Abstract
Purpose
To investigate whether embryo rebiopsy increases the yield of in vitro fertilization (IVF) cycles.
Methods
Retrospective study including 18,028 blastocysts submitted for trophectoderm biopsy and preimplantation genetic testing for aneuploidy (PGT-A) between January 2016 and December 2021 in a private IVF center. Out of the 517 embryos categorized as inconclusive, 400 survived intact to the warming procedure, re-expanded, and were suitable for rebiopsy. Of them, 71 rebiopsied blastocysts were transferred. Factors affecting the probability of obtaining an undiagnosed blastocyst and clinical outcomes from blastocysts biopsied once and twice were investigated.
Results
The overall diagnostic rate was 97.1%, with 517 blastocysts receiving inconclusive reports. Several blastocyst and laboratory features, such as the day of the biopsy, the stage of development, and the biopsy methodology, were related to the risk of obtaining an inconclusive diagnosis after PGT-A. A successful diagnosis was obtained in 384 of the rebiopsied blastocysts, 238 of which were chromosomally transferable. A total of 71 rebiopsied blastocysts were transferred, resulting in 32 clinical pregnancies [(clinical pregnancy rate (CPR)=45.1%], 16 miscarriages [(miscarriage rate (MR)=41%], and, until September 2020, 12 live births [(live birth rate (LBR)=23.1%]. A significantly lower LBR and higher MR were obtained after transferring rebiopsied blastocysts compared to those biopsied once.
Conclusion
Although an extra round of biopsy and vitrification may cause a detrimental effect on embryo viability, re-analyzing the test-failure blastocysts contributes to increasing the number of euploid blastocysts available for transfer and the LBR.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02875-z.
Keywords: Preimplantation genetic testing for aneuploidy, Trophectoderm biopsy, Inconclusive result, Undiagnosed blastocyst, Rebiopsy
Introduction
Embryo biopsy has become a standard procedure in most in vitro fertilization (IVF) clinics. However, it is also one of the most invasive techniques performed, and therefore care must be taken to avoid compromising embryonic viability. At the past congress of the European Society of Human Reproduction and Embryology, the Preimplantation Genetic Testing (PGT) Consortium presented the data collected in 2020 from 37 reporting centers across Europe, Japan, Israel, and Taiwan [1]. A total of 10,393 embryos were biopsied and analyzed for PGT. However, not all embryos received a genetic diagnosis. Specifically, 736 (7%) had an inconclusive chromosomal assessment after embryo biopsy.
Occasionally, this group of embryos that fail the initial chromosomal diagnosis may be blindly transferred. However, since rebiopsy is not considered a regular strategy to obtain a valid diagnosis when the first attempt fails, these embryos are typically discarded due to their uncertain chromosomal status, thus contributing to embryo wastage.
The retesting strategy is not only time-consuming but also implies additional effort and expense for the embryology unit. Moreover, it involves an extra round of warming, biopsy, and vitrification, which could impair the competence of the rescued blastocysts. Although evidence suggests that vitrified-warmed and fresh embryo transfers lead to equivalent pregnancy rates [2–4], it is unclear whether multiple rounds of cryopreservation are detrimental to embryo development, as conflicting findings have been published [5–7]. In addition, little information is available on the outcomes of twice-biopsied blastocysts since removing too many trophectoderm (TE) cells could affect embryo implantation [8].
Regarding the prevalence of inconclusive diagnoses after TE biopsy in PGT cycles, several works have been presented at various annual meetings of the American Society for Reproductive Medicine, mainly retrospective [9–13]. However, to date, there are limited reports investigating the clinical outcomes after a second round of biopsy [14–19].
On the one hand, the present study aimed to identify blastocyst features and laboratory procedures related to the probability of obtaining an inconclusive result after preimplantation genetic testing for aneuploidy (PGT-A). On the other hand, we aimed to evaluate the impact of a second round of biopsy, vitrification, and warming in terms of survival, efficiency, and clinical outcomes in euploid embryos with an inconclusive result after the first TE biopsy.
Material and methods
Study population, participants, and design
This retrospective study was conducted in a single IVF laboratory and included all blastocysts that underwent TE biopsy for PGT-A from January 2016 to December 2021.
Indications for PGT-A included advanced maternal age, recurrent miscarriage, severe male factor, recurrent implantation failure, and aneuploidy screening. Blastocysts biopsied on day 7 were excluded from the analysis due to their low number. Patients eligible for preimplantation genetic testing for chromosomal structural rearrangements (PGT-SR) or preimplantation genetic testing for monogenic disorders (PGT-M) were also excluded from the study.
During the study timeframe, a total of 5171 PGT-A cycles were performed by 4295 patients. This led to a total of 18,028 biopsied blastocysts, 517 of which had inconclusive results. The mean age of patients providing oocytes was 37.4 ± 3.7 (range 18–45 years old).
IVF-related procedures
Ovarian stimulation, oocyte retrieval, intracytoplasmic sperm injection (ICSI), and embryo culture were performed as described previously by Bori [20].
Blastocyst grading
Before embryo biopsy, blastocysts were scored according to guidelines from the Spanish Association for the Study of Biology of Reproduction (ASEBIR), which are based on the evaluation of the degree of expansion and the appearance of TE and inner cell mass (ICM) cells [21]. Regarding the degree of expansion, all biopsied blastocysts were hatching blastocysts (iHB) or hatched blastocysts (HB). Extrapolating to Gardner’s criteria, the degree of expansion of iHB and HB is 5 and 6, respectively [22].
