Abstract
Purpose
Evaluation of the variabilities in apparent diffusion coefficient (ADC) measurements of the spleen (ADCspleen) and the paraspinal muscles (ADCmuscle) to identify the reference organ for normalizing the ADC from the abdominal diffusion weighted imaging (DWI).
Methods
Two MRI scanners, with 314 abdominal exams on the GE and 929 on the Siemens system, were used for MRI examinations including DWI (b-values, 50 and 800 s/mm2). For a subset of 73 exams on the Siemens system a second exam was conducted. Four regions of interest (ROIs) in each exam were placed to measure the ADCspleen and the bilateral ADCmuscle. ADC variability between patients (on each scanner separately), ADC variability due to ROI placement between the two ROIs in each organ, and variability in the subset between the first and second exams were assessed.
Results
The ADCspleen was more scattered and variable than the ADCmuscle in the comparability (n = 929 and 314 for two MRI scanners, respectively) and repeatability (n = 73) datasets. The Bland-Altmann bias and limits of agreement (LoAs) for the ADCspleen (ICC, 0.47; CV, 0.070) and ADCmuscle (ICC, 0.67; CV, 0.023) in the repeatability datasets (n = 73) were −0.1 (−25.7%–25.6%) and −0.3 (−8.8%–8.1%), respectively. For the Siemens system, the Bland-Altmann bias and LoAs for the ADCspleen (ICC, 0.72; CV, 0.061) and ADCmuscle (ICC, 0.53; CV, 0.030) in the comparability datasets (n = 929) were 2.1 (−20.0%–24.2%) and 0.7 (−10.0%–11.4%), respectively. Similar findings have been found in the GE system (n = 314). The CVs for the ADCmuscle measurements were lower than those of the ADCspleen both in the repeatability and the comparability analyses (all p < 0.001).
Conclusion
Paraspinal muscles demonstrate better reference characteristics than the spleen in estimating ADC variability of abdominal DWI.
Keywords: Diffusion weighted imaging, Apparent diffusion coefficient, Spleen, Paraspinal muscle, Normalization
1. Introduction
Diffusion-weighted imaging (DWI) is a quantitative MRI technique to measure the changes in the extent and direction of random water motion in the tissue microenvironment modified by interactions with the hydrophobic cell membranes, intracellular organelles, and macromolecules under physiological and pathological conditions [1]. The apparent diffusion coefficient (ADC) derived from the DWI data is a quantitative measure of promising clinical value for the diagnosis and treatment evaluation of diseases [2,3]. Multiple studies have reported that the ADC is lower for malignant lesions compared to benign tissues [[4], [5], [6]]. However, ADC of the normal and neoplastic tissues vary significantly across studies [7,8]. The variations in ADC of abdominal organs and neoplastic lesions between studies can be attributed to the use of different MRI scanners, DWI sequences, acquisition parameters, motion artifacts, susceptibility factors, regions of interest (ROIs), and others [1,4].
Therefore, normalization would be useful to decrease variations in the ADC measurements of normal tissues and tumors [[8], [9], [10]]. In abdominal DWI studies, the spleen and paraspinal muscles are commonly used as references for normalizing the ADC. Currently, there is no consensus on whether the spleen or paraspinal muscle is a better choice as a reference organ for DWI studies of abdominal tissues [[11], [12], [13], [14], [15], [16], [17], [18]]. In this study, we compared the variability in ADC measurements of the spleen and paraspinal muscles in a large cohort of patients to determine the preferred reference organ that can be used to normalize the ADC of the abdominal tissues.
2. Methods
2.1. Patients
The Biomedical Research Ethics Committee of our institution approved this study and the requirement for informed consent from the patients was waived. This study enrolled 1243 patients (835 males, 408 females; mean age, 57.0 ± 12.8 years; age range: 18–91 years) with a total of 1321 liver MRI examinations between June 2019 and May 2020. Patients younger than 18 years old, patients with DWI artifacts, and patients with spleen deficiency or spleen diseases were excluded. Fig. 1 shows the details of the patient enrollment including the inclusion and exclusion criteria. Among the enrolled participants, 73 patients underwent two follow-up MRI examinations with a mean time interval of 134 ± 78 days (range, 1–306 days) in an MRI scanner (Magnetom Skyra scanner) to measure the repeatability of the ADC for the spleen (ADCspleen) and the paraspinal muscle (ADCmuscle). Two MR scanners, with 314 abdominal exams on the GE (Discovery MR750) and 929 on the Siemens (Magnetom Skyra) systems, respectively, were used for the comparability analysis, which is assessed from the difference between two regions of interest (ROIs) for the paraspinal muscle (left and right in the muscle) and the spleen.
Fig. 1.
Flowchart of the patient enrollment criteria in this study.
