Abstract
Background:
Data regarding the risk of ischemic stroke within 1 year after the post-acute phase of COVID-19 remain scant. We assess the risk of ischemic stroke in COVID-19 survivors after SARS-CoV-2 infection by performing a systematic review and meta-analysis of the available data.
Methods:
Following the PRISMA guidelines, we searched Medline and Scopus to locate all articles published up to February 11, 2023, reporting the risk of incident ischemic stroke in adult patients recovered from COVID-19 infection compared to non-infected patients (controls) defined as those who did not experience the infection over the same follow-up period. Ischemic stroke risk was evaluated using the Mantel–Haenszel random effects models with adjusted Hazard ratio (HR) as the effect measure with 95% confidence interval (CI) while heterogeneity was assessed using Higgins I2 statistic.
Results:
Overall, 23,559,428 patients (mean age 56, 1 year, 54.3% males), of whom 1,595,984 had COVID-19, were included. Over a mean follow-up of 9.2 months, ischemic stroke occurred in 4.40 [95% CI: 4.36–4.43] out of 1000 patients survived to COVID-19 compared to 3.25 [95% CI:3.21–3.29] out of 1000 controls. Recovered COVID-19 patients presented a higher risk of ischemic stroke ((HR: 2.06, 95% CI: 1.75–2.41, p < 0.0001, I2 = 63.7%) compared to people who did not have COVID-19. COVID-19 patients hospitalized at the time of the infection have a subsequent higher risk of stroke during the follow-up compared to those non-hospitalized.
Conclusions:
Recovered COVID-19 patients have a higher risk of ischemic stroke compared to subjects from the general population within 9 months from the index infection.
Keywords: Stroke, COVID-19, long-COVID
Introduction
Soon after the identification of the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and the onset of the Coronavirus Disease 19 (COVID-19) pandemic, clinicians observed that SARS-CoV-2 infection did not only produce acute respiratory problems. In fact, since the virus presents its receptors in different body systems, acute cutaneous, cardiovascular, hepatic, and renal complications were observed.1 –4 This burden of complications had a synergistic effect when overlapped to the underlying patient’s comorbidities. 5
Moreover, recent investigations demonstrated that during COVID-19 also the risk of developing an acute ischemic stroke is increased, especially in patients who are severely ill and/or have pre-existing cardiovascular risk factors.6,7 Although the exact pathogenetic mechanisms linking the SARS-CoV-2 infection with the occurrence of ischemic stroke remain largely unknown, some recent evidence suggested that COVID-stroke affects predominantly males, the anterior cerebral circulation and may be due to a complex and multifactorial pathophysiological process.8 –10 Different underlying pathophysiological pathways, including the hypercoagulable state, the cytokine storm induced by the immune response against the viral infection as well as a pre-existent endotheliopathy and blood brain barrier permeability seem to play a pivotal role in promoting the occurrence of ischemic cerebral events in COVID-19 patients.11 –14 For these reasons, since the first phases of COVID-19 pandemic, clinicians implemented anticoagulation at different dosages, trying to counteract the COVID-19 associated coagulopathy. 15 However, the larger part of studies examining the relationship between stroke and SARS-CoV-2 infection have mainly focused on the potential pathophysiological mechanisms underlying this relationship in the acute phase of the infection.16,17 Conversely, data regarding the risk of ischemic stroke as a post-acute COVID-19 sequelae (PACS)18,19 remain scant and discordant. Therefore, this work aims at assessing the risk of incident ischemic stroke as post – acute sequelae of SARS-CoV-2 infection by performing a systematic review and meta-analysis of all available data.
Material and methods
Study design
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (Supplementary File 1). 20 Data were obtained by searching the MEDLINE and Scopus database for all the studies published at any time up to February 14th, 2023, reporting the risk of incident ischemic stroke in COVID-19 survivors diagnosed between 4 months (minimum follow-up length of revised investigations) and a maximum of 48 months after the index infection (maximum follow-up length of revised studies). In the revised manuscripts, the group of patients who developed stroke, had COVID-19 and then recovered from the acute infection (study group) was compared to a contemporary well-matched cohort of subjects who did not experience the SARS-CoV-2 infection, but some of them experienced ischemic stroke during the same follow-up period (control group).
