Abstract
The current treatment of patients with human epidermal growth factor receptor 2 (HER2)-positive advanced breast cancer (ABC) has been greatly impacted in the past decade by the introduction of antibody–drug conjugates (ADCs), which represent a relatively novel therapeutic class with the peculiar ability to deliver otherwise overtly toxic chemotherapeutics to tumor sites by exploiting the specificities of monoclonal antibodies. Indeed, drug engineering refinements in ADC design, such as through the introduction of cleavable linkers and hydrophobic payloads, resulted in improved patient outcomes in recent years. Two different ADCs, namely trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd), have already entered clinical practice for the treatment of HER2-positive ABC. In this scenario, T-DXd has shown to portend better survival outcomes compared to T-DM1, while leaving a large unsought area of unmet medical need upon T-DXd failure. Treatment decision and benefit of cancer drugs following T-DXd still represent an area of clinical controversy, where a preclinical investigation and clinical development should be prioritized. As the pace of innovation is currently accelerating, and with novel ADC formulations advancing in early-phase clinical trials, the whole BC field is changing at an unprecedented rate, with potential broadenings of therapeutic indications. In this review, we present the clinical landscape of HER2-positive advanced BC and discuss our vision on how to tackle T-DXd resistance, providing a perspective on the priority areas of the cancer research in this setting.
Key words: antibody–drug conjugates, breast cancer, HER2, trastuzumab deruxtecan, resistance
Highlights
-
•
Three different ADCs have been granted regulatory approval in metastatic BC (mBC).
-
•
T-DXd has demonstrated superior clinical benefit in HER2-positive mBC in the second-line setting.
-
•
Optimal sequential therapies after T-DXd currently represent an important area of (pre-)clinical investigation.
-
•
Refined ADC constructs and novel clinical trial designs may portend improved outcomes in mBC in the post-T-DXd setting.
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer in women, as well as the leading cause of death from cancer.1 While most BC diagnoses are made at an early disease stage, which is associated with a 5-year survival probability of 96% in Europe,2 advanced BC (ABC) remains an incurable disease. BC is a highly heterogeneous disease with different clinical behaviors and molecular drivers, necessitating complex and various therapeutic approaches based on the elucidation of biological underpinnings.3 At a glance, BC can be subclassified based on hormone receptor (HR) expression, namely estrogen (ER) and progesterone receptors (PgR), and human epidermal growth factor receptor 2 (HER2) overexpression and/or gene amplification assessed by immunohistochemistry (IHC) and in situ hybridization (ISH).4, 5, 6 Such biological characterization is essential to define BC patients’ prognosis, as well as to guide therapeutic decision making.7
Deeper knowledge in BC biology coupled with innovations in drug development and clinical research led to the introduction of highly active agents with a clear impact on patients’ survival and quality of life.7 In this scenario, the current anticancer therapeutic armamentarium has been substantially and greatly expanded in the past 10-15 years, thanks to the remarkable clinical results obtained with newer antibody–drug conjugates (ADCs). Indeed, while earlier works in the 1980s had been largely disappointing,8,9 refined ADC designs led to the identification of clinically active compounds with more favorable toxicity profiles,10,11 ultimately leading to the regulatory approval of five different ADCs for the treatment of solid malignancies (Table 1) and >140 ADCs in clinical development.12
Table 1.
List of currently approved antibody–drug conjugates for the treatment of solid malignancies
| Target | Indication | Payload | Mechanism of action | DAR | Linker | Payload t1/2 | Cycle dose | |
|---|---|---|---|---|---|---|---|---|
| Trastuzumab emtansine | HER2 | Breast cancer | DM1 emtansine | Tubulin inhibitor | 3.5 | Non-cleavable | NA | 3.6 mg/kg |
| Trastuzumab deruxtecan | HER2 | Breast, gastric, non-small-cell lung cancer | Exatecan derivative | Topoisomerase inhibitor | 8 | Cleavable | 5.8 days | 5.4 mg/kg |
| Sacituzumab govitecan | TROP2 | Breast, urothelial cancer | SN38 | Topoisomerase inhibitor | 7.6 | Cleavable | 0.75 days | 20 mg/kg |
| Enfortumab vedotin | Nectin-4 | Urothelial cancer | MMAE | Tubulin inhibitor | 4 | Cleavable | 2.4 days | 3.75 mg/kg |
| Tisotumab vedotin | Tissue factor | Cervical cancer | MMAE | Tubulin inhibitor | 4 | Cleavable | NA | 2 mg/kg |
| Mirvetuximab soravtansine | Folate receptor alpha | Ovarian cancer | DM4 | Tubulin inhibitor | 3.4 | Cleavable | NA | 6 mg/kg |
DAR, drug-to-antibody ratio; DM1/DM4, maytansinoids; HER2, human epidermal growth factor receptor 2; MMAE, Monomethyl auristatin E; NA, not available; t1/2, half-life; TROP2, trophoblast cell surface antigen 2.
The remarkable clinical results obtained with novel ADCs have represented a turning point in the BC field, impacting current therapeutic algorithms across BC subtypes. Three ADCs, namely trastuzumab emtansine (T-DM1), trastuzumab deruxtecan (T-DXd), and sacituzumab govitecan (SG), got regulatory approval (Figure 1), with most indications in the HER2-positive ABC scenario.13,14 Taking into account the fast-evolving clinical scenario as well as the superiority of T-DXd over T-DM1 in the HER2-positive ABC scenario,15 the ideal management of patients progressing on T-DXd remains an unsought area of clinical investigation. Herein, we describe the current clinical landscape for the treatment of HER2-positive ABC, with a particular focus on the post-T-DXd setting.
Figure 1.
Chronologicaloutline of FDA approvals of ADCs for the treatment of breast cancer. ADC, antibody–drug conjugate; FDA, Food and Drug Administration; HER2, human epidermal growth factor receptor 2; mBC, metastatic breast cancer; pts, patients; ChT, chemotherapy; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan; TNBC, triple-negative breast cancer; SG, sacituzumab govitecan; eBC, early breast cancer. Created with BioRender.com.
