Abstract
Global-scale ecological changes and intensifying habitat destruction and have caused alarming declines in wildlife populations, resulting in a great need for concerted efforts towards their conservation. Despite this, animals are frequently overlooked in restoration and management initiatives and therefore populations often do not reassemble following disturbance without re-establishing habitat that meets their abiotic and biotic requirements. However, restoration ecologists broadly lack insight into the physiological mechanisms that can govern the responses of fauna to environmental change and management. Therefore, we conducted a literature search for studies reporting a mechanistic understanding of faunal habitat suitability and selection in restored landscapes to deliver an updated perspective on the integration of animal ecophysiology and restoration ecology. Of the 75,442 studies that we identified discussing ecological restoration in the last 50 years, only 8,627 (11.4%) did so in the context of fauna from which 912 studies (1.2%) examined habitat selection, 35 studies (0.05%) integrated physiology and only 15 studies (0.02%) explored thermal biology, despite temperature being one of the most pervasive drivers of physiological functioning. To combat this, we developed a conceptual framework that can guide restoration ecophysiology and promote innovative, multidisciplinary research through an established adaptive management structure. While physiological tools and approaches are currently underutilised in restoration practice, integrating them into ecological restoration, and environmental management more broadly, will offer exciting new opportunities to describe, explain and predict the responses of fauna to environmental change occurring, and that yet to come.
Keywords: Environmental management, Habitat suitability, Monitoring, Thermal biology, Thresholds, Wildlife conservation
Introduction
Intensifying anthropogenic pressures have led to global-scale ecological changes that will lead to cascading impacts on landscapes, biodiversity, and human well-being into the future (Crutzen 2006, IPBES 2019). Changing land-use is a leading cause of biodiversity losses with 75% of land having been significantly altered by anthropogenic pressures such as agricultural, industrial, or urban development (IPBES 2019).There has been an average global decline in abundance of 68% across monitored wildlife populations representing 4,392 species in the last 50 years (WWF 2020). Furthermore, without mitigation or reduction of biodiversity and habitat loss, one million species are potentially facing extinction in the coming decades (IPBES 2019). Fortunately, ecological restoration can ameliorate, or even reverse, biodiversity and habitat loss (Suding et al. 2015). The core objective of ecological restoration is to assist the recovery of damaged, degraded or destroyed habitat to a self-sustaining, functioning and resilient ecosystem (Miller et al. 2017). Degradation can result in substantial biological, physical and chemical change (Heneghan et al. 2008). Therefore, mitigation efforts require collaborations between scientific expertise across several ecological disciplines but coupling the expanding knowledge around ecological restoration with practical implementation can be a complex and long-term enterprise (Miller et al. 2017, Bertuol-Garcia et al. 2018). Adding to this challenge is the limited understanding of species’ abiotic and biotic requirements for development, reproduction, and survival, that ultimately drive their successful recovery in restored landscapes.
Topographical, vegetative and hydrological factors frequently dominate the restoration literature and monitoring schemes (McAlpine et al. 2016), while animals are overlooked, owing largely to the assumption that their recolonisation of restored environments occurs without facilitation (Palmer et al. 1997, Hilderbrand et al. 2005, Majer 2009). Animals play critical roles in the delivery of ecological services such as nutrient cycling, pollination, and seed dispersal (Kremen et al. 2007, Noriega et al. 2018). Robust, functional and resilient faunal communities lay the foundations for the continuity of ecosystem function and, consequently, the long-term outcomes for restored ecosystems (Montoya et al. 2012). However, recent meta-analyses conclude that while restoration can improve biodiversity values, fauna compositions and ecological services in degraded landscapes, the resulting communities fail to resemble intact or ‘reference’ ecosystems (Benayas et al. 2009, Crouzeilles et al. 2016, Shimamoto et al. 2018). Additionally, several independent reviews have consistently demonstrated that animals are poorly considered in the restoration ecology literature (Majer 2009, Cristescu et al. 2012, Cross et al. 2019).
To obtain a comprehensive, scalable understanding of fauna recolonisation in restoration, it is necessary to define and explore the potential mechanisms that may explain their responses to environmental change and selection for certain habitats. In this context, mechanism can refer to lower-level biological processes (e.g., respiration) that give rise to higher-level biological processes (e.g., development) as a function of a known environmental variable (e.g., temperature). Understanding causal mechanisms should form the foundation for decision-making and monitoring protocols (Kearney et al. 2009), but the mechanisms underpinning patterns in ecological restoration are often unexplored (Suding 2011, Brudvig 2017). We suggest that understanding the responses of animals to restoration may be best achieved by combining traditional biodiversity surveys with ecophysiology to better describe habitat suitability and selection. This knowledge can be generated by experimental trials under both in situ and ex situ contexts that determine species performance and tolerance to a range of controlled conditions related to biological, chemical, or physical factors in restored landscapes (Cooke et al. 2021). This mechanistic approach can describe the processes underpinning the patterns that emerge throughout restoration, allowing prediction of restoration trajectories to better inform management.