Data extraction and quality assessment
We included both prospective and retrospective case–control studies in which the primary outcome was the occurrence of ischemic stroke after COVID-19 infection. The selection of studies included in our analysis was independently conducted by two authors (M.Z., G.R.) in a blinded fashion. Any discrepancies in study selection were resolved by consulting a third author (M.M). The following MeSH terms were used for the search: “Ischemic stroke” AND “COVID-19 sequelae” OR “Ischemic stroke” AND “COVID-19” OR “Ischemic stroke” AND “SARS-COV-2 sequelae” OR “Ischemic stroke” AND “SARS-CoV-2.” The full search strategy is showed in Supplemental File 2. Moreover, we searched the bibliographies of the target studies for additional references. Potential overlaps among the sources used by the reviewed studies were evaluated and excluded.
Specifically, inclusion criteria were: (i) studies enrolling subjects with previous confirmed COVID-19 infection and recovered from the disease (ii) providing the adjusted hazard ratio (HR) and relative 95% confidence interval (CI) for the risk of incident ischemic stroke after the index infection. Conversely, case reports, review articles, abstracts, and editorials/letters were excluded. Data extraction was independently conducted by two authors (M.Z., G.R). For all the reviewed investigations we extracted, when provided, the number of enrolled patients, the mean age, the gender, the prevalence of cardiovascular comorbidities such as arterial hypertension (HT), diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), obesity, pre-existing heart failure (HF), cerebrovascular disease and the length of follow-up. The quality of included studies was graded using the Newcastle-Ottawa quality assessment scale (NOS). 21
Data synthesis and analysis
Continuous variables were expressed as mean while categorical variables were presented as numbers and relative percentages. Ischemic stroke risk data were pooled using the Mantel–Haenszel random effects models with Hazard ratio (HR) as the effect measure with 95% confidence interval (CI). Heterogeneity among studies was assessed using Higgins and Thomson I2 statistic. Specifically, the I2 values correspond to the following levels of heterogeneity: low (<25%), moderate (25%−75%) and high (>75%). The presence of potential publication bias was verified by visual inspection of the funnel plot. Due to the low number of the included studies (<10), small-study bias was not examined as our analysis was underpowered to detect such bias. However, a predefined sensitivity analysis (leave-one-out analysis) was performed removing one study at the time, to evaluate the stability of our results regarding the risk of ischemic stroke. To further appraise the impact of potential baseline confounders, a meta-regression analysis was also performed. All meta-analyses were conducted using Comprehensive Meta-Analysis software, version 3 (Biostat, USA).
Results
Search results and included studies
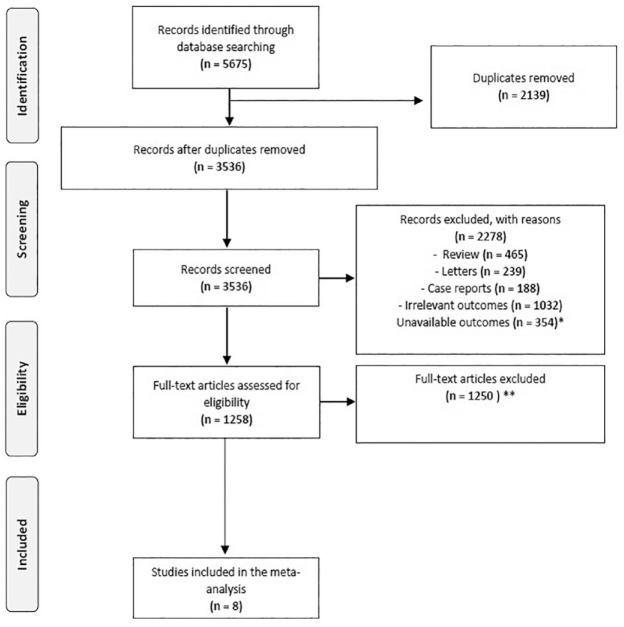
A total of 5675 articles were obtained using our search strategy. After excluding duplicates and preliminary screening, 1258 full-text articles were assessed for eligibility, 1250 studies were excluded for not meeting the inclusion criteria, leaving eight investigations fulfilling the inclusion criteria (Figure 1).22 –29
Figure 1.
PRISMA flowchart.
