Abstract
Degranulation of peripheral blood polymorphonuclear leukocytes (PMNLs) was monitored in human immunodeficiency virus (HIV) type 1 (HIV-1)-infected individuals with or without pulmonary tuberculosis (HIV/TB and HIV groups, respectively) by measuring the release of β-glucuronidase induced by interleukin-8 (IL-8). This was increased in a dose-dependent manner in the control groups consisting of healthy blood donors and patients with pulmonary tuberculosis. In contrast, PMNLs from the HIV and HIV/TB groups responded reciprocally in the same assay; that is, higher IL-8 input concentrations resulted in the release of less enzyme than lower IL-8 input concentrations. The degranulation response of PMNLs from HIV-1-infected individuals was similarly altered for another agonist, N-formyl-methionyl-leucyl-phenylalanine, suggesting that impairment of the nonoxidative armature of PMNL was a more generalized phenomenon. However, impaired IL-8-induced degranulation was found to be associated with the reduced expression of both IL-8 receptors, A and B, on whole-blood PMNLs from HIV-1-infected patients compared with that on whole-blood PMNLs from healthy persons. The density of IL-8RA, in particular, was most reduced on the surfaces of PMNLs from those patients with the poorest degranulation in response to IL-8. Inefficient agonist-induced degranulation may contribute to the increased susceptibility of HIV-1-infected persons to secondary microbial infections, this being further exacerbated in HIV/TB patients who, in addition, display defects in phagocytosis and oxidative burst.
Polymorphonuclear neutrophils are key effector cells in host defense and as a result of infection or tissue injury are recruited to sites of inflammation in large numbers from the bloodstream (26). The five principal neutrophil chemotactic factors are CXC-chemokine interleukin-8 (IL-8), N-formyl-methionyl-leucyl-phenylalanine (fMLP), platelet-activating factor, anaphylotoxin C5a, and leukotriene B4 (LTB4), and in addition to being involved in the extravasion of neutrophils, these agents act in conjunction with other cytokines and chemotactic agonists in the priming of neutrophils to respond more effectively once they are at the site of inflammation (4, 6, 17, 30). Opsonized microorganisms are avidly ingested by neutrophils and are killed by an oxygen-independent mechanism, which is characterized by the production of toxic reactive oxygen intermediates, and an oxygen-dependent mechanism, which involves the action of potent antimicrobial polypeptides contained within cytoplasmic granules (15), which are released into phagolysosomes. Primary granules, also referred to as azurophilic granules (5), contain a number of antimicrobial effector proteins and hydrolases such as elastase, collagenase, and β-glucuronidase which disrupt microbial functions or structural components.
Patients infected with human immunodeficiency virus (HIV) type 1 (HIV-1) display a variety of immune abnormalities, including various defects in the microbicidal responses of phagocytic cells. These abnormalities could contribute to the impaired host defense against the various opportunistic pathogens that characterize AIDS. Infection with HIV-1 has been shown to be the largest known risk factor for the development of tuberculosis (22), and individuals infected with both HIV-1 and Mycobacterium tuberculosis also have an increased risk of acquiring new opportunistic infections (34). In addition to tuberculosis, opportunistic infections frequently found in HIV-1-infected patients include those caused by bacterial pathogens such as Streptococcus pneumoniae, Salmonella spp., and Pseudomonas aeruginosa, fungal infections such as cryptococcal meningitis and Pneumocystis carinii pneumonia, and infections caused by parasitic pathogens such as Toxoplasma gondii (10).
Functional defects have been observed in neutrophils from HIV-1-infected patients and include defects in phagocytosis (13), chemotaxis (8, 19, 32), bacterial killing (8, 21), and oxidative burst (1, 24). We have previously reported on the enhanced ability of whole-blood polymorphonuclear leukocytes (PMNLs) from HIV-1-infected individuals to phagocytose Escherichia coli and on the unaltered oxidative burst of PMNLs in response to E. coli as an agonist. Both of these functions, on the other hand, were significantly depressed in patients with pulmonary tuberculosis with or without concurrent HIV-1 infection (29). In a study by Wenisch et al. (33), neutrophils from HIV-1-infected individuals were shown to have an inability to kill Candida spp., despite enhanced phagocytosis and unimpaired oxidative burst. These results suggest that defective microbial killing by neutrophils from HIV-1-infected individuals is likely to be the result of an ineffective nonoxidative defense armature. In order to test this hypothesis, we measured the degranulation responses of PMNLs from HIV-1-infected individuals in response to IL-8, a CXC-chemokine essential to a number of PMNL antimicrobial functions. Furthermore, we sought to determine if impaired responses to IL-8 were associated with reduced expression of either one or both of the IL-8 receptors A (CXCR-1) and B (CXCR-2) on PMNLs in a way similar to that which we have previously described for calcium mobilization and chemotaxis (19).
MATERIALS AND METHODS
Reagents.
