Abstract
This is the first report of the production of monoclonal antibodies against elk coronavirus. The nucleoprotein gene of elk coronavirus was amplified by PCR and was cloned and expressed in a prokaryotic expression vector. Recombinant nucleocapsid protein was used to immunize mice for the production of hybridomas. Twelve hybridomas that produced monoclonal antibodies against the nucleocapsid protein of elk coronavirus were selected by an indirect fluorescent-antibody test, an enzyme-linked immunosorbent assay, and a Western blot assay. Ten of the monoclonal antibodies were of the immunoglobulin G1 (IgG1) isotype, one was IgG2a, and one was IgM. All had kappa light chains. By immunohistochemistry four monoclonal antibodies detected bovine coronavirus and elk coronavirus in formalin-fixed intestinal tissues. Antinucleoprotein monoclonal antibodies were found to be better at ruminant coronavirus detection than the anti-spike protein monoclonal antibodies. Because nucleoprotein is a more abundant antigen than spike protein in infected cells, this was not an unexpected finding.
Elk (Cervus elephus) is a large species of the family Cervidae, and about 1 million live in North America. Elk are raised commercially for their antlers, which are used as aphrodisiacs. The elk industry generates profits of about a $120 million a year. One previous report identified an elk coronavirus (ECV) that causes enteritis and pneumonia in elk calves with high morbidity and mortality (11). Coronaviruses isolated from wild ruminants are antigenically indistinguishable from bovine coronavirus (BCV) (14). ECV is genetically and antigenically closely related to BCV and belongs to antigenic group II of the family Coronaviridae (11). There is 99% homology between the nucleoprotein gene sequence of ECV and BCV.
Nucleocapsid (N) protein is a structural protein of coronaviruses that forms a helical nucleocapsid with genomic RNA (13). N protein plays an important role in viral pathogenesis and replication (10). The open reading frame coding for the N protein is located at the 3′ end of the RNA genome (7). Monoclonal antibodies against the N protein protect mice from lethal infection and inhibit viral transcription in vitro (12). The monoclonal antibodies against the N protein of coronaviruses are generally nonneutralizing (3, 6).
This is the first study in which monoclonal antibodies against the N protein of ECV have been produced and characterized (there are no previous reports on the detection and pathogenesis of ECV). We have found these monoclonal antibodies to be very effective for use with immunohistochemistry (IHC) for the detection of BCV and ECV in formalin-fixed tissues. The lesions caused by ruminant coronaviruses are subtle and are similar to those caused by other ruminant viruses, such as bovine viral diarrhea virus, a pestivirus. It is difficult to make a confirmed diagnosis on the basis of histopathology alone. Thus, IHC could provide a useful adjunct tool for the confirmation of coronavirus infections.
MATERIALS AND METHODS
Virus and cells.
ECV WY-29 was propagated in human rectal tumor-18 cells with trypsin and pancreatin in the culture medium (8, 9) and was plaque purified as described previously (11).
Cloning of the nucleoprotein gene of ECV in prokaryotic expression vector.
Reverse transcription and PCR were performed with a forward primer (5′-TCTGGCATGGACACCGCATT-3′) and a reverse primer (5′-CCAGGTGCCGACATAAGGTT-3′). The PCR product was ligated into pBluescript-SK (+) and was then subcloned into a prokaryotic expression vector (pQE-30; Qiagen Inc., Chatsworth, Calif.). The nucleoprotein inserts were sequenced by using the Sequitherm EXCEL Cycle Sequencing kit (Epicentre Technologies, Madison, Wis.) to confirm the exactness of the N protein sequence and proper in-frame ligation. The complete sequence of ECV N protein cDNA has been published previously (11).
Expression and purification of recombinant ECV N protein.
