Abstract
Aim
Animal models of Extracorporeal Cardiopulmonary Resuscitation (ECPR) focusing on neurological outcomes are required to further the development of this potentially life-saving technology. The aim of this review is to summarize current animal models of ECPR.
Methods
A comprehensive database search of PubMed, EMBASE, and Web of Science was undertaken. Full-text publications describing animal models of ECPR between January 1, 2000, and June 30, 2022, were identified and included in the review. Data describing the conduct of the animal models of ECPR, measured variables, and outcomes were extracted according to pre-defined definitions.
Results
The search strategy yielded 805 unique reports of which 37 studies were included in the final analysis. Most studies (95%) described using a pig model of ECPR with the remainder (5%) describing a rat model. The most common method for induction of cardiac arrest was a fatal ventricular arrhythmia through electrical stimulation (70%). 10 studies reported neurological assessment of animals using physical examination, serum biomarkers, or electrophysiological findings, however, only two studies described a multimodal assessment. No studies reported the use of brain imaging as part of the neurological assessment. Return of spontaneous circulation was the most reported primary outcome, and no studies described the neurological status of the animal as the primary outcome.
Conclusion
Current animal models of ECPR do not describe clinically relevant neurological outcomes after cardiac arrest. Further work is needed to develop models that more accurately mimic clinical scenarios and can test innovations that can be translated to the application of ECPR in clinical medicine.
Keywords: Extracorporeal cardiopulmonary resuscitation, Cardiac arrest, Animal model, Neurological assessment, Scoping review
Introduction
Extracorporeal cardiopulmonary resuscitation (ECPR) is the rapid deployment of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) during refractory cardiac arrest.1, 2, 3, 4, 5, 6 Current survival rates for out-of-hospital cardiac arrest (OHCA) are low, with approximately 10% of patients surviving to hospital discharge.7, 8, 9, 10 Studies of ECPR for OHCA, using highly organised systems, have produced survival rates of around 30% in selected patients.1, 2 Resuscitation guidelines now recommend considering ECPR as a salvage therapy and are likely to lead to increased utilization of this technology in the future.11, 12
Neurological impairment after cardiac arrest directly correlates with disability after hospital discharge and places significant burdens on patients, families, and the health care system.7, 8, 9, 13, 14 The importance of investigating the neurological outcomes of cardiopulmonary resuscitation (CPR) and ECPR has been extensively discussed,3, 15, 16 however, interventions to improve survival after cardiac arrest have yet to translate into improved neurological outcomes.3, 4, 5, 6
The optimal application of ECPR to improve neurological outcomes after cardiac arrest requires further investigation. Conducting clinical studies of ECPR is difficult due to significant practical and ethical barriers, and only a few clinical trials of ECPR have been successfully completed.17 In this context, an appropriate animal model of ECPR is essential to advancing its clinical application. These models can provide insights into the pathophysiology of neurological and other organ injuries sustained during cardiac arrest, help investigate the effects of new therapies, and inform the design of future clinical trials facilitating the development of effective treatments to improve neurological outcomes in ECPR patients.18 The clinical relevance of current animal models of ECPR, and their ability to assess neurological injury and detect the effects of interventions to improve neurological outcomes remains unclear.19, 20 The aim of this scoping review is to summarize and compare current animal models of ECPR and to report their methodology and assessment of neurological outcomes.
Materials and Methods
Design
This scoping review was performed following the requirements of the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) (Supplemental Fig. 1).21
Eligibility criteria
An ECPR animal model was defined as: An animal model of cardiac arrest in which there was intentional induction of cardiac arrest with subsequent extracorporeal membrane oxygenation with or without CPR including manual or mechanical chest compressions. Cardiac arrest was defined as the appearance of ventricular fibrillation, pulseless electrical activity, or asystole on the electrocardiogram, and the simultaneous absence of fluctuation in arterial pressure.
This scoping review comprised studies of all types which matched the following PICO approach: (P) population: defined as all animal models with cardiac arrest; (I) intervention: defined as animals cannulated to ECMO during cardiac arrest; (C) controls: defined as animals compared to the intervention group during cardiac arrest regardless of ECMO use; (O) outcomes: defined as any outcome.
The International Liaison Committee on Resuscitation guidelines first proposing the concept of time-flow in the management of resuscitation were published in the year 2000, and in vivo studies of all types, prognostic, interventional, or pathophysiological were included from that year.22 The search was not restricted by the publication language. Case reports, reviews, letters, commentaries, editorials, comments, and solitary abstracts were excluded. Additionally, animal models utilising ECMO configurations or therapeutic interventions that are not used clinically for ECPR such as: central ECMO by median sternotomy; arterial return via cannulation of the carotid artery with the associated increased the risk of stroke23; the use of two return cannulae at the initiation of ECMO; the use of cardiopulmonary bypass as used for cardiovascular surgery but not for cardiac arrest; or the use of temperature management with extracorporeal cooling prior to the start of oxygenation via the membrane oxygenator, were also excluded.
Search strategy and selection process
Considering that ECPR is a relatively new technique, with a rapid increase in the number of ECPR patients since the early 2000s,24, 25 the search period was limited to the last 20 years. PubMed, Excerpta Medica dataBASE (EMBASE), and Web of Science databases were searched for articles reporting animal models of ECPR from January 1st, 2000, to June 30th, 2022. The search strategy was designed in conjunction with trained medical librarians and contained keywords relevant to cardiac arrest and ECPR. The full search strategy is provided in Supplemental Figs. 2–4.