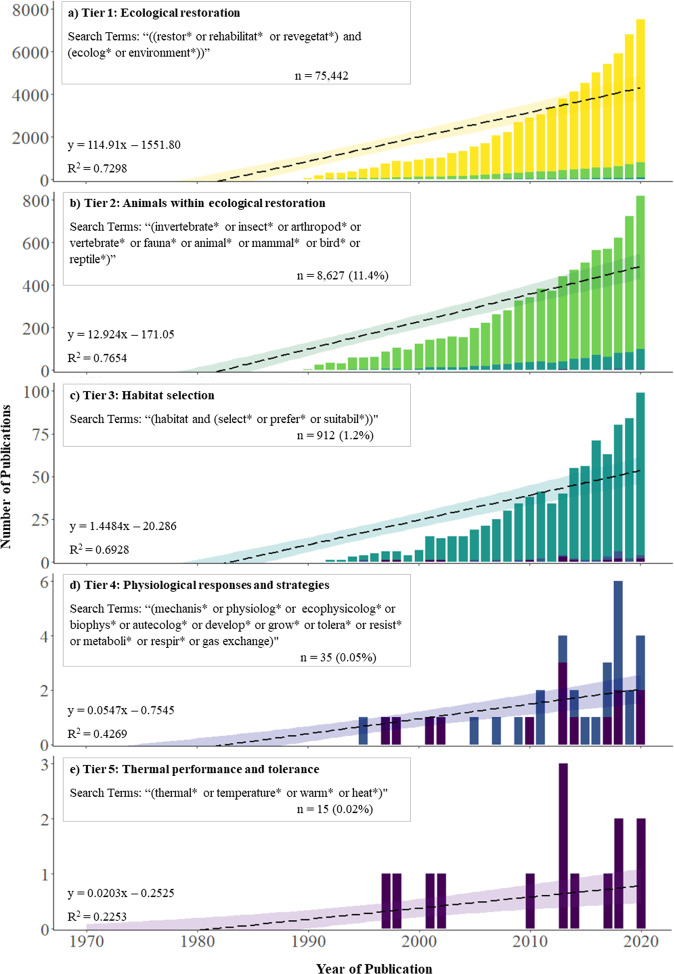
Our aim was to deliver an updated perspective on the integration of animal ecophysiology into restoration ecology, and specifically to identify ways to inform practice and management. We conducted a literature review of studies that examined ecophysiological mechanisms underpinning habitat suitability and selection for fauna in restored landscapes catalogued by the ISI Web of Science (Core Collection; Last searched 18th May 2021). We refined our search terms across five ‘tiers’ that systematically narrowed our searches from restoration ecology, to studies specifically exploring thermal biology as a key driver of patterns and processes in ecological restoration (Fig. 1). While the physiology of abiotic stress is multifaceted, we narrowed our physiological context to thermal biology as temperature is a fundamental driver of most physiological and biochemical processes in need of greater consideration (Huey 1991, Tuff et al. 2016). We used simple linear regression models to quantify research output over time to compare publication trajectories for each search ‘tier’ and limited our search to studies dealing with terrestrial fauna (i.e., insects, birds, mammals, and reptiles). However, we acknowledge that many aquatic ecosystems also require restoration and believe many concepts discussed here will also translate into current and future freshwater or marine management.
Fig. 1.
Five-tiered literature search demonstrating (a) the development of the restoration ecology literature over the last 50 years (yellow). b The representativeness of fauna in the restoration ecology literature (green). c The consideration of fauna habitat preference, selection and suitability in restoration ecology (teal). d The application of ecophysiological approaches and mechanisms in explaining habitat selection (blue). e The assessment of temperature as a driving mechanism for faunal responses to restoration (purple), as an exemplar of a physiologically motivating environmental characteristic. Dashed lines represent linear regression models with colour corresponding banding representing confidence intervals
The place of fauna in ecological restoration
Restoration ecology aims to inform, guide, and support the practice of reinstating functional ecosystems to degraded landscapes (Miller et al. 2017, Wainwright et al. 2018, Tomlinson et al. 2022). Over the last 50 years, restoration ecology has amassed over 75,000 indexed scientific publications (Fig. 1a; Tier 1). Annual research output has grown significantly (Fig. 1a; t1, 49 = 11.5, P < 0.001) with the last decade alone accounting for 54,768 (72.5%) publications (t1,9 = 18.0, P < 0.001), representing a 165% increase in publications compared with the four preceding decades combined (20,674 publications). This substantial increase in research highlights the developing empiricism of restoration science and contribution of restoration research to basic ecological theory (Bradshaw 1983, Jordan et al. 1988, Perring et al. 2015). However, persistent biases in research agendas and monitoring protocols in ecological restoration has been identified repeatedly (Palmer et al. 1997, Hilderbrand et al. 2005). Ecological restoration dominated by consideration of vegetation communities and structure, with comparatively little attention directed towards other elements of ecosystem health and functionality. Arguably one of the most frequently acknowledged shortcomings is failure to consider fauna (Cross et al. 2019).
Fauna are often assumed to return to restored landscapes unaided following the reestablishment of vegetation (Palmer et al. 1997, Hilderbrand et al. 2005). This bias towards the restoration of the floral community overlooks key components of a functioning ecosystem, as restoring vegetation does not always support the return of fauna, nor the associated services they provide (Jones and Davidson 2016). This trend, recognised over three decades ago (Butcher et al. 1989, Majer 1989), is persistent with only 12.4% of papers reporting ecological restoration in the first decade of the 21st century have focused on fauna (Majer 2009), and of these studies, birds typically received the most attention. To examine the place of fauna within terrestrial restoration ecology we refined our first-tier search by the terms “invertebrate* or insect* or arthropod* or vertebrate* or fauna* or animal* or mammal* or bird* or reptile*’, where the asterisk represents a Boolean operator to expand the search to the broadest extent of relevant papers. Publications dedicated to fauna have increased steadily in the last 50 years (Fig. 1b; t1, 49 = 12.6, P < 0.001) and, and the last decade, represents a 139% increase in publications compared to the combined output of the previous four decades (t1,9 = 12.5, P < 0.001). Proportionally, however, the representation by fauna-oriented studies has decreased since the assessment by Majer (2009), and only accounted for 11.4% of the restoration literature that we identified. Despite several reports explicitly advocating for greater consideration of fauna (Majer 2009, Cross et al. 2019), the disparity between animal and plant-oriented studies has increased. This is a substantial oversight considering the critical roles that fauna often play in plant recruitment (Catterall 2018), and ultimately, successful restoration through pollination and seed dispersal. When fauna are considered in restoration, however, assessments tend to be largely descriptive, by associating patterns of taxonomic composition and diversity indices with different ecological states or management practices (Miller et al. 2017). While these approaches provide insights into the spatiotemporal variation of species and community assemblages, correlative associations are limited in their capacity to explain habitat selection and suitability (Weiner 1995, Lawton 1999). Habitat selection is a driving process structuring animal populations in restored landscapes and therefore, successful restoration is dependent upon facilitating suitable habitat that fauna can access and use appropriately (Miller et al. 2017).
