Abstract
Macrophage-mediated inflammation drives autoimmune and chronic inflammatory diseases. Treatment with anti-inflammatory agents can be an effective strategy to reduce this inflammation; however, high concentrations of these agents can have immune-dampening and other serious side effects. Synergistic combination of anti-inflammatory agents can mitigate dosing by requiring less drug. Multiple anti-inflammatory agents were evaluated in combination for synergistic inhibition of macrophage inflammation. The most potent synergy was observed between dexamethasone (DXM) and fumaric acid esters (e.g., monomethyl fumarate (MMF)). Furthermore, this combination was found to synergistically inhibit inflammatory nuclear factor κB (NF-κB) transcription factor activity. The optimal ratio for synergy was determined to be 1:1, and DXM and MMF were conjugated by esterification at this molar ratio. The DXM–MMF conjugate displayed improved inhibition of inflammation over the unconjugated combination in both murine and human macrophages. In the treatment of human donor monocyte-derived macrophages, the combination of DXM and MMF significantly inhibited inflammatory gene expression downstream of NF-κB and overall performed better than either agent alone. Further, the DXM–MMF conjugate significantly inhibited expression of NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome-associated genes. The potent anti-inflammatory activity of the DXM–MMF conjugate in human macrophages indicates that it may have benefits in the treatment of autoimmune and inflammatory diseases.
Graphical Abstract
INTRODUCTION
Chronic inflammatory diseases are the most significant cause of death worldwide, with inflammation-associated diseases accounting for more than half of human deaths since 1980.1 Among these inflammatory diseases are autoimmune diseases. In 2005, up to 23.5 million people were affected by autoimmune diseases in the United States, and the prevalence of autoimmune diseases has been increasing over several decades both in the United States and worldwide.2–4 Activated inflammatory macrophages are key drivers of damage in autoimmune and chronic inflammatory diseases (e.g., multiple sclerosis, rheumatoid arthritis, psoriasis), making them an important therapeutic target.5–8
Macrophages drive inflammatory responses through the secretion of inflammatory cytokines and reactive oxygen species, directly causing inflammation and recruiting inflammatory immune cells.5,9 Key signaling pathways in suppressing the inflammatory response of macrophages include nuclear factor κB (NF-κB) and glucocorticoid receptor signaling. NF-κB is the upstream transcription factor of most inflammatory cytokine expression and reactive oxygen species production in macrophages, including tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), interleukin 1β (IL-1β), and nitric oxide (NO). Furthermore, NF-κB activity also promotes expression of NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome component genes, which can also lead to the production of inflammatory cytokines. Increased NF-κB activity has been implicated in patients with autoimmune diseases, and inhibition of this signaling pathway has been shown to be a viable treatment strategy.10–14 Antagonism of NF-κB through activation of the glucocorticoid receptor has many anti-inflammatory functions and has been shown to be a critical pathway in the modulation of macrophage anti-inflammatory responses.10,15
Anti-inflammatory agents that target NF-κB and/or glucocorticoid receptor signaling pathways have been effective in treating multiple autoimmune diseases. The most commonly used are glucocorticoids (e.g., dexamethasone (DXM)), which activate the glucocorticoid receptor.16–18 Other classes of anti-inflammatory agents known to antagonize NF-κB signaling include fumaric acid esters (FAE) and retinoids (e.g., all-trans-retinoic acid (ATRA)).19,20 Both FAE and retinoids are common treatments for autoimmune diseases.18,21–23 One of the most common FAE is dimethyl fumarate (DMF), which is approved for use in multiple sclerosis and psoriasis.18,21 DMF’s active metabolite, monomethyl fumarate (MMF), is also approved for the treatment of multiple sclerosis.24,25 Another inhibitor of NF-κB signaling is the dietary supplement curcumin, which has been assessed for the treatment of autoimmune disease.26,27 However, curcumin has poor bioavailability, and it may require administration in combination with other anti-inflammatory agents.28 While these anti-inflammatory agents can represent effective treatments for autoimmunity and inflammation, long-term side effects from many of these therapies include opportunistic infections, organ toxicity, osteoporosis, and cardiovascular disease, particularly at higher doses and/or sustained doses.18,29,30
Combining drugs can increase therapeutic efficacy and mitigate side effects. Combination therapy is often used in cases where monotherapy is not effective and is clinically used to treat multiple autoimmune diseases, including psoriasis and rheumatoid arthritis.16,18,31 The best candidates for combination therapies are those that exhibit synergy. Synergistic combinations usually involve drugs with parallel functions or parallel targets in a pathway, which results in increased effectiveness over monotherapy. Synergistic combinations can be dose-sparing, lowering the overall amount of drug required for therapeutic benefit, thereby mitigating side effects observed with higher doses. Due to the severe side effects of continued glucocorticoid therapy, dose-sparing combination therapies are often used in the clinic to minimize the amount of glucocorticoid administered to patients with autoimmunity.32,33 The combination of glucocorticoid and retinoid improves efficacy and tolerability in the treatment of psoriasis.34 Additionally, the combination of DXM and the nonsteroidal anti-inflammatory drug naproxen has shown dose-sparing effects in a rodent model of rheumatoid arthritis.35
A potential method of combination therapy is the chemical conjugation of two compounds into a drug–drug conjugate, also referred to as a mutual prodrug. Drugs can be conjugated together by direct fusion or by a stable or cleavable linker. A cleavable linker may be required or beneficial in regulating either drug’s conversion into its active form. Besides simplifying a combination therapy into one compound, mutual prodrugs can improve stability, bioavailability, membrane permeability, and biocompatibility over unconjugated forms.36–39 In the case of synergistic combinations, mutual prodrugs can have the particular benefit of promoting delivery of each compound to the same target at an optimal ratio. In some cases, conjugation can lead to improved or unique drug properties.40 Indeed, there are mutual prodrugs that have been shown to increase anti-inflammatory activity over their unconjugated forms.41,42 Sulfasalazine, used in the clinical treatment of several autoimmune diseases, is a mutual prodrug of 5-aminosalicylic acid and sulfapyridine conjugated by an azo bond.43 Additionally, combination of sulfasalazine with another anti-inflammatory agent, methotrexate, can improve treatment of rheumatoid arthritis over either drug alone.44 Another clinical example of a mutual prodrug used as an anti-inflammatory is benorylate, which is an ester conjugate of paracetamol and aspirin.45 Clearly, combinations of anti-inflammatory agents and their mutual prodrugs show promise in the treatment of autoimmune and inflammatory diseases.
In the present study, we assessed multiple combinations of anti-inflammatory compounds for synergistic inhibition of macrophage inflammation and NF-κB signaling. Synergy between two agents was determined quantitatively using the Chou–Talalay equation for the combination index (CI).46 After identification of the best synergistic pair, a drug–drug conjugate was synthesized and assessed for inhibition inflammation and NF-κB signaling in mouse and human macrophages.
RESULTS AND DISCUSSION
Dexamethasone Synergizes with Other Anti-inflammatory Agents.
We selected five anti-inflammatory agents based on their clinical applications and inflammatory macrophage suppressive properties: DXM, DMF, MMF, curcumin, and ATRA.47–51 To identify synergistic combinations, agents were combined pairwise in various ratios and assessed for inhibition of NO production from lipopolysaccharide (LPS)-activated murine macrophages in vitro. The degree to which each combination yielded synergistic inhibition was graphically represented as an isobologram (Figure 1). In addition, a combination index (CI) was calculated for each drug ratio based on the concentration in which NO production was inhibited 50% (IC50). A CI < 1 value indicates a synergistic combination, while lower CI values indicate more robust synergy.46 For screening purposes, a stringent cutoff of CI < 0.7 was considered robust synergy, while CI values closer to 1 were considered additive. Four combinations displayed substantial synergy, with minimum CIs ranging from 0.49 to 0.03. Three combinations were found to be additive.
Figure 1.
