Abstract
The coronavirus disease 2019 (COVID-19) pandemic has had enormous implications for the care of patients with chronic liver disease (CLD), cirrhosis, and liver transplant (LT). Clinical outcomes of COVID-19 vary in patients with CLD and cirrhosis compared to healthy controls, and in patients with LT compared to patients without LT. Several special considerations apply to the approach to vaccination and treatment in patients with CLD and LT. The practice of liver transplantation has also been heavily impacted by the pandemic, including persistent reductions in living donor LT and increases in LT for an indication of alcohol-related liver disease. Recent medical society guidelines strive to standardize severe acute respiratory syndrome coronavirus 2 testing in donors and recipients and the approach to transplantation after recovered from COVID-19 infection, but certain controversies remain.
Keywords: cirrhosis, COVID-19, liver transplant, vaccination
Introduction
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had substantial unique implications for patients with chronic liver disease (CLD), cirrhosis, and liver transplant (LT). Patients with CLD and cirrhosis may have more severe disease and higher COVID-19-associated mortality than patients without CLD.1–5 Especially with the development of novel SARS-CoV-2 vaccines and antiviral medications over the past years since the onset of the pandemic, there has been substantial progress in prevention and management of COVID-19 in patients with CLD and LT. However, several limitations or special considerations exist. As detailed below, vaccine efficacy varies in this population,6–9 and several therapeutics developed for COVID-19 may affect liver function tests (LFTs), interact with immunosuppressants taken after LT, or be contraindicated in patients with advanced cirrhosis.10–14 Management of immunosuppressive medications after LT in the face of COVID-19 infection is nuanced and necessitates a patient-centered approach.15,16
The practice of liver transplantation has been heavily impacted by the pandemic. After initial substantial reductions in LT volume, rates of deceased donor LT recovered over the course of 2020, nearing pre-pandemic volumes.17,18 LT in the pandemic era has required additional measures to minimize the risks of SARS-CoV-2 in LT recipients and live donors. Certain decisions surrounding LT, such as timing after COVID-19 infection and mandatory recipient vaccination, remain controversial. Current society guidelines and areas of ongoing consideration will be discussed here in detail.
Clinical outcomes of COVID-19 in patients with CLD and LT
Outcomes in patients with CLDs and cirrhosis
Acute liver injury has been reported in 14–53% of patients hospitalized for COVID-19, ranging from mild-to-severe transaminase elevations.19–21 While severe cases account for a small proportion of those affected by acute liver injury in COVID-19, they are associated with an increased inflammatory state and a more severe disease course, including higher rates of intensive care unit (ICU) admission, mechanical ventilation, renal replacement therapy, and death.22–24 Other less common but more severe forms of liver disease, including acute liver failure and progressive cholangiopathy, have also been reported in the context of COVID-19 infection.25,26
Given the prevalence of hepatic involvement in COVID-19 and its potential for increased morbidity and mortality, numerous studies have sought to characterize the clinical outcomes of COVID-19 in patients with preexisting CLD and cirrhosis. Compared to those without liver disease, patients with CLD are at increased risk of severe COVID-19 disease and death.1,2 Among individuals with CLD, patients with nonalcoholic fatty liver disease (NAFLD) are overrepresented among those hospitalized for COVID-19, accounting for up to 20% of admitted patients. 27 NAFLD has been identified as an independent risk factor for ICU admission and mechanical ventilation, consistent with other medical comorbidities associated with metabolic syndrome such as diabetes, hypertension, and cardiovascular disease.19,28 Specific predictors of mortality associated with COVID-19 mortality include alcohol-related liver disease, decompensated cirrhosis, and hepatocellular carcinoma. 27 The reason for increased mortality in these specific populations remains unclear but is suspected to be driven by a constellation of factors including a heightened baseline inflammatory state and impaired immune function.
Among patients with cirrhosis and COVID-19, multicenter studies across the United States, Europe, and China have found a mortality rate of up to 35%, significantly higher than in patients without cirrhosis.2–5 Furthermore, studies have consistently shown that increasing severity of liver disease is associated with increased mortality in COVID-19. For example, in a multicenter international cohort study of 745 patients with CLD and COVID-19, increasing Child-Turcotte-Pugh (CTP) Class – indicating more severe underlying disease – was independently associated with increased mortality. 29 Similarly, a retrospective study in Italy found that patients with higher presenting Model for End-Stage Liver Disease (MELD) and Chronic Liver Failure Consortium-Organ Failure scores experienced higher mortality rates. 4 These findings underscore the importance of recognizing cirrhosis, especially with severe disease, as a high-risk comorbid condition when caring for patients hospitalized with COVID-19.
As in patients without liver disease, morbidity and mortality from COVID-19 among those with CLD are driven largely by cardiopulmonary complications including respiratory failure, sepsis, and shock. 29 This was demonstrated in a study comparing patients with cirrhosis hospitalized for COVID-19 to patients with cirrhosis hospitalized for bacterial infection; mortality was significantly increased in those hospitalized for COVID-19. 4 In another multicenter age- and gender-matched cohort study, patients with cirrhosis and COVID-19 experienced higher rates of ICU admission, mechanical ventilation, and shock compared to those with cirrhosis alone, suggesting that patients with cirrhosis hospitalized for COVID-19 are more likely to develop complications related to the viral infection rather than their underlying disease. 3 However, this study also found that patients with cirrhosis and COVID-19 experienced higher mortality rates than those with COVID-19 without cirrhosis, but similar mortality rates compared to those with cirrhosis without COVID-19, suggesting that cirrhosis itself continues to drive high inpatient mortality rates.
Outcomes in LT recipients
LT recipients are faced with unique infectious complications compared to the general population, particularly due to the use of immunosuppressive medications. As the prevalence of COVID-19 infection continues to grow worldwide, there have been an increasing number of studies evaluating clinical outcomes of COVID-19 in LT and other solid organ transplant (SOT) recipients. Early reports described an increased risk of severe disease and mortality in SOT recipients hospitalized for COVID-19 compared to non-transplant patients, with a mortality rate approaching 20%. 30 Subsequent reports have identified a similar risk of death in SOT transplant patients hospitalized for COVID-19, with a trend toward worse outcomes during the pandemic’s first wave, potentially skewed by early testing limitations leading to underrepresentation of mild or asymptomatic cases.31,32 Older age, male sex, and increasing BMI in the SOT population are independently associated with increased mortality, paralleling what has been described in the general population.
