Abstract
Fungal diseases are a leading threat to human health, especially in individuals with compromised immunity. Although there have been recent important advances in antifungal drug development, antifungal resistance, drug-drug interactions and difficulties in delivery remain major challenges. Among its pleiotropic actions, nitric oxide (NO) is a key molecule in host defense. We have developed a flexible nanoparticle platform that delivers sustained release of NO and have demonstrated the platform’s efficacy against diverse bacteria as well as some fungal species. In this work, we investigate the effects of two NO-releasing particles against a panel of important human yeast. Our results demonstrate that the compounds are both effective against diverse yeast, including ascomycota and basidiomycota species, and that NO-releasing particles may be a potent addition to our armamentarium for the treatment of focal and disseminated mycoses.
Keywords: novel, medical mycology, candida, cryptococcus, drug discovery
Introduction
According to the last comprehensive population study in 2013, approximately 2.8% of the United States population was estimated to be immunocompromised (Harpaz et al., 2016), and it is expected this frequency to stay the same or increase due to wider indications for immunomodulators and up trending yearly transplants. While immunocompromised patients are prone to a broad range of infections, invasive fungal infections (IFI) are relatively more common in this population. IFIs carry high morbidity, due to fungal endocarditis and valvular damage, and high mortality, estimated with candidemia to be between 19 to 40% (Kullberg & Arendrup, 2015; Liu & Nosanchuk, 2020; Morgan et al., 2005). Among Candida species, there are primary resistance and acquired antifungal resistant mechanisms such as drug target overexpression or alteration and drug efflux pump overexpression (Berkow & Lockhart, 2017). On the other hand, Cryptococcus neoformans is innately resistant to echinocandins and reports of azole resistance complicating treatment course have been described (Feldmesser et al., 2000; Mpoza et al., 2017). Mold pathogens’ resistance mechanisms exhibited similar mechanisms in Aspergillus fumigatus, while Sporothrix species also utilizes melanin (Berger et al., 2017; Waller et al., 2021). Given the difficulties with treatment of these diverse mycoses, researchers are investigating new therapeutic agents in attempts to keep pace with fungal resistance evolution.
Nitric Oxide (NO) is a lipophilic, diatomic molecule that serves various functions within the body. Pertaining to the immune system, innate immunity utilizes NO with cidal or static effects in response to pathogen invasion (De Groote & Fang, 1995). Endogenous NO is produced via inducible NO synthase activity upon L-arginine (O W Griffith & Stuehr, 1995). In 2008, a silane-based hydrogel nanoparticle (NP) that allows for steady nitric oxide (NO) release was created in which the NPs are NO donors via thermal reduction of nitrites by glucose (Friedman et al., 2008). Formulations of the silicone-based compound were highly effective against a select set of pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), Candida albicans and Pseudomonas aeruginosa under planktonic or biofilm growth conditions (Ahmadi et al., 2016; Duong et al., 2014; Han et al., 2009; Macherla et al., 2012; Martinez et al., 2009; Mihu et al., 2016; Mihu et al., 2010). The efficacy of the NPs has been further tested and shown to be potently active against a range of Gram-positive and Gram-negative bacteria, including strains that were resistant to common antibiotics (Friedman et al., 2011). The NP platform is flexible, allowing for the incorporation of compounds such as S-Nitrosothiol (SNO) and N-acetylcysteine (NAC). SNO was found to improve the stability of the NP while keeping the effects of NO (Al-Sa’doni & Ferro, 2000; Al-Sa’doni & Ferro, 2004; Hornyák et al., 2012; Hu & Chou, 2006; Richardson & Benjamin, 2002). SNO goes through S-transnitrosation, where NO is transferred from one thiol to another thiol-containing surface on intracellular or plasma proteins (Nacharaju et al., 2012).
In 2012, the NP platform was further optimized to encapsulate NAC-SNO forming NAC-SNO-NP to achieve a slow sustained production of NO and NAC associated products (Nacharaju et al., 2012). Like NO, NAC also demonstrates antimicrobial properties, particularly exhibiting anti-mycobacterial and anti-biofilm properties (Amaral et al., 2016; Costa et al., 2017; De Groote & Fang, 1995; Moon et al., 2016; Parry & Neu, 1977). We have recently tested NAC-SNO-NP’s antifungal activities against Candida auris, where it effectively eradicated planktonic C. auris while also exhibiting anti-biofilm activities (Cleare LG, 2020). The compound was also tested in a murine burn model that demonstrated no obvious cytotoxicity on histology (Landriscina et al., 2015). In 2018, Abuzeid et al developed a porous organosilica microparticles (MP) that contained SNO (SNO-MP) that exhibited similar NO release and antibacterial effects (Abuzeid et al., 2018). This MP also did not display cytotoxicity at 5 and 10 mg/mL to sinonasal epithelial cells (Li et al., 2022).
Thus, in this study, we tested two S-nitrosothiol containing hydrogel-based particles, NAC-SNO NPs and SNO-MPs at concentrations established with testing of C. albicans and C. auris. We tested these compounds against additional Candida species and C. neoformans as well as several Sporothrix species, which cause subcutaneous infections. Historically, S. schenckii was considered as one species until Marimon et al demonstrated that it is a complex made up of S. mexicana, S. schenckii sensu stricto, S. brasiliensis, and S. globosa. The latter three are known to be associated with infections in humans (Marimon et al., 2007). This study significantly expands the study of NO-releasing particles against important human pathogens, including Sporothrix spp. The work further demonstrates the value of expanding efforts to harness NO-releasing compounds as broad-spectrum antifungals for cutaneous, subcutaneous, and disseminated mycoses, including biofilm associated disease.
Material and Methods
Preparation of Sol-Gel
The sol-gel was prepared with tetramethylorthosilicate (TMOS), as described (Brinker et al., 1982; Girish et al., 2019).Briefly, 3 mL of TMOS were hydrolyzed by 0.37 mL of 40 mM HCl in the presence of 2.5 mL MeOHat 60 °C for 1.5 h. This mixture was diluted with 200 μL of water and 1 mL of similarly hydrolyzed 3-aminopropyltrimethoxysilane (APTS). APTS introduces amino groups into the matrix and promoted sustained release of the enclosed contents in our previous studies (Brinker et al., 1982). The polymerization of this hydrolyzed TMOS was carried out at 40 °C, and a clear gel formed within 30 min.