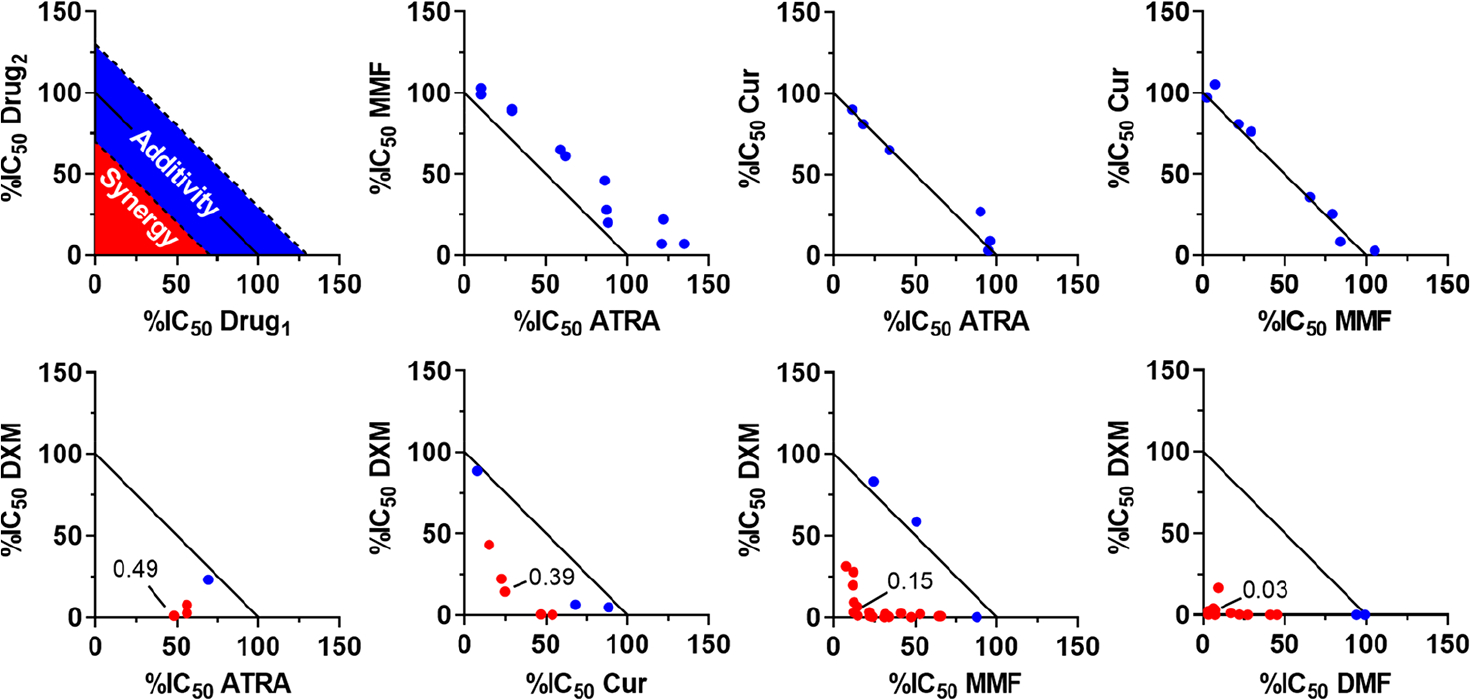
Isobolograms representing inhibition of nitric oxide (NO) production of combined anti-inflammatory agents from murine macrophages. Agents include dexamethasone (DXM), all-trans-retinoic acid (ATRA), curcumin (Cur), monomethyl fumarate (MMF), and dimethyl fumarate (DMF). Inhibition is normalized to untreated cells. Each point represents various concentrations of drug combinations evaluated by comparing the 50% inhibitory concentration (IC50) to the IC50 of each agent alone. As shown in the model isobologram, points within the red-shaded region represent a synergistic combination and are colored red in the isobolograms, whereas points in the blue-shaded region represent additive combinations and are colored blue. For screening purposes, combinations were considered synergistic at a combination index of <0.7.
Combining any two of ATRA, MMF, and curcumin was found to be additive, suggestive of a common or similar mechanism between these agents. Retinoids, fumarates, and curcumin are all known to have direct antioxidant functions, which may explain their additive relationship.52–54 Notably, the glucocorticoid DXM displayed a synergistic relationship with all other agents evaluated. Glucocorticoids have commonly been used to treat autoimmune disease in combination with other therapies.16,31 Additionally, other agents have been shown to have anti-inflammatory synergy with DXM, including L-arginine and pentoxifylline.55,56 One of the synergistic combinations found here was between DXM and ATRA. This result was encouraging, as glucocorticoids and retinoids have already shown combinatorial benefit in the topical treatment of psoriasis.34,57,58 The lowest CIs and greatest synergy were observed when DXM was combined with a fumaric acid ester (MMF or DMF). Due to their clinical significance, we focused further study on the combination of DXM and FAE.
Because DXM and DMF displayed the highest level of synergy in the inhibition of NO from murine macrophages, we explored if this combination could synergistically inhibit the activity of the transcription factor NF-κB. It was suspected that NF-κB activity may be synergistically inhibited because both DXM and FAE are known to inhibit distinct targets within the NF-κB pathway (Figure 2). Through binding the glucocorticoid receptor, DXM can inhibit NF-κB activity by inducing IκBα synthesis, preventing NF-κB complex translocation to the nucleus, and increasing p65 export from the nucleus.59–61 DMF inhibits MSK1 from phosphorylating p65, and inhibits NF-κB translocation to the nucleus.14,62 This is especially relevant to synergy between DMF and DXM, as DXM synergizes with an inhibitor of an upstream activator of MSK1, p38 MAP kinase, in the inhibition of human macrophage inflammation.63 DXM can also inhibit NF-κB transcriptional activity by promoting export of MSK1 from the nucleus into the cytoplasm.64 Additionally, both DMF and MMF are thought to inhibit NF-κB activity through interfering with intracellular redox mechanisms, and DMF has been reported to synergize with other NF-κB inhibitors.22,65 With the use of NF-κB luciferase reporter murine macrophages, it was observed that the IC50 was reduced up to 4000-fold for DXM and 15-fold for DMF when the two agents were combined in various ratios (Figure 3A–D). The greatest synergy was observed with treatment in a 1:1 molar ratio, with a CI of 0.09 ± 0.04 (Figure 3E). Because drug–drug conjugates can involve one molecule of each component, we considered whether a conjugation strategy could be applied to DXM and FAE combination treatment.
Figure 2.
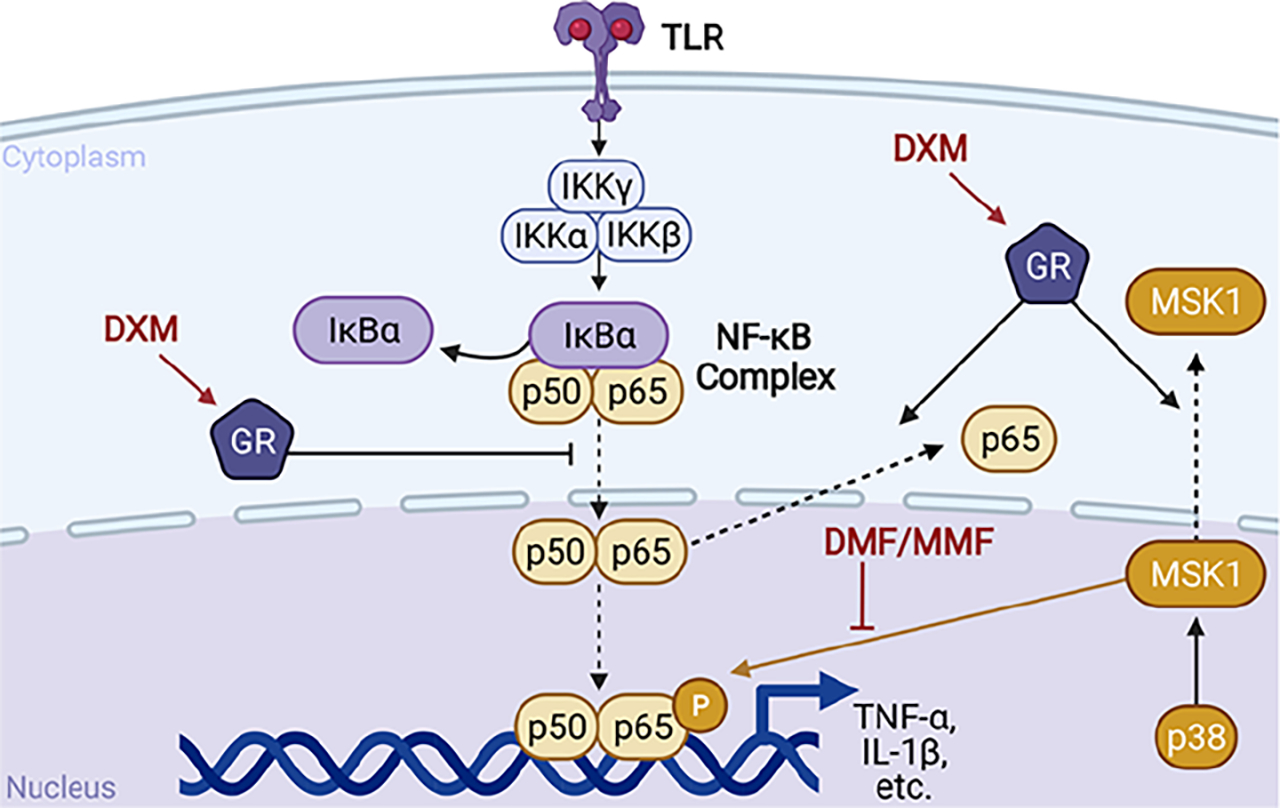
Simplified nuclear factor κB (NF-κB) activation pathway with dexamethasone (DXM) and dimethyl fumarate (DMF)/monomethyl fumarate (MMF) inhibitory targets from the literature. TLR = toll-like receptor. IκBα = inhibitor of κB. IKK = IκB kinase. GR = glucocorticoid receptor. MSK1 = mitogen- and stress-activated protein kinase 1. p38 = p38 MAP kinase. IL-1β = interleukin 1β. TNF-α = tumor necrosis factor α.
