Abstract
Five new NNN pincer-type ligands and their palladium complexes were successfully synthesised and characterised by FT-IR, 1H NMR, 13C NMR, and UV–vis analyses. TEM analysis was used to observe the morphological character of the black residues obtained from the fourth cycle of the reusability test. Furthermore, suitable crystals of the N2,N6-bis(2-tert-butylphenyl)pyridine-2,6-dicarboxamide and its palladium complex were elucidated with the X-ray single crystal diffraction method. Both the ligand and its palladium complex crystallise in a monoclinic system with space group P21/c for the H2L4 and C2/c for the palladium complex. The structure of the pincer ligand and its palladium complex were stabilised by intramolecular and intermolecular C–H⋅⋅⋅O, C–H⋅⋅⋅N, and N–H⋅⋅⋅N contacts. A Suzuki-Miyaura cross-coupling reaction between aryl halides and phenylboronic acid was used to assess the catalytic abilities of the palladium pincer complexes. All of the prepared complexes exhibited considerable catalytic activity. However, complexes 4 (Acetonitrile-N2,N6-bis(2-tert-butylphenyl)pyridine-2,6-dicarboxamidopalladium(II)) and 5 (Acetonitrile-N2,N6-bis(2-nitrophenyl)pyridine-2,6-dicarboxamidopalladium(II)) provided almost 100% conversion with nearly 100% yield in the reaction between 4-bromotoluene and phenylboronic acid. Furthermore, these active complexes catalysed the reaction of the sterically hindered and deactivated substrates (1-Bromo-4-izobutylbenzene and 2-bromo-6-methoxynaphthalene) with phenylboronic acid, and complete conversion and yields up to 100% were achieved in a short time with the 2-bromo-6-methoxynaphthalene.
Keywords: Synthesis, Pincer type ligands, Pd(II) complexes, Crystal structure, Catalytic activity, Suzuki-Miyaura cross coupling
Graphical abstract
Highlights
-
•
Five novel NNN pincer-type ligands, along with their corresponding Pd(II) complexes, were synthesised and characterised using FT-IR, 1H NMR, 13C NMR, and UV–vis techniques.
-
•
The single crystal X-ray diffraction method was used to characterise the crystal structure of both H2L4 and its Pd(II) complex.
-
•
The stabilization of both the pincer ligand and its Pd(II) complex was achieved through intermolecular and intramolecular interactions such as N–H⋅⋅⋅N, C–H⋅⋅⋅N, and C–H⋅⋅⋅O interactions.
-
•
The catalytic activities of the Pd(II) complexes were examined in the Suzuki-Miyaura cross-coupling reaction.
1. Introduction
Some of the versatile organometallic catalysts utilized in organic synthesis for the production of carbon-carbon and carbon-heteroatom bonds include organopalladium compounds. An ideal organometallic catalyst should have strong catalytic activity even at low catalyst loadings, together with stability and selectivity [1]. Carbon-carbon bond formation reactions are among the important reactions in chemistry as they provide key steps in the construction of complex bioactive molecules, such as pharmaceuticals and agrochemicals. These reactions are also essential in the development of innovative organic materials with new electronic, optical, or mechanical effects that probably play an important role in the evolving branches of nanotechnology [2]. Over the past 40 years, the most important methodologies for the formation of carbon-carbon bonds have involved the use of transition metals to mediate reactions in a controlled and selective manner. C–C cross-coupling reactions are described as carbon-carbon bond reactions that occur between an organic electrophile and an organometallic nucleophile in the presence of a metal catalyst. The catalysts used in these reactions are generally transition-metal complexes, especially nickel and palladium metals. These two metals are involved in the wide majority of cross-coupling reactions, because of the ease of interchange of redox steps, that is, conversions such as Ni(+2)/Ni(0) and Pd(+2)/Pd(0), which is a basic necessity to complete the catalytic cycle [3]. The Pd(0)/Pd(II) catalytic cycles are prone to the inherent drawbacks of β-hydride elimination from Pd (II), often resulting in irreversible rupture of the palladium-carbon bond and subsequent decomposition of the complex. Various research groups have reported that, in many instances, the true catalysts involved in C–C bond coupling utilising palladacycles are not necessarily the palladacycles or pincer palladium complexes themselves, but rather palladium nanoparticles or other weakly low-ligated palladium species. However, Milstein and his colleagues demonstrated that under mild reaction conditions, the palladium(II) center in pincer complexes can undergo reversible Pd(II) to Pd(IV) transformations, as evidenced by their study [4]. In contrast to the Pd(0)/Pd(II) cycle, the Pd(II)/Pd(IV) cycle offers greater promise for the facile reductive elimination step from a Pd(IV) center and exhibits enhanced chemoselectivity for the oxidative addition on Pd(II). However, the oxidation of Pd(II) to Pd(IV) poses a thermodynamically unfavourable process as a result of the electronic deficiency of the Pd(IV) center. In this context, taking into account the strong electron-donating nature of pincer ligands, they appear to be a suitable choice for supporting and stabilising Pd(IV) complexes. Until now, numerous studies have reported Pd(IV) pincer complexes as catalytic intermediates, thus broadening the application range of palladium-based catalysts [[5], [6], [7], [8]].
Since the first work on palladium pincer complex catalysts was published by Milstein and coworkers as part of the Heck reaction, numerous studies on cross-linking reactions have been published. The most extensive studies in this field are Heck-coupling/Heck-type reactions and Suzuki-Miyaura cross-coupling reactions [9]. Usually homogeneous catalytic systems previously reported are phosphine ligand-based systems. Though the catalytic activity of transition metal complexes with phosphine ligands is well, the sensibility of ligands and complexes to air makes them less attractive [[10], [11], [12]]. The electronic and structural properties of the metal center used in catalysis can be improved by the appropriate selection of the chelating arms of the pincer ligand [13]. The notable stability achieved by employing tridentate ligands is a fundamental characteristic of pincer complexes, allowing their use as catalysts under high-temperature conditions [14]. Palladium(II) complexes based on the NNN pincer type ligand have become very remarkable due to their favourable balance between stability and reactivity and their high catalytic activity in C–C bond formation reactions [[15], [16], [17], [18]]. Furthermore, many of these complexes are stable to air and moisture, making them easy to transport and store, providing long-lasting catalysts and broad reaction coverage [9]. In view of these studies, the aim of our study is to investigate the new types of pincer complexes and their catalytic roles in the Suzuki-Miyaura cross-coupling reaction. For this purpose, we synthesised five new pincer-type pyridine-2,6-dicarboxamide NNN ligands and their palladium complexes and characterised by various methods. Furthermore, the catalytic activities of the palladium complexes were tested in the Suzuki-Miyaura cross-coupling reaction.
2. Experimental
2.1. Instrumentations
The synthesised compounds were subjected to various analytical techniques to elucidate their properties. 1H and 13C NMR analysis was carried out using a Bruker UPB Avance-III 400 MHz NMR spectrometer. TMS was used as an internal standard in dimethyl sulfoxide-d6 on the scale in ppm. The pincer ligands were analyzed using FT-IR spectroscopy with a PerkinElmer Frontier Spectrum100 ATR spectrometer. Using a Shimadzu GC-2030 instrument with a 30 m × 0.25 mm RTX-5 capillary column and FID detector, the catalytic activity of Pd complexes in the C–C cross coupling process was assessed. UV–vis spectroscopy was employed to examine the spectra of the pincer ligands and their palladium complexes on a Shimadzu UV-3100 spectrometer. With Cu-Kα radiation, X-ray single-crystal analysis (SCXRD) was performed using a Bruker CCD APEX-II diffractometer. The crystals were kept at room temperature throughout the analysis, and the solution was achieved using the OLEX2 [19] program, the Superflip [[20], [21], [22]] structure resolution program, the Charge Flipping resolution technique, and the full matrix least squares method on F2 by ShelXL [23], with the refinement of F2 against all reflections. Hydrogen atoms were refined isotropically, and all non-H atoms were refined anisotropically, while the refinement of the molecule's molecular structure graphics was created with OLEX2 program [19].
2.2. Synthesis of pyridine-2,6-dicarboxamide NNN pincer ligands
2.2.1. Synthesis method for H2L1-3
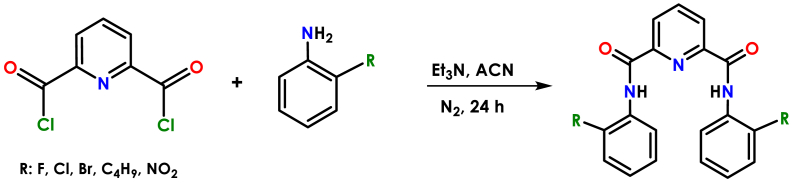
All experimental process were carried out in open atmosphere. First, the precursor compound pyridine-2,6-dicarbonylchloride was synthesised from pyridine-2,6-dicarboxylic acid (the synthesis details and characterization data were given in supplementary material) [24]. Et3N (10 mmol, 1.01 g) and related aniline derivatives (2-fluoroaniline, 2-chloroaniline, and 2-bromoaniline, 10 mmol) were added to the solution of the compound of pyridine-2,6-dicarbonylchloride (5 mmol) in acetonitrile (50 mL), under N2 atmosphere, respectively. The mixture was stirred at room temperature for 24 h. The precipitated solid was filtered off, washed with ether two times, and dried in vacuo (Scheme 1).
Scheme 1.
Synthesis of H2L1-5 ligands.
N2,N6-bis(2-Fluorophenyl)pyridine-2,6-dicarboxamide (H2L1): Color: White. Yield: 77%. 1H NMR (400 MHz, DMSO‑d6): δ 10.93 (s, 2H, NH), 8.40 (t, 2H, HAr), 8.36–8.32 (m, 1H, HAr), 7.72 (t, 2H, HAr), 7.39 (t, 4H, HAr), 7.34–7.31 (m, 2H, HAr) ppm. 13C NMR (100 MHz, DMSO‑d6): δ 161.97 (C O), 155.37, 154.92, 148.27, 140.15, 125.39, 124.92, 124.54 (CAr) ppm. FTIR (ν, cm−1): 3360, 3272 (N–H), 3091 (Ar–H), 1672 (C O).
N2,N6-bis(2-Chlorophenyl)pyridine-2,6-dicarboxamide (H2L2): Color: White. Yield: 80%. 1H NMR (400 MHz, DMSO‑d6): δ 10.90 (s, 2H, NH), 8.42 (t, 2H, HAr), 8.37–8.33 (m, 1H, HAr), 7.80 (d, 2H, HAr), 7.65 (dd, 2H, HAr), 7.48 (td, 2H, HAr), 7.38 (td, 2H, HAr) ppm. 13C NMR (100 MHz, DMSO‑d6): δ 161.81 (C O), 148.60, 140.26, 134.18, 127.74, 124.06 (CAr) ppm. FTIR (ν, cm−1): 3363, 3270 (N–H), 3110 (ArH), 1673 (C O).