The ICM of blastocysts was classified as “good” (if well defined, compacted, and consisting of many cells, which refers to grades A and B of Gardner and ASEBIR classification criteria); or “poor” (if consisting of a lower number of cells, which corresponds to grade C in Gardner and ASEBIR scoring systems). Following the same criteria, the TE was defined as “good” when it was uniform, well defined, and consisting of many cohesive cells (grades A and B of Gardner and ASEBIR classification criteria); or “poor” when it was formed by few cells and had an irregular appearance (grade C in Gardner and ASEBIR scoring systems).
Blastocyst biopsy, tubing, and chromosomal assessment
On the morning of day 3 of embryo development, a laser-assisted hole was made in the zona pellucida to create a small opening and facilitate hatching [23]. The day of blastocyst biopsy depended exclusively on blastocyst development and quality. In all cases, only iHB or HB with a defined ICM and a cohesive TE epithelium were considered eligible for biopsy. Blastocysts not reaching the guide mark on day 5 (D5) [116 ± 2-h post-insemination (hpi)] were given one more day in culture and biopsied on day 6 (D6) (140 ± 2 hpi) if meeting the established criteria.
The TE biopsy was performed by the pulling or the flicking method. The policy for choosing the biopsy methodology depended on blastocyst appearance at the operator’s discretion. HB were always biopsied by flicking. Approximately 5 to 8 TE cells with visible nuclei opposite the ICM were collected from the blastocyst with a biopsy pipette.
Briefly and as previously described [24, 25], when performing the pulling method, blastocysts were held firmly with the holding pipette, and the biopsy pipette was used to pull TE cells away while intercellular laser pulses were applied. Alternatively, with the flicking method, TE cells were given laser pulses and were subsequently excised with a quick movement of the biopsy pipette against the holding pipette. Laser pulses were performed with OCTAX NavilaseTM (Olympus, Tokyo, Japan) using the TE biopsy mode (intensity 1.8 ms, ø13 μm).
Aspirated TE cells were collected into DNase-free polymerase chain reaction tubes using UV-sterilized material and sterile gloves. The presence of the biopsied cells inside the tube was confirmed using a stereomicroscope. All samples were labeled, immediately stored at −20 °C, and shipped to the same genetics laboratory [26] to ensure consistency and uniformity in the analysis. Blastocysts were moved back to a tri-gas incubator in hypoxic conditions and subsequently cryopreserved using Kitazato® vitrification protocol following standard procedures [27, 28].
TE biopsies were analyzed by next-generation sequencing (NGS) technology. Library preparation and sequencing were performed as previously described [29]. Plots were inspected by a certified geneticist. Possible calls from the PGT-A assay were either conclusive or inconclusive diagnoses. Embryos with conclusive diagnoses were either transferable (including euploid and transferable mosaic embryos) or non-transferable (including aneuploid and non-transferable mosaic embryos). Embryos with inconclusive chromosomal assessments were those lacking an auto-call. These “no calls” were either due to a lack of amplification (indicating the presence of insufficient genetic material in the TE biopsy) or due to the data being widely scattered and not meeting quality control standards.
Rebiopsy of blastocysts with inconclusive results
Embryos with inconclusive results were routinely rebiopsied at no additional cost to the patients. After warming according to our standard Kitazato® protocol, blastocysts were incubated for 2–6 h in a culture medium under 6% CO2, 5% O2, and 89% N2. Only the surviving blastocysts that were fully re-expanded and had sufficient quality, with no signs of degeneration, were suitable to undergo a second biopsy.
Rebiopsy and revitrification were performed following the same laboratory procedures described above. The rebiopsied specimens were submitted for PGT-A reanalysis utilizing the same platform and quality control metrics for interpreting the results. Embryos that were rebiopsied were further classified as transferable or non-transferable. A percentage of embryos remained undiagnosed.
Warming and transfer of biopsied and rebiopsied blastocysts
The embryo warming and transfer protocols were those previously described [27, 30]. Briefly, embryo warming was performed on the morning of the day of the cryotransfer. After warming, blastocyst survival was evaluated based on embryo morphology and the degree of re-expansion, and blastocysts were cultured for 2–4 h until embryo transfer. Endometrial preparation was conducted under hormonal replacement therapy, and embryo transfer was performed under ultrasound guidance with luteal phase support. Only single embryo transfers (SET) were performed, and blastocysts diagnosed in the first round of biopsy were prioritized.
Retrospective analysis
Factors contributing to the risk of inconclusive results after PGT-A
The main parameters that could affect the chance of obtaining an inconclusive result after PGT-A were investigated. For this purpose, the rate of inconclusive results was compared based on: (I) day of blastocyst biopsy (D5 vs. D6), (II) developmental stage (iHB vs. HB), (III) ICM grade (good vs. poor), (IV) TE grade (good vs. poor), and (V) biopsy methodology (flicking vs. pulling). The influence of these variables and maternal age was assessed through logistic regression analysis.
In addition, the rate of inconclusive results among the embryologists performing the biopsies was compared and evaluated along with the biopsy methodology. The analysis included a total of 13 biopsy operators, excluding those who had performed fewer than 20 cases.