Two independent reviewers (SI and SR) initially screened articles based on their titles and abstracts. Following the initial screening, relevant full-text articles identified were reviewed by the two independent reviewers (SI and SR). Any disagreement regarding the inclusion or exclusion of an article was resolved by discussion with a 3rd independent reviewer (KL).
Data collection
Data extracted from the selected papers were classified into three categories: 1) Characteristics of an ECPR animal model; 2) Primary and secondary outcomes measured in the study; 3) Details of neurological assessment.
Characteristics of an ECPR animal model included the following: the year of publication; number of animals used in the study; sex; weight; the method of induction of cardiac arrest; the time course of cardiac arrest including: the duration of cardiac arrest without CPR or ECMO support (no flow time), and the duration from the initiation of CPR to the start of ECMO support (low flow time); the method of CPR; the observation period, defined as: the duration from induction of cardiac arrest to the end of the experiment; ECMO period, defined as: the duration from the start to the end of ECMO; and targeted temperature management (TTM) after the start of ECMO.
Primary and secondary outcomes included: the return of spontaneous circulation (ROSC); survival rate; imaging assessment; successful weaning rate from ECMO; physical assessment; haemodynamics; physiological assessment such as: echocardiography, coronary blood flow measurement, regional O2 saturation, electroencephalogram recording, and microcirculation assessment; biomarkers of tissues injury, inflammation and neuronal injury; neurological assessment; and histological findings.
Details of neurological assessments extracted included: physical assessment; measurement of biomarkers of neuronal injury; physiological assessments such as electroencephalogram, somatosensory evoked potential, and brain regional oxygen saturation recordings; brain imaging including: computed tomography, and magnetic resonance imaging. The selection of these neurological assessment parameters was based on their reported use for prognostic neurological assessment after cardiac arrest in recent publications.26, 27, 28
Information on procedures during the experiments such as anesthesia and ventilation strategy, and ECMO management is provided in Supplemental Tables 1 and 2.
Studies were assessed for compliance with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines which provide specific recommendations for methodology and results in animal studies (Supplemental Tables 3 and 4).29
Data synthesis and analysis
Continuous variables were presented as median and interquartile range (IQR), whereas categorical variables were presented as numbers and percentages. In addition, the extracted data were categorized by animal species. Statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan).30
Results
Study selection
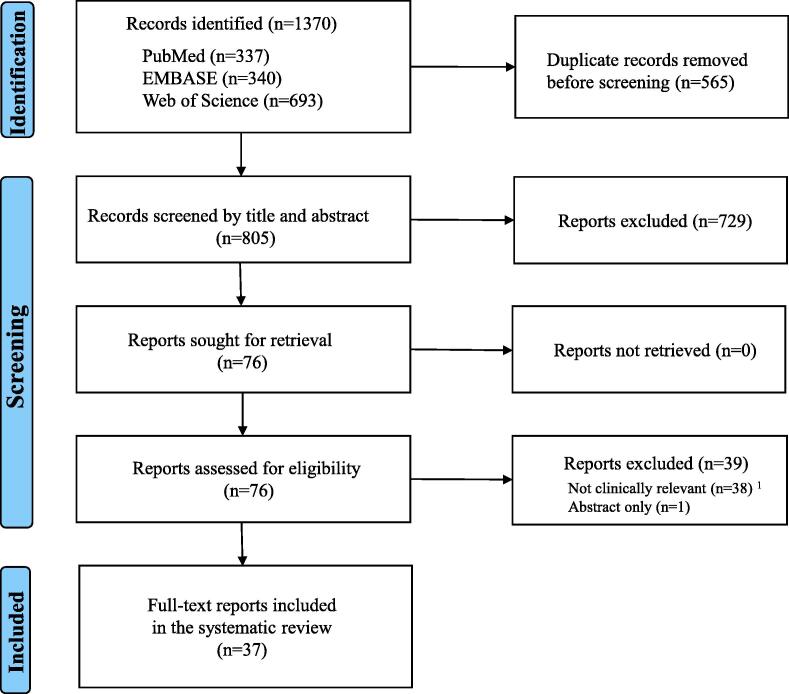
A total of 1370 studies were retrieved from PubMed, EMBASE, and Web of Science. After excluding duplicates, 805 studies were screened by titles and abstracts. Of these, 76 studies passed the first screening and had full-text articles retrieved. Following secondary screening, 39 studies were excluded: 38 were excluded because the experimental configuration or conduct of ECMO was not considered clinically relevant to ECPR, and one study was excluded as it was only reported in abstract form. The remaining 37 studies were included in the final analysis. Fig. 1 shows the PRISMA flow diagram for study inclusion and exclusion. Compliance with the Essential 10 set of the ARRIVE guidelines for manuscripts describing animal research was found to be poor, with calculation of sample size, exclusion criteria and reason for exclusion, blinding, and experimental procedure (when, where, and why) frequently unreported.
Fig. 1.
Flow chart of study selection,1 Central ECMO: 4, Configuration: 13 (cannulation of carotid artery: 7, direct cannulation to right atrium:5, two return cannulae used: 1), Cardiopulmonary bypass use including reservoir: 19, the use of temperature management with extracorporeal cooling prior to the start of oxygenation via the membrane oxygenator: 2, ECMO: extracorporeal membrane oxygenation.
