Abstract
The development of sustainable biorefineries and bioeconomy has been the mandate of most of the governments with major focus on restricting the climate change concerns and finding new strategies to maintain the global food supply chain. Xylooligosaccharides (XOS) are short-chain oligomers which due to their excellent prebiotic potential in the nutraceutical sector has attracted intense research focus in the recent years. The agro-industrial crop and food waste can be utilized for the production of XOS which are derived from hemicellulose fraction (xylan) of the lignocellulosic materials. The extraction of xylan, is traditionally achieved by acidic and alkaline pretreatments which, however, have limited industrial applications. The inclusion of cutting-edge and environmentally beneficial pretreatment methods and technologies such as deep eutectic solvents and green catalysts are preferred. Moreover, the extraction of xylans from biomass using combinatorial pretreatment approaches may help in economizing the whole bioprocess. The current review outlines the factors involved in the xylan extraction and depolymerization processes from different lignocellulosic biomass and the subsequent enzymatic hydrolysis for XOS production. The different types of oligosaccharides and their prebiotic potential for the growth of healthy gut bacteria have also been explained. The introduction of modern molecular technologies has also made it possible to identify enzymes and microorganisms with the desired characteristics for usage in XOS industrial production processes.
Keywords: Bioeconomy, Human health, Prebiotics, Xylooligosaccharides, Lignocellulosic biomass, Pretreatment, Xylan
1. Introduction
The concept of circular bioeconomy has grown over the last few years for the production of valuable biomaterials and biofuels, owing to the concerns over the increasing organic waste pollution [1,2]. Different policies and regulations have been framed by different international organizations for the proper valorization, reuse and sustainability of biomass. The circular bioeconomy and the idea of integrated biorefineries are being cohesively used, incorporating an intriguing technological method in the utilization of organic waste for the sustainable synthesis of industrially relevant biochemicals [3,4]. Among all the organic wastes, lignocellulosic biomass is the most abundantly generated plant-based waste that is a potential bioresource for the production of value-added materials in the food and biofuel industry [[5], [6], [7]]. Different lifestyle changes in the 21st century have made people more aware in consuming healthy, nutritious food [8]. Consumers are moving towards adding more naturally occurring foods that are rich in nutrients and have positive health effects in their regular meals [9]. Among many healthy foods, functional foods, such as non-digestible prebiotic oligosaccharides, are becoming increasingly popular [10]. Prebiotics are recognized as food ingredients that support the maintenance of a balanced human intestinal flora. Prebiotics promote the growth of beneficial bacteria in the gut, which has a range of positive health benefits [11]. Consequently, the food industry has added prebiotics as valuable components to a variety of goods, including dairy and meat products, whole wheat bread, cereal bars and chocolates.
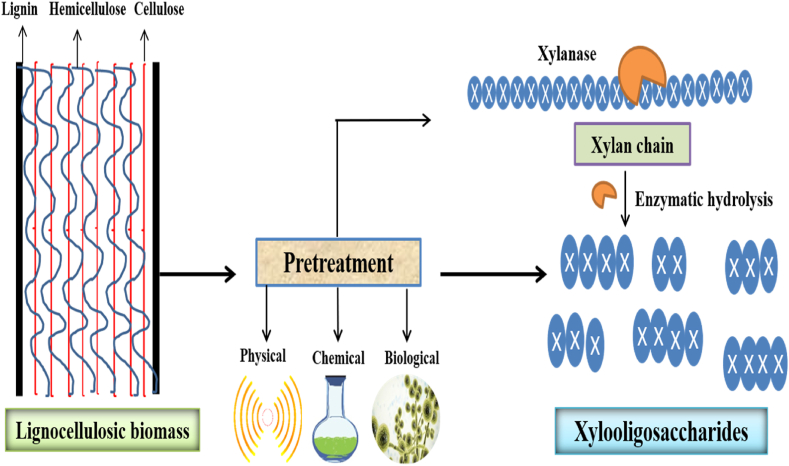
Xylooligosaccharides (XOS), due to their capacity to increase the activity of the valuable gut microbiota have recently attracted interest as potential prebiotic substances in the field of food research [12]. To address the expanding market demands, the food industry is working to create new XOS production bioprocesses that are more sustainable and efficient. XOS are sugar oligomers primarily containing β-d-xylopyranosyl (xylose) units that are linked via β (1 → 4)-xylosidic linkages with numerous substitutions [13]. Some of the health benefits of XOS include antioxidant effects, reduced blood sugar and cholesterol, decreased risk of colon cancer, increased calcium absorption, improved gastrointestinal health and cytotoxic effect on human leukemic cells [14]. Because of its high carbohydrate content, lignocellulosic biomass (LCB) is the most promising, cost-effective, and long-term source of a variety of valuable products, including lignocellulose-derived prebiotic xylooligosaccharides [15]. Each year, tons of LCB (such as crop residues, food waste, and agricultural waste) is generated from various post-harvest and processing operations [2,[16], [17], [18], [19]]. According to previous research studies, LCB can be effectively converted to XOS through enzymatic hydrolysis or microbial fermentation (Fig. 1) [6,13,20,21]. XOS have the same beneficial properties as other common oligosaccharides, such as short-chain carbohydrates digestible by gut flora, resistance to low pH and high temperature, and potential health benefits [22,23].
Fig. 1.
Bioconversion of lignocellulosic biomass into xylooligosaccharides for health benefits.
The LCB is constituted majorly by cellulose, hemicellulose and lignin biopolymers that can be valorized into an array of biochemicals [1,5,[24], [25], [26]]. The hemicelluloses are majorly composed of xylans, xyloglucans, mannans, and glucomannans, all linked by a β-(1 → 4) backbone. XOS are commonly derived from the debranching and depolymerization of xylan component after it is extracted from LCB [14]. The highly recalcitrant nature of LCB, restricts the accessibility of xylans for the production of XOS. Therefore, pretreatment is an intrinsic step to remove the recalcitrance and extraction of xylan from LCB [27]. The lignocellulose pretreatment, as an initial step, disintegrates the complex lignin-carbohydrate linkage to finally release xylan in solubilized form, which can be recovered later. The conventional methods of XOS production from the xylan-rich lignocellulosic feedstocks usually involve autohydrolysis or chemical treatments using acids or alkalis. Over the last decade, acid and alkali pretreatment approaches have been employed to valorize LCB for the extraction of xylans. However, there are hazards associated with the use of these conventional chemicals, such as acid pretreatment may degrade xylan in to xylose and therefore the recovery of xylan from LCB by acid pretreatment becomes difficult [28,29]. The drawbacks of these traditional pretreatment chemicals has necessitated the exploration of environment friendly and effective pretreatment agents [16,24,30]. The introduction of ‘green’ pretreatment methods such as those involving deep eutectic solvents (DESs), ionic liquids and organosolvs have created room for the development of economical lignocellulosic biorefineries [27,[31], [32], [33], [34]]. Moreover, the low-cost physical pretreatments such as ultrasonic waves and microwave irradiations can be employed as assistive technologies with the chemical pretreatment methods to augment the effectiveness of the overall bioconversion process of LCB for the production of XOS. However, for the successful production of XOS on commercial scale, various LCB raw materials, xylan extraction techniques, and enzyme sources for xylan hydrolysis are still being evaluated for optimum performance under the most environmentally friendly circumstances.
The current review provides a comprehensive summary of the key technological factors affecting the production of XOS from diverse lignocellulose materials. The review aims to explore current developments in the bioprocessing of LCB into XOS with focus on state-of-the-art pretreatment techniques and xylan enzymatic hydrolysis. Moreover, the production of XOS using exciting technological approaches such as protein engineering, metagenomics and cell surface engineering is also briefly presented.
2. Oligosaccharides as healthy food supplements
2.1. General aspects of prebiotics
Prebiotics are food that induces the activity of important microorganisms such as bacteria and fungi. They are non-digestible fibre compounds which can pass through the upper part of the gastrointestinal tract undigested and stimulate the growth of some bacteria by acting as a substrate for them [35]. Prebiotics can also have a significant impact on the gut microbiota, which in turn affects the metabolic processes and immersion of the intestine, suppresses pathogenic bacteria, modifies the mortal susceptible system, and reduces the availability of adhesion sites to harmful organisms. Different prebiotics have known to provide positive effects on the body's systems, including the gut, immune system, blood pressure, and anti-obesity effects (Fig. 2). They also lower the risk of cancer and promote heart health. Gibson and Roberfroid [36] are credited with coining the term "prebiotic," which they define as ‘a non-digestible food element that benefits the host by selectively enhancing the growth/activity of one or a small number of bacteria in the colon, and thereby enhances host health.’ A number of microorganisms in the human gut play an important role in improving digestion and overall health of their human hosts, owing to their ability to break down complex food ingredients that remain undigested in the upper digestive tract. Such microbes obtain their energy by fermenting non-digestible substances in the diet and converting them to beneficial metabolites over time, boosting overall gut health [35]. There are two ways to improve the health-promoting activities of these microorganisms, either by their intake in live form as a food supplement or by promoting the growth of these existing microbes through increased dietary intake of non-digestible food ingredients called in the form of prebiotics [36].
Fig. 2.
Different health benefits of prebiotics.
Prebiotic has been defined as “a selectively fermented ingredient that allows specific changes, both in the composition and/or activity of the gastrointestinal microbiota that confers health benefits” [22]. In order to be regarded as prebiotics, the ingredients should demonstrate the following:
-
i.
Non-digestible by the endogenous enzymes in the human gut. In fact, it should possess resistance towards gastric acidity, hydrolysis by digestive enzymes and gastrointestinal tract absorption.
-
ii.
Stimulatory towards the growth and/or activity of specific bacteria responsible for health and wellbeing, thus, conferring health benefits to the host.
-
iii.
Undergo selective fermentation by specific intestinal microbiota.
-
iv.
Have been synthesized by microorganism or their enzymes, or have plant origin.
-
v.
Compatible with other food/feed ingredients.
Prebiotics can be derived artificially or naturally, and can also be used as functional foods owing to the fact that these are natural substance found in foods that ordinarily wouldn't contain them. When consumed in the recommended amounts, prebiotics can improve health or lower the risk of disease while also enhancing a consumer's quality of life [37]. However, despite the fact that prebiotics contain carbohydrates of various sizes, the non-digestible oligosaccharides with short sugar units have the greatest commercial interest, since they provide the requisite level of fermentation selectivity. Prebiotics have a particular metabolic action in the large intestine which serves as a food for good bacteria, as they are not digested in the small intestine. By creating a comfortable environment and encouraging the growth of beneficial bacteria, an exaggerated alteration in the composition of the gut microbiota is generated [38]. Many good bacteria such as those belonging to Bifidobacterium sp., Bacteroides, Lactobacillus sp., Saccharomyces, Enterococcus, and Propionibacterium are known to have effective properties in breaking high molecular weight carbohydrates [37,39].
