Abstract
The American white ibis (Eudocimus albus), a common bird species in Florida, has become increasingly urban, with many populations relying heavily on urban and suburban habitats, which may alter parasite transmission. Parasites of ibis, especially haemosporidians, are understudied. Avian haemosporidia can have a wide range of impacts on birds, including decreased reproductive success or increased mortality. Because southern Florida is subtropical and has a high diversity of potential vectors for haemosporidia, we hypothesized that there will be a high prevalence and genetic diversity of haemosporidia in white ibis. A total of 636 ibis from South Florida were sampled from 2010 to 2022, and blood samples were tested for haemosporidia by examination of Giemsa-stained thin blood smears and/or nested PCRs targeting the cytochrome b gene. A total of 400 (62.9%, 95% CI 59–66.7%) ibis were positive for parasites that were morphologically identified as Haemoproteus plataleae. Sequences of 302 positives revealed a single haplotype of Haemoproteus (EUDRUB01), which was previously reported from white ibis in South Florida and captive scarlet ibis (E. ruber) in Brazil. No Plasmodium or Leucocytozoon infections were detected. Parasitemias of the 400 positive birds were very low (average 0.084%, range 0.001%-2.16% [although only 2 birds had parasitemias >1%]). Prevalence and parasitemias were similar for males and females (68% vs. 61.6% and 0.081% vs. 0.071%, respectively). Prevalence in juveniles was lower compared with adults (52% vs. 67.4%) but parasitemias were higher in juveniles (0.117% vs. 0.065%). This data shows that H. plataleae is common in ibis in South Florida. Although parasitemias were generally low, additional research is needed to determine if this parasite has subclinical effects on ibis, if additional haplotypes or parasite species infect ibis in other regions of their range, or if H. plataleae is pathogenic for other sympatric avian species.
Keywords: Avian malaria, Birds, Haemosporida, Molecular characterization, Pelecaniformes, Threskiornithidae, Wading birds, Wildlife
Graphical abstract
Highlights
-
•
High prevalence (63%) of Haemoproteus plataleae in 636 white ibis in South Florida.
-
•
Only a single H. plataleae lineage (EUDRUB01) detected in 302 PCR positive ibis.
-
•
Prevalence was higher in adults, but average parasitemia was higher in juvenile ibis.
-
•
1- and 2-year-olds had higher parasitemia compared with 3-year-olds and adults.
-
•
Parasitemia was higher in spring and summer periods compared with the fall season.
1. Introduction
The American white ibis (Eudocimus albus) (Pelicaniformes: Threskiornithidae) ranges throughout the southeastern United States, Mexico, and Central America. In southern Florida, this medium-sized wading bird is a year-round resident that historically breeds and forages in wetlands, particularly the Greater Everglades ecosystem. However, the white ibis is now commonly observed foraging in urbanized areas. Rapid urbanization of Florida may impact the health of urban-dwelling wildlife and may alter the exposure of ibis and other wading birds to pathogens and their ability to respond to infections, as is seen in other avifauna (Delahay et al., 2009; Evans et al., 2009; Zylberberg et al., 2013; Boal and Mannan, 2014; Cornet et al., 2014; Hernandez et al., 2016). For example, white ibis in urban settings are becoming habituated to inappropriate food handouts from humans (e.g., bread), which may impact their health due to poor food quality. This habituation and increased use of provisioned food comes with trade-offs and has been associated with changes in pathogen prevalence and decreased immunity (Murray et al., 2018, 2021; Cummings et al., 2020; Seixas et al., 2022). Finally, changes in habitat use and movement patterns could also influence ibis exposure to pathogens and vectors. For example, ibis that use urban habitats more during the non-breeding seasons are more sedentary and tend to have longer non-breeding seasons and shorter breeding seasons that begin earlier in the year compared to ibis that primarily use wetland habitats (Kidd-Weaver et al., 2020).
Avian haemosporidian parasites infect the blood cells of birds and are transmitted by different groups of dipteran vectors (Valkiunas, 2005). The three most common genera in birds are Plasmodium, Leucocytozoon, and Haemoproteus, which are transmitted by mosquitoes, black flies, and biting midges or hippoboscid flies, respectively (Valkiunas, 2005). In natural hosts, avian haemosporidian infections are often chronic and, although they are generally considered nonpathogenic, decreased survival and fecundity, poorer post-breeding body condition, and hindered mate selection have been documented (Wiehn et al., 1997; Dawson and Bortolotti, 2000; Sanz et al., 2001). Importantly, research on reed warblers (Acrocephalus arundinaceus) over a 25-year period showed that birds with haemoparasite infections had significantly decreased lifespans and lower lifetime reproductive success (Asghar et al., 2015).
Few studies have been conducted on haemosporidians of white ibis, but infections with Haemoproteus have been reported from both northern and southern Florida and prevalence tends to be high in adult birds (Forrester, 1980; Telford Jr. et al., 1992; Coker et al., 2017). These studies morphologically identified the parasites as H. plataleae, a widespread parasite of numerous Ciconiiformes and Pelicaniformes species nearly worldwide. In addition, one molecular-based study found 1 of 4 white ibis from South Florida positive for a Plasmodium sp. (Bryan et al., 2015). Genetic characterization of H. plataleae from a small number of white ibis revealed only a single haplotype (called hWHIB01 at the time) (Coker et al., 2017). However, a higher diversity of parasites may occur in ibis as Plasmodium elongatum (haplotye GRW06) and Plasmodium sp. MYCAME02 have been reported from closely related Threskiornithidae species (a roseate spoonbill (Platalea ajaja) and a glossy ibis (Plegadis falcinellus), respectively) (Bryan et al., 2015; Coker et al., 2017). In addition, three novel Plasmodium lineages within the MYCAMPE02 group (likely representing P. paranucleophilum) and one novel Haemoproteus lineage (PLATAJH1) have been reported from roseate spoonbills in Brazil (Chahad-Ehlers et al., 2018).
The primary goals of the current study were to determine the prevalence, parasitemia, and genetic diversity of blood parasites in white ibis in South Florida and determine how these factors varied by individual demographic parameters. A high diversity of haemosporidia has been reported in conspecific wading birds in the Order Pelecaniformes (Family Ardeidae); therefore, we hypothesized that unrecognized diversity of blood parasites is present in white ibis. In addition, because white ibis are using an increasing number of urban sites, we hypothesized that parasite exposure would vary by habitat type.
2. Methods
2.1. Animal capture and sampling
White ibis were captured using a leg lasso or mist nets at sites in four southern Florida counties from 2010 to 2022 encompassing urban and natural areas (Table 1). At urban sites, birds were well habituated to the presence of humans and in residential parks and neighborhoods, so these birds were captured with leg lassos made of clear fishing line (Adams et al., 2019). At rural sites, 12 m mist nests were positioned in a “V” near the edge of water bodies or in shallow water and white, plastic flamingo decoys were used to lure in flocks (Heath and Frederick, 2003).
