = 0.28%/year, 95% CI 0.24 to 0.32) (Table 1, Fig. 2). No regression model was built for NM because of heterogeneity from subjectivity and lack of uniform criteria. A scatter plot of NM was drawn in Supplementary Fig. SII. Around 2000, NM began to become “strict” probably due to changes in criteria.
Table 1.
Trends in semen quality outcomes in weighted linear regression models
| Outcomes | Start year–end year | Number of studies | Regression coefficient (95% CI) | R2 | P value |
|---|---|---|---|---|---|
| TSC | 1978–2021 | 93 | − 3.06 (− 3.28, − 2.84) | 0.94 | < 0.001 |
| SC | 1978–2021 | 147 | − 0.47 (− 0.51, − 0.43) | 0.91 | < 0.001 |
| PR | 1992–2021 | 110 | − 0.15 (− 0.20, − 0.09) | 0.44 | < 0.001 |
| TM | 1978–2021 | 73 | 0.28 (0.24, 0.32) | 0.79 | < 0.001 |
TSC total sperm counts, SC sperm concentration, PR progressive motile sperm, TM total motile sperm
Fig. 2.
Trends in semen quality of total sperm counts (TSCd), sperm concentration (SC), progressive motile sperm (PR), and total motile sperm (TM)
In the meta-regression analyses, the potential covariates were adjusted for TSC, SC, PR, and TM to investigate heterogeneity and impact on semen quality. The results of the regression indicated that age (P < 0.001), continent (P < 0.001), WHO criteria (P < 0.001), and abstinence time (P = 0.01) significantly impacted on TSC; age (P < 0.001) and continent (P < 0.001) significantly impacted on SC; age (P = 0.003), continent (P = 0.04, income (P = 0.002), and abstinence time (P = 0.02) significantly impacted on PR; continent (P < 0.001) and WHO criteria (P = 0.01) significantly impacted on TM (Supplementary Tables SIII–SVI).
Subgroup analyses in semen quality
Considering that the statistically significant covariates (age, continent, income, abstinence time, except for WHO criteria because of its interaction with year) were similar in outcomes, subgroup analyses were based on the same covariates (Table 2). The positive regression coefficients were observed in Oceania (regression coefficient = 2.76, 95% CI 1.34 to 4.17), 2–3 days of abstinence (regression coefficient = 2.04, 95% CI 1.97 to 2.12), and 2–4 days of abstinence (regression coefficient = 3.73, 95% CI 3.69 to 3.77) for TSC; 2–3 days of abstinence (regression coefficient = 0.38, 95% CI 0.29 to 0.46) for SC; Europe (regression coefficient = 0.15, 95% CI − 0.01 to 0.30, P = 0.06), North America (regression coefficient = 0.31, 95% CI 0.12 to 0.50), and high income (regression coefficient = 0.59, 95% CI 0.33 to 0.85) for PR; and Oceania (regression coefficient = 0.48, 95% CI 0.48 to 0.48), high income (regression coefficient = 0.43, 95% CI 0.34 to 0.52), and 2–4 days of abstinence (regression coefficient = 0.63, 95% CI 0.48 to 0.77) for TM. Positive regression coefficients for some categories suggested that outcomes might not be declining and even increasing in these subgroups. Equally important was that most outcomes in these subgroups were negative regression coefficients, suggesting that the downward trends were still major.
Table 2.
