Highlights
-
•
The major depressive disorder patients (MDD) showed decreased cortical volume in the superior temporal gyrus (STG) and middle temporal gyrus (MTG) than controls.
-
•
The MDD patients showed decreased surface area in the bilateral STG, temporal pole gyrus, entorhinal cortex, left inferior temporal gyrus, and fusiform gyrus than the controls.
-
•
The MDD patients exhibited lower functional connectivity (FC) between STG/MTG and regions of the visual network than controls.
-
•
The structural and functional findings involving the sensory processing areas showed a good classification between the MDD patients and controls.
Keywords: Multi-modal MRI, Surface-based analysis, Surface area, Cortical volume, Functional connectivity
Abstract
Background
Multi-modal magnetic resonance imaging (MRI) measures are supposed to be able to capture different brain neurobiological aspects of major depressive disorder (MDD). A fusion analysis of structural and functional modalities may better reveal the disease biomarker specific to the MDD disease.
Methods
We recruited 30 MDD patients and 30 matched healthy controls (HC). For each subject, we acquired high-resolution brain structural images and resting-state fMRI (rs-fMRI) data using a 3 T MRI scanner. We first extracted the brain morphometric measures, including the cortical volume (CV), cortical thickness (CT), and surface area (SA), for each subject from the structural images, and then detected the structural clusters showing significant between-group differences in each measure using the surface-based morphology (SBM) analysis. By taking the identified structural clusters as seeds, we performed seed-based functional connectivity (FC) analyses to determine the regions with abnormal FC in the patients. Based on a logistic regression model, we performed a classification analysis by selecting these structural and functional cluster-wise measures as features to distinguish the MDD patients from the HC.
Results
The MDD patients showed significantly lower CV in a cluster involving the right superior temporal gyrus (STG) and middle temporal gyrus (MTG), and lower SA in three clusters involving the bilateral STG, temporal pole gyrus, and entorhinal cortex, and the left inferior temporal gyrus, and fusiform gyrus, than the controls. No significant difference in CT was detected between the two groups. By taking the above-detected clusters as seeds to perform the seed-based FC analysis, we found that the MDD patients showed significantly lower FC between STG/MTG (CV’s cluster) and two clusters located in the bilateral visual cortices than the controls. The logistic regression model based on the structural and functional features reached a classification accuracy of 86.7% (p < 0.001) between MDD and controls.
Conclusion
The present study showed sensory abnormalities in MDD patients using the multi-modal MRI analysis. This finding may act as a disease biomarker distinguishing MDD patients from healthy individuals.
1. Introduction
Major depressive disorder (MDD) is a severe psychiatric disorder that severely impacts individual life quality and has a high worldwide prevalence (Malhi and Mann, 2018). As ranked by World Health Organization (WHO), depression has been one of the leading causes of the global burden of disease (WHO, 2008). The 12-month and prevalence lifetime prevalence for MDD were observed to be 6.6% and 16.2% respectively. Moreover, MDD was more commonly reported in women compared to men, indicating a twice higher occurrence rate (Kupfer et al., 2012). Several hypotheses involving the monoamine, hypothalamic–pituitary–adrenal axis, inflammation, neuroplasticity, genes, and brain structural/functional changes have been proposed to understand the pathophysiology of depression (Jeon and Kim, 2016, Kennis et al., 2020, Kupfer et al., 2012, Malhi and Mann, 2018, Miller and Raison, 2016, Price and Drevets, 2012). Among them, neuroimaging techniques comprise a powerful method to detect brain structural and functional changes. Many studies have already analyzed neuroimaging biomarkers specific to depression and subtypes of depression (Cohen et al., 2021, Kube et al., 2020, Sui et al., 2020). However, despite extensive research, the lack of spatially converged findings across modalities in MDD still poses challenges to its diagnosis and treatment and calls for more investigation (Cash et al., 2023, Gray et al., 2020, Malhi and Mann, 2018, Müller et al., 2017).
Magnetic resonance imaging (MRI) has been widely used to delineate brain structural and functional properties in vivo. Brain structural and resting-state functional MRI techniques are largely used to explore neuroimaging biomarkers specific to MDD (Pilmeyer et al., 2022). The structural MRI (sMRI) modality enables the depiction of neuroanatomical morphological changes, and the resting-state fMRI (rs-fMRI) modality enables the measurement of brain functional abnormalities, like abnormal functional relationships between brain regions. The voxel-based morphometry (VBM) (Good et al., 2001), surface-based morphometry (SBM) (Pantazis et al., 2004), and rs-fMRI functional connectivity (FC) (Mulders et al., 2015) are commonly used to analyze brain anatomical and functional characteristics in MDD. These methods have revealed large-scale alterations in brain regions associated with cognitive and affective functions (Dichter et al., 2015, Disner et al., 2011, Wang et al., 2012). Previous sMRI studies (Arnone et al., 2012, Bora et al., 2012, Du et al., 2012, Koolschijn et al., 2009, Li et al., 2020, Zhang et al., 2016, Zhao et al., 2014, Zhu et al., 2022) reported abnormal gray matter (GM) volume and thickness in MDD in regions of the prefrontal cortex, orbitofrontal cortex, temporal cortex, insular cortex, cingulate cortex, hippocampus, striatum, amygdala, and thalamus. In addition, previous rs-fMRI studies (Briley et al., 2022, Javaheripour et al., 2021, Long et al., 2020, Mulders et al., 2015, Veer et al., 2010) frequently reported resting-state FC abnormalities in regions involved in the default mode network, salience network, dorsal attention network, visual network, and somatosensory network. Although promising progress has been made in MDD research, the findings from different MRI modalities are not well-converged (Cash et al., 2023, Gray et al., 2020, Müller et al., 2017). The spatially heterogeneous distribution of these findings across multiple MRI modalities (Liu et al., 2016, Zhang et al., 2022) may limit the knowledge of neuroimaging markers for diagnoses and treatments of MDD (Zhuo et al., 2019).
A considerable solution for this heterogeneity is to include multidisciplinary technologies such as multimodality MRI and artificial intelligence approaches (Zhuo et al., 2019). Multi-modal MRI measures can capture different aspects of brain structural and functional properties (Glasser, Coalson, et al., 2016), and a fusion analysis of them can be powerful in identifying the disease markers (Calhoun and Sui, 2016, Liu et al., 2018, Meng et al., 2017, Sui et al., 2015, Sui et al., 2018). To detect confirmative imaging results, previous studies overlapped regions showing between-group differences across measures (Hong et al., 2017, Liu et al., 2016) and used multivariate algorithms such as the canonical correlation analysis (Liu et al., 2018, Meng et al., 2017, Sui et al., 2015, Sui et al., 2018) to characterize the association between brain structural/functional properties and clinical symptoms and behaviors.
Surface-based analysis (SBA) is considered to provide a better framework to analyze multi-modal measurements than volume-based analysis (Glasser et al., 2013) for two main reasons. First, the SBA approach enables simultaneously analyzing multiple structural measures such as cortical thickness (CT), surface area (SA), and cortical volume (CV) (Peng et al., 2015). Second, due to subject-wise variabilities in cortical folding patterns (Glasser, Smith, et al., 2016), the SBA approach may provide better cortical spatial localizations (Coalson et al., 2018, Glasser et al., 2016b), alignments (Glasser et al., 2016b, Robinson et al., 2018, Van Essen, 2004), morphological identification and visualization (Dale et al., 1999, Xu et al., 2016) relative to the voxel-based analysis (VBA). A recent study (Brodoehl et al., 2020) suggested that the surface-based smoothing process reduced signal contamination and enhanced brain activity and FC results compared to the volumetric smoothing process. Hence, we were motivated to perform a surface-based multi-modal fusion analysis, involving the detection of the structural alteration and the FC alterations based on structural findings in MDD.