Animal characteristics
Characteristics of animal ECPR models are summarized in Table 1. All included studies were published from 2012 onwards. The majority of studies reported using a pig model (95%), and the remainder reported using a rat model (5%) of ECPR. The median number of animals used per study, intervention group, and control group, was 14 (IQR: 10–18), 8 (6–10), and 8 (6–10) respectively, with the median weight of animal being 48 kg in the pig models (IQR: 35–53).
Table 1.
Characteristics of an animal model of Extracorporeal Cardiopulmonary Resuscitation.
| Variables |
Total (N = 37) |
Pig (N = 35) |
Rat (N = 2) |
|---|---|---|---|
| Year of publication | |||
| 2021∼2022 | 11 (29) | 10 (29) | 1 (50) |
| 2016∼2020 | 18 (49) | 17 (48) | 1 (50) |
| 2011∼2015 | 8 (22) | 8 (23) | 0 (0) |
| 2000∼2010 | 0 (0) | 0 (0) | 0 (0) |
| Number of animals used in the article | 14 [10–18] | 14 [9–18] | 15 [16–17] |
| Sex | |||
| Male | 6 (16) | 5 (14) | 1 (50) |
| Female | 10 (27) | 10 (29) | 0 (0) |
| Both | 5 (14) | 5 (14) | 0 (0) |
| Not reported | 16 (43) | 15 (43) | 1 (50) |
| Weight (kg) 1 | 48 [35–53] | 48 [37–54] | 0.45 [0.43–0.48] |
| Induction of cardiac arrest | |||
| Electrical stimulation | 26 (70) | 26 (74) | 0 (0) |
| Occlusion of coronary artery + Electrical stimulation | 3 (8) | 3 (8) | 0 (0) |
| Asphyxia | 5 (14) | 3 (8) | 2 (100) |
| Hypothermia | 2 (5) | 2 (6) | 0 (0) |
| Administration of potassium | 1 (3) | 1 (3) | 0 (0) |
| Time course of cardiopulmonary arrest (min) | |||
| No flow time 2 | 10 [5–15] | 10 [5–15] | 13 [10–17] |
| Low flow time 3 | 0 [0–6] | 0 [0–6] | 0 [0–0] |
| Method of cardiopulmonary resuscitation | |||
| Manual chest compression by hand | 3 (8) | 3 (9) | 0 (0) |
| Mechanical chest compression with device | 13 (35) | 13 (37) | 0 (0) |
| No cardiopulmonary resuscitation 4 | 20 (54) | 18 (51) | 2 (100) |
| Not reported | 1 (3) | 1 (3) | 0 (0) |
| Observation period from induction of cardiac arrest tothe end of the experiment (minutes) |
180 [100–345] | 180 [95–352] | 210 [195–225] |
| ECMO period from the start to the end of ECMO | 174 [60–300] | 150 [60–300] | 197 [185–208] |
| Temperature targeted management 5 | 17 (46) | ||
| Normothermia (from 35 to 38 °C) | 11 (30) | 10 (29) | 1 (50) |
| Hypothermia (from 32 to 35 °C) | 6 (16) | 6 (17) | 0 (0) |
| Not reported | 20 (54) | 19 (54) | 1 (50) |
Data are presented as median [interquartile range] and as number (percentage).
ECMO: extracorporeal membrane oxygenation.
Not reported in two pig studies.
No-flow time was defined as the duration of cardiac arrest without cardiopulmonary resuscitation (CPR) or extracorporeal membrane oxygenation (ECMO) support.
Low-flow time was defined as the duration from the initiation of CPR to the start of ECMO circulation.
No CPR means that ECMO was already set up and started after no-flow time.
Two of 17 articles are evaluated by comparing hypothermia (33 °C) using ECMO and normothermia (37 °C), and these articles were included in the hypothermia group.
Methods used to induce cardiac arrest are shown in Table 1 and Fig. 2. Of the studies using a pig model, 26 (74%) induced cardiac arrest by electrical stimulation using pacing. Three studies performed percutaneous coronary balloon occlusion of a coronary artery prior to electrical stimulation. The position of balloon occlusion was the ostial left anterior descending branch in one study; the proximal left circumflex artery in one study; and not reported in the remaining study. Other methods of inducing cardiac arrest included: asphyxia; hypothermia using external cold packs; and administration of intravenous potassium. In all (n = 2) studies reporting the use of a rat model, cardiac arrest was induced by asphyxia.
Fig. 2.
Methods used to induce cardiac arrest in animal models of extracorporeal cardiopulmonary resuscitation,1 Two articles reported percutaneous acute coronary occlusion using a balloon before electrical pacing (one reported balloon occlusion of the ostial branch of the left anterior descending artery, and the other reported balloon occlusion of the proximal left circumflex artery).
The median no-flow time was 10 min (IQR: 5–15), and the median low flow time was 0 min (IQR: 0–6). Four studies (11%) did not report no-flow times and 20 studies (54%) did not report low flow times.
