Highlights
-
•
MucoUp®, a hyaluronic acid, was used for the first time as a spacer in brachytherapy for uterine cervical cancer.
-
•
The study found that MucoUp® can be safely used in gynecologic brachytherapy to create space between CTVHR and OARs, without causing any significant side effects.
-
•
MucoUp® insertion enabled the median total CTVHR D90 to exceed 80 Gy while achieving dose constraints of OARs.
Keywords: Brachytherapy, Uterine cervical cancer, Spacer, MucoUp®
Abstract
We first used MucoUp®, a hyaluronic acid used in endoscopic resection, as a spacer in brachytherapy. In five cervical cancer patients, MucoUp® insertion increased a 90% dose of the high-risk CTV to over 80 Gy while decreasing the dose of organs at risk. No related adverse events were observed.
Introduction
Spacers can be used in radiation oncology to create a physical space between the clinical target volume (CTV) and the surrounding normal tissues to prevent late radiation-related toxicities while increasing the dose to the CTV [1], [2], [3], [4]. In radiotherapy for prostate cancer, a multi-center prospective clinical trial showed SpaceOAR® (Boston Scientific, Marlborough, MA, USA) reduced rectal irradiation and reduced rectal toxicity [2]. While its use is covered by medical insurance in Japan for prostate radiotherapy, it is not covered for uterine cervical cancer radiotherapy. Kishi et al. first reported the usefulness of hyaluronic acid, Suvenyl® (Chugai Pharmaceutical Co., Tokyo, Japan), which is covered by medical insurance for knee osteoarthritis in Japan, for high-dose rate brachytherapy (HDR-BT) in re-irradiation settings for various anatomical sites such as the head and neck, axilla, skeleton, breast, pelvis, and abdominal wall[1]. Then, another Japanese group reported a series of articles regarding the usefulness of Suvenyl® after obtaining institutional off-label application [5], [6], [7], [8], [9], [10]. However, because viscosupplementation for knee osteoarthritis could not show a clinically meaningful improvement over injecting saline [11], it was announced in January 2023 that Chugai Pharmaceutical Co. would stop the production of Suvenyl®. MucoUp® (Seikagaku Co., Tokyo, Japan) is also made of hyaluronic acid, which is used as a submucosal injectate during endoscopic resection for superficial gastrointestinal tract tumors [12]. While Suvenyl® is categorized as a pharmaceutical agent, MucoUp® is categorized as a medical device, and since it is easier to obtain official approval of an off-label application by the Pharmaceuticals and Medical Devices Agency (PMDA) for medical devices than pharmaceutical agents, we obtained institutional approval of an off-label application of MucoUp® for gynecologic brachytherapy from our hospital. This is the first-in-human experience of MucoUp® as a spacer in brachytherapy for uterine cervical cancer.
Materials and methods
We used MucoUp® for the first five patients who had locally advanced cervical carcinoma without direct invasion of the bladder or rectum (cT4) to create space between CTV and the organs at risk (OARs). Even though there is posterior parametrial involvement towards the uterosacral ligament, if there is no direct tumor invasion to the rectal wall and there is a fine fat tissue that can be visualized as a bright white layer on the transrectal ultrasound (TRUS), we usually do not exclude such patients.
The prior whole pelvic radiotherapy (WPRT) was 45–50 Gy/25–28 fractions, with no center shielding. For each time of external radiation, 350 ml of drinking water and an hour of urine storage were used to minimize the inter-fraction movement of the uterine and reduce the unneeded irradiation for the bowel. Patients with a tumor size of > 4 cm or N1 and no renal impairment had concurrent chemotherapy with 5–6 cycles of weekly Cisplatin (wCDDP) 40 mg/m2. Pelvic examinations were performed once a week to assess the tumor response. Informed consent was acquired for brachytherapy, sedation, and the use of MucoUp®. One week before brachytherapy, a pelvic Magnetic Resonance Imaging (MRI) examination was done to assess the residual gross tumor volume before brachytherapy.
