Abstract
Purpose
Trehalose is a non-permeable protectant that is the key to preserve live cells in a dry state for potential storage at ambient temperatures. After intracellular trehalose delivery via cold-responsive nanoparticles (CRNPs), the objective was to characterize the tolerance of cat cumulus-oocyte complexes (COCs) to different levels of microwave-assisted dehydration.
Methods
Trehalose was first encapsulated in CRNPs. After exposure to trehalose-laden CRNPs, different water amounts were removed from cat COCs by microwave drying. After each dehydration level, meiotic and developmental competences were evaluated via in vitro maturation, fertilization, and embryo culture. In addition, expressions of critical genes were assessed by quantitative RT-PCR.
Results
CRNPs effectively transported trehalose into COCs within 4 h of co-incubation at 38.5 °C followed by a cold-triggered release at 4 °C for 15 min. Intracellular presence of trehalose enabled the maintenance of developmental competence (formation of blastocysts) as well as normal gene expression levels of HSP70 and DNMT1 at dehydration levels reaching up to 63% of water loss.
Conclusion
Intracellular trehalose delivery through CRNPs improves dehydration tolerance of COCs, which opens new options for oocyte storage and fertility preservation at ambient temperatures.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02831-x.
Keywords: Cold-responsive nanoparticles, Cumulus-oocyte complex, Microwave-assisted dehydration, Trehalose, Fertility preservation
Introduction
Germ cell preservation for the long term is a powerful tool to extend fertility in human, livestock, and endangered species [1]. Currently, sperm cells and oocytes are cryopreserved and stored in liquid nitrogen in specialized facilities. However, the high costs and maintenance requirement of cryobanking render it less accessible for some regions of the world and human populations. In the meantime, dry preservation is being developed as a more economical alternative to cryopreservation. It would allow storage of biomaterials in the dry state at non-cryogenic temperatures, therefore, eliminating the need and expenses for maintaining biological samples in liquid nitrogen.
Several drying techniques have been developed for long-term preservation, including air drying, convective drying, microwave-assisted drying, and freeze drying [2]. While extensive injuries in the membrane and cytosolic structures severely compromise the survival of the whole cells, successful preservation of the nuclear genome has been reported for sperm, oocytes, and somatic cells in various mammalian species [2, 3]. However, the use of dried/rehydrated nuclear genome requires additional technologies, such as intracytoplasmic sperm injection, germinal vesicle (oocyte’s nucleus) transfer, or somatic cell nuclear transfer [4–7]. These micromanipulations are labor intensive, often result in low success rate, and are likely to introduce unintended long-term effects. Developing methods to protect whole cells, especially germ cells, could simplify the downstream usage of dry-preserved biomaterials.
Lessons learned from natural anhydrobiotic organisms are guiding the research explorations to preserve whole cells and tissues from vertebrate species. Collective findings in small organisms also highlight the protective properties of trehalose, a non-reducing disaccharide sugar [8]. Trehalose increases dehydration tolerance of cells by maintaining three-dimensional conformation of macromolecules, preventing collapse or aggregation of cellular structures, and stabilizing cellular components in amorphous trehalose glass formed after dehydration [8]. Unlike these anhydrobiotic species, mammalian cells do not synthesize trehalose and sugars do not permeate cell membranes. Intracellular delivery of trehalose prior to dehydration therefore is a critical prerequisite. When focusing solely on preserving nuclei, disruptions of plasma membranes (with hemolysin, for instance [9, 10]) have been applied to ensure trehalose incorporation. However, new approaches are required to deliver trehalose when targeting the whole gamete while retaining membrane integrity as well as proper cellular and molecular functions.
Cold-responsive nanoparticles (CRNPs) are considered a promising approach to naturally deliver and incorporate trehalose into the living cells prior to dehydration. CRNPs consist of poly(lactic-co-glycolic acid) (PLGA), pluronic F127 (PF127), and poly(N-isopropylacrylamide-co-butyl acrylate) (pNIPAM-B), all are FDA-approved polymers for their biocompatibility and biodegradability [11]. Specifically, pNIPAM-B is thermally responsive and can serve as a switch for CRNP disassembly when exposed to temperatures below 16 °C. Previous studies showed that trehalose-encapsulated CRNPs can enter cells via natural endocytosis and then disassemble upon cold treatment at 4 °C to release trehalose intracellularly [11].
Regarding dehydration, our laboratory has previously established microwave-assisted dehydration procedure for domestic cat germinal vesicles with promising outcome, including the preservation of structural ad function properties [10, 12]. We now aim to expand the preservation targets to the cumulus-oocyte complexes (COCs), the functional unit for subsequent oocyte maturation. The first step toward this aim is to explore noninvasive trans-membrane transport methods of trehalose. The objectives of the present study therefore were to (1) evaluate the use of CRNP-assisted trehalose delivery into cat COCs and (2) measure the beneficial effect of trehalose during microwave-assisted dehydration. We specifically examined the COC functions and selected gene expressions to characterize the impacts of trehalose delivery and subsequent drying.
Materials and methods
All chemicals were purchased from Sigma-Aldrich unless otherwise indicated.
Experimental design
We first characterized CRNP-facilitated intracellular delivery of encapsulated cargos into COCs using doxorubicin hydrochloride (DOX) as a fluorescent marker. COCs (n = 178 in 3 replicates) were incubated with DOX-laden CRNPs at 38.5 ˚C for 1, 2, 3, or 4 h. A portion of the COCs underwent cold treatment (15 min at 4 °C) after the incubation periods while the others did not. Fluorescent uptakes of the COCs and denuded oocytes were examined immediately after incubation or cold treatment.
Before we utilize CRNPs for dry preservation, it was critical to ensure that CRNP exposure was not cytotoxic to COCs. We therefore investigated the effect of CRNP exposure and cold treatment on the ability of COCs to reach the metaphase II (MII) stage and support early embryo development. COCs (n = 297 in 5 replicates) were randomly allocated into three groups and incubated without CRNPs, with blank CRNPs, or with trehalose-encapsulated CRNPs. Half of the COCs from each group underwent cold treatment while the other half did not. All the COCs were then in vitro matured, fertilized, and cultured to assess meiotic and developmental competence.
After investigating the safety of CRNP exposure, we used CRNPs as a vehicle to transport trehalose into COCs and evaluated the impact of intracellular trehalose on dehydration tolerance. A drying curve of trehalose solution was first established to determine the level of water removal at different microwave drying times (n = 6 per time point). COCs (n = 350 in 5 replicates) were exposed to either blank or trehalose-encapsulated CRNPs, cold triggered, and then underwent microwave-assisted drying for 0, 15, 20, or 30 min (corresponding to removal of 0, 47, 63, and 88% of water, respectively) (Supplemental Fig. 1). COCs then were rehydrated, and their meiotic and developmental competence was assessed.
