Abstract
A 43-year-old man developed headache, dizziness, abdominal pain, and vomiting. His blood pressure was 203/121 mmHg, heart rate 122 beats/min, body temperature 39.1°C, and respiratory rate 24/min. He had elevated levels of creatinine at 2.95 mg/dL and lipase at 1,364 U/L as well as an extremely low calcium level at 5.2 mg/dL. Hypertriglyceridemia and hyperglycemia were seen. Chest and abdominal computed tomography showed interstitial pneumonia, severe pancreatitis, and a right adrenal tumor. The patient also developed vertebral artery dissection and medullary infarction. After right adrenalectomy, the patient was diagnosed with pheochromocytoma multisystem crisis (PMC). Acute pancreatitis might augment numerous life-threatening manifestations of PMC.
Keywords: pheochromocytoma multisystem crisis, acute pancreatitis
Introduction
Pheochromocytoma is a catecholamine-producing tumor that causes paroxysmal episodes of hypertension, palpitation, headache, hyperthermia, and sweating (1). Pheochromocytoma crisis occurs as hypertensive emergency due to extraordinary high-level secretion of catecholamine, and pheochromocytoma multisystem crisis (PMC) is an unusual presentation of pheochromocytoma that results in multiple organ damage (1-3).
PMC is defined as the presence of hypertension or hypotension, a high or subnormal fever, encephalopathy, and multi-organ failure (1-3). PMC is associated with numerous symptoms, thus making it difficult to diagnose. In addition, acute pancreatitis is also a life-threatening disease (4) and is not often complicated with PMC.
We herein report an unusual case of PMC concomitant with the development of acute pancreatitis. The successful treatment of PMC and acute pancreatitis depends on a prompt diagnosis.
Case Report
A 43-year-old man developed nocturnal intense abdominal pain, headache, dizziness, and vomiting over a couple of days. He was a drinker (beer, 3,500 mL/day) but lacked any other remarkable personal or family history. On admission, his consciousness was alert. His blood pressure was 203/121 mmHg, heart rate, 122 beats/min (regular), body temperature 39.1°C, respiratory rate 24/min, body height 173 cm, and body weight 73 kg. His heart and lung sounds were normal, and rebound tenderness was not observed on an abdominal examination. No lower limb edema was seen.
A laboratory examination revealed an elevated white blood cell count at 24,300 /μL (neutrophils dominant), creatinine level of 2.95 mg/dL, amylase level of 534 U/L (reference range: 44-132), and lipase level of 1,364 U/L (13-60) (Table 1). An extremely low calcium level of 5.2 mg/dL was also noted.
Table 1.
Laboratory Tests on Admission.
| Value | Unit | Reference ranges | |
|---|---|---|---|
| White blood cells | 24,300 | /µL | 3,300-8,600 |
| Neutrophils | 86.1 | % | 38.5-80.5 |
| Hb | 16.9 | g/dL | 13.7-16.8 |
| Hct | 49.7 | % | 40.7-50.1 |
| Plt | 31.5 | ×104/µL | 15.8-34.8 |
| TP | 6.8 | g/dL | 6.6-8.1 |
| Alb | 2.9 | g/dL | 4.1-5.1 |
| BUN | 14 | mg/dL | 8-20 |
| Cr | 2.95 | mg/dL | 0.65-1.07 |
| AST | 53 | U/L | 13-30 |
| ALT | 79 | U/L | 10-42 |
