Abstract
Abstract
Purpose
To assess the effects of the oocyte on mRNA abundance of FSHR, AMH and major genes of the maturation cascade (AREG, EREG, ADAM17, EGFR, PTGS2, TNFAIP6, PTX3, and HAS2) in bovine cumulus cells.
Methods
(1) Intact cumulus-oocyte complexes, (2) microsurgically oocytectomized cumulus-oolema complexes (OOX), and (3) OOX + denuded oocytes (OOX+DO) were subjected to in vitro maturation (IVM) stimulated with FSH for 22 h or with AREG for 4 and 22 h. After IVM, cumulus cells were separated and relative mRNA abundance was measured by RT-qPCR.
Results
After 22 h of FSH-stimulated IVM, oocytectomy increased FSHR mRNA levels (p=0.005) while decreasing those of AMH (p=0.0004). In parallel, oocytectomy increased mRNA abundance of AREG, EREG, ADAM17, PTGS2, TNFAIP6, and PTX3, while decreasing that of HAS2 (p<0.02). All these effects were abrogated in OOX+DO. Oocytectomy also reduced EGFR mRNA levels (p=0.009), which was not reverted in OOX+DO. The stimulatory effect of oocytectomy on AREG mRNA abundance (p=0.01) and its neutralization in OOX+DO was again observed after 4 h of AREG-stimulated IVM. After 22 h of AREG-stimulated IVM, oocytectomy and addition of DOs to OOX caused the same effects on gene expression observed after 22 h of FSH-stimulated IVM, except for ADAM17 (p<0.025).
Conclusion
These findings suggest that oocyte-secreted factors inhibit FSH signaling and the expression of major genes of the maturation cascade in cumulus cells. These may be important actions of the oocyte favoring its communication with cumulus cells and preventing premature activation of the maturation cascade.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-023-02822-y.
Keywords: Oocyte-secreted factors (OSFs), Cumulus cells, FSH, EGF-like maturation cascade, mRNA
Introduction
Oocyte maturation and cumulus cell differentiation occurs in a gradual and synchronous manner within the follicle [1]. Disruption of intrafollicular homeostasis by stimulatory hormonal treatments or removal of the cumulus-oocyte complex (COC) from the follicle for in vitro maturation (IVM) can accelerate oocyte chromatin condensation, oocyte transcriptional silencing, loss of cumulus-oocyte communication, and meiotic resumption, threateaning oocyte developmental competence [2–5]. Understanding the mechanisms that promote nuclear-cytoplasmic synchronicity may provide important tools to improve in vivo and in vitro strategies utilized to treat female subfertility that rely on oocyte quality.
Studies in mice and cattle have revealed that the oocyte is not a passive passenger in the developing follicle, but regulates cumulus metabolism, differentiation, and expansion through oocyte-secreted factors (OSF) [6–11]. The participation of the oocyte in mechanisms regulating its communication with cumulus cells and the activation of the maturation cascade constitutes, however, a valid and interesting hypothesis not yet directly investigated.
Elegant studies in mice demonstrated that the delivery of cumulus-derived cyclic guanosine monophosphate (cGMP) through gap junctions communicating cumulus trans-zonal projections (TZPs) with the ooplasm prevents meiotic progression beyond prophase I before the LH ovulatory surge [12, 13]. cGMP is produced upon activation of the natriuretic peptide receptor 2 by natriuretic peptide C (NPPC) in mural (mice and cattle) and cumulus (cattle) granulosa cells and flows into the oocyte to decrease the activity of phosphodiesterase 3 on cyclic adenosine monophosphate (cAMP), thus preventing its degradation and maintaining its inhibitory action on the maturation promoting factor [11–13]. Somehow paradoxically, while sustained relatively high levels of cAMP are required to maintain the meiotic arrest in the bovine oocyte, supraphysiological cAMP levels induced by FSH can disrupt gap-junction-mediated communication between bovine cumulus cells, thus reducing cGMP delivery into the oocyte and precipitating oocyte chromatin compaction and transcription silencing [14–18].
Studies in mice, cattle, and primates converge to indicate that in order to trigger the ovulatory cascade and final oocyte maturation, LH first stimulates the expression and release of epidermal growth factor (EGF)–like family members from mural granulosa cells, particularly amphiregulin (AREG) and epiregulin (EREG) [19–23], as well as ADAM17, a member of the disintegrin and metalloproteinase family that cleaves transmembrane precursors of EGF-like factors, releasing the active forms for autocrine and paracrine action [24]. Studies in mice demonstrated that, subsequently, EGF-like factors bind to EGF receptors in cumulus cells and, through the mitogen activated protein kinase 3 and 1 (MAPK3/1), stimulate the expression of prostaglandin synthase 2 (PTGS2), triggering a positive feedback loop involving prostaglandin E2 (PGE2), ADAM17, and EGF-like factors [25–27]. Increased MAPK3/1 signaling then promotes meiotic resumption by interrupting the delivery of cumulus-derived cGMP into the oocyte through gap junction closure and TZP retraction [17, 28].
FSH also strongly stimulates AREG and EREG expression in murine, swine, and bovine cumulus cells [22, 29, 30] and because of the absence of functional LH receptors in cumulus cells, IVM is commonly induced with FSH in livestock ART and animal models reviewed by [31]. In cattle and mice, the activation of the ovulatory cascade by either FSH or EGF-like factors increases the expression of genes crucial for cumulus expansion, including hyaluronan synthase 2 (HAS2) that encodes a key enzyme for the conversion of glucose into hyaluronan, the major component of the extracellular matrix, and tumor necrosis factor-alpha-induced protein-6 (TNFAIP6) and pentraxin-3 (PTX3), both encoding major structural proteins of the expanding [32–35].
Interestingly, studies in mice and cattle provided robust evidence that, apart from stimulating the EGF/ADAM17/PGE2 feedback loop and decreasing gap-junction-mediated transport [16, 27], increased FSH activity can also reduce TZP density between cumulus cells and the oocyte [2, 3]. We have recently integrated a large body of data from different animal models and women suggesting that TZP loss induced by excessive FSH signaling may compromise key processes for oocyte developmental competence such as energetic metabolism, oxidative stress control, DNA damage repair, and extra and intra-spindle actin dynamics [36]. Indeed, intrafollicular levels of FSH are negatively correlated with oocyte competence to achieve a live birth in women, while those of AMH, an intra-ovarian inhibitor of FSH signaling, are positively correlated [37–39].
We have previously observed that OSFs stimulate NPPC expression in bovine cumulus cells, suggesting that the oocyte participates in the mechanisms sustaining the meiotic arrest through cumulus cells [11]. Herein, we investigated whether the oocyte controls the expression of genes regulating FSH signaling and the maturation cascade in bovine cumulus cells. More specifically, we tested the hypothesis that OSFs modulate the expression of FSHR, AMH, and participants of the EGF-like cascade in cumulus cells, in a way that favors cumulus-oocyte communication and oocyte nuclear-cytoplasmic synchronicity.