Table 1.
Sample size, prevalence of Haemoproteus plataleae detection, and parasitemia (median and range) for each of the sites in south Florida where white ibis (Eudocimus albus) were sampled from 2010 to 2022.
| Site | County | Parasite Prevalence |
Parasitemia (%) |
||
|---|---|---|---|---|---|
| No. Positive/No. Sampled (%) | 95% Confidence Interval (%) | Median | Range | ||
| Wildlife Rehabilitation Center | Palm Beach | 1/1 (100%) | 25.0–100.0 | 0.01 | 0.01–0.01 |
| Wildlife Rehabilitation Center | Lee | 1/1 (100%) | 25.0–100.0 | 0.004 | 0.004–0.004 |
| Dreher urban park | Palm Beach | 41/63 (65.1%) | 52.0–76.7 | 0.044 | 0.002–0.842 |
| Urban park (DUP) | Palm Beach | 10/11 (90.9%) | 58.7–99.8 | 0.037 | 0.004–0.22 |
| Fisheating Creek Outpost | Glades | 0/1 (0%) | 0.0–97.5 | NA | NA |
| Green Cay wetlands | Palm Beach | 27/44 (61.4%) | 45.5–75.6 | 0.024 | 0.002–0.148 |
| Garden Lakes business park | Palm Beach | 2/3 (66.7%) | 9.4–99.2 | 0.075 | 0.05–0.1 |
| Urban park (GP) | Palm Beach | 20/35 (57.1%) | 39.4–73.7 | 0.023 | 0.002–0.27 |
| Indian Creek urban park | Palm Beach | 55/82 (67.1%) | 55.8–77.1 | 0.015 | 0.001–0.74 |
| Juno Beach urban park | Palm Beach | 32/61 (52.5%) | 39.3–65.4 | 0.030 | 0.003–2.16 |
| Wildlife Management Area | Palm Beach | 5/11 (45.4%) | 16.8–76.6 | 0.044 | 0.004–0.42 |
| Kitching Creek | Martin | 8/15 (53.3%) | 26.6–78.7 | 0.021 | 0.002–0.296 |
| Lion Country Safari park | Palm Beach | 35/53 (66%) | 51.7–78.5 | 0.019 | 0.001–0.48 |
| Loxahatchee NE | Palm Beach | 11/16 (68.8%) | 41.3–89.0 | 0.046 | 0.006–0.09 |
| Loxahatchee Slough | Palm Beach | 8/12 (66.7%) | 34.9–90.1 | 0.031 | 0.002–0.19 |
| Loxahatchee Wildlife Refuge | Palm Beach | 6/7 (85.7%) | 42.1–99.6 | 0.026 | 0.016–0.158 |
| Lake Worth | Palm Beach | 15/30 (50%) | 31.3–68.7 | 0.012 | 0.002–0.89 |
| Urban park at Assisted Living Facility (PO) | Palm Beach | 17/21 (81%) | 58.1–94.6 | 0.01 | 0.002–0.442 |
| Strip Plaza parking lot (PP) | Palm Beach | 7/9 (77.8%) | 40.0–97.2 | 0.009 | 0.005–0.163 |
| Royal Palm urban park | Palm Beach | 0/4 (0%) | 0.0–60.2 | NA | NA |
| Apartment complex (SM) | Palm Beach | 1/2 (50%) | 1.3–98.7 | 0.007 | 0.007–0.007 |
| Solid Waste site | Palm Beach | 89/140 (63.6%) | 55.0–71.5 | 0.03 | 0.001–1.34 |
| Park at industrial site (TT) | 7/12 (58.3%) | 27.7–84.8 | 0.058 | 0.002–0.092 | |
| Palm Beach Zoo | Palm Beach | 2/2 (100%) | 15.8–100.0 | 0.26 | 0.12–0.4 |
After capture, the birds were placed in pillowcases prior to blood sampling and no bird was handled for longer than 1 h. Age (juvenile [collective term for 1, 2, or 3 yr old] or adult; based on plumage; Heath et al., 2020), standard morphometric measurements (weight, wing chord, tarsus length and width, and culmen length), body condition (scale of 0.5=severely emaciated–5=obese) and ectoparasite loads were recorded. Blood samples were collected from the jugular or metatarsal vein. Two thin blood smears were prepared, dried on site, and fixed in methanol within 24 h. Remaining blood was stored in heparinized tubes (Microtainer, Becton Dickinson, Frankin Lakes, NJ) and kept on ice in the field prior to being frozen at −20 °C. Although some birds can be sexed based on skin color and morphology (Heath and Fredrick, 2006), sex was determined using molecular methods as previously described (Griffiths et al., 1998).
Additional information on the study sites and methods is available in Hernandez et al. (2016), Kidd-Weaver et al. (2020), and Murray et al. (2021). All capture and sampling techniques were reviewed and approved by the University of Georgia's IACUC (# A2011 08–018, A2013- 10-016, A2016 11-019, and A2019 10-009). This work required permits from both the Florida Fish and Wildlife Conservation Commission (LSSC-11-00119F) and the U. S. Fish and Wildlife Service (MB779238-0), and both permits required prior approval by an IACUC.
2.2. Blood smear analysis
Thin blood smears were stained with Geimsa stain per manufacturers’ directions (Dipquick, Jorgensen Laboratories, Inc., Loveland, Colorado). Approximately 20,000-50,000 erythrocytes were examined at 100x using a compound microscope (Olympus CH30, Olympus Optical Co., Japan) as suggested by Godfrey et al. (1987) when anticipating extremely low parasitemias. The parasites were morphologically identified using published descriptions and a dichotomous key (Bennett et al., 1975; Valkiunas, 2005; Valkiunas and Iezhova, 2022).