| γ-GTP | 239 | U/L | 13-64 |
| ALP | 77 | U/L | 38-113 |
| LDH | 520 | U/L | 124-222 |
| T-Bil | 0.5 | mg/dL | 0.4-1.5 |
| Amylase | 534 | U/L | 44-132 |
| Lipase | 1,364 | U/L | 13-60 |
| Na | 122 | mmol/L | 138-145 |
| K | 3.2 | mmol/L | 3.6-4.8 |
| Cl | 77 | mmol/L | 101-108 |
| Ca | 5.2 | mg/dL | 5.8-10.1 |
| P | 1.8 | mg/dL | 2.7-4.6 |
| CRP | 12.49 | mg/dL | <0.30 |
| Plasma glucose | 189 | mg/dL | 73-109 |
| HbA1c | 6.5 | % | 4.9-6.0 |
| Triglyceride | 5,478 | mg/dL | 40-234 |
| HDL-c | 19 | mg/dL | 48-103 |
| LDL-c | 28 | mg/dL | 65-163 |
| NEFA | 1,019 | μEq/L | 172-586 |
| Apoprotein C-II | 28.0 | mg/dL | 1.8-4.6 |
| Lipoprotein lipase | 61 | ng/mL | 45-63 |
| IgG4 | 50.3 | mg/dL | 11-121 |
| Anti-GAD antibody | <5.0 | U/mL | <5.0 |
| D-dimer | 2.11 | µg/mL | <0.49 |
Artery blood gas examination on room air:
| Value | Unit | Reference ranges | |
|---|---|---|---|
| pH | 7.349 | 7.35-7.45 | |
| pCO2 | 23.6 | mmHg | 35-45 |
| pO2 | 131 | mmHg | 80-100 |
| SpO2 | 99.0 | % | 94-99 |
| HCO3- | 12.7 | mmol/L | 22-26 |
| Base excess | -10.8 | mmol/L | -2.2-1.2 |
| Lactate | 13.1 | mmol/L | <1.3 |
Hb: hemoglobin, Hct: hematocrit, Plt: platelet, TP: total protein, Alb: albumin, T-Bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, γ-GTP: γ-glutamyl transpeptidase, BUN: blood urea nitrogen, Cr: creatine, Na: sodium, K: potassium, Cl: chloride, Ca: calcium, P: phosphate, CRP: C-reactive protein, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol, NEFA: non-esterified fatty acid, Anti-GAD antibody: anti-glutamic acid decarboxylase antibody
Underline denotes abnormal values.
In addition, marked hypertriglyceridemia, 5,478 mg/dL (40-234) was observed. The levels of low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol were decreased. Since the concentrations of apoprotein C-II and lipoprotein lipase (LPL) were not decreased, primary hyperchylomicronemia was not suspected. Serum non-esterified fatty acid (NEFA) levels were increased to 1,019 μEq/L (172-586). Both levels of chylomicron (CM) and very-low-density lipoprotein (VLDL) were increased, and World Health Organization (WHO) classification type V dyslipidemia was suspected (Fig. 1). In addition, the plasma glucose level was elevated at 189 mg/dL, and the HbA1c level was supranormal at 6.5%. Increased plasma renin activity (PRA) at 20.6 ng/mL/h (0.2-3.9) and a high concentration of plasma aldosterone at 64.5 pg/mL (4.0-82.1) indicated dehydration (Table 2).
Figure 1.
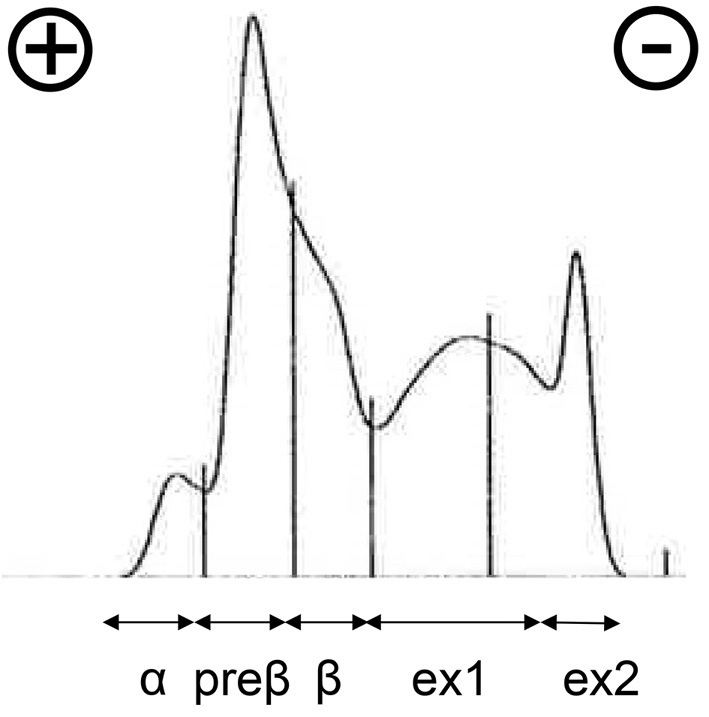
A serum lipoprotein analysis by agarose gel electrophoresis of the patient. Approximate proportions of each fraction; α: 5.2% (reference ranges for men: 26.9-50.5%), preβ: 31.3% (7.9-23.8%), and β: 18.6% (35.3-55.5%). Ex1+ex2=44.9% (extra fractions). Generally, fraction α represents HDL predominance, fraction preβ includes mostly VLDL and LDL, and fraction β mostly contains LDL. Ex1 (tailing) was considered to be CM, and ex2 (residue) was also thought to be CM. HDL: high-density lipoprotein, VLDL: very-low-density lipoprotein, LDL: low-density lipoprotein, CM: chylomicron
Table 2.