Lastly, we further explored the molecular response to CRNP exposure and dehydration within cumulus cells and oocytes after treatment conditions that were selected from the previous experiments. A portion of fresh COCs (n = 90 in 6 replicates) were collected from each collection batch to serve as controls. The rest of the COCs (n = 360 in 6 replicates) were exposed to either blank or trehalose-laden CRNPs followed by cold treatment. Half of the COCs from each group was collected without dehydration (time 0) and the other half was collected after 20 min of dehydration followed by rehydration (time 20). COCs then were mechanically denuded to extract RNA and assess RNA integrity in oocytes and cumulus cells separately. For quantitative real-time PCR (qPCR) analysis, 3 genes were selected as markers based on their critical roles in these cells, including caspase-3 (CASP3; apoptosis), heat shock protein 70 (HSP70; stress response, chaperone), and DNA methyltransferase 1 (DNMT1; epigenetics, embryo development) (Table 1).
Table 1.
| Genes accession number | Primer sequences (5′–3′) | Amplicon size (bp) | Annealing temperature (°C) | Primers designed in |
|---|---|---|---|---|
| CASP3 | F: ACCGGCAAACCCAAACTC | 91 | 60.5 | Amelkina et al. 2015 |
| NM_001009338 | R: CTGACAGGCGATGTCATCC | |||
| HSP70 | F: AGCAGGTGTGTAACCCCATC | 135 | 57.5 | This study |
| XM_019830386.3 | R: AAGGATCCTAATCCACCTCCTC | |||
| DNMT1 | F: ACGTCAGACCATCGGGCATT | 185 | 61.0 | This study |
| XM_019817312.2 | R: GCTGTTGACACACCTCGCAG | |||
| ACTB | F: ACCCACACTGTGCCCATCTA | 169 | CCs: 61.2 | Hachen et al. 2012 |
| ON164672 | R: CTCCAGGGAGGACGAGGAC | Oocytes: 59.7 | ||
| CYPA | F: CCTTCTGTAGCTCGGGTGAG | 113 | CCs: 61.2 | Siemieniuch et al. 2012 |
| NM_001009370 | R: CTTGGAGGGGAGGTAAGGAG | Oocytes: 59.0 | ||
| RPS7 | F: GCAGAAGCGTCCCAGAAGC | 129 | 63.0 | Braun et al. 2012 |
| AY800278 | R: ACCTTTATGAGCCGGCTGC |
Synthesis of CRNPs
CRNPs were prepared by a double emulsion (water-in-oil-in-water or W–O-W) according to a previously reported protocol [11]. Briefly, a stock solution of 1.3 M trehalose in deionized (DI) water was prepared as the first water phase. For the oil phase, 15 mg of PLGA, 30 mg of pNIPAM-B, and 10 mg of PF127 were dissolved in 2 mL of dichloromethane (DCM). To form the first W–O emulsion, 300 µL of trehalose solution was added dropwise to the oil phase and emulsified at an amplitude of 18% for 1 min using a Branson 450 sonifier. The first W–O emulsion was then added to the second water phase, 10 mL of 2% polyvinyl alcohol (PVA) solution (in DI water), and emulsified at an amplitude of 24% for 1 min to obtain the double emulsion (W–O-W). After rotary evaporation at 37 °C to remove DCM, the nanoparticle suspension was stirred for 15 min at 1200 rpm. The nanoparticles were collected by centrifugation at 10,000 × g for 10 min at room temperature and washed once with DI water.
Collection of COCs
The Animal Care and Use Committee from the Smithsonian’s National Zoo and Conservation Biology Institute granted a waiver of the animal care and use approval for that study because testes and ovaries were collected at local veterinary clinics as byproducts from owner-requested routine neutering and spaying. Ovaries from adult domestic cats were recovered after routine ovariohysterectomy and stored in Dulbecco’s PBS (DPBS) supplemented with 100 IU/mL penicillin and 100 μg/mL streptomycin at 4 °C until processing within 24 h. COCs were mechanically isolated into HMEM medium (HEPES-buffered MEM supplemented with 2 mM L-glutamine, 1 mM pyruvate, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 4 mg/mL bovine serum albumin (BSA). Only grade 1 (uniformly dark cytoplasm, 5 or more layers of cumulus cells) and grade 2 (same as grade 1, but with < 5 cell layers) COCs were collected based on standard classification criteria [17].
Cell uptake of nanoparticles and cold-triggered release
To prepare CRNPs for cell uptake, nanoparticles were rinsed with culture medium (MEM supplemented with 1 mM pyruvate, 2 mM L-glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 4 mg/mL BSA), centrifuged at 9600 × g for 10 min, and resuspended in culture medium to 1 mg/mL via sonication and pipetting. To allow cell uptake of nanoparticles, COCs were incubated in resuspended CRNPs for desired duration at 38.5 ˚C under a controlled humidified atmosphere with 5% CO2. Additionally, 250 nM of milrinone was added to the medium to prevent meiosis resumption during exposure [18]. To trigger release of encapsulated materials after CRNP exposure, COCs underwent a cold treatment by incubating them at 4 ˚C for 15 min.
Assessment of cell uptake of DOX-laden nanoparticles
DOX is an intrinsically fluorescent molecule with an emission signal at 595 nm upon excitation [19]. Its fluorescent property and high DNA affinity makes it an ideal probe for tracking cellular uptake and release of nanoparticles. To assess cell uptake and cold-triggered release of DOX-laden nanoparticles, COCs were placed on a concave slide after CRNP exposure/cold-treatment and examined using an Olympus BX41 epifluorescence microscope with SPOT advanced software 5.0 (Diagnostic Instruments, Inc.). After images were taken, COCs were immediately denuded and fixed in 4% paraformaldehyde (PFA) at room temperature for 30 min. Denuded oocytes were then rinsed twice with PBS, mounted with Vectashield mounting medium with DAPI (Vector Laboratories) and examined under an Olympus BX41 epifluorescence microscope. Images of COCs or denuded oocytes from the same cohort were taken under the same configurations and focal plans to allow comparisons. To measure fluorescent intensity of DOX on the images, the limits of COCs or oocytes were delineated, and the mean fluorescent intensity (sum of fluorescent values of all the pixels in the region of interest divided by the number of pixels) was recorded using ImageJ software (National Institutes of Health). The mean fluorescent intensity of the background area in the same image was also recorded. Final DOX intensity was presented as mean fluorescent intensity of COC or oocyte subtracted by mean background intensity.