Antibody–Drug Conjugates: Structural Principles
ADCs have been designed with the aim of obtaining higher therapeutic indexes by delivering cytotoxic agents active in the nanomolar range specifically to tumor cells.16,17 In principle, it is obtained by exploiting the target specificity of monoclonal antibodies (mAbs), thus reducing off-target toxicities.18 Structurally, ADCs are composed of a mAb, a linker, and cytotoxic payloads. Each of these components is crucial in determining pharmacokinetic (PK) and pharmacodynamic (PD) profiles, impacting clinical efficacy.19
Antibody
The IgG1 backbone confers specificity, typically for tumor-associated antigens (TAAs), including HER2 and trophoblast cell surface antigen 2 (TROP2), and it can retain complement fixation and immune cell engagement via Fcγ receptors (FcγRs). Given that TAAs are typically just being overexpressed in tumor tissues, with some levels of expression being retained in other non-malignant tissues, their differential expression mainly determines ADC specificity, hence their toxicity and efficacy profiles.16 In addition, ADC activity is also greatly impacted by target heterogeneity, turnover rate, as well as intracellular lysosomal modifications. ‘Ideal’ cancer targets are represented by functionally oncogenic proteins, with widespread and selective cancer overexpression, to potentially act upon a growth signaling switch and to reduce negative selection pressure, with limited intratumoral heterogeneity, to minimize ‘on-target off-tumor’ and ‘off-target off-tumor’ toxicities, while maximizing anticancer activity.16 Interestingly, novel ADC designs utilizing dual targeting (i.e. biparatropic or bispecific), or targeting tumor-specific antigens represent an expanding area of drug development.20
Linker
The function of the linker is at least dual: avoiding plasmatic payload detachment, while promoting cytotoxic drug delivery in tumor tissues. To date, there are two main types of molecular linkers: non-cleavable (NC; e.g. thioether or maleimidocaproyl, MC) or cleavable (C; e.g. disulfide, dipeptide, hydrazone, as well as MC-containing). In terms of ADC processing, the main difference between NC and C linkers is that the former require whole ADC degradation in late endosomes or lysosomes resulting in a payload–linker complex, whereas the latter are also degraded by proteolytic enzymes (i.e. cathepsins), an acidic or reducing microenvironment.19 A functional property of NC linkers is their higher plasma stability, resulting in altered PK and tolerability. Of note, only relatively small molecules with discrete distance between the pharmacophore and the conjugation site can tolerate thioether-based modifications needed for the formation of NC linkers.12
Payload
Among the six ADCs currently approved for the treatment of solid tumors (Table 1), all of them utilize a chemotherapeutic agent as payload. While early ADC formulations utilizing standard cytotoxic agents did not yield clinically meaningful activity,21 the introduction of more potent agents that are pharmacologically active at sub-nanomolar concentrations led to more pronounced clinical responses. For example, camptothecin analogs (i.e. SN-38, DXd) inhibit topoisomerase I (TOPO1); maytansinoids (i.e. DM1) disrupt microtubule instability; whereas auristatins [i.e. monomethyl auristatin E (MMAE); monomethyl auristatin F (MMAF)] destabilize microtubules.16 A key feature of ADCs that has a clear impact on their activity, PK, and safety profiles is the drug-to-antibody ratio (DAR), which refers to the average number of payloads per mAb molecules. DAR values of the Food and Drug Administration (FDA)-approved ADC range from 2 to 8, with higher values correlating with higher in vitro cytotoxicity. Of note, ADC with hydrophobic payloads and high DAR values undergo faster hepatic clearance, while newer ADC designs with reduced hydrophobicity and high DAR have been shown to improve PK and therapeutic indices.22 Moreover, novel classes of payloads other than chemotherapeutic agents are currently being investigated, such as radionuclides or immune-stimulatory molecules.19
In general, a crucial feature of different ADC designs is the use of C linkers with hydrophobic payloads, which are thus able to passively diffuse into nearby cellular membranes beyond their target specificity. Such a phenomenon, termed ‘bystander effect’, is thought to underpin ADC antitumor activity also to ‘antigen-negative’ cancer cells and to possibly reduce the issue of intratumor heterogeneity.23 The implementation of innovative C linker designs seems to be a crucial aspect determining the antitumor activity of next-generation ADCs. In these regards, it will be critical to promote real-time interactions among clinical and preclinical research to address critical and/or unresolved issues in the field.
Antibody–Drug Conjugates for the Treatment of Her2-Positive Advanced Breast Cancer
ADC development in ABC stemmed from HER2-targeting compounds in the HER2-positive disease, primarily with T-DM1 and T-DXd. While both T-DM1 and T-DXd are composed of trastuzumab as their mAb portion (anti-HER2 IgG1), T-DM1 is linked via an NC linker to the maytansinoid microtubule inhibitor DM1, with a mean DAR of 3.5.16 T-DXd is linked via a C-linker tetrapeptidic linker to an exatecan-derived topoisomerase inhibitor, with a mean DAR of 8.16 Originating from such structural differences, these two agents have demonstrated distinct clinical behaviors, while both representing valuable therapeutic options in different clinical circumstances. In this section, we will describe the most relevant clinical data obtained with ADCs in ABC, both in the HER2-positive and in the HER2-low subgroups.
HER2 targeting is a cornerstone of ABC treatment, both in the early and in the advanced setting, for HER2-positive disease.24 HER2 activation feeds proliferative and pro-survival signaling cascades via phosphoinositide 3-kinases/Ak strain transforming/mammalian target of rapamycin and rapidly accelerated fibrosarcoma/mitogen-activated protein kinase kinase/mitogen-activated protein kinase pathways, respectively, and also mediates therapy resistance mechanisms.25,26 Stemming from the previous experience obtained with anti-HER2 mAbs, such as trastuzumab and pertuzumab,27, 28, 29, 30 and anti-HER2 tyrosine kinase inhibitors, such as tucatinib, lapatinib, and neratinib,31, 32, 33 clinical testing of anti-HER2 ADCs emerged as an immediate consequence for drug developers, clinical researchers, and patients.
The current upfront standard-of-care (SOC), first-line treatment of HER2-positive ABC is represented by taxane-based chemotherapy in combination with dual HER2 blockade with trastuzumab and pertuzumab (Figure 2). The phase III CLEOPATRA clinical trial demonstrated both a median progression-free survival (mPFS) and a median overall survival (mOS) benefit compared to the docetaxel–trastuzumab.28 In the upfront setting, T-DXd is currently being evaluated in the phase III DESTINY-Breast09 clinical trial either alone or in combination with pertuzumab versus SOC, as well as in the phase Ib/II DESTINY-Breast07 clinical trial either alone or in combination with durvalumab, pertuzumab, paclitaxel, or tucatinib, including in patients with untreated brain metastases (BMs).
Figure 2.
Treatment algorithm for the treatment of HER2-positive mBC. ADC, antibody–drug conjugate; ChT, chemotherapy; CT, clinical trial; H, trastuzumab; HER2, human epidermal growth factor receptor 2; mBC, metastatic breast cancer; P, pertuzumab; T, taxole; T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan. Created with BioRender.com.
Upon progression to taxane–trastuzumab–pertuzumab combination, or in case of disease relapse within 6 months from the end of adjuvant therapy, T-DXd represents the current SOC based on the DESTINY-Breast03 results.34 Early on in its clinical development, heavily pre-treated ABC patients receiving T-DXd at the recommended doses for expansion (5.4 and 6.5 mg/kg) in the phase I clinical trial already showed objective responses (59.5%, 95% confidence interval (CI): 49.7%-68.7%). Concerning safety, two treatment-related deaths due to pneumonitis occurred, and 17% of patients had either interstitial lung disease (ILD), organising pneumonia, or pneumonitis.35 Exposure-to-efficacy/safety modeling analysis from a phase I clinical trial revealed both dose–response and dose–toxicity significant relationships, identifying 5.4 mg/kg as the recommended dose level for further clinical testing.36 Indeed, the phase II DESTINY-Breast01 clinical trial, investigating T-DXd at 5.4 mg/kg, in pre-treated HER2-positive ABC, demonstrated an objective response rate (ORR) of 60.9% (53.4%-68.0%), with an mPFS of 19.4 months (95% CI: 12.7 months-not reached).37 Based on these data, on 20 December 2019, the FDA issued the first approval for T-DXd for patients with HER2-positive ABC who had received ≥2 prior anti-HER2-based regimens. Recently, the DESTINY-Breast02 trial provided evidence of superior clinical activity of T-DXd over capecitabine–trastuzumab/lapatinib in patients with HER2-positive ABC previously treated with T-DM1, without new safety concerns.38 Ultimately, the phase III DESTINY-Breast03 trial provided the first clinical data regarding a head-to-head comparison of T-DXd versus T-DM1 in patients with HER2-positive ABC previously treated with a taxane and trastuzumab. In this setting, T-DXd greatly outperformed T-DM1 in terms of mPFS and mOS with a HR of 0.33 (0.26-0.43, P < 0.0001) and 0.64 (0.47-0.87, P = 0.0037), respectively.15,34 The most common grade ≥3 treatment-related adverse events (TRAEs) for T-DXd were neutropenia (19.1%) and thrombocytopenia (7%), while those for T-DM1 were thrombocytopenia (24.9%) and transaminase increase (5.0%). ILD was detected in 10.5% patients, with no grade 4/5 events. These data led to the recent FDA approval on 6 May 2022, drastically re-designing the second-line setting and leaving ample debate on the role of T-DM1 in subsequent therapeutic options. T-DXd is also being investigated either in combination with tucatinib in the phase II HER2CLIMB-04 clinical trial, or alone in the phase III DESTINY-Breast12 trial in the setting of BM. Moreover, the small phase I DASH trial also explores the combination of T-DXd and the oral ataxia telangiectasia and Rad3-related protein inhibitor ceralasertib (Table 2).
