Abstract
PURPOSE/OBJECTIVE:
To compare toxicity profiles of low-dose rate (LDR) and high-dose rate (HDR) brachytherapy boost combined with ultra-hypofractionated external beam radiation therapy (UH-EBRT).
MATERIALS/METHODS:
99 patients with intermediate-risk prostate cancer underwent an HDR ( n =59) or LDR (n=40) boost combined with UH-EBRT (5 Gy × 5) . HDR (Ir-192) was delivered a single dose (15 Gy) and LDR (Pd-103) prescription dose was 100 Gy. Median baseline IPSS was 5 for both cohorts. Median follow-up was 29.3mos. Cumulative incidences were calculated for toxicity. Fisher exact tests were used to evaluate associations.
RESULTS:
Overall incidence of grade 2 genitourinary toxicity for the entire cohort at 12 and 24 months was 21% and 29%, respectively. The incidence of grade 2 genitourinary toxicity at 12 and 24 months was higher for LDR cohort compared with HDR cohort (45% vs 5.1% and 55% vs 11%; p<0.001). On MVA, only treatment regimen (LDR versus HDR) was associated with grade 2+ genitourinary toxicity (p<0.001). Two patients experienced grade 2 rectal toxicity in each cohort. No grade > 3 toxicities were observed.
CONCLUSIONS:
Both LDR and HDR brachytherapy combined with UH-EBRT had favorable toxicity profiles, but significantly less grade 2 + genitourinary toxicity was observed in patients receiving HDR. © 2022 American Brachytherapy Society. Published by Elsevier Inc. All rights reserved.
Keywords: LDR brachytherapy, HDR brachytherapy, Gu toxicity, Ultra-hypofractionated external beam radiotherapy, Stereotactic radiotherapy
Introduction
Prostate brachytherapy is an important tool that can facilitate dose escalation to the prostate while minimizing normal tissue exposure. For patients with National Comprehensive Cancer Network (NCCN) intermediate-risk and high-risk prostate cancer, trials have consistently shown improved relapse-free survival outcomes when a brachytherapy boost is added to external beam radiation therapy (EBRT) (1–3) compared to external beam radiation therapy alone. Brachytherapy-based dose escalation can be achieved using either low-dose-rate (LDR) or high-dose-rate (HDR) techniques, and the isotope choice is primarily based on availability, provider discretion, and clinical experience. The advantages of LDR include a minimal need for operative shielding, relatively short procedure length, and reliable post-implant dosimetry for quality assurance purposes. The advantages of HDR include minimal posttreatment radiation precautions, more reliable dose delivery, and improved potential for extra-prostatic disease coverage. Currently, both techniques are well accepted approaches for use when combined with EBRT for patients with higher-risk disease.
One important concern raised about the integration of brachytherapy with external beam radiotherapy is the potential for increased genitourinary (GU) toxicity as seen in the ASCENDE-RT trial ( 4 ). While a component of the toxicity found in the aforementioned trial has been ascribed to treatment technique including the use of generous treatment margins during brachytherapy planning, it is unclear as to the contribution of the brachytherapy technique and isotope selection on toxicity results. Of note, this trial reported appreciably higher GU toxicity rates as compared with other prospective and retrospective data when using combined brachytherapy with external beam radiotherapy (5–7).
Typically, external beam radiation therapy after brachytherapy involves 37.5–45 Gy in 1.8–2.6 Gy fractions delivered daily; however, shorter courses (five treatments) increase convenience for patients and may provide a biologic advantage. In a prospective Phase II trial, we reported that LDR (Pd-103) combined with 25 Gy in 5 Gy fractions was well tolerated: rate of grade 2 genitourinary toxicity at 12 months was 25%, and rate of bowel toxicity at 12 months was 5% ( 6 ). We also recently reported our results using HDR brachytherapy (15 Gy) followed by stereotactic body radiation therapy (25 Gy in 5 fractions to the prostate and/or seminal vesicles and/or pelvic lymph nodes), observing similarly favorable acute and late grade 2 or higher GU toxicity outcomes in 5.9% and 9.9% of patients, respectively ( 7 ). These results seem comparable to conventionally fractionated regimens; however, optimal isotope selection remains uncertain. Recently, Dhere et al. also reported improved acute GU toxicity in patients undergoing an HDR compared with an LDR boost in a secondary analysis of a prospective study using conventional fractionation to the prostate and/or pelvis ( 8 ). While reported rates of GU toxicity are overall low in these studies, there remains uncertainty regarding optimal isotope selection and brachytherapy technique when applied as a boost.