Comparison of clinical outcomes between biopsied and rebiopsied blastocysts
In embryos with inconclusive diagnoses, the impact of a second round of biopsy, vitrification, and warming was assessed. The following outcome measures were compared between biopsied and rebiopsied embryos: (I) clinical pregnancy rate (CPR), defined as the percentage of embryo transfers resulting in a gestational sac observed during the ultrasound examination scan at >5 weeks gestation; (II) miscarriage rate (MR), calculated as the number of miscarriages up to the 20th week of pregnancy divided by the number of gestations with positive β-hCG; and (III) live birth rate (LBR), defined as the percentage of embryo transfers resulting in a fetus born alive beyond the 24th week of pregnancy. Regarding LBR, only data from 2016 to September 2020 were included, as live births were not fully updated in our database after this date.
Statistical analysis
Continuous variables were presented as mean ± standard deviation and range. Categorical variables were reported as rates with a 95% confidence interval. The Shapiro-Wilk test was used to examine the normal distribution of the data. The Student’s t test was used to compare continuous characteristics between groups, while the chi-square test was performed to assess statistically significant differences in categorical variables. Following univariate analysis, logistic regression was conducted, and adjusted odds ratios were calculated to determine the risk of a TE biopsy resulting in an inconclusive diagnosis. All statistical analyses were carried out using the SPSS software, and a p value < 0.05 was considered statistically significant.
Ethical approval
All procedures were approved by our institutional review board (#2202-VLC-010-MN), the ethics committee of Clinical Research IVI-RMA Valencia, which regulates and approves the analysis of databases for research purposes. The project complies with the Spanish law governing assisted reproductive technologies (14/2006), and, given its retrospective nature, formal consent from study participants was not required.
Results
Incidence of inconclusive results after PGT-A
The overall diagnostic rate was 97.1% (17,511/18,028), while the remaining 2.9% of biopsied blastocysts (517/18,028) failed to yield a conclusive result.
Factors contributing to the risk of inconclusive results after PGT-A
The assessment of variables related to blastocyst evaluation revealed that D5-biopsied embryos exhibited a significantly lower rate of inconclusive results (2.7%) compared to slower-growing embryos biopsied on D6 (3.3%) (P<0.05). Similarly, the blastocyst stage was strongly correlated with the chance of obtaining inconclusive diagnoses, as evidenced by the significantly lower rate of inconclusive results observed in iHB (2.7%) compared to HB (4.2%) (P<0.05) (Table 1).
Table 1.
Rate of inconclusive results depending on blastocyst features and the biopsy methodology
| Parameter | Rate of inconclusive results after PGT-A | 95% CI | P value |
|---|---|---|---|
| Biopsy day | 0.02 | ||
| Day 5 | 2.67% (313/11,744) | 2.37–2.85 | |
| Day 6 | 3.25% (204/6284) | 2.91–3.69 | |
| Stage | 5.30E-05 | ||
| iHB | 2.67% (420/15,704) | 2.42–2.92 | |
| HB | 4.17% (97/2324) | 3.36–4.98 | |
| ICM quality | NS | ||
| Good (grades A+B) | 2.76% (301/10,916) | 2.45–3.07 | |
| Poor (grade C) | 3.04% (216/7112) | 2.63–3.43 | |
| TE quality | NS | ||
| Good (grades A+B) | 2.76% (266/9630) | 2.43–3.09 | |
| Poor (grade C) | 2.99% (251/8398) | 2.62–3.34 | |
| Biopsy methodology | 3.88E-21 | ||
| Flicking | 2.33% (348/14,920) | 2.09–2.57 | |
| Pulling | 5.44% (169/3108) | 4.61–6.19 |
PGT-A, preimplantation genetic testing for aneuploidy; CI, confidence interval; NS, not significant; iHB, hatching blastocyst; HB, hatched blastocyst; ICM, inner cell mass; TE, trophectoderm
In contrast, embryo quality did not correlate with the risk of obtaining an inconclusive diagnosis. Paradoxically, embryos with a poor-quality TE had similar rates of inconclusive results (3%) compared to those with a good-quality TE (2.8%) (P>0.05). Similarly, no significant correlation was found between the rate of inconclusive results and ICM scores (3% vs. 2.8%, respectively) (P>0.05) (Table 1).
Regarding the biopsy methodology, the rate of inconclusive results was significantly higher in the pulling group (5.4%) compared to the flicking group (2.3%) (P<0.05) (Table 1). Additionally, when assessing the operators’ performance, most embryologists displayed similar rates of inconclusive results (2–4%) (P>0.05), suggesting comparable overall biopsy skill levels (Supplementary Table 1).
Consistently, logistic regression analysis showed no correlation between blastocyst quality (ICM and TE scores) or maternal age and the risk of inconclusive results. In contrast, the day of blastocyst biopsy, the blastocyst stage, and the biopsy methodology were factors significantly affecting the risk of having an inconclusive chromosomal assessment (Table 2).
Table 2.
