Abstract
Background
Epinephrine is currently the only recommended cardio-resuscitative medication for use in neonatal cardiopulmonary resuscitation (CPR), as per the consensus of science and treatment recommendations. An alternative medication, vasopressin, might be beneficial in neonatal CPR due to its combined pulmonary vasodilation and systemic vasoconstriction properties.
Aim
We aimed to compare the time to return of spontaneous circulation (ROSC) with administration of vasopressin or epinephrine during CPR of asphyxiated post-transitional piglets.
Methods
Newborn piglets (n = 8/group) were anesthetized, tracheotomized and intubated, instrumented, and exposed to 50 min normocapnic hypoxia followed by asphyxia and cardiac arrest. Piglets were randomly allocated to receive vasopressin (Vaso, 0.4 U/kg) or epinephrine (Epi, 0.02 mg/kg) during CPR. Piglets were resuscitated with chest compressions superimposed with sustained inflations, and were administered either Vaso or Epi intravenously every 3 min until ROSC (max. 3 doses). Hemodynamic and cardiac function parameters were collected.
Main Results
The median (IQR) time to ROSC was 106 (93–140) s with Vaso and 128 (100–198) s with Epi (p = 0.28). The number of piglets that achieved ROSC was 8 (100%) with Vaso and 7 (88%) with Epi (p = 1.00). Vaso-treated piglets had a significantly longer post-resuscitation survival time (240 (240–240) min) than Epi-treated piglets (65 (30–240) min, p = 0.02). Vaso-treated piglets had significantly improved carotid blood flow immediately after ROSC (p < 0.05), had longer duration of post-resuscitation hypertension (p = 0.05), and had significantly improved heart rate, arterial pressure, and cerebral blood oxygen saturation 4 h after ROSC (p < 0.05).
Conclusions
Vasopressin improved post-resuscitation survival and hemodynamics, and might be an alternative cardio-resuscitative medication during neonatal CPR, but further studies are warranted.
Keywords: Infant, Newborn, Vasopressor, Chest Compression, Sustained Inflation, Adrenaline
Introduction
In the delivery room, the incidence of cardiopulmonary resuscitation (CPR) is 0.06% to 0.12% (0.1% of term and up to 15% of preterm infants),1, 2 while during neonatal intensive care unit (NICU) admission CPR occurs in 0.25% to 1%.3 The current consensus of science and treatment recommendations (CoSTR) are focused on delivery room resuscitation and there are no specific recommendations for NICU CPR,4 however, the delivery room resuscitation recommendations are routinely applied in the NICU.
Cardiac arrest in newborn infants is mainly a consequence of hypoxia/asphyxia,5 and successful resuscitation requires delivery of high-quality chest compressions (CC) and the most effective cardio-resuscitative agent.6, 7 The current CoSTR states that epinephrine should be given at a dose of 0.01–0.03 mg/kg, preferably given intravenously, with repeated doses every 3–5 min during CPR until return of spontaneous circulation (ROSC).5 Epinephrine is an endogenous catecholamine, which causes vasoconstriction (α1 receptors), coronary vasoconstriction (α2 receptors), β1 receptors stimulation [increases heart rate (chronotropy), conduction velocity (dromotropy), contractility (inotropy), rate of myocardial relaxation (lusitropy)], and smooth muscle relaxation and increases myocardial contractility (β2 receptor).8, 9 However, epinephrine also increases myocardial oxygen demand and respiratory and metabolic acidosis and inhibits hemodynamic responses (e.g., aggravated hypertension or tachycardia after ROSC).10
Although epinephrine has been used for decades during neonatal CPR, the optimal timing, dose, and route are unknown.8, 9, 11 High-quality evidence (i.e., large randomized clinical trials) to better guide healthcare providers in resuscitative effort are lacking, and arises from i) the relatively infrequent need of CC and epinephrine during neonatal CPR, and ii) the inability to consistently anticipate which newborn infants are at high risk of requiring CPR. Guidelines for neonatal CPR recognize the lack of neonatal data (a recent systematic review from the International Liaison Committee on Resuscitation identified only four cohort studies including 117 patients reporting on epinephrine).12 Neonatal guidelines extrapolate data from studies with adult patients and animals, which may not apply wholly to neonates.5, 13
Vasopressin, an antidiuretic hormone, might be an alternative as it causes systemic vasoconstriction with pulmonary vasodilation, does not worsen respiratory and metabolic acidosis, and does not increase myocardial oxygen demand.14 In adults with out-of-hospital cardiac arrest, vasopressin was associated with significantly higher rates of survival as compared with epinephrine until hospital admission (29% vs. 20% p = 0.02) and hospital discharge (5% vs. 2%, p = 0.04).15 Duncan et al. reported that pediatric patients who received vasopressin as a rescue medication after epinephrine administration during in-hospital cardiac arrest had similar survival at 24 h or at discharge compared to patients only receiving epinephrine.16 Although vasopressin might be beneficial when asystole is the leading cause for cardiac arrest, there is currently insufficient data about vasopressin during neonatal resuscitation. We aimed to compare vasopressin and epinephrine during CPR of asphyxiated post-transitional piglets. The systemic vasoconstriction and pulmonary vasodilation characteristics of vasopressin may be beneficial in a neonatal CPR setting, especially in the presence of high pulmonary vascular resistance. Therefore, we hypothesized that in asphyxiated newborn post-transitional piglets receiving CPR, vasopressin compared to epinephrine will reduce time to ROSC and improve survival.