Recombinant human IL-8 was obtained from Boehringer Mannheim (Mannheim, Germany). fMLP, cytochalasin B, p-nitrophenyl-β-d-glucuronide, and Histopaque-Ficoll were from Sigma Chemical Co. (St. Louis, Mo.). Mouse monoclonal antibodies to IL-8RA (9H1) and IL-8RB (10H2) were supplied by Genentech, Inc., San Francisco, Calif. (2). Mouse immunoglobulin G (IgG) antibodies of the IgG1 and IgG2a isotypes were from Serotec (Oxford, England) and were used as controls for IL-8RA and IL-8RB, respectively. Secondary antibody was fluorescein isothiocyanate-conjugated goat anti-mouse (GAM-FITC) antibody obtained from Dako (Denmark). FACS lysing solution (10× concentrate) was from Becton Dickinson (San Jose, Calif.). The cell fixative was 1.5% (vol/vol) formaldehyde (Merck, Darmstadt, Germany) with 2% (wt/vol) bovine serum albumin (Sigma Chemical Co.).
Patient samples.
Subjects in four groups were recruited for studies on PMNL degranulation and included seven healthy blood donors (ND group), 11 patients with pulmonary tuberculosis (TB group), 11 HIV-1-seropositive patients (HIV group), and 9 individuals coinfected with HIV-1 and M. tuberculosis (HIV/TB group). A further 6 patients were recruited in the ND group and a further 12 patients were recruited in the HIV/TB group for the concurrent analysis of each of the IL-8 receptors on whole-blood PMNLs by flow cytometry and functional analysis of purified PMNLs by the β-glucuronidase assay. There were approximately equivalent numbers of male and female subjects in each group, and all were between 25 and 45 years of age. All patients in the TB and HIV/TB groups had received standard anti-TB treatment for between 6 weeks and 4 months. None of the patients in the HIV and HIV/TB groups had received any antiretroviral therapy. Blood was collected by venipuncture and placed into Vacutainer tubes (Becton Dickinson) containing EDTA. The blood was processed immediately for assays of PMNL function and was analyzed by flow cytometry within 6 h of collection. This study was approved by the University of the Witwatersrand Ethical Committee, and patients were recruited after informed consent had been obtained and the confidentiality of all records had been ensured.
Separation of neutrophils.
Anticoagulated blood was centrifuged at 200 × g for 10 min at room temperature, and the plasma was removed. PMNLs were isolated from buffy coats by first centrifuging phosphate-buffered saline (PBS)-diluted whole blood (1:1) on a primary Histopaque-Ficoll gradient at 1,000 × g for 30 min at room temperature. After removal of the mononuclear cell layer, the remaining Ficoll and PMNL layers were layered onto a secondary Ficoll gradient and centrifuged as described above. The PMNL layer was then removed, and the residual erythrocytes were lysed with a solution of 0.15 M NH4Cl–10 mM KHCO3–1 mM sodium EDTA (pH 7.2). Purified PMNL suspensions were >98% viable as determined by trypan blue exclusion.
β-Glucuronidase assay.
PMNL degranulation was measured in response to IL-8 by the release of β-glucuronidase as described by Schröder et al. (28). Briefly, the PMNL concentration was adjusted to 107/ml, and cytochalasin B was added to a final concentration of 5 μg/ml. Aliquots of 100 μl of the cell suspension were placed in a 96-well round-bottom plate, and the plate was incubated for 15 min at 37°C. The human IL-8 test samples (at twofold serial dilutions for dose-response curves [final concentrations, 7.8 to 500 ng/ml] or at one low [15.63 ng/ml] and one high [500 ng/ml] input concentration), each in a total volume of 100 μl, were added to separate wells, and the plate was incubated for a further 30 min at 37°C. The cells were then pelleted at 1,000 rpm (Sorvall H1000B rotor) for 10 min at 4°C, and 100 μl of the supernatant was transferred to the wells of a 96-well flat-bottom plate containing 100 μl of 0.01 M p-nitrophenyl-β-d-glucuronide in 0.1 M sodium acetate (pH 4.0). The plate was incubated overnight at 20°C, the reaction was stopped with 100 μl of 0.4 M glycine buffer (pH 10), and the absorbance was read at 405 nm. For the determination of total β-glucuronidase content in PMNLs, 5 × 105 and 1 × 106 cells were lysed in 100 μl of 0.4% (vol/vol) Triton X-100–PBS. The release of β-glucuronidase at different IL-8 concentrations was calculated as the optical density at 405 nm (OD405) obtained at a particular IL-8 concentration divided by the total OD405 of the PMNL lysate and expressed as a percentage.
Fluorescence labelling of PMNLs in whole blood.
Staining was performed in whole blood from study subjects as described previously (19). Briefly, 5 μl of appropriately diluted IL-8RA or IL-8RB antibody was added to 50 μl of whole blood (final concentration, 2.5 μg/ml). Control antibodies were mouse IgG1 or IgG2a, respectively. The samples were then incubated with the primary antibodies for 20 min at room temperature and washed twice with 3 ml of wash solution. A total of 5 μl of GAM-FITC was then added to each of the samples, which were again incubated for 20 min at room temperature. Samples were then washed, the erythrocytes were lysed with 2 ml of 1× FACS lysing solution, and washed again, and the cells were resuspended in 200 μl of fixative.
Flow cytometry.
A Becton Dickinson FACSort flow cytometer with a 488-nm argon laser was used for all analyses. Forward light scatter (FSC) and side light scatter (SSC) characteristics were used in the gating of the granulocyte population. The data were analyzed with Cellquest, version 1.0, software (Becton Dickinson) and were expressed as the percentage of cells expressing IL-8RA or IL-8RB and their respective fluorescence intensities or median channel shift values (median channel number for the sample stained with IL-8 receptor antibodies minus the median channel number for the corresponding isotype antibody control sample).