Figure 3.

Evaluation of NF-κB inhibition in murine macrophages when treated with drug combinations. Dexamethasone (DXM) and dimethyl fumarate (DMF) were combined in various molar ratios and used to treat activated NF-κB luciferase reporter murine macrophages. Inhibition is normalized to untreated cells. Concentration at which 50% inhibition of NF-κB was observed (IC50) of each combination with respect to (A, B) DXM or (C, D) DMF at 24 h. A one-way ANOVA with Dunnett’s test for multiple comparisons was used to determine significance. *, p-value < 0.05; **, p-value < 0.01. (E) Combination index (CI) for each ratio. + +, p-value < 0.01 for one-sample t test compared to 1. Data are presented as mean ± standard deviation. Each plot represents at least three independent experiments.
Conjugation of Monomethyl Fumarate to Dexamethasone Improves Anti-inflammatory Activity in Macrophages.
To promote delivery of both DXM and FAE in the optimal 1:1 ratio, MMF was conjugated to DXM (Scheme 1). The 1:1 ratio was achieved by esterification between the carboxyl group on MMF and the primary hydroxyl group on DXM. DMF lacks a carboxyl group for esterification by this scheme and would have required conversion to MMF by ester hydrolysis and yielded the same resulting conjugate molecule. Consequently, of the two FAE, only MMF was used for conjugation to DXM. The conjugation was confirmed by nuclear magnetic resonance (NMR) and mass spectrometric analyses (Figure S1, Figure S2). In aqueous environments, including intracellular spaces, hydrolysis would cleave the ester bond and release the components DXM and MMF in a 1:1 ratio. Additionally, hydrolysis would likely be catalyzed via esterase activity in vivo. The reported in vivo half-life of DMF is about 12 min, and the ester hydrolysis of glucocorticoid conjugates at the C-21 position is also reported to be within this time frame.66,67 Additionally, conjugation at the C-21 position on DXM is not required for glucocorticoid activity, making it an idea point for chemistry.68
Scheme 1. Conjugation of Dexamethasone and Monomethyl Fumaratea.

aEDC = N-(3-(dimethylamino)propyl)-N′-ethylcarbodiimide; DMAP = 4-dimethylaminopyridine.
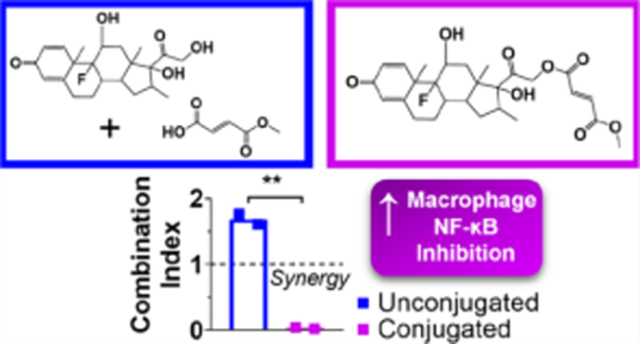
The anti-inflammatory activity of the DXM–MMF conjugate was compared to DXM and MMF unconjugated, in the same 1:1 ratio (Figure 4A,B). Surprisingly, the combinatorial benefit of DXM and MMF was not only retained with conjugation, but was significantly improved. In the assessment of NO inhibition from activated murine macrophages, the IC50 of the DXM–MMF conjugate was >25-fold lower than with unconjugated DXM and MMF delivered in the same ratio (Figure 4C, p-value = 0.01). The level of synergy was also seemingly improved by conjugation, with a 30-fold reduction in CI over unconjugated DXM and MMF, although this comparison was not significant (Figure 4D, p-value = 0.1).
Figure 4.

Nitric oxide inhibition in murine macrophages when treated with monomethyl fumarate (MMF) and dexamethasone (DXM) conjugate. Conjugated or unconjugated DXM and MMF were added to activated murine macrophages and were compared to (A) DXM or (B) MMF content. Inhibition is normalized to untreated cells. (C) Concentration at which NO production is inhibited 50% (IC50) relative to DXM and (D) combination index (CI) between unconjugated and conjugated DXM/MMF. Data are presented as mean ± standard deviation and represent two individual experiments. *, p-value < 0.05.
Formulations involving FAE showed cytotoxicity in murine macrophages starting at concentrations of >10 μM, while no cytotoxicity was observed for DXM at any tested concentration (Figure S3A,B). This range of tolerability is similar to cytotoxicity data reported by others for the treatment of murine macrophages with FAE.69 The DXM–MMF conjugate maintained anti-inflammatory activity and no apparent cytotoxicity at concentrations ranging from less than 100 nM to greater than 10 μM. Though unconjugated DXM and MMF showed no cytotoxicity at >10 μM, it was only up to 6-fold higher than its IC50, relative to FAE content. The DXM–MMF conjugate showed no cytotoxicity up to >13-fold its IC50. Nonetheless, both combinations of DXM and MMF were tolerated in the murine macrophages within an active range.
Given that the DXM–MMF conjugate significantly improved NO inhibition relative to the unconjugated components, it was examined whether NF-κB inhibition was similarly enhanced using activated NF-κB luciferase reporter murine macrophages (Figure 5A,B). We observed that the DXM–MMF conjugate improved NF-κB inhibition 2-fold (Figure 5C, p-value = 0.03). Additionally, a high level of synergy between DXM and MMF in the inhibition of NF-κB activity was observed for both conjugated and unconjugated treatments, with CIs of 0.04 ± 0.02 and 0.08 ± 0.03, respectively (Figure 5D). The 2-fold difference between conjugated and unconjugated DXM and MMF in NF-κB inhibition contrasts the >25-fold difference observed in NO inhibition. It is possible that a small difference in NF-κB activity had a larger effect on downstream NO production, or the conjugated form additionally inhibits another upstream regulator of NO, such as AP-1.70,71 Further work is needed to elucidate the mechanistic basis of this observation, but importantly, the DXM–MMF conjugate remains a potent inhibitor of macrophage inflammation and NF-κB activity.
Figure 5.
