Abstract
Purpose
We aimed to assess the humoral response to and reactogenicity of coronavirus disease 2019 (COVID-19) vaccination according to the vaccine type and to analyze factors associated with immunogenicity in actively treated solid cancer patients (CPs).
Materials and Methods
Prospective cohorts of CPs, undergoing anticancer treatment, and healthcare workers (HCWs) were established. The participants had no history of previous COVID-19 and received either mRNA-based or adenovirus vector–based (AdV) vaccines as the primary series. Blood samples were collected before the first vaccination and after 2 weeks for each dose vaccination. Spike-specific binding antibodies (bAbs) in all participants and neutralizing antibodies (nAbs) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) wild-type, Delta, and Omicron variants in CPs were analyzed and presented as the geometric mean titer.
Results
Age-matched 20 HCWs and 118 CPs were included in the analysis. The bAb seroconversion rate and antibody concentrations after the first vaccination were significantly lower in CPs than in HCWs. After the third vaccination, antibody levels in CPs with a primary series of AdV were comparable to those in HCWs, but nAb titers against the Omicron variant did not quantitatively increase in CPs with AdV vaccine as the primary series. The incidence and severity of adverse reactions post-vaccination were similar between CPs and HCWs.
Conclusion
CPs displayed delayed humoral immune response after SARS-CoV-2 vaccination. The booster dose elicited comparable bAb concentrations between CPs and HCWs, regardless of the primary vaccine type. Neutralization against the Omicron variant was not robustly elicited following the booster dose in some CPs, implying the need for additional interventions to protect them from COVID-19.
Keywords: Immunogenicity, Reactogenicity, COVID-19 vaccine, Solid cancer, Chemotherapy
Introduction
Actively treated cancer patients (CPs) are at higher risk of contracting the coronavirus disease 2019 (COVID-19) and developing severe COVID-19 complications because of their compromised immune systems as a result of cancer itself, age, treatment, or comorbidities [1]. The adjusted odds ratio for contracting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was 7.14 (95% confidence interval [CI], 6.91 to 7.39) in patients recently diagnosed with cancer in comparison to patients without cancer [2]. Cancer is significantly associated with severity and high mortality in patients with COVID-19 [3,4]. Therefore, as the efficacy of the novel SARS-CoV-2 vaccines has been proved in clinical trials [5,6], CPs were being prioritized for COVID-19 vaccination.
Initially authorized SARS-CoV-2 vaccines were mRNA-based and adenovirus vectored vaccines targeting the SARS-CoV-2 binding receptor to prevent viral entry into human cells. Specifically, mRNA-based vaccines targeting the spike (S) protein of SARS-CoV-2 are BNT162b2 (Comirnaty, BioNTech/Pfizer, Mainz, Germany) and mRNA-1273 (Moderna, Cambridge, MA), and the chimpanzee adenovirus vector–based vaccine with full-length SARS-CoV-2 spike insert is AZD1222 (Vaxzevria, AstraZeneca, Oxford, UK); all of these were approved as two-dose series.
Many studies have reported immune responses following two doses of SARS-CoV-2 vaccination in CPs compared to those in the general population. In a meta-analysis, the first vaccine dose showed a 55 % reduced likelihood of humoral response in CPs than in healthy controls, and even after the completion of the vaccination regimen, a lower seroconversion rate remained in CPs [7]. However, the analysis included only two studies in which patients received adenoviral-vectored vaccines.
Waning acquired immunity against SARS-CoV-2 and the emergence of variants that can escape immune responses weaken the effectiveness of COVID-19 vaccines [8]. Therefore, mRNA-based vaccines have been recommended as a third dose, which demonstrated the effectiveness in preventing symptomatic COVID-19 infection caused by SARS-CoV-2 variants [9,10]. As of October 2022, extended primary three-dose vaccination series plus a booster dose are recommended to the immunocompromised to reduce the risk of hospitalization for COVID-19 [11,12]. However, risk factors affecting vaccine response and the extent of the immunosuppression are variable in CPs [13], and studies for immune response against SARS-CoV-2 among actively treated solid CPs are still lacking.
In this study, we aimed to analyze humoral immune responses with various vaccine types, including spike-specific antibodies (also called binding antibody [bAb]) and neutralizing antibody (nAb) responses against the wild-type virus, Delta, and Omicron variants; identify factors associated with high immunogenicity; and determine solicited adverse reactions (ARs) in actively treated solid CPs.