Methods
All experiments were conducted after approval from the Animal Care and Use Committee, University of Alberta (AUP00002920), according to the ARRIVE guidelines,17 and registered at preclinicaltrials.eu (PCTE0000368). The study protocol is graphically presented in Fig. 1.
Fig. 1.
Study flow diagram.
Randomization
Piglets were randomly allocated to either vasopressin (Vaso) or epinephrine (Epi). Allocation was block-randomized 1:1 with variable block sizes using a computer-generated randomization program (https://www.randomizer.org). Sequentially numbered, sealed, brown envelopes containing the group allocation were opened during the experiments (Fig. 1).
Sample size and power estimates
The primary outcome measure was resuscitation time to achieve ROSC. Our previous studies reported a mean (standard deviation (SD)) time to ROSC of 200 (20) s during resuscitation using CC with sustained inflations (CC + SI) and an intravenous epinephrine dose of 0.02 mg/kg.18 We hypothesized that an intravenous vasopressin dose of 0.4 U/kg during resuscitation with CC + SI would reduce time to achieve ROSC. A sample size of 16 piglets (eight per group) would be sufficient to detect a clinically important (10%) reduction in time to achieve ROSC (i.e., 200 vs. 180 s), with 80% power and a 2-tailed alpha error of 0.05. We used 0.02 mg/kg of epinephrine and 0.4 U/kg of vasopressin as these doses were currently or previously recommended.
Blinding
One investigator (TFL) opened the randomization envelope and prepared the study drug. The content of the drug syringe was only known to TFL to conceal group allocation. GMS assessed cardiac arrest (confirmed asystole) and was blinded to group allocation. All other group members were also blinded to group allocation. The statistical analysis was blinded to group allocation, and the investigators were unblinded following completion of the analysis.
Inclusion and exclusion criteria
Newborn mixed breed piglets (0–3 days old) obtained on the day of experimentation from the University Swine Research Technology Centre were included. There was no exclusion criterion.
Animal preparation
Piglets were instrumented as previously described with some modifications.19, 20 Following induction of anaesthesia using isoflurane, piglets were intubated via a tracheostomy, and mechanical ventilation (Sechrist Infant Ventilator Model IV-100; Sechrist Industries, Anaheim, California) was commenced at a respiratory rate of 20 breaths/min with peak inspiratory pressure of 25 cm H2O and positive end expiratory pressure of 5 cm H2O. Oxygen saturation was kept within 90–100%, and glucose level and hydration was maintained with an intravenous infusion of 5% dextrose at 10 mL/kg/hr. During the experiment, anaesthesia was maintained with intravenous propofol 5–10 mg/kg/hr and morphine 0.1 mg/kg/hr. Additional doses of propofol (1–2 mg/kg) and morphine (0.05–0.1 mg/kg) were given as needed and body temperature was maintained at 38.5–39.5 °C using an overhead warmer and a circulating water heat pad.19, 20
Hemodynamic and cardiac function parameters
A 5-French Argyle® (Klein-Baker Medical Inc. San Antonio, Texas) double-lumen catheter was inserted into the right femoral vein for administration of fluids and medications and to measure central venous pressure. A 5-French Argyle® single-lumen catheter was inserted above the right renal artery via the femoral artery for continuous arterial blood pressure monitoring and arterial blood gas measurements. The right common carotid artery was exposed and encircled with a real-time ultrasonic flow probe (2 mm; Transonic Systems Inc., Ithaca, New York) to measure carotid blood flow. A Millar® catheter (MPVS Ultra®, ADInstruments, Houston, Texas) was inserted into the left ventricle via the left common carotid artery for continuous measurement of left ventricular pressure, composite, and segmental volumes, which were used for cardiac output calculation.21
Piglets were placed in supine position and allowed to recover from surgical instrumentation until baseline hemodynamic measures were stable (minimum of one hour). Ventilator rate was adjusted to keep the partial arterial carbon dioxide (CO2) between 35 and 45 mmHg, as determined by periodic arterial blood gas analysis. Arterial blood pressure, central venous pressure, heart rate, and percutaneous oxygen saturation were continuously measured and recorded throughout the experiment with a Hewlett Packard 78833B monitor (Hewlett Packard Co., Palo Alto, California).19, 20 Post-resuscitation hypertension was defined as a mean arterial pressure (MAP) higher than baseline value. Lack time to peak was defined as the time required following ROSC for hypertensive values to be reached. The duration was defined as the time spent in a hypertensive state before MAP returned to baseline values. End MAP was defined as values at the end of the 4-hour post-resuscitation observation period.