2.2. Types and classification of oligosaccharides
Oligosaccharides are a type of polymeric carbohydrates that are midway between simple sugars and polysaccharides. Xylooligosaccharides (XOS), galacto-oligosaccharides (GOS), fructo-oligosaccharides (FOS), malto-oligosaccharides (IMOS), and mannan oligosaccharides (MOS) are amongst the most common functional oligosaccharides. Oligosaccharides can be found in a variety of foods, including honey, lentils, milk, wheat, sugar beet, sugarcane juice, fruits, and vegetables [40]. FOS, GOS, lactulose derived galacto-oligosaccharides (LDGOS), XOS, arabino-oligosaccharides (AOS), and algae-derived marine oligosaccharides (ADMO) are the most popular oligosaccharides with a wide range of applications, including having prebiotic properties.
2.2.1. Xylooligosaccharides (XOS)
Xylooligosaccharides are oligomers having 1,4-linked xylose backbone and substitutes such as uronic acids, acetyl groups and arabinose units. Xylooligosaccharides are incredibly variable in terms of degree of polymerization (DP), degree of substitutions (ratio of arabinose to xylose) and linkages. The physical and biological characteristics of XOS depend upon the type of substitutions, their pattern of occurrence and molecular weight distribution on xylan backbone [37]. They are often utilized as food additions in dairy and confectionary items because of their moderate sweetness, besides being used as medications [41]. Due to their resistance to heat and acidity, which enables them to be used in the creation of low pH meals, XOS are favored in food processing over fructooligosaccharides and inulin. Xylooligosaccharides have attracted a lot of interest as a prebiotic due to its multifaceted benefits, particularly in lowering gastrointestinal issues [42,43]. Factors like XOS linkages and ring configurations affect XOS stability. Although studies have shown XOS can be produced from a number of plants, microbial based sources, its large-scale production from lignocellulose materials is required, owing to the large amount of XOS’s precursor (xylan) being present in the biomass. Numerous studies have focused on the selective hydrolysis of xylans from LCB to produce prebiotic XOS with DP between 2 and 4, which exhibit speedier fermentation as a result of their consumption by probiotic microorganisms in functional meals [13,15,44]. The bacteria that live in the human gut, including Lactobacillus sp., Bifidobacterium, Clostridium and Bacteroides can produce energy from polysaccharides and xylooligosaccharides when consumed.
2.2.2. Galactooligosaccharide (GOS)
Galactooligosaccharides are lactose-derived compounds containing β-linked galactose moieties with galactose or glucose at the reducing end. GOS contain different β linked galactose moieties (Such as β-(1 → 2), β-(1 → 3), β-(1 → 4), β-(1 → 6) with a degree of polymerization (DP) from 2 to 8. GOS are a beneficial prebiotic because gut bacteria can ferment them despite not being digested in the upper digestive system. GOS retain β-1, 6- linked galactose chains ending with a reducing β-linked glucose half [40]. The synthesis of GOS involves the action of β-galactosidase enzyme through transgalactosylation reaction using lactose as the primary substrate. According to reports, the degree of selectivity relies on the source of the enzyme, besides the amount of lactose present during the process. Typically, high lactose concentrations enhance its enzymatic transgalactosylation with β-galactosidases, which results in increased GOS production yields [45,46]. It is possible to produce GOS from lactose using microbial cells or an enzymatic reaction, or agro-industrial waste additives (like whey) instead may also be used for their production [47]. A study was conducted on the synthesis of GOS as a prebiotic by partially purified β-galactosidase from Enterobacter aerogenes employing lactose substrate [48]. It was found that the synthesized GOS promoted the growth of three microbial strains with probiotic activity such as Bifidobacterium bifidum, Lactobacillus delbrueckii and Lactiplantibacillus plantarum. Moreover, the symbiotic combinations of the prebiotic strains displayed excellent antioxidant activities. Huang et al. [46] reported the production of a novel β-galactosidase from Klebsiella oxytoca and expressed the genes of the enzymes in E. coli bacterium. While purified β-gal 2 had a strong transgalactosylation capacity for GOS synthesis, β-gal 1 possessed a greater enzyme activity for lactose hydrolysis. Though, β-galactosidase-based GOS synthesis is a well-known method, the high-purity and mass-scale GOS production is still a challenge which needs to be addressed for higher product yields on industrial levels.
2.2.3. Fructooligosaccharide (FOS)
Fructo-oligosaccharides are primarily derived from garlic, onion, chicory, asparagus, dragon fruit, rye, banana, and artichoke [49]. Dietary FOS are not easily hydrolyzed in the small intestine; therefore, it enters the cecum intact. Intestinal bacteria break them down into short-chain carboxylic acids, L-lactate, CO2, H2, and other metabolites. The purpose of FOS is to selectively stimulate the growth and/or activity of one or a few beneficial bacterial species of the colon to promote host health. The FOS structure contains one glucose (F) moiety and 2–60 fructose (F) moieties linked by (2–1)/(2–6) glycosidic linkages, such as in 1-kestose (1G, 2F), nystose (1G, 3F) and fructofuranosyl nystose (1G, 4F) [50]. As the DP increases, the sweetness of FOS diminishes. FOS reduces the level of fatty acids, phospholipids, cholesterol and triglycerides in the intestine and improves the absorption of minerals like Ca+2, K+ and Mg+2 [51]. By using isolated enzymes from microorganisms or their whole cells, FOS can be produced commercially. The fungal enzymes are preferred over bacterial enzymes due to their higher titer and extracellular nature. Fungal strains such as Aspergillus niger, Aureobasidum pullulans that release the enzyme β-fructofuranosidase or fructosyltransferase are known to synthesize FOS [45]. The synthesis of products with a low DP and the concentration of FOS could both be raised by increasing the substrate (sucrose) concentration. Wienberg et al. [52] using inulosucrase enzyme isolated from Lactobacillus gasseri synthesized inulin-type fructooligosaccharide (I-FOS) at elevated levels of sucrose concentration (570–800 g/L). The results depicted an enhanced bioconversion of sucrose with 401 ± 7 g/L I-FOS concentration and 53 ± 1% (w/w) I-FOS purity. Similarly, I-FOS was extracted from red onion using neokestose sugar that selectively promoted the growth of probiotic lactobacilli [53]. A productive and effective platform for FOS production can be provided by further improvement in the biotechnological methods.
2.2.4. Cello-oligosaccharides (COS)
Cello-oligosaccharides are a new class of non-digestible oligosaccharides oligomers studied in recent times that possess D-glucose units with β-1,4 linkages [54]. The shorter COS or disaccharides are water soluble and contain three (cellotriose) to six (cellohexose) glucose monomer units, whereas the longer COS form insoluble cellulose material. Studies show that the soluble COS have prebiotic potential that encourage the growth of numerous Lactobacillus and Bifidobacterium strains in vitro even if humans cannot digest them [55]. The recent trends show that lignocellulosic biomass has been seen as a potential resource for the industrial production of COS. The production of COS from LCB often involves physical, chemical as well as enzymatic processes. A novel study was carried out to show the synergism between enzymes lytic polysaccharide monooxygenase, endoglucanases, cellobiose dehydrogenase and additives for COS production after the enzymatic hydrolysis of organosolv pretreated sugarcane straw biomass [54]. A 2.7-fold of enhance COS was produced from the biomass after the optimization of enzymatic hydrolysis using copper and lactose as additives. In another study by Zhong et al. [56], a 82 mol % yield with a scale up to 93 g/L for cello-oligosaccharides production from 0.5 M sucrose was achieved. The COS were comprised of DP 3 (33 wt %), DP 4 (34 wt %), DP 5 (24 wt %), and DP 6 (9 wt %) and involved minimal loss (≤10 mol %) to insoluble fractions. After isolation (≥95% purity; ≥90% yield), the COS were examined for growth promotion of probiotic strains. Compared to inulin, trans-galacto-oligosaccharides, and cellobiose, COS demonstrated up to 4.1-fold increase in cell density for Lactobacillus rhamnosus, Lactococcus lactis, Lactobacillus paracasei and Clostridium butyricum showing that COS acts as a useful functional prebiotic carbohydrate. These findings suggest new perceptions on the industrial production of COS and its commercial use.
3. Xylooligosaccharides as potential prebiotics
3.1. Physicochemical properties of XOS
XOS is naturally found in a certain food in limited amounts such as fruits, vegetables, milk, honey, and bamboo shoots. However, to enhance the economic suitability, large-scale production and purification of XOS is required. Prebiotic XOS with fewer than 4 monomers are thought to be useful but possibly not essential. More significantly, XOS stand out for their general pH and thermal stability, being able to survive pH fluctuations between 2.5 and 8 and stable at temperatures above 100 °C [57]. XOS have a variable molecular mass and their sweetness is comparable to around 30% of sucrose, with no off-taste. Xylan is transformed into high molecular weight xylooligomers, branching or linear XOs, high and low molecular weight xylooligomers, xylose, or is degraded to furfural during the depolymerization process. The degree of polymerization, their pattern of substitution on the xylose chain, presence of side groups, ratio of arabinose to xylose, as well as the types of bonds existing in XOS vary significantly depending on the source of xylan and the production procedure [13,58].
3.2. Biological properties of XOS
The ability of XOS to regulate body weight, insulin sensitivity, reduce colon inflammation and glucose and lipid homeostasis can have a positive impact on metabolic aspects of diabetes. Diabetes has been considered one of the most prevalent lifestyle diseases with prolonged negative health effects in the body [59]. XOS may have positive effects on the metabolic disorders linked to diabetes [60]. Moreover, XOS reduces the degradation of muscle protein during energy production and are known to have antioxidant and antiallergic properties [61]. Since XOS have beneficial effects on animal health, they can potentially be used as an alternatives to antibiotics in animal feed without running the risk of generating resistance to pathogenic microorganisms [62]. Additionally, they can be utilized as essential ingredients in the pharmaceutical industry for the drug production to regulate obesity and cure gastrointestinal infections. Owing to an array of healthy biological properties, XOS can be added to foods as components to support the health improvement. However, due to the suggested daily consumption amount of 2–5 g, its dosage should be made cautiously [61,63].
4. Xylooligosaccharides production from lignocellulosic biomass
The use of lignocellulosic biomass (LCB) for the production of XOS has been considered as an abundant source that could be an intriguing possibility for meeting future XOS demands without causing any negative environmental consequences. Lignin, hemicellulose, and cellulose make up lignocellulose in the form of a complex three-dimensional network. These components are bonded to one another with numerous linkages, giving cell walls their well-behaved properties of recalcitrance and stiffness. The chemical characteristics of these lignocellulosic constituents make them biotechnologically valuable substrates. Forestry and agricultural operations, paper-pulp companies, and numerous agro industries all generate large volumes of lignocellulosic waste [27,37]. The different lignocellulosic wastes for XOS production include sugarcane bagasse, corn cob, wheat straw, rice straw, poplar wood, forestry waste, and many other plant or crop wastes. These wastes are however, a source of pollution, since the biomass is burnt widely to dispose of lignocellulose waste. This practice is not only limited to underdeveloped nations, but now has become a global phenomenon [1]. However, vast quantities of "waste" plant biomass can be turned into a variety of value-added products, including XOS. The agricultural industry in India itself creates a large amount of lignocellulosic waste, which is a huge source of renewable feedstock for biorefineries. Only if enough biomass and desirable characteristics are available to generate bio-based products, the strategy toward bioeconomy will be successful [64]. The composition of different polymers in the LCB vary (Table 1) which depends upon the type of biomass, harvesting time, growth conditions, climate, and many others.
