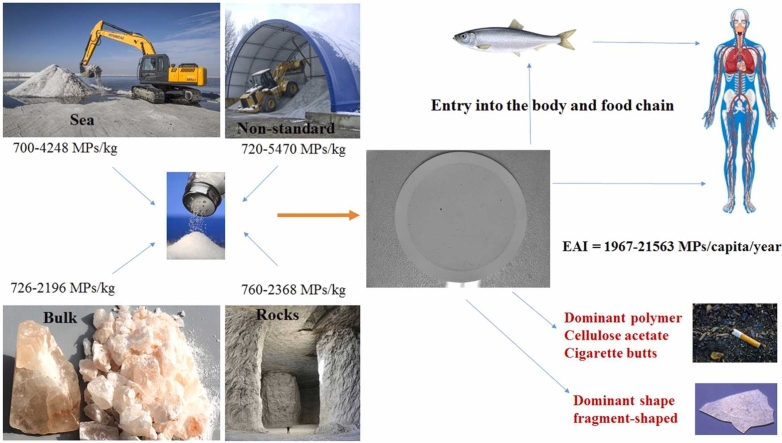
Abstract
Plastics and microplastics (MPs) are toxic, pervasive and threatening the biotic and abiotic components of the earth, and they threaten food safety and food security by moving in the food chain. In this study, the amounts and characteristics of 40 table salt samples with different brands, including sea salt (No = 13), rock (No = 13), bulk (No = 8) and non-standard (No = 6), were investigated with a combination of sieving, filtration, observation and FTIR, Micro-Raman and SEM techniques. The results showed that all the salts were contaminated with MPs. In general, the abundance range of detected particles was 700–5470 MPs/kg. The abundance of MPs was higher in counterfeit and non-standard salts (1825 ± 1808 MPs/kg). Investigating the relationship between the effect of the purification process (Kruskal-Wallis Test, P = 0.841), the type of packaging (Kruskal-Wallis Test, P = 0.609), and the type of salt (Kruskal-Wallis Test, P = 0.942), on the abundance of MPs using a comparison test Kruskal-Wallis was not significant. However, the numerical difference was recognizable. The most identified polymer in the salts was cellulose acetate, which probably causes by unmanaged plastic litter in the environment (especially cigarette butts). The dominant form of particles was fragment-shaped, which is the most abundant form of identified MPs in the environment. Both environmental pollution and secondary pollution (during production and packaging), respectively, contribute to the contamination of salts with MPs. The estimated human dietary intake (EDI) and the amount of estimated annual intake (EAI) for different ages in Iran were obtained EDI = 5–59 MPs/capita/day and EAI = 1967–21563 MPs/capita/year. The surface morphology of the particles showed that the MPs were affected by continuous weathering, mechanical fracture and oxidation. MPs are a threat to human health due to the absorption and transmission of dangerous pollutants and their inherent toxicity. Therefore, a solution must be thought of to prevent the contamination of the food chain through salts by MPs, (with protective measures at the salt source, and by improving its production processes.
Keywords: Table salt, Microplastics, Daily intake, Iran
Graphical Abstract
Highlights
-
•
All the salt samples were contaminated with MPs.
-
•
The abundance of particles detected in salts was 700–5470 MPs/kg.
-
•
The average abundance of MPs was higher in counterfeit and non-standard salts (1825 ± 1808 MPs/kg).
-
•
Environmental pollution more than secondary pollution contributes to the contamination of salts with MPs.
-
•
According to the EDI results, adults are more at risk of MPs.
1. Introduction
Plastic is a material designed to be durable and cost-effective and has a wide variety of uses [1]. Since the mass production of plastic in the 1950s, its global production has been increasing, and currently, its annual production has reached more than 400 million tons [2]. Most plastics are very resistant, and depending on their type, they can remain in the environment for hundreds of years. Plastics have caused the pollution of all environmental ecosystems [3], [4], [5]. These pollutants turn into MP particles (mm > 5) after destruction and decomposition. MPs are toxic, pervasive and threatening biotic and abiotic components of the earth [6], [7]. Various studies have shown that the contamination of various ecosystems with MP particles has caused these polluting particles to enter the human body through the consumption of contaminated food and water as well as air inhalation [8], [9], [10], [11]. Recent studies have confirmed the presence of this pollutant in the digestive system [12], [13], blood [14] and lungs of humans [15], [16]. MP particles are inherently polluting due to the additives used in their production [17], [18]. After entering the body, these pollutants are able to move and accumulate in different organs and tissues [19], [20]. MPs can lead to endocrine disruptions and Increased oxidative stress, and can affect mobility, reproduction, development, and carcinogenesis [4], [21], [22].
Recently, MPs have attracted much attention as emerging food contaminants due to their effects on human health and food safety [23], [24], [25]. Therefore, determining the amount of MPs intake by the human body is an important issue. Because in addition to assessing health risks, these data can also be used to recommend a consumption guideline to effectively reduce pollution and the entry of MPs into the human body [26]. Salts are essential nutritional elements for humans and are also used in food preservation. Therefore, like water and air, daily consumption of salt is inevitable [27], [28]. It has been reported that the global consumption of salt in 2018 was about 300 million tons, of which approximately 11.6 % (including table salts and food processing) was for human consumption [29]. Salts are mainly produced from the evaporation of salty waters such as the sea, lakes and, wells and also rock salt [30], and these salt extraction sources are usually affected by some human activities. Consequently, salts are generally exposed to different types of pollutants (especially plastics and MPs) [31]. Despite the relatively low daily salt consumption compared to other exposure routes, salt contamination with MPs is very significant [23]. The recommended limit of salt consumption by the World Health Organization (WHO) and the United States Food and Agriculture Organization (FAO) is less than 5 g/d [32]. However, according to the reports presented, the average salt consumption in Iran is more than 10 g/d [33]. The WHO report also estimated the amount of salt consumed by adults in Iran at 8–12 g/d, which is almost twice the recommended amount [34]. MPs in table salt can enter the digestive system and human body through swallowing and hand-to-mouth contact [23]. It has been reported that 94 % of salt in different countries contains MPs. The results of various studies have shown that the amount of MPs particles in salt is 140.2 MPs/kg on average, and due to the annual consumption of 3.75 kg of salt per person, several hundreds of MPs can enter a person's body yearly [35]. It should be considered that there is limited information about the contamination of table salt with MPs in the world and there is no complete and up-to-date study in this regard in Iran. On the other hand, due to the confirmation of the entry of these dangerous pollutants into the human body and their possible carcinogenic effect, it is necessary to determine the number of MPs in salts as one of the main ways of daily exposure to these pollutants. Therefore, the purpose of this research is to investigate the types of table salt offered in the Iranian market in terms of contamination with MPs and to estimate the amount of MPs' daily intake by citizens in the country.
2. Materials and methods
2.1. Sampling method
For the present study, 40 samples of table salt with different brands were collected, and all were from Iranian salt production. The prepared samples included sea salt (No = 13), rock salt (No = 13), bulk salt (No = 8) and non-standard salts (No = 6) that were collected from supermarkets and various stores across the country (four provinces in Iran). Bulk samples were in 10 kg packages (without the brand name, the trade name, and other specifications), and the rest of the samples were selected in different weights of 0.5, 1, 2, 2.5, 3, and 5 kg. The type of packaging of the samples included single-layer and double-layer plastic bags, plastic cans and woven plastic packaging. Table 1 shows the specifications of each salt sample. Unfortunately, in Iran, salts with fake (non-standard) brands are also sold in the market in different packages and are consumed by people. For this purpose, different samples of these brands were also prepared, and the abundance of MP particles was investigated. It is necessary to be explained that non-standard (fake) salts are salts that do not have the approval of the Ministry of Health. Failure to receive approval may be due to improper purification conditions, problems in the salt packing process, the presence of various impurities and pollution in the salt, or the manufacturer's failure to request a permit.
Table 1.