Subgroup analyses of TSC, SC, PR, and TM based on age, continent, income, and abstinence time
| Outcomes | Age (years) | Continent | Income | Abstinence time (days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18–29 | 30–39 | Asia | Europe | North America | Oceania | High income | Upper Middle income | 2–3 | 2–4 | 2–7 | ||
| TSC | R2 | 0.91 | 0.77 | 0.79 | 0.57 | 0.73 | 0.48 | 0.93 | 0.71 | 0.99 | 1.00 | 0.90 |
| Regression coefficient (95% CI) | − 2.54 (− 2.75, − 2.32) | − 2.54 (− 3.46, − 1.62) | − 2.16 (− 2.43, − 1.89) | − 2.72 (− 3.02, − 2.43) | − 5.00 (− 6.74, − 3.27) | 2.76 (1.34, 4.17) | − 4.47 (− 4.74, − 4.20) | − 1.58 (− 1.83, − 1.33) | 2.04 (1.97, 2.12) | 3.73 (3.69, 3.77) | − 4.64 (− 5.00, − 4.31) | |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| SC | R2 | 0.87 | 0.70 | 0.94 | 0.98 | 0.88 | / | 0.96 | 0.66 | 0.78 | 1.00 | 0.69 |
| Regression coefficient (95% CI) | − 0.93 (− 0.97, − 0.81) | − 0.73 (− 0.94, − 0.52) | − 1.16 (− 1.21, − 1.10) | − 0.80 (− 0.83, − 0.77) | − 2.80 (− 3.32, − 2.27) | / | − 0.72 (− 0.75, − 0.68) | − 0.99 (− 1.12, − 0.86) | 0.38 (0.29, 0.46) | − 2.69 (− 2.84, − 2.54) | − 1.18 (− 1.32, − 1.05) | |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | / | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| PR | R2 | 0.92 | 0.95 | 0.54 | 0.14 | 0.44 | / | 0.33 | 0.38 | 0.74 | / | 0.26 |
| Regression coefficient (95% CI) | − 0.69 (− 0.74, − 0.64) | − 0.66 (− 0.72, − 0.59) | − 0.27 (− 0.32, − 0.22) | 0.15 (− 0.01, 0.30) | 0.31 (0.12, 0.50) | / | 0.59 (0.33, 0.85) | − 0.26 (− 0.32, − 0.20) | − 0.25 (− 0.34, − 0.16) | / | − 0.22 (− 0.28, − 0.15) | |
| P value | < 0.001 | < 0.001 | < 0.001 | 0.06 | 0.003 | / | < 0.001 | < 0.001 | < 0.001 | / | < 0.001 | |
| TM | R2 | 0.77 | 0.69 | 0.94 | 0.97 | / | 1.00 | 0.64 | 0.77 | / | 0.80 | 0.14 |
| Regression coefficient (95% CI) | − 0.56 (− 0.66, − 0.46) | − 0.61 (− 0.87, − 0.36) | − 0.75 (− 0.80, − 0.70) | − 0.45 (− 0.49, − 0.42) | / | 0.48 (0.48, 0.48) | 0.43 (0.34, 0.52) | − 0.43 (− 0.49, − 0.36) | / | 0.63 (0.48, 0.77) | − 0.17 (− 0.27, − 0.07) | |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | / | < 0.001 | < 0.001 | < 0.001 | / | < 0.001 | 0.001 | |
*Since the number of data points for the subgroups of age (40–45 year old), continent (Africa, South America), income (lower middle income, low income), and abstinence time (2–5 days, 2–6 days) were less than 10, they were not included in the subgroup analyses
/: No data
Sensitivity analyses
Sensitivity analyses were performed for the weighted linear regression models in the whole population (Table 3). For the mode of TSC, SC, and PR, they were stable regardless of changes in different covariates. It should be noted that when removing studies with sample size < 100, the regression coefficients of TSC (regression coefficient = − 0.42, 95% CI − 0.60 to − 0.24) change the most significantly compared to other covariates.
Table 3.
Sensitivity analyses of weighted linear regression models
| Covariates | TSC | SC | PR | TM | ||||
|---|---|---|---|---|---|---|---|---|
| Regression coefficient (95% CI) | P value | Regression coefficient (95% CI) | P value | Regression coefficient (95% CI) | P value | Regression coefficient (95% CI) | P value | |
| Only cross-sectional studies were included | − 2.85 (− 2.96, − 2.75) | < 0.001 | − 0.55 (− 0.62, − 0.48) | < 0.001 | − 0.28 (− 0.36, − 0.20) | < 0.001 | 0.33 (0.32, 0.35) | < 0.001 |
| Removing studies of sperm donors | − 1.10 (− 1.70, − 0.50) | < 0.001 | − 0.57 (− 0.67, − 0.46) | < 0.001 | − 0.27 (− 0.29, − 0.25) | < 0.001 | − 0.36 (− 0.46, − 0.27) | < 0.001 |
| Removing studies not reporting start years and end years | − 2.92 (− 3.10, − 2.74) | < 0.001 | − 0.43 (− 0.54, − 0.32) | < 0.001 | − 0.19 (− 0.28, − 0.10) | < 0.001 | 0.43 (0.34, 0.53) | < 0.001 |
| Removing years before 1999 | − 3.27 (− 3.66, − 2.88) | < 0.001 | − 0.91 (− 0.92, − 0.90) | < 0.001 | − 0.25 (− 0.32, − 0.18) | < 0.001 | − 0.37 (− 0.43, − 0.30) | < 0.001 |
| Removing studies with poor quality | − 3.00 (− 3.20, − 2.81) | < 0.001 | − 0.46 (− 0.51, − 0.41) | < 0.001 | − 0.16 (− 0.20, − 0.12) | < 0.001 | 0.26 (0.21, 0.32) | < 0.001 |
| Removing studies with sample size < 100 | − 0.42 (− 0.60, − 0.24) | < 0.001 | − 0.27 (− 0.32, − 0.22) | < 0.001 | − 0.23 (− 0.28, − 0.17) | < 0.001 | − 0.37 (− 0.41, − 0.32) | < 0.001 |
Nevertheless, the regression coefficients of TM became negative when removing 20 studies of sperm donors (regression coefficient = − 0.36, 95% CI − 0.46 to − 0.27), 8 studies of years before 1999 (regression coefficient = − 0.37, 95% CI − 0.43 to − 0.30), and 17 studies with sample size < 100 (regression coefficient = − 0.37, 95% CI − 0.41 to − 0.32).