Synthesis of NAC-SNO-NP
The synthesis of the NAC-SNO-NP using sol-gel was previously described (Cleare LG, 2020). In brief, two grams of sol-gel were mashed and cooled on ice. NAC-SNO was prepared by mixing 900 μmol of nitrite and 1080 μmol of NAC, then cooled on ice. Two ml of freshly made NAC-SNO were added to the measured mashed sol-gel. The mixture was mixed on a lab rotator for 3 h at 4 °C. The mixture was then centrifuged at 1900 × g for 5 min, the supernatant separated, and the particles lyophilized. The dried powder was ground into finer particles with a mortar and pestle (NAC-SNO-NP). The NAC-SNO-NPs were stored in aliquots at −80 °C. The final concentration of NAC-SNO in NP was 1.2 μmol/mg. The concentration of NAC in the particles was not determined. The particle size ranged from 200 to 2000 nm, as determined by dynamic light scattering (Cleare LG, 2020). NO and NAC-SNO release profiles were measured as described (Cleare LG, 2020) using an NO analyzer (Sievers 280i; GE Instruments, Boulder, CO, USA). The NAC-SNO-NP NO release profile exhibits a logarithmic decay with rapid release of NO particles at 4 parts per million (ppm) then quickly dissipates to approximately 2.5 ppm by 1 hour, but sustains 1.7 ppm to the end of 280 min experiment (Cleare LG, 2020).
Synthesis of SNO-microparticles (SNO-MPs)
SNO-MPs were synthesized as described (Abuzeid et al., 2018). In brief, an organosilica sol-gel monolith was formed containing many covalently attached nitrosated thiol groups. Hydrolyzed trimethyl orthosilicate, hydrolyzed/nitrosated mercaptopropyl-trimethoxysilane, polyethylene glycol, and sodium phosphate were mixed and maintained at room temperature to form a spanning network of siloxane (Si-O-Si) linkages through condensation. The resultant porous sol-gel monolith was lyophilized, dry-milled, and then pestled to a micron-sized powder with particle diameters of 5 to 10 μm (Abuzeid et al., 2018). Blank MPs incapable of NO release (B-MP) were prepared by performing the aforementioned steps, with the exception of nitrosation.
Nitrosation of SNO-MP
Un-nitrosated (blank) SNO-MP were measured out and suspended in 1 ml of sterile deionized water via vortex for 30 seconds then placed on ice. Five Molar of sodium nitrite were prepared with 345 mg of sodium nitrite in 1 ml of de-ionized water then stored at 4 °C, replaced every 7 days. Cold 5M sodium nitrite were added to the SNO-MP water suspension at a ratio of 0.967 μl of sodium nitrite for every 1 mg of SNO-MP. The new solution was then vortexed for 30 seconds, and 1M of cold HCl was then added. The solution turned pink immediately, indicative of NO presence, and the solution was vortexed for 30 seconds to ensure complete mixing. Nitrosated SNO-MPs were then spun down with a tabletop centrifuge. The supernatant was decanted, then washed with another 1 ml of deionized water. Finally, the product was suspended in cold minimal media with 5 % fetal bovine serum (FBS; Atlanta Biologicals) to achieve the desired particle concentration. NO kinetics were described previously (Abuzeid et al., 2018). In brief, NO analyzer (Sievers NO Analyzer 280i; GE Analytical Instruments, Boulder, CO) was used. NO flux peaked at 1.8 nmol/mg/min within first 10 minutes, then followed a logarithmic decay and sustained a 0.4 nmol/mg/min for approximately 64 hours.
Planktonic culture preparation
Candida albicans SC5314 (ATCC MYA-2876), Candida parapsilosis ATCC22019, Candida krusei ATCC6258, and Cryptococcus neoformans H99 (ATCC 208821) were stored at −80 °C. Aliquots were removed from the frozen vials and cells were cultivated in Sabouraud Dextrose (SD) broth then seeded onto SD agar plates. These plates were then stored at 4 °C and renewed on a biweekly basis. Prior to each experiment, one colony was picked and then inoculated in 10 ml of SD broth and incubated overnight at their respective temperatures (Table 1) on a rotator at 200 rpm.
Table 1.
This table displays the medium and incubation temperature for each fungal pathogen.
| Organism | Plating Agar/ Planktonic Media | Incubation Temperature (°C) |
|---|---|---|
| Candida albicans SC5314 | SD | 30 |
| Candida parapsilosis ATCC22019 | SD | 30 |
| Candida krusei ATCC6258 | SD | 30 |
| Cryptococcus neoformans H99 | BHI | 37 |
| Sporothrix schenckii 1099–18 | BHI | 35 |
| Sporothrix globosa CFP1021 | BHI | 35 |
| Sporothrix brasiliensis CFP0551 | BHI | 35 |
Sporothrix schenckii 1099–18, Sporothrix globosa CFP1021, and Sporothrix brasiliensis CFP0551 were stored at −80 °C. The Sporothrix isolates were acquired from a fungal culture collection maintained at Fundação Oswaldo Cruz, Brazil. Cells removed from the frozen stock were cultivated in SD broth then seeded onto SD agar plates. Conversion to the yeast form was performed on Brain Heart Infusion (BHI) agar incubated at 35 °C. These plates were then stored at 4 °C and renewed monthly. Prior to each experiment, one colony was inoculated in 10 ml BHI medium and incubated overnight at their respective temperatures (Table 1) on a rotator at 200 rpm.
Planktonic cells viability and treatment
After washing with phosphate-buffered saline (PBS), the targeted fungal cells were suspended in chemically defined minimal media (20 mg/mL of thiamine, 30 mM glucose, 26 mM glycine, 20 mM MgSO4·7H2O, and 58.8 mM KH2PO4) supplemented with 5% FBS. Cell concentrations were measured via hemocytometer and solutions were diluted to make a cell suspension at 2 × 106 cells/ml from which 100 μl were added to round bottomed 2 ml test tubes. One glass bead (1× 4 mm) was added to each tube to reduce particle sedimentation. For each tube, 100 μl of 10 mg/mL and 20 mg/mL were added to achieve either a 5 mg/mL or 10 mg/mL final particle concentration, respectively. Concentrations are chosen based on previous in vitro studies with associated NO release kinetics (Abuzeid et al., 2018; Cleare LG, 2020). Cell-particle suspensions were incubated in the dark, at the desired temperature (Table 1) for 24 hours, rotating at 200 rpm.