N2,N6-bis(2-Bromophenyl)pyridine-2,6-dicarboxamide (H2L3): Color: White. Yield: 75%. 1H NMR (400 MHz, DMSO‑d6): δ 10.93 (s, 2H, NH), 8.41 (t, 2H, HAr), 8.34–8.30 (m, 1H, HAr), 7.78 (dd, 2H, HAr), 7.69 (dd, 2H, HAr), 7.50 (td, 2H, HAr), 7.29 (td, 2H, HAr) ppm. 13C NMR (100 MHz, DMSO‑d6): δ 161.83 (C O), 148.36, 140.25, 132.78, 128.30, 125.39 (CAr) ppm. FTIR (ν, cm−1): 3343, 3263 (N–H), 3113 (ArH), 1673 (C O).
2.2.2. Synthesis method for H2L4,5
The solution of aniline (2-tert-butylaniline, 2-nitroaniline, 10 mmol) and Et3N (10 mmol) in acetonitrile (10 mL) was kept in an ice bath for 2 h and added dropwise for 3 h over the solution of compound pyridine-2,6 dicarbonylchloride (5 mmol) in acetonitrile (50 mL). The solution was stirred under N2 atmosphere. At the end of the reaction, which lasted for 24 h under room conditions, the solution was evaporated. The resulting white solid product was washed with ether and dried in vacuo (Scheme 1).
N2,N6-bis(2-tert-Butylphenyl)pyridine-2,6-dicarboxamide (H2L4): Color: White. Yield: 80%. 1H NMR (400 MHz, DMSO‑d6): δ 10.87 (s, 2H, NH), 8.39 (t, 2H, HAr), 8.33–8.29 (m, 1H, HAr), 7.52 (dd, 2H, HAr), 7.36–7.29 (m, 4H, HAr), 7.17 (dd, 2H, HAr), 1.36 (s, 18H, HCH3) ppm. 13C NMR (100 MHz, DMSO‑d6): δ 162.76 (C O), 148.97, 147.44, 139.91, 135.77, 131.74, 124.81 (CAr), 34.96 (CCH3), 31.02 (CH3) ppm. FTIR (ν, cm −1): 3341 (N–H), 3078 (ArH), 1671 (C O).
N2,N6-bis(2-Nitrophenyl)pyridine-2,6-dicarboxamide (H2L5): Color: Yellow. Yield: 84%. 1H NMR (400 MHz, DMSO‑d6): δ 11.75 (s, 2H, NH), 8.44–8.36 (m, 3H, HAr), 8.20 (t, 4H, HAr), 7.88 (t, 2H, HAr), 7.50 (t, 2H, HAr) ppm. 13C NMR (100 MHz, DMSO‑d6): δ 161.66 (C O), 147.85, 141.30, 134.91, 125.95, 124.94 (CAr) ppm. FTIR (ν, cm −1): 3372, 3329 (N–H), 3094 (ArH), 1666 (C O).
2.3. General method for the synthesis of NNN palladium(II) pincer complexes (1–5)
Palladium(II) acetate salt (1.2 mmol) dissolved in hot acetonitrile was added dropwise to the solution of the ligand (1 mmol) in hot acetonitrile under reflux. When the reaction colour changed from red to yellow, the temperature was reduced and the reaction was stirred at room temperature for one week. At the end of this period, the solution was evaporated and the oily product obtained was crystallized from acetonitrile and diethyl ether mixture (Scheme 2).
Scheme 2.
Synthesis of NNN palladium(II) pincer complexes.
Acetonitrile-N2,N6-bis(2-fluorophenyl)pyridine-2,6-dicarboxamidopalladium(II) (1): Color: Yellow. Yield: 80%. 1H NMR (400 MHz, DMSO‑d6): δ 8.34 (t, 1H, HAr), 7.83 (d, 2H, HAr), 7.26–7.12 (m, 8H, HAr), 2.07 (s, 3H, CH3CN) ppm. 13C NMR (100 MHz, DMSO‑d6): δ 166.55 (C O), 157.12 (CCN), 154.68, 150.16, 141.09, 132.84, 128.30, 122.85, 114.55 (CAr), 1.14 (CCH3) ppm.
Acetonitrile-N2,N6-bis(2-chlorophenyl)pyridine-2,6-dicarboxamidopalladium(II) (2): Color: Yellow. Yield: 75%. 1H NMR (400 MHz, DMSO‑d6): δ 8.35 (t, 1H, HAr), 7.84 (d, 2H, HAr), 7.45 (d, 2H, HAr), 7.28 (t, 4H, HAr), 7.22–7.18 (m, 2H, HAr), 2.07 (s, 3H, CH3CN) ppm. 13C NMR (100 MHz, DMSO‑d6): δ 166.10 (C O), 150.27 (CCN), 142.79, 141.10, 129.72, 128.39, 128.12, 126.13, 125.24, 124.67 (CAr), 1.13 (CCH3) ppm.
Acetonitrile-N2,N6-bis(2-bromophenyl)pyridine-2,6-dicarboxamidopalladium(II) (3): Color: Yellow. Yield: 84%. 1H NMR (400 MHz, DMSO‑d6): δ 8.34 (t, 1H, HAr), 7.85 (d, 2H, HAr), 7.62 (dd, 2H, HAr), 7.35–7.29 (m, 4H, HAr), 7.12 (td, 2H, HAr). 2.07 (s, 3H, CH3CN) ppm. 13C NMR (100 MHz, DMSO‑d6): δ 166.81 (C O), 151.23 (CCN), 145.47, 129.41, 127.77, 125.89, 121.78 (CAr), 1.12 (CCH3) ppm.
Acetonitrile-N2,N6-bis(2-tert-butylphenyl)pyridine-2,6-dicarboxamidopalladium(II) (4): Color: Yellow. Yield: 78%. 1H NMR (400 MHz, DMSO‑d6): δ 8.33 (t, 1H, HAr), 7.80 (d, 2H, HAr), 7.36 (dd, 2H, HAr), 7.19 (dd, 1H, HAr), 7.15 (dd, 2H, HAr), 7.12 (td, 3H, HAr), 2.10 (s, 3H, CH3CN), 1.51 (s, 18H, HCH3) ppm. 13C NMR (100 MHz, DMSO‑d6): δ 167.67 (C O), 152.35 (CCN), 146.70, 145.66,141.77, 130.28, 126.98, 126,48, 125.21, 125.10 (CAr), 35.53 (CCH3), 31.43 (CH3), 1.12 (CCH3) ppm.
Acetonitrile-N2,N6-bis(2-nitrophenyl)pyridine-2,6-dicarboxamidopalladium(II) (5): Color: Yellow. Yield: 82%. 1H NMR (400 MHz, DMSO‑d6): δ 8.40 (t, 1H, HAr), 7.91 (t, 4H, HAr), 7.69 (td, 2H, HAr), 7.41 (td, 4H, HAr), 2.10 (s, 3H, CH3CN) ppm. 13C NMR (100 MHz, DMSO‑d6): δ 167.58 (C O), 150.94 (CCN), 145.52, 142.51, 133.14, 126.38, 125.41, 124.32 (CAr), 1.12 (CCH3) ppm.
2.4. Suzuki-Miyaura cross-coupling reaction method
The reaction of phenylboronic acid (1.2 mmol), arylbromide (1.0 mmol), organic solvent (2 mL), base (1.2 mmol), and palladium catalyst (0.001 mmol) was carried out in a closed tube at 110 °C for the required duration. The resulting mixture was subsequently treated with brine and saturated ammonium chloride solution, and the organic phase was separated and dried using anhydrous Na2SO4. The dried mixture was then filtered and evaporated [25,26]. To isolate the product, the crude mixture was mixed with ethyl acetate (15 mL) and subjected to chromatography on silicagel. The resulting isolated product was then characterized using 1H NMR and GC analysis, with dodecane added as an internal standard prior to the GC analysis.
3. Results and discussion
3.1. Synthesis
In the first step of the synthesis part of this study, new pyridine-2,6-dicarboxamide, NNN pincer-type ligands were synthesised and the synthesised compounds were characterised by 1H NMR, 13C NMR, COSY, HMQC and FT-IR techniques. In the second step of the synthesis, palladium (II) complexes of these NNN pincer-type ligands were synthesised by the method specified in the synthesis part and their structures were elucidated by 1H NMR, 13C NMR, and FT-IR methods. Characterisation data for all prepared compounds are in agreement with the values in the literature [[27], [28], [29]].
FTIR measurements of the synthesised ligands were performed in the range of 450–4000 cm−1. When the frequencies of the functional groups in the structure of the ligands were examined, the stretching frequencies of the NH groups were recorded in the range of 3263–3372 cm−1 for the H2L1-5 compounds [30,31]. The stretching vibrations detected at 1672, 1673, 1673, 1671, and 1666 cm−1 for the H2L1-5 ligands, respectively, are assigned to the carbonyl ν(C O) stretching vibration mode and these results are in agreement with previous studies [[32], [33], [34]]. In agreement with previous work [34], the IR frequencies of the ν(CAr-H) group in the structure of the compounds were monitored in the range of 3078–3113 cm−1. The data obtained from the FTIR study are compatible with the literature [[35], [36], [37]] and these data confirmed the structure of the prepared NNN pincer type compounds.
In the 1H NMR spectrum of pincer ligands, amide proton signals appeared at δ 10.93, 10.90, 10.93, 10.87, 11.75 ppm, respectively, for the H2L1-5 compounds [38]. Due to the electronegative nature of the nitro group, the amide proton of the H2L5 compound was resonated in the lower field (δ 11.75 ppm). The protons of the pyridine ring, which are common in the structure of the compounds, resonate in common, except for the H2L5 compound, in the triplet form of two protons in the range of δ 8.37–8.29 ppm, and the remaining proton as a multiplet in specific intervals for each compound. In the compound H2L5, three protons in the pyridine ring were observed as multiplets in the range of δ 8.44–8.36 ppm. According to the data from the literature [39], other aromatic protons were found in the predicted range of δ 8.20–7.17 ppm. The methyl protons in the tert-butyl group in the H2L4 compound appeared as a singlet at δ 1.36 ppm. The signals from the carbonyl group were found in the 13C NMR spectra at 161.97, 161.81, 161.83, 162.76 and 161.66 ppm for the H2L1−5 compounds, respectively. The aromatic carbons in the structure of the ligands emerged between the range of δ 155.37–124.06 ppm. Although the quaternary carbon of the tert-butyl group in the H2L4 compound resonated at δ 34.96 ppm, the carbons of the methyl group were also observed at δ 31.02 ppm [40].