*Articles excluded because not provided data on ischemic stroke events.
**Articles excluded because not provided Hazard ratio for acute ischemic stroke.
Characteristics of the population and quality assessment
Overall, 23,559,428 adult patients were included in this analysis.22 –29 Among them 1,595,984 had confirmed COVID-19 infection (Table 1). Although the demographic characteristics and the pre-existing comorbidities were not systematically recorded in all the investigations, the cohorts mainly consisted of middle-aged patients who were more frequently males (mean age 56, 1 year, 54.3% males). The mean length of follow-up was 9.2 months ranging between 4 and 18 months, respectively. Over the follow-up period, incident ischemic stroke occurred to 4.40 [95% CI: 4.36–4.43] out of 1000 patients with a previous COVID-19 infection. Conversely, the incidence of stroke in the control group was 3.25 [95% CI: 3.21–3.29] out of 1000 patients among controls. Quality assessment showed that all studies were of moderate-high quality according to the NOS scale (Table 1). 21
Table 1.
General characteristics of the population reviewed.
| Authors | Country (year of enrollment) | Study design | Sample size n | Cases n | Controls n | Age (years) | Males n (%) | HT n (%) | DM n (%) | COPD n (%) | CKD n (%) | Obesity n (%) | Pre-existing HF n (%) | Cancer n (%) | Pre-existing Cerebrovascular disease n (%) | FW-length (months) | NOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohen et al. 22 | US (2020) | R | 2,895,943 | 133,366 | 2,762,577 | 75.7 | 1,227,545 (42.0) | 2,081,772 (72.0) | 938,043 (32.7) | 578,650 (20),* | 528,314 (14.0) | 478,902 (17.0) | 334,654 (12.0) | 418,700 (14.4) | 364,782 (13.0) | 4 | 8 |
| Daugherty et al. 23 | US (2020) | R | 9,247,505 | 266,586 | 8,980,919 | 42.4 | 4,640,393 (50.2) | NR | 521,699 (5.6) | NR | NR | NR | NR | NR | NR | 6 | 6 |
| Mizrahi et al. 24 | Israel (2020–2021) | R | 2,113,007 | 320,857 | 1,792,150 | 25.0*** | 148,095 (49.4)*** | 22,490 (7.5)*** | 11,412 (3.8)*** | 1519 (0.5)*** | 4756 (1.6)*** | 63,418 (21.1)*** | NR | 6953 (2.3)*** | NR | 6 | 8 |
| Raisi- Estabragh et al. 25 | UK (2021) | R | 471,227 | 18,564 | 452,663 | 69.0 | 210,730 (44.7) | 157,538 (33.4) | 34,039 (7.2) | NR | NR | NR | 10,260 (2.2) | NR | NR | 4 | 7 |
| Wan et al. 26 | UK (2020) | P | 78,435 | 7139 | 71,296 | 65.8 | 37,744 (48.1) | 27,981 (35.6) | 7976 (10.1) | NR | NR | NR | 2352 (2.9) | NR | 3680 (4.6) | 18 | 8 |
| Wang et al. 27 | US (2020] | R | 2.940.988 | 691,455 | 2,249,533 | 43.8 | 1,241,483 (42.2) | 440,998 (14.9) | 188,488 (6.4)** | 51,592 (1.7) | 59,177 (2.0) | 286,338 (9.7)** | NR | NR | NR | 12 | 7 |
| Xu et al. et al. 28 | US (2020–2021) | R | 5,792,8631 | 154,068 | 5,638,795 | 62.5 | 5,288,431 (90.2) | 1,525,944 (26.3) | 1,321, 907 (22.8) | 633,000 (10.9) | 970, 057 (16.7) | 2.468.617 (42.6) | NR | 357,192 | NR | 12 | 8 |
| Tisler et al. 29 | Estonia (2020–2021) | R | 19,460 | 3949 | 15,511 | 65.1 | 8900 (45.7) | 10,601 (54.4) | 3341 (17.1) | 2205 (13.9),* | 870 (4.4) | 1496 (7.6) | NR | 3998 (20.5) | 578 (2.9) | 12 | 8 |
HT: arterial hypertension; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; HF: heart failure; FW: follow-up; NR: not reported; R: retrospective; P: prospective; NOS: Newcastle-Ottawa quality assessment scale.