2.3. Molecular detection and characterization
DNA was extracted from whole blood (10 μl) using a Qiagen DNeasy blood extraction kit (Qiagen, Valencia, California) per the manufacturer's instructions. A 480 base pair fragment of the mitochondrial cytochrome b (cytb) gene of Haemoproteus and Plasmodium and a 478 bp fragment of Leucocytozoon were targeted with a nested PCR: the primers HaemNFI (5′-CATATATTAAGAGAAITATGGAG-3′) and HaemNR3 (5′-ATAGAAAGATAAGAAATACCATTC-3′) were used in the primary PCR to amplify a 617 bp fragment (including primers) of all three genera (Hellgren et al., 2004), and two separate secondary PCR reactions were run using primers HaemF (5′- ATGGTG-CTTTCGATATATGCATG - 3′) and HaemR2 (5′- GCATTATCTGGATGTGATAATGGT - 3′) to amplify Haemoproteus and Plasmodium and primers HaemFL (5′- ATGGTGTTTTAGATACTTACATT - 3′) and HaemR2L (5′- CATTATCTGGATGAGATAATGGIGC - 3′) to amplify Leucocytozoon (Hellgren et al., 2004; Waldenstrom et al., 2004). All PCR reactions were run for 20 cycles (primary reaction) or 35 cycles (for both secondary reactions) of 94 °C for 30 s, 50 °C for 30 s, and 72 °C for 45 s in 25 μl volumes with 1.25 mM dNTPs (Promega, Madison, Wisconsin), 3.125 mM MgCl2, 1.25 u GoTaq® Flexi DNA polymerase (Promega), and 1.25 μM of each primer, combined with 5 μl of DNA for the primary reaction or 1 μl of the primary product for the secondary reactions.
Products were visualized in 2% agarose gels stained with Gel Red (Biotium Inc., Hayward, California). Amplicons were gel purified using the Qiagen QIAquick gel extraction kit (Qiagen), and bi-directionally sequenced at the Georgia Genomics Facility (Athens, Georgia) or GENEWIZ (Azenta Life Sciences, South Plainfield, New Jersey). Consensus sequences were generated using Geneious Prime (Dotmatics, San Diego, California). Related sequences in the GenBank and the MalAvi databases were obtained for comparison (Bensch et al., 2009).
DNA from a blood sample of a duck infected with Haemoproteus nettionis and Leucocytozoon simondi was used as positive control in each set of PCR reactions. DNA extraction, primary and secondary amplification, and product analysis were completed in separate dedicated areas to avoid and detect contamination. A negative molecular grade water control was included in each set of extractions and every 10-12 extractions within larger sets. Additional negative water controls were included in each set of primary and secondary PCR reaction sets.
2.4. Data analysis
Prevalence of parasite detection was calculated, with 95% confidence intervals, based on year (2010-2017, 2022), sex, bird age class (adult vs juvenile [further divided for a subset of birds into 1–3 years]), habitat type (natural vs urban as reported by Murray et al. (2018)), season (fall, spring, summer), and body condition score (0.5–5). Body condition was determined as defined in Murray et al. (2018) with the addition of a 0.5 category for two ibis that were severely emaciated. Seasons were defined as fall (non-breeding period from October-December and January), spring (pre-breeding period from February and March), and summer (breeding season from June-August).
Fisher's Exact and ChiSquare tests were used to compare prevalence across categories. Only results for birds with parasites detected were used to compare parasitemia across the categories listed above. Log10 of the percent of red blood cells with parasites present was calculated for each observation to correct for non-normally distributed data and one-way Analysis of Variance (ANOVA) was used to compare the calculated values across categories. Post-hoc pairwise comparisons were conducted using a Tukey adjustment for multiple comparisons. All analyses were conducted in JMP® PRO version 16.0.0, with statistical significance assessed at α = 0.05.
3. Results
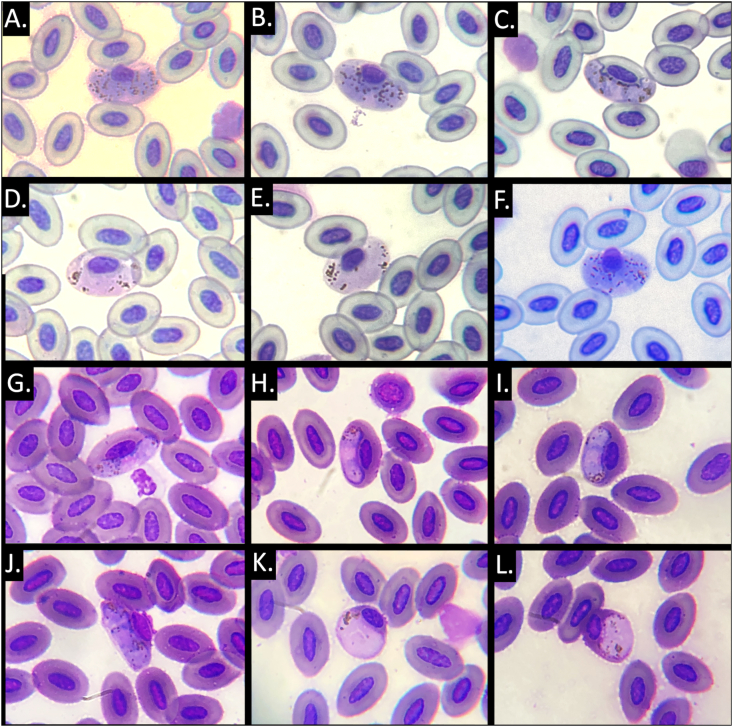
Overall, Haemoproteus was detected in 400 of 636 (62.9%, 95% CI 59–66.7%) white ibis examined (Table 1). All parasites were morphologically consistent with H. plataleae; the length and width of 50 parasites was 14.1 (12–14.8) x 4.8 (4.6–4.9) μm and large numbers (>30) of small to medium-sized granules were present in macrogametocytes (Fig. 2) (Bennett et al., 1975; Valkiunas 2005; Valkiunas and Iezhova, 2022). These forms were clearly distinct from other parasites infecting Pelecaniformes including H. pelouroi (i.e., we had no markedly irregular irregular forms, our parasites had >30 pigment granules) and H. herodiadis (our parasites had >30 pigment granules and our gametocytes were not thin). Most parasites were microhalteridial and halterdial, but rare circumnuclear forms were also observed. Host nuclei were typically displaced. A single H. plataleae-infected white ibis had, in addition to many typical gametocytes, a few round forms. The white ibis with the round forms only had a single cytb sequence that was 100% similar to other H. plataleae sequences from other white ibis and rare round forms have also been reported by de Mello (1937) from hosts in India.
Fig. 2.
Typical Haemoproteus plataleae stages from three infected white ibis (Eudocimus alba) from South Florida. All ibis were genetically confirmed to be infected with the EUDRUB01 lineage. A-E, an ibis from Juno Beach urban park; F, an ibis from Indian Creek urban park; and G-L, an ibis from the Solid Waste site. The latter bird had rare round forms (K-L), which were absent from other H. plataleae-infected ibis. Younger stages (C, H, I) had a an evident ‘cleft’ between the gametocyote and erythrocyte nucleus.