In the 1H NMR spectra of the complexes, after complexation, the absence of resonance of the amide protons confirmed the coordination of amide nitrogen atoms with the Pd(II) ion after deprotonation [41]. In addition to this observation, the resonance values of the aromatic protons shifted as a consequence of coordination with the palldium (II) ion. In the 1H NMR spectra of the palladium complex 4, the methyl protons shifted to a downfield (δ 1.51 ppm) different from the free ligand, due to electron withdrawal by the metal ion, leading to a decrease in the electron density [42]. The chemical shift value of CH3CN methyl protons that have attached to palladium were detected at δ 2.07 and 2.10 ppm in the 1H NMR spectra of the complexes [43]. In the 13C NMR spectra of the complexes, carbons of the CN and CH3 groups of acetonitrile coordinating with palladium were observed in the range of δ 150.27–157.12 and 1.12–1.14 ppm, respectively [44]. Carbonyl carbons of the palladium complexes were detected in the lower region between the δ 166.10–167.67 ppm different from the free ligands. The resonance values of the aromatic carbons shifted for each palladium complex after the complexation. In the 13C NMR spectra of the palladium complex 4, the resonance values of the tert-butyl group shifted to downfield. As a result of this, the quaternary carbon of the ligand (δ 34.96 ppm) emerged at δ 35.53 ppm and the methyl carbon (δ 31.02 ppm) appeared at δ 31.43 ppm.
3.2. The analysis of molecular structure
The good quality block white crystals of the N2,N6-bis(2-tert-butylphenyl)pyridine-2,6-dicarboxamide (H2L4) ligand and the block yellow crystals of its NNN Pd pincer complex 4 were obtained in acetonitrile and the appropriate crystals were chosen for the X-ray single crystal analysis. H2L4 crystallized in the monoclinic space group P21/c with Z = 4 while complex 4 generated in the monoclinic space group C2/c with Z = 8. Detailed crystallographic data and structure refinement parameters for both H2L4 and complex 4 can be found in Table 1, and the molecular structure of the synthesised H2L4 ligand and its Pd complex 4 are depicted in Fig. 1a (H2L4) and 1b (Complex 4).
Table 1.
Crystal structure refinement parameters of the H2L4 and complex 4.
| Parameters | H2L4 | Complex 4 |
|---|---|---|
| Empirical formula | C27H31N3O2 | C29H32N4O2Pd |
| Formula weight | 429.55 | 574.98 |
| Temperature | 273.15 K | 298.15 K |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | C2/c |
| a | 12.8772(5) Å | 28.276(5) Å |
| b | 14.1350(6) Å | 15.649(2) Å |
| c | 13.0122(5) Å | 14.735(2) Å |
| β | 91.093(2)ᵒ | 112.481(9)ᵒ |
| Volume | 2368.04(16) Å3 | 6024.7(16) Å3 |
| Z | 4 | 8 |
| ρ | 1.205 g/cm3 | 1.268 g/cm3 |
| μ | 0.604 mm−1 | 5.196 mm−1 |
| F(000) | 920.0 | 2368.0 |
| Crystal size | 0.317 × 0.297 × 0.156 mm3 | 0.295 × 0.276 × 0.134 mm3 |
| Radiation | CuKα (λ = 1.54178 Å) | CuKα (λ = 1.54178 Å) |
| 2Θ range for data collection | 6.866°–136.464° | 6.584°–136.48° |
| Index ranges | −15 ≤ h ≤ 15, −16 ≤ k ≤ 17, −15 ≤ l ≤ 15 | −34 ≤ h ≤ 34, −18 ≤ k ≤ 17, −17 ≤ l ≤ 17 |
| Reflections collected | 13492 | 47331 |
| Independent reflections | 4292 [Rint = 0.1523, Rsigma = 0.1227] | 5508 [Rint = 0.1209, Rsigma = 0.0515] |
| Data/restraints/parameters | 4292/0/296 | 5508/0/333 |
| Goodness-of-fit on F2 | 1.029 | 1.065 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0935, wR2 = 0.2287 | R1 = 0.0607, wR2 = 0.1611 |
| Final R indexes [all data] | R1 = 0.1547, wR2 = 0.2685 | R1 = 0.0792, wR2 = 0.1749 |
| Largest diff. peak/hole | 0.48/-0.43 e.Å−3 | 0.88/-1.10 e.Å−3 |
| CCDC no* | 2259839 | 2259840 |
*Supplementary crystallographic data (CCDC 2259839 and 2259840) can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
Fig. 1.
Crystal structure of (a) H2L4 and (b) complex 4.
In the crystal structure of the ligand, the carbonyl groups have a specific bond length for a double bond such as C1-02 1.213(4) and O1–C17 1.228(4) Å [45,46]. All C–N bonds in the structure are shorter than the average length of the C–N bond, which is 1.48 Å (Table 2) [27,45]. The angles, which are C12–N1–C16 118.4(2) and C17–N3–C18 124.0(3)° in the structure of the pincer ligand, which are close to 120°, show the sp2 hybridization of the N1 and N3 atoms (Table 2).
Table 2.
Selected bond distance (Å) and bond angles, (°) for pincer ligand H2L4 and complex 4.
| Bond | Distance (Å) | Bond | Angle (°) |
|---|---|---|---|
| N2,N6-bis(2-tert-Butylphenyl)pyridine-2,6-dicarboxamide(H2L4) | |||
| O1–C17 | 1.228(4) | C1–N2–C2 | 130.2(3) |
| O2–C1 | 1.213(4) | C12–N1–C16 | 118.4(2) |
| N2–C2 | 1.418(4) | C17–N3–C18 | 124.0(3) |
| N2–C1 | 1.341(4) | C6–C5–C4 | 120.1(3) |
| N1–C12 | 1.330(4) | C5–C6–C7 | 119.4(4) |
| N1–C16 | 1.341(4) | C6–C7–C2 | 120.9(3) |
| N3–C17 | 1.348(4) | C7–C2–N2 | 119.2(3) |
| N3–C18 | 1.427(4) | C7–C2–C3 | 121.2(3) |
| C5–C6 | 1.356(6) | C3–C2–N2 | 119.6(3) |
| C5–C4 | 1.388(5) | O2–C1–N2 | 126.8(3) |
| C6–C7 | 1.382(5) | O2–C1–C12 | 120.3(3) |
| C7–C2 | 1.394(4) | N2–C1–C12 | 112.8(2) |
| C2–C3 | 1.411(4) | N1–C12–C1 | 117.8(2) |
| C1–C12 | 1.512(4) | N1–C12–C13 | 122.8(3) |
| C12–C13 | 1.383(4) | C13–C12–C1 | 119.4(3) |
| C16–C17 | 1.485(4) | N1–C16–C17 | 117.4(2) |
| C16–C15 | 1.393(4) | N1–C16–C15 | 122.0(3) |
| C18C19 | 1.401(4) | C15–C16–C17 | 120.5(3) |
| C18–C23 | 1.382(5) | O1–C17–N3 | 123.6(3) |
| C19–C24 | 1.538(4) | O1–C17–C16 | 121.6(3) |
| C19–C20 | 1.396(4) | N3–C17–C16 | 114.7(3) |
| Acetonitrile-N2,N6-bis(2-tert-butylphenyl) pyridine-2,6-dicarboxamidopalladium(II)(Complex 4) | |||
| Pd1–N1 | 1.917(5) | N1–Pd1–N2 | 80.59(19) |
| Pd1–N2 | 2.041(4) | N1–Pd1–N3 | 81.01(19) |
| Pd1–N3 | 2.021(5) | N1–Pd1–N4 | 174.1(2) |
| Pd1–N4 | 2.022(5) | N3–Pd1–N2 | 161.45(19) |
| O2–C7 | 1.220(7) | N3–Pd1–N4 | 95.8(2) |
| O1–C1 | 1.243(7) | N4–Pd1–N2 | 102.8(2) |
| N1–C2 | 1.347(8) | C2–N1–Pd1 | 117.8(4) |
| N1–C6 | 1.334(7) | C6–N1–Pd1 | 118.3(4) |
| N2–C7 | 1.354(7) | C6–N1–C2 | 123.6(5) |
| N2–C8 | 1.444(7) | C7–N2–Pd1 | 114.6(4) |
| N3–C18 | 1.446(7) | C7–N2–C8 | 120.5(5) |
| N3–C1 | 1.336(8) | C8–N2–Pd1 | 123.8(3) |
| N4–C28 | 1.134(8) | C18–N3–Pd1 | 125.3(4) |
| C7–C6 | 1.507(8) | C1–N3–Pd1 | 114.8(4) |
| C2–C1 | 1.513(8) | C1–N3–C18 | 119.1(5) |
| C2–C3 | 1.384(8) | C28–N4–Pd1 | 162.0(6) |
| C18–C19 | 1.373(8) | O2–C7–N2 | 127.1(6) |
| C18–C23 | 1.404(8) | O2–C7–C6 | 121.0(5) |
| C6–C5 | 1.379(8) | N2–C7–C6 | 111.9(5) |
| C19–C20 | 1.383(9) | N1–C2–C1 | 113.1(5) |
| C8–C13 | 1.404(9) | N1–C2–C3 | 118.9(6) |
| C4–C3 | 1.386(9) | N1–C6–C7 | 114.2(5) |
| C4–C5 | 1.385(9) | N1–C6–C5 | 119.0(6) |
| C11–C10 | 1.361(10) | C13–C8–N2 | 123.8(5) |
| C23–C22 | 1.401(9) | C9–C8–N2 | 114.3(6) |
The Pd complex was formed by the coordination of the Pd(II) ion with the pyridine nitrogen of the ligand and the nitrogen of the two amide groups in the ligand and the nitrogen of an acetonitrile compound. The sum of the angles around the Pd(II) metal center is 360°, which is indicating a square planar geometry (Table 2) [47]. Four-coordination of Pd has resulted in the formation of two five-membered chelate rings such as Pd1–N3–C1–C2–N1 and Pd1–N2–C7–C6–N1 (Fig. 1b). The chelate bite angles of these five-membered rings in complex 4 are N1–Pd1–N3 = 81.01(19) and N1–Pd1–N2 = 80.59(19)°. In addition, the bite angles of N(amide)-Pd-NAcetonitrile are found as N3–Pd1–N4 = 95.8(2) and N2–Pd1–N4 = 102.8(2)°. Furthermore, the angle of the NPyridine-Pd-NAcetonitrile bond was detected as N1–Pd1–N4 = 174.1(2)° (Table 2). The dihedral angle of N–C–C–N (Pd1–N1–C2–C1–N3 and Pd1–N1–C6–C7–N2) which contains amide nitrogen and pyridyl rings in the complex is 1.46°, demonstrates that the rings are almost coplanar [48]. Furthermore, the maximum deviation from the Pd1–N1–C2–C1–N3 plane was found to be 0.046 Å for the N3 atom, while it was determined to be 0.039 Å for the N2 atom in the Pd1–N1–C6–C7–N2 plane.
The length of the Pd-NAcetonitrile bond, which is Pd1–N4 = 2.022(5) Å in complex 4 is compatible with the length of the Pd-NAcetonitrile bond found in previous studies [44,49]. It was determined that the length of the carbonyl bond (C1–O1 = 1.243(7) Å) in the Pd complex was longer than that of the free ligand (C1–O2 = 1.213(4) Å) due to complexation. Some of the C–N bond lengths (C7–N2 = 1.354(7) and C2–N1 = 1.347(8) Å) of the Pd complex are longer than the corresponding bond lengths in the free ligand (C17–N3 = 1.348(4) and C12–N1 = 1.330(4) Å) as a consequence of coordination with the Pd metal (Table 2).