Endocrinological Examination on Admission.
| Value | Unit | Reference ranges | |||
|---|---|---|---|---|---|
| Insulin | 75.3 | µU/mL | <18.7 | ||
| TSH | 0.495 | µIU/mL | 0.5-5.0 | ||
| FT4 | 1.13 | ng/dL | 0.9-1.7 | ||
| *Cortisol | 18.8 | µg/dL | 7.1-19.6 | ||
| *PRA | 20.6 | ng/mL/h | 0.2-3.9 | ||
| *Aldosterone | 64.5 | pg/mL | 4.0-82.1 | ||
| DHEA-S | 74 | µg/dL | 70-495 | ||
| Plasma catecholamines and metanephrine/normetanephrine | |||||
| Noradrenaline | 1,550 | pg/mL | 100-450 | ||
| Adrenaline | 1,136 | pg/mL | <100 | ||
| Dopamine | 54 | pg/mL | <20 | ||
| Metanephrine | 308 | pg/mL | <130 | ||
| Normetanephrine | 474 | pg/mL | <506 | ||
| Urinary catecholamines and metanephrine/normetanephrine | |||||
| Noradrenaline | 613 | µg/day | 48.6-168.4 | ||
| Adrenaline | 736 | µg/day | 3.4-26.9 | ||
| Dopamine | 468.8 | µg/day | 365.0-961.5 | ||
| Metanephrine | 3.31 | mg/day | 0.04-0.19 | ||
| Normetanephrine | 1.73 | mg/day | 0.09-0.33 | ||
*Cortisol, PRA, and aldosterone were measured on the next day (Day 1) of admission.
TSH: thyrotropin, FT4: free thyroxine, PRA: plasma renin activity, DHEA-S: dehydroepiandrosterone sulfate
Underline denotes abnormal values.
An electrocardiogram showed sinus tachycardia, 117. Chest computed tomography (CT) showed a region suggesting interstitial pneumonia in the right lobe (Fig. 2). Pleural and pericardial effusions were not seen. Abdominal CT showed severe pancreatitis with diffuse edematous inflammation reaching under the kidneys (Fig. 3). A considerable amount of ascites was seen, and no gallstones were observed. Abdominal CT also showed an enlarged (45-mm) right adrenal tumor (Fig. 3).
Figure 2.
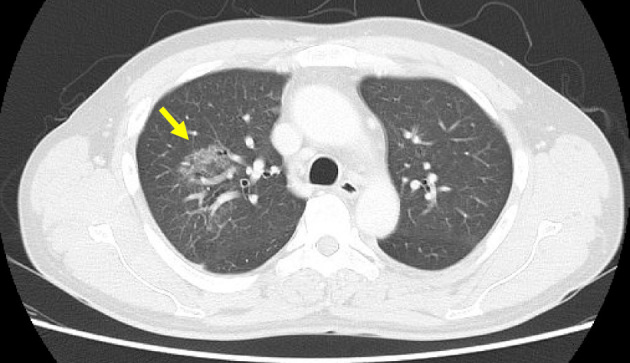
Chest computed tomography findings showing ground-glass opacity and interstitial pneumonia in the right lobe. No pleural or pericardial effusion was observed.
Figure 3.

Abdominal computed tomography findings showing severe pancreatitis with diffuse edematous inflammation (arrowheads) and an enlarged (45-mm diameter, arrow) right adrenal tumor. Ascites was seen, but no gallstones were observed.
Based on the acute pancreatitis classification (4,5), critically low level of base excess of artery blood gas (-10.8 mmol/L), elevated serum creatinine (2.95 mg/dL) and lactate dehydrogenase (LDH) levels (520 U/L), and low serum calcium level (5.2 mg/dL), we concluded that he had severe acute pancreatitis. In addition, the increased WBC count, elevated heart rate, and respiratory condition suggested systemic inflammatory response syndrome (SIRS) (4).
Intravenous fluid resuscitation: Ringer's solution of 4,500 mL/day with enteral nutrition (ElentalⓇ; EA Pharma, Tokyo, Japan) of 900 kcal/day as well as analgesia (intravenous infusion of buprenorphine, 0.6 mg/day), an intravenous proton-pump inhibitor (omeprazole, 40 mg/day), and antibiotics [ampicillin/sulbactam (ABPC/SBT), 6 g/day] gradually ameliorated the pancreatitis (Fig. 4). The abdominal pain, hypertension, right lung shadow, and acute kidney damage also improved after a week.