Biofilm viability and treatment
Individual colonies of C. albicans SC5314 were picked from SD agar and inoculated into SD broth overnight at 30 °C on a rotator at 200 rpm. Culture suspensions were spun down 2500 rpm at 7 minutes, washed with PBS twice and suspended with minimum media (MM) with 5% FBS. The suspension was then diluted 1:500 in formalin, and cell counts were done via hemocytometer. Cells were then diluted to 106 cells/ml. 200 μl of said dilution were placed in a well of a 96 flat bottom plate and then incubated for 24 hours at 30 °C. The supernatant was carefully replaced with 200 μl of MM with 5% FBS with another 24 hours of incubation. The supernatant was then replaced again without disruption of the formed biofilm with 200 μl of appropriate concentration of blank or nitrosated particles and incubated at 30 °C for 24 hours in the dark. Biofilms were disrupted via pipetting, diluted, and plated onto agar plates for colony-forming units (CFU) counting.
Measuring Planktonic and Biofilm Viability by CFU Killing Assay
Treatment groups for each pathogen included 2 arms (NAC-SNO-NP and SNO-MP) where each arm had no particle (control), blank particles, or NO-loaded particles at 5 mg/mL or 10 mg/mL, suspended in previously defined MM with 5% FBS. Blank particles consisted of NP and MP scaffolding without the addition of NAC, NAC-SNO, or SNO. Each experiment was done in triplicates, and each experiment was repeated at least 3 times. There were at least 9 data points per particle per pathogen. After 24 hours of incubation with particles, the suspensions of planktonic cultures were serially diluted and plated onto agar plates (see Table 1). The plates were incubated at 37 °C for at least 48 hours until colonies become countable for CFU.
Surviving Colony susceptibility test
To rule out yeast cells developing de novo resistance in biofilm conditions in the presence of the 10mg/ml SNO-MP, we assessed C. albicans SC5314 biofilm viability through picking one surviving colony for clonal expansion for repeat susceptibility testing. The colony was then cultivated overnight in SD broth and then seeded onto SD agar plate, which was then stored at 4°C for 2 weeks. Prior to each experiment, one colony was picked and then inoculated in 10ml SD broth overnight at 30°C on a rotator at 200 rpm. SNO-MP was then tested following planktonic viability and treatment protocol previously described.
Statistical Analysis
All graphs present average and S.E.M. relative to at least 3 independent experiments. Statistical analysis was performed using GraphPad Prism (Version 9, GraphPad Software, La Jolla California, USA). One-way ANOVA, followed by Bonferroni’s multiple comparison test was used to analyze the data. Statistical significance was set at the standard p < 0.05, and * = p < 0.05, ** = p < 0.01, *** = p < 0.001, and **** = p < 0.0001.
Results
Planktonic cells of C. albicans were similarly killed by both NAC-SNO-NP and SNO-MP and blanks from both particles had no effect on the cells (Figure 1A). The same pattern was observed for biofilms treated with SNO-MP. For NAC-SNO-NP however, the biofilm, 10 mg/mL of NAC-SNO-NP particle concentration was markedly more effective than 5 mg/mL, whereas this lower concentration sowed similar effect as blank particles that curiously were able to inhibit the viability of C. albicans cells in the biofilm (Figure 1B). The SNO-MP experiments demonstrated that both concentrations of the MP reduced C. albicans CFUs (Figure 1b). There were significant reductions starting at 5 mg/mL concentration with CFU formation 4.9 % and 1.9 % of control for planktonic and biofilm conditions, respectively. At 10 mg/mL, SNO-MP reduced growth to 2.4% of control for planktonic and eliminated 100% of yeast cells under the biofilm condition. To demonstrate that the few surviving colonies from the 10 mg/mL SNO-MP-treated biofilm remained susceptible to the compound, cells from surviving colonies were treated under planktonic condition and the SNO-MP efficiently killed the cells (Figure 1c). These results suggest that both NAC-SNO-NP and SNO-MP are efficient against C. albicans grown in planktonic and biofilm cultures, and surviving cells from treated biofilm do not become resistant to SNO-MPs.
Figure 1. Effects of NO-releasing particles on C. albicans.
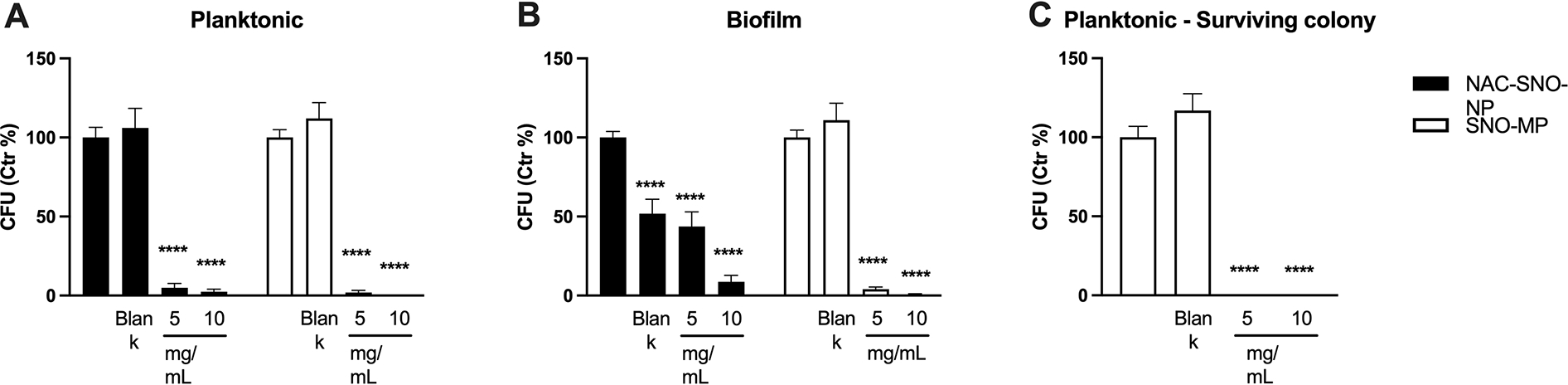
(A) Both NAC-SNO-NP and SNO–MP in both concentrations significantly reduced viability of planktonic C. albicans. (B) Both concentrations of NPs and MPs reduced C. albicans viability in biofilms. (C) Surviving colonies from the biofilm (10 mg/mL SNO-MP) were susceptible to SNO-MP on planktonic growth. Graphs show average and SEM relative to at least 3 independent experiments. * = p < 0.05 by one-way ANOVA followed by Bonferroni’s multiple comparison test. C = control, B = blank.