Statistical analysis.
Comparison of IL-8RA and IL-8RB percentages, fluorescence intensities, immunological parameters, and degranulation capacities between groups was done by use of the nonparametric Mann-Whitney U test. For paired analyses the Wilcoxon ranks sum test was applied. Spearman rank correlations were applied when comparing data within groups.
RESULTS
PMNL β-glucuronidase response to IL-8.
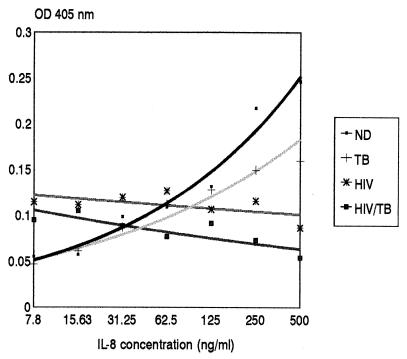
The exocytosis of primary granules, and hence β-glucuronidase, from cytochalasin-treated PMNLs occurs in response to IL-8 in a dose-dependent manner. The release of β-glucuronidase in response to twofold dilutions of IL-8 and in response to PBS (control for spontaneous release) was determined for PMNLs isolated from the peripheral blood of four study groups: 7 individuals in the ND group, 11 individuals in the TB group, 11 individuals in the HIV group, and 9 individuals in the HIV/TB group. The immunological characteristics of these patient groups are presented in Table 1. The IL-8 dose-response graphs, determined as mean OD405 values for all individuals, were similar for the ND and TB groups (Fig. 1). PMNLs from patients in the HIV and HIV/TB groups, however, showed a nearly reciprocal relationship between IL-8 concentration and the amount of β-glucuronidase released, in that increasing IL-8 concentrations resulted in decreased enzyme release.
TABLE 1.
Immunological characteristics of study groupsa
| Characteristic | Study group
|
||
|---|---|---|---|
| TB | HIV | HIV/TB | |
| No. of subjects | 11 | 9 | 11 |
| Leukocyte count (103/μl) | 10.3 ± 0.9 | 4.7 ± 0.5 | 6.1 ± 0.6 |
| Granulocytes | |||
| Count (103/μl) | 6.2 ± 1.2 | 2.2 ± 0.2 | 3.2 ± 0.4 |
| Percentage of cells | 64.6 ± 2.5 | 48.4 ± 4.8 | 55.7 ± 4.1 |
| CD4 cells | |||
| Count (cells/μl) | 776 ± 115 | 412 ± 109 | 286 ± 76 |
| Percentage of cells | 42.2 ± 2.5 | 17.6 ± 2.9 | 15.3 ± 2.4 |
| CD8 cells | |||
| Count (cells/μl) | 565 ± 75 | 1,524 ± 248 | 994 ± 126 |
| Percentage of cells | 34.5 ± 1.2 | 53.5 ± 3.5 | 61.7 ± 2.1 |
| CD4:CD8 | 1.29 ± 0.12 | 0.38 ± 0.09 | 0.26 ± 0.05 |
Results are expressed as the mean ± standard error of the mean. Percentile P25 and P75 values for healthy adults (as absolute counts and percentages, respectively) were as follows: for leukocytes, 3.6 to 10.0; for granulocytes, 1.4 to 6.5 and 42.2 to 75.2; for CD4 cells, 700 to 1,100 and 38 to 46; for CD8 cells, 500 to 900 and 31 to 40; for CD4:CD8, 1.0 to 1.5.
FIG. 1.
Induced release of β-glucuronidase from PMNLs in response to different doses of IL-8. PMNLs were isolated from whole blood from subjects in the ND (n = 7), TB (n = 11), HIV (n = 11), and HIV/TB (n = 9) study groups and were assayed immediately for the release of β-glucuronidase as described in Materials and Methods. The OD450 values represent enzyme release that is due to IL-8, i.e., the total OD450 at a particular IL-8 input concentration minus the OD450 obtained for the unstimulated control. Results are expressed as the mean OD405 at each IL-8 concentration for each group of individuals.