Figure 4.
The clinical course of the patient. The patient was admitted, and on Day 13, pheochromocytoma attack was seen. Hypertension was well controlled with the α-blocker doxazosin (gradually increased to 16 mg/day), and pheochromocytoma attack ceased. After adrenalectomy, all symptoms and laboratory abnormalities, including hyperamylasemia, hyperglycemia, and hypertriglyceridemia, were improved. AMY: amylase, PG: plasma glucose, LDL-C: low-density lipoprotein cholesterol, HDL-C: high-density lipoprotein cholesterol, TG: triglyceride
However, the patient still felt headache, dizziness, and nausea. On Day 13, the patient suddenly suffered from headache and severe dizziness with vomiting (Fig. 4; pheochromocytoma attack). His blood pressure increased to 220/123 mmHg, his heart rate was 130/min, and his body temperature was 39.2°C. His casual plasma glucose level was 757 mg/dL, and his serum amylase level was 165 U/L (Fig. 4). Intense insulin therapy (>50 units of insulin/day) and supportive therapy were commenced. The persistent headache, nausea, and dizziness were suggestive of cerebrovascular disorders and encephalopathy. Brain magnetic resonance imaging revealed right vertebral artery dissection (Fig. 5A) and a right medullary infarction (Fig. 5B). Medication with drip infusion of argatroban at 60 mg/day and oral aspirin at 200 mg/day was commenced.
Figure 5.

Brain MRI showed right vertebral artery dissection on T1 FLAIR imaging (A, arrow) and right medullary infarction on diffusion-weighted imaging (B, arrow).
Subsequently, the right adrenal tumor showed a trace uptake on an 123I-MIBG scintigram (Fig. 6). Plasma levels of noradrenaline, adrenaline, dopamine, and metanephrine increased, and the urinary levels of noradrenaline, adrenaline, metanephrine, and normetanephrine were also markedly elevated (Table 2). Therefore, right adrenal pheochromocytoma was strongly suspected. The patient's hypertension was well controlled with the α-blocker doxazosin (gradually increased to 16 mg/day), and the pheochromocytoma attack ceased. The preoperative levels of PRA (1.5 ng/mL/h) and aldosterone [4.5 (4.0-82.1) pg/mL] were normal.
Figure 6.

The right adrenal tumor showed uptake of the trace on an 123I-MIBG scintigram.
On Day 75, right laparoscopic adrenalectomy was performed, and the patient was histologically diagnosed with pheochromocytoma (Fig. 7).
Figure 7.
Macroscopically, the right adrenal mass (4×3.5 mm) had a brown-colored appearance (A, arrows). The tumor showed a zellballen pattern, low cellularity, the absence of comedo-necrosis, the presence of vascular invasion, and the absence of capsular invasion, indicating pheochromocytoma (Hematoxylin and Eosin staining, ×100) (B). Ki67 labelling index 6/223 (2.7%) (C, ×100). Immunohistochemical staining was positive for synaptophysin (D, ×100), chromogranin (E, ×100), and neuron-specific enolase (NSE) (F, ×100).
The resected tumor showed a zellballen pattern, low cellularity, the absence of comedo-necrosis, the presence of vascular invasion, the absence of capsular invasion, a Ki67 labelling index of 6/223 (2.7%), and adrenaline type (Fig. 7A-C). Therefore, the Grading of Adrenal Pheochromocytoma and Paraganglioma (GAPP) score was 2/10, indicating that the tumor was ‘well-differentiated low risk’ (6). Immunohistochemical staining was positive for synaptophysin, chromogranin, and neuron-specific enolase (NSE) (Fig. 7D-F). An immunohistochemical analysis or genetic examination of succinate dehydrogenase complex iron sulfur subunit B (SDHB) has not yet been conducted.
After tumor resection, the serum amylase levels tentatively increased to 915 U/L but then subsided (Fig. 4), and the urine catecholamine levels normalized (metanephrine, 0.04 mg/day and normetanephrine, 0.01 mg/day). Hypotension after surgery was not observed, and his blood pressure was normalized. The patient no longer complained of hypertensive or neurological symptoms. All laboratory manifestations also improved, including hyperamylasemia, hyperglycemia, and hypertriglyceridemia (Fig. 4). The fasting serum levels of triglyceride (TG) decreased to 179 mg/dL on Day 19 after the pheochromocytoma attack. The patient currently requires no medications except for oral aspirin (100 mg/day) for the right artery dissection and right medullary infarction.