Prevalence varied by age class, with adults having significantly higher prevalence (67.4%) compared to juveniles (52.0%, Fisher's Exact p = 0.0005, Table 2). Among juveniles, prevalence was lowest for 2 yr olds (36.6%) (Table 2). Prevalence varied by year, with significantly higher prevalence in 2016 (70.4%) compared to 2012 (45.2%) and 2017 (51.5%, ChiSquare p = 0.0086, Table 2). Prevalence also varied by season, with significantly higher prevalence in ibis captured in the spring (69.7%), compared to the fall (56.2%, ChiSquare p = 0.0066, Table 2). Prevalence did not significantly differ based on sex, habitat type, or body condition score.
Table 2.
Prevalence and parasitemia of Haemoproteus plataleae detected based on age, sex, habitat type, year, and season for white ibis (Eudocimus albus) sampled in south Florida from 2010 to 2022.
| Parasite Prevalence |
Parasitemia (%) |
|||
|---|---|---|---|---|
| No. Positive/No. Sampled (%) | 95% Confidence Interval (%) | Median | Range | |
| Age Class | ||||
| Adult | 294/436 (67.4) | 62.8–71.8 | 0.02 | 0.001–1.34 |
| Juvenile | 93/179 (52.0) | 44.4–59.5 | 0.06 | 0.002–0.74 |
| Age | ||||
| 1 yr | 13/22 (59.1) | 36.4–79.3 | 0.38 | 0.008–0.74 |
| 2 yr | 15/41 (36.6) | 22.1–53.1 | 0.13 | 0.006–0.4 |
| 3 yr | 50/82 (61.0) | 49.6–71.6 | 0.037 | 0.002–0.19 |
| 4+ (adult) | 157/228 (68.8) | 62.4–74.8 | 0.024 | 0.001–0.84 |
| Sex | ||||
| Female | 196/318 (61.6) | 56.0–67.0 | 0.026 | 0.001–0.84 |
| Male | 100/147 (68.0) | 59.8–75.5 | 0.027 | 0.002–0.89 |
| Habitat Type | ||||
| Natural | 74/120 (61.7) | 52.4–70.4 | 0.027 | 0.002–0.42 |
| Urban | 326/516 (63.2) | 58.8–67.4 | 0.024 | 0.001–2.16 |
| Year | ||||
| 2010 | 15/22 (68.2) | 45.1–86.1 | 0.021 | 0.001–2.16 |
| 2011 | 1/4 (25) | 0.63–80.6 | 0.225 | 0.225–0.225 |
| 2012 | 19/42 (45.2) | 29.8–61.3 | 0.010 | 0.002–0.442 |
| 2013 | 59/90 (65.6) | 54.8–75.3 | 0.030 | 0.002–0.890 |
| 2014 | 70/104 (67.3) | 57.4–76.2 | 0.024 | 0.001–1.34 |
| 2015 | 36/62 (58.1) | 44.8–70.5 | 0.022 | 0.002–0.158 |
| 2016 | 140/199 (70.4) | 63.5–76.6 | 0.026 | 0.002–0.42 |
| 2017 | 53/103 (51.5) | 41.4–61.4 | 0.066 | 0.002–0.842 |
| 2022 | 7/10 (70) | 34.8–93.3 | 0.009 | 0.001–0.13 |
| Season | ||||
| Fall | 113/201 (56.2) | 49.1–63.2 | 0.01 | 0.002–1.26 |
| Spring | 189/271 (69.7) | 63.9–75.2 | 0.036 | 0.001–1.34 |
| Summer | 98/164 (59.8) | 51.8–67.3 | 0.054 | 0.001–0.890 |
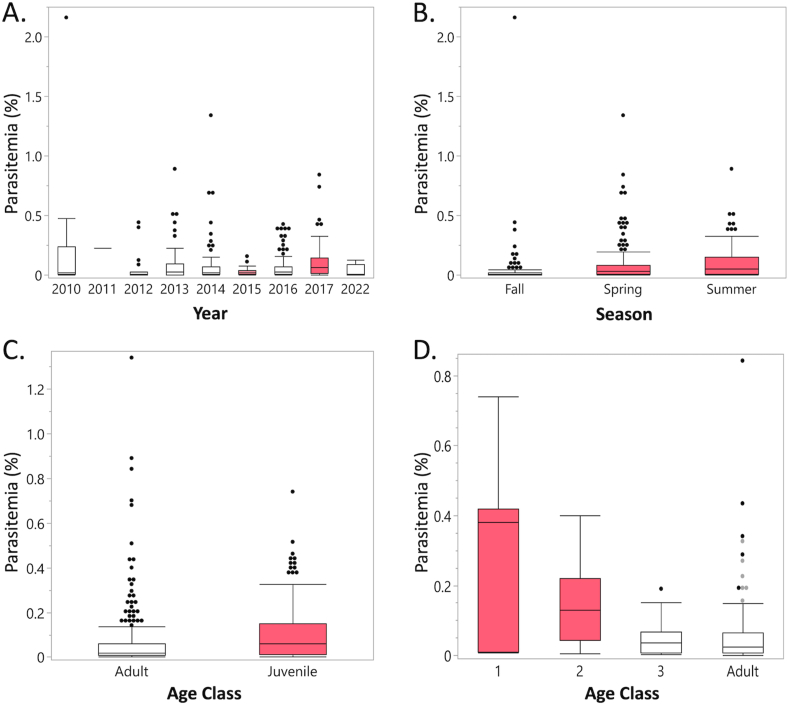
Of the 400 samples with parasites detected, parasitemia was generally low and varied from 0.001% to 2.16% (Table 1, Table 2). There were significant differences in parasitemia based on age, year, and season (Table 2). Juveniles (birds ≤3 yrs of age) had higher parasitemia compared to adults (p < 0.0001), and for the subset of juveniles that were divided by year, the 1 and 2 yr olds had higher parasitemia compared to 3 yr olds and adults (p ≤ 0.02) (Fig. 1, Table 2). Across years, parasitemia was significantly higher in 2017, compared to 2015 (p = 0.0362), and across seasons, parasitemia was higher in spring and summer, compared to fall (p < 0.0001) (Fig. 1, Table 2).
Fig. 1.
Box plots of parasitemia values of Haemoproteus plataleae in white ibis (Eudocimus albus) sampled from South Florida from 2010 to 2022 by year (A.), season (B.), and age (C. and D.). C. shows all ibis with general adult vs. juvenile age class designations and D. shows data for the subset of ibis that were aged to specific year for juveniles (1, 2, or 3 yrs old). Years 2015 and 2017 were significantly different from each other, but both were similar to other years. For remaining figures, factors that are differently colored are significantly different from each other. Note that the x-axis maximum varies between plots.