Following these initial reports, larger studies have further refined our understanding of the relationship between COVID-19 and LT specifically. Among LT recipients, several studies in the United States and Europe have described a mortality rate nearing 20%, in line with rates seen among all SOT recipients (Table 1).33,34 In a multicenter cohort study across 18 countries, LT recipients with COVID-19 were hospitalized at similar rates compared to age- and gender-matched non-transplant controls but experienced higher rates of ICU admission and mechanical ventilation. 34 In this and other studies, LT did not significantly increase the risk of death in patients with COVID-19, but older age and medical comorbidities – including renal dysfunction and non-liver cancer – did.34–36 In a systematic review of 1481 LT recipients from 17 studies, there was no difference in mortality among LT recipients hospitalized for COVID-19 when compared to non-transplant patients with similar comorbidities. 37 Another meta-analysis of 12 studies comprising 517 LT recipients hospitalized for COVID-19 found that time since LT did not significantly impact the risk of death. 38 In all, despite initial concerns that immunocompromised transplant recipients would be more severely impacted by COVID-19, non-transplant-associated medical comorbidities seem to drive clinical outcomes.
Table 1.
Outcomes of COVID-19 among LT recipients.
| Study | Cohort and comparator group | Outcomes in LT group |
|---|---|---|
| Becchetti et al.
39
June 2020 |
- 57 LT recipients - No comparator group - 12 centers across Europe |
- Mortality rate of 12.0% - Immunosuppression reduction in 38.6% and discontinuation in 7.0% of patients, without impact on clinical outcomes |
| Colmenero et al.
15
August 2020 |
- 111 LT recipients - Comparator group: matched patients without LT - 25 centers in Spain |
- Mortality rate of 18.0% - Severe disease in 31.5% of patients - Increased risk of severe disease with mycophenolate but not calcineurin inhibitors or everolimus |
| Webb et al.
34
August 2020 |
- 151 LT recipients - Comparator group: 627 patients without LT - International registry |
- Mortality rate of 18.5% - ICU admission in 28% of patients - Increased age, serum creatinine, and non-liver cancer were associated with death - LT not associated with death |
| Mansoor et al.
40
September 2020 |
- 126 LT recipients - Comparator group: propensity score-matched patients without LT - U.S. health research network |
- Risk of hospitalization 40.0% - No increased risk of mortality, thrombosis, or ICU admission |
| Rabiee et al.
33
September 2020 |
- 112 LT recipients - Comparator group: age- and gender-matched patients with CLD without LT - 15 centers in the United States |
- Mortality rate of 22.3% - Acute liver injury in 34.6% of patients - Immunosuppression changes not associated with acute liver injury or mortality |
| Belli et al.
16
March 2021 |
- 103 LT recipients - No comparator group - 56 centers across Europe |
- Mortality rate of 15.0% - Increased age associated with death - Tacrolimus use associated with survival |
| Jayant et al.
38
April 2021 |
- Meta-analysis of 12 studies comprising 517 LT recipients | - Mortality rate of 20.0% - Immunosuppression change in 38.0% of patients - Time since transplant not an independent risk factor of death |
| Dumortier et al.
41
July 2021 |
- 67 inpatient and 37 outpatient LT recipients - No comparator group - French nationwide registry |
- Mortality rate of 20.0% overall (28.1% in hospitalized patients) - Severe disease in 33.0% of all patients - Increased age associated with death |
| Kulkrani et al.
37
August 2021 |
- Meta-analysis of 18 studies comprising 1522 LT recipients | - Mortality rate of 17.4% - Graft dysfunction in 2.3% of patients - No difference in mortality between those infected within 1 year versus after 1 year following LT |
| Shafiq and Gibson
42
June 2022 |
- 39 LT recipients - Comparator group: 78 patients without LT of similar age, demographics, and comorbidities - Single-center study |
- No difference in hospitalization, length of stay, need for supplemental oxygen, effect on liver enzymes, or death |
CLD, chronic liver disease; ICU, intensive care unit; LT, liver transplant.
COVID-19 prevention in patients with CLD and LT
There has been significant interest in understanding and optimizing preventive measures, particularly vaccination, to reduce the risk of SARS-CoV-2 infection and severe COVID-19 disease in patients with CLD, cirrhosis, and LT. Studies of vaccine efficacy include those assessing serologic immune response and those evaluating real-world outcomes including incident SARS-CoV-2 infection, hospitalization, or mortality.
Immune response to vaccination
Patients with CLD have been found to have decreased response to vaccination against other infections, such as hepatitis B virus (HBV). 43 In response to SARS-CoV-2 vaccination, rates of initial antibody seroconversion in patients with CLD appear to be comparable to those in healthy controls; however, patients with CLD have a more rapid decline in antibody levels over time.6,44 A systematic review and meta-analysis of 19 studies including 1700 LT recipients, 1624 patients with CLD, and more than 800 healthy controls compared pooled rates of detectable humoral immune response to SARS-CoV-2 vaccination and found no difference between patients with CLD and healthy controls, or between cirrhotic and non-cirrhotic patients. 45 The study found that immune response depended on vaccine type, with two doses of mRNA vaccine yielding positive humoral immune response in 99% of patients with CLD, compared to 91% in those who received inactivated vaccines. 45 In studies evaluating spike-specific T-cell response to vaccination, on the other hand, patients with CLD had a significantly lower response than healthy controls, but a significantly greater response than LT recipients. 7 Therefore, it has been posited that despite comparable antibody seroconversion rates, ineffective T-cell response may result in decreased protection from SARS-CoV-2 in patients with CLD and LT compared to healthy controls.
Consistent with prior studies of response to non-SARS-CoV-2 vaccination in SOT recipients, LT recipients are significantly less likely to demonstrate an immune response to COVID-19 vaccinations than healthy controls. Antibody seropositivity rates after two vaccine doses range from 38.7% to 71% for LT recipients.8,46–51 Risk factors for a poor immunologic response in SOT recipients overall and in LT recipients specifically have included diabetes mellitus and specific immunosuppressant exposures including mycophenolate, high-dose prednisone, or two or more immunosuppressant agents concurrently.8,45,49,52 Interestingly, calcineurin inhibitors (CNIs), older age, and short time from vaccination to transplant were also found to be risk factors for failed response to SARS-CoV-2 vaccination among SOT recipients in general, 9 but have not consistently been found to be risk factors among LT recipients in particular. 45 A third dose of mRNA vaccine has been found to increase antibody titers as well as T-cell response in SOT recipients.53,54
Clinical outcomes after vaccination
Beyond laboratory-based assays of immune response, studies of clinical outcomes in patients vaccinated against SARS-CoV-2 have largely demonstrated that vaccination is effective in preventing severe COVID-19 in patients with CLD. In a propensity-matched cohort study of Veterans Affairs patients with cirrhosis, those who received at least one dose of an mRNA vaccine had a modest reduction in incident COVID-19 infection and a 100% reduction in COVID-19-related hospitalization or death. Subgroup analyses also demonstrated a greater risk reduction in COVID-19 infection among patients with compensated cirrhosis, compared to those without decompensated cirrhosis. 55 A case series drawing from international reporting registries of patients with CLD or LT who had received at least one vaccine dose prior to testing positive for SARS-CoV-2 found that all severe COVID-19 cases were patients who had received a single dose within the preceding 1–2 weeks. Among LT recipients who had received two vaccine doses, no breakthrough infections resulted in mechanical ventilation, ICU admission, or death. 56
Protection against SARS-CoV-2 infection in SOT recipients may vary according to vaccine type, though findings have not been consistent.57,58 Studies have demonstrated that SOT recipients have greater protection against severe COVID-19 after three doses of mRNA vaccine than after two doses, although the efficacy of vaccination still does not reach that of immunocompetent controls. 59 Further data regarding long-term protection against SARS-CoV-2 and severe COVID-19 afforded by fourth or booster doses in patients with CLD and LT are needed.