Material and methods
Unless specified, all chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Experimental design
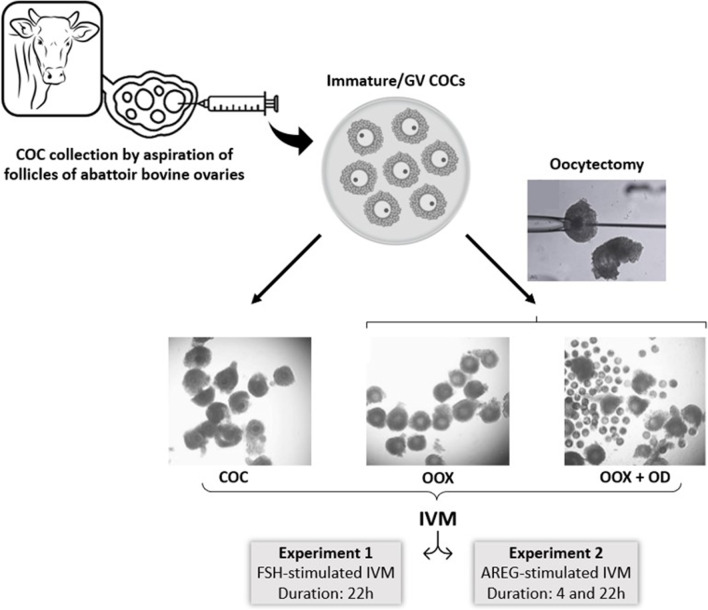
The effects of the oocyte on cumulus cell gene expression and their mediation by OSFs were assessed through the comparison of three different groups of COCs subjected to IVM: (1) intact COCs; (2) oocytectomized COCs (COCs with ooplasm removed by micromanipulation; OOX); and (3) oocytectomized COCs cultured with denuded oocytes (OOX+DO; Fig. 1). In experiment 1, the above groups were subjected to IVM stimulated with FSH (1 μg/mL) for 22 h (n=4 independent replicates per group), a timepoint chosen in accordance with previous timecourse studies [22, 40]. Since higher AMH and lower FSHR expression levels were observed together with decreased expression of AREG, EREG, ADAM17, PGTS2, TNFAIP6, and PTX3 in COC and OOX+DO, in experiment 2, we tested the hypotheses (1) that the inhibitory effect of the oocyte on genes regulating the ovulatory cascade does not depend on decreased FSH signaling and involves a direct and acute inhibition of the EGF-like cascade, and (2) that the inhibitory effect of the oocyte on genes regulating FSH signaling also occurs during EGF-like stimulated IVM. For this, we compared FSHR, AMH, AREG, EREG, ADAM17, EGFR, PTGS2, TNFAIP6, PTX3, and HAS2 mRNA abundance in cumulus cells from COC, OOX, and OOX+DO groups, subjected to IVM stimulated with AREG (100 ng/mL) for 4 and 22 h (n=4 independent replicates per group per culture duration). These two timepoints were chosen in order to allow a more accurate assessment of mRNA levels of genes involved in the activation of the maturation cascade, as well as of downstream genes crucial to COC final maturation [22, 40].
Fig. 1.
Illustrative experimental design. Immature GV cumulus-oocyte complexes (COCs) were recovered through the aspiration of follicles of bovine ovaries obtained at an abattoir. Intact COCs (COC), oocytectomized COCs (OOX), and oocytectomized COCs together with denuded oocytes (OOX+DO) were subjected to FSH-stimulated IVM for 22h in experiment 1, and AREG-stimulated IVM for 4 and 22h in experiment 2
In vitro maturation and oocytectomy
Ovaries of adult cows (predominantly Nellore, Bos indicus) were obtained from nearby slaughterhouses and transported to the laboratory in saline (0.9% NaCl) at 37 °C. Germinal vesicle stage/immature COCs were aspirated from 2- to 8-mm diameter follicles with an 18-gauge needle and then selected using a stereomicroscope (Nikon, SMZ800, Tokyo, Japan). Only COCs with a homogeneous ooplasm and a compact multilayer of cumulus cells were used (grades I and II) [41]. Selected COCs (20–25 COCs/group) were then washed and transferred to four-well plates containing TCM199 supplemented with 1 μg/mL porcine FSH (equivalent to 1.6 mU/mL; Folltropin-V® Bioniche Animal Health, Belleville ON, Canada) or 100 ng/mL recombinant human AREG (R&D Systems), 22 μg/mL sodium pyruvate, 75 μg/mL amikacin, and 4 mg/mL fatty acid-free bovine serum albumin. COCs were incubated at 38.5 °C in 5% CO2 in humidified air.
In order to remove the influence of the oocyte on cumulus differentiation during IVM, COCs were placed in 200 μL drops of TCM199 partially covered with mineral oil and the ooplasm was aspirated with a micromanipulator as previously described [42, 43] (Fig. 1). The resulting ooplasm-free cumulus complexes (OOX) were cultured exactly as COCs in accordance with the experimental design and IVM description above. Denuded oocytes (DOs) were obtained from pools of 100 grade 1 and 2 immature COCs subjected to mechanical cell separation by repeated pipetting, and added to ooplasm-free cumulus complexes at the start of IVM at the ratio of 100 oocytes per 20–25 ooplasm-free COCs. This DO/OOX ratio had been previously demonstrated to neutralize the effects of oocytectomy on mRNA abundance of KITL (kit ligand), NPPC, and FGF2 in bovine OOXs [10, 11].
Sham controls were performed by penetrating COCs with a micro-aspiration needle as far as the oolemma but without removing any cellular material. These COCs were subjected to FSH-stimulated IVM for 22 h alongside COC and OOX groups to measure PTGS2 mRNA abundance in cumulus cells (n=3 independent replicates per group). Abundance of PTGS2 mRNA was increased in OOX compared to intact COCs (COC), but did not differ between SHAM and COC groups (Supplementary Fig. 1).
