Abstract
Purpose
Unlike other cells in the body, in sperm, telomere length (TL) increases with age. TL can regulate nearby genes, and the subtelomeric region is rich in retrotransposons. We hypothesized that age-related telomere lengthening in sperm might suppress Long Interspersed Element 1 (LINE-1/L1), the only competent retrotransposon in humans.
Methods
We measured L1 copy number (L1-CN) and sperm telomere length (STL) from young and older men to evaluate the relationship between age, TL and L1-CN. We also evaluated L1-CN and TL in individual sperm to determine whether these variables influence sperm morphology. STL was assayed by Multiplex quantitative polymerase chain reaction method (mmqPCR) and L1-CN by Quantitative polymerase chain reaction (qPCR).
Results
We found that STL increased, and L1-CN decreased significantly with paternal age. STL in normal single sperm was significantly higher than in abnormal sperm. L1-CN did not differ between normal and abnormal sperm. Furthermore, morphologically normal sperm have longer telomeres than abnormal sperm.
Conclusions
Elongation of telomeres in the male germline could repress retrotransposition, which tends to increase with cellular aging. More studies in larger cohorts across a wide age span are needed to confirm our conclusions and explore their biological and clinical significance.
Supplementary information
The online version contains supplementary material available at 10.1007/s10815-023-02857-1.
Keywords: Telomere length, LINE-1 copy number, Human sperm, Aging
Introduction
Transposable elements (TEs) are genomic units capable of moving within practically all eukaryotic organisms [1]. The mammalian genome hosts transposable elements, representing up to 40% of the genome, and can propagate into new locations throughout the genome. Long interspersed element 1 (L1) retrotransposon, the only autonomous, active element in humans, comprises 17% of the human genome [2]. L1 elements encode the necessary “machinery” for their mobilization [3] by retrotransposition, which involves transcription of L1, translation of open reading frames (ORFs), and translocating L1 transcripts to the nucleus for their reverse transcription [4]. Cytosine demethylation, which occurs during early embryonic development and aging, reduces repressive epigenetic marks [5–7] and promotes L1 retrotransposition [8–10]. De novo insertion of L1s can disrupt oocyte and embryo development [4, 8, 11–15]. During aging, retrotransposons also become dysregulated and contribute to age-related genomic instability [16].
Spermatozoa of practically all species can spontaneously take up exogenous DNA or RNA molecules and internalize them into nuclei [16–18]. L1 derived reverse transcriptase (RT), present in sperm heads, can reverse-transcribe internalized molecules in cDNA copies [17]. Besides being stored in mature spermatozoa, L1 encoded RT also is abundantly expressed in early embryos and is related to the genesis and propagation of extra-chromosomal information [17]. The reverse transcription occurs early after fertilization and is triggered separately in both pronuclei suggesting that L1 RNA preexists in both gametes and is immediately available for retrotranscription [19]. RT activity to support reverse transcription observed in female pronuclei is provided by spermatozoa and delivered to oocytes at fertilization [19]. Maintaining germline integrity is crucial for the accurate transmission of genetic information to the next generation. Recent studies have reported several defense mechanisms to repress germline retrotransposon, including transcriptional and posttranscriptional silencing of retrotransposons by PIWI-interacting RNA (piRNA) and DNA methylation [20].
As couples postpone childbearing, understanding paternal and maternal reproductive aging becomes an urgent priority [21]. Telomeres are repetitive sequences and associated proteins that cap and protect chromosome ends, undergo attrition with age, and provide a cellular aging clock. Studies have revealed that sperm telomeres play a significant role in reproductive function and that telomere length is shorter in sperm of males with infertility problems [22–30]. The telomere length has also been reported associated positively with sperm count, progressive motility and vitality, and negatively with sperm DNA fragmentation and protamination [31–33]. In meiosis, telomere ensures the synapsis, recombination and segregation of homologs [34]. In addition, the migration of sperm telomeres during spermatogenesis plays an important role on fertilization and pronucleus formation [35, 36]. Shortening of telomeres can lead to the segregation of chromosomes from the nuclear membrane, the consequences of which are defects in the process of spermatogenesis, formation of pronuclei and delay or interruption of the cell cycle after ICSI [35].
Unlike other tissues, sperm telomeres increase rather than decrease with paternal age [37]. The biological significance of age-related telomere elongation in sperm remains poorly understood. The telomere position effect (TPE) occurs when telomeres silence nearby genes as described in yeast, mice, and humans. [38]. Sperm telomere shortening may not result from aging itself, but rather from processes, such as oxidative stress, that accumulate with age. Aging also is associated with altered DNA methylation and increased L1 copy number in many cell types [16, 39]. We hypothesize that sperm telomere elongation suppresses L1 retrotransposon with age, which would otherwise increase as cells age [13]. To test this hypothesis we compared mean L1 copy number and sperm telomere length (STL) in ejaculated human sperm from young and older men [21]. Furthermore, we assessed L1 copy number and STL between normal and abnormal individual human sperm to evaluate the relationship between sperm morphology and these two variables.
Material and methods
Study population and sample preparation
This prospective case–control pilot study first assessed L1 copy number and STL in fresh ejaculated sperm samples from men of various ages (Experiment 1, Fig. 1). Sperm samples (n = 36) were donated for research by consenting men aged 29–55 undergoing infertility treatment at NYU Langone Fertility Center (NYUFC). Baseline semen parameters were evaluated, and samples were processed by swim-up. First we assessed the relationship between age and STL or L1 copy number. In addition we investigated whether STL or L1 differ between young (≤ 40 years) and old (≥ 45 years) men.
Fig. 1.