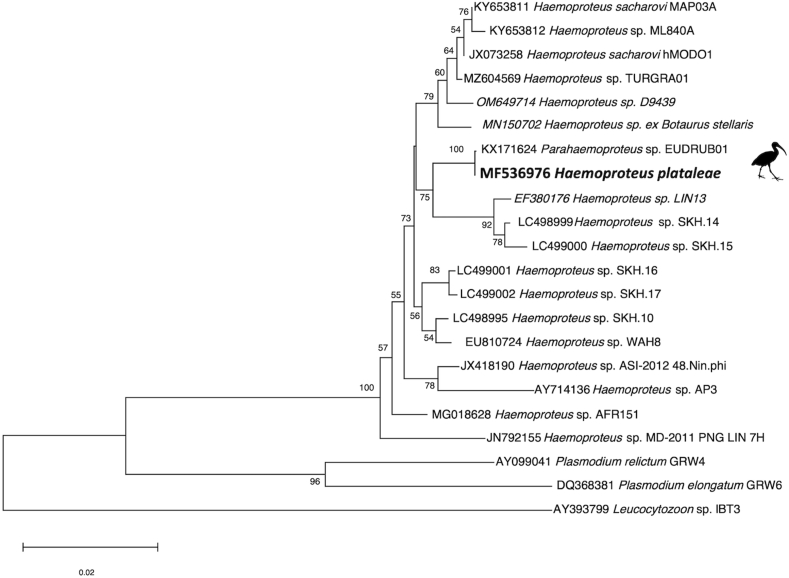
A random set of 349 blood smear-positive birds were tested by PCR; 336 (96.3%) were PCR positive for the protocol that amplifies Haemoproteus and Plasmodium. High quality sequences were obtained for 302 (89.9%) of these samples. The sequences were identical and were a 100% match to Haemoproteus haplotype (hWHIB01) that had been initially detected in white ibis in South Florida (Coker et al., 2017) and subsequently from a captive scarlet ibis in Brazil (EUDRUB01) (Chagas et al., 2017). Phylogenetically, H. plataleae grouped with, but was distinct from, other Haemoproteus spp. and was in a sister group to several sequences from South Korean hosts (Fig. 3). There was no evidence of Plasmodium in the sequences (i.e., polymorphic bases) (Kistler et al., 2013) and all birds were PCR negative for Leucocytozoon.
Fig. 3.
Phylogenetic relationship of Haemoproteus plataleae from white ibis (Eudocimus albus) with other Haemoproteus spp.
4. Discussion
To date, this is the largest study to genetically characterize and morphologically identify haemoparasites in white ibis. Similar to previous studies, we detected a high prevalence of H. plataleae that was confirmed genetically to be a single lineage across numerous white ibis populations in South Florida. Because of the high prevalence and the low parasitemia levels we detected, white ibis likely develop chronic infections with H. plataleae (Forrester, 1980; Telford Jr. et al., 1992; Stevenson and Anderson, 1994; Dorn et al., 2011). Prevalence and/or parasitemias were related to several factors including age, season, and year but no differences were noted based on sex, habitat type, or body condition.
Blood parasite diversity in the family Threskiornithidae, in particular the ibis, appears to be low. Only two species of Haemoproteus have been reported from the Threskiornithidae, H. plataleae and H. pelouroi. To date, no Leucocytozoon spp. have been reported from the Threskiornithidae, and an uncharacterized Plasmodium sp. has recently been reported from a single white ibis from Florida (Bryan et al., 2015). Haemoproteus pelouroi was first reported from the Hadada ibis (Bostrychia hagedash) and the African sacred ibis (Threskiornis aethiopicus) in 1946 from West Africa. Numerous morphologic characteristics of the gametocytes, even immature ones, can be used to distinguish H. pelouroi from H. plataleae, which was first described from India in a Eurasian spoonbill (Platalea leucorodia leucorodia) (de Mello, 1935). Although H. plataleae has been reported from numerous hosts around the world, Bennet et al. (1975) formally redescribed the species from the white ibis. Based on morphologic identification, other hosts include the scarlet ibis, saddle-billed stork (Ephippiorhynchus senegalensis), marabou stork (Leptoptilos crumeniferus), yellow-billed stork (Mycteria ibis), glossy ibis (Plegadis falcinellus), red-naped ibis (Pseudibis papillosa), and Australian white ibis (Threskiornis molucca) (Valkiunas, 2005). Thus, H. plataleae, based on morphology, has been reported from five continents, which is one of the widest distributions of any known blood parasite. However, only the scarlet ibis has been genetically confirmed to be infected with the same lineage we detected in the white ibis (Chagas et al., 2017). Thus, genetic characterization of H. plataleae from hosts in the Americas, Europe, Africa, Asia, and Australia is needed to determine if it is a species complex in this wide range of hosts.
Only one previous study has genetically characterized blood parasites in white ibis. In that study, Coker et al. (2017) only detected a single haplotype (hEUDRUB01) of H. plataleae that was subsequently reported from a captive scarlet ibis in a Brazilian zoo (it is unknown if this bird acquired the infection in Brazil or elsewhere) (Chagas et al., 2017). To date, this genotype has been found in several southern Florida counties in this study (Glades, Lee, Martin, and Palm Beach) and we have also detected it in Broward County (Yabsley, unpublished data); however, morphologically similar parasites have been found throughout Florida. White ibis are widespread in the southern United States, Central America, and northern South America, so ibis from these regions should be examined to determine if other species or haplotypes infect ibis in other regions. The lack of Plasmodium infection in any of the >600 ibis tested was surprising considering the positive ibis from Bryan et al. (2015) was from Palm Beach County, which is where most of the ibis in this study originated. Unfortunately, the sequence for this Plasmodium is not available so the species is not known. If Plasmodium infections do occur in ibis, the lack of detection in this study could be due to short-term detectable parasitemia for this Plasmodium sp. in ibis (although we sampled across multiple seasons and age classes) or rare transmission of this Plasmodium sp. to ibis in southern Florida. Additionally, ibis migrate so the Plasmodium-infected ibis could have acquired the infection at another site (Heath et al., 2020). Of note, several Plasmodium spp. have been reported in sympatric wading birds and many of these Plasmodium species have low host specificity, so changing habitat use by ibis may alter their parasite exposure in the future (Bensch et al., 2000; Coker et al., 2017).
The low parasitemia we detected supports that infection with H. plataleae is a chronic infection or, less likely, that reinfections are common (Forrester, 1980; Jarvi et al., 2003; Valkiunas, 2005). The only previous study that reported parasitemia was Coker et al. (2017), who reported data from only six white ibis of unknown age; parasitemias were also low (0.004–0.575%). Low level, chronic infections have historically been considered of little clinical consequence, but an increasing number of studies have associated chronic blood parasite infections with decreased survival and fecundity, poorer post-breeding body condition, hindered mate selection, and decreased life span due to telomere shortening (Wiehn et al., 1997; Dawson and Bortolotti, 2000; Sanz et al., 2001; Asghar et al., 2015). Therefore, it is important to understand factors associated with infection of white ibis with blood parasites, even if they have not been associated with acute clinical disease, although the latter has not been evaluated in chicks.