In vitro maturation (IVM), in vitro fertilization (IVF), and embryo culture
Control or CRNP-loaded COCs were cultured in protein plus blastocyst medium (SAGE) containing 1 µg/mL of ovine luteinizing hormone (National Hormone and Pituitary Program) and 50 AU/µL of porcine follicle-stimulating hormone for 26 h in 38.5 ˚C incubators with 5% CO2 and humidified air atmosphere. After rinsing with blastocyst medium, COCs were incubated with 1 × 106/mL of fresh or frozen-thawed motile cat epididymal spermatozoa for IVF. At 20 h post-insemination, COCs were denuded and cleaned by gentle pipetting. Presumptive zygotes were cultured in blastocyst medium for up to 7 days before fixation with 4% PFA. The fixed samples were then mounted with Vectashield mounting medium with DAPI. Maturation of oocyte was defined by MII chromosomal alignment in non-fertilized oocytes. Final percentages of MII were determined retrospectively by including oocytes that had been fertilized and/or formed embryos. Final embryo stages were determined by number of blastomeric nuclei as well as the presence or absence of a blastocoele [20]. Percentages of cleaved embryos and those that had reached at least the 8-cell stage were determined retrospectively.
Assessment of water content of trehalose solution during microwave drying
As water molecules evaporate during microwave drying, the amount of water left in the samples decreases. Measuring the residual water levels in microwaved samples thus allowed us to determine the level of water removal during the dehydration process. To this end, kinetics of water content of 0.3 M trehalose solution (in Tris–EDTA buffer) during microwave drying was determined as previously described [9]. Briefly, 40 µL of trehalose solution was deposited onto each glass coverslip and dried with a SAM 255 microwave (CEM) at 20% power with upper temperature threshold set at 40 ˚C. Water weight was measured with a Karl Fisher titrator (Mettler-Toledo V20) at 5 min intervals for up to 40 min. To obtain the dried weight of 40 µL of 0.3 M trehalose solution, the total weight of the trehalose solution was measured with analytical balance (XP-150, Mettler Toledo) immediately before water content measurement. The dried weight was calculated by subtracting water weight from the total weight of the sample. The water content was expressed as gram H2O per gram dried weight (gH2O/gDW) and as percentage of water weight without drying.
Microwave-assisted drying and rehydration of COCs
Microwave drying was adapted from previously described procedures [20]. CRNP-loaded COCs were transferred to 0.3 M trehalose in Tris–EDTA buffer. Suspension of COCs (up to 10) in 40 µL trehalose solution was deposited on each glass coverslip and dried for 0, 15, 20, or 30 min in the microwave with the same setting as previously mentioned. For rehydration, 500 µL of HMEM was deposited on top of the samples and incubated at 38.5 ˚C for 30 min.
RNA isolation and cDNA synthesis
For each sample, 15 COCs were pooled together, and total RNA was isolated separately from cumulus cells (n = 6) and oocytes (n = 6) using Arcturus PicoPure RNA Isolation Kit (Thermo Fisher) with RNase-Free DNase Set (Qiagen) following manufacturer protocol. In brief, after COCs were mechanically denuded with a stripper pipette, oocytes were rinsed with PBS and stored in extraction buffer at – 80 ˚C until RNA isolation. Dissociated cumulus cells were collected by centrifugation, rinsed with PBS, and stored in extraction buffer at – 80 ˚C until RNA isolation. Concentration and integrity of isolated RNA were assessed with RNA ScreenTape (cumulus cells) and High Sensitivity RNA ScreenTape (oocytes) using TapeStation System (Agilent). Either 150 ng (cumulus cells) or 3 ng (oocytes) of isolated RNA was reverse transcribed into single-stranded (ss) cDNA using SuperScript™ III First-Strand Synthesis SuperMix for qRT-PCR (Invitrogen).
Quantitative real-time PCR
Primers for qPCR were either designed in this study or taken from previous studies on domestic cat reproductive tissues (Table 1). The qPCR was performed using the CFX Opus 96 Real-Time PCR System (Bio-Rad). For this, diluted ss cDNA (4 µL, corresponding to 15 ng and 600 pg of total RNA for cumulus cells and oocytes, respectively) was analyzed in a 10 µL reaction volume including SsoAdvanced™ Universal SYBR® Green Supermix (Bio-Rad). The qPCR conditions were 95 °C for 2 min and 40 cycles of 8 s at 95 °C and 10 s at different annealing temperatures (Table 1). Additional melt curve step was added at the end for quality control and optimization. Quantification of qPCR products and validation of reference genes was performed using the CFX Maestro Software 2.3 (Bio-Rad). Standard curves built using serial dilutions of pooled ss cDNA separately for cumulus cells and oocytes were used for optimization and efficiency calculation. Ribosomal protein s7 (RPS7), β-actin (ACTB), and cyclophilin A (CYPA) were validated as optimal reference genes for our experiment and were used to calculate relative normalized expression for genes of interest with ΔΔCq method [21, 22].
Statistical analyses
Fluorescent intensity values of DOX were assessed by Shapiro–Wilk normality tests. Data were analyzed by Kruskal–Wallis test followed by Dunn’s multiple tests. For assessment of meiotic and developmental competence, data from all replicates were pooled and analyzed by chi-square analyses. RNA integrity number (RIN) was assessed by analysis of variance (ANOVA). Kruskal–Wallis rank sum test was used to determine changes in relative mRNA levels of these genes in cumulus cells and oocytes throughout different treatments. Wilcoxon rank sum test was used for post-hoc pairwise comparison of treatment groups (p-value adjustment: Benjamini-Hochberg). One replicate from trehalose-exposed cumulus cells (time 20 group) and two from blank CRNP-exposed oocytes groups (one from time 0 and one from time 20) were outliers and were excluded from analyses. In all test, differences were considered significant at p < 0.05 (GraphPad Prism 7.03 or R version 4.1.3).
Results
Intracellular cargo delivery into COCs by CRNPs
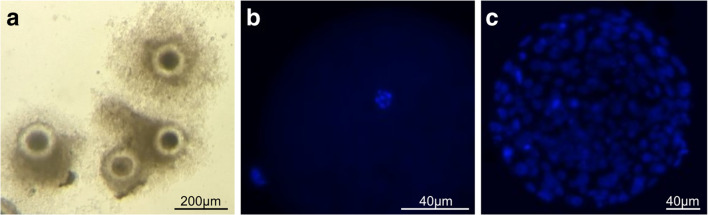
CRNPs encapsulated with DOX, a fluorescent probe, was utilized to monitor the kinetics of intracellular cargo uptake and release into COCs. There was no detectable background fluorescent signal in cat COCs before exposure (Fig. 1a and b). Strong DOX signal was detected in the whole COCs after only 1 h of incubation with CRNPs (p < 0.05) and was maintained at the similar intensity thereafter (Fig. 1c and e). In the oocytes (after removal of the surrounding cumulus cells), DOX signal remained weak in the first 2 h of incubation, but the intensity gradually increased between 2 and 4 h duration (Fig. 1d and e). Importantly, DOX signal was observed in the nuclei of the oocytes (germinal vesicle) after cold treatment due to its high DNA affinity (Fig. 1d), indicating successful cargo release from disassembled CRNPs.