The crystal structure of the NNN pincer ligand and its Pd complex is consolidated through the intramolecular and intermolecular contacts that are formed between C–H⋅⋅⋅N, C–H⋅⋅⋅O and N–H⋯N. While an intermolecular interaction (C5–H5⋅⋅⋅O1i, with symmetry code i = x, y, 1 + z) contributing to crystal packing was detected in the H2L4 ligand, three intermolecular connections (C4–H4⋅⋅⋅O2 ii, C20–H20⋅⋅⋅O2 iii and C29–H29C⋅⋅⋅O1 iv, with symmetry codes ii = x, 2-y, −1/2 + z; iii = 1/2-x, 3/2-y, 1-z; iv = x, 1-y, 1/2 + z) were found in the Pd complex. The crystal packing of the pincer ligand H2L4 and the Pd complex 4 are illustrated in Fig. 2a (H2L4) and 2b (Complex 4). The intramolecular and intermolecular interactions of the H2L4 and Pd complex 4 are illustrated in Fig. 3a (H2L4) and 3b (Complex 4) and Fig. 4a (H2L4) and 4b (Complex 4), respectively, and the parameters of the intermolecular and intramolecular H-bonds for the H2L4 and complex 4 are given in Table 3.
Fig. 2.
The crystal packing diagrams of the (a) pincer ligand H2L4 along the a–axis and (b) Pd complex 4 throughout the crystallographic b–axis.
Fig. 3.
The intramolecular hydrogen bonds of the (a) H2L4 and (b) Pd complex 4 generated by the N–H⋯N, C–H⋅⋅⋅O, and C–H⋅⋅⋅N interactions.
Fig. 4.
(a) The intermolecular hydrogen bonds via C–H⋅⋅⋅O interactions led to formation of zig-zag motif in H2L4 and (b) intermolecular hydrogen bonds generated by the C–H⋅⋅⋅O contacts in Pd complex 4.
Table 3.
Inter molecular and intramolecular hydrogen bonds for H2L4 and complex 4 (Å, °) *.
| D-H⋅⋅⋅A | d(D-H) | d(H⋅⋅⋅A) | d(D⋅⋅⋅A) | ∠ (D-H⋅⋅⋅A) |
|---|---|---|---|---|
| H2L4 | ||||
| N2–H2⋅⋅⋅N1 | 0.86 | 2.19 | 2.654(3) | 114 |
| N3–H3⋅⋅⋅N1 | 0.86 | 2.31 | 2.689 (3) | 107 |
| C5–H5⋅⋅⋅O1i | 0.93 | 2.54 | 3.405(5) | 155 |
| C7–H7⋅⋅⋅O2 | 0.93 | 2.22 | 2.871(5) | 126 |
| C9–H9C⋅⋅⋅N2 | 0.96 | 2.52 | 3.162(5) | 124 |
| C11–H11A⋅⋅⋅N2 | 0.96 | 2.50 | 3.109(5) | 121 |
| C25–H25B⋅⋅⋅N3 | 0.96 | 2.52 | 3.131(5) | 122 |
| C26–H26B⋅⋅⋅N3 | 0.96 | 2.55 | 3.163(5) | 121 |
| Complex 4 | ||||
| C4–H4⋅⋅⋅O2ii | 0.93 | 2.54 | 3.365(9) | 149 |
| C16–H16A⋅⋅⋅O2 | 0.96 | 2.37 | 3.185(14) | 142 |
| C17–H17C⋅⋅⋅N2 | 0.96 | 2.44 | 3.093(12) | 125 |
| C20–H20⋅⋅⋅O2iii | 0.93 | 2.59 | 3.363(10) | 140 |
| C25–H25A⋅⋅⋅N3 | 0.96 | 2.50 | 3.165(16) | 126 |
| C26–H26C⋅⋅⋅O1 | 0.96 | 2.48 | 3.392(17) | 158 |
| C26–H26C⋅⋅⋅N3 | 0.96 | 2.54 | 3.218(17) | 128 |
| C29–H29C⋅⋅⋅O1iv | 0.96 | 2.42 | 3.219(11) | 141 |
*Symmetry code: i = x, y, 1 + z; ii = x, 2-y, −1/2 + z; iii = 1/2-x, 3/2-y, 1-z; iv = x, 1-y, 1/2 + z.
The pincer ligand H2L4 contains intermolecular ring interactions, which are in the offset face-to-face manner. The convenient π⋅⋅⋅π contacts are generally described with interplanar distances between 3.3 and 3.8 Å and the displacement angle up to 20° [50,51]. The acceptable π⋅⋅⋅π interactions in H2L4 occurred between the Cg(1)⋅⋅⋅Cg(2)i 3.885(2) Å (Symmetry code: i = 1-x, 1-y, 1-z) and Cg(3)⋅⋅⋅Cg(3)v 3.8087(18) Å (Symmetry code: v = -x, 1-y, -z) with displacement angles 22.2 and 24.9°, respectively. Other π⋅⋅⋅π interactions found in H2L4 are above 3.8 Å and show weaker interactions. The detailed geometrical data of π⋅⋅⋅π interactions of the H2L4 ligand is listed in Table 4 and the Cg⋅⋅⋅Cg interactions of the H2L4 ligand are shown in Fig. 5a.
Table 4.
Geometrical parameters of π⋅⋅⋅π stacking interactions for H2L4 and C–H⋅⋅⋅π contacts for the complex 4 (Å, °).
|
H2L4 | |||||
|---|---|---|---|---|---|
| Rings I–Ja,b,l | Cg(I)⋅⋅⋅Cg(J)c | γ d | Cg(I)-perpe | Cg(J)-perpf | |
| Cg(1)⋅⋅⋅Cg(2)i | 3.885(2) | 23.6 | 3.5599(14) | 3.5966(16) | |
| Cg(1)⋅⋅⋅Cg(2)ii | 4.096(2) | 30.8 | −3.5198(14) | −3.9255(16) | |
| Cg(2)⋅⋅⋅Cg(1)i | 3.885(2) | 22.2 | 3.5966(16) | 3.5600(14) | |
| Cg(2)⋅⋅⋅Cg(1)iii | 4.096(2) | 16.6 | −3.9255(16) | −3.5198(14) | |
| Cg(3)⋅⋅⋅Cg(2)iv | 5.930(2) | 72.3 | 1.8025(16) | 3.5276(14) | |
| Cg(3)⋅⋅⋅Cg(3)v | 3.8087(18) | 24.9 | −3.4559(13) | −3.4558(13) | |
| Complex 4 | |||||
| C–H⋅⋅⋅Cg(J)g | H⋅⋅⋅Cg | H-perph | < C–H⋅⋅⋅Cgi | γ j | C⋅⋅⋅Cgk |
| C19 –H19⋅⋅⋅Cg(2)vi | 2.84 | −2.83 | 147 | 5.33 | 3.649(7) |
| C29–H29B⋅⋅⋅Cg(5)vii | 2.62 | −2.58 | 143 | 10.64 | 3.442(10) |
H2L4: Cg(1), Cg(2) and Cg(3) are the centroid of the rings, (N1–C12–C13–C14–C15–C16), (C2–C7), and (C18–C23), respectively. Complex 4: Cg(2) and Cg(5) are the centroid of the rings, (Pd1–N1–C6–C7–N2) and (C18–C23), respectively.
Ring number I and J (ring number mentioned over).
Centroid margin among ring I and ring J.
Angle among the centroid vector Cg(I)⋅⋅⋅Cg(J) and the normal to plane J.
Vertical margin of Cg(I) on ring J (Å).
Vertical margin of Cg(J) on ring I (Å).
Center of gravitation of ring J (ring number mentioned over).
Vertical margin of H to the ring plane J.
Angle among C–H and centroid Cg.
Angle among Cg-H vector and ring J normal.
Margin among C-atom and the proximate carbon atom in the benzene ring.
Symmetry: i = 1-x, 1-y, 1-z; ii = x, 1/2-y, −1/2+ z; iii = x, 1/2-y, 1/2 + z; iv = -x, 1-y, 1-z; v = -x, 1-y, -z; vi = 1/2-x, 3/2-y, 1-z; vii = x, 1-y, 1/2 + z.
Fig. 5.
(a) π⋅⋅⋅π interactions in H2L4 and (b) C–H⋅⋅⋅π contacts for complex 4.
The C–H … π interactions comprise the interaction of an aromatic ring's electron-rich π-cloud with a partially positive H atom. According to previous studies, the distance of meaningful C–H⋅⋅⋅π contact is in the range of 2.8–3.2 Å [52]. Two stacking C–H⋅⋅⋅π interactions were found in complex 4. These interactions are occurred among the carbon atom of the molecule, which acts a hydrogen bond donor to the centroid of the phenyl ring in the next molecule [53]. The existed C–H⋅⋅⋅π stacking interactions in complex 4 are C19–H19⋅⋅⋅Cg(2)vi 2.84 Å (Sym: vi = 1/2-x, 3/2-y, 1-z) and C29–H29B⋅⋅⋅Cg(5)vii 2.62 Å (Sym: vii = x, 1-y, 1/2 + z), in addition the C–H⋅⋅⋅π angles are 147 and 143°, for these interactions, respectively. The C–H⋅⋅⋅π angles are under the favourable value, which is 180°, and this may be generated from the steric hindrance in the complex [54]. The detailed geometric information of C–H···π stacking contacts of the complex 4 is given in Table 4 and the illustration of the C–H···π stacking contacts of the complex 4 is shown in Fig. 5b.
3.3. UV–Visible study
UV–vis analyses of NNN pincer ligands and their Pd(II) complexes were performed at the 200–800 nm range in acetonitrile at room temperature. An absorption band was determined for the H2L2 and H2L3 ligands at 278 nm. Although two distinct shoulders were detected in 220 and 280 nm for H2L1 and 221 and 268 nm for H2L4 spectra, three shoulders were observed at 223, 261, and 309 nm, respectively, for the H2L5 ligand. These observed peaks in the ligands are assigned to intraligand n → π* and π → π* transitions in the UV region [27,55]. After complexation, the peaks that were detected in the 220–223 and 261–280 nm range in the ligands were slightly blue-shifted to 218–222 and 260–266 nm in their Pd(II) complex spectra. Furthermore, the new absorption bands that formed between 311 and 321 nm belong to Ligand → Metal charge transfer transitions [56]. The UV absorption spectra of the NNN pincer ligands and their Pd(II) complexes are shown in Fig. 6a and b, respectively.
Fig. 6.
The superposed UV spectrum of the (a) ligands (H2L1-5) and (b) Pd(II) complexes in CH3CN.