Cerebral oxygenation
Cerebral oxygenation (crSO2) was measured using the InvosTM Cerebral/Somatic Oximeter Monitor (Invos 5100, Somanetics Corp., Troy, MI). The sensor was placed on the right forehead of the piglet and secured with wrap and tape. Light shielding was achieved with a slim cap. The InvosTM Cerebral/Somatic Oximeter Monitor calculates crSO2, which is expressed as the percentage of oxygenated haemoglobin (oxygenated haemoglobin/total haemoglobin). Values of regional oxygen saturation are stored every second with a sample rate of 0.13 Hz.22
Automated chest compression (CC) machine
The automated CC machine was specifically designed in our laboratory. The CC machine delivers CC rates (50–200/min),18, 23 anterior-posterior chest compression depths (10–70%),24, 25 acceleration of compressions (100–1000 cm/s2), speed of recoil (1–100 cm/s), steps per revolution (400–1,200 steps/revolution), and varying duty cycles.
Experimental protocol
Post-transitional piglets were randomized into two groups: epinephrine (Epi, 0.02 mg/kg) and vasopressin (Vaso, 0.4 U/kg). Following surgical instrumentation and stabilization, piglets were placed onto the automated CC machine, which was placed in the surgical bed. The piglets’ anterior-posterior chest diameter was measured from the sternum to the vertebrae touching the bed (anterior to posterior) with a measuring tape and the CC depth of 33% was calculated.24, 25 Piglets were then exposed to 50 min of normocapnic hypoxia, which was followed by asphyxia. Asphyxia was achieved by disconnecting the ventilator and clamping the endotracheal tube until asystole. Asystole was defined as zero arterial blood flow and no audible heartbeat during auscultation. Fifteen seconds after confirmation of asystole, positive pressure ventilation was provided for 30 s with a Neopuff T-Piece (Fisher & Paykel, Auckland, New Zealand) with 21% oxygen, peak inspiratory pressure of 30 cm H2O, positive end expiratory pressure of 5 cm H2O, and gas flow of 10 L/min. After 30 s of positive pressure ventilation, mechanical CC were started, with 100% oxygen and CC during sustained inflation (CC + SI) was delivered with a peak inspiratory pressure of 30 cm H2O for 30 s.20 The sustained inflation was interrupted for 1 s before a further 30 s of sustained inflation was provided, which was continued until ROSC. The following were the settings of the automated CC machine: CC rate of 90/min, acceleration of compression of 500 cm/s2, speed of recoil of 50 cm/s, a simulated two-thumb technique, and an anterior-posterior depth of 33%. Cardio-resuscitative drug Epi (0.02 mg/kg) or Vaso (0.4 U/kg), according to group allocation, was administered intravenously 1 min after the start of CC and thereafter every 3 min until ROSC, with a maximum of three doses and a maximum resuscitation time of 8 min. ROSC was defined as an unassisted heart rate > 100 beats per min for at least 15 s. After ROSC, the piglets recovered for four hours before being euthanized with an intravenous overdose of sodium pentobarbital (120 mg/kg). If there was no ROSC, piglets were euthanized immediately with an intravenous overdose of sodium pentobarbital (120 mg/kg). Autopsies were performed in all piglets to assess for injuries to the sternum, ribs, heart or lungs (e.g., bruising, abrasions, contusions, fractures).
Data collection and statistical analysis
The demographics of the study piglets were recorded. Transonic flow probe, heart rate and pressure transducer outputs were digitized and recorded with the LabChart® programming software (ADInstruments, Houston, Texas). Airway pressure, gas flow, tidal volume, and end-tidal CO2 were measured and analyzed using Flow Tool Physiologic Waveform Viewer (Philips Healthcare, Wallingford, Connecticut). Hemodynamic data until time to ROSC and post-resuscitation was analyzed (i.e., arterial blood pressure, central venous pressure, carotid blood flow). Data are presented as mean (standard deviation – SD) for normally distributed continuous variables and median (interquartile range - IQR) when the distribution was skewed. Data were tested for normality (Shapiro-Wilk and Kolmogorov-Smirnov test) and compared using either Student-T-Test (data normally distributed) or Rank Sum if data were skewed. P-values are 2-sided and p < 0.05 was considered statistically significant. Statistical analyses were performed with SigmaPlot (Systat Software Inc, San Jose, California).
Results
Sixteen mixed breed newborn post-transitional piglets 0–3 days old, ranging in weight from 1.7-2.4 kg, were obtained on the day of the experiment and were randomly assigned to Vaso (0.4 U/kg; n = 8) or Epi (0.02 mg/kg; n = 8). There were no differences in baseline parameters between groups (Table 1). Blood gas parameters before and after resuscitation are presented in Table 2.
Table 1.
Baseline characteristics.
| Vaso 0.4 U/kg (n = 8) | Epi 0.02 mg/kg (n = 8) | p-value | |
|---|---|---|---|
| Age (days) | 1.5 (1.0–2.8) | 1.0 (0.3–2.0) | 0.17 |
| Weight (kg) | 2.2 (1.7–2.3) | 2.2 (1.9–2.4) | 0.47 |
| Gender (male/female) | 4/4 | 2/6 | 0.61 |
| Heart rate (bpm) | 154 (148–181) | 162 (146–185) | 0.79 |
| MAP (mmHg) | 61 (54–66) | 59 (48–70) | 0.59 |
| Carotid flow (mL/kg/min) | 34 (24–47) | 37 (29–46) | 0.96 |
| Cerebral oxygenation (%) | 41 (39–41) | 43 (39–47) | 0.38 |
| pH | 7.53 (7.47–7.56) | 7.52 (7.49–7.59) | 0.63 |
| PaCO2 (torr) | 36 (28–37) | 34 (31–36) | 0.98 |
| PaO2 (torr) | 68 (61–77) | 73 (68–84) | 0.29 |
| Base excess (mmol/L) | 2 (1–4) | 3 (0–7) | 0.84 |
| Lactate (mmol/L) | 3.5 (2.9–5.7) | 3.2 (2.0–4.5) | 0.30 |
Data are presented as median (IQR); MAP- Mean arterial blood pressure.