Experimental design: Two experiments were defined according to the type of sperm sample: Experiment 1- Ejaculated sperm samples were collected. Firstly, a correlation coefficient of STL and LINE-1 copy number was performed including all samples (N = 36). In addition, we compared STL and L1 in samples from men (≤ 40 years) and (≥ 45 years). Experiment 2- Fresh ejaculated sperm samples were collected and a comparison between STL and L1 in individual normal (N = 49) and abnormal sperm (N = 58) was performed
Sperm genomic DNA was extracted using DNeasy Blood & Tissue Kit, QIAGEN, following the manufacturer’s recommended protocol, with an additional lysis step using X2 buffer (20 mM Tris–Cl, pH 8.0, 20 mM EDTA, 200 mM NaCl, 4% SDS); 80 mM dithiothreitol (DTT) and 12.5 μL/mL Proteinase K).
For single cell analysis (Experiment 2, Fig. 1), fresh ejaculated sperm samples (n = 7) from men with different ages (30–48), presenting normal motility and concentration [40], were accessioned to investigate if there is a statistical difference between sperm morphology in single sperm cells (normal and abnormal group) and STL or L1. Baseline semen parameters were evaluated, and samples washed for 10 min and resuspended in global G1 medium. An aliquot was added to a 5 ul of polyvinylpyrrolidone (PVP) drop to an ICSI dish. A micromanipulator coupled to an inverted microscope (Nikon, TE300) was used to select 20 individual sperm, 10 with normal morphology (control) and 10 with abnormal morphology, according to World Health Organization (WHO) criteria [40] from each specimen. Individual sperm were captured using an ICSI micropipette (Origio, Trumbull, CT, USA), and placed in individual drops containing 2 μL PVP/PBS (PBS Invitrogen, Grand Island, NY, USA) containing 0.1% of PVP (Sigma-Aldrich, St. Louis, MO, USA). Individual sperm were transferred to qPCR microtubes in 1 ul PVP/PBS and stored at -80 °C until single cell analysis. STL was assayed by mmqPCR and [41] L1 copy number by qPCR as follows.
Sperm telomere length assay
After sperm genomic DNA was obtained, samples were diluted to 1 ng/µl. Placenta gDNA was serially diluted (10, 2, 0.4, 0.08, 0.016) for standard curve calculation. Samples were run in triplicate with a target-specific non-template control (NTC) and DNA from embryonic stem cells obtained from Rockefeller University (RUES) as a positive control (PTC) [42]. Primers were synthesized by Integrated DNA Technologies (Table 1) according to the assay described below. mmqPCR reaction was set up with 10 μL 2xSYBR green supermix, 1.8 μL of each mix containing telomere forward and reverse primer (10 μM), 1.8 μL of each mix of single copy gene Albumin forward and reverse primer (10 μM), 5 μL of the sample diluted to 1 ng/µl and water to a 20 μL final volume. The thermal cycling program was set according to the original article description [41] as 15 min at 95° C; 2 cycles of 15 s at 94° C, 15 s at 49° C; 32 cycles of 15 s at 94° C, 10 s at 62° C, 15 s at 74° C with Telomere signal acquisition, 10 s at 84° C, 15 s at 88° C with albumin signal acquisition. Relative telomere length was calculated by the 2−ΔΔCT method compared to the positive control sample.
Table 1.
Sequences of all primers
| Primer sequences - ejaculated sperm experiments | ||
| Primer | Sequence of forward primer | Sequence of reverse primer |
| Telomere | ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT | TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA |
| Albumin | CGGCGGCGGGCGGCGCGGGCTGGGCGGAAATGCTGCACAGAATCCTTG | GCCCGGCCCGCCGCGCCCGTCCCGCCGGAAAAGCATGGTCGCCTGTT |
|
LINE-1 5’-UTR [43] |
ACAGCTTTGAAGAGAGCA-GTGGTT | AGTCTGCCCGTTCTCAGA-TCT |
| 5srDNA | CTCGTCTGATCTCGGAAGCTAAG | GCGGTCTCCATCCAAGTAC |
| Primer sequences - single cell experiments | ||
| Primer | Sequence of forward primer | Sequence of reverse primer |
| Telomere | CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT | GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT |
| Alu [44] | GACCATCCCGGCTAAAACG | GGGTTCACGCCATTCTC |
| LINE-1 ORF2 [43] | CAAACACCGCATATTCTCACTCA | CTTCCTGTGTCCATGTGATCTCA |
| 5S rDNA [45] | CTCGTCTGATCTCGGAAGCTAAG | GCGGTCTCCATCCAAGTAC |
Sperm LINE-1 copy number assay
Similarly, to analyze the L1 copy number in ejaculated sperm, samples were run in triplicate, and the qPCR plate was divided in two to run 2 primers in each. Each reaction was set up with 10 μL 2xSYBR green supermix, 1 μL of each mix of L1 forward and reverse primer (10 μM) or 1 μL of each mix of 5srDNA reference gene forward and reverse primer (10 μM), 5 μL of sample diluted to 1 ng/µl and water to a 20 μL final volume. Thermal cycler reaction conditions were set at 95° C for 10 min, followed by 40 data collection cycles at 95° C for 15 s and 60° C for 1 min. Relative LINE-1 copy number was calculated by the 2−ΔΔCT method when comparing to the positive control sample RUES.