2.2. DWI
MRI examinations were performed on two 3.0-T scanners, namely, MAGNETOM Skyra (Siemens Healthcare, Erlangen, Germany) and Discovery MR750 (GE Healthcare, Wisconsin, USA). Both were equipped with embedded body coils for signal transmission. Magnetom Skyra was equipped with a combination of a 32-channel posterior spine coil and an 18-channel anterior body coil, whereas the Discovery MR750 was equipped with a 32-channel anterior body coil. DWI was performed using the axial single-shot echo-planar-imaging sequence with three orthogonal diffusion gradients. Patients underwent routine clinical liver MRI protocols including free breathing DWI with b values of 50 and 800 s/mm2. The DWI acquisition parameters in the Magnetom Skyra were as follows: time of repetition = 5000 ms, echo time = 54 ms, matrix = 128 × 104 (reconstruction matrix = 256 × 208), slice/gap thickness = 6 mm/1.2 mm, field of view = 340–400 mm2, pixel bandwidth = ∼2300 Hz, partial Fourier Factor = 6/8, use of parallel imaging = 2, measurement time = ∼90 s, average = 1 and 4 for b50 and b800, number of slices = 26, respectively. The DWI acquisition parameters in the Discovery MR750 were as follows: time of repetition = 3586 ms, echo time = 77.5 ms, matrix = 128 × 128 (reconstruction matrix = 256 × 256), and slice/gap thickness = 6 mm/1 mm, field of view = 340–400 mm2, pixel bandwidth = ∼1953 Hz, partial Fourier Factor = none, use of parallel imaging = 2, measurement time = ∼80 s, average = 1 and 6 for b50 and b800, number of slices = 30, respectively. Written informed consent for the publication of the MRI images was obtained from patient.
2.3. DWI data analysis
The ADC maps were generated inline during DWI acquisition using a mono-exponential model. ADCspleen and ADCmuscle were measured on a PACS system version 6.0 (GE Healthcare, Milwaukee, WI, USA). Four regions of interest (ROIs) (mean area, 152 ± 3 mm2; range 144–167 mm2) [Fig. 2(a-d)]were drawn within the homogenous parts of the spleen (two ROIs) and the bilateral paraspinal muscles for each patient to measure the ADCspleen and ADCmuscle, which were also used to calculate the normalized ADC including ADCspleen to ADCmuscle ratio (rADCsp/m) and the ADCmuscle to ADCspleen ratio (rADCm/sp). In the repeatability analysis, two DWIs were acquired in the Magnetom Skyra MRI scanner for each of the 73 patients, and average ADCs of twice measurements of the spleen or the paraspinal muscles in each patient were used to evaluate the repeatability of the ADCs and the rADC. In the comparability analysis, the ADCs of bilateral paraspinal muscles and ADCspleen with two different ROIs in each patient were used to evaluate the comparability of the ADCs and the rADC.
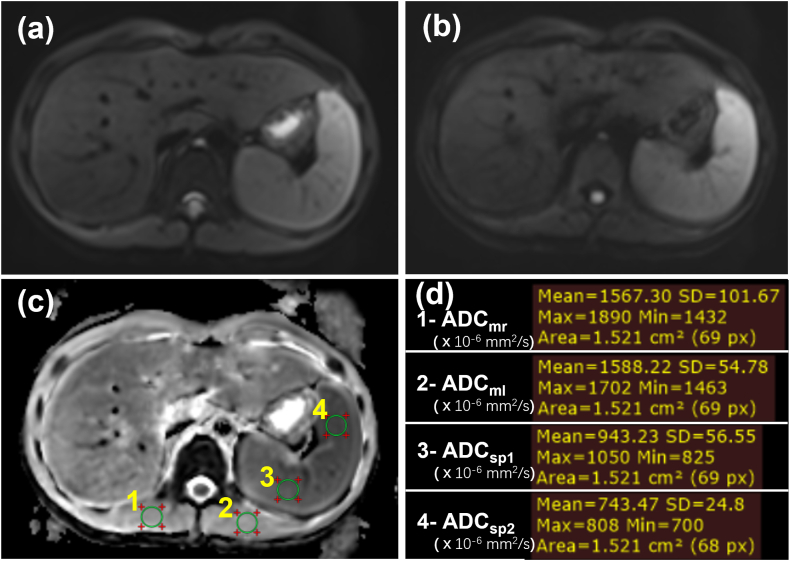
Fig. 2.
ADC measurements in the paraspinal muscle and the Spleen. (a) DWI image with b0. (b) DWI image with b800. (c) Four same-size regions of interest (ROIs) for ADC measurements. (d) Results of ADCm and ADCsp measurements. The unit of ADC values is 10−6 mm2/s.
ADC = apparent diffusion coefficient, DWI = diffusion weighted imaging, ADCm = ADC values of the paraspinal muscles, ADCsp = ADC values of the spleen, ADCmr = ADCm in the right paraspinal muscle, ADCml = ADCm in the left paraspinal muscle, ADCsp1 = ADCsp of the first ROI in the spleen, ADCsp2 = ADCsp of the second ROI in the spleen.
2.4. Statistical analysis
Statistical analysis was performed using the MedCalc software version 13.0.0.0 (MedCalc Software, Ostend, Belgium). In order to better reveal which one of the paraspinal muscle and the spleen is a better reference for normalizing the ADC of tissues, we further compared the variabilities of the relative values (rADC) in addition to the variabilities of the ADC values of the paraspinal muscle and the spleen. The variability in the ADC and rADC were analyzed by calculating the intraclass correlation coefficient (ICC) for single measurements, Bland-Altman analysis, %repeatability coefficient (%RC) [4], histogram analysis, and the coefficient of variation (CV). The multiple regressions of standard deviations (SDs) for the ADCs (ADCspleen and ADCmuscle) of repeated DWI scans were performed with time delay, mean ADC, age, and sex, to demonstrate the absence of biological changes in spleen and muscle. Linear regression analysis and correlation coefficient were performed to determine the correlations between age/gender and the ADCs as well as the rADC of the spleen and paraspinal muscle. Independent samples t-tests were used to compare the differences in the ADC and rADC of the spleen and the paraspinal muscles between the Magnetom Skyra and Discovery MR750 scanners as well as those between different genders. A p-value below 0.05 was considered statistically significant.