Habitat selection by animals is defined by competing environmental costs and benefits to an organism’s performance and fitness (Mayor et al. 2009), and has a profound influence on population dynamics (Pulliam and Danielson 1991), biotic interactions (Martin 2001), and community reassembly (Binckley, Resetarits (2005)). Therefore, it represents a significant, spatially dependent process driving fauna recovery (Hale and Swearer 2017). The number of studies exploring habitat selection have increased steadily since 1970 (Fig. 1c; t1,9 = 10.5, P < 0.001), and significantly over the last decade (t1,9 = 9.4, P < 0.001), but represented less than 1.3% of the broader restoration ecology literature that we identified (Fig. 1a) and only 10.6% of restoration science dedicated to fauna (Fig. 1b). The negligible amount of research dedicated to understanding habitat selection by animals highlights a considerable omission in a field seeking to ameliorate the risks and consequences of habitat loss. Restoration landscapes could be specifically constructed to meet the requirements of animals if the mechanistic drivers of habitat selection were understood (Hale et al. 2019). Yet in many cases, population-level proxies for demography (e.g., abundance) serve as metrics to evaluate the successful or unsuccessful return of fauna to restored habitats. Unfortunately, when such patterns are assessed without reference to function, causality is difficult to infer and if barriers to recovery occur, they are harder to identify and address effectively. In this instance, ecophysiology can help establish links between pattern and process thereby providing critical insight into the mechanisms underpinning species’ responses to ecological restoration. Insights into habitat suitability can characterise physical site conditions and resources necessary for returning functional and sustainable populations and identify potential environmental stressors that may be limiting to restoration success (e.g., temperature and moisture availability).
The integration of animal ecophysiology and restoration
Cooke and Suski (2008) called for the development and validation of ecophysiological models incorporating laboratory and field approaches to evaluate the relationships between habitat quality, organism performance, and population dynamics. While some studies have recently advanced these efforts by providing theory, model and test in a single restoration context (e.g., Tomlinson et al. 2014, Tomlinson et al. 2017, Tomlinson et al. 2018), our search for terms “mechanis* or physiolog* or ecophysiolog* or biophys*or autecolog* or develop* or grow* or tolera* or resist* or metaboli* or respir* or gas exchange” yielded a proportionally low number of publications in the field of ecological restoration. We identified only 209 research reports (0.27%) and only 35 (0.05%) empirical studies incorporated ecophysiological or biophysical assessments of fauna re-assembly in restoration (Fig. 1d). Though high-profile appeals for the integration of restoration and ecophysiology were expressed over a decade ago (Cooke and Suski 2008), and despite the increasingly accessible methodologies available to do so (Cooke and Suski 2008, Tomlinson et al. 2014, Madliger et al. 2018), there has been negligible increase in research productivity in this space following this call to action (Fig. 1d; t1,9 = 1.8, P < 0.10).
Where ecophysiology has been applied to answer questions about fauna in changing environments, it has generally proven to be readily incorporated, transparent, and insightful. For example, high-resolution distribution models that integrate insect thermal constraints have been used to identify how different pollinating guilds (i.e., bees, wasps, and beetles) respond to fragmentation and thermal variability in a restoration landscape (Tomlinson et al. 2018, Tomlinson 2020). Such models can guide the reconstruction of habitat to ameliorate physiological stress and satisfy the energetic requirements of the species. Similarly, the suitability of translocation sites for the critically endangered Western Swamp Tortoise (Pseudemydura umbrina Siebenrock, 1901), predicted through biophysical simulation of the species’ thermodynamic niche (Mitchell et al. 2013), identified recipient sites for assisted colonisation in response to climate change. Most recently, such modelling has been used to identify sites where Tasmanian Devils (Sarcophilus harrisii Boitard, 1841) could be reintroduced to reinstate predator prey relationships, supress cat predation, and advance otherwise unassisted ecological restoration (Morris et al. 2022). However, models such as these are effectively hypotheses without the validation obtained from field-based studies of free-ranging animals in situ.
The minimal maintenance energetic requirements of an animal can be readily measured and standardised for both ectotherms and endotherms (Withers 1992). The relationship between this fundamental currency of ecology (Kleiber 1961), and abiotic conditions such as ambient temperature, pH, and salinity forms the basis of many of the biophysical models that seek to explain how animals interact with their environment in space and time (Kearney and Porter 2009, Kearney et al. 2010, Kearney et al. 2013). Though rarely explored, when such models have been tested by measuring the cost of living of an animal in situ (e.g., through field metabolic rates; FMR), energetic requirements and expenditure can be remarkably similar to the modelled expectations (e.g., Tomlinson et al. 2017). While some isotopic and telemetric techniques for measuring FMR may not be universally suitable across taxa and environments (Cooke et al. 2004, Cooke 2008, Tomlinson et al. 2014), where feasible, such techniques can present important insights into tolerance constraints on organisms under environmentally relevant conditions. In cases where measurements of true FMR are not feasible, there alternative approaches that provide insight into energetic constraints. Thompson et al. (2018) combined simple ecophysiological experiments with field studies to understand how temperature drives habitat occupancy of two anoles (Norops humilis Peters, 1863 and N. limifrons Cope, 1862) across forest regeneration stages. Here, thermal constraints of the two species likely caused their avoidance of early stages of restoration. Such approaches are relatively simple to apply broadly and provide mechanistic insights into habitat selection to increase the value of restoration monitoring.
Using temperature as a driving mechanism in restoration science
The influence of temperature on most physiological and biochemical processes, such as metabolic, growth, and developmental rates, make it one of the most pervasive abiotic drivers of the biology and ecology of both plant and animal taxa (Angilletta 2006, Kooijman and Kooijman 2010, Gilbert et al. 2014, Buckley et al. 2015). Consequently, calls to explore the thermal drivers of habitat selection are not new (Huey 1991), and have been reiterated recently (Tuff et al. 2016, Tomlinson et al. 2018, Garcia and Clusella-Trullas 2019). Therefore, we further refined our search to “thermal* or temperature* or warm* or heat*”, identifying only 15 studies (0.02% of the restoration ecology literature) that incorporate thermal physiology into fauna reassembly research (Fig. 1e). There has been no significant increase in publications in the last decade, contrasting with the trends observed in the broader literature (Fig. 1a–c). To our knowledge, thermal performance has not been used to explain patterns of population or community reassembly in the context of ecological restoration. By examining thermal performance, restoration ecologists may be able to better predict potential demographic bottlenecks and forecast which species will persist, decline, or drop out of the modified systems, and those that have the capacity to recover with ongoing restoration management.