Planktonic yeast cells from non-albicans species of C. krusei (Figure 2A) and C. parapsilosis (Figure 2B) were killed by both the NAC-SNO-NP and SNO-MP, at both concentrations 5 mg/mL and 10 mg/mL each. There were no differences between treatment concentrations and particle platforms as the treatments eradicated all yeast cells. However, we observed an interesting increase in viability in both species treated with blank MPs when compared to their control conditions.
Figure 2. Susceptibility of non-albicans Candida spp. against NAC-SNO-NP and SNO-MP.
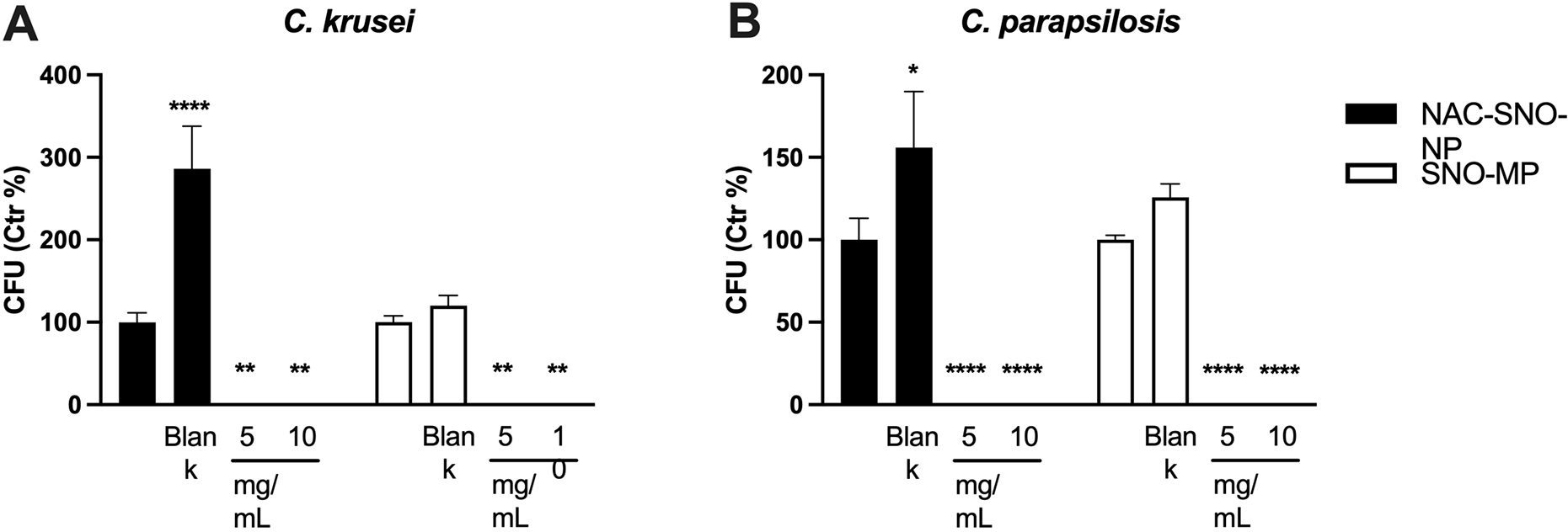
C. krusei (A) and C. parapsilosis (B) yeast cells were eradicated by the NO-releasing particles. Graphs show average and SEM relative to at least 3 independent experiments. * = p < 0.05 by one-way ANOVA followed by Bonferroni’s multiple comparison test. C = control, B = blank.
The incubation with particles from both platforms and in both concentrations induced death of planktonic cultures of C. neoformans (Figure 3). As observed in C. albicans biofilms, treatment of C. neoformans planktonic cultures with blank NPs partially inhibited growth.
Figure 3. C. neoformans is susceptible against the treatment with NAC-SNO-NP and SNO-MP.
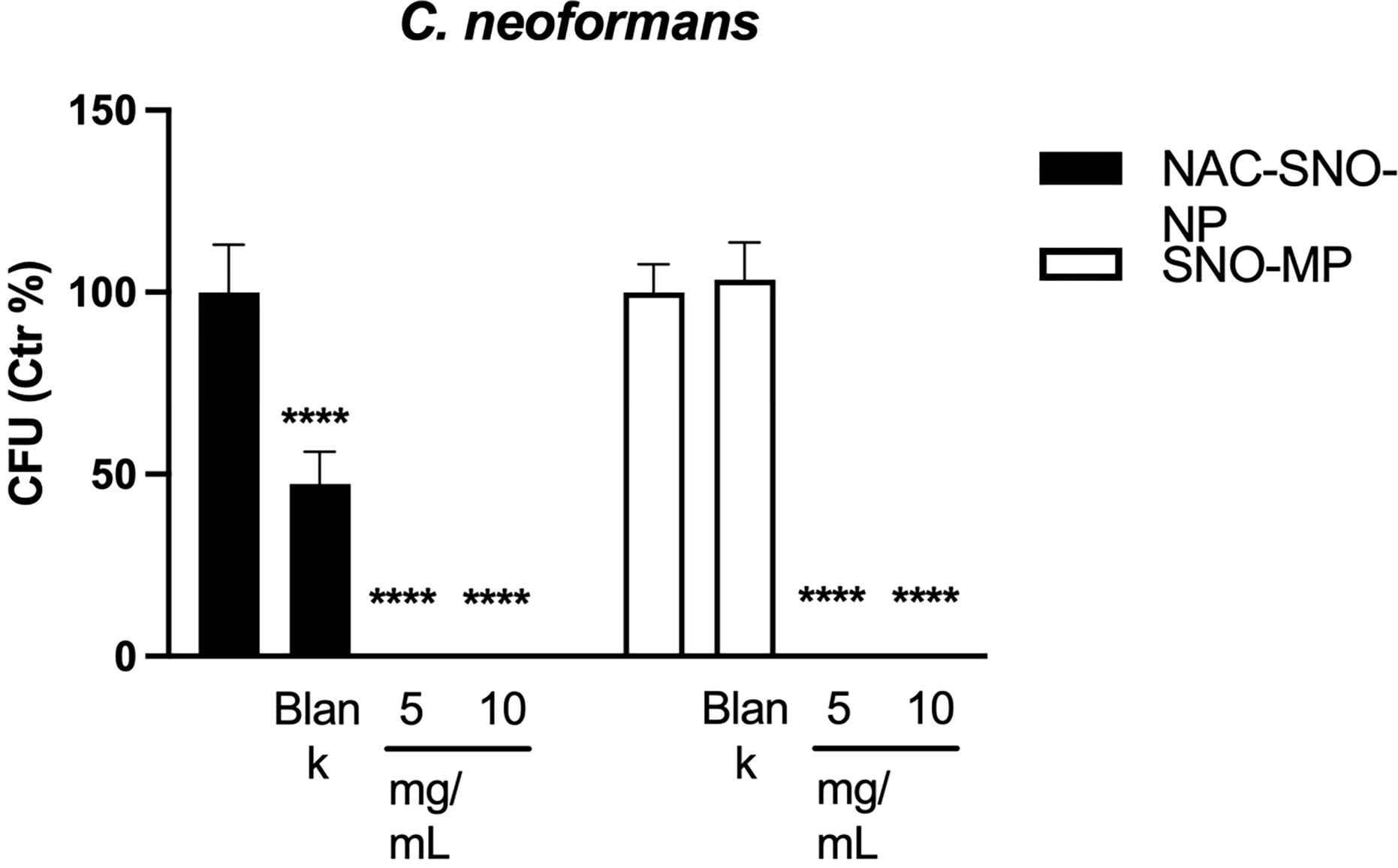
Both the NAC-SNO-NP and SNO-MP killed C. neoformans in both concentrations tested. The graph shows averages and SEM relative to 3 independent experiments. * = p < 0.05 by one-way ANOVA followed by Bonferroni’s multiple comparison test. C = control, B = blank.