3. Results
3.1. Repeatability analysis of the ADC and rADC
The multiple regressions of SDs for the ADCspleen and ADCmuscle of repeated DWI scans were performed with time-delay, mean ADC, age, and sex, with p values of 0.70 and 0.25, respectively. The results demonstrate the absence of biological changes in the spleen and muscle for ADC measurements except for no abnormal image changes. Histograms show the ADCs (Fig. 3a) and the normalized ADCs (Fig. 3b) of the spleen and the paraspinal muscle for the 73 patients based on two DWI acquisitions. The %RC for the ADCmuscle and ADCspleen were 8.4% and 25.4%, respectively. The SDs of ADC and rADC values vs. the mean values were shown in Fig. 4(a–d). Bland-Altman data for the repeatability analyses of the ADCs and the normalized ADCs is summarized in Table 1. The ICC values for the ADCmuscle and ADCspleen were 0.67 and 0.47, respectively. The ICC values for the rADCm/sp and rADCsp/m were 0.40 and 0.39, respectively. In comparison with the raw ADC data, rADCm/sp values with the normalized ADC showed lower repeatability with the Bland-Altman bias, and limits of agreement (LoAs) for the ADCmuscle and rADCm/sp were −0.3 (−8.8%–8.1%) and −0.3 (−28.6%–29.1%), respectively. The Bland-Altman bias and LoAs for the ADCspleen and rADCsp/m were −0.1 (−25.7%–25.6%) and 0.3 (−29.1%–28.6%), respectively. The CV for the ADCmuscle was lower than that of ADCspleen, rADCm/sp, and rADCsp/m (all p < 0.001; Table 1).
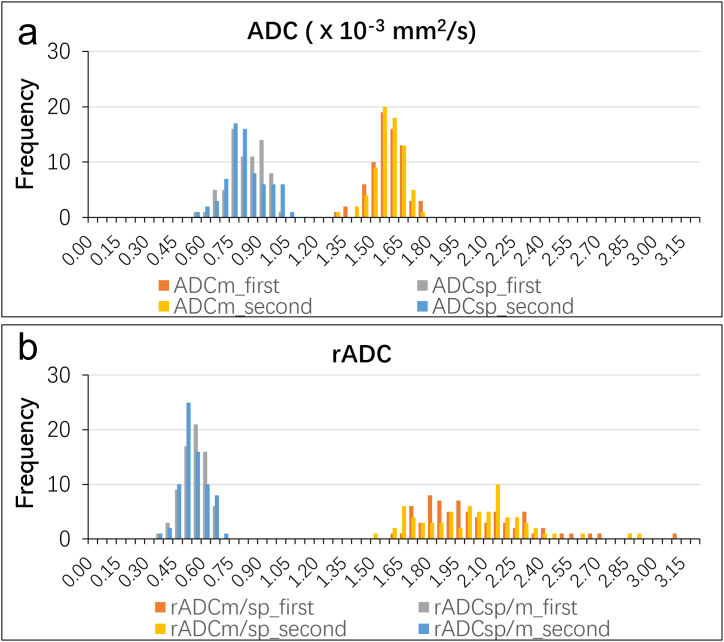
Fig. 3.
Histograms of the ADCs ( × 10−3 mm2/s) and normalized ADCs of the spleen and the paraspinal muscle from the two follow-up DWI of 73 patients. (a) The mean ADCs ( × 10-3 mm2/s) for the spleen and the paraspinal muscle of 73 patients from the two follow-up DWIs. (b) The normalized ADCs for the spleen and the paraspinal muscles of 73 patients from the two follow-up DWIs. The histogram was generated using 65 bins with a bin size of 0.05.
ADC = apparent diffusion coefficient, DWI = diffusion weighted imaging, ADCm = ADC of the paraspinal muscles, ADCsp = ADC of the spleen, rADCm/sp = ADCm to ADCsp ratio, rADCsp/m = ADCsp to ADCm ratio.
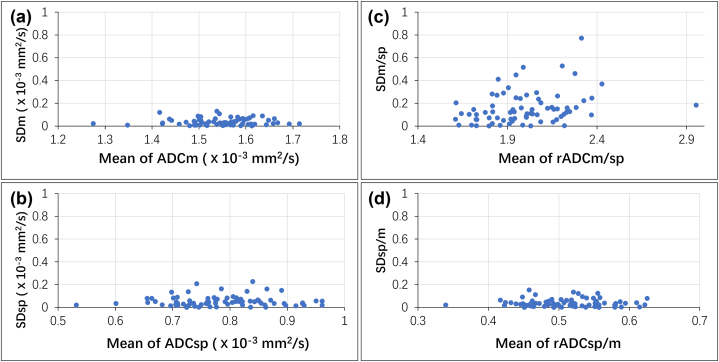
Fig. 4.
Plots for the standard deviations (SDs) of the ADC ( × 10−3 mm2/s) and rADC of the repeatability DWI scans vs. the mean ADC and rADC values (n = 73). (a) The ADCmuscle from the two DWIs of 73 patients. (b) The ADCspleen from two DWIs of 73 patients; (c) The ratios of the ADCmuscle to the ADCspleen (rADCm/sp); (d) The ratios of the ADCspleen to the ADCmuscle (rADCsp/m). Y-axis: SD in ADC or normalized ADC measurements; X-axis: mean ADC or normalized ADC.
ADC = apparent diffusion coefficient, DWI = diffusion weighted imaging, ADCm = ADC of the paraspinal muscles, ADCsp = ADC of the spleen, rADCm/sp = ADCm to ADCsp ratio, rADCsp/m = ADCsp to ADCm ratio.
Table 1.