Chest compressions were performed in 16 studies (46%), all of which used pigs as the animal model. Chest compressions were manually performed in three studies (8%), while the use of a mechanical chest compression device, such as LUCAS (Physio-Control/ Jolife AB, Lund, Sweden), was reported in 13 studies (35%).31, 32 In 20 studies (54%), no CPR was performed.
The median observation period was 180 minutes (IQR: 100–345) and the median ECMO period was 174 minutes (IQR: 60–300). 17 studies (46%) performed TTM as post-cardiac arrest care, of which 11 studies (30%) targeted normothermia and 6 (16%) targeted therapeutic hypothermia.
With respect to ECMO management, cannulation for drainage was via the femoral vein (50%) or the jugular vein (50%), however all return cannulae were inserted in a femoral artery. 35 studies (95%) used an intravenous infusion of heparin as anti-coagulation (Supplemental Table 1). Additional information on details of ECMO management, and anesthesia and ventilation strategy are provided in Supplemental Tables 1 and 2.
Outcome
Table 2 shows the details of reported outcomes. Of the 37 studies, seven studies (19%) specified a primary outcome including: rate of ROSC (n = 5: 14%); survival rate (n = 1: 3%); and cardiac magnetic resonance imaging (MRI) findings (n = 1: 3%). A number of other experimental endpoints were reported, and of these ROSC rate (n = 10: 27%), and haemodynamic measures such as blood pressure, heart rate, central venous pressure, pulmonary artery pressure, and cardiac output (n = 15: 40%) were most commonly reported.
Table 2.
Outcomes.
| Variables |
Total (N = 37) |
Pig (N = 35) |
Rat (N = 2) |
|---|---|---|---|
| Primary outcome | |||
| ROSC rate | 5 (13) | 5 (14) | 0 (0) |
| Survival rate | 1 (3) | 1 (3) | 0 (0) |
| Imaging (Cardiac MRI) | 1 (3) | 1 (3) | 0 (0) |
| Primary outcome was not clearly mentioned | 30 (81) | 28 (80) | 2 (100) |
| Other outcomes | |||
| ROSC rate | 10 (27) | 8 (23) | 2 (100) |
| Survival rate | 2 (5) | 1 (3) | 1 (50) |
| Successful weaning rate from ECMO | 3 (8) | 3 (9) | 0 (0) |
| Haemodynamics 1 | 15 (40) | 14 (40) | 1 (50) |
| Physiological assessment | |||
| Echocardiography 2 | 4 (11) | 4 (11) | 0 (0) |
| Coronary blood flow | 3 (8) | 3 (9) | 0 (0)- |
| Carotid blood flow | 2 (5) | 2 (6) | 0 (0) |
| Regional O2 saturation | 3 (8) | 2 (6) | 1 (50) |
| Microcirculation 3 | 1 (3) | 0 (0)- | 1 (50) |
| Imaging (cardiac MRI and left ventriculography) | 2 (5) | 2 (6) | 0 (0) |
| Biomarker 4 | 6 (16) | 5 (14) | 1 (50) |
| Neurological assessment | 15 (40) | 13 (37) | 2 (100) |
| Histology 5 | 12 (32) | 11 (30) | 1 (50) |
ROSC: return of spontaneous circulation, MRI: magnetic resonance imaging, ECMO: extracorporeal membrane oxygenation.
Data are presented as median [interquartile range] or number (percentage).
Haemodynamics include such as blood pressure, heart rate, central venous pressure, pulmonary artery pressure, and cardiac output.
Echocardiography was assessed with ventricular ejection fraction, cardiac output.
Microcirculation was assessed with mesenteric blood flow.
Biomarkers include High mortality group box-1 (HMGB-1), Neutrophil gelatinase-associated lipocalin (NGAL), and pulmonary surfactant protein.
Histological assessments include brain (5/15, 33%), lung (1/15, 7%), heart (4/15, 27%), and kidney (5/15, 33%).
Details of neurological outcomes
A total of 10 studies (27%) reported neurological assessment (Table 3). Three studies (8%) reported physical neurological assessment using a neurological deficit scoring (NDS) system. The NDS assessed level of consciousness, respiratory pattern, and cranial nerve function (scored from 0% (normal) to 100% (brain dead),33 or the response to the toe pinch and corneal reflex. Several studies reported the measurement of biomarkers of neuronal injury including: neuron-specific enolase (NSE) (n = 5: 14%) and S-100B (n = 1: 3%). Only one study reported two types of biomarkers. Electroencephalogram (n = 2: 5%), somatosensory evoked potentials (n = 1: 3%), and brain regional oxygen saturation (rSO2) (n = 3: 8%) were reported as electrophysiological assessments. There were no reports of brain imaging. Multimodal neurological assessment was reported in four studies and included: physical assessment and biomarker; physical assessment and EEG; and biomarker and brain rSO2. The time points at which neurological assessments were undertaken are shown in Supplemental Table 5.
Table 3.
