Abstract
Nano and micro-technologies are used for therapeutic delivery of biologics and small molecules in formulations ranging in size from one nanometer to 100 microns or more. Here we review the unique physiochemical properties of these technologies and how they lead to more beneficial drug pharmacokinetics and toxicity over conventional formulations.
In the past 75 years, the ability to manipulate matter at the nano (~1–100 nm) and micro (~100 nm–100 μm) scales with increasing precision has led to stunning progress in an array of fields, including medicine. Therapeutics, which previously have been classified as either small molecule drugs or macromolecule biologics, now include nano/microparticles (NPs) (Figure 1) with therapeutic value partly or wholly attributable to their nanoscale molecular arrangement. These formulations are often comprised of materials such as lipids, polymers, and/or surfactants, and form a variety of NPs which have many advantages as well as drawbacks (Figure 2). Moreover, NPs have illustrated unique physicochemical properties that lead to amore desirable pharmacokinetic profile, increased drug solubility, improved drug stability, superior cellular trafficking, and control of the timing and location of therapeutic release, and thereby reduced toxicity.
Figure 1. Timeline of FDA Approval of Therapeutic Nano- and Microparticles (NPs).
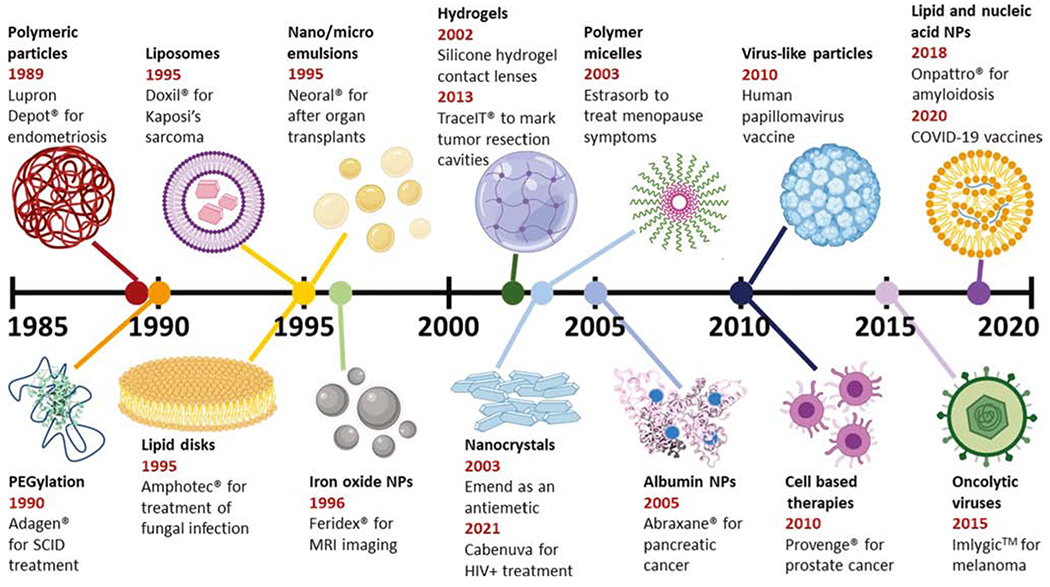
The earliest date indicates the year of first FDA approval for that formulation, with commercial name and indication also given. Additional influential formulations using that technology are also indicated. Figures are not to scale with each other. Abbreviation: COVID-19, coronavirus disease 2019; MRI, magnetic resonance imaging; SCID, severe combined immunodeficiency disease.
Figure 2. Materials Commonly Employed in Nano- and Microparticle Therapeutics.