Cefmetazole 2 g/100 ml was administered one hour before brachytherapy for antibacterial. Anapeine 7 mg/20 ml was used to give sacral anesthesia in the prone position. Sedatives and analgesics were given while patients were placed in the lithotomy position (Midazolam 3–5 mg /3–5 ml, Atarax-P 25 mg/ 50 ml, and Fentanyl 0.2 mg/ 20 ml at 1–2 ml/hour). The bladder was injected with 100 ml of saline.
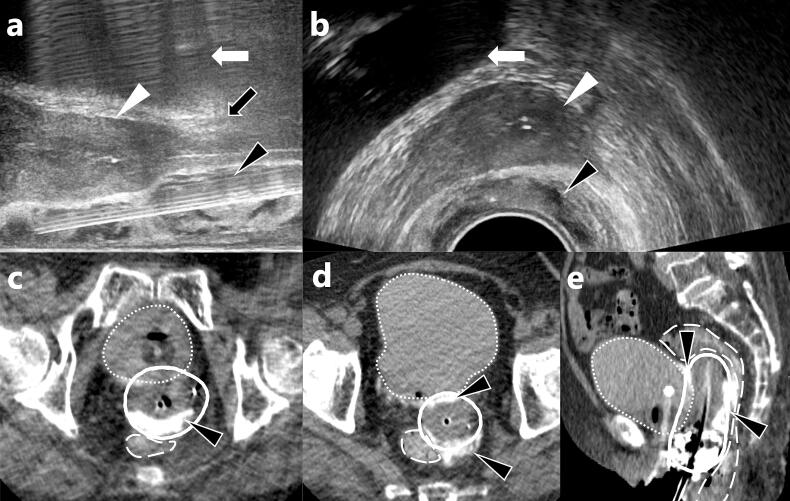
First, an eighteen-gauge needle (Create Medic Co., Ltd., Kanagawa, Japan) was inserted between the anterior vaginal wall and bladder (vesicovaginal septum) with TRUS guidance, taking care not to damage the urethra and bladder wall. MucoUp® 5–10 ml was injected after hydrodissection with the saline solution to confirm the correct anatomical location (Fig. 1).
Fig. 1.
Figures (a) and (b) show axial and sagittal views of transrectal ultrasound during MucoUp® insertion at the rectal side. MucoUp® (black arrowhead) is inserted between the rectum and cervical cancer (white arrowhead). The bladder is indicated by a white arrow, and the vagina by a black arrow. Figures (c, d) and (e) show axial and sagittal CT images after the insertion of MucoUp® and brachytherapy applicators. MucoUp® (black arrowhead) is mixed with contrast enhancement; consequently, it is visualized as a white layer both anteriorly and posteriorly to the uterus. The emphasized white line represents the 6 Gy isodose line. Due to MucoUp®, the 6 Gy isodose line does not cross the outlines of the bladder (dotted line) and rectum (long-dashed line).
Next, a needle was inserted between the posterior wall of the vagina and rectum (rectovaginal septum), and 10–30 ml of MucoUp® was inserted in the same way under TRUS guidance to avoid damaging the anterior rectal mucosa and the small intestine or sigmoid colon beyond the peritoneum membrane.
Following MucoUp® insertion, tandem, ovoid, or cylinder applicators were positioned: selection of the vaginal applicator whether ovoid or cylinder was according to the extent of vaginal wall invasion. Additionally, we packed gauze into the anterior and posterior sides of the ovoid, according to a cadaver study showing that the maximum dose reduction could be achieved by the combination of the hydrogel spacer and gauze packing [13].
In patients with parametrial invasion, needles for interstitial brachytherapy were inserted transperineally and implanted in the tumor with TRUS guidance. When adequate attention is paid to avoiding air bubble contamination in the preparation of the spacer material, TRUS images are usually unaffected, and it is possible to insert additional interstitial needles after spacer injection.