Table 1.
Polymeric composition of different lignocellulosic feedstocks.
| Biomass | Cellulose (%) | Lignin (%) | Xylan (%) | References |
|---|---|---|---|---|
| Wheat straw | 38–41 | 20–22 | 21.2 | [65] |
| Switchgrass | 37 | 19 | 20.4 | [66] |
| Sugarcane bagasse | 40–43 | 23–28 | 21.1 | [18,65] |
| Birch wood | 40 | 24 | 24 | [67] |
| Poplar sawdust | 40 | 28 | 17.8 | [68] |
| Rice straw | 43 | 23.14 | 16.33 | [69] |
| Corn straw | 30.24 | 19.57 | 20.02 | [70] |
| Bamboo powder | 44.29 | 27.23 | 18.37 | [71] |
| Corn stover | 30.70 ± 1.23 | 24.38 ± 0.65 | 21.17 ± 0.83 | [72] |
| Banana peel | 52.43 ± 2.81 | 7.74 ± 0.93 | 37.19 ± 1.64 | [73] |
| Mango seed husks | 55.93 ± 4.58 | 11.91 ± 2.34 | 18.14 ± 5.07 | [74] |
| Brewers’ spent grain | 15.2 ± 0.5 | 12.5 ± 0.8 | 16.9 ± 0.7 | [75] |
| Pineapple leaf | 46.24 ± 0.32 | 26.01 ± 0.14 | 19.48 ± 0.02 | [76] |
| Sorghum biomass | 34.8 | 14.3 | 29.7 | [77] |
| Parthenium hysterophorous | 49.98 | 17.6 | 14.18 | [17] |
| Sunflower stalk | 27.22 | – | 10.7 | [19] |
| Agave tequilana | 26.0 ± 1.2 | 13.8 ± 1.3 | 22.8 ± 1.2 | [78] |
The hemicellulose structure is related to xylose, arabinose, galactose, mannose, rhamnose, and acetylated groups that after fractionation of LCB allows for the co-production of oligosaccharides and monomeric sugars. Xylan, which is a major hemicellulosic sugar extracted from hardwoods, is predominantly depolymerized into XOS (Fig. 3). The xylans from agro-industrial crop or food residues are divided into several classes based on the types of lateral groups and the degree of substitution. The class of arabinoxylans, which is typically present in tissues of sugarcane and the major cereals, including barley, wheat, corn, rye, oat, sorghum and rice is particularly well-represented [37]. The xylan which is extracted from grasses is comparable to that found in hardwood, with an exception of L-arabinose which is higher in grasses. The degree of polymerization and the kind of linkage present in the xylose structure vary depending on the various sources used to produce XOS.
Fig. 3.
Process of xylooligosaccharides production from lignocellulosic biomass.
5. Methods for the extraction of xylan/XOS from LCB
The recalcitrance of lignocellulosic biomass, which is tightly attached to other cell wall components including cellulose and lignin by chemical bonds and physical barriers, presents a significant obstacle during the extraction of xylan or XOS [3,32,79]. The majority of the H-bonds between hemicellulose and cellulose are weak, whereas lignin generates ester linkages that are susceptible to hydrolysis with just a small number of resistant ester/ether linkages [60]. Therefore, pretreatment of LCB becomes a step of utmost importance to reduce its recalcitrance and releasing the complex sugars including xylan to ultimately get XOS. Drying and grinding are the first steps in the biomass fractionation process because they have a direct impact on the material's crystallinity and increase its contact surface [19,30,80]. From there, these operations serve as a pretreatment for the subsequent processes, which include chipping, dry crushing and ball milling [81]. However, these initial steps of biomass deconstruction are not enough to valorize and separate the components of the agro-industrial waste, which results in creation of multi-stage processes for their transformation and a significant increase in production costs. The pretreatment is required to increase the biomass's availability for subsequent operations and hence, is regarded as a crucial step that significantly affects LCB’s digestibility. Due to the fact that pretreatment accounts for 40% of the overall processing cost, it has a significant impact on downstream processing costs involving enzyme loading, detoxification, waste treatment requirements, among others [82].
Over the last decade, a number of pretreatments have been exploited for the extraction od complex sugars such as xylan and thereby LCB valorization into biofuels and commercial biomaterials including physical (ultrasound and microwaves), chemical (acid, alkali, ionic liquids, deep eutectic solvents), physicochemical (steam explosion), biological (using fungi and bacteria) [16,30,32,80,[83], [84], [85]]. These pretreatment methods can produce XOS from different LCB sources.
5.1. Acid pretreatment
Traditionally acid and alkali pretreatment methods have been explored for the extraction of xylan using solubilization method by a number of researchers around the globe. The acid pretreatment of LCB results in the cleavage of the glycosidic linkages in hemicellulose chain yielding different oligosaccharides and monosaccharides [61,65,86,87]. Han et al. [88] employed an integrated biorefinery process for the production of XOS from the valorization of corncob biomass using gluconic acid (0.6 mol/L) pretreatment that resulted in a maximum XOS yield of 56.2% under optimized conditions. Similarly, in another study, sugarcane bagasse biomass was subjected to xylonic acid (0.64 M) pretreatment that under optimized conditions of pretreatment provided an augmented XOS yield of 44.5% [89].
Organic acids have also been employed for the extraction of prebiotic XOS from xylan residues of LCB. Yan et al. [90] used citric acid and maleic acid for the production of XOS from industrial derived xylan residue, in which citric acid was found to be effective in giving higher XOS yield (52.3%) compared to that from maleic acid (48.9%). Recently, Zahoor et al. [91] used a novel technology for the co-production of oligosaccharide (XOS) and monosaccharide (glucose) from wheat straw and Monterey pine sawdust biomass after the pentanol-dilute acid pretreatment. A maximum XOS of 48.65% and 46.85% was released from pine sawdust and wheat straw biomass, respectively after the lignin removal from these biomass sources. Moreover, upon enzymatic hydrolysis of pretreated solid biomass residues resulted in higher glucose yields of 93.34% and 88.65% from wheat straw and pine sawdust, respectively. Similarly, in an attempt to reduce the production cost for the valorization of sugarcane bagasse biomass for nutraceutical industries, a novel furoic acid pretreatment method was developed by Dai et al. [92] for the co-production of glucose (335 g/kg biomass) and XOS (120 g/kg biomass). The acid pretreatment though is process for the fractionation of biomass, it however requires high temperatures and pressures, which encourage the production of hazardous byproducts and therefore, necessitate further XOS purification steps.
5.2. Alkali pretreatment for XOS production
Contrary to the acid treatment for the deconstruction of LCB, the alkali pretreatment methods cause the solubilization of hemicellulose by disrupting the H-bonds between cellulose and hemicellulose in presence of OH- ions from alkali [27,30,79]. The alkaline pretreatment has been widely used as a valuable biomass pretreatment technique to increase the enzymatic conversion of lignocellulose. The saponification of ester and ether bonds is one of the primary chemical reactions involved in the extraction process. The cellulose and hemicellulose structural linkages separate as a result of alkaline extraction, preventing the hemicellulose polymer from fragmenting. Due to the xylan’s pH stability and the ease with which the solubilized fraction can be recovered from the liquid, the extraction of xylan from LCB under alkaline conditions is favorable. Previous studies demonstrated that the lignin could be successfully removed by the alkali pretreatment, and the intricate structure of the lignocellulosic network could be broken down [15,30,93].
Rogoski et al. [94] recently pretreated cassava peel residues with NaOH for lignin removal and xylan extraction which was followed by enzymatic hydrolysis of xylan using xylanase enzyme to produce XOS. A lignin removal percentage of as high as 34.20% and XOS yield of 396.5 mg XOS/g xylan after the alkaline hydrolysis and subsequent enzymatic hydrolysis, respectively was achieved. Alternatively, sugarcane bagasse biomass was the sustainably utilized for XOS production alongside bioethanol using alkali pretreatment. The results demonstrated that 6.26 g of XOS, 1.44g xylitol and 15.95 g bioethanol could be produced from the alkaline bioconversion of 100 g raw biomass [95]. These investigations exhibit that using an integrated process, the LCB can be effectively bio-converted into fuels and prebiotic oligomers (such as XOS) by maximum utilization of the polysaccharides of LCB fractions using hydrolyzing enzymes. However, several drawbacks of alkali pretreatment such as low solid-to-liquid ratio, the formation of inhibitors, long residence time, relatively high reaction temperature, and formation of salts from the extracted xylan that need to be purified, restrict the development of an accepted alkali-based pretreatment technology for XOS production.
5.3. Ionic liquid pretreatment
Ionic liquids (ILs) over the years have been recognized as ‘green’ pretreatment solvents carrying environment friendly properties for the fractionation of LCB into different biochemicals and biofuels [19,25,27]. The use of ILs and DESs to break up the substantial intrinsic bonds between cellulose, lignin and hemicellulose fractions and transforming the polymeric sugars into amorphous form, has been strongly supported by prior studies from the literature [[16], [17], [18],25]. In a research study by Avila et al. [96], the concentration of commercial hemicellulases (endoxylanases) was optimized for increased XOS production from IL pretreated sugarcane bagasse and straw. The combinatorial pretreatment strategies using ILs with other green treatment methods have been tried to effectively fractionate lignocellulosic biomass for low cost-production of XOS. The work of Bhatia et al. [97] demonstrated the use of combined IL (1-ethyl-3-methylimidazolium acetate) and steam explosion pretreatments for effective delignification and deacetylation of Miscanthus biomass to produce high-value XOS and gluco-oligosaccharides. Pretreatment methods for ionic liquids range from lignin and hemicellulose extraction to a reduction in degree of polymerization of hemicellulose reduction for XOS, reducing native biomass recalcitrance. These requirements depend on the ILs' properties, the process parameters (duration of treatment, temperature, particle size and biomass loading) and the particular interactions of the ILs with the LCB such as grasses, hardwoods and softwoods. However, the high operational costs, the requirement for energy-efficient and affordable ways for the recovery and reusability of ILs, the viscosity of ILs, as well as equipment handling are among the list of some of the limitations that has restricted their wide scale applications in biorefineries.