The NO-releasing compounds were lethal to S. globosa (Figure 4A). Although the SNO-MP eradicated S. schenckii and NAC-SNO-NP reduced growth, the changes were not significantly different from controls, likely due to the low growth of the strain (Figure 4B). Of note, there were marked variations in S. schenckii across the experiments resulting in large variance, such that the treatments were not statistically different than controls, but no cells were detected in either concentration of SNO-MP or for the vast majority of 10 mg/mL treatment with NAC-SNO-NP (Figure 4b). On the other hand, S. brasiliensis was resistant to the 5 mg/mL concentration of NPs but susceptible to the other treatments (Figure 4C). As observed for non-albicans species of Candida, blank NP induced growth of S. globosa and S. brasiliensis, and blank MPs induced growth of S. globosa and S. schenckii.
Figure 4. Efficacy of NAC-SNO- NP and SNO-MP against Sporothrix spp.
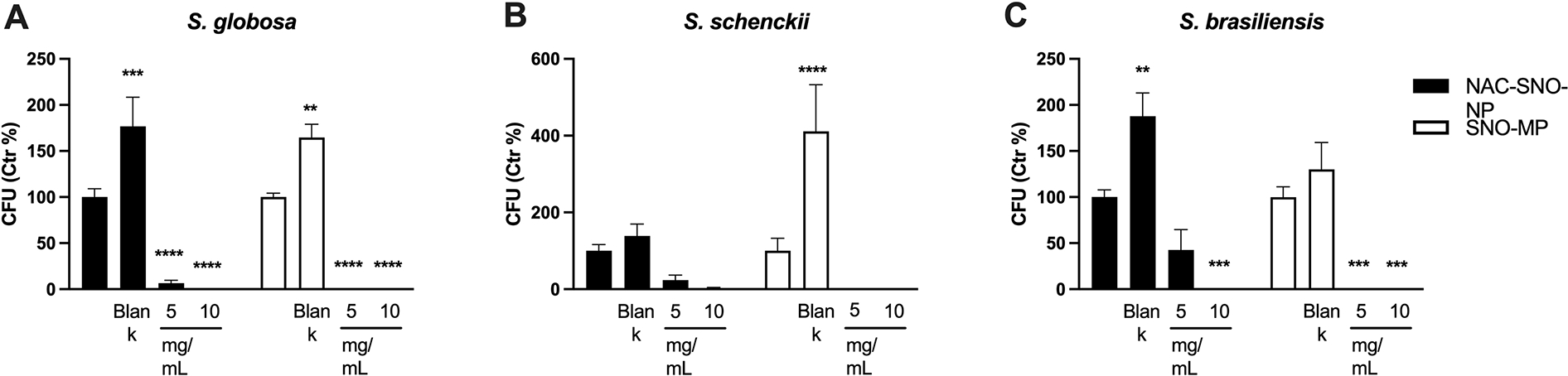
Planktonic cultures of S. globosa (A), S. schenckii (B), and S. brasiliensis (C) were treated with NAC-SNO-NP and SNO-MP in the indicated concentrations and the resulting suspensions were plated for CFU counting. Graphs show average and SEM relative to at least 3 independent experiments. * = p < 0.05 by one-way ANOVA followed by Bonferroni’s multiple comparison test. C = control, B = blank.
Discussion
Both the NPs and MPs exhibited strong antifungal activities against planktonic C. albicans, C. krusei, C. parapsilosis, and C. neoformans, and against C. albicans biofilms. We chose C. albicans to study the particles’ effect in the setting of biofilm growth as it exhibits a heterogenous structure composed of robust extracellular polysaccharide matrix with blastophores and hyphae (Chandra et al., 2001). On the other hand, C. parapsilosis forms biofilm that has minimal extracellular matrix (ECM) made primarily from clusters of yeast cells (Lattif et al., 2010). C. krusei biofilm composition has been described as thick with ECM with embedded pseudohyphal forms of the organism (Chandra & Mukherjee, 2015). Cell populations in biofilm are metabolically different from their planktonic counterparts with biofilm-associated up-regulation of efflux pump genes, quorum-sensing molecules, and surface contact factors (Brinker et al., 1982). Our prior studies demonstrated that 5 mg/mL NO-NP significantly reduced C. albicans’ biofilm but did not eliminate the cells (Ahmadi et al., 2016), and this is consistent with our study (Fig. 1b). Notably, SNO-MP showed great antifungal activity on biofilm at 5 mg/mL concentration and virtually 100% elimination at 10 mg/mL. The surviving colonies from biofilms treated with 10 mg/mL of SNO-MP were susceptible to the same treatment once re-cultivated in a planktonic setting, suggesting that some cells were protected by ECM or dead yeast from the NO, but that true resistance did not occur. Thus, a re-exposure to the SNO-MPs would be effective in eradicating the yeast. More experiments should be done to further clarify the survival mechanism of yeasts in biofilm against NO generating particles.