Materials and Methods
1. Study design and participants
A prospective cohort was recruited including CPs > 19 years of age, with solid malignancy, undergoing anticancer treatment for less than a month. Exclusion criteria were patients with a previously confirmed COVID-19, positive results for SARS-CoV-2 nucleocapsid antibody, and less than 3 months of life expectancy at baseline. This cohort was established in a 2,700-bed tertiary hospital in Korea.
In the Republic of Korea, BNT162b2 and AZD1222 were initially approved and administered to healthcare workers (HCWs) in March 2021. Therefore, a multicenter prospective cohort was first established among healthy HCWs who received two doses of BNT162b2 at 3-week intervals or AZD1222 at 11-week intervals [14]. In our analysis, age-matched healthy controls were recruited from a multicenter HCW cohort and included as a comparison group.
Later, mRNA-1273 and Ad26.COV2 (Janssen [Johnson & Johnson], New Brunswick, NJ) were subsequently approved and administered to the population as per the priority order. Due to supply chain issues, most of these vaccines had been administered according to a national vaccination strategy; researchers did not intervene in the vaccination schedule of the participants.
Blood samples were collected before the first dose of vaccination as a baseline study and after 2 weeks of each vaccine dose administered in enrolled patients. The booster dose was recommended by the government, and the interval between the second and third vaccine doses was recommended as 6 months for healthy individuals and 2 months for immunocompromised patients, including patients with solid cancers.
Data regarding demographics, medical history, vaccines administered, previous SARS-CoV-2 infection, cancer history, cancer treatment, laboratory findings, and post-vaccination reactions were obtained from examination histories and review of electronic medical records.
2. Specific binding antibody response
Spike-specific bAb responses were analyzed in the blood samples of CPs and HCWs collected before vaccination and after each vaccination dose using a commercial immunoassay. The Elecsys Anti-SARS-CoV-2 S protein assay (Roche Diagnostics, Mannheim, Germany) is an electrochemiluminescence immunoassay used to detect IgG to the SARS-CoV-2 specific-spike protein of the receptor-binding domain on the Cobas e801 analyzer (Roche Diagnostics), with a measuring range from 0.4–250 U/mL (up to 2,500 U/mL with onboard 1:10 dilution, and up to 25,000 U/mL with onboard 1:100 dilution). The antigens in the reagent primarily capture anti-SARS-CoV-2 IgG, but also IgA, and IgM [15]. Values higher than 0.8 U/mL were considered positive.
The presence of anti-nucleocapsid (N) antibody was used as a surrogate marker of history of COVID-19. The Elecsys Anti–SARS-CoV-2 N protein assay was performed on baseline samples to exclude participants with a history of COVID-19. A cutoff index greater than or equal to 1.0 is considered positive [16].
3. Neutralizing response using plaque reduction neutralization test
The plaque reduction neutralization test was performed on the plasma samples from each CP for the SARS-CoV-2 wild-type virus (BetaCoV/Korea/KCDC03/2020), Delta strain (hCoV-19/Korea/KDCA119861/2021), and Omicron strain (hCoV-19/Korea/KDCA447321/2021, lineage B.1.1.529). The nAb responses following the second and third vaccine doses were analyzed in the sera of CPs. The reasons why only the specific group and time points were included in the analysis are as follows: (1) Peak bAb titers were confirmed after two or three doses of vaccination, suggesting the need for nAb analysis. (2) The HCW cohort was established for another study; therefore, the samples were inadequate to perform the neutralization assay.
The virus was grown on VeroE6 cells in a biosafety level 3 laboratory at the Avison Biomedical Research Center, Seoul, Korea. The inactivated serum samples were serially diluted two-fold (1:40 to 1:1,280), and the virus diluted at a concentration of 3×103 plaque-forming unit (PFU)/mL was equally distributed to the diluted serum at a concentration of 50 PFU (30 to 60 plaques observed). The two-fold serially diluted inactivated serum samples (135 μL) were added to an equal volume of 100 median tissue culture infectious dose (TCID50) of the virus. The isolates containing 100 TCID50 were incubated in 96-well plates for 60 minutes at 37°C. The virus-serum mixtures were incubated for 60 minutes at 37°C in a CO2 incubator. These mixtures were added to the Vero E6 cells seeded in 24-well plates with 1×105 cells/well and incubated for 1 hour at 37°C; overlay media composed of 2% fetal bovine serum Dulbecco’s modified eagle medium and 1% agar were added to E6 cells and incubated for 3 days in a 37°C CO2 incubator. After plaque formation, 10% formaldehyde solution and 0.33 % neutral red/in phosphate buffered saline solution were used to fix and stain the cell culture layer. The serum SARS-CoV-2 isolate level was measured using a cytopathic effect assay. The 50% neutralization dilution (ND50) was expressed as the reciprocal dilution of serum, which resulted in a 50% reduction in plaque numbers compared to those with the positive virus control. The Spearman-Kärber method was used to calculate the ND50 titer values.