3.4. Suzuki-Miyaura cross-coupling reaction
To obtain the optimal Suzuki-Miyaura cross-coupling reaction conditions, the effects of solvent, catalyst ratio, base, temperature, and time were examined for the Suzuki-Miyaura cross-coupling reaction in an optimisation study. For this purpose, 4-bromotoluene was used as the substrate and complex 3 as the model catalyst. 0.005 mmol of catalyst 3 was used for preliminary tests. All reactions were performed under aerobic conditions. To find the appropriate base, the reactions were carried out with KOH, K2CO3, K3PO4, Na2CO3, Cs2CO3 and Et3N, as the base and DMF were used as solvent in this stage. At the end of the reaction, K2CO3 provided the highest conversion (47.2%, Table 5, entry 2). Among other bases. Although inorganic bases provide varying amounts of conversion, as is known, the least coupling product (2.2% yield, Table 5, entry 6) was obtained when Et3N, an organic base, was used. As determined in previous studies, potassium-containing bases with a “cation effect” are extremely important for the activation of boronic acid. In addition, being easily accessible and inexpensive has allowed bases such as potassium-containing K2CO3 and KOH to be widely used in coupling reactions [[57], [58], [59], [60]]. Optimisation reactions were carried out at 90, 100, 110 and 120 °C, with K2CO3 as the base. Since there was no significant difference between 110 and 120 °C (Table 5, entries 9 and 10) according to the conversions and it is also important to perform the reactions under milder conditions, the optimal reaction temperature for the coupling reactions was accepted as 110 °C. DMF, toluene, EtOH, ACN, 1,4-dioxane, H2O, EtOH–H2O (v/v: 1:1 mL), 1,4-dioxane-H2O (v/v = 1:1 mL) were evulated as solvent, in the presence of K2CO3 at 110 °C. EtOH was found to be the best solvent as we obtained a 100% conversion with 98.6% yield (Table 5, Entry 12). In Suzuki C–C coupling reactions, it's crucial to ensure that the catalyst and substrates are as soluble as possible in polar solvents like ethanol [61]. The reactions were then carried out in different time intervals with different mole ratios of the model catalyst under the optimum base, solvent, and temperature determined for the Suzuki-Miyaura cross-coupling reaction. The effect of catalyst amount was investigated, entries 23, 25 and 26 in Table 5, indicate the effect of catalyst loading on the performance of complex 3. When catalyst loading decreased to 0.001 mmol, 86% conversion with 86% yield was obtained in half an hour (Table 5, entry 23) and more than 97% conversion with over 97% yield, in 5 h (Table 5, entry 24). When the catalyst loading continued to be reduced, 89% conversion and 89% coupling products were obtained with 0.0002 mmol catalyst in 12 h, while 86% conversion and 86% coupling product were obtained in 12 h when the catalyst ratio was reduced to 0.0001 mmol (Table 5, entries 26 and 25).
Table 5.
The obtained optimisation parameters for the Suzuki C–C coupling reactiona.
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst (mmol) | Base | Solvent | Temperature (°C) | Time (h) | Conversion (%)b | Yield (%)b | Selectivity (%) | TONc |
| 1 | 0.005 | KOH | DMF | 80 | 12 | >13 | >1 | >11 | >27 |
| 2 | 0.005 | K2CO3 | DMF | 80 | 12 | >47 | >28 | >60 | >94 |
| 3 | 0.005 | K3PO4 | DMF | 80 | 12 | >14 | >1 | >11 | >28 |
| 4 | 0.005 | Na2CO3 | DMF | 80 | 12 | >41 | >20 | >48 | >83 |
| 5 | 0.005 | Cs2CO3 | DMF | 80 | 12 | >13 | >0 | >3 | >27 |
| 6 | 0.005 | Et3N | DMF | 80 | 12 | >43 | >2 | >5 | >87 |
| 7 | 0.005 | K2CO3 | DMF | 90 | 12 | >46 | 28 | >60 | >92 |
| 8 | 0.005 | K2CO3 | DMF | 100 | 12 | >50 | >35 | >65 | >100 |
| 9 | 0.005 | K2CO3 | DMF | 110 | 12 | 51 | 36 | 70 | 102 |
| 10 | 0.005 | K2CO3 | DMF | 120 | 12 | 52 | 36 | 69 | 104 |
| 11 | 0.005 | K2CO3 | H2O | 110 | 12 | 82 | >76 | >93 | 164 |
| 12 | 0.005 | K2CO3 | EtOH | 110 | 12 | 100 | >98 | >98 | 200 |
| 13 | 0.005 | K2CO3 | 1,4-Dioxane | 110 | 12 | 91 | >85 | 94 | 182 |
| 14 | 0.005 | K2CO3 | Toluene | 110 | 12 | >98 | >92 | >93 | >197 |
| 15 | 0.005 | K2CO3 | CH3CN | 110 | 12 | 100 | 94 | 94 | 200 |
| 16 | 0.005 | K2CO3 | Et4NBr-DMF | 110 | 12 | >58 | >49 | 84 | >197 |
| 17 | 0.005 | K2CO3 | EtOH– H2O | 110 | 12 | 100 | >94 | >94 | 200 |
| 18 | 0.005 | K2CO3 | 1,4-Dioxane-H2O | 110 | 12 | >97 | >94 | >97 | >194 |
| 19 | 0.005 | K2CO3 | EtOH | 110 | 2 | 96 | 95 | >98 | 192 |
| 20 | 0.005 | K2CO3 | EtOH–Et4NBr | 110 | 2 | 98 | 97d | >98 | 196 |
| 21 | 0.005 | K2CO3 | EtOH | 110 | 4 | 98 | 97 | >98 | 196 |
| 22 | 0.005 | K2CO3 | EtOH–Et4NBr | 110 | 4 | 98 | 97d | >98 | 196 |
| 23 | 0.001 | K2CO3 | EtOH | 110 | 0.5 | 86 | 86 | 100 | 860 |
| 24 | 0.001 | K2CO3 | EtOH | 110 | 5 | >97 | >97 | 100 | >970 |
| 25 | 0.0001 | K2CO3 | EtOH | 110 | 12 | 86 | 86 | 100 | 8600 |
| 26 | 0.0002 | K2CO3 | EtOH | 110 | 12 | 89 | 89 | 100 | 4450 |
Reaction circumstances: 4-Bromotoluene (1.0 mmol), phenylboronic acid (1.2 mmol), base (1.2 mmol), solvent (2 mL).
Yield and conversion rates were determined by GC analysis based on aryl halides in the presence of dodecane used as internal standard.
Mole product/mole catalyst.
Et4NBr (0.02 mmol).
Quaternary ammonium salts are known to exert an activating effect on the reaction of arylhalides with phenylboronic acids [62]. Based on this, the effect of tetraethylammonium bromide salt on activity was investigated in this study, but no contribution was observed to increase conversion (Table 5, entry 22).
As a result of the optimisation study with the chosen model catalyst, the optimum base, solvent, time, temperature, and catalyst ratio were investigated for the Suzuki-Miyaura cross-coupling reaction; according to the results the best conditions are identified as follows: K2CO3, EtOH, 5 h, 110 °C and 0.001 mmol of catalyst. After the optimum reaction conditions were determined, the activity analyses of the other catalysts were carried out under these determined parameters.
The catalytic activities of the other Pd complexes 1, 2, 4, and 5, which contain different ligand substitutions, were tested under optimised conditions. The results are shown in Table 6. To determine the catalytic activity of Pd complexes, 4-bromotoluene was first used as a substrate in the Suzuki coupling reaction with phenylboronic acid. All palladium complexes were utilized as catalyst in the reaction, conversion rates were determined for each hour, with a maximum of 5 h. All complexes were found to catalyse the coupling of phenylboronic acid with 4-bromotoluene to form the coupling product 4-methylbiphenyl in good yields. In general, the use of deactivated aryl bromides, substituted with electron-donating groups such as -methyl or -methoxy, for the Suzuki C–C coupling reactions decelerates the reaction [[63], [64], [65]]. However, the 1, 2, 4, and 5 Pd complexes which have ortho-substituted F, Cl, tert-butyl, and NO2 groups, respectively, served as an effective catalyst for the coupling reaction and formed good yields with 4-bromotoluene, which has a donating group at the para position on the phenyl ring. In 1 h, 80% conversion with 80% yield and 79% conversion with 79% yield were obtained from 1 to 2 complexes, respectively. The highest conversion and yield of these complexes were obtained at the fourth hour (Table 6, entries 1 and 2). However, there was an increase in the conversion rates provided by these two complexes until the first 4 h, but a slight decrease was observed in the last hour. This is most likely because the catalyst may be saturated after the first 4 h. An excellent yield was achieved from the 4 and 5 complexes, with more than 99% conversion for complex 4 and more than 98% conversion for complex 5 in 1 h (Table 6, entries 3 and 4). Complex 4 contains sterically hindered bulky tert-butyl group, which accelerates the oxidative addition of aryl bromides [66]. In the coupling reaction of 4-bromotoluene with phenylboronic acid, the catalytic activity of the 4 and 5 complexes, which provide better conversion than other Pd complexes, was then investigated in coupling reactions using substrates of 1-bromo-4-isobutylbenzene and 2-bromo-6-methoxynaphthalene. The complexes showed good conversion and ensured high yields of coupling products from these bulky deactivated and sterically hindered substrates. In the reaction of 1-bromo-4-isobutyl benzene, complex 4 produced more than 98% conversion and over 98% yield in 1 h (Table 6, entry 5), while complex 5 formed more than 94% conversion and more than 94% coupling product in 1 h (Table 6, entry 6). Furthermore, complex 5 reached more than 96% conversion and generated over 96% yield of the coupling product in 2 h (Table 6, entry 7). In the Suzuki coupling of 2-bromo-6-methoxynaphthalene, both complexes 4 and 5 gave almost %100 excellent yields in 1 h (Table 6, entries 8 and 9).
Table 6.
The catalytic activities of NNN Pd pincer complexes in Suzuki-Miyaura C–C coupling reactiona.
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Catalyst | Substrate | Time (h) | Conversion (%)b | Yield (%)b | TONc |
| 1 | Complex 1 | 4-Bromotoluene | 4 | >91 | >91 | >910 |
| 2 | Complex 2 | 4-Bromotoluene | 4 | >93 | >93 | >930 |
| 3 | Complex 4 | 4-Bromotoluene | 1 | >99 | >99 | >990 |
| 4 | Complex 5 | 4-Bromotoluene | 1 | >98 | >98 | >980 |
| 5 | Complex 4 | 1-Bromo-4-izobutylbenzene | 1 | >98 | >98 | >980 |
| 6 | Complex 5 | 1-Bromo-4-izobutylbenzene | 1 | >94 | >94 | >940 |
| 7 | Complex 5 | 1-Bromo-4-izobutylbenzene | 2 | >96 | >96 | >960 |
| 8 | Complex 4 | 2-Bromo-6-methoxynaphthalene | 1 | 100 | >99 | 1000 |
| 9 | Complex 5 | 2-Bromo-6-methoxynaphthalene | 1 | 100 | >99 | 1000 |
Reaction circumstances: Ar–Br (1.0 mmol), phenylboronic acid (1.2 mmol), K2CO3 (1.2 mmol), catalysis (0.001 mmol), EtOH (2 mL) at 110 °C.
Yield and conversion rates were determined by GC analysis based on aryl halides in the presence of dodecane used as internal standard.
Mole product/mole catalyst.