Single cell sperm telomere length and L1 copy number assay
Sperm single-cell genomic DNA was obtained by adding 1 μL 2 × Lysis buffer (100 ul mM Tris–HCl ph: 7.4; 300 mM NaCl; 0.8 mM EDTA, 2% NP-40; 5 mM DTT) to a qPCR microtube and heated at 75° C for 10 min. A single-cell telomere length assay (SC-qPCR) [37, 42] was used to measure STL and L1 copy number in each sperm. A key feature of this assay is a pre-amplification step performed before quantitative polymerase chain reaction (qPCR). Briefly, pre-qPCR was performed using the Takara Taq Hot Start pre-amplification kit. The reactions were set up by aliquoting 38 μL of master mix into the qPCR microtubes containing 1 μL sperm single-cell genomic DNA plus 1 μL of Lysis buffer. Each reaction was set up with 4 μL 10xqPCR buffer, 4 μL 2.5 mM dNTP, 0.25 μL DNA polymerase, 1 μL of each mix of telomere forward and reverse primer (10 μM′), 1 μL of each mix of ALU reference genes forward and reverse primer (10 μm), 1 ul of each mix of L1-ORF2 forward and reverse primer (10 μM,), 1 ul of each mix of 5sr DNA forward and reverse primer (10 μM) and water to a 40 μL final volume. Thermal cycler reaction conditions were set at 94° C for 5 min, followed by 18 cycles of 94° C for 30 s, 60° C annealing for 30 s, and extension at 72° C for 30 s, with a final extension for 10 min at 72° C. PCR products were purified following the protocol of the purification kit (Agencourt AMPure XP beads) and were eluted in 50 μL of double distilled water.
Finally, to perform the real-time PCR, each reaction included 10 µL 2 × SYBR Green mix (Bio-Rad), 1 µL of each of 10 µM forward and reverse primers, 4 µL molecular-grade water, and 5 µL genomic DNA to yield a 20µL reaction. DNA samples were placed in a 96-well plate for telomere primers, and reference gene (ALU) primers, L1 primers and reference gene (5sr) primers, respectively. A thermocycler (CFX96 system—Bio-Rad) was used with reaction conditions of 95° C for 10 min, followed by 40 cycles of data collection at 95° C for 15 s, 60° C for annealing, and extension for 1 min). Samples were run in triplicate along with a target-specific non-template control (NTC). DNA from RUES cells was used as a positive control in each plate to assure concordance between plates. After thermal cycling, the CFX manager software was used to generate standard curves and Ct values for telomere and L1 (ORF2) and the reference genes (ALU) and (5srDNA) signals, respectively. To ensure high reproducibility of samples, only assays with real-time qPCR efficiencies between 95 and 105% and intra-assay coefficient of variation (CoV) less than 1% were included in the analysis. Relative telomere length and LINE-1 copy number were calculated by the 2−ΔΔCT method compared to positive control samples [46].
Statistical analysis
Pearson's correlation coefficient was performed to verify the relationship between age and STL or L1 copy number. The Wilcoxon nonparametric test for independent samples was applied to verify whether there is a statistical difference between STL, L1 copy number, and the stratified variable age (≤ 40 years and ≥ 45 years). To verify correlation between the STL and L1 copy number, the correlation coefficient was estimated using Partial Pearson’s correlation coefficients controlling for the variable age.
The Wilcoxon nonparametric test was applied for independent samples to analyze if there is a statistical difference between sperm morphology (normal and abnormal group) and STL or L1. Analyzes were implemented in the SAS version 9.4 program. A P-value < 0.05 was considered significant.
Results
Experiment 1
Sample characteristics are shown in Table 2 as median (interquartile range – IQR; minimum–maximum). The median age of the 36 ejaculated samples included in this study was 41 (36–44; 29–55) years old, the volume was 2.6 (1.70–3.95; 0.45–6.6) ml, the concentration of spermatozoa per ml was 77 (40.50–96.50; 0.3–178), and the percentage of progressive motility was 56.5 (50–65; 20–86).
Table 2.
Clinical characteristics of the samples included in this study
| Samples | N | Age (years) | Volume (ml) | Concentration per ml | Progressive motility (%) |
|---|---|---|---|---|---|
| Ejaculated | 36 | 41 (36–44; 29–55) | 2.6 (1.70–3.95; 0.45–6.6) | 77 (40.50–96.50; 0.3–178) | 56.5 (50–65; 20–86) |
| ≤ 40 years | 16 | 35.5 (32.5–36.5; 29–38) | 2.50 (1.90–3.10; 0.5 -5.5) | 80.75 (46.0–99.0; 6–160) | 60.0 (54.5–70.0; 40–79) |
| ≥ 45 years | 7 | 49.0 (48.0–55.0; 47–55) | 2.3 (0.90–4.70; 0.45–4.8) | 53.0 (42.0–80.0; 0.3–90) | 44.0 (31.0–51.0; 20–65) |
| Single sperm | 7 | 41 (36–47; 30–48) | 3.0 (2.0–4.3; 1.7–5.0) | 60 (20–99; 20–120) | 52 (50–65; 45–82) |
Data presented as median (interquartile range – IQR; minimum—maximum); N: number, concentration (× 106/mL); %: percentage
Sperm telomere length and LINE-1 copy number in ejaculated sperm samples
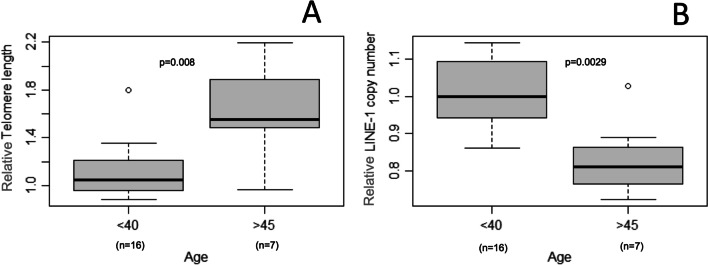
Amplification and standard calibration curves for 5S rDNA and L1 5’UTR primers using serially diluted placenta gDNA are presented in Supplementary Figure 1 A-D. STL was positively correlated with age (n: 36; r = 0.4096; p = 0.0131) (Fig. 2A). STL from older (age ≥ 45 years, n: 7; Median, IQR: 1.55, 1.46–2.11) was significantly longer than younger men (age ≤ 40 years, n: 16, Median, IQR: 1.05, 0.96–1.21) (p = 0.008; Fig. 3A). L1 copy number in sperm decreased significantly with paternal age (n: 36; r = -0.3443; p = 0.0397; Fig. 2B), being significantly lower in older (age ≥ 45 years, n: 7; Median, IQR: 0.81, 0.75–0.89) compared to younger men (≤ 40 years, n: 16, Median, IQR: 1.00, 0.94–1.09) (p = 0.0029; Fig. 3B.). No correlation was observed between STL and L1 when we controlled for the variable age p = 0.67 (data not shown).