The present study aimed at detecting brain abnormalities in MDD patients using surface-based multi-modal MRI analysis. We used 30 MDD patients and 30 age-, sex- and education-level-matched healthy controls (HC). We first detect the clusters with significant differences in the CV, CT, and SA measures between the MDD and HC groups. We then determined brain FC alterations associated with structural changes that we found in MDD patients. Finally, we investigated whether these structural and functional abnormalities can successfully distinguish MDD from HC or not by using a logistical regression model.
2. Materials and methods
2.1. Subjects
Thirty patients with MDD and thirty age-, gender- and education-matched HCs were recruited from the Department of Psychiatry at the Affiliated Brain Hospital of Guangzhou Medical University. All subjects who qualified for this study were right-handed and aged from 18 to 60 years. All MDD patients were clinically diagnosed according to the Structured Clinical Interview for DSM-IV Patient Edition (Glasofer et al., 2017) and none of them had any other comorbid psychotic or neurological diseases. The severity of depression was evaluated using the 24-item Hamilton Depression Rating Scale (HDRS) (Williams, 1988) and each of the MDD patients had at least 21 scores on HDRS. In this study, 26 MDD patients never received antidepressant treatment (i.e., medication-free), and the other 4 MDD patients took no medication for three months before participating in this study (i.e., medical-naive). The healthy subjects were screened with the DSM-IV Non-Patient Edition to confirm the lifetime absence of Axis I illnesses and had no history of psychiatric illness in any two lines of first- to third-degree biological relatives. All the MDD patients and healthy controls reported no lifetime history of seizures, head trauma, serious medical or surgical illness, substance abuse or dependence, or contraindications for MRI. Moreover, none of the subjects had abnormal MRI signals in the brain checked based on the clinical T1-weighted and T2-weighted FLAIR MRI images. The study protocol was approved by the Ethics Committee of Affiliated Brain Hospital of Guangzhou Medical University, and all subjects gave their written informed consent.
2.2. MRI data acquisition
All MRI data were acquired from a 3.0 Tesla MRI system (Achieva X-series scanner; Philips, Medical Systems, Best, Netherlands) with an 8-channel phased-array head-coil at the Department of Radiology in the Affiliated Brain Hospital of Guangzhou Medical University. Tight but comfortable foam padding was used to reduce head motion, and earplugs were provided to muffle scanner noise.
The high-resolution brain structural images were obtained with T1-weighted (T1w) 3D turbo field-echo (TFE) and T2-weighted (T2w) 3D turbo spin-echo (TSE) sequences. The T1w TFE sequence parameters were as follows: TR = 8.2 ms, TE = 3.7 ms, flip angle = 7°, the field of view (FOV) = 256 × 256 mm2, data matrix = 256 × 256, slice thickness = 1 mm, voxel size = 1.0 mm3, and 188 sagittal slices. The T2w TSE sequence parameters were: TR = 2,500 ms, TE = 250 ms, flip angle = 90°, FOV = 256 × 256 mm2, data matrix = 256 × 256, slice thickness = 1 mm, voxel size = 1.0 mm3, and 188 sagittal slices. The rs-fMRI was obtained with a gradient-echo echo-planar-imaging (EPI) sequence with the following parameters: TR = 2,000 ms, TE = 30 ms, flip angle = 90°, FOV = 220 × 220 mm2, data matrix = 64 × 64, slice thickness = 4 mm with interslice gap = 0.6 mm, voxel size = 3.4 mm × 3.4 mm × (4.0 + 0.6 mm), and 33 axial interleaved slices. The rs-fMRI scanning lasted about 8 min and acquired 240 time points for each subject. During the rs-fMRI scanning, subjects were instructed to relax with their eyes closed and not to sleep or think about things in particular. All of the images were acquired in the same session for each subject.
2.3. Structural MRI data pre-processing
The structural MRI (sMRI) data, including T1w and T2w images, were visually inspected and rated on a four-point quality scale (poor, fair, good, and excellent) by a neuroradiologist (HW). Only the “good” or “excellent” images were included in this study. No imaging datasets were excluded according to this threshold. All the eligible images were preprocessed by using the structural Human Connectome Project (HCP) pipeline (version 3.27) (Glasser et al., 2013).
The sMRI data were preprocessed by following partial sessions of HCP structural pipelines which involved the PreFreeSurfer and FreeSurfer. In the PreFreeSurfer section, the T1w images were cropped, AC-PC aligned, and brain extracted. PreFreeSurfer produced an undistorted “native” structural volume space for each subject, aligned the T1w and T2w images, performed a bias field correction, and registered the subject's native structural volume to the MNI space. Due to the lack of structural field maps, the gradient nonlinearity correction was not included. In the FreeSurfer section, we conducted the procedures of the auto-recon pipeline of FreeSurfer, which used the brain mask from PreFreeSurfer as an input, performed brain extraction, brain segmentations, surface tessellation, spheric registration, and morphometric measurements. From these procedures, we obtained the cortical measurements of the CT, SA, and CV.
2.4. Resting-state fMRI preprocessing
The rs-fMRI data were preprocessed with the pipeline of DPABI (https://rfmri.org/dpabi). The pipeline included the following steps: exclusion of the first 10 volumes, slice-timing and head motion correction, detrend, regression of Friston-24 motion parameters and signals of the white matter and cerebrospinal fluid, and temporal filtering (0.01–0.1 Hz). In the preprocessing, we set the threshold of the rs-fMRI images as the head movement < 1.5 mm in any plane or rotation < 1.5° in any axis. No subject was excluded according to this threshold.
2.5. Cortical measurement generation
We first extracted three structural morphological measures including the cortical thickness (CT, lh/rh.thickness), surface area (SA, lh/rh.area), and cortical volume (CV, lh/rh.volume) from the FreeSurfer section described above. Then, we took a surface-based down-resampling procedure to normalize these measures. The normalization steps (Glasser et al., 2013, Zhang et al., 2022) were as follows: (1) individual midthickness surfaces were averaged from white matter (WM) and pial surfaces and were resampled to the fs_LR 164 k surface and then to the fs_LR 32 k surface (2-mm vertex spacing), (2) CT, SA, and CV were separately resampled to fs_LR 32 k surface via the fs_LR 164 k surface, and (3) all measures were smoothed (Glasser et al., 2013, Liu et al., 2016, Zhang et al., 2022) with a Gaussian kernel of full-width-at-half-maximum (FWHM) = 10 mm (σ = 4.25 mm, ) for keeping a consistency in smoothing effects. Finally, we examined the differences in each of these measures between MDD and HC groups, which were denoted as structural alterations, and the significant clusters were used as seeds in the following functional analysis.
After the identification of structural alterations, we performed the seed-based FC analysis on the cortical surface with the following steps: (a) the functional images (timeseries) were co-registered to the cortical surface using a boundary-based registration (BBR). Individual mean functional images were registered to the preprocessed structural images to generate transformation matrices. The other functional images were projected to the cortical surface by sampling along the midthickness surface using the BBR transformation matrix. (b) The projected functional images were resampled to fs_LR 32 k surface via fs_LR 164 k, and smoothed with a Gaussian kernel of 10-mm FWHM. (c) The FC between the seed region and rest vertices of bilateral surfaces were calculated by taking each structural cluster as the seed (wb_command -cifti-average-roi-correlation). Finally, we examined the difference in the seed-based FC between MDD and HC groups.
2.6. Statistical analyses
2.6.1. Demographical statistics
We assessed group differences in gender, age, and education years between MDD and HC groups. In the demographical statistics, a χ2 test was used for comparing the gender differences, while independent t-tests were used for comparing age and years of education between the two groups. The significance level was set at p < 0.05.
2.6.2. Multidimensional structural analyses
To assess structural morphological differences between the MDD and HC groups, we applied the Permutation Analysis of Linear Models (PALM) (Winkler et al., 2016), a tool that allows inference using permutation methods, to the structural measures of the left- and right-hemisphere surfaces separately. In specific, we calculated between-group differences in each structural measure on each vertex by using the PALM (5,000 permutations) with gender, age, and educational level as covariates. Cluster-forming threshold (cluster) and family-wise error (FWE) methods were applied for the multiple-comparison correction (pcluster+FWE < 0.05). To determine the significant clusters of each measure after the corrections, we split measure-specific results into independent clusters by using Workbench/wb_command -cifti-find-clusters. These clusters were reported based on the Desikan-Killiany atlas (Desikan et al., 2006).