Vaccination recommendations
Current vaccination recommendations vary slightly among countries. The European Association for the Study of the Liver (EASL) recommends that patients with CLD or LT receive three doses of any SARS-CoV-2 vaccine, one of which can be replaced by SARS-CoV-2 infection, with consideration of a fourth dose in LT recipients or in patients with CLD who received initial doses in short intervals. The American Association for the Study of Liver Diseases (AASLD) recommends that any vaccine with a U.S. Food and Drug Administration (FDA) Emergency Use Authorization (EUA) may be used, in accordance with Centers for Disease Control recommendations, 60 but preferentially recommends mRNA vaccines for primary vaccination series. The AASLD recommends two doses of mRNA vaccines for patients with CLD and three doses for LT recipients, as well as mRNA boosters 2 months after completion of the primary series.61,62
Liver-related vaccination safety and adverse effects
Liver-related adverse effects following SARS-CoV-2 vaccination have been rare. While these are important to evaluate in post-vaccination monitoring, they should not deter immunization efforts. In the largest case series to date, 275 cases of liver-related adverse events following SARS-CoV-2 vaccination were identified. More than half of these were cases of autoimmune hepatitis (AIH), the majority of which were new onset cases with only a few being confirmed relapses or occurring in patients with other preexisting liver disease. The median time to presentation was 14 days after vaccination, the majority occurred following mRNA vaccinations, and cases were highly responsive to standard treatment with steroids and azathioprine. 63 The long-term risk of further relapse is not yet known.
In addition to AIH, portal vein thrombosis has been infrequently observed following SARS-CoV-2 vaccination. This has been seen predominantly in middle-aged females without preexisting liver disease, with median time to presentation 10 days after vaccination. Nearly 90% occurred with adenovirus vector vaccination. 63 Even less common liver-related adverse effects reported include transaminase elevations without further specified explanation and splanchnic vein thrombosis. Four cases of post-vaccination acute liver failure have been reported, including one case in a LT recipient who required re-transplantation. 63 Six cases of post-vaccination acute cellular rejection in LT recipients have been reported, all of whom recovered.64–67
Passive immunization and pre-exposure prophylaxis
The neutralizing monoclonal antibody combination tixagevimab/cilgavimab was shown to effectively reduce the risk of SARS-CoV-2 infection in unvaccinated patients, 68 including SOT recipients. 10 As such, the AASLD recommended tixagevimab/cilgavimab treatment in LT recipients with contraindications to vaccination (e.g. history of severe adverse reaction), and the EASL recommended treatment in immunocompromised patients, including those with decompensated cirrhosis.61,62 However, emerging variants were found not to be neutralized by tixagevimab/cilgavimab, prompting the FDA to rescind its EUA, suspending its use in the United States. 69
Management of COVID-19 in patients with CLD and LT
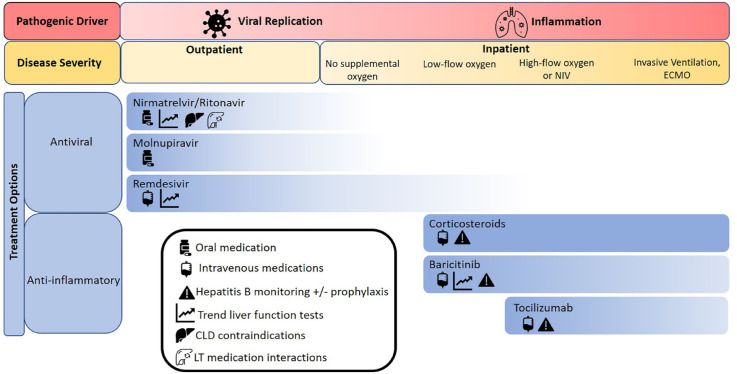
The approach to management of COVID-19 in CLD patients and LT recipients largely resembles that in the general population, with special attention to medication interactions, adverse effects, and hepatic clearance. As in the treatment of all comers with COVID-19, management of early or mild illness is aimed at limiting viral replication, while interventions for severe or prolonged disease target the inflammatory response to SARS-CoV-2. Special treatment considerations for patients with CLD and LT are summarized in Figure 1.
Figure 1.
Available treatments for COVID-19 and special considerations for patients with CLD and LT.
CLD, chronic liver disease; LT, liver transplant; NIV, noninvasive ventilation.
Antiviral treatment
Considerations regarding specific antiviral therapies in patients with CLD and LT are detailed below. Large-scale data in these patients are limited, as they were excluded from most initial drug trials.
Nirmatrelvir/ritonavir is an oral combination protease inhibitor that can significantly reduce the risk of COVID-19-related hospitalization or death in patients treated within 5 days of symptom onset, and has been approved for use in patients with mild-to-moderate COVID-19 who are at risk for disease progression. 11 Nirmatrelvir 300 mg with ritonavir 100 mg twice daily for 5 days, initiated as soon as possible after COVID-19 diagnosis, is recommended. 11 Ritonavir has hepatic clearance and is not recommended in patients with CTP Class C cirrhosis. Ritonavir is also an inhibitor of cytochrome P450 3A4 (CYP3A4), limiting its use in patients receiving CNI, mammalian target of rapamycin inhibitors (mTORi), or direct-acting antiviral agents for hepatitis C virus (HCV). 12
Molnupiravir is an oral nucleoside analog (NA) shown to reduce COVID-19-related hospitalization and mortality in patients without a supplemental oxygen requirement when given within 5 days of symptom onset. 13 Molnupiravir 800 mg twice per day for 5 days, initiated within 5 days of symptom onset, is recommended. 70 No drug interactions relevant to CLD patients or LT recipients have been reported, and there are no liver disease-specific contraindications.
Remdesivir is an intravenous NA prodrug that has been shown to reduce the risk of hospitalization and death in patients with mild-to-moderate COVID-19 who are at high risk for progression to severe disease, including hospitalized patients requiring minimal supplemental oxygen.12,71,71 Remdesivir 200 mg on day 1, followed by 100 mg daily for 3 days in non-hospitalized patients and for 5 days in hospitalized patients is recommended. 73 Patients may develop elevated aminotransferases during therapy. LFT monitoring is recommended during treatment with remdesivir, and the medication should be discontinued if alanine aminotransferase (ALT) rises to more than 10 times the upper limit of normal.