Characteristics of salt samples (in terms of salt type, purification method and packaging type).
| Sample | Salt type | Package type | Purification method |
|---|---|---|---|
| 1 | Rock salt | double layer (inner layer – thin plastic and outer layer - thick plastic) | Crystallized salt |
| 2 | Crystallized salt | ||
| 3 | plastic bag | Crystallized salt | |
| 4 | plastic bag | Crystallized salt | |
| 5 | Plastic can | Crystallized salt | |
| 6 | plastic bag | Crystallized salt | |
| 7 | plastic bag | Crystallized salt | |
| 8 | Plastic woven | Crystallized salt | |
| 9 | Plastic woven | Refined salt - Iodized | |
| 10 | plastic bag | Refined salt - Iodized | |
| 11 | Plastic can | Refined salt - Iodized | |
| 12 | plastic bag | Refined salt - Iodized | |
| 13 | plastic bag | Refined salt - Iodized | |
| 14 | Sea salt | plastic bag | Refined salt - Iodized |
| 15 | plastic bag | Refined salt - Iodized | |
| 16 | Plastic can | Refined salt - Iodized | |
| 17 | plastic bag | Refined salt - Iodized | |
| 18 | plastic bag | Crystallized salt | |
| 19 | plastic bag | Crystallized salt | |
| 20 | plastic bag | Crystallized salt | |
| 21 | plastic bag | Crystallized salt | |
| 22 | plastic bag | Crystallized salt | |
| 23 | plastic bag | Crystallized salt | |
| 24 | Plastic can | Crystallized salt | |
| 25 | Plastic woven | Crystallized salt | |
| 26 | Plastic woven | Crystallized salt | |
| 27 | Bulk salt - No commercial brand |
No packing | Unrefined (Large and heavy pieces of salt that are powdered manually or industrially) |
| 28 | |||
| 29 | |||
| 30 | |||
| 31 | |||
| 32 | |||
| 33 | |||
| 34 | |||
| 35 | Non-standard | plastic bag | Non-standard |
| 36 | plastic bag | ||
| 37 | double layer | ||
| 38 | plastic bag | ||
| 39 | plastic bag | ||
| 40 | double layer |
2.2. Sample processing
About 200–250 g of each type of salt was dissolved in 1 liter of distilled water. The density of these mixtures was between 1.2 and 1.3 g mL-1. Then, the digestion process was performed using H2O2 30 % (20 mL, for 24 h and 50 °C) to remove the possible biological substances in the salts. After the digestion stage, the samples were sieved using sieves (Mesh = 18 and 150). Then, for the convenience of counting MP particles, the residue on each sieve, and also the material passing through the sieve (Mesh = 150) was filtered by a cellulose nitrate filter. The filters were kept in a clean and covered glass petri dish to continue the analysis. Visual analysis of particles (size, shape and color determination) was done using a microscope. After that, to determine the chemical structure of MP particles and the characteristics of chemical species, fourier transform infrared spectroscopy (FTIR) and Micro-Raman spectroscopy were used. FTIR spectroscopy was used to determine the polymer structure of packaging bags and cans, as well as large polymer particles (> 150 µm), and Micro-Raman spectroscopy was used to determine the polymer structure of small MP particles (< 150 µm). Determining the surface morphology of MPs was done using a scanning electron microscope (SEM). Additional information on microscopy, FTIR, Micro-Raman and SEM characteristics and the number of samples analyzed is given in the Supplementary text (Text S1).
2.3. Estimated daily intake (EDI) and estimated annual intake (EAI)
Using Eq. (1), daily intake (EDI) and Eq. (2), annual intake (EAI) of MPs through salt consumption by citizens was estimated. In this study, the ingestion rate (IR) was determined according to the per capita salt consumption per person per day in Iran. C is the concentration of MPs (Particles/kg).
| EDI (MP/capita/day) = (C × IR) × 1 day | (1) |
| EAI (MP/capita/year) = (C × IR) × 365 day | (2) |
2.4. Quality control and quality assurance
The blank test was performed to avoid and assess the contamination of laboratory tools and equipment due to the commute, air, chemicals and tester during the test process. For this purpose, samples of ultrapure distilled water (5 samples) were poured into the beaker and kept for 1–5 days in the laboratory space and in the sample storage area. Also, cellulose nitrate filters (5 filters on glass plates) were used as a negative control (blank control), which were placed near the microscope, hood and surrounding environment of the experiment [36].
To avoid errors and contamination during sample preparation, testing and analysis, the doors and windows were closed and the fan was turned off. Commute restrictions to the laboratory were applied at the time and place of the test. Polymer-free coats and gloves were always used during testing. Glass and steel tools and equipment were used. The tools and equipment were washed regularly; the glass tools (with nitric acid 65 % and ultrapure water) and stainless steel equipment (with ultrapure water). Finally, they were dried and sterilized in the oven (170 °C, for 1 h). Aluminum foil was used to cover and package clean and sterilized equipment and tools. Forearms, hands, and work surfaces were regularly washed and wiped to prevent contamination with hair particles, dust, dirt, and more [37]. For visual analysis and to reduce particle counting errors, usually, three people did the particle counting.
2.5. Confidentiality
Any identifying information was not made available or accessed by anyone other than the project team. In addition, ethical issues based on the institution's ethics were considered in the research policy.
2.6. Hot needle examination
A hot needle test was performed to ensure the extracted particles are plastic. With this test, the plastic particles melt and become sticky. This method was performed for larger particles [38].
2.7. Recovery rate
Recovery rate tests were performed to verify the extraction process, using a standard method (with minor modifications). For this purpose, three types of MPs (PE, PP and PS) were used. For each MPs, 150 approximately spherical and fragment-shaped particles with sizes between 20 and 45 µm, 75–105 µm, and 125–150 µm were added to ultrapure water, respectively [39]. PE, PP and PS particles were obtained from a local plastic factory. Then they were classified by sieves with meshes of 100, 120, 150, 200, 325, and 600 in different sizes. The recovery rate for each MPs (PE, PP and PS) was evaluated separately. Two samples were considered for each size class, and the recovery rate was determined by two methods (method 1 = the entire filter area, method 2 = the checkerboard pattern method). In total, 36 samples (18 samples for each method) were used to evaluate the recovery rate.
2.8. Statistical analysis
The Kruskal-Wallis comparison test was used to investigate the effect of the purification process, type of packaging, and type of salt on the abundance of MPs. To investigate the effect of different factors on the recovery rate [(including the counting method (counting the entire filter area and the checkerboard pattern method), polymer type (PE, PP and PS) and particle size (20–45 µm, 75–105 µm and 125–150 µm)], Three-Way ANOVA test was performed; in the following, Bonferroni-adjusted significance tests were presented for pairwise comparisons.
3. Results and discussion
3.1. Quality control and quality assurance
3.1.1. Blank
On average, 9 ± 2.5 MPs particles per blank were detected (blank = cellulose nitrate membrane filter). Background contamination was negligible compared to the abundance of MPs detected in the samples. (Background contamination was 0.2–0.3 %) (Table S1). Also, in other blank samples (blank samples = ultra-pure distilled water), in order to control contamination in the sample storage area, the detected particles were, on average, 10.8 ± 1.6, which is insignificant. Considering the low blank values that have been obtained, it can be said that effective prevention of errors and contamination of samples has occurred in the process of study and analysis.
3.1.2. Recovery rates
For PP particles in the size class 20–45 µm, 75–105 µm and 125–150 µm, the recovery rate when counting 100 % of the filter area was about 67.5 ± 5.7 %, 72.3 ± 7.1 %, and 75.7 ± 7.1 %, respectively, and when counting checkerboard pattern was obtained, 70.7 ± 5.6 %, 73.3 ± 1.8 %, and 78.7 ± 5.6 %, respectively. (Table S2). For PE particles, in the size class of 20–45 µm, 75–105 µm and 125–150 µm, the recovery rate when counting 100 % of the filter area was respectively about 64.3 ± 5.2 %, 66.7 ± 5.7 %, and 72.3 ± 5.2 % and when counted by checkerboard pattern, it was estimated as 68 ± 1.9 %, 70.7 ± 1.9 %, and 73.3 ± 1.9 %, respectively (Table S2).
For PS particles in the size class of 20–45 µm, 75–105 µm and 125–150 µm, the recovery rate when counting 100 % of the filter area was respectively 61 ± 5 %, 61.7 ± 0.5 %, and 67.3 ± 2 %, and when counted by checkerboard pattern, 64 ± 7.5 %, 65.3 ± 5.6 % and 69.3 ± 7 % were obtained, respectively (Table S2). The effect of different factors influencing the recovery rate was investigated using the Three-Way ANOVA test and the results are presented in Table S2. The results showed that the recovery rate of MPs was significantly different based on the counting method (entire filter area and checkerboard pattern method) (p = 0.0001), based on the type of polymer (p = 0.005) and particle size (p = 0.014). Therefore, all the investigated factors had an effect on the recovery rate. Bonferroni adjusted significance tests were performed for pairwise comparisons, and the results are presented in Table S3.
3.2. MPs abundance
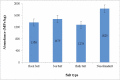
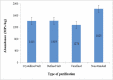
In this study, 4 types of salt (sea salt, rock salt, bulk salt and non-standard salt) with different brands were evaluated regarding MPs contamination. The results indicated that MPs are present in all salt samples. The distribution results of the abundance of MPs based on the type of salt, purification method and type of packaging are presented in Table 2, Table 3, Table 4, respectively. The abundance of MPs was obtained: (760–2368 MPs/kg) in rock salt, (700–4248 MPs/kg) in sea salt, (726–2196 MPs/kg) in bulk salt, and (720–5470 MPs/kg) in counterfeit or non-standard salts. As the results show, in different brands of salt (especially two brands 18 and 40), a significant numerical difference is observed in the abundance of MPs. Investigating the effect of the purification process (Kruskal Wallis Test, p = 0.841), the type of packaging (Kruskal Wallis Test, p = 0.609), and the type of salt (Kruskal Wallis Test, p = 0.942), on the abundance of MPs was performed, using the Kruskal Wallis comparison test. The test results showed that although the numerical difference in the abundance of MPs particles in the salt samples is significant, there is no statistically significant difference. The lack of difference in the average abundance of MPs in salts can be due to the pollution of all ecosystems with plastics and MPs, as well as secondary pollution (during production and packaging). Nevertheless, in terms of the type of salt, the highest and lowest averages of MPs were found in non-standard (fake) salt samples (1824.7 ± 1808.1 MPs/kg) and bulk salts (uncharacterized and packaged) (1278.3 ± 553.2 MPs/kg), respectively. In terms of the purification process, the highest and lowest average of MPs were respectively obtained in samples of non-standard salt (1824.7 ± 1808.1 MPs/kg) and unrefined salts (bulk salts) (1278.3 ± 553.2 MPs/kg). The difference in the mean of crystallized and refined salts was not significant.