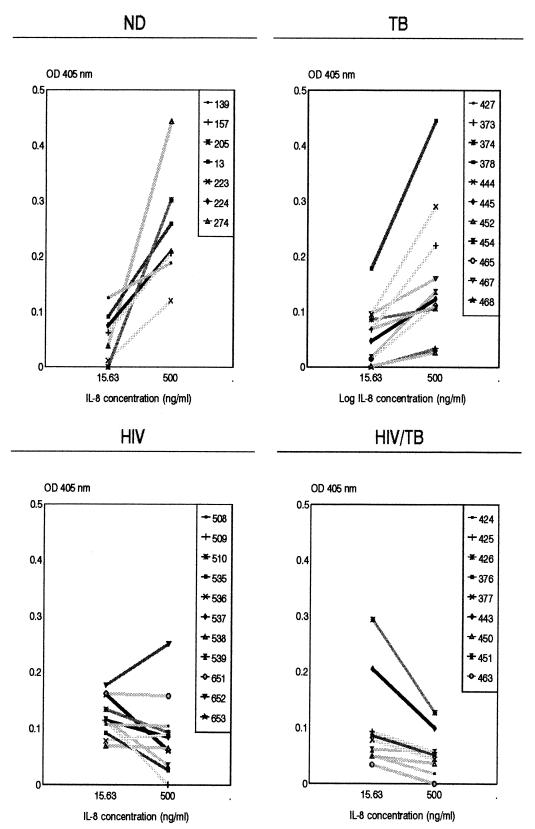
Figure 2 shows the β-glucuronidase released with a low (15.63 ng/ml) and a high (500 ng/ml) IL-8 input concentration for all the same individuals in each of the four groups whose data are presented in Fig. 1 in order to demonstrate the patterns of the individual responses. PMNLs from 7 of the 11 HIV patients in the HIV group showed a reciprocal response to IL-8, whereas patients 508, 651, and 538 had flat responses, and patient 652 had a positive-slope dose-response graph, as found for the ND group. Interestingly, the latter patient had a CD4 T cell count of only 53 cells/μl. Overall, there was no relationship between absolute CD4 T-cell counts and either the type of response or the magnitude of enzyme release obtained in the HIV or the HIV/TB group. When the results within the groups obtained with the 15.63- and 500-ng/ml IL-8 concentrations are compared, the amount of β-glucuronidase released with 500 ng/ml was significantly higher than the amount released with 15.63 ng/ml for the ND (P < 0.02) and TB (P < 0.01) groups, whereas for both the HIV and HIV/TB groups, for which the overall response was reciprocal, the release of β-glucuronidase was significantly lower with 500 ng of IL-8 per ml than 15.63 ng of IL-8 per ml (P < 0.05). When the results between the groups obtained with the different IL-8 concentrations are compared, significant differences in the release of enzyme in response to 15.63 ng IL-8 per ml were obtained between the ND and HIV groups (P < 0.05) and the TB and HIV groups (P < 0.01). With 500 ng of IL-8 per ml, significant differences were observed between the ND and HIV groups (P < 0.01), the ND and HIV/TB groups (P < 0.01), the TB and HIV groups (P = 0.05), and the TB and HIV/TB groups (P < 0.02).
FIG. 2.
β-Glucuronidase released with a low and a high input concentration of IL-8 and degranulation responses for each individual within each of the four study groups described in the legend to Fig. 1. Results are the OD450 values due to IL-8 only, i.e., the amount of enzyme released by unstimulated controls are subtracted from the total amount of enzyme released.
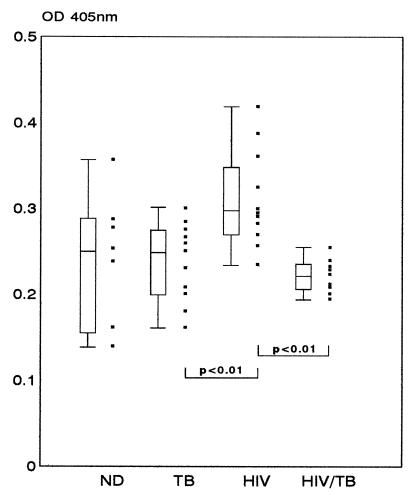
In order to determine if there was any difference in the ability of PMNLs to spontaneously degranulate as a result of disease, we compared the amount of β-glucuronidase released from PMNLs from the different groups in the absence of any stimulus (Fig. 3). This was significantly increased in the HIV group compared to that in the TB group (P < 0.01) and that in the HIV/TB group (P < 0.001). Although not significant (P > 0.05), there was a definite trend toward an increase in the release of enzyme in the HIV group relative to that in the ND group. The presence of IL-8 significantly increased the amount of enzyme released above that found for the corresponding PBS controls at a concentration of 15.63 ng of IL-8 per ml for the ND (P = 0.05) and TB (P < 0.01) groups and a concentration of 500 ng of IL-8 per ml for the HIV (P < 0.01) and HIV/TB (P < 0.01) groups (data not shown).
FIG. 3.
Spontaneous release of β-glucuronidase from PMNLs from patients in the ND, TB, HIV, and HIV/TB study groups. The amount of β-glucuronidase released from PMNLs in the absence of any stimulus (PBS controls) was determined for each of the study groups, as for Fig. 1 and 2. Solid squares, individual values; error bars, 10th and 90th percentiles. Boxes represent values between the 25th and 75th percentiles, with the median indicated. Significant differences between groups are indicated.
PMNL β-glucuronidase response to FMLP.
Because degranulation of PMNLs from HIV-1-infected individuals was clearly impaired in response to IL-8, we questioned whether this was specifically an IL-8-dependent phenomenon or whether impairment of degranulation was a more generalized phenomenon of HIV-1 infection. We therefore tested, using the same assay system described above, the ability of PMNLs from HIV-1-infected individuals to release β-glucuronidase in response to another agonist, fMLP. Degranulation in response to fMLP (concentration range, 10−6 to 10−9 M) showed results similar to those found for IL-8 in HIV-1-infected patients (data not shown).
Reduced IL-8-induced degranulation is not the result of reduced levels of β-glucuronidase in primary granules.