A variety of monoclonal antibodies have been used for both prevention and treatment of mild-to-moderate COVID-19 in unvaccinated patients and those with suboptimal vaccination response. The presence of new viral variants with mutations in the spike protein has made it difficult to predict the efficacy of these therapies for emerging dominant strains. Bebtelovimab was previously recommended for COVID-19 treatment for LT recipients by the AASLD and the EASL, but its EUA was revoked by the FDA due to limited efficacy with emerging strains.74,75 Monoclonal antibodies do not carry specific safety concerns relating to CLD or LT.
Immunomodulatory treatment
With severe COVID-19, treatments targeting the inflammatory response are used in place of antiviral therapies. Immunomodulatory treatments have been found to improve mortality, but come with additional considerations in patients with CLD – particularly those with HBV infection – as well as LT recipients taking immunosuppressants. Corticosteroids, specifically dexamethasone, have been found to have a significant mortality benefit among hospitalized patients with COVID-19, particularly those requiring mechanical ventilation. 76 In addition to risks of non-SARS-CoV-2 infection and uncontrolled hyperglycemia, patients with HBV infection bear the additional potential risk of HBV reactivation with the use of high-dose steroids. 77 HBV surface antigen (HBsAg) and core antibody (anti-HBc) should be checked prior to administration, and HBsAg-positive patients should have HBV DNA levels monitored and receive NA therapy. Anti-HBc-positive patients should also have HBV DNA levels monitored and should receive NA therapy if detectable. 61
More targeted immunomodulatory therapies used for critically ill COVID-19 patients who are already receiving corticosteroids can be beneficial in patients with CLD and LT. Baricitinib, a Janus kinase (JAK)-1 and -2 inhibitor, was found to reduce mortality in hospitalized patients with COVID-19 treated with corticosteroids, without a significant increase in adverse events.78,79 A theoretical increased risk of bacterial super-infection in immunocompromised patients has been proposed. 61 JAK inhibitors as a class have been associated with mild, transient transaminase elevations and LFT monitoring is recommended, but baricitinib itself has not been linked to significant liver injury. 80 HBV reactivation is also a known risk, and testing and treatment, if indicated, are recommended.61,77,81
Tocilizumab, an interleukin-6 inhibitor, was found to reduce all-cause mortality in patients with COVID-19 when added to corticosteroids. 82 It is associated with mild, transient ALT elevations as well as rare, severe liver injury. LFTs should be monitored during treatment with tocilizumab, and treatment should be stopped if ALT elevations greater than five times the upper limit of normal are observed. 83 There is a risk of HBV reactivation, and serologies should be checked prior to treatment initiation. 61 Data on outcomes following tocilizumab use among SOT or LT recipients are mixed, with some studies showing shortened hospital stay but others showing no impact on ICU admission or mortality.14,84–86 The risk of concomitant non-SARS-CoV-2 infection in patients treated with tocilizumab has ranged widely in studies of non-SOT patients with COVID-19, ranging from 5% to 50%,14,87,88 and data on bacterial super-infection in patients with CLD or LT are lacking.
Antithrombotic treatment
Coagulopathy is recognized as a major contributor to disease severity in COVID-19. Therapeutic anticoagulation or intensified prophylaxis has not been demonstrated to impact mortality, major venous thromboembolism (VTE) events, or major organ failure in the general population of patients with COVID-19, but therapeutic dosing has been associated with increased risk of bleeding complications. 89 Despite historical concerns about increased bleeding risk in patients with CLD, patients with CLD are known to be at increased risk of VTE, 90 and anticoagulation in CLD patients has not been associated with increased bleeding complications. 91 In the setting of COVID-19, patients with cirrhosis who received VTE prophylaxis were not found to have an increase in major bleeding events. 4
Immunosuppression management after LT
Immunosuppressant management in SOT recipients with COVID-19 must balance the benefits of protective immune activation with the risks of excessive inflammation and transplant rejection. 61 In the early phases of the COVID-19 pandemic, SARS-CoV-2 infection frequently led to changes in immunosuppressive regimens in patients with LT. In a European study including 57 LT recipients, immunosuppression was reduced in 39% of patients and discontinued in 7% of patients. 39 Subsequent data from the U.S. multicenter COVID-19 in CLD consortium found that immunosuppression was modified in 49% of LT recipients with COVID-19, particularly in those who had severe illness requiring ICU admission, mechanical ventilation, or vasopressors. 33 In these studies, change in immunosuppression – most commonly discontinuation of mycophenolate – was not associated with adverse outcomes including acute liver injury or mortality.33,41 In fact, a prospective study of 111 LT recipients with COVID-19 found that baseline immunosuppression containing mycophenolate was an independent predictor of severe COVID-19 infection, especially at doses greater than 1000 mg/day, potentially due to a synergistic effect of mycophenolate and COVID-19 on T lymphocytes.15,92 In contrast, CNI and mTORi have not been associated with COVID-19 severity and are thought to have a neutral or even beneficial impact on disease course.16,93–95 CNI use has been associated with improved survival among LT recipients with COVID-19, 16 although these patients were younger and had fewer comorbidities. CNIs may have direct antiviral properties and mTORi are also postulated to suppress COVID-19 replication directly.95,96 As such, CNI and mTORi are not routinely modified in patients with COVID-19, as supported by major society recommendations.61,62
Overall, current evidence suggests that the management of immunosuppression in LT recipients with COVID-19 should be tailored to the individual needs of each patient. For those with severe COVID-19 infection or at high risk of disease progression, reduction in immunosuppression may be appropriate; in such cases, it may be reasonable to preferentially decrease the dose of mycophenolate. This management strategy is supported by major society recommendations.61,62 In patients receiving tacrolimus, special attention should be placed on monitoring medication levels, as COVID-19 has been shown to increase serum tacrolimus concentrations in SOT recipients. 16
Trends in liver transplantation in the COVID-19 era
LT activity has been significantly and variably impacted since the start of the COVID-19 pandemic. Globally, there was an initial decline in LT volume, but the degree of this initial reduction as well as return to pre-COVID-19 transplant volume has varied significantly with subsequent waves of COVID-19 and with heterogeneous local responses. During the first year of the COVID-19 pandemic, both the length of waitlists and waitlist mortality generally increased compared to 2019, albeit with significant geographic variability.97,98 An international retrospective study of 22 nationwide transplant cohorts comparing the early COVID-19 era in 2020 to the analogous period in 2019 found a reduction in LT volume by 11% (ranging from +9% to −57%) in 2020, and an estimated 7370 waitlisted patient life-years lost. Regions with greater proportion of living donation experienced a more substantial decrease in LT volume. 97
In the United States, there was a 38% initial reduction in LT volume in early 2020 compared to 2019, 17 but only a 1.2% decrease in LT volume by the end of 2020.18,98 Figure 2 shows weekly LT volume (a) and waitlist additions (b) in the United States based on Organ Procurement and Transplantation Network (OPTN) data since the beginning of 2020, and demonstrate an overall stabilization in liver transplantation activity since the early pandemic period. There have remained slight seasonal reductions in LT volume corresponding to rises in COVID-19 cases during ‘waves’ of the pandemic. 99 Rates of living donor LT (LDLT) in particular remained persistently decreased and have still not returned to pre-pandemic levels in 2022.99,100
Figure 2.