Measurement of mRNA abundance
After IVM, cumulus cells were mechanically separated by repeated pipetting in PBS, transferred to 1.5-mL tubes, collected by centrifugation for 5 min at 700g, and frozen at −80 °C in 350 μL of RNA extraction lysis buffer (RNeasy® kit; Qiagen, Mississauga, ON, Canada). Total RNA was extracted using the RNeasy® kit as recommended by the manufacturer, after which RNA samples were eluted in 30 μL of RNAse-free water. Total RNA concentrations were measured by spectrophotometry using a NanoDrop ND® 1000 (Thermo Scientific, Wilmington, DE, USA). Total RNA (100 ng/reaction) was incubated with DNAse I (1 U/μg; Invitrogen, São Paulo, Brazil) and then reverse transcribed using random primers (High-Capacity kit, Applied Biosystems, Waltham, MA, USA). Relative mRNA abundance of the selected target genes was assessed by RT-qPCR analysis using the StepOnePlus™ Real-Time PCR System (Applied Biosystems) and the Power Sybr Green PCR Master Mix (Applied Biosystems). The final volume of the PCR reaction was 20 μL and thermocycling conditions were 95 °C for 10 min (1 cycle), denaturing at 95 °C for 15 s followed by annealing at 60 °C for 1 min (40 cycles). Primers for the reference (CYCA and RPL15) and target genes (FSHR, AMH, ADAM17, EGFR, AREG, EREG, PTGS2, HAS2, PTX3, and TNFAIP6; Table 1) were as previously used and validated [22, 40, 44––46]. Relative expression values for each gene were calculated using the 2ΔΔCt method [44].
Table 1.
Genes analyzed in CCs samples by RT-qPCR
| GENE | SEQUENCE | REFERENCE |
|---|---|---|
| CYCA |
F: 5′-GCC ATG GAG CGC TTT GG-3′ R: 5′-CCA CAG TCA GCA ATG GTG ATC T-3′ |
[40] |
| RPL15 |
F: 5′-CTC ATC GTT GGT GCC AAT GCA AGT-3′ R: 5′-TCA CAT CCA CCC TGG GAA ACA GAA-3′ |
[70] |
| FSHR |
F: 5′-AGC CCC TTG TCA CAA CTC TAT GTC-3′ R: 5′-GTT CCT CAC CGT GAG GTA GAT GT-3′ |
[71] |
| AMH |
F: 5′-AAG AAG TCT TCA GCA CCT CAG CCT-3′ R: 5′-AGT CCC AGG CTT GCT GAA AGA TGA-3′ |
NM_173890.1 |
| EGFR |
F: 5′-AAA GTT TGC CAA GGG ACA AG-3′ R: 5′-AAA GCA CAT TTC CTC GGA TG-3′ |
[40] |
| PTX3 |
F: 5′-CCT CAG CTA TCG GTC CAT AA-3′ R: 5′-ATT GAA GCC TGT GAG GTC TGC-3′ |
[40] |
| PTGS2 |
F: 5′-AAG CCT AGC ACT TTC GGT GGA GAA-3′ R: 5′-TCC AGA GTG GGA AGA GCT TGC ATT-3′ |
[40] |
| HAS2 |
F: 5′-ACA CAG ACA GGC TGA GGA CAA CTT-3′ R: 5′-AAG CAG CTG TGA TTC CAA GGA GGA-3′ |
[40] |
| TNFAIP6 |
F: 5′-GCA AAG GAG TGT GGT GGT GTG TTT-3′ R: 5′-ACT GAG GTG AAT GCG CTG ACC ATA-3′ |
[40] |
| AREG |
F: 5′-CTT TCG TCT CTG CCA TGA CCT T-3′ R: 5′-CGT TCT TCA GCG ACA CCT TCA-3′ |
[40] |
| EREG |
F: 5′-ACT GCA CAG CAT TAG TTC AAA CTG A-3′ R: 5′-TGT CCA TGC AAA CAG TAG CCA TT-3′ |
[40] |
| ADAM17 |
F: 5′-TGG GAT GTG AAG ATG TTG CTA GA-3′ R: 5′-ATC CAA GTG TTC CCA TAT CAA AAT C-3′ |
[40] |
F forward primer, R reverse primer
Statistical analysis
The distribution of gene expression data was first assessed and, when not normally distributed, expression values were log-transformed. The effects of oocytectomy and of OSF replacement (addition of denuded oocytes to culture) on cumulus mRNA abundance were tested by analysis of variance, and in the face of a significant effect, gene expression values from the 3 experimental groups were compared with the Tukey-Kramer test. The statistical analysis was performed using the JMP software (SAS Institute, Cary, NC, USA) and differences were considered significant when p<0.05.
Results
Experiment 1
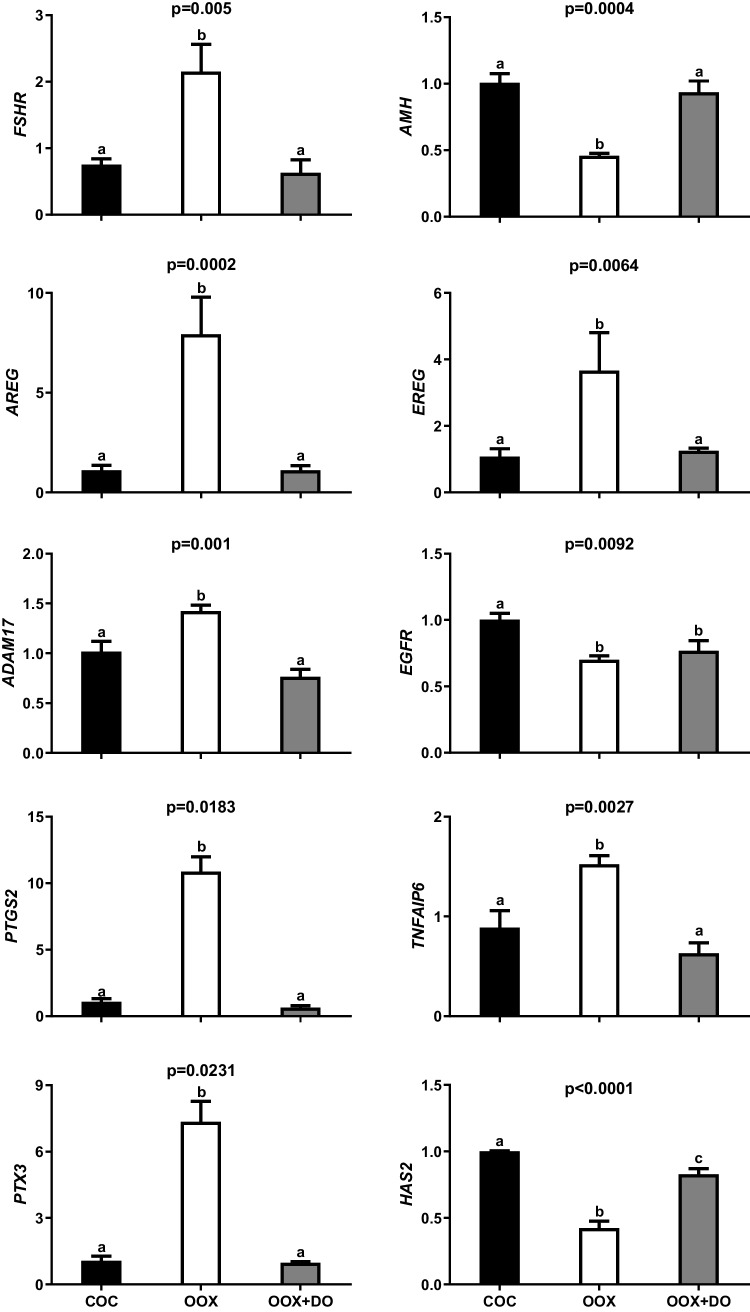
The removal of the ooplasm from the COC by oocytectomy increased FSHR, AREG, EREG, ADAM17, PTGS2, TNFAIP6, and PTX3 mRNA abundance in cumulus cells after 22 h of FSH-stimulated IVM, which was entirely reverted by the addition of denuded oocytes to culture (Fig. 2). Oocytectomy caused 40 and 70% increases in ADAM17 and TNFAIP6 mRNA abundance, respectively, while approximately tripling the abundance of FSHR and EREG mRNA. The most drastic changes were observed, however, in AREG, PTX3 and PTGS2 mRNA levels, which increased between 7 to 10 times after oocytectomy. In contrast, oocytectomy halved the abundance of AMH and HAS2 mRNA, which was again completely neutralized by supplementation with denuded oocytes. Oocytectomy also decreased mRNA levels of EGFR, but this 30% reduction was not reverted by the addition of denuded oocytes to IVM.