Because the level of IL-8-induced release of β-glucuronidase from PMNLs from HIV-1-infected individuals was decreased relative to the level released from PMNLs from healthy controls, we questioned whether PMNLs from HIV-1-infected individuals contained fewer granules, perhaps due to altered PMNL maturation in infected patients rather than an altered ability to exocytose granule contents. This we determined by calculating the amount of β-glucuronidase released by PMNLs when they were induced with IL-8 as a proportion of the total amount present in 106 PMNLs. PMNLs from healthy individuals released only 20 to 34% of their total available β-glucuronidase with the highest IL-8 concentration (500 ng/ml) used in this study. On the other hand, by use of the concentration of IL-8 which allowed maximum enzyme release in HIV-1-infected individuals (15.63 ng/ml), the mean percentage of enzyme released calculated for 20 patients in the HIV/TB group was 15.8% and ranged from 10.6 to 23% (data not shown). Thus, the reduced release of enzyme from PMNLs from HIV-1-infected individuals was not due to a limited β-glucuronidase content in primary granules.
Relationship between impaired degranulation and IL-8 receptor expression.
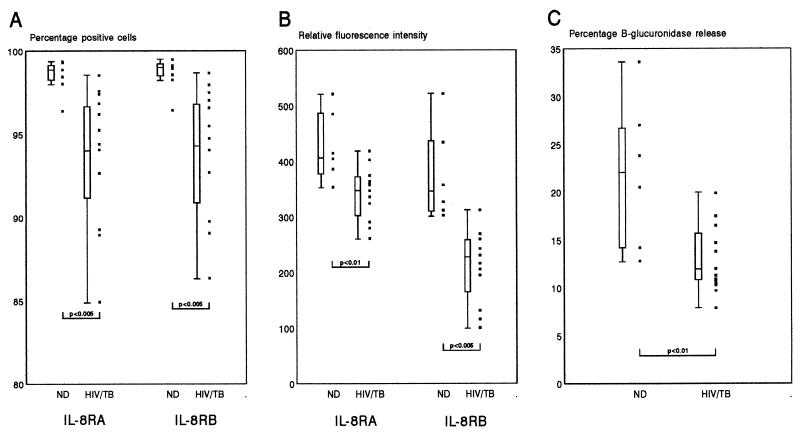
We have previously shown that the expression of both IL-8 receptors is downregulated on PMNLs from HIV-1-infected individuals (19). Because IL-8 mediates its activities through these receptors, we compared IL-8RA and IL-8RB expression on PMNLs and their subsequent degranulation ability in response to IL-8. Figure 4 shows a composite of the results obtained from a comparison of IL-8R expression and subsequent degranulation capacity in response to IL-8 for a group of 6 individuals in the ND group and 12 individuals in the HIV/TB group. Impaired degranulation of PMNLs from the HIV/TB group, measured as percent release of β-glucuronidase in response to 500 ng of IL-8 per ml, was associated with significant decreases both in the proportions of IL-8RA- and IL-8RB-expressing PMNLs and in the intensities of IL-8RA and IL-8RB fluorescence. When further stratified into a group of individuals in the HIV/TB group who had a capacity to release enzyme in the range that fell above the 25th percentile calculated for the ND group (n = 4) and a group for whom the capacity fell below that percentile (n = 8), the major predictor for reduced degranulation function was a significantly reduced fluorescence intensity for IL-8RA (P < 0.05).
FIG. 4.
Relationship between expression of IL-8 receptors A and B on whole-blood PMNLs and IL-8-induced degranulation responses of PMNLs isolated from a group of individuals in the ND (n = 6) and HIV/TB (n = 12) groups. The proportion of IL-8RA- and IL-8RB-expressing PMNLs (A), their relative fluorescence intensities (B), and the degranulation ability of the isolated PMNLs in response to 500 ng of IL-8 per ml (C) are shown as individual values (solid squares) and 10th and 90th percentiles (error bars). Boxes represent values between the 25th and 75th percentiles, with the median indicated. Significant differences between the ND and HIV/TB groups are indicated.
DISCUSSION
Recent work (29, 33) has suggested that defective functioning of the nonoxidative armature of PMNLs may be what is responsible for the reduced microbial killing seen by PMNLs from HIV-1-infected individuals. Here we present results in support of this hypothesis. PMNLs from HIV-1-infected individuals, whether coinfected with M. tuberculosis or not, had a significantly altered degranulation ability in response to IL-8. Most striking was the reciprocal nature of the response compared to the normal response, in that higher IL-8 concentrations induced the release of significantly lower amounts of β-glucuronidase than lower input concentrations. Even with the lower IL-8 concentrations, PMNLs from HIV-1-infected persons did not attain enzyme release comparable to the normal maximal enzyme release. The type of enzyme release response or its magnitude was unrelated to the stage of disease in these patients, as determined by the CD4 T-cell count, suggesting that this defect occurs early in HIV-1 infection.
