Abstract
Purpose
We determined the sperm retrieval rate in men with persistent azoospermia post-chemotherapy in relation to cyclophosphamide equivalent dose (CED), a unit for quantifying alkylating agent exposure.
Methods
Medical records were retrospectively reviewed of 1098 patients diagnosed with non-obstructive azoospermia who had undergone microdissection testicular sperm extraction (mTESE) between January 2010 and 2021 at our institution. Twenty-three patients with a prior history of chemotherapy were included in the study. Oncological data, chemotherapy regime, and dosage were reviewed. The pretreatment hormone profile, CED, and mTESE outcomes were analyzed.
Results
Testicular spermatozoa were successfully retrieved from 11 patients (47%). The mean patient age was 37.3 years (range, 27–41 years), and mean time interval from chemotherapy to mTESE, 11.8 years (range, 1–45 years). Patients exposed to alkylating agents had significantly lower sperm retrieval rates than those not exposed to alkylating agents (1/9, 11% vs. 10/14, 71%, p = 0.009). No men with CED > 4000 mg/m2 (n = 6) had viable sperm in the testes during mTESE. Moreover, patients diagnosed with testicular non-seminomatous germ cell tumors had a favorable sperm retrieval rate (67%) compared to patients with lymphoma (20%) or leukemia (33%).
Conclusion
Patients with permanent azoospermia post-chemotherapy have a lower testicular sperm retrieval rate when the chemotherapy regimen included alkylating agents. In cases where patients have undergone more intensive gonadotoxic treatments, such as higher CED, the likelihood of successful sperm retrieval is low. It is advisable to counsel such patients using the CED model prior to considering surgical sperm retrieval.
Keywords: Azoospermia, Microdissection testicular sperm extraction, Post-chemotherapy, Cyclophosphamide
Introduction
Chemotherapy has been widely used both alone and in combination with other treatment modalities, to treat a range of pathologies including cancers, bone marrow disease, and immune system disorders [1]. While cytotoxic chemotherapy can be particularly effective, a certain class of cytotoxic chemotherapy drugs appears to potentiate gonadal toxicity, potentially resulting in permanent infertility [2]. Aside from the fact reproductive alterations are influenced by various treatment modalities, cancer can also adversely affect spermatogenesis prior to gonadotoxic treatments. Specifically, testicular cancer patients are reported to have inferior semen parameters compared to men with other malignancies [3]. This is likely attributed to local tumor effects, paracrine action, endocrine disruption, or genetic predisposition, theoretically limiting the efficacy of sperm cryopreservation before treatment commences in men with malignancies [3, 4].
Approximately 40,000 males under the age of 40 years develop cancer in the USA annually [5, 6]. Owing to an increase in cancer incidence in children and young adults, and a declining mortality rate, an increase in male long-term cancer survivors of reproductive age with reduced fertility potential is expected [6]. There has been an increase in cancer incidence in men of reproductive age in Taiwan, a trend also observed in other Western countries, such as the USA [7]. Notably, the incidence of testicular cancer among children and adolescents in Taiwan is higher than that of some Western countries [8]. This highlights the necessity for providers to offer prompt fertility preservation options at the time of cancer diagnosis for patients in Taiwan.
Alkylating agents used in chemotherapy regimens act by covalently binding an alkyl group to the guanine base of deoxyribonucleic acid (DNA), preventing cell division. Consequently, alkylating agents pose the greatest threat to gonadal function and spermatogenesis compared with other antineoplastic agents. The St. Jude Lifetime Cohort study used the cyclophosphamide equivalent dose (CED) to quantify the alkylating exposure for each individual patient. The study demonstrated a negative correlation between CED and sperm concentration; in addition, azoospermic men who had undergone chemotherapy had a significantly higher CED than normozoospermic men [2].
Recovery of spermatogenesis following gonadotoxic treatment is dependent on the cumulative alkylating agent and radiation dose used. For men with persistent azoospermia post-chemotherapy, microdissection testicular sperm extraction (mTESE) is considered a last resort to obtain viable sperm suitable for intracytoplasmic sperm injection (ICSI). This enables these men to have the best chance to biologically father a child [9]. Despite the recovery of viable testicular sperm with mTESE being uncertain in this population, we have used minimal CED (mCED), to quantify the minimal estimated alkylating agent exposure, according to the specific chemotherapy regimen given [10]. In our previous work, we observed patient exposure to alkylating agents was associated with a lower likelihood of successful sperm retrieval (SSR), and more importantly, no patient had SSR if the mCED exceeded 4000 mg/m2 [10]. Obtaining the exact alkylating dosage exposure for a given patient is difficult and this information is key to predicting the outcome of mTESE. Therefore, by studying a cohort with a known chemotherapy regimen and dosage, we aimed to analyze the sperm retrieval rate in relation to CED in post-chemotherapy azoospermic men, using their exact alkylating agent dose to quantify CED and evaluate the validity and application of CED.