Fig. 2.
Effects of oocyte removal with or without replacement of OSFs on FSHR, AMH, AREG, EREG, ADAM17, EGFR, PTGS2, TNFAIP6, PTX3, and HAS2 mRNA abundance in cumulus cells from COCs subjected to FSH-stimulated IVM for 22 h. COCs were cultured intact (COC), oocytectomized (OOX), or oocytectomized with denuded oocytes (OOX + DO). Data represent mRNA abundance of a given target gene relative to the reference genes (RPL15 and CYCA). Bars with different letters are significantly different (p<0.05). Data derive from four independent replicates
Experiment 2
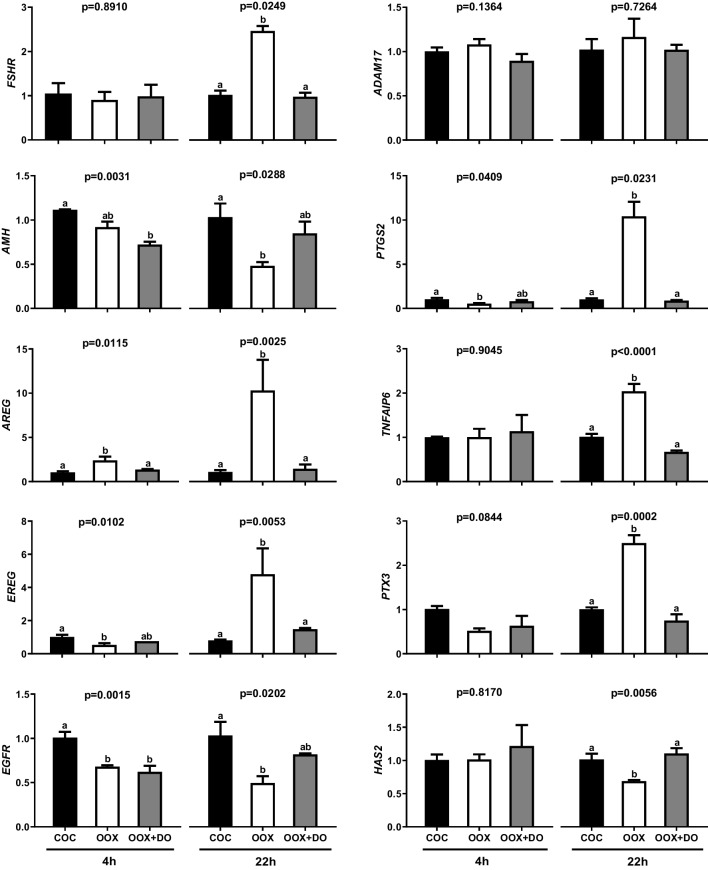
Since in experiment 1, in FSH-stimulated IVM, oocytectomy decreased the expression of genes participating in the EGF/ADAM17/PGE2 positive feedback loop in parallel with changes in FSHR and AMH mRNA levels suggestive of a reduction in FSH signaling, we then assessed whether these effects are dependent on reduced FSH signaling by repeating the same experimental design with AREG-stimulated IVM for 4 and 22 h (Fig. 3). Oocytectomy doubled AREG mRNA levels after 4 h, which was neutralized by the addition of denuded oocytes to culture. In contrast, oocytectomy decreased EREG, EGFR, and PTGS2 mRNA abundance, which was not reverted by the addition of denuded oocytes to culture. Furthermore, oocytectomy did not significantly alter FSHR and AMH mRNA abundance after 4 h of AREG-stimulated IVM, but the addition of denuded oocytes to culture decreased AMH mRNA levels in relation to the intact/control group.
Fig. 3.
Effects of oocyte removal with or without replacement of OSFs on FSHR, AMH, AREG, EREG, ADAM17, EGFR, PTGS2, TNFAIP6, PTX3, and HAS2 mRNA abundance in cumulus cells from COCs subjected to AREG-stimulated IVM for 4 and 22 h. COCs were cultured intact (COC), oocytectomized (OOX), or oocytectomized with denuded oocytes (OOX + DO). Data represent mRNA abundance of a given target gene relative to the reference genes (RPL15 and CYCA). Bars with different letters are significantly different (p<0.05). Data derive from four independent replicates
After 22 h of AREG-stimulated IVM, consistently with experiment 1, oocytectomy increased AREG (10-fold), EREG (5-fold), PTGS2 (10-fold), TNFAIP6 (2-fold), and PTX3 (2.5fold) mRNA levels. Also in agreement with experiment 1, oocytectomy augmented FSHR mRNA abundance (2.5-fold), while approximately halving that of AMH, EGFR, and HAS2 mRNA. All effects of oocytectomy observed at 22 h of IVM were reverted by the addition of denuded oocytes to culture. In contrast to experiment 1, oocytectomy did not significantly alter ADAM17 mRNA abundance after 22 h of AREG-stimulated IVM.
Discussion
Understanding the mechanisms that control oocyte developmental competence is crucial for the improvement of female subfertility treatments. Data indicative of the participation of OSFs in mechanisms regulating cumulus cell metabolism and differentiation have accumulated for more than three decades [8, 9, 42, 45]. We have previously provided evidence that OSFs may also control oocyte nuclear maturation through cumulus cells [11]. Herein, we provide novel evidence that, through secreted factors, the oocyte controls cumulus cell gene expression in such a way that would protect its companion cells from excessive FSH signaling and prevent premature activation of the ovulatory cascade.