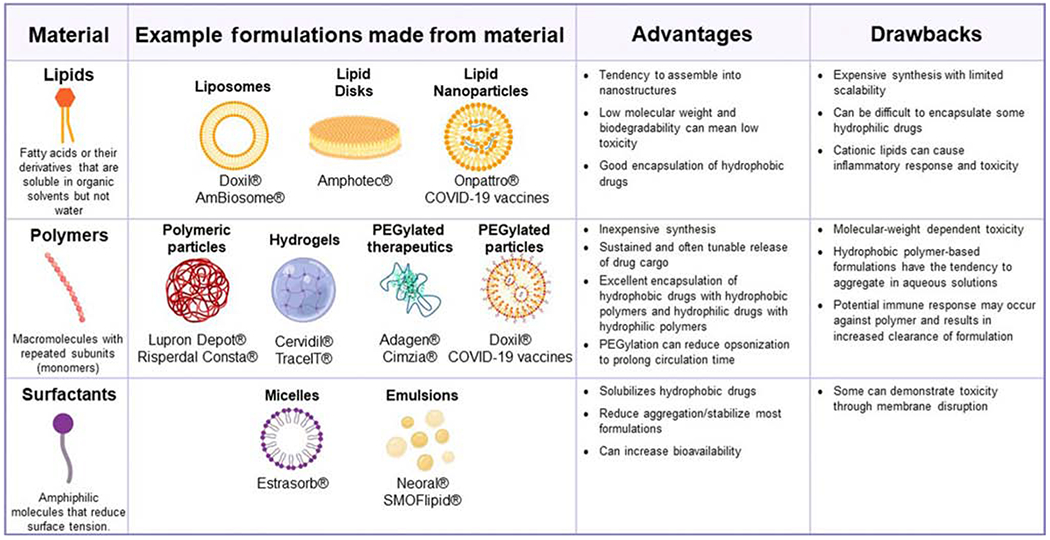
Abbreviations: COVID-19, coronavirus disease 2019; PEG, polyethylene glycol.
The first FDA-approved synthetic NP therapy was Lupron Depot®, designed to better control release of leuprorelin, a gonadotropin-releasing hormone (GnRH) receptor agonist, over time. Leuprorelin is encapsulated within 1–100 micron diameter spherical particles made from poly(lactic-co-glycolic acid) (PLGA). PLGA is a hydrophobic polyester polymer which hydrolytically degrades into lactic acid and glycolic acid, releasing encapsulated cargo through degradation and diffusion. Lupron Depot® permits intramuscular administration at monthly or longer intervals, rather than the daily administration required with the conventional formulation. This concept of sustained-release PLGA microparticle formulations has led to over ten FDA-approved formulations [1].
Nanocrystal formulations are also useful as injectable formulations, as demonstrated by the recent FDA approval of Cabenuva, an intramuscular injection of a nanocrystalline suspension of cabotegravir and rilpivirine. These two anti-HIV drugs can be taken orally, but the need to take them every day can lead to poor compliance, increasing the risk of developing drug-resistant strains of HIV. The injectable nanocrystalline suspension, consisting of nanosized crystals of the drug stabilized by surfactants, requires only monthly injections. While it accomplishes a similar goal to PLGA microspheres of sustained release after injection, the nanocrystal formulation for these drugs was used because their poor solubility means the crystals will have an inherently slow rate of release. Moreover, the comparatively high drug doses required in the small volume of a single injection necessitates the use of high loadings of drugs with minimal excipients (15–27% mass excipient per mass total formulation according to prescribing information) [2].
Orally-administered drugs exhibiting low solubility and/or low permeability can exhibit poor or highly variable bioavailability. NPs have been employed to address this problem. Neoral® is a self-microemulsifying drug delivery system (SMEDDS) comprised of the poorly water-soluble immunosuppressant cyclosporine, propylene glycol (a surfactant), and ethanol, which spontaneously forms surfactant-stabilized oily droplets under 200 nm in diameter upon exposure to the aqueous environment of the gut. These oily droplets better solubilize the therapy, resulting in enhanced oral absorption [3].