Materials and methods
Study participants and study variables
We retrospectively reviewed the medical records of all men diagnosed with non-obstructive azoospermia (n = 1098) who underwent mTESE between January 2010 and 2021. The study included men with a history of chemotherapy prior to mTESE. Each patient underwent a thorough andrological evaluation which included, history-taking, physical examination, and reproductive hormone measurements (follicle-stimulating hormone [FSH], luteinizing hormone [LH], testosterone, prolactin, and estradiol [E2]). The patients underwent at least 2 separate semen analyses. Diagnosis of azoospermia was made in accordance with WHO guidelines, based on a complete absence of spermatozoa after centrifugation [11].
For each eligible patient, data on the cytotoxic chemotherapy regimen, dosage, age at the time of receiving, time interval from chemotherapy to mTESE, exposure to platinum-based drugs, and cumulative radiation exposure were recorded. The cumulative dose of alkylating agents was quantified using the validated CED score [12]. The study was approved by the Medical Ethics Review Committee (IRB protocol number: 2021–11-001CC).
Microdissection testicular sperm extraction
A standard midline raphe longitudinal incision of approximately 3 cm was made through the scrotum, the testes were freed and mobilized after layering and opening the tunica vaginalis as described by Schlegel [13]. An operating microscope at 20–25 × magnification was used to perform the rest of the procedure. Following the opening of the tunica albuginea, the testicular parenchyma was widely exposed for direct examination of the larger and more opaque seminiferous tubules. Meticulous dissection of seminiferous tubules was performed to identify and recover small samples. These samples were deemed the most likely to contain sperm for further analysis by an experienced embryologist, prior to undergoing ICSI. If no viable spermatozoa were identified in the initial sample, subsequent samples were extracted from the same testis or contralateral testis, as required.
Assisted reproduction program
Progestin-primed ovarian stimulation (PPOS) protocols were used for controlled ovarian stimulation in all patients. The PPOS protocol consists of ovarian stimulation with gonadotropin injection (corifollitropin alfa and/or human menopausal gonadotropin (hMG), dosage was determined by the patient’s condition), from the early follicular phase. Pituitary suppression was induced via medroxyprogesterone acetate (MPA), 5 mg twice a day, from the start of ovarian stimulation. Transvaginal sonography and serum hormone tests (for FSH, LH, E2, and progesterone [P]) were performed on the starting day prior to gonadotropin injection, and again, 1 week after controlled ovarian stimulation. Patients were triggered at night provided at least 3 leading follicles reached over 17 mm in diameter. If the follicle development was not adequate for the trigger, additional hMG (150–225 IU per day) was administered for days depending on the prediction following measurement on stimulation day 8. If required, additional follicle monitoring was performed every 2–3 days to assess whether the trigger threshold was reached. Triggering was induced by subcutaneous injection of triptorelin (0.2 mg), with or without human chorionic gonadotropin (hCG) (1500–6500 IU), which was subject to a risk evaluation for early-onset ovarian hyperstimulation syndrome. Oocytes were retrieved 34–38 h after triggering and were cryopreserved. During the menstruation following the oocyte retrieval, endometrial preparation was performed via estradiol administration. If the endometrial thickness reached above 7 mm after 2 weeks of estradiol administration, sperm retrieval (mTESE) was arranged. If sperm was obtained on the day of mTESE, oocytes were thawed for ICSI, and luteal support was started on the same day. Properly developed embryos were transferred under synchronization between the endometrium and embryo status.