Comparisons of the apparent diffusion coefficients (ADCs) ( × 10−3 mm2/s) and the normalized ADCs (rADC) for the spleen and the paraspinal muscle based on the twice diffusion weighted imaging (DWI) measurements (n = 73).
| Paraspinal muscle | Spleen | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | First DWI | Second DWI | ICC | CV of the two measurementsb | First DWI | Second DWI | ICC | CV of the two measurementsb |
| ADC ( × 10−3 mm2/s) | 1.546 ± 0.084 (1.260–1.706) | 1.551 ± 0.079 (1.290–1.728) | 0.67 | 0.023 ± 0.019 (0.001–0.085) *,† | 0.786 ± 0.095 (0.517–0.979) | 0.787 ± 0.104 (0.547–1.000) | 0.47 | 0.070 ± 0.060 (0.001–0.281) * |
| rADC | 1.996 ± 0.277 (1.557–3.084) | 2.003 ± 0.278 (1.468–2.858) | 0.40 | 0.078 ± 0.068 (0.001–0.333)† | 0.510 ± 0.064 (0.324–0.642) | 0.509 ± 0.070 (0.350–0.681) | 0.39 | 0.078 ± 0.068 (0.001–0.333) |
| CV of ADCa | 0.052 × 10−3 mm2/s | 0.126 × 10−3 mm2/s | ||||||
| CV of rADCa | 0.139 | 0.131 | ||||||
Data are represented as means ± standard deviation; Data in parentheses represent the range of the values.
ADC, apparent diffusion coefficient; CV, coefficient of variation, rADC, ratio of ADC.
*, †P values < 0.001; aThe mean CV values of all the measured ADC values for paraspinal muscle or spleen; bCV was calculated from the two ADC measurements for paraspinal muscle or spleen from each of the 73 patients.
3.2. Comparability analysis of the ADC and rADC
We analyzed the ADCs and normalized ADCs of the spleen and the paraspinal muscle for 929 patients based on the DWIs acquired on the Magnetom Skyra. The ICC values for the ADCmuscle and ADCspleen were 0.53 and 0.72, respectively. The ICC values for the rADCm/sp and the rADCsp/m were 0.93 and 0.73, respectively. The Bland-Altman bias and LoAs for the ADCmuscle and rADCm/sp were 0.7 (−10.0%–11.4%). The Bland-Altman bias and LoAs for the ADCspleen and rADCsp/m were 2.1 (−20.0%–24.2%). The %RC for the ADCmuscle and ADCspleen were 10.8% and 22.5%, respectively. We then analyzed the ADCs and the normalized ADCs of the spleen and the paraspinal muscle for 314 patients based on the DWIs acquired on the Discovery MR750. The ICC values for the ADCmuscle and rADCm/sp were 0.43 and 0.85, respectively. The Bland-Altman bias and LoAs for the ADCmuscle and the rADCm/sp were 0.9 (−8.7%–10.6%). The ICC values for the ADCspleen and the rADCsp/m were 0.57 and 0.62, respectively. The Bland-Altman bias and LoAs for the ADCspleen and the rADCsp/m were −0.3 (−16.8%–16.2%). The %RC for the ADCmuscle and ADCspleen were 9.8% and 16.5%, respectively. The above results are shown in Table 2 and Fig. 5(a–d). The CVs for the ADCmuscle and rADCm/sp were lower than the CVs for the ADCspleen and rADCsp/m (p < 0.001).
Table 2.
Comparisons of the two measurements of the ADC ( × 10−3 mm2/s) and rADC for the spleen and the paraspinal muscles of the 2 patient cohorts.
| Parameter | Magnetom Skyra (n = 929) |
Discovery MR750 (n = 314) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Paraspinal muscle |
Spleen |
Paraspinal muscle |
Spleen |
|||||||||||||
| Right | Left | ICC | CV of two measurementsb | First ROI | Second ROI | ICC | CV of two measurementsb | Right | Left | ICC | CV of two measurementsb | First ROI | Second ROI | ICC | CV of two measurementsb | |
| ADC ( × 10−3 mm2/s) | 1.558 ± 0.092 (1.123–1.912) | 1.546 ± 0.081 (1.127–1.811) | 0.53 | 0.030 ± 0.025 (0.000–0.149) | 0.801 ± 0.123 (0.506–1.329) | 0.783 ± 0.116 (0.446–1.357) | 0.72 | 0.061 ± 0.053 (0.000–0.284) | 1.500 ± 0.066 (1.339–1.740) | 1.486 ± 0.071 (1.312–1.675) | 0.43 | 0.029 ± 0.021 (0–0.101) | 0.820 ± 0.069 (0.625–0.993) | 0.823 ± 0.078 (0.607–0.996) | 0.57 | 0.047 ± 0.036 (0–0.224) |
| rADC | 2.005 ± 0.295 (1.146–3.021) | 1.989 ± 0.286 (1.201–3.040) | 0.93 | 0.030 ± 0.025 (0.000–0.149) | 0.517 ± 0.081 (0.324–0.850) | 0.505 ± 0.077 (0.292–0.887) | 0.73 | 0.061 ± 0.053 (0.000–0.284) | 1.836 ± 0.158 (1.500–2.413) | 1.820 ± 0.168 (1.448–2.306) | 0.85 | 0.029 ± 0.021 (0–0.101) | 0.550 ± 0.051(0.389–0.689) | 0.552 ± 0.053 (0.441–0.670) | 0.62 | 0.047 ± 0.036 (0–0.224) |
| CV of ADCa | 0.049 × 10−3 mm2/s | 0.140 × 10−3 mm2/s | 0.039 × 10−3 mm2/s | 0.079 × 10-3 mm2/s | ||||||||||||
| CV of rADCa | 0.143 | 0.144 | 0.086 | 0.085 | ||||||||||||
Data are represented as means ± standard deviation; Data in parentheses represent the range of values.