In this study, we sought to explore whether any notable differences in GU or GI toxicity were observed between LDR and HDR brachytherapy boosts when combined with ultra-hypofractionated external beam radiotherapy (UH-EBRT) to the prostate and seminal vesicles among patients with intermediate-risk prostate cancer.
Patients & methods
Following IRB approval, we retrospectively reviewed the records of 99 patients treated at our institution between 2014 and 2019 with clinically localized intermediate-risk prostate cancer who underwent definitive radiation therapy consisting of interstitial brachytherapy (low-dose-rate ( n =40) or high-dose-rate (n=59)) followed by ultra-hypofractionated external beam radiotherapy (25 Gy in five fractions) delivered to the prostate and seminal vesicles. The LDR cohort included patients enrolled on a prospective trial ( 6 ). In 2016, we established an intraoperative treatment planning and delivery workflow for HDR brachytherapy; following that timepoint, an HDR boost was more frequently used in combination with brachytherapy for higher-risk disease.
Baseline patient characteristics for both cohorts are presented in Table 1 . The median age for the entire cohort was 66 years. Overall, 90% of patients had ≤T2a disease and 55% had Gleason 3 +4 disease. However, 75% were classified as NCCN unfavorable intermediate-risk, primarily due to ≥50% cores positive and/or multiple intermediate-risk factors. There were no significant differences in IPSS or use of genitourinary medication at baseline amongst the cohorts. A trend toward higher prostate volume (median 38 cc vs. 32 cc; p =0.05) at baseline was noted for the HDR cohort compared to the LDR cohort. Additionally, more patients in the HDR cohort received neoadjuvant and concurrent androgen deprivation therapy compared to the LDR cohort (32% vs. 10%; p =0.01).
Table 1.
Baseline patient characteristics
| LDR cohort (n = 40) | HDR cohort (n = 59) | P = | |
|---|---|---|---|
| Age (median, range) | 66 (36–85) | 67(50–78) | >0.9 |
| T stage | >0.9 | ||
| ≤T2a | 36 (90%) | 53 (90%) | |
| >T2a | 4 (10%) | 6 (10%) | |
| Gleason score | 0.033 | ||
| 3 + 4 | 27 | 27 | |
| 4 + 3 | 13 | 32 | |
| Initial PSA (median, range) | 7.8 (2.7–19.7) | 8.03(1.9–18.3) | >0.9 |
| NCCN Risk Group | 0.021 | ||
| Favorable | 15 | 10 | |
| Unfavorable | 25 | 49 | |
| Prostate volume (median, range) | 32cc (17–66) | 38cc (16–87cc) | 0.05 |
| Baseline IPSS (median, range) | 4 (0–15) | 5 (0–18) | 0.10 |
| Baseline use of urinary meds | 0.3 | ||
| No | 34 (85%) | 54 (92%) | |
| Yes | 6 (15%) | 5 (8.5%) | |
| ADT use | 0.01 | ||
| No | 36 (90%) | 40 (68%) | |
| Yes | 4 (10%) | 19 (32%) | |
| ADT duration (median) | 6 m | 6m | |
| Use of rectal spacer | <0.001 | ||
| No | 33 (82%) | 16 (27%) | |
| Yes | 7 (18%) | 43 (73%) |
HDR = high-dose-rate; LDR = low-dose-rate; NCCN = national comprehensive cancer network; ADT = androgen deprivation therapy.
Treatment
The details of the brachytherapy procedure for LDR and HDR have been previously described (7, 8). All patients underwent transrectal ultrasound-guided, transperineal interstitial brachytherapy with real-time intraoperative treatment planning. LDR patients underwent a permanent Pd-103 radioactive seed implant with a prescribed dose of 100 Gy to the prostate. All patients underwent CT- or MRI-based post-implant dosimetry on the day of the procedure (D0). The details of post-implant D0 dosimetry for the LDR cohort are presented in Table 2 . HDR patients were prescribed 15 Gy in a single fraction using Ir-192. The intraoperative dosimetry details for the HDR cohort are also detailed in Table 2. In 2015, we initiated a program offering hydrogel rectal spacer placement (SpaceOAR, Augmenix Inc Waltham, MA) at the time of brachytherapy for appropriately selected patients (i.e., patients without posterior extracapsular disease on pretreatment MRI). Significantly more patients in the HDR cohort underwent hydrogel rectal spacer placement at the time of brachytherapy (73% vs. 18%, p < 0.001), as shown in Table 1
Table 2.