As a consequence, the NNN pincer-type-based Pd complexes that we synthesised proved to be very capable in the Suzuki-Miyaura coupling reaction because they exhibited high activity, good selectivity, and excellent yields of the coupling product. When the results obtained in this study are evaluated, it is determined that the catalytic activity of our Pd complexes in the Suzuki-Miyaura coupling reaction was better than the activities of the Pd complexes that have similar NNN ligand structure, which was previously reported by Jerome et al. [44]. Furthermore, the Pd complexes we synthesised as catalysts achieved high conversion in a much shorter time.
3.4.1. Hg poisoning test
The mercury poisoning test is a very significant way to determine whether the reaction is homogeneous or heterogeneous [67]. To understand the nature of the Pd complexes, the Suzuki reaction was performed under optimised conditions. 4-Bromotoluene (1.0 mmol), phenylboronic acid (1.2 mmol), K2CO3 (1.2 mmol), EtOH (2 mL), and palladium catalyst (0.001 mmol) and excess Hg (200 molar equivalents of the Pd catalyst) were mixed in a closed reaction tube, and the reaction mixture obtained was stirred for the required time at 110 °C. The procedure for the Suzuki reaction was then repeated, and the isolated yields were analyzed by GC.
The Hg (0) poisoning test is the most well‐known test to reveal the nature of the complexes and is easy to perform on C–C coupling reactions. Hg(0) amalgamates connect very strongly with Pd(0), Rh(0), and Ni(0) based complexes, thus blocking the access of the active site to the substrate [63,67]. According to the results of the mercury test, when the conversion rates of the complexes are examined, it is seen that there is a serious decrease, although not completely suppressed (Table 7). These findings suggest that catalytic activity is produced by Pd complexes containing the NNN pincer ligand. Because the Pd(0) species are protected by the ligand, the Hg(0) could not fully extinguish the reaction. However, the fact that Hg(0) does not completely stop the reaction and causes extremely low conversions under the same conditions can be explained by ligand dissociation [68]. It is important to point that, studies have reported, not only colloidal Pd(0) but also molecular Pd(0) complexes can be exterminated by elemental mercury [69,70]. Furthermore, Hg(0) poisoning experiments indicate that soluble Pd(0) species are most likely decomposition products of palladacyclines. Therefore, it can be said that catalytic process based on a homogeneous catalytic mechanism including intermediates Pd(0) and Pd(II) [71].
Table 7.
Activation of catalysts in Suzuki-Miyaura coupling reaction between 4-bromotoluene and phenylboronic acid under Hg(0) poisoning effecta.
 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst | Time (h) | Conversion (%)b | Yield (%)b | TONc |
| 1 | Complex 1 | 4 | >18 | >16 | >180 |
| 2 | Complex 2 | 4 | >38 | >36 | >380 |
| 3 | Complex 3 | 5 | >37 | >34 | >370 |
| 4 | Complex 4 | 1 | >39 | >39 | >390 |
| 5 | Complex 5 | 1 | >16 | >16 | >160 |
Reaction circumstances: Ar–Br (1.0 mmol), phenylboronic acid (1.2 mmol), K2CO3 (1.2 mmol), catalysis (0.001 mmol), EtOH (2 mL) and Hg (200 molar equivalents of the Pd catalyst) at 110 °C.
Yield and conversion rates were determined by GC analysis based on aryl halides in the presence of dodecane used as internal standard.
Mole product/mole catalyst.
The formed ligand-free molecular paladium species continued to react with Hg until these catalytic species were deactivated [72]. Therefore, ligand-free species or colloids are likely to be active species for the catalytic reaction [73]. These results have shown that NNN ligands in the structures of Pd complexes are extremely important for the stabilization of active catalytic species. At the same time, NNN Pd complexes are intermediate substances required for the generation of effective Pd(0) species in the reaction condition [63,69,74].
3.4.2. Reusability analysis
According to the results of the Hg test, Complex 5 showed a significant decrease in conversion rate. As a result of this, it has been determined that Complex 5, with its more heterogeneous character, is more suitable for the reusability test. For this purpose, the catalytic activity of the black precipitates formed during the reaction of Complex 5 was investigated under optimised conditions for the reusability test. The procedure for the reusability test is as follows; after each cycle was completed, the mixture was diluted with EtOAc (15 mL) and the black precipitates obtained were collected by centrifugation and subsequently rinsed with methanol, water, and diethylether, respectively. Then, dried at 80 °C before using for the next reaction with fresh 4-bromotoluene and phenylboronic acid [41,75,76]. After four cycles, we observed a considerably decrease in the activity of the complex (from %87 conversion for the first run to %34 conversion for the fourth run). In fact, the black residues formed at the end of each reaction could be obtained under very difficult conditions with a significant loss of yield. These results showed how important NNN-pincer ligands are to the catalytic activity of our Pd complexes, considering their role in stabilising the catalytic species formed in the reaction medium. TEM analysis was performed for the morphological characterisation of the resulting black residues. TEM images obtained from the fourth cycle (Fig. 7a and b) indicate that the Pd species are well dispersed. Furthermore, Complex 5 contains nanosized spherical particles.
Fig. 7.
TEM images for complex 5 at (a) 20 nm and (b) 5 nm scales.
4. Conclusions
The NNN pincer-type novel five-ligand and their Pd(II) complexes were synthesised and characterised with several methods. The crystal structure of one ligand and its Pd(II) complex (H2L4 and complex 4) was also able to be elucidated with a single-crystal XRD analysis. The single-crystal XRD data provided us to better understand the molecular structures of the NNN pincer ligand and its Pd complex. Single crystal XRD results revealed that both the ligand and its Pd complex contain supramolecular interactions. These intramolecular and intermolecular contacts that are formed between C–H⋅⋅⋅N, C–H⋅⋅⋅O, N–H⋯N, π⋅⋅⋅π and C–H … π are contributing to stabilization of the crystal system. In line with previous studies in the literature, pincer complexes are known to be effective in C–C cross-coupling reactions. On the basis of all of these studies, the catalytic activities of the synthesised Pd(II) complexes were tested in the Suzuki-Miyaura cross-coupling reaction. According to the results of the catalytic study, it was determined that our pincer-structured Pd(II) complexes were highly capable of the Suzuki C–C cross-coupling reaction between the sterically hindered and deactivated aryl bromides and phenylbronic acid at the required reaction times. Furthermore, these Pd(II) complexes demonstrated good conversion and selectivity at short times with even low catalyst loadings. Among the synthesised complexes, when evaluated on the basis of their high conversion and selectivity that they showed in short time periods, it was determined that complexes 4 and 5 performed higher activity. For example, complete conversion and nearly 100% yield were achieved from the reaction of the 2-bromo-6-methoxynaphthalene substrate with phenylboronic acid, which Complex 4 and 5 used as catalyst. Reusability analysis was performed with the black residues of complex 5. Unfortunately, progressively lower conversion rates were obtained as a result of each cycle (from %87 conversion for the first run to %34 conversion for the fourth run). On the basis of the results of the Hg(0) poisoning test, although a serious decrease in the activity of all Pd(II) complexes was observed, it was not completely suppressed, and the reaction continued at a certain conversion rate. This showed us that our NNN pincer-type ligands are extremely important in terms of stabilising the active catalytic species formed during the reaction in the medium and are also the distributors of the generated active particles. To contribute to the number of catalytic studies in the scope of cross-coupling reactions, in our current studies, the synthesis of these and similar pincer complexes and their catalytic applicability in various catalytic reactions continues to be investigated.
Authorship contribution statement
Hakan Arslan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ebru Keskin: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Funding
Mersin University
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study is part of the Ph.D. thesis of Ebru Keskin and was supported by the Research Fund of Mersin University, Mersin, Türkiye (Grant number: 2019-1-TP3-3475).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17608.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shinde V.N., Bhuvanesh N., Kumar A., Joshi H. Design and syntheses of palladium complexes of NNN/CNN pincer ligands: catalyst for cross dehydrogenative coupling reaction of heteroarenes. Organometallics. 2020;39:324–333. doi: 10.1021/acs.organomet.9b00695. [DOI] [Google Scholar]
- 2.Suzuki A. Cross-coupling reactions of organoboranes: an easy way to construct C-C bonds (Nobel Lecture) Angew Chem. Int. Ed. Engl. 2011;50:6722–6737. doi: 10.1002/anie.201101379. [DOI] [PubMed] [Google Scholar]
- 3.D'Alterio M.C., Casals-Cruañas È., Tzouras N.V., Talarico G., Nolan S.P., Poater A. Mechanistic aspects of the palladium-catalyzed Suzuki-Miyaura cross-coupling reaction. Chemistry. 2021;27:13481–13493. doi: 10.1002/chem.202101880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frech C.M., Shimon L.J.W., Milstein D. Redox-induced collapse and regeneration of a pincer-type complex framework: a nonplanar coordination mode of palladium(II) Angew. Chem. Int. Ed. 2005;44:1709–1711. doi: 10.1002/anie.200462386. [DOI] [PubMed] [Google Scholar]