Single colonies of transformants were grown in Luria-Bertani medium (Difco, Detroit, Mich.) with ampicillin (100 μg/ml) and kanamycin (25 μg/ml). Protein expression was induced with 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) according to the instructions provided by the manufacturer (Qiagen Inc.). After 4 h of induction, the cells were harvested by centrifugation at 4,000 × g for 15 min and lysed by sonification in buffer B (8 M urea, 0.1 M NaH2PO4, 0.01 M and Tris-HCl [pH 8.0]). The recombinant N proteins were analyzed on a sodium dodecyl sulfate (SDS)–10% linear polyacrylamide gel. Recombinant ECV N proteins were purified with Ni-NTA columns (the polyhistidine tag at the amino terminus of the recombinant N protein binds to Ni-NTA resin). The recombinant N fusion protein was detected by Western blot analysis with mouse antipolyhistidine as the primary antibody and horse anti-mouse horseradish peroxidase (HRPO) labeled as the secondary antibody. 4-Chloro-1-naphthol (4-CN) (Pierce, Rockford, Ill.) chromogen was used to detect the bands.
Hybridoma production.
Six-week-old BALB/c mice (n = 3) were immunized with purified recombinant N protein mixed with an equal volume of Ribi adjuvant (RIBI Immunochem Research Inc., Hamilton, Mont.). The spleen cells were fused with a myeloma cell line (Ag8) with 50% polyethylene glycol (Sigma, St. Louis, Mo.). Positive hybridoma clones were selected and cloned by single-step limiting dilution as described previously (15).
Enzyme-linked immunosorbent assay (ELISA).
Immulon-1 microtiter plates (Dynatech Laboratories Inc., Alexandria, Va.) were coated with purified recombinant N antigen (50 ng/well) and blocked with casein enzymatic hydrolysate (Sigma). Cell culture supernatant incubation was followed by the addition of anti-mouse HRPO as a secondary antibody. All incubations were performed at 37°C for 30 min. Between steps, six washes with phosphate-buffered saline–Tween buffer were performed. Tetramethylbenzidine was used as the substrate, and the absorbance was measured at a 620-nm wavelength.
Western immunoblot assay.
Purified recombinant ECV N protein was separated on SDS-polyacrylamide gels and was transferred to nitrocellulose membranes (Micron Separations Inc., Westboro, Mass.) by electroblotting. The membranes were incubated with monoclonal antibody from hybridoma culture supernatants. After being washed with Tris-buffered saline, the membranes were incubated with horse anti-mouse immunoglobulin G (IgG) labeled with HRPO. Chromogen was developed with 4-CN (Pierce).
Immunoprecipitation test.
ECV-infected cells were washed and harvested in ice-cold phosphate-buffered saline, pelleted, and resuspended in 400 μl of extraction buffer (10 mM HEPES, 10 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride). The cells were swollen on ice for 15 min, and 25 μl of 10% Nonidet P-40 was added to release the antigen. The mixture was centrifuged, and the cytoplasmic protein extract containing ECV antigen was collected. The antigens were immunoprecipitated overnight at 4°C by the addition of 20 μl of cell culture supernatant containing monoclonal antibodies against ECV N protein to 200 μl of lysate. The resulting immune complexes were captured on formalin-fixed Staphylococcus aureus Cowan I cells, and the cells were incubated on ice for 2 h. The bacterial cells were pelleted by centrifugation at 4,000 × g for 10 min and washed once with TSA (1% Triton X-100 and 1% sodium deoxycholic acid) and once with 10 mM Tris-HCl (pH 7.5) and 1 mM EDTA. The cells were centrifuged, and the bacterial pellet was resuspended in 20 μl of 1% SDS sample loading buffer and then electrophoresed on an SDS–10% polyacrylamide gel. The complexes were transferred to nitrocellulose membranes by electroblotting. The membranes were incubated with bovine anti-BCV serum as the primary antibody, followed by goat anti-bovine HRPO as the secondary antibody. The color was developed with 4-CN.
IHC.
Spiral colon sections taken from calves experimentally infected with BCV were used for IHC. Tissues were formalin fixed and paraffin embedded. Tissues were sectioned at 4 μm and heat fixed at 55°C for 30 min. Then, the slides were prepared by previously described procedures (15). Anti-nucleoprotein monoclonal antibodies were used as primary antibodies, and anti-mouse HRPO was used as the secondary antibody. The slides were washed with distilled water and were counterstained with hematoxylin for 30 s. The sections were dehydrated and then mounted with Permount (Fisher, St. Louis, Mo.) and examined by light microscopy.