Table 2.
Blood gas changes before and after resuscitation.
| Vaso 0.4 U/kg | Epi 0.02 mg/kg | p-value | |
|---|---|---|---|
| pH | |||
| Baseline | 7.53 (7.47–7.56) | 7.52 (7.49–7.59) | 0.63 |
| After asphyxiation | 6.65 (6.56–6.81)# | 6.59 (6.58–6.72)# | 0.74 |
| 1 h after resuscitation | 7.31 (7.01–7.33)# | 7.07 (6.76–7.17)# | 0.26 |
| 4 h after resuscitation | 7.46 (7.32–7.47) | 7.11 (7.05–7.17)# | 0.09 |
| PaCO2 | |||
| Baseline | 36 (28–37) | 34 (31–36) | 0.98 |
| After asphyxiation | 83 (78–100)# | 100 (90–123)# | 0.16 |
| 1 h after resuscitation | 33 (28–43) | 36 (32–46) | 0.42 |
| 4 h after resuscitation | 33 (28–39) | 33 (29–36) | 0.30 |
| PaO2 | |||
| Baseline | 68 (61–77) | 73 (68–84) | 0.29 |
| After asphyxiation | 16 (11–19) | 14 (7–24) | 0.94 |
| 1 h after resuscitation | 80 (67–123) | 80 (64–96) | 0.86 |
| 4 h after resuscitation | 68 (62–86) | 72 (63–79) | 0.86 |
| Base excess | |||
| Baseline | 2 (1 ∼ 4) | 3 (0 ∼ 7) | 0.84 |
| After asphyxiation | −27 (−29 ∼ −22)# | −29 (−30 ∼ −23)# | 0.63 |
| 1 h after resuscitation | −11 (−20 ∼ −10)# | −21 (−29 ∼ −15)# | 0.26 |
| 4 h after resuscitation | −1 (−10 ∼ 1.5) | −18 (−24 ∼ −11)# | 0.17 |
| Lactate | |||
| Baseline | 3.5 (2.9–5.7) | 3.2 (2.0–4.5) | 0.30 |
| After asphyxiation | 19.6 (18.2–20.0)# | 16.1 (15.5–19.1)# | 0.09 |
| 1 h after resuscitation | 15.5 (14.6–17.6)# | 18 (16.8–18.2)# | 0.13 |
| 4 h after resuscitation | 6.3 (5.0–9.6) | 13.4 (8.9–17.8)# | 0.19 |
Data are presented as median (IQR); # Significantly different from baseline values.
Resuscitation and primary outcome
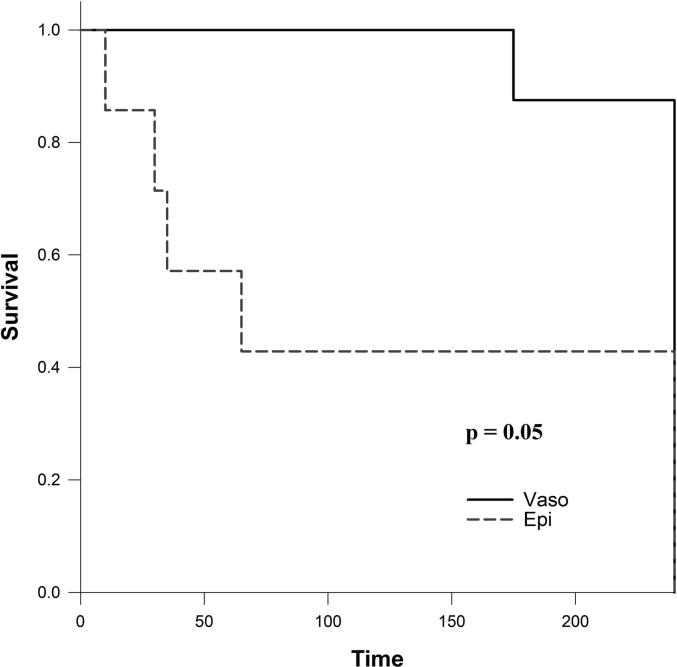
Table 3 presents a summary of asphyxia and resuscitation outcome measures. The median (IQR) duration of asphyxia before commencement of CC was not different between groups, with 367 (196–525) s with Vaso and 410 (275–533) s with Epi (p = 0.53). The median (IQR) time to achieve ROSC was 106 (93–140) s with Vaso and 128 (100–198) s with Epi (p = 0.28). The number of piglets that achieved ROSC was 8 (100%) with Vaso and 7 (88%) with Epi (p = 1.00). Although the proportion of piglets that survived 4 hours after ROSC was not statistically higher with Vaso compared to Epi (7/8 (88%) vs. 3/7 (43%), p = 0.12), the median (IQR) survival time after ROSC of the Vaso group was longer than that of the Epi group (240 (240–240) vs. 65 (30–240) min, respectively, p = 0.02). Fig. 2 presents the Kaplan-Meier survival curves from both groups (p = 0.05).