ADC, apparent diffusion coefficient; CV, coefficient of variation; rADC, the ratio of ADCs.
aThe mean CV values of all the measured ADC values for the paraspinal muscle or spleen; bCV was calculated from the two ADC measurements for Paraspinal muscle or spleen from each of the 73 patients.
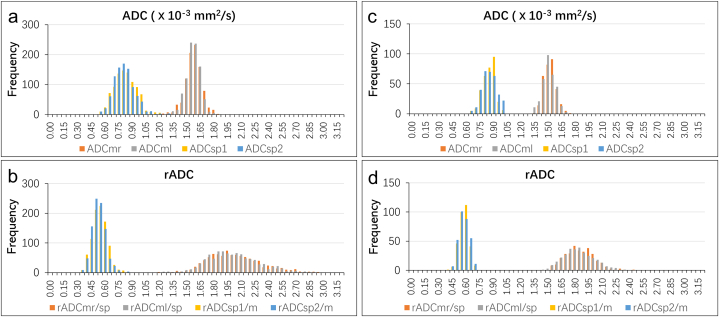
Fig. 5.
Histograms of the ADCs ( × 10−3 mm2/s) and the normalized ADCs for the spleen and the paraspinal muscle of each patient in two cohorts with DWI acquisitions on two MRI scanners. (a) The mean ADC ( × 10−3 mm2/s) of the spleen and the paraspinal muscle of the 929 patients (Magnetom Skyra). (b) The normalized ADC of the spleen and the paraspinal muscle of the 929 patients (Magnetom Skyra). (c) The mean ADC ( × 10−3 mm2/s) of the spleen and the paraspinal muscles derived from the DWIs of the 314 patients (Discovery MR750). (d) The normalized ADC of the spleen and the paraspinal muscle derived from the DWIs of the 314 patients (Discovery MR750). The histogram was generated using 65 bins with a bin size of 0.05. The indices “1” and “2” or “r”/“l” refer to the different ROIs described in Fig. 2.
ADC = apparent diffusion coefficient, DWI = diffusion weighted imaging ADCm = ADC of the paraspinal muscles, ADCsp = ADC of the spleen, rADCm/sp = ADCm to ADCsp ratio, rADCsp/m = ADCsp to ADCm ratio.
3.3. Comparisons of ADC and rADC between the two MRI scanners
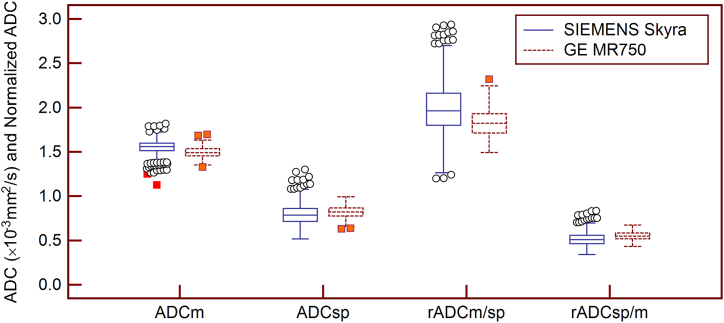
The mean ADCmuscle (1.493x10−3 mm2/s vs. 1.552x10−3 mm2/s, CV = 3.9%) and rADCm/sp (1.828 vs. 1.997, CV = 8.8%) based on the DWIs acquired with Discovery MR750 were lower than those based on the DWIs acquired with the Magnetom Skyra. However, the mean ADCspleen (0.822x10−3 mm2/s vs. 0.792x10−3 mm2/s, CV = 3.7%) and rADCsp/m (0.551 vs. 0.511, CV = 7.5%) based on the DWIs acquired with the Discovery MR750-derived DWIs were higher than those based on the DWIs acquired with the Magnetom Skyra (Fig. 6).
Fig. 6.
The Box-and-Whisker plots show the mean ADCs ( × 10−3 mm2/s) and rADCs of the spleen and paraspinal muscle from the two MRI scanners. The central box represents the values from the lower to the upper quartile (25th percentile to 75th percentile). The middle line represents the median. The plots with solid blue lines represent the mean ADC and rADC values of the 929 patients from the DWIs acquired using the Magnetom Skyra. The plots with red-dotted lines represent the mean ADC and rADC values of the 314 patients from the DWIs acquired using the Discovery MR 750 scanner. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
ADC = apparent diffusion coefficient, ADCm = ADC values of the paraspinal muscles, ADCsp = ADC values of the spleen, rADCm/sp = ADCm to ADCsp ratio, rADCsp/m = ADCsp to ADCm ratio.
3.4. Effects of gender and age on ADC and rADC
We analyzed the correlations of the ADC and the rADC values of the spleen and the paraspinal muscle with the age and gender of 1243 patients. The ADCmuscle and rADCm/sp for the males were higher than those for the females (both P < 0.001; Table 3). In both males and females, linear regression analyses and correlation coefficient results showed that the ADCmuscle (correlation coefficient = −0.155, P < 0.001; slope coefficients, −0.001) and the rADCm/sp (correlation coefficient = −0.097, P < 0.001; slope coefficients, −0.002) were inversely related to the age of the patients (Fig. 7).
Table 3.