Age was a significant factor for both prevalence and parasitemia for H. plataleae. The prevalence was highest in adults, which is similar to data from Forrester (1980), Telford et al. (1992), Bryan et al. (2015), and Coker et al. (2017); however, these studies sampled nestlings whereas the juveniles that we sampled were older (after fledging). Forrester (1980) found that only four of 40 nestlings were positive, and infections were only noted at a single site. Similar to prevalence, parasitemias were also associated with age and were highest in juveniles, specifically the 1-yr-old and 2-yr-old groups. Combined, these data support the hypothesis that ibis become infected soon after leaving the nest, at which time they have relatively high parasitemias, then parasitemias decrease over time, and then after their first two years of life they have developed chronic infections or are frequently re-infected.
Season was also significantly associated with prevalence, with white ibis sampled in the spring being more likely to be infected than those sampled in the fall. Prevalence was also higher in the spring compared with summer. Similarly, parasitemia levels of H. plataleae were higher during the spring and summer compared to fall. The reason(s) for this increased prevalence and parasitemias are unknown but could be due to several factors. Hormone fluctuations, in particular testosterone levels, are often associated with increased infection risk; however, increased testosterone levels have not previously been linked to increased susceptibility to haemosporida in birds or reptiles (Buttemer and Astheimer, 2000; Oppliger et al., 2004). An increase in vector abundance during the breeding season may cause an increase in prevalence, but it could also be attributed to a decreased ability to clear infections due to the stress associated with reproduction that begins during the spring season (Allander, 1997; Tschirren et al., 2003; Garvin and Schoech, 2006). Furthermore, many species experience a phenomenon termed “spring relapses,” which is synonymous to a seasonal idiopathic increase in parasitemia, which could result in increased detection (Valkiunas, 2005).
In this study, similar to Forrester (1980), we did not find a difference in prevalence between male and female ibis sampled. In addition, we did not find a difference in parasitemias between the sexes. In general, there is most often a male bias in prevalence and/or parasitemia of blood parasites compared to females which is often attributed to differences in hormone levels, immunity, behavior, or other unknown factors (Zuk, 1996; Zuk and McKean, 1996). However, potential differences in blood parasite prevalence between the sexes are highly variable by host-parasite system. For example, a high prevalence of Haemoproteus infection has been reported in male ruby-crowned kinglets (Regulus calendula), white-crowned sparrows (Zonotrichia leucophrys), and Wilson's warblers (Cardellina pusilla) (Rodriguez et al., 2021), but the opposite has been reported for female house sparrows (Passer domesticus) (Bichet et al., 2014), and there are numerous studies that failed to find differences in prevalence between the sexes (Astudillo et al., 2013; Popescu et al., 2020).
The long-term consequences of urbanization of white ibis populations have only begun to be evaluated. One of our major ongoing research questions is how the use of urban environments impacts the risk of parasite and vector exposure for ibis and, ultimately, for sympatric bird species. Ibis have only relatively recently begun to use urban parks and suburban neighborhoods for foraging and possibly breeding. One of our initial objectives was to use parasite assemblages or specific parasite species as ‘biological tags’ to determine where ibis may be foraging, roosting or breeding, similar to what has been done with parasites of fish (Catalano et al., 2014; Gagne et al., 2022). Although the only parasite we detected was H. plataleae and infection status was not associated with habitat use, Teitelbaum et al. (2020) found that using GPS tracking data from ibis during their non-breeding season, urban-urban and natural-natural connections were strong and that ibis using urban sites had the least-variable habitat use. They also found that a few ibis used both habitats, suggesting that ibis were beginning to specialize in urban vs. natural habitat use, which may result in altered pathogen and parasite exposure in the future. For example, urban or suburban environments may have increased or decreased numbers of vectors that may impact pathogen transmission (e.g., West Nile virus, Seixas et al., 2022). Specifically, H. plataleae, a member of the Parahaemoproteus subgenus, is likely transmitted by biting midges (Culicoides spp.), which vary significantly between habitat types (van Hoesel et al., 2020). Finally, because ibis roost and breed in multi-species colonies that include some species of conservation concern (e.g., wood storks (Mycteria americana), roseate spoonbills, and others), another unknown is the importance of ibis as maintenance hosts of H. plataleae to sympatric birds. Besides the related scarlet ibis in Brazil, Coker et al. (2017) found a single infection in a green heron (Butorides virescens), but the outcome of this infection is unknown. Surveillance for this parasite in these sympatric species, especially chicks who are often more susceptible to disease, is warranted.
Data availability
All relevant data are within the paper and its Supporting Information files.
Declaration of conflicting interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing, we confirm that we have followed the regulations of our institutions concerning intellectual property. We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from myabsley@uga.edu.