Planning CT was taken after the insertion of spacer gel and the brachytherapy applicators with patients in the lithotomy position. MucoUp® was combined with a contrast agent (3 ml of contrast agent was mixed with 20 ml of MucoUp®) to visualize itself on CT. Due to the leaking of the contrast agent over time, planning CT images are advised to be taken within 30 min of the insertion of MucoUp®.
The bladder, rectum, sigmoid colon, and small intestine were contoured as OARs, and a high-risk clinical target volume (CTVHR) was contoured referring to MRI taken immediately before brachytherapy, physical examination findings taken during brachytherapy, and TRUS findings. Dose calculations were performed in Oncentra Brachy (Elekta Solutions AB, Stockholm, Sweden). We planned for a 90% dose of CTVHR (CTVHR D90) to be 80–85 Gy or higher in total equivalent dose in 2 Gy fractions (EQD2) based on planning CT images. The total dose tolerance limits of OARs were 75 Gy to the rectum, 90 Gy to the bladder, 75 Gy to the sigmoid colon, 70 Gy to the small bowel in EQD2.
If enough doses could be supplied to CTVHR D90 while adhering to the OARs’ dose restrictions, brachytherapy could be conducted three or four times with a single fraction dose of 6 Gy. In one case, the patient strongly requested to terminate the treatment after the second brachytherapy session, therefore, the second brachytherapy dose was set at 9.6 Gy. Adverse events during chemoradiotherapy, brachytherapy, and spacer injection were evaluated with Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. In addition, we examined the actual volume of MucoUp® inserted and determined the maximum separation distance between the rectum/bladder wall and the tumor formed by MucoUp® insertion using planning CT during brachytherapy.The study was approved by the medical ethics management committee of our hospital on January 27, 2023.
Results
Between February 2 and March 16, 2023, five patients were inserted as the first-in-human study. The patients' background is summarized in Table 1.
Table 1.
Patients' backgrounds, inserted volume of MucoUp®, and the total dose to each clinical target volume and organs at risk.
| Patient No. | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age (years old) | 66 | 75 | 79 | 78 | 58 |
| Body Mass Index | 22.9 | 25.4 | 23.6 | 23.2 | 20.6 |
| TNM, Stage (FIGO 2018) | T3aN0M0, IIIA | T2bN0M0, IIB | T2bN0M0, IIB | T2bN1M0, IIIC1 | T2bN1M0, IIIC1 |
| Initial tumor diameter (mm), CTVHR volume at the first brachytherapy (ml) | 61, 89 | 27, 42 | 43, 16 | 44, 31 | 57, 41 |
| Whole pelvic irradiation (Gy/fractions) | 50.4/28 | 45/25 | 45/25 | 45/25 | 45/25 |
| MucoUp® volume (bladder/rectal side) | 5 ml/15–30 ml | 8–9 ml/15 ml | 5–8 ml/15–22 ml | 8–10 ml/14–15 ml | 10 ml/30 ml |
| Total CTVHR D90 (Gy) | 80 | 74.8 | 81.8 | 82.3 | 80.2 |
| Total Rectum D2cc (Gy) | 64.2 | 64.8 | 64.9 | 59.4 | 62.5 |
| Total Bladder D2cc (Gy) | 73.1 | 69.9 | 72.5 | 64.5 | 70.8 |
CTVHR = a high-risk clinical target volume.
The median age was 75 years (58–79 years), Eastern Cooperative Oncology Group Performance Status (ECOG PS) was 0 in 4 patients and 1 in 1, and the median Body Mass Index was 23.2 (20.6–25.4).
T classification was T2b in 4 patients and T3a in 1, N classification was N1 in 2 patients and N0 in others, and M0 in all patients; 2018 FIGO Staging Classification was IIB in 2 patients, IIIA in 1, and IIIC1 in 2. The median size of the primary tumor at the first visit was 44 mm (27–61 mm).