The association between apparent diffusion coefficients (ADCs) ( × 10−3 mm2/s) and the ratio of ADCs (rADCs) for the spleen and paraspinal muscles and gender (n = 1243).
| Paraspinal muscle (n = 1243) | Spleen (n = 1243) | |||||
|---|---|---|---|---|---|---|
| Parameter | Male (n = 835) | Female (n = 408) | P* | Male (n = 835) | Female (n = 408) | P* |
| ADC ( × 10−3 mm2/s) | 1.544 ± 0.073 (1.125–1.819) | 1.522 ± 0.079 (1.247–1.760) | <0.001 | 0.793 ± 0.104 (0.515–1.276) | 0.812 ± 0.098 (0.539–1.300) | 0.003 |
| rADC | 1.981 ± 0.279 (1.199–2.938) | 1.900 ± 0.239 (1.203–2.905) | <0.001 | 0.515 ± 0.071 (0.340–0.834) | 0.534 ± 0.065 (0.344–0.831) | <0.001 |
Data are represented as means ± standard deviation; Data in parentheses represent the range of values.
ADC, apparent diffusion coefficient; rADC, the ratio of ADC.
*Independent samples t-test.
Fig. 7.
The scatter diagram shows the ADCs ( × 10−3 mm2/s) and rADCs of the paraspinal muscle and the spleen versus the age of patients (n = 1243). Y-axis: ADC ( × 10−3 mm2/s) or rADC; X-axis: Patient age. The lines represent the linear regression lines.
ADC = apparent diffusion coefficient, ADCm = ADC of the paraspinal muscles, ADCsp = ADC of the spleen, rADCm/sp = ADCm to ADCsp ratio, rADCsp/m = ADCsp to ADCm ratio.
The ADCspleen and rADCsp/m for the males were lower than those for the females (P = 0.003 and P < 0.001, respectively). Furthermore, linear regression analysis and correlation coefficient results showed that the ADCspleen (correlation coefficient = 0.06, P = 0.035; slope coefficients, 0.001) and rADCsp/m (correlation coefficient = 0.116, P < 0.001; slope coefficients, 0.001) values increased with the age of patients (Fig. 7).
4. Discussion
Our study demonstrated that the ADCspleen was more scattered and variable than the ADCmuscle both in the comparability and repeatability analyses. This was confirmed by the larger range of LoAs, %RC and CVs for the two measurements of ADCspleen. The CVs for ADCmuscle in the repeatability (2.3%) and the comparability (3.0% for Magnetom Skyra and 2.9% for Discovery MR750) analyses and the analysis between MRI scanners (3.9%) were consistent with those reported in the in vitro experiments using DWI phantoms [[19], [20], [21]]. The %RC of ADCmuscle was 8.4%, which was lower than that of the organs of the liver (26%) and prostate (47%) [4]. Therefore, the paraspinal muscle is a better choice as an internal reference for investigating the variability in the ADC measurements during multicenter longitudinal studies with different MRI scanners. Furthermore, in our study, the mean ADCspleen and ADCmuscle were 0.80 x10−3 mm2/s and 1.54 x10−3 mm2/s, respectively. These values were consistent with the previously reported ADCs for these tissues [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]].
DWI is a commonly used clinical tool for the diagnostic and treatment evaluation of various diseases. However, ∼10% variability is observed in the ADCs between MRI centers because of differences in the DWI protocols, pulse sequences, and scanner operator et al. [2,4,7,22]. Previous reports have demonstrated significant differences in the ADC between abdominal tumors and normal abdominal tissues [2,7]. Therefore, the normalized ADC of the target tissues is more reliable than the raw ADC [8,15,16,18]. Some studies have shown that the paraspinal muscle is a good option as a reference organ because of low variability in its ADC under various abdominal disease conditions [13,23]. The spleen is another reference organ for normalizing the ADC of abdominal organs [15,16,18]. Song et al. suggested that the spleen was a better reference organ than the paraspinal muscle for decreasing the variability in the ADC measurements of the abdominal organs based on the ICC values [16]. Koc et al. found that the area under the receiver operating characteristic curve values of the normalized ADCs using paraspinal muscle as a reference organ are higher than those normalized using the spleen as a reference organ for distinguishing the malignant and the benign abdominal lesions [17]. Additionally, Ding et al. also reported that the paraspinal muscle was a better choice as a reference organ than the spleen for normalizing the ADC of the pancreas [13]. In this study, the comparability of the rADCm/sp value was satisfactory with the LoA ranging from −10.0% to 11.4%. However, the repeatability of the rADCm/sp value was not satisfactory because the LoA ranged widely from −29.1% to 28.6%. Furthermore, rADCm/sp was more scattered than the ADCmuscle in the repeatability analysis. This can be attributed to a larger variation of the ADCspleen compared to the variation in the ADCmuscle. Therefore, the CV of the rADCm/sp was higher than that of the ADCmuscle. If the variability in the ADCs for the target tissues or tumors was lower than that of the spleen, the errors would be magnified if the spleen is used as the reference organ. Despite a good correlation with the ICC of 0.72, ADCspleen was highly variable with the LoA ranging from −20.0% to 24.2%. In comparison, ADCmuscle was less variable with the LoA ranging from −10.0% to 11.4% despite a moderate correlation with the ICC of 0.53. Nearly 25.4% (424/1670) of patients were excluded from this study because of splenic deficiency or diseases. In such cases, the spleen could not be used for normalizing the ADC of the abdominal organs. Several consensus guidelines have been proposed regarding the use of quantitative DWI sequences, parameters, and ROI methods for the abdomen to improve the accuracy, repeatability, and comparability of the absolute ADC and normalized ADC [[24], [25], [26], [27]].