Logistic regression analysis for the risk of a blastocyst to be diagnosed as inconclusive
| Parameter | OR | 95% CI | P value |
|---|---|---|---|
| Biopsy day | |||
| Day 5 | REf | Ref | 0.006 |
| Day 6 | 1.328 | 1.084–1.627 | |
| Stage | |||
| iHB | Ref | Ref | 0.000 |
| HB | 1.797 | 1.395–2.315 | |
| ICM quality | |||
| Good (grades A+B) | Ref | Ref | NS |
| Poor (grade C) | 1.066 | 0.877–1.296 | |
| TE quality | |||
| Good (grades A+B) | Ref | Ref | NS |
| Poor (grade C) | 1.073 | 0.885–1.301 | |
| Biopsy methodology | |||
| Flicking | Ref | Ref | 0.000 |
| Pulling | 2.986 | 2.441–3.652 | |
| Oocyte age (years) | 0.990 | 0.976–1.004 | NS |
OR, odds ratio; CI, confidence interval; NS, not significant; iHB, hatching blastocyst; HB, hatched blastocyst; ICM, inner cell mass; TE, trophectoderm
Chromosomal assessment of rebiopsied blastocysts
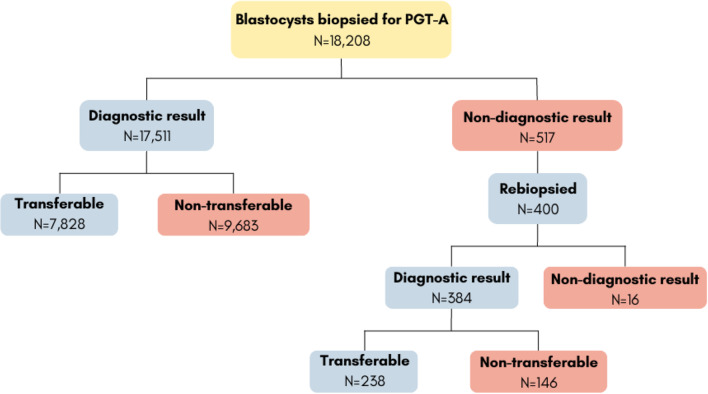
77.4% of vitrified embryos with inconclusive diagnoses (400/517) survived intact to the warming procedure, re-expanded, and had enough quality to be biopsied, vitrified, and analyzed in a second round. A successful diagnosis was obtained in 96% of the rebiopsied embryos (384/400), yet still, 4% of them led to a repeated non-diagnostic result (16/400). This means that only 0.1% of all initially biopsied embryos (16/18,028) remained with “no diagnosis” as final chromosomal status (Fig. 1).
Fig. 1.
Study workflow
Fifty-six percent of the rebiopsied embryos were classified as euploid (224/400) (Table 3). Interestingly, the euploidy rate was significantly higher in rebiopsied embryos than in embryos biopsied once (42.2%=7603/18,028) (P<0.0001). These differences were observed across all maternal age groups investigated except in the >42 years group (probably due to the low numbers of the rebiopsy group). Consistently, the percentage of transferable mosaic embryos was higher in the rebiopsy compared to the biopsy group (data not shown), which contributed to the increase in the rate of total transferable blastocysts (59.5% vs. 43.4%, respectively) (P<0.0001) (Table 3).
Table 3.
Comparison of diagnostic metrics and clinical outcomes between blastocysts biopsied once and twice
| Outcome | Biopsy | Rebiopsy | P value |
|---|---|---|---|
| Euploid blastocysts | 42.16% (7603/18,028) | 56.00% (224/400) | 4.97E-08 |
| Maternal age | |||
| <35 | 58.97% (3069/5202) | 73.64% (81/110) | 0.002 |
| 35–37 | 50.36% (1491/2966) | 63.64% (49/77) | 0.02 |
| 38–40 | 37.16% (2278/6126) | 49.29% (69/140) | 0.004 |
| 41–42 | 22.48% (630/2805) | 38.18% (21/55) | 0.008 |
| >42 | 14.54% (135/929) | 22.22% (4/18) | NS |
| Transferable blastocysts | 43.42% (7828/18,028) | 59.50% (238/400) | 1.94E-10 |
| Clinical pregnancy | 55.26% (2521/4562) | 45.07% (32/71) | NS |
| Miscarriage | 24.00% (673/2804) | 41.02% (16/39) | 0.01 |
| Live birth (until Sept 2020) | 43.58% (1476/3387) | 23.08% (12/52) | 0.003 |
Transferable blastocysts included euploid and transferable mosaic blastocysts. NS, not significant
Comparison of clinical outcomes between biopsied and rebiopsied blastocysts
A total of 71 transferable, rebiopsied blastocysts were warmed and underwent SET. Subsequently, their clinical outcomes were compared to those of the 4562 blastocysts biopsied once and transferred during the study timeframe. The CPR was comparable between blastocysts biopsied once (55.3%=2521/4562) and those rebiopsied (45.1%=32/71) (P=0.08). However, rebiopsied blastocysts had a significantly lower LBR (23.1%=12/52) and a significantly higher MR (41%=16/39) compared to blastocysts biopsied once (43.58%=1476/3387 and 24%=673/2804, respectively) (P<0.05) (Table 3).
Discussion
Blastocyst biopsy for PGT usually involves a relatively low rate of embryos with inconclusive results. However, the absolute numbers can still be significant, especially in busy laboratories. According to previous data, around 2–7% of biopsied blastocysts lead to a no-result assessment [14, 15, 17, 19, 31]. This percentage is consistent with our prevalence of inconclusive results in PGT-A cycles (2.9%), which may be considered acceptable, especially after rebiopsy (0.1%). In this work, we focused our efforts on investigating, from both technical and clinical perspectives, the potential use of blastocysts with inconclusive diagnoses after the initial PGT-A biopsy.
Regarding laboratory procedures, the Preimplantation Genetic Diagnosis International Society has warned that poor biopsy technique, laboratory practice, or sample handling may cause extra cell damage and affect the outcome of PGT [32]. For this reason, the expertise and skill of embryologists have always been a matter of concern since not all operators are equally trained, and their performance is not always regularly evaluated. It is not only important to avoid harming the blastocyst but also to ensure the integrity of the biopsied cells. A logical approach is to find the optimal balance between obtaining good-quality data and the minimal invasion that preserves embryonic competence.