Post-implant dosimetry for LDR cohort ( n = 40) and intraoperative dosimetry for HDR cohort ( n = 59)
| LDR cohort | HDR cohort | |
|---|---|---|
| Median (IQR) | Median (IQR) | |
| Prostate V150 (%) | 65.0 (61.25–71.0) | 41.3 (38.2–45.3) |
| Prostate V100 (%) | 93.5 (91–96.0) | 97.0 (95.6–97.8) |
| Prostate D90 (%) | 106.53 (102.0–117.75 | 110.9 (108.3–112.7) |
| Urethra D05 (%) | 132.0 (120.25–140.95) | 0.0 (0.0–0.0) |
| Urethra D20 (%) | 118.50(110–129.0) | 115.2 (112.0–118.7) |
| Rectum V100 (cc) | 0 (0–0.006) | 112.6 (108.6–116.1) |
V150 = volume receiving 150% of prescription dose; V100 = volume receiving 100% of prescription dose; D90 = dose received by 90% of volume; D05 = dose received by 5% of volume;
Approximately 2 weeks following brachytherapy, all patients underwent MRI-based treatment planning for ultra- hypofractionated external beam radiation therapy. Simulation and treatments were performed with a full bladder and empty rectum. The prostate and seminal vesicles were contoured, and a planning margin of 0.5 cm was utilized, except posteriorly where a 0.3 cm margin was used. Organs at risk were delineated on treatment planning software and included the urethra, bladder, and rectum. The prescription UH-EBRT dose was 25 Gy delivered in daily 5 Gy fractions. The treatment planning details, including goals and constraints for UH-EBRT, are shown in Table 3. Daily cone-beam CT imaging and Intrafraction motion monitoring was performed using implanted fiducial markers placed at the time of brachytherapy.
Table 3.
Ultra-hypofractionated external beam radiation treatment planning constraints
| Goal/constraint | |
|---|---|
| PTV D95% | 90% |
| Urethra max Urethra D1cc | 100% of Rx 90% of Rx |
| Bladder wall max Bladder wall D5cc Bladder wall mean dose | 106% of Rx 103% of Rx 1650cGy |
| Rectal wall max Rectal wall D1cc Rectal wall mean dose | 106% of Rx 103% of Rx 1550cGy |
| Small/large bowel dose max | 2500/2650cGY |
| Femur max | 2000cGy |
D95 = dose received by 95% of volume; D1cc = dose received by 1cc of volume; D5cc = dose received by 5cc of volume.
Follow-up
Patients were followed every 3–6 months for the first 2 years following treatment, including toxicity assessment using the Common Terminology Criteria for Adverse Events version 4.0, International Prostate Symptom Score (IPSS), and bowel symptom assessment. Acute toxicity was defined as any toxicity occurring within 3 months of treatment end and late toxicity was defined as any toxicity that occurred thereafter. Prostate-specific antigen (PSA) testing was performed at each follow-up visit. All patients had a minimum follow-up of 12 months. The median follow-up for the entire cohort was 29.3 months (range 13.2–79.4 months) and was 36.3 months and 23.1 months for the LDR and HDR cohorts, respectively.
Statistical methods
Unadjusted two-sided p -values < 0.05 were considered statistically significant. All analyses were performed with SAS 9.4 TS1M6 software (The SAS Institute, Cary, NC). Descriptive statistics were used to summarize patient characteristics. Cumulative incidences were calculated for toxicity outcomes. Fisher exact tests were used to evaluate associations.
Results
The overall incidence of grade 2 genitourinary toxicity for the entire cohort at 12 and 24 months was 21% and 29%, respectively. The most common grade 2 genitourinary toxicities noted were frequency/urgency requiring medication (65%) and genitourinary obstructive symptoms requiring medication (45%). No patient experienced urinary obstruction requiring catheterization in either cohort. There were no grade 3 or 4 genitourinary toxicities noted. The incidence of grade 2 genitourinary toxicity at 12 and 24 months was higher for LDR cohort compared with HDR cohort (45% vs. 5.1% and 55% vs. 11%; p < 0.001). The time to grade 2 genitourinary toxicities for both the LDR and HDR cohorts is depicted in Fig. 1 . On univariate and multivariate analysis, only the use of an LDR boost was associated with the development of grade 2 or higher GU toxicity (Table 4).