Fig. 2.
Pearson Correlation coefficient of sperm telomere length and LINE-1 copy number. Note: Pearson Correlation coefficient showed that sperm telomere length (A) increased and LINE-1 copy number (B) decreased as paternal age advancing. Relative telomere length and LINE-1 copy number are presented as a ratio
Fig. 3.
Comparison of sperm telomere length and LINE-1 copy number between older and younger men. Note: Comparison of sperm telomere length (A) and LINE-1 copy number (B) between older (≥ 45 years) and younger (≤ 40 years) men. Relative telomere length and LINE-1 copy number are presented as a ratio. Patient ≤ 40 years had a telomere length greater than 1.8, being considered an outlier (A) and a patient ≥ 45 year had a L1 copy number greater than 1, also being considered an outlier (B)
Experiment 2
From samples (n = 7) included in the single cell experiment, median (interquartile range – IQR; minimum–maximum) age was 41 (36–47; 30–48) years old. The volume of the samples included was 3.0 (2.0–4.3; 1.7–5.0) ml. The concentration of spermatozoa per ml was 60 (20–99, 20–120), and the percentage of progressive motility was 52 (50–65; 45–82) (Table 2).
Sperm telomere length and L1 copy number in single sperm cells
STL in sperm with normal morphology (n = 49; Median, IQR: 8.55, 6.09–21.95) was significantly higher than in morphologically abnormal sperm (n: 58, Median, IQR: 4.89, 2.74–8.97) (p = 0.0015) (Fig. 4A). L1 copy number did not differ between normal (n = 49, Median, IQR: 1.84, 1.01–3.05) and abnormal sperm (n = 58, Median, IQR: 1.80, 1.01–2.94) (p = 0.7239) (Fig. 4B). Sperm abnormalities were mainly midpiece defects, crooked tail, piriform head, fusiform head, macrocephalic and microcephalic heads. Due to the small sample size, we were unable to separate and compare sperm for each defect individually.
Fig. 4.
Sperm telomere length and L1 copy number in single sperms with normal and abnormal morphology. Note: Comparison of sperm telomere length (A) and L1 copy number (B) in single sperms between normal and abnormal morphology. Relative telomere length and LINE-1 copy number are presented as a ratio
Discussion
We found increasing STL with advancing men’s age, as reported previously in bulk specimens of sperm. Furthermore, STL in individual sperm with normal morphology was significantly higher than in abnormal sperm. In contrast, L1 copy number in ejaculated sperm decreased significantly with paternal age, as sperm telomeres lengthen. L1 copy number did not differ between normal and abnormal morphology individual sperm samples. However, when we controlled for the variable age no correlation was observed between STL and L1.
Other studies have reported increasing STL with advancing paternal age [37, 47, 48], though the mechanisms underlying age-dependent telomere elongation remain poorly understood. Epigenetic factors and/or selective survival of germ-line stem cells have been proposed to explain how STL increases while telomere length in most other cells decreases with age [49]. Sperm with longer telomeres might arise from a subset of germ-line stem cells that sustained less aging-related oxidative stress and/or fewer replications prior to entering meiosis [49]. Telomerase counteracts chromosome erosion by adding repetitive sequences to terminal ends [50] and remains active in spermatogonial stem cells, though the kinetics of telomerase suggest that typically it maintains rather than elongates telomeres [51–55].
Regardless of the underlying mechanism, the biological and clinical significance of telomere elongation in sperm from older men remains unclear. We looked for a possible link between STL and L1 because telomere elongation and retrotransposons activation occurs during gametogenesis, during early development [56] and are involved in aging, suggesting that common pathways could drive both processes [57]. Retroelements are not only evolutionary ancestors of telomeres, but also active players in telomere maintenance in many species [57]. Indeed, telomeres of some fungi [58], algae [59], moths [60], crustaceans [61], and DNA repair–deficient mammalian cells [62] are comprised not only of telomere repeats, but also transposable elements (TEs) that insert preferentially at chromosome ends. These telomeric mobile elements typically are derived from a single class of TE-non-long terminal repeat (“non-LTR”) retrotransposons [63], such as L1, which mobilize via reverse transcription and preferentially insert into telomeres [64]. Retrotransposon expression is repressed during most stages of development, ensuring normal cell function. DNA methylation, the primary regulator of endogenous retroelement repression [65, 66], peaks in mature mammalian spermatozoa [67]. Despite a high level of DNA methylation, L1 is transcriptionally expressed in ejaculated human spermatozoa of both normozoospermic and oligozoospermic men [68].
Increased somatic L1 copy number is found in several neuropsychiatric syndromes for which advanced men’s age is a risk factor, including Ataxia-telangiectasia, Autism spectrum disorder, and Schizophrenia [3]. The brain appears to be an especially susceptible target for L1 even in healthy adults, with approximately 80 copies per cell in the hippocampus [69]. Cellular senescence also increases L1 copy number. In cultured human fibroblasts, L1 becomes de-repressed and actively transposes during replicative senescence [70]. Relaxation of retrotransposable element silencing with age may contribute to age-related DNA damage [3].