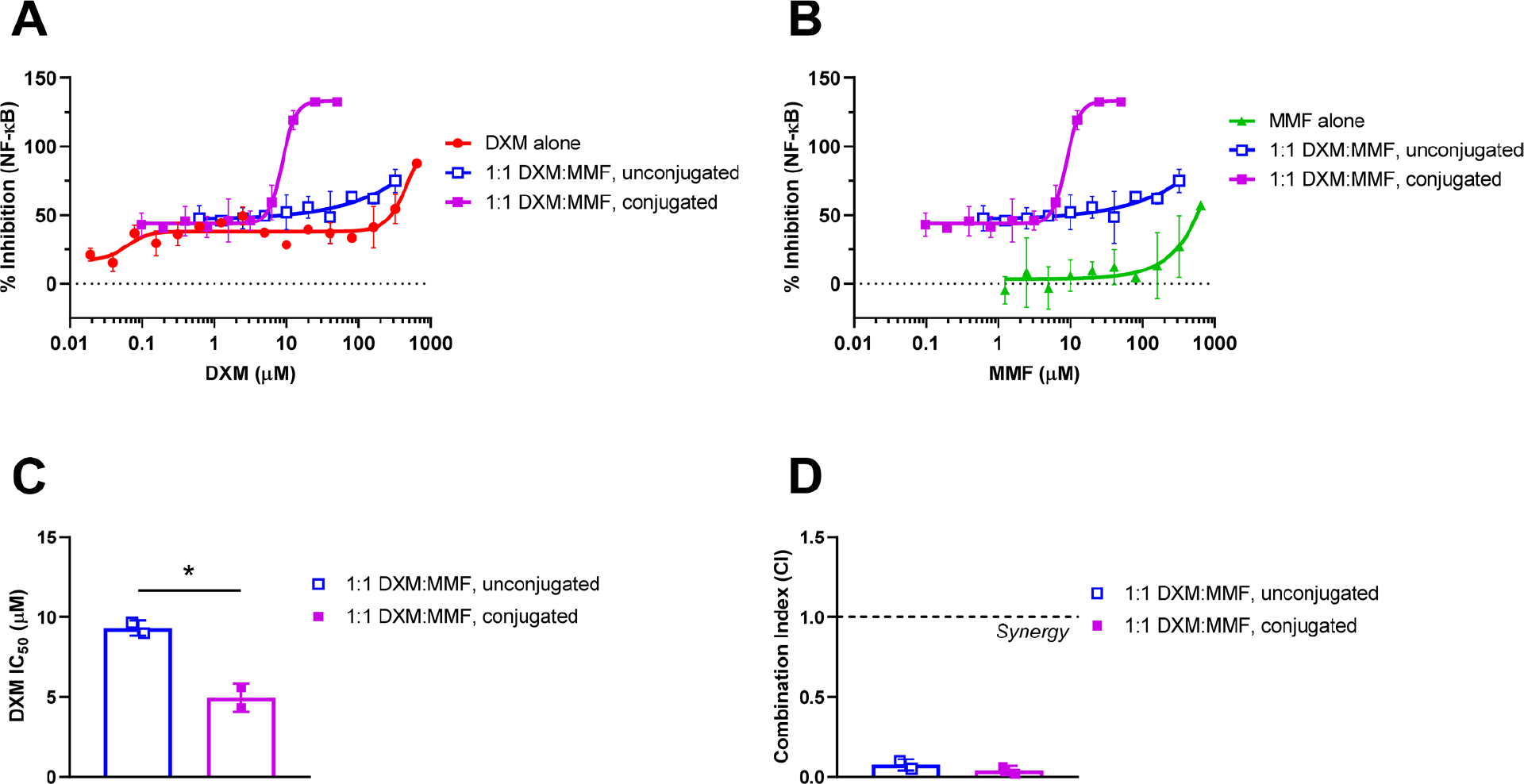
Inhibition of NF-κB by conjugated or unconjugated dexamethasone (DXM) and monomethyl fumarate (MMF) in activated luciferase reporter murine macrophages. Conjugated and unconjugated DXM and MMF were compared relative to (A) DXM or (B) MMF content. Inhibition is normalized to untreated cells. (C) Concentration at which NO production is inhibited 50% (IC50) relative to DXM and (D) combination index (CI) compared between unconjugated and conjugated DXM and MMF. Data are presented as mean ± standard deviation and represent two individual experiments. *, p-value < 0.05.
To determine if the robust synergy of DXM and FAE anti-inflammatory activity in murine macrophages could be translated to human macrophages, we first compared conjugated and unconjugated DXM and MMF in macrophages derived from a human monocyte cell line. NF-κB reporter THP-1 monocytes were differentiated into macrophages, activated with LPS, and treated with conjugated and unconjugated DXM and MMF (Figure 6A,B). Unlike the murine macrophage cell line, in human macrophages, NF-κB inhibition by the combination of DXM and MMF was greatly improved by conjugation. The IC50 of DXM was improved >60-fold by conjugation to MMF compared to the unconjugated combination (Figure 6C, p-value = 0.02). Notably, DXM had little activity on its own in this assay, with an IC50 determined to be >650 μM. Interestingly, unconjugated DXM and MMF did not display synergistic inhibition of NF-κB in the human macrophage cell line. Instead, we observed a high CI (>1.5) and lower range of activity for the unconjugated combination of DXM and MMF than MMF alone, suggestive of an antagonistic relationship. In fact, conjugation of DXM and MMF was required to obtain synergy in NF-κB inhibition using the human macrophage cell line (Figure 6D). The CI of conjugated DXM and MMF was 0.3 ± 0.1, similar to what was observed for NF-κB inhibition in the murine macrophages. The DXM–MMF conjugate was even better tolerated in the treatment of THP-1 macrophages than the murine macrophages (Figure S3C,D). Only two tested concentrations of the conjugate had more than 5% cytotoxicity, with no more than 17% cytotoxicity observed overall. Other formulations including FAE at similar doses showed greater cytotoxicity, up to 45%.
Figure 6.

Inhibition of NF-κB in human NF-κB luciferase reporter THP-1 derived macrophages when treated with conjugated and unconjugated monomethyl fumarate (MMF) and dexamethasone (DXM). Conjugated and unconjugated DXM and MMF were compared relative to (A) DXM or (B) MMF content. Inhibition is normalized to untreated cells. (C) Concentration at which NO production is inhibited 50% (IC50) relative to DXM and (D) combination index (CI) compared between unconjugated and conjugated DXM and MMF. Data are presented as mean ± standard deviation and represent two individual experiments. *, p-value < 0.05; **, p-value < 0.01.
Given the high level of NF-κB inhibition observed by treatment with the DXM–MMF conjugate, human blood monocyte-derived macrophages (hMDMs) were examined for the inhibition of inflammatory genes downstream of NF-κB transcriptional activity (Figure S4). Similar to the previous assays, hMDMs were activated with LPS and treated with either conjugated or unconjugated DXM–MMF in a 1:1 ratio. These comparisons were made across a 3-log range of concentrations and were further compared to DXM or MMF alone relative to their respective contents in the combined treatment groups. Unlike DXM or MMF alone, the combination of DXM and MMF significantly reduced expression of mRNA encoding the inflammatory cytokine TNF-α at all tested concentrations (Figure 7A). The combination of DXM and MMF displayed significantly improved inhibition over either DXM or MMF alone at 0.1 μM. Similar trends were seen in the inhibition of TNF-α protein production, with combined DXM and MMF treatment showing significant inhibition at 10-fold lower concentrations than DXM or MMF alone (Figure 7B). In addition to TNF-α, combined DXM and MMF treatment significantly inhibited expression of mRNA encoding inflammatory cytokines IL-6 and IL-1β compared to untreated or MMF-treated cells at all tested concentrations (Figure S5A,B). Compared to DXM, a potential trend of increased inhibition of IL-1β expression was observed at the highest tested concentration for the combined treatment. While conjugation of DXM to MMF was required for synergy in the treatment of THP-1 monocyte-derived macrophages, this phenomenon was not wholly observed in the treatment of primary hMDMs. We instead observed similar levels of cytokine inhibition between both conjugated and unconjugated DXM and MMF in the treatment of hMDMs. However, we did observe that only the DXM–MMF conjugate was able to significantly reduce expression of NLRP3, the key component of the NLRP3 inflammasome (Figure 7C). Notably, NLRP3 expression was less sensitive to anti-inflammatory treatment than other NF-κB-related genes tested in hMDMs, which may indicate the increased potency of the conjugate observed in the other two cell lines. Inhibition of the NLRP3 inflammasome in macrophages has been implicated as a potential strategy for autoimmune therapy, indicating that inhibition of this pathway would also be therapeutic.72,73 Overall, the DXM–MMF conjugate significantly inhibited the expression of both IL-1β and NLRP3, two key components of inflammasome activity downstream of NF-κB transcription. These findings demonstrate that the DXM–MMF conjugate is a potent suppressor of inflammatory signaling in hMDMs.
Figure 7.

Anti-inflammatory activity in human macrophages when treated with conjugated and unconjugated dexamethasone (DXM) and monomethyl fumarate (MMF). DXM and MMF were assessed for TNF-α (A) mRNA and (B) protein in cell supernatant or (C) NLRP3 mRNA after treatment. Expression was normalized to DMSO-treated wells. #, p-value < 0.05; ##, p-value < 0.01; ###, p-value < 0.001, with respect to DMSO-treated wells. *, p-value < 0.05; **, p-value <0.01; ***, p-value < 0.001. Data are presented as mean ± standard deviation and represent two individual experiments.