Discussion
Summary of findings
In our study, we found significant decreases in TSC (− 3.06 million/year), SC (− 0.47 million/ml/year), and PR (− 0.15%/year), but a seemingly upward trend in TM (0.28%/year), NM concentrating on “strict” range after 2000 among global 18–45 years old young men during 1978–2021. However, there might not be downward trends in some subgroups, including TSC in Oceania (regression coefficient = 2.76), 2–3-day abstinence time (regression coefficient = 2.04), and 2–4-day abstinence time (regression coefficient = 3.73), SC in 2–3-day abstinence time (regression coefficient = 0.38), PR in North America (regression coefficient = 0.31), and high income (regression coefficient = 0.59), and TM in Oceania (regression coefficient = 0.48), high income (regression coefficient = 0.43), and 2–4-day abstinence time (regression coefficient = 0.63). According to sensitivity analyses, modes of TSC, SC, and PR showed good stability in different covariates, but poor stability in the mode of TM.
Comparison with similar studies
There were several similar systematic review studies focused on trends in semen quality [4, 8, 12, 15, 24, 25]. We compared these studies with our study in Supplementary Table SVII. The major differences between our study and these similar studies were year range and demographics. Both TSC and SC in our study had downward trends consistent with other similar studies, except for no significant trends in Cipriani [24]. We found seemingly upward trends in the Oceania subgroup and 2–3-day and 2–4-day abstinence time subgroup in TSC and SC. None of the studies mentioned above reported similar results. It should be noted that the Oceania subgroup only included one retrospective study in Australia between 1983 and 2001 [26]. The upward trend in the 2–3-day and 2–4-day abstinence time subgroup differs from the longer abstinence time subgroup possibly because TSC and SC increase with abstinence time, peaking at around seventh days and showing clear decreasing trends [27, 28]. However, the decline of TSC and SC was the main trend in most subgroups. Especially, TSC and SC in the North America subgroup and TSC in the high-income subgroup were steep downward trends, which were also demonstrated by Levine et al.
Both PR and TM were added to our study two outcomes that have not been reported in other studies because sperm motility is a critical indicator of semen quality and fertility potential [29]. PR and TM had worse goodness of fit than TSC and SC due to the subjectivity in the absence of quality controls of methods, and this problem was exacerbated in sperm morphology analysis [30]. Although the positive coefficient of TM indicated that there might be an upward trend, we cannot be sure that this result is reliable. In the subgroup analysis, we noted that the downward trends of PR and TM were not obvious or had a very small slope, while there were upward trends in North America and Oceania. Most of the countries in these continents are high-income countries, which was consistent with the coefficient of the high-income subgroup. Similar results were also found in some studies [31, 32].
Explore heterogeneity and covariates
Published literature on trends in human semen quality around the world varies wildly in populations and study designs. It is important to consider causes of heterogeneity between studies and covariates affecting outcomes, such as geographical and ethnic differences, different analytical methods, and selection bias and measurement bias [33, 34]. The target population of our study was restricted to healthy men aged 18–45 years, to reduce the influence of age on semen quality [35, 36]. However, no differences in outcomes were observed among subgroups by age between 18 and 45 years in our study. Because semen collection is so personal and potentially embarrassing, highly selected volunteers constitute a biased sample that cannot be effectively extrapolated to the whole healthy men [33, 37]. Another very important covariate is geographical differences. Many multicenter studies have confirmed this issue [38–40]. Geographical differences exist at different scales (e.g., continent, country, province, urban, and rural). For geographic differences, we only consider the continental level, not the country level. Because dozens of countries were included, subgroup analysis for country level could not be completed. The relationship between geographical position and semen quality is complex and superficial. The deeper factors should be taken into account, including lifestyle, diet, climate, and even environmental pollution [34, 41, 42], but these factors are often sophisticated and poorly reported in the text.