Regarding external irradiation prior to brachytherapy, 4 patients underwent total pelvic irradiation of 45 Gy/25 fractions (3DCRT) and 1 of 50.4 Gy/28 fractions (IMRT). Two patients with positive lymph nodes received irradiation boosts of 14–14.4 Gy/7–8 fractions to the positive lymph nodes. Weekly Cisplatin 40 mg/m2 for 6 cycles in 1 patient and 5 cycles in 3 was used as concurrent chemotherapy. One patient only received 4 cycles of wCDDP due to Grade 3 diarrhea. Four patients completed brachytherapy in three sessions, and one in two sessions as mentioned before. One patient experienced an antimicrobial-induced Grade 3 maculopapular skin rash that delayed the third session of brachytherapy.
MucoUp® insertion took about 15 min for both bladder and rectal sides in all patients, and the total brachytherapy time including MucoUp® insertion was close to 2 h. The median insertion volumes of MucoUp® were 8 ml (5–10 ml) on the bladder side and 15 ml (14–30 ml) on the rectal side. The median maximum separation distances by MucoUp® were 7 mm (5–10 mm) between the anterior vaginal wall and the bladder wall, and 8 mm (5–19 mm) between the posterior vaginal wall and the rectal wall on the planning CT.
The target volume and OARs doses for each session and total doses combined with external irradiation were on the blow; the median CTVHR D90 was 11.3 Gy (9.4–18.5 Gy) for each session and 80.2 Gy (74.8–82.3 Gy) for total dose, the median Rectum D2cc was 6.6 Gy (4.6–13.0 Gy) and 64.3 Gy (59.4–64.9 Gy), the median Bladder D2cc was 7.9 Gy (6.0–18.9 Gy) and 70.9 Gy (64.5–73.1 Gy), the median Sigmoid colon D2cc was 3.7 Gy (0.4–5.5 Gy) and 54.2 Gy (46.2–57.2 Gy), and the median small bowel D2cc was 2.6 Gy (1.5–7.1 Gy) and 48.4 Gy (43.2–56.9 Gy). The OARs' dose constraints were achieved in all patients.
Regarding adverse events related to brachytherapy, one patient had a vaginal laceration that did not require sutures and continued bleeding from the tumor that was controlled by pressure hemostasis, which may be caused by chemotherapy-related grade 2 thrombocytopenia. No other adverse events were noted. And there were no specific adverse effects associated with MucoUp® injection.
Discussion
In 2021, Potter et al. reported results from a prospective multi-institutional observational study of Image-Guided Adaptive Brachytherapy (IGABT) for uterine cervical cancer involving over one thousand patients, in which 5-year local control was as good as 90% regardless of T stage when > 85 Gy was delivered to the high-risk CTV D90 (CTVHR D90) [14]. However, these favorable results were at the cost of severe late radiation-related toxicities as much as 14.6% of patients experienced grade 3–5 late radiation-related toxicities. In additional information provided by a response to the letter to the editor, it was shown that dose constraint observation rate for rectum D2cc < 70 Gy in 84% and < 65 Gy in 64.2% in the EMBRACE-I study [15], showing that substantial percentage of patients could not observe the dose constraint even though 43% of patients used IC/IS technique [14]. In contrast, by combining spacers with interstitial implantations, it was possible to achieve dose constraints in all cases in this study.
In contrast in our country, because the Japanese treatment guidelines recommend the application of central shielding in the latter part of WPRT [16], the rate of late radiation-related toxicities was lower than that of Potter et al. Though some of them still showed excellent local control, local controls of which were slightly worse [17], [18], [19], [20], [21], supposedly due to the lower total dose goal for CTVHR D90 [22]. In such circumstances, the authors think that gel spacers can play an important role in delivering CTVHR D90 high dose while keeping dose constraints within the recommendations of the gynecological (Gyn) GEC-ESTRO working group [10], [23], [24].