In the current study, even though the correlation of the ADCspleen with age is significant, the correlation coefficient is low suggesting negligible ADC dependence. These findings are inconsistent with Li et al. reports [28] but consistent with Nazarlou et al.'s findings [29]. Furthermore, our results demonstrated that the mean ADCs and normalized ADCs correlated with gender for both the spleen and the paraspinal muscle. The findings are inconsistent with Nazarlou et al.'s reports [29]. The main reason for this discrepancy may be due to the differences in the sample size and the MRI parameters used in these studies. These findings should be taken into consideration in future applications.
This study has a few limitations. Firstly, our hospital performed DWI using the free-breathing method to optimize the imaging time and the signal-to-noise ratio. The free-breathing method is versatile in abdomen MRI [30]. However, abdominal DWI is also performed in many cases using the breath-hold and respiratory-triggered methods. Therefore, in future studies, the variability in ADCspleen and ADCmuscle should be investigated for DWI studies with breath-hold and respiratory-triggered methods. Secondly, only two scanners were used, and longitudinal scans were on a single system, we did not investigate the diagnostic performance of the normalized ADC for distinguishing between different pathological types of abdominal lesions. Future studies need to investigate this because it may be clinically significant and are necessary to confirm our findings for abdominal tumors.
5. Conclusions
Our study demonstrated that the ADCmuscle was less variable than ADCspleen and is a better choice as the internal reference to investigate ADC variability across MRI scanners. The spleen is not a suitable reference for normalizing ADC of abdominal tissues or tumors with low ADC variability.
Author contribution statement
Yukun Chen & Panpan Yang; Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Caixia Fu; Yun Bian & Chengwei Shao: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Chao Ma & Jianping Lu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the National Natural Science Foundation of China [82171915, 82171930, 62173252]; 234 Platform Discipline Consolidation Foundation Project of Changhai Hospital [2020YPT001]; Shanghai Science and Technology Innovation Action Plan Medical Innovation Research Project [20Y11912500].
Data availability statement
Data will be made available on request.
Additional information
Supplementary content related to this article has been published online at [URL].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hori M., Kamiya K., Murata K. Technical basics of diffusion-weighted imaging, Magn. Reason. Imaging. Clin. N. Am. 2021;29(2):129–136. doi: 10.1016/j.mric.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Taouli B., Beer A.J., Chenevert T., et al. Diffusion-weighted imaging outside the brain: consensus statement from an ISMRM-sponsored workshop. J. Magn. Reson. Imag. 2016;44(3):521–540. doi: 10.1002/jmri.25196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dreher C., Kuder T.A., König F., et al. Advanced diffusion-weighted abdominal imaging: qualitative and quantitative comparison of high and ultra-high b-Values for lesion detection and image quality. Invest. Radiol. 2020;55(5):285–292. doi: 10.1097/RLI.0000000000000639. [DOI] [PubMed] [Google Scholar]
- 4.Shukla-Dave A., Obuchowski N.A., Chenevert T.L., et al. Quantitative imaging biomarkers alliance (QIBA) recommendations for improved precision of DWI and DCE-MRI derived biomarkers in multicenter oncology trials. J. Magn. Reason. Imaging. 2019;49(7):e101–e121. doi: 10.1002/jmri.26518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang L., Zhou X.J. Diffusion MRI of cancer: from low to high b-values. J. Magn. Reason. Imaging. 2019;49(1):23–40. doi: 10.1002/jmri.26293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ni P., Lin Y., Zhong Q., Chen Z., Sandrasegaran K., Lin C. Technical advancements and protocol optimization of diffusion-weighted imaging (DWI) in liver. Abdom. Radiol. 2016;41(1):189–202. doi: 10.1007/s00261-015-0602-x. [DOI] [PubMed] [Google Scholar]
- 7.Jafar M.M., Parsai A., Miquel M.E. Diffusion-weighted magnetic resonance imaging in cancer: reported apparent diffusion coefficients, in-vitro and in-vivo reproducibility, World. J. Radiol. 2016;8(1):21–49. doi: 10.4329/wjr.v8.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmeel F.C. Variability in quantitative diffusion-weighted MR imaging (DWI) across different scanners and imaging sites: is there a potential consensus that can help reducing the limits of expected bias? Eur. Radiol. 2019;29(5):2243–2245. doi: 10.1007/s00330-018-5866-4. [DOI] [PubMed] [Google Scholar]
- 9.Soyer P., Kanematsu M., Taouli B., et al. ADC normalization: a promising research track for diffusion-weighted MR imaging of the abdomen. Diagn. Interv. Imaging. 2013;94(6):571–573. doi: 10.1016/j.diii.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Do R.K., Chandarana H., Felker E., Hajdu C.H., Babb J.S., Kim D., Taouli B. Diagnosis of liver fibrosis and cirrhosis with diffusion-weighted imaging: value of normalized apparent diffusion coefficient using the spleen as reference organ. AJR Am. J. Roentgenol. 2010;195(3):671–676. doi: 10.2214/AJR.09.3448. [DOI] [PubMed] [Google Scholar]
- 11.Pawluś A., Szymańska K., Łasecki M., et al. Which organ should be considered a reference in diffusion weighted imaging of the abdomen?: the reproducibility of ADC measurements of the spleen and the renal cortex on a 1.5T MR. Adv. Clin. Exp. Med. 2017;26(5):811–816. doi: 10.17219/acem/60877. [DOI] [PubMed] [Google Scholar]