Differences in study design often lead to methodological heterogeneity in systematic reviews of prevalence [43, 44]. Although cohort studies, case-control studies, and cross-sectional studies are all observational studies, they are different in terms of objective, population eligibility criteria, data processing, statistical analysis, and follow-up periods. Meanwhile, the result of the quality assessment also indicated that most studies did not do well enough in sample size justification, exposure measurement, and confounding adjustment so that the number of studies with good quality assessment was small (35.80%). When performing the regression analysis, we also found that the published studies were not evenly spatiotemporal distribution. These issues reduced our confidence in changes in global trends of semen quality.
Strengths and limitations
Our study assessed global trends in semen quality among young men over a broader time frame, and focused on more outcomes not only TSC and SC but also sperm motility and normal sperm morphology. Subgroup analyses and sensitivity analyses were used to explore possible sources of heterogeneity and explain part of the covariates. Although previous similar studies did not include sperm motility and morphology in their analysis due to significant variability of criteria [4, 12, 15], our study found that variability in sperm motility was acceptable and data points were not significant dispersion. The data points of sperm morphology were discrete but a general trend of change with the year of 2000 as the boundary. There are several limitations as well, including the uneven distribution of data across regions, particularly the severe paucity of data in Africa and South America, and the large amount of literature in Europe and Asia; hard to precisely obtain outcomes at each year point due to year range were reported in most studies; and missing data, the common problem in almost all systematic reviews. We also considered including “race” (ethnic distribution) as a covariate when designing the statistical analysis scheme. Unfortunately, this analysis was canceled because most of the articles did not report the race data of the included population. The analysis of population types was excluded due to a lack of sufficient evidence regarding the influence of various population types on semen quality.
Conclusion
Between 1978 and 2021, downward trends in semen quality among global young men were observed in our study, including total sperm count, sperm concentration, and progressive motile sperm. But total motile sperm did not appear to be trending down or even to be leveling off. At the same time, there were some variabilities in different subgroups, including different continents, income, and duration of abstinence, showing different trends. In the future, well-designed cross-sectional studies are needed, and more representative populations are included. The results of studies should be more adequate and clearer.
Supplementary information
Author contribution
XFL and XCP designed this meta-analysis together. XFL and CYY were responsible for the inclusion and exclusion of the article. CYY and YQS gathered and processed the data. XFL and CCD made the quality assessment. XCP was responsible for data acquisition and performed data analysis with CYY. XFL and XCP drafted the article and revised the manuscript. All authors read and approved the final manuscript.
Declarations
Ethics approval
Registration number CRD 42022352085
Conflict of interest
The authors declare no competing interests.
Footnotes
Key questions
What is already known on this topic?
Many studies have reported declines in TSC and SC, while some authors hold the opinion that worldwide sperm counts are not declining. What is more, sperm motility and morphology are not taken into consideration.
What are the new findings?
In this study, we performed a comprehensive meta-analysis to explore the trend in semen quality of young men. Total 162 eligible studies including 264,665 men from 28 countries were got between 1978 and 2021. The downward trends in semen quality among global young men were observed in our study, including TSC, SC, and PR, but TM did not appear to be trending down or even to be leveling off.
What do the new findings imply?