In our hands, the day of blastocyst biopsy, the stage of blastocyst development, and the biopsy methodology were the main factors affecting the success of the diagnosis.
The day of blastocyst biopsy correlated with the chance of obtaining an inconclusive result and was significantly higher in embryos biopsied on D6 than in those biopsied on D5. This differs from previous data suggesting that D6 is the ideal timing for embryo biopsy [17] or manifesting that the incidence of inconclusive results is independent of the day of blastocyst biopsy [15]. Such discrepancies may be explained by different laboratory and embryo conditions. In addition, Neal et al. [15] also reported a higher risk of no-result in blastocysts with worse TE quality. Although this may make intuitive sense, our data and others’ [17] have failed to show such a correlation. This may be attributed to different policies for blastocyst biopsy among the reporting centers and to the subjectivity by which TE quality is evaluated. Despite the lower TE quality, these blastocysts contain good-quality DNA, and their biopsy is justified. What all studies appear to agree on is that the ICM quality is not related to the risk of obtaining an inconclusive result after the PGT biopsy.
The stage of blastocyst development was also considered in this work. The higher rate of inconclusive results found in HB compared to iHB is consistent with the fact that HB are more frequently seen on D6 than on D5 (data not shown). Being the hatching process a very objective feature, embryologists may see HB as more fragile specimens and the biopsied cells as material more likely to be degraded, which may affect the risk of obtaining inconclusive results.
Regarding the biopsy methodology, we report a higher incidence of inconclusive results when performing the pulling method than with the flicking method. This contrasts with previous findings suggesting that flicking is a more aggressive and rougher technique [35]. However, in this particular study [35], the euploidy rate was similar regardless of the biopsy method. Relatedly, Coll et al. [24] discarded the relationship between the biopsy methodology and the prevalence of mosaicism after PGT-A. Besides the application of different methodologies for TE excision, different applications of laser technology can affect the integrity of the DNA contained within the biopsy. In our study, most biopsies were obtained after applying around four laser pulses. However, a higher number of pulses may have been applied with the use of the pulling method. In this regard, data seem controversial, as there is no clear relationship between the number and intensity of laser pulses and the level of DNA damage on the biopsied cells [33, 34]. Regrettably, the number of pulses was not annotated at the time of embryo biopsy, as it was not mandatory for our electronic medical records. As manifested by other authors [17], these studies are necessary to understand the consequences of the techniques used in daily IVF activity.
Other factors potentially affecting the risk of inconclusive diagnosis are the tubing procedure and the time gap between tubing and DNA analysis. These factors were not considered in the present work. As other authors suggested, the quality of the biopsy specimen could be affected by the presence of dead TE cells, which is associated with a poor quality of the TE [19], or by the biopsy of too few cells, which may alter chromosomal profiles [24]. Conversely, a lack of result is unlikely due to the loss of the biopsied specimen if its presence is confirmed after the tubing procedure, as we routinely do.
Focusing on the laboratory aspects related to clinical outcomes, several reports have evidenced the high ability of blastocysts to recover from a double round of vitrification and warming, although the survival rate is usually not 100% [7, 18, 19, 36]. However, little information is available regarding the blastocyst’s capacity to survive a second round of biopsy combined with vitrification [14, 15]. In our study, only 77.4% of embryos with a former inconclusive diagnosis could be rebiopsied. Indeed, their ability to re-expand and survive a second round of biopsy was lower than that of single-biopsied blastocysts from our PGT program. Similar findings have been reported previously (75.4% vs. 95.5%, respectively) [16, 19]. Again, the embryologist’s criteria for performing a second biopsy based on TE quality may condition informativity and survival rates.
Despite the vast majority of blastocysts yielding an interpretable result after the second biopsy round, a small percentage remained undiagnosed (4% of the rebiopsied blastocysts or 0.1% of all initially biopsied embryos). It has been suggested that this could be an inherent limitation of PGT-A [15].
In addition, our data show that blastocysts with inconclusive results are more likely to be euploid when rebiopsied, as reported previously [11, 13]. This may be because embryos harboring chromosomal alterations are less likely to survive to a subsequent round of vitrification and warming and retain sufficient quality to be rebiopsied. Previous works have reported variable euploidy rates in rebiopsied blastocysts, ranging from 44 to 67% [13, 17, 19]. However, these percentages could be confounded by the PGT indication [14]. Also, as stated previously [16], the use of stringent criteria in the evaluation of DNA profiles may lead to a significant increase in the risk of obtaining inconclusive results by reporting as inconclusive samples that would otherwise be called euploid. Although not shown in the present study, euploidy rates decreased significantly with the poorer quality of the TE and the ICM in both biopsied and rebiopsied blastocysts, as previously shown [37].
Finally, rebiopsied blastocysts had a significantly lower LBR and a higher MR compared to those biopsied once. This contributes to the idea that a second round of biopsy, vitrification, and warming exerts an additive effect against embryo viability and its developmental potential. Since the beginning of the implementation of the rebiopsy strategy, its potential impact on embryo development was laid on the table [36]. However, there are controversial results in this regard. Some works have reported differences in CPR and LBR, indicating a detrimental effect of rebiopsy on blastocysts [15, 16, 18]. In addition, double vitrification coupled with a single biopsy was reported to increase MR and diminish LBR [5]. In contrast, other works either observed no differences [17, 19] or differences were not statistically significant, possibly due to the low sample size [14].