2.6.3. Seed-based FC analyses
To detect brain FC alterations associated with structural alterations in MDD patients, we used clusters showing significant differences between MDD and HC groups in the Multidimensional structural analyses as seed regions. We then applied PALM (5,000-time permutations) with seed-based FC as the dependent variable, group as the independent variable, and gender, age, and educational level as the covariates. Afterward, the seed-based FC results were thresholded by a cluster-forming FWE method (pcluster+FWE < 0.05) and split into the clusters using the Workbench. FC results after multiple-comparison corrections were reported based on the network parcellation atlas (Yeo et al., 2011).
2.6.4. Classification analysis
To distinguish MDD from controls based on our multi-modal results, we applied a cross-validated logistic regression model by using the classification module of Matlab 2020a. The steps were as follows. (1) Feature selection: The cluster-wise averaged values from both structural morphological and seed-based FC analyses were taken as features. (2) Model selection: A logistic regression model was used to detect the model classification based on both structural and functional features. (3) A k-fold cross-validation: A 10-fold cross-validation procedure was applied to evaluate the accuracy of the logistic regression model. In each of the cross-validation iterations, 90% of the subjects were selected as the training set, while the rest 10% of the subjects were selected as the test set. (4) Statistical significance: For confirming the significance of our model and its probability distance to the chance level, a label-shuffled permutation was applied (5,000 times). In each permutation loop, the group labels (MDD/HC) of subjects were randomly assigned and re-entered into the model space to compute the classification accuracy. After all permutation loops (5,000 times), the null accuracy distribution based on the label-shuffled permutation was calculated and then the model performance was identified by seeking the position of the accuracy gotten from our model in the generated null accuracy distribution.
To assess the performance of the logistic regression model, we generated classification accuracy and statistical significance. For the model performance, a confusion matrix and a receiver-operating characteristics (ROC) plot were created to show the model's classification accuracy and sensitivity.
3. Results
3.1. Demographics
Table 1 lists the demographical information of the MDD and HC groups. No significant difference was found in gender, age, and educational level between MDD and HC groups.
Table 1.
Demographical information of the Major Depressive Disorder (MDD) and healthy controls (HC) groups.
| Parameter | MDD (n = 30) |
HC (n = 30) |
p-value (two-tailed) |
|---|---|---|---|
| Gender | 12 M/18F | 12 M/18F | 1.00 a |
| Age (years old) | 26.7 ± 5.9 | 26.3 ± 5.6 | 0.82b |
| Educational level (years) | 13.9 ± 3.5 | 13.9 ± 2.4 | 0.98b |
| Onset age (years old) | 24.5 ± 6.5 | N/A | |
| Duration time (years) | 2.23 ± 2.6 | N/A | |
| HDRS | 31.6 ± 7.3 | N/A |
aχ2-test. btwo-sample t-tests.
Abbreviations: HDRS, Hamilton Depression Rating Scale; MDD, Major Depressive disorder; HC, healthy controls; N/A, non-applicable; M, male; F, female.
3.2. Surface-based structural properties
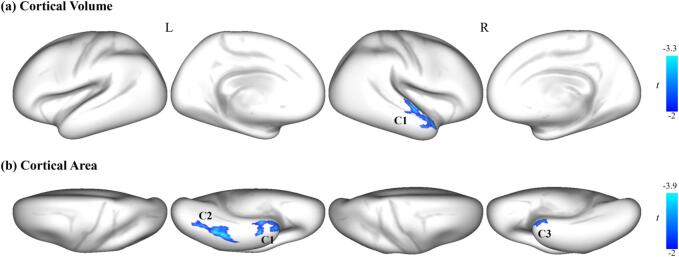
To detect the abnormal brain structure in MDD, we compared the morphological properties between the two groups. We found that the MDD patients had significantly lower cortical volume (CV) and surface area (SA) in the temporal and occipital cortices than the HC (Fig. 1, pcluster+FWE < 0.05). However, no significant between-group difference was found in the cortical thickness (CT). Fig. 1A shows that the MDD patients had significantly lower CV than the HC group in a cluster, involving the superior temporal gyrus (STG) and middle temporal gyrus (MTG). Fig. 1B shows that the MDD patients had significantly lower SA in three clusters than the HC group. The first cluster involves vertices of the left inferior temporal lobe (ITG), STG, temporal pole gyrus (TPG), entorhinal cortex (EC), and fusiform gyrus (FG). The second cluster includes vertices of the left FG and ITG. The third cluster involves vertices of the right STG, TPG, and EC. The detailed information for these clusters is listed in Table 2.
Fig. 1.
Regions with significantly decreased brain structural morphological properties obtained from the surface-based analysis in the MDD group relative to the HC group (MDD < HC). (a) Cortical volume, and (b) Surface area. The color bar indicates the vertex-wise t values survived under a cluster-forming thresholded family-wise error (FWE) correction (p < 0.05).
Table 2.
The cortical structural alterations of the Major Depressive Disorder (MDD) group compared with the healthy control (HC) group.
| Cluster | MNI | t | Vertices | Brain Areas | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| CV | ||||||
| C1 | 60 | −4 | 0 | −3.33 | 374 | R.STG, R.MTG |
| SA | ||||||
| C1 | −42 | −10 | −41 | −3.65 | 297 | L.ITG, L.TPG, L.EC, L.FG, L.STG |
| C2 | −40 | −48 | −20 | −3.89 | 190 | L.FG, L.ITG |
| C3 | 50 | 2 | −12 | −3.06 | 212 | R.STG, R.TPG, R.EC |
This table lists the MNI coordinates of the clusters and the associated areas showing significantly decreased cortical volume (CV) and surface area (SA) in major depressive disorder (MDD) patients relative to the healthy controls (HC). These clusters were reported based on the Desikan-Killiany atlas (Desikan et al., 2006).
Abbreviations: STG, superior temporal gyrus; MTG, middle temporal gyrus; ITG, inferior temporal gyrus; TPG, temporal pole gyrus; EC, entorhinal cortex; FG, fusiform gyrus. C1, C2, and C3 refer the 1st, 2nd, and 3rd cluster, respectively.
The MNI coordinates were on the mid-thickness surface (fs_LR 32 k).
3.3. Seed-based functional connectivity
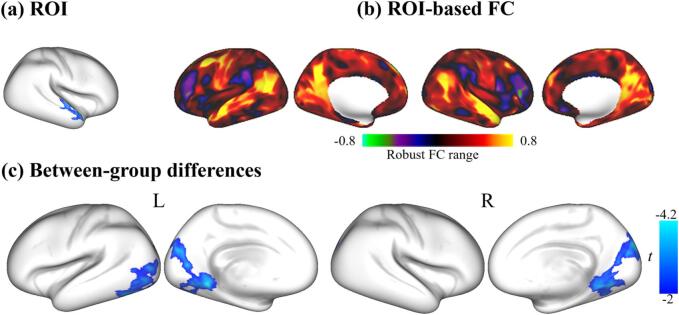
To determine the FC alterations in the MDD, we took the significant structural clusters as the seeds and compared the seed-based FC between the two groups. Fig. 2 shows that the MDD patients had significantly lower FC in two regions than the controls when we took the cluster at STG/MTG (obtained from CV comparison) as the seed. These regions are mainly distributed in the visual network according to the network parcellation atlas (Yeo et al., 2011).
Fig. 2.
The seed-based functional connectivity (FC) analysis in the MDD group relative to the HC group. (a) The selected seed was the region showing significant between-group differences in the cortical volume. (b) An example of the ROI-based FC maps for an individual. The color bar shows the robust range of Fisher’s r-to-z transformed FC. (c) Clusters showed significantly decreased FC in the MDD group relative to the HC group. The color bar indicates the vertex-wise t values survived under a cluster-forming thresholded family-wise error (FWE) correction (p < 0.05).