Table 2.
Distribution of MPs particles among contaminated salt brands, on type of salt.
| NO | Abundance (MPs/kg) |
Salt type [average - (MPs/kg)] |
Chart | |
|---|---|---|---|---|
| Mean | SD | |||
| 1 | 1034.0 | 138.6 | Rock Salt (1356 ± 533) |
 |
| 2 | 1092.0 | 62.2 | ||
| 3 | 788.0 | 50.9 | ||
| 4 | 1056.0 | 33.9 | ||
| 5 | 1948.0 | 39.6 | ||
| 6 | 2368.0 | 113.1 | ||
| 7 | 932.0 | 62.2 | ||
| 8 | 1172.0 | 39.6 | ||
| 9 | 1892.0 | 96.2 | ||
| 10 | 1352.0 | 56.6 | ||
| 11 | 1136.0 | 22.6 | ||
| 12 | 760.0 | 22.6 | ||
| 13 | 2092.0 | 62.2 | ||
| 14 | 1808.0 | 181.0 | Sea Salt (1475 ± 902) |
|
| 15 | 1736.0 | 181.0 | ||
| 16 | 1092.0 | 73.5 | ||
| 17 | 900.0 | 50.9 | ||
| 18 | 4248.0 | 124.5 | ||
| 19 | 700.0 | 39.6 | ||
| 20 | 1636.0 | 50.9 | ||
| 21 | 1056.0 | 22.6 | ||
| 22 | 1040.0 | 79.2 | ||
| 23 | 962.0 | 87.7 | ||
| 24 | 1264.0 | 45.3 | ||
| 25 | 1576.0 | 101.8 | ||
| 26 | 1156.0 | 62.2 | ||
| 27 | 1856.0 | 124.5 | Bulk Salt - No commercial brand (1278 ± 553) |
|
| 28 | 1016.0 | 67.9 | ||
| 29 | 780.0 | 17.0 | ||
| 30 | 2196.0 | 28.3 | ||
| 31 | 726.0 | 70.7 | ||
| 32 | 1064.0 | 113.1 | ||
| 33 | 1680.0 | 67.9 | ||
| 34 | 908.0 | 79.2 | ||
| 35 | 720.0 | 22.6 | Non-Standard (1825 ± 1808) |
|
| 36 | 808.0 | 33.9 | ||
| 37 | 1452.0 | 209.3 | ||
| 38 | 1286.0 | 2.8 | ||
| 39 | 1212.0 | 28.3 | ||
| 40 | 5470.0 | 99.0 | ||
Table 3.
Distribution of MPs particles among contaminated salt brands, on type of purification.
| NO | Abundance (MPs/kg) |
Type of purification [average - (MPs/kg)] |
Chart | |
|---|---|---|---|---|
| Mean | SD | |||
| 1 | 1034 | 138.6 | Crystallized Salt (1413 ± 845) |
 |
| 2 | 1092 | 62.2 | ||
| 3 | 788 | 50.9 | ||
| 4 | 1056 | 33.9 | ||
| 5 | 1948 | 39.6 | ||
| 6 | 2368 | 113.1 | ||
| 7 | 932 | 62.2 | ||
| 8 | 1172 | 39.6 | ||
| 9 | 4248 | 124.5 | ||
| 10 | 700 | 39.6 | ||
| 11 | 1636 | 50.9 | ||
| 12 | 1056 | 22.6 | ||
| 13 | 1040 | 79.2 | ||
| 14 | 962 | 87.7 | ||
| 15 | 1264 | 45.3 | ||
| 16 | 1576 | 101.8 | ||
| 17 | 1156 | 62.2 | ||
| 18 | 1892 | 96.2 | Refined Salt (1419 ± 477) |
|
| 19 | 1352 | 56.6 | ||
| 20 | 1136 | 22.6 | ||
| 21 | 760 | 22.6 | ||
| 22 | 2092 | 62.2 | ||
| 23 | 1808 | 181.0 | ||
| 24 | 1736 | 181.0 | ||
| 25 | 1092 | 73.5 | ||
| 26 | 900 | 50.9 | ||
| 27 | 1856 | 124.5 | Unrefined (1278 ± 553) |
|
| 28 | 1016 | 67.9 | ||
| 29 | 780 | 17.0 | ||
| 30 | 2196 | 28.3 | ||
| 31 | 726 | 70.7 | ||
| 32 | 1064 | 113.1 | ||
| 33 | 1680 | 67.9 | ||
| 34 | 908 | 79.2 | ||
| 35 | 720 | 22.6 | Non-Standard (1825 ± 1808) |
|
| 36 | 808 | 33.9 | ||
| 37 | 1452 | 209.3 | ||
| 38 | 1286 | 2.8 | ||
| 39 | 1212 | 28.3 | ||
| 40 | 5470 | 99.0 | ||
Table 4.
Distribution of MPs particles among contaminated salt brands, on type of packaging.
| NO | Abundance (MPs/kg) |
Package Type [average - (MPs/kg)] |
Chart | |
|---|---|---|---|---|
| Mean | SD | |||
| 1 | 1034 | 138.6 | Double Layer (2262 ± 2146) |
 |
| 2 | 1092 | 62.2 | ||
| 3 | 1452 | 209.3 | ||
| 4 | 5470 | 99.0 | ||
| 5 | 788 | 50.9 | Plastic Bag (1373 ± 827) |
|
| 6 | 1056 | 33.9 | ||
| 7 | 2368 | 113.1 | ||
| 8 | 932 | 62.2 | ||
| 9 | 1352 | 56.6 | ||
| 10 | 760 | 22.6 | ||
| 11 | 2092 | 62.2 | ||
| 12 | 1808 | 181.0 | ||
| 13 | 1736 | 181.0 | ||
| 14 | 900 | 50.9 | ||
| 15 | 4248 | 124.5 | ||
| 16 | 700 | 39.6 | ||
| 17 | 1636 | 50.9 | ||
| 18 | 1056 | 22.6 | ||
| 19 | 1040 | 79.2 | ||
| 20 | 962 | 87.7 | ||
| 21 | 720 | 22.6 | ||
| 22 | 808 | 33.9 | ||
| 23 | 1286 | 2.8 | ||
| 24 | 1212 | 28.3 | ||
| 25 | 1856 | 124.5 | No Packing (1278 ± 553) |
|
| 26 | 1016 | 67.9 | ||
| 27 | 780 | 17.0 | ||
| 28 | 2196 | 28.3 | ||
| 29 | 726 | 70.7 | ||
| 30 | 1064 | 113.1 | ||
| 31 | 1680 | 67.9 | ||
| 32 | 908 | 79.2 | ||
| 33 | 1948 | 39.6 | Plastic Can (1360 ± 399) |
|
| 34 | 1136 | 22.6 | ||
| 35 | 1092 | 73.5 | ||
| 36 | 1264 | 45.3 | ||
| 37 | 1576 | 101.8 | Plastic Woven (1449 ± 353) |
|
| 38 | 1156 | 62.2 | ||
| 39 | 1172 | 39.6 | ||
| 40 | 1892 | 96.2 | ||
As mentioned above, non-standard (fake) salts do not have the approval of the Ministry of Health, due to inappropriate purification, problems in the production and packaging of salts, or the presence of various impurities and pollution in salt, the lack of request and follow-up for permission. A part of MPs pollution, especially in non-standard salts, might be related to secondary pollution (during the production, purification and packaging process) [30], and a huge part of these impurities and pollution may be caused by the source of salt extraction. (environmental pollution) [40]. Considering that bulk salts (unrefined and unpackaged) have the lowest amount of MPs, it can be said with a strong probability that the pollution caused by the process of production, purification and packaging (secondary pollution) had an effect in increasing the abundance of MPs in other studied salts.
After non-standard salts, most MPs contamination is related to sea salts (1474.9 ± 901.7 MPs/kg). The results of various studies in the world have confirmed that plastics eventually enter aquatic ecosystems [41], [42]. Based on studies, approximately 15–40 % of mismanaged plastics end up in the sea [41], [42]. Hence, it can be stated that the high contamination of sea salts with MPs may be caused by the accumulation of these particles in the seas [40].
In terms of the type of packaging, the highest average of MPs was observed in salt samples with double-layer packaging (2262 ± 2146.6 MPs/kg). The high abundance of particles in these samples shows that the packaging process (especially double-layer packaging) has been effective in the number of particles [30]. On the other hand, the low number of MPs in bulk salts indicates the low effect of secondary pollution, especially the pollution caused by the packaging process in this type of salts.