Table 2.
List of active and recruiting clinical trials investigating T-DXd in advanced breast cancer, as of 22 October 2022
| CT full name | CT name | CT code | CT phase | Patients | HER2 |
|---|---|---|---|---|---|
| A Study of T-DXd in Participants With or Without Brain Metastasis Who Have Previously Treated Advanced or Metastatic HER2+ BC | DESTINY-Breast12 | NCT04739761 | Phase III | 500 | POS |
| A Study of Tucatinib Plus T-DXd in HER2+ BC | HER2CLIMB-04 | NCT04539938 | Phase II | 70 | POS |
| Study of T-DXd versus Investigator's Choice Chemotherapy in HER2-low, HR+, Metastatic BC | DESTINY-Breast06 | NCT04494425 | Phase III | 850 | Low |
| T-DXd With or Without Pertuzumab Versus Taxane, Trastuzumab and Pertuzumab in HER2+ Metastatic BC | DESTINY-Breast09 | NCT04784715 | Phase III | 1134 | POS |
| A Phase 1b/2 Study of T-DXd Combinations in HER2+ Metastatic BC | DESTINY-Breast07 | NCT04538742 | Phase I/II | 450 | POS |
| A Phase 1b Study of T-DXd Combinations in HER2-low Advanced or Metastatic BC | DESTINY-Breast08 | NCT04556773 | Phase I | 182 | Low |
| T-DXd and Pembrolizumab in Participants With Locally Advanced/Metastatic Breast or Non-Small Cell Lung Cancer | NCT04042701 | Phase I | 115 | Both | |
| Testing the Biological Effects of T-DXd on Patients With Advanced Cancer | NCT04294628 | Phase I | 37 | Both | |
| Testing the Combination of Two Anti-cancer Drugs, T-DXd and AZD6738, for The Treatment of Patients With Advanced Solid Tumors Expressing the HER2 Protein or Gene | DASH | NCT04704661 | Phase I | 15 | POS |
| Study of AZD5305 as Monotherapy and in Combination With Anti-cancer Agents in Patients With Advanced Solid Malignancies | PETRA | NCT04644068 | Phase I/II | 715 | NEG |
ABC, advanced breast cancer; BC, breast cancer; CT, clinical trial; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; NEG, negative; POS, positive; T-DXd, trastuzumab deruxtecan.
Source: clinicaltrials.gov.
Importantly, clinical activity of T-DXd in patients with active BMs has also been demonstrated in the phase II TUXEDO-1 trial, with an intracranial ORR of 73.3% (95% CI: 48.1%-89.1%) and two complete responses (CRs; 13.3%) out of 15 enrolled patients,39 in the phase II DEBBRAH trial (46.2%, 95% CI: 19.2%-74.9%), in the retrospective analysis from untreated/progressive BMs (70%, 7/10), as well as in a series of patients with leptomeningeal disease.40, 41, 42 In patients with stable BMs, both the DESTINY-Breast01 as well as the DESTINY-Breast03 demonstrated remarkable levels of intracranial ORR, with 58.3% (95% CI: 36.6%-77.9%) and 67.4%, respectively.43,44 These data further confirmed T-DXd as a valid therapeutic option in this patient population with an unmet medical need.
What to do after Progression on Trastuzumab Deruxtecan?
While the clinical scenario of the first and second treatment lines is clearly defined, there is currently no consensus on subsequent treatment lines, as currently there are no available clinical data of therapies beyond T-DXd progression. Among various available treatment options, decision making must be supported by patient- and disease-related factors, including overall tolerability, clinical benefit to prior therapies, disease burden, and eventual central nervous system (CNS) involvement.7
In the HER2-positive setting, the preferred treatment options are currently represented by T-DM1 and tucatinib–capecitabine–trastuzumab. Based on the results of the EMILIA trial, in which T-DM1 was shown to prolong the mOS from 25.9 months (95% CI: 22.7-28.3 months) to 29.9 months (95% CI: 26.3-34.1 months) (hazard ratio 0.75, 95% CI: 0.64-0.88), and mPFS, with a favorable safety profile,14 T-DM1 obtained FDA approval on 22 February 2013.13 Also in patients progressing on ≥2 HER2-directed regimens in the advanced setting, the phase III TH3RESA clinical trial demonstrated an mOS benefit with T-DM1 over SOC (hazard ratio 0.68, 95% CI: 0.54-0.85, P = 0.0007).45 Interestingly, in the KAMILLA phase IIIb clinical trial, T-DM1 was also shown to retain clinical activity in patients with baseline BM. In this trial, the reported mOS was 18.9 months (95% CI: 17.1-21.3 months) and the mPFS was 5.5 months (95% CI: 5.3-5.6 months), without evidencing new safety issues.46 The capecitabine–trastuzumab–tucatinib regimen, instead, was tested in the phase III HER2CLIMB trial, showing an mOS benefit (hazard ratio 0.73, 95% CI: 0.59-0.90, P = 0.004) with 24.7 versus 19.2 months upon addition of tucatinib,31 including patients with active BM. The phase III NALA trial compared capecitabine + neratinib or lapatinib and evidenced an mPFS improvement with neratinib (hazard ratio 0.76, 95% CI: 0.63-0.93, P = 0.0059), with demonstrated CNS activity, albeit without a significant mOS benefit (hazard ratio 0.88, 95% CI: 0.72-1.07, P = 0.2098).47 The SOPHIA trial, instead, compared chemotherapy combination to either trastuzumab or margetuximab, a chimeric, Fc-engineered, anti-HER2 mAb with increased affinity for activating FcγRs (CD16A) and decreased for inhibitory FcγRs (CD32B). Margetuximab improved mPFS over trastuzumab (hazard ratio 0.76, 95% CI: 0.59-0.98, P = 0.03), with an mPFS of 5.8 months (95% CI: 5.5-7.0 months) versus 4.9 months (95% CI: 4.2-5.6 months).48 Interestingly, while mOS was not prolonged by margetuxumab (hazard ratio 0.95, 95% CI: 0.77-1.17, P = 0.620), the CD16A genotype suggested an mOS benefit of margetuximab in CD16A-158FF patients (23.6 versus 19.2 months, hazard ratio 0.72, 95% CI: 0.52-1.00), and an mOS benefit of trastuzumab in CD16A-158VV patients (31.1 versus 22.0 months, hazard ratio 1.77, 95% CI: 1.01-3.12).49 Also, the lapatinib–trastuzumab combination showed clinical activity in both the HR-positive and -negative sub-populations,50,51 while lapatinib–capecitabine was superior to capecitabine alone in patients previously treated with a taxane, an anthracycline, and trastuzumab.32 Of note, aside from the SOC in the third-line setting, disitamab vedotin (RC-48), a humanized anti-HER2 mAb coupled to MMAE via a C linker with a DAR of 4, is currently entering phase III clinical testing. A pooled analysis from phase I clinical trials showed clinical activity in both the HER2-low (ORR 39.6%, 95% CI: 25.8%-54.7%) and HER2-positive (ORR 42.9%, 95% CI: 21.8%-66.0%) setting at a recommended phase II dose (RP2D) of 2 mg/kg, with a manageable toxicity profile.52 Indeed, based on these results, phase III clinical trials are currently testing RC-48 in the HER2-low (NCT04400695) and HER2-positive settings (NCT03500380).
