Abstract
Melanoma is the most aggressive and deadliest type of skin cancer. In the last 10 years, immune checkpoint blockades (ICBs) including PD-1/PD-L1 and CTLA-4 inhibitor has been shown to be effective against melanoma. PD-1/PD-L1 and CTLA-4 inhibitors have shown varying degrees of drug resistance in the treatment of melanoma patients. Furthermore, the clinical benefits of ICBs are also accompanied by severe immune toxicity. Therefore, there is an urgent need to develop new immune checkpoint inhibitors to optimize melanoma therapy and reduce cytotoxicity. T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain (TIGIT) is thought to activate inhibitory receptors in T cells, natural killer (NK) cells, and regulatory T cells (Tregs), and has become a promising target for immunotherapy. Studies have found that TIGIT can be detected in different stages of melanoma, which is closely related to the occurrence, development, and prognosis of melanoma. This review mainly describes the immunosuppressive mechanism of TIGIT and its role in antitumor immunity of melanoma, so as to provide new ideas and schemes for the clinical treatment of melanoma with targeted TIGIT.
Subject terms: Tumour immunology, Skin cancer
Facts
TIGIT binds CD155 and CD112 to create immune suppression; CD226 binds CD155 to deliver a positive signal; CD96 binds CD155 to create immune suppression.
TIGIT exerts inhibitory effects on innate and adaptive immunity through multiple mechanisms, including triggering T/NK cell-intrinsic inhibition, inducing immunosuppressive DCs, inhibiting CD226 signaling, enhancing immunosuppression of Tregs and promoting Fap2-induced T/NK cells inhibition.
Blockade of TIGIT on CD8+ T cells, Tregs, and NK cells augments antitumor immunity.
Open question
How to find and intervene in targets involved in the immunosuppressive effect of TIGIT?
How to develop TIGIT blockers to restore antitumor immunity in melanoma?
What drugs can improve the efficacy and safety of TIGIT blockers in the treatment of melanoma?
Introduction
Melanoma is the most aggressive and deadliest type of skin cancer. Worldwide, melanoma represents 1.7% of all newly diagnosed cancers, and 0.6% of cancer-related deaths [1]. The incidence of melanoma has doubled in the last 30 years [2]. The main cause of death in melanoma patients is the extensive spread of tumors to the liver, lung, brain, bone, lymphatic system, and other organs [3]. The most effective treatment for melanoma is surgery resection, and for unresectable metastatic melanoma, radiotherapy, and chemotherapy have traditionally been used. These last two therapies, however, have shown many inconveniences like resistance, secondary cancers, or toxicity to healthy tissues [4].
In the last ten years, immune checkpoint blockades (ICBs) including PD-1/PD-L1 and CTLA-4 inhibitor, designed to restore immune cell activity against pathogens and cancer cells, has been shown to be effective against many types of cancer [5]. Currently, PD-1/PD-L1 inhibitors are approved by the FDA for the treatment of more than a dozen tumors, including melanoma [6]. However, ~40%–65% of melanoma patients have intrinsic resistance to PD-1-based therapy [7, 8], and 43% of responders develop secondary resistance within 3 years [9]. In addition, anti-CTLA-4 antibody has also been used in the clinical treatment of melanoma [10]. Unfortunately, anti-CTLA-4 has a low clinical response rate for melanoma [11]. Furthermore, the clinical benefits of ICBs are also accompanied by severe immune toxicity, including cardiotoxicity [12], pneumonia [13], hepatitis, colitis [14], pancreatitis [15], and endocrine dysfunction [16]. Additionally, other new ICBs have also been approved, such as anti-LAG3 in melanoma [17].
As T cells and natural killer (NK) cells are central parts of the immune system [18, 19], an increasing number of studies have focused on the inhibitory immune checkpoints they express on their surfaces. T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibition motif domain (TIGIT) is thought to activate inhibitory receptors in T cells, NK cells, and regulatory T cells (Tregs), and has become a promising target for immunotherapy [18, 20, 21]. Studies have found that TIGIT can be detected in different stages of melanoma [22], which is closely related to the occurrence, development, and prognosis of melanoma [23, 24]. However, it is not known whether TIGIT-based immunotherapy could induce better treatment results and less toxicity compared to anti-PD-1/PD-L1 or anti-CTLA-4 immunotherapy in melanoma, which needs to be further explored. This review mainly describes the immunosuppressive mechanism of TIGIT and its role in antitumor immunity of melanoma, so as to provide new ideas and schemes for the clinical treatment of melanoma with targeted TIGIT, and discusses whether anti-TIGIT may be an alternative to anti-PD-1/PD-L1 or anti-CTLA-4.
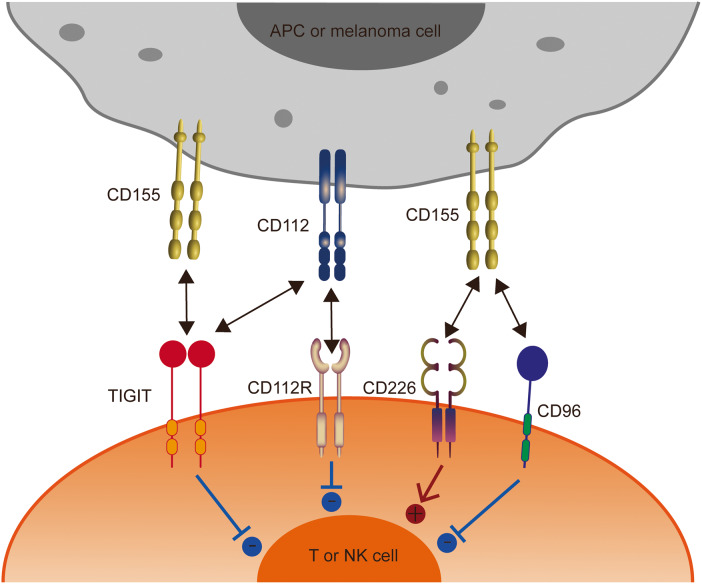
TIGIT structure and ligands
TIGIT, also known as WUCAM [20], Vstm3 [25], VSIG9 [26], is a co-inhibitory receptor belonging to the immunoglobulin superfamily [27] that was first identified in 2009 [21]. TIGIT is composed of an extracellular immunoglobulin variable region (IgV) domain, a type 1 transmembrane domain, and an intracellular domain containing an immunoreceptor tyrosine-based inhibitory motif (ITIM) and an Ig tail-tyrosine (ITT)-like motif constitute [21, 27–29]. The TIGIT molecule is relatively conservative, and the amino acid sequence of human TIGIT shares 88%, 67%, and 58% homology with those of rhesus monkeys, dogs, and mice, respectively [21].