NPs incorporating cancer chemotherapies can overcome poor drug bioavailability and reduce drug associated toxicity. Liposomes are spherical lipid bilayer particles with an aqueous interior. Doxil®, approved in 1995, consists of a liposome encapsulating doxorubicin with a surface polyethylene glycol (PEG) corona. PEG is a common surface modification for NPs because it reduces protein adsorption and uptake into cells, significantly prolonging circulation time. Doxil® exhibits a much greater half-life and reduced cardiotoxicity compared with the conventional formulation. Enrichment of Doxil® in tumor tissue has been attributed to the enhanced permeability and retention (EPR) effect, wherein intravenously-administered NPs more easily enter the tumor due to poor vessel integrity. However, this central ‘dogma’ of cancer nanomedicine has been questioned, especially as most liposomal chemotherapeutic formulations do not improve overall survival, but rather primarily mitigate toxicity [4].
In the past decade, the rise of nucleic acid-based therapeutics has demanded better delivery, and researchers have turned to NPs to overcome this challenge. With DNA and RNA therapies, formulation into NPs has become the dominant method for delivery to the relevant cellular compartments (cytosol for RNA and nucleus for DNA). Licensed therapies for nucleic acid delivery have predominantly consisted of lipid nanoparticles (LNPs) which are NPs consisting of lipids in complex with their nucleic acid cargo. Cationic or ionizable groups incorporated into the NPs serve multiple roles in delivering nucleic acids. They complex negatively-charged nucleic acids for efficient encapsulation, particle formation, and cellular uptake, as well as facilitate endosomal release into the cytosol through interaction with the negatively charged head groups of the endosomal lipid membrane. Onpattro®, a PEGylated lipid NP containing siRNA that was FDA approved in 2018, is used to treat transthyretin-mediated amyloidosis [5]. Because cationic groups induce significant immune activation and toxicity, Onpattro® has ionizable lipids which are positively charged at acidic pH while being largely neutral at physiological pH. In the reduced endosomal pH, the lipids become charged to promote endosomal disruption. Employing lipid NPs for RNA delivery skyrocketed during the coronavirus disease 2019 (COVID-19) pandemic, when two vaccines (mRNA-1273 from Moderna and BNT162b2 from Pfizer/BioNTech) were used to deliver mRNA encoding severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein and were issued Emergency Use Authorization by the FDA.
While there have been many successes in translating NPs into the clinic, past failures can provide insight into issues which remain unresolved. Among these was the bankruptcy of Bind Therapeutics, who used PEGylated polylactic acid (PLA-PEG) NPs to encapsulate the chemotherapeutic docetaxel. The PEG surface was modified with a small molecule to bind to prostate-specific membrane antigen, putatively permitting targeted accumulation in cancer tissues [6]. However, they did not reach their specified endpoints during their Phase III trial and subsequently declared bankruptcy, selling their assets to Pfizer in 2017. In addition to demonstrating the potential discrepancies between promising preclinical data and results in large-scale clinical trials, this incident also prompted critical reevaluation of the EPR effect and targeting moieties for nanomedicines. Future approaches may benefit from incorporating precision medicine principles, such as administering NP imaging agents to demonstrate EPR or targeting susceptibility as a selection criterion for nanomedicine treatment.
As NP therapies require purity and uniformity not only of their constituent molecules, but also in the specific way these molecules are arranged at the nano scale, clinical scale use of NPs can also present unique manufacturing challenges. The unique requirements for the manufacture of liposomes, for example, led to a worldwide Doxil® shortage in 2012 and 2013 after one of the only plants manufacturing it was shut down. Methods to achieve more scalable and reproducible manufacturing include the use of self-assembling molecules to achieve bottom-up rather than top-down NP formation, or the use of microfluidics to allow for efficient mixing and continuous manufacturing. The application of these methods to the manufacture of emerging NP therapies is needed to help ensure clinical success.