Building on previous experience with trastuzumab, whereby it has been shown that maintenance of HER2 blockade beyond progression in the second- and third-line settings provides clinical benefit and prolongs survival, it is advisable to maintain HER2 blockade also upon T-DXd failure.53, 54, 55, 56 In this context, preferred chemotherapeutic regimens with recognized activity in HER2-positive BC are represented by anthracyclines, eribulin, and vinorelbine.7 In addition, given the lack of data regarding common resistance mechanisms, it might be also advisable to carry out tumor re-biopsy upon T-DXd failure in order to better guide treatment decision making, as well as optimally refer patients to clinical trials, upon availability and feasibility. Lastly, real-world data concerning the activity of the aforementioned regimens upon T-DXd failure could further aid in the identification of most active compounds, as in the previous case of T-DM1 in pertuzumab pre-treated patients.56
Addressing Trastuzumab Deruxtecan Resistance
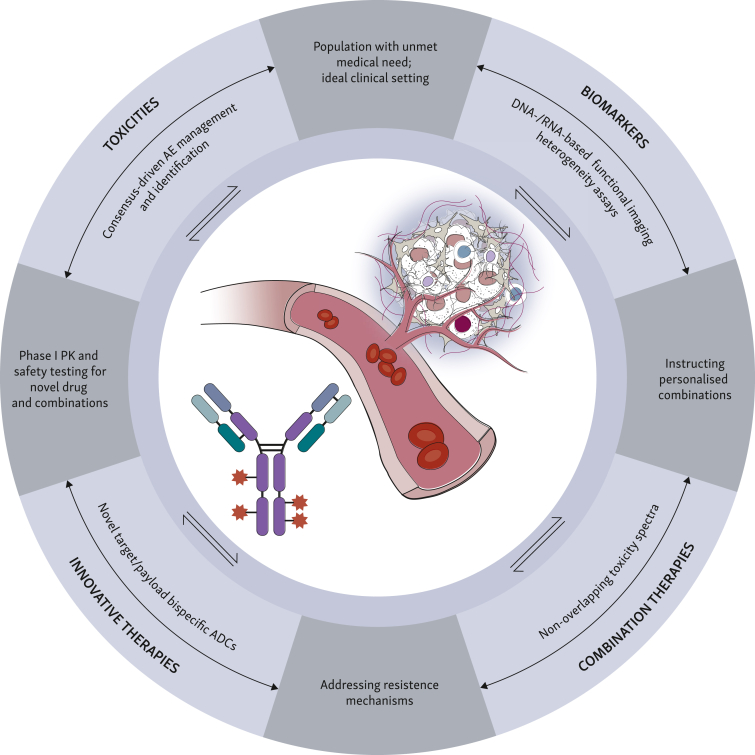
The remarkable clinical data obtained so far with the introduction of ADCs, and T-DXd in particular, already changed current treatment algorithms in different solid malignancies in various clinical settings.19 In the BC scenario only, there have been six regulatory approvals in the past decade, of which five were within the past 3 years.16 This growing momentum is fostering ADC clinical testing in various disease stages, and it will not only provide with newer agents or indications, but it may also challenge currently utilized BC classification systems. In this context, four major pillars should guide clinical management in ABC progressing to T-DXd (Figure 3): prior toxicities, biomarker assessments, novel agents, and combinatorial regimens.
Figure 3.
Four (pre-)clinical research pillars to guide the post-T-DXd clinical scenario. AE, adverse event; ADC, antibody–drug conjugate; PK, pharmacokinetics; T-DXd, trastuzumab deruxtecan. Created with BioRender.com.
Toxicity assessment and management
Firstly, a major step forward has been the elucidation of critical ADC-related toxicities and the definition of shared clinical practice guidelines for their management.57,58 For example, ILD has been recognized as an AE of special interest upon treatment with T-DXd in ∼15.4% patients across different solid tumors.58 Based on early-phase clinical trial data, a consensus guideline has been reached for the management of T-DXd-related ILD, further promoting clinical development and clinical trial design.57,59 Nonetheless, many unresolved questions are still a matter of debate, such as the issue of implementation of better monitoring techniques, the optimization of monitoring schedules, or the identification of patients who recovered after a grade 2 ILD toxicity and could be safely re-challenged with T-DXd. Importantly, the identification of such AEs of special interest would also better instruct novel combinatorial regimens.
Biomarker identification and validation
Biomarker analyses from the DAISY clinical trial revealed reduction of expression of HER2 in approximately two-third of patients upon T-DXd resistance, besides a relevant contribution to T-DXd response by the spatial distribution of HER2-negative cells, possibly suggesting avoiding another anti-HER2 ADC upon progression.60 Moreover, ERBB2 mRNA expression has been shown to positively correlate with response to T-DM1, as well as PFS and OS, providing both prognostic and predictive information regardless of IHC levels.61 In addition, genomic analysis from patients enrolled in the DAISY trial showed the presence of recurrent mutations to the SLX4 gene in ∼20% of patients upon T-DXd progression as compared to 2% of patients at baseline, warranting for further validation as a mechanism of acquired resistance.62
Certainly, patients’ enrollment into clinical trials should always be considered in these scenarios to promote access to innovative therapies as well as combination treatments. Upon progression on ADC-based therapies, current options include the use of another or a novel ADC with different targets or payload or DAR, or combinatorial strategies, in order to potentially overcome resistance to previous ADCs, either by referring patients to clinical trials or by choosing currently standard treatment regimens.
In order to further boost ADC activity, to gain insight into the most common mechanisms of resistance, as well as to identify the most suitable combinatorial drugs, a compelling effort in preclinical and translational research should be conducted in parallel and concomitantly to ongoing clinical trials. Putative resistance mechanisms being acknowledged so far are either ‘mAb related’ (i.e. reduction of expression of target, epitope masking, ‘binding-site barrier’) or ‘payload related’ (i.e. efflux pumps, defective internalization/lysosomal processing).16,18,63 Moreover, early preclinical studies have so far failed to show recurrent baseline driver alterations at the basis of T-DXd resistance, while showing different transcriptomic responses according to HER2 status.60 In addition, intratumor heterogeneity is thought to play a key role in altering ADC tissue penetration, tumor response to therapy, and hence tumor resistance mechanisms.60 HER2 heterogeneity, also within HER2-positive patients, has also been shown to influence therapy responses.64,65 Of note, HER2 heterogeneity has been associated with inferior pathological CR (pCR) rates upon T-DM1 and pertuzumab therapy among HER2-positive early BC (eBC) patients compared to HER2 non-heterogeneous ones (0% versus 55%, P < 0.0001).66 Building on these notions, the ZEPHIR trial investigated the use of a HER2–positron emission tomography (PET) computed tomography assay as a prediction tool for T-DM1 activity in the ABC setting,67 and, similarly, fluorine-18-2fluoro-2-deoxy-D-glucose (18F-FDG) PET has been shown to identify patients with HER2-positive eBC (PHERGain) who could benefit from chemotherapy-free regimens,68 providing a fertile ground for innovative trials with ADCs as well.
Innovative therapeutic agents
Novel drugs entering the clinical scenario, in particular innovative ADCs with diverse designs, may represent newer treatment lines upon T-DXd failure or combination partners in forthcoming clinical trials.