Overall, our data encourage the rescue of undiagnosed blastocysts for clinical use. Although an extra round of biopsy and vitrification compromised LBR and increased MR, it resulted in an increased number of embryos available for transfer, which still have reproductive potential and may contribute to increasing the total number of babies born. Also, despite the potential differences in blastocysts’ competence to implant and grow properly, the evidence suggests that rebiopsy does not impact neonatal outcomes [5, 9, 17, 18]. However, the neonatal follow-up of children born from rebiopsied blastocysts was not conducted in the present study. Therefore, further studies are needed to reassure the safety of the technique and draw more definitive conclusions.
In our view, this study addresses a clinically relevant topic, and makes a valuable contribution to the literature by providing new insights into the factors contributing to the risk of inconclusive results and the clinical outcomes derived from rebiopsied blastocysts. By encompassing 6 years of experience in PGT-A cycles, our data demonstrate the success of rebiopsy in yielding euploid results compatible with live birth. This information guides clinical decision-making regarding embryos with non-informative results and facilitates the development of improved strategies for selecting those with higher chances of successful implantation and pregnancy. However, some study limitations should also be acknowledged. First, due to its retrospective nature, a selection bias may exist due to the lack of randomization. Additionally, it was a single-center study; therefore, the generalization of our findings to other PGT-A programs should be made with caution. Finally, although biopsy operators were routinely evaluated to ensure compliance with standard quality control metrics, the potential influence of the biopsy technician as a confounding factor in assessing the risk of inconclusive results must be recognized.
In summary, we believe that blastocyst rebiopsy is a valid approach to offer to patients and should be implemented as a regular strategy, at least if no more euploid embryos are available and patients refuse to undergo a blind embryo transfer. Otherwise, with the increasing use of PGT-A in IVF units worldwide, the number of embryos with an inconclusive diagnosis will continue to rise year after year.
Supplementary information
Supplemental Table S1. Incidence of inconclusive results after PGT-A and frequency of technique usage across biopsy operators. Column proportions were compared using a Chi-square test with Bonferroni correction at the 95% confidence level. The same letters for each parameter indicate homogeneous subsets. PC: pairwise comparisons. PGT-A: Preimplantation Genetic Testing for Aneuploidy.
Acknowledgements
The authors thank all the embryologists and technicians of the IVF laboratory at IVI-RMA Valencia (Spain), especially the biopsy team members, for their clinical and technical support in this study.
Author contributions
All authors have materially participated in the research and/or article preparation. (1) M. Nohales, A. Coello, A. Martin, F. Insua, and MJ. De los Santos: the conception and design of the study, or acquisition of data, or analysis and interpretation of data. (2) M. Nohales, A. Martin, and M. Meseguer: drafting the article or revising it critically for important intellectual content. (3) A. Martin and M. Nohales: final approval of the version to be submitted.
Data availability
Data regarding any of the subjects in the study have not been previously published.
Declarations
Ethics approval
All procedures were approved by our institutional review board (#2202-VLC-010-MN), the ethics committee of Clinical Research IVI-RMA Valencia, which regulates and approves the analysis of databases for research purposes. The project complies with the Spanish law governing assisted reproductive technologies (14/2006).
Consent to participate
Given its retrospective nature, formal consent of study participants was not required.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van Montfoort A, De Rycke M, Carvalho F, Rubio C, Bronet F, Spinella F, Goossens V. O-041 Data from the ESHRE PGT consortium – year 2020. Hum Reprod. 2022;37:deac104–deac047. doi: 10.1093/humrep/deac104.047. [DOI] [Google Scholar]
- 2.Maheshwari A, Raja EA, Bhattacharya S. Obstetric and perinatal outcomes after either fresh or thawed frozen embryo transfer: an analysis of 112,432 singleton pregnancies recorded in the Human Fertilisation and Embryology Authority anonymized dataset. Fertil Steril. 2016;106:1703–1708. doi: 10.1016/j.fertnstert.2016.08.047. [DOI] [PubMed] [Google Scholar]
- 3.Roy TK, Bradley CK, Bowman MC, SJ MA. Single-embryo transfer of vitrified-warmed blastocysts yields equivalent live-birth rates and improved neonatal outcomes compared with fresh transfers. Fertil Steril. 2014;101:1294–1301. doi: 10.1016/j.fertnstert.2014.01.046. [DOI] [PubMed] [Google Scholar]