At large scales, climate change has prioritised research into temperature-driven contributions to conservation priorities (Tylianakis et al. 2008, Gilman et al. 2010, Lister and Garcia 2018). However, habitat degradation and subsequent restoration can cause localised shifts in the thermal environment (Meyer et al. 2001). Depending on the context and stages of ecological restoration some sites can be warmer, more exposed, and more desiccating environments (Cross et al. 2020), or they can be cooler and less suitable for native animals to manage their thermal biology (Garcia and Clusella-Trullas 2019). Consequently, the recovery of fauna in a restoration landscape depends, in part, on the ability of individuals to tolerate novel temperature regimes arising from degraded and restored ecosystems (Meyer and Sisk 2001, Meyer et al. 2001). Therefore, it is essential for scientists and practitioners to be aware of the potential thermal implications associated with habitat degradation, restoration, and management to effectively ameliorate thermal stress and maximise thermal suitability for wildlife populations. Integrating ecophysiology with thermal biology and other disciplines, such as community and population ecology, offers exciting new pathways to increase restoration success by developing mechanistic insights to guide the design, implementation and on-going management of ecological restoration.
Experimental adaptive management: The opportunity to integrate ecophysiology into restoration ecology
While the importance of interdisciplinary research integrating physiology and restoration ecology has been long insisted upon (Cooke and Suski 2008), translating ecophysiological techniques into tools for practical ecological restoration remains a major challenge. However, criticism has been directed at ecophysiologists for primarily communicating their findings to other scientists through peer-reviewed literature (Cooke and O’Connor 2010). This risks the field of physiology becoming an echo-chamber, and the value of the research being poorly communicated to practitioners (Cooke and O’Connor 2010, Seavy and Howell 2010). Conversely, the practical outcomes of restoration projects are often not published in peer-reviewed journals, restricting feedback from practitioners to the scientific community (Sunderland et al. 2009). This also restricts the communication of lessons learned only to local groups, regardless of the global challenge that ecological restoration represents. Practitioners have also been criticised for not seeking and using the most appropriate, evidenced-based support for their management actions (Sutherland et al. 2004, Cooke and O’Connor 2010). As a science, however, ecophysiology has been criticised for operating at inadequate biological, spatial and temporal scales (Cooke and O’Connor 2010), and of having objectives that do not align with the knowledge needs of practitioners and decision-makers (Cooke and Suski 2008, Cooke and O’Connor 2010). We suggest that increased communication between both physiologists and ecologists, and between science and practice can be beneficial to all parties. In this regard, practitioners and ecologists should collaborate further and facilitate bi-directional flows of knowledge to maximise restoration success (Baker et al. 2014, Young et al. 2014, David et al. 2016).
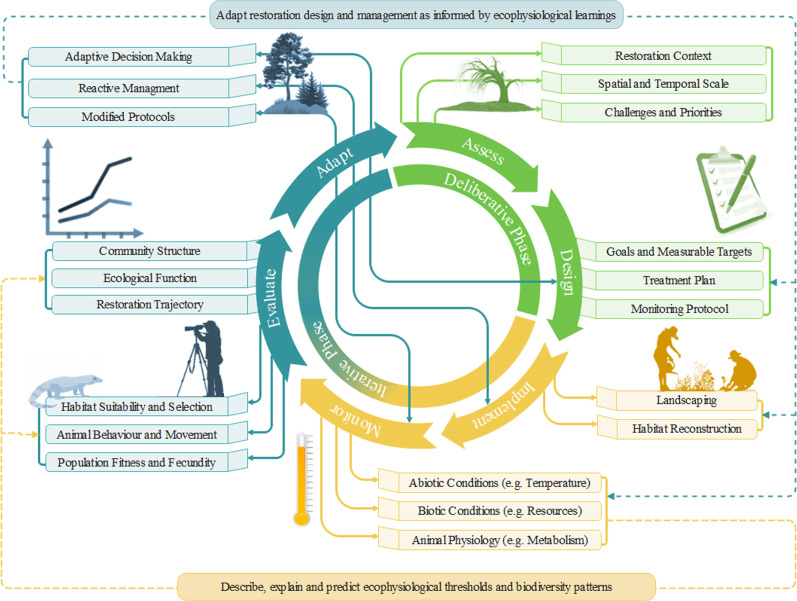
One way to foster greater collaboration between animal ecophysiology and restoration ecology is to embed ecophysiology into adaptive management (Fig. 2). Adaptive management is a structured, cyclical process of decision-making that accounts for change and uncertainty in a “learning by doing” fashion where actions are undertaken iteratively to minimise uncertainty and improve upon previous efforts (Williams 2011, McDonald et al. 2016). Adaptive management is regarded as the standard approach to ecological restoration (McDonald et al. 2016, SERA 2017), and should be designed with the input of both researchers and practitioners (Taylor et al. 1997, Morghan et al. 2006). Adaptive management generally conducts regular monitoring and evaluation to assess whether current management actions are achieving set goals and modifying these actions to address any shortcomings identified and minimising uncertainty through ongoing acquisition of knowledge (Fig. 2; Murray and Marmorek 2003). However, the implementation of an adaptive management framework can be optimised with experimental designs that test explicit hypotheses (Bormann et al. 2007, Williams 2011).
Fig. 2.
Schematic overview representing how animal physiology can be used across an adaptive management cycle to describe, predict, and explain organism responses to ecological restoration, contribute to the evaluation of restoration trajectories and inform management actions to feed back into the design, implementation, and ongoing management of ecological restoration in a flexible, iterative process of decision making and knowledge acquisition
Species-specific ecophysiology and site-specific conditions (and manipulation of such) have high applicability in the design, implementation, monitoring and adaptive management of ecological restoration (Fig. 2; Tomlinson et al. 2022). While traditional biodiversity monitoring (e.g., species richness, abundance and composition) can describe patterns of spatial and temporal variation, they are limited in their capacity to provide causal interpretation of restoration successes and failures (Lawton 1999, Verberk et al. 2013). Integrating experimental physiology into restoration management can delineate cause-and-effect relationships between organism performance (e.g., developmental rate, metabolic rate, reproductive output) and limiting environmental factors and predict the responses of fauna to environmental change or management practices (Cooke and Suski 2008, Tarszisz et al. 2014). For example, using ecophysiological approaches to assess pre-disturbance conditions of an ecosystem (e.g., before mining or clearing) can establish a reference system that maximises habitat suitability for returning fauna or the suitability of translocation sites as a mitigation strategy (“Assess”; Fig. 2; Tarszisz et al. 2014). This information can then be used to design restoration goals and treatment plans that maximise the necessary resources and physical characteristics to support the physiological and energetic constraints of key functional groups and target taxa (“Design”; Fig. 2). For example, initial seed blends may be selected to provide greater nutritional and provisioning resources for keystone pollinators (Tomlinson et al. 2018), or landforms may be specifically designed to maximise microhabitat variability, creating different niches across a restoration landscape (“Implement”; Fig. 2; Milling et al. 2018). Ecophysiological insight can also inform management actions that increase barriers to fitness to minimise species invasion or optimise biological control practices (Schmitz and Barton 2014, Tougeron et al. 2016).