It was expected that bulk salts (unrefined and unpackaged) would have high contamination. However, contrary to expectations, in this study, the least MPs contamination was related to these salts. Bulk salts were respectively 5 %, 13 % and 30 % less contaminated than rock salts, sea salt and non-standard salt. Also, they were contaminated almost less than 10 %, compared to refined salts and about 6–43 % less compared to packaged salts. According to the obtained results (Tables 2–4), it can be concluded that apart from the pollution of salt extraction sources (sea, rock, and more) and environmental pollution, the packaging process (especially double-layer packaging) has a great impact on salt pollution and the presence of MPs. In other words, if the purification of salts and their packaging are not done in a standard way, they will have a significant impact on the abundance of MPs. It is necessary to explain that the purification process can reduce the presence of these physical pollutants [43], but these purification processes are not able to remove MPs particles completely. Because as mentioned before, the number of MPs in refined and packaged salt studied is more than in bulk salt. To clarify this issue, another study needs to be done regarding the tracking of MPs contamination in salts from the source to consumption so that the role of different processes of salt extraction, purification and packaging on the amount of contamination can be determined.
3.3. Characteristics of MPs particles
The typical MP shapes were presented in Fig. S1. The types of MPs observed in the present study were fragments, pellets, fibers and films. Fig. S2 displays the shape distribution of MPs based on the type of salt, type of packaging, and purification method. According to the results of the study, fragment-shaped MPs (about 51–61 %) had the most abundance. Then were fiber-shaped (approximately 22–28 %), film-shaped (12–19 %) and pellets-shaped particles (less than 3 %). The high abundance of fiber-shaped and fragment-shaped particles in the samples indicates both environmental and secondary pollution [44]. According to the studies of other researchers, the most abundant shapes of MPs detected in different environments are fibrous and fragmented [45], [46]. Consequently, these particles have been able to enter the salt production cycle. Of course, on the other hand, the process of fragmentation of particles may have occurred. Most of the particles are fragmented-shaped in the fragmentation process (except fibrous particles) [44]. Hence, it is likely that the abundance of fragment-shaped particles is greater than that of fiber-shaped. Among the purified salts, the abundance of particles was as follows: fragment > fiber > film > pellet. Also, with a low probability, it can be said that there is a relationship between the shape of MPs and the ability to remove them through different purification processes [47].
The colors of the MPs particles identified in this study were mainly black, white, and red and a small percentage were green, brown, and others (Fig. S3). According to the evidence related to the color of the identified particles, it can be said that a part of the contamination (although relatively minor) of salt occurs in the processing and packaging stages. This possibility has also been reported by other studies [31], [40], [48]. Therefore, probably these particles (red, green and brown) can be caused by the packaging process (secondary pollution).
The investigation of the chemical structure of MPs particles’ and also the chemical structure of the packaging was performed by using Micro-Raman and FT-IR, and the results are presented in Fig. S4. The most common types of packaging included polyethylene (PE), polypropylene (PP), high-density polyethylene (HDPE) and polyethylene terephthalate (PET), which was about 37.5 %, 30 %, 17.5 % and 15 %, respectively (Fig. S5). The most dominant polymers (MPs) identified in salt samples were cellulose acetate (32.5 %), PE (20 %), PS (17.5 %), and PP (15 %), PET (5 %), and acrylonitrile butadiene styrene (ABS) (10 %) (Fig. S4, S5). It should be explained that on a global scale, the production and using of PE (use in packaging, disposable containers, bottles and bags) is the most dominant [43]. In fact, the most produced and used among all types of plastics in worldwide is related to PE (36 %) and PP (17 %), respectively. Consequently, most plastics left in the environment and environmental pollution are related to these two polymers. In addition, PE and PP are less dense than water. During salt extraction, they float on the surface of the water and can become a residue in the salt after the water evaporates [43]. On the other hand, the identification of packaging chemical structure in this study also confirmed that these two polymers have been used in the packaging of salts. Therefore, salt contamination of MPs can be caused both by the packaging and by environmental pollution, which has also been reported in previous studies [43], [49]. Of course, as other studies have reported, the contribution of environmental pollution can be higher. In the present study, the presence of high amounts of cellulose acetate (CA) and acrylonitrile butadiene styrene (ABS) (more than 42 % in total) in the salts, can be attributed to environmental pollution. The polymer used in none of the salt packaging in the current study was CA and ABS. Therefore, as mentioned probably the main reason for the presence of these two types of polymers in salt is environmental pollution. Among the most important sources of environmental pollution with cellulose acetate, we can mention cigarette filters and then textiles. It is reported that about 5.7 trillion cigarettes are smoked worldwide annually, and it was reported that more than 4.5 trillion cigarette butts become garbage and are discarded in natural environments every year. Also, it has been reported that cigarette butts make up 38 % of the total litter discarded in the environment [50], a large part of which ends up in the oceans and seas [51].
The total solid waste generated in the world and Iran, has been estimated at > 2 billion tons [52] and > 50 million tons annually [53], respectively, and the percentage of textiles in the wastes of the world and Iran reported about 4 % and 3 % [54]. Only 25 % of textiles [55] and 9 % of plastic in the world are recycled, and about 12 % of plastic in the world is managed by the waste-to-energy method [56]. Therefore, about 75 % of textiles and 79 % of plastics worldwide are mismanaged and are finally scattered and abandoned in natural environment. Recycling of textiles is almost not performed in Iran, plastic recycling is about 5 %, and more than 70 % of the waste is mismanaged [53]. It is also reported that more than 50 billion cigarettes are consumed in Iran every year. This number is still increasing, and there is no management on the generated cigarette butts [57]. Therefore, cigarette butts and textiles are present in the environment, especially in marine ecosystems, and by exposure to the chemical compounds of seawater, they can become microfibers [50]. Hence, the presence of MPs in the environment (especially marine ecosystem) and environmental pollution can be one of the main factors of salt pollution.
Moreover, the results of the present study indicated that bulk salts (without packaging) have less MPs pollution than packaged salts (6–43 % less pollution). Therefore, it can be said that the main source of bulk salt pollution is probably related to environmental pollution. Also, various studies in Iran have shown that the most considerable amount of environmental pollution (especially marine pollution) related to MPs was related to PE, PET, PS and PP particles [50], [53], [58], [59]. However, secondary contamination (caused by the process of production, purification and packaging) may also have occurred. The raw data and additional descriptions of about production and use of the polymers are provided in Table S4.
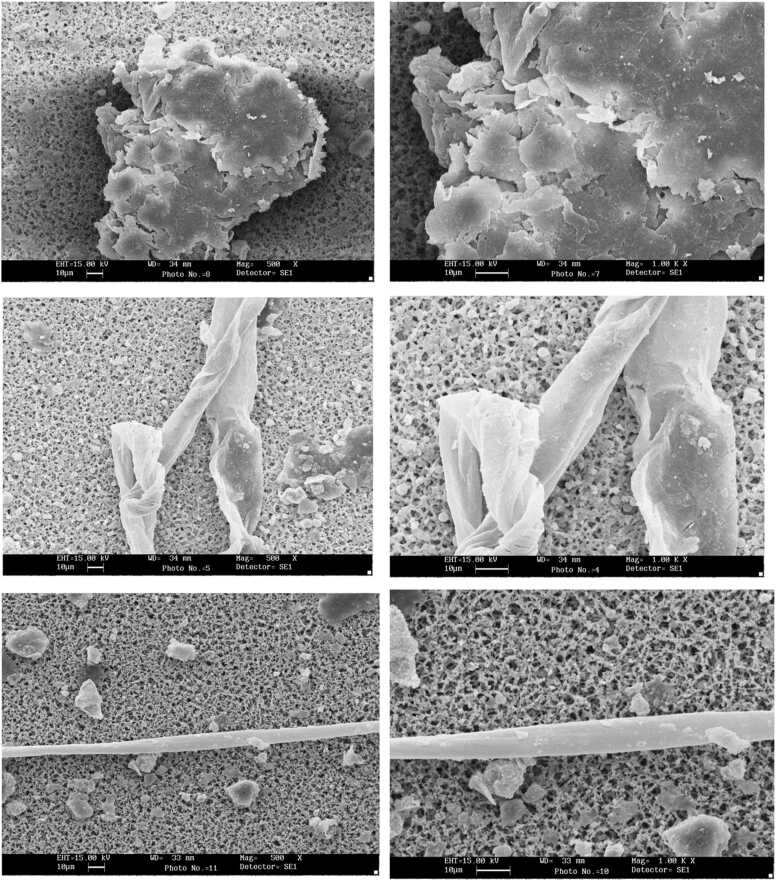
Fig. 1 illustrates the analysis of the surface morphology of MPs using SEM. The surface morphology of the particles shows that some particles have uneven, brittle, rough and grooved surfaces. Film particles show the shape of crushed, brittle, cracked and deteriorating surfaces. Some fiber-shaped particles are interwoven, and in some parts, they have fractures and grooves, which can indicate the continuation of degradation and turn into smaller particles. Fragment-shaped particles have smooth surfaces with sharp edges. The smooth surface and sharp edges indicate a new fracture of the larger pieces of plastic waste and can show secondary contamination from packaging degradation. Pellet particles are like fine and smooth particles in which the continuation of degradation is less visible. The particle surface and its surrounding smaller particles are visible. Hence, the presence of small particles indicates the continuation of degradation and destruction.