- 4.Kato O, Kawasaki N, Bodri D, Kuroda T, Kawachiya S, Kato K, Takehara Y. Neonatal outcome and birth defects in 6623 singletons born following minimal ovarian stimulation and vitrified versus fresh single embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2012;161:46–50. doi: 10.1016/j.ejogrb.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Li W, Jia H, Gao Y, Shi W, Bai H. Double vitrification-warming cycles, coupled with blastocyst biopsy, impair live birth but do not affect neonatal outcomes. Int J Gynaecol Obstet. 2022;160(3):806–813. doi: 10.1002/ijgo.14355. [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Jiang J, Xi Q, Li D, Ren X, Li Z, Zhu L, Jin L. Repeated cryopreservation process impairs embryo implantation potential but does not affect neonatal outcomes. Reprod Biomed Online. 2021;42:75–82. doi: 10.1016/j.rbmo.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Cobo A, Castello D, Vallejo B, Albert C, de los Santos JM, Remohi J. Outcome of cryotransfer of embryos developed from vitrified oocytes: double vitrification has no impact on delivery rates. Fertil Steril. 2013;99:1623–1630. doi: 10.1016/j.fertnstert.2013.01.106. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Luo K, Cheng D, Tan Y, Lu C, He H, Gu Y, Lu G, Gong F, Lin G. Number of biopsied trophectoderm cells is likely to affect the implantation potential of blastocysts with poor trophectoderm quality. Fertil Steril. 2016;105:1222–1227.e4. doi: 10.1016/j.fertnstert.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Neal SA, Morin SJ, Tiegs AW, Sun L, Franasiak JM, Kaser DJ, Hong KH, Werner MD, Scott RT., Jr Repeat biopsy for preimplantation genetic screening (PGS) reanalysis does not adversely impact obstetrical outcomes. Fertil Steril. 2018;109:e41. doi: 10.1016/j.fertnstert.2018.02.080. [DOI] [Google Scholar]
- 10.Lee H, McCulloh DH, Olivares R, Goldstein-Tufaro A, McCaffrey C, Grifo J. Live births after transfer of rebiopsy and revitrification of blastocyst that had “no diagnosis” following trophectoderm biopsy. Fertil Steril. 2016;106:e164. doi: 10.1016/j.fertnstert.2016.07.483. [DOI] [Google Scholar]
- 11.Kaing A, Kroener L, Brower M, Hill D, Danzer H, Barritt J. Rebiopsy and preimplanation genetic screening (PGS) reanalysis demonstrate the majority of originally “no diagnosis” embryos are euploid with comparable pregnancy rates. Fertil Steril. 2015;104:e277. doi: 10.1016/j.fertnstert.2015.07.869. [DOI] [Google Scholar]
- 12.Swain JE, Schoolcraft WB, Katz-Jaffe M. Dual trophectoderm biopsy on the same blastocyst does not impair clinical outcomes. Fertil Steril. 2015;104:e186. doi: 10.1016/j.fertnstert.2015.07.577. [DOI] [Google Scholar]
- 13.Brower M, Hill D, Danzer H, Surrey M, Ghadir S, Chang W, Wambach C, Alexander C, Barritt J. “No diagnosis” embryos after PGS should not be discarded: rebiopsy and reanalysis demonstrate the majority are euploid. Fertil Steril. 2014;102:e31. doi: 10.1016/j.fertnstert.2014.07.114. [DOI] [Google Scholar]
- 14.De Vos A, Van Landuyt L, De Rycke M, Verdyck P, Verheyen G, Buysse A, Belva F, Keymolen K, Tournaye H, Verpoest W. Multiple vitrification-warming and biopsy procedures on human embryos: clinical outcome and neonatal follow-up of children. Hum Reprod. 2020;35:2488–2496. doi: 10.1093/humrep/deaa236. [DOI] [PubMed] [Google Scholar]
- 15.Neal SA, Sun L, Jalas C, Morin SJ, Molinaro TA, RTJ S. When next-generation sequencing-based preimplantation genetic testing for aneuploidy (PGT-A) yields an inconclusive report: diagnostic results and clinical outcomes after re biopsy. J Assist Reprod Genet. 2019;36:2103–2109. doi: 10.1007/s10815-019-01550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parriego M, Coll L, Vidal F, Boada M, Devesa M, Coroleu B, Veiga A. Inconclusive results in preimplantation genetic testing: go for a second biopsy? Gynecol Endocrinol. 2019;35:90–92. doi: 10.1080/09513590.2018.1497153. [DOI] [PubMed] [Google Scholar]
- 17.Cimadomo D, Rienzi L, Romanelli V, Alviggi E, Levi-Setti PE, Albani E, Dusi L, Papini L, Livi C, Benini F, Smeraldi A, Patassini C, Ubaldi FM, Capalbo A. Inconclusive chromosomal assessment after blastocyst biopsy: prevalence, causative factors and outcomes after re-biopsy and re-vitrification. A multicenter experience. Hum Reprod. 2018;33:1839–1846. doi: 10.1093/humrep/dey282. [DOI] [PubMed] [Google Scholar]
- 18.Bradley CK, Livingstone M, Traversa MV, SJ MA. Impact of multiple blastocyst biopsy and vitrification-warming procedures on pregnancy outcomes. Fertil Steril. 2017;108:999–1006. doi: 10.1016/j.fertnstert.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Tan K, Gong F, Gu Y, Tan Y, Lu C, Luo K, Lu G, Lin G. Blastocysts can be rebiopsied for preimplantation genetic diagnosis and screening. Fertil Steril. 2014;102:1641–1645. doi: 10.1016/j.fertnstert.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Bori L, Meseguer F, Valera MA, Galan A, Remohi J, Meseguer M. The higher the score, the better the clinical outcome: retrospective evaluation of automatic embryo grading as a support tool for embryo selection in IVF laboratories. Hum Reprod. 2022;37:1148–1160. doi: 10.1093/humrep/deac066. [DOI] [PubMed] [Google Scholar]
- 21.Ardoy M, Calderón G, Arroyo G, Cuadros J, Figueroa M, Herrer R. ASEBIR criteria for the morphological evaluation of human oocytes, early embryos and blastocysts. In: ASEBIR clinical embryology papers; 2008. [Google Scholar]