TIGIT is expressed on activated traditional αβ T cells, but also on memory T cells, Tregs, follicular helper cells, and NKT cells [30, 31]. In addition to T cells, TIGIT is also expressed in NK cells [27], which is induced in mouse NK cells and constitutively expressed in human NK cells. For cancers, compared to CD226, TIGIT is weakly expressed by naive T cells and co-expressed with PD-1 on mouse and human tumor-infiltrating CD8+ T cells [32, 33]. Additionally, TIGIT is highly expressed in peripheral blood Tregs of healthy donors and cancer patients and is further upregulated in the tumor microenvironment [34]. Studies have shown that the hypomethylation of TIGIT loci is a characteristic of human Treg, and the expression of TIGIT makes the activated Treg better different from the activated effector T cells in vitro or in vivo [35]. The ligands of TIGIT include CD112, and poliovirus receptor (PVR), of which PVR is the high-affinity cognate receptor of TIGIT, and PVR is also known as CD155, Necl-5, and Tage4 [36]. Furthermore, TIGIT competes for ligands with CD226 (DNAM-1) and CD96 (TACTILE) [37]. Studies have shown that TIGIT can effectively block the binding of CD155 and CD96 [38] or CD226 [39], which further proves that TIGIT has the highest affinity for CD155 (Table 1). Notably, TIGIT not only competes with CD226 for ligands, but also can directly cis-bind to CD226 to prevent its homo-dimerization, so that CD226 cannot bind to CD155 to play a co-stimulatory role. However, the degree of co-expression of TIGIT and CD226 on T cells in the inflamed tissue is still unclear.
Table 1.
Ligand-binding affinities for TIGIT, CD226, and CD112R.
| Ligand/receptor affinity | TIGIT | CD226 | CD112R | CD96 |
|---|---|---|---|---|
| CD155 | 1–3 nM | 114-199 nM | / | 37.6 nM |
| CD112 | Not measurable | 0.31–8.97 µM | 88 nM | / |
CD155 is mainly expressed on the surface of dendritic cells (DCs), T cells, B cells, and macrophages, and also in nonhematopoietic tissues such as the kidney, nervous system, and intestine to varying degrees [31]. In addition, CD155 has been reported to be highly expressed in a variety of human malignancies, including melanoma [40], pancreatic cancer [41], colon cancer [42], lung adenocarcinoma [43], and glioblastoma [44]. CD155 is a cell adhesion molecule that affects cell proliferation, migration, invasion, and adhesion through tumor-associated signaling pathways. It also interacts with CD226, TIGIT, and CD96 on immune cells, affecting the function of tumor-infiltrating T cells and NK cells [45]. In melanoma patient, high CD155 expression in tumors is also associated with resistance to anti-PD-1 therapy [46]. Braun et al. also revealed that CD155 on melanoma cells drives resistance to immunotherapy by inducing degradation of the activating receptor CD226 in CD8+ T cells [47]. These data suggest that CD155 expression in tumors has a dual pro-tumor effect, both tumor-intrinsic and through inhibition of antitumor immunity.
CD112 is expressed on DCs and monocytes [48] and is highly expressed in various cancers [49–51], but is rare in melanoma cell lines [51]. Furthermore, CD112 has a higher affinity for CD112R (PVRIG) than for TIGIT, and suppresses T cells mainly via binding to CD112R [52, 53] and not via TIGIT [53]. However, in melanoma, whether TIGIT works primarily by binding to CD155 rather than CD112 requires more direct evidence, which needs to be further explored in the future. Details of TIGIT structure and ligands are shown in Fig. 1.
Fig. 1. The interaction of TIGIT family receptors and ligands.
TIGIT, CD226, CD96, and CD112R are expressed in T cells and NK cells. The ligands CD155 and CD112 are expressed on tumor cells or APCs. TIGIT delivers inhibitory signals by binding to CD155 and CD112, with the highest affinity for CD155. CD226 and CD96 compete with TIGIT for binding to CD155, but with lower affinity than TIGIT. CD226 delivers activating signals. However, whether CD96 triggers inhibitory or activating signals remains to be determined. CD112R and CD226 also competitively bind to CD112, with higher affinity with CD112R. APCs, antigen-presenting cells.
TIGIT in cancer progression
TIGIT overexpression has been found in the cellular microenvironment of several cancers and is associated with a poor prognosis for cancer, including melanoma [54, 55].
In aggressive breast cancer, a large-scale transcriptome data analysis found that TIGIT was highly specifically expressed in aggressive breast cancer, and its pro-tumor activity was associated with immune-related genes. TIGIT expression was positively correlated with gene expression related to inflammation and immune response 33721026. Therefore, TIGIT expression appears to be strongly associated with advanced malignant pathological types of breast cancer and may be a potential biomarker of breast cancer progression. In renal cell carcinoma, immunohistochemical and flow cytometry results showed that TIGIT expression in cancer tissues was increased compared with adjacent cancer, but the number of TIGIT+T cells and TIGIT+NK cells was not related to clinicopathological features. In addition, high TIGIT expression was associated with the clinicopathological characteristics of lung adenocarcinoma, which was associated with advanced TNM staging, lymphoid metastasis, distant metastasis, and low expression of antitumor immunity-related genes [56]. Similarly, CD8+T-cell populations with high TIGIT expression in peripheral blood in patients with hepatocellular carcinoma were inversely correlated with overall survival (OS) and progression-free survival (PFS) [57]. Interestingly, increased TIGIT expression in gastric cancer appears to be a favorable event. TIGIT expression correlates with an active immune landscape, survival and immunotherapeutic sensitivity, and favorable prognosis. Patients with high TIGIT expression respond better to immunotherapy than those with low TIGIT expression [58].
In addition, in addition to the above-mentioned solid tumors, the high expression of TIGIT on immune cells also plays an important role in the progression of hematological tumors. An increase in the number of TIGIT-expressing CD4+ T cells and CD8+ T cells in tumors in patients with follicular lymphoma is associated with poor prognosis and survival [59]. In addition, high expression of TIGIT in peripheral blood CD8+ T cells in patients with acute myeloid leukemia is associated with the development of primary refractory disease [59].
These data suggest that TIGIT has an inhibitory effect in antitumor immunity in cancer patients, but whether TIGIT’s role in melanoma is different from other cancers is not clear, and it is worth further exploration in the future.
The mechanisms of TIGIT co-inhibition
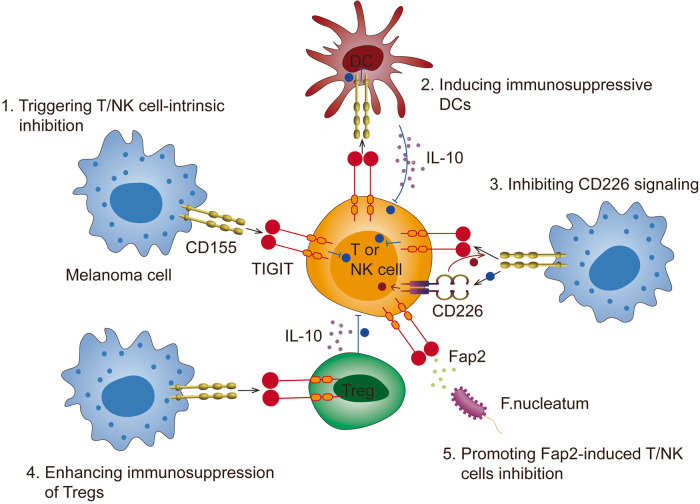
TIGIT exerts inhibitory effects on innate and adaptive immunity through multiple mechanisms, including triggering T/NK cell-intrinsic inhibition, inducing immunosuppressive DCs, inhibiting CD226 signaling, enhancing immunosuppression of Tregs and promoting Fap2-induced T/NK cells inhibition (Fig. 2).