Table 3.
Characteristics of asphyxia, resuscitation, and survival of asphyxiated piglets.
| Vaso 0.4 U/kg | Epi 0.02 mg/kg | p-value | ||
|---|---|---|---|---|
| Asphyxia time (sec) † | 367 (196 ∼ 525) | 410 (275 ∼ 533) | 0.53 | |
| Resuscitation | Number of doses required # | 1 (1–1) | 1 (1–3) | 0.96 |
| Achieving ROSC | 8 (100) | 7 (88) | 1.00 | |
| ROSC time (sec) † | 106 (93 ∼ 140) | 128 (100 ∼ 198) | 0.28 | |
| Survival 4 h after ROSC (% change after ROSC) | 7 (88) | 3 (43) | 0.12 | |
| Survival time after ROSC (min) | 240 (240 ∼ 240)* | 65 (30 ∼ 240) | 0.02 |
Data are presented as n (%), unless indicated †median (IQR), #median (range); * Significantly different from Epi group.
Fig. 2.
Survival after resuscitation.
Hemodynamic parameters
Hemodynamic parameters at baseline and at commencement of resuscitation were not different between groups. Carotid artery blood flow following ROSC was significantly higher within the first 10 min after ROSC in piglets receiving Vaso compared to Epi (Fig. 3). Piglets treated with Vaso during resuscitation had significantly higher heart rate, mean arterial pressure, and oxygen saturation in the brain compared to piglets receiving Epi over the 4-hour recovery period (Fig. 4, online supplement).
Fig. 3.
Post-resuscitation changes in carotid artery blood flow, # Significantly different from Epi group (2-way ANOVA); * Significantly different at current time point.
Characteristics of post-resuscitation hypertension are presented in Table 4 (online supplement). More piglets receiving Vaso had post-resuscitation hypertension after ROSC (8/8 (100%) Vaso group vs. 5/6 (83%) Epi group). The duration of post-resuscitation hypertension was also longer with Vaso compared to Epi (990 (855–2490) s vs. 480 (450–690) s, respectively, p = 0.05).
The dP/dtmax and dP/dtmin represent the maximum and minimum rate of pressure change in the left ventricle and have generally been used as an index of ventricular performance. Left ventricle contractile function (dP/dtmax and dP/dtmin) parameters were significantly improved throughout the 4-hour post-resuscitation period with Vaso compared to Epi (Fig. 5, online supplement).
Discussion
In the current study, we compared epinephrine with vasopressin during neonatal CPR in a post-transitional asphyxiated piglet model. The results of our study can be summarized as follows: 1) time to ROSC and number of piglets achieving ROSC was not different (Table 3), 2) vasopressin significantly improved post-resuscitation survival-time (Table 3) and survival (Fig. 2, Kaplan-Meier survival curves), 3) vasopressin significantly improved carotid blood flow immediately after ROSC (Fig. 3) but had longer post-resuscitation hypertension with higher blood pressure (Table 4), 4) vasopressin significantly improved heart rate, arterial pressure, and cerebral blood oxygen saturation at 4 hours after ROSC (Fig. 4), and 5) vasopressin significantly improved left ventricle contractile function (dP/dtmax and dP/dtmin) throughout the 4-hour post-resuscitation period (Fig. 5).
To our knowledge, this is the 3rd animal study comparing vasopressin with epinephrine, and our results are somewhat different from the previous studies. Previous studies either used a transitional near-term-sheep model to compare vasopressin (0.4 U/kg) and epinephrine (0.03 mg/kg) and reported time to ROSC [13 ± 6 min vs. 8 ± 2 min, no p-value reported] and survival rates [3/9 vs. 7/10, no p-value reported] between vasopressin and epinephrine26 or a a post-transitional cardiac arrest piglet model comparing high and low dose vasopressin (0.2 and 0.4 U/kg) and epinephrine (0.01 and 0.03 mg/kg) but did not report time to ROSC however that study reported a significantly higher survival rate with vasopressin (0.4 U/kg) vs. low-dose epinephrine (0.01 mg/kg) [9/10 vs. 5/13 (p < 0.05)], but no significant difference in survival when compared to high-dose epinephrine (0.03 mg/kg, 6/11 survival).27 he discrepancy can be due to different i) animal models (post-transitional piglet27 and transitional near-term lambs,26 ii) cause of cardiac arrest (asphyxia,26, 27 or hypoxia followed by asphyxia[current study]), iii) doses of epinephrine (0.01,27 0.02 [current study], and 0.03 mg/kg,26 while the vasopressin dose of 0.4 U/kg was the same in all three studies, iv) resuscitation techniques (manual CC and ventilations with defibrillation,27 manual CC using coordinated 3:1 compression-to-ventilation ratio,26 mechanical CC with sustained inflations[current study]), and v) post-resuscitation observation periods (20 min,26 120 min,27 and 240 min[current study]).