RESULTS
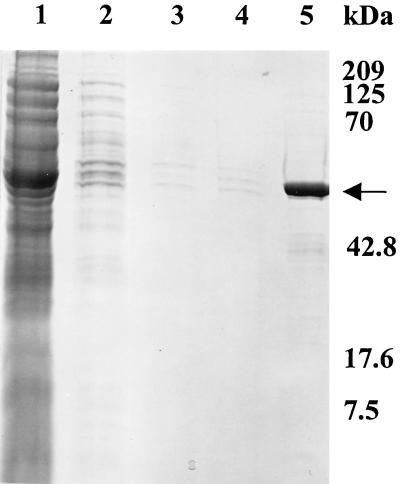
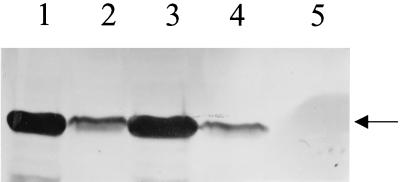
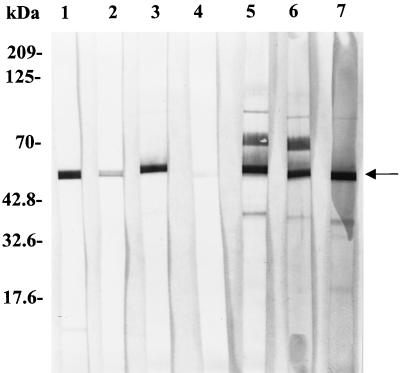
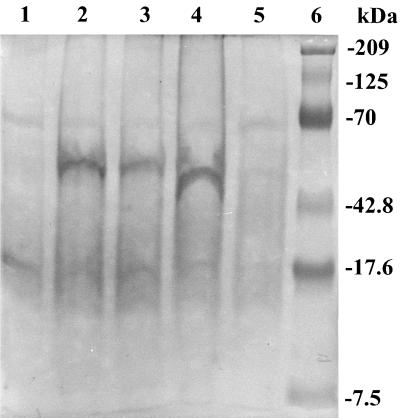
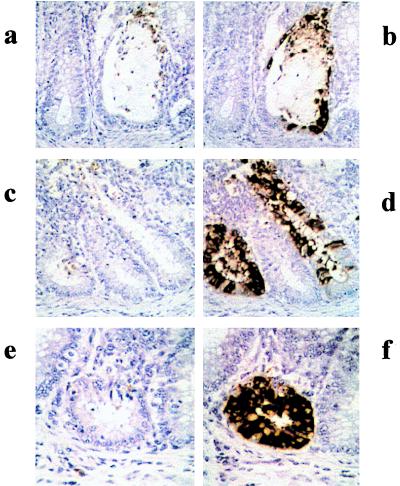
The ECV nucleoprotein gene was subcloned in the pQE-30 expression vector, and the recombinant N protein with a polyhistidine tag at the amino terminus was induced by the addition of 2 mM IPTG to the bacterial culture. A protein band corresponding to the expected molecular mass of 50 kDa, which was not present in uninduced cultures, was revealed in IPTG-induced cultures. The recombinant N protein bound to Ni-NTA columns was eluted with 100 mM imidazole (Fig. 1). Recombinant N protein reacted with bovine anti-BCV polyclonal serum in a Western blot assay (Fig. 2). The smaller bands seen in Fig. 2, in addition to the band for the N protein, may have been produced as premature translation termination products. One cell fusion of spleen from immunized mice with Ag8 myeloma cells produced 30 hybridoma lines, of which 12 cell lines were selected on the basis of their reactivities with the ECV N protein shown by ELISA and Western blots under denaturing conditions. With the exception of one monoclonal antibody (monoclonal antibody 7A4), all bound to SDS-denatured antigen by Western immunoblotting (Fig. 3) and ELISA (Table 1). The monoclonal antibody isotyping was performed with the mouse isotyper kit. Ten of the monoclonal antibodies had the IgG1 chain, one had IgM, and one had IgG2a (Table 1). All the monoclonal antibodies had a kappa light chain and were negative for a lambda light chain. Anti-N protein monoclonal antibodies immunoprecipitated a 50-kDa protein from BCV-infected lysates (Fig. 4), further establishing the specificities of the antibodies. Four monoclonal antibodies (monoclonal antibodies 9B8, 8F2, 5E6, and 4B12) detected BCV antigen in intestinal sections by IHC staining. BCV antigen was detected in the cytoplasm of crypt enterocytes (Fig. 5). The biological properties of different anti-N protein monoclonal antibodies are summarized in Table 1.