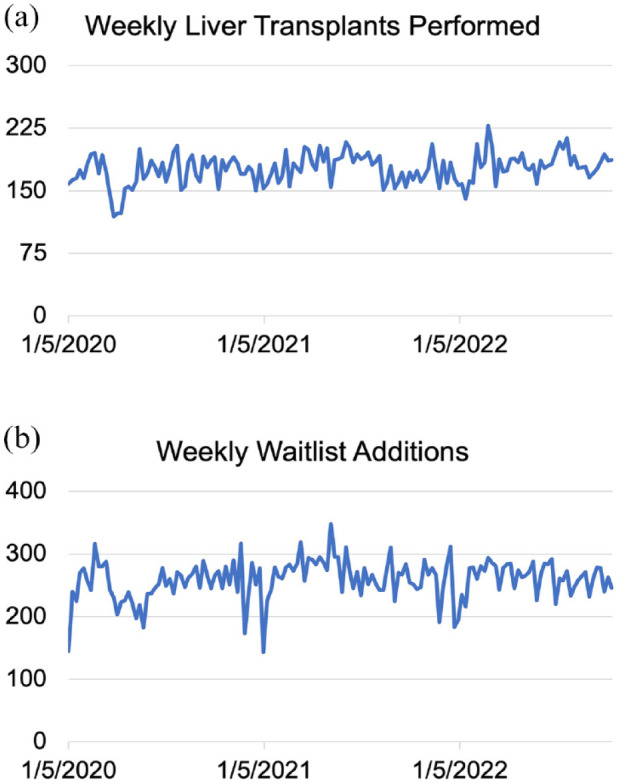
(a) Weekly liver transplant volume and (b) weekly liver transplant waitlist additions in the United States between 5 January 2020 and 16 October 2022 based on data from the Organ Procurement and Transplantation Network.
The increase in alcohol use and alcohol-related liver disease (ALD) associated with the COVID-19 pandemic have significantly influenced LT in the United States. Frequency of alcohol consumption and heavy drinking, particularly for women, increased early in the pandemic compared to the year before. 101 Similar trends were seen in other parts of the world, with increases in harmful alcohol consumption in China as well as increased high-risk drinking in the setting of fewer abstinence resources in England.102,103 This trend corresponded with a rise in prevalence and severity of ALD,104,105 resulting in significant increases in LT waitlist additions and transplants for an indication of ALD.18,106 By the end of 2020, ALD accounted for 40.1% of all LT listings, more than HCV and nonalcoholic steatohepatitis combined. ALD patients presented with higher acuity of illness than patients with other LT indications and were more likely to be listed and transplanted with MELD above 30. The greatest increases in ALD-related listing and transplantation were seen among young adults. 106 Waitlist registrations and LT for acute alcohol-related hepatitis (AH) also nearly doubled in 2020 compared to 2019, surpassing pre-COVID-19 trends and correlating with retail alcohol sales.107,108 The rise in listings and transplants for AH was most pronounced among women. 107 Despite potential challenges of transplanting more patients with ALD, 3-month post-LT patient survival has not changed relative to the pre-COVID era and remains above 96%. 18
SARS-CoV-2 testing and prevention considerations in LT candidates and recipients
LT candidate testing
The AASLD and the American Society of Transplantation (AST) currently recommend testing for SARS-CoV-2 prior to transplantation, regardless of vaccination status. If candidates have active COVID-19 infection, transplantation is usually not recommended, but exactly how long after infection is the ideal time to undergo transplantation is not yet well defined.109,110 Concerns about transplanting patients with active COVID-19 include increased surgical risk and risk of worsening COVID-19 illness with the initiation of immunosuppression. The COVIDSurg and GlobalSurg Collaboratives found that surgeries performed at least 7 weeks after COVID diagnosis had the same mortality risk as those without prior COVID infection. 111 The AST recommends deferring transplantation until the candidate has complete symptom resolution and ideally a negative respiratory tract PCR test. When candidates have persistently positive PCR tests following symptom resolution, it can be particularly difficult to determine when transplantation is safest; in these cases, the AST and AASLD suggest that quantitative cycle threshold testing and/or local infectious diseases expertise be utilized.109,110
Deceased donor testing
Similar to candidate testing, it is recommended that all donors be screened for signs and symptoms of COVID-19 and undergo a PCR test no more than 72 h prior to organ procurement. 110 The risk of SARS-CoV-2 transmission via LT is not known. A review of United Network for Organ Sharing data from March 2020 to December 2021 identified 106 LTs from SARS-CoV-2-positive donors. LT recipients from SARS-CoV-2-positive donors had shorter length of stay after transplant, but higher rate of graft rejection before discharge, compared to LT recipients from SARS-CoV-2-negative donors. By 30 days post-LT, no difference in graft failure or mortality was found. 112 A study of 147 LTs from SARS-CoV-2-positive donors also found no difference in overall graft survival at 6 months compared to LTs from SARS-CoV-2-negative donors. 113 Post-transplant liver biopsies from 10 consecutive recipients from SARS-CoV-2-positive donors did not demonstrate the presence of viral particles in any recipients. 114 Altogether, there is growing evidence that LT using SARS-CoV-2-positive donors may be safe. This is reflected in current AST and OPTN guidance on deceased donor testing. Respiratory tract PCR testing within 72 h of procurement is recommended and organs from COVID-19-positive organs can be considered after appropriate patient counseling.110,115 Further studies of long-term outcomes following LT with SARS-CoV-2-positive donors will be needed to inform standardized protocols.