In the HER2-positive/-low settings, trastuzumab duocarmazine (SYD985) is an anti-HER2 mAb coupled via a C linker to a duocarmycin alkylating agent, with a DAR of 2.6. In the dose-escalation and -expansion study (NCT02277717), SYD985 was administered to patients with HER2 IHC expression of 1+ or more in multiple cancer types including heavily pre-treated ABC. Of note, after witnessing a dose-limiting toxicity (DLT) at 2.4 mg/kg (death due to pneumonitis), the RP2D for the dose expansion was established at 1.2 mg/kg. Interestingly, in the dose expansion, 16/48 and 15/47 patients with HER2-positive and HER2-low ABC obtained an objective response, with grade 3 or more AEs occurring in 35% of patients (i.e. neutropenia, fatigue, and conjunctivitis).69 The subsequent phase III TULIP trial randomized HER2-positive ABC pre-treated patients (≥2 lines) to either SYD985 or treatment of physician’s choice. At the latest data presentation, the mPFS was of 7.0 months (95% CI: 5.4-7.2 months) for SYD985 and 4.9 months (95% CI: 4.0-5.5 months) in the control arm, with a hazard ratio of 0.64 (95% CI: 0.49-0.84, P = 0.002), despite showing similar ORR.70 The rate of ILD/pneumonitis for SYD985 was 7.6% (22/288), while no events had been reported in the control arm. Of particular relevance, grade ≥3 ocular toxicities occurred in 21.2% of patients in the SYD985 arm, leading to treatment discontinuation in 20.8% of patients. Based on these data, on 12 July 2022, the FDA had accepted a Biologics License Application for HER2-positive ABC. Ongoing trials are currently investigating SYD985 in combination with paclitaxel for ABC (ISPY-P1.01). Concerning RC-48, a pooled analysis from phase I clinical trials showed clinical activity in both the HER2-low (ORR 39.6%, 95% CI: 25.8%-54.7%) and HER2-positive (ORR 42.9%, 95% CI: 21.8%-66.0%) setting at an RP2D of 2 mg/kg, with a manageable toxicity profile.52 Indeed, based on these results, phase III clinical trials are currently testing RC-48 in the HER2-low (NCT04400695) and HER2-positive settings (NCT03500380). Other anti-HER2 ADCs are currently being tested in earlier phases of clinical development and include, for example, A166 and ALT-P7.71 Of note, bispecific ADCs are also being tested in refractory ABC, such as zanidatamab zovodotin (ZW49). ZW49 is a biparatropic ADC targeting HER2 with the binding-site specificities of both trastuzumab and pertuzumab and coupled to an auristatin payload via a C linker, with a DAR of 2. First clinical data regarding the phase I dose-escalation study (NCT03821233) in patients with HER2-positive advanced solid tumors have shown two DLTs (keratitis, grade 2) at the 1.75 mg/kg and 2.5 mg/kg cohorts, with treatment-related keratitis in 43% patients, warranting for mandatory ocular prophylaxis. Interestingly, at the dose-expansion dose of 2.5 mg/kg, the disease control rate in patients with HER2-positive ABC was 50% (95% CI: 15.7%-84.3%).72
Patritumab deruxtecan (HER3-DXd) is an anti-HER3 mAb coupled to a TOPO1 inhibitor via a C linker, with a median DAR of 8. HER3 belongs to the ErbB receptor family, and it is expressed on approximately half of ABC.73 Of note, HER3 does not retain its own kinase activity, although it dimerizes with other ErbB family members, including HER2, to unleash proliferative signaling cascades.74 In the phase I/II trial (NCT02980341), HER3-DXd demonstrated clinical activity across all BC subtypes, with a remarkable ORR of 42.9% (95% CI: 17.7%-71.1%) in the HER2-positive subgroup, as well as a tolerable safety profile at the 6.4 mg/kg dose, with 71.4% patients developing grade ≥3 TEAEs, the most common being hematologic.75 Further investigation of HER3-DXd in the ABC setting is currently ongoing (NCT04965766, NCT04699630). Ongoing trials will evaluate HER3-DXd in the triple-negative BC setting (TOT-HER3), as well as in the HR-positive/HER2-negative setting alone or in combination with endocrine therapy (SOLTI-VALENTINE).
Also, immune-stimulator antibody conjugates composed of a toll-like receptor (TLR) 7/8 agonist and an anti-HER2 antibody, such as NJH395 and BDC-1001, are being tested in early-stage clinical trials in advanced HER2-positive malignancies, either alone (NCT03696771) or in combination with anti-PD1 mAb (NCT04278144).76,77
Combinatorial therapeutic regimens
Another crucial aspect is represented by combinatorial therapies with ADCs. Table 3 outlines ongoing phase III clinical trials assessing ADC combinations in HER2-positive ABC, investigating the addition of target, or immunotherapeutic, agents. Of note, the phase III MARIANNE clinical trial, which investigated T-DM1 in combination with pertuzumab in the HER2-positive settings, did not show its primary superiority endpoints, albeit demonstrating the non-inferiority of T-DM1 compared to trastuzumab–taxane.78 Although currently we can utilize more active ADCs (i.e. T-DXd) with higher DARs and different linker technologies, these data also highlight the importance of identification of ideal clinical scenarios to test possible combinatorial strategies. Indeed, a crucial step forward for the development of ADC in clinical practice is also represented by the identification of patients’ population of interest with unmet medical need, such as patients with active BM. Expanding research within these patients’ subpopulation is of utmost importance and needs to be further implemented also with other treatment options utilized in this context. In patients with HER2-positive ABC with unstable BM, the preferred treatment option might be represented by tucatinib–capecitabine–trastuzumab or the enrollment in clinical trials investigating combinatorial strategies, such as tucatinib plus T-DM1 (HER2CLIMB-02) or T-DXd (HER2CLIMB-04).31 Of note, the phase II MonarcHER trial showed that, in HR-positive/HER2-positive ABC patients, the combination of abemaciclib–fulvestrant–trastuzumab improves mPFS (8.3 months, 95% CI: 5.9-12.6 months) over trastuzumab chemotherapy (5.7 months, 95% CI: 5.4-7.0 months) with hazard ratio of 0.67 (95% CI: 0.45-1.00, P = 0.051), with an interesting trend for improved mOS among patients with luminal versus non-luminal subtypes (31.7 versus 19.7 months, hazard ratio 0.68, 95% CI: 0.46-1.00).75 Altogether, these trials evidenced the importance of implementing tailored therapeutic options for different patients’ populations. In addition, numerous phase III clinical trials are ongoing also for SG (ASCENT-03, SASCIA) and Dato-DXd (TROPION-Breast01 and TROPION-Breast02).
Table 3.
List of phase III clinical trials investigating ADC combination therapies in HER2-positive advanced breast cancer, as of 22 October 2022
| CT full name | CT name | CT code | Setting | ADC | Combo |
|---|---|---|---|---|---|
| A Study of T-DM1 in Combination With Atezolizumab or Placebo as a Treatment for Participants With HER2+ and PD-L1+ Locally Advanced or metastatic BC | KATE3 | NCT04740918 | HER2+ | T-DM1 | Atezolizumab/placebo |
| A Study of Tucatinib versus Placebo in Combination With T-DM1 for Patients With Advanced or Metastatic HER2+ BC | HER2CLIMB 02 | NCT03975647 | HER2+ | T-DM1 | Tucatinib/placebo |
| T-DXd With or Without Pertuzumab Versus Taxane, Trastuzumab and Pertuzumab in HER2+ Metastatic BC | DESTINY-Breast09 | NCT04784715 | HER2+ | T-DXd | Pertuzumab/placebo |
ADC, antibody–drug conjugate; BC, breast cancer; CT, clinical trial; HER2, human epidermal growth factor receptor 2; PD-L1, programmed death-ligand 1; T-DM1, ado-trastuzumab emtansine; T-DXd, trastuzumab deruxtecan.