Fig. 2. The mechanisms of TIGIT co-inhibition.
TIGIT exerts inhibitory effects on innate and adaptive immunity through multiple mechanisms. 1. TIGIT binds CD155 to trigger T/NK cell-intrinsic inhibition; 2. TIGIT binds CD155 to induce immunosuppressive DCs; 3. TIGIT binds CD155 to inhibit CD226 signaling; 4. TIGIT binds CD155 to enhance immunosuppression of Tregs; 5. Fap2 protein from Fusobacterium nucleatum binds TIGIT to induce T/NK cell inhibition.
Triggering T/NK cell-intrinsic inhibition
At first, TIGIT on T cells can act directly on T cells by attenuating T-cell receptor (TCR) -driven activation signals [60]. Specifically, TIGIT can inhibit the expression of TCR itself by inducing downregulation of the TCR-α chain and the molecules that make up the TCR complex, thereby inhibiting the proliferation and activation of CD8+ T cells [60]. In addition, TIGIT can reduce TCR-induced p-ERK signaling in CD8+ T cells [61]. However, no studies have confirmed that TIGIT plays a role in melanoma immune evasion by directly downregulating TCR signaling.
In NK cells, upon binding of TIGIT to CD155, the ITT-like motif in the tail is phosphorylated at Tyr225 and binds to the cytoplasmic adaptor Grb2, which recruits SH domain-containing inositol-5-phosphatase (SHIP1) to inhibit PI3K and MAPK signaling cascades, thereby downregulating NK cell activity [28]. Phosphorylated ITT-like motifs also bind to β-arrestin2, follow by recruiting SHIP1 to disrupt TRAF6 autoubiquitination, thereby inhibiting NF-κB activity and IFN-γ production in NK cells [29].
Inducing immunosuppressive DCs
In addition to directly inhibiting T cells, Tigit on T cells can also bind to CD155 on DCs to indirectly inhibit the activation of T cells. TIGIT induces phosphorylation of CD155 on DCs, thereby enhancing interleukin (IL)-10 production and diminishing IL-12 production by dendritic cells [21]. However, whether this DC-dependent indirect regulation exists in the immune response of melanoma remains to be explored in the future.
Inhibiting CD226 signaling
As TIGIT competes with CD226 for CD155 ligands, blocking CD226-mediated T cells and NK cells co-stimulation helps promote immunosuppression by TIGIT [62]. Inozume et al. reported that TIGIT upregulation and CD226 downregulation of melanoma-specific cytotoxic T lymphocytes (CTLs) were induced by tumor stimulation. These findings suggested that an imbalance in CD226 and TIGIT expression is a novel mechanism of T-cell suppression in the effector phase of the antitumor CTL response [63]. Further, CD226 blockade abrogates the effects of dual PD-1 and TIGIT blockade on the proliferation and cytokine production of tumor antigen-specific CD8+ T cells in melanoma [32]. In addition, TIGIT can prevent co-stimulatory signaling via CD226 by blocking CD226 homo-dimerization [33].
Enhancing immunosuppression of Tregs
Tregs express a variety of inhibitory receptors that support their inhibitory function, including TIGIT. TIGIT is highly expressed by a subset of natural Tregs in mice [34] and the majority of Tregs in humans [34, 64, 65]. Notably, studies have found that TIGIT+ Tregs were more inhibitory than TIGIT- Tregs in melanoma patients [64, 65]. Interestingly, Fourcade et al. [64] showed that in melanoma patients, Tregs showed increased expression of TIGIT and decreased expression of the competitive co-stimulatory receptor CD226 compared with CD4+ effector T cells, resulting in an increased TIGIT/CD226 ratio. A high TIGIT/CD226 ratio in Tregs correlates with increased Treg frequencies in tumors and poor clinical outcome upon ICBs. The future challenge lies in determining whether the TIGIT/CD226 ratio in Tregs can be used as a biomarker of clinical response to ICB in melanoma patients.
Promoting Fap2-induced T/NK cells inhibition
Bacteria, such as Fusobacterium nucleatum, are present in the tumor microenvironment of various cancers, including melanoma [66]. F. nucleatum has been shown to directly interact with TIGIT in NK and T-cells through its Fap2 protein to inhibit NK cell cytotoxicity and suppress T-cell activity [67]. Although it has not been demonstrated whether this mechanism is involved in immune escape in melanoma, the possibility of such a mechanism is not excluded due to the presence of F. nucleatum in melanoma.
TIGIT in melanoma immunotherapy
TIGIT is expressed on human tumor-infiltrating CD8+ T cells, NK cells, Th, and Treg cells in melanoma [32, 63]. Decreased TIGIT expression in CD8+ T cells was associated with inhibition of tumor growth in melanoma cells [68]. Lee et al. evaluated the expression of TIGIT in 124 melanoma patients by immunohistochemistry and analyzed their clinicopathological features and survival. The results showed that high expression of TIGIT was associated with worse survival. These results suggest that TIGIT has an inhibitory effect on antitumor immunity in melanoma patients [69]. In the following sections, we will discuss which tumor-infiltrating immune cell populations are inhibited by TIGIT to cause immune escape from melanoma and possible therapeutic strategies (Table 2).
Table 2.
TIGIT in melanoma immunotherapy.
