Abstract
A 67-year-old woman with a 2-day history of a fever, headache and disturbed consciousness was admitted to our hospital. Bacillus subtilis was isolated from both the cerebrospinal fluid and blood. She was cured by the administration of vancomycin. Next-generation sequencing identified the strain as B. subtilis var. natto, the same strain found in natto, which this patient ate daily. We suspected that some of the B. subtilis that caused the infection may have actually been B. subtilis var. natto.
Keywords: meningitis, Bacillus subtilis, Bacillus subtilis subsp. subtilis, Bacillus subtilis var. natto, nattokinase, probiotics
Introduction
Natto is a popular traditional Japanese fermented food made by fermenting steamed soybeans with Bacillus subtilis var. natto and has high nutritional value and various functional effects (1,2). B. subtilis var. natto was discovered by Sawamura in 1906 (3) and has the ability to produce the fibrinolytic enzyme “nattokinase." Strains Miyagino, Takahashi, and Naruse are three major natto starter strains. The BEST195 strain, whose genome has been used as a reference genome, is the Miyagino strain (4).
We herein report the first case of B. subtilis var. natto (represented by the BEST195 strain) bacteremia with meningitis in a patient who ingested natto daily.
Case Report
A 67-year-old woman presented to the emergency department with a 2-day history of a fever, headache and disturbed consciousness. She had vomited blood two months before admission and had erosive esophagitis diagnosed by esophagogastroduodenoscopy. Her medical history included systemic scleroderma, polymyositis and reflux esophagitis. She was being treated with 5 mg of prednisolone per day. She had no significant travel history. She was a life-long nonsmoker and had never consumed alcohol or used illicit drugs. She ate one pack of natto every day.
On admission, her vital signs revealed a temperature of 38.0°C, a blood pressure of 140/70 mmHg, a pulse of 95 beats/min, and a respiratory rate of 22 breaths/min. A physical examination revealed a lethargic patient with a Glasgow coma scale (GCS) score of 12/15 (E3V4M5). A neurological examination revealed nuchal rigidity and a positive Kernig sign; otherwise, there were no focal neurologic deficits. The rest of the physical examination was normal. She had no untreated dental decay and no wounds in her oral cavity.
Laboratory examinations showed a normal platelet count, decreased hemoglobin (10.8 g/mL) and serum sodium levels (134 mEq/L) and elevated values of leukocytes (14,310 /μL), aspartate aminotransferase (79 IU/mL), alanine aminotransferase (45 IU/mL), alkaline phosphatase (533 IU/mL), lactate dehydrogenase (261 IU/mL), D-dimer (2.34 μg/mL) and C-reactive protein (6.09 mg/L). Lumbar puncture yielded whitish cerebrospinal fluid (CSF) containing 31,536 cells/μL (88% polymorphonuclear cells, 12% mononuclear cells), an elevated protein content of 1,231 mg/dL, and a low glucose level of 23 mg/dL (serum glucose level 97 mg/dL). Head computed tomography (CT) revealed no abnormalities. The patient was diagnosed with bacterial meningitis and treated immediately with empirical antibiotic therapy with meropenem and vancomycin.
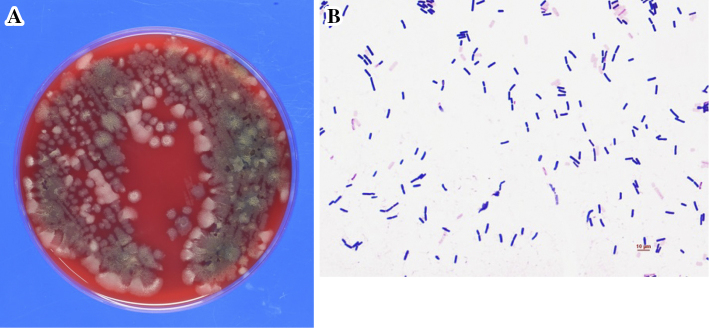
B. subtilis was isolated from two sets of blood and CSF culture. Sputum and urine cultures were negative. The colonies formed sticky strings and smelled like natto (Fig. 1A, B), so the strain of B. subtilis was suspected to be B. subtilis var. natto. The colonies were susceptible to penicillin G, imipenem/cilastatin, gentamicin, erythromycin, clindamycin, vancomycin, levofloxacin and trimethoprim/sulfamethoxazole according to the Clinical and Laboratory Standards Institute (CLSI) Guidelines (document M45).