It is possible that the increased activity observed with treatment by the DXM–MMF conjugate is from a new function unique to the conjugate molecule. For example, melatonin–tamoxifen conjugation improves the treatment of breast cancer through the creation of a unique pharmacophore.40 Also, the increased activity of the DXM–MMF conjugate may be in line with other studies that have shown an enhancement of anti-inflammatory activity when electron-withdrawing groups are located on a heterocyclic backbone.74 However, a more likely explanation for the increased activity of the DXM–MMF conjugate becomes apparent in the comparison of MMF and DMF. Though MMF is the active form of DMF, in our assays DMF consistently had a lower IC50 than MMF and showed better synergy with DXM. This aligns with others who have shown that DMF, but not MMF, can inhibit inflammation in hMDMs.48 MMF is a metabolic product of DMF, as it breaks down into MMF through hydrolysis of one of its terminal esters into the more negatively charged carboxylate.75 The more charged MMF is significantly less permeable to cellular membranes than DMF, which limits its interaction with its intracellular targets, as supported by multiple in vitro studies with the two FAE.48,75,76 Like DMF, the DXM–MMF conjugate is neutrally charged and releases MMF via ester hydrolysis. It is therefore likely that the DXM–MMF conjugate more easily permeates the cellular membrane than MMF alone. Indeed, a conjugate between raloxifene and FAE led to increased NF-κB inhibition in MCF-7 breast cancer cells, though raloxifene showed no NF-κB inhibitory activity on its own.42 The enhanced activity of the raloxifene–FAE conjugate was attributed to increased permeability. In our studies, the DXM–MMF conjugate displayed activity similar to that of the combination of DXM and DMF in the inhibition of NO production and NF-κB activity in murine macrophages (Figure S6A,B). This observation supports the idea that MMF conjugated to another molecule by ester bond functions similarly to DMF as a prodrug. Additionally, ester conjugation at the C-21 position of a glucocorticoid may improve its lipophilicity, which could result in improved uptake of the conjugate across the cell membrane.68 However, increased binding to the glucocorticoid receptor in macrophages has been shown for a DXM C-21 acetate derivative, so it is possible that conjugation to DXM at the C-21 position leads to increased receptor binding and glucocorticoid activity.77
While the combination of DXM and MMF had a substantial benefit in murine macrophages and hMDMs, the unconjugated combination displayed antagonistic inhibition of NF-κB in THP-1 macrophages. It has been reported that DXM and DMF do not synergistically inhibit NF-κB in airway smooth muscle cells, which suggests that the synergistic effects may be cell type specific.78 Unlike in the other cell types used here, DXM only showed minimal inhibitory capacity at the highest tested doses in THP-1 cells. In fact, others have reported DXM to enhance NF-κB activation in THP-1 macrophages, which may be related to THP-1 maturation processes.79 Interestingly, conjugation was required to observe synergy in NF-κB inhibition between DXM and MMF in THP-1 macrophages. Indeed, even the combination of DXM and DMF showed an antagonistic relationship in these cells (Figure S6C). However, this antagonistic relationship was not observed with the DXM–MMF conjugate, which may indicate it has other unique properties or targets. In our study, outcomes with RAW264.7 macrophages more resembled hMDMs than did the THP-1 human cell line, suggesting that RAW264.7 may be an appropriate macrophage cell line for an initial screen. Nonetheless, the conjugate consistently showed potent anti-inflammatory activity in all tested cells, showing that it is indeed effective in multiple types of macrophages.
Both the conjugated and unconjugated combinations of DXM and MMF displayed significant inhibition of NF-κB-related genes in hMDMs. Notably, only the DXM–MMF conjugate showed inhibitory activity in the inhibition of NLRP3 expression in hMDMs. Conjugation may therefore be required for the synergistic benefit of DXM and MMF in the inhibition of at least some inflammatory signaling pathways in macrophages. Nonetheless, conjugation of DXM and MMF could promote the cytosolic delivery of both compounds in the optimal 1:1 synergistic ratio. Further study is warranted to determine the potential therapeutic benefit of the DXM–MMF conjugate in the treatment of autoimmune and inflammatory diseases.
CONCLUSION
DXM was found to synergize with four other anti-inflammatory agents in the inhibition of macrophage inflammation. Potent synergy was observed between DXM and FAE, including the inhibition of both NO production and inflammatory NF-κB transcription factor activity. Combination glucocorticoid and FAE treatment may therefore have noteworthy clinical relevance in decreasing the amount of each drug needed for therapeutic efficacy. A drug–drug conjugate was synthesized from DXM and MMF by esterification to promote delivery at the optimal synergistic ratio of 1:1. The DXM–MMF conjugate exhibited improved NF-κB inhibitory activity and synergy over the unconjugated combination in both murine and human macrophages. We also showed that conjugation between DXM and MMF retained robust anti-inflammatory activity in primary human macrophages and enabled significant inhibition of NLRP3 expression. Potent anti-inflammatory activity and minimal cytotoxicity in human macrophages suggest that the DXM–MMF conjugate may have significant translational potential for the treatment of autoimmune and chronic inflammatory diseases.
EXPERIMENTAL PROCEDURES
Materials.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise indicated. Chemicals used include dexamethasone (DXM; Alfa Aesar, Tewksbury, MA), monomethyl fumarate (MMF), dimethyl fumarate (DMF), all-trans-retinoic acid (ATRA), curcumin (Cur), lipopolysaccharide (LPS; isolated from E. coli O111:B4 or O55:B5), 4-dimethylaminopyridine (DMAP), and N-(3-(dimethylamino)propyl)-N′-ethylcarbodiimide hydrochloride (EDC).
Cell Culture and Treatment.
RAW 264.7 (TIB-71; ATCC Manassas, VA) murine macrophages were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin; 3 μg/mL puromycin was added to the media for cultures of NF-κB Renilla luciferase reporter RAW 264.7 macrophages (Crown Bioscience, San Diego, CA). THP-1 NF-κBLuc-2 (TIB-202-NFkB-LUC2; ATCC) human monocytes were cultured according to the supplier’s protocol and differentiated into macrophages with 150 nM phorbol myristate acetate (PMA) for 72 h. To test inhibition of inflammatory responses, cells were plated in 96-well culture plates (Murine macrophages 50 000 cells/well; NF-κB reporter 5000 cells/well; THP-1 macrophages 40 000 cells/well) and activated with 5 ng/mL LPS (E. coli O111:B4) for 24 h. Drugs were added after the first hour. For the screening of combinations, treatments were given at concentrations near or lower than the experimentally determined IC50 for each agent to detect potential combinatorial benefit. Each agent’s activity is presented as percent inhibition relative to controls, as indicated in the figure captions. Cells not activated with LPS were used to establish background activity.
Peripheral blood mononuclear cells (PBMCs) were obtained from healthy donors for the isolation of human monocytes. Informed consent was obtained from all volunteers under The Ohio State University Institutional Review Board approval number 2017H0040. For hMDMs, monocytes were isolated from healthy donor PBMCs by Ficoll gradient and differentiated into macrophages as previously described.80,81 PBMCs were cultured on Teflon wells for 5 days in RPMI-1640 (Gibco) containing 10% autologous serum. On day 5, cells were collected and hMDMs were purified via adherence to tissue culture plates at 5 × 106 cells/mL in the presence of RPMI-1640 and 10% autologous serum for 2 h at 37 °C. After incubation, nonadherent cells were eliminated by washing the hMDMs monolayer. On day 6, hMDMs were activated with LPS (E. coli O55:B5) at 100 ng/mL by 24 h in the presence of drug after the first hour. On day 7, supernatants and RNA were collected for further analysis compared to no drug treatment.
Inhibition of Nitric Oxide Production.
After 24 h treatment, NO was quantified in cell supernatants using the Griess Reagent System assay kit (Promega, Madison, WI) according to the manufacturer’s protocol.
Inhibition of NF-κB Activity.
To assess LUC-2 firefly luciferase activity, treated cells were washed with PBS and lysed with 9 mM MgCl2 and 0.125% Nonidet P-40 substitute in PBS for 15 min at room temperature with shaking. Using half of the lysate, luciferin (PerkinElmer, Waltham, MA) was added to 150 μg/mL for 10 min before the luminescence was read. For Renilla luciferase activity, cells were lysed and luminescence was assessed by using the Renilla Luciferase Glow Assay Kit (Thermo Fisher Scientific, Waltham, MA) and the manufacturer’s protocol.
Synergy Determination.
To assess synergistic combinations, anti-inflammatory agents were combined to treat cells in fixed ratios and titrated to determine the IC50 and compared to the IC50 of each agent alone. Drug combinations were considered synergistic if the CI was significantly less than 1. For screening purposes, CI ≤ 0.7 was used as a stringent cutoff for synergy, and combinations with 0.7 < CI < 1.3 were considered additive.
Synthesis of Dexamethasone–Monomethyl Fumarate Conjugate.
DMAP (460 mg, 3.28 mmol) was dissolved in 4 mL of dry dimethylformamide and added at room temperature to a 2 mL solution of monomethyl fumarate (MMF; 230 mg, 2.3 mmol) in dry dimethylformamide. The reaction mixture was stirred for 5 min at room temperature. EDC (430 mg, 2.3 mmol) in 2 mL of dry dimethylformamide (solution was cloudy) was then added at 0 °C, and the resulting suspension was stirred for 10 min at 0 °C. Dexamethasone (DXM; 100 mg, 0.259 mmol) in 3 mL of dry dimethylformamide was then added at 0 °C. The cooling bath was removed, and the colorless solution was stirred at room temperature for 1.5 h and then heated to 40 °C for 20 h. The mixture was diluted by the addition of HCl (1 M, 50 mL) and CH2Cl2 (50 mL) at room temperature. Vigorous stirring of the biphasic mixture was continued at room temperature for 30 min. The phases were separated, and the aqueous layer was extracted with CH2Cl2 (2×). The combined organic phases were concentrated under reduced pressure and purified by flash chromatography (3:1 v/v CH2Cl2/EtOAc) to afford the target compound. The product was further purified by recrystallization from DCM:hexane to give a white powder (54 mg, 41.3% yield). The product was further characterized by 1H and 13C NMR (Figure S1), as well as by electrospray ionization mass spectrometry (Figure S2).