Trigger points and clear measurable indicators can also be established empirically through physiological metrics such as tolerance thresholds or performance parameters. Regular site monitoring informed by these metrics can establish whether restoration actions achieve restoration goals by comparing changes in animal physiology from an established baseline or comparing mean responses between restored and ‘reference’ populations (“Monitor”; Fig. 2; Madliger et al. 2018). The inclusion of stress, nutritional and reproductive physiology into monitoring protocols provides valuable insights into the synergistic links between animal behaviour, movement and ultimately habitat selection restoration initiatives (Tarszisz et al. 2014, Tomlinson et al. 2018, Tomlinson 2020). However, biotelemetric approaches (Cooke et al. 2004), in conjunction with measures of traditional biodiversity values (e.g., species abundance, richness, and diversity) or functional responses (e.g., fecundity or behaviour), can greatly enhance our understanding of the patterns of reassembly that emerge in restored landscapes (Seebacher and Franklin 2012). Doing so can provide mechanistic perspectives to traditional monitoring outcomes and determine whether restoration practice needs to be adjusted.
Coupling in situ biodiversity surveys, ex situ physiological measurements and landscape ecology can deliver unparalleled insight into which restoration activities can maximise habitat suitability, organism performance and ultimately, function (“Evaluate”; Fig. 2). Ecophysiology can assist in the evaluation of restoration practices that support the transition of degraded sites towards a reference target, and those that do not, while delivering a unique capacity to predict future responses by drawing cause-and-effect relationships (Cooke and Suski 2008). For example, comparative behavioural, reproductive, and developmental physiology can be conducted ex situ and in situ to explain variation in physiological performance relative to the biotic and abiotic conditions of different successional ages, structure, or quality (Tudor 2021). Such insights can describe ecophysiological variation, explain emerging patterns of biodiversity and predict future responses to changing environments. Collectively, ecophysiology can provide evidence-based guidance to evaluate the consequences and uncertainties of management actions and feed into the adaptive decision making of how management can be modified to improve future outcomes. Following this, ecophysiology can iteratively feed back into the design, implementation and monitoring of restoration in such a way that balances between the acquisition of knowledge to minimise uncertainties to improve future outcomes and achieving the best short term results based on current knowledge (“Adapt”; Fig. 2; Murray and Marmorek 2003).
While such high-resolution physiological data are not essential for all species, applying this framework to key focal groups, such as keystone species or ecosystem service providers, provides a strategic avenue for maximising physiological insight that can be used to guide restoration initiatives (Tomlinson et al. 2022). The adaptive management schema that we have developed here is not necessarily a new framework; it instead identifies how to incorporate novel ecophysiological measurements into an established management structure to gain new insights into ecological restoration. While this framework will not be enough to close the gap between science and practice, it offers a tangible tool to foster the integration between the two emerging fields of restoration ecology and conservation physiology. This integrative, interdisciplinary approach may have been an overlooked element in allowing ecophysiology to play a substantial and informative role in ecological restoration, and we are hopeful that applying this framework to other ecophysiological traits will offer exciting new pathways to increase restoration success and respond to the challenges that we have set for ourselves in the Anthropocene.
Conclusions
Our literature review showed that, while restoration ecology is a rapidly growing field of research (Fig. 1a), fauna remain poorly integrated (Fig. 1b). In fact, fauna are considered proportionally less than they were a decade ago (Majer 2009). Despite the intrinsic value in empirically describing the interactions between fauna and restoration practices, physiological studies are rare in restoration ecology, and responses to the most pervasive abiotic driver, temperature, are hardly considered at all (Fig. 1e). This is a substantial oversight given that preference or avoidance of different habitats is often motivated largely by physiological constraints and that physiological requirements can profoundly alter the interaction between animals and their biotic niche (Nowakowski et al. 2018). Ecophysiological approaches exist to bridge this knowledge gap and they can be rapidly integrated into any restoration context to help understand complex organism-environment dynamics. Collaborations between restoration practitioners and scientists should aim to not only optimise protocols to better describe, explain and predict responses of biological systems, but to also engage with the iterative improvement of long-term management and research to maximise the value of restored landscapes for faunal communities.
Acknowledgements
This research was supported by an Australian Government Research Training Program Scholarship and Curtin University.
Author Contributions
EPT, WL and ST contributed to the conceptualisation and ideas in this manuscript. EPT led the writing of the manuscript. EPT and WL conducted literature search and analysis. All authors contributed critically to subsequent drafts and gave final approval for publication.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Compliance with Ethical Standards
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
2/22/2023
The original online version of this article was revised: Missing Open Access funding information has been added in the Funding Note.