Defined as chronic pulmonary disease.
Only DM type 2. R: retrospective.
Referred to propensity matched cohort.
Risk of ischemic stroke after COVID-19 recovery: overall population
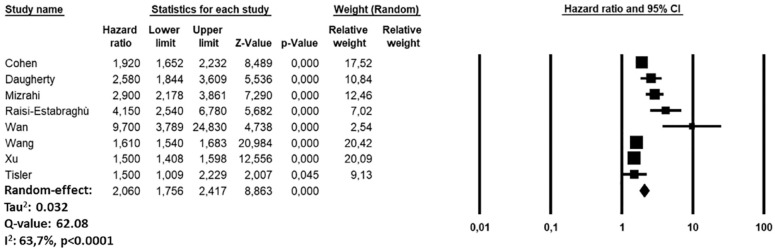
During the follow-up period, recovered COVID-19 patients showed an increased risk of ischemic stroke (HR: 2.06, 95% CI: 1.75–2.41, p < 0.0001, I2 = 63.7%) compared to subjects who did not experience COVID-19 infection but developed ischemic stroke over the same period (Figure 2). The funnel plot is showed in Supplemental Figure 2). The sensitivity analysis confirmed yielded results reporting an HR ranging between 1.95 (95% CI: 1.68–2.26, p < 0.0001, I2:67.5%) and 2.13 (95% CI: 1.80–2.53, p < 0.0001, I2: 67.3%), indicating that the obtained results were not driven by any single study.
Figure 2.
Forest plot investigating the risk of ischemic stroke in COVID-19 recovered patients.
Risk of ischemic stroke after COVID-19 recovery stratified by care setting during the index infection
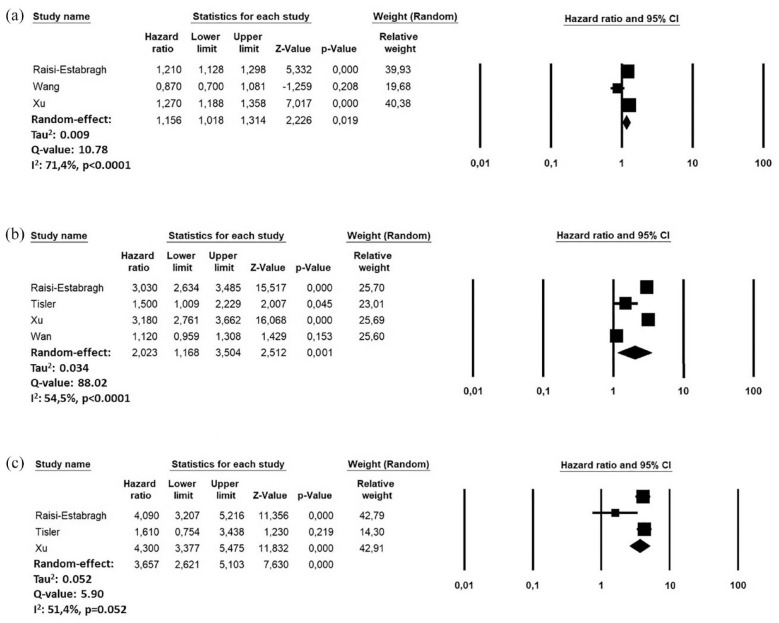
A further sub-analysis revealed an increased risk of incident stroke in patient who have a more severe infection. Indeed, during the follow-up period, the risk of stroke was lower in patients who not required hospitalization at the time of the infection (HR: 1.15, 95% CI: 1.01–1.31, I2: 71.4%; n = 9,205,078)25 –27 compared to those hospitalized (HR: 2.02, 95% CI: 1.16–3.50, I2: 54.5%; n = 9,224,548)25 –27,29 or requiring intensive care admission (HR: 3.65, 95% CI: 2.62–5.10, I2: 51.4: n = 6,283,550)25,26,29(Figure 3).
Figure 3.
Forest plots investigating the risk of ischemic stroke in COVID-19 recovered patients, according to the care setting during the acute phase of the infection: (a) Non-hospitalized, (b) Hospitalized, and (c) Hospitalized ICU.