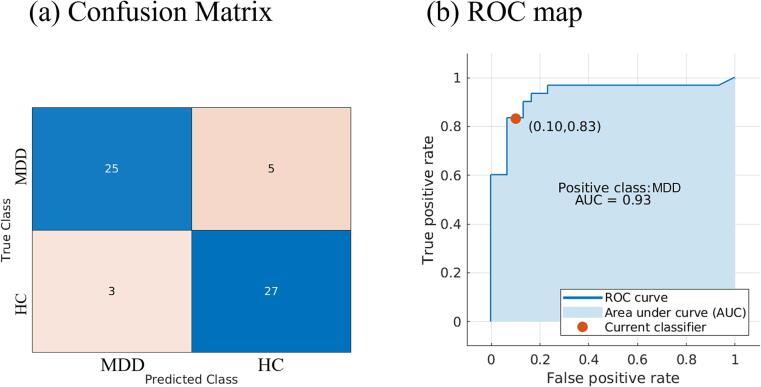
3.4. Classification
The logistic regression model analysis showed an 86.7% classification accuracy in distinguishing the MDD patients from the HC (Fig. 3). The area under the curve (AUC) was 0.93, the true positive rate (TPR) was 83%, and the false positive rate (FPR) was 10%.
Fig. 3.
The confusion matrix and receiver operating characteristic (ROC) curve of the logistic regression model. (a) The confusion matrix lists the classification performance of the logistic regression model. (b) The area under the curve (AUC) was 0.93, the true positive rate (TPR) was 0.83, and the false positive rate (FPR) was 0.1.
4. Discussion
The present study applied a multi-modal fusion analysis, which included structural analysis and seed-based FC analysis, to detect the cortical morphological vulnerabilities and functional network alterations associated with structural abnormalities in MDD. The MDD patients had significantly lower CV and SA in the temporal and occipital cortices than the controls. The seed-based FC analysis revealed lower FC between STG/MTG and regions in the visual network relative to the controls. In addition, structural values (CV and SA) in those regions and FC between them provided a high classification accuracy in distinguishing MDD patients from controls.
4.1. Structural alterations
The surface-based analyses showed that the values of CV and SA were lower in the regions involving the STG, MTG, EC, FG, ITG, and TPC in the MDD patients than in the HC (Fig. 1 and Table 2). These regions largely overlapped with brain sensory cortices (i.e., visual and auditory cortices). The STG, MTG, ITG, and TPC are located in the lateral temporal cortices, which are believed to be engaged in the auditory, visual, facial cognition, and language-associated functions (Acheson and Hagoort, 2013, Cheng et al., 2016, Ishai et al., 1999, Kellogg, 2003, Sakurai et al., 2008). The abnormality in these regions was found to be associated with symptom severity and suicidal behaviors in MDD patients (Cao et al., 2016, Fitzgerald et al., 2008, Hwang et al., 2010, Kocsis et al., 2021, Li et al., 2010, Niu et al., 2017, Pan et al., 2015, Peng et al., 2015, Phillips et al., 2015, Takahashi et al., 2010). The FG is involved in face cognition and social communication (Haxby et al., 2002, Hoffman and Haxby, 2000), and its alteration may influence the vulnerability to depression (Papmeyer et al., 2015). The EC acts as the gateway between the hippocampus and neocortex, and its volume was found to be decreased in patients with depression (Furtado et al., 2008). Moreover, optogenetic activation in the entorhinal-visual circuit was found to promote depressive behaviors in mice (Lu et al., 2022). Although depression is not a typical psychiatric disorder characterized by disturbed sensory perception, the sensory abnormality (e.g., visual, auditory, and touching systems) was found to contribute to the anhedonia symptoms and individual emotional experiences in MDD (Bubl et al., 2012, Fitzgerald, 2013, Qiao et al., 2013, Schwenzer et al., 2012). The deficits in auditory and visual perception may potentially lead to attentional and control deficits in MDD patients (Grange and Rydon-Grange, 2022, Zweerings et al., 2019). Moreover, accumulating evidence reported interactions between sensory perception and depressive symptoms in MDD patients (Canbeyli, 2010, Canbeyli; Canbeyli, 2013). For instance, a bidirectional relationship was suggested that sensory stimulation can modulate depression and in turn, depression impairs sensory perception (Canbeyli, 2022, Canbeyli).
4.2. Seed-based FC alterations
The present study found decreased FC between the cluster at MTG/STG and clusters in the bilateral occipital cortices (Fig. 2C). The impaired activity of visual cortices was found to be related to abnormal cognitive and emotional functions in MDD patients (Le et al., 2017). A previous study also found that the FC of the visual network may contribute to distinguishing MDD patients from controls (Zeng et al., 2012). Moreover, repetitive transcranial magnetic stimulation to the visual cortices was reported to alleviate depressive symptoms (Zhang et al., 2021) and its activation was found to correlate with treatment responses in MDD patients (Furey et al., 2013). Our study reported significantly decreased FC between the STG/MTG and bilateral visual network, suggesting a disrupted connection between the auditory and visual cortices in MDD patients, which is consistent with previous studies (He et al., 2016, Lu et al., 2020). Taken together, the structural and functional alterations observed in the present study may suggest a disrupted sensory system in MDD patients.
4.3. Classification
The present study showed that the patients had brain structural and functional alterations in regions related to sensory processing functions (Fig. 1, Fig. 2). Based on these multi-modal MRI alterations, we detected a relatively high classification rate (86.7%) in distinguishing the MDD patients from the healthy controls (Fig. 3). These findings indicated sensory processing abnormalities in individuals with MDD, which are consistent with previous studies (Bubl et al., 2010, Chen et al., 2022, Fitzgerald, 2013, Gong et al., 2020, Xia et al., 2022, Zweerings et al., 2019).
Furthermore, altered activity and connectivity of sensory areas have been identified as indicators of treatment responses and factors modulating depressive symptoms in individuals with MDD (Canbeyli, 2022, Canbeyli, Dichter et al., 2015, Farb et al., 2011, Furey et al., 2013, Gao et al., 2018, Li et al., 2021, Rolle et al., 2020). Although the classification model performance is varied by modalities, cross-validation schemes, sample sizes, and classification algorithms, our classification result aligns with previous research that classified MDD patients from controls or predicted treatment outcomes (Aleem et al., 2022, Cohen et al., 2021, Gao et al., 2018). Previous reviews reported that the overall classification accuracy was 82% (Lee et al., 2018) and AUC was 0.84 (Cohen et al., 2021) for predicting treatments in MDD patients. The classification accuracy between MDD and HC reviewed by (Gao et al., 2018) was discovered to be ranged from 45% to 99% (Lord et al., 2012, Sundermann et al., 2017). In line with these studies, our cross-validated logistic regression model has shown a good classification performance, suggesting that sensory processing abnormalities may potentially serve as one of the disease biomarkers in MDD (Lu et al., 2020, Xia et al., 2022).
5. Conclusion
The present study showed that MDD patients had structural and functional alterations in the sensory system that contributed to a high classification accuracy in distinguishing the MDD patients from controls. The findings of structural and functional alterations in the sensory system may act as a neuroimaging biomarker in MDD disease.
6. Limitations
Several limitations should be considered in our study. First, the sample size in the current study is relatively small, which may bias the results and impact the result reproducibility. Future studies should address this issue by employing a larger sample size or including an independent sample for validation. Second, the subcortical areas were not considered in the SBA approach. In fact, the cortico-subcortical (limbic) circuits have been proposed to be involved in the pathophysiology of MDD (Disner et al., 2011, Kupfer et al., 2012). Future studies should consider the effect of subcortical areas on the mechanisms of MDD. Third, the removal of global signals can reduce physiological noise and alter neural signal distribution, and this approach is still in debate (Aquino et al., 2020, Murphy and Fox, 2017, Power et al., 2017). Future multi-modal MRI studies may also consider the effect of global signals.