References
- Angilletta MJ. Estimating and comparing thermal performance curves. J Therm Biol. 2006;31:541–545. [Google Scholar]
- Baker S, Eckerberg K, Zachrisson A. Political science and ecological restoration. Environ Politics. 2014;23:509–524. [Google Scholar]
- Benayas JMR, Newton AC, Diaz A, Bullock JM. Enhancement of biodiversity and ecosystem services by ecological restoration: a meta-analysis. Science. 2009;325:1121–1124. doi: 10.1126/science.1172460. [DOI] [PubMed] [Google Scholar]
- Bertuol-Garcia D, Morsello C, El-Hani CN, Pardini R. A conceptual framework for understanding the perspectives on the causes of the science–practice gap in ecology and conservation. Biol Rev. 2018;93:1032–1055. doi: 10.1111/brv.12385. [DOI] [PubMed] [Google Scholar]
- Binckley CA, Resetarits WJ., Jr. Habitat selection determines abundance, richness and species composition of beetles in aquatic communities. Biol Lett. 2005;1:370–374. doi: 10.1098/rsbl.2005.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann BT, Haynes RW, Martin JR. Adaptive management of forest ecosystems: Did some rubber hit the road? BioSci. 2007;57:186–191. [Google Scholar]
- Bradshaw AD. The reconstruction of ecosystems. J Appl Ecol. 1983;20:1–17. [Google Scholar]
- Brudvig LA. Toward prediction in the restoration of biodiversity. J Appl Ecol. 2017;54:1013–1017. [Google Scholar]
- Buckley LB, Ehrenberger JC, Angilletta MJ., Jr. Thermoregulatory behaviour limits local adaptation of thermal niches and confers sensitivity to climate change. Funct Ecol. 2015;29:1038–1047. [Google Scholar]
- Butcher J, Majer J, Unsworth P. Bibliography of fauna studies in reclaimed lands. In: Majer J, editor. Animals in Primary Succession: the Role of Fauna in Reclaimed Lands. Cambridge, United Kingdom: Cambridge University Press; 1989. [Google Scholar]
- Catterall CP. Fauna as passengers and drivers in vegetation restoration: A synthesis of processes and evidence. Ecol Manag Restor. 2018;19:54–62. [Google Scholar]
- Cooke SJ. Biotelemetry and biologging in endangered species research and animal conservation: relevance to regional, national, and IUCN Red List threat assessments. Endanger species Res. 2008;4:165–185. [Google Scholar]
- Cooke SJ, Bergman JN, Madliger CL, Cramp RL, Beardall J, Burness G, Clark TD, Dantzer B, De La Barrera E, Fangue NA. One hundred research questions in conservation physiology for generating actionable evidence to inform conservation policy and practice. Conserv Physiol. 2021;9:coab009. doi: 10.1093/conphys/coab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke SJ, Hinch SG, Wikelski M, Andrews RD, Kuchel LJ, Wolcott TG, Butler PJ. Biotelemetry: A mechanistic approach to ecology. Trends Ecol Evol. 2004;19:334–343. doi: 10.1016/j.tree.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Cooke SJ, O’Connor CM. Making conservation physiology relevant to policy makers and conservation practitioners. Conserv Lett. 2010;3:159–166. [Google Scholar]
- Cooke SJ, Suski CD. Ecological restoration and physiology: An overdue integration. BioSci. 2008;58:957–968. [Google Scholar]
- Cristescu RH, Frère C, Banks PB. A review of fauna in mine rehabilitation in Australia: Current state and future directions. Biol Conserv. 2012;149:60–72. [Google Scholar]
- Cross SL, Tomlinson S, Craig MD, Bateman PW. The Time Local Convex Hull method as a tool for assessing responses of fauna to habitat restoration: a case study using the perentie (Varanus giganteus: Reptilia: Varanidae) Aust J Zool. 2020;67:27–37. [Google Scholar]
- Cross SL, Tomlinson S, Craig MD, Dixon KW, Bateman PW. Overlooked and undervalued: The neglected role of fauna and a global bias in ecological restoration assessments. Pacific. Conserv Biol. 2019;25:331–341. [Google Scholar]
- Crouzeilles R, Curran M, Ferreira MS, Lindenmayer DB, Grelle CE, Benayas JMR. A global meta-analysis on the ecological drivers of forest restoration success. Nat Commun. 2016;7:1–8. doi: 10.1038/ncomms11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutzen PJ (2006) The “Anthropocene”. Pages 13-18 in E. Ehlers and T. Krafft, editors. Earth System Science in the Anthropocene. Springer Berlin Heidelberg, Berlin, Heidelberg.
- David E, Dixon KW, Menz MH. Cooperative extension: A model of science–practice integration for ecosystem restoration. Trends plant Sci. 2016;21:410–417. doi: 10.1016/j.tplants.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Garcia RA, Clusella-Trullas S. Thermal landscape change as a driver of ectotherm responses to plant invasions. Proc R Soc B. 2019;286:20191020. doi: 10.1098/rspb.2019.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert B, Tunney TD, McCann KS, DeLong JP, Vasseur DA, Savage V, Shurin JB, Dell AI, Barton BT, Harley CDG, Kharouba HM, Kratina P, Blanchard JL, Clements C, Winder M, Greig HS, O’Connor MI. A bioenergetic framework for the temperature dependence of trophic interactions. Ecol Lett. 2014;17:902–914. doi: 10.1111/ele.12307. [DOI] [PubMed] [Google Scholar]
- Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. A framework for community interactions under climate change. Trends Ecol Evol. 2010;25:325–331. doi: 10.1016/j.tree.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Hale R, Mac Nally R, Blumstein DT, Swearer SE. Evaluating where and how habitat restoration is undertaken for animals. Restor Ecol. 2019;27:775–781. [Google Scholar]
- Hale R, Swearer SE. When good animals love bad restored habitats: how maladaptive habitat selection can constrain restoration. J Appl Ecol. 2017;54:1478–1486. [Google Scholar]
- Heneghan L, Miller SP, Baer S, Callaham MA, Jr, Montgomery J, Pavao‐Zuckerman M, Rhoades CC, Richardson S. Integrating soil ecological knowledge into restoration management. Restor Ecol. 2008;16:608–617. [Google Scholar]
- Hilderbrand RH, Watts AC, Randle AM (2005) The myths of restoration ecology. Ecol Soc 10:19
- Huey RB. Physiological consequences of habitat selection. Am Naturalist. 1991;137:S91–S115. [Google Scholar]
- IPBES (2019) Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services.in S. Díaz, J. Settele, E. S. Brondízio, H. T. Ngo, M. Guèze, J. Agard, A. Arneth, P. Balvanera, K. Brauman, and S. H. Butchart, editors. Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. IPBES secretariat, Bonn, Germany.