FIG. 1.
SDS-polyacrylamide gel electrophoresis analysis of N protein purified through Ni-NTA columns. Lane 1, crude bacterial lysate; lanes 2 to 4, washes with 1, 2.5, and 5 mM imidazole, respectively; lane 5, purified recombinant N protein eluted by using 100 mM imidazole (indicated by arrow). Molecular masses (in kilodaltons) are indicated at the left.
FIG. 2.
Western blot analysis of induced bacterial cell lysates from different clones. Lanes 1 and 3, induced positive clones (clones 752 and 753, respectively) that express the recombinant ECV N protein; lanes 2 and 4, uninduced bacterial cell lysate controls; lane 5, negative control (induced bacterial lysate without the nucleoprotein gene). The arrow indicates the recombinant 50-kDa nucleocapsid protein.
FIG. 3.
Western blotting analysis of monoclonal antibodies to ECV N protein. Purified N protein was separated on a 10% polyacrylamide gel and electroblotted onto a nitrocellulose membrane. The strips were incubated with different anti-N protein monoclonal antibodies. Lanes 1 and 2, anti-histidine antibody and mouse anti-ECV polyclonal serum, respectively; lanes 3 to 7, different monoclonal antibody clones (clones 3C9, 7A4, 3C10, 8F2, and 9B8, respectively). The arrow indicates a 50-kDa N-protein band. The higher bands may be aggregates of ECV nucleoprotein expressed in E. coli.
TABLE 1.
Monoclonal antibodies to ECV: isotypes and reactivities in ELISA, Western blot assay, and IHC
| Monoclonal antibody | Isotype | ELISA resulta | Western blot result | IHC resultb |
|---|---|---|---|---|
| 1A8 | IgG1 | + | Weak | − |
| 3C9 | IgG1 | +++ | Medium | − |
| 3C10 | IgG2a | ++++ | Strong | − |
| 4B6 | IgG1 | ++ | Medium | − |
| 4B12 | IgG1 | +++ | Strong | + |
| 5E6 | IgG1 | ++ | Medium | + |
| 6B3 | IgG1 | + | Weak | − |
| 7A4 | IgM | +++ | Negative | − |
| 8F2 | IgG1 | ++++ | Strong | + |
| 9B8 | IgG1 | +++ | Strong | + |
| 9D2 | IgG1 | ++ | Medium | − |
| 9E9 | IgG1 | ++++ | Strong | − |
−, negative result; +, 0.1 to 0.5 optical density units; ++, 0.5 to 1 optical density units; −−−, 1 to 2 optical density units; ++++, >2 optical density units (the optical density was measured at 620 nm).
−, negative; +, positive.
FIG. 4.
Immunoprecipitation of ECV-infected cytoplasmic lysates. Human rectal tumor-18 cells were infected with ECV, and infected cytoplasmic extracts were immunoprecipitated with different anti-N protein monoclonal antibodies. The lysates were immunoprecipitated with monoclonal antibodies 7A4 (lane 1), 9B8 (lane 2), and 8F2 (lane 3). Lane 4, polyclonal serum against ECV used as a positive control; lane 5, mock-infected lysate used as a negative control; lane 6, protein molecular mass standard (in kilodaltons).
FIG. 5.