5.4. Deep eutectic solvent pretreatment
DESs on the other side have edge over ILs owing to their least demerits in the bioprocessing of LCB into XOS. These are the ‘green’ eutectic mixtures that can be formed by mixing two or more chemical substances in a specific molar ratio, one of them being a hydrogen bond donor (HBD) and another is hydrogen bond acceptor (HBA) [21,81,98]. They have beneficial qualities such as non-toxicity, low melting point, low viscosity, low volatility, high conductivity, high thermal stability, high surface tension, and biocompatibility [20,99]. In DES pretreatment, the DES is prepared at a fixed molar ratio of its HBA and HBD, heated at certain temperature for 2–3 h to make them liquid at room temperatures. Thereafter, the solid dry biomass is added to DES in definite solid to liquid ratio, followed by pretreatment in the temperature range of 60–130 °C. After pretreatment, the solution is allowed to cool, washed, filtered and hydrolyzed to extract XOS (Fig. 4) [16]. The DES used in the pretreatment can be reused again for subsequent pretreatment cycles after its recycling and recovery, to economize the process. Some studies have suggested that the efficiency of DES pretreatment for LCB could well be synergistically enhanced by combining them with other pretreatment approaches. Recently, Ma et al. [100] carried out a synergistic DES (isopropanolamine/choline chloride (ChCl))-hydrogen peroxide pretreatment for the breakdown of xylose residue that resulted in increased lignin removal (97.1%), highest glucose yield (95.9%), with potential for XOS synthesis.
Fig. 4.
Process of DES based pretreatment of LCB for the xylooligosaccharide production.
The assistive green pretreatment technology, i.e., microwave irradiations has found to be quire beneficial to improve the efficiency of DES pretreatment (Table 2). A microwave assisted DES (ChCl/formic acid) pretreatment of wheat straw resulted in successful production of XOS with a high xylose and XOS yield of 20% and 32%, respectively [21]. Acid autohydrolysis assisted DES pretreatment of LCB for XOS production is also considered as a suitable green method due to less consumption of chemical residues. Ying et al. [101] recently used acetic acid hydrolyzed wheat bran as raw material for further treatment with DES (ChCl/lactic acid) that proved to be effective in providing a total XOS yield of 62.9%, high arabinose (76.1%), glucose (83.8%) and xylose yields (54.8%) respectively. In the current biorefinery process, the integrated pretreatment systems involving DESs could boost the possibility of valorization of LCB, maximize the xylan extraction and hence, XOS production.
Table 2.
Production of XOS from LCB employing different pretreatment methods.
| Lignocellulosic feedstock | Pretreatment method | Pretreatment conditions | Significant results | References |
|---|---|---|---|---|
| Banana pseudostem | Hydrogen peroxide | 200 mL 20% (m/m) H2O2, 150 rpm, 25 °C, 4 h | High XOS yield (50.40 g/L) | [102] |
| Industrial derived xylan | Maleic acid (MA), Citric acid (CA) | 0.5 M of CA and 0.1 M of MA at 150 °C for 40 min | XOS yield of 52.3% (CA) and 48.9% (MA) | [90] |
| Sugarcane bagasse | Xylonic acid | 3.0 ± 0.1 g biomass mixed with 30 mL xylonic acid (0.64 M) | XOS yield of 44.5% | [89] |
| Pine sawdust, wheat straw | Dilute acid/pentanol | 1% sulfuric acid, 60% pentanol; 1:10 solid to liquid ratio, 170 °C, 45 min | XOS yield of 46.85% (wheat straw) and 48.65% (pine sawdust) | [91] |
| Cassava peel | NaOH pretreatment | 2% (w/v) NaOH for 30 min, 121 °C, 1:100/solid: liquid | 396.5 mg XOS/g xylan | [94] |
| Corn stover | Alkaline H2O2-Ball milling | 0.45 g/g (H2O2: substrate), 1:3 (w/v) solid-to-liquid ratio, 1h ball-milling | High XOS yield of 69.65% | [103] |
| Miscanthus | 1-ethyl-3-methylimidazolium acetate ([C2mim][OAc]) | 300 mg biomass mixed with 9.7 g of [C2mim][OAc], 160 °C, 3 h | 90% (w/w) of xylan converted to XOS | [97] |
| Tamarix austromongolica | Hydrothermal-IL (1-butyl-3-methylimidazoliumacesulfamate) | Hydrothermal pretreatment: 180 °C for 30 min; IL pretreatment: 5% loading at 110 °C for 3 h | 352 mg/g glucose, 60.98 mg/g XOS and 178.2 mg/g lignin extraction | [104] |
| Poplar sawdust | Hydrothermal-DES (ChCl/Lactic acid) | Hydrothermal pretreatment: 180 °C, 30 min; DES pretreatment: 10 g biomass mixed with 100 g DES, 50–130 °C, 90 min | 53.2% XOS extraction | [20] |
| Wheat straw | Microwave-DES (ChCl/formic acid) | Microwave: 209 W, 80 s (1309 J/s); 1 g biomass in 10 mL DES | 32% and 20% XOS and xylan yield, respectively | [21] |
6. Enzymatic hydrolysis of xylan for xylooligosaccharides production
For the bioconversion of organic wastes including agro-industrial lignocellulose wastes, hydrolytic enzymes called hydrolases are often utilized [23,26,32,105,106]. Since the enzymatic process operates under mild reaction conditions without the use of hazardous chemicals, it is more conducive to the idea of a biodegradable process for XOS production. Furthermore, the utilization of xylanolytic enzymes exhibits high specificity and efficiency, enabling a greater control over degree of polymerization and a decreased formation of unwanted xylose and other by-products [23,58,107]. Some of the most commonly used hydrolases for plant cell wall disintegration are cellulase, laccase, β-glucosidase, xylanases, and mannanase. Hemicellulose is hydrolyzed by endo-enzymes within the main chain, and exo-enzymes act on the endo-enzyme hydrolysis byproducts to generate monosaccharides. Two steps are quite essential for the enzymatic hydrolysis step to produce XOS. The hemicellulose found in the LCB should first be separated using various pretreatments, as discussed in the upper sections. To do this, it's critical to remember that the pretreatment should be chosen based on the desired final hemicellulose recovered features, such as polysaccharide, oligosaccharide, or monosaccharide [61]. The second step, which is enzymatic saccharification, involves xylan hydrolysis with the help of xylanolytic enzymes such as endo-1,3-β-xylanases and endo-1,4-β-xylanases [108]. The xylose-releasing enzymes or the endo-xylanases, exo-xylanase/β-xylosidase and enzymes reducing the degree of polymerization of xylose or xylans have been known to be isolated from fungal and bacterial strains [23,58,107]. Enzyme preparations with low exo-xylanase and/or β-xylosidase activity are preferred for preventing the production of xylose.
Short XOS are released when glycosidic connections are broken by endoxylanases. Reduced xylan polymerization is caused by endo-1,4-xylanases' action on glycosidic bonds [10]. The bonds chosen for the cleavage are chosen based on factors such the chain's length, the degree of branching, and the number of substituents. Small molecules like mono, di, and tri-saccharides of D-xylopyranosyl may be produced at a later stage of hydrolysis after the synthesis of D-xylopyranosyl oligomers. After the xylan has been sufficiently broken down into short oligomers, xylosidases are activated [109]. The aggregation of such oligomers may hinder the action of endoxylanase, and therefore as a result, β-xylosidase hydrolyzes these oligomers into xylose monomer, minimizing the inhibitory effects of oligomers and increasing xylan hydrolysis efficiency [82]. However, endo-1,4-xylanase without β-xylosidases is desirable for XOS production as it stops xylose release, increasing XOS yield. Debranching enzymes that remove arabinose, acetyl and glucuronic groups from the xylan backbone include arabinofuranosidase, acid esterase, glucuronidase, respectively.
In recent years, xylooligosaccharides synthesis has made substantial use of fungal xylanases. In a study by Pereira et al. [110], Aspergillus nidulans A773 xylanase was used to enzymatically hydrolyze soybean fiber, producing significant levels of XOS. After 12 h of enzymatic hydrolysis of xylan-rich wheat husk, a crude extracellular xylanase from Aspergillus fumigatus R1 achieved a maximum XOS yield of 1 gm% [111]. In another study, xylan recovered via alkaline extraction of sugarcane bagasse was converted into 27.1% XOS by A. fumigatus xylanase [112]. It has been observed that to produce a more cost-effective bioprocess with a greater yield, the enzymatic hydrolysis conditions of hemicellulose in XOS should be improved. The synthesis of XOS can be affected by some biochemical or microbiological aspects of the enzymatic hydrolysis, hence optimizing this bioprocess requires achieving the appropriate enzyme and substrate concentration, temperature and reaction time [61]. The improvements in the enzyme extraction processes, employing novel enzyme immobilization strategies may create a room for further economizing the XOS production process.
7. Advances in lignocellulosic biorefineries for xylooligosaccharides production
The development of a sustainable lignocellulosic biorefinery with the best possible exploitation of lignocellulosic waste calls for the inclusion of effective pretreatment approaches, efficient enzyme cocktails and unique microorganisms for fermentation [27,113]. Utilizing cutting-edge molecular technologies, it has become possible to find enzymes and microbes with the desired properties for use in industrial production processes for XOS. Technologies involving such as metagenomics, protein engineering and cell surface engineering are being applied by researchers to improve the efficiency of a lignocellulosic biorefinery.
7.1. Protein engineering approaches
Protein engineering or genetic engineering is an essential tool for alterations in the microbial genes or enzymes polypeptide chains, thereby producing functionally stable enzymes. Enzymes such as xylanases with improved properties for XOS production can be altered by site-directed mutagenesis or directed evolution. In a research study, a xylanase displaying improved enzyme properties was produced from the expression of a mutant baxA gene from Bacillus amyloliquefaciens [114]. The purified enzyme exhibited 3.5 times higher specific activity and that gave a good yield of XOS from beechwood xylans, oat spelt and birchwood. A mutant xylanase (XynAR) was generated from Streptomyces rameus that exhibited improved acid tolerance, thermostability and catalytic efficiency for the potential production of XOS [115]. Enhancing the catalytic activity of xylanase is more important for robust enzyme engineering than improving the pH and temperature characteristics. By adding the single mutation for xylanase (T74Y) from Thermotoga maritima, Yang et al. [116] achieved an increased 1.3-fold catalytic activity of xylanase for XOS production. Similarly, Boonyapakron et al. [117] disclosed that by eliminating a carbohydrate-binding module from a thermophilic GH11 xylanase generated from a metagenomic library, a 6.5-fold enhancement in catalytic performance was achieved. Such mutant variations can be employed in lignocellulosic biorefineries to produce XOS on commercial levels.