Quantitative Real-Time PCR.
Analysis of mRNA expression from hMDMs was performed with Taqman quantitative real-time PCR of cDNA templates, as previously described.80 The following assay IDs for primers were used to assess human gene expression, all obtained from Thermo Fisher Scientific: HS00174131 (IL-6), HS00174128 (TNF-α), HS00174097 (IL-1β), HS02800695 (HPRT, housekeeping gene), and HS00918082 (NLRP3).
Cytokine ELISA.
Analysis of TNF-α protein released from hMDMs was performed with a sandwich ELISA, as previously described.82 Purified and biotin-labeled antihuman TNF-α and protein standards were purchased from BioLegend (San Diego, CA).
Statistical Analysis.
Unless otherwise indicated, comparisons between two groups were made by using a t test and comparisons made between two or more groups were made by using a one-way ANOVA with Tukey’s post-test for multiple comparisons. Statistical significance was considered with a p-value < 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the following: National Academies of Science Engineering and Medicine (NASEM) Ford Foundation Pre-Doctoral Fellowship, NIH NIAID R01AI141333, NIH NIAID T32AI007273, and NIH NIAID R01AI137525. Figure 2 was created with BioRender. The authors would like to acknowledge Isabella Young, Cole Batty, Dr. Sean Simpson, and the Ainslie Lab at UNC for guidance and laboratory support.
ABBREVIATIONS
- AP-1
activator protein 1
- ATRA
all-trans-retinoic acid
- CI
combination index
- DMAP
4-dimethylaminopyridine
- DMF
dimethyl fumarate
- DXM
dexamethasone
- EDC
N-(3-(dimethylamino)propyl)-N′-ethylcarbodiimide hydrochloride
- FAE
fumaric acid ester
- hMDMs
human monocyte-derived macrophages
- IC50
50% inhibitory concentration
- IL-1β
interleukin 1β
- IL-6
interleukin 6
- LPS
lipopolysaccharide
- MMF
monomethyl fumarate
- MSK1
mitogen- and stress-activated protein kinase 1
- NF-κB
nuclear factor κB
- NO
nitric oxide
- NLRP3
NOD-, LRR-, and pyrin domain-containing protein 3
- PBMCs
peripheral blood mononuclear cells
- TNF-α
tumor necrosis factor α
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.bioconjchem.1c00200.
Supporting methods; NMR spectra; ESI-MS spectrum; cytotoxicity assays; characterization of human macrophages; IL-6 and IL-1β expression from human macrophages; comparison of conjugate to dimethyl fumarate (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.bioconjchem.1c00200
Contributor Information
Christopher J. Genito, Department of Microbiology and Immunology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States
Meital Eckshtain-Levi, Division of Pharmacoengineering and Molecular Pharmaceutics, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States.
Zayda L. Piedra-Quintero, Division of Medical Laboratory Science, School of Health and Rehabilitation Sciences, College of Medicine, Wexner Medical Center, The Ohio State University, Columbus, Ohio 43210, United States
Sai Archana Krovi, Division of Pharmacoengineering and Molecular Pharmaceutics, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States.
Abriana Kroboth, Division of Medical Laboratory Science, School of Health and Rehabilitation Sciences, College of Medicine, Wexner Medical Center, The Ohio State University, Columbus, Ohio 43210, United States.
Rebeca T. Stiepel, Division of Pharmacoengineering and Molecular Pharmaceutics, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States
Mireia Guerau-de-Arellano, Division of Medical Laboratory Science, School of Health and Rehabilitation Sciences, College of Medicine, Wexner Medical Center, The Ohio State University, Columbus, Ohio 43210, United States.
Eric M. Bachelder, Division of Pharmacoengineering and Molecular Pharmaceutics, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States
Kristy M. Ainslie, Department of Microbiology and Immunology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States Division of Pharmacoengineering and Molecular Pharmaceutics, Eshelman School of Pharmacy, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, United States; Joint Department of Biomedical Engineering, University of North Carolina at Chapel Hill and North Carolina State University, Chapel Hill, North Carolina 27599, United States.
REFERENCES
- (1).Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, et al. (2019) Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Dinse GE, Parks CG, Weinberg CR, Co CA, Wilkerson J, Zeldin DC, Chan EKL, and Miller FW (2020) Increasing Prevalence of Antinuclear Antibodies in the United States. Arthritis Rheumatol. 72, 1026–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Lerner A, Jeremias P, and Matthias T (2015) The World Incidence and Prevalence of Autoimmune Diseases is Increasing. International Journal of Celiac Disease 3, 151–155. [Google Scholar]
- (4).Progress in Autoimmune Diseases Research (2005), National Institute of Allergy and Infectious Diseases. [Google Scholar]
- (5).Ma WT, Gao F, Gu K, and Chen DK (2019) The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 10, 1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Moore KJ, and Tabas I (2011) Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Mulherin D, Fitzgerald O, and Bresnihan B (1996) Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 39, 115–24. [DOI] [PubMed] [Google Scholar]
- (8).Haringman JJ, Gerlag DM, Zwinderman AH, Smeets TJ, Kraan MC, Baeten D, McInnes IB, Bresnihan B, and Tak PP (2005) Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann. Rheum. Dis. 64, 834–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Fujiwara N, and Kobayashi K (2005) Macrophages in inflammation. Curr. Drug Targets: Inflammation Allergy 4, 281–6. [DOI] [PubMed] [Google Scholar]
- (10).Eggert M, Klüter A, Zettl UK, and Neeck G (2004) Transcription factors in autoimmune diseases. Curr. Pharm. Des. 10, 2787–96. [DOI] [PubMed] [Google Scholar]
- (11).Christophi GP, Panos M, Hudson CA, Christophi RL, Gruber RC, Mersich AT, Blystone SD, Jubelt B, and Massa PT (2009) Macrophages of multiple sclerosis patients display deficient SHP-1 expression and enhanced inflammatory phenotype. Lab. Invest. 89, 742–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lisi S, Sisto M, Lofrumento DD, and D’Amore M (2012) Altered IκBα expression promotes NF-κB activation in monocytes from primary Sjögren’s syndrome patients. Pathology 44, 557–61. [DOI] [PubMed] [Google Scholar]
- (13).Mohammadi A, Sharifi A, Pourpaknia R, Mohammadian S, and Sahebkar A (2018) Manipulating macrophage polarization and function using classical HDAC inhibitors: Implications for autoimmunity and inflammation. Crit Rev. Oncol Hematol 128, 1–18. [DOI] [PubMed] [Google Scholar]
- (14).Gesser B, Rasmussen MK, and Iversen L (2020) Dimethyl Fumarate Targets MSK1, RSK1, 2 and IKKα/β Kinases and Regulates NF-κB /p65 Activation in Psoriasis: A Demonstration of the Effect on Peripheral Blood Mononuclear Cells, Drawn from Two Patients with Severe Psoriasis Before and After Treatment with Dimethyl Fumarate. Psoriasis: Targets Ther. 10, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Ray A, and Prefontaine KE (1994) Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc. Natl. Acad. Sci. U. S. A. 91, 752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Singh JA, Saag KG, Bridges SL, Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH, et al. (2016) 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 68, 1–26. [DOI] [PubMed] [Google Scholar]
- (17).Flammer JR, and Rogatsky I (2011) Minireview: Glucocorticoids in autoimmunity: unexpected targets and mechanisms. Mol. Endocrinol. 25, 1075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Menter A, and Griffiths CEM (2007) Current and future management of psoriasis. Lancet 370, 272–284. [DOI] [PubMed] [Google Scholar]