Impaired degranulation was detected not only in response to IL-8 but also in response to another agonist, fMLP. Ellis et al. (8) conducted a series of experiments to evaluate neutrophil functions, including degranulation, in patients with AIDS or AIDS-related complex. Degranulation was assayed by a methodology similar to that used in this study. In contrast to our results, they found no difference between degranulation of PMNLs in response to fMLP in HIV-1-infected patients and healthy controls, although in their study, release of β-glucuronidase was measured with only one concentration of fMLP (10−7 M). From our results obtained with IL-8 as an agonist, it can be seen that had we used only one concentration in the range where there is a crossover of the dose-response graphs for the different study groups (Fig. 1), then we would not have detected a defective response in HIV-1-infected individuals. Similarly, Valone et al. (32) studied PMNL degranulation in HIV-1-infected patients with persistent generalized lymphadenopathy using fMLP or LTB4 as the stimulus in the β-glucuronidase assay. β-Glucuronidase release in response to fMLP was similar for PMNLs from HIV-infected patients and PMNLs from control subjects. Differences were, however, observed when LTB4 was used as an agonist, in that there was a significantly reduced release of β-glucuronidase with various LTB4 input concentrations from patient PMNLs compared to that from control PMNLs.
Levels of IL-8 are known to be raised in the peripheral circulation of HIV-1-infected individuals (16, 20, 31), and as IL-8 dynamically regulates its own receptors on PMNLs (27), this could be one mechanism by which the nonoxidative processes of peripheral PMNLs could be altered. Several lines of evidence suggest that PMNLs from HIV-1-infected individuals are primed in vivo, and this is borne out by studies showing altered surface marker expression, such as increased CD11b (23) and reduced FcγRIII (CD16) expression (18), and altered PMNL functions, such as enhanced phagocytosis of E. coli (29) and Candida sp. (33) and enhanced apoptosis of PMNLs upon their isolation (25). The tendency toward enhanced spontaneous release of β-glucuronidase from PMNLs from the HIV group shown here would further support this. Exposure to the HIV-1 proteins, various proinflammatory mediators, circulating bacterial products, and cytokines which activate neutrophils (3, 14) that may be present in the peripheral circulation of HIV-1-infected persons may contribute to a primed PMNL phenotype conducive to an increased rate of subsequent apoptosis.
IL-8 exerts its effects on PMNLs, in particular, degranulation and chemotaxis, by binding to specific receptors, CXCR-1 (IL-8RA) and CXCR-2 (IL-8RB). IL-8RA and IL-8RB have been shown to be functionally different, and neutrophil responses such as the release of granule enzymes are mediated by both IL-8RA and IL-8RB (11). We have recently shown that PMNLs from patients with HIV-1 infection, patients with pulmonary TB, and patients with dual infections have significantly diminished expression of both IL-8RA and IL-8RB compared to that of PMNLs from uninfected individuals, and, as a consequence, impaired calcium mobilization and chemotaxis in response to IL-8 (19). Here, we show that impaired degranulation of PMNLs in response to IL-8 in patients in the HIV/TB group is yet another consequence of significantly reduced IL-8RA and IL-8RB expression (in terms of both percentage and fluorescence intensity) in HIV-1-infected individuals. Furthermore, HIV-1-infected persons who had the poorest ability to respond to a high IL-8 concentration were also those who had the lowest density of IL-8RA on their PMNLs.
We have previously shown a reduced level of expression of CD16 on the surfaces of PMNLs from HIV-1-seropositive patients with pulmonary tuberculosis compared to that on the surfaces of PMNLs from subjects in the ND, TB, and HIV groups (18). Although there was a trend toward a reduced level of CD16 expression compared with the normal level of expression for both the TB and the HIV groups, with the HIV group further having a lower median level of expression than the TB group, this was not significant. Evidence suggests that a reduction in the level of CD16 expression on PMNLs with time in culture is associated with apoptosis (7). Neutrophil degranulation has been found to be triggered through CD16, resulting in the exocytosis of granule proteins (9). An interesting association between the function of CD16 and that of the fMLP receptor has been reported (12), in that chemotaxis of PMNLs in response to fMLP could be inhibited by anti-CD16 antibodies or removal of CD16 from the cell surface. Because both IL-8 receptors belong to the same family of 7-transmembrane G-protein coupled receptors and mediate functions similar to that mediated by the fMLP receptor, it is possible that a similar relationship might exist between CD16 or another receptor and IL-8 receptors. Of particular interest in this regard is the fact that the patients with TB used as controls in this study had a tendency only toward a reduced degranulation response at high IL-8 concentrations with a normal dose-response graph. However, because expression of both IL-8RA and IL-8RB on whole-blood PMNLs from patients with TB is significantly reduced compared with the normal level of expression (19), although not to the same extent as that for PMNLs from HIV-1-infected patients, one might have expected a greater impairment of degranulation in response to IL-8 than what we observed. Although there is clearly an association between IL-8R expression and subsequent IL-8-specific responses, these findings suggest the involvement of an indirect mechanism in degranulation that may be at play in HIV-1-infected persons but not in patients with pulmonary TB. It is therefore possible that decreased expression of a receptor such as CD16 on PMNLs may indirectly affect function through the modulation of other receptors, including IL-8 receptors, and may provide an explanation for reduced IL-8R expression in the TB group (19) but nearly normal degranulation responses to IL-8. Another explanation may be that, as demonstrated in this study, the intensity of IL-8RA expression provides the major determining factor in altered degranulation in response to IL-8, and its expression in particular is significantly higher in the TB group than in the HIV or HIV/TB group (19).