7.2. Metagenomics
Metagenomics is one of the many powerful omics technologies which is employed for the discovery of novel enzymes that don’t require the isolation and culturing of the microorganism. Metagenomic techniques have been established to discover the genes for desired enzymes with unique properties in order to discover some of the uncultivable and unexplored microflora. The method involves the collection and screening of total DNA from the microbe to identify novel genes' open reading frames encoding novel enzymes. The screening of metagenomic libraries is carried out by three strategies, viz., direct phenotypic detection, gene expression and heterologous complementation. For the conversion of lignocellulosic biomass, metagenomics offers a platform for the discovery of novel hydrolytic enzymes with novel properties, such as ionic stability, pH stability and thermostability [9]. Sun et al. [43] cloned a gene in Bacillus megaterium for thermostable xylanase expression from the metagenomic DNA of compost made from cow dung and reported a high extracellular xylanase activity (106 IU/ml) for XOS production. In another study, a novel xylanase (XynM1) was discovered using the metagenomic resource created from extreme temperature of an aquatic habitat by cloning the metagenomic DNA fragment and expressing it in Escherichia coli [118]. The results exhibited an increased catalytic activity of the novel xylanase in hydrolyzing the xylan and catalytically releasing XOS from beechwood, corncob, wheat and sweet sorghum. However, many more details on the exploring the role of these state-of-the art technologies for XOS production on industrial scale are yet to be revealed.
8. Future prospects and conclusions
Scientists from all over the world are interested in XOS production from LCB because of its many functional characteristics as prebiotics. The potential for XOS to develop unique functional foods is diverse, and the industry has a bright future in the food business. The market for XOS as prebiotics has been expanding steadily around the world due to the rise in interest in functional substances and life quality, with a growth of US$ 94 million in 2018 and a projected US$ 130 million by 2025 [37,119]. However, despite the great health benefits of xylooligosaccharides, the lack of an efficient bioprocess for converting lignocellulosic xylan into XOS hinders large-scale production. There is still scope for improvements in research and development, and sustainable commercialization of XOS. The study of lignocellulosic biomass for the production of high-value products like XOS has proliferated in recent years. Utilizing LCB gives agro-industrial wastes more value and enables their conversion into useful industrial products. It is also necessary to determine the technological and economic viability of XOS production. The extraction of xylan followed by hydrolysis is a typical method for producing XOS. An appropriate pretreatment which not only is inexpensive and eco-friendly, must also be effective in yielding the satisfactory results. In addition to existing physical and chemical pretreatments, additional techniques like high pressure, organosolvs and deep eutectic solvents might be mentioned as alternatives to increase enzyme accessibility to hemicellulose depolymerization. Enzymes have been suggested as a platform to produce XOS without hazardous by-products, however more knowledge about how to employ enzymes properly is still needed. As the non-digestible oligosaccharides are mostly metabolized/fermented by the intestinal microbiota, it is important to investigate the structure-function connection and the bioavailability of XOS in order to develop metabolites that have health favouring biological effects.
Funding statement
Dr. Vishal Sharma was supported by the National Science and Technology Council project, Taiwan (Ref. No. 112-2222-E-992-005). Prof. Cheng-Di Dong also thankfully acknowledges the Taiwan MOST for funding support (Ref. No. 109-2222-E-992-002).
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
All the authors thankfully acknowledge the National Kaohsiung University of Science and Technology, Kaohsiung City, Taiwan for technical support.
References
- 1.Sharma V., Tsai M.-L., Nargotra P., Chen C.-W., Sun P.-P., Singhania R.R., Patel A.K., Dong C.-D. Journey of lignin from a roadblock to bridge for lignocellulose biorefineries: a comprehensive review. Sci. Total Environ. 2022 doi: 10.1016/j.scitotenv.2022.160560. [DOI] [PubMed] [Google Scholar]
- 2.Sharma V., Tsai M.-L., Nargotra P., Chen C.-W., Kuo C.-H., Sun P.-P., Dong C.-D. Agro-Industrial food waste as a low-cost substrate for sustainable production of industrial enzymes: a Critical Review. Catalysts. 2022;12:1373. doi: 10.3390/catal12111373. [DOI] [Google Scholar]
- 3.Sharma S., Tsai M.-L., Sharma V., Sun P.-P., Nargotra P., Bajaj B.K., Chen C.-W., Dong C.-D. Environment friendly pretreatment approaches for the bioconversion of lignocellulosic biomass into biofuels and value-added products. Environments. 2023;10:6. doi: 10.3390/environments10010006. [DOI] [Google Scholar]
- 4.Yadav A., Sharma V., Tsai M.-L., Chen C.-W., Sun P.-P., Nargotra P., Wang J.-X., Dong C.-D. Development of lignocellulosic biorefineries for the sustainable production of biofuels: towards circular bioeconomy. Bioresour. Technol. 2023 doi: 10.1016/j.biortech.2023.129145. [DOI] [PubMed] [Google Scholar]
- 5.Sharma V., Bhat B., Gupta M., Vaid S., Sharma S., Nargotra P., Singh S., Bajaj B.K. In: Sustainable Biotechnology- Enzymatic Resources of Renewable Energy. Singh O.V., Chandel A.K., editors. Springer International Publishing; Cham: 2018. Role of systematic biology in biorefining of lignocellulosic residues for biofuels and chemicals production; pp. 5–55. [DOI] [Google Scholar]
- 6.Nargotra P., Sharma V., Lee Y.-C., Tsai Y.-H., Liu Y.-C., Shieh C.-J., Tsai M.-L., Dong C.-D., Kuo C.-H. Microbial lignocellulolytic enzymes for the effective valorization of lignocellulosic biomass: a review. Catalysts. 2023;13:83. doi: 10.3390/catal13010083. [DOI] [Google Scholar]
- 7.Sharma V., Tsai M.-L., Chen C.-W., Sun P.-P., Nargotra P., Dong C.-D. Advances in machine learning technology for sustainable biofuel production systems in lignocellulosic biorefineries. Sci. Total Environ. 2023;886 doi: 10.1016/j.scitotenv.2023.163972. [DOI] [PubMed] [Google Scholar]
- 8.Sharma V., Tsai M.-L., Sun P.-P., Chen C.-W., Nargotra P., Dong C.-D. Sequential ultrasound assisted deep eutectic solvent-based protein extraction from Sacha inchi meal biomass: towards circular bioeconomy. J. Food Sci. Technol. 2023 doi: 10.1007/s13197-023-05689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar V., Bahuguna A., Ramalingam S., Kim M. Developing a sustainable bioprocess for the cleaner production of xylooligosaccharides: an approach towards lignocellulosic waste management. J. Clean. Prod. 2021;316 doi: 10.1016/j.jclepro.2021.128332. [DOI] [Google Scholar]
- 10.Singh R.D., Banerjee J., Arora A. Prebiotic potential of oligosaccharides: a focus on xylan derived oligosaccharides. Bioact. Carbohydr. Diet. 2015;5:19–30. doi: 10.1016/j.bcdf.2014.11.003. [DOI] [Google Scholar]
- 11.Quigley E.M.M. Prebiotics and probiotics in digestive health. Clin. Gastroenterol. Hepatol. 2019;17:333–344. doi: 10.1016/j.cgh.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 12.You S., Ma Y., Yan B., Pei W., Wu Q., Ding C., Huang C. The promotion mechanism of prebiotics for probiotics: a review. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.1000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta M., Bangotra R., Sharma S., Vaid S., Kapoor N., Dutt H.C., Bajaj B.K. Bioprocess development for production of xylooligosaccharides prebiotics from sugarcane bagasse with high bioactivity potential. Ind. Crops Prod. 2022;178 doi: 10.1016/j.indcrop.2022.114591. [DOI] [Google Scholar]
- 14.Santibáñez L., Henríquez C., Corro-Tejeda R., Bernal S., Armijo B., Salazar O. Xylooligosaccharides from lignocellulosic biomass: a comprehensive review. Carbohydr. Polym. 2021;251 doi: 10.1016/j.carbpol.2020.117118. [DOI] [PubMed] [Google Scholar]
- 15.Lehuedé L., Henríquez C., Carú C., Córdova A., Mendonça R.T., Salazar O. Xylan extraction from hardwoods by alkaline pretreatment for xylooligosaccharide production: a detailed fractionation analysis. Carbohydr. Polym. 2023;302 doi: 10.1016/j.carbpol.2022.120381. [DOI] [PubMed] [Google Scholar]
- 16.Sharma V., Nargotra P., Sharma S., Bajaj B.K. Efficacy and functional mechanisms of a novel combinatorial pretreatment approach based on deep eutectic solvent and ultrasonic waves for bioconversion of sugarcane bagasse. Renew. Energy. 2021;163:1910–1922. doi: 10.1016/j.renene.2020.10.101. [DOI] [Google Scholar]
- 17.Nargotra P., Sharma V., Bajaj B.K. Consolidated bioprocessing of surfactant-assisted ionic liquid-pretreated Parthenium hysterophorus L. biomass for bioethanol production. Bioresour. Technol. 2019;289 doi: 10.1016/j.biortech.2019.121611. [DOI] [PubMed] [Google Scholar]
- 18.Sharma V., Nargotra P., Bajaj B.K. Ultrasound and surfactant assisted ionic liquid pretreatment of sugarcane bagasse for enhancing saccharification using enzymes from an ionic liquid tolerant Aspergillus assiutensis VS34, Bioresour. Technol. 2019;285 doi: 10.1016/j.biortech.2019.121319. [DOI] [PubMed] [Google Scholar]
- 19.Nargotra P., Sharma V., Gupta M., Kour S., Bajaj B.K. Application of ionic liquid and alkali pretreatment for enhancing saccharification of sunflower stalk biomass for potential biofuel-ethanol production. Bioresour. Technol. 2018;267:560–568. doi: 10.1016/j.biortech.2018.07.070. [DOI] [PubMed] [Google Scholar]