Intriguingly, we report here that L1 copy number decreases with age in human sperm, unlike the increased L1 observed in aging somatic cells [70, 71]. This mirrors the observation of telomere elongation in sperm; in contrast to the telomere shortening that takes place in all somatic cells. These two sperm-specific genomic processes may represent independent manifestations of germline aging. Alternatively, they may be linked. The retrotransposon origin of telomeres suggests that mechanisms of transposon control could be adopted for telomere regulation [57]. Also, L1 knockdown leads to telomere dysfunction in human cancer cells [72, 73]. L1 retrotransposition poses a significant threat to the germline, and many redundant strategies have evolved to repress it. As discussed above, cytosine and histone methylation repress L1 transcription. Piwi and piRNA silence retrotransposons selectively in the male germline [74, 75]. We cannot rule out the possibility that elongation of telomeres in sperm with advancing age counters the age-related L1 de repression, which occurs in most tissues, and that disruption of this regulatory process contributes to the paternal age effect. However, since partial correlation between STL and L1 was not observed after eliminating the effect of age we cannot exclude the independence of the two observed effects.
Our pilot study has some obvious limitations. The study’s small sample size and narrow range of men’s ages available in our patient population limit interpretation of the men’sage effect on TL and L1 copy number. In addition, L1 RNA is maintained in mature spermatozoa throughout spermatogenesis, but we did not investigate whether L1 RNA stored in mature sperm decreases with age. Also, qPCR is just one of many techniques to measure telomere DNA. It remains the most widely used method for TL measurement, due to its low cost, ease of use, requirement for low starting DNA quantity, and advantages for high throughput analyses, qPCR does not, however, accurately measure the shortest telomere or chromosome-specific telomere lengths.
Older men comprise a growing proportion of fathers. The potential impact of men’s age on the germline and subsequent development, and even on the offspring longevity, therefore, merit further study.
Conclusion
Elongation of telomeres in the male germline may repress retrotransposition, which tends to increase with cellular aging. More studies in larger cohorts across a wide age span are needed to confirm our conclusions and explore their biological and clinical significance.
Supplementary information
Below is the link to the electronic supplementary material.
Acknowledgements
Coordination for the Improvement of Higher Education Personnel (CAPES, Finance Code: 001, Brazil; process n. 88887.371487/2019-00 and process n. 88887.597054/2021-00 to TSB), Brazilian National Council for Scientific and Technological Development (CNPq, Brazil; Grant number 204747/2018-0 to FBK) and the Stanley H. Kaplan Fund of the NYU Grossman School of Medicine (to DLK).
Abbreviations
- CoV
Coefficient of variation
- DTT
Dithiothreitol
- EDTA
Ethylenediaminetetraacetic acid
- gDNA
Genomic DNA
- ICSI
Intracytoplasmic sperm injection
- IRB
Institutional Review Board
- IQR
Interquartile range
- L1
Long interspersed element 1
- L1-CN
L1 copy number
- Long Interspersed Element 1
LINE-1/L1
- mmqPCR
Multiplex quantitative polymerase chain reaction method
- non-LTR
TE-non-long terminal repeat
- NTC
Non-template control
- NYU
New York University
- NYUFC
New York University Langone Fertility Center
- ORFs
Open reading frames
- piRNA
PIWI-interacting RNA
- PTC
Positive control
- PVP
Polyvinylpyrrolidone
- qPCR
Quantitative polymerase chain reaction
- rDNA
Recombinant DNA
- RT
Reverse transcriptase
- RUES
Rockefeller University
- SC-qPCR
Single-cell telomere length assay
- STL
Sperm telomere length
- TEs
Transposable elements
- TL
Telomere length
- TPE
Telomere position effect
- WHO
World Health Organization
Data availability
The data underlying this study will be shared on reasonable request to the corresponding author.
Declarations
Ethics approval
The New York University Langone Medical Center Institutional Review Board (IRB) approved this study under the IRB number S#16–00154.
Consent to participate
Each participant provided written informed consent.