Fig. 1.
High-resolution morphological characteristics of some MPs particles.
3.4. Comparison of MPs in different salts of the world
Recently, the presence of MP particles in table salt has attracted much attention. So, the results of some studies are compared with the present study and presented in Table 5. As can be seen in this table, all the samples in this study, like all the salts of other countries, including China [30], India [40], Thailand [35], Korea [26], Lebanon [43], Turkey [60], and Italy [61], were contaminated with MPs. It is necessary to explain that because there is still no standard method for determining the abundance of MPs [62], and the methods of recognizing and identifying MPs are different in various studies. This has affected the abundance of identifiable MPs [62],and it makes comparison difficult. Besides that, the geographical and extraction origin of salts [31], the amounts of production and use of plastics in each area, the management and non-management of plastics, the quality of packaging and secondary pollution can affect the abundance of MPs in salts [53], [58], [63]. However, comparing the results shows that the abundance of particles identified in the present study is higher than in some other studies. One of the main reasons can be related to the size of the detected particles. Selvam et al. concluded that 60 % of MP particles detected in sea salts were smaller than 100 µm [64]. Also, Sharifi et al. reported that 94 % of refined sea salt and 84 % of unrefined sea salt are smaller than 100 µm [62]. In the present study, the abundance of existing MPs in the salts was obtained less than the existing MPs in the salts of Italy and Croatia [48]. The type of salt examined in Italy and Croatia was only sea salt.
Table 5.
Comparison between the results of this study and other studies (MPs abundance, dominant MPs, and MPs type).
| References | Origin | Salt type | No of salt brand | MP abundance (MP/kg) | MP identification technique |
Filter pore size (μm) |
Dominant MP types |
Human intake (MPs capita− 1 Salt yr− 1) |
|
|---|---|---|---|---|---|---|---|---|---|
| Shape | Polymer | ||||||||
| (Yang et al., 2015) | China | Sea salt Lake salt Rock/well salt |
5 | 550–681 | Visual/FTIR | 100–200 | Fiber | PET > PE > cellophane | - |
| (Karami et al., 2017) | 6 countries | Sea salt | 15 | 0–10 | Raman | 149 | Fragment | PP, PE, poly-acrylonitrile | 37 |
| 2 countries | Lake salt | 2 | 0 | ||||||
| (Renzi et al., 2019) | Italy, Croatia | Sea salt | 11 | 1570–39,800 | Visual | 0.45 | Fragment for Italy Fiber for Croatia |
- | 36.5–36,172 |
| (Seth and Shriwastav, 2018) | India | Sea salt | 8 | 56–103 | μ-FTIR | 0.45 | Fragment | PES, PE, PA | 188 |
| (Kim et al., 2018) | 16 countries | Sea salt | 28 | 0–13,629 | 0–13,629 | 100–500 | Fragment | PE > PP > PET | - |
| (Lee et al., 2019) | 6 countries | Sea salt | 10 | 2.5–20 | μ-FTIR | 5 | Fragment | PP, PE, PS | 35.8 |
| Thailand | Rock salt | 1 | 12.5 | Fragment | PP | ||||
| (Lee et al., 2021) | Korea | Sea salt | 1 | 2395 | μ-FTIR | 20 | Fragment | PP, PE | 12,000 |
| (Sharifi and Movahedian Attar, 2021) | Iran | Crystallized salt | - | 151.4 ± 48.8 | Micro-Raman | 0.45 | Fiber, Fragment | PP, PE, PET | - |
| Refined sea salt | 406.7 ± 93.3 | ||||||||
| Unrefined sea salt | 1288.6 ± 184.9 | ||||||||
| Rock salt | 283.4 ± 97 | ||||||||
| (Nakat et al., 2023) | Lebanon | 16 | 0–635.2 | FTIR | 0.7 | - | PP, PS, PE, thermoplastic elastomers | 2372 | |
| (Özçifçi et al., 2023) | Turkey | Sea salt Lake salt Rock salt |
36 | 39 ± 30 | FTIR | fiber, granulated, film | CPE | 150 | |
| This study | Iran | Sea salt | 13 | 700–4248 | > 100 (FTIR) < 100 (Micro-Raman) |
0.45 | Fragment, Fiber | CA, PE, PS, PP, ABS, PET | - |
| Rock salt | 13 | 760–2368 | |||||||
| Bulk salt | 8 | 726–2196 | |||||||
| Non-standard | 6 | 720–5470 | |||||||
As can be observed in Table 5, the dominant shape of MPs and the dominant type of MPs in the salts of various areas of the world are almost similar. Considering that most salt pollution is caused by the environment and based on studies reported in other countries, the dominant type of MPs detected in the environment are PE and PP, and also, the material of the packages used for salts is often PE and PP. Therefore, these two types of polymers are the most abundant particles in the salts of various areas of the world. But based on the results of the present study (Fig. S5), CA (32.5 %) was the most abundant compared to other polymers, and after that were PE and PS. As mentioned above, cellulose acetate is mainly used in cigarette filters and textiles. Given that, textile waste and cigarette butts are mismanaged in Iran, therefore, the possibility of leaving these wastes in the environment is very high [50]. On the other hand, due to the poor chemical resistance of cigarettes and textiles, as well as, their low mechanical strength, low thermal resistance, and poor resistance to UV, they are easily be destroyed and turned into small particles [65]. Hence, the presence of these types of MPs was more observed in the present study.
3.5. The calculation of estimated daily intake (EDI) and annual intake (EAI)
According to the per capita salt consumption in Iran, EDI and EAI values were determined in adults, teenager, and children, as well as in urban and rural areas of Iran, and the results are presented in Table 6. The average salt consumption in Iran varies from 7.7 g/d for children [66] to 9.8 g/d for teenagers and adults [66], and exceeding the recommended World Health Organization (WHO)’s daily intake of 5 g, and is almost twice the world standard [32]. As can be seen in Table 6, the EDI for adults, teenagers and children were obtained 6.5–53.6 MP/capita/day, 6.8–53.1 MP/capita/day, and 5.4–42.1 MP/capita/day, respectively. EAI for adults, teenagers and children were estimated at 2376–19,566 MP/capita/year, 2478–19,366 MP/capita/year and 1967–15,373 MP/capita/year, respectively. In general, EDI and EAI for urban areas of Iran were obtained 6.6–51.4 MP/capita/day and 2402–18,768 MP/capita/year, respectively, and for rural areas of Iran were obtained 7.6–59.1 MP/capita/day and 2759–21,562 MP/capita/year, respectively. The difference in EDI and EAI in urban and rural areas is due to the different per capita salt consumption of in cities and villages.
Table 6.
Comparative EDI and EAI for adults, teenagers, and children for different levels of consumption.
| Group | IR | C = n/kg |
C = n/g |
EDI a |
EAI b |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Average | Max | Min | Average | Max | Min | Average | Max | Min | Average | Max | |||
| Adults | Male | 9.8 | 700 | 1449.3 | 5470 | 0.70 | 1.45 | 5.47 | 6.9 | 14.2 | 53.6 | 2503.9 | 5184.1 | 19,566.2 |
| Female | 9.3 | 700 | 1449.3 | 5470 | 0.70 | 1.45 | 5.47 | 6.5 | 13.5 | 50.9 | 2376.2 | 4919.6 | 18,567.9 | |
| Teenager | 13–20 Years | 9.7 | 700 | 1449.3 | 5470 | 0.70 | 1.45 | 5.47 | 6.8 | 14.1 | 53.1 | 2478.4 | 5131.2 | 19,366.5 |
| Children | < 12 Years | 7.7 | 700 | 1449.3 | 5470 | 0.70 | 1.45 | 5.47 | 5.4 | 11.2 | 42.1 | 1967.4 | 4073.3 | 15,373.4 |
| Area | Urban | 9.4 | 700 | 1449.3 | 5470 | 0.70 | 1.45 | 5.47 | 6.6 | 13.6 | 51.4 | 2401.7 | 4972.5 | 18,767.6 |
| Rural | 10.8 | 700 | 1449.3 | 5470 | 0.70 | 1.45 | 5.47 | 7.6 | 15.7 | 59.1 | 2759.4 | 5713.1 | 21,562.7 | |
(Microplastic Particles (MPs) capita−1 day−1) = (daily salt intake per person (g capita−1 day−1) × Microplastic Particles count per kg (MPs kg−1) × 1).
(Microplastic Particles (MPs) capita−1 year−1) = (daily salt intake per person (g capita−1 day−1) × Microplastic Particles count per kg (MPs kg−1) × 365).