Fig. 1.
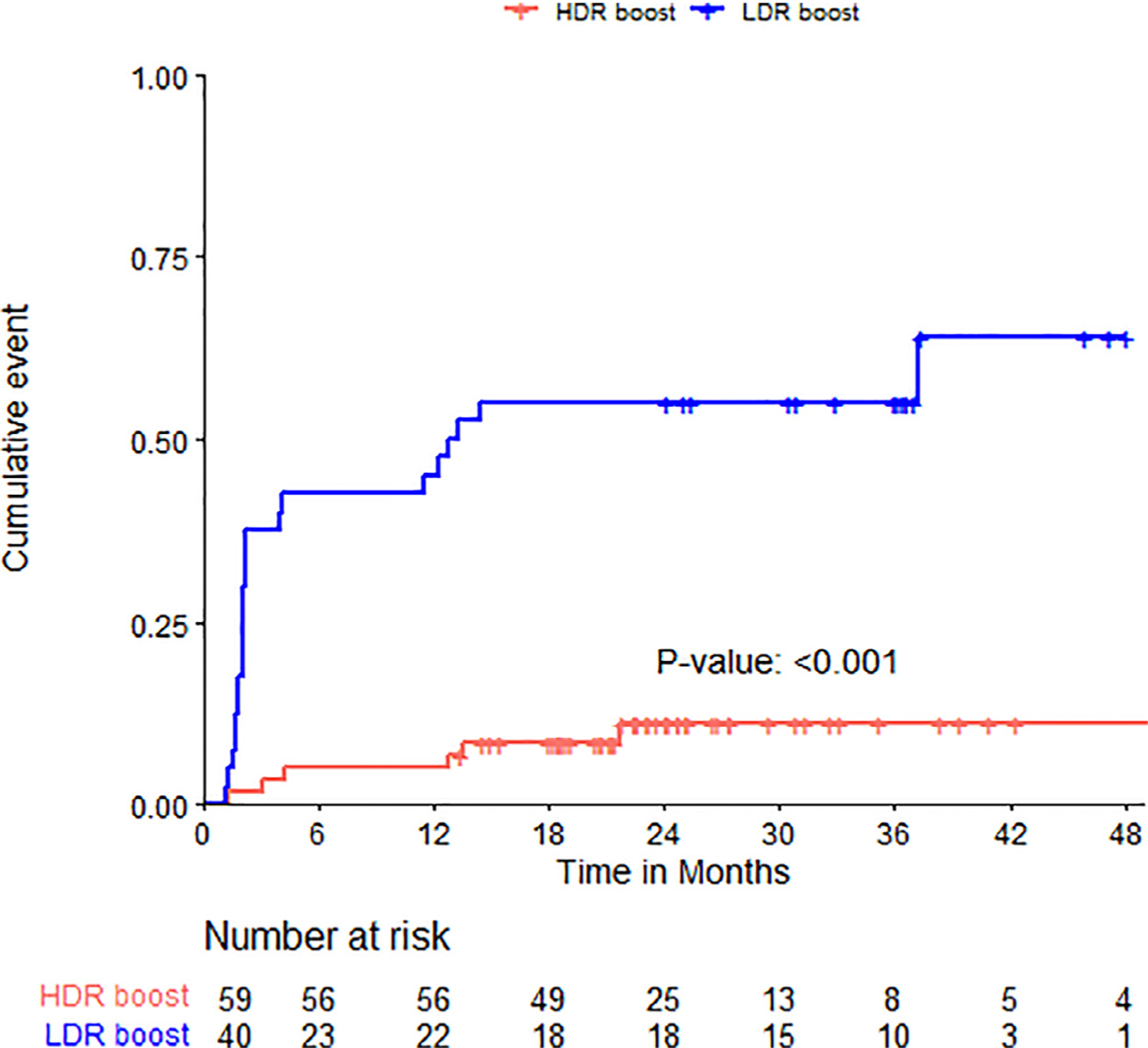
Time to grade 2 genitourinary toxicity in LDR and HDR cohort
Table 4.