Source: clinicatrials.gov.
Conclusions
Technological refinement of ADC constructs led to unprecedented clinical results for the treatment of solid malignancies, and of HER2-positive ABC in particular.16 Moreover, the rapid pace of clinical innovations brought upon by these agents is staggering and unprecedented, with three regulatory approvals in this scenario since 2013. With the recognition of T-DXd as the preferred second-line treatment regimen of HER2-positive ABC, and with ongoing clinical trials investigating its role in the frontline setting, there is a great unmet medical need for the management of patients progressing to T-DXd, as no clinical data exist so far.
Indeed, a comprehensive insight regarding ADCs’ mechanisms of action, together with their PK/PD profiles and molecular drivers of resistance, is largely lacking, mainly due to the lack of preclinical studies, the absence of reliable in vivo models, and/or biosimilar drug products.79 Therefore, there is no standardized consensus regarding the optimal management of patients experiencing resistance to ADCs, in particular T-DXd, and clinical trial referral being often considered the best approach, if feasible. Combinatorial strategies with registered regimens may also be envisaged, especially upon identification of patients’ population of interest with unmet medical needs. Diversely, the most suitable therapeutic regimen should be identified among available standard therapies, according to prior toxicities and clinical history.
In this scenario, both preclinical research efforts aiming at dissecting T-DXd mechanisms of action and resistance, as well as clinical research programs investigating novel ADC compounds and combinatorial strategies, are greatly encouraged.7 Clinical data so far have unexpectedly shown that ADCs fail to increase their payloads’ maximum tolerated dose, as previously thought.80 Indeed, a thorough elucidation of ADC mechanisms of action, as well as their PK and PD profiles, is largely lacking, warranting for the introduction of more reliable in vivo and in vitro models. In addition, research and innovations in the field of ADC design are also greatly envisaged. In particular, future developments may involve the use of bispecific, or tumor-specific antigen-targeting, mAbs, or novel payloads with immune-stimulatory/radioactive molecules, or the use of dual payloads within the same construct.19
In the field of BC, instead, a crucial missing understanding relates to the impact of both intrinsic and acquired patterns of BC intratumor heterogeneity, as well as the identification of reliable biomarkers predictive of either toxicities or efficacy.79 Indeed, a better elucidation of these aspects would greatly aid in the design of more appropriate clinical trials, as well as in the identification of populations of interest that would benefit the most from ADC-based therapies, and in the management of treatment-related toxicities.
Overall, the full potential of ADCs for the treatment of HER2-positive ABC is yet to be unleashed, especially by means of a more profound understanding of their mechanisms of action and their interactions with diverse tumor microenvironments, as well as by innovations in drug engineering and clinical trials design.
Funding
None declared.
Disclosure
ST reports the following consulting or advisory role: Novartis, Pfizer, Merck, Lilly, Nektar, NanoString Technologies, AstraZeneca, Puma Biotechnology, Genentech/Roche, Eisai, Sanofi, Bristol Myers Squibb, Seattle Genetics, Odonate Therapeutics, OncoPep, Kyowa Hakko Kirin, Samsung Bioepis, CytomX Therapeutics, Daiichi Sankyo, Athenex, Gilead, Mersana, Cer-tara, Chugai Pharma, Ellipses Pharma, Infinity, 4D Pharma, OncoSec Medical Inc., BeyondSpring Pharmaceuticals, OncXerna, Zymeworks, Zentalis, Blueprint Medicines, Reveal Genomics, ARC Therapeutics; Institutional Research Funding: Genentech/Roche, Merck, Exelixis, Pfizer, Lilly, Novartis, Bristol Myers Squibb, Eisai, AstraZeneca, NanoString Technologies, Cyclacel, Nektar, Gilead, Odonate Therapeutics, Sanofi, Seattle Genetics. All the competing interests were outside the submitted work. JC has received institutional grants or contracts from Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer Healthcare, Eisai, F Hoffman-La Roche, Guardant Health, Merck Sharp & Dohme, Pfizer, Piqur Therapeutics, Puma C, and Queen Mary University of London; has received consulting fees from Roche, Celgene, Cellestia, AstraZeneca, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, Merck Sharp & Dohme, GSK, Leuko, Bioasis, Clovis Oncology, Boehringer Ingelheim, Ellipses, Hibercell, BioInvent, Gemoab, Gilead, Menarini, Zymeworks, and Reveal Genomics; has received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Roche, Novartis, Celgene, Eisai, Pfizer, Samsung Bioepis, Lilly, Merck Sharp & Dohme, and Daiichi Sankyo; has received support for attending meetings or travel from Roche, Novartis, Eisai, Pfizer, Daiichi Sankyo, AstraZeneca, and Gilead; has patents planned, issued, or pending (WO 2014/199294 A; US 2019/ 0338368 A1); and has stock or stock options with MedSIR, Nektar Pharmaceuticals, and Leuko. GC received honoraria for speaker's engagement: Roche, Seattle Genetics, Novartis, Lilly, Pfizer, Foundation Medicine, NanoString, Samsung, Celltrion, BMS, MSD; honoraria for providing consultancy: Roche, Seattle Genetics, NanoString; honoraria for participating in the advisory board: Roche, Lilly, Pfizer, Foundation Medicine, Samsung, Celltrion, Mylan; honoraria for writing engagement: Novartis, BMS; honoraria for participation in Ellipsis Scientific Affairs Group; institutional research funding for conducting phase I and II clinical trials: Pfizer, Roche, Novartis, Sanofi, Celgene, Servier, Orion, AstraZeneca, Seattle Genetics, AbbVie, Tesaro, BMS, Merck Serono, Merck Sharp Dome, Janssen-Cilag, Philogen, Bayer, Medivation, Medimmune. All other authors have declared no conflicts of interest.