In conclusion, this study has shown, in further support of our previous study (19), that PMNL functions mediated through IL-8 receptors are compromised in HIV-1-infected individuals. Virus-induced changes or indirect immune processes as a result of HIV-1 infection may render the polymorphonuclear phagocytic cell relatively ineffectual with respect to one or more of its antimicrobial activities. Furthermore, it is clear that individuals who are coinfected with HIV-1 and M. tuberculosis show the greater impairment of PMNL function compared to the PMNL function of those infected only with HIV-1, because in addition to the defects in the nonoxidative armature described here, their PMNLs have reduced capacities to phagocytose E. coli and to mount a respiratory burst in response to an agonist (29). This is consistent with clinical findings of an increased susceptibility to secondary infections in patients with HIV-1 infection and TB compared to that in persons infected with HIV-1 alone and may therefore contribute to the increased morbidity and mortality in patients coinfected with HIV-1 and M. tuberculosis (34). An understanding of the underlying mechanisms that bring about changes in PMNL function in HIV-1-infected individuals could facilitate the development of rational and effective therapeutic approaches that could help curb the enhanced risk of superinfections in immunosuppressed individuals.
ACKNOWLEDGMENTS
This work was supported by the Poliomyelitis Research Foundation and Medical Research Council of South Africa.
We thank D. Spencer at the HIV outpatient clinic and L. Page-Shipp from Rietfontein Hospital, Johannesburg, South Africa, for cooperation in this study. IL-8R-specific monoclonal antibodies were kindly supplied by A. Chuntharapai and K. Jin Kim from the Department of Bioanalytical Technology, Genentech, Inc. (San Francisco, Calif.).
REFERENCES
- 1.Chen T P, Roberts R L, Wu K G, Ank B J, Stiehm E R. Decreased superoxide anion and hydrogen peroxide production by neutrophils and monocytes in human immunodeficiency virus-infected children and adults. Pediat Res. 1993;34:544–550. doi: 10.1203/00006450-199310000-00032. [DOI] [PubMed] [Google Scholar]
- 2.Chuntharapai A, Lee J, Hébert C A, Kim K J. Monoclonal antibodies detect different distribution patterns of IL-8 receptor A and IL-8 receptor B on human peripheral blood leukocytes. J Immunol. 1994;153:5682–5688. [PubMed] [Google Scholar]
- 3.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 4.Dahinden C A, Zingg J, Maly F E, de Weck A L. Leukotriene production in human neutrophils primed by recombinant human granulocyte macrophage colony-stimulating factor and stimulated with complement component C5a and FMLP as second signals. J Exp Med. 1988;167:1281–1295. doi: 10.1084/jem.167.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damiano V V, Kucich U, Murer E, Laudenslager N, Weinbaum G. Ultrastructural quantitation of peroxidase- and elastase-containing granules in human neutrophils. Am J Pathol. 1988;131:235–245. [PMC free article] [PubMed] [Google Scholar]
- 6.Daniels R H, Finnen M J, Hill M E, Lackie M J. Recombinant human monocyte IL-8 primes NADPH-oxidase and phospholipase A2 activation in human neutrophils. Immunology. 1992;75:157–163. [PMC free article] [PubMed] [Google Scholar]
- 7.Dransfield I, Buckle A-M, Savill J S, McDowall A, Haslett C, Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (FcγRIII) expression. J Immunol. 1994;153:1254–1263. [PubMed] [Google Scholar]
- 8.Ellis M, Gupta S, Galant S, Hakim S, VandeVen C, Toy C, Cairo M S. Impaired neutrophil function in patients with AIDS or AIDS-related complex: a comprehensive evaluation. J Infect Dis. 1988;158:1268–1276. doi: 10.1093/infdis/158.6.1268. [DOI] [PubMed] [Google Scholar]
- 9.Huizinga T W J, Dolman K M, van der Linden N J M, Kleijer M, Nuijens J H, von dem Borne A E G K, Roos D. Phosphatidylinositol-linked FcRIII mediates exocytosis of neutrophil granule proteins, but does not mediate initiation of the respiratory burst. J Immunol. 1990;144:1432–1437. [PubMed] [Google Scholar]
- 10.Jentsch U. A review of opportunistic infections in Africa. South Afr J Epidemiol Infect. 1997;12:28–32. [Google Scholar]
- 11.Jones S A, Wolf M, Qin S, Mackay C R, Baggiolini M. Different functions for the interleukin-8 receptors (IL-8R) of human neutrophil leukocytes: NADPH oxidase and phospholipase D are activated through IL-8R1 but not IL-8R2. Proc Natl Acad Sci USA. 1996;93:6682–6686. doi: 10.1073/pnas.93.13.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kew R R, Grimaldi C M, Furie M B, Fleit H B. Human neutrophils FcγRIIIB and formyl peptide receptors are functionally linked during formyl-methionyl-leucyl-phenylalanine-induced chemotaxis. J Immunol. 1992;149:989–997. [PubMed] [Google Scholar]
- 13.Lazzerin A, Uberti Foppa C, Galli M, Mantovani A, Poli G, Franzetti F, Novati R. Impairment of polymorphonuclear leukocyte function in patients with acquired immunodeficiency syndrome and lymphadenopathy syndrome. Clin Exp Immunol. 1986;65:105–111. [PMC free article] [PubMed] [Google Scholar]