| TIGIT-expressing immune cells | Treatment strategies | Mechanism | Ref. |
|---|---|---|---|
| CD8+T cells | Elraglusib (9-ING-41) | Reducing TIGIT expression on CD8+ T cells | [68] |
| CD8+T cells | Dual PD-1/TIGIT blockade | Enhancing the proliferation and function of tumor antigen-specific CD8+ T cells | [32, 63] |
| CD8+T cells | Dual PD-1/TIGIT blockade | Promoting the activation of CD226 signaling pathway | [80] |
| CD8+T cells | Dual anti-CD96/TIGIT | Restoring melanoma-infiltrating CD8+ T-cell antitumor immunity | [83] |
| Tregs | Activating CD226 in Tregs together with TIGIT blockade | Counteracting Tregs suppression | [64] |
| Tregs | Dual anti-TIM-3/TIGIT | Reducing immunosuppression of Tregs | [86] |
| NK cells | IL-15 stimulation together with TIGIT blockade | Augmenting antitumor immunity of NK cells | [95] |
| NK cells | Dual anti-CTLA-4/TIGIT | Improving the immunosuppression of NK cells against melanoma | [96] |
| NK cells | Deletion of CISH | Optimizing NK cell killing properties and decreasing TIGIT immune checkpoint receptor expression | [98] |
Blockade of TIGIT on CD8+ T cells augments antitumor immunity
CD8+ T cells can not only kill tumor cells immediately by secreting factors such as granzyme B, perforin, and INF-γ, but also generate immune memory and reside in peripheral tissues to maintain antitumor immune response and inhibit tumor growth [70]. Thus, augmenting the CD8+ T-cell antitumor response is a major strategy in most cancer immunotherapies [71]. Blocking TIGIT in the co-culture system of melanoma cells and CD8+ T cells in vitro restored the production of IFN-γ in CD8+ T cells [72]. In addition to anti-TIGIT mAb, a recent clinical trial found that Elraglusib (9-ING-41) also reduced TIGIT expression on CD8+ T cells, thus exerting an inhibitory effect on melanoma [68]. Elraglusib is a reversible ATP-competitive small-molecule inhibitor of glycogen synthase kinase-3β, a serine/threonine kinase with multiple roles in tumor growth, cell invasion, and metastasis [73–75]. Nevertheless, single TIGIT blockade achieved no or moderate antitumor efficacy in experimental tumor models [33, 76–78] and in enhancing in vitro functionality of human tumor-infiltrating CD8+ T cells [79]. Similarly, dual PD-1/TIGIT blockade also enhanced the proliferation and function of tumor antigen-specific CD8+ T cells and CD8+ tumor-infiltrating lymphocytes (TILs) in melanoma patients compared with single TIGIT blockade [32, 63]. However, dual blockade of TIGIT and PD-1 should be further explored to induce potent antitumor CD8+ T cells responses in patients with advanced melanoma.
Notably, blocking CD226 in vitro and in a murine melanoma model nullifies the dual blocking effect of PD-1/TIGIT, suggesting that TIGIT blockade promotes CD155 binding to CD226 to activate CD8+ T-cell immune activity [32]. In addition, PD-1 inhibition rescued CD226 activity by preventing PD-1-SHP2 dephosphophorylation of the CD226 intracellular domain [80]. This indicates that dual PD-1/TIGIT blockade may enhance antitumor immunity by promoting the activation of CD226 signaling pathway. However, Chauvin et al. found that CD8+ TILs downregulated CD226 expression in melanoma, which may be an important barrier to limit the dual blocking effect of PD-1/TIGIT in melanoma patients [32]. Since CD155 plays a critical role in mediating the downregulation of CD226 expression on melanoma-infiltrating immune cells [81], reducing CD155 expression in melanoma may be a potential strategy to enhance the dual blocking effect of PD-1/TIGIT on melanoma [82].
Besides PD-1 blockade, other ICBs combined with TIGIT blockade also enhance antitumor immune responses in melanoma. For example, Mittal et al. [83] observed that CD96 co-expressed with TIGIT in CD8+ melanoma TILs, and dual anti-CD96/TIGIT combination therapy was superior to anti-TIGIT monotherapy in suppressing tumor growth and improving mouse survival in B16F10 melanoma. Further study also found that anti-PD-1 combined with CD96/TIGIT dual blockade on melanoma growth inhibition effect is significantly better than the dual anti-CD96/TIGIT combined treatment. This provides a new strategy for restoring melanoma-infiltrating CD8+ T-cell antitumor immunity by blocking TIGIT.
Blockade of TIGIT on Tregs augments antitumor immunity
Tregs, as an important mechanism for regulating homeostasis of the immune system and the immune tolerance of the body, play an important role in tumor immune escape [84]. In contrast to the effects on CD8+ T cells, TIGIT expression on Tregs enhanced the suppressor function of Tregs [85]. In melanoma, activation of CD226 opposes TIGIT to disrupt the suppression and stability of Tregs [64], which provide the rationale for novel immunotherapies to activate CD226 in Tregs together with TIGIT blockade to counteract Treg suppression in melanoma patients. Additionally, Kurtulus et al. [86] revealed that TIGIT+ Tregs upregulated the expression of the co-inhibitory receptor TIM-3 in tumor tissues. Then, a TIGIT-null melanoma mouse model was constructed and anti-TIM-3 was found to have a higher survival rate than observed with TIGIT deficiency alone, suggesting that TIM-3 and TIGIT synergized to suppress antitumor immune responses in melanoma. These results support the combined use of ICBs targeting Tregs in melanoma immunotherapy.
Blockade of TIGIT on NK cells augments antitumor immunity
NK cells are derived from bone marrow lymphoid stem cells, their differentiation and development depend on the bone marrow and thymus microenvironment, and are mainly distributed in bone marrow, peripheral blood, liver, spleen, lung, and lymph nodes [87]. Different from T and B cells, NK cells are a type of lymphocyte that can non-specifically kill tumor cells and virus-infected cells without prior sensitization [88]. NK cell-based cancer immunotherapy, which refers to the activation of NK function and showing substantial therapeutic effects on tumors [89], is increasingly used in melanoma [90].
Single-cell characteristics of the melanoma cell landscape identified the high expression of TIGIT on tumor-infiltrating NK cells [91], offering new options for clinical translation. The signal balance between co-stimulatory and co-inhibitory signal molecules expressed in NK cells regulates the immune activity of NK cells [92]. Consistent with CD8+ T cells, CD226 is an activating receptor, while TIGIT and CD96 are inhibitory receptors that bind to tumor-derived CD155 to regulate NK cell-mediated tumor immunotherapy [93]. Notably, NK cell-based therapies represent a powerful approach to kill MHC class I-deficient tumors that may arise upon CD8+ T-cell-mediated immune destruction of MHC class I-presenting tumor cells [94]. Chauvin et al. [81] found that membrane-bound CD155 triggers CD226 internalization and degradation in NK cells, while IL-15 promoted increased expression of TIGIT and CD226 on tumor-infiltrating NK cells in melanoma. The study further revealed that IL-15 stimulation together with TIGIT blockade promotes NK cell-mediated destruction of MHC class I-deficient melanoma, while CD226 blockade decreases the effects of IL-15 and TIGIT blockade. In addition, another study also showed that CD155 inhibits the CD226-mediated cytotoxic activity of NK cells, thus promoting the lung colonization of B16/BL6 melanoma [95].
Interestingly, other ICBs combined with TIGIT blockade also enhanced antitumor immune responses of NK cells in melanoma. For example, Rethacker et al. [96] found decreased CTLA-4 and TIGIT expression in blood NK cells from 16 patients who received ipilimumab, which is a fully humanized anti-CTLA-4 monoclonal antibody approved by FDA for late-stage melanoma [97], suggesting that the combination of CTLA-4 and TIGIT blockade may improve the immunosuppression of NK cells against melanoma. A recent study also found that the deletion of cytokine-inducible SH2-containing protein (CISH), a critical immune checkpoint, favors NK cell accumulation to the primary tumor, optimizes NK cell killing properties, and decreases TIGIT immune checkpoint receptor expression, limiting NK cell exhaustion [98]. This makes dual targeting of CISH and TIGIT a potential strategy to activate NK cell-dependent melanoma immunotherapy.