Prior work has shown that C. neoformans can be eliminated via NO mediated killing (Ghosn et al., 2006), and our experiments demonstrated that both the NP and MP are viable platforms that can deliver sufficient concentrations of NO to induce clearance of the pathogen in vitro. Preliminary in vivo data involving hamsters hint that NO particle platforms could be safely used systemically, albeit there was a decrease in mean arterial pressure (MAP) (Nacharaju et al., 2012). Given that C. neoformans meningitis remains a cause of high mortality and morbidity in patients with AIDS, and the first line treatment of liposomal amphotericin B and flucytosine (Perfect et al., 2010) often carry terrible toxicity, our particle platforms could be explored as alternative or additive therapeutic options.
In murine models, S. brasiliensis seems to be the most virulent of the Sporothrix species as it causes mortality at a low inoculum concentration. In contrast, S. schenckii induces mortality at high inoculum, while mice usually survive from S. globosa infection. Mice infected with S. brasiliensis displayed the greatest infiltration of fungal cells (Arrillaga-Moncrieff et al., 2009). In Brazil, there has been outbreaks of feline sporotrichosis with associated high numbers of human infections in Rio de Janeiro, with majority of the patients reported to have had contact with cats (Barros et al., 2011; Barros et al., 2004; Schubach et al., 2008). The feline-transmitted sporotrichosis has been caused by S. brasiliensis (Sanchotene et al., 2015). Itraconazole remains as the first line treatment, but it interacts with many drugs and cannot be used during pregnancy (Kauffman et al., 2007), suggesting that a non-azole treatment could be a good alternative to this azole. The NO-releasing MP and NP particles demonstrated activity against Sporothrix species. Both particles eradicated S. globosa. For S. brasiliensis, the SNO-MP had greater efficacy than the NAC-SNO-NP at 5 mg/mL concentration, but both particles achieved complete clearance at the higher concentration. Both particles reduced the growth of S. schenckii, although the controls had low growth, which contributed to their statistical non-significance.
The blank MPs induced growth of S. globosa and S. schenckii, but they did not show activity against the other tested fungi. The blank NPs also induced growth in non-albicans Candida species and S. globosa and S. brasiliensis, but they inhibited growth of C. albicans (biofilm) and C. neoformans. These alterations in growth may be from a yet unknown interference between particles and cells, such as stress responses from contact with the particles or other effects due to the particles’ material; although there is no known effect from the sol-gel that NAC-SNO-NP or SNO-MP were derived from on microbe growth. Nevertheless, it is important to mention that although the blank particles promoted fungal growth, the nitrosation process negated this growth effect and instead resulted in fungal death.
In conclusion, we have demonstrated that both NAC-SNO-NP and SNO-MP have potent antifungal activity against a variety of pathogenic fungal organisms, such as Candida spp, Sporothrix spp, and C. neoformans. At this stage, these compounds are mainly tested in vitro studies, thus side effects observed in vivo experiments are quite limited. However, other NO generating compounds have been shown to lower blood pressure in mice, which can theoretically translate to our platform as well (Garcia et al., 2008). On the other hand, the NO-releasing compounds’ antifungal effects are quite notable, and this work expands therapeutic potential of these platforms and supports future work to translate these NO-releasing particles from the bench to the bedside.
Highlights.
Nitric oxide-loaded particles have antifungal properties
NO-releasing systems can be associated with nanoparticles and microparticles
The NO-loaded particles are effective against planktonic ascomycetes and basidiomycetes species
Candida albicans biofilm cells are susceptible to the NO-loaded particles
Acknowledgements
This article is part of the “Fungal growth, development, and stress responses” special issue for the XIII International Fungal Biology Conference (IFBC) & IV International Symposium on Fungal Stress (ISFUS), which is supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grant 2021/13614-3 and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) grant 88887.680665/2022-00. J.D.N. and D.Z.M. were partially supported by NIH R21 AI156104-01. R.A.P. was partially supported by Programa Jovens Pesquisadores (INI/Fiocruz), grant number INI-003-FIO-19-2-7, and CAPES 88887.372494/2019-00. L.B.R.S. was supported by BEPE FAPESP 2019/20622-2.
Footnotes
Declarations of interest
The authors have no conflicts to declare.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT author statement.
Sichen Liu: methodology, formal analysis, investigation, writing-original draft, writing – review & editing Daniel Zamith: methodology, visualization, writing – review & editing Rodrigo Almeida-Paes: investigation Leandro Buffoni Roque da Silva: investigation Parimala Nacharaju: resources Joshua D. Nosanchuk: conceptualization, methodology, resources, writing – review & editing, supervision, funding acquisition.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abuzeid WM, Girish VM, Fastenberg JH, Draganski AR, Lee AY, Nosanchuk JD, & Friedman JM (2018, Oct). Nitric oxide-releasing microparticles as a potent antimicrobial therapeutic against chronic rhinosinusitis bacterial isolates. Int Forum Allergy Rhinol, 8(10), 1190–1198. 10.1002/alr.22185 [DOI] [PubMed] [Google Scholar]
- Ahmadi MS, Lee HH, Sanchez DA, Friedman AJ, Tar MT, Davies KP, Nosanchuk JD, & Martinez LR (2016). Sustained Nitric Oxide-Releasing Nanoparticles Induce Cell Death in Candida albicans Yeast and Hyphal Cells, Preventing Biofilm Formation In Vitro and in a Rodent Central Venous Catheter Model. Antimicrobial Agents and Chemotherapy, 60(4), 2185–2194. 10.1128/AAC.02659-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sa’doni H, & Ferro A (2000, May). S-Nitrosothiols: a class of nitric oxide-donor drugs. Clin Sci (Lond), 98(5), 507–520. [PubMed] [Google Scholar]