Another major impediment to successful clinical application of NPs is the potential for development of deleterious immune responses against excipients that comprise the formulation. Many of the characteristics listed earlier which make NPs favorable as a therapy or vaccine can provoke an immune response against the particle itself upon repeated administration to patients. This phenomenon is observed with therapeutics whose surfaces are decorated with PEG. For example, both Doxil® [7] and PEGylated LNPs used to deliver RNA [8] can undergo accelerated blood clearance upon repeated administration, a phenomenon generally attributed to increased levels of anti-PEG and anti-phosphorylcholine IgM in serum. An anti-PEG immune response has also been proposed as a possible cause of the exceedingly rare anaphylactic events observed with COVID-19 RNA vaccines [9]. To overcome accelerated blood clearance, strategies have been employed to remove the PEG from the particle upon administration, whether via abstraction of PEGylated lipids from the particle by plasma lipoproteins [10] or by engineering a labile linker between PEG and the NP. Other groups have investigated substitution of other hydrophilic polymers for PEG and found that other polymers were less likely to induce accelerated clearance with repeat administration [11]. Disrupting NP interactions with charged amino acids by surface modification with zwitterions can also reduce protein adsorption similarly to hydrophilic polymers [12]. Regardless of the solution, PEG’s immunogenicity underscores the critical nature of NPs’ interaction with the immune system and characterizing this interaction should be a priority in the development of any NP therapeutic.
Despite the concerns noted with NPs, there is clearly a compelling future for NP therapeutics. Their continued refinement has the potential to enable even greater control of the physicochemical and pharmacokinetic properties of small molecules and biologics. They are a primary enabling factor in the rapid rise of the new class of nucleic acid therapeutics, and there is significant promise for further developments in this area, be it the NP-mediated delivery of mRNA which itself encodes for NPs [13], or the use of LNPs to allow for in vivo CRISPR-Cas mediated gene editing [14]. As the potential applications continue to expand, it is important that we consider lessons of the past to best use the tools of NP technology to better human health.
Acknowledgments
The images presented were created with BioRender.com. We would also like to acknowledge our funding sources National institutes of Health (NIH) National Institute of Allergy and infectious Diseases (NIAID) R01AI147497.
Footnotes
Declaration of Interests
There are no interests to declare.
References
- 1.Anselmo AC and Mitragotri S (2014) An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 190, 15–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams PE et al. (2015) Formulation and pharmacology of long-acting rilpivirine. Curr. Opin. HIV AIDS 10, 233–238 [DOI] [PubMed] [Google Scholar]
- 3.Min DI (1996) Neoral: a microemulsion cyclosporine. J. Transpl. Coord 6, 5–8 [DOI] [PubMed] [Google Scholar]
- 4.Sindhwani S and Chan WCW (2021) Nanotechnology for modern medicine: next step towards clinical translation. J. Intern. Med Published online January 22, 2021. 10.1111/joim.13254 [DOI] [PubMed] [Google Scholar]
- 5.Akinc A et al. (2019) The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol 14, 1084–1087 [DOI] [PubMed] [Google Scholar]
- 6.Hrkach J et al. (2012) Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med 4, 128ra39. [DOI] [PubMed] [Google Scholar]
- 7.Abu Lila AS et al. (2013) The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage. J. Control. Release 172, 38–47 [DOI] [PubMed] [Google Scholar]
- 8.Besin G et al. (2019) Accelerated blood clearance of lipid nanoparticles entails a biphasic humoral response of B-1 followed by B-2 lymphocytes to distinct antigenic moieties. Immunohorizons 3, 282–293 [DOI] [PubMed] [Google Scholar]
- 9.Klimek L et al. (2020) ARIA-EAACI statement on severe allergic reactions to COVID-19 vaccines - an EAACI-ARIA position paper. Allergy Published online December 30, 2020. 10.1111/all.14726 [DOI] [PubMed] [Google Scholar]
- 10.Mui BL et al. (2013) Influence of polyethylene glycol lipid desorption rates on pharmacokinetics and pharmacodynamics of siRNA lipid nanoparticles. Mol. Ther. Nucleic Acids 2, e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kierstead PH et al. (2015) The effect of polymer backbone chemistry on the induction of the accelerated blood clearance in polymer modified liposomes. J. Control. Release 213, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlenoff JB (2014) Zwitteration: coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 30, 9625–9636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melo M et al. (2019) Immunogenicity of RNA replicons encoding HIV env immunogens designed for self-assembly into nanoparticles. Mol. Ther 27, 2080–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng Q et al. (2020) Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol 15, 313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]