4. Reactogenicity
In all participants, local and systemic reactions following SARS-CoV-2 vaccination were solicited using a standardized questionnaire [17]. The questionnaire consisted of local and systemic symptoms, including injection site swelling, pain, erythema, fever, headache, diarrhea, fatigue, arthralgia, and myalgia. Participants were asked about the duration and severity of each symptom with grades within 7 days following each vaccine dose. To calculate the cumulative symptom scores, the scores were summed and adapted to severity: asymptomatic (grade 0), mild (below 4 scores, grade 1), moderate (below 8 scores, grade 2), and severe (> 8 scores, grade 3). The participants were also asked to report any other symptoms, and researchers regularly checked the condition of the participants in the clinics or via phone calls.
5. Study endpoint and statistical analyses
The primary endpoints of this study were the seroconversion rates and concentrations of spike-specific antibodies in CPs compared to those in HCWs. The secondary endpoints included the neutralization titer against SARS-CoV-2 in CPs and vaccine reactogenicity. Continuous variables were presented as means and standard deviations or as medians and interquartile ranges. Categorical variables are presented as numbers and percentages. Antibody levels were analyzed as geometric mean titers (GMTs) with 95% CI. Antibody titers between groups were tested using the 2-tailed Mann-Whitney test, with p < 0.05 considered significant. Variables were compared using the Pearson’s chi-squared test or Fisher’s exact method for categorical data and Mann-Whitney U test for continuous data in analyzing responses. Statistical analyses were performed both on R Studio (ver. 4.2.1, R Foundation for Statistical Computing, Vienna, Austria) using packages and GraphPad Prism (ver. 8, GraphPad Software Inc., San Diego, CA).
Results
1. Baseline characteristics of the study population
Between April 2021 and November 2021, 133 CPs were enrolled in the study. All participants received BNT162b2, AZD1222, and mRNA-1273 as the first vaccination. Among these, 14 participants were excluded because they could not be sampled or vaccinated. One patient who received a heterologous vaccination was also excluded from our analysis; 118 CPs were eventually included for the analysis. Twenty age-matched healthy participants from the control group were included in the HCW cohort (S1 Fig.). All studies were approved by the institutional review board of Severance Hospital (4-2020-0076) and written informed consent was obtained from all participants.
The CP cohort was divided into two groups depending on the type of the primary vaccine series: “Ca-mRNA” (CPs receiving two doses of either BNT162b2 or mRNA-1273) and “Ca-AdV” (CPs receiving two doses of AZD1222 as a primary series). The baseline characteristics of CPs and HCWs are presented in Table 1.
Table 1.