In the current study, we compared vasopressin (0.4 U/kg) and epinephrine (0.02 mg/kg) in our post-transitional model of asphyxial cardiac arrest and observed no difference in time to ROSC or the number of piglets achieving ROSC. Rawat et al. used a transitional near-term-sheep model to compare vasopressin (0.4 U/kg) and epinephrine (0.03 mg/kg) during asphyxial cardiac arrest induced by umbilical cord occlusion and reported no difference in time to ROSC between vasopressin and epinephrine [13 ± 6 min vs. 8 ± 2 min, no p-value reported].26 McNamara et al. compared high and low dose vasopressin (0.2 and 0.4 U/kg) and epinephrine (0.01 and 0.03 mg/kg) in a post-transitional cardiac arrest piglet model with asphyxia induced by disconnection of mechanical ventilator (no hypoxia)27 but did not report time to ROSC.27 Of note, McNamara et al. diagnosed cardiac arrest with ultrasound and observed ventricular fibrillation in a third of piglets.
Rawat et al. reported no difference in survival rates with vasopressin vs. epinephrine [3/9 vs. 7/10, no p-value reported], however, the post-resuscitation observation period was only 20 min, which might have been too short to observe longer effects of both drugs. In comparison, McNamara et al. reported a significantly higher survival rate with vasopressin (0.4 U/kg) vs. low-dose epinephrine (0.01 mg/kg) [9/10 vs. 5/13 (p < 0.05)], but no significant difference in survival when compared to high-dose epinephrine (0.03 mg/kg, 6/11 survival). Similarly, in our study the survival (4-hour post-resuscitation observation period) was significantly improved with vasopressin compared to epinephrine (Fig. 2).
Except for an increased heart rate following ROSC in the epinephrine group, Rawat et al. reported no difference in hemodynamic parameters between vasopressin- and epinephrine-treated lambs (carotid artery blood flow, systolic and diastolic blood pressures).26 However, because lambs were only monitored for 20 min after achieving ROSC, no substantial post-resuscitation effects of vasopressin vs. epinephrine can be deduced. In the current study, we observed that although immediate survival rate and time to achieve ROSC following resuscitation using vasopressin and epinephrine are comparable, survival during the 4-hour post-resuscitation period was significantly greater in vasopressin-treated piglets (Fig. 2). Furthermore, by four hours’ post-resuscitation vasopressin-treated piglets presented with significantly higher heart rate, mean arterial blood pressure, and brain oxygen saturation, likely a result of increased left ventricle contractile function and increased carotid artery flow rate (Figs. 4 and 5). An increase in dP/dtmin is associated with an increase in diastolic function during isovolumic relaxation, suggestive of improved coronary artery perfusion pressure.
Hypertension following CPR and ROSC is a post-resuscitation occurrence that we commonly observe in our piglet asphyxia model. This episode, also known as rebound hypertension, has also been reported in near-term lambs following fetal asphyxia, C-section delivery with immediate umbilical cord clamping, and resuscitation with positive pressure ventilation.28 The rapid and marked overshoot in mean systemic arterial blood pressure was accompanied by increased cerebrovascular injury, both of which were mostly absent when lambs remained attached to the cord.28 In the current study, post-resuscitation hypertension was present in both vasopressin and epinephrine groups with vasopressin-treated piglets remaining in this phase 2-times longer than epinephrine-treated with less fluctuation in mean arterial blood pressure. While less fluctuations in blood pressure could be protective against cerebral injury, the twice as long post-resuscitation hypertension is concerning and could lead to increased cerebral injury. We did not examine brain injury in our experiment, which is a limitation.
Studies in newborn rats reported that vasopressin-induced pulmonary vasodilation is absent likely due to lower tissue V1a expression.29 Rawat et al. demonstrated in an in-vitro vessel study that vasopressin has more pulmonary vasodilation compared to epinephrine, however epinephrine seems to relax coronary arteries while vasopressin causes vasoconstriction in coronary arteries, which could be the cause of lower rates of ROSC in their model.26 In comparison, McNamara et al. reported lower pulmonary vascular resistance with vasopressin compared to epinephrine.27 Similar, Cheung et al. reported improved pulmonary blood flow without deterioration of systemic, carotid, or mesenteric hemodynamics with continuous vasopressin infusion.30 These data suggest that there might be a difference in how different animal species react to vasopressin.
Limitations
In the current study, we administered cardiopulmonary resuscitation using continuous CC during sustained inflation (CC + SI), which although is mentioned in the “knowledge gap” section of the neonatal resuscitation guidelines,5 is not the current recommended clinical practice.13 Our use of a piglet asphyxia model is a strength of this translational study, as this model closely simulates delivery room events, with the gradual onset of severe asphyxia leading to bradycardia and eventual asystole. A further strength of this study is the use of our custom-designed automated CC machine, which allowed consistent delivery of CC rates and reduced potential bias (e.g., fatigue during CC, or inability to constantly achieve rate and/or depth of CC).18, 23, 24, 25 Our asphyxia model uses piglets that have already undergone the fetal-to-neonatal transition, were sedated/anesthetized, and uses tracheostomy with a tightly sealed endotracheal tube to prevent leak; which does not occur in the delivery room, and are limitations of our model.31, 32
Conclusions
Although time to ROSC and number of piglets achieving ROSC was not different between vasopressin and epinephrine, the post-resuscitation survival-time and survival was significantly improved with vasopressin. Vasopressin might be an alternative to epinephrine during neonatal CPR and further studies are warranted.