In order to homogenize and compare the results of EDI and EAI in the present study with the study conducted in Lebanon, the calculations were carried out again with the recommended limit of salt consumption by the WHO (< 5 g/d). Based on the recommended limit of salt consumption by WHO, the average EAI in Lebanon has been reported as about 290 MP/capita/year [43], and the average EAI in this study was estimated at approximately 2645 MP/capita/year, that EAI in Iran was obtained about 9 times higher than in Lebanon. Of course, it is necessary to explain that many parameters may affect the results of EAI and this comparison; including can be pointed environmental factors, sources of salt extraction (such as sea salt, rock, etc), processing and packaging steps, consumption behavior and methods of sampling, extraction, identification and detection [31], [40], [48].
It should be noted that so far, there are no exact and complete information and data about the toxicology of MPs. Therefore, accurate assessment of health and human risks is still complex and ambiguous. Although some of the identified plastic polymers have a lower risk scale (such as PE, PS, PET and PP) or are known as less toxic polymers [67], [68]. But some plastics, such as ABS, have a much higher risk scale, and even small amounts can have mutagenic and carcinogenic effects. In addition, in some studies, have been reported MPs as vectors and carriers of other pollutants (heavy metals and organic substances), which can increase the risk of exposure to these toxic substances and can also have a synergistic effect [67], [68]. Recently, has been confirmed plastic particle pollution in human blood [14]. After entering the body, these pollutants are able to move and accumulate in different organs and tissues [19]. Recent studies on various animals have reported several problems with exposure to micro and nano-plastics; endocrine disruptions, effect on mobility and development, interference with immune response, impaired reproduction, carcinogenesis, transgenerational effects, and cytotoxicity in cerebral and epithelial human cells [4], [21], [69], [70]. Therefore, there is a possibility that the mentioned problems will be revealed even in humans.
3.6. Proposed strategies to reduce the contamination of MPs in salt
Based on the findings of this research, salts can be contaminated with MPs mainly due to environmental pollution and also during the stages of production, moving and transfer, processing and packaging (secondary pollution). Considering the daily consumption of salt and its role in the diet and food industry, as well as the impact on public health, the quality control of table salt is of particular importance and should be taken into account. So, to reduce the environmental pollution of salts, detailed and regular planning for plastic waste management; should be done Prohibition of producing, using, abandoning, and disposing of plastic in the environment, source reduction, replacement, reuse, and recycling of plastics, supervision and enforcement of laws, management of urban runoff, proper treatment of wastewater, cleaning of beaches, raising awareness and people's attitudes, lifestyle changes, incentives (e.g. reduction of taxes on the manufacture of permanent bags, biodegradable polymers, cellulosic disposable containers, etc), implementation of extended producer responsibility (EPR), are among those actions that can be suggested to reduce the environmental pollution and eventually salt pollution [71], [72], [73].
On the other hand, better methods and technologies should be considered to reduce the contamination of salts during the production, processing and packaging steps. Prohibiting the sale and supply of unrefined and non-standard salts in the market should be considered, and the main sources of distribution of these salts should be identified and sealed. In Iran, sea salts are usually prepared by evaporation and purification, and rock salts are generally prepared by crystal purification [74]. Evaporation is accomplished naturally (sunlight and wind), which itself can be the cause of pollution. The purification process is usually carried out; by grinding, adding chemicals, sand filtration, evaporation, centrifugation, final washing, drying and packaging, respectively [75]. The crystal purification method also includes grinding, dissolution stage, purification and creating a saturated solution, filtering, circulating and creating a supersaturated solution and removing some ions, dewatering from salt, iodizing, drying, siloing, and packaging [75]. Therefore, it is suggested to use appropriate tools, equipment and ponds to reduce physical and biological pollution in the evaporation stage along with a tailor-made food safety training program [43]. Actually, it is critical that salt producers and handlers follow good manufacturing practices (GMP) and good hygienic practices (GHP) to improve the safety of salt (presence of hair, ant and skin) [43]. In order to prevent the possible contamination of salts by MPs in the air during the evaporation and drying stage, it is also suggested that the ponds be away from sources of plastic pollution (e.g. landfills, dumping waste (plastic), polluted beaches, and like) [76]. In the next stages of purification, including adding water and washing the salts, should be used treated water (with minimum MPs) [43], and during the drying, the control of possible pollution by MPs in the air should be performed as much as possible [43], [77]. In the silo and storage stage, good storage practices and good hygiene practices should be considered to improve the safety of salt [43]. Biodegradable plastics, plastics with a lower risk scale or non-plastic packaging such as glass should be used in the salt packaging.
4. Conclusion
The present study is the first complete study regarding the investigation of MPs in Iranian salts. The results of the study showed that all the studied salts were contaminated with MPs. The analyzed MPs particles had different colors and shapes as well as different polymers. The average particles detected in the studied salts were obtained at about 1449 ± 918 MPs/kg. The most common type of salts packaging included PE, PP, HDPE and PET, which constituted about 37.5 %, 30 %, 17.5 %, and 15 %, respectively. However, the main polymers identified in salts, include CA (32.5 %), PE (20 %), PS (17.5 %), PP (15 %), PET (5 %) and ABS (10 %). Among the studied salts, the abundance of particles in terms of shape was as follows: fragment > fiber > film > pellet. The main origin of salt contamination with MPs is most likely related to environmental pollution, and another part of it can be attributed to salt processing and packaging. The surface morphology of MPs displayed that the particles were affected by continuous weathering, mechanical fracture and oxidation. According to the results of EDI in this study, there is a possibility that Iranians are more exposed to contamination compared to some countries. The findings of this study can be a source for policymakers and responsible authorities to improve and promote the rules and regulations of the production and supply of table salt, to ensure the production of safe salt-free from MPs and other pollutants. Also, other responsible organizations, including the Iranian Ministry of Health, can help to reduce exposure to MPs by providing advice and training regarding compliance with the permissible limit of salt consumption in the country, in addition to preventing some diseases related to its excessive consumption (such as high blood pressure). It is necessary to explain that more research is needed to assess the potential health risks associated with MPs consumption and its related biomarkers. It is also necessary to accurately identify and track MPs from the source of salt extraction to the table. Finally, considering the role of environmental pollution in the contamination of salts with MPs, it is suggested to implement proper plastic management and disposal in addition to reducing their production and consumption. Moreover, in order to reduce salt contamination in the production process, better and more advanced technologies should be used in the processing and packaging of salts. At the same time, the production and supply of unauthorized and non-standard salts should be prevented.
Ethical Approval
Approval was obtained from the institutional-level Medical Ethics Board of Trustees (MEBoT) at Tabriz University of Medical Sciences (Ethics Approval no.: IR.TBZMED.REC.1400.885).
Funding
This work was supported by Tabriz University of Medical Sciences under Grant no. = 68589.
CRediT authorship contribution statement
Taghipour: Investigation, Methodology, Software, Visualization, Validation, Formal analysis, Writing – review & editing. Ghayebzadeh: Investigation, Methodology, Formal analysis, Software, Visualization, Writing – original draft, Writing – review & editing. Seyed Mousavi: Writing – original draft, Conducting tests. Sharifi: Writing – original draft, Conducting tests. Payandeh: Software, Analysis and/or Interpretation of Data.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors desire to thank financial suppliers and others who had logistical support.
Handling Editor: Dr. L.H. Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2023.07.003.
Appendix A. Supplementary material
Supplementary material
Data Availability
Data will be made available on request.