Living donor considerations
LDLT volume in the United States was heavily impacted by the COVID-19 pandemic. Given the additional consideration of donor safety, supplementary protections have been considered. The AST currently recommends that living donors undergo respiratory tract PCR testing within 72 h of donation. Vaccination, including booster doses, is strongly encouraged, but not universally required. Other recommended preventative strategies including masking, physical distancing, and hand hygiene, while self-quarantine for 14 days prior to scheduled LDLT should be strongly considered. At present, the AST recommends against performing LDLT using SARS-CoV-2-positive donors and instead recommends deferring donation until 6 weeks after COVID-19 illness, at which point excess surgical risk and active viral replication are thought to be limited. If interval PCR testing remains positive, local infectious disease expertise consultation is recommended. 110
Vaccination mandates
Decisions by some centers to enforce mandatory SARS-CoV-2 vaccination for transplant candidates and living donors was one of the most publicized issues facing the transplant community. Transplant center policies regarding vaccination mandates are highly heterogeneous across the United States, with 35% of centers reporting a SARS-CoV-2 vaccination mandate for transplant candidates and only a small fraction of those centers mandating vaccination for living donors. 116 Neither the AST nor the AASLD currently recommends universal vaccination mandates.109,117
Conclusion
COVID-19 has introduced a significant burden of morbidity and mortality in patients with CLD, cirrhosis, and LT. As the pandemic has evolved and especially with the development of novel vaccines and medications, COVID-19 can be effectively prevented and treated in patients with CLD and LT. Several specific considerations apply in these patients, including extended vaccination series and attention to treatment regimen drug interactions and adverse effects. Trends and policies in liver transplantation have also been substantially impacted by the pandemic. As understanding of SARS-CoV-2 transmission and risk have evolved, academic societies have issued specific guidance to standardize SARS-CoV-2 testing and management leading up to LT, although questions regarding SARS-CoV-2-positive organ donors, transplant timing after resolved recipient COVID-19 infection, and universal vaccination mandates remain. Further studies evaluating long-term clinical outcomes in patients with CLD and LT will help to guide future practice.
Acknowledgments
None.
Footnotes
ORCID iD: Yael R. Nobel  https://orcid.org/0000-0001-5759-3730
https://orcid.org/0000-0001-5759-3730
Contributor Information
Gabriel Perreault, Division of Digestive and Liver Diseases, Columbia University Irving Medical Center, New York, NY, USA.
Charlotte Ching, Department of Medicine, Columbia University Irving Medical Center, New York, NY, USA.
Yael R. Nobel, Instructor in Medicine, Division of Digestive and Liver Diseases, Columbia University Irving Medical Center, 161 Ft. Washington Ave., Suite 862, New York, NY 10032, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Gabriel Perreault: Conceptualization; Data curation; Writing – original draft.
Charlotte Ching: Data curation; Writing – original draft.
Yael R. Nobel: Conceptualization; Methodology; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Sarin SK, Choudhury A, Lau GK, et al. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; the APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int 2020; 14: 690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh S, Khan A. Clinical characteristics and outcomes of coronavirus disease 2019 among patients with preexisting liver disease in the United States: a multicenter research network study. Gastroenterology 2020; 159: 768–771.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bajaj JS, Garcia-Tsao G, Biggins SW, et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut 2021; 70: 531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iavarone M, D’Ambrosio R, Soria A, et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol 2020; 73: 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qi X, Liu Y, Wang J, et al. Clinical course and risk factors for mortality of COVID-19 patients with pre-existing cirrhosis: a multicentre cohort study. Gut 2021; 70: 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Willuweit K, Frey A, Passenberg M, et al. Patients with liver cirrhosis show high immunogenicity upon COVID-19 vaccination but develop premature deterioration of antibody titers. Vaccines 2022; 10: 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruether DF, Schaub GM, Duengelhoef PM, et al. SARS-CoV2-specific humoral and T-cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol 2022; 20: 162–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hall VG, Ferreira VH, Ierullo M, et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant 2021; 21: 3980–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li JJ, Ayada I, Wang YN, et al. Factors associated with COVID-19 vaccine response in transplant recipients: a systematic review and meta-analysis. Transplantation 2022; 106: 2068–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al Jurdi A, Morena L, Cote M, et al. Tixagevimab/cilgavimab pre-exposure prophylaxis is associated with lower breakthrough infection risk in vaccinated solid organ transplant recipients during the omicron wave. Am J Transplant 2022; 22: 3130–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med 2022; 386: 1397–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gottlieb RL, Vaca CE, Paredes R, et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med 2022; 386: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bernal AJ, da Silva MMG, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med 2022; 386: 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fernandez-Ruiz M, Aguado JM. Immunomodulatory therapies for COVID-19 in solid organ transplant recipients. Curr Transplant Rep 2020; 7: 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Colmenero J, Rodriguez-Peralvarez M, Salcedo M, et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol 2021; 74: 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belli LS, Fondevila C, Cortesi PA, et al. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with Covid-19: results from the ELITA/ELTR multi-center European study. Gastroenterology 2021; 160: 1151–1163.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yuan Q, Haque O, Coe TM, et al. The heterogenous effect of COVID-19 on liver transplantation activity and waitlist mortality in the United States. Front Surg 2021; 8: 669129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuo YF, Kwo P, Wong RJ, et al. Impact of COVID-19 on liver transplant activity in the USA: variation by etiology and cirrhosis complications. J Clin Transl Hepatol 2023; 11: 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020; 26: 1017–1032. [DOI] [PubMed] [Google Scholar]
- 20. Fan Z, Chen L, Li J, et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol 2020; 18: 1561–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferm S, Fisher C, Pakala T, et al. Analysis of gastrointestinal and hepatic manifestations of SARS-CoV-2 infection in 892 patients in queens, NY. Clin Gastroenterol Hepatol 2020; 18: 2378–2379.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phipps MM, Barraza LH, LaSota ED, et al. Acute liver injury in COVID-19: prevalence and association with clinical outcomes in a large U.S. Cohort. Hepatology 2020; 72: 807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piano S, Dalbeni A, Vettore E, et al. Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19. Liver Int 2020; 40: 2394–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mao R, Qiu Y, He J-S, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020; 5: 667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Melquist S, Estepp K, Aleksandrovich Y, et al. COVID-19 presenting as fulminant hepatic failure: a case report. Medicine (Baltimore) 2020; 99: e22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roth NC, Kim A, Vitkovski T, et al. Post-COVID-19 cholangiopathy: a novel entity. Am J Gastroenterol 2021; 116: 1077–1082. [DOI] [PubMed] [Google Scholar]