Early studies in mice utilizing oocytectomy to assess the participation of the oocyte in cumulus cell differentiation demonstrated that OSFs contribute to generate the cumulus cell phenotype by suppressing the expression of the LH receptor [46]. In the present study, oocytectomy increased FSHR mRNA levels in cumulus cells, while decreasing those of AMH, both in FSH and in AREG-stimulated IVM. Since these effects were abrogated when oocytectomized COCs were cultured together with denuded oocytes known to release peptides in culture [42, 45], it is logical to assume these changes were mediated by OSFs. The stimulatory effect of OSFs on AMH mRNA levels is in line with previous studies in sheep and women showing higher AMH expression in cumulus cells as compared with mural granulosa cells, as the first are closer to the oocyte and thus more susceptible to its paracrine influence [47, 48]. Since AMH was shown to inhibit FSH signaling, both at the level of FSHR expression and cAMP response, in human granulosa cells [37, 38], the present data suggest that the oocyte reduces FSH activity in surrounding cumulus cells. During antral follicle growth, FSH signaling in mural granulosa cells is crucial to stimulate steroidogenesis and final follicle growth in all mammalian species [49]. On the other hand, concomitant intense FSH signaling in cumulus cells could prematurely activate the maturation cascade by stimulating EGF-like expression while precipitating loss of TZP-mediated cumulus-oocyte communication [2, 3, 22, 36]. Therefore, through the modulation of FSHR and AMH expression in cumulus cells, while trying to assure synchronous maturation of its cytoplasmic and nuclear compartments, the oocyte possibly makes an effort to preserve its communication with cumulus cells, thus maximizing the incorporation of cumulus-derived metabolites and mRNA essential for its homeostasis and developmental competence [36, 50].
The identity of the OSFs promoting these changes remains to be clarified, but previous studies point to bone morphogenetic protein 15 (BMP15), growth and differentiation factor 9 (GDF9), and fibroblast growth factor 10 (FGF10) as plausible candidates. Both BMP15 and GDF9 decreased FSHR expression in rat granulosa cells [51, 52], and together stimulated AMH expression in human and mouse cumulus cells [53, 54]. FGF10 has been more recently characterized as an important OSF in the bovine model, in which it was found to inhibit FSHR expression and estradiol production in cultured granulosa cells [40, 55, 56].
The present data indicate that the oocyte also inhibits the expression of major genes of the ovulatory cascade. Oocytectomy increased mRNA levels of AREG, EREG, ADAM17, and PTGS2 in cumulus cells, genes known to compose a positive feedback loop triggered by EGF signaling that is required for final COC maturation [27], during FSH-stimulated IVM. Once again, the effects of oocytectomy were neutralized by the addition of denuded oocytes to culture, indicating the mediation by OSFs. Importantly, the manifestation of the inhibitory effect of OSFs on AREG mRNA abundance after 4 h, and on AREG, EREG, and PTGS2 mRNA levels at 22 h of AREG-stimulated IVM indicates that this action is acute and independent of FSH signaling. Consistently with these changes, OSFs also suppressed TNFAIP6 and PTX3 transcription in cumulus cells in our study, genes encoding structural proteins of the extracellular matrix, known to be activated downstream from EGF-like signaling [33, 57, 58]. Interestingly, these findings suggest that, through OSFs, the oocyte inhibits precocious/excessive activation of the maturation cascade, likely preserving TZP-mediated communication with cumulus cells [28] and preventing premature meiotic resumption [16], both actions in favor of developmental competence. This suggestion is in line with studies in pigs and cattle, in which supplemention with Neuregulin 1 (NGR1), a modulatory EGF-like factor that attenuates AREG intracellular responses and slows meiotic progression [59–61], improved post-IVF embryo development [61, 62].
The identity of the OSFs mediating the inhibitory influence of the oocyte on the maturation cascade remains to be revealed. Intriguingly, the most investigated OSFs to date, BMP15 and GDF9, appear to act in consonance, both in opposition to the effects of OSFs observed in this study. BMP15 was previously shown to increase AREG, EREG, PTGS2, TNPAIP6, and PTX3 expression in mice and cows [40, 63, 64]. Although no study to date has demonstrated a direct effect of GDF9 on the expression of EGF-like factors, suppression of oocyte GDF9 expression by RNA interference decreased the expression of PTGS2 in the murine COC [65], and GDF9 combined with BMP15 increased EGF signaling in swine cumulus cells [66].
Interestingly and paradoxically, our data suggest that, although to a much lesser extent in relation to the aforementioned inhibitory changes, the oocyte stimulates EGFR expression. This finding is in agreement with a previous study in mice, in which the negative impact of oocytectomy on EGFR mRNA levels was nevertheless far more intense [67]. It is tempting to speculate that, by increasing EGFR expression and paradoxically inhibiting FSH signaling and AREG/EREG transcription in cumulus cells, the oocyte may stimulate responsiveness to the maturation trigger, while trying to assure that it occurs at the right time, only following LH-stimulated secretion of EGF-like factors from mural granulosa cells, and at an appropriate speed.
Finally, the present data indicate that, through OSFs, the oocyte stimulates HAS2 transcription in cumulus cells, which is somehow in contrast with its effects on genes triggering the maturation cascade (AREG, EREG, and ADAM17), as well as on downstream genes known to respond to MAPK3/1 signaling (PTGS2, TPNFA1, and PTX3) [26, 68]. This finding is nevertheless in agreement with previous evidence that OSFs regulate cumulus expansion and potently stimulate HAS2 expression in mice (BMP15 and GDF9) [65, 69] and cattle (BMP15) [40].
Our study is limited by the assessment of mRNA abundance as the only endpoint reflecting gene expression. In addition, we cannot rule out that denudation may alter oocyte secretion activity thus impacting the physiologycal accuracy of the effects attributed to OSFs herein. Therefore, further studies are needed to confirm the suggestions emerging from the present data. On the other hand, the present design including different IVM stimulators and culture times allowed the production of novel data shedding light on the actions of the oocyte in the regulation of cumulus cell gene expression in a mono-ovulatory species. Moreover, the present evidence of inhibitory influences of the oocyte on FSH signaling and on the maturation cascade in cumulus cells represent new important parameters for the optimization of ART strategies, indicating that excessive FSH intrafollicular activity and premature activation of oocyte maturation may be detrimental to oocyte developmental competence.
In conclusion, the present findings suggest that, through the secretion of paracrine factors, the oocyte controls cumulus cells gene expression in an apparent effort to restrict FSH signaling and to prevent premature activation of the maturation cascade. In the light of the converging literature discussed herein, we propose that these influences may be important in the determination of the cumulus cell phenotype and critical to safeguard cumulus-oocyte communication and nuclear-cytoplasmic synchronicity during final antral follicle development. Therefore, the present data and insights may contribute for a better understanding of the oocyte biology and stimulate clinical thinking towards the improvement of subfertility treatments through the optimization of cumulus-oocyte homeostasis.