Figure 1.
(A) Subculture bacterial colonies on blood agar plates after 24-hour incubation of the isolated organism from blood culture. (B) Gram staining from the patient’s blood and CSF culture revealed the presence of Gram-positive rods.
She was diagnosed with meningitis due to B. subtilis, and the gastrointestinal tract, specifically erosion of the esophagus, was suspected as a portal of entry for B. subtilis var. natto. She was treated with meropenem and vancomycin, and the meningeal signs disappeared after three days of treatment. In addition, blood culture was negative after 3 days of treatment, and the CSF culture was also negative after 11 days of treatment. The CSF containing 5 cells/μL (100% mononuclear cells), an elevated protein content of 61 mg/dL, and a low glucose level of 38 mg/dL (serum glucose level 80 mg/dL). Therefore, meropenem was discontinued after 5 days, and vancomycin was continued for 19 days of treatment. As the clinical course was favorable, she was discharged after 24 days of hospitalization.
To confirm that the strain of B. subtilis was B. subtilis var. natto, we purchased the same brand of natto that the patient had been eating every day and applied some of it to culture on a blood agar medium (Nippon Becton Dickinson, Tokyo, Japan). Whole-genome sequencing of each B. subtilis strain detected in the patient's blood and natto consumed by the patient was performed with an iSeq 100 system (Illumina, San Diego, USA). We compared the genome of each B. subtilis with the nattokinase reference sequence (5) and confirmed the presence of the same sequence of a gene that encodes nattokinase in the genome of the clinical strain, the strain of the bought natto, and Miyagino (B. subtilis var. natto BEST195) (Table 1). Other B. subtilis excluding B. subtilis subsp. subtilis strains did not contain the sequence of the gene that encodes nattokinase. Furthermore, whole-genome sequencing of each B. subtilis strain from the patient's blood and natto consumed by the patient showed high similarity to the chromosome alignment of B. subtilis var. natto BEST195 by BLASTN search of the NCBI nr/nt database. Furthermore, no single-nucleotide variants between the B. subtilis strain detected in the patient's blood and the natto consumed by the patient were detected in the core-genome region on a comparison with the reference genome (B. subtilis var. natto BEST195) (Fig. 2). These results indicate that 1) the bacteria detected in the patient's blood culture was B. subtilis var. natto, and 2) the strains of B. subtilis var. natto detected in the blood and natto consumed by the patient were identical.
Table 1.
A Comparison of Each Bacillus subtilis Genome with the Reference Sequence of Nattokinase.
| NCBI accession No. | Length | %Ident | ||||
|---|---|---|---|---|---|---|
| B. subtilis strain from the patient’s blood | - | 1,146 | 100 | |||
| B. subtilis var. natto BEST195 | NC_017196.2 | 1,146 | 100 | |||
| B. subtilis subsp. subtilis | NC_000964.3 | 1,146 | 99.215 | |||
| B. subtilis subsp. spizizeni | NZ_CP034943.1 | 1,146 | 93.382 | |||
| B. subtilis subsp. inaquosorum | NZ_CP013984.1 | 1,146 | 96.161 |
Figure 2.
Whole-genome sequencing of each B. subtilis strain detected in the patient’s blood and natto consumed by the patient. We observed a guanine-to-adenine nucleotide change in coding DNA No.315 between the B. subtilis strain detected in the patient’s blood, natto consumed by the patient, and the reference genome (B. subtilis var. natto BEST195), although the amino acid sequence was not affected.