- 27. Kim D, Adeniji N, Latt N, et al. Predictors of outcomes of COVID-19 in patients with chronic liver disease: US multi-center Study. Clin Gastroenterol Hepatol 2021; 19: 1469–1479.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hashemi N, Viveiros K, Redd WD, et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: a multicentre United States experience. Liver Int 2020; 40: 2515–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marjot T, Moon AM, Cook JA, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol 2021; 74: 567–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant 2020; 20: 1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Softeland JM, Friman G, von Zur-Muhlen B, et al. COVID-19 in solid organ transplant recipients: a national cohort study from Sweden. Am J Transplant 2021; 21: 2762–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jering KS, McGrath MM, McCausland FR, et al. Excess mortality in solid organ transplant recipients hospitalized with COVID-19: a large-scale comparison of SOT recipients hospitalized with or without COVID-19. Clin Transplant 2022; 36: e14492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rabiee A, Sadowski B, Adeniji N, et al. Liver injury in liver transplant recipients with coronavirus disease 2019 (COVID-19): U.S. Multicenter experience. Hepatology 2020; 72: 1900–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Webb GJ, Marjot T, Cook JA, et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol 2020; 5: 1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rinaldi M, Bartoletti M, Bussini L, et al. COVID-19 in solid organ transplant recipients: no difference in survival compared to general population. Transpl Infect Dis 2021; 23: e13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pereira MR, Arcasoy S, Farr MA, et al. Outcomes of COVID-19 in solid organ transplant recipients: a matched cohort study. Transpl Infect Dis 2021; 23: e13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kulkarni AV, Tevethia HV, Premkumar M, et al. Impact of COVID-19 on liver transplant recipients-a systematic review and meta-analysis. EClinicalMedicine 2021; 38: 101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jayant K, Reccia I, Virdis F, et al. COVID-19 in hospitalized liver transplant recipients: an early systematic review and meta-analysis. Clin Transplant 2021; 35: e14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Becchetti C, Zambelli MF, Pasulo L, et al. COVID-19 in an international European liver transplant recipient cohort. Gut 2020; 69: 1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mansoor E, Perez A, Abou-Saleh M, et al. Clinical characteristics, hospitalization, and mortality rates of coronavirus disease 2019 among liver transplant patients in the United States: a multicenter research network study. Gastroenterology 2021; 160: 459–462.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dumortier J, Duvoux C, Roux O, et al. Covid-19 in liver transplant recipients: the French SOT COVID registry. Clin Res Hepatol Gastroenterol 2021; 45: 101639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shafiq M, Gibson C. Clinical outcomes of coronavirus disease 2019 in liver transplant recipients. World J Hepatol 2022; 14: 1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aggeletopoulou I, Davoulou P, Konstantakis C, et al. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol 2017; 27: e1942. [DOI] [PubMed] [Google Scholar]
- 44. Bakasis AD, Bitzogli K, Mouziouras D, et al. Antibody responses after SARS-CoV-2 vaccination in patients with liver diseases. Viruses-Basel 2022; 14: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Luo D, Chen XP, Du J, et al. Immunogenicity of COVID-19 vaccines in chronic liver disease patients and liver transplant recipients: a systematic review and meta-analysis. Liver Int 2023: 43; 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Timmermann L, Globke B, Lurje G, et al. Humoral immune response following SARS-CoV-2 vaccination in liver transplant recipients. Vaccines 2021; 9: 1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rabinowich L, Grupper A, Baruch R, et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol 2021; 75: 435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Herrera S, Colmenero J, Pascal M, et al. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant 2021; 21: 3971–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marion O, del Bello A, Florence Abravanel F, et al. Safety and immunogenicity of anti–SARS-CoV-2 Messenger RNA vaccines in recipients of solid organ transplants. Ann Int Med 2021; 174: 1336–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Huang HJ, Yi SG, Mobley CM, et al. Early humoral immune response to two doses of severe acute respiratory syndrome coronavirus 2 vaccine in a diverse group of solid organ transplant candidates and recipients. Clin Transplant 2022; 36: e14600. [DOI] [PubMed] [Google Scholar]
- 51. Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol 2021; 75: 1434–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ou MT, Boyarsky BJ, Motter JD, et al. Safety and reactogenicity of 2 doses of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation 2021; 105: 2170–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hall VG, Ferreira VH, Ku TC, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med 2021; 385: 1244–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Del Bello A, Abravanel F, Marion O, et al. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant 2022; 22: 322–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. John BV, Deng YY, Scheinberg A, et al. Association of BNT162b2 mRNA and mRNA-1273 vaccines with COVID-19 infection and hospitalization among patients with cirrhosis. JAMA Int Med 2021; 181: 1306–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moon AM, Webb GJ, Garcia-Juarez I, et al. SARS-CoV-2 infections among patients with liver disease and liver transplantation who received COVID-19 vaccination. Hepatol Commun 2022; 6: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Callaghan CJ, Mumford L, Curtis RMK, et al. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation 2022; 106: 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Prendecki M, Clarke C, Edwards H, et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis 2021; 80: 1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kwon JH, Tenforde MW, Gaglani M, et al. mRNA Vaccine effectiveness against coronavirus disease 2019 hospitalization among solid organ transplant recipients. J Infect Dis 2022; 226: 797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Centers for Disease Control. (2022, accessed 24 Oct 2022). [Google Scholar]
- 61. Marjot T, Eberhardt CS, Boettler T, et al. Impact of COVID-19 on the liver and on the care of patients with chronic liver disease, hepatobiliary cancer, and liver transplantation: an updated EASL position paper. J Hepatol 2022; 77: 1161–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology 2020; 72: 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alhumaid S, Al Mutair A, Rabaan AA, et al. New-onset and relapsed liver diseases following COVID-19 vaccination: a systematic review. BMC Gastroenterol 2022; 22: 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Valsecchi M, Lauterio A, Crocchiolo R, et al. New-onset antibodies to platelet factor 4 following liver transplantation from a donor with vaccine-induced thrombotic thrombocytopenia. Liver Transplant 2022; 28: 314–316. [DOI] [PubMed] [Google Scholar]
- 65. Hughes DL, Brunn JA, Jacobs J, et al. Guillain-Barre syndrome after COVID-19 mRNA vaccination in a liver transplantation recipient with favorable treatment response. Liver Transplant 2022; 28: 134–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sarwar R, Adeyi O, Lim N. Acute cellular rejection in liver transplant recipients following vaccination against Covid-19. Am J Transplant 2022; 22: 560–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vyhmeister R, Enestvedt CK, VanSandt M, et al. Steroid-resistant acute cellular rejection of the liver after severe acute respiratory syndrome coronavirus 2 mRNA vaccination. Liver Transplant 2021; 27: 1339–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Levin MJ, Ustianowski A, De Wit S, et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of Covid-19. N Engl J Med 2022; 386: 2188–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. FDA announces Evusheld is not currently authorized for emergency use in the U.S. U.S. Food and Drug Administration. https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use-us#update01062 (2023, accessed 31 May 2023).
- 70. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med 2022; 386: 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19-Final report. N Engl J Med 2020; 383: 1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ader F, Bouscambert-Duchamp M, Hites M, et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis 2022; 22: 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/ (2022, accessed 29 September 2022). [PubMed] [Google Scholar]
- 74. Montgomery H, Hobbs FDR, Padilla F, et al. Efficacy and safety of intramuscular administration of tixagevimab–cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2022; 10: 985–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. FDA Announces Bebtelovimab is Not Currently Authorized in Any US Region. U.S. Food and Drug Administration. https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-bebtelovimab-not-currently-authorized-any-us-region (2022, accessed 23 May 2023).