Details of neurological assessments.
| Neurological variables |
Total (N = 37) |
Pig (N = 35) |
Rat (N = 2) |
|---|---|---|---|
| Physical assessment 1 | 3 (8) | 2 (6) | 1 (50) |
| Biomarker 2 | |||
| Neuron-specific enolase (NSE) | 5 (14) | 5 (14) | 0 (0) |
| S-100B | 1 (3) | 1 (3) | 0 (0) |
| Neurofilament light chain (NFL) | 0 (0) | 0 (0) | 0 (0) |
| Glial fibrillary acidic protein (GLAP) | 0 (0) | 0 (0) | 0 (0) |
| Tau protein | 0 (0) | 0 (0) | 0 (0) |
| Ubiquitin carboxy-terminal hydrolase-L1 (UCH-L1) | 0 (0) | 0 (0) | 0 (0) |
| Electrophysiological assessment | |||
| Electroencephalogram (EEG) | 2 (5) | 1 (3) | 1 (50) |
| Somatosensory evoked potentials (SSEPs) | 1 (3) | 1 (3) | 0 (0) |
| Brain regional oxygen saturation (rSO2) | 3 (8) | 3 (8) | 0 (0) |
| Imaging (Brain) | |||
| Computed Tomography (CT) | 0 (0) | 0 (0) | 0 (0) |
| Magnetic Resonance Imaging (MRI) | 0 (0) | 0 (0) | 0 (0) |
| Multimodal 2 | 4 (10) | 3 (3) | 1 (50) |
Data are presented as median [interquartile range] and as number (percentage).
3 Multimodal includes; Physical assessment + Biomarker: 2, Physical assessment + EEG: 1, Biomarker + rSO2: 1.
Physical assessment was evaluated by neurological deficit scoring (NDS) system or the response to the toe pinch and corneal reflex. (NDS assessed by level of consciousness, respiratory pattern, and cranial nerve function and scored from 0% (normal) to 100% (brain dead). Forbess JM, et al. J Thorac Cardiovasc Surg 2010;139(5): 1325–32).
One study reported both NSE and S-100B.
Discussion
In this scoping review, we have provided a comprehensive overview of reported preclinical models of ECPR. The main findings of the pooled data can be summarized as follows: (1) neurological outcomes have been overlooked, or inadequately and inconsistently assessed, and poorly reported; (2) the most popular method for inducing cardiac arrest, fatal arrhythmia by electrical stimulation using pacing, is not representative of the primary cause of cardiac arrest in a public setting; (3) ECMO management that does not reflect clinical practice occurs often in published animal models. From these results, it appears that there is currently no appropriate animal model for ECPR that mimics the clinical application of this resuscitation technique.
Primary outcome
In this review, no animal models of ECPR have reported neurological outcomes as the primary outcome. The importance of neurological outcomes as the primary endpoint for resuscitation after cardiac arrest has been discussed in several articles.3, 15, 16, 34 Unfavourable neurological outcomes directly correlate with disability after hospital discharge, and cause significant burdens on patients, families, and the health care system.7, 8, 9, 13, 14 High-quality post-resuscitation care, including TTM which avoids hypertension in acute phase contribute to the improved neurological outcomes of survivors after cardiac arrest.26, 27, 28, 35 In addition, the prediction of neurological outcomes is based on multi-modal assessments incorporating the findings of clinical examination, biomarkers, electrophysiological assessment (EEG and SSEPs), and brain imaging such as CT or MRI.25, 26, 27
In a systematic review of animal models of cardiac arrest, it was observed that neurological outcomes, evaluated by brain histology, imaging, and electrophysiology, were rarely used in models with a short observation period (<24 h) when compared to models with a long observation period (>24 h).36 In this review of animal models of ECPR, the median observation time was 180 min, only three studies observed the animals for more than 24 h. Neuronal injuries associated with ischaemia after cardiac arrest require time to become established, and for the findings of investigations to correlate with neurological outcome.26, 27, 28 Clinical neurological assessment such as brain imaging, biomarker evaluation (e.g. NSE, S-100B), and electrophysiological assessments (EEG, SSEPs, and rSO2) are commonly performed from 24 to 72 h after cardiac arrest26, 27, 28 (Supplemental Fig. 5). Therefore, there is a significant gap in current ECPR animal models, such that their timeframes do not allow for adequate assessment of neurological outcomes and do not reflect assessments undertaken clinically in humans. While it is acknowledged that prolonged cardiac arrest animal models with high-quality, post-resuscitation care are difficult, resource intensive, and expensive to perform, they are necessary in order for the animal models to be clinically relevant to neurological outcomes. Given that a significant proportion of patients undergoing ECPR suffer unfavourable neurological outcomes,3, 4, 5, 6 it is important for animal models to have extended observation periods so that appropriate neurological evaluation can be incorporated. As the preclinical model of cardiac arrest with long observation periods including post-resuscitation care without ECMO has been reported,37, 38, 39 similar observations are expected for an ECPR model.
Method for inducing cardiac arrest
Fatal arrhythmia induced by electrical stimulation using pacing was the most common method utilized in animal models of ECPR. Induction of cardiac arrest by coronary artery occlusion has only been reported in 2% of non-ECPR animal models of cardiac arrest,41, 42 and in only 3 (8%) of the ECPR animal models identified in this review. This differs from the observed aetiology of sudden cardiac death in out of hospital cardiac arrest where acute coronary artery occlusion leading to myocardial infarction is most common.10, 11, 12
The method for inducing cardiac arrest may be significant to the neurological outcome. Delayed coronary reperfusion and severely unstable haemodynamics following cardiac arrest due to an acute coronary artery occlusion may exacerbate myocardial and systemic inflammation,19, 40 with the systemic inflammatory response potentially worsening neurological injury. ECPR models using electrical stimulation to induce cardiac arrest may not elicit the same systemic inflammatory response as models using coronary artery occlusion, therefore affecting the degree of neurological injury observed.