Clinical application of anti-TIGIT monoclonal antibodies in melanoma
At present, anti-TIGIT monoclonal antibodies have been conducted in multiple clinical trials in melanoma, however, these clinical trials are still in the stage of recruiting patients, and the results of the study have not been reported. Details of the clinical trials that have been conducted are shown in Table 3.
Table 3.
Clinical trials of anti-TIGIT monoclonal antibodies in melanoma.
| Registration number | Study phase | Primary outcome measures | Arms and interventions | Research status |
|---|---|---|---|---|
| NCT05130177 | II | ORR | Zimberelimab (anti-PD-1) plus Domvanalimab (anti-TIGIT) | Recruiting |
| NCT05665595 | III | RFS | Pembrolizumab (anti-PD-1) plus Vibostolimab (anti-TIGIT)/Pembrolizumab | Recruiting |
| NCT04305054 | II | ORR, AE | Pembrolizumab plus Vibostolimab/Pembrolizumab plus Quavonlimab (anti-CTLA-4)/Pembrolizumab+Favezelimab (anti-LAG3)/Pembrolizumab | Recruiting |
| NCT04305041 | II | ORR, AE | Pembrolizumab plus Quavonlimab plus Vibostolimab | Recruiting |
| NCT05483400 | II | pCR, ORR | Tiragolumab (anti-TIGIT) plus Atezolizumab (anti-PD-1) | Not yet recruiting |
| NCT04303169 | II | pCR, AE | Pembrolizumab/Pembrolizumab plus Vibostolimab/Favezelimab plus Pembrolizumab | Recruiting |
| NCT05060432 | II | ORR, AE | EOS-448 (anti-TIGIT) plus Pembrolizumab/Dostarlimab (anti-PD-1) | Recruiting |
RFS recurrence-free survival, ORR objective response rate, AE adverse event; pCR pathological complete response.
Conclusion and prospects
Prior to the introduction of immunotherapy for the treatment of advanced melanoma, outcomes were generally poor despite the application of many cytotoxic agents and combinations [99]. Melanoma is a highly malignant tumor, most of which are found at an advanced stage due to its rapid development and evolution, which makes the operation impossible [100, 101]. Therefore, it is necessary to further explore the application of immunotherapy in melanoma and explore the relevant mechanisms. TIGIT exerts inhibitory effects on innate and adaptive immunity through multiple mechanisms, including triggering T/NK cell-intrinsic inhibition, inducing immunosuppressive DCs, inhibiting CD226 signaling, enhancing immunosuppression of Tregs and promoting Fap2-induced T/NK cells inhibition. However, these mechanisms have not all been confirmed in melanoma immune response and need to be further explored in the future.
Decreased TIGIT expression in immune cells was associated with the inhibition of tumor growth in melanoma patients, making TIGIT a promising target in melanoma immunotherapy. Blockade of TIGIT on CD8+ T cells, Tregs, and NK cells augment antitumor immunity. However, single TIGIT blockade has minimal effects on melanoma growth in most experimental tumor models and is also insufficient to reinvigorate functions of human tumor-infiltrating CD8+ T cells. TIGIT blockade synergizes with PD-1/PDL-1 blockade or CD96 blockade to enhance antitumor CD8+ T-cell immunity in preclinical models. Additionally, dual targeting of CTLA-4 or CISH and TIGIT may be a potential strategy to activate NK cell-dependent melanoma immunotherapy. However, direct clinical and preclinical evidence is lacking. In addition, future studies need to carry out relevant clinical trials to compare the efficacy and toxicity of anti-TIGIT with anti-PD-1/PD-L1 or anti-CTLA-4 in melanoma. Furthermore, CD226 plays a critical role as a master regulator of dual PD-1/TIGIT blockade. Its downregulation by CD8+ T cells and NK cells in melanoma may represent a major obstacle to the success of dual PD-1/TIGIT blockade in the clinic. Therefore, it appears essential to design novel strategies to augment CD226 expression and signaling or prevent its downregulation in melanoma immunotherapy.
Supplementary information
Acknowledgements
This study was funded by the National Natural Science Foundation of China (grant no. 81472806) and the Natural Science Foundation of Liaoning Province (grant no. 2010225032 and 2022-YGJC-30).
Author contributions
WT and JC wrote the manuscript and created the figures. TJ and XC collected and prepared the related papers. TJ and XC conceived the final approval of the version to be submitted and obtaining of the funding. All authors read and approved the final manuscript.
Data availability
All data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Consent for publication
All authors are aware of and agree to the content of the paper and their being listed as co-author of the paper.
Footnotes
Edited by Professor Hans-Uwe Simon
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Wei Tang, Jun Chen.
Contributor Information
Tianlong Ji, Email: 18040095029@163.com.