Fig. 1.

Uptake and release of DOX-laden CRNPs in cat COCs. Representative images of COCs before CRNP exposure (a brightfield and b fluorescent) and after 4-h exposure to CRNPs followed by cold treatment (c) and after removal of most cumulus cells (d). e Fluorescent intensity (mean ± SEM) after exposure to CRNPs in COCs or oocytes. Within each cell type, values with different letters differ across time points, p < 0.05. Scale bars = 50 µm. Arrowhead: germinal vesicle
Effect of CRNP exposure and cold treatment on meiotic and developmental competence of COCs
To determine the safety of applying CRNPs to COCs, we examined the effect on the meiotic and developmental competence of COCs after 4 h of CRNP exposure and/or 15 min of cold treatment at 4 ˚C. Cumulus cell expansion (Fig. 2a) was observed in all groups, regardless of treatment. Without cold treatment, percentages of COCs reaching MII, cleaving after IVF, and developing to 8-cell or blastocyst stages were similar among the control group without nanoparticles and the groups exposed to either blank or trehalose-laden CRNPs (p > 0.05, Fig. 2b and c, Table 2). After cold treatment, a lower proportion of COCs exposed to blank CRNPs reached the MII stage compared to the control group without CRNPs or with CRNP and no cold treatment (p < 0.05, Table 2). Both blank and trehalose-laden CRNP-exposed groups also led to higher proportions of cleaved embryos than their non-exposed counterpart after cold treatment (p < 0.05, Table 2). Percentages of 8-cell embryos were not affected by the treatments (p > 0.05, Table 2). Percentages of blastocysts were lower only after exposure to blank CRNP and cold treatment (p < 0.05, Table 2).
Fig. 2.
Assessment of meiotic and developmental competence of COCs. Representative images of COCs with expanded cumulus (a), metaphase II oocyte with first polar body (b), and resulting blastocyst after 7 days of in vitro culture (c)
Table 2.
Meiotic and developmental competence of COCs after CRNP exposure and/or cold treatment (15 min of cold treatment at 4 °C). Data were pooled from 5 replicates. Within column, values with different letters differ (p < 0.05)
| CRNP | Cold treatment | N | MII %* | Cleaved embryos %# | 8-cell embryos %# | Blastocysts %# |
|---|---|---|---|---|---|---|
| None | − | 46 | 61% (28/46)a | 79% (22/28)ab | 79% (22/28) | 36% (10/28)a |
| + | 48 | 56% (27/48)ab | 59% (16/27)a | 59% (16/27) | 19% (5/27)ab | |
| Blank | − | 48 | 60% (29/48)a | 69% (20/29)ab | 69% (20/29) | 24% (7/29)ab |
| + | 55 | 40% (22/55)b | 91% (20/22)b | 82% (18/22) | 5% (1/22)b | |
| Trehalose | − | 50 | 52% (26/50)ab | 81% (21/26)ab | 77% (20/26) | 38% (10/26)a |
| + | 50 | 44% (22/50)ab | 86% (19/22)b | 82% (18/22) | 14% (3/22)ab |
* Proportions of metaphase II (MII) oocytes out of the total number of COCs (N) in each treatment group
# Proportions of embryos out of the total number of matured oocytes in each treatment group
N, total number of COCs; MII, oocytes reaching metaphase II
Effect of CRNP-delivered trehalose on dehydration tolerance of COCs
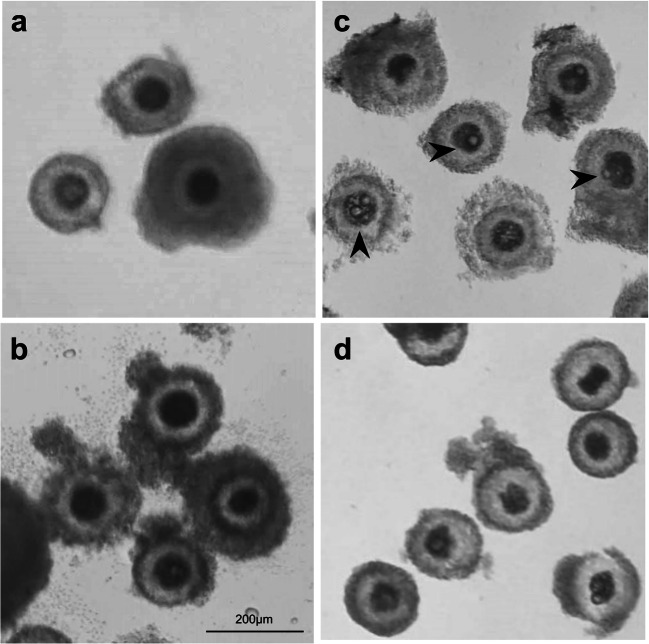
To assess the effect of CRNP-delivered trehalose on dehydration tolerance of COCs, we examined the effect on the meiotic and developmental competence of trehalose-loaded COCs rehydrated after different microwave drying times. After 0, 15, or 20 min of drying in a 40-µL drop of 0.3 M trehalose, trehalose was partially dehydrated and remained in the liquid state (Fig. 3a). Oocyte morphology was normal and cumulus cell expansion was observed after all treatments (both blank and trehalose CRNP-loaded groups) following rehydration and IVM (Fig. 4a and b). After 30 min of drying, 0.3 M trehalose had transformed into glass state (Fig. 3b). Abnormal morphology and/or large cellular vacuoles were observed in all oocytes from the 30-min drying groups (both blank and trehalose CRNP-loaded groups) after rehydration, and cumulus cell expansion did not take place after IVM (Fig. 4c and d). In the groups of COCs loaded with blank CRNPs, percentage of COCs reaching MII decreased after 20 min of drying compared to 0 min (p < 0.05, Table 3). In the groups of COCs loaded with CRNPs containing trehalose, the percentage of COCs reaching MII decreased after 15 and 20 min compared to the 0 min controls (p < 0.05, Table 3). After 30 min of drying, none of the COCs reached MII stage. Among matured oocytes loaded with blank CRNPs, incidence of embryos cleaving and reaching 8-cell stage decreased after 20 min microwaving compared to the controls without dehydration (p < 0.05, Table 3). None of these 20-min-dehydrated oocytes were able to form blastocysts (Table 3). However, statistical significance was not met, likely due to relatively small number of matured oocytes after partial dehydration. On the other hand, among the trehalose-loaded, mature oocytes, the percentages of embryos cleaving, reaching 8-cell stage, and forming blastocysts were similar to the controls without dehydration (p > 0.05, Table 3).