In animal models of cardiac arrest, the method for inducing cardiac arrest varies depending on the type of animal.36 It has been reported that electrical stimulation using pacing is the most popular method in pig models because this method is technically easy and does not affect the other findings such as electrolyte levels unlike drug use.36, 43 On the other hand, in most rat studies, cardiac arrest is induced by a bolus of potassium and asphyxia.36 While this is an easy method for induction of cardiac arrest, and can allow for the assessment of post-cardiac arrest syndromes such as ischaemia–reperfusion injury, its clinical applicability may be limited.
Management of ECMO
In this review, there are some important differences in the management of animals on ECMO compared to the clinical situation. Firstly, in humans it is recommended that the no-flow time should be less than 5 min prior to commencing CPR, and in addition, it is reasonable to consider commencing ECMO cannulation after 10–20 min of failed CPR.12, 35 Although low flow time has been reported from 50 to 60 min in the real world,6 in this review only two studies reported more than 45 min of low flow time. Supplemental Fig. 6 shows the relationship between the time course and survival rate.
In preclinical studies, a high survival rate is required to investigate the neurological outcome of any intervention. Previous experiments using pig models of cardiac arrest have reported that an 8-minute no-flow time resulted in a 30–40% animal death rate within 60 h of post-resuscitation care,44 while a 6-minute no-flow time did not cause any mortality.45 Other experiments have shown that the low flow time in animal models of conventional cardiac arrest should be at least 12 min to get relevant neurological injury46 and that pig models with 9 minutes of no-flow and 8 min of low-flow time induced only mild neurological injury.37 Considering these results, the optimal no-flow and low-flow times are unclear. It is likely that a minimum of 10–15 min each of no-flow time and low-flow time will be required to reproducibly induce an assessable degree of neurological injury, with the time course being titrated to produce the extent of neurological injury that is sought for each experimental set-up.
Secondly, ECPR is initiated in the clinical setting for refractory cardiac arrest, and it is recommended to consider the start of ECMO cannulation after 10–20 min for patients with refractory CPA.12 In no clinical situation is the low flow time zero, since there is always an attempt at CPR prior to the decision to initiate ECPR, however in 20 reports of animal models of ECPR, the low flow time was set to zero minutes.
Thirdly, the duration of ECMO in animal models is short when compared with clinical situations. Post-resuscitation care usually involves patient management for 72 h or more.26, 27 However, in reported animal models of ECPR, the mean time of ECMO management was 174 min making it difficult to apply these models to the clinical situation.
Limitations
Our study has several limitations. Firstly, data extraction into pre-defined categories may result in a simplification of the data presented in the studies. Secondly, this study did not include formal risk of bias assessments because of the scoping nature of this study. Thirdly, the summary category of all studies may be biased within some variables as not all procedures can be performed in small animals (e.g. measurement of coronary blood flow, basic life support). Fourthly, the study did not include an assessment of the methodological quality in the individual studies. Lastly in the study, we defined adult animals not categorized as pediatric. Hence the age span of the adult group is wide and some of the animals may not be regarded at true adults according to their biological development.
Conclusions
In this scoping review, we provided an overview of preclinical models of ECPR. Current animal models of ECPR fail to mimic the clinical situation of human ECPR and do not include clinically relevant neurological assessments or outcomes. Further work is required to develop a standardized animal model of ECPR for cardiac arrest that can be used to test innovations in this field that may translate into improved neurological outcomes for ECPR patients.
CRediT authorship contribution statement
Shinichi Ijuin: Investigation, Writing – original draft. Keibun Liu: Conceptualization, Investigation, Supervision, Writing – review & editing. Denzil Gill: Investigation, Supervision, Writing – review & editing. Sun Kyun Ro: Investigation, Supervision. Jana Vukovic: Investigation, Supervision. Satoshi Ishihara: Investigation, Supervision. Jan Belohlavek: Investigation, Supervision. Gianluigi Li Bassi: Investigation, Supervision. Jacky Y Suen: Conceptualization, Investigation, Supervision. John F Fraser: Conceptualization, Investigation, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to acknowledge Janine Brookes and Jana Waldmann in The Prince Charles Hospital Library for study selection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2023.100426.
Contributor Information
Shinichi Ijuin, Email: shinichiijuin821@gmail.com.