- 20.Shen B., Hou S., Jia Y., Yang C., Su Y., Ling Z., Huang C., Lai C., Yong Q. Synergistic effects of hydrothermal and deep eutectic solvent pretreatment on co-production of xylo-oligosaccharides and enzymatic hydrolysis of poplar. Bioresour. Technol. 2021;341 doi: 10.1016/j.biortech.2021.125787. [DOI] [PubMed] [Google Scholar]
- 21.Isci A., Thieme N., Lamp A., Zverlov V., Kaltschmitt M. Production of xylo-oligosaccharides from wheat straw using microwave assisted deep eutectic solvent pretreatment. Ind. Crops Prod. 2021;164 doi: 10.1016/j.indcrop.2021.113393. [DOI] [Google Scholar]
- 22.Davani-Davari D., Negahdaripour M., Karimzadeh I., Seifan M., Mohkam M., Masoumi S.J., Berenjian A., Ghasemi Y. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8:92. doi: 10.3390/foods8030092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma S., Sharma V., Nargotra P., Bajaj B.K. Process desired functional attributes of an endoxylanase of GH10 family from a new strain of Aspergillus terreus S9. Int. J. Biol. Macromol. 2018;115:663–671. doi: 10.1016/j.ijbiomac.2018.04.096. [DOI] [PubMed] [Google Scholar]
- 24.Sharma S., Nargotra P., Sharma V., Bangotra R., Kaur M., Kapoor N., Paul S., Bajaj B.K. Nanobiocatalysts for efficacious bioconversion of ionic liquid pretreated sugarcane tops biomass to biofuel. Bioresour. Technol. 2021;333 doi: 10.1016/j.biortech.2021.125191. [DOI] [PubMed] [Google Scholar]
- 25.Vaid S., Nargotra P., Bajaj B.K. Consolidated bioprocessing for biofuel-ethanol production from pine needle biomass. Environ. Prog. Sustain. 2018;37:546–552. doi: 10.1002/ep.12691. [DOI] [Google Scholar]
- 26.Sharma S., Sharma P., Sharma V., Bajaj B.K. Environmental and Agricultural Microbiology. John Wiley & Sons, Ltd; 2021. Polyhydroxybutyrate as an eco-friendly alternative of synthetic plastics; pp. 101–149. [DOI] [Google Scholar]
- 27.Sharma V., Tsai M.-L., Chen C.-W., Sun P.-P., Patel A.K., Singhania R.R., Nargotra P., Dong C.-D. Deep eutectic solvents as promising pretreatment agents for sustainable lignocellulosic biorefineries: a review. Bioresour. Technol. 2022;360 doi: 10.1016/j.biortech.2022.127631. [DOI] [PubMed] [Google Scholar]
- 28.Li R., Zheng Y., Zhao X., Yong Q., Meng X., Ragauskas A., Huang C. Recent advances in biomass pretreatment using biphasic solvent systems. Green Chem. 2023;25:2505–2523. doi: 10.1039/D3GC00271C. [DOI] [Google Scholar]
- 29.Huang C., Jiang X., Shen X., Hu J., Tang W., Wu X., Ragauskas A., Jameel H., Meng X., Yong Q. Lignin-enzyme interaction: a roadblock for efficient enzymatic hydrolysis of lignocellulosics. Renew. Sustain. Energy Rev. 2022;154 doi: 10.1016/j.rser.2021.111822. [DOI] [Google Scholar]
- 30.Sharma V., Nargotra P., Sharma S., Bajaj B.K. Efficient bioconversion of sugarcane tops biomass into biofuel-ethanol using an optimized alkali-ionic liquid pretreatment approach. Biomass Conv. Bioref. 2020:1–14. doi: 10.1007/s13399-020-01123-z. 10. [DOI] [Google Scholar]
- 31.Nargotra P., Sharma V., Sharma S., Kapoor N., Bajaj B.K. Development of consolidated bioprocess for biofuel-ethanol production from ultrasound-assisted deep eutectic solvent pretreated Parthenium hysterophorus biomass. Biomass Conv. Bioref. 2020;10:1–16. doi: 10.1007/s13399-020-01017-0. [DOI] [Google Scholar]
- 32.Nargotra P., Sharma V., Sharma S., Bangotra R., Bajaj B.K. Purification of an ionic liquid stable cellulase from Aspergillus aculeatus PN14 with potential for biomass refining. Environ. Sustain. 2022;5:313–323. doi: 10.1007/s42398-022-00232-x. [DOI] [Google Scholar]
- 33.Sharma V., Nargotra P., Sharma S., Sawhney D., Vaid S., Bangotra R., Dutt H.C., Bajaj B.K. Microwave irradiation-assisted ionic liquid or deep eutectic solvent pretreatment for effective bioconversion of sugarcane bagasse to bioethanol. Energ. Ecol. Environ. 2023;8:141–156. doi: 10.1007/s40974-022-00267-0. [DOI] [Google Scholar]
- 34.Ortizo R.G.G., Sharma V., Tsai M.-L., Wang J.-X., Sun P.-P., Nargotra P., Kuo C.-H., Chen C.-W., Dong C.-D. Extraction of novel bioactive peptides from fish protein hydrolysates by enzymatic reactions. Appl. Sci. 2023;13:5768. doi: 10.3390/app13095768. [DOI] [Google Scholar]
- 35.Hutkins R.W., Krumbeck J.A., Bindels L.B., Cani P.D., Fahey G., Goh Y.J., Hamaker B., Martens E.C., Mills D.A., Rastal R.A., Vaughan E., Sanders M.E. Prebiotics: why definitions matter. Curr. Opin. Biotechnol. 2016;37:1–7. doi: 10.1016/j.copbio.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 37.Corim Marim A.V., Gabardo S. Xylooligosaccharides: prebiotic potential from agro-industrial residue, production strategies and prospects. Biocatal. Agric. Biotechnol. 2021;37 doi: 10.1016/j.bcab.2021.102190. [DOI] [Google Scholar]
- 38.Sanders M.E., Merenstein D.J., Reid G., Gibson G.R., Rastall R.A. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019;16:605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- 39.Kumar D., Lal M.K., Dutt S., Raigond P., Changan S.S., Tiwari R.K., Chourasia K.N., Mangal V., Singh B. Functional fermented probiotics, prebiotics, and synbiotics from non-dairy products: a perspective from nutraceutical. Mol. Nutr. Food Res. 2022;66 doi: 10.1002/mnfr.202101059. [DOI] [PubMed] [Google Scholar]
- 40.Saini R., Patel A.K., Saini J.K., Chen C.-W., Varjani S., Singhania R.R., Di Dong C. Recent advancements in prebiotic oligomers synthesis via enzymatic hydrolysis of lignocellulosic biomass. Bioengineered. 2022;13:2139–2172. doi: 10.1080/21655979.2021.2023801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samanta A.K., Jayapal N., Jayaram C., Roy S., Kolte A.P., Senani S., Sridhar M. Xylooligosaccharides as prebiotics from agricultural by-products: production and applications. Bioact. Carbohydr. Diet. Fibre. 2015;5:62–71. doi: 10.1016/j.bcdf.2014.12.003. [DOI] [Google Scholar]
- 42.Baker J.T., Duarte M.E., Holanda D.M., Kim S.W. Friend or Foe? Impacts of dietary xylans, xylooligosaccharides, and xylanases on intestinal health and growth Performance of Monogastric Animals. Animals. 2021;11:609. doi: 10.3390/ani11030609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun M., Zheng H., Meng L., Sun J., Song H., Bao Y., Pei H., Yan Z., Zhang X., Zhang J., Liu Y., Lu F. Direct cloning, expression of a thermostable xylanase gene from the metagenomic DNA of cow dung compost and enzymatic production of xylooligosaccharides from corncob. Biotechnol. Lett. 2015;37:1877–1886. doi: 10.1007/s10529-015-1857-6. [DOI] [PubMed] [Google Scholar]
- 44.Zhang W., Zhang B., Lei F., Li P., Jiang J. Coproduction xylo-oligosaccharides with low degree of polymerization and glucose from sugarcane bagasse by non-isothermal subcritical carbon dioxide assisted seawater autohydrolysis. Bioresour. Technol. 2022;349 doi: 10.1016/j.biortech.2022.126866. [DOI] [PubMed] [Google Scholar]
- 45.Mano M.C.R., Neri-Numa I.A., da Silva J.B., Paulino B.N., Pessoa M.G., Pastore G.M. Oligosaccharide biotechnology: an approach of prebiotic revolution on the industry. Appl. Microbiol. Biotechnol. 2018;102:17–37. doi: 10.1007/s00253-017-8564-2. [DOI] [PubMed] [Google Scholar]
- 46.Huang J., Zhu S., Zhao L., Chen L., Du M., Zhang C., Yang S.-T. A novel β-galactosidase from Klebsiella oxytoca ZJUH1705 for efficient production of galacto-oligosaccharides from lactose. Appl. Microbiol. Biotechnol. 2020;104:6161–6172. doi: 10.1007/s00253-020-10679-9. [DOI] [PubMed] [Google Scholar]
- 47.Moreno F.J., Montilla A., Villamiel M., Corzo N., Olano A. Analysis, structural characterization, and bioactivity of oligosaccharides derived from lactose. Electrophoresis. 2014;35:1519–1534. doi: 10.1002/elps.201300567. [DOI] [PubMed] [Google Scholar]
- 48.Maity M., Majumdar S., Bhattacharyya D.K., Bhowal J., Das A., Barui A. Evaluation of prebiotic properties of galactooligosaccharides produced by transgalactosylation using partially purified β-galactosidase from Enterobacter aerogenes KCTC2190. Appl. Biochem. Biotechnol. 2023;195:2294–2316. doi: 10.1007/s12010-022-04073-6. [DOI] [PubMed] [Google Scholar]
- 49.Bhatia L., Sharma A., Bachheti R.K., Chandel A.K. Lignocellulose derived functional oligosaccharides: production, properties, and health benefits. Prep. Biochem. Biotechnol. 2019;49:744–758. doi: 10.1080/10826068.2019.1608446. [DOI] [PubMed] [Google Scholar]
- 50.Singh S.P., Jadaun J.S., Narnoliya L.K., Pandey A. Prebiotic oligosaccharides: special focus on fructooligosaccharides, its biosynthesis and bioactivity. Appl. Biochem. Biotechnol. 2017;183:613–635. doi: 10.1007/s12010-017-2605-2. [DOI] [PubMed] [Google Scholar]
- 51.de la Rosa O., Flores-Gallegos A.C., Muñíz-Marquez D., Nobre C., Contreras-Esquivel J.C., Aguilar C.N. Fructooligosaccharides production from agro-wastes as alternative low-cost source. Trends Food Sci. Technol. 2019;91:139–146. doi: 10.1016/j.tifs.2019.06.013. [DOI] [Google Scholar]
- 52.Wienberg F., Hövels M., Deppenmeier U. High-yield production and purification of prebiotic inulin-type fructooligosaccharides. Amb. Express. 2022;12:144. doi: 10.1186/s13568-022-01485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aisara J., Wongputtisin P., Deejing S., Maneewong C., Unban K., Khanongnuch C., Kosma P., Blaukopf M., Kanpiengjai A. Potential of inulin-fructooligosaccharides extract produced from red onion (Allium cepa var. viviparum (Metz) Mansf.) as an alternative prebiotic product. Plants. 2021;10:2401. doi: 10.3390/plants10112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barbosa F.C., Kendrick E., Brenelli L.B., Arruda H.S., Pastore G.M., Rabelo S.C., Damasio A., Franco T.T., Leak D., Goldbeck R. Optimization of cello-oligosaccharides production by enzymatic hydrolysis of hydrothermally pretreated sugarcane straw using cellulolytic and oxidative enzymes. Biomass Bioenergy. 2020;141 doi: 10.1016/j.biombioe.2020.105697. [DOI] [Google Scholar]