8. Data availability statement
Data and code are available from the corresponding author upon reasonable request.
9. Competing interests statement
The authors declare that they have no competing financial interests.
CRediT authorship contribution statement
Shufei Zhang: Conceptualization, Methodology, Formal analysis, Writing – original draft, Writing – review & editing. Shenglin She: Conceptualization, Methodology, Data curation, Formal analysis, Writing – review & editing. Yidan Qiu: Writing – review & editing. Zezhi Li: Data curation. Xiaoyan Wu: Writing – review & editing. Huiqing Hu: Data curation, Writing – review & editing. Wei Zheng: Data curation. Ruiwang Huang: Conceptualization, Methodology, Formal analysis, Supervision, Writing – original draft, Writing – review & editing. Huawang Wu: Conceptualization, Methodology, Formal analysis, Supervision, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported in part by the Natural Science Foundation of China (Grants 81871338, 81571333, 82171914), Guangzhou Municipal Key Discipline in Medicine (2021-2023), Guangzhou High-level Clinical Key Specialty, Guangzhou Research-oriented Hospital.
Contributor Information
Ruiwang Huang, Email: ruiwang.huang@gmail.com.
Huawang Wu, Email: huawangwu@126.com.
Data availability
Data will be made available on request.
References
- Acheson D.J., Hagoort P. Stimulating the Brain’s Language Network: Syntactic ambiguity resolution after TMS to the inferior frontal gyrus and middle temporal gyrus. J. Cogn. Neurosci. 2013;25(10):1664–1677. doi: 10.1162/jocn_a_00430. [DOI] [PubMed] [Google Scholar]
- Aleem S., Huda N.u., Amin R., Khalid S., Alshamrani S.S., Alshehri A. Machine learning algorithms for depression: diagnosis, insights, and research directions. Electronics. 2022;11(7):1111. [Google Scholar]
- Aquino K.M., Fulcher B.D., Parkes L., Sabaroedin K., Fornito A. Identifying and removing widespread signal deflections from fMRI data: Rethinking the global signal regression problem. Neuroimage. 2020;212 doi: 10.1016/j.neuroimage.2020.116614. [DOI] [PubMed] [Google Scholar]
- Arnone D., McIntosh A.M., Ebmeier K.P., Munafò M.R., Anderson I.M. Magnetic resonance imaging studies in unipolar depression: Systematic review and meta-regression analyses. Eur. Neuropsychopharmacol. 2012;22(1):1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Bora E., Fornito A., Pantelis C., Yücel M. Gray matter abnormalities in Major Depressive Disorder: A meta-analysis of voxel based morphometry studies. J. Affect. Disord. 2012;138(1):9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Briley P.M., Webster L., Boutry C., Cottam W.J., Auer D.P., Liddle P.F., Morriss R. Resting-state functional connectivity correlates of anxiety co-morbidity in major depressive disorder. Neurosci. Biobehav. Rev. 2022;138 doi: 10.1016/j.neubiorev.2022.104701. [DOI] [PubMed] [Google Scholar]
- Brodoehl S., Gaser C., Dahnke R., Witte O.W., Klingner C.M. Surface-based analysis increases the specificity of cortical activation patterns and connectivity results. Sci. Rep. 2020;10(1):5737. doi: 10.1038/s41598-020-62832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubl E., Kern E., Ebert D., Bach M., Tebartz van Elst L. Seeing Gray When Feeling Blue? Depression Can Be Measured in the Eye of the Diseased. Biol. Psychiatry. 2010;68(2):205–208. doi: 10.1016/j.biopsych.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Bubl E., Ebert D., Kern E., van Elst L.T., Bach M. Effect of antidepressive therapy on retinal contrast processing in depressive disorder. Br. J. Psychiatry. 2012;201(2):151–158. doi: 10.1192/bjp.bp.111.100560. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Sui J. Multimodal Fusion of Brain Imaging Data: A Key to Finding the Missing Link(s) in Complex Mental Illness. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2016;1(3):230–244. doi: 10.1016/j.bpsc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R. Canbeyli Sensorimotor Modulation of Mood and Depression: In Search of an Optimal Mode of Stimulation Front. Hum. Neurosci. 7. [DOI] [PMC free article] [PubMed]
- Canbeyli R. Sensorimotor modulation of mood and depression: An integrative review. Behav. Brain Res. 2010;207(2):249–264. doi: 10.1016/j.bbr.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Canbeyli, R. (2022). Sensory stimulation via the visual, auditory, olfactory and gustatory systems can modulate mood and depression [https://doi.org/10.1111/ejn.15507]. European Journal of Neuroscience, 55(1), 244-263. https://doi.org/https://doi.org/10.1111/ejn.15507. [DOI] [PubMed]
- Cao J., Chen X., Chen J., Ai M., Gan Y., Wang W.o., Lv Z., Zhang S., Zhang S., Wang S., Kuang L.i., Fang W. Resting-state functional MRI of abnormal baseline brain activity in young depressed patients with and without suicidal behavior. J. Affect. Disord. 2016;205:252–263. doi: 10.1016/j.jad.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Cash R.F.H., Müller V.I., Fitzgerald P.B., Eickhoff S.B., Zalesky A. Altered brain activity in unipolar depression unveiled using connectomics. Nature Mental Health. 2023;1(3):174–185. doi: 10.1038/s44220-023-00038-8. [DOI] [Google Scholar]
- Chen G., Chen P., Gong JiaYing, Jia Y., Zhong S., Chen F., Wang J., Luo Z., Qi Z., Huang L.i., Wang Y. Shared and specific patterns of dynamic functional connectivity variability of striato-cortical circuitry in unmedicated bipolar and major depressive disorders. Psychol. Med. 2022;52(4):747–756. doi: 10.1017/S0033291720002378. [DOI] [PubMed] [Google Scholar]
- Cheng W., Rolls E.T., Qiu J., Liu W., Tang Y., Huang C.-C., Wang XinFa, Zhang J., Lin W., Zheng L., Pu JunCai, Tsai S.-J., Yang A.C., Lin C.-P., Wang F., Xie P., Feng J. Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain. 2016;139(12):3296–3309. doi: 10.1093/brain/aww255. [DOI] [PubMed] [Google Scholar]
- Coalson T.S., Van Essen D.C., Glasser M.F. The impact of traditional neuroimaging methods on the spatial localization of cortical areas. Proc. Natl. Acad. Sci. 2018;115(27):E6356. doi: 10.1073/pnas.1801582115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S.E., Zantvoord J.B., Wezenberg B.N., Bockting C.L.H., van Wingen G.A. Magnetic resonance imaging for individual prediction of treatment response in major depressive disorder: a systematic review and meta-analysis. Transl. Psychiatry. 2021;11(1):168. doi: 10.1038/s41398-021-01286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical Surface-Based Analysis: I. Segmentation and Surface Reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dichter G.S., Gibbs D., Smoski M.J. A systematic review of relations between resting-state functional-MRI and treatment response in major depressive disorder. J. Affect. Disord. 2015;172:8–17. doi: 10.1016/j.jad.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner S.G., Beevers C.G., Haigh E.A.P., Beck A.T. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 2011;12(8):467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Du M.-Y., Wu Q.-Z., Yue Q., Li J., Liao Y.i., Kuang W.-H., Huang X.-Q., Chan R.C.K., Mechelli A., Gong Q.-Y. Voxelwise meta-analysis of gray matter reduction in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;36(1):11–16. doi: 10.1016/j.pnpbp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Farb N.A.S., Anderson A.K., Bloch R.T., Segal Z.V. Mood-Linked Responses in Medial Prefrontal Cortex Predict Relapse in Patients with Recurrent Unipolar Depression. Biol. Psychiatry. 2011;70(4):366–372. doi: 10.1016/j.biopsych.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P.J. Gray colored glasses: Is major depression partially a sensory perceptual disorder? J. Affect. Disord. 2013;151(2):418–422. doi: 10.1016/j.jad.2013.06.045. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.B., Laird A.R., Maller J., Daskalakis Z.J. A meta-analytic study of changes in brain activation in depression [https://doi.org/10.1002/hbm.20426] Hum. Brain Mapp. 2008;29(6):683-695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey M.L., Drevets W.C., Hoffman E.M., Frankel E., Speer A.M., Zarate C.A., Jr. Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA Psychiat. 2013;70(3):280–290. doi: 10.1001/2013.jamapsychiatry.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtado C.P., Maller J.J., Fitzgerald P.B. A magnetic resonance imaging study of the entorhinal cortex in treatment-resistant depression. Psychiatry Res. Neuroimaging. 2008;163(2):133–142. doi: 10.1016/j.pscychresns.2007.11.005. [DOI] [PubMed] [Google Scholar]
- S. Gao V.D. Calhoun J. Sui Machine learning in major depression: From classification to treatment outcome prediction [https://doi.org/10.1111/cns.13048] CNS Neuroscience & Therapeutics 24 11 2018 1037-1052 10.1111/cns.13048. [DOI] [PMC free article] [PubMed]
- D.R. Glasofer A.J. Brown M. Riegel Structured Clinical Interview for DSM-IV (SCID) T. Wade Encyclopedia of Feeding and Eating Disorders 2017 Springer Singapore 799 802 10.1007/978-981-287-104-6_80.