- Jones ME, Davidson N. Applying an animal‐centric approach to improve ecological restoration. Restor Ecol. 2016;24:836–842. [Google Scholar]
- Jordan WR, Peters RL, Allen EB. Ecological restoration as a strategy for conserving biological diversity. Environ Manag. 1988;12:55–72. [Google Scholar]
- Kearney M, Porter W. Mechanistic niche modelling: combining physiological and spatial data to predict species’ ranges. Ecol Lett. 2009;12:334–350. doi: 10.1111/j.1461-0248.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- Kearney M, Shine R, Porter WP. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc Natl Acad Sci. 2009;106:3835–3840. doi: 10.1073/pnas.0808913106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney M, Simpson SJ, Raubenheimer D, Helmuth B. Modelling the ecological niche from functional traits. Philos Trans R Soc B: Biol Sci. 2010;365:3469–3483. doi: 10.1098/rstb.2010.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney MR, Simpson SJ, Raubenheimer D, Kooijman SALM. Balancing heat, water and nutrients under environmental change: a thermodynamic niche framework. Funct Ecol. 2013;27:950–966. [Google Scholar]
- Kleiber M (1961) The fire of life: an introduction to animal energetics/Max Kleiber. New York: Wiley, New York.
- Kooijman B, Kooijman S. Dynamic energy budget theory for metabolic organisation. Cambridge, New York: Cambridge University Press; 2010. [Google Scholar]
- Kremen C, Williams NM, Aizen MA, Gemmill‐Herren B, LeBuhn G, Minckley R, Packer L, Potts SG, Roulston TA, Steffan‐Dewenter I. Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land‐use change. Ecol Lett. 2007;10:299–314. doi: 10.1111/j.1461-0248.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- Lawton JH. Are there general laws in ecology? Oikos. 1999;84:177–192. [Google Scholar]
- Lister BC, Garcia A. Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc Natl Acad Sci. 2018;115:E10397. doi: 10.1073/pnas.1722477115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madliger CL, Love OP, Hultine KR, Cooke SJ. The conservation physiology toolbox: status and opportunities. Conserv Physiol. 2018;6:coy029. doi: 10.1093/conphys/coy029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer JD. Animals in primary succession: the role of fauna in reclaimed lands. Cambridge, United Kingdom: Cambridge University Press; 1989. [Google Scholar]
- Majer JD. Animals in the restoration process—progressing the trends. Restor Ecol. 2009;17:315–319. [Google Scholar]
- Martin TE. Abiotic vs. biotic influences on habitat selection of coexisting species: climate change impacts? Ecology. 2001;82:175–188. [Google Scholar]
- Mayor SJ, Schneider DC, Schaefer JA, Mahoney SP. Habitat selection at multiple scales. Ecoscience. 2009;16:238–247. [Google Scholar]
- McAlpine C, Catterall CP, Nally RM, Lindenmayer D, Reid JL, Holl KD, Bennett AF, Runting RK, Wilson K, Hobbs RJ. Integrating plant‐and animal‐based perspectives for more effective restoration of biodiversity. Front Ecol Environ. 2016;14:37–45. [Google Scholar]
- McDonald TG Gann J Jonson, Dixon K (2016) International standards for the practice of ecological restoration–including principles and key concepts. Society for Ecological Restoration, Washington, D.C.
- Meyer CL, Sisk TD. Butterfly response to microclimatic conditions following ponderosa pine restoration. Restor Ecol. 2001;9:453–461. [Google Scholar]
- Meyer CL, Sisk TD, Covington WW. Microclimatic changes induced by ecological restoration of ponderosa pine forests in northern Arizona. Restor Ecol. 2001;9:443–452. [Google Scholar]
- Miller BP, Sinclair EA, Menz MH, Elliott CP, Bunn E, Commander LE, Dalziell E, David E, Davis B, Erickson TE. A framework for the practical science necessary to restore sustainable, resilient, and biodiverse ecosystems. Restor Ecol. 2017;25:605–617. [Google Scholar]
- Milling CR, Rachlow JL, Olsoy PJ, Chappell MA, Johnson TR, Forbey JS, Shipley LA, Thornton DH. Habitat structure modifies microclimate: An approach for mapping fine‐scale thermal refuge. Methods Ecol Evol. 2018;9:1648–1657. [Google Scholar]
- Mitchell N, Hipsey MR, Arnall S, McGrath G, Tareque HB, Kuchling G, Vogwill R, Sivapalan M, Porter WP, Kearney MR. Linking eco-energetics and eco-hydrology to select sites for the assisted colonization of Australia’s rarest reptile. Biology. 2013;2:1–25. doi: 10.3390/biology2010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya D, Rogers L, Memmott J. Emerging perspectives in the restoration of biodiversity-based ecosystem services. Trends Ecol Evol. 2012;27:666–672. doi: 10.1016/j.tree.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Morghan KJR, Sheley RL, Svejcar TJ. Successful adaptive management—the integration of research and management. Rangel Ecol Manag. 2006;59:216–219. [Google Scholar]
- Morris, SD, MR Kearney, CN Johnson, and BW Brook (2022) Too hot for the devil? Did climate change cause the mid‐Holocene extinction of the Tasmanian devil Sacrophilus harrisii from mainland Australia? Ecography 2022.
- Murray C, Marmorek D (2003) Adaptive management and ecological restoration. Ecological restoration of southwestern ponderosa pine forests:417–428.
- Noriega JA, Hortal J, Azcárate FM, Berg MP, Bonada N, Briones MJ, Del Toro I, Goulson D, Ibanez S, Landis DA. Research trends in ecosystem services provided by insects. Basic Appl Ecol. 2018;26:8–23. [Google Scholar]
- Nowakowski AJ, Frishkoff LO, Agha M, Todd BD, Scheffers BR. Changing thermal landscapes: merging climate science and landscape ecology through thermal biology. Curr Landsc Ecol Rep. 2018;3:57–72. [Google Scholar]
- Palmer MA, Ambrose RF, Poff NL. Ecological theory and community restoration ecology. Restor Ecol. 1997;5:291–300. [Google Scholar]
- Perring MP, Standish RJ, Price JN, Craig MD, Erickson TE, Ruthrof KX, Whiteley AS, Valentine LE, Hobbs RJ. Advances in restoration ecology: rising to the challenges of the coming decades. Ecosphere. 2015;6:1–25. [Google Scholar]
- Pulliam HR, Danielson BJ. Sources, sinks, and habitat selection: a landscape perspective on population dynamics. Am Naturalist. 1991;137:S50–S66. [Google Scholar]
- Schmitz OJ, Barton BT. Climate change effects on behavioral and physiological ecology of predator–prey interactions: implications for conservation biological control. Biol Control. 2014;75:87–96. [Google Scholar]
- Seavy NE, Howell CA. How can we improve information delivery to support conservation and restoration decisions? Biodivers Conserv. 2010;19:1261–1267. [Google Scholar]
- Seebacher F, Franklin CE. Determining environmental causes of biological effects: the need for a mechanistic physiological dimension in conservation biology. Philos Trans R Soc B: Biol Sci. 2012;367:1607–1614. doi: 10.1098/rstb.2012.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERA (2017) National Standards for the Practice of Ecological Restoration in Australia. Second Edition. Society for Ecological Restoration Australasia. Washington, D.C.