Overall, our study demonstrated that the semen quality of global young men is declining, and more studies are needed to focus on the causes of the declines on the future.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Agarwal A, Baskaran S, Parekh N, et al. Male infertility. Lancet. 2021;397(10271):319–333. doi: 10.1016/S0140-6736(20)32667-2. [DOI] [PubMed] [Google Scholar]
- 2.Choy JT, Eisenberg ML. Male infertility as a window to health. Fertil Steril. 2018;110(5):810–814. doi: 10.1016/j.fertnstert.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Sun H, Gong TT, Jiang YT, Zhang S, Zhao YH, Wu QJ. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990-2017: results from a global burden of disease study, 2017. Aging (Albany NY). 2019;11(23):10952–10991. doi: 10.18632/aging.102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lv MQ, Ge P, Zhang J, Yang YQ, Zhou L, Zhou DX. Temporal trends in semen concentration and count among 327 373 Chinese healthy men from 1981 to 2019: a systematic review. Hum Reprod. 2021;36(7):1751–1775. doi: 10.1093/humrep/deab124. [DOI] [PubMed] [Google Scholar]
- 5.Ferlin A, Garolla A, Ghezzi M, et al. Sperm count and hypogonadism as markers of general male health. Eur Urol Focus. 2021;7(1):205–213. doi: 10.1016/j.euf.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Swerdloff RS. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil Steril. 2014;102(6):1502–1507. doi: 10.1016/j.fertnstert.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villani MT, Morini D, Spaggiari G, et al. Are sperm parameters able to predict the success of assisted reproductive technology? A retrospective analysis of over 22,000 assisted reproductive technology cycles. Andrology. 2022;10(2):310–321. doi: 10.1111/andr.13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305(6854):609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorgensen N, Lamb DJ, Levine H, et al. Are worldwide sperm counts declining? Fertil Steril. 2021;116(6):1457–1463. doi: 10.1016/j.fertnstert.2021.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez ND, Tissera A, Molina R, Gaggino P, Mangeaud A, Martini AC. Is seminal quality worsening? A 20-year experience in Cordoba, Argentina. J Assist Reprod Genet. 2022;39(5):1125–1134. doi: 10.1007/s10815-022-02458-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jorgensen N, Joensen UN, Jensen TK, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012;2(4) 10.1136/bmjopen-2012-000990. [DOI] [PMC free article] [PubMed]
- 12.Levine H, Jorgensen N, Martino-Andrade A, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23(6):646–659. doi: 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sengupta P, Borges E, Jr, Dutta S, Krajewska-Kulak E. Decline in sperm count in European men during the past 50 years. Hum Exp Toxicol. 2018;37(3):247–255. doi: 10.1177/0960327117703690. [DOI] [PubMed] [Google Scholar]
- 14.Sengupta P, Nwagha U, Dutta S, Krajewska-Kulak E, Izuka E. Evidence for decreasing sperm count in African population from 1965 to 2015. Afr Health Sci. 2017;17(2):418–427. doi: 10.4314/ahs.v17i2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine H, Jorgensen N, Martino-Andrade A, et al. Temporal trends in sperm count: a systematic review and meta-regression analysis of samples collected globally in the 20th and 21st centuries. Hum Reprod Update. 2022; 10.1093/humupd/dmac035. [DOI] [PubMed]
- 16.Jeong M, Kim SK, Kim H, Lee JR, Jee BC, Kim SH. Predictive value of sperm motility before and after preparation for the pregnancy outcomes of intrauterine insemination. Clin Exp Reprod Med. 2021;48(3):255–261. doi: 10.5653/cerm.2021.04469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren W, Qu J, Xue B, Hu J, Zu X. Infertility duration and pre-operative sperm progressive motility are significant factors of spontaneous pregnancy after varicocele repair. Am J Reprod Immunol. 2020;84(6):e13318. doi: 10.1111/aji.13318. [DOI] [PubMed] [Google Scholar]
- 18.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022) John Wiley & Sons; 2022. [Google Scholar]
- 20.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 22.Mutiu S. Application of weighted least squares regression in forecasting. Int J Recent Res Interdiscip Sci. 2015;1(1):45–54. [Google Scholar]
- 23.World Bank . World Bank Country Classifications by Income Level. World Bank; 2022. [Google Scholar]
- 24.Cipriani S, Ricci E, Chiaffarino F, et al. Trend of change of sperm count and concentration over the last two decades: a systematic review and meta-regression analysis. Andrology. 2023; 10.1111/andr.13396. [DOI] [PubMed]