Cryopreservation of retrieved testicular sperm
Patients desiring to preserve their sperm for future use can choose cryopreservation of testicular sperm. The utilization of ICSI injection pipettes (CooperSurgical, CT, USA), equipped with a micromanipulator, enables the retrieval of the spermatozoa. Subsequently, these collected spermatozoa are stored in a volume of basic medium of less than 0.1 ml while awaiting the vitrification process. During vitrification, the individual sperm cells are carefully transferred to a droplet of sperm freezing medium (1 μL) on the Cryotech strip (Reprolife, Tokyo, Japan) using the ICSI pipette [14]. The strip is then inserted into a cover and enclosed within a rigid plastic straw with utmost care. The end of the straw is sealed and immediately positioned approximately 4 cm above the surface of the liquid nitrogen for a duration of 2 min (− 120 °C). Finally, the entire assembly is directly immersed in liquid nitrogen.
Statistical analyses
Both categorical and nominal variables were expressed as absolute numbers with percentages and analyzed using Fisher’s exact test. Continuous variables were presented as means ± standard deviations and analyzed using the Mann–Whitney U test. SSR was the primary outcome. A 2-tailed p-value < 0.05 was considered significant. All statistical analyses were performed using IBM SPSS software (version 24.0, IBM Corp.)
Results
Patient characteristics
A total of 23 patients were included in the study; their baseline characteristics are summarized in Table 1. The mean age of the cohort receiving chemotherapy and mTESE was 24.9 years (range 2–38) and 37.3 years (range 27–41). The mean duration from chemotherapy to mTESE was 11.8 ± 10.7 years. The distribution of different types of cancer among the study population was as follows: non-seminomatous germ cell tumors (NSGCT) (n = 6, 26%), non-Hodgkin’s lymphoma (NHL) (n = 4, 17%), seminoma (n = 3, 13%), leukemia (n = 3, 13%), osteosarcoma (n = 1, 4%), nasopharyngeal carcinoma (NPC) (n = 1, 4%), Hodgkin’s lymphoma (n = 1, 4%), and colon cancer (n = 1, 4%). Langerhans cell histiocytosis, aplastic anemia, and hypergammaglobulinemia were the underlying medical conditions of the 3 patients who received cytotoxic chemotherapy without an underlying malignancy.
Table 1.
Demographic data of azoospermic patients after chemotherapeutic treatment
| No | Indication for chemotherapy | Age at receiving C/T(years) | Chemotherapy regimen | CED dose (mg/m2) | Regimen contained platinum-based agent | Cisplatin dose (mg/m2) | Time from C/T to mTESE(yrs) | FSH (mU/ml) | LH (mU/ml) | Testosterone (ng/ml) | Prolactin (ng/ml) | Estradiol (pg/ml) | Testis size (ml) | Sperm ( +) on TESE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Colon cancer | 30 | FOLFOX | 0 | Yes | 0 | 4 | 18.96 | 6.59 | 5.9 | 10.6 | 19.37 | 20 | Yes |
| 2 | NSGCT | 27 | BEP | 0 | Yes | 487.2 | 7 | 26.42 | 14.65 | 4.95 | 5.72 | 46.97 | 18 | Yes |
| 3 | NPC | 33 | PFL, PTX, Cis | 0 | Yes | 294.1 | 6 | 10.4 | 6.37 | 1.3 | 6.76 | 10 | 13 | Yes |
| 4 | NSGCT | 34 | BEP | 0 | Yes | 120 | 1 | 7.5 | 3.9 | 0.27 | 5.18 | 10 | 5 | No |
| 5 | NHL | 24 | CHOP, ESHAP | 8786.1 | Yes | 45.3 | 15 | 26.3 | 11.8 | 2.1 | 5.02 | 19 | 5 | No |
| 6 | NSGCT | 17 | BEP | 0 | Yes | 400 | 14 | 16.5 | 9.37 | 5.84 | 7.0 | 36 | 10 | No |