Because SpaceOAR® was already approved by Japanese medical insurance for prostate radiotherapy, the authors requested that Boston Scientific conduct a clinical trial to expand the indication of SpaceOAR® for gynecologic radiotherapy. However, it was rejected due to the high costs of conducting such a clinical trial. As mentioned earlier, it was announced that Suvenyl® would not be produced soon because it was shown that injecting hyaluronic acid into the knee joint did not improve clinical outcomes compared to a saline injection [11]. MucoUp®, a compound that is used to lift the submucosal tissue during endoscopic resection for superficial malignancies in the gastrointestinal tract, has the same ingredient as Suvenyl®; though, according to the package insert, while the molecular weight of Suvenyl® ranges from 1500 to 3900 kDa, that of MucoUp® is 500 to 1200 kDa.
In addition, MucoUp® is easier to expand the indication of medical insurance for medical devices than pharmaceutical agents in Japan. Because MucoUp® is categorized as a medical device compared to Suvenyl® which is categorized as a pharmaceutical agent, we obtained off-label application approval from our hospital for using MucoUp® during gynecological brachytherapy and reported the clinical outcomes of the initial five patients who received MucoUp® injection during brachytherapy. To the best of our knowledge, this is the first report on using MucoUp® as a spacer during brachytherapy for gynecologic malignancies.
The procedure was the same as SpaceOAR® for prostate cancer, which can be carried out safely in each session. Unlike SpaceOAR®, which remains for 2–3 months, the hyaluronic acid gel with modest molecular weight is absorbed within a few days, making it suitable for brachytherapy because even if it is injected into the wrong anatomic sites, it will not cause any harm. Therefore, MucoUp® injections should be performed in every brachytherapy. If the spacer is injected into an appropriate position [7], it is possible to expect the same effects.
Usually, it is possible to insert TRUS up to the level of the peritoneal reflection. Therefore, it is possible to create a space cranially until the rectum enters the peritoneal cavity. However, when the needle is inserted into the peritoneal cavity, the spacer material dissolves in the peritoneum, and a meaningful space cannot be obtained only with 20–40 ml of hydrogel. In such a case, artificial ascites injection can be used to create a space higher than the peritoneal reflection [25].
On the other hand, possibly due to its modest molecular weight, it appeared to have a lesser capacity to expand the distance between the target volume and the OARs than Suvenyl®. In this study, the average dose of CTVHR D90 was 80 Gy, which is a moderately high dose, although it did not reach the recommendation of > 85 Gy. Due to the logistical problem, we do not perform MRI-based IGABT but CT-based IGABT. As reported by Viswanathan et al., CT-based CTVHR usually overestimates the volume, especially in the lateral direction [26]. Therefore, CTVHRD90 85 Gy in CT-based IGABT is much higher than 85 Gy in MRI-based IGABT. As such, ASTRO guidelines do not recommend prescribing > 85 Gy for all cases and recommend > 80 Gy for ≤ 4 cm residual disease, partly because CT-based IGABT is more popular in the United States [27]. Therefore, the average dose of CTVHR D90 80 Gy in CT-based IGABT could be much higher in MRI-IGABT.
As mentioned above, due to logistical problems, we do not perform MRI-based IGABT. As such, it was necessary to mix contrast enhancement agents to visualize the location of the MucoUp®. However, it is conceivable that MucoUp® may be visible without the use of contrast agents in MRI imaging. Therefore, the evaluation of the space and the assessment of MucoUp® could potentially differ or remain consistent between CT- and MRI-based planning. This discrepancy or similarity in assessment warrants further investigation and should be addressed in future studies to gain a better understanding of the implications and potential solutions for this issue.