Contributor Information
J. Cortes, Email: javier.cortes@maj3.health.
G. Curigliano, Email: giuseppe.curigliano@ieo.it.
References
- 1.Giaquinto A.N., Sung H., Miller K.D., et al. Breast Cancer Statistics, 2022. CA Cancer J Clin. 2022;72(6):524–541. doi: 10.3322/caac.21754. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C., Matsuda T., Di Carlo V., et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan P.H., Ellis I., Allison K., et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77(2):181–185. doi: 10.1111/his.14091. [DOI] [PubMed] [Google Scholar]
- 5.Allison K.H., Hammond M.E.H., Dowsett M., et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38(12):1346–1366. doi: 10.1200/JCO.19.02309. [DOI] [PubMed] [Google Scholar]
- 6.Wolff A.C., Hammond M.E.H., Allison K.H., et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. 2018;142(11):1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 7.Gennari A., André F., Barrios C.H., et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Saleh M.N., Sugarman S., Murray J., et al. Phase I trial of the anti-Lewis Y drug immunoconjugate BR96-doxorubicin in patients with Lewis Y-expressing epithelial tumors. J Clin Oncol. 2000;18(11):2282–2292. doi: 10.1200/JCO.2000.18.11.2282. [DOI] [PubMed] [Google Scholar]
- 9.Jackson D., Stover D. Using the lessons learned from the clinic to improve the preclinical development of antibody drug conjugates. Pharm Res. 2015;32(11):3458–3469. doi: 10.1007/s11095-014-1536-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sievers E.L., Larson R.A., Stadtmauer E.A., et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol. 2001;19(13):3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 11.Younes A., Gopal A.K., Smith S.E., et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu Z., Li S., Han S., Shi C., Zhang Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther. 2022;7(1):93. doi: 10.1038/s41392-022-00947-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma S., Miles D., Gianni L., et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diéras V., Miles D., Verma S., et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732–742. doi: 10.1016/S1470-2045(17)30312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurvitz S.A., Hegg R., Chung W.P., et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401(10371):105–117. doi: 10.1016/S0140-6736(22)02420-5. [DOI] [PubMed] [Google Scholar]
- 16.Drago J.Z., Modi S., Chandarlapaty S. Unlocking the potential of antibody-drug conjugates for cancer therapy. Nat Rev Clin Oncol. 2021;18(6):327–344. doi: 10.1038/s41571-021-00470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicolò E., Zagami P., Curigliano G. Antibody-drug conjugates in breast cancer: the chemotherapy of the future? Curr Opin Oncol. 2020;32(5):494–502. doi: 10.1097/CCO.0000000000000656. [DOI] [PubMed] [Google Scholar]
- 18.Nessler I., Menezes B., Thurber G.M. Key metrics to expanding the pipeline of successful antibody-drug conjugates. Trends Pharmacol Sci. 2021;42(10):803–812. doi: 10.1016/j.tips.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarantino P., Carmagnani Pestana R., Corti C., et al. Antibody-drug conjugates: Smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. 2022;72(2):165–182. doi: 10.3322/caac.21705. [DOI] [PubMed] [Google Scholar]
- 20.Corti C., Antonarelli G., Valenza C., et al. Histology-agnostic approvals for antibody-drug conjugates in solid tumours: is the time ripe? Eur J Cancer. 2022;171:25–42. doi: 10.1016/j.ejca.2022.04.039. [DOI] [PubMed] [Google Scholar]
- 21.Tolcher A.W., Sugarman S., Gelmon K.A., et al. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J Clin Oncol. 1999;17(2) doi: 10.1200/JCO.1999.17.2.478. [DOI] [PubMed] [Google Scholar]
- 22.Lyon R.P., Bovee T.D., Doronina S.O., et al. Reducing hydrophobicity of homogeneous antibody-drug conjugates improves pharmacokinetics and therapeutic index. Nat Biotechnol. 2015;33(7):733–735. doi: 10.1038/nbt.3212. [DOI] [PubMed] [Google Scholar]
- 23.Giugliano F., Corti C., Tarantino P., Michelini F., Curigliano G. Bystander effect of antibody-drug conjugates: fact or fiction? Curr Oncol Rep. 2022;24(7):809–817. doi: 10.1007/s11912-022-01266-4. [DOI] [PubMed] [Google Scholar]
- 24.Martínez-Sáez O., Prat A. Current and future management of HER2-positive metastatic breast cancer. JCO Oncol Pract. 2021;17(10):594–604. doi: 10.1200/OP.21.00172. [DOI] [PubMed] [Google Scholar]
- 25.Agus D.B., Akita R.W., Fox W.D., et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Saenz A., Dreyer C., Campbell M.R., Steri V., Gulizia N., Moasser M.M. HER2 amplification in tumors activates PI3K/Akt signaling independent of HER3. Cancer Res. 2018;78(13):3645–3658. doi: 10.1158/0008-5472.CAN-18-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slamon D.J., Leyland-Jones B., Shak S., et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 28.Swain S.M., Baselga J., Kim S.B., et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piccart M., Procter M., Fumagalli D., et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J Clin Oncol. 2021;39(13):1448–1457. doi: 10.1200/JCO.20.01204. [DOI] [PubMed] [Google Scholar]
- 30.Cameron D., Piccart-Gebhart M.J., Gelber R.D., et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389(10075):1195–1205. doi: 10.1016/S0140-6736(16)32616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curigliano G., Mueller V., Borges V., et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): final overall survival analysis. Ann Oncol. 2022;33(3):321–329. doi: 10.1016/j.annonc.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Geyer C.E., Forster J., Lindquist D., et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 33.Chan A., Moy B., Mansi J., et al. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the phase III ExteNET trial. Clin Breast Cancer. 2021;21(1):80–91.e7. doi: 10.1016/j.clbc.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Cortés J., Kim S.B., Chung W.P., et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386(12):1143–1154. doi: 10.1056/NEJMoa2115022. [DOI] [PubMed] [Google Scholar]
- 35.Tamura K., Tsurutani J., Takahashi S., et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 2019;20(6):816–826. doi: 10.1016/S1470-2045(19)30097-X. [DOI] [PubMed] [Google Scholar]
- 36.Tamura K., Modi S., Tsurutani J., et al. Abstract P6-17-10: Dose justification for DS-8201a, a HER2-targeted antibody-drug conjugate, for HER2-positive breast cancer: observed clinical data and exposure-response analyses. Cancer Res. 2019;79(suppl 4):P6-17–P10. [Google Scholar]
- 37.Modi S., Saura C., Yamashita T., et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–621. doi: 10.1056/NEJMoa1914510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.André F., Shahidi J., Lee C., Wang K., Krop I.E. Abstract OT1-07-04: [Fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) vs investigator’s choice of treatment in subjects with HER2-positive, unresectable and/or metastatic breast cancer who previously received T-DM1: a randomized, phase 3 trial (DESTINY-Breast02) Cancer Res. 2020;80(suppl 4):OT1-07–OT04. [Google Scholar]
- 39.Bartsch R., Berghoff A.S., Furtner J., et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022;28(9):1840–1847. doi: 10.1038/s41591-022-01935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pérez-García J.M., Batista M.V., Cortez P., et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro Oncol. 2023;25(1):157–166. doi: 10.1093/neuonc/noac144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kabraji S., Ni J., Sammons S., et al. Preclinical and clinical efficacy of trastuzumab deruxtecan in breast cancer brain metastases. Clin Cancer Res. 2023;29(1):174–182. doi: 10.1158/1078-0432.CCR-22-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alder L., Trapani D., Van Swearingen A., et al. Abstract 5257: Durable clinical and radiographic responses in a series of patients with HER2+ Breast Cancer (BC) Leptomeningeal Disease (LMD) treated with trastuzumab deruxtecan (T-DXd) Cancer Res. 2022;82(suppl 12):5257. [Google Scholar]
- 43.Jerusalem G., Park Y.H., Hurvitz S.A., et al. Trastuzumab deruxtecan in HER2-positive metastatic breast cancer patients with brain metastases: a DESTINY-Breast01 subgroup analysis. Cancer Discov. 2022;12(12):2754–2762. doi: 10.1158/2159-8290.CD-22-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jacobson A. Trastuzumab deruxtecan improves progression-free survival and intracranial response in patients with HER2-positive metastatic breast cancer and brain metastases. Oncologist. 2022;27(suppl 1):S3–S4. doi: 10.1093/oncolo/oyac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krop I.E., Kim S.B., Martin A.G., et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18(6):743–754. doi: 10.1016/S1470-2045(17)30313-3. [DOI] [PubMed] [Google Scholar]