Xiufeng Cong, Email: cara_cong@163.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41419-023-05961-3.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Weiss SA, Wolchok JD, Sznol M. Immunotherapy of melanoma: facts and hopes. Clin Cancer Res. 2019;25:5191–201. doi: 10.1158/1078-0432.CCR-18-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berciano-Guerrero MA, Guardamagna M, Perez-Ruiz E, Jurado JM, Barragan I, Rueda-Dominguez A. Treatment of metastatic melanoma at first diagnosis: review of the literature. Life (Basel) 2022;12:1302. [DOI] [PMC free article] [PubMed]
- 4.Sood S, Jayachandiran R, Pandey S. Current advancements and novel strategies in the treatment of metastatic melanoma. Integr Cancer Ther. 2021;20:1534735421990078. doi: 10.1177/1534735421990078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Twomey JD, Zhang B. CaNcer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 2021;23:39. doi: 10.1208/s12248-021-00574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Keersmaecker B, Claerhout S, Carrasco J, Bar I, Corthals J, Wilgenhof S, et al. TriMix and tumor antigen mRNA electroporated dendritic cell vaccination plus ipilimumab: link between T-cell activation and clinical responses in advanced melanoma. J Immunother Cancer 2020;8:e000957. [DOI] [PMC free article] [PubMed]
- 7.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 9.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N. Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 10.Eggermont AM, Testori A, Maio M, Robert C. Anti-CTLA-4 antibody adjuvant therapy in melanoma. Semin Oncol. 2010;37:455–9. doi: 10.1053/j.seminoncol.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Savoia P, Astrua C, Fava P. Ipilimumab (Anti-Ctla-4 Mab) in the treatment of metastatic melanoma: effectiveness and toxicity management. Hum Vaccin Immunother. 2016;12:1092–101. doi: 10.1080/21645515.2015.1129478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel RP, Parikh R, Gunturu KS, Tariq RZ, Dani SS, Ganatra S, et al. Cardiotoxicity of immune checkpoint inhibitors. Curr Oncol Rep. 2021;23:79. doi: 10.1007/s11912-021-01070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalisz KR, Ramaiya NH, Laukamp KR, Gupta A. Immune checkpoint inhibitor therapy-related pneumonitis: patterns and management. Radiographics. 2019;39:1923–37. doi: 10.1148/rg.2019190036. [DOI] [PubMed] [Google Scholar]
- 14.Reddy HG, Schneider BJ, Tai AW. Immune checkpoint inhibitor-associated colitis and hepatitis. Clin Transl Gastroenterol. 2018;9:180. doi: 10.1038/s41424-018-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Sbeih H, Tang T, Lu Y, Thirumurthi S, Altan M, Jazaeri AA, et al. Clinical characteristics and outcomes of immune checkpoint inhibitor-induced pancreatic injury. J Immunother Cancer. 2019;7:31. doi: 10.1186/s40425-019-0502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4:173–82. doi: 10.1001/jamaoncol.2017.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakish HH, Ahmed FA, Elshami M, Loftus AW, Hoehn RS, Ammori JB, et al. Trends in melanoma phase 3 clinical trials since 2010: is there hope for advanced melanoma therapies beyond approved treatment mechanisms? Cancers (Basel) 2022;14:5184. [DOI] [PMC free article] [PubMed]
- 18.Walsh SR, Simovic B, Chen L, Bastin D, Nguyen A, Stephenson K, et al. Endogenous T cells prevent tumor immune escape following adoptive T cell therapy. J Clin Invest. 2019;129:5400–10. doi: 10.1172/JCI126199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol. 2011;11:645–57. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boles KS, Vermi W, Facchetti F, Fuchs A, Wilson TJ, Diacovo TG, et al. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. Eur J Immunol. 2009;39:695–703. doi: 10.1002/eji.200839116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 22.Shaffer T, Natarajan A, Gambhir SS. PET imaging of TIGIT expression on tumor-infiltrating lymphocytes. Clin Cancer Res. 2021;27:1932–40. doi: 10.1158/1078-0432.CCR-20-2725. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JA, Zhou XY, Huang D, Luan C, Gu H, Ju M, et al. Development of an immune-related gene signature for prognosis in melanoma. Front Oncol. 2020;10:602555. doi: 10.3389/fonc.2020.602555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrow NE, Holl EK, Jung J, Gao J, Jung SH, Al-Rohil RN, et al. Characterization of sentinel lymph node immune signatures and implications for risk stratification for adjuvant therapy in melanoma. Ann Surg Oncol. 2021;28:3501–10. doi: 10.1245/s10434-020-09277-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, et al. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol. 2011;41:902–15. doi: 10.1002/eji.201041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong M, Chen J, Deng Y, Zhang D, Dong L, Sun D. H2AFZ is a prognostic biomarker correlated to TP53 mutation and immune infiltration in hepatocellular carcinoma. Front Oncol. 2021;11:701736. doi: 10.3389/fonc.2021.701736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci USA. 2009;106:17858–63. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, Zhang H, Li M, Hu D, Li C, Ge B, et al. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell Death Differ. 2013;20:456–64. doi: 10.1038/cdd.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Xia P, Du Y, Liu S, Huang G, Chen J, et al. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling. J Biol Chem. 2014;289:17647–57. doi: 10.1074/jbc.M114.572420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue C, Gao S, Li S, Xing Z, Qian H, Hu Y, et al. TIGIT as a promising therapeutic target in autoimmune diseases. Front Immunol. 2022;13:911919. doi: 10.3389/fimmu.2022.911919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harjunpaa H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol. 2020;200:108–19. doi: 10.1111/cei.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, et al. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest. 2015;125:2046–58. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26:923–37. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40:569–81. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Maksimovic J, Naselli G, Qian J, Chopin M, Blewitt ME, et al. Genome-wide DNA methylation analysis identifies hypomethylated genes regulated by FOXP3 in human regulatory T cells. Blood. 2013;122:2823–36. doi: 10.1182/blood-2013-02-481788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manieri NA, Chiang EY, Grogan JL. TIGIT: a key inhibitor of the cancer immunity cycle. Trends Immunol. 2017;38:20–28. doi: 10.1016/j.it.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, et al. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol. 2014;15:431–8. doi: 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- 38.Chiang EY, de Almeida PE, de Almeida Nagata DE, Bowles KH, Du X, Chitre AS, et al. CD96 functions as a co-stimulatory receptor to enhance CD8(+) T cell activation and effector responses. Eur J Immunol. 2020;50:891–902. doi: 10.1002/eji.201948405. [DOI] [PubMed] [Google Scholar]
- 39.Shibuya A, Shibuya K. DNAM-1 versus TIGIT: competitive roles in tumor immunity and inflammatory responses. Int Immunol. 2021;33:687–92. doi: 10.1093/intimm/dxab085. [DOI] [PubMed] [Google Scholar]