It is possible that because most patients included in this study received three-dimensional conformal radiation therapy (3DCRT) with the four-field box technique, not IMRT, it might be possible to expect better gastrointestinal toxicities with better dosimetry. In the near future, when we can afford adequate human resources, such as a sufficient number of radiation oncologists or medical physicists, we are willing to shift all the EBRT components to IMRT. However, even with IMRT, because an adequate margin is usually added around the clinical target volume to compensate for the inter-fraction and intra-fraction organ movements, whether EBRT technique is 3DCRT or IMRT, its influence on total rectal D2cc or bladder D2cc is not significant compared to the dose contribution from brachytherapy. Therefore, we believe that the key to reducing radiation exposure to the bladder and rectum lies in the sophisticated delivery of brachytherapy doses through the use of a gel spacer. According to the findings reported by Vittrup et al., based on the long-term late severe toxicity analysis from the EMBRACE-I study [28], it is advised that the total dose of pelvic external beam radiation therapy (EBRT) should be 45 Gy, rather than 50 Gy. This lower pelvic EBRT dose recommendation aims to reduce the risk of late severe gastrointestinal toxicities, such as late diarrhea and pelvic bone fractures. Additionally, maintaining the pelvic EBRT dose at 45 Gy allows for the potential of brachytherapy dose escalation, which can further optimize treatment outcomes. These recommendations underscore the importance of balancing treatment efficacy and toxicity management in pelvic radiation therapy.
This initial report solely serves as a first-in-human study, demonstrating the feasibility of MucoUp® as a spacer for gynecological brachytherapy; therefore, no data regarding local control or late toxicity was demonstrated. While it provides valuable insights into the potential applications of MucoUp® as a spacer, it does not provide conclusive evidence regarding its long-term effectiveness or address the specific patient population for whom spacer injection may be inappropriate. The determination of the long-term efficacy of the spacer and the patient’s eligibility for spacer injection requires further investigation and should be addressed in future studies. These subsequent studies will help establish the guidelines and criteria for identifying patients who may not be suitable candidates for spacer injection in gynecological brachytherapy, while also evaluating the overall effectiveness of the technique. We are planning to confirm the long-term adverse events and to report on dosimetry and effectiveness for a larger number of patients. The authors hope that this article will serve as a foundation for future multicenter prospective clinical trials for expanding the indication of Mucoup® for brachytherapy in the future, in which the bladder and rectal D2cc and late genitourinary and gastrointestinal toxicities would be the endpoints. Prior to conducting a randomized trial, it is preferable to conduct a multicenter phase II study, and preparations are currently underway to be approved for medical insurance.
Declaration of generative AI and AI-assisted technologies in the writing process.
During the preparation of this work the author(s) used [ChatGPT] in order to correct and sophisticate the English sentences. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Funding
The article receives no funding to support.
Author contribution
NM conceived the idea for this manuscript, and YM wrote the draft of the manuscript. YM, NM, TK, SS, JT, MO, YK, TK, TH, KF, YT, and NS contributed to patient treatment and data collection. NM reviewed and revised the manuscript. Finally, YM, NM, TK, SS, JT, MO, YK, TK, TH, KF, YT, and NS read the manuscript and approved it.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Yoichi Muramoto, Email: y.muramoto.kq@juntendo.ac.jp.
Naoya Murakami, Email: n.murakami.zi@juntendo.ac.jp.