John F Fraser, Email: fraserjohn001@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sakamoto T., Morimura N., Nagao K., et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults out-of-hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85:762–768. doi: 10.1016/j.resuscitation.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 2.Holmberg M.J., Granfeldt A., Guerguerian A.M., et al. Extracorporeal cardiopulmonary resuscitation for cardiac arrest: An updated systematic review. Resuscitation. 2023;182 doi: 10.1016/j.resuscitation.2022.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Bartos J.A., Grunau B., Carlson C., et al. Improved survival with extracorporeal cardiopulmonary resuscitation despite progressive metabolic derangement associated with prolonged resuscitation. Circulation. 2020;141:877–886. doi: 10.1161/CIRCULATIONAHA.119.042173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakashima T., Noguchi T., Tahara Y., et al. Patients with refractory out-of-cardiac arrest and sustained ventricular fibrillation as candidates for extracorporeal cardiopulmonary resuscitation-prospective multi-center observational study. Circ J. 2019;83:1011–1018. doi: 10.1253/circj.CJ-18-1257. [DOI] [PubMed] [Google Scholar]
- 5.Yannopoulos D., Bartos J., Raveendran G., et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): a phase 2, single centre, open-label, randomised controlled trial. Lancet. 2020;396:1807–1816. doi: 10.1016/S0140-6736(20)32338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue A., Hifumi T., Sakamoto T., et al. Extracorporeal cardiopulmonary resuscitation in adult patients with out-of-hospital cardiac arrest: a retrospective large cohort multicenter study in Japan. Crit Care. 2022;26:129. doi: 10.1186/s13054-022-03998-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fugate J.E., Brinjikji W., Mandrekar J.N., et al. Post-cardiac arrest mortality is declining: a study of the US National Inpatient Sample 2001 to 2009. Circulation. 2012;126:546–550. doi: 10.1161/CIRCULATIONAHA.111.088807. [DOI] [PubMed] [Google Scholar]
- 8.Drennan I.R., Lin S., Sidalak D.E., Morrison L.J. Survival rates in out-of-hospital cardiac arrest patients transported without prehospital return of spontaneous circulation: an observational cohort study. Resuscitation. 2014;85:1488–1493. doi: 10.1016/j.resuscitation.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 9.De Graaf C., Beesems S.G., Koster R.W. Time of on-scene resuscitation in out of-hospital cardiac arrest patients transported without return of spontaneous circulation. Resuscitation. 2019;138:235–242. doi: 10.1016/j.resuscitation.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 10.Panchal A.R., Bartos J.A., Cabanas J.G., et al. Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular care. Circulation. 2020;16:366–S468. doi: 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 11.Panchal A.R., Berg K.M., Hirsch K.G., et al. 2019 American Heart Association focused update on advanced cardiovascular life support: use of advanced airways, vasopressors, and extracorporeal cardiopulmonary resuscitation during cardiac arrest: An update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2019;140:e881–e894. doi: 10.1161/CIR.0000000000000732. [DOI] [PubMed] [Google Scholar]
- 12.Richardson A.S.C., Tonna J.E., Nanjayya V., et al. Extracorporeal Cardiopulmonary Resuscitation in Adults. Interim Guideline Consensus Statement From the Extracorporeal Life Support Organization. ASAIO J. 2021;67:221–228. doi: 10.1097/MAT.0000000000001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen N., Wetterslev J., Cronberg T., et al. TTM Trial Investigators. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 14.Perkins G.D., Graesner J.T., Semeraro F., et al. executive summary. Resuscitation. 2021;2021:1–60. doi: 10.1016/j.resuscitation.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Geocadin R.G., Callaway C.W., Fink E.L., et al. American Heart Association Emergency Cardiovascular Care Committee. Standards for studies of neurological prognostication in comatose survivors of cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2019;140:e517–e542. doi: 10.1161/CIR.0000000000000702. [DOI] [PubMed] [Google Scholar]
- 16.Sonneville R., Schmidt M. Extracorporeal Cardiopulmonary Resuscitation for Adults With Refractory Out-of-Hospital Cardiac Arrest: Towards Better Neurological Outcomes. Circulation. 2020;1447:887–890. doi: 10.1161/CIRCULATIONAHA.119.044969. [DOI] [PubMed] [Google Scholar]
- 17.Belohlavek J., Smalcova J., Rob D., et al. Effect of Intra-arrest Transport, Extracorporeal Cardiopulmonary Resuscitation, and Immediate Invasive Assessment and Treatment on Functional Neurologic Outcome in Refractory Out-of-Hospital Cardiac Arrest A Randomized Clinical Trial. JAMA. 2022;327:737–747. doi: 10.1001/jama.2022.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matute-Bello G., Frevert C.W., Martin T.R. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:379–399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uchino H., Ogihara Y., Fukui H., et al. Brain injury following cardiac arrest: pathophysiology for neurocritical care. J Intensive Care. 2016;27:31. doi: 10.1186/s40560-016-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Andersson L.W., Isbye D., Kjaergaard J., et al. Effect of Vasopressin and Methylprednisolone vs Placebo on Return on Spontaneous Circulation in Patients With In-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA. 2021;326:1586–1594. doi: 10.1001/jama.2021.16628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tricco A.C., Lillie E., Zarin W., et al. PRISMA Extension for Scoping Reviews (PRISMAScR): Checklist and Explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 22.Introduction to the International Guidelines for CPR and ECC. Circulation. 2000;2000:102. doi: 10.1161/01.cir.102.suppl_1.i-1. [DOI] [PubMed] [Google Scholar]
- 23.Di Gennaro J.L., Chan T., Farris R.W.D., Weiss N.S., McMullan D.M. Increased Stroke Risk in Children and Young Adults on Extracorporeal Life Support with Carotid Cannulation. ASAIO J. 2019;65:718–724. doi: 10.1097/MAT.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 24.Conrad S.A., Rycus P.T., Dalton H. Extracorporeal Life Support Registry Report 2004. ASAIO J. 2005;51:4–10. doi: 10.1097/01.mat.0000151922.67540.e9. [DOI] [PubMed] [Google Scholar]
- 25.Extracorporeal Life Support Organization Registry Report: International Summary, 2022. Extracorporeal Life Support Organization (ELSO) International Summary of Statistics ECMO ECLS 2022. Available online: https://www.elso.org/Registry/InternationalSummaryandReports/InterbnatonalSummar.aspx.