| 7 | Leukemia | 22 | A5D3, A5I2, A5N2 | 0 | No | 0 | 19 | 22.3 | 11.13 | 1.83 | 10.26 | 19 | 12 | Yes |
| 8 | HGG | 30 | Chl, P | 53,878.2 | No | 0 | 3 | 24.05 | 7.57 | 2.22 | 8.62 | 18 | 7 | No |
| 9 | LCH | 2 | Vb, P | 0 | No | 0 | 19 | 11.35 | 23 | 9.73 | 8.55 | 94 | 20 | No |
| 10 | NHL | 38 | RCHOP | 7828.4 | No | 0 | 3 | 35.7 | 13.41 | 1.38 | 5.77 | 14 | 30 | No |
| 11 | NSGCT | 15 | BEP, Vb | 0 | Yes | 623.2 | 19 | 0.1 | 0.06 | 17.96 | 18.6 | 53 | 7 | Yes |
| 12 | NHL | 37 | ICE, SMILE, DHAP | 7612.8 | Yes | 720 | 4 | 24 | 4.83 | 1.98 | 10.17 | 24.7 | 17 | No |
| 13 | NSGCT | 28 | BEP, Vb | 0 | Yes | 381.1 | 5 | 25.2 | 6.82 | 3.47 | 12.58 | 22.4 | 13 | Yes |
| 14 | Leukemia | 6 | CTX, V, EPI, Ara-C | 0 | No | 0 | 26 | 15 | 7.56 | 4.24 | 11.4 | 13.9 | 10 | No |
| 15 | Seminoma | 33 | BEP | 0 | Yes | 313.9 | 5 | 18.6 | 7.22 | 4.63 | 14.87 | 21.5 | 8 | Yes |
| 16 | Seminoma | 36 | BEP | 0 | Yes | 336.2 | 6 | 27.1 | 5.84 | 2.75 | 10.31 | 17.4 | 18 | No |
| 17 | OGS | 21 | MTX, IFO, Cis, DOX | 11,358.9 | Yes | 407.9 | 10 | 10.2 | 4.01 | 2.36 | 4.61 | 16.8 | 8 | No |
| 18 | NHL | 33 | RCHOP, ESHAP | 8470.6 | Yes | 148.6 | 7 | 19.6 | 7.42 | 3.04 | 3.04 | 19.7 | 11 | Yes |
| 19 | NSGCT | 30 | BEP | 0 | Yes | 301.2 | 0.4 | 24.4 | 5.90 | 4.75 | 18.5 | 19.5 | 14 | No |
| 20 | Seminoma | 27 | Carboplatin | 0 | Yes | 0 | 8 | 59.9 | 16.9 | 3.55 | 16 | 5.7 | 20 | Yes |
| 21 | Leukemia | 2 | CTX, V, EPI, Ara-C | 1293.2 | No | 0 | 45 | 17.4 | 7.8 | 1.18 | 20.5 | 18.7 | 13 | No |
| 22 | Anaplastic anemia | 34 | CTX | 2738.9 | No | 0 | 18 | 24.5 | 10.7 | 3.08 | 8.2 | 19.1 | 7 | Yes |
| 23 | Hodgkin’s lymphoma | 14 | Vb, DOX, MTX, P | 0 | No | 0 | 27 | 15.5 | 9.1 | 4.41 | 9.7 | 36.8 | 15 | Yes |
NSGCT, non-seminomatous germ cell tumor; NPC, nasopharyngeal cancer; NHL, non-Hodgkin’s lymphoma; HGG, hypergammaglobulinemia; LCH, Langerhans cell histiocytosis; OGS, osteogenic sarcoma; FOLFOX, folinic acid, fluorouracil, oxaliplatin; BEP, bleomycin, etoposide, cisplatin; PFT, cisplatin, leucovorin, 5-fluorouracil; PTX, paclitaxel; Cis, cisplatin; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; ESHAP, etoposide, methylprednisolone, cytarabine, cisplatin; A5D3, cytarabine, daunomycin; A5I2, cytarabine, idarubicin; A5N2, cytarabine, mitoxantrone; Chl, chlorambucil; P, prednisolone; Vb, vinblastine; ICE, ifosfamide, cisplatin, etoposide; SMILE, dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide; DHAP, dexamethasone, cytarabine, cisplatin; CTX, cyclophosphamide; V, vincristine; EPI, epirubicin; Ara-C, cytarabine; MTX, methotrexate; IFO, ifosfamide; DOX, doxorubicin; RCHOP, rituximab plus CHOP
Parameters associated with mTESE success
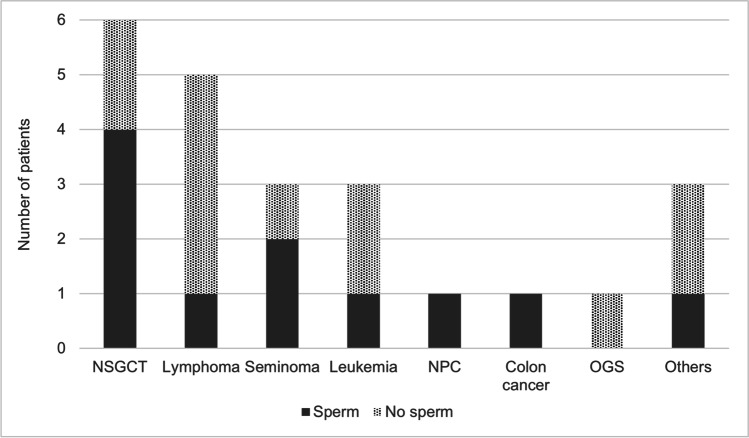
Sperm was successfully retrieved in 47.8% of men. SSR rates were 66% for NSGCT (4/6), 0% for NHL (0/4), 66% for seminoma (2/3), 33% for leukemia (1/3), 0% for osteosarcoma (0/1), 100% for NPC (1/1), 100% for Hodgkin’s lymphoma (1/1), and 100% for colon cancer survivors (1/1) (Fig. 1). Nine men in the study cohort were treated with alkylating agents. Although there was a wide range of CED scores, 1293 to 53,878 mg/m2, only one patient treated with alkylating agents had successful mTESE, with a CED score of 2739 mg/m2 (Fig. 2).