References
- 1.Singh P., Sharma V.P. Integrated plastic waste management: environmental and improved health approaches. Procedia Environ. Sci. 2016;35:692–700. doi: 10.1016/j.proenv.2016.07.068. [DOI] [Google Scholar]
- 2.Oberoi G., Garg A. Single-use plastics: a roadmap for sustainability? Supremo Amicus. 2021;24:585. [Google Scholar]
- 3.Geyer R., Jambeck J.R., Law K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes D.K.A., Galgani F., Thompson R.C., Barlaz M. Accumulation and fragmentation of plastic debris in global environments. Philos. Trans. R. Soc. B: Biol. Sci. 2009;364:1985–1998. doi: 10.1098/rstb.2008.0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Süssmann J., Krause T., Martin D., Walz E., Greiner R., Rohn S., Fischer E.K., Fritsche J. Evaluation and optimisation of sample preparation protocols suitable for the analysis of plastic particles present in seafood. Food Control. 2021;125 [Google Scholar]
- 6.Kabir A.H.M.E., Sekine M., Imai T., Yamamoto K., Kanno A., Higuchi T. Microplastics in the sediments of small-scale Japanese rivers: abundance and distribution, characterization, sources-to-sink, and ecological risks. Sci. Total Environ. 2022;812 doi: 10.1016/j.scitotenv.2021.152590. [DOI] [PubMed] [Google Scholar]
- 7.Rochman C.M., Brookson C., Bikker J., Djuric N., Earn A., Bucci K., Athey S., Huntington A., McIlwraith H., Munno K. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019;38:703–711. doi: 10.1002/etc.4371. [DOI] [PubMed] [Google Scholar]
- 8.Carbery M., O’Connor W., Palanisami T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environ. Int. 2018;115:400–409. doi: 10.1016/j.envint.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Smith M., Love D.C., Rochman C.M., Neff R.A. Microplastics in seafood and the implications for human health. Curr. Environ. Health Rep. 2018;5:375–386. doi: 10.1007/s40572-018-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liebezeit G., Liebezeit E. Non-pollen particulates in honey and sugar. Food Addit. Contam.: Part A. 2013;30:2136–2140. doi: 10.1080/19440049.2013.843025. [DOI] [PubMed] [Google Scholar]
- 11.Usman S., Razis A.F.A., Shaari K., Azmai M.N.A., Saad M.Z., Isa N.M., Nazarudin M.F. Polystyrene microplastics induce gut microbiome and metabolome changes in Javanese medaka fish (Oryzias javanicus Bleeker, 1854) Toxicol. Rep. 2022;9:1369–1379. doi: 10.1016/j.toxrep.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwabl P., Köppel S., Königshofer P., Bucsics T., Trauner M., Reiberger T., Liebmann B. Detection of various microplastics in human stool: a prospective case series. Ann. Intern. Med. 2019;171:453–457. doi: 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- 13.Mičušík M., Kleinová A., Oros M., Šimon P., Dubaj T., Procházka M., Omastová M. Plastic ingestion by the Wels catfish (Silurus glanis L.): detailed chemical analysis and degradation state evaluation. Toxicol. Rep. 2021;8:1869–1876. doi: 10.1016/j.toxrep.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leslie H.A., Van Velzen M.J., Brandsma S.H., Vethaak A.D., Garcia-Vallejo J.J., Lamoree M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022;163 doi: 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- 15.Jenner L.C., Rotchell J.M., Bennett R.T., Cowen M., Tentzeris V., Sadofsky L.R. Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 2022;831 doi: 10.1016/j.scitotenv.2022.154907. [DOI] [PubMed] [Google Scholar]
- 16.Huang S., Huang X., Bi R., Guo Q., Yu X., Zeng Q., Huang Z., Liu T., Wu H., Chen Y. Detection and analysis of microplastics in human sputum. Environ. Sci. Technol. 2022;56:2476–2486. doi: 10.1021/acs.est.1c03859. [DOI] [PubMed] [Google Scholar]
- 17.Ranjani M., Veerasingam S., Venkatachalapathy R., Mugilarasan M., Bagaev A., Mukhanov V., Vethamony P. Assessment of potential ecological risk of microplastics in the coastal sediments of India: a meta-analysis. Mar. Pollut. Bull. 2021;163 doi: 10.1016/j.marpolbul.2021.111969. [DOI] [PubMed] [Google Scholar]
- 18.Thompson R.C., Swan S.H., Moore C.J., Saal F.S. Vom. Our plastic age. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009 doi: 10.1098/rstb.2009.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng Y., Zhang Y., Lemos B., Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci. Rep. 2017;7:1–10. doi: 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahu E., Datsomor W.G., Folorunsho R., Fisayo J., Crane R., Marchant R., Montford J., Boateng M.C., Oti M.E., Oguguah M.N. Human health risk and food safety implications of microplastic consumption by fish from coastal waters of the eastern equatorial Atlantic Ocean. Food Control. 2023;145 [Google Scholar]
- 21.Lithner D., Damberg J., Dave G., Larsson Å. Leachates from plastic consumer products–screening for toxicity with Daphnia magna. Chemosphere. 2009;74:1195–1200. doi: 10.1016/j.chemosphere.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Natarajan L., Soupam D., Dey S., Chandrasekaran N., Kundu R., Paul S., Mukherjee A. Toxicity of polystyrene microplastics in freshwater algae Scenedesmus obliquus: effects of particle size and surface charge. Toxicol. Rep. 2022;9:1953–1961. doi: 10.1016/j.toxrep.2022.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q., Xu E.G., Li J., Chen Q., Ma L., Zeng E.Y., Shi H. A review of microplastics in table salt, drinking water, and air: direct human exposure. Environ. Sci. Technol. 2020;54:3740–3751. doi: 10.1021/acs.est.9b04535. [DOI] [PubMed] [Google Scholar]
- 24.Rainieri S., Barranco A. Microplastics, a food safety issue? Trends Food Sci. Technol. 2019;84:55–57. [Google Scholar]
- 25.Rubio-Armendáriz C., Alejandro-Vega S., Paz-Montelongo S., Gutiérrez-Fernández Á.J., Carrascosa-Iruzubieta C.J., Hardisson-de la Torre A. Microplastics as emerging food contaminants: a challenge for food safety. Int. J. Environ. Res. Public Health. 2022;19:1174. doi: 10.3390/ijerph19031174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H.-J., Song N.-S., Kim J.-S., Kim S.-K. Variation and uncertainty of microplastics in commercial table salts: critical review and validation. J. Hazard. Mater. 2021;402 doi: 10.1016/j.jhazmat.2020.123743. [DOI] [PubMed] [Google Scholar]
- 27.W.H. Organization, Guideline: Sodium Intake for Adults and Children, World Health Organization, 2012. [PubMed]
- 28.Barboza L.G.A., Vethaak A.D., Lavorante B.R.B.O., Lundebye A.-K., Guilhermino L. Marine microplastic debris: an emerging issue for food security, food safety and human health. Mar. Pollut. Bull. 2018;133:336–348. doi: 10.1016/j.marpolbul.2018.05.047. [DOI] [PubMed] [Google Scholar]
- 29.I. Ore, I.O. Pigments, P. Rock, Q. Crystal, R. Earths, S. Ash, Mineral Commodity Summaries 2020, 2021.
- 30.Yang D., Shi H., Li L., Li J., Jabeen K., Kolandhasamy P. Microplastic pollution in table salts from China. Environ. Sci. Technol. 2015;49:13622–13627. doi: 10.1021/acs.est.5b03163. [DOI] [PubMed] [Google Scholar]
- 31.Kim J.-S., Lee H.-J., Kim S.-K., Kim H.-J. Global pattern of microplastics (MPs) in commercial food-grade salts: sea salt as an indicator of seawater MP pollution. Environ. Sci. Technol. 2018;52:12819–12828. doi: 10.1021/acs.est.8b04180. [DOI] [PubMed] [Google Scholar]
- 32.Mozaffarian D., Fahimi S., Singh G.M., Micha R., Khatibzadeh S., Engell R.E., Lim S., Danaei G., Ezzati M., Powles J. Global sodium consumption and death from cardiovascular causes. N. Engl. J. Med. 2014;371:624–634. doi: 10.1056/NEJMoa1304127. [DOI] [PubMed] [Google Scholar]
- 33.Motlagh Z., Mazloomy S., Mozaffari Khosravi H., Morowatisharifabad M., Askarshahi M. Salt intake among women refer to medical health centers, Yazd, Iran, 2011. SSU_Journals. 2011;19:550–560. [Google Scholar]
- 34.Pourkhajoei S., Yazdi‐Feyzabadi V., Amiresmaeili M., Nakhaee N., Goudarzi R. Mean population salt intake in Iran: a systematic review and meta‐analysis. Health Sci. Rep. 2022;5 doi: 10.1002/hsr2.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee H., Kunz A., Shim W.J., Walther B.A. Microplastic contamination of table salts from Taiwan, including a global review. Sci. Rep. 2019;9:10145. doi: 10.1038/s41598-019-46417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldwin A.K., Corsi S.R., Mason S.A. Plastic debris in 29 Great Lakes tributaries: relations to watershed attributes and hydrology. Environ. Sci. Technol. 2016;50:10377–10385. doi: 10.1021/acs.est.6b02917. [DOI] [PubMed] [Google Scholar]
- 37.Taghipour H., Ghayebzadeh M., Ganji F., Mousavi S., Azizi N. Tracking microplastics contamination in drinking water in Zahedan, Iran: from source to consumption taps. Sci. Total Environ. 2023;872 doi: 10.1016/j.scitotenv.2023.162121. [DOI] [PubMed] [Google Scholar]
- 38.De Witte B., Devriese L., Bekaert K., Hoffman S., Vandermeersch G., Cooreman K., Robbens J. Quality assessment of the blue mussel (Mytilus edulis): comparison between commercial and wild types. Mar. Pollut. Bull. 2014;85:146–155. doi: 10.1016/j.marpolbul.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Weber F., Kerpen J., Wolff S., Langer R., Eschweiler V. Investigation of microplastics contamination in drinking water of a German city. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.143421. [DOI] [PubMed] [Google Scholar]