- 46.Montemurro F., Delaloge S., Barrios C.H., et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann Oncol. 2020;31(10):1350–1358. doi: 10.1016/j.annonc.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 47.Saura C., Oliveira M., Feng Y.H., et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138–3149. doi: 10.1200/JCO.20.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rugo H.S., Im S.A., Cardoso F., et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(4):573–584. doi: 10.1001/jamaoncol.2020.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rugo H.S., Im S.A., Cardoso F., et al. Margetuximab versus trastuzumab in patients with previously treated HER2-positive advanced breast cancer (SOPHIA): final overall survival results from a randomized phase 3 trial. J Clin Oncol. 2023;41(2):198–205. doi: 10.1200/JCO.21.02937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blackwell K.L., Burstein H.J., Storniolo A.M., et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 51.Johnston S.R.D., Hegg R., Im S.A., et al. Phase III, randomized study of dual human epidermal growth factor receptor 2 (HER2) blockade with lapatinib plus trastuzumab in combination with an aromatase inhibitor in postmenopausal women with HER2-positive, hormone receptor-positive metastatic breast cancer: updated results of ALTERNATIVE. J Clin Oncol. 2021;39(1):79–89. doi: 10.1200/JCO.20.01894. [DOI] [PubMed] [Google Scholar]
- 52.Wang J., Liu Y., Zhang Q., et al. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: a pooled analysis of two studies. J Clin Oncol. 2021;39(suppl 15) [Google Scholar]
- 53.Sanglier T., Ross R., Shi T., Mouta J., Swain S., Cardoso F. Trastuzumab-based regimens beyond progression: a crucial treatment option for HER2+ advanced/metastatic breast cancer. Breast. 2022;66:262–271. doi: 10.1016/j.breast.2022.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrelli F., Barni S. A pooled analysis of 2618 patients treated with trastuzumab beyond progression for advanced breast cancer. Clin Breast Cancer. 2013;13(2):81–87. doi: 10.1016/j.clbc.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 55.von Minckwitz G., du Bois A., Schmidt M., et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03-05 study. J Clin Oncol. 2009;27(12):1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 56.Omarini C., Piacentini F., Sperduti I., et al. T-DM1 efficacy in trastuzumab-pertuzumab pre-treated HER2 positive metastatic breast cancer patients: a meta-analysis. BMC Cancer. 2022;22(1):623. doi: 10.1186/s12885-022-09556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tarantino P., Modi S., Tolaney S.M., et al. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: a review. JAMA Oncol. 2021;7(12):1873–1881. doi: 10.1001/jamaoncol.2021.3595. [DOI] [PubMed] [Google Scholar]
- 58.Powell C.A., Modi S., Iwata H., et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open. 2022;7(4) doi: 10.1016/j.esmoop.2022.100554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goto K., Sang-We K., Kubo T., et al. LBA55 Trastuzumab deruxtecan (T-DXd) in patients (Pts) with HER2-mutant metastatic non-small cell lung cancer (NSCLC): interim results from the phase 2 DESTINY-Lung02 trial. Ann Oncol. 2022;33:S1422. [Google Scholar]
- 60.Mosele M.F., Lusque A., Dieras V., et al. LBA1 Unraveling the mechanism of action and resistance to trastuzumab deruxtecan (T-DXd): biomarker analyses from patients from DAISY trial. Ann Oncol. 2022;33:S123. [Google Scholar]
- 61.Brasó-Maristany F., Griguolo G., Chic N., et al. HER2DX ERBB2 mRNA expression in advanced HER2-positive breast cancer treated with T-DM1. J Natl Cancer Inst. 2023;115(3):332–336. doi: 10.1093/jnci/djac227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andre F., Fernanda M., Deluche E., et al. 2022. Mechanism of action and resistance to Trastuzumab Deruxtecan in patients with metastatic breast cancer: the DAISY trial. [DOI] [Google Scholar]
- 63.García-Alonso S., Ocaña A., Pandiella A. Trastuzumab emtansine: mechanisms of action and resistance, clinical progress, and beyond. Trends Cancer. 2020;6(2):130–146. doi: 10.1016/j.trecan.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 64.Loi S., Dafni U., Karlis D., et al. Effects of estrogen receptor and human epidermal growth factor receptor-2 levels on the efficacy of trastuzumab: a secondary analysis of the HERA trial. JAMA Oncol. 2016;2(8):1040–1047. doi: 10.1001/jamaoncol.2016.0339. [DOI] [PubMed] [Google Scholar]
- 65.Okines A.F.C., Turner N.C. Heterogeneous HER2 amplification-a new clinical category of HER2-positive breast cancer? Cancer Discov. 2021;11(10):2369–2371. doi: 10.1158/2159-8290.CD-21-0936. [DOI] [PubMed] [Google Scholar]
- 66.Filho O.M., Viale G., Stein S., et al. Impact of HER2 heterogeneity on treatment response of early-stage HER2-positive breast cancer: phase II neoadjuvant clinical trial of T-DM1 combined with pertuzumab. Cancer Discov. 2021;11(10):2474–2487. doi: 10.1158/2159-8290.CD-20-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gebhart G., Lamberts L.E., Wimana Z., et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): the ZEPHIR trial. Ann Oncol. 2016;27(4):619–624. doi: 10.1093/annonc/mdv577. [DOI] [PubMed] [Google Scholar]
- 68.Pérez-García J.M., Gebhart G., Ruiz Borrego M., et al. Chemotherapy de-escalation using an 18F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): a multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021;22(6):858–871. doi: 10.1016/S1470-2045(21)00122-4. [DOI] [PubMed] [Google Scholar]
- 69.Banerji U., van Herpen C.M.L., Saura C., et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2019;20(8):1124–1135. doi: 10.1016/S1470-2045(19)30328-6. [DOI] [PubMed] [Google Scholar]
- 70.Manich C.S., O’Shaughnessy J., Aftimos P.G., et al. LBA15 Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann Oncol. 2021;32:S1288. [Google Scholar]
- 71.Swain S.M., Shastry M., Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov. 2023;22(2):101–126. doi: 10.1038/s41573-022-00579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jhaveri K., Han H., Dotan E., et al. 460MO Preliminary results from a phase I study using the bispecific, human epidermal growth factor 2 (HER2)-targeting antibody-drug conjugate (ADC) zanidatamab zovodotin (ZW49) in solid cancers. Ann Oncol. 2022;33:S749–S750. [Google Scholar]
- 73.Luhtala S., Staff S., Kallioniemi A., Tanner M., Isola J. Clinicopathological and prognostic correlations of HER3 expression and its degradation regulators, NEDD4-1 and NRDP1, in primary breast cancer. BMC Cancer. 2018;18(1):1045. doi: 10.1186/s12885-018-4917-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drago J.Z., Ferraro E., Abuhadra N., Modi S. Beyond HER2: targeting the ErbB receptor family in breast cancer. Cancer Treat Rev. 2022;109 doi: 10.1016/j.ctrv.2022.102436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krop I.E., Masuda N., Mukohara T., et al. Results from the phase 1/2 study of patritumab deruxtecan, a HER3-directed antibody-drug conjugate (ADC), in patients with HER3-expressing metastatic breast cancer (MBC) J Clin Oncol. 2022;40(suppl 16) [Google Scholar]
- 76.Sharma M., Carvajal R.D., Hanna G.J., et al. Preliminary results from a phase 1/2 study of BDC-1001, a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), in patients (Pts) with advanced HER2-expressing solid tumors. J Clin Oncol. 2021;39(suppl 15) [Google Scholar]
- 77.Janku F., Han S.W., Doi T., et al. Preclinical characterization and phase I study of an anti-HER2-TLR7 immune-stimulator antibody conjugate in patients with HER2+ malignancies. Cancer Immunol Res. 2022;10(12):1441–1461. doi: 10.1158/2326-6066.CIR-21-0722. [DOI] [PubMed] [Google Scholar]
- 78.Perez E.A., Barrios C., Eiermann W., et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35(2):141–148. doi: 10.1200/JCO.2016.67.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Corti C., Giugliano F., Nicolò E., Ascione L., Curigliano G. Antibody-drug conjugates for the treatment of breast cancer. Cancers (Basel) 2021;13(12):2898. doi: 10.3390/cancers13122898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colombo R., Rich J.R. The therapeutic window of antibody drug conjugates: a dogma in need of revision. Cancer Cell. 2022;40(11):1255–1263. doi: 10.1016/j.ccell.2022.09.016. [DOI] [PubMed] [Google Scholar]