- 40.Bevelacqua V, Bevelacqua Y, Candido S, Skarmoutsou E, Amoroso A, Guarneri C, et al. Nectin like-5 overexpression correlates with the malignant phenotype in cutaneous melanoma. Oncotarget. 2012;3:882–92. doi: 10.18632/oncotarget.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishiwada S, Sho M, Yasuda S, Shimada K, Yamato I, Akahori T, et al. Clinical significance of CD155 expression in human pancreatic cancer. Anticancer Res. 2015;35:2287–97. [PubMed] [Google Scholar]
- 42.Masson D, Jarry A, Baury B, Blanchardie P, Laboisse C, Lustenberger P, et al. Overexpression of the CD155 gene in human colorectal carcinoma. Gut. 2001;49:236–40. doi: 10.1136/gut.49.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakai R, Maniwa Y, Tanaka Y, Nishio W, Yoshimura M, Okita Y, et al. Overexpression of Necl-5 correlates with unfavorable prognosis in patients with lung adenocarcinoma. Cancer Sci. 2010;101:1326–30. doi: 10.1111/j.1349-7006.2010.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sloan KE, Eustace BK, Stewart JK, Zehetmeier C, Torella C, Simeone M, et al. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer. 2004;4:73. doi: 10.1186/1471-2407-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhan M, Zhang Z, Zhao X, Zhang Y, Liu T, Lu L, et al. CD155 in tumor progression and targeted therapy. Cancer Lett. 2022;545:215830. doi: 10.1016/j.canlet.2022.215830. [DOI] [PubMed] [Google Scholar]
- 46.Lepletier A, Madore J, O'Donnell JS, Johnston RL, Li XY, McDonald E, et al. Tumor CD155 expression is associated with resistance to anti-PD1 immunotherapy in metastatic melanoma. Clin Cancer Res. 2020;26:3671–81. doi: 10.1158/1078-0432.CCR-19-3925. [DOI] [PubMed] [Google Scholar]
- 47.Braun M, Aguilera AR, Sundarrajan A, Corvino D, Stannard K, Krumeich S, et al. CD155 on tumor cells drives resistance to immunotherapy by inducing the degradation of the activating receptor CD226 in CD8(+) T Cells. Immunity. 2020;53:805–823 e815. doi: 10.1016/j.immuni.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Pende D, Castriconi R, Romagnani P, Spaggiari GM, Marcenaro S, Dondero A, et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood. 2006;107:2030–6. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez-Correa B, Gayoso I, Bergua JM, Casado JG, Morgado S, Solana R, et al. Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol. 2012;90:109–15. doi: 10.1038/icb.2011.15. [DOI] [PubMed] [Google Scholar]
- 50.El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW, et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007;67:8444–9. doi: 10.1158/0008-5472.CAN-06-4230. [DOI] [PubMed] [Google Scholar]
- 51.Casado JG, Pawelec G, Morgado S, Sanchez-Correa B, Delgado E, Gayoso I, et al. Expression of adhesion molecules and ligands for activating and costimulatory receptors involved in cell-mediated cytotoxicity in a large panel of human melanoma cell lines. Cancer Immunol Immunother. 2009;58:1517–26. doi: 10.1007/s00262-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu Y, Paniccia A, Schulick AC, Chen W, Koenig MR, Byers JT, et al. Identification of CD112R as a novel checkpoint for human T cells. J Exp Med. 2016;213:167–76. doi: 10.1084/jem.20150785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murter B, Pan X, Ophir E, Alteber Z, Azulay M, Sen R, et al. Mouse PVRIG has CD8(+) T cell-specific coinhibitory functions and dampens antitumor immunity. Cancer Immunol Res. 2019;7:244–56. doi: 10.1158/2326-6066.CIR-18-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chauvin JM, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020;8:e000957. [DOI] [PMC free article] [PubMed]
- 55.Kaminska P, Buszka K, Galus L, Jankowski M, Nowicki M, Mackiewicz J, et al. Circulating melanoma cell numbers correlate with TIGIT-positive cytotoxic T cell counts in advanced-stage melanoma patients. Cells. 2023;12:856. [DOI] [PMC free article] [PubMed]
- 56.Sun Y, Luo J, Chen Y, Cui J, Lei Y, Cui Y, et al. Combined evaluation of the expression status of CD155 and TIGIT plays an important role in the prognosis of LUAD (lung adenocarcinoma) Int Immunopharmacol. 2020;80:106198. doi: 10.1016/j.intimp.2020.106198. [DOI] [PubMed] [Google Scholar]
- 57.Liu X, Li M, Wang X, Dang Z, Jiang Y, Wang X, et al. PD-1(+) TIGIT(+) CD8(+) T cells are associated with pathogenesis and progression of patients with hepatitis B virus-related hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68:2041–54. doi: 10.1007/s00262-019-02426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma J. Bioinformatics-guided analysis uncovers TIGIT as an epigenetically regulated immunomodulator affecting immunotherapeutic sensitivity of gastric cancer. Cancer Biomark. 2022;33:349–58. doi: 10.3233/CBM-210159. [DOI] [PubMed] [Google Scholar]
- 59.Yang ZZ, Kim HJ, Wu H, Jalali S, Tang X, Krull JE, et al. TIGIT expression is associated with T-cell suppression and exhaustion and predicts clinical outcome and anti-PD-1 response in follicular lymphoma. Clin Cancer Res. 2020;26:5217–31. doi: 10.1158/1078-0432.CCR-20-0558. [DOI] [PubMed] [Google Scholar]
- 60.Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, et al. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. J Immunol. 2011;186:1338–42. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Josefsson SE, Huse K, Kolstad A, Beiske K, Pende D, Steen CB, et al. T cells expressing checkpoint receptor TIGIT are enriched in follicular lymphoma tumors and characterized by reversible suppression of T-cell receptor signaling. Clin Cancer Res. 2018;24:870–81. doi: 10.1158/1078-0432.CCR-17-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. J Immunol. 2012;188:3869–75. doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Inozume T, Yaguchi T, Furuta J, Harada K, Kawakami Y, Shimada S. Melanoma cells control antimelanoma CTL responses via Interaction between TIGIT and CD155 in the effector phase. J Invest Dermatol. 2016;136:255–63. doi: 10.1038/JID.2015.404. [DOI] [PubMed] [Google Scholar]
- 64.Fourcade J, Sun Z, Chauvin JM, Ka M, Davar D, Pagliano O, et al. CD226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight. 2018;3:e121157. [DOI] [PMC free article] [PubMed]
- 65.Fuhrman CA, Yeh WI, Seay HR, Saikumar Lakshmi P, Chopra G, Zhang L, et al. Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J Immunol. 2015;195:145–55. doi: 10.4049/jimmunol.1402381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yusuf E, Wybo I, Pierard D. Case series of patients with Fusobacterium nucleatum bacteremia with emphasis on the presence of cancer. Anaerobe. 2016;39:1–3. doi: 10.1016/j.anaerobe.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 67.Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42:344–55. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shaw G, Cavalcante L, Giles FJ, Taylor A. Elraglusib (9-ING-41), a selective small-molecule inhibitor of glycogen synthase kinase-3 beta, reduces expression of immune checkpoint molecules PD-1, TIGIT and LAG-3 and enhances CD8(+) T cell cytolytic killing of melanoma cells. J Hematol Oncol. 2022;15:134. doi: 10.1186/s13045-022-01352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee WJ, Lee YJ, Choi ME, Yun KA, Won CH, Lee MW, et al. Expression of lymphocyte-activating gene 3 and T-cell immunoreceptor with immunoglobulin and ITIM domains in cutaneous melanoma and their correlation with programmed cell death 1 expression in tumor-infiltrating lymphocytes. J Am Acad Dermatol. 2019;81:219–27. doi: 10.1016/j.jaad.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 70.Han J, Khatwani N, Searles TG, Turk MJ, Angeles CV. Memory CD8(+) T cell responses to cancer. Semin Immunol. 2020;49:101435. doi: 10.1016/j.smim.2020.101435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aoki H, Shichino S, Matsushima K, Ueha S. Revealing clonal responses of tumor-reactive T-cells through T cell receptor repertoire analysis. Front Immunol. 2022;13:807696. doi: 10.3389/fimmu.2022.807696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Such L, Zhao F, Liu D, Thier B, Le-Trilling VTK, Sucker A, et al. Targeting the innate immunoreceptor RIG-I overcomes melanoma-intrinsic resistance to T cell immunotherapy. J Clin Invest. 2020;130:4266–81. doi: 10.1172/JCI131572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borden BA, Baca Y, Xiu J, Tavora F, Winer I, Weinberg BA, et al. The landscape of glycogen synthase kinase-3 beta genomic alterations in cancer. Mol Cancer Ther. 2021;20:183–90. doi: 10.1158/1535-7163.MCT-20-0497. [DOI] [PubMed] [Google Scholar]