- 5.Pilarski L.T., Selander N., Böse D., Szabó K.J. Catalytic allylic C-H acetoxylation and benzoyloxylation via suggested (η3-Allyl)palladium(IV) intermediates. Org. Lett. 2009;11(23):5518–5521. doi: 10.1021/ol9023369. [DOI] [PubMed] [Google Scholar]
- 6.Ohff M., Ohff A., van der Boom M.E., Milstein D. Highly active Pd(II) PCP-type catalysts for the heck reaction. J. Am. Chem. Soc. 1997;119:11687–11688. doi: 10.1021/ja9729692. [DOI] [Google Scholar]
- 7.Asay M., Morales-Morales D. vol. 54. Springer; Cham: 2015. Recent advances on the chemistry of POCOP–nickel pincer compounds. (Topics in Organometallic Chemistry). [DOI] [Google Scholar]
- 8.Aydin J., Larsson J.M., Selander N., Szabó K.J. Pincer complex-catalyzed redox coupling of alkenes with iodonium salts via presumed palladium(IV) intermediates. Org. Lett. 2009;11(13):2852–2854. doi: 10.1021/ol9010739. [DOI] [PubMed] [Google Scholar]
- 9.Selander N., J Szabó K. Catalysis by palladium pincer complexes. Chem. Rev. 2011;111:2048–2076. doi: 10.1021/cr1002112. [DOI] [PubMed] [Google Scholar]
- 10.Balaraman E., Gnanaprakasam B., Shimon L.J.W., Milstein D. Direct hydrogenation of amides to alcohols and amines under mild conditions. J. Am. Chem. Soc. 2010;132:16756–16758. doi: 10.1021/ja1080019. [DOI] [PubMed] [Google Scholar]
- 11.Barrios-Francisco R., Balaraman E., Diskin-Posner Y., Leitus G., Shimon L.J.W., Milstein D. PNN ruthenium pincer complexes based on phosphinated 2,2′-dipyridinemethane and 2,2′-oxobispyridine. Metal–ligand cooperation in cyclometalation and catalysis. Organometallics. 2013;32:2973–2982. doi: 10.1021/om400194w. [DOI] [Google Scholar]
- 12.Spasyuk D., Smith S., Gusev D.G. From esters to alcohols and back with ruthenium and osmium catalysts. Angew Chem. Int. Ed. Engl. 2012;51:2772–2775. doi: 10.1002/anie.201108956. [DOI] [PubMed] [Google Scholar]
- 13.Peris E., Crabtree R.H. Key factors in pincer ligand design. Chem. Soc. Rev. 2018;47:1959–1968. doi: 10.1039/c7cs00693d. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Sebastian L., Morales-Morales D. Cross-coupling reactions catalysed by palladium pincer complexes. A review of recent advances. J. Organomet. Chem. 2019;893:39–51. doi: 10.1016/j.jorganchem.2019.04.021. [DOI] [Google Scholar]
- 15.Ramalingam B.M., Ramakrishna I., Baidya M. Nickel-catalyzed direct alkenylation of methyl heteroarenes with primary alcohols. J. Org. Chem. 2019;84:9819–9825. doi: 10.1021/acs.joc.9b01517. [DOI] [PubMed] [Google Scholar]
- 16.Meghdadi S., Amirnasr M., Amiri A., Musavizadeh Mobarakeh Z., Azarkamanzad Z. Benign synthesis of N-(8-quinolyl)pyridine-2-carboxamide) ligand (Hbpq), and its Ni(II) and Cu(II) complexes. A fluorescent probe for direct detection of nitric oxide in acetonitrile solution based on Hbpq copper(II) acetate interaction. C. R. Chim. 2014;17:477–483. doi: 10.1016/j.crci.2013.10.003. [DOI] [Google Scholar]
- 17.Ni Z.-H., Kou H.-Z., Zhang L.-F., Ni W.-W., Jiang Y.-B., Cui A.-L., Ribas J., Sato O. mer-[Fe(pcq)(CN)3]-: a novel cyanide-containing building block and its application to assembling cyanide-bridged trinuclear FeIII2MnII complexes [pcq- = 8-(pyridine-2-carboxamido)quinoline anion] Inorg. Chem. 2005;44:9631–9633. doi: 10.1021/ic051385v. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W., Wang Y., Cao J., Wang L., Pan Y., Redshaw C., Sun W.-H. Synthesis and characterization of dialkylaluminum amidates and their ring-opening polymerization of ε-caprolactone. Organometallics. 2011;30:6253–6261. doi: 10.1021/om2008343. [DOI] [Google Scholar]
- 19.Dolomanov O.V., Bourhis L.J., Gildea R.J., Howard J.A.K., Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009;42:339–341. doi: 10.1107/s0021889808042726. [DOI] [Google Scholar]
- 20.Palatinus L., Chapuis G. SUPERFLIP– a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007;40:786–790. doi: 10.1107/s0021889807029238. [DOI] [Google Scholar]
- 21.Palatinus L., van der Lee A. Symmetry determination following structure solution inP1. J. Appl. Crystallogr. 2008;41:975–984. doi: 10.1107/s0021889808028185. [DOI] [Google Scholar]
- 22.Palatinus L., Prathapa S.J., van Smaalen S. EDMA: a computer program for topological analysis of discrete electron densities. J. Appl. Crystallogr. 2012;45:575–580. doi: 10.1107/s0021889812016068. [DOI] [Google Scholar]
- 23.Sheldrick G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015;71:3–8. doi: 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arrieta A., Aizpurua J.M., Palomo C. N,N-Dimethylchlorosulfitemethaniminium chloride (SOCl2-DMF) a versatile dehydrating reagent. Tetrahedron Lett. 1984;25(31):3365–3368. doi: 10.1016/s0040-4039(01)81386-1. [DOI] [Google Scholar]
- 25.Gu S., Xu H., Zhang N., Chen W. Triazole-functionalized N-heterocyclic carbene complexes of palladium and platinum and efficient aqueous Suzuki-Miyaura coupling reaction. Chem. Asian J. 2010;5:1677–1686. doi: 10.1002/asia.201000071. [DOI] [PubMed] [Google Scholar]
- 26.Camacho-Espinoza M., Penieres-Carrillo J.G., Rios-Guerra H., Lagunas-Rivera S., Ortega-Jiménez F. An efficient and simple imidazole-hydrazone ligand for palladium-catalyzed Suzuki-Miyaura cross-coupling reactions in water under infrared irradiation. J. Organomet. Chem. 2019;880:386–391. doi: 10.1016/j.jorganchem.2018.11.016. [DOI] [Google Scholar]
- 27.Solmaz U., Ince S., Yilmaz M.K., Arslan H. Conversion of monodentate benzoylthiourea palladium(II) complex to bidentate coordination mode: synthesis, crystal structure and catalytic activity in the Suzuki-Miyaura cross-coupling reaction. J. Organomet. Chem. 2022;973–974 doi: 10.1016/j.jorganchem.2022.122374. [DOI] [Google Scholar]
- 28.Solmaz U., Arslan H. Spectral, crystallographic, theoretical, and catalytic activity studies of the PdII complexes in different coordination modes of benzoylthiourea ligand. J. Mol. Struct. 2022;1269 doi: 10.1016/j.molstruc.2022.133839. [DOI] [Google Scholar]
- 29.Keskin E., Solmaz U., Gumus I., Arslan H. Di- and tetra-nuclear oxorhenium(V) complexes of benzoylthiourea derivative ligands: synthesis, structural characterization, and catalytic applications. Polyhedron. 2022;219 doi: 10.1016/j.poly.2022.115786. [DOI] [Google Scholar]
- 30.Solmaz U., Gumus I., Binzet G., Celik O., Balci G.K., Dogen A., Arslan H. Synthesis, characterization, crystal structure, and antimicrobial studies of novel thiourea derivative ligands and their platinum complexes. J. Coord. Chem. 2018;71:200–218. doi: 10.1080/00958972.2018.1427233. [DOI] [Google Scholar]
- 31.Gumus I., Solmaz U., Binzet G., Keskin E., Arslan B., Arslan H. Hirshfeld surface analyses and crystal structures of supramolecular self-assembly thiourea derivatives directed by non-covalent interactions. J. Mol. Struct. 2018;1157:78–88. doi: 10.1016/j.molstruc.2017.12.017. [DOI] [Google Scholar]
- 32.Gumus I., Solmaz U., Celik O., Binzet G., Balcı G.K., Arslan H. Synthesis, characterization and crystal structure of cis-bis[4-fluoro-N-(diethylcarbamothioyl)benzamido-κ2O,S]platinum(II) Eur. J. Chem. 2015;6:237–241. doi: 10.5155/eurjchem.6.3.237-241.1265. [DOI] [Google Scholar]
- 33.Keskin E., Solmaz U., Binzet G., Gumus I., Arslan H. Synthesis, characterization and crystal structure of platinum(II) complexes with thiourea derivative ligands. Eur. J. Chem. 2018;9:360–368. doi: 10.5155/eurjchem.9.4.360-368.1774. [DOI] [Google Scholar]
- 34.Gumus I., Gonca S., Arslan B., Keskin E., Solmaz U., Arslan H. N-(Dibenzylcarbamothioyl)-3-methylbutanamide: crystal structure, Hirshfeld surfaces and antimicrobial activity. Eur. J. Chem. 2017;8:410–416. doi: 10.5155/eurjchem.8.4.410-416.1650. [DOI] [Google Scholar]
- 35.Solmaz U., Keskin E., Gumus I., Cevik P.K., Binzet G., Arslan H. Platinum(ii) complex containing n-(bis(-2,4-dimethoxy-benzyl)carbamothioyl)- 4-methylbenzamide ligand: synthesis, crystal structure, Hirshfeld surface analysis, and antimicrobial activity. J. Struct. Chem. 2022;63:62–74. doi: 10.1134/s0022476622010073. [DOI] [Google Scholar]
- 36.Gumus I., Solmaz U., Gonca S., Arslan H. Molecular self-assembly in indole-based benzamide derivative: crystal structure, Hirshfeld surfaces and antimicrobial activity. Eur. J. Chem. 2017;8:349–357. doi: 10.5155/eurjchem.8.4.349-357.1637. [DOI] [Google Scholar]
- 37.Ozer C.K., Solmaz U., Arslan H. Crystal structure, Hirshfeld surface analysis, and DFT studies of N-(2-chlorophenylcarbamothioyl)cyclohexanecarboxamide. Eur. J. Chem. 2021;12:439–449. doi: 10.5155/eurjchem.12.4.439-449.2196. [DOI] [Google Scholar]
- 38.Keskin E., Turunc E., Arslan H. Synthesis, characterization, electrochemical behavior, and catalytic activity of cobalt(II) metal complexes with pincer-type methylbenzamide derivative ligands. Polyhedron. 2022;221 doi: 10.1016/j.poly.2022.115846. [DOI] [Google Scholar]
- 39.Arslan B., Binzet G. Synthesis, crystal structure analysis, DFT calculations, antioxidant and antimicrobial activity of N,N-di-2,4-dimethoxybenzyl-N’-2-nitrobenzoylthiourea. J. Mol. Struct. 2022;1267 doi: 10.1016/j.molstruc.2022.133579. [DOI] [Google Scholar]
- 40.Ilis M., Batalu D., Pasuk I., Cîrcu V. Cyclometalated palladium(II) metallomesogens with Schiff bases and N-benzoyl thiourea derivatives as co-ligands. J. Mol. Liq. 2017;233:45–51. doi: 10.1016/j.molliq.2017.02.114. [DOI] [Google Scholar]
- 41.Solmaz U., Gumus I., Yilmaz M.K., Ince S., Arslan H. Palladium complexes derived from benzoylthiourea ligands: synthesis, crystal structure, and catalytic application in Suzuki C–C coupling reactions. Appl. Organomet. Chem. 2021;35 doi: 10.1002/aoc.6348. [DOI] [Google Scholar]