Acknowledgements
We thank Dorren Chaussadas, Sarah Dean, and R. Ethan Cooper for assistance in field captures and sampling of ibis. We truly appreciate the cooperation and assistance of staff at the various collection sites, the towns for permission and help in accessing urban ibises, and the participation of several private landowners that allowed sampling. This work was primarily supported by the National Science Foundation, Ecology, and Evolution of Infectious Diseases (grant number DEB-1518611). Additional financial support was provided by the American Association of Zoo Veterinarians to SMH, the Arnett C. Mace Jr. Distinguished Professorship through the Warnell School of Forestry and Natural Resources, the wildlife management agencies of the Southeastern Cooperative Wildlife Disease Study member states through the Federal Aid to Wildlife Restoration Act (50 Stat. 917), and SCWDS federal agency partners including the United States Geological Survey Ecosystems Mission Area and the United States Fish and Wildlife Service National Wildlife Refuge System. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Adams H., Murray M.H., Welch C., Kidd-Weaver A., Ellison T., Curry S., Hepinstall‐Cymerman J., Hernandez S.M. Capturing American white ibises in urban South Florida using two novel techniques. J. Field Ornithol. 2019;90:373–381. [Google Scholar]
- Allander K. Reproductive investment and parasite susceptibility in the Great Tit. Funct. Ecol. 1997;11:358–364. [Google Scholar]
- Asghar M., Hasselquist D., Hansson B., Zehtindjiev P., Westerdahl H., Bensch S. Chronic infection. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science. 2015;347:436–438. doi: 10.1126/science.1261121. [DOI] [PubMed] [Google Scholar]
- Astudillo V.G., Hernandez S.M., Kistler W.M., Boone S.L., Lipp E.K., Shrestha S., Yabsley M.J. Spatial, temporal, molecular, and intraspecific differences of haemoparasite infection and relevant selected physiological parameters of wild birds in Georgia, USA. Int. J. Parasitol. Parasites Wildl. 2013;2:178–189. doi: 10.1016/j.ijppaw.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G.F., Greiner E.C., Campell A.B. Avian haemoproteidae 5. The haemoproteids of the family Threskiornithidae. Can. J. Zool. 1975;53:634–638. doi: 10.1139/z75-076. [DOI] [PubMed] [Google Scholar]
- Bensch S., Stjernman M., Hasselquist D., Ústman Ú., Hansson B., Westerdahl H., Pinheiro R.T. Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. Roy. Soc. Lond. B. 2000;267:1583–1589. doi: 10.1098/rspb.2000.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch S., Hellgren O., Pérez-Tris J. MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour. 2009;9:1353–1358. doi: 10.1111/j.1755-0998.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- Bichet C., Sorci G., Robert A., Julliard R., Lendvai A.Z., Chastel O., Garnier S., Loiseau C. Epidemiology of Plasmodium relictum infection in the house sparrow. J. Parasitol. 2014;100:59–65. doi: 10.1645/12-24.1. [DOI] [PubMed] [Google Scholar]
- Boal C.W., Mannan R.W. Comparative breeding ecology of cooper's hawks in urban and exurban areas of southeastern Arizona. J. Wildl. Manag. 2014;63:77–84. [Google Scholar]
- Bryan A.L., Jr., Love C.N., Mills G.L., Borkhataria R.R., Lance S.L. Testing for associations between hematozoa infection and mercury in wading bird nestlings. J. Wildl. Dis. 2015;51:222–226. doi: 10.7589/2013-12-332. [DOI] [PubMed] [Google Scholar]
- Buttemer W.A., Astheimer L.B. Testosterone does not affect basal metabolic rate or blood parasite load in captive male white-plumed honeyeaters Lichenostomus penicillatus. J. Avian Biol. 2000;31:479–488. [Google Scholar]
- Catalano S.R., Whittington I.D., Donnellan S.C., Gillanders B.M. Parasites as biological tags to assess host population structure: guidelines, recent genetic advances and comments on a holistic approach. Int. J. Parasitol. Parasites Wildl. 2014;3:220–226. doi: 10.1016/j.ijppaw.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagas C.R., Valkiunas G., de Oliveira Guimaraes L., Monteiro E.F., Guida F.J., Simoes R.F., Rodrigues P.T., de Albuquerque Luna E.J., Kirchgatter K. Diversity and distribution of avian malaria and related haemosporidian parasites in captive birds from a Brazilian megalopolis. Malar. J. 2017;16:83. doi: 10.1186/s12936-017-1729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahad-Ehlers S., Fushita A.T., Lacorte G.A., Assis P.C.P., Del Lama S.N. Effects of habitat suitability for vectors, environmental factors and host characteristics on the spatial distribution of the diversity and prevalence of haemosporidians in waterbirds from three Brazilian wetlands. Parasites Vectors. 2018;11:276. doi: 10.1186/s13071-018-2847-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker S.M., Hernandez S.M., Kistler W.M., Curry S.E., Welch C.N., Barron H.W., Harsch S., Murray M.H., Yabsley M.J. Diversity and prevalence of hemoparasites of wading birds in southern Florida, USA. Int. J. Parasitol. Parasites Wildl. 2017;6:220–225. doi: 10.1016/j.ijppaw.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet S., Bichet C., Lacombe S., Faivre B., Sorci G. Impact of host nutritional status on infection dynamics and parasite virulence in bird-malaria system. J. Anim. Ecol. 2014;83:256–265. doi: 10.1111/1365-2656.12113. [DOI] [PubMed] [Google Scholar]
- Cummings C.R., Hernandez S.M., Murray M., Ellison T., Adams H.C., Cooper R.E., Curry S., Navara K.J. Effects of an anthropogenic diet on indicators of physiological challenge and immunity of white ibis nestlings raised in captivity. Ecol. Evol. 2020;10:8416–8428. doi: 10.1002/ece3.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R.D., Bortolotti G.R. Effects of hematozoan parasites on condition and return rates of American kestrels. Auk. 2000;117:373–380. [Google Scholar]
- de Mello I.F. New haemoproteids of some Indian birds. Proc. Natl. Acad. Sci. India B. 1935:460–475. [Google Scholar]
- de Mello I.F. On haemoprotozoa of Indian birds. C.R. 12th Internatl. Congr. Zool. 1937;2:1391–1445. [Google Scholar]
- Delahay R.J., Smith G.C., Hutchings M.R. In: Management of Disease in Wild Mammals. Delahay R.J., Smith G.C., Hutchings M.R., editors. Springer; Tokya: 2009. The science of wildlife disease management; pp. 1–8. [Google Scholar]
- Dorn N.J., Cook M.I., Herring G., Boyle R.A., Nelson J., Gawlik D.E. Aquatic prey switching and urban foraging by the white ibis Eudocimus albus are determined by wetland hydrological conditions. Ibis. 2011;153:323–335. [Google Scholar]
- Evans K.L., Gaston K.J., Sharp S.P., McGowan A., Simeoni M., Hatchwell B.J. Effects of urbanisation on disease prevalence and age structure in blackbird Turdus merula populations. Oikos. 2009;118:774–782. [Google Scholar]
- Forrester D.J. Hematozoa and mallophaga from the white ibis, Eudocimus albus L., in Florida. J. Parasitol. 1980;66:58. [PubMed] [Google Scholar]