- Al-Sa’doni HH, & Ferro A (2004, Oct). S-nitrosothiols as nitric oxide-donors: chemistry, biology and possible future therapeutic applications. Curr Med Chem, 11(20), 2679–2690. 10.2174/0929867043364397 [DOI] [PubMed] [Google Scholar]
- Amaral EP, Conceição EL, Costa DL, Rocha MS, Marinho JM, Cordeiro-Santos M, D’Império-Lima MR, Barbosa T, Sher A, & Andrade BB (2016, 2016/10/28). N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiology, 16(1), 251. 10.1186/s12866-016-0872-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrillaga-Moncrieff I, Capilla J, Mayayo E, Marimon R, Mariné M, Gené J, Cano J, & Guarro J (2009, Jul). Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect, 15(7), 651–655. 10.1111/j.1469-0691.2009.02824.x [DOI] [PubMed] [Google Scholar]
- Barros MB, de Almeida Paes R, & Schubach AO (2011, Oct). Sporothrix schenckii and Sporotrichosis. Clin Microbiol Rev, 24(4), 633–654. 10.1128/cmr.00007-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros MB, Schubach Ade O, do Valle AC, Gutierrez Galhardo MC, Conceição-Silva F, Schubach TM, Reis RS, Wanke B, Marzochi KB, & Conceição MJ (2004, Feb 15). Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis, 38(4), 529–535. 10.1086/381200 [DOI] [PubMed] [Google Scholar]
- Berger S, El Chazli Y, Babu AF, & Coste AT (2017, 2017-June-07). Azole Resistance in Aspergillus fumigatus: A Consequence of Antifungal Use in Agriculture? [Mini Review]. Frontiers in microbiology, 8. 10.3389/fmicb.2017.01024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkow EL, & Lockhart SR (2017). Fluconazole resistance in Candida species: a current perspective. Infect Drug Resist, 10, 237–245. 10.2147/idr.S118892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker CJ, Keefer KD, Schaefer DW, & Ashley CS (1982, March 01, 1982). Sol-gel transition in simple silicates. Journal of Non Crystalline Solids, 48, 47. https://ui.adsabs.harvard.edu/abs/1982JNCS...48...47B [Google Scholar]
- Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, & Ghannoum MA (2001). Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. Journal of bacteriology, 183(18), 5385–5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra J, & Mukherjee PK (2015). Candida Biofilms: Development, Architecture, and Resistance. Microbiology spectrum, 3(4), 10.1128/microbiolspec.MB-0020-2015. 10.1128/microbiolspec.MB-0020-2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleare LG LK, Abuzeid WM, Nacharaju P, Friedman JM, Nosanchuk JD. (2020). NO Candida auris: Nitric Oxide in Nanotherapeutics to Combat Emerging Fungal Pathogen Candida auris. Journal of Fungi, 6(2), 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F, Sousa DM, Parreira P, Lamghari M, Gomes P, & Martins MCL (2017, 2017/12/12). N-acetylcysteine-functionalized coating avoids bacterial adhesion and biofilm formation. Scientific Reports, 7(1), 17374. 10.1038/s41598-017-17310-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote MA, & Fang FC (1995). NO inhibitions: antimicrobial properties of nitric oxide. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 21 Suppl 2, S162–S165. 10.1093/clinids/21.supplement_2.s162 [DOI] [PubMed] [Google Scholar]
- Duong HTT, Jung K, Kutty SK, Agustina S, Adnan NNM, Basuki JS, Kumar N, Davis TP, Barraud N, & Boyer C (2014). Nanoparticle (star polymer) delivery of nitric oxide effectively negates Pseudomonas aeruginosa biofilm formation. Biomacromolecules, 15(7), 2583–2589. 10.1021/bm500422v [DOI] [PubMed] [Google Scholar]
- Feldmesser M, Kress Y, Mednick A, & Casadevall A (2000, Dec). The effect of the echinocandin analogue caspofungin on cell wall glucan synthesis by Cryptococcus neoformans. J Infect Dis, 182(6), 1791–1795. 10.1086/317614 [DOI] [PubMed] [Google Scholar]
- Friedman A, Blecher K, Sanchez D, Tuckman-Vernon C, Gialanella P, Friedman JM, Martinez LR, & Nosanchuk JD (2011, May-Jun). Susceptibility of Gram-positive and -negative bacteria to novel nitric oxide-releasing nanoparticle technology. Virulence, 2(3), 217–221. 10.4161/viru.2.3.16161 [DOI] [PubMed] [Google Scholar]
- Friedman AJ, Han G, Navati MS, Chacko M, Gunther L, Alfieri A, & Friedman JM (2008, 2008/08/01/). Sustained release nitric oxide releasing nanoparticles: Characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide, 19(1), 12–20. 10.1016/j.niox.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Garcia JAD, dos Santos L, Moura AL, Ricardo KFS, Wanschel ACB, Shishido SM, Spadari-Bratfisch RC, de Souza HP, & Krieger MH (2008). S-Nitroso-N-Acetylcysteine (SNAC) Prevents Myocardial Alterations in Hypercholesterolemic LDL Receptor Knockout Mice by Antiinflammatory Action. Journal of Cardiovascular Pharmacology, 51(1), 78–85. 10.1097/FJC.0b013e31815c39d4 [DOI] [PubMed] [Google Scholar]
- Ghosn E, Russo M, & Almeida S (2006, 08/01). Nitric oxide-dependent killing of Cryptococcus neoformans by B-1-derived mononuclear phagocyte. Journal of leukocyte biology, 80, 36–44. 10.1189/jlb.1005603 [DOI] [PubMed] [Google Scholar]
- Girish VM, Liang H, Aguilan JT, Nosanchuk JD, Friedman JM, & Nacharaju P (2019, 2019/08/01/). Anti-biofilm activity of garlic extract loaded nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine, 20, 102009. 10.1016/j.nano.2019.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G, Martinez LR, Mihu MR, Friedman AJ, Friedman JM, & Nosanchuk JD (2009). Nitric oxide releasing nanoparticles are therapeutic for Staphylococcus aureus abscesses in a murine model of infection. PloS one, 4(11), e7804–e7804. 10.1371/journal.pone.0007804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpaz R, Dahl R, & Dooling K (2016). The Prevalence of Immunocompromised Adults: United States, 2013. Open Forum Infectious Diseases, 3(suppl_1). 10.1093/ofid/ofw172.1141 [DOI] [Google Scholar]