- Shimamoto CY, Padial AA, da Rosa CM, Marques MC. Restoration of ecosystem services in tropical forests: A global meta-analysis. PloS one. 2018;13:e0208523. doi: 10.1371/journal.pone.0208523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suding K, Higgs E, Palmer M, Callicott JB, Anderson CB, Baker M, Gutrich JJ, Hondula KL, LaFevor MC, Larson BM. Committing to ecological restoration. Science. 2015;348:638–640. doi: 10.1126/science.aaa4216. [DOI] [PubMed] [Google Scholar]
- Suding KN. Toward an era of restoration in ecology: Successes, failures, and opportunities ahead. Annu Rev Ecol, Evol, Syst. 2011;42:465–487. [Google Scholar]
- Sunderland T, Sunderland‐Groves J, Shanley P, Campbell B. Bridging the gap: How can information access and exchange between conservation biologists and field practitioners be improved for better conservation outcomes? Biotropica. 2009;41:549–554. [Google Scholar]
- Sutherland WJ, Pullin AS, Dolman PM, Knight TM. The need for evidence-based conservation. Trends Ecol Evol. 2004;19:305–308. doi: 10.1016/j.tree.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Tarszisz E, Dickman CR, Munn AJ (2014) Physiology in conservation translocations. Conserv Physiol 2:cou054 [DOI] [PMC free article] [PubMed]
- Taylor B, Kremsater L, Ellis R (1997) Adaptive management of forests in British Columbia. British Columbia, Forest Practices Branch, Victoria, BC, Canada.
- Thompson ME, Halstead BJ, Donnelly MA. Thermal quality influences habitat use of two anole species. J Therm Biol. 2018;75:54–61. doi: 10.1016/j.jtherbio.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Tomlinson S. The construction of small-scale, quasi-mechanistic spatial models of insect energetics in habitat restoration: A case study of beetles in Western Australia. Divers Distrib. 2020;26:1016–1033. [Google Scholar]
- Tomlinson S, Arnall SG, Munn A, Bradshaw SD, Maloney SK, Dixon KW, Didham RK. Applications and implications of ecological energetics. Trends Ecol Evol. 2014;29:280–290. doi: 10.1016/j.tree.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Tomlinson S, Dixon KW, Didham RK, Bradshaw SD. Landscape context alters cost of living in honeybee metabolism and feeding. Proc R Soc B: Biol Sci. 2017;284:20162676. doi: 10.1098/rspb.2016.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson S, Tudor EP, Turner SR, Cross S, Riviera F, Stevens J, Valliere J, Lewandrowski W (2022) Leveraging the value of conservation physiology for ecological restoration. Restor Ecol 30:e13616
- Tomlinson S, Webber BL, Bradshaw SD, Dixon KW, Renton M. Incorporating biophysical ecology into high-resolution restoration targets: insect pollinator habitat suitability models. Restor Ecol. 2018;26:338–347. [Google Scholar]
- Tougeron K, Van Baaren J, Burel F, Alford L. Comparing thermal tolerance across contrasting landscapes: first steps towards understanding how landscape management could modify ectotherm thermal tolerance. Insect Conserv Divers. 2016;9:171–180. [Google Scholar]
- Tudor EP (2021) The patterns and processes of insect pollinator re-assembly across a post-mining restoration landscape. Master by Research Thesis. Curtin University.
- Tuff K, Tuff T, Davies K. A framework for integrating thermal biology into fragmentation research. Ecol Lett. 2016;19:361–374. doi: 10.1111/ele.12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylianakis JM, Didham RK, Bascompte J, Wardle DA. Global change and species interactions in terrestrial ecosystems. Ecol Lett. 2008;11:1351–1363. doi: 10.1111/j.1461-0248.2008.01250.x. [DOI] [PubMed] [Google Scholar]
- Verberk WC, Van Noordwijk C, Hildrew AG. Delivering on a promise: integrating species traits to transform descriptive community ecology into a predictive science. Freshw Sci. 2013;32:531–547. [Google Scholar]
- Wainwright CE, Staples TL, Charles LS, Flanagan TC, Lai HR, Loy X, Reynolds VA, Mayfield MM. Links between community ecology theory and ecological restoration are on the rise. J Appl Ecol. 2018;55:570–581. [Google Scholar]
- Weiner J. On the Practice of Ecology. J Ecol. 1995;83:153–158. [Google Scholar]
- Williams BK. Adaptive management of natural resources—framework and issues. J Environ Manag. 2011;92:1346–1353. doi: 10.1016/j.jenvman.2010.10.041. [DOI] [PubMed] [Google Scholar]
- Withers PC (1992) Comparative animal physiology. Saunders College Pub. Philadelphia.
- WWF (2020) Living Planet Report 2020-Bending the curve of biodiversity loss. World Wildlife Fund, Gland, Switzerland.
- Young JC, Waylen KA, Sarkki S, Albon S, Bainbridge I, Balian E, Davidson J, Edwards D, Fairley R, Margerison C, McCracken D, Owen R, Quine CP, Stewart-Roper C, Thompson D, Tinch R, van den Hove S, Watt A. Improving the science-policy dialogue to meet the challenges of biodiversity conservation: Having conversations rather than talking at one-another. Biodivers Conserv. 2014;23:387–404. [Google Scholar]