- 40.Seth C.K., Shriwastav A. Contamination of Indian sea salts with microplastics and a potential prevention strategy. Environ. Sci. Pollut. Res. 2018;25:30122–30131. doi: 10.1007/s11356-018-3028-5. [DOI] [PubMed] [Google Scholar]
- 41.Jambeck J.R., Geyer R., Wilcox C., Siegler T.R., Perryman M., Andrady A., Narayan R., Law K.L. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- 42.Siegfried M., Koelmans A.A., Besseling E., Kroeze C. Export of microplastics from land to sea. A modelling approach. Water Res. 2017;127:249–257. doi: 10.1016/j.watres.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 43.Nakat Z., Dgheim N., Ballout J., Bou-Mitri C. Occurrence and exposure to microplastics in salt for human consumption, present on the Lebanese market. Food Control. 2023;145 [Google Scholar]
- 44.Toumi H., Abidli S., Bejaoui M. Microplastics in freshwater environment: the first evaluation in sediments from seven water streams surrounding the lagoon of Bizerte (Northern Tunisia) Environ. Sci. Pollut. Res. 2019;26:14673–14682. doi: 10.1007/s11356-019-04695-0. [DOI] [PubMed] [Google Scholar]
- 45.Burns E.E., Boxall A.B.A. Microplastics in the aquatic environment: evidence for or against adverse impacts and major knowledge gaps. Environ. Toxicol. Chem. 2018;37:2776–2796. doi: 10.1002/etc.4268. [DOI] [PubMed] [Google Scholar]
- 46.Kooi M., Koelmans A.A. Simplifying microplastic via continuous probability distributions for size, shape, and density. Environ. Sci. Technol. Lett. 2019;6:551–557. [Google Scholar]
- 47.Pivokonsky M., Cermakova L., Novotna K., Peer P., Cajthaml T., Janda V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018;643:1644–1651. doi: 10.1016/j.scitotenv.2018.08.102. [DOI] [PubMed] [Google Scholar]
- 48.Renzi M., Blašković A. Litter & microplastics features in table salts from marine origin: Italian versus Croatian brands. Mar. Pollut. Bull. 2018;135:62–68. doi: 10.1016/j.marpolbul.2018.06.065. [DOI] [PubMed] [Google Scholar]
- 49.Fadare O.O., Okoffo E.D., Olasehinde E.F. Microparticles and microplastics contamination in African table salts. Mar. Pollut. Bull. 2021;164 doi: 10.1016/j.marpolbul.2021.112006. [DOI] [PubMed] [Google Scholar]
- 50.Soleimani F., Dobaradaran S., Vazirizadeh A., Mohebbi G., Ramavandi B., De-la-Torre G.E., Nabipour I., Schmidt T.C., Novotny T.E., Maryamabadi A. Chemical contents and toxicity of cigarette butts leachates in aquatic environment: a case study from the Persian Gulf region. Chemosphere. 2023;311 doi: 10.1016/j.chemosphere.2022.137049. [DOI] [PubMed] [Google Scholar]
- 51.Kurmus H., Mohajerani A. The toxicity and valorization options of cigarette butts. Waste Manag. 2020;104:104–118. doi: 10.1016/j.wasman.2020.01.011. [DOI] [PubMed] [Google Scholar]
- 52.Kaza S., Yao L., Bhada-Tata P., Van Woerden F. World Bank Publications; 2018. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. [DOI] [Google Scholar]
- 53.Ghayebzadeh M., Aslani H., Taghipour H., Mousavi S. Estimation of plastic waste inputs from land into the Caspian Sea: a significant unseen marine pollution. Mar. Pollut. Bull. 2020;151 doi: 10.1016/j.marpolbul.2019.110871. [DOI] [PubMed] [Google Scholar]
- 54.Esmaeilizadeh S., Shaghaghi A., Taghipour H. Key informants’ perspectives on the challenges of municipal solid waste management in Iran: a mixed method study. J. Mater. Cycles Waste Manag. 2020;22:1284–1298. [Google Scholar]
- 55.Juanga-Labayen J.P., Labayen I.V., Yuan Q. A review on textile recycling practices and challenges. Textiles. 2022;2:174–188. [Google Scholar]
- 56.Zaman A., Newman P. Plastics: are they part of the zero-waste agenda or the toxic-waste agenda? Sustain. Earth. 2021;4:1–16. [Google Scholar]
- 57.Alimohammadi M., Jafari-Mansoorian H., Hashemi S.Y., Momenabadi V., Ghasemi S.M., Karimyan K. Review on the implementation of the Islamic Republic of Iran about tobacco control, based on MPOWER, in the framework convention on tobacco control by the World Health Organization. Addict. Health. 2017;9:183. [PMC free article] [PubMed] [Google Scholar]
- 58.Ghayebzadeh M., Taghipour H., Aslani H. Estimation of plastic waste inputs from land into the Persian Gulf and the Gulf of Oman: an environmental disaster, scientific and social concerns. Sci. Total Environ. 2020;733 doi: 10.1016/j.scitotenv.2020.138942. [DOI] [PubMed] [Google Scholar]
- 59.Naji A., Nuri M., Amiri P., Niyogi S. Small microplastic particles (S-MPPs) in sediments of mangrove ecosystem on the northern coast of the Persian Gulf. Mar. Pollut. Bull. 2019;146:305–311. doi: 10.1016/j.marpolbul.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 60.Özçifçi Z., Basaran B., Akçay H.T. Microplastic contamination and risk assessment in table salts: Turkey. Food Chem. Toxicol. 2023;175 doi: 10.1016/j.fct.2023.113698. [DOI] [PubMed] [Google Scholar]
- 61.Renzi M., Grazioli E., Bertacchini E., Blašković A. Microparticles in table salt: levels and chemical composition of the smallest dimensional fraction. J. Mar. Sci. Eng. 2019;7:310. [Google Scholar]
- 62.Sharifi H., Movahedian Attar H. Quantitative and qualitative evaluation of microplastics in different salts from Iran. Int. J. Environ. Health Eng. 2021;2021:1–6. [Google Scholar]
- 63.Ryan P.G., Moore C.J., Van Franeker J.A., Moloney C.L. Monitoring the abundance of plastic debris in the marine environment. Philos. Trans. R. Soc. B: Biol. Sci. 2009;364:1999–2012. doi: 10.1098/rstb.2008.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Selvam S., Manisha A., Venkatramanan S., Chung S.Y., Paramasivam C.R. Microplastic presence in commercial marine sea salts: a baseline study along Tuticorin Coastal salt pan stations, Gulf of Mannar, South India. Mar. Pollut. Bull. 2020;150 doi: 10.1016/j.marpolbul.2019.110675. [DOI] [PubMed] [Google Scholar]
- 65.Song J., Liu M., Yang Z., Xu S., Cheng B., Fei P. Synthesis and characterization of cellulose acetate naphthoate with good ultraviolet and chemical resistance. E-Polymers. 2017;17:333–340. [Google Scholar]
- 66.Emamian M.H., Ebrahimi H., Hashemi H., Fotouhi A. Salt intake and blood pressure in Iranian children and adolescents: a population-based study. BMC Cardiovasc. Disord. 2021;21:1–10. doi: 10.1186/s12872-021-01876-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rochman C.M. Marine Anthropogenic Litter. Springer; Cham: 2015. The complex mixture, fate and toxicity of chemicals associated with plastic debris in the marine environment; pp. 117–140. [Google Scholar]
- 68.Lithner D., Larsson Å., Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011;409:3309–3324. doi: 10.1016/j.scitotenv.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 69.Akdogan Z., Guven B. Microplastics in the environment: a critical review of current understanding and identification of future research needs. Environ. Pollut. 2019;254 doi: 10.1016/j.envpol.2019.113011. [DOI] [PubMed] [Google Scholar]
- 70.Miranda T., Vieira L.R., Guilhermino L. Neurotoxicity, behavior, and lethal effects of cadmium, microplastics, and their mixtures on Pomatoschistus microps juveniles from two wild populations exposed under laboratory conditions―implications to environmental and human risk assessment. Int. J. Environ. Res. Public Health. 2019;16:2857. doi: 10.3390/ijerph16162857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Critchell K., Bauer-Civiello A., Benham C., Berry K., Eagle L., Hamann M., Hussey K., Ridgway T. In: Coasts and Estuaries. Wolanski E., Day J.W., Elliott M., Ramachandran R., editors. Elsevier; 2019. Chapter 34 – plastic pollution in the coastal environment: current challenges and future solutions; pp. 595–609. [DOI] [Google Scholar]
- 72.Pettipas S., Bernier M., Walker T.R. A Canadian policy framework to mitigate plastic marine pollution. Mar. Policy. 2016;68:117–122. doi: 10.1016/j.marpol.2016.02.025. [DOI] [Google Scholar]
- 73.Xanthos D., Walker T.R. International policies to reduce plastic marine pollution from single-use plastics (plastic bags and microbeads): a review. Mar. Pollut. Bull. 2017;118:17–26. doi: 10.1016/j.marpolbul.2017.02.048. [DOI] [PubMed] [Google Scholar]
- 74.S.A.A. Mehrdad, S. Azadi, M.N. Lashkari, Removing of sulfate impurity from culinary salt in salex process, 2010.
- 75.Cheraghali A.M., Kobarfard F., Faeizy N. Heavy metals contamination of table salt consumed in Iran. Iran. J. Pharm. Res.: IJPR. 2010;9:129. [PMC free article] [PubMed] [Google Scholar]
- 76.Kazour M., Terki S., Rabhi K., Jemaa S., Khalaf G., Amara R. Sources of microplastics pollution in the marine environment: importance of wastewater treatment plant and coastal landfill. Mar. Pollut. Bull. 2019;146:608–618. doi: 10.1016/j.marpolbul.2019.06.066. [DOI] [PubMed] [Google Scholar]
- 77.Shaban A. Use of satellite images to identify marine pollution along the Lebanese coast. Environ. Forensics. 2008;9:205–214. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.