Discussion
B. subtilis is a Gram-positive spore-forming bacteria. B. subtilis contains B. subtilis subsp. subtilis, B. subtilis subsp. spizizeni and B. subtilis subsp. inaquosorum. It is commonly found in the environment and in the human gut and is generally considered non-hazardous to human health (6). Bacteremia can be difficult to distinguish from infection and contamination. B. subtilis was isolated from two sets of blood cultures in this patient, so we diagnosed true infection of B. subtilis. Most Bacillus spp. isolates are susceptible to vancomycin, clindamycin, fluoroquinolones, aminoglycosides, carbapenems and penicillins. Imipenem and some extended-spectrum β-lactams are sometimes seen to be inactive by the β-lactamase of Bacillus spp. The bacteria in the present case were susceptible to imipenem/cilastatin and penicillin G, and the isolate did not produce β-lactamase.
On reviewing the literature, we identified six previous cases of B. subtilis meningitis (Table 2) (7-12). Septicemia, pneumonia, endocarditis, wound infection and intraocular inflammation caused by B. subtilis have been reported, as well as meningitis (13). Rarely, B. subtilis cause infections in humans, mainly being seen in patients with underlying diseases or an immunocompromised state. Systemic corticosteroid administration is a risk factor for the onset of B. subtilis bacteremia (13,14). Long-term steroid treatment is thought to have been a risk factor in the present case of B. subtilis bacteremia. We suspect that the lesion in the intestinal tract was also a risk factor for B. subtilis bacteremia. Notably, previously reported cases were not subjected to whole-genome sequencing between B. subtilis and B. subtilis var. natto.
Table 2.
Previous Reports of Meningitis Caused by B. Subtilis.
| Year | Disease | Source | Ref | |||
|---|---|---|---|---|---|---|
| 1913 | Meningitis | Lumbar puncture | 7 | |||
| 1916 | Brain abscess | Brain surgery | 8 | |||
| 1926 | Meningitis | Chronic infection of the middle ear | 9 | |||
| 1949 | Meningitis | Urinary tract infection | 10 | |||
| 1950 | Meningitis | Lumbar puncture | 11 | |||
| 2018 | Brain abscess | Bad oral hygiene | 12 | |||
| 2020 | Meningitis | Eat natto | - |
Nattokinase is an extracellular enzyme secreted by B. subtilis var. natto (4). The B. subtilis strain detected in our patient's blood contained the sequence of a gene that encodes nattokinase. Furthermore, the B. subtilis strain detected in the patient's blood, natto consumed by the patient, and B. subtilis var. natto BEST195 were the same variant. We also observed a guanine-to-adenine nucleotide change in coding DNA no. 315 between the B. subtilis strain detected in the patient's blood, natto consumed by the patient, and B. subtilis var. natto BEST195. Nevertheless, the amino acid sequence was not affected.
A previous study in B. subtilis subsp. subtilis other than B. subtilis var. natto observed loss of function or hypofunction because of a single-nucleotide substitution in the degQ and swrA (15). This phenomenon is important in distinguishing B. subtilis var. natto from other B. subtilis. We noted that the sequences in the degQ and swrA regions were identical between the B. subtilis strain detected in the patient's blood and B. subtilis var. natto BEST195 (Fig. 3A, B).
Figure 3.
The sequences in the degQ (A) and swrA (B). The sequences in the degQ and swrA from the bacteria detected in the patient’s blood culture and natto consumed by the patient are shown on the upper side. The sequences from B. subtilis subsp. subtilis are shown on the lower side.
Natto is a popular traditional Japanese fermented food and a favorite among elder people as a probiotic (2,16). While probiotic-related bacteremia is rare, Boyle et al. reported that risk factors for probiotics-related sepsis include compromised immunity, premature infants, central venous catheter, impaired intestinal epithelial barrier, administration of probiotics by jejunostomy, concomitant administration of broad-spectrum antibiotics to which probiotics are resistant, probiotics with high mucosal adhesion or known pathogenicity, and cardiovascular disease (17). In recent years, Kato et al. reported a case of B. subtilis var. natto bacteremia caused by ingestion of natto (2).