Meta-regression for the risk of incident ischemic stroke
A meta-regression analysis showed a significant direct relationship for the risk of stroke using age (p = 0.001), HT (p = 00.001) and obesity (p = 0.002) as moderators, while an indirect association was observed when male sex (p = 0.01), and the follow-up length (p = 0.003) were adopted as moderating variables (Table 2).
Table 2.
Meta-regression analysis for the risk of ischemic stroke after COVID-19 infection.
| Items | Coeff. | 95% CI | p |
|---|---|---|---|
| Age (years) | 0.002 | 0.105 to 0.154 | 0.001 |
| Male sex (%) | –0.012 | –0.059 to –0.028 | 0.01 |
| HT (%) | 0.007 | 0.006 to 0.009 | 0.001 |
| DM (%) | –0.016 | –0.039 to 0.006 | 0.16 |
| COPD (%) | –0.005 | –0.029 to 0.019 | 0.69 |
| CKD (%) | –0.015 | –0.051 to 0.019 | 0.37 |
| Obesity (%) | 0.005 | 0.014 to 0.025 | 0.002 |
| Cancer (%) | –0.041 | –0.100 to 0.018 | 0.17 |
| Follow-up length (months) | –0.045 | –0.047 to –0.137 | 0.003 |
CI: confidence interval; HT: arterial hypertension; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease.
Discussion
The present analysis, based on a large population of more than 20 million adult subjects, showed that ischemic stroke occurred in 4.40 per 1000 individuals who had experienced COVID-19 infection as compared to 3.25 per 1000 among individuals without COVID-19. Furthermore, after COVID-19 recovery, subjects had two-fold increased risk of ischemic stroke, especially in the early phase of the follow-up, which vary according to the severity of the index infection, as reflected by the different hazards observed comparing inpatients and outpatients.
The long-term incidence of ischemic stroke was like that observed in previous epidemiological studies base on subjects from the general population for which the crude incidence was approximately 3.29 cases per 1000 person-year. However, most of the epidemiological studies reported the stroke incidence as number of events per years; conversely, our follow-up was shorter, so a direct epidemiological comparison is difficult.
Presented results were associated with a moderate heterogeneity level which was probably multifactorial. Indeed, the different demographical and clinical characteristics of the population revised, the follow-up length and the geographical location of the studies have concurred to the compressive heterogeneity level observed. Meta-regression analysis significantly contributed to define the observed heterogeneity revealing that the risk of stroke was directly influenced by age, female sex, HT, and obesity while it resulted inversely related with the length of the follow-up. These findings are in accordance with previous investigations showing that the risk of stroke during the acute phase of SARS-CoV-2 infection was more likely to occur in older, males and hypertensive patients.30 –33 However, it should be noted that when the death rate is high from causes other than the disease of interest, the incidence rates of the illness are generally overestimated in traditional Kaplan–Meier survival analysis due to existence of competing risks.34,35 Unfortunately, the revised investigations did not systematically report data regarding previous cerebrovascular disease or they onset during the acute phase of the disease, limiting the possibility to perform dedicated sub-analysis. Similarly, the revised investigations did not systematically report data regarding the existence of previous cerebrovascular disease or they onset during the acute phase of the disease, limiting the possibility to perform dedicated sub-analysis.
The exact pathophysiological mechanism underlying the onset of stroke in recovered COVID-19 patients is not yet fully understood. It seems that the resultant persisting inflammation and cytokine storm may lead to platelet, tissue factor and coagulation cascade activation contributing to the onset of cerebrovascular accidents.10,36,37 Moreover, it is also plausible that factors such as hypoxia or hypotension, which have been reported as a part of long-COVID syndrome, may have resulted in acute decompensation of cerebrovascular autoregulation, which was previously preserved in the setting of chronic vascular occlusion or severe stenosis. 38 It is just possible that SARS-CoV-2 endotheliitis may have destabilized a mild pre-existent chronic atherosclerotic plaque which triggered the onset of ischemic cerebrovascular injuries. 39 Moreover, we cannot exclude that some individuals may have experienced symptomatic or asymptomatic atrial fibrillation events, which have been reported as potential SARS-CoV-2 sequelae.24 –26
Over the latest years, several evidence have demonstrated that, in the early period after COVID-19 infection, several acute and chronic cardiovascular conditions may occur, including acute pulmonary embolism, deep vein thrombosis, heart failure, arterial hypertension, atrial fibrillation and diabetes. All these conditions, especially if remain undiagnosed and untreated, may represent concurrent risk factors for the onset of ischemic stroke.2,22 –25,40,41 However, if the onset of these novel cardiovascular disease represents a direct consequence of COVID-19 or if the viral infection may act only as a triggering factor on an unknown clinical condition/predisposition, remains unknown.