References
- 1.Kishi K., Sonomura T., Shirai S., Sato M., Tanaka K. Critical organ preservation in reirradiation brachytherapy by injectable spacer. Int J Radiat Oncol Biol Phys. 2009;75(2):587–594. doi: 10.1016/j.ijrobp.2009.03.072. [DOI] [PubMed] [Google Scholar]
- 2.Mariados N., Sylvester J., Shah D., Karsh L., Hudes R., Beyer D., et al. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015;92:971–977. doi: 10.1016/j.ijrobp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Serizawa I., Kusano Y., Kano K., Shima S., Tsuchida K., Takakusagi Y., et al. Three cases of retroperitoneal sarcoma in which bioabsorbable spacers (bioabsorbable polyglycolic acid spacers) were inserted prior to carbon ion radiotherapy. J Radiat Res. 2022;63:296–302. doi: 10.1093/jrr/rrac002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viswanathan A.N., Damato A.L., Nguyen P.L. Novel use of a hydrogel spacer permits reirradiation in otherwise incurable recurrent gynecologic cancers. J Clin Oncol. 2013;31(34):e446–e447. doi: 10.1200/JCO.2012.47.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kashihara T., Murakami N., Tselis N., Kobayashi K., Tsuchida K., Shima S., et al. Hyaluronate gel injection for rectum dose reduction in gynecologic high-dose-rate brachytherapy: initial Japanese experience. J Radiat Res. 2019;60:501–508. doi: 10.1093/jrr/rrz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murakami N., Shima S., Kashihara T., Tselis N., Kato T., Takagawa Y., et al. Hyaluronic gel injection into the vesicovaginal septum for high-dose-rate brachytherapy of uterine cervical cancer: an effective approach for bladder dose reduction. J Contemp Brachytherapy. 2019;11(1):1–7. doi: 10.5114/jcb.2019.82612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iijima K., Murakami N., Nakamura S., Nishioka S., Chiba T., Kuwahara J., et al. Configuration analysis of the injection position and shape of the gel spacer in gynecologic brachytherapy. Brachytherapy. 2021;20(1):95–103. doi: 10.1016/j.brachy.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Murakami N., Nakamura S., Kashihara T., Kato T., Shibata Y., Takahashi K., et al. Hyaluronic acid gel injection in rectovaginal septum reduced incidence of rectal bleeding in brachytherapy for gynecological malignancies. Brachytherapy. 2020;19(2):154–161. doi: 10.1016/j.brachy.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi R., Murakami N., Chiba T., Okuma K., Inaba K., Takahashi K., et al. Effect of Hyaluronate Acid Injection on Dose-Volume Parameters in Brachytherapy for Cervical Cancer. Advances. Radiat Oncol. 2022;7(3):100918. doi: 10.1016/j.adro.2022.100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakami N., Okuma K., Kato T., Igaki H. Now is it time to implement spacers in cervical cancer brachytherapy? J Radiat Res. 2022;63(4):696–698. doi: 10.1093/jrr/rrac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira T.V., Juni P., Saadat P., Xing D., Yao L., Bobos P., et al. Viscosupplementation for knee osteoarthritis: systematic review and meta-analysis. BMJ. 2022;378 doi: 10.1136/bmj-2022-069722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kishihara T., Chino A., Uragami N., Yoshizawa N., Imai M., Ogawa T., et al. Usefulness of sodium hyaluronate solution in colorectal endoscopic mucosal resection. Dig Endosc. 2012;24(5):348–352. doi: 10.1111/j.1443-1661.2012.01244.x. [DOI] [PubMed] [Google Scholar]
- 13.Damato A.L., Kassick M., Viswanathan A.N. Rectum and bladder spacing in cervical cancer brachytherapy using a novel injectable hydrogel compound. Brachytherapy. 2017;16(5):949–955. doi: 10.1016/j.brachy.2017.04.236. [DOI] [PubMed] [Google Scholar]
- 14.Pötter R., Tanderup K., Schmid M.P., Jürgenliemk-Schulz I., Haie-Meder C., Fokdal L.U., et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. Lancet Oncol. 2021;22(4):538–547. doi: 10.1016/S1470-2045(20)30753-1. [DOI] [PubMed] [Google Scholar]
- 15.Vittrup A.S., Spampinato S., Jensen N.B.K., Tanderup K., Kirchheiner K., Pötter R., et al. In Reply to Murakami et al. Int J Radiat Oncol Biol Phys. 2023;116(4):964–965. doi: 10.1016/j.ijrobp.2023.03.070. [DOI] [PubMed] [Google Scholar]