To our knowledge, the present case is the first in the literature describing bacteremia and meningitis induced by B. subtilis var. natto by ingestion of natto (18). Clinicians should be aware that some B. subtilis that cause infection may actually be B. subtilis var. natto.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Terada A, Yamamoto M, Yoshimura E. Effect of the fermented soybean product “natto" on the composition and metabolic activity of the human fecal flora. Jpn J Food Microbiol 16: 221-230, 1999. [Google Scholar]
- 2. Kato A, Yoshifuji A, Komori K, et al. A case of Bacillus subtilis var. natto bacteremia caused by ingestion of natto during COVID-19 treatment in a maintenance hemodialysis patient with multiple myeloma. J Infect Chemother 28: 1212-1215, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sawamura S. On the micro-organisms of natto. Bull Coll Agric Tokyo Imp Univ 7: 107-110, 1906. [Google Scholar]
- 4. Kamada M, Hase S, Fujii K, et al. Whole-genome sequencing and comparative genome analysis of Bacillus subtilis strains isolated from non-salted fermented soybean foods. PLoS One 10: e0141369, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sumi H. [Natto as a Functional Food]. Bull Okayama Prefect Jr Coll 35: 46-52, 1991(in Japanese). [Google Scholar]
- 6. Lampropoulos PK, Gkentzi D, Tzifas S, Dimitriou G. Neonatal sepsis due to Bacillus subtilis. Cureus 13: e17692, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Senge J. Meningitis purulenta et Encephalitis haemorrhagica nach Lumbalanästhesie, verursacht durch einen eigenartigen Sporenbildner. Cent f Bakt I Abt Orig 70: 353-368, 1913. [Google Scholar]
- 8. Lindberg G. Meningeal hemorrhage with infection by B. Subtilis in an infant. Hygiea 78: 1089-1112, 1916. [Google Scholar]
- 9. Sanderson DD. Bacillus subtilis in pure culture complicating mastoiditis and meningitis. Nebraska State M J 11: 318, 1926. [Google Scholar]
- 10. Hull ER, Howie JE, Bean H. An uncommon infection of the urinary tract with terminal septicemia. Lancet 230: 189-190, 1937. [Google Scholar]
- 11. Weinstein L, Colburn CG. Bacillus subtilis meningitis and bacteremia; report of a case and review of the literature on subtilis infections in man. AMA Arch Intern Med 86: 585-594, 1950. [DOI] [PubMed] [Google Scholar]
- 12. Tsonis I, Karamani L, Xaplanteri P, et al. Spontaneous cerebral abscess due to Bacillus subtilis in an immunocompetent male patient: a case report and review of literature. World J Clin Cases 6: 1169-1174, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ozkocaman V, Ozcelik T, Ali R, et al. Bacillus spp. among hospitalized patients with haematological malignancies: clinical features, epidemics and outcomes. J Hosp Infect 64: 169-176, 2006. [DOI] [PubMed] [Google Scholar]
- 14. Gaur AH, Patrick CC, McCullers JA, et al. Bacillus cereus bacteremia and meningitis in immunocompromised children. Clin Infect Dis 32: 1456-1462, 2001. [DOI] [PubMed] [Google Scholar]
- 15. Nishito Y, Osana Y, Hachiya T. Whole genome assembly of a natto production strain Bacillus subtilis natto from very short read data. BMC Genomics 11: 243, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Costa RL, Moreira J, Lorenzo A, Lamas CC. Infectious complications following probiotic ingestion: a potentially underestimated problem? A systematic review of reports and case series. BMC Complement Altern Med 18: 329, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boyle RJ, Robins-Browne RM, Tang MLK. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr 83: 1256-1264, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Tarumoto N, Imai K, Sakai J, et al. A case of bacterial meningitis caused by B. subtilis var. natto. J Jpn Assoc Infect Dis 94: 264-265, 2020. [Google Scholar]