- 26.Sandroni C., D’Arrigo S., Cacciola S., et al. Prediction of poor neurological outcome in comatose survivors of cardiac arrest: a systematic review. Intensive Care Med. 2020;46:1803–1851. doi: 10.1007/s00134-020-06198-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolan J.P., Sandroni C., Bottiger B.W., et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: post-resuscitation care. Resuscitation. 2021;161:220–269. doi: 10.1016/j.resuscitation.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Nolan J.P., Sandroni C., Bottiger B.W., et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: post-resuscitation care. Intensive Care Med. 2021;47:369–421. doi: 10.1007/s00134-021-06368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Percie du Sert N., Ahluwalia A., Alam S., et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tranberg T., Lassen J.F., Kaltoft A.K., et al. Quality of cardiopulmonary resuscitation in out-of-hospital cardiac arrest before and after introduction of a mechanical chest compression device, LUCAS-2; a prospective, observational study. Scand J Trauma Resusc Emerg Med. 2015;23:37. doi: 10.1186/s13049-015-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klosiewicz T., Puslecki M., Zalewski R., et al. Impact of automatic chest compression devices in out-of-hospital cardiac arrest. J Thorac Dis. 2020;12:2220–2227. doi: 10.21037/jtd.2020.04.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trummer G., Foerster K., Buckberg G.D., et al. Successful resuscitation after prolonged periods of cardiac arrest: a new field in cardiac surgery. J Thorac Cardiovasc Surg. 2010;139:1325–1332. doi: 10.1016/j.jtcvs.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 34.Grunau B., Kime N., Leroux B., et al. Association of intra-arrest transport vs continued on-scene resuscitation with survival to hospital discharge among patients with out-of-hospital cardiac arrest. JAMA. 2020;324:1058–1067. doi: 10.1001/jama.2020.14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations: Summary From the Basic Life Support, Advanced Life Support, Neonatal Life Support, Education, Implementation, Teams, First Aid Task Forces, the COVID-19 Working Group Circulation. 2022;145:e645–e721. doi: 10.1161/CIR.0000000000001017. [DOI] [PubMed] [Google Scholar]
- 36.Vognsen M., Fabian-Jessing B.K., Secher N., et al. Contemporary animal models of cardiac arrest: a systematic review. Resuscitation. 2017;113:115–123. doi: 10.1016/j.resuscitation.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 37.Vammen L., Johannsen C.M., Baltsen C.D., et al. Thiamine for the treatment of cardiac arrest-induced neurological injury: a randomized, blinded, placebo-controlled experimental study. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.028558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rysz S., Lundberg J., Nordberg P., et al. The effect of levosimendan on survival and cardiac performance in an ischemic cardiac arrest model – a blinded randomized placebo-controlled study in swine. Resuscitation. 2020;150:113–120. doi: 10.1016/j.resuscitation.2020.02.032. [DOI] [PubMed] [Google Scholar]
- 39.Ruggeri L., Nespoli F., Ristagno G., et al. Esmolol during cardiopulmonary resuscitation reduces neurological injury in a porcine model of cardiac arrest. Sci Rep. 2021;11:10635. doi: 10.1038/s41598-021-90202-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li D., Ni H., Rui Q., Gao R., Chen G. Mst1: Function and mechanism in brain and myocardial ischemia reperfusion injury. Curr Neuropharmacol. 2018;16:1358–1364. doi: 10.2174/1570159X16666180516095949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spaulding C.M., Joly L.M., Rosenberg A., et al. Immediate coronary angiography in survivors of out-of-hospital cardiac arrest. N Engl J Med. 1997;336:1629–1633. doi: 10.1056/NEJM199706053362302. [DOI] [PubMed] [Google Scholar]
- 42.Sunde K., Pytte M., Jacobsen D., et al. Implementation of a standardised treatment protocol for post-resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73:29–39. doi: 10.1016/j.resuscitation.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 43.Lind C.L., Johannsen C.M., Vammen L., Magnussen A., Andersen L.W., Granfeldt A. Translation from animal studies of novel pharmacological therapies to clinical trials in cardiac arrest: A systematic review. Resuscitation. 2021;158:258–269. doi: 10.1016/j.resuscitation.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 44.Gong P., Hua R., Zhang Y., et al. Hypothermia-induced neuroprotection is associated with reduced mitochondrial membrane permeability in a swine model of cardiac arrest. J Cereb Blood Flow Metab. 2013;33:928–934. doi: 10.1038/jcbfm.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suh G.J., Kwon W.Y., Kim K.S., et al. Prolonged therapeutic hypothermia is more effective in attenuating brain apoptosis in a Swine cardiac arrest model. Crit Care Med. 2014;42:e132–e142. doi: 10.1097/CCM.0b013e3182a668e4. [DOI] [PubMed] [Google Scholar]
- 46.Babini G., Grassi L., Russo I., et al. Duration of untreated cardiac arrest and clinical relevance of animal experiments: The relationship between the “No-Flow” duration and the severity of post-cardiac arrest syndrome in a porcine Model. Shock. 2018;49:205–212. doi: 10.1097/SHK.0000000000000914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.