- 76. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yip TCF, Gill M, Wong GLH, et al. Management of hepatitis B virus reactivation due to treatment of COVID-19. Hepatol Int 2022; 16: 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo- controlled phase 3 trial. Lancet Respir Med 2021; 9: 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ely EW, Ramanan AV, Kartman CE, et al. Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial. Lancet Respir Med 2022; 10: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gatti M, Turrini E, Raschi E, et al. Janus kinase inhibitors and coronavirus disease (COVID)-19: rationale, clinical evidence and safety issues. Pharmaceuticals 2021; 14: 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Harigai M, Winthrop K, Takeuchi T, et al. Evaluation of hepatitis B virus in clinical trials of baricitinib in rheumatoid arthritis. RMD Open 2020; 6: e001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19. A meta-analysis. JAMA 2021; 326: 499–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schiff MH, Kremer JM, Jahreis A, et al. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther 2011; 13: R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Avery RK. Update on COVID-19 therapeutics for solid organ transplant recipients, including the omicron surge. Transplantation 2022; 106: 1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yamani AH, Alraddadi BM, Almaghrabi RS, et al. Early use of tocilizumab in solid organ transplant recipients with COVID-19: a retrospective cohort study in Saudi Arabia. Immun Inflamm Dis 2022; 10: e587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bodro M, Cofan F, Rios J, et al. Use of anti-cytokine therapy in kidney transplant recipients with COVID-19. J Clin Med 2021; 10: 1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Price CC, Altice FL, Shyr Y, et al. Tocilizumab treatment for cytokine release syndrome in hospitalized patients with coronavirus disease 2019 survival and clinical outcomes. Chest 2020; 158: 1397–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis 2021; 73: E445–E454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. REMAP-CAP A-a and ATTACC Investigators. Therapeutic anticoagulation with heparin in critically Ill patients with Covid-19. N Engl J Med 2021; 385: 777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Søgaard KK, Horváth-Puhó E, Grønbæk H, et al. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol 2009; 104: 96–101. [DOI] [PubMed] [Google Scholar]
- 91. Loffredo L, Pastori D, Farcomeni A, et al. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta-analysis. Gastroenterology 2017; 153: 480–487.e1. [DOI] [PubMed] [Google Scholar]
- 92. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis 2020; 221: 1762–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Gálvez-Romero JL, Palmeros-Rojas O, Real-Ramírez FA, et al. Cyclosporine A plus low-dose steroid treatment in COVID-19 improves clinical outcomes in patients with moderate to severe disease: pilot study. J Int Med 2021; 289: 906–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Belli LS, Perricone G, Adam R, et al. Impact of DAAs on liver transplantation: major effects on the evolution of indications and results. An ELITA study based on the ELTR registry. J Hepatol 2018; 69: 810–817. [DOI] [PubMed] [Google Scholar]
- 95. Solanich X, Antolí A, Rocamora-Blanch G, et al. Methylprednisolone pulses plus tacrolimus in addition to standard of care vs. standard of care alone in patients with severe COVID-19. A randomized controlled trial. clinical trial. Front Med 2021; 8: 691712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. de Wilde AH, Zevenhoven-Dobbe JC, van der Meer Y, et al. Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol 2011; 92: 2542–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Russo FP, Izzy M, Rammohan A, et al. Global impact of the first wave of COVID-19 on liver transplant centers: a multi-society survey (EASL-ESOT/ELITA-ILTS). J Hepatol 2022; 76: 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Aubert O, Yoo D, Zielinski D, et al. COVID-19 pandemic and worldwide organ transplantation: a population-based study. Lancet Public Health 2021; 6: E709–E719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Network OPaT. Data from: Organ procurement and transplantation network national data, https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#, 2022.
- 100. Strauss AT, Boyarsky BJ, Garonzik-Wang JM, et al. Liver transplantation in the United States during the COVID-19 pandemic: national and center-level responses. Am J Transplant 2021; 21: 1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pollard MS, Tucker JS, Green HD., Jr. Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Network Open 2020; 3: e2022942–e2022942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ahmed MZ, Ahmed O, Aibao Z, et al. Epidemic of COVID-19 in China and associated psychological problems. Asian J Psychiatr 2020; 51: 102092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jackson SE, Garnett C, Shahab L, et al. Association of the COVID-19 lockdown with smoking, drinking and attempts to quit in England: an analysis of 2019-20 data. Addiction 2021; 116: 1233–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jain A, Sobotka LA, Allen KD, et al. S1067 Alcohol-related liver disease increased in severity during the COVID-19 pandemic. Am J Gastroenterol 2021; 116: S506. [Google Scholar]
- 105. Mahmud N, Hubbard RA, Kaplan DE, et al. Declining cirrhosis hospitalizations in the wake of the COVID-19 pandemic: a national cohort study. Gastroenterology 2020; 159: 1134–1136.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cholankeril G, Goli K, Rana A, et al. Impact of COVID-19 pandemic on liver transplantation and alcohol-associated liver disease in the USA. Hepatology 2021; 74: 3316–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Anderson MS, Valbuena VSM, Brown CS, et al. Association of COVID-19 with new waiting list registrations and liver transplantation for alcoholic hepatitis in the United States. JAMA Network Open 2021; 4: e2131132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bittermann T, Mahmud N, Abt P. Trends in liver transplantation for acute alcohol-associated hepatitis during the COVID-19 pandemic in the US. JAMA Network Open 2021; 4: e2118713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. AASLD. AASLD expert panel consensus statement: COVID-19 clinical best practice advice for hepatology and liver transplant providers. Alexandria, Virginia: American Association for the Study of Liver Diseases, 2022. [Google Scholar]
- 110. American Society of Transplantation. SARS-CoV-2: recommendations and guidance for organ donor testing and evaluation. American Society of Transplantation, Mt. Laurel, New Jersey, 2022. [Google Scholar]
- 111. Collaborative C, Collaborative G. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia 2021; 76: 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dhand A, Okumura K, Nabors C, et al. Solid organ transplantation from COVID positive donors in the United States: analysis of United Network for organ sharing database. Transpl Infect Dis 2022; 25: e13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Schold JD, Koval CE, Wee A, et al. Utilization and outcomes of deceased donor SARS-CoV-2-positive organs for solid organ transplantation in the United States. Am J Transplant 2022; 22: 2217–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Romagnoli R, Gruttadauria S, Tisone G, et al. Liver transplantation from active COVID-19 donors: a lifesaving opportunity worth grasping? Am J Transplant 2021; 21: 3919–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Network OPaT. Summary of current evidence and information– Donor SARS-CoV-2 testing & organ recovery from donors with a history of COVID-19. OPTN, 22 August 2022. [Google Scholar]
- 116. Hippen BE, Axelrod DA, Maher K, et al. Survey of current transplant center practices regarding COVID-19 vaccine mandates in the United States. Am J Transplant 2022; 22: 1705–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. American Society of Transplantation. COVID-19: FAQs for Organ Transplantation. American Society of Transplantation, 2022. [Google Scholar]