Supplementary Information
Supplementary Fig. 1 PTGS2 mRNA abundance in cumulus cells from COCs subjected to FSH-stimulated IVM for 22 hours. COCs were cultured intact (COC), oocytectomized (OOX) or after penetration of the cumulus with a micro-aspiration needle as far as the oolemma, identically as performed in OOX except for oolemma perforation and ooplasm aspiration (SHAM). Bars with different letters are significantly different (p<0.05). Data derive from three independent replicates. (DOCX 16 kb)
Author contribution
JB: definition of the objectives, study design, data analysis and interpretation, manuscript writing. TTD and PFL: performance of experiments and data analysis. MDC, MMR, and CAP data interpretation and discussion and critical reading during manuscript preparation.
Funding
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP grant number 2019/14588-6) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES, code 0001).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jose Buratini, Email: jburatini@eugin.it.
Thaisy Tino Dellaqua, Email: thaisy.dellaqua@unesp.br.
Paula Fernanda de Lima, Email: paulafld@yahoo.com.br.
Mario Mignini Renzini, Email: mmignini@eugin.it.
Mariabeatrice Dal Canto, Email: bdalcanto@eugin.it.
Christopher A. Price, Email: christopher.price@umontreal.ca
References
- 1.Luciano AM, Sirard MA. Successful in vitro maturation of oocytes: a matter of follicular differentiation. Biol Reprod. 2018;98:162–169. doi: 10.1093/biolre/iox149. [DOI] [PubMed] [Google Scholar]
- 2.Hyttel P, Callesen H, Greve T. Ultrastructural features of preovulatory oocyte maturation in superovulated cattle. Reproduction. 1986;76:645–656. doi: 10.1530/jrf.0.0760645. [DOI] [PubMed] [Google Scholar]
- 3.Combelles CM, Carabatsos MJ, Kumar TR, Matzuk MM, Albertini DF. Hormonal control of somatic cell oocyte interactions during ovarian follicle development. Mol Reprod Dev. 2004;69:347–355. doi: 10.1002/mrd.20128. [DOI] [PubMed] [Google Scholar]
- 4.Hyttel P. Bovine cumulus-oocyte disconnection in vitro. Anat Embryol. 1987;176:41–44. doi: 10.1007/BF00309750. [DOI] [PubMed] [Google Scholar]
- 5.Lodde V, Modina S, Galbusera C, Franciosi F, Luciano AM. Large-scale chromatin remodeling in germinal vesicle bovine oocytes: interplay with gap junction functionality and developmental competence. Mol Reprod Dev. 2007;74:740–749. doi: 10.1002/mrd.20639. [DOI] [PubMed] [Google Scholar]
- 6.Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. doi: 10.1530/rep.0.1220829. [DOI] [PubMed] [Google Scholar]
- 7.Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Anim Reprod Sci. 2004;82-83:431–446. doi: 10.1016/j.anireprosci.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–177. doi: 10.1093/humupd/dmm040. [DOI] [PubMed] [Google Scholar]
- 9.Eppig JJ. Reproduction: oocytes call, granulosa cells connect. Curr Biol. 2018;28:R354–R3R6. doi: 10.1016/j.cub.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Barros RG, Lima PF, Soares ACS, Sanches L, Price CA, Buratini J. Fibroblast growth factor 2 regulates cumulus differentiation under the control of the oocyte. J Assist Reprod Genet. 2019;36:905–913. doi: 10.1007/s10815-019-01436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima PF, Ormond CM, Caixeta ES, Barros RG, Price CA, Buratini J. Effect of kit ligand on natriuretic peptide precursor C and oocyte maturation in cattle. Reproduction. 2016;152:481–489. doi: 10.1530/REP-16-0155. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–369. doi: 10.1126/science.1193573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol. 2012;356:65–73. doi: 10.1016/j.mce.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luciano AM, Modina S, Vassena R, Milanesi E, Lauria A, Gandolfi F. Role of intracellular cyclic adenosine 3',5'-monophosphate concentration and oocyte-cumulus cells communications on the acquisition of the developmental competence during in vitro maturation of bovine oocyte. Biol Reprod. 2004;70:465–472. doi: 10.1095/biolreprod.103.020644. [DOI] [PubMed] [Google Scholar]
- 15.Modina S, Luciano AM, Vassena R, Baraldi-Scesi L, Lauria A, Gandolfi F. Oocyte developmental competence after in vitro maturation depends on the persistence of cumulus-oocyte comunications which are linked to the intracellular concentration of cAMP. Ital J Anat Embryol. 2001;106:241–248. [PubMed] [Google Scholar]
- 16.Luciano AM, Franciosi F, Modina SC, Lodde V. Gap junction-mediated communications regulate chromatin remodeling during bovine oocyte growth and differentiation through cAMP-dependent mechanism(s) Biol Reprod. 2011;85:1252–1259. doi: 10.1095/biolreprod.111.092858. [DOI] [PubMed] [Google Scholar]
- 17.Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135:3229–3238. doi: 10.1242/dev.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atef A, Francois P, Christian V, Marc-Andre S. The potential role of gap junction communication between cumulus cells and bovine oocytes during in vitro maturation. Mol Reprod Dev. 2005;71:358–367. doi: 10.1002/mrd.20281. [DOI] [PubMed] [Google Scholar]
- 19.Park JY, Su YQ, Ariga M, Law E, Jin SLC, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 20.Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84. doi: 10.1210/en.2004-0588. [DOI] [PubMed] [Google Scholar]
- 21.Conti M, Hsieh M, Park JY, Su YQ. Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol. 2006;20:715–723. doi: 10.1210/me.2005-0185. [DOI] [PubMed] [Google Scholar]
- 22.Caixeta ES, Machado MF, Ripamonte P, Price C, Buratini J. Effects of FSH on the expression of receptors for oocyte-secreted factors and members of the EGF-like family during in vitro maturation in cattle. Reprod Fertil Dev. 2013;25:890–899. doi: 10.1071/RD12125. [DOI] [PubMed] [Google Scholar]
- 23.Fru KN, Cherian-Shaw M, Puttabyatappa M, VandeVoort CA, Chaffin CL. Regulation of granulosa cell proliferation and EGF-like ligands during the periovulatory interval in monkeys. Hum Reprod. 2007;22:1247–1252. doi: 10.1093/humrep/del519. [DOI] [PubMed] [Google Scholar]
- 24.Yamashita Y, Kawashima I, Yanai Y, Nishibori M, Richards JS, Shimada M. Hormone-induced expression of tumor necrosis factor alpha-converting enzyme/A disintegrin and metalloprotease-17 impacts porcine cumulus cell oocyte complex expansion and meiotic maturation via ligand activation of the epidermal growth factor receptor. Endocrinology. 2007;148:6164–6175. doi: 10.1210/en.2007-0195. [DOI] [PubMed] [Google Scholar]