- 12.Charatcharoenwitthaya P., Sukonrut K., Korpraphong P., Pongpaibul A., Saiviroonporn P. Diffusion-weighted magnetic resonance imaging for the assessment of liver fibrosis in chronic viral hepatitis. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0248024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding X., Xu H., Zhou J., Xu J., Mei H., Long Q., Wang Y. Reproducibility of normalized apparent diffusion coefficient measurements on 3.0-T diffusion-weighted imaging of normal pancreas in a healthy population. Medicine (Baltim.) 2019;98(14) doi: 10.1097/MD.0000000000015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang W., Song J.S., Kwak H.S., Hwang S.B., Paek M.Y. Intra-individual comparison of conventional and simultaneous multislice-accelerated diffusion-weighted imaging in upper abdominal solid organs: value of ADC normalization using the spleen as a reference organ. Abdom. Radiol (NY) 2019;44(5):1808–1815. doi: 10.1007/s00261-019-01924-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim B.R., Song J.S., Choi E.J., Hwang S.B., Hwang H.P. Diffusion-weighted imaging of upper abdominal organs acquired with multiple b-value combinations: value of normalization using spleen as the reference organ. Korean J. Radiol. 2018;19(3):389–396. doi: 10.3348/kjr.2018.19.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song J.S., Kwak H.S., Byon J.H., Jin G.Y. Diffusion-weighted MR imaging of upper abdominal organs at different time points: apparent diffusion coefficient normalization using a reference organ. J. Magn. Reason. Imaging. 2017;45(5):1494–1501. doi: 10.1002/jmri.25456. [DOI] [PubMed] [Google Scholar]
- 17.Koc Z., Erbay G., Karadeli E. Internal comparison standard for abdominal diffusion-weighted imaging. Acta. Radiol. 2017;58(9):1029–1036. doi: 10.1177/0284185116681040. [DOI] [PubMed] [Google Scholar]
- 18.Song J.S., Hwang S.B., Chung G.H., Jin G.Y. Intra-individual, inter-vendor comparison of diffusion-weighted MR imaging of upper abdominal organs at 3.0 Tesla with an emphasis on the value of normalization with the spleen. Korean J. Radiol. 2016;17(2):209–217. doi: 10.3348/kjr.2016.17.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malyarenko D.I., Wilmes L.J., Arlinghaus L.R., et al. QIN DAWG validation of gradient nonlinearity bias correction workflow for quantitative diffusion-weighted imaging in multicenter trials. Tomography. 2016;2(4):396–405. doi: 10.18383/j.tom.2016.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malyarenko D., Galbán C.J., Londy F.J., et al. Multi-system repeatability and reproducibility of apparent diffusion coefficient measurement using an ice-water phantom. J. Magn. Reason. Imaging. 2013;37(5):1238–1246. doi: 10.1002/jmri.23825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulkern R.V., Ricci K.I., Vajapeyam S., Chenevert T.L., Malyarenko D.I., Kocak M., Poussaint T.Y. Pediatric brain tumor consortium multisite assessment of apparent diffusion coefficient z-axis variation assessed with an ice-water phantom. Acad. Radiol. 2015;22(3):363–369. doi: 10.1016/j.acra.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chenevert T.L., Galbán C.J., Ivancevic M.K., et al. Diffusion coefficient measurement using a temperature-controlled fluid for quality control in multicenter studies. J. Magn. Reason. Imaging. 2011;34(4):983–987. doi: 10.1002/jmri.22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corona-Villalobos C.P., Pan L., Halappa V.G., Bonekamp S., Lorenz C.H., Eng J., Kamel I.R. Agreement and reproducibility of apparent diffusion coefficient measurements of dual-b-value and multi-b-value diffusion-weighted magnetic resonance imaging at 1.5 Tesla in phantom and in soft tissues of the abdomen. J. Comput. Assist. Tomogr. 2013;37(1):46–51. doi: 10.1097/RCT.0b013e3182720e07. [DOI] [PubMed] [Google Scholar]
- 24.Baltzer P., Mann R.M., Iima M., et al. EUSOBI international breast diffusion-weighted imaging working group. Diffusion-weighted imaging of the breast-a consensus and mission statement from the EUSOBI international breast diffusion-weighted imaging working group. Eur. Radiol. 2020;30(3):1436–1450. doi: 10.1007/s00330-019-06510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ljimani A., Caroli A., Laustsen C., et al. Consensus-based technical recommendations for clinical translation of renal diffusion-weighted MRI. Magma. 2020;33(1):177–195. doi: 10.1007/s10334-019-00790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beets-Tan R.G.H., Lambregts D.M.J., Maas M., et al. Magnetic resonance imaging for clinical management of rectal cancer: updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur. Radiol. 2018;28(4):1465–1475. doi: 10.1007/s00330-017-5026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes A., Alonzi R., Blackledge M., et al. UK quantitative WB-DWI technical workgroup: consensus meeting recommendations on optimisation, quality control, processing and analysis of quantitative whole-body diffusion-weighted imaging for cancer. Br. J. Radiol. 2018;91(1081) doi: 10.1259/bjr.20170577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li G., Xu P., Pan X., Qin H., Chen Y. The effect of age on apparent diffusion coefficient values in normal spleen: a preliminary study. Clin. Radiol. 2014;69(4):e165–e167. doi: 10.1016/j.crad.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Nazarlou A.K., Abdolmohammadi J. A study of the relationship between gender/age and apparent diffusion coefficient values in spleen of healthy adults using diffusion-weighted magnetic resonance imaging. Electron. Physician. 2015;7(1):1005–1009. doi: 10.14661/2015.1005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taouli B., Koh D.M. Diffusion-weighted MR imaging of the liver. Radiology. 2010;254(1):47–66. doi: 10.1148/radiol.09090021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.