Current data may be useful for minimizing the risk ischemic stroke events after hospital discharge in COVID-19 survivors, although our results must be considered preliminary and cannot be directly translated into clinical practice giving recommendations regarding the type and regimen of thrombophylactic strategies. Further studies are needed to determine the potential benefit of therapeutic anticoagulation for the risk of ischemic stroke in recovered COVID-19 patients. Unfortunately, reviewed manuscripts, being based on the assessment of generic risk of clinical consequences after COVID-19 infection, they did not provide any data regarding the location of the cerebrovascular accident or potential chronic anticoagulant treatments during the acute phase or at discharge, limiting our possibility to analyze the potential etiology and/or the impact of such treatment on the long-term risk of ischemic stroke. Therefore, our preliminary results must be carefully interpreted and considered in the light of a hypothesis generating study. Indeed, further larger prospective studies are needed to confirm our findings.
Limitations
Our study has several limitations related to the observational nature of the reviewed studies with all their inherited biases. Potential underestimation on the ischemic stroke incidence and relative risk could derive from the absence of a specific and dedicated follow-up; indeed, reviewed articles obtained the data larger medical records dataset using the relative ICD-10 codes without performing a clinical follow-up. As known, such methodology may be prone to misdiagnosis due to miscoding. Furthermore, only one of the revised studies was prospective; therefore, the retrospective design of the remaining investigations included into the analysis may have partially biased the results. Few investigations have analyzed the risk of ischemic stroke in patients recovered from COVID-19 infection, limiting the number of the studies included in the meta-analysis; however, the number of patients enrolled mitigates, at least partially, this limit. Furthermore, the analyses reviewed did not provide any data regarding the administration of anticoagulant treatment or atrial fibrillation at discharge as well as concerning the location and severity of stroke or administration of thrombolytic drugs. At the same manner, we cannot exclude that sampling bias by the competing risk of death may also have led to the underestimation of the real cumulative incidence of ischemic stroke. Lastly, no data regarding the type and number of vaccinations against SARS-CoV-2 as well as the patients experiencing stroke during the acute phase of the infection were systematically reported, making impossible any type of sub-analysis. Despite these limitations, to the best of our knowledge, this study is the first systematic review and meta-analysis providing a provisional estimation on the incidence and the risk of ischemic stroke in patients who recovered from COVID-19.
Conclusions
Ischemic stroke represents a potential sequela of SARS-CoV-2 infection within 9 months from the acute infection. Further studies are needed to determine the potential benefit of therapeutic anticoagulation for the risk of ischemic stroke in recovered COVID-19 patients.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873231190432 for Risk of ischemic stroke in patients recovered from COVID-19 infection: A systematic review and meta-analysis by Marco Zuin, Maria Mazzitelli, Gianluca Rigatelli, Claudio Bilato and Anna Maria Cattelan in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873231190432 for Risk of ischemic stroke in patients recovered from COVID-19 infection: A systematic review and meta-analysis by Marco Zuin, Maria Mazzitelli, Gianluca Rigatelli, Claudio Bilato and Anna Maria Cattelan in European Stroke Journal
Supplemental material, sj-jpg-3-eso-10.1177_23969873231190432 for Risk of ischemic stroke in patients recovered from COVID-19 infection: A systematic review and meta-analysis by Marco Zuin, Maria Mazzitelli, Gianluca Rigatelli, Claudio Bilato and Anna Maria Cattelan in European Stroke Journal
Acknowledgments
We have no acknowledgments to disclose.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent was not required given the nature of the study as a systematic review and meta-analysis.
Ethical approval: No ethical approval is required given the nature of the study as a systematic review and meta-analysis.
Guarantor: MM.