- Glasser M.F., Sotiropoulos S.N., Wilson J.A., Coalson T.S., Fischl B., Andersson J.L., Xu J., Jbabdi S., Webster M., Polimeni J.R., Van Essen D.C., Jenkinson M. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Coalson T.S., Robinson E.C., Hacker C.D., Harwell J., Yacoub E., Ugurbil K., Andersson J., Beckmann C.F., Jenkinson M., Smith S.M., Van Essen D.C. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536(7615):171–178. doi: 10.1038/nature18933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser M.F., Smith S.M., Marcus D.S., Andersson J.L.R., Auerbach E.J., Behrens T.E.J., Coalson T.S., Harms M.P., Jenkinson M., Moeller S., Robinson E.C., Sotiropoulos S.N., Xu J., Yacoub E., Ugurbil K., Van Essen D.C. The Human Connectome Project's neuroimaging approach. Nat. Neurosci. 2016;19(9):1175–1187. doi: 10.1038/nn.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J., Wang J., Qiu S., Chen P., Luo Z., Wang J., Wang Y. Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: voxel-based meta-analysis. Transl. Psychiatry. 2020;10(1):353. doi: 10.1038/s41398-020-01036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N.A., Friston K.J., Frackowiak R.S.J. A Voxel-Based Morphometric Study of Ageing in 465 Normal Adult Human Brains. Neuroimage. 2001;14(1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grange J.A., Rydon-Grange M. Computational modelling of attentional selectivity in depression reveals perceptual deficits. Psychol. Med. 2022;52(5):904–913. doi: 10.1017/S0033291720002652. [DOI] [PubMed] [Google Scholar]
- Gray J.P., Müller V.I., Eickhoff S.B., Fox P.T. Multimodal Abnormalities of Brain Structure and Function in Major Depressive Disorder: A Meta-Analysis of Neuroimaging Studies. Am. J. Psychiatry. 2020;177(5):422–434. doi: 10.1176/appi.ajp.2019.19050560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby J.V., Hoffman E.A., Gobbini M.I. Human neural systems for face recognition and social communication. Biol. Psychiatry. 2002;51(1):59–67. doi: 10.1016/S0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- He Z., Cui Q., Zheng J., Duan X., Pang Y., Gao Q., Han S., Long Z., Wang Y., Li J., Wang X., Zhao J., Chen H. Frequency-specific alterations in functional connectivity in treatment-resistant and -sensitive major depressive disorder. J. Psychiatr. Res. 2016;82:30–39. doi: 10.1016/j.jpsychires.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Hoffman E.A., Haxby J.V. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat. Neurosci. 2000;3(1):80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Hong S.-J., Valk S.L., Di Martino A., Milham M.P., Bernhardt B.C. Multidimensional Neuroanatomical Subtyping of Autism Spectrum Disorder. Cereb. Cortex. 2017;28(10):3578–3588. doi: 10.1093/cercor/bhx229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.-P., Lee T.-W., Tsai S.-J., Chen T.-J., Yang C.-H., Lirng J.-F., Tsai C.-F. Cortical and Subcortical Abnormalities in Late-Onset Depression With History of Suicide Attempts Investigated With MRI and Voxel-Based Morphometry. J. Geriatr. Psychiatry Neurol. 2010;23(3):171–184. doi: 10.1177/0891988710363713. [DOI] [PubMed] [Google Scholar]
- Ishai A., Ungerleider Leslie G., Martin A., Schouten Jennifer L., Haxby James V. Distributed representation of objects in the human ventral visual pathway. Proc. Natl. Acad. Sci. 1999;96(16):9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheripour N., Li M., Chand T., Krug A., Kircher T., Dannlowski U., Nenadić I., Hamilton J.P., Sacchet M.D., Gotlib I.H., Walter H., Frodl T., Grimm S., Harrison B.J., Wolf C.R., Olbrich S., van Wingen G., Pezawas L., Parker G., Hyett M.P., Sämann P.G., Hahn T., Steinsträter O., Jansen A., Yuksel D., Kämpe R., Davey C.G., Meyer B., Bartova L., Croy I., Walter M., Wagner G. Altered resting-state functional connectome in major depressive disorder: a mega-analysis from the PsyMRI consortium. Transl. Psychiatry. 2021;11(1) doi: 10.1038/s41398-021-01619-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S.W., Kim Y.K. Neuroinflammation and cytokine abnormality in major depression: Cause or consequence in that illness? World J Psychiatry. 2016;6(3):283–293. doi: 10.5498/wjp.v6.i3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg R.T. Vol. 2. Sage; 2003. (Cognitive psychology). [Google Scholar]
- Kennis M., Gerritsen L., van Dalen M., Williams A., Cuijpers P., Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol. Psychiatry. 2020;25(2):321–338. doi: 10.1038/s41380-019-0585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocsis K., Holczer A., Kazinczi C., Boross K., Horváth R., Németh L.V., Klivényi P., Kincses Z.T., Must A. Voxel-based asymmetry of the regional gray matter over the inferior temporal gyrus correlates with depressive symptoms in medicated patients with major depressive disorder. Psychiatry Res Neuroimaging. 2021;317:111378. doi: 10.1016/j.pscychresns.2021.111378. [DOI] [PubMed] [Google Scholar]
- P.C.M.P. Koolschijn N.E.M. van Haren G.J.L.M. Lensvelt-Mulders H.E. Hulshoff Pol R.S. Kahn Brain volume abnormalities in major depressive disorder: A meta-analysis of magnetic resonance imaging studies [https://doi.org/10.1002/hbm.20801] Human brain mapping 30 11 2009 3719-3735 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed]
- Kube T., Schwarting R., Rozenkrantz L., Glombiewski J.A., Rief W. Distorted Cognitive Processes in Major Depression: A Predictive Processing Perspective. Biol. Psychiatry. 2020;87(5):388–398. doi: 10.1016/j.biopsych.2019.07.017. [DOI] [PubMed] [Google Scholar]