Immunohistochemical detection of BCV-antigen in formalin-fixed, paraffin-embedded tissue sections of bovine rectum. Plates a and b, plates c and d, and plates e and f are serial sections of the same region. Plates a, c, and e are immunostained with monoclonal antibody Z3A5, which recognizes the spike protein of BCV (16). Plates b, d, and f are immunostained with monoclonal antibody 8F2, which recognizes the nucleoprotein. Positive staining (red-brown stain) is present in the cytoplasms of infected crypt epithelial cells. The chromogen was 3,3′-diaminobenzidine; hematoxylin counterstain was used. Magnifications: ×328 (a to d) and ×374 (e and f).
DISCUSSION
Ruminant coronaviruses (BCV and ECV) cause enteritis and pneumonia in young calves of domesticated cattle and elk, respectively (2, 11). Accurate diagnosis of infections caused by these viruses is important for the prevention of outbreaks of ECV and BCV. Both viruses are detected routinely by transmission electron microscopic examination of fecal samples, direct fluorescent-antibody assay with frozen sections of the spiral colon, and ELISA (5). Immunofluorescence is a rapid technique, but its nonspecific fluorescence and the fading of the fluorescence on exposure to normal light limits its application. Transmission electron microscopy detects virus only when the number of particles is very large and only a small number of samples can be examined. Also, transmission electron microscopy sometimes gives false-positive results because of its detection of nonviral coronavirus-like particles (5). Other constraints include the requirement for fresh samples and large number of virus particles. Therefore, the use of IHC with enzyme-labeled antibodies for the detection of viral antigens in formalin-fixed tissues provides a useful alternative and an adjunct for the detection of BCV and ECV. In addition to aiding in the study of viral pathogenesis, IHC detects viral antigen in infected crypt enterocytes before histological lesions can be seen in these cells. Moreover, most ruminant coronaviruses produce subtle lesions (crypt dilation and Peyer’s patch lymphoid depletion), which are difficult to distinguish from the lesions caused by ruminant pestiviruses. Several tests have been developed for the detection of BCV and other bovine pathogens by using monoclonal antibodies against spike protein and other structural proteins (1, 4, 15). In our study we have used recombinant N protein for the production of monoclonal antibodies.
N protein is the predominant antigen produced in coronavirus-infected cells, thus making it a major viral target. Therefore, N protein-based assays will provide better sensitivity. In an Escherichia coli expression system, we found that the level of expression of N protein of ECV was higher compared to the level of expression of the N protein of BCV. Because BCV and ECV are very closely related biologically, genetically, and antigenically (11), we chose to use the recombinant N protein of ECV for the production of monoclonal antibodies.
This is the first report in which monoclonal antibodies against the N protein of ECV have been produced and characterized. Among the 12 anti-N protein monoclonal antibodies, 1 did not react against the N protein when an ELISA and a Western blot assay were used for screening (Table 1). This could be explained by reactivity against hidden epitopes or by a weak affinity of the monoclonal antibody. The majority of the monoclonal antibodies had the IgG heavy chain. Because of an abundance of the N antigen, the anti-N protein monoclonal antibodies are highly sensitive and specific for the detection of BCV and ECV antigens. Compared to an earlier study in which monoclonal antibodies against the spike protein were used for IHC to detect BCV antigen (15), we found that our anti-N protein monoclonal antibodies were much more sensitive and specific for the detection of viral antigen (Fig. 5). Currently, no established tests are available for the diagnosis of ECV infection. Thus, our anti-N protein monoclonal antibodies serve as better tools for the diagnosis of both BCV and ECV infections when formalin-fixed tissues are submitted for diagnostic investigations.
The N protein is believed to play a role in the packaging and replication of BCV; however, its exact role in packaging has not yet been defined. The panel of anti-N protein monoclonal antibodies described here may also be used to analyze the role of N protein in the pathogenesis of BCV and for the study of its RNA binding properties to gain insight into the encapsidation and packaging of BCV RNAs.
ACKNOWLEDGMENTS
We thank Shawn Torrez for technical assistance.