Contributorship: MZ, GR, MM researched literature, conceived the study, performed data analysis. MZ, GR, performed data acquisition. MZ, MM. MZ, MM wrote the manuscript’s first draft. MM was as guarantor involved in all steps of this clinical study from research idea to conceptualization, data acquisition, and analysis as well as writing the manuscript’s first draft. CB and AMC reviewed and edited the manuscript. All the authors approved the final version of the manuscript.
ORCID iD: Marco Zuin  https://orcid.org/0000-0002-4559-1292
https://orcid.org/0000-0002-4559-1292
Supplemental material: Supplemental material for this article is available online.
References
- 1. Drake TM, Riad AM, Fairfield CJ, et al.; ISARIC4C investigators. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO clinical characterisation protocol UK: a prospective, multicentre cohort study. Lancet 2021; 398: 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zuin M, Rigatelli G, Bilato C, et al. Heart failure as a complication of COVID-19 infection: systematic review and meta-analysis. Acta Cardiol 2022; 77: 107–113. [DOI] [PubMed] [Google Scholar]
- 3. Mensah GA, Vaduganathan M, Roth GA. Acute cardiovascular complications of COVID-19: the high risk of underlying heart disease. J Am Coll Cardiol 2023; 81: 570–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan BWL, Tan BWQ, Tan ALM, et al. Long-term kidney function recovery and mortality after COVID-19-associated acute kidney injury: an international multi-centre observational cohort study. EClinicalMedicine 2022; 55: 101724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polverino F, Stern DA, Ruocco G, et al.; ItaliCO Study Group. Comorbidities, cardiovascular therapies, and COVID-19 mortality: a nationwide, Italian Observational Study (ItaliCO). Front Cardiovasc Med 2020; 7: 585866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nannoni S, de Groot R, Bell S, et al. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke 2021; 16: 137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chavda V, Chaurasia B, Fiorindi A, et al. Ischemic stroke and SARS-CoV-2 infection: the bidirectional pathology and risk morbidities. Neurol Int 2022; 14: 391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aghayari Sheikh Neshin S, Shahjouei S, Koza E, et al. Stroke in SARS-CoV-2 infection: a pictorial overview of the pathoetiology. Front Cardiovasc Med 2021; 8: 649922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Finsterer J, Scorza FA, Scorza CA, et al. Ischemic stroke in 455 COVID-19 patients. Clinics 2022; 77: 100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wijeratne T, Gillard Crewther S, Sales C, et al. COVID-19 pathophysiology predicts that ischemic stroke occurrence is an expectation, not an exception—A systematic review. Front Neurol 2021; 11: 607221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sánchez KE, Rosenberg GA. Shared inflammatory pathology of stroke and COVID-19. Int J Mol Sci 2022; 23: 5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Di Castelnuovo A, Costanzo S, Antinori A, et al. Heparin in COVID-19 patients is associated with reduced In-hospital mortality: the multicenter Italian CORIST study. Thromb Haemost 2021; 121: 1054–1065. [DOI] [PubMed] [Google Scholar]
- 13. McAlpine LS, Zubair AS, Maran I, et al. Ischemic stroke, inflammation, and endotheliopathy in COVID-19 patients. Stroke 2021; 52: e233–e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kakarla V, Kaneko N, Nour M, et al. Pathophysiologic mechanisms of cerebral endotheliopathy and stroke due to Sars-CoV-2. J Cereb Blood Flow Metab 2021; 41: 1179–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zuin M, Rigatelli G, Zuliani G, et al. Age-adjusted D-dimer cutoffs to guide anticoagulation in COVID-19. Lancet 2021; 398: 1303–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li S, Ren J, Hou H, et al. The association between stroke and COVID-19-related mortality: a systematic review and meta-analysis based on adjusted effect estimates. Neurol Sci 2022; 43: 4049–4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang L, Sun W, Wang Y, et al. Clinical course and mortality of stroke patients with coronavirus disease 2019 in Wuhan, China. Stroke 2020; 51: 2674–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Estiri H, Strasser ZH, Brat GA, et al. Evolving phenotypes of non-hospitalized patients that indicate long COVID. BMC Med 2021; 19: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tleyjeh IM, Saddik B, AlSwaidan N, et al. Prevalence and predictors of post-acute COVID-19 syndrome (PACS) after hospital discharge: a cohort study with 4 months median follow-up. PLoS One 2021; 16: e0260568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, et al.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses, https://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf (2012, accessed 29 August 2022).