- Kupfer D.J., Frank E., Phillips M.L. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379(9820):1045–1055. doi: 10.1016/S0140-6736(11)60602-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.M., Borghi J.A., Kujawa A.J., Klein D.N., Leung H.-C. Alterations in visual cortical activation and connectivity with prefrontal cortex during working memory updating in major depressive disorder. NeuroImage: Clinical. 2017;14:43–53. doi: 10.1016/j.nicl.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Ragguett R.-M., Mansur R.B., Boutilier J.J., Rosenblat J.D., Trevizol A., Brietzke E., Lin K., Pan Z., Subramaniapillai M., Chan T.C.Y., Fus D., Park C., Musial N., Zuckerman H., Chen V.-H., Ho R., Rong C., McIntyre R.S. Applications of machine learning algorithms to predict therapeutic outcomes in depression: A meta-analysis and systematic review. J. Affect. Disord. 2018;241:519–532. doi: 10.1016/j.jad.2018.08.073. [DOI] [PubMed] [Google Scholar]
- Li C.-T., Lin C.-P., Chou K.-H., Chen I.Y., Hsieh J.-C., Wu C.-L., Su T.-P. Structural and cognitive deficits in remitting and non-remitting recurrent depression: A voxel-based morphometric study. Neuroimage. 2010;50(1):347–356. doi: 10.1016/j.neuroimage.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang Y., Meng C., Zhang C., Zhao W., Zhu D.-M., Zhu J. Functional stability predicts depressive and cognitive improvement in major depressive disorder: A longitudinal functional MRI study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2021;111 doi: 10.1016/j.pnpbp.2021.110396. [DOI] [PubMed] [Google Scholar]
- Li Q., Zhao Y., Chen Z., Long J., Dai J., Huang X., Lui S.u., Radua J., Vieta E., Kemp G.J., Sweeney J.A., Li F., Gong Q. Meta-analysis of cortical thickness abnormalities in medication-free patients with major depressive disorder. Neuropsychopharmacology. 2020;45(4):703–712. doi: 10.1038/s41386-019-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Bernhardt B.C., Hong S.-J., Caldairou B., Bernasconi A., Bernasconi N. The superficial white matter in temporal lobe epilepsy: a key link between structural and functional network disruptions. Brain. 2016;139(9):2431–2440. doi: 10.1093/brain/aww167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Wang H., Song M., Lv L., Cui Y., Liu Y., Fan L., Zuo N., Xu K., Du Y., Yu Q., Luo N.a., Qi S., Yang J., Xie S., Li J., Chen J., Chen Y., Wang H., Guo H., Wan P., Yang Y., Li P., Lu L., Yan H., Yan J., Wang H., Zhang H., Zhang D., Calhoun V.D., Jiang T., Sui J. Linked 4-Way Multimodal Brain Differences in Schizophrenia in a Large Chinese Han Population. Schizophr. Bull. 2018;45(2):436–449. doi: 10.1093/schbul/sby045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Z., Du L., Zhao J., Wu S., Zheng Q., Lei X. Prediction on treatment improvement in depression with resting state connectivity: A coordinate-based meta-analysis. J. Affect. Disord. 2020;276:62–68. doi: 10.1016/j.jad.2020.06.072. [DOI] [PubMed] [Google Scholar]
- Lord A., Horn D., Breakspear M., Walter M., Zang Y.-F. Changes in Community Structure of Resting State Functional Connectivity in Unipolar Depression. PLoS One. 2012;7(8):e41282. doi: 10.1371/journal.pone.0041282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F., Cui Q., Huang X., Li L., Duan X., Chen H., Pang Y., He Z., Sheng W., Han S., Chen Y., Yang Y., Luo W., Yu Y., Jia X., Tang Q., Li D.i., Xie A., Chen H. Anomalous intrinsic connectivity within and between visual and auditory networks in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2020;100:109889. doi: 10.1016/j.pnpbp.2020.109889. [DOI] [PubMed] [Google Scholar]
- Lu J., Zhang Z., Yin X., Tang Y., Ji R., Chen H., Guang Y.u., Gong X., He Y., Zhou W., Wang H., Cheng K.e., Wang Y., Chen X., Xie P., Guo Z.V. An entorhinal-visual cortical circuit regulates depression-like behaviors. Mol. Psychiatry. 2022;27(9):3807–3820. doi: 10.1038/s41380-022-01540-8. [DOI] [PubMed] [Google Scholar]
- Malhi G.S., Mann J.J. Depression. Lancet. 2018;392(10161):2299–2312. doi: 10.1016/S0140-6736(18)31948-2. [DOI] [PubMed] [Google Scholar]
- Meng X., Jiang R., Lin D., Bustillo J., Jones T., Chen J., Yu Q., Du Y., Zhang Y.u., Jiang T., Sui J., Calhoun V.D. Predicting individualized clinical measures by a generalized prediction framework and multimodal fusion of MRI data. Neuroimage. 2017;145:218–229. doi: 10.1016/j.neuroimage.2016.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders P.C., van Eijndhoven P.F., Schene A.H., Beckmann C.F., Tendolkar I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci. Biobehav. Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Müller V.I., Cieslik E.C., Serbanescu I., Laird A.R., Fox P.T., Eickhoff S.B. Altered Brain Activity in Unipolar Depression Revisited: Meta-analyses of Neuroimaging Studies. JAMA Psychiat. 2017;74(1):47–55. doi: 10.1001/jamapsychiatry.2016.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Fox M.D. Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage. 2017;154:169–173. doi: 10.1016/j.neuroimage.2016.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu M., Wang Y., Jia Y., Wang J., Zhong S., Lin J., Sun Y., Zhao L., Liu X., Huang L.i., Huang R. Common and Specific Abnormalities in Cortical Thickness in Patients with Major Depressive and Bipolar Disorders. EBioMedicine. 2017;16:162–171. doi: 10.1016/j.ebiom.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization W.H. The global burden of disease: 2004 update. World Health. 2008 Organization. [Google Scholar]
- Pan L.A., Ramos L., Segreti A., Brent D.A., Phillips M.L. Right superior temporal gyrus volume in adolescents with a history of suicide attempt. Br. J. Psychiatry. 2015;206(4):339–340. doi: 10.1192/bjp.bp.114.151316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis, D., Leahy, R. M., Nichols, T. E., & Styner, M. (2004, 18-18 April 2004). Statistical surface-based morphometry using a nonparametric approach. 2004 2nd IEEE International Symposium on Biomedical Imaging: Nano to Macro (IEEE Cat No. 04EX821).