Funding sources
We would like to thank the public for donating money to our funding agencies: The study was supported by a Grant-in-Aid Grant from the Heart and Stroke Foundation Canada (G-22-0031980).
Author’s contribution
Conception and design: GMS, PYC, MOR, TFL.
Collection and assembly of data: GMS, PYC, MOR, TFL.
Analysis and interpretation of the data: GMS, PYC, MOR, TFL.
Drafting of the article: GMS, PYC, MOR, TFL.
Critical revision of the article for important intellectual content: GMS, PYC, MOR, TFL.
Final approval of the article: GMS, PYC, MOR, TFL.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2023.100427.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Changes of heart rate (A), mean arterial pressure (MAP) (B), Carotid Flow (C), and Brain oxygenation (SO2) (D)(online supplement).
Changes in cardiac function parameters Stroke volume (A), dp/dt max (B)End diastolic volume (C), and dp/dt min (D) (online supplement).
Post-resuscitation hypertension (online supplement).
References
- 1.Barber C.A., Wyckoff M.H. Use and efficacy of endotracheal versus intravenous epinephrine during neonatal cardiopulmonary resuscitation in the delivery room. Pediatrics. 2006;118:1028–1034. doi: 10.1542/peds.2006-0416. [DOI] [PubMed] [Google Scholar]
- 2.Halling C., Raymond T., Brown L.S., et al. Neonatal delivery room CPR: An analysis of the Get with the Guidelines®—Resuscitation Registry. Resuscitation. 2021;158:236–242. doi: 10.1016/j.resuscitation.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Handley S.C., Passarella M., Raymond T.T., Lorch S.A., Ades A., Foglia E.E. Epidemiology and outcomes of infants after cardiopulmonary resuscitation in the neonatal or pediatric intensive care unit from a national registry. Resuscitation. 2021;165:14–22. doi: 10.1016/j.resuscitation.2021.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalaniti K., Schmölzer G.M., McNamara P.J. Neonatal resuscitation beyond the delivery room - does one protocol fit all? Acta Paediatr. 2015;104:971–973. doi: 10.1111/apa.13116. [DOI] [PubMed] [Google Scholar]
- 5.Wyckoff M.H., Wyllie J.P., Aziz K., et al. Neonatal life support: 2020 International consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2020;142:329–337. doi: 10.1161/cir.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 6.Solevåg A.L., Cheung P.-Y., O’Reilly M., Schmölzer G.M. A review of approaches to optimise chest compressions in the resuscitation of asphyxiated newborns. Arch Dis Child – Fetal Neonatal Ed. 2016;101:F272. doi: 10.1136/archdischild-2015-309761. [DOI] [PubMed] [Google Scholar]
- 7.Kapadia V.S., Wyckoff M.H. Chest compressions for bradycardia or asystole in neonates. Clin Perinatol. 2012;39:833–842. doi: 10.1016/j.clp.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Pinto M., Solevåg A., OʼReilly M., Aziz K., Cheung P.-Y., Schmölzer G.M. Evidence on adrenaline use in resuscitation and its relevance to newborn infants: a non-systematic review. Neonatology. 2017;111:37–44. doi: 10.1159/000447960. [DOI] [PubMed] [Google Scholar]
- 9.Kapadia V.S., Wyckoff M.H. Epinephrine use during newborn resuscitation. Front Pediatr. 2017;5:97. doi: 10.3389/fped.2017.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Preziosi M.P., Roig J.C., Hargrove N., Burchfield D.J. Metabolic acidemia with hypoxia attenuates the hemodynamic responses to epinephrine during resuscitation in lambs. Crit Care Med. 2006;21:1901–1907. doi: 10.1097/00003246-199312000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Vali P., Sankaran D., Rawat M., Berkelhamer S., Lakshminrusimha S. Epinephrine in neonatal resuscitation. Children. 2019;6:51–115. doi: 10.3390/children6040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isayama T., Mildenhall L., Schmölzer G.M., et al. The route, dose, and interval of epinephrine for neonatal resuscitation: a systematic review. Pediatrics. 2020;146 doi: 10.1542/peds.2020-0586. [DOI] [PubMed] [Google Scholar]
- 13.Aziz K., Lee H.C., Escobedo M.B., et al. Part 5: Neonatal Resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:1–27. doi: 10.1161/cir.0000000000000902. [DOI] [PubMed] [Google Scholar]
- 14.Aung K., Htay T. Vasopressin for cardiac arrest: a systematic review and meta-analysis. Arch Intern Med. 2005;165:17–24. doi: 10.1001/archinte.165.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel V., Krismer A.C., Arntz H.R., et al. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. New England J. 2004;350:105–113. doi: 10.1056/nejmoa025431. [DOI] [PubMed] [Google Scholar]
- 16.Duncan J.M., Meaney P., Simpson P., et al. Vasopressin for in-hospital pediatric cardiac arrest: results from the American Heart Association National Registry of Cardiopulmonary Resuscitation. Pediatr Crit Care Med: J Soc Crit Care Med World Federation Pediatric Intensive Crit Care Soc. 2009;10:191–195. doi: 10.1097/pcc.0b013e31819a36f2. [DOI] [PubMed] [Google Scholar]