Fig. 1.
Sperm retrieval stratified by cancer subtypes. NSGCT, non-seminomatous germ cell tumor; NPC, nasopharyngeal carcinoma; OGS, osteogenic sarcoma
Fig. 2.
Relation between CED and sperm retrieval outcome using mTESE (means indicated by horizontal lines with each group)
Exposure to a platinum-based regimen, history of radiation therapy, age at the time of chemotherapy, age at mTESE, testes size, and reproductive hormone parameters (LH, FSH, testosterone, prolactin, and estradiol), were not predictive factors for the outcome of mTESE (Table 2). However, sperm was more frequently successfully retrieved from patients treated with chemotherapy without alkylating agents (10/14, 71% vs 1/9, 11%). In addition, there was a notable difference in CED scores between men with and without SSR; men with SSR had a significantly lower CED (249.0 vs. 8523.2 mg/m2, p = 0.012). For patients with no sperm obtained by mTESE, the histological findings of their testicular samples showed SCO.
Table 2.
Patients’ characteristics stratified by sperm retrieval outcome
| Median (IQR) | p value | Mean (SD) | p value | |||
|---|---|---|---|---|---|---|
| mTESE outcome | mTESE outcome | |||||
| Successful (n = 11) | Unsuccessful (n = 12) | Successful (n = 11) | Unsuccessful (n = 12) | |||
| Male age at TESE, years | 38 (34–40.5) | 40 (33–41) | .740 | 38.0 (5.7) | 36.7 (5.7) | .740 |
| Male FSH, mIU/ml | 22.3 (17.1–24.9) | 19.6 (15–24.1) | .651 | 22.4 (14.7) | 19.6 (8.2) | .651 |
| Male LH, mIU/ml | 7.2 (6.5–10.9) | 7.6 (5.8–11.1) | .928 | 8.7 (4.6) | 8.9 (5.3) | .928 |
| Male testosterone, ng/ml | 4.4 (3.3–4.9) | 2.2 (1.8–3.0) | .079 | 5.1 (4.5) | 3.1 (2.5) | .079 |
| Male prolactin, ng/ml | 10.6 (8.9–15.4) | 8.5 (5.2–10.3) | .059 | 10.6 (4.7) | 9.6 (5.1) | .059 |
| Male estradiol, pg/ml | 19.5 (19.0–29.6) | 18.7 (16.8–21.5) | .316 | 24.9 (14.7) | 25.2 (22.6) | .316 |
| Time from C/T to mTESE, years | 7 (5–18.5) | 10 (4–19) | .928 | 10.8 (8.5) | 12.7 (12.7) | .928 |
| Testis volume, ml | 13 (10–16.5) | 11 (8–17) | .525 | 13.4 (4.7) | 12.8 (7.3) | .525 |
| Alkylating agents exposure, number | 1 (9%) | 8 (67%) | .009 | 1 (9%) | 8 (67%) | .009 |
| CED dose, mg/m2 | 0 (0–0) | 3049.8 (0–8470.6) | .012 | 249.0 (825.8) | 8523.2 (14,891.5) | .012 |
| Platinum-based agents exposure, number | 8 (73%) | 7 (58%) | .667 | 8 (73%) | 7 (58%) | .667 |
| Accumulative cisplatin dose, mg/m2 | 294.1 (0–347.5) | 45.3 (0–336.2) | .928 | 218.2 (228.6) | 181.5 (228.6) | .928 |
Reproductive outcomes
To date, 8 ICSI cycles with testicular sperm have been carried out in 7 couples in our institution. As a result, 134 oocytes were obtained and 104 were MII mature oocytes. The fertilization rate was 67.3% (70/104), and all female partners of cancer survivors undergoing ICSI had received at least 1 cycle of embryo transfer. The overall biochemical (defined as serum level of β-hCG > 10 mUI/ml) and clinical pregnancy rates (presence of fetal heartbeat at 6–8 weeks of gestation) per cycle were 50% (4/8) and 37.5% (3/8), respectively. As of the current update, there is an ongoing pregnancy that has successfully reached the second trimester, and two pregnancies have successfully reached full-term delivery, resulting in the birth of a healthy boy and a healthy girl. Additionally, two couples received ICSI cycles at another institution, and two couples banked sperm from the male partner but did not undergo further ICSI cycles, primarily due to financial reasons.