Fig. 3.
Dehydration of COCs in 0.3 M trehalose solution. Representative images showing trehalose in liquid state (15 min) (a) and glass state (30 min) (b). Insets: Magnification showing the edge of the trehalose drops
Fig. 4.
Cumulus-oocyte complex morphology following rehydration (a, c) and cumulus cell expansion after IVM (b, d). Representative images of normal oocyte morphology after partial dehydration and rehydration (a) and expanded cumulus cells after IVM (b). Representative images of abnormal oocyte morphology and large vacuoles (arrowheads) after 30 min of drying and rehydration (c) and lack of cumulus cell expansion after IVM (d)
Table 3.
Meiotic and developmental competence of COCs after CRNP exposure, cold treatment, different drying times, and rehydration. Data were pooled from 5 replicates. Within column, values with different letters differ (p < 0.05)
| Drying time | CRNP | N | MII %* | Cleaved embryos %# | 8-cell embryos %# | Blastocysts %# |
|---|---|---|---|---|---|---|
| 0 min | Blank | 33 | 67% (22/33)a | 77% (17/22)a | 77% (17/22)a | 18% (4/22) |
| Trehalose | 36 | 61% (22/36)a | 82% (18/22)a | 77% (17/22)a | 14% (3/22) | |
| 15 min | Blank | 47 | 51% (24/47)ab | 75% (18/24)a | 75% (18/24)a | 8% (2/24) |
| Trehalose | 46 | 41% (19/46)b | 68% (13/19)ab | 68% (13/19)ab | 16% (3/19) | |
| 20 min | Blank | 50 | 38% (19/50)b | 42% (8/19)b | 37% (7/19)b | 0% (0/19) |
| Trehalose | 47 | 40% (19/47)b | 68% (13/19)ab | 58% (11/19)ab | 11% (2/19) | |
| 30 min | Blank | 44 | 0% (0/44)c | - | - | - |
| Trehalose | 47 | 0% (0/47)c | - | - | - |
* Proportions of metaphase II (MII) oocytes out of the total number of COCs (N) in each treatment group
# Proportions of embryos out of the total number of matured oocytes in each treatment group
N, total number of COCs; MII, oocytes reaching metaphase II
Effect of CRNP-delivered trehalose followed by dehydration on RNA integrity and gene expression of COCs
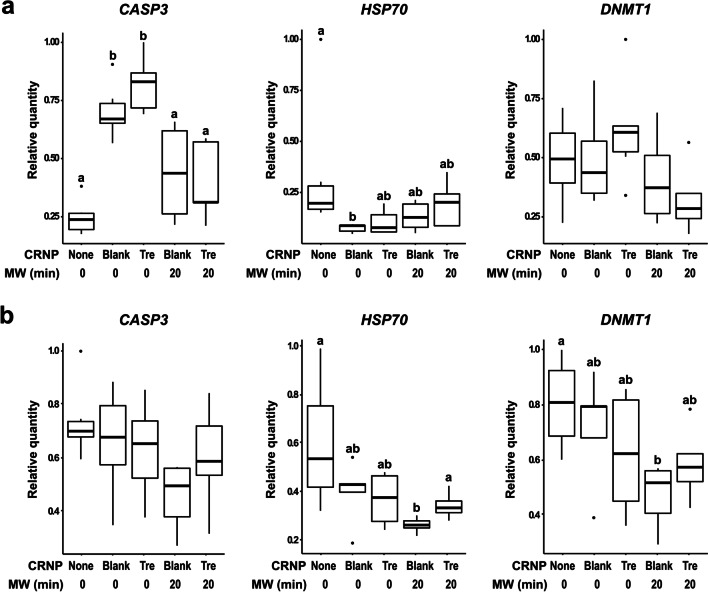
To better understand the cellular response to CRNP-delivering of trehalose and dehydration, RNA was extracted and expression of CASP3, HSP70, and DNMT1 was examined in the cumulus cells and oocytes separately. No significant difference in RNA integrity was found in either cumulus cells (RIN score range: 8.5–9.2; p > 0.05) or oocytes (RIN score range: 6.0–6.4; p > 0.05) from different groups. CASP3 expression was significantly elevated (p < 0.05) in the cumulus cells after CRNP (either blank- or trehalose-encapsulated) exposure and cold treatment, compared to the fresh control (Fig. 5a). The expression level recovered following 20 min of drying and rehydration. The alteration in CASP3 expression was not observed in the oocytes (Fig. 5b). In the cumulus cells, HSP70 expression decreased after exposure to blank, but not trehalose encapsulated, CRNPs. The expression level recovered after dehydration and rehydration. For DNMT1, no significant change (p > 0.05) in expression level was observed in the cumulus cells of any treatment groups compared to the fresh control (Fig. 5a). In the oocytes, the expression of HSP70 and DNMT1 was not significantly affected (p > 0.05) by CRNP exposure and cold treatment. However, expression levels of both genes were significantly lower (p < 0.05) after dehydration in the blank CRNP-exposed group, but not their trehalose-enclosed counterparts (Fig. 5b).
Fig. 5.
Relative mRNA expression levels of CASP3, HSP70, and DNMT1 in cumulus cells (a) and oocytes (b) after different treatments. Box plots depict the distribution of data from lower to upper quartile values; whiskers indicate minimum and maximum values; black dots indicate outliers; horizontal lines within the boxes indicate the median values. Y-axis indicates relative mRNA quantity of target genes after normalization with reference genes. Cumulus cells and oocytes from fresh COCs (without CRNP exposure, cold treatment, or drying) were used as controls. Data were from 6 replicates with cumulus cells and oocytes from 15 COCs per treatment group per replicates. MW, microwave; Blank, blank CRNPs; Tre, trehalose-laden CRNPs. Values with different letters differ (p < 0.05)
Discussion
Previous studies on oocyte dehydration have mainly targeted the nucleus of immature oocyte, the germinal vesicle, because of its higher resilient to non-physiological conditions than the whole germ cell [23, 24]. Here, we present the first effort demonstrating that the whole COC can survive partial dehydration (equivalent to 63% of water loss). Following incorporation of trehalose with CRNPs, we assessed COC functions. CRNPs effectively and safely transported and released the encapsulated cargos into COCs with 4 h of co-incubation at 38.5 °C followed by a cold treatment at 4 °C for 15 min. Intracellular delivery of trehalose via CRNPs protected developmental competence as well as gene expression levels of HSP70 and DNMT1of COCs during partial dehydration.