- 74.Sahin I, Eturi A, De Souza A, Pamarthy S, Tavora F, Giles FJ, et al. Glycogen synthase kinase-3 beta inhibitors as novel cancer treatments and modulators of antitumor immune responses. Cancer Biol Ther. 2019;20:1047–56. doi: 10.1080/15384047.2019.1595283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walz A, Ugolkov A, Chandra S, Kozikowski A, Carneiro BA, O'Halloran TV, et al. Molecular pathways: revisiting glycogen synthase kinase-3beta as a target for the treatment of cancer. Clin Cancer Res. 2017;23:1891–7. doi: 10.1158/1078-0432.CCR-15-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiu DK, Yuen VW, Cheu JW, Wei LL, Ting V, Fehlings M, et al. Hepatocellular carcinoma cells up-regulate PVRL1, stabilizing PVR and inhibiting the cytotoxic T-cell response via TIGIT to mediate tumor resistance to PD1 inhibitors in mice. Gastroenterology. 2020;159:609–23. doi: 10.1053/j.gastro.2020.03.074. [DOI] [PubMed] [Google Scholar]
- 77.Ostroumov D, Duong S, Wingerath J, Woller N, Manns MP, Timrott K, et al. Transcriptome profiling identifies TIGIT as a marker of T-cell exhaustion in liver cancer. Hepatology. 2021;73:1399–418. doi: 10.1002/hep.31466. [DOI] [PubMed] [Google Scholar]
- 78.Hung AL, Maxwell R, Theodros D, Belcaid Z, Mathios D, Luksik AS, et al. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology. 2018;7:e1466769. doi: 10.1080/2162402X.2018.1466769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ge Z, Zhou G, Campos Carrascosa L, Gausvik E, Boor PPC, Noordam L, et al. TIGIT and PD1 co-blockade restores ex vivo functions of human tumor-infiltrating CD8(+) T cells in hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol. 2021;12:443–64. doi: 10.1016/j.jcmgh.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang B, Zhang W, Jankovic V, Golubov J, Poon P, Oswald EM, et al. Combination cancer immunotherapy targeting PD-1 and GITR can rescue CD8(+) T cell dysfunction and maintain memory phenotype. Sci Immunol 2018;3:eaat7061. [DOI] [PubMed]
- 81.Chauvin JM, Ka M, Pagliano O, Menna C, Ding Q, DeBlasio R, et al. IL15 stimulation with TIGIT blockade reverses CD155-mediated NK-cell dysfunction in melanoma. Clin Cancer Res. 2020;26:5520–33. doi: 10.1158/1078-0432.CCR-20-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fang L, Zhao F, Iwanowycz S, Wang J, Yin S, Wang Y, et al. Anticancer activity of emodin is associated with downregulation of CD155. Int Immunopharmacol. 2019;75:105763. doi: 10.1016/j.intimp.2019.105763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mittal D, Lepletier A, Madore J, Aguilera AR, Stannard K, Blake SJ, et al. CD96 is an immune checkpoint that regulates CD8(+) T-cell antitumor function. Cancer Immunol Res. 2019;7:559–71. doi: 10.1158/2326-6066.CIR-18-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Itahashi K, Irie T, Nishikawa H. Regulatory T-cell development in the tumor microenvironment. Eur J Immunol. 2022;52:1216–27. doi: 10.1002/eji.202149358. [DOI] [PubMed] [Google Scholar]
- 85.Ge Z, Peppelenbosch MP, Sprengers D, Kwekkeboom J. TIGIT, the next step towards successful combination immune checkpoint therapy in cancer. Front Immunol. 2021;12:699895. doi: 10.3389/fimmu.2021.699895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MW, et al. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2015;125:4053–62. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Huntington ND, Cursons J, Rautela J. The cancer-natural killer cell immunity cycle. Nat Rev Cancer. 2020;20:437–54. doi: 10.1038/s41568-020-0272-z. [DOI] [PubMed] [Google Scholar]
- 88.Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18:671–88. doi: 10.1038/s41577-018-0061-z. [DOI] [PubMed] [Google Scholar]
- 89.Yu Y. The function of NK cells in tumor metastasis and NK cell-based immunotherapy. Cancers (Basel) 2023;15:2323. [DOI] [PMC free article] [PubMed]
- 90.Cappello S, Sung HM, Ickes C, Gibhardt CS, Vultur A, Bhat H, et al. Protein signatures of NK cell-mediated melanoma killing predict response to immunotherapies. Cancer Res. 2021;81:5540–54. doi: 10.1158/0008-5472.CAN-21-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li J, Smalley I, Chen Z, Wu JY, Phadke MS, Teer JK, et al. Single-cell characterization of the cellular landscape of acral melanoma identifies novel targets for immunotherapy. Clin Cancer Res. 2022;28:2131–46. doi: 10.1158/1078-0432.CCR-21-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–58. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sanchez-Correa B, Valhondo I, Hassouneh F, Lopez-Sejas N, Pera A, Bergua JM, et al. DNAM-1 and the TIGIT/PVRIG/TACTILE axis: novel immune checkpoints for natural killer cell-based cancer immunotherapy. Cancers (Basel) 2019;11:877. [DOI] [PMC free article] [PubMed]
- 94.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819–29. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okumura G, Iguchi-Manaka A, Murata R, Yamashita-Kanemaru Y, Shibuya A, Shibuya K. Tumor-derived soluble CD155 inhibits DNAM-1-mediated antitumor activity of natural killer cells. J Exp Med. 2020;217:1. doi: 10.1084/jem.20191290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rethacker L, Roelens M, Bejar C, Maubec E, Moins-Teisserenc H, Caignard A. Specific patterns of blood ILCs in metastatic melanoma patients and their modulations in response to immunotherapy. Cancers (Basel) 2021;13:1446. [DOI] [PMC free article] [PubMed]
- 97.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bernard PL, Delconte R, Pastor S, Laletin V, Costa Da Silva C, Goubard A, et al. Targeting CISH enhances natural cytotoxicity receptor signaling and reduces NK cell exhaustion to improve solid tumor immunity. J Immunother Cancer 2022;10:e004244. [DOI] [PMC free article] [PubMed]
- 99.Knight A, Karapetyan L, Kirkwood JM. Immunotherapy in melanoma: recent advances and future directions. Cancers (Basel) 2023;15:1106. [DOI] [PMC free article] [PubMed]
- 100.Yamazaki N, Isei T, Kiyohara Y, Koga H, Kojima T, Takenouchi T, et al. A phase I study of the safety and efficacy of talimogene laherparepvec in Japanese patients with advanced melanoma. Cancer Sci. 2022;113:2798–806. doi: 10.1111/cas.15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reijers ILM, Menzies AM, van Akkooi ACJ, Versluis JM, van den Heuvel NMJ, Saw RPM, et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: the PRADO trial. Nat Med. 2022;28:1178–88. doi: 10.1038/s41591-022-01851-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this study are available from the corresponding author upon reasonable request.