Discussion
The present patient developed nocturnal abdominal pain concomitant with hypertensive crisis, suggesting multiple organ damage. Abdominal CT showed marked pancreatitis and a right adrenal mass, and the laboratory findings, including elevated levels of amylase and catecholamine, supported the diagnosis of pheochromocytoma and acute pancreatitis. Catecholamine excess due to PMC was thought to have induced life-threatening vasoconstriction/hyperinflammatory syndrome, leading to hypertensive, neurological, cerebro-cardiovascular, respiratory, renal, gastrointestinal, and metabolic emergencies in the current case (1,2). In addition, acute pancreatitis might have augmented those symptoms (4,5).
Interleukin-6 (IL-6) production from pheochromocytoma may play an important role in acute inflammatory syndrome (7). Although the serum levels and tissue staining of IL-6 were not evaluated in the current case, we speculate that IL-6 might have been involved in the development of PMC.
PMC is a rare, life-threatening entity associated with numerous symptoms that is also difficult to diagnose (1,2). Ando et al. reported that PMC accounted for 19.0% of all pheochromocytoma crisis cases, and the mortality rate was more than 10% (1). The current case exhibited neurological symptoms, possibly because of cerebrovascular disease or encephalopathy due to vasoconstriction and hyperinflammatory disorder induced by pheochromocytoma. The acute kidney damage improved within a week after fluid resuscitation, suggesting that initial volume depletion had been induced by PMC and acute pancreatitis (1,3,8). Heart complications were not suggested, possibly because of the volume reduction. Nomoto et al. reported an autopsy case of PMC, showing a region mimicking interstitial pneumonia with acute extravasation (3). Since the cardiac function was not deteriorated in the current case, the etiology of the interstitial shadow was not likely cardiogenic. The lung shadow subsided without antibiotics and thus was suggested to be affected by complex vascular and humoral factors due to PMC and acute pancreatitis (1,3,9).
Generally, in patients with pheochromocytoma, lipolysis is promoted by excess catecholamines, and serum TG levels tend to be decreased (10). Serum NEFA levels were also increased in the present case. Curiously, several factors associated with hypertriglyceridemia (lipogenesis) obviously surpassed lipolysis. Dietary habits, glucose intolerance, excess alcohol drinking of the patient, and impaired clearance of VLDL and CM as well as their remnants due to acute kidney damage may have contributed to the elevation of the serum TG levels (11). Furthermore, hyperinflammatory syndrome due to PMC and acute pancreatitis might have impaired the LPL activity (despite normal serum concentrations) and other possible enzymatic activities associated with the metabolism of CM and VLDL to LDL or HDL. The presence of multiple organ damage, including that of the liver, muscles, and adipose tissue, was speculated to be related to the downregulation of VLDL intake and the increase of VLDL output, so marked hypertriglyceridemia and extremely low levels of LDL and HDL were observed (Fig. 8) (12). Supporting this speculation, the imbalance in the TG, LDL, and HDL levels was partly improved after admission.
Figure 8.
Schematic image of hypertriglyceridemia in this patient. TG: triglyceride, PMC: pheochromocytoma multisystem crisis, CM: chylomicron, VLDL: very low-density lipoprotein, LDL: low-density lipoprotein, HDL: high-density lipoprotein, LPL: lipoprotein lipase
Extreme hyperglycemia and hypertriglyceridemia as metabolic emergencies were partly ameliorated after the improvement of acute pancreatitis and completely improved after adrenalectomy. As a treatment of PMC, appropriate blood pressure control by medications, such as α- or β-blockers, followed by the surgical removal of the adrenal mass is recommended, as was conducted in the current case to prevent intra- and post-operative hypotension (9).