CRNPs possess two key features that could facilitate loading of nonpermeable protectants: the ability to enter cells through natural endocytosis and controllable release of enclosed cargos after cold-triggered disassembly. Previous research has demonstrated successful cargo loading using CRNPs into cultured cell lines [11]. However, the female gamete is a more difficult target due to its protective barrier, zona pellucida. Using a fluorescent probe (DOX), we confirmed the intracellular uptake and release of CRNP-encapsulating cargos into COCs. Importantly, DOX was detectable in the oocytes. Previous studies exposed COCs or denuded oocytes to PLGA nanoparticles and demonstrated that these nanoparticles could enter oocytes via two routes: direct uptake by oocytes (paracellular) and indirect transport via transzonal projections from cumulus cells into oocytes (transcellular) [25, 26]. CRNPs are mainly composed of PLGA and similar cellular uptake mechanisms likely apply. We speculate that the weak DOX signal in the oocytes in the first 2 h of CRNP exposure primarily came from direct endocytosis. Additional transcellular transport of CRNPs from cumulus cells then increased overtime and resulted in substantial elevation of DOX signal in the oocytes after 2 h. More experiments will be needed to elucidate the transport mechanisms.
With increasing usage of nanoparticles in industrial and biomedical fields, the potential influence of nanoparticles on fertility has become a topic of interest. Numerous studies have reported adverse effect on female germ cells after exposure to metal- or carbon-based nanoparticles [27–32]. On the other hand, some polymeric nanoparticles, such as PLGA, are considered biocompatible and have been widely used for drug delivery into mammalian cells [33]. Recent studies showed that exposing COCs or oocytes to PLGA nanoparticles had no negative impact on oocyte maturation and subsequent embryo development in mouse and cattle [25, 26]. PF127 and pNIPAM-B, the other two components of CRNPs, have been shown to be non-toxic in cell lines [34, 35], but their effects have not been evaluated in oocytes. Here we confirmed that CRNP exposure was not detrimental to the meiotic and developmental competence of cat oocytes. However, our results also suggest that current cold treatment condition was not optimal for the function of the cells. The cold treatment condition was determined based on previous CRNP research [11] as well as the wide availability of 4 °C refrigerators in most laboratories. Although ovaries can be kept in the refrigerator before processing, short-term cold treatment of collected COCs appeared to affect both maturation and embryo development processes. Moreover, CASP3 expression was significantly elevated after cold treatment in the cumulus cells, suggesting potential triggering of the apoptosis cascade in these cells. Although similar elevation of the pro-apoptotic gene was not observed in the oocytes, the bidirectional communications between oocyte and cumulus cells that are critical for oocyte competence acquisition [36] could be disrupted if large portion of cumulus cells undergo apoptosis. Previous research has shown that damage in cumulus cells during cryopreservation negatively impacted porcine oocyte fertilization and subsequent embryo development [37], confirming the importance to maintain cumulus cell quality during gamete preservation. Former studies suggest that mammalian oocytes have the ability to tolerate a certain level of chilling [38–40]. Therefore, we are currently evaluating alternative cold treatment temperatures (14–16 °C) in the follow-up studies to minimize the negative impact on oocytes while allowing adequate cold-triggered release.
Dehydration stress can induce various damages in the structures and functions of gametes [3]. This is evident in our data that without protectant, the capability of COCs to mature and sustain early embryo development noticeably decreased as over 60% of water (20-min drying) was removed. The functional damages may in part link to the change in gene expression in the oocytes without affecting overall RNA integrity. Hsp70 is a chaperone protein that plays a key role in maintaining protein stability, often in response to stressed conditions. In natural desiccation-tolerant organisms (such as brine shrimp and tardigrades), Hsp70 facilitates cellular recovery from the dried state [41, 42]. The decline in HSP70 levels in the oocytes after 20 min of drying may jeopardize recovery of proteostasis during rehydration and lead to reduced meiotic and developmental competence. DNMT1 is an essential maternal effect gene involved in the epigenetic regulations of both oocytes and early embryos [43, 44]. Disruption in maternal DNMT1 expression resulted in defects in embryo development [45]. Decreased DNMT1 expression we observed after drying may affect oocyte maturation and embryo development through aberrant epigenetic controls. It is interesting to note that CASP3 expression in the cumulus cells was no longer elevated after 20 min of drying. This suggest that the upregulation of CASP3 after CRNP exposure was temporary, and the cells were able to recover from the cold shock even after partial dehydration. When 88% of water was removed (30-min drying), structural integrity of oocytes could no longer be maintained and COCs were likely not viable or functional. This could partly result from fragmentation or disorganization of membranes and cytoskeletons, knowing that both are vulnerable to dehydration stress [3].
Trehalose has been used as a protectant against dehydration stress in gametes [3]. Studies focused on germinal vesicles demonstrated that drying in the presence of trehalose preserved DNA integrity, meiotic competence, and developmental potential of the nuclei in cats and pigs [5, 6, 10]. Expanding the target to the whole COC unit, we found that trehalose exposure did not improve the ability of COCs to reach MII stage. Encouragingly, the deficiency in developmental potential in partially dried (20 min) COCs was mitigated in the trehalose-loaded group. Corroborating with the findings, declined expression of both HSP70 and DNMT1 in partially dried oocytes was mitigated in the presence of trehalose while CASP3 expression remained unaltered. Taken together, our work suggests that the protective properties of trehalose to mitigate damages from partial dehydration and sustain developmental potential of the female gametes could be extended from germinal vesicle to the whole COC unit. Expression of these selected genes under protective partial dehydration of COCs appears comparable to the outcome of vitrification (current standard cryopreservation method for COCs). It has been reported that vitrification did not affect expression of HSP70 and DNMT1 in canine COCs [46]. Similarly, microarray analysis revealed that expression of DNMT1 and CASP3 was unaltered in vitrified-warmed bovine COCs [47]. Moreover, several studies have shown that COC vitrification either had no effect on the expression of apoptotic genes or led to upregulation of anti-apoptotic genes in pig, sheep, cattle, and dog [46, 48–50]. There is currently limited information on the molecular response to dehydration stress and mitigation in the gametes [3]. A more comprehensive transcriptomic study is warranted to understand the underlying mechanisms that lead to the dehydration injuries and recoveries.
Preserving genome through dehydration and storage at ambient temperatures is the next frontier for biobanking. The present study is the first effort to dry preserve whole COC unit with assistance of nanotechnologies. Although COCs likely did not survive in trehalose glass (i.e., 30 min of drying) under current protocol, our overall results demonstrated feasibility to enhance dehydration tolerance of COCs under partially dehydrated conditions and provided directions for future explorations.
Supplementary Information
High resolution image (TIF 12966 kb)
Kinetics of water content during microwave drying. A 40 µl drop of 0.3 M trehalose was microwave dried for 0 to 40 min (5 min interval). Water contents were expressed as gH2O/gDW (left Y axis) and percentage of water weight (right Y axis). Values are mean ± SEM. (PNG 87 kb)
Acknowledgements
We thank Dr. Keiko Antoku (Last Chance Animal Rescue) and Dr. Joy Lewis (Spay Now Animal Surgery Clinic), and their staff for providing domestic cat testes and ovaries.