Discussion
Post-chemotherapy, the risk of primary gonadal failure in men of reproductive age is highly variable and difficult to predict. We evaluated the importance of CED for quantifying alkylating agent exposure. CED has been previously used to stratify individual risk of azoospermia development in childhood cancer survivors. This helps to predict the success of sperm retrieval in men who remain persistently azoospermic post-chemotherapy. Our results show that surgical sperm retrieval was successful in 47% of men with azoospermia and a history of chemotherapy. Moreover, findings show that alkylating agent exposure and CED are independently and negatively associated with successful testicular sperm retrieval.
In cancer-directed therapy using a chemotherapy regimen, alkylating agents are among the most widely used chemotherapeutic agents. These pose the greatest risk of inducing primary gonadal failure and compromising future fertility [2]. As a result, azoospermia is one of the most enduring negative consequences of antineoplastic treatment. Using CED to quantify the cumulative exposure to alkylating agents of each individual, the St. Jude Lifetime Cohort study identified that the risk of azoospermia increases with the increment of CED. Moreover, spermatogenesis was less common at a cumulative CED of < 4000 mg/m2. However, no uniform and safe threshold of alkylating agent for gonadotoxicity has been established [2]. Our research team has previously used minimal CED as a novel approach for estimating a patient’s minimum alkylating agent exposure. This approach considers both the chemotherapy regimen and cancer etiology due to the inability to obtain the exact alkylating dosage given. In this previous study, SSR by mTESE was only seen among cancer survivors receiving mCED less than 4000 mg/m2 [10]. In the present study, the majority of patients (n = 19) received chemotherapy years prior to the sperm retrieval procedure in our institution. This allowed precise calculation of CED through recording the exact dosage of each alkylating agent administered. In total, 4 patients received chemotherapy at other medical facilities. However, we were also able to obtain details of their treatment, which included chemotherapy regimen and dosage, radiation type, and exposure dosage.
Results suggest that success in sperm retrieval cannot be predicted by patient age at diagnosis, age at assessment, preoperative reproductive hormone profile, or testicular size, as in existing reports [9, 15, 16]. However, exposure to alkylating agents was associated with a significantly lower sperm retrieval rate, which correlates with one of the largest cohort studies to date reported by Hsiao et al. [9]. In this study, the researchers performed a SSR analysis in 73 post-chemotherapy azoospermic cases. Importantly, they indicated that the sperm retrieval rate was the lowest (14.3%, 1/7) in those with sarcoma etiology [9]. This may be due, in part, to the sarcoma survivors being more likely to have received a high cumulative alkylating dose. In this study, the only osteosarcoma patient in the cohort was treated with 11,358.9 mg/m2 CED, high-dose alkylating agent exposure.