Consent for publication
The author agree to its submission to the Journal of Assisted Reproduction and Genetics and, if accepted, to its publication in this journal. We warrant that this article is original, does not infringe on any copyright or other proprietary right of any third party, is not under consideration by another journal and has not been previously published. Ethical approval has been sought and obtained as necessary and any conflicts of interest stated.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mita P, Boeke JD. How retrotransposons shape genome regulation. Curr Opin Genet Dev. 2016;37:90–100. doi: 10.1016/j.gde.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belan E. LINEs of evidence: noncanonical DNA replication as an epigenetic determinant. Biol Direct. 2013;8:22. doi: 10.1186/1745-6150-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Terry DM, Devine SE. Aberrantly High Levels of Somatic LINE-1 Expression and Retrotransposition in Human Neurological Disorders. Front Genet. 2019;10:1244. doi: 10.3389/fgene.2019.01244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuroki R, Murata Y, Fuke S, Nakachi Y, Nakashima J, Kujoth GC, et al. Establishment of Quantitative PCR Assays for Active Long Interspersed Nuclear Element-1 Subfamilies in Mice and Applications to the Analysis of Aging-Associated Retrotransposition. Front Genet. 2020;11:519206. doi: 10.3389/fgene.2020.519206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293(5532):1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 6.Seisenberger S, Peat JR, Hore TA, Santos F, Dean W, Reik W. Reprogramming DNA methylation in the mammalian life cycle: building and breaking epigenetic barriers. Philos Trans R Soc Lond B Biol Sci. 2013;368(1609):20110330. doi: 10.1098/rstb.2011.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos F, Hendrich B, Reik W, Dean W. Dynamic reprogramming of DNA methylation in the early mouse embryo. Dev Biol. 2002;241(1):172–182. doi: 10.1006/dbio.2001.0501. [DOI] [PubMed] [Google Scholar]
- 8.Kano H, Godoy I, Courtney C, Vetter MR, Gerton GL, Ostertag EM, et al. L1 retrotransposition occurs mainly in embryogenesis and creates somatic mosaicism. Genes Dev. 2009;23(11):1303–1312. doi: 10.1101/gad.1803909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solyom S, Kazazian HH., Jr Mobile elements in the human genome: implications for disease. Genome Med. 2012;4(2):12. doi: 10.1186/gm311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazazian HH, Jr, Moran JV. Mobile DNA in Health and Disease. N Engl J Med. 2017;377(4):361–370. doi: 10.1056/NEJMra1510092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns KH, Boeke JD. Human transposon tectonics. Cell. 2012;149(4):740–752. doi: 10.1016/j.cell.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazazian HH., Jr Genetics L1 retrotransposons shape the mammalian genome. Science. 2000;289(5482):1152–1153. doi: 10.1126/science.289.5482.1152. [DOI] [PubMed] [Google Scholar]
- 13.Kohlrausch FB, Berteli TS, Wang F, Navarro PA, Keefe DL. Control of LINE-1 Expression Maintains Genome Integrity in Germline and Early Embryo Development. Reprod Sci. 2022;29(2):328–340. doi: 10.1007/s43032-021-00461-1. [DOI] [PubMed] [Google Scholar]
- 14.Kazazian HH. Retrotransposon insertions in germ cells and somatic cells. Dev Biol (Basel). 2001;106:307–13. [PubMed] [Google Scholar]
- 15.Kazazian HH., Jr Mobile elements: drivers of genome evolution. Science. 2004;303(5664):1626–1632. doi: 10.1126/science.1089670. [DOI] [PubMed] [Google Scholar]
- 16.Gorbunova V, Seluanov A, Mita P, McKerrow W, Fenyö D, Boeke JD, et al. The role of retrotransposable elements in ageing and age-associated diseases. Nature. 2021;596(7870):43–53. doi: 10.1038/s41586-021-03542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spadafora C. Sperm-Mediated Transgenerational Inheritance. Front Microbiol. 2017;8:2401. doi: 10.3389/fmicb.2017.02401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith K, Spadafora C. Sperm-mediated gene transfer: applications and implications. BioEssays. 2005;27(5):551–562. doi: 10.1002/bies.20211. [DOI] [PubMed] [Google Scholar]
- 19.Vitullo P, Sciamanna I, Baiocchi M, Sinibaldi-Vallebona P, Spadafora C. LINE-1 retrotransposon copies are amplified during murine early embryo development. Mol Reprod Dev. 2012;79(2):118–127. doi: 10.1002/mrd.22003. [DOI] [PubMed] [Google Scholar]
- 20.Newkirk SJ, Lee S, Grandi FC, Gaysinskaya V, Rosser JM, Vanden Berg N, et al. Intact piRNA pathway prevents L1 mobilization in male meiosis. Proc Natl Acad Sci USA. 2017;114(28):E5635–E5644. doi: 10.1073/pnas.1701069114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bray I, Gunnell D, Davey SG. Advanced paternal age: how old is too old? J Epidemiol Community Health. 2006;60(10):851–853. doi: 10.1136/jech.2005.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biron-Shental T, Wiser A, Hershko-Klement A, Markovitch O, Amiel A, Berkovitch A. Sub-fertile sperm cells exemplify telomere dysfunction. J Assist Reprod Genet. 2018;35(1):143–148. doi: 10.1007/s10815-017-1029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasilopoulos E, Fragkiadaki P, Kalliora C, Fragou D, Docea AO, Vakonaki E, et al. The association of female and male infertility with telomere length (Review) Int J Mol Med. 2019;44(2):375–389. doi: 10.3892/ijmm.2019.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Q, Zhang N, Zhao F, Zhao W, Dai S, Liu J, et al. Processing of semen by density gradient centrifugation selects spermatozoa with longer telomeres for assisted reproduction techniques. Reprod Biomed Online. 2015;31(1):44–50. doi: 10.1016/j.rbmo.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Anifandis G, Samara M, Simopoulou M, Messini CI, Chatzimeletiou K, Thodou E, et al. Insights into the Role of Telomeres in Human Embryological Parameters. Opinions Regarding IVF. J Dev Biol. 2021;9(4). 10.3390/jdb9040049. [DOI] [PMC free article] [PubMed]