Author contribution
PL: conceptualization, project administration, methodology, investigation, formal analysis, visualization, and writing—original draft preparation; SS: methodology and writing—methodology; OA: investigation, analysis, writing—methodology, and review; HS: methodology and writing—review; XH: resources and writing—review; PC: conceptualization, funding acquisition, resources, supervision, and writing—review and editing.
Funding
This work was supported by the Office of the Director, National Institutes of Health, grant/award number: R01OD023139. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data availability
Data are available upon request to the corresponding author.
Declarations
Ethical disclosure
The Animal Care and Use Committee from the Smithsonian’s National Zoo and Conservation Biology Institute granted a waiver of the animal care and use approval for that study because testes and ovaries were collected at local veterinary clinics as byproducts from owner-requested routine neutering and spaying.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Devi L, Goel S. Fertility preservation through gonadal cryopreservation. Reprod Med Biol. 2016;15(4):235–251. doi: 10.1007/s12522-016-0240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comizzoli P, He X, Lee PC. Long-term preservation of germ cells and gonadal tissues at ambient temperatures. Reprod Fertil. 2022;3(2):R42–R50. doi: 10.1530/RAF-22-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Comizzoli P, Amelkina O, Lee PC. Damages and stress responses in sperm cells and other germplasms during dehydration and storage at nonfreezing temperatures for fertility preservation. Mol Reprod Dev. 2022;89(12):565–578. doi: 10.1002/mrd.23651. [DOI] [PubMed] [Google Scholar]
- 4.Saragusty J, Loi P. Exploring dry storage as an alternative biobanking strategy inspired by Nature. Theriogenology. 2019;126:17–27. doi: 10.1016/j.theriogenology.2018.11.027. [DOI] [PubMed] [Google Scholar]
- 5.Graves-Herring JE, Wildt DE, Comizzoli P. Retention of structure and function of the cat germinal vesicle after air-drying and storage at suprazero temperature. Biol Reprod. 2013;88(6):139. doi: 10.1095/biolreprod.113.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dang-Nguyen TQ, Nguyen HT, Nguyen MT, Somfai T, Noguchi J, Kaneko H, et al. Maturation ability after transfer of freeze-dried germinal vesicles from porcine oocytes. Anim Sci J. 2018;89(9):1253–1260. doi: 10.1111/asj.13067. [DOI] [PubMed] [Google Scholar]
- 7.Wakayama S, et al. Healthy cloned offspring derived from freeze-dried somatic cells. Nat Commun. 2022;13(1):3666. doi: 10.1038/s41467-022-31216-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hibshman JD, Clegg JS, Goldstein B. Mechanisms of desiccation tolerance: themes and variations in brine shrimp, roundworms, and tardigrades. Front Physiol. 2020;11:592016. doi: 10.3389/fphys.2020.592016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patrick JL, Elliott GD, Comizzoli P. Structural integrity and developmental potential of spermatozoa following microwave-assisted drying in the domestic cat model. Theriogenology. 2017;103:36–43. doi: 10.1016/j.theriogenology.2017.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Elliott GD, et al. Resilience of oocyte germinal vesicles to microwave-assisted drying in the domestic cat model. Biopreserv Biobank. 2015;13(3):164–171. doi: 10.1089/bio.2014.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, et al. Cold-responsive nanoparticle enables intracellular delivery and rapid release of trehalose for organic-solvent-free cryopreservation. Nano Lett. 2019;19(12):9051–9061. doi: 10.1021/acs.nanolett.9b04109. [DOI] [PubMed] [Google Scholar]
- 12.Lee PC, Comizzoli P. Desiccation and supra-zero temperature storage of cat germinal vesicles lead to less structural damage and similar epigenetic alterations compared to cryopreservation. Mol Reprod Dev. 2019;86(12):1822–1831. doi: 10.1002/mrd.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amelkina O, et al. Apoptosis-related factors in the luteal phase of the domestic cat and their involvement in the persistence of Corpora lutea in lynx. PLoS ONE. 2015;10(11):e0143414. doi: 10.1371/journal.pone.0143414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hachen A, Jewgenow K, Braun BC. Sequence analysis of feline oviductin and its expression during the estrous cycle in the domestic cat (Felis catus) Theriogenology. 2012;77(3):539–549. doi: 10.1016/j.theriogenology.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Siemieniuch MJ, et al. Steroidogenic capacity of the placenta as a supplemental source of progesterone during pregnancy in domestic cats. Reprod Biol Endocrinol. 2012;10:89. doi: 10.1186/1477-7827-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun BC, Zschockelt L, Dehnhard M, Jewgenow K. Progesterone and estradiol in cat placenta--biosynthesis and tissue concentration. J Steroid Biochem Mol Biol. 2012;132(3–5):295–302. doi: 10.1016/j.jsbmb.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Wood TC, Wildt DE. Effect of the quality of the cumulus-oocyte complex in the domestic cat on the ability of oocytes to mature, fertilize and develop into blastocysts in vitro. J Reprod Fertil. 1997;110(2):355–360. doi: 10.1530/jrf.0.1100355. [DOI] [PubMed] [Google Scholar]
- 18.Grupen CG, Fung M, Armstrong DT. Effects of milrinone and butyrolactone-I on porcine oocyte meiotic progression and developmental competence. Reprod Fertil Dev. 2006;18(3):309–317. doi: 10.1071/RD05125. [DOI] [PubMed] [Google Scholar]
- 19.Shah S, et al. Fluorescence properties of doxorubicin in PBS buffer and PVA films. J Photochem Photobiol B. 2017;170:65–69. doi: 10.1016/j.jphotobiol.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee PC, et al. Desiccated cat spermatozoa retain DNA integrity and developmental potential after prolonged storage and shipping at non-cryogenic temperatures. J Assist Reprod Genet. 2021. [DOI] [PMC free article] [PubMed]
- 21.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):ESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comizzoli P, Wildt DE, Pukazhenthi BS. In vitro compaction of germinal vesicle chromatin is beneficial to survival of vitrified cat oocytes. Reprod Domest Anim. 2009;44(Suppl 2):269–274. doi: 10.1111/j.1439-0531.2009.01372.x. [DOI] [PubMed] [Google Scholar]