- Gagne R.B., Crooks K.R., Craft M.E., Chiu E.S., Fountain-Jones N.M., Malmberg J.L., Carver S., Funk W.C., VandeWoude S. Parasites as conservation tools. Conserv. Biol. 2022;36 doi: 10.1111/cobi.13719. [DOI] [PubMed] [Google Scholar]
- Garvin M.C., Schoech S.J. Hormone levels and infection of Haemoproteus danilewskyi in free-ranging blue jays (Cyanocitta cristata) J. Parasitol. 2006;92:659–662. doi: 10.1645/GE-759R.1. [DOI] [PubMed] [Google Scholar]
- Godfrey R.D., Jr., Fedynich A.M., Pence D.B. Quantification of hematozoa in blood smears. J. Wildl. Dis. 1987;23:558–565. doi: 10.7589/0090-3558-23.4.558. [DOI] [PubMed] [Google Scholar]
- Griffiths R., Double M.C., Orr K., Dawson R.J. A DNA test to sex most birds. Mol. Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- Heath J.A., Frederick P., Kushlan J., Bildstein K. In: Birds of the World. Poole A.F., editor. Cornell Lab of Ornithology; Ithaca, NY, USA: 2020. White ibis (Eudocimus albus), version 1.0. [Google Scholar]
- Heath J.A., Frederick P.C. Trapping White Ibises with rocket nets and mist nets in the Florida Everglades. J. Field Ornithol. 2003;74:187–192. [Google Scholar]
- Heath J.A., Fredrick P.C. White ibis integument color during the breeding season. J. Field Ornithol. 2006;77:141–150. [Google Scholar]
- Hellgren O., Waldenstrom J., Bensch S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004;90:797–802. doi: 10.1645/GE-184R1. [DOI] [PubMed] [Google Scholar]
- Hernandez S.M., Welch C.N., Peters V.E., Lipp E.K., Curry S., Yabsley M.J., Sanchez S., Presotto A., Gerner-Smidt P., Hise K.B., Hammond E., Kistler W.M., Madden M., Conway A.L., Kwan T., Maurer J.J. Urbanized white ibises (Eudocimus albus) as carriers of Salmonella enterica of significance to public health and wildlife. PLoS One. 2016;11 doi: 10.1371/journal.pone.0164402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvi S.I., Farias M.E.M., Baker H., Freifeld H.B., Baker P.E., Gelder E., Massey J.G., Atkinson C.T. Detection of avian malaria (Plasmodium spp.) in nativeland birds of American Samoa. Conserv. Genet. 2003;4:629–637. [Google Scholar]
- Kidd-Weaver A., Hepinstall-Cymerman J., Welch C.N., Murray M.H., Adams H.C., Ellison T.J., Yabsley M.J., Hernandez S.M. The movements of a recently urbanized wading bird reveal changes in season timing and length related to resource use. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler W.M., Hernandez S.M., Gibbs S.E., Ballard J.R., Arnold S.L., Johnson T., Yabsley M.J. Evaluation of a restriction fragment length enzyme assay for differentiation of Haemoproteus and Plasmodium across a standard region of the mitochondrial genome. J. Parasitol. 2013;99:1133–1136. doi: 10.1645/13-211.1. [DOI] [PubMed] [Google Scholar]
- Murray M.H., Hernandez S.M., Rozier R.S., Kidd A.D., Hepinstall-Cymerman J., Curry S.E., Yabsley M.J., Adams H., Ellison T., Welch C.N., Lipp E.K. Site fidelity is associated with food provisioning and Salmonella in an urban wading bird. EcoHealth. 2021;18:345–358. doi: 10.1007/s10393-021-01543-x. [DOI] [PubMed] [Google Scholar]
- Murray M.H., Kidd A.D., Curry S.E., Hepinstall-Cymerman J., Yabsley M.J., Adams H.C., Ellison T., Welch C.N., Hernandez S.M. From wetland specialist to hand-fed generalist: shifts in diet and condition with provisioning for a recently urbanized wading bird. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373 doi: 10.1098/rstb.2017.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppliger A., Giorgi M.S., Conelli A., Nembrini M., John-Alder H.B. Effect of testosterone on immunocompetence, parasite load, and metabolism in the common wall lizard (Podarcis muralis) Can. J. Zool. 2004;82:1713–1719. [Google Scholar]
- Popescu M., Trychta M., Jackson E., Selman J., Houston A., Collins M. Avian haemosporidian prevalence and its relationship to host traits in Western Tennessee. J. Ornithol. 2020;161:995–1010. [Google Scholar]
- Rodriguez M.D., Doherty P.F., Piaggio A.J., Huyvaert K.P. Sex and nest type influence avian blood parasite prevalence in a high-elevation bird community. Parasites Vectors. 2021;14:145. doi: 10.1186/s13071-021-04612-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz J.J., Arriero E., Moreno J., Merino S. Female Hematozoan infection reduces hatching success but not fledging success in Pied Flycatchers Ficedula hypoleuca. Auk. 2001;118:750–755. [Google Scholar]
- Seixas J.S., Hernandez S.M., Kunkel M.R., Weyna A.A.W., Yabsley M.J., Shender L., Nemeth N.M. West nile virus infections in an urban colony of American white ibises (Eudocimus albus) in South Florida, USA. J. Wildl. Dis. 2022;58:205–210. doi: 10.7589/JWD-D-21-00030. [DOI] [PubMed] [Google Scholar]
- Stevenson H.M., Anderson B.H. University Press of Florida; Gainesville: 1994. Birdlife of Florida. [Google Scholar]
- Teitelbaum C.S., Hepinstall-Cymerman J., Kidd-Weaver A., Hernandez S.M., Altizer S., Hall R.J. Urban specialization reduces habitat connectivity by a highly mobile wading bird. Mov. Ecol. 2020;8:49. doi: 10.1186/s40462-020-00233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford S.R., Jr., Spalding M.G., Forrester D.J. Hemoparasites of wading birds (Ciconiiformes) in Florida. Can. J. Zool. 1992;70:1397–1408. [Google Scholar]
- Tschirren B., Fitze P.S., Richner H. Sexual dimorphism in susceptibility to parasites and cell-mediated immunity in great tit nestlings. J. Anim. Ecol. 2003;72:839–845. [Google Scholar]
- Valkiunas G. CRC Press; Boca Raton, Florida: 2005. Avian Malaria Parasites and Other Haemosporidia. [Google Scholar]
- Valkiunas G., Iezhova T.A. Keys to the avian Haemoproteus parasites (haemospirida, haemoproteidae) Malar. J. 2022;21:269. doi: 10.1186/s12936-022-04235-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoesel W., Santiago-Alarcon D., Marzal A., Renner S.C. Effects of forest structure on the interaction between avian hosts, dipteran vectors and haemosporidian parasites. BMC Ecol. 2020;20:47. doi: 10.1186/s12898-020-00315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldenstrom J., Bensch S., Hasselquist D., Ostman O. A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J. Parasitol. 2004;90:191–194. doi: 10.1645/GE-3221RN. [DOI] [PubMed] [Google Scholar]
- Wiehn J., Korpimáki E., Bildstein K.L., Sorjonen J. Mate choice and reproductive success in the American Kestrel: a role for blood parasites? Ethology. 1997;103:304–317. [Google Scholar]
- Zuk M. Disease, endocrine–immune interactions, and sexual selection. Ecology. 1996;77:1037–1042. [Google Scholar]
- Zuk M., McKean K.A. Sex differences in parasite infections: patterns and processes. Parasitol. Int. 1996;26:1009–1023. [PubMed] [Google Scholar]
- Zylberberg M., Lee K.A., Klasing K.C., Wikelski M. Variation with land use of immune function and prevalence of avian pox in Galapagos finches. Conserv. Biol. 2013;27:103–112. doi: 10.1111/j.1523-1739.2012.01944.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.