- 14.Lee A, Whyte M K B, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukocyte Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- 15.Lehrer R I, Ganz T. Antimicrobial polypeptides in human neutrophils. Blood. 1990;76:2169–2181. [PubMed] [Google Scholar]
- 16.Matsumoto T, Miike T, Nelson R P, Trudeau W L, Lockey R F, Yodoi J. Elevated serum levels of IL-8 in patients with HIV infection. Clin Exp Immunol. 1993;93:149–151. doi: 10.1111/j.1365-2249.1993.tb07957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McColl S R, Beauseigle D, Gilbert C, Naccache P H. Priming of the human neutrophil respiratory burst by granulocyte macrophage colony-stimulating factor and tumour necrosis factor-α involves regulation at a post cell-surface receptor level. J Immunol. 1990;145:3047–3053. [PubMed] [Google Scholar]
- 18.Meddows-Taylor S, Martin D J, Tiemessen C T. Altered expression of FcγRIII (CD16) on polymorphonuclear neutrophils from individuals with human immunodeficiency virus type 1 disease and pulmonary tuberculosis. Clin Diagn Lab Immunol. 1997;4:789–791. doi: 10.1128/cdli.4.6.789-791.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meddows-Taylor S, Martin D J, Tiemessen C T. Reduced expression of interleukin-8 receptors A and B on polymorphonuclear neutrophils from persons with human immunodeficiency virus type 1 disease and pulmonary tuberculosis. J Infect Dis. 1998;177:921–930. doi: 10.1086/515232. [DOI] [PubMed] [Google Scholar]
- 20.Meddows-Taylor, S., D. J. Martin, and C. T. Tiemessen. Dysregulated production of interleukin-8 in individuals infected with human immunodeficiency virus type 1 and Mycobacterium tuberculosis. Infect. Immun. 67:1251–1260. [DOI] [PMC free article] [PubMed]
- 21.Murphy P M, Lane H C, Fauci A S, Gallin J I. Impairment of neutrophil bactericidal capacity in patients with AIDS. J Infect Dis. 1988;158:627–630. doi: 10.1093/infdis/158.3.627. [DOI] [PubMed] [Google Scholar]
- 22.Murray J F. The white plague: down and out, or up and coming. Am Rev Respir Dis. 1989;140:1788–1795. doi: 10.1164/ajrccm/140.6.1788. [DOI] [PubMed] [Google Scholar]
- 23.Palmer S, Hamblin A S. Increased CD11/CD18 expression on the peripheral blood leukocytes of patients with HIV disease: relationship to disease severity. Clin Exp Immunol. 1993;93:344–349. doi: 10.1111/j.1365-2249.1993.tb08183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitrak D L, Bak P M, DeMarais P, Novak R M, Anderson B R. Depressed neutrophil superoxide production in human immunodeficiency virus infection. J Infect Dis. 1993;167:1406–1410. doi: 10.1093/infdis/167.6.1406. [DOI] [PubMed] [Google Scholar]
- 25.Pitrak D L, Chie Tsai H, Mullane K M, Sutton S H, Stevens P. Accelerated neutrophil apoptosis in the acquired immunodeficiency syndrome. J Clin Invest. 1996;98:2714–2719. doi: 10.1172/JCI119096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roitt I, Brostoff J, Male D. Adaptive and innate immunity. In: Roitt I, Brostoff J, Male D, editors. Immunology. New York, N.Y: Glower Medical Publishing; 1989. p. 1.1. [Google Scholar]
- 27.Samanta A K, Oppenheim J J, Matsushima K. Interleukin-8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J Biol Chem. 1990;265:183–189. [PubMed] [Google Scholar]
- 28.Schröder J-M, Mrowietz U, Morita E, Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987;139:3474–3483. [PubMed] [Google Scholar]
- 29.Shalekoff S, Tiemessen C T, Gray C M, Martin D J. Depressed phagocytosis and oxidative burst in polymorphonuclear leukocytes from individuals with pulmonary tuberculosis with or without HIV-1 infection. Clin Diagn Lab Immunol. 1998;5:41–44. doi: 10.1128/cdli.5.1.41-44.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart A G, Harris T, De Nichilo M, Lopez A F. Involvement of leukotriene B4 and platelet activating factor in cytokine priming of human polymorphonuclear neutrophils. Immunology. 1991;72:206–212. [PMC free article] [PubMed] [Google Scholar]
- 31.Thea D M, Porat R, Nagimbi K, Baangi M, St. Louis M E, Kaplan G, Dinarello C A, Keusch G T. Plasma cytokines, cytokine antagonists, and disease progression in African women infected with HIV-1. Ann Intern Med. 1996;124:757–762. doi: 10.7326/0003-4819-124-8-199604150-00009. [DOI] [PubMed] [Google Scholar]
- 32.Valone F H, Payan D G, Abrams D I, Goetzl E J. Defective polymorphonuclear leukocyte chemotaxis in homosexual men with persistent lymph node syndrome. J Infect Dis. 1984;150:267–271. doi: 10.1093/infdis/150.2.267. [DOI] [PubMed] [Google Scholar]
- 33.Wenisch C, Parschalk B, Zedwitz-Liebenstein K, Graninger W, Rieger A. Dysregulation of the polymorphonuclear leukocyte-Candida spp. interaction in HIV-positive patients. AIDS. 1996;10:983–987. doi: 10.1097/00002030-199610090-00008. [DOI] [PubMed] [Google Scholar]
- 34.Whalen C, Horsburgh C R, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med. 1995;151:129–135. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]