Despite platinum-based chemotherapy regimens not being included in the CED calculation, platinum compounds can reduce spermatogenesis and alter the structure of seminiferous tubules. This is associated with a lower likelihood of paternity, especially when these agents are administered at higher doses [17]. Results demonstrate platinum agent-treated patients did not show inferior sperm retrieval outcomes (53.3%, 8/15), compared to those without platinum drug exposure (37.5%, 3/8). This could be due to these patients receiving a mean cisplatin dose of 402 ± 220 mg/m2, a low dose of cisplatin to impact on fertility potential [11]. Four of the 11 patients with unsuccessful sperm retrieval had zero CED. This included 2 cases of NSGCT, 1 case of testicular seminoma, and 1 case of Langerhans cell histiocytosis. The 2 patients with NSGCT received cumulative cisplatin doses of 120 and 400 mg/m2, whereas the seminoma patient was treated with a 336 mg/m2 cisplatin dose. The patient with Langerhans cell histiocytosis did not receive cisplatin treatment before mTESE. Notably, none of the patients was exposed to radiation. This evidence indicates that cisplatin susceptibility varies among individuals. Moreover, cisplatin can initiate germ stem cell apoptosis, thereby completely depleting spermatozoa in the testes, even at low cisplatin doses.
Intense radiation exposure has also been implicated in infertility. Such treatment can diminish fertility when aimed at or around a male reproductive organ or targeted at the pituitary gland, hindering the production of hormones necessary for reproduction. Compared to single-dose radiation, the administration of fractionated radiation induced more significant gonadal toxicity [18]. Generally, at doses totaling no more than fractionated 1.2 Gy, the resulting azoospermia may be reversible [19]. However, doses over fractionated 2.5 Gy typically result in irreversible destruction of spermatogonium stem cells, resulting in permanent azoospermia [20]. Though, a large single dose of ionizing radiation, exceeding 6 Gy, is required to predispose azoospermia development [21]. In total, 2 patients in our cohort with unsuccessful sperm retrieval were treated with a single high dose of radiation (12 Gy) to the testes.
This study had a few limitations. The main limitation of the study is the retrospective design. Therefore, the results are highly dependent on the quality of medical records. In addition, despite a significant difference in the CED between groups, the sample size was small. Moreover, the majority of patients who underwent SSR had zero CED, meaning that they had not been previously exposed to alkylating agents as part of their treatment regimen. This makes it difficult to determine a cutoff for CED, above which would indicate an individual is unlikely to have SSR. Furthermore, although CED is useful for quantifying individual exposure to alkylating agents, it is difficult to exclude the gonadotoxic effects of other chemotherapy agents during cancer treatment. This is due to the lack of uniform standardized measurements. Additionally, there is a range of cytotoxic chemotherapies used in treating hematological and other solid tumors, such as cisplatin, vinca alkaloids, and antimetabolites, that are not included in the CED calculation, yet may also affect spermatogenesis. This limits to an extent, the practicality of such an approach.
Conclusion
Our findings indicate that patients with permanent azoospermia post-chemotherapy have a lower testicular sperm retrieval rate when the chemotherapy regimen contains alkylating agents. However, while such information may provide clinically useful information to counsel patients regarding their probability of SSR, the utility of CED among these patients provides a better strategy for predicting SSR. In cases where patients have undergone more intensive gonadotoxic treatments, such as higher CED, the likelihood of successful sperm retrieval is low. Therefore, alternatives such as the use of donor sperm should be considered within the context of assisted reproductive technology.
Acknowledgements
The authors express their gratitude to Howard En-Hao Tien and Irene Yea-Lan Chang for their assistance with statistical analysis and to Li-Jung Chang for her generous technical support.
Author contribution
Conceptualization: S-J. Tsai, I-S. Huang. Data curation: S-J. Tsai, I-S. Huang, W-J. Chen, William J. Huang. Formal analysis: all authors.
Funding acquisition: I-Shen Huang, William J. Huang. Investigation: I-S. Huang, L–H. Li, William J. Huang. Title page (with ALL authors’ information). Methodology: I-S. Huang. Project administration: I-S. Huang. Resources: William J. Huang. Software: S-J. Tsai, I-S. Huang. Supervision: I-S. Huang. Validation: I-S. Huang. Visualization: I-S. Huang. Writing—original draft: all authors. Writing—review and editing: all authors.
Funding
This work was supported by the Taipei Veterans General Hospital (VGH 109-C-132 and V112B-034).
Data Availability
All relevant data supporting the findings of this study will be made available upon request to qualified researchers for the purpose of replication and further scientific investigation.