Acute pancreatitis is the most frequent gastrointestinal cause of hospitalization and one of the main causes of in-hospital death (4,5,8). In the absence of gallstones, the etiology of pancreatitis in the current case seemed to be excess alcohol drinking, hypertriglyceridemia, or stress. Acute pancreatitis is an inflammatory disease caused by activation of proteases in the pancreas. Pancreatic enzyme activation triggers local and systemic inflammatory responses that are associated with the recruitment of inflammatory cells into the pancreas and the widespread up-regulation of inflammatory markers in distant tissues. The current case was classified as severe acute pancreatitis characterized by SIRS and systemic complications (4,5). Severe acute pancreatitis develops in around 20% of patients with acute pancreatitis and may be associated with multiorgan failure, similar to the current case (4,5). Increased serum triglyceride levels early in the course of acute pancreatitis have been reported to constitute an independent risk for the development of persistent organ failure (13). Wei et al. revealed that a high fasting index (TG×plasma glucose) was associated with a poor prognosis in patients with acute pancreatitis (14). Therefore, acute pancreatitis with hypertriglyceridemia should be diagnosed and treated promptly.
Gan et al. reported that pheochromocytoma presents with acute hyperamylasemia and multiorgan failure, as was seen on admission, during pheochromocytoma attack, and at adrenalectomy in our case (2). Considering these findings, our case might have developed PMC that subsequently triggered acute pancreatitis, or vice versa. The exact pathophysiology of PMC concomitant with severe acute pancreatitis remains unclear, and a further investigation is required.
In conclusion, a rare case of PMC complicated with acute pancreatitis was successfully treated based on an accurate and appropriate diagnosis. Clinicians should pay attention to the occurrence of both life-threatening diseases in the clinical settings.
The authors state that they have no Conflict of Interest (COI).
Hidefumi Inaba and Yosuke Kaido contributed equally to this work.
Acknowledgement
We are indebted to Dr. Noriyuki Itoh for performing laparoscopic adrenalectomy (Japanese Red Cross Wakayama Medical Center).
References
- 1. Ando Y, Ono Y, Sano A, Fujita N, Ono S, Tanaka Y. Clinical characteristics and outcomes of pheochromocytoma crisis: a literature review of 200 cases. J Endocrinol Invest 2022 Jul 20. [DOI] [PubMed] [Google Scholar]
- 2. Gan TJ, Miller RF, Webb AR, et al. Phaeochromocytoma presenting as acute hyperamylasaemia and multiple organ failure. Can J Anaesth 41: 244-247, 1994. [DOI] [PubMed] [Google Scholar]
- 3. Nomoto Y, Kawano K, Fujisawa N, et al. Pheochromocytoma multisystem crisis behaving like interstitial pneumonia: an autopsy case. Intern Med 56: 149-152, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banks PA, Bollen TL, Dervenis C, et al. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut 62: 102-111, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Takada T, Isaji S, Mayumi T, et al. JPN clinical practice guidelines 2021 with easy-to-understand explanations for the management of acute pancreatitis. J Hepatobiliary Pancreat Sci 29: 1057-1083, 2022. [DOI] [PubMed] [Google Scholar]
- 6. Kimura N, Takekoshi K, Naruse M. Risk stratification on pheochromocytoma and paraganglioma from laboratory and clinical medicine. J Clin Med 7: 242, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meijs AC, Schroijen MA, Snel M, Corssmit EPM. Interleukin-6 producing pheochromocytoma/paraganglioma: case series from a tertiary referral centre for pheochromocytomas and paragangliomas. J Endocrinol Invest 44: 2253-2259, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nassar TI, Qunibi WY. AKI associated with acute pancreatitis. Clin J Am Soc Nephrol 14: 1106-1115, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luo S, Cui Q, Wang D. Case report: surgical intervention under pheochromocytoma multisystem crisis: timing and approach. Front Oncol 12: 908039, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Erlic Z, Beuschlein F. Metabolic alterations in patients with pheochromocytoma. Exp Clin Endocrinol Diabetes 127: 129-136, 2019. [DOI] [PubMed] [Google Scholar]
- 11. Kaysen GA. New insights into lipid metabolism in chronic kidney disease. J Ren Nutr 21: 120-123, 2011. [DOI] [PubMed] [Google Scholar]
- 12. Feingold KR. Introduction to lipids and lipoproteins. In: Endotext [Internet]. Feingold KR, Anawalt B, Boyce A, et al. , Eds. MDText.com, South Dartmouth, MA, 2000: PMID: 26247089. [Google Scholar]
- 13. Nawaz H, Koutroumpakis E, Easler J, et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol 110: 1497-1503, 2015. [DOI] [PubMed] [Google Scholar]
- 14. Wei Y, Guo J. High triglyceride-glucose index is associated with poor prognosis in patients with acute pancreatitis. Dig Dis Sci. Forthcoming. [DOI] [PubMed] [Google Scholar]