This work was supported by grants from the Kansas Agricultural Experiment Station (1443 proposal), Kansas State University, College of Veterinary Medicine dean’s grant research projects, U.S. Department of Agriculture (NC-62) regional hatch funds, and Shering-Plough Animal Health, Omaha, Nebr.
Footnotes
This is contribution no. 99-169-J from the Kansas Agricultural Experiment Station.
REFERENCES
- 1.Baszler T V, Evermann J F, Kaylor P S. Diagnosis of naturally occurring bovine viral diarrhea virus infections in ruminants using monoclonal antibody-based immunohistochemistry. Vet Pathol. 1995;32:609–618. doi: 10.1177/030098589503200601. [DOI] [PubMed] [Google Scholar]
- 2.Clark M A. Bovine coronavirus. Br Vet J. 1993;49:51–70. doi: 10.1016/S0007-1935(05)80210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corapi W V, Darteil R J, Audonnet J C, Chappuis G E. Localization of antigenic sites of feline infectious peritonitis virus involved in neutralization and antibody-dependent enhancement. J Virol. 1995;69:2858–2862. doi: 10.1128/jvi.69.5.2858-2862.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crouch C F, Raybould T J G, Acres S D. Monoclonal antibody capture enzyme-linked immunosorbent assay for detection of bovine enteric coronavirus. J Clin Microbiol. 1984;19:388–393. doi: 10.1128/jcm.19.3.388-393.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dar A M, Kapil S, Goyal S M. Comparison of immunohistochemistry, electron microscopy, and direct fluorescent antibody test for the detection of bovine coronavirus. J Vet Diagn Invest. 1998;10:152–157. doi: 10.1177/104063879801000206. [DOI] [PubMed] [Google Scholar]
- 6.Deregt D, Babiuk L A. Monoclonal antibodies to bovine coronavirus: characteristics and topographical mapping of neutralizing epitopes on the E2 and E3 glycoproteins. Virology. 1987;161:410–420. doi: 10.1016/0042-6822(87)90134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guy J S, Brian D A. Bovine coronavirus genome. J Virol. 1979;29:293–300. doi: 10.1128/jvi.29.1.293-300.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapil S, Chard-Bergstrom C, Bolin P, Landers D. Plaque variations in clinical isolates of bovine coronavirus. J Vet Diagn Invest. 1995;7:538–539. doi: 10.1177/104063879500700420. [DOI] [PubMed] [Google Scholar]
- 9.Kapil S, Richardson K L, Radi C, Chard-Bergstrom C. Factors affecting isolation and propagation of bovine coronavirus in human rectal tumor-18 cell line. J Vet Diagn Invest. 1996;8:96–99. doi: 10.1177/104063879600800115. [DOI] [PubMed] [Google Scholar]
- 10.King B, Brian D A. Bovine coronavirus structural proteins. J Virol. 1982;42:700–707. doi: 10.1128/jvi.42.2.700-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majhdi F, Minocha H C, Kapil S. Isolation and characterization of a coronavirus from elk calves with diarrhea. J Clin Microbiol. 1997;35:2937–2942. doi: 10.1128/jcm.35.11.2937-2942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakanaga K, Yamanouchi K, Fujiwara K. Protective effect of monoclonal antibodies on lethal mouse hepatitis virus infection in mice. J Virol. 1986;59:168–171. doi: 10.1128/jvi.59.1.168-171.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sturman L S, Holmes K V. The molecular biology of coronaviruses. Adv Virus Res. 1983;28:35–112. doi: 10.1016/S0065-3527(08)60721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsunemitsu H, El-Kanawati Z R, Smith D R, Reed H H, Saif L J. Isolation of coronaviruses antigenically indistinguishable from bovine coronavirus from wild ruminants with diarrhea. J Clin Microbiol. 1995;33:3264–3269. doi: 10.1128/jcm.33.12.3264-3269.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Andrews G A, Chard-Bergstrom C, Minocha H C, Kapil S. Application of immunohistochemistry and in situ hybridization for detection of bovine coronavirus in paraffin-embedded, formalin-fixed intestines. J Clin Microbiol. 1997;35:2964–2965. doi: 10.1128/jcm.35.11.2964-2965.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]