Declarations
Ethics approval
The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Approval was granted by the Medical Ethics Review Committee (IRB protocol number: 2021–11-001CC).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15–25. doi: 10.1038/cdd.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green DM, Liu W, Kutteh WH, Ke RW, Shelton KC, Sklar CA, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. Lancet Oncol. 2014;15:1215–1223. doi: 10.1016/S1470-2045(14)70408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams DH, 4th, Karpman E, Sander JC, Spiess PE, Pisters LL, Lipshultz LI. Pretreatment semen parameters in men with cancer. J Urol. 2009;181:736–740. doi: 10.1016/j.juro.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Sineath RC, Mehta A. Preservation of fertility in testis cancer management. Urol Clin North Am. 2019;46:341–351. doi: 10.1016/j.ucl.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 6.Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70:443–459. doi: 10.3322/caac.21637. [DOI] [PubMed] [Google Scholar]
- 7.Huang YC, Chen YH. Cancer incidence characteristic evolution based on the National Cancer Registry in Taiwan. J Oncol. 2020;2020:1408793. doi: 10.1155/2020/1408793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hung GY, Horng JL, Lee YS, Yen HJ, Chen CC, Lee CY. Cancer incidence patterns among children and adolescents in Taiwan from 1995 to 2009: a population-based study. Cancer. 2014;120:3545–3553. doi: 10.1002/cncr.28903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsiao W, Stahl PJ, Osterberg EC, Nejat E, Palermo GD, Rosenwaks Z, et al. Successful treatment of postchemotherapy azoospermia with microsurgical testicular sperm extraction: the Weill Cornell experience. J Clin Oncol. 2011;29:1607–1611. doi: 10.1200/JCO.2010.33.7808. [DOI] [PubMed] [Google Scholar]
- 10.Huang IS, Huang WJ, Wren J, Bennett NE, Brannigan RE. The minimal cyclophosphamide equivalent (MCED) dose as an approach to predict outcome of microdissection testicular sperm extraction in patients with persistent azoospermia after chemotherapy. Fertil Steril. 2018;110:e160. doi: 10.1016/j.fertnstert.2018.07.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corea M, Campagnone J, Sigman M. The diagnosis of azoospermia depends on the force of centrifugation. Fertil Steril. 2005;83:920–922. doi: 10.1016/j.fertnstert.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 12.Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod. 1999;14:131–135. doi: 10.1093/humrep/14.1.131. [DOI] [PubMed] [Google Scholar]
- 14.Maleki B, Khalili MA, Gholizadeh L, Mangoli E, Agha-Rahimi A. Single sperm vitrification with permeable cryoprotectant-free medium is more effective in patients with severe oligozoospermia and azoospermia. Cryobiology. 2022;104:15–22. doi: 10.1016/j.cryobiol.2021.11.176. [DOI] [PubMed] [Google Scholar]
- 15.Shin T, Kobayashi T, Shimomura Y, Iwahata T, Suzuki K, Tanaka T, et al. Microdissection testicular sperm extraction in Japanese patients with persistent azoospermia after chemotherapy. Int J Clin Oncol. 2016;21:1167–1171. doi: 10.1007/s10147-016-0998-5. [DOI] [PubMed] [Google Scholar]
- 16.Shiraishi K, Matsuyama H. Microdissection testicular sperm extraction and salvage hormonal treatment in patients with postchemotherapy azoospermia. Urology. 2014;83:100–106. doi: 10.1016/j.urology.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 17.Chow EJ, Stratton KL, Leisenring WM, Oeffinger KC, Sklar CA, Donaldson SS, et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2016;17:567–576. doi: 10.1016/S1470-2045(16)00086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howell SJ, Shalet SM. Spermatogenesis after cancer treatment: damage and recovery. J Natl Cancer Inst Monogr. 2005;34:12–17. doi: 10.1093/jncimonographs/lgi003. [DOI] [PubMed] [Google Scholar]
- 19.Centola GM, Keller JW, Henzler M, Rubin P. Effect of low-dose testicular irradiation on sperm count and fertility in patients with testicular seminoma. J Androl. 1994;15:608–613. [PubMed] [Google Scholar]
- 20.Sandeman TF. The effects of x irradiation on male human fertility. Br J Radiol. 1966;39:901–907. doi: 10.1259/0007-1285-39-468-901. [DOI] [PubMed] [Google Scholar]
- 21.Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril. 2013;100:1180–1186. doi: 10.1016/j.fertnstert.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data supporting the findings of this study will be made available upon request to qualified researchers for the purpose of replication and further scientific investigation.