Univariate and multivariate analyses for time to grade 2 or higher GU toxicity
| Characteristic | HR | 95% CI; p value |
|---|---|---|
| Univariate analysis Cohort | ||
| HDR | - | |
| LDR | 7.86 | 3.19,19.4; <0.001 |
| Age | 1.02 | 0.97, 1.07; 0.4 |
| Prostate volume | 0.98 | 0.95,1.01; 0.2 |
| Baseline IPSS | 0.94 | 0.85–1.04; 0.2 |
| Baseline urinary medication | 2.03 | 0.77–5.32; 0.2 |
| ADT use | ||
| No | - | |
| Yes | 0.46 | 0.16–1.33; 0.2 |
| Multivariate analysis Cohort | ||
| HDR | - | - |
| LDR | 8.14 | 2.82–23.5; <0.001 |
HR = hazard ratio; HDR = high-dose-rate; LDR = low-dose-rate; IPSS = international prostate symptom score; ADT = androgen deprivation therapy.
The mean and median baseline IPSS scores were 4.7 and 4.0 for the LDR cohort and 6.3 and 5.0 for the HDR cohort, respectively ( Table 1 ). The mean IPSS at 12 months ( ±3 months) for the LDR cohort was 10.2 and for the HDR cohort was 7.5 ( p =0.05). The mean change in IPSS from baseline (∆IPSS)=at 12 months (±3months) was +5 for the LDR cohort compared with −0.5 for the HDR cohort ( p =0.001). Insufficient IPSS data −was available to compare IPSS between cohorts beyond 12 months.
At 12 months, 4 patients developed grade 2 rectal toxicity (all rectal bleeding). This represented 5% (2/40) in LDR cohort and 3% (2/59) in the HDR cohort. There were no grade 3 GI toxicities noted for either cohort.
The overall 2- and 3-year PSA relapse-free survival outcome for the entire cohort was 97%. No significant differences between the LDR and HDR cohorts were observed.
Discussion
In this study, we found a significantly higher rate of grade 2 genitourinary toxicity among patients who underwent an LDR brachytherapy boost compared with an HDR boost for patients treated with a combination of brachytherapy and UH-EBRT. To our knowledge, this is the first reported analysis comparing a LDR and HDR boost in this treatment setting.
Our findings are important as they provide clarity on the genitourinary toxicity associated with isotope and brachytherapy technique selection when combining brachytherapy and ultra-hypofractionated radiation therapy. Our findings are consistent with other reports reflecting more favorable genitourinary tolerability for HDR as compared to LDR brachytherapy when used as either monotherapy or combined with conventional fractionation regimens. In a phase II randomized pilot study of HDR and LDR used as monotherapy for low-risk and intermediate-risk prostate cancer, Hathout et al. reported significantly improved IPSS and Expanded Prostate Cancer Index Composite scores at timepoints within 12 months of treating with a HDR brachytherapy boost (9). Additionally, time to IPSS symptom resolution was significantly shorter (3.3 vs. 6.5 months; p =0.013) in the HDR cohort. Similarly, in a retrospective study comparing patient-reported genitourinary outcomes up to 3 years following HDR (Ir-192 15 Gy x 1) or LDR (Pd-103 100 Gy or I–125 110 Gy) when combined with EBRT (37.5–45 Gy), the LDR cohort experienced a significantly greater worsening of IPSS and Expanded Prostate Cancer Index Composite genitourinary domain scores (8).
Concerns regarding GU toxicity when using brachytherapy as part of a combined modality therapy were amplified with the findings of ASCENDE-RT, a randomized trial comparing dose escalated EBRT (DE-EBRT) compared with LDR brachytherapy combined with external beam radiation (LDR-EBRT) for intermediate-risk and high-risk prostate cancer (3,4). In this trial, acute grade 2 GU toxicity was 30% in the LDR-EBRT cohort compared with 15.8% in the DE-EBRT, which was persistent at 5 years. Additionally, the cumulative incidence of late grade 3 GU toxicity was significantly higher for the LDR-EBRT cohort (18.4% vs. 5.2%; p < 0.001). Most of these grade 3 events ultimately resolved; however, the prevalence of late grade 3 toxicity remained higher in the LDR cohort even at 2 years (7% vs. 1%; p =0.005). Of note, membranous urethral strictures accounted for approximately half of all grade 3 events in the LDR cohort in the ASCENDE-RT trial, which is likely related to the generous inferior margin used on the planning ultrasound. It is also notable that these grade 3 toxicity rates were significantly higher than other published studies. In the current study, our patient cohorts did not experience grade 3 genitourinary toxicity with a median follow-up of approximately 2 years, suggesting it is not likely that striking differences in toxicity outcomes between the LDR and HDR cohorts will arise with longer follow-up. Indeed, most of the GU toxicity observed in the ASCENDE-RT trial manifested approximately 2 years after treatment (4). However, continued monitoring of these cohorts is needed to confirm this.