- 25.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20:1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- 26.Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. doi: 10.1126/science.1171396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita Y, Shimada M. The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process. J Reprod Dev. 2012;58:510–514. doi: 10.1262/jrd.2012-056. [DOI] [PubMed] [Google Scholar]
- 28.Abbassi L, El-Hayek S, Carvalho KF, Wang W, Yang Q, Granados-Aparici S, Mondadori R, Bordignon V, Clarke HJ. Epidermal growth factor receptor signaling uncouples germ cells from the somatic follicular compartment at ovulation. Nat Commun. 2021;12:01–13. doi: 10.1038/s41467-021-21644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prochazka R, Petlach M, Nagyova E, Nemcova L. Effect of epidermal growth factor-like peptides on pig cumulus cell expansion, oocyte maturation, and acquisition of developmental competence in vitro: comparison with gonadotropins. Reproduction. 2011;141:425–435. doi: 10.1530/REP-10-0418. [DOI] [PubMed] [Google Scholar]
- 30.Downs SM, Chen J. EGF-like peptides mediate FSH-induced maturation of cumulus cell-enclosed mouse oocytes. Mol Reprod Dev. 2008;75:105–114. doi: 10.1002/mrd.20781. [DOI] [PubMed] [Google Scholar]
- 31.Buratini J, Soares ACS, Barros RG, Dellaqua TT, Lodde V, Franciosi F, Dal Canto M, Renzini MM, Luciano AM. Physiological parameters related to oocyte nuclear differentiation for the improvement of IVM/IVF outcomes in women and cattle. Reprod Fertil Dev. 2021:34. 10.1071/rd21278. [DOI] [PubMed]
- 32.Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13:289–312. doi: 10.1093/humupd/dml062. [DOI] [PubMed] [Google Scholar]
- 33.Salustri A, Yanagishita M, Hascall VC. Synthesis and Accumulation of hyaluronic acid and proteoglycans in the mouse cumulus cell-oocyte complex during follicle-stimulating hormone-induced mucification. J Biol Chem. 1989;264:13840–13847. doi: 10.1016/s0021-9258(18)80077-1. [DOI] [PubMed] [Google Scholar]
- 34.Kimura N, Konno Y, Miyoshi K, Matsumoto H, Sato E. Expression of hyaluronan synthases and CD44 messenger RNAs in porcine cumulus-oocyte complexes during in vitro maturation. Biol Reprod. 2002;66:707–717. doi: 10.1095/biolreprod66.3.707. [DOI] [PubMed] [Google Scholar]
- 35.Schoenfelder M, Einspanier R. Expression of hyaluronan synthases and corresponding hyaluronan receptors is differentially regulated during oocyte maturation in cattle. Biol Reprod. 2003;69:269–277. doi: 10.1095/biolreprod.102.011577. [DOI] [PubMed] [Google Scholar]
- 36.Buratini J, Dellaqua TT, Dal Canto M, La Marca A, Carone D, Mignini Renzini M, Webb R. The putative roles of FSH and AMH in the regulation of oocyte developmental competence: from fertility prognosis to mechanisms underlying age-related subfertility. Hum Reprod Update. 2022;28:232–254. doi: 10.1093/humupd/dmab044. [DOI] [PubMed] [Google Scholar]
- 37.Pellatt L, Rice S, Dilaver N, Heshri A, Galea R, Brincat M, Brown K, Simpson ER, Mason HD. Anti-Mullerian hormone reduces follicle sensitivity to follicle-stimulating hormone in human granulosa cells. Fertil Steril. 2011;96:1246–1251. doi: 10.1016/j.fertnstert.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Chang HM, Klausen C, Leung PC. Antimullerian hormone inhibits follicle-stimulating hormone-induced adenylyl cyclase activation, aromatase expression, and estradiol production in human granulosa-lutein cells. Fertil Steril. 2013;100:585–592. doi: 10.1016/j.fertnstert.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Ciepiela P, Duleba AJ, Kario A, Chelstowski K, Branecka-Wozniak D, Kurzawa R. Oocyte matched follicular fluid anti-Mullerian hormone is an excellent predictor of live birth after fresh single embryo transfer. Hum Reprod. 2019;34:2244–2253. doi: 10.1093/humrep/dez186. [DOI] [PubMed] [Google Scholar]
- 40.Caixeta ES, Sutton-McDowall ML, Gilchrist RB, Thompson JG, Price CA, Machado MF, Lima PF, Buratini J. Bone morphogenetic protein 15 and fibroblast growth factor 10 enhance cumulus expansion, glucose uptake, and expression of genes in the ovulatory cascade during in vitro maturation of bovine cumulus-oocyte complexes. Reproduction. 2013;146:27–35. doi: 10.1530/REP-13-0079. [DOI] [PubMed] [Google Scholar]
- 41.Khurana NK, Niemann H. Effects of oocyte quality, oxygen tension, embryo density, cumulus cells and energy substrates on cleavage and morula/blastocyst formation of bovine embryos. Theriogenology. 2000;54:741–766. doi: 10.1016/S0093-691X(00)00387-3. [DOI] [PubMed] [Google Scholar]
- 42.Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor (s) secreted by the oocyte. Dev Biol. 1990;138:16–25. doi: 10.1016/0012-1606(90)90172-F. [DOI] [PubMed] [Google Scholar]
- 43.Paradis F, Moore HS, Pasternak JA, Novak S, Dyck MK, Dixon WT, Foxcroft GR. Pig preovulatory oocytes modulate cumulus cell protein and gene expression in vitro. Mol Cell Endocrinol. 2010;320:87–96. doi: 10.1016/j.mce.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 44.Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanderhyden BC, Caron PJ, Buccione R, Eppig JJ. Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev Biol. 1990;140:307–317. doi: 10.1016/0012-1606(90)90081-S. [DOI] [PubMed] [Google Scholar]
- 46.Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod. 1997;56:976–984. doi: 10.1095/biolreprod56.4.976. [DOI] [PubMed] [Google Scholar]
- 47.Grondahl ML, Nielsen ME, Dal Canto MB, Fadini R, Rasmussen IA, Westergaard LG, Kristensen SG, Yding AC. Anti-Mullerian hormone remains highly expressed in human cumulus cells during the final stages of folliculogenesis. Reprod BioMed Online. 2011;22:389–398. doi: 10.1016/j.rbmo.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Campbell BK, Clinton M, Webb R. The role of anti-Mullerian hormone (AMH) during follicle development in a monovulatory species (sheep) Endocrinology. 2012;153:4533–4543. doi: 10.1210/en.2012-1158. [DOI] [PubMed] [Google Scholar]
- 49.Webb R, Buratini J, Hernandez-Medrano JH, Gutierrez CG, Campbell BK. Follicle development and selection: past, present and future. Anim Reprod. 2016;13:234–249. doi: 10.21451/1984-3143-ar883. [DOI] [Google Scholar]