- 17.du Sert N.P., Hurst V., Ahluwalia A., et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Exp Physiol. 2020;105:1459–1466. doi: 10.1113/ep088870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruckner M., Neset M., Garcia-Hidalgo C., et al. Chest Compression Rates of 90/min versus 180/min during Neonatal Cardiopulmonary Resuscitation: A Randomized Controlled Animal Trial. Children. 2022;9:1838. doi: 10.3390/children9121838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung P.-Y., Gill R.S., Bigam D.L. A swine model of neonatal asphyxia. J Visual Exp: JoVE. 2011 doi: 10.3791/3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmölzer G.M., O’Reilly M., LaBossiere J., et al. Cardiopulmonary resuscitation with chest compressions during sustained inflations: a new technique of neonatal resuscitation that improves recovery and survival in a neonatal porcine model. Circulation. 2013;128:2495–2503. doi: 10.1161/circulationaha.113.002289. [DOI] [PubMed] [Google Scholar]
- 21.Wagner H., Götberg M., Hardig B.M., et al. Repeated epinephrine doses during prolonged cardiopulmonary resuscitation have limited effects on myocardial blood flow: a randomized porcine study. BMC Cardiovascular Disorders. 2014;14:199–209. doi: 10.1186/1471-2261-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pichler G., Binder C., Avian A., Beckenbach E., Schmölzer G.M., Urlesberger B. Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J Pediatrics. 2013;163:1558–1563. doi: 10.1016/j.jpeds.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Bruckner M., Neset M., O’Reilly M., Lee T.-F., Cheung P.-Y., Schmölzer G.M. Haemodynamic changes with varying chest compression rates in asphyxiated piglets. Archives Dis Child - Fetal Neonatal Ed. 2022 doi: 10.1136/archdischild-2021-323271. fetalneonatal-2021-323271. [DOI] [PubMed] [Google Scholar]
- 24.Bruckner M., O’Reilly M., Lee T.-F., Neset M., Cheung P.-Y., Schmölzer G.M. Effects of varying chest compression depths on carotid blood flow and blood pressure in asphyxiated piglets. Archives Dis Child - Fetal Neonatal Ed. 2021 doi: 10.1136/archdischild-2020-319473. fetalneonatal-2020-319473. [DOI] [PubMed] [Google Scholar]
- 25.Bruckner M., Kim S.Y., Shim G.H., et al. Assessment of optimal chest compression depth during neonatal cardiopulmonary resuscitation: a randomised controlled animal trial. Archives Dis Child - Fetal Neonatal Ed. 2021 doi: 10.1136/archdischild-2021-321860. fetalneonatal-2021-321860. [DOI] [PubMed] [Google Scholar]
- 26.Rawat M., Gugino S., Koenigsknecht C., et al. Masked Randomized Trial of Epinephrine versus Vasopressin in an Ovine Model of Perinatal Cardiac Arrest. Children. 2023;10:349. doi: 10.3390/children10020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNamara P.J., Engelberts D., Finelli M., Adeli K., Kavanagh B.P. Vasopressin improves survival compared with epinephrine in a neonatal piglet model of asphyxial cardiac arrest. Pediatric Res. 2014;75:738–748. doi: 10.1038/pr.2014.38. [DOI] [PubMed] [Google Scholar]
- 28.Polglase G.R., Blank D.A., Barton S.K., et al. Physiologically based cord clamping stabilises cardiac output and reduces cerebrovascular injury in asphyxiated near-term lambs. Arch Disease Childhood-Fetal. 2017 doi: 10.1136/archdischild-2017-313657. fetalneonatal-2017-313657-10. [DOI] [PubMed] [Google Scholar]
- 29.Enomoto M., Pan J., Shifrin Y., Belik J. Age dependency of vasopressin pulmonary vasodilatory effect in rats. Pediatr Res. 2014;75:315–321. doi: 10.1038/pr.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheung D.C., Gill R.S., Liu J.-Q., et al. Vasopressin improves systemic hemodynamics without compromising mesenteric perfusion in the resuscitation of asphyxiated newborn piglets: a dose–response study. Intensive Care Med. 2012;38:491–498. doi: 10.1007/s00134-011-2437-4. [DOI] [PubMed] [Google Scholar]
- 31.Hooper S.B., te Pas A.B., Polglase G.R., Wyckoff M.H. Animal models in neonatal resuscitation research: What can they teach us? Sem Fetal Neonatal Med. 2018;23:300–305. doi: 10.1016/j.siny.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Solevåg A., Cheung P.-Y., Lie H., et al. Chest compressions in newborn animal models: a review. Resuscitation. 2015;96:151–155. doi: 10.1016/j.resuscitation.2015.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Changes of heart rate (A), mean arterial pressure (MAP) (B), Carotid Flow (C), and Brain oxygenation (SO2) (D)(online supplement).
Changes in cardiac function parameters Stroke volume (A), dp/dt max (B)End diastolic volume (C), and dp/dt min (D) (online supplement).
Post-resuscitation hypertension (online supplement).