- Papmeyer M., Giles S., Sussmann J.E., Kielty S., Stewart T., Lawrie S.M., McIntosh A.M. Cortical Thickness in Individuals at High Familial Risk of Mood Disorders as They Develop Major Depressive Disorder. Biol. Psychiatry. 2015;78(1):58–66. doi: 10.1016/j.biopsych.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Peng D., Shi F., Li G., Fralick D., Shen T., Qiu M., Liu J., Jiang K., Shen D., Fang Y., He Y. Surface vulnerability of cerebral cortex to major depressive disorder. PLoS One. 2015;10(3):e0120704. doi: 10.1371/journal.pone.0120704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J.L., Batten L.A., Tremblay P., Aldosary F., Blier P. A Prospective, Longitudinal Study of the Effect of Remission on Cortical Thickness and Hippocampal Volume in Patients with Treatment-Resistant Depression. Int. J. Neuropsychopharmacol. 2015;18(8):pyv037. doi: 10.1093/ijnp/pyv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilmeyer, J., Huijbers, W., Lamerichs, R., Jansen, J. F. A., Breeuwer, M., & Zinger, S. (2022). Functional MRI in major depressive disorder: A review of findings, limitations, and future prospects [https://doi.org/10.1111/jon.13011]. Journal of Neuroimaging, 32(4), 582-595. https://doi.org/https://doi.org/10.1111/jon.13011. [DOI] [PMC free article] [PubMed]
- Power J.D., Plitt M., Laumann T.O., Martin A. Sources and implications of whole-brain fMRI signals in humans. Neuroimage. 2017;146:609–625. doi: 10.1016/j.neuroimage.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn. Sci. 2012;16(1):61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Qiao Z., Yu Y., Wang L., Yang X., Qiu X., Zhang C., Ning N., Shi J., Chen L.u., Li Z., Liu J., Xu J., Zhao L., Yang Y. Impaired pre-attentive change detection in major depressive disorder patients revealed by auditory mismatch negativity. Psychiatry Res. Neuroimaging. 2013;211(1):78–84. doi: 10.1016/j.pscychresns.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Robinson E.C., Garcia K., Glasser M.F., Chen Z., Coalson T.S., Makropoulos A., Bozek J., Wright R., Schuh A., Webster M., Hutter J., Price A., Cordero Grande L., Hughes E., Tusor N., Bayly P.V., Van Essen D.C., Smith S.M., Edwards A.D., Hajnal J., Jenkinson M., Glocker B., Rueckert D. Multimodal surface matching with higher-order smoothness constraints. Neuroimage. 2018;167:453–465. doi: 10.1016/j.neuroimage.2017.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolle C.E., Fonzo G.A., Wu W., Toll R., Jha M.K., Cooper C., Chin-Fatt C., Pizzagalli D.A., Trombello J.M., Deckersbach T., Fava M., Weissman M.M., Trivedi M.H., Etkin A. Cortical Connectivity Moderators of Antidepressant vs Placebo Treatment Response in Major Depressive Disorder: Secondary Analysis of a Randomized Clinical Trial. JAMA Psychiat. 2020;77(4):397. doi: 10.1001/jamapsychiatry.2019.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y., Mimura I., Mannen T. Agraphia for kanji resulting from a left posterior middle temporal gyrus lesion. Behav. Neurol. 2008;19(3):93–106. doi: 10.1155/2008/393912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenzer M., Zattarin E., Grözinger M., Mathiak K. Impaired pitch identification as a potential marker for depression. BMC Psychiatry. 2012;12(1):32. doi: 10.1186/1471-244X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Pearlson G.D., Du Y., Yu Q., Jones T.R., Chen J., Jiang T., Bustillo J., Calhoun V.D. In Search of Multimodal Neuroimaging Biomarkers of Cognitive Deficits in Schizophrenia. Biol. Psychiatry. 2015;78(11):794–804. doi: 10.1016/j.biopsych.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Qi S., van Erp T.G.M., Bustillo J., Jiang R., Lin D., Turner J.A., Damaraju E., Mayer A.R., Cui Y., Fu Z., Du Y., Chen J., Potkin S.G., Preda A., Mathalon D.H., Ford J.M., Voyvodic J., Mueller B.A., Belger A., McEwen S.C., O’Leary D.S., McMahon A., Jiang T., Calhoun V.D. Multimodal neuromarkers in schizophrenia via cognition-guided MRI fusion. Nat. Commun. 2018;9(1) doi: 10.1038/s41467-018-05432-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Jiang R., Bustillo J., Calhoun V. Neuroimaging-based Individualized Prediction of Cognition and Behavior for Mental Disorders and Health: Methods and Promises. Biol. Psychiatry. 2020;88(11):818–828. doi: 10.1016/j.biopsych.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundermann B., Feder S., Wersching H., Teuber A., Schwindt W., Kugel H., Heindel W., Arolt V., Berger K., Pfleiderer B. Diagnostic classification of unipolar depression based on resting-state functional connectivity MRI: effects of generalization to a diverse sample. J. Neural Transm. 2017;124(5):589–605. doi: 10.1007/s00702-016-1673-8. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Yücel M., Lorenzetti V., Walterfang M., Kawasaki Y., Whittle S., Suzuki M., Pantelis C., Allen N.B. An MRI study of the superior temporal subregions in patients with current and past major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(1):98–103. doi: 10.1016/j.pnpbp.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Thomas Yeo B.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zöllei L., Polimeni J.R., Fischl B., Liu H., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C. Surface-based approaches to spatial localization and registration in primate cerebral cortex. Neuroimage. 2004;23:S97–S107. doi: 10.1016/j.neuroimage.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Veer I.M., Beckmann C., Van Tol M.-J., Ferrarini L., Milles J., Veltman D., Rombouts S. Whole Brain Resting-State Analysis Reveals Decreased Functional Connectivity in Major Depression [Original Research] Front. Syst. Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Hermens D.F., Hickie I.B., Lagopoulos J. A systematic review of resting-state functional-MRI studies in major depression. J. Affect. Disord. 2012;142(1):6–12. doi: 10.1016/j.jad.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Williams J.B.W. A Structured Interview Guide for the Hamilton Depression Rating Scale. Arch. Gen. Psychiatry. 1988;45(8):742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Webster M.A., Brooks J.C., Tracey I., Smith S.M., Nichols T.E. Non-parametric combination and related permutation tests for neuroimaging. Hum. Brain Mapp. 2016;37(4):1486–1511. doi: 10.1002/hbm.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Liu J., Mechelli A., Sun X., Ma Q., Wang X., Wei D., Chen Y., Liu B., Huang C.-C., Zheng Y., Wu Y., Chen T., Cheng Y., Xu X., Gong Q., Si T., Qiu S., Lin C.-P., Cheng J., Tang Y., Wang F., Qiu J., Xie P., Li L., He Y. Connectome gradient dysfunction in major depression and its association with gene expression profiles and treatment outcomes. Mol. Psychiatry. 2022;27(3):1384–1393. doi: 10.1038/s41380-022-01519-5. [DOI] [PubMed] [Google Scholar]
- Xu T., Opitz A., Craddock R.C., Wright M.J., Zuo X.-N., Milham M.P. Assessing Variations in Areal Organization for the Intrinsic Brain: From Fingerprints to Reliability. Cereb. Cortex. 2016;26(11):4192–4211. doi: 10.1093/cercor/bhw241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L.-L., Shen H., Liu L.i., Wang L., Li B., Fang P., Zhou Z., Li Y., Hu D. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain. 2012;135(5):1498–1507. doi: 10.1093/brain/aws059. [DOI] [PubMed] [Google Scholar]
- Zhang H., Li L., Wu M., Chen Z., Hu X., Chen Y., Zhu H., Jia Z., Gong Q. Brain gray matter alterations in first episodes of depression: A meta-analysis of whole-brain studies. Neurosci. Biobehav. Rev. 2016;60:43–50. doi: 10.1016/j.neubiorev.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Zhang S., Wang Y., Zheng S., Seger C., Zhong S., Huang H., Hu H., Chen G., Chen L., Jia Y., Huang L.i., Huang R. Multimodal MRI reveals alterations of the anterior insula and posterior cingulate cortex in bipolar II disorders: A surface-based approach. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2022;116:110533. doi: 10.1016/j.pnpbp.2022.110533. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zhang H., Xie C.-M., Zhang M., Shi Y., Song R., Lu X., Zhang H., Li K., Wang B.i., Yang Y., Li X., Zhu J., Zhao Y., Yuan T.-F., Northoff G. Task-related functional magnetic resonance imaging-based neuronavigation for the treatment of depression by individualized repetitive transcranial magnetic stimulation of the visual cortex. Sci. China Life Sci. 2021;64(1):96–106. doi: 10.1007/s11427-020-1730-5. [DOI] [PubMed] [Google Scholar]
- Zhao Y.-J., Du M.-Y., Huang X.-Q., Lui S., Chen Z.-Q., Liu J., Luo Y., Wang X.-L., Kemp G.J., Gong Q.-Y. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychol. Med. 2014;44(14):2927–2937. doi: 10.1017/S0033291714000518. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Zhao Y., Wen K., Li Q., Pan N., Fu S., Li F., Radua J., Vieta E., Kemp G.J., Biswa B.B., Gong Q. Cortical thickness abnormalities in patients with bipolar disorder: A systematic review and meta-analysis. J. Affect. Disord. 2022;300:209–218. doi: 10.1016/j.jad.2021.12.080. [DOI] [PubMed] [Google Scholar]
- Zhuo C., Li G., Lin X., Jiang D., Xu Y., Tian H., Song X. The rise and fall of MRI studies in major depressive disorder. Transl. Psychiatry. 2019;9(1):335. doi: 10.1038/s41398-019-0680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- J. Zweerings M. Zvyagintsev B.I. Turetsky M. Klasen A.A. König E. Roecher K. Mathiak Fronto-parietal and temporal brain dysfunction in depression: A fMRI investigation of auditory mismatch processing [https://doi.org/10.1002/hbm.24623] Human brain mapping 40 12 2019 3657-3668 10.1002/hbm.24623. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and code are available from the corresponding author upon reasonable request.
Data will be made available on request.