- 26.Franken DR, Oehninger S. Semen analysis and sperm function testing. Asian J Androl. 2012;14(1):6–13. doi: 10.1038/aja.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pizzol D, Ferlin A, Garolla A, Lenzi A, Bertoldo A, Foresta C. Genetic and molecular diagnostics of male infertility in the clinical practice. Front Biosci (Landmark Ed) 2014;19(2):291–303. doi: 10.2741/4208. [DOI] [PubMed] [Google Scholar]
- 28.Tahamtan S, Tavalaee M, Izadi T, Barikrow N, Zakeri Z, Lockshin RA, et al. Reduced sperm telomere length in individuals with varicocele is associated with reduced genomic integrity. Sci Rep. 2019;9(1):4336. doi: 10.1038/s41598-019-40707-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thilagavathi J, Kumar M, Mishra SS, Venkatesh S, Kumar R, Dada R. Analysis of sperm telomere length in men with idiopathic infertility. Arch Gynecol Obstet. 2013;287(4):803–807. doi: 10.1007/s00404-012-2632-8. [DOI] [PubMed] [Google Scholar]
- 30.Lafuente R, Bosch-Rue E, Ribas-Maynou J, Alvarez J, Brassesco C, Amengual MJ, et al. Sperm telomere length in motile sperm selection techniques: A qFISH approach. Andrologia. 2018;50(2). 10.1111/and.12840. [DOI] [PubMed]
- 31.Amirzadegan M, Sadeghi N, Tavalaee M, Nasr-Esfahani MH. Analysis of leukocyte and sperm telomere length in oligozoospermic men. Andrologia. 2021;53(10):e14204. doi: 10.1111/and.14204. [DOI] [PubMed] [Google Scholar]
- 32.Thilagavathi J, Venkatesh S, Dada R. Telomere length in reproduction. Andrologia. 2013;45(5):289–304. doi: 10.1111/and.12008. [DOI] [PubMed] [Google Scholar]
- 33.Rocca MS, Speltra E, Menegazzo M, Garolla A, Foresta C, Ferlin A. Sperm telomere length as a parameter of sperm quality in normozoospermic men. Hum Reprod. 2016;31(6):1158–1163. doi: 10.1093/humrep/dew061. [DOI] [PubMed] [Google Scholar]
- 34.Siderakis M, Tarsounas M. Telomere regulation and function during meiosis. Chromosome Res. 2007;15(5):667–679. doi: 10.1007/s10577-007-1149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darmishonnejad Z, Tavalaee M, Izadi T, Tanhaei S, Nasr-Esfahani MH. Evaluation of sperm telomere length in infertile men with failed/low fertilization after intracytoplasmic sperm injection. Reprod Biomed Online. 2019;38(4):579–587. doi: 10.1016/j.rbmo.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 36.Powell D, Cran DG, Jennings C, Jones R. Spatial organization of repetitive DNA sequences in the bovine sperm nucleus. J Cell Sci. 1990;97(Pt 1):185–191. doi: 10.1242/jcs.97.1.185. [DOI] [PubMed] [Google Scholar]
- 37.Antunes DM, Kalmbach KH, Wang F, Dracxler RC, Seth-Smith ML, Kramer Y, et al. A single-cell assay for telomere DNA content shows increasing telomere length heterogeneity, as well as increasing mean telomere length in human spermatozoa with advancing age. J Assist Reprod Genet. 2015;32(11):1685–1690. doi: 10.1007/s10815-015-0574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baur JA, Zou Y, Shay JW, Wright WE. Telomere position effect in human cells. Science. 2001;292(5524):2075–2077. doi: 10.1126/science.1062329. [DOI] [PubMed] [Google Scholar]
- 39.Richardson B. Impact of aging on DNA methylation. Ageing Res Rev. 2003;2(3):245–261. doi: 10.1016/s1568-1637(03)00010-2. [DOI] [PubMed] [Google Scholar]
- 40.WHO. World Health Organization laboratory manual for the examination and processing of human semen. 5th ed: World Health Organization; 2010.
- 41.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang F, Pan X, Kalmbach K, Seth-Smith ML, Ye X, Antumes DM, et al. Robust measurement of telomere length in single cells. Proc Natl Acad Sci U S A. 2013;110(21):E1906–E1912. doi: 10.1073/pnas.1306639110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang P, Ludwig AK, Hastert FD, et al. L1 retrotransposition is activated by Ten-eleven-translocation protein 1 and repressed by methyl-CpG binding proteins. Nucleus. 2017;8(5):548–562. doi: 10.1080/19491034.2017.1330238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt H, Taubert H, Lange H, Kriese K, Schmitt WD, Hoffmann S, Bartel F, Hauptmann S. Small polydispersed circular DNA contains strains of mobile genetic elements and occurs more frequently in permanent cell lines of malignant tumors than in normal lymphocytes. Oncol Rep. 2009;22(2):393–400. [PubMed] [Google Scholar]
- 45.Macia A, Widmann TJ, Heras SR, Ayllon V, Sanchez L, Benkaddour-Boumzaouad M, Muñoz-Lopez M, Rubio A, Amador-Cubero S, Blanco-Jimenez E, Garcia-Castro J, Menendez P, Ng P, Muotri AR, Goodier JL, Garcia-Perez JL. Engineered LINE-1 retrotransposition in nondividing human neurons. Genome Res. 2017;27(3):335–348. doi: 10.1101/gr.206805.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 47.Eisenberg DT, Hayes MG, Kuzawa CW. Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc Natl Acad Sci USA. 2012;109(26):10251–10256. doi: 10.1073/pnas.1202092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Q, Zhao F, Dai S, Zhang N, Zhao W, Bai R, et al. Sperm telomere length is positively associated with the quality of early embryonic development. Hum Reprod. 2015;30(8):1876–1881. doi: 10.1093/humrep/dev144. [DOI] [PubMed] [Google Scholar]
- 49.Kimura M, Cherkas LF, Kato BS, Demissie S, Hjelmborg JB, Brimacombe M, et al. Offspring's leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4(2):e37. doi: 10.1371/journal.pgen.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zakian VA. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]
- 51.Keefe DL, Liu L. Telomeres and reproductive aging. Reprod Fertil Dev. 2009;21(1):10–14. doi: 10.1071/rd08229. [DOI] [PubMed] [Google Scholar]