- 22. Cohen K, Ren S, Heath K, et al. Risk of persistent and new clinical sequelae among adults aged 65 years and older during the post-acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2022; 376: e068414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Daugherty SE, Guo Y, Heath K, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ 2021; 373: n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mizrahi B, Sudry T, Flaks-Manov N, et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. BMJ 2023; 380: e072529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raisi-Estabragh Z, Cooper J, Salih A, et al. Cardiovascular disease and mortality sequelae of COVID-19 in the UK Biobank. Heart 2022; 109: 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wan EYF, Mathur S, Zhang R, et al. Association of COVID-19 with short- and long-term risk of cardiovascular disease and mortality: a prospective cohort in UK Biobank. Cardiovasc Res 2023; 119: 1718–1727. [DOI] [PubMed] [Google Scholar]
- 27. Wang W, Wang CY, Wang SI, et al. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: a retrospective cohort study from the TriNetX US collaborative networks. eClinicalMed 2022; 53: 101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med 2022; 28: 2406–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tisler A, Stirrup O, Pisarev H, et al. Post-acute sequelae of COVID-19 among hospitalized patients in Estonia: nationwide matched cohort study. PLoS One 2022; 17: e0278057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taquet M, Geddes JR, Husain M, et al. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 2021; 8: 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koton S, Schneider ALC, Rosamond WD, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA 2014; 312: 259–268. [DOI] [PubMed] [Google Scholar]
- 32. Luo W, Liu X, Bao K, et al. Ischemic stroke associated with COVID-19: a systematic review and meta-analysis. J Neurol 2022; 269: 1731–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qureshi AI, Baskett WI, Huang W, et al. Acute ischemic stroke and COVID-19: an analysis of 27 676 patients. Stroke 2021; 52: 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke 2020; 51: 2002–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Walraven C, Hawken S. Competing risk bias in Kaplan-Meier risk estimates can be corrected. J Clin Epidemiol 2016; 70: 101–105. [DOI] [PubMed] [Google Scholar]
- 36. Cevik M, Tate M, Lloyd O, et al. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe 2021; 2: e13–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mbonde AA, O'Carroll CB, Grill MF, et al. Stroke features, risk factors, and pathophysiology in SARS-CoV-2-infected patients. Mayo Clin Proc Innov Qual Outcomes 2022; 6: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Esenwa C, Cheng NT, Lipsitz E, et al. COVID-19-associated carotid atherothrombosis and stroke. AJNR Am J Neuroradiol 2020; 41: 1993–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. John S, Kesav P, Mifsud VA, et al. Characteristics of large-vessel occlusion associated with COVID-19 and ischemic stroke. AJNR Am J Neuroradiol 2020; 41: 2263–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zuin M, Rigatelli G, Bilato C, et al. Risk of incident new-onset arterial hypertension after COVID-19 recovery: a systematic review and meta-analysis. High Blood Press Cardiovasc Prev 2023; 30: 227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zuin M, Barco S, Giannakoulas G, et al. Risk of venous thromboembolic events after COVID-19 infection: a systematic review and meta-analysis. J Thromb Thrombolysis 2023; 55: 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873231190432 for Risk of ischemic stroke in patients recovered from COVID-19 infection: A systematic review and meta-analysis by Marco Zuin, Maria Mazzitelli, Gianluca Rigatelli, Claudio Bilato and Anna Maria Cattelan in European Stroke Journal
Supplemental material, sj-docx-2-eso-10.1177_23969873231190432 for Risk of ischemic stroke in patients recovered from COVID-19 infection: A systematic review and meta-analysis by Marco Zuin, Maria Mazzitelli, Gianluca Rigatelli, Claudio Bilato and Anna Maria Cattelan in European Stroke Journal
Supplemental material, sj-jpg-3-eso-10.1177_23969873231190432 for Risk of ischemic stroke in patients recovered from COVID-19 infection: A systematic review and meta-analysis by Marco Zuin, Maria Mazzitelli, Gianluca Rigatelli, Claudio Bilato and Anna Maria Cattelan in European Stroke Journal