- 24.Comizzoli P, Wildt DE, Pukazhenthi BS. Impact of anisosmotic conditions on structural and functional integrity of cumulus-oocyte complexes at the germinal vesicle stage in the domestic cat. Mol Reprod Dev. 2008;75(2):345–354. doi: 10.1002/mrd.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goncalves DR, et al. Cellular uptake of polymeric nanoparticles by bovine cumulus-oocyte complexes and their effect on in vitro developmental competence. Eur J Pharm Biopharm. 2021;158:143–155. doi: 10.1016/j.ejpb.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, et al. TRITC-loaded PLGA nanoparticles as drug delivery carriers in mouse oocytes and embryos. ACS Appl Mater Interfaces. 2021;13(5):5975–5988. doi: 10.1021/acsami.0c19792. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Wu D, Khan FA, Wang Y, Xu J, Luo C, et al. Zinc oxide nanoparticle causes toxicity to the development of mouse oocyte and early embryo. Toxicol Lett. 2022;358:48–58. doi: 10.1016/j.toxlet.2022.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Sribna VO, Voznesenska TY, Blashkiv TV. The influence of zero-valent iron nanoparticles on oocytes and surrounding follicular cells in mice. Appl Nanosci. 2019;9(6):1395–1403. doi: 10.1007/s13204-019-00978-7. [DOI] [Google Scholar]
- 29.Lin YH, et al. The effects of graphene quantum dots on the maturation of mouse oocytes and development of offspring. J Cell Physiol. 2019;234(8):13820–13831. doi: 10.1002/jcp.28062. [DOI] [PubMed] [Google Scholar]
- 30.Wang R, et al. Potential adverse effects of nanoparticles on the reproductive system. Int J Nanomedicine. 2018;13:8487–8506. doi: 10.2147/IJN.S170723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lei R, et al. Effects of fullerenol nanoparticles on rat oocyte meiosis resumption. Int J Mol Sci. 2018;19(3). [DOI] [PMC free article] [PubMed]
- 32.Das J, Choi YJ, Song H, Kim JH. Potential toxicity of engineered nanoparticles in mammalian germ cells and developing embryos: treatment strategies and anticipated applications of nanoparticles in gene delivery. Hum Reprod Update. 2016;22(5):588–619. doi: 10.1093/humupd/dmw020. [DOI] [PubMed] [Google Scholar]
- 33.Kim YS, et al. PLGA nanoparticles with multiple modes are a biologically safe nanocarrier for mammalian development and their offspring. Biomaterials. 2018;183:43–53. doi: 10.1016/j.biomaterials.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 34.Adekiya TA, et al. In vivo evaluation of praziquantel-loaded solid lipid nanoparticles against S. mansoni infection in preclinical murine models. Int J Mol Sci. 2022;23(16):1. doi: 10.3390/ijms23169485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naziris N, et al. Thermoresponsive chimeric nanocarriers as drug delivery systems. Colloids Surf B Biointerfaces. 2021;208:112141. doi: 10.1016/j.colsurfb.2021.112141. [DOI] [PubMed] [Google Scholar]
- 36.Turathum B, Gao EM, Chian RC. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells. 2021;10(9). [DOI] [PMC free article] [PubMed]
- 37.Lopez A, et al. DNA damage in cumulus cells generated after the vitrification of in vitro matured porcine oocytes and its impact on fertilization and embryo development. Porcine Health Manag. 2021;7(1):56. doi: 10.1186/s40813-021-00235-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suttirojpattana T, Somfai T, Matoba S, Nagai T, Parnpai R, Geshi M. The effect of temperature during liquid storage of in vitro-matured bovine oocytes on subsequent embryo development. Theriogenology. 2016;85(3):509–518.e1. doi: 10.1016/j.theriogenology.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 39.Han D, Zhao BT, Liu Y, Li JJ, Wu YG, Lan GC, et al. Interactive effects of low temperature and roscovitine (ROS) on meiotic resumption and developmental potential of goat oocytes. Mol Reprod Dev. 2008;75(5):838–46. [DOI] [PubMed]
- 40.Martino A, Pollard JW, Leibo SP. Effect of chilling bovine oocytes on their developmental competence. Mol Reprod Dev. 1996;45(4):503–512. doi: 10.1002/(SICI)1098-2795(199612)45:4<503::AID-MRD13>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 41.Iryani MTM, et al. Cyst viability and stress tolerance upon heat shock protein 70 knockdown in the brine shrimp Artemia franciscana. Cell Stress Chaperones. 2020;25(6):1099–1103. doi: 10.1007/s12192-020-01113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alterio T, et al. Heat shock proteins in encysted and anhydrobiotic eutardigrades. J Limnol. 2012;71(1).
- 43.Menezo Y, et al. Methylation: an ineluctable biochemical and physiological process essential to the transmission of life. Int J Mol Sci. 2020;21(23). [DOI] [PMC free article] [PubMed]
- 44.Huan Y, et al. A novel role for DNA methyltransferase 1 in regulating oocyte cytoplasmic maturation in pigs. PLoS ONE. 2015;10(5):e0127512. doi: 10.1371/journal.pone.0127512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell LE. Maternal effect genes: update and review of evidence for a link with birth defects. HGG Adv. 2022;3(1):100067. doi: 10.1016/j.xhgg.2021.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turathum B, et al. Effects of vitrification on nuclear maturation, ultrastructural changes and gene expression of canine oocytes. Reprod Biol Endocrinol. 2010;8:70. doi: 10.1186/1477-7827-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang J, et al. Dynamic changes in the global transcriptome of bovine germinal vesicle oocytes after vitrification followed by in vitro maturation. Reprod Fertil Dev. 2018;30(10):1298–1313. doi: 10.1071/RD17535. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz-Conca M, et al. Apoptosis and glucocorticoid-related genes mRNA expression is modulated by coenzyme Q10 supplementation during in vitro maturation and vitrification of bovine oocytes and cumulus cells. Theriogenology. 2022;192:62–72. doi: 10.1016/j.theriogenology.2022.08.030. [DOI] [PubMed] [Google Scholar]
- 49.Somfai T, et al. Vitrification of porcine cumulus-oocyte complexes at the germinal vesicle stage does not trigger apoptosis in oocytes and early embryos, but activates anti-apoptotic Bcl-XL gene expression beyond 4-cell stage. J Reprod Dev. 2020;66(2):115–123. doi: 10.1262/jrd.2019-094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebrahimi B, et al. In vitro maturation, apoptotic gene expression and incidence of numerical chromosomal abnormalities following cryotop vitrification of sheep cumulus-oocyte complexes. J Assist Reprod Genet. 2010;27(5):239–246. doi: 10.1007/s10815-010-9401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High resolution image (TIF 12966 kb)
Kinetics of water content during microwave drying. A 40 µl drop of 0.3 M trehalose was microwave dried for 0 to 40 min (5 min interval). Water contents were expressed as gH2O/gDW (left Y axis) and percentage of water weight (right Y axis). Values are mean ± SEM. (PNG 87 kb)
Data Availability Statement
Data are available upon request to the corresponding author.