- 50.Richani D, Dunning KR, Thompson JG, Gilchrist RB. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum Reprod Update. 2021;27:27–47. doi: 10.1093/humupd/dmaa043. [DOI] [PubMed] [Google Scholar]
- 51.Vitt UA, Hayashi M, Klein C, Hsueh AJW. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod. 2000;62:370–377. doi: 10.1095/biolreprod62.2.370. [DOI] [PubMed] [Google Scholar]
- 52.Otsuka F, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem. 2001;276:11387–11392. doi: 10.1074/jbc.M010043200. [DOI] [PubMed] [Google Scholar]
- 53.Convissar S, Armouti M, Fierro MA, Winston NJ, Scoccia H, Zamah AM, Stocco C. Regulation of AMH by oocyte-specific growth factors in human primary cumulus cells. Reproduction. 2017;154:745–753. doi: 10.1530/REP-17-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roy S, Gandra D, Seger C, Biswas A, Kushnir VA, Gleicher N, Kumar TR, Sen A. Oocyte-derived factors (GDF9 and BMP15) and FSH regulate AMH expression via modulation of H3K27AC in granulosa cells. Endocrinology. 2018;159:3433–3445. doi: 10.1210/en.2018-00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buratini J, Pinto MGL, Castilho AC, Amorim RL, Giometti IC, Portela VM, Nicola ES, Price CA. Expression and function of fibroblast growth factor 10 and its receptor, fibroblast growth factor receptor 213, in bovine follicles. Biol Reprod. 2007;77:743–750. doi: 10.1095/biolreprod.107.062273. [DOI] [PubMed] [Google Scholar]
- 56.Castilho ACS, Price CA, Dalanezi F, Ereno RL, Machado MF, Barros CM, Gasperin BG, Goncalves PBD, Buratini J. Evidence that fibroblast growth factor 10 plays a role in follicle selection in cattle. Reprod Fertil Dev. 2015;29:234–243. doi: 10.1071/RD15017. [DOI] [PubMed] [Google Scholar]
- 57.Camaioni A, Klinger FG, Campagnolo L, Salustri A. The influence of Pentraxin 3 on the ovarian function and its impact on fertility. Front Immunol. 2018;9:2808. doi: 10.3389/fimmu.2018.02808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ochsner SA, Day AJ, Rugg MS, Breyer RM, Gomer RH, Richards JS. Disrupted function of tumor necrosis factor-alpha-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology. 2003;144:4376–4384. doi: 10.1210/en.2003-0487. [DOI] [PubMed] [Google Scholar]
- 59.Noma N, Kawashima I, Fan HY, Fujita Y, Kawai T, Tomoda Y, Mihara T, Richards JS, Shimada M. LH-induced neuregulin 1 (NRG1) type III transcripts control granulosa cell differentiation and oocyte maturation. Mol Endocrinol. 2011;25:104–116. doi: 10.1210/me.2010-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawashima I, Umehara T, Noma N, Kawai T, Shitanaka M, Richards JS, Shimada M. Targeted disruption of Nrg1 in granulosa cells alters the temporal progression of oocyte maturation. Mol Endocrinol. 2014;28:706–721. doi: 10.1210/me.2013-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dellaqua TT, Vigaro RA, Janini LCZ, Dal Canto M, Renzini MM, Lodde V, Luciano AM, Buratini J. Neuregulin 1 (NRG1) modulates oocyte nuclear maturation during IVM and improves post-IVF embryo development. Theriogenology. 2023;195:209–216. doi: 10.1016/j.theriogenology.2022.10.041. [DOI] [PubMed] [Google Scholar]
- 62.Mao J, Whitworth KM, Spate LD, Walters EM, Zhao J, Prather RS. Regulation of oocyte mitochondrial DNA copy number by follicular fluid, EGF, and neuregulin 1 during in vitro maturation affects embryo development in pigs. Theriogenology. 2012;78:887–897. doi: 10.1016/j.theriogenology.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshino O, McMahon HE, Sharma S, Shimasaki S. A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc Natl Acad Sci. 2006;103:10678–10683. doi: 10.1073/pnas.0600507103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Q, Rajanahally S, Edson MA, Matzuk MM. Stable expression and characterization of N-terminal tagged recombinant human bone morphogenetic protein 15. Mol Hum Reprod. 2009;15:779–788. doi: 10.1093/molehr/gap062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gui LM, Joyce IM. RNA interference evidence that growth differentiation factor-9 mediates oocyte regulation of cumulus expansion in mice. Biol Reprod. 2005;72:195–199. doi: 10.1095/biolreprod.104.033357. [DOI] [PubMed] [Google Scholar]
- 66.Sugimura S, Ritter LJ, Rose RD, Thompson JG, Smitz J, Mottershead DG, Gilchrist RB. Promotion of EGF receptor signaling improves the quality of low developmental competence oocytes. Dev Biol. 2015;403:139–149. doi: 10.1016/j.ydbio.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 67.Su YQ, Sugiura K, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ. Mouse oocytes enable LH-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol Endocrinol. 2010;24:1230–1239. doi: 10.1210/me.2009-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shrestha K, Lukasik K, Baufeld A, Vanselow J, Moallem U, Meidan R. Regulation of ovulatory genes in bovine granulosa cells: lessons from siRNA silencing of PTGS2. Reproduction. 2015;149:21–29. doi: 10.1530/REP-14-0337. [DOI] [PubMed] [Google Scholar]
- 69.Peng J, Li Q, Wigglesworth K, Rangarajan A, Kattamuri C, Peterson RT, Eppig JJ, Thompson TB, Matzuk MM. Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci U S A. 2013;110:E776–E785. doi: 10.1073/pnas.1218020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Franchi FF, Satrapa RA, Fontes PK, Santos PH, Razza EM, Emanuelli IP, Ereno RL, Mareco EA, Nogueira MFG, Barros CM, et al. Equine chorionic gonadotropin drives the transcriptional profile of immature cumulus-oocyte complexes and in vitro-produced blastocysts of superstimulated Nelore cows. Mol Reprod Dev. 2019;86:1639–1651. doi: 10.1002/mrd.23251. [DOI] [PubMed] [Google Scholar]
- 71.Caixeta ES, Ripamonte P, Franco MM, Buratini J, Dode MAN. Effect of follicle size on mRNA expression in cumulus cells and oocytes of Bos indicus: an approach to identify marker genes for developmental competence. Reprod Fertil Dev. 2009;21:655–664. doi: 10.1071/RD08201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1 PTGS2 mRNA abundance in cumulus cells from COCs subjected to FSH-stimulated IVM for 22 hours. COCs were cultured intact (COC), oocytectomized (OOX) or after penetration of the cumulus with a micro-aspiration needle as far as the oolemma, identically as performed in OOX except for oolemma perforation and ooplasm aspiration (SHAM). Bars with different letters are significantly different (p<0.05). Data derive from three independent replicates. (DOCX 16 kb)