In our study, we found no significant differences in GI toxicity between the LDR and HDR cohorts; however, limited conclusions can be drawn due to our limited number of GI events and the fact that HDR patients were significantly more likely to have a rectal spacer placed. Overall, rectal toxicity with brachytherapy combined with UH-EBRT was low with or without a rectal spacer.
There are several limitations of this work, including the retrospective nature of our report. First, our relatively short follow-up may underestimate differences in late GU toxicity that may occur > 3 years posttreatment, particularly with respect to urethral stricture. Second. the brachytherapy technique was per physician preference and some confounding factors regarding isotope selection may not have been accounted for in our analysis. Thirdly, utilization of medication for genitourinary symptoms may vary amongst providers, which may impact grade 2 toxicity scoring when using Common Terminology Criteria for Adverse Events. Finally, the small number of patients in this report may under power our ability to discern more subtle toxicity differences amongst cohorts.
Conclusions
Our study demonstrates that an HDR-boost compared with an LDR-boost combined with ultra-hypofractionated external beam radiation therapy is associated with less genitourinary morbidity. Thus, an HDR boost may be preferable to minimize impact on genitourinary quality of life when utilizing combined modality radiotherapy with UH-RT.
Acknowledgment
We acknowledge Dr Jennifer Huber for her technical editing assistance during the preparation of this manuscript.
Disclosures:
This research was funded in part through the National Institute of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748.
References
- [1].Sathya JR, Davis IR, Julian JA, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol 2005;23:1192–1199. [DOI] [PubMed] [Google Scholar]
- [2].Hoskin PJ, Rojas AM, Ostler PJ, et al. Randomised trial of external-beam radiotherapy alone or with high-dose-rate brachytherapy for prostate cancer: Mature 12-year results. Radiother Oncol 2021;154:214–219. [DOI] [PubMed] [Google Scholar]
- [3].Morris WJ, Tyldesley S, Rodda S, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017;98:275–285. [DOI] [PubMed] [Google Scholar]
- [4].Rodda S, Morris WJ, Hamm J, et al. ASCENDE-RT: An analysis of health-related quality of life for a randomized trial comparing low-dose-rate brachytherapy boost with dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017;98:581–589. [DOI] [PubMed] [Google Scholar]
- [5].Spratt DE, Zumsteg ZS, Ghadjar P, et al. Comparison of high-dose (86.4 Gy) IMRT vs combined brachytherapy plus IMRT for intermediate-risk prostate cancer. BJU Int 2014;114:360–367. [DOI] [PubMed] [Google Scholar]
- [6].Kollmeier MA, McBride S, Varghese M, et al. Low-dose-rate brachytherapy combined with ultrahypofractionated radiation therapy for clinically, localized, intermediate-risk prostate cancer: Results from a prospective trial. Int J Radiat Oncol Biol Phys 2020;108:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gorovets D, Hopkins M, Kollmeier M, et al. Early outcomes of high-dose-rate brachytherapy combined with ultrahypofractionated radiation in higher-risk prostate cancer. Brachy 2021;26:S1538–S4721. [DOI] [PubMed] [Google Scholar]
- [8].Dhere VR, Fischer-Valuck BW, Goyal S, et al. Toxicity outcomes after low-dose-rate vs high-dose-rate brachytherapy boost in combination with external beam radiation for intermediate and high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2021;111:e272. [DOI] [PubMed] [Google Scholar]
- [9].Hathout L, Mahmoud O, Wang Y, et al. A phase 2 randomized pilot study comparing high-dose-rate brachytherapy and low-dose-rate brachytherapy as monotherapy in localized prostate cancer. Adv Radiat Oncol 2019;4:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]