- 25.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Environ Health Perspect. 2000;108(10):961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costello MF, Sjoblom P, Haddad Y, Steigrad SJ, Bosch EG. No decline in semen quality among potential sperm donors in Sydney, Australia, between 1983 and 2001. J Assist Reprod Genet. 2002;19(6):284–290. doi: 10.1023/a:1015729314081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levitas E, Lunenfeld E, Weiss N, et al. Relationship between the duration of sexual abstinence and semen quality: analysis of 9,489 semen samples. Fertil Steril. 2005;83(6):1680–1686. doi: 10.1016/j.fertnstert.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 28.Hanson BM, Aston KI, Jenkins TG, Carrell DT, Hotaling JM. The impact of ejaculatory abstinence on semen analysis parameters: a systematic review. J Assist Reprod Genet. 2018;35(2):213–220. doi: 10.1007/s10815-017-1086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nallella KP, Sharma RK, Aziz N, Agarwal A. Significance of sperm characteristics in the evaluation of male infertility. Fertil Steril. 2006;85(3):629–634. doi: 10.1016/j.fertnstert.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 30.Tomlinson MJ. Uncertainty of measurement and clinical value of semen analysis: has standardisation through professional guidelines helped or hindered progress? Andrology. 2016;4(5):763–770. doi: 10.1111/andr.12209. [DOI] [PubMed] [Google Scholar]
- 31.Cannarella R, Condorelli RA, Gusmano C, et al. Temporal trend of conventional sperm parameters in a sicilian population in the decade 2011-2020. J Clin Med. 2021;10(5) 10.3390/jcm10050993. [DOI] [PMC free article] [PubMed]
- 32.Perheentupa A, Sadov S, Ronka R, et al. Semen quality improves marginally during young adulthood: a longitudinal follow-up study. Hum Reprod. 2016;31(3):502–510. doi: 10.1093/humrep/dev328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merzenich H, Zeeb H, Blettner M. Decreasing sperm quality: a global problem? BMC Public Health. 2010;10:24. doi: 10.1186/1471-2458-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Auger J, Eustache F, Chevrier C, Jegou B. Spatiotemporal trends in human semen quality. Nat Rev Urol. 2022;19(10):597–626. doi: 10.1038/s41585-022-00626-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone BA, Alex A, Werlin LB, Marrs RP. Age thresholds for changes in semen parameters in men. Fertil Steril. 2013;100(4):952–958. doi: 10.1016/j.fertnstert.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 36.Johnson SL, Dunleavy J, Gemmell NJ, Nakagawa S. Consistent age-dependent declines in human semen quality: a systematic review and meta-analysis. Ageing Res Rev. 2015;19:22–33. doi: 10.1016/j.arr.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Muller A, De La Rochebrochard E, Labbe-Decleves C, et al. Selection bias in semen studies due to self-selection of volunteers. Hum Reprod. 2004;19(12):2838–2844. doi: 10.1093/humrep/deh521. [DOI] [PubMed] [Google Scholar]
- 38.Feferkorn I, Azani L, Kadour-Peero E, et al. Geographic variation in semen parameters from data used for the World Health Organization semen analysis reference ranges. Fertil Steril. 2022;118(3):475–482. doi: 10.1016/j.fertnstert.2022.05.037. [DOI] [PubMed] [Google Scholar]
- 39.Palani A, Sengupta P, Agarwal A, Henkel R. Geographical differences in semen characteristics: comparing semen parameters of infertile men of the United States and Iraq. Andrologia. 2020;52(3):e13519. doi: 10.1111/and.13519. [DOI] [PubMed] [Google Scholar]
- 40.Jorgensen N, Andersen AG, Eustache F, et al. Regional differences in semen quality in Europe. Hum Reprod. 2001;16(5):1012–1019. doi: 10.1093/humrep/16.5.1012. [DOI] [PubMed] [Google Scholar]
- 41.Salas-Huetos A, Bullo M, Salas-Salvado J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: a systematic review of observational studies. Hum Reprod Update. 2017;23(4):371–389. doi: 10.1093/humupd/dmx006. [DOI] [PubMed] [Google Scholar]
- 42.Gabrielsen JS, Tanrikut C. Chronic exposures and male fertility: the impacts of environment, diet, and drug use on spermatogenesis. Andrology. 2016;4(4):648–661. doi: 10.1111/andr.12198. [DOI] [PubMed] [Google Scholar]
- 43.Hoffmann F, Eggers D, Pieper D, Zeeb H, Allers K. An observational study found large methodological heterogeneity in systematic reviews addressing prevalence and cumulative incidence. J Clin Epidemiol. 2020;119:92–99. doi: 10.1016/j.jclinepi.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Gagnier JJ, Moher D, Boon H, Beyene J, Bombardier C. Investigating clinical heterogeneity in systematic reviews: a methodologic review of guidance in the literature. BMC Med Res Methodol. 2012;12:111. doi: 10.1186/1471-2288-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.