- 55.Ávila P.F., Silva M.F., Martins M., Goldbeck R. Cello-oligosaccharides production from lignocellulosic biomass and their emerging prebiotic applications. World J. Microbiol. Biotechnol. 2021;37:73. doi: 10.1007/s11274-021-03041-2. [DOI] [PubMed] [Google Scholar]
- 56.Zhong C., Ukowitz C., Domig K.J., Nidetzky B. Short-Chain cello-oligosaccharides: intensification and scale-up of their enzymatic production and selective growth promotion among probiotic bacteria. J. Agric. Food Chem. 2020;68:8557–8567. doi: 10.1021/acs.jafc.0c02660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pinales-Márquez C.D., Rodríguez-Jasso R.M., Araújo R.G., Loredo-Treviño A., Nabarlatz D., Gullón B., Ruiz H.A. Circular bioeconomy and integrated biorefinery in the production of xylooligosaccharides from lignocellulosic biomass: a review. Ind. Crops Prod. 2021;162 doi: 10.1016/j.indcrop.2021.113274. [DOI] [Google Scholar]
- 58.Amorim C., Silvério S.C., Prather K.L.J., Rodrigues L.R. From lignocellulosic residues to market: production and commercial potential of xylooligosaccharides. Biotechnol. Adv. 2019;37 doi: 10.1016/j.biotechadv.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Tran C.T.H., Nargotra P., Pham H.T.C., Lieu D.M., Huynh P.K., Wang H.-M.D., Dong C.-D., Kuo C.-H. The effect of carboxymethyl cellulose and β-cyclodextrin as debittering agents on bitterness and physicochemical properties of bitter gourd extract. J. Food Sci. Technol. 2023;60:1521–1529. doi: 10.1007/s13197-023-05693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palaniappan A., Antony U., Emmambux M.N. Current status of xylooligosaccharides: production, characterization, health benefits and food application. Trends Food Sci. Technol. 2021;111:506–519. doi: 10.1016/j.tifs.2021.02.047. [DOI] [Google Scholar]
- 61.de Freitas C., Carmona E., Brienzo M. Xylooligosaccharides production process from lignocellulosic biomass and bioactive effects. Bioact. Carbohydr. Diet. Fibre. 2019;18 doi: 10.1016/j.bcdf.2019.100184. [DOI] [Google Scholar]
- 62.Chen Y., Xie Y., Zhong R., Liu L., Lin C., Xiao L., Chen L., Zhang H., Beckers Y., Everaert N. Effects of xylo-oligosaccharides on growth and gut microbiota as potential replacements for antibiotic in weaning piglets. Front. Microbiol. 2021;12:1–12. doi: 10.3389/fmicb.2021.641172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gobinath D., Madhu A.N., Prashant G., Srinivasan K., Prapulla S.G. Beneficial effect of xylo-oligosaccharides and fructo-oligosaccharides in streptozotocin-induced diabetic rats. Br. J. Nutr. 2010;104:40–47. doi: 10.1017/S0007114510000243. [DOI] [PubMed] [Google Scholar]
- 64.Patil P.S., Fernandes C.G., Sawant S.C., Lali A.M., Odaneth A.A. High-throughput system for carbohydrate analysis of lignocellulosic biomass. Biomass Conv. Bioref. 2022 doi: 10.1007/s13399-022-02304-8. [DOI] [Google Scholar]
- 65.Poletto P., Pereira G.N., Monteiro C.R.M., Pereira M.A.F., Bordignon S.E., de Oliveira D. Xylooligosaccharides: transforming the lignocellulosic biomasses into valuable 5-carbon sugar prebiotics. Process Biochem. 2020;91:352–363. doi: 10.1016/j.procbio.2020.01.005. [DOI] [Google Scholar]
- 66.Otieno D.O., Ahring B.K. The potential for oligosaccharide production from the hemicellulose fraction of biomasses through pretreatment processes: xylooligosaccharides (XOS), arabinooligosaccharides (AOS), and mannooligosaccharides (MOS) Carbohydr. Res. 2012;360:84–92. doi: 10.1016/j.carres.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 67.Huang X., Ouyang X., Hendriks B.M.S., Morales Gonzalez O.M., Zhu J., Korányi T.I., Boot M.D., Hensen E.J.M. Selective production of mono-aromatics from lignocellulose over Pd/C catalyst: the influence of acid co-catalysts. Faraday Discuss. 2017;202:141–156. doi: 10.1039/C7FD00039A. [DOI] [PubMed] [Google Scholar]
- 68.Ying W., Fang X., Xu Y., Zhang J. Combined acetic acid and enzymatic hydrolysis for xylooligosaccharides and monosaccharides production from poplar. Biomass Bioenergy. 2022;158 doi: 10.1016/j.biombioe.2022.106377. [DOI] [Google Scholar]
- 69.Baramee S., Siriatcharanon A., Ketbot P., Teeravivattanakit T., Waeonukul R., Pason P., Tachaapaikoon C., Ratanakhanokchai K., Phitsuwan P. Biological pretreatment of rice straw with cellulase-free xylanolytic enzyme-producing Bacillus firmus K-1: structural modification and biomass digestibility. Renew. Energy. 2020;160:555–563. doi: 10.1016/j.renene.2020.06.061. [DOI] [Google Scholar]
- 70.Guo J., Cao R., Huang K., Xu Y. Comparison of selective acidolysis of xylan and enzymatic hydrolysability of cellulose in various lignocellulosic materials by a novel xylonic acid catalysis method. Bioresour. Technol. 2020;304 doi: 10.1016/j.biortech.2020.122943. [DOI] [PubMed] [Google Scholar]
- 71.Xiong B., Ma S., Chen B., Feng Y., Peng Z., Tang X., Yang S., Sun Y., Lin L., Zeng X., Chen Y. Formic acid-facilitated hydrothermal pretreatment of raw biomass for co-producing xylo-oligosaccharides, glucose, and lignin. Ind. Crops Prod. 2023;193 doi: 10.1016/j.indcrop.2022.116195. [DOI] [Google Scholar]
- 72.Luo H., Shi Y., Xie F., Zhou T., Gao L., Yang R., Wang Z. Efficient co-production of fermentable sugars and biobutanol from corn stover based on a novel butyric acid pretreatment strategy. Ind. Crops Prod. 2023;191 doi: 10.1016/j.indcrop.2022.115976. [DOI] [Google Scholar]
- 73.Achinas S., Krooneman J., Euverink G.J.W. Enhanced biogas production from the anaerobic batch treatment of banana peels. Engineering. 2019;5:970–978. doi: 10.1016/j.eng.2018.11.036. [DOI] [Google Scholar]
- 74.Siacor F.D.C., Lobarbio C.F.Y., Taboada E.B. Pretreatment of Mango (Mangifera indica L. Anacardiaceae) seed husk for bioethanol production by dilute acid treatment and enzymatic hydrolysis. Appl. Biochem. Biotechnol. 2021;193:1338–1350. doi: 10.1007/s12010-020-03387-7. [DOI] [PubMed] [Google Scholar]
- 75.Rojas-Chamorro J.A., Romero I., López-Linares J.C., Castro E. Brewer’s spent grain as a source of renewable fuel through optimized dilute acid pretreatment. Renew. Energy. 2020;148:81–90. doi: 10.1016/j.renene.2019.12.030. [DOI] [Google Scholar]
- 76.Do N.H.N., Ho K.H., Nguyen V.V., Le P.K. Novel recycling of pineapple leaves into cellulose microfibers by two-step grinding of ball milling and high-speed rotor–stator homogenization. J. Polym. Res. 2022;29:1–9. doi: 10.1007/s10965-022-03081-8. [DOI] [Google Scholar]
- 77.Deshavath N.N., Mohan M., Veeranki V.D., Goud V.V., Pinnamaneni S.R., Benarjee T. Dilute acid pretreatment of sorghum biomass to maximize the hemicellulose hydrolysis with minimized levels of fermentative inhibitors for bioethanol production. 3 Biotech. 2017;7:139. doi: 10.1007/s13205-017-0752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang L., Lu M., Carl S., Mayer J.A., Cushman J.C., Tian E., Lin H. Biomass characterization of Agave and Opuntia as potential biofuel feedstocks. Biomass Bioenergy. 2015;76:43–53. doi: 10.1016/j.biombioe.2015.03.004. [DOI] [Google Scholar]
- 79.Vaid S., Sharma S., Dutt H.C., Mahajan R., Bajaj B.K. One pot consolidated bioprocess for conversion of Saccharum spontaneum biomass to ethanol-biofuel. Energy Convers. Manag. 2021;250 doi: 10.1016/j.enconman.2021.114880. [DOI] [Google Scholar]
- 80.Singh N., Singhania R.R., Nigam P.S., Dong C.-D., Patel A.K., Puri M. Global status of lignocellulosic biorefinery: challenges and perspectives. Bioresour. Technol. 2022;344 doi: 10.1016/j.biortech.2021.126415. [DOI] [PubMed] [Google Scholar]
- 81.Kumar B., Bhardwaj N., Agrawal K., Chaturvedi V., Verma P. Current perspective on pretreatment technologies using lignocellulosic biomass: an emerging biorefinery concept. Fuel Process. Technol. 2020;199 doi: 10.1016/j.fuproc.2019.106244. [DOI] [Google Scholar]
- 82.Sindhu R., Binod P., Pandey A. Biological pretreatment of lignocellulosic biomass – an overview. Bioresour. Technol. 2016;199:76–82. doi: 10.1016/j.biortech.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 83.Singhania R.R., Patel A.K., Raj T., Chen C.-W., Ponnusamy V.K., Tahir N., Kim S.-H., Dong C.-D. Lignin valorisation via enzymes: a sustainable approach. Fuel. 2022;311 doi: 10.1016/j.fuel.2021.122608. [DOI] [Google Scholar]
- 84.Haldar D., Dey P., Patel A.K., Dong C.-D., Singhania R.R. A critical review on the effect of lignin redeposition on biomass in controlling the process of enzymatic hydrolysis. Bioenerg. Res. 2022;15:863–874. doi: 10.1007/s12155-021-10374-1. [DOI] [Google Scholar]
- 85.Tsai M.-F., Huang C.-Y., Nargotra P., Tang W.-R., Liao K.-T., Lee Y.-C., Lin C.-M., Lin C., Shieh C.-J., Kuo C.-H. Green extraction and purification of chondroitin sulfate from jumbo squid cartilage by a novel procedure combined with enzyme, ultrasound and hollow fiber dialysis. J. Food Sci. Technol. 2023;60:1711–1722. doi: 10.1007/s13197-023-05701-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nargotra P., Sharma V., Tsai M.-L., Hsieh S.-L., Dong C.-D., Wang H.-M.D., Kuo C.-H. Recent advancements in the valorization of agro-industrial food waste for the production of nanocellulose. Appl. Sci. 2023;13:6159. doi: 10.3390/app13106159. [DOI] [Google Scholar]
- 87.Vaid S., Bhat N., Nargotra P., Bajaj B.K. Combinatorial application of ammonium carbonate and sulphuric acid pretreatment to achieve enhanced sugar yield from pine needle biomass for potential biofuel–ethanol production. Energ. Ecol. Environ. 2018;3:126–135. doi: 10.1007/s40974-018-0083-1. [DOI] [Google Scholar]