- (19).Mazzola MA, Raheja R, Regev K, Beynon V, von Glehn F, Paul A, Pierre I, Kivisakk P, Weiner HL, and Gandhi R (2019) Monomethyl fumarate treatment impairs maturation of human myeloid dendritic cells and their ability to activate T cells. Mult Scler 25, 63–71. [DOI] [PubMed] [Google Scholar]
- (20).Rafa H, Benkhelifa S, AitYounes S, Saoula H, Belhadef S, Belkhelfa M, Boukercha A, Toumi R, Soufli I, Moraès O, et al. (2017) All-Trans Retinoic Acid Modulates TLR4/NF-κB Signaling Pathway Targeting TNF-α and Nitric Oxide Synthase 2 Expression in Colonic Mucosa during Ulcerative Colitis and Colitis Associated Cancer. Mediators Inflammation 2017, 7353252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Burness CB, and Deeks ED (2014) Dimethyl Fumarate: A Review of Its Use in Patients with Relapsing-Remitting Multiple Sclerosis. CNS Drugs 28, 373–387. [DOI] [PubMed] [Google Scholar]
- (22).Mrowietz U, and Asadullah K (2005) Dimethylfumarate for psoriasis: more than a dietary curiosity. Trends Mol. Med. 11, 43–8. [DOI] [PubMed] [Google Scholar]
- (23).Ruggieri S, Tortorella C, and Gasperini C (2014) Pharmacology and clinical efficacy of dimethyl fumarate (BG-12) for treatment of relapsing-remitting multiple sclerosis. Ther. Clin. Risk Manage. 10, 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Litjens NHR, Burggraaf J, van Strijen E, van Gulpen C, Mattie H, Schoemaker RC, van Dissel JT, Thio HB, and Nibbering PH (2004) Pharmacokinetics of oral fumarates in healthy subjects. Br. J. Clin. Pharmacol. 58, 429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Lategan TW, Wang L, Sprague TN, and Rousseau FS (2021) Pharmacokinetics and Bioavailability of Monomethyl Fumarate Following a Single Oral Dose of Bafiertam (Monomethyl Fumarate) or Tecfidera. CNS Drugs 35, 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Weisberg SP, Leibel R, and Tortoriello DV (2008) Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 149, 3549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Yang M, Akbar U, and Mohan C (2019) Curcumin in Autoimmune and Rheumatic Diseases. Nutrients 11, 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Hewlings SJ, and Kalman DS (2017) Curcumin: A Review of Its Effects on Human Health. Foods 6, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Oray M, Abu Samra K, Ebrahimiadib N, Meese H, and Foster CS (2016) Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 15, 457–465. [DOI] [PubMed] [Google Scholar]
- (30).Naldi L, and Griffiths CE (2005) Traditional therapies in the management of moderate to severe chronic plaque psoriasis: an assessment of the benefits and risks. Br. J. Dermatol. 152, 597–615. [DOI] [PubMed] [Google Scholar]
- (31).Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, Karpouzas GA, Merrill JT, Wallace DJ, Yazdany J, et al. (2012) American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. 64, 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Heaphy MR, Albrecht J, and Werth VP (2005) Dapsone as a glucocorticoid-sparing agent in maintenance-phase pemphigus vulgaris. Arch. Dermatol. 141, 699–702. [DOI] [PubMed] [Google Scholar]
- (33).Del Rosso J, and Friedlander SF (2005) Corticosteroids: options in the era of steroid-sparing therapy. J. Am. Acad. Dermatol. 53, S50–8. [DOI] [PubMed] [Google Scholar]
- (34).Lebwohl MG, Breneman DL, Goffe BS, Grossman JR, Ling MR, Milbauer J, Pincus SH, Sibbald RG, Swinyer LJ, Weinstein GD, et al. (1998) Tazarotene 0.1% gel plus corticosteroid cream in the treatment of plaque psoriasis. J. Am. Acad. Dermatol. 39, 590–596. [DOI] [PubMed] [Google Scholar]
- (35).Li X, DuBois DC, Song D, Almon RR, Jusko WJ, and Chen X (2017) Modeling Combined Immunosuppressive and Anti-inflammatory Effects of Dexamethasone and Naproxen in Rats Predicts the Steroid-Sparing Potential of Naproxen. Drug Metab. Dispos. 45, 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Huang P, Wang D, Su Y, Huang W, Zhou Y, Cui D, Zhu X, and Yan D (2014) Combination of small molecule prodrug and nanodrug delivery: amphiphilic drug-drug conjugate for cancer therapy. J. Am. Chem. Soc. 136, 11748–56. [DOI] [PubMed] [Google Scholar]
- (37).Redasani VK, and Bari SB (2012) Synthesis and evaluation of mutual prodrugs of ibuprofen with menthol, thymol and eugenol. Eur. J. Med. Chem. 56, 134–8. [DOI] [PubMed] [Google Scholar]
- (38).Yuan Z, Chen S, Sun Q, Wang N, Li D, Miao S, Gao C, Chen Y, Tan C, and Jiang Y (2017) Olaparib hydroxamic acid derivatives as dual PARP and HDAC inhibitors for cancer therapy. Bioorg. Med. Chem. 25, 4100–4109. [DOI] [PubMed] [Google Scholar]
- (39).Aljuffali IA, Lin CF, Chen CH, and Fang JY (2016) The codrug approach for facilitating drug delivery and bioactivity. Expert Opin. Drug Delivery 13, 1311–25. [DOI] [PubMed] [Google Scholar]
- (40).Hasan M, Marzouk MA, Adhikari S, Wright TD, Miller BP, Matossian MD, Elliott S, Wright M, Alzoubi M, Collins-Burow BM, et al. (2019) Pharmacological, Mechanistic, and Pharmacokinetic Assessment of Novel Melatonin-Tamoxifen Drug Conjugates as Breast Cancer Drugs. Mol. Pharmacol. 96, 272–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Melagraki G, Afantitis A, Igglessi-Markopoulou O, Detsi A, Koufaki M, Kontogiorgis C, and Hadjipavlou-Litina DJ (2009) Synthesis and evaluation of the antioxidant and anti-inflammatory activity of novel coumarin-3-aminoamides and their alpha-lipoic acid adducts. Eur. J. Med. Chem. 44, 3020–3026. [DOI] [PubMed] [Google Scholar]
- (42).Kastrati I, Siklos MI, Brovkovych SD, Thatcher GRJ, and Frasor J (2017) A Novel Strategy to Co-target Estrogen Receptor and Nuclear Factor κB Pathways with Hybrid Drugs for Breast Cancer Therapy. Horm. Cancer 8, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Rains CP, Noble S, and Faulds D (1995) Sulfasalazine. A review of its pharmacological properties and therapeutic efficacy in the treatment of rheumatoid arthritis. Drugs 50, 137–56. [DOI] [PubMed] [Google Scholar]
- (44).Capell HA, Madhok R, Porter DR, Munro RA, McInnes IB, Hunter JA, Steven M, Zoma A, Morrison E, Sambrook M, et al. (2006) Combination therapy with sulfasalazine and methotrexate is more effective than either drug alone in patients with rheumatoid arthritis with a suboptimal response to sulfasalazine: results from the double-blind placebo-controlled MASCOT study. Ann. Rheum. Dis. 66, 235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Wright V (1976) A Review of Benorylate-A New Antirheumatic Drug. Scand. J. Rheumatol. 4, 5–8. [PubMed] [Google Scholar]
- (46).Chou TC (2006) Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 58, 621–81. [DOI] [PubMed] [Google Scholar]
- (47).Korhonen R, Lahti A, Hämäläinen M, Kankaanranta H, and Moilanen E (2002) Dexamethasone inhibits inducible nitric-oxide synthase expression and nitric oxide production by destabilizing mRNA in lipopolysaccharide-treated macrophages. Mol. Pharmacol. 62, 698–704. [DOI] [PubMed] [Google Scholar]
- (48).Michell-Robinson MA, Moore CS, Healy LM, Osso LA, Zorko N, Grouza V, Touil H, Poliquin-Lasnier L, Trudelle AM, Giacomini PS, et al. (2016) Effects of fumarates on circulating and CNS myeloid cells in multiple sclerosis. Ann. Clin. Transl. Neurol. 3, 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Parodi B, Rossi S, Morando S, Cordano C, Bragoni A, Motta C, Usai C, Wipke BT, Scannevin RH, Mancardi GL, et al. (2015) Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol. 130, 279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Ben P, Liu J, Lu C, Xu Y, Xin Y, Fu J, Huang H, Zhang Z, Gao Y, Luo L, et al. (2011) Curcumin promotes degradation of inducible nitric oxide synthase and suppresses its enzyme activity in RAW 264.7 cells. Int. Immunopharmacol. 11, 179–86. [DOI] [PubMed] [Google Scholar]
- (51).Mehta K, McQueen T, Tucker S, Pandita R, and Aggarwal BB (1994) Inhibition by all-trans-retinoic acid of tumor necrosis factor and nitric oxide production by peritoneal macrophages. J. Leukocyte Biol. 55, 336–42. [DOI] [PubMed] [Google Scholar]