- Hornyák I, Marosi K, Kiss L, Gróf P, & Lacza Z (2012, Feb). Increased stability of S-nitrosothiol solutions via pH modulations. Free Radic Res, 46(2), 214–225. 10.3109/10715762.2011.647692 [DOI] [PubMed] [Google Scholar]
- Hu TM, & Chou TC (2006, Jul 28). The kinetics of thiol-mediated decomposition of S-nitrosothiols. Aaps j, 8(3), E485–492. 10.1208/aapsj080357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman CA, Bustamante B, Chapman SW, & Pappas PG (2007). Clinical Practice Guidelines for the Management of Sporotrichosis: 2007 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases, 45(10), 1255–1265. 10.1086/522765 [DOI] [PubMed] [Google Scholar]
- Kullberg BJ, & Arendrup MC (2015). Invasive Candidiasis. New England Journal of Medicine, 373(15), 1445–1456. 10.1056/NEJMra1315399 [DOI] [PubMed] [Google Scholar]
- Landriscina A, Musaev T, Rosen J, Ray A, Nacharaju P, Nosanchuk JD, & Friedman AJ (2015, Jul). N-acetylcysteine S-nitrosothiol Nanoparticles Prevent Wound Expansion and Accelerate Wound Closure in a Murine Burn Model. J Drugs Dermatol, 14(7), 726–732. [PubMed] [Google Scholar]
- Lattif AA, Mukherjee PK, Chandra J, Swindell K, Lockhart SR, Diekema DJ, Pfaller MA, & Ghannoum MA (2010). Characterization of biofilms formed by Candida parapsilosis, C. metapsilosis, and C. orthopsilosis. International Journal of Medical Microbiology, 300(4), 265–270. [DOI] [PubMed] [Google Scholar]
- Li KL, Miranda DZ, Cleare LG, Akbar NA, Friedman JM, Draganski A, Nosanchuk JD, & Abuzeid WM (2022, Oct 8). Nitric oxide-generating microparticles: An in vitro evaluation of anti-biofilm efficacy and sinonasal epithelial cell cytotoxicity. Int Forum Allergy Rhinol. 10.1002/alr.23096 [DOI] [PubMed] [Google Scholar]
- Liu S, & Nosanchuk JD (2020). Fungal Cardiac Infections. In Reference Module in Life Sciences Elsevier. 10.1016/B978-0-12-809633-8.21020-0 [DOI] [Google Scholar]
- Macherla C, Sanchez DA, Ahmadi MS, Vellozzi EM, Friedman AJ, Nosanchuk JD, & Martinez LR (2012). Nitric oxide releasing nanoparticles for treatment of Candida albicans burn infections. Frontiers in microbiology, 3, 193–193. 10.3389/fmicb.2012.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marimon R, Cano J, Gené J, Sutton DA, Kawasaki M, & Guarro J (2007). Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. Journal of clinical microbiology, 45(10), 3198–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, Friedman AJ, Nosanchuk JD, & Friedman JM (2009, 2009/10/01/). Antimicrobial and Healing Efficacy of Sustained Release Nitric Oxide Nanoparticles Against Staphylococcus Aureus Skin Infection. Journal of Investigative Dermatology, 129(10), 2463–2469. 10.1038/jid.2009.95 [DOI] [PubMed] [Google Scholar]
- Mihu MR, Cabral V, Pattabhi R, Tar MT, Davies KP, Friedman AJ, Martinez LR, & Nosanchuk JD (2016). Sustained Nitric Oxide-Releasing Nanoparticles Interfere with Methicillin-Resistant Staphylococcus aureus Adhesion and Biofilm Formation in a Rat Central Venous Catheter Model. Antimicrobial Agents and Chemotherapy, 61(1), e02020–02016. 10.1128/AAC.02020-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, & Martinez LR (2010, Mar-Apr). The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence, 1(2), 62–67. 10.4161/viru.1.2.10038 [DOI] [PubMed] [Google Scholar]
- Moon J-H, Choi Y-S, Lee H-W, Heo JS, Chang SW, & Lee J-Y (2016). Antibacterial effects of N-acetylcysteine against endodontic pathogens. Journal of microbiology (Seoul, Korea), 54(4), 322–329. 10.1007/s12275-016-5534-9 [DOI] [PubMed] [Google Scholar]
- Morgan J, Meltzer MI, Plikaytis BD, Sofair AN, Huie-White S, Wilcox S, Harrison LH, Seaberg EC, Hajjeh RA, & Teutsch SM (2005, Jun). Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect Control Hosp Epidemiol, 26(6), 540–547. 10.1086/502581 [DOI] [PubMed] [Google Scholar]
- Mpoza E, Rhein J, & Abassi M (2017). Emerging fluconazole resistance: Implications for the management of cryptococcal meningitis. Medical mycology case reports, 19, 30–32. 10.1016/j.mmcr.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacharaju P, Tuckman-Vernon C, Maier KE, Chouake J, Friedman A, Cabrales P, & Friedman JM (2012). A nanoparticle delivery vehicle for S-nitroso-N-acetyl cysteine: sustained vascular response. Nitric oxide : biology and chemistry, 27(3), 150–160. 10.1016/j.niox.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O W Griffith a., & Stuehr DJ. (1995). Nitric Oxide Synthases: Properties and Catalytic Mechanism. Annual Review of Physiology, 57(1), 707–734. 10.1146/annurev.ph.57.030195.003423 [DOI] [PubMed] [Google Scholar]
- Parry MF, & Neu HC (1977). Effect of N-acetylcysteine on antibiotic activity and bacterial growth in vitro. Journal of clinical microbiology, 5(1), 58–61. https://pubmed.ncbi.nlm.nih.gov/401831 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC274532/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O, Nguyen M-H, Pappas PG, Powderly WG, Singh N, Sobel JD, & Sorrell TC (2010). Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clinical Infectious Diseases, 50(3), 291–322. 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson G, & Benjamin N (2002, Jan). Potential therapeutic uses for S-nitrosothiols. Clin Sci (Lond), 102(1), 99–105. [PubMed] [Google Scholar]
- Sanchotene KO, Madrid IM, Klafke GB, Bergamashi M, Della Terra PP, Rodrigues AM, de Camargo ZP, & Xavier MO (2015, Nov). Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses, 58(11), 652–658. 10.1111/myc.12414 [DOI] [PubMed] [Google Scholar]
- Schubach A, Barros MB, & Wanke B (2008, Apr). Epidemic sporotrichosis. Curr Opin Infect Dis, 21(2), 129–133. [DOI] [PubMed] [Google Scholar]
- Waller SB, Dalla Lana DF, Quatrin PM, Ferreira MRA, Fuentefria AM, & Mezzari A (2021, Mar). Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol, 52(1), 73–80. 10.1007/s42770-020-00307-z [DOI] [PMC free article] [PubMed] [Google Scholar]