- 88.Han J., Cao R., Zhou X., Xu Y. An integrated biorefinery process for adding values to corncob in co-production of xylooligosaccharides and glucose starting from pretreatment with gluconic acid. Bioresour. Technol. 2020;307 doi: 10.1016/j.biortech.2020.123200. [DOI] [PubMed] [Google Scholar]
- 89.Zhou X., Xu Y. Eco-friendly consolidated process for co-production of xylooligosaccharides and fermentable sugars using self-providing xylonic acid as key pretreatment catalyst. Biotechnol. Biofuels. 2019;12:272. doi: 10.1186/s13068-019-1614-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan B., Huang C., Lai C., Ling Z., Yong Q. Production of prebiotic xylooligosaccharides from industrial-derived xylan residue by organic acid treatment. Carbohydr. Polym. 2022;292 doi: 10.1016/j.carbpol.2022.119641. [DOI] [PubMed] [Google Scholar]
- 91.Madadi M., Zahoor, Shah S.W.A., Sun C., Wang W., Ali S.S., Khan A., Arif M., Zhu D. Efficient co-production of xylooligosaccharides and glucose from lignocelluloses by acid/pentanol pretreatment: synergetic role of lignin removal and inhibitors. Bioresour. Technol. 2022;365 doi: 10.1016/j.biortech.2022.128171. [DOI] [PubMed] [Google Scholar]
- 92.Dai L., Huang T., Jiang K., Zhou X., Xu Y. A novel recyclable furoic acid-assisted pretreatment for sugarcane bagasse biorefinery in co-production of xylooligosaccharides and glucose. Biotechnol. Biofuels. 2021;14:35. doi: 10.1186/s13068-021-01884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jin Y., Shi Z., Xu G., Yang H., Yang J. A stepwise pretreatment of sugarcane bagasse by alkaline and hydroxymethyl reagent for bioethanol production. Ind. Crops Prod. 2020;145 doi: 10.1016/j.indcrop.2020.112136. [DOI] [Google Scholar]
- 94.Rogoski W., Pereira G.N., Cesca K., da Silva M.A., Zanella E., Stambuk B.U., Ávila P.F., Goldbeck R., de Oliveira D., de Andrade C.J. Production of cassava peel-based xylooligosaccharides using endo-1,4-β-xylanase from Trichoderma longibrachiatum: the effect of alkaline pretreatment. Biomass Conv. Bioref. 2022 doi: 10.1007/s13399-022-03287-2. [DOI] [Google Scholar]
- 95.Patel A., Divecha J., Shah A. A sustainable process for co-production of xylooligosaccharides and ethanol from alkali treated sugarcane bagasse: a strategy towards waste management. Prep. Biochem. Biotechnol. 2022;0:1–11. doi: 10.1080/10826068.2022.2119575. [DOI] [PubMed] [Google Scholar]
- 96.Ávila P.F., Franco Cairo J.P.L., Damasio A., Forte M.B.S., Goldbeck R. Xylooligosaccharides production from a sugarcane biomass mixture: effects of commercial enzyme combinations on bagasse/straw hydrolysis pretreated using different strategies. Food Res. Int. 2020;128 doi: 10.1016/j.foodres.2019.108702. [DOI] [PubMed] [Google Scholar]
- 97.Bhatia R., Lad J.B., Bosch M., Bryant D.N., Leak D., Hallett J.P., Franco T.T., Gallagher J.A. Production of oligosaccharides and biofuels from Miscanthus using combinatorial steam explosion and ionic liquid pretreatment. Bioresour. Technol. 2021;323 doi: 10.1016/j.biortech.2020.124625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amesho K.T.T., Lin Y.-C., Mohan S.V., Halder S., Ponnusamy V.K., Jhang S.-R. Deep eutectic solvents in the transformation of biomass into biofuels and fine chemicals: a review. Environ. Chem. Lett. 2022 doi: 10.1007/s10311-022-01521-x. [DOI] [Google Scholar]
- 99.Gong W.-H., Zhang C., He J.-W., Gao Y.-Y., Li Y.-J., Zhu M.-Q., Wen J.-L. A synergistic hydrothermal-deep eutectic solvents (DES) pretreatment for acquiring xylooligosaccharides and lignin nanoparticles from Eucommia ulmoides wood. Int. J. Biol. Macromol. 2022;209:188–197. doi: 10.1016/j.ijbiomac.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 100.Ma C.-Y., Sun Q., Zuo C., Xu L.-H., Sun S.-N., Wen J.-L., Yuan T.-Q. Efficient fractionation and targeted valorization of industrial xylose residue by synergistic and mild alkaline deep eutectic solvent-hydrogen peroxide pretreatment. Fuel Process. Technol. 2023;241 doi: 10.1016/j.fuproc.2022.107591. [DOI] [Google Scholar]
- 101.Ying W., Li X., Lian Z., Xu Y., Zhang J. An integrated process using acetic acid hydrolysis and deep eutectic solvent pretreatment for xylooligosaccharides and monosaccharides production from wheat bran. Bioresour. Technol. 2022;363 doi: 10.1016/j.biortech.2022.127966. [DOI] [PubMed] [Google Scholar]
- 102.de Freitas C., Brienzo M. Enzymatic Hydrolysis Applied to banana pseudostem biomass compared to solubilized xylan for xylooligosaccharides production with high substrate concentration. Bioenerg. Res. 2022 doi: 10.1007/s12155-022-10508-z. [DOI] [Google Scholar]
- 103.Zhang F., Lan W., Li Z., Zhang A., Tang B., Wang H., Wang X., Ren J., Liu C. Co-production of functional xylo-oligosaccharides and fermentable sugars from corn stover through fast and facile ball mill-assisted alkaline peroxide pretreatment. Bioresour. Technol. 2021;337 doi: 10.1016/j.biortech.2021.125327. [DOI] [PubMed] [Google Scholar]
- 104.Xu J., Hou H., Hu J., Liu B. Coupling of hydrothermal and ionic liquid pretreatments for sequential biorefinery of Tamarix austromongolica. Appl. Energy. 2018;229:745–755. doi: 10.1016/j.apenergy.2018.08.038. [DOI] [Google Scholar]
- 105.Singh S., Gupta P., Sharma V., Koul S., Kour K., Bajaj B.K. Multifarious potential applications of keratinase of Bacillus subtilis K-5. Biocatal. Biotransform. 2014;32:333–342. doi: 10.3109/10242422.2014.978306. [DOI] [Google Scholar]
- 106.Chen C.-C., Nargotra P., Kuo C.-H., Liu Y.-C. High-Molecular-Weight exopolysaccharides production from Tuber borchii cultivated by submerged fermentation. Int. J. Mol. Sci. 2023;24:4875. doi: 10.3390/ijms24054875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma S., Sharma V., Nargotra P., Bajaj B.K. Bioprocess development for production of a process-apt xylanase with multifaceted application potential for a range of industrial processes. SN Appl. Sci. 2020;2:739. doi: 10.1007/s42452-020-2541-6. [DOI] [Google Scholar]
- 108.Rico X., Gullón B., Alonso J.L., Parajó J.C., Yáñez R. Valorization of peanut shells: manufacture of bioactive oligosaccharides. Carbohydr. Polym. 2018;183:21–28. doi: 10.1016/j.carbpol.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 109.Wang F., Yao Z., Zhang X., Han Z., Chu X., Ge X., Lu F., Liu Y. High-level production of xylose from agricultural wastes using GH11 endo-xylanase and GH43 β-xylosidase from Bacillus sp. Bioproc. Biosyst. Eng. 2022;45:1705–1717. doi: 10.1007/s00449-022-02778-w. [DOI] [PubMed] [Google Scholar]
- 110.Pereira G.F., de Bastiani D., Gabardo S., Squina F., Ayub M.A.Z. Solid-state cultivation of recombinant Aspergillus nidulans to co-produce xylanase, arabinofuranosidase, and xylooligosaccharides from soybean fibre. Biocatal. Agric. Biotechnol. 2018;15:78–85. doi: 10.1016/j.bcab.2018.05.012. [DOI] [Google Scholar]
- 111.Jagtap S., Deshmukh R.A., Menon S., Das S. Xylooligosaccharides production by crude microbial enzymes from agricultural waste without prior treatment and their potential application as nutraceuticals. Bioresour. Technol. 2017;245:283–288. doi: 10.1016/j.biortech.2017.08.174. [DOI] [PubMed] [Google Scholar]
- 112.de Figueiredo F.C., Carvalho A.F.A., Brienzo M., Campioni T.S., de Oliva-Neto P. Chemical input reduction in the arabinoxylan and lignocellulose alkaline extraction and xylooligosaccharides production. Bioresour. Technol. 2017;228:164–170. doi: 10.1016/j.biortech.2016.12.097. [DOI] [PubMed] [Google Scholar]
- 113.Huang C., Li R., Tang W., Zheng Y., Meng X. Improve Enzymatic Hydrolysis of lignocellulosic biomass by modifying lignin structure via sulfite pretreatment and using lignin blockers. Fermentation. 2022;8:558. doi: 10.3390/fermentation8100558. [DOI] [Google Scholar]
- 114.Xu X., Liu M., Huo W., Dai X. Obtaining a mutant of Bacillus amyloliquefaciens xylanase A with improved catalytic activity by directed evolution. Enzym. Microb. Technol. 2016;86:59–66. doi: 10.1016/j.enzmictec.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 115.Wu Q., Zhang C., Zhu W., Lu H., Li X., Yang Y., Xu Y., Li W. Improved thermostability, acid tolerance as well as catalytic efficiency of Streptomyces rameus L2001 GH11 xylanase by N-terminal replacement. Enzym. Microb. Technol. 2023;162 doi: 10.1016/j.enzmictec.2022.110143. [DOI] [PubMed] [Google Scholar]
- 116.Yang J., Ma T., Shang-guan F., Han Z. Improving the catalytic activity of thermostable xylanase from Thermotoga maritima via mutagenesis of non-catalytic residues at glycone subsites. Enzym. Microb. Technol. 2020;139 doi: 10.1016/j.enzmictec.2020.109579. [DOI] [PubMed] [Google Scholar]
- 117.Boonyapakron K., Chitnumsub P., Kanokratana P., Champreda V. Enhancement of catalytic performance of a metagenome-derived thermophilic oligosaccharide-specific xylanase by binding module removal and random mutagenesis. J. Biosci. Bioeng. 2021;131:13–19. doi: 10.1016/j.jbiosc.2020.09.008. [DOI] [PubMed] [Google Scholar]
- 118.Joshi N., Sharma M., Singh S.P. Characterization of a novel xylanase from an extreme temperature hot spring metagenome for xylooligosaccharide production. Appl. Microbiol. Biotechnol. 2020;104:4889–4901. doi: 10.1007/s00253-020-10562-7. [DOI] [PubMed] [Google Scholar]
- 119.Singh R.D., Nadar C.G., Muir J., Arora A. Green and clean process to obtain low degree of polymerisation xylooligosaccharides from almond shell. J. Clean. Prod. 2019;241 doi: 10.1016/j.jclepro.2019.118237. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.