- 42.Lal R.A., Choudhury S., Ahmed A., Borthakur R., Asthana M., Kumar A. Synthesis of homobimetallic molybdenum(VI) complex of bis(2-hydroxy-1-naphthaldehyde)malonoyldihydrazone and its reaction with electron and proton bases. Spectrochim. Acta Mol. Biomol. Spectrosc. 2010;75:212–224. doi: 10.1016/j.saa.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q.-Q., Begum R.A., Day V.W., Bowman-James K. Chemical mustard containment using simple palladium pincer complexes: the influence of molecular walls. J. Am. Chem. Soc. 2013;135:17193–17199. doi: 10.1021/ja408770u. [DOI] [PubMed] [Google Scholar]
- 44.Jerome P., Sathishkumar P.N., Bhuvanesh N.S.P., Karvembu R. Towards phosphine-free Pd(II) pincer complexes for catalyzing Suzuki-Miyaura cross-coupling reaction in aqueous medium. J. Organomet. Chem. 2017;845:115–124. doi: 10.1016/j.jorganchem.2017.03.045. [DOI] [Google Scholar]
- 45.Allen F.H., Kennard O., Watson D.G., Brammer L., Orpen A.G., Taylor R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc., Perkin Trans. 1987;2:S1–S19. doi: 10.1039/P298700000S1. [DOI] [Google Scholar]
- 46.Arslan H., Külcü N., Flörke U. Synthesis and characterization of copper(II), nickel(II) and cobalt(II) complexes with novel thiourea derivatives. Transit. Met. Chem. 2003;28:816–819. doi: 10.1023/a:1026064232260. [DOI] [Google Scholar]
- 47.Cirera J., Alemany P., Alvarez S. Mapping the stereochemistry and symmetry of tetracoordinate transition-metal complexes. Chemistry. 2004;10:190–207. doi: 10.1002/chem.200305074. [DOI] [PubMed] [Google Scholar]
- 48.Latheef L., Prathapachandra Kurup M.R. Spectral and structural studies of nickel(II) complexes of salicylaldehyde 3-azacyclothiosemicarbazones. Polyhedron. 2008;27:35–43. doi: 10.1016/j.poly.2007.08.048. [DOI] [Google Scholar]
- 49.Yadav S., Singh A., Rashid N., Ghotia M., Roy T.K., Ingole P.P., Ray S., Mobin S.M., Dash C. Phosphine-free bis(pyrrolyl)pyridine based NNN-pincer palladium(II) complexes as efficient catalysts for Suzuki-miyaura cross-coupling reactions of aryl bromides in aqueous medium. ChemistrySelect. 2018;3:9469–9475. doi: 10.1002/slct.201801647. [DOI] [Google Scholar]
- 50.Yang L., Powell D.R., Houser R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007:955–964. doi: 10.1039/b617136b. [DOI] [PubMed] [Google Scholar]
- 51.Janiak C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000:3885–3896. doi: 10.1039/B003010O. [DOI] [Google Scholar]
- 52.Roy S., Drew M.G.B., Bauzá A., Frontera A., Chattopadhyay S. Estimation of conventional C–H⋯π (arene), unconventional C–H⋯π (chelate) and C–H⋯π (thiocyanate) interactions in hetero-nuclear nickel(ii)–cadmium(ii) complexes with a compartmental Schiff base. Dalton Trans. 2017;46:5384–5397. doi: 10.1039/c6dt04906k. [DOI] [PubMed] [Google Scholar]
- 53.Gumus I., Solmaz U., Binzet G., Keskin E., Arslan B., Arslan H. Supramolecular self-assembly of new thiourea derivatives directed by intermolecular hydrogen bonds and weak interactions: crystal structures and Hirshfeld surface analysis. Res. Chem. Intermed. 2019;45:169–198. doi: 10.1007/s11164-018-3596-5. [DOI] [Google Scholar]
- 54.Jiang Y.-F., Xi C.-J., Liu Y.-Z., Niclós-Gutiérrez J., Choquesillo-Lazarte D. Intramolecular “CH···π(metal chelate ring) interactions” as structural evidence for metalloaromaticity in bis(pyridine‐2,6‐diimine)Ru II complexes: intramolecular interactions as evidence for metalloaromaticity. Eur. J. Inorg. Chem. 2005:1585–1588. doi: 10.1002/ejic.200400864. 2005. [DOI] [PubMed] [Google Scholar]
- 55.Pratiwi R.A., Nandiyanto A.B.D. How to read and interpret UV-VIS spectrophotometric results in determining the structure of chemical compounds. Indonesian J. Edu. Res. Technol. 2022;2(1) doi: 10.17509/ijert.v2i1.35171. 1–20. [DOI] [Google Scholar]
- 56.Akbari A., Alinia Z. Synthesis, characterization, and DFT calculation of a Pd(II) Schiff base complex. Turk. J. Chem. 2013;37:867–878. doi: 10.3906/kim-1207-74. [DOI] [Google Scholar]
- 57.Scrivanti A., Bertoldini M., Matteoli U., Antonaroli S., Crociani B. [PdCl2{8-(di-tert-butylphosphinooxy)quinoline)}]: a highly efficient catalyst for Suzuki–Miyaura reaction. Tetrahedron. 2009;65:7611–7615. doi: 10.1016/j.tet.2009.06.099. [DOI] [Google Scholar]
- 58.Bedford R.B., Hazelwood S.L., Welch) neé, Horton P.N., Hursthouse M.B. Orthopalladated phosphinite complexes as high-activity catalysts for the Suzuki reaction. Dalton Trans. 2003;21:4164–4174. doi: 10.1039/B303657J. [DOI] [Google Scholar]
- 59.Bedford R.B., Hazelwood S.L., Limmert M.E., Albisson D.A., Draper S.M., Scully P.N., Coles S.J., Hursthouse M.B. Orthopalladated and -platinated bulky triarylphosphite complexes: synthesis, reactivity and application as high-activity catalysts for Suzuki and Stille coupling reactions. Chemistry. 2003;9:3216–3227. doi: 10.1002/chem.200304997. [DOI] [PubMed] [Google Scholar]
- 60.So C.M., Yeung C.C., Lau C.P., Kwong F.Y. A new family of tunable indolylphosphine ligands by one-pot assembly and their applications in Suzuki-Miyaura coupling of aryl chlorides. J. Org. Chem. 2008;73:7803–7806. doi: 10.1021/jo801544w. [DOI] [PubMed] [Google Scholar]
- 61.Wolfson A., Biton S., Levy-Ontman O. Study of Pd-based catalysts within red algae-derived polysaccharide supports in a Suzuki cross-coupling reaction. RSC Adv. 2018;8:37939–37948. doi: 10.1039/c8ra08408d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selvakumar K., Zapf A., Beller M. New palladium carbene catalysts for the Heck reaction of aryl chlorides in ionic liquids. Org. Lett. 2002;4:3031–3033. doi: 10.1021/ol020103h. [DOI] [PubMed] [Google Scholar]
- 63.Mahamo T., Mogorosi M.M., Moss J.R., Mapolie S.F., Chris Slootweg J., Lammertsma K., Smith G.S. Neutral palladium(II) complexes with P,N Schiff-base ligands: synthesis, characterization and application as Suzuki–Miyaura coupling catalysts. J. Organomet. Chem. 2012;703:34–42. doi: 10.1016/j.jorganchem.2011.12.021. [DOI] [Google Scholar]
- 64.Yilmaz M.K. Palladium(II) complexes with new bidentate phosphine-imine ligands for the Suzuki C C coupling reactions in supercritical carbon dioxide. J. Supercrit. Fluids. 2018;138:221–227. doi: 10.1016/j.supflu.2018.04.022. [DOI] [Google Scholar]
- 65.Yılmaz M.K., Güzel B. Iminophosphine palladium(II) complexes: synthesis, characterization, and application in Heck cross-coupling reaction of aryl bromides: heck cross-coupling reactions with iminophosphine Pd(II) complexes. Appl. Organomet. Chem. 2014;28:529–536. doi: 10.1002/aoc.3158. [DOI] [Google Scholar]
- 66.Ulusoy M., Birel Ö., Şahin O., Büyükgüngör O., Cetinkaya B. Structural, spectral, electrochemical and catalytic reactivity studies of a series of N2O2 chelated palladium(II) complexes. Polyhedron. 2012;38:141–148. doi: 10.1016/j.poly.2012.02.035. [DOI] [Google Scholar]
- 67.Şengül A., Hanhan M.E. Water soluble benzimidazole containing ionic palladium(II) complex for rapid microwave-assisted Suzuki reaction of aryl chlorides: water soluble benzimidazole - Pd(II) complexes for Suzuki reaction. Appl. Organomet. Chem. 2018;32:e4288. doi: 10.1002/aoc.4288. [DOI] [Google Scholar]
- 68.Nobre S.M., Monteiro A.L. Pd complexes of iminophosphine ligands: a homogeneous molecular catalyst for Suzuki–Miyaura cross-coupling reactions under mild conditions. J. Mol. Catal. Chem. 2009;313:65–73. doi: 10.1016/j.molcata.2009.08.003. [DOI] [Google Scholar]
- 69.Olsson D., Wendt O.F. Suzuki reaction catalysed by a PCsp3P pincer Pd(II) complex: evidence for a mechanism involving molecular species. J. Organomet. Chem. 2009;694:3112–3115. doi: 10.1016/j.jorganchem.2009.05.025. [DOI] [Google Scholar]
- 70.van Asselt R., Elsevier C.J. Homogeneous catalytic hydrogenation of alkenes by zero-valent palladium complexes of cis-fixed dinitrogen ligands. J. Mol. Catal. 1991;65 doi: 10.1016/0304-5102(91)85057-9. L13–L19. [DOI] [Google Scholar]
- 71.Sabounchei S.J., Ahmadi M., Panahimehr M., Bagherjeri F.A., Nasri Z. Phosphine mono- and bis-ylide palladacycles as homogeneous molecular precatalysts: simple and efficient protocol greatly facilitate Suzuki and Heck coupling reactions. J. Mol. Catal. Chem. 2014:383–384. doi: 10.1016/j.molcata.2014.01.002. 249–259. [DOI] [Google Scholar]
- 72.Phan N.T.S., Van Der Sluys M., Jones C.W. On the nature of the active species in palladium catalyzed mizoroki–heck and Suzuki–miyaura couplings – homogeneous or heterogeneous catalysis, A critical review. Adv. Synth. Catal. 2006;348:609–679. doi: 10.1002/adsc.200505473. [DOI] [Google Scholar]
- 73.Joshi H., Prakash O., Sharma A.K., Sharma K.N., Singh A.K. Suzuki coupling reactions catalyzed with palladacycles and palladium(II) complexes of 2‐thiophenemethylamine‐based Schiff bases: examples of divergent pathways for the same ligand: Suzuki coupling reactions. Eur. J. Inorg. Chem. 2015:1542–1551. doi: 10.1002/ejic.201403176. 2015. [DOI] [Google Scholar]
- 74.Kumar S., Rao G.K., Kumar A., Singh M.P., Saleem F., Singh A.K. Efficient catalytic activation of Suzuki–Miyaura C–C coupling reactions with recyclable palladium nanoparticles tailored with sterically demanding di-n-alkyl sulfides. RSC Adv. 2015;5:20081–20089. doi: 10.1039/c5ra00441a. [DOI] [Google Scholar]
- 75.Mohammadi E., Movassagh B. Polystyrene-resin supported N-heterocyclic carbene-Pd(II) complex based on plant-derived theophylline: a reusable and effective catalyst for the Suzuki-Miyaura cross-coupling reaction of arenediazonium tetrafluoroborate salts with arylboronic acids. J. Organomet. Chem. 2016;822:62–66. doi: 10.1016/j.jorganchem.2016.08.017. [DOI] [Google Scholar]
- 76.Akkoç M., Buğday N., Altın S., Kiraz N., Yaşar S., Özdemir İ. N-heterocyclic carbene Pd(II) complex supported on Fe3O4@SiO2: highly active, reusable and magnetically separable catalyst for Suzuki-Miyaura cross-coupling reactions in aqueous media. J. Organomet. Chem. 2021;943 doi: 10.1016/j.jorganchem.2021.121823. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.