- 16.Ebina Y., Mikami M., Nagase S., Tabata T., Kaneuchi M., Tashiro H., et al. Japan Society of Gynecologic Oncology guidelines 2017 for the treatment of uterine cervical cancer. Int J Clin Oncol. 2019;24(1):1–19. doi: 10.1007/s10147-018-1351-y. [DOI] [PubMed] [Google Scholar]
- 17.Aoshika T., Noda S.-e., Abe T., Kumazaki Y.u., Hirai R., Igari M., et al. Results of computer tomography-based adaptive brachytherapy in combination with whole-pelvic- and central-shielding-external beam radiotherapy for cervical cancer. Brachytherapy. 2022;21(6):783–791. doi: 10.1016/j.brachy.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Kusada T., Toita T., Ariga T., Kudaka W., Maemoto H., Makino W., et al. Definitive radiotherapy consisting of whole pelvic radiotherapy with no central shielding and CT-based intracavitary brachytherapy for cervical cancer: feasibility, toxicity, and oncologic outcomes in Japanese patients. Int J Clin Oncol. 2020;25(11):1977–1984. doi: 10.1007/s10147-020-01736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murakami N., Ando K., Murata M., Murata K., Ohno T., Aoshika T., et al. An Asian multi-national multi-institutional retrospective study comparing intracavitary versus the hybrid of intracavitary and interstitial brachytherapy for locally advanced uterine cervical carcinoma. J Radiat Res. 2022;63(3):412–427. doi: 10.1093/jrr/rrac014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami N., Watanabe M., Uno T., Sekii S., Tsujino K., Kasamatsu T., et al. Phase I/II prospective clinical trial for the hybrid of intracavitary and interstitial brachytherapy for locally advanced uterine cervical cancer. J Gynecol Oncol. 2022;34(3) doi: 10.3802/jgo.2023.34.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohno T., Noda S.E., Okonogi N., Murata K., Shibuya K., Kiyohara H., et al. In-room computed tomography-based brachytherapy for uterine cervical cancer: results of a 5-year retrospective study. J Radiat Res. 2017;58:543–551. doi: 10.1093/jrr/rrw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami N., Okuma K., Takahashi A., Kato T., Igaki H. Is it time to modify the Japanese Uterine Cervical Cancer Guidelines to recommend a higher dose for radio-resistant tumors? Jpn J Clin Oncol. 2022;53(2):179–181. doi: 10.1093/jjco/hyac171. [DOI] [PubMed] [Google Scholar]
- 23.Pötter R., Haie-Meder C., Limbergen E.V., Barillot I., Brabandere M.D., Dimopoulos J., et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78(1):67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Pötter R., Tanderup K., Kirisits C., de Leeuw A., Kirchheiner K., Nout R., et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol. 2018;9:48–60. doi: 10.1016/j.ctro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karube M., Murakami N., Okamoto H., Okuma K., Kashihara T., Takahashi K., et al. Transvaginal artificial ascites infusion as a spacer in gynecological brachytherapy: a novel technique. J Contemp Brachytherapy. 2020;12(5):487–491. doi: 10.5114/jcb.2020.100382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viswanathan A.N., Dimopoulos J., Kirisits C., Berger D., Pötter R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68(2):491–498. doi: 10.1016/j.ijrobp.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 27.Chino J., Annunziata C.M., Beriwal S., Bradfield L., Erickson B.A., Fields E.C., et al. Radiation Therapy for Cervical Cancer: Executive Summary of an ASTRO Clinical Practice Guideline. Pract Radiat Oncol. 2020;10(4):220–234. doi: 10.1016/j.prro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vittrup A.S., Kirchheiner K., Pötter R., Fokdal L.U., Jensen N.B.K., Spampinato S., et al. Overall Severe Morbidity After Chemo-Radiation Therapy and Magnetic Resonance Imaging-Guided Adaptive Brachytherapy in Locally Advanced Cervical Cancer: Results From the EMBRACE-I Study. Int J Radiat Oncol Biol Phys. 2023;116(4):807–824. doi: 10.1016/j.ijrobp.2023.01.002. [DOI] [PubMed] [Google Scholar]