- 22.Gardner DK. Schoolcraft WB In vitro culture of human blastocysts: towards reproductive certainty: infertility and genetics beyond. Carnforth: Parthenon Press; 1999. [Google Scholar]
- 23.Boada M, Carrera M, De La Iglesia C, Sandalinas M, Barri PN, Veiga A. Successful use of a laser for human embryo biopsy in preimplantation genetic diagnosis: report of two cases. J Assist Reprod Genet. 1998;15:302–307. doi: 10.1023/a:1022548612107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coll L, Parriego M, Carrasco B, Rodriguez I, Boada M, Coroleu B, Polyzos NP, Vidal F, Veiga A. The effect of trophectoderm biopsy technique and sample handling on artefactual mosaicism. J Assist Reprod Genet. 2022;39:1333–1340. doi: 10.1007/s10815-022-02453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizobe Y, Kuwatsuru Y, Kuroki Y, Fukumoto Y, Tokudome M, Moewaki H, Watanabe M, Iwakawa T, Takeuchi K. The effects of differences in trophectoderm biopsy techniques and the number of cells collected for biopsy on next-generation sequencing results. Reprod Med Biol. 2022;21:e12463. doi: 10.1002/rmb2.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin A, Rodrigo L, Beltran D, Meseguer M, Rubio C, Mercader A, de Los Santos MJ. The morphokinetic signature of mosaic embryos: evidence in support of their own genetic identity. Fertil Steril. 2021;116:165–173. doi: 10.1016/j.fertnstert.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 27.Cobo A, Vajta G, Remohi J. Vitrification of human mature oocytes in clinical practice. Reprod Biomed Online. 2009;19(Suppl 4):4385. [PubMed] [Google Scholar]
- 28.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300–308. doi: 10.1016/s1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 29.Tiegs AW, Tao X, Zhan Y, Whitehead C, Kim J, Hanson B, Osman E, Kim TJ, Patounakis G, Gutmann J, Castelbaum A, Seli E, Jalas C, RTJ S. A multicenter, prospective, blinded, nonselection study evaluating the predictive value of an aneuploid diagnosis using a targeted next-generation sequencing-based preimplantation genetic testing for aneuploidy assay and impact of biopsy. Fertil Steril. 2021;115:627–637. doi: 10.1016/j.fertnstert.2020.07.052. [DOI] [PubMed] [Google Scholar]
- 30.Coello A, Nohales M, Meseguer M, de Los Santos MJ, Remohi J, Cobo A. Prediction of embryo survival and live birth rates after cryotransfers of vitrified blastocysts. Reprod Biomed Online. 2021;42:881–891. doi: 10.1016/j.rbmo.2021.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Schoolcraft WB, Treff NR, Stevens JM, Ferry K, Katz-Jaffe M, RTJ S. Live birth outcome with trophectoderm biopsy, blastocyst vitrification, and single-nucleotide polymorphism microarray-based comprehensive chromosome screening in infertile patients. Fertil Steril. 2011;96:638–640. doi: 10.1016/j.fertnstert.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 32.Cram DS, Leigh D, Handyside A, Rechitsky L, Xu K, Harton G, Grif RC, Fragouli E, Kahraman S, Forman E, Katz-Jaffe M, Tempest H, Thornhill A, Strom C, Escudero T, Qiao J, Munne S, Simpson JL, Kuliev A. PGDIS Position Statement on the Transfer of Mosaic Embryos 2019. Reprod Biomed Online. 2019;39(Suppl 1):e1–e4. doi: 10.1016/j.rbmo.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Kelk DA, Sawarkar SS, Liu Y, Dufton M, Ribustello L, Munne S. Does laser assisted biopsy introduce mosaic or chaotic changes to biopsied cells? Fertil Steril. 2017;108:e88. doi: 10.1016/j.fertnstert.2017.07.272. [DOI] [Google Scholar]
- 34.Johnson D, Haimowitz Z, Arifova M, Akopians A, Welch C, Barritt J. Repeated high intensity laser biopsy pulses do not alter genetic testing results nor increases mosaicism following human embryo blastocyst biopsy of trophectoderm cells. Reprod Biomed. 2019;39:e4–e5. doi: 10.1016/j.rbmo.2019.07.013. [DOI] [Google Scholar]
- 35.Benavent M, Escriba M, Miret C, Vanrell I, Costa-Borges N, Calderón G, Crespo J, Teruel J. Evaluation of the impact of the pulling and flicking trophectoderm biopsy procedures on the integrity of the biopsied cells and their correlation to PGT-A results. Fertil Steril. 2019;112:e242. doi: 10.1016/j.fertnstert.2019.07.1377. [DOI] [Google Scholar]
- 36.Taylor TH, Patrick JL, Gitlin SA, Michael Wilson J, Crain JL, Griffin DK. Outcomes of blastocysts biopsied and vitrified once versus those cryopreserved twice for euploid blastocyst transfer. Reprod Biomed Online. 2014;29:59–64. doi: 10.1016/j.rbmo.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, Katz-Jaffe MG, Wells D. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95:520–524. doi: 10.1016/j.fertnstert.2010.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Incidence of inconclusive results after PGT-A and frequency of technique usage across biopsy operators. Column proportions were compared using a Chi-square test with Bonferroni correction at the 95% confidence level. The same letters for each parameter indicate homogeneous subsets. PC: pairwise comparisons. PGT-A: Preimplantation Genetic Testing for Aneuploidy.
Data Availability Statement
Data regarding any of the subjects in the study have not been previously published.