- 52.Riethman H. Human telomere structure and biology. Annu Rev Genomics Hum Genet. 2008;9:1–19. doi: 10.1146/annurev.genom.8.021506.172017. [DOI] [PubMed] [Google Scholar]
- 53.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 54.Reig-Viader R, Brieño-Enríquez MA, Khoriauli L, Toran N, Cabero L, Giulotto E, et al. Telomeric repeat-containing RNA and telomerase in human fetal oocytes. Hum Reprod. 2013;28(2):414–422. doi: 10.1093/humrep/des363. [DOI] [PubMed] [Google Scholar]
- 55.Reig-Viader R, Vila-Cejudo M, Vitelli V, Buscà R, Sabaté M, Giulotto E, et al. Telomeric repeat-containing RNA (TERRA) and telomerase are components of telomeres during mammalian gametogenesis. Biol Reprod. 2014;90(5):103. doi: 10.1095/biolreprod.113.116954. [DOI] [PubMed] [Google Scholar]
- 56.Richardson SR, Gerdes P, Gerhardt DJ, Sanchez-Luque FJ, Bodea GO, Muñoz-Lopez M, et al. Heritable L1 retrotransposition in the mouse primordial germline and early embryo. Genome Res. 2017;27(8):1395–1405. doi: 10.1101/gr.219022.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kordyukova M, Olovnikov I, Kalmykova A. Transposon control mechanisms in telomere biology. Curr Opin Genet Dev. 2018;49:56–62. doi: 10.1016/j.gde.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 58.Starnes JH, Thornbury DW, Novikova OS, Rehmeyer CJ, Farman ML. Telomere-targeted retrotransposons in the rice blast fungus Magnaporthe oryzae: agents of telomere instability. Genetics. 2012;191(2):389–406. doi: 10.1534/genetics.111.137950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higashiyama T, Noutoshi Y, Fujie M, Yamada T. Zepp, a LINE-like retrotransposon accumulated in the Chlorella telomeric region. EMBO J. 1997;16(12):3715–3723. doi: 10.1093/emboj/16.12.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osanai-Futahashi M, Fujiwara H. Coevolution of telomeric repeats and telomeric repeat-specific non-LTR retrotransposons in insects. Mol Biol Evol. 2011;28(11):2983–2986. doi: 10.1093/molbev/msr135. [DOI] [PubMed] [Google Scholar]
- 61.Gladyshev EA, Arkhipova IR. Telomere-associated endonuclease-deficient Penelope-like retroelements in diverse eukaryotes. Proc Natl Acad Sci U S A. 2007;104(22):9352–9357. doi: 10.1073/pnas.0702741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morrish TA, Garcia-Perez JL, Stamato TD, Taccioli GE, Sekiguchi J, Moran JV. Endonuclease-independent LINE-1 retrotransposition at mammalian telomeres. Nature. 2007;446(7132):208–212. doi: 10.1038/nature05560. [DOI] [PubMed] [Google Scholar]
- 63.Beck CR, Garcia-Perez JL, Badge RM, Moran JV. LINE-1 elements in structural variation and disease. Annu Rev Genomics Hum Genet. 2011;12:187–215. doi: 10.1146/annurev-genom-082509-141802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saint-Leandre B, Nguyen SC, Levine MT. Diversification and collapse of a telomere elongation mechanism. Genome Res. 2019;29(6):920–931. doi: 10.1101/gr.245001.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lavie L, Kitova M, Maldener E, Meese E, Mayer J. CpG methylation directly regulates transcriptional activity of the human endogenous retrovirus family HERV-K(HML-2) J Virol. 2005;79(2):876–883. doi: 10.1128/jvi.79.2.876-883.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schulz WA, Steinhoff C, Florl AR. Methylation of endogenous human retroelements in health and disease. Curr Top Microbiol Immunol. 2006;310:211–250. doi: 10.1007/3-540-31181-5_11. [DOI] [PubMed] [Google Scholar]
- 67.Smallwood SA, Kelsey G. De novo DNA methylation: a germ cell perspective. Trends Genet. 2012;28(1):33–42. doi: 10.1016/j.tig.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 68.Lazaros L, Kitsou C, Kostoulas C, Bellou S, Hatzi E, Ladias P, et al. Retrotransposon expression and incorporation of cloned human and mouse retroelements in human spermatozoa. Fertil Steril. 2017;107(3):821–830. doi: 10.1016/j.fertnstert.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 69.Coufal NG, Garcia-Perez JL, Peng GE, Yeo GW, Mu Y, Lovci MT, et al. L1 retrotransposition in human neural progenitor cells. Nature. 2009;460(7259):1127–1131. doi: 10.1038/nature08248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Cecco M, Criscione SW, Peckham EJ, Hillenmeyer S, Hamm EA, Manivannan J, et al. Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell. 2013;12(2):247–256. doi: 10.1111/acel.12047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Cecco M, Ito T, Petrashen AP, Elias AE, Skvir NJ, Criscione SW, et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature. 2019;566(7742):73–78. doi: 10.1038/s41586-018-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Aschacher T, Wolf B, Enzmann F, Kienzl P, Messner B, Sampl S, et al. LINE-1 induces hTERT and ensures telomere maintenance in tumour cell lines. Oncogene. 2016;35(1):94–104. doi: 10.1038/onc.2015.65. [DOI] [PubMed] [Google Scholar]
- 73.Aschacher T, Wolf B, Aschacher O, Enzmann F, Laszlo V, Messner B, et al. Long interspersed element-1 ribonucleoprotein particles protect telomeric ends in alternative lengthening of telomeres dependent cells. Neoplasia. 2020;22(2):61–75. doi: 10.1016/j.neo.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gainetdinov I, Skvortsova Y, Kondratieva S, Funikov S, Azhikina T. Two modes of targeting transposable elements by piRNA pathway in human testis. RNA. 2017;23(11):1614–1625. doi: 10.1261/rna.060939.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reznik B, Cincotta SA, Jaszczak RG, Mateo LJ, Shen J, Cao M, et al. Heterogeneity of transposon expression and activation of the repressive network in human fetal germ cells. Development. 2019;146(12). 10.1242/dev.171157. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study will be shared on reasonable request to the corresponding author.