- (52).Siddikuzzaman, and Grace VMB (2013) Antioxidant potential of all-trans retinoic acid (ATRA) and enhanced activity of liposome encapsulated ATRA against inflammation and tumor-directed angiogenesis. Immunopharmacol. Immunotoxicol. 35, 164–173. [DOI] [PubMed] [Google Scholar]
- (53).Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, Zeng W, Hronowsky X, Buko A, Chollate S, et al. (2011) Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134, 678–92. [DOI] [PubMed] [Google Scholar]
- (54).Menon VP, and Sudheer AR (2007) Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 595, 105–25. [DOI] [PubMed] [Google Scholar]
- (55).Chatterjee S, Premachandran S, Shukla J, and Poduval TB (2007) Synergistic therapeutic potential of dexamethasone and L-arginine in lipopolysaccharide-induced septic shock. J. Surg. Res. 140, 99–108. [DOI] [PubMed] [Google Scholar]
- (56).Speer EM, Dowling DJ, Xu J, Ozog LS, Mathew JA, Chander A, Yin D, and Levy O (2018) Pentoxifylline, dexamethasone and azithromycin demonstrate distinct age-dependent and synergistic inhibition of TLR- and inflammasome-mediated cytokine production in human newborn and adult blood in vitro. PLoS One 13, No. e0196352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Stücker M, Hoffmann M, and Altmeyer P (2002) Instrumental evaluation of retinoid-induced skin irritation. Skin Res. Technol. 8, 133–40. [DOI] [PubMed] [Google Scholar]
- (58).Tanghetti EA, and Tazarotene Stable Plaque Psoriasis Trial Study Group. (2000) An observation study evaluating the treatment of plaque psoriasis with tazarotene gels, alone and with an emollient and/or corticosteroid. Cutis 66, 4–11. [PubMed] [Google Scholar]
- (59).Auphan N, DiDonato JA, Rosette C, Helmberg A, and Karin M (1995) Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 270, 286–90. [DOI] [PubMed] [Google Scholar]
- (60).Aghai ZH, Kumar S, Farhath S, Kumar MA, Saslow J, Nakhla T, Eydelman R, Strande L, Stahl G, Hewitt C, et al. (2006) Dexamethasone suppresses expression of Nuclear Factor-kappaB in the cells of tracheobronchial lavage fluid in premature neonates with respiratory distress. Pediatr. Res. 59, 811–5. [DOI] [PubMed] [Google Scholar]
- (61).Nelson G, Wilde GJ, Spiller DG, Kennedy SM, Ray DW, Sullivan E, Unitt JF, and White MR (2003) NF-kappaB signalling is inhibited by glucocorticoid receptor and STAT6 via distinct mechanisms. J. Cell Sci. 116, 2495–503. [DOI] [PubMed] [Google Scholar]
- (62).Peng H, Guerau-De-Arellano M, Mehta VB, Yang Y, Huss DJ, Papenfuss TL, Lovett-Racke AE, and Racke MK (2012) Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor κB (NF-κB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J. Biol. Chem. 287, 28017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Armstrong J, Harbron C, Lea S, Booth G, Cadden P, Wreggett KA, and Singh D (2011) Synergistic effects of p38 mitogen-activated protein kinase inhibition with a corticosteroid in alveolar macrophages from patients with chronic obstructive pulmonary disease. J. Pharmacol. Exp. Ther. 338, 732–40. [DOI] [PubMed] [Google Scholar]
- (64).Beck IM, Vanden Berghe W, Vermeulen L, Bougarne N, Vander Cruyssen B, Haegeman G, and De Bosscher K (2008) Altered subcellular distribution of MSK1 induced by glucocorticoids contributes to NF-kappaB inhibition. EMBO J. 27, 1682–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Hund AC, Lockmann A, and Schön MP (2016) Mutually enhancing anti-inflammatory activities of dimethyl fumarate and NF-κB inhibitors–implications for dose-sparing combination therapies. Exp Dermatol 25, 124–30. [DOI] [PubMed] [Google Scholar]
- (66).Mrowietz U, Christophers E, and Altmeyer P (1999) Treatment of severe psoriasis with fumaric acid esters: scientific background and guidelines for therapeutic use. The German Fumaric Acid Ester Consensus Conference. Br. J. Dermatol. 141, 424–429. [DOI] [PubMed] [Google Scholar]
- (67).Park KK, Ko DH, You Z, and Lee HJ (2003) Metabolism of steroidal anti-inflammatory antedrugs in vitro: methyl 3,20-dioxo-11beta,17alpha,21-trihydroxy-1,4-pregnadiene-16alpha-carboxylate; its 9alpha-fluorinated, and their 21-O-acyl derivatives. Steroids 68, 315–9. [DOI] [PubMed] [Google Scholar]
- (68).Shroot B, Caron JC, and Ponec M (1982) Glucocorticoid specific binding: structure-activity relationships. Br. J. Dermatol. 107 (23), 30–4. [DOI] [PubMed] [Google Scholar]
- (69).Yamaguchi Y, Kanzaki H, Katsumata Y, Itohiya K, Fukaya S, Miyamoto Y, Narimiya T, Wada S, and Nakamura Y (2017) Dimethyl fumarate inhibits osteoclasts via attenuation of reactive oxygen species signalling by augmented antioxidation. J. Cell Mol. Med. 22, 1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Seidel P, Merfort I, Tamm M, and Roth M (2010) Inhibition of NF-κB and AP-1 by dimethylfumarate correlates with down-regulated IL-6 secretion and proliferation in human lung fibroblasts. Swiss Med. Wkly. 140, No. w13132. [DOI] [PubMed] [Google Scholar]
- (71).Herrlich P (2001) Cross-talk between glucocorticoid receptor and AP-1. Oncogene 20, 2465–75. [DOI] [PubMed] [Google Scholar]
- (72).Shin TH, Kim HS, Kang TW, Lee BC, Lee HY, Kim YJ, Shin JH, Seo Y, Won Choi S, Lee S, et al. (2016) Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis. 7, No. e2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Inoue M, Williams KL, Gunn MD, and Shinohara ML (2012) NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U. S. A. 109, 10480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Ullas BJ, Rakesh KP, Shivakumar J, Gowda DC, and Chandrashekara PG (2020) Multi-targeted quinazolinone-Schiff’s bases as potent bio-therapeutics. Results in Chemistry 2, 100067. [Google Scholar]
- (75).Mrowietz U, Morrison PJ, Suhrkamp I, Kumanova M, and Clement B (2018) The Pharmacokinetics of Fumaric Acid Esters Reveal Their In Vivo Effects. Trends Pharmacol. Sci. 39, 1–12. [DOI] [PubMed] [Google Scholar]
- (76).Blewett MM, Xie J, Zaro BW, Backus KM, Altman A, Teijaro JR, and Cravatt BF (2016) Chemical proteomic map of dimethyl fumarate-sensitive cysteines in primary human T cells. Sci. Signaling 9, No. rs10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Heiman AS, Hickman F, Ko DH, and Lee HJ (1998) New steroidal anti-inflammatory antedrugs bind to macrophage glucocorticoid receptors and inhibit nitric oxide generation. Steroids 63, 644–9. [DOI] [PubMed] [Google Scholar]
- (78).Seidel P, Merfort I, Hughes JM, Oliver BG, Tamm M, and Roth M (2009) Dimethylfumarate inhibits NF-{kappa}B function at multiple levels to limit airway smooth muscle cell cytokine secretion. Am. J. Physiol Lung Cell Mol. Physiol 297, L326–39. [DOI] [PubMed] [Google Scholar]
- (79).Wang Y, Zhang JJ, Dai W, Lei KY, and Pike JW (1997) Dexamethasone potently enhances phorbol ester-induced IL-1beta gene expression and nuclear factor NF-kappaB activation. J. Immunol. 159, 534–537. [PubMed] [Google Scholar]
- (80).Amici SA, Young NA, Narvaez-Miranda J, Jablonski KA, Arcos J, Rosas L, Papenfuss TL, Torrelles JB, Jarjour WN, and Guerau-De-Arellano M (2018) CD38 Is Robustly Induced in Human Macrophages and Monocytes in Inflammatory Conditions. Front. Immunol. 9, 1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Kang BK, and Schlesinger LS (1998) Characterization of mannose receptor-dependent phagocytosis mediated by Mycobacterium tuberculosis lipoarabinomannan. Infect. Immun. 66, 2769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Guerau-De-Arellano M, Smith KM, Godlewski J, Liu Y, Winger R, Lawler SE, Whitacre CC, Racke MK, and Lovett-Racke AE (2011) Micro-RNA dysregulation in multiple sclerosis favours pro-inflammatory T-cell-mediated autoimmunity. Brain 134, 3578–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