Demographics and clinical characteristics of patients with solid cancers and health care workers
| Ca-mRNAa) (n=80) | Ca-AdVb) (n=38) | Healthcare workers (n=20) | |
|---|---|---|---|
| Demographics | |||
| Age (yr) | 54 (48–59) | 64 (61–68) | 58 (57–59) |
| Male sex | 51 (63.8) | 31 (81.6) | 9 (45.0) |
| BMI | 23.0±3.3 | 22.9±3.0 | 23.4±2.4 |
| Underlying disease | |||
| Hypertension | 19 (23.8) | 16 (42.1) | 3 (15.0) |
| Diabetes mellitus | 18 (22.5) | 9 (23.7) | 2 (10.0) |
| Lung disease | 4 (5.0) | 8 (21.1) | 0 |
| Renal disease | 3 (3.75) | 4 (10.5) | 0 |
| Liver disease | 14 (17.5) | 3 (7.9) | 0 |
| Vaccination | |||
| Vaccine type (1st and 2nd doses) | |||
| BNT162b2 | 65 (81.2) | - | 6 (30.0) |
| ChAdOx1 | - | 38 (100) | 14 (70.0) |
| mRNA-1273 | 15 (18.8) | - | 0 |
| Participants receiving the 3rd dose | 27 (33.8) | 29 (76.3) | 19 (95) |
| Vaccine type (3rd dose), if received the 3rd dose | |||
| BNT162b2 | 25 (31.3) | 15 (39.5) | 19 (95.0) |
| mRNA-1273 | 2 (2.5) | 14 (36.8) | 1 (5.0) |
| Days between 2nd and 3rd vaccine | 98 (81–137) | 92 (82–113) | 182 (176–201) |
| Cancer characteristic | |||
| Cancer type | |||
| Stomach | 52 (65.0) | 26 (68.4) | - |
| Pancreas | 4 (5.3) | 2 (5.6) | - |
| Colorectum | 3 (3.9) | 4 (11.1) | - |
| Melanoma | 5 (6.6) | 1 (2.8) | - |
| Biliary | 5 (6.6) | 0 | - |
| Other | 11 (9.2) | 5 (13.2) | - |
| ECOG PS at the enrollment | |||
| 0 | 46 (58.2) | 17 (44.7) | - |
| 1 | 31 (39.2) | 21 (55.3) | - |
| 2 | 2 (2.5) | 0 | - |
| Aim of anticancer treatment | |||
| Neo-adjuvant | 1 (1.2) | 0 | - |
| Adjuvant | 20 (25.0) | 10 (26.3) | - |
| Palliative | 59 (73.8) | 28 (73.7) | - |
| Anticancer treatment | |||
| Cytotoxic agents | 47 (58.8) | 22 (57.9) | - |
| Cytotoxic+immunotherapy | 9 (11.2) | 4 (10.5) | - |
| Cytotoxic+target therapy | 7 (8.8) | 4 (10.5) | - |
| Immunotherapy | 4 (5.0) | 3 (7.9) | - |
| Target therapy | 8 (10.0) | 3 (7.9) | - |
| Other combinations | 5 (6.3) | 2 (5.3) | - |
Values are presented as median (IQR), number (%), or mean±SD. BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; PS, performance status; SD, standard deviation.
Cancer patients who received a primary series of mRNA vaccine (either BNT162b2 or mRNA-1273),
Cancer patients who received a primary series of adenovirus vector vaccine (ChAdOx1).
In this study, the median age of the study group was 54, 64, and 58 years, and male was 63.8%, 81.6%, and 45% in Ca-mRNA, Ca-AdV, and HCW groups, respectively. In the Ca-mRNA cohort, 65 (81.2%) patients received BNT162b2 and 15 (18.8%) received mRNA-1273. In the HCW cohort, six participants (30.0%) received BNT162b2 and 14 participants (70.0%) received mRNA-1273.
Stomach cancer had the highest prevalence in the included CPs (66.1%). The Eastern Cooperative Oncology Group status of the most CPs (97.5%) was 0 or 1. Seventy-four percent of patients were subjected to an anticancer regimen with a palliative aim. Regarding the type of anticancer treatment, 78.8% of the CPs were treated with cytotoxic agents, followed by immunotherapy (16.9%), targeted therapy (18.6%), and others (5.9%), some of which were administered either alone or in combination. During the study period, there were none of reported COVID-19 infection among study participants.
2. Seroconversion
The spike-specific bAb seroconversion rate after the first vaccination dose was 54%, 39%, and 85% in the Ca-mRNA, Ca-AdV, and HCW groups, respectively. The seroconversion rate was significantly lower in CPs than in HCWs, regardless of the vaccine type (49% vs. 85%, p < 0.001). After the second vaccine dose, all participants, including CPs and HCWs, showed seroconversion. To compare the seroconversion rates stratified by vaccine type, both CPs and HCWs were divided into the following four groups: Ca-mRNA, Ca-AdV, HCW-mRNA (HCWs with mRNA vaccine), and HCW-AdV (HCWs with AdV vaccine as the primary series). After the first vaccine dose, spike-specific IgG seroconversion rates were 54.3% and 39.5% in Ca-mRNA and Ca-AdV, respectively, and the difference was not statistically significant (p=0.162). Similarly, seroconversion rates in HCW-mRNA (100%) were higher than those in the HCW-AdV group (78.6%); however, the difference was not statistically significant (p=0.521). The seroconversion rate was significantly lower in the CP group than in the HCW group (p < 0.001 and p=0.005 in the mRNA and AdV-vaccinated, respectively). After the second vaccine dose, the seroconversion rates in all the groups increased to 100%, which was maintained after the booster dose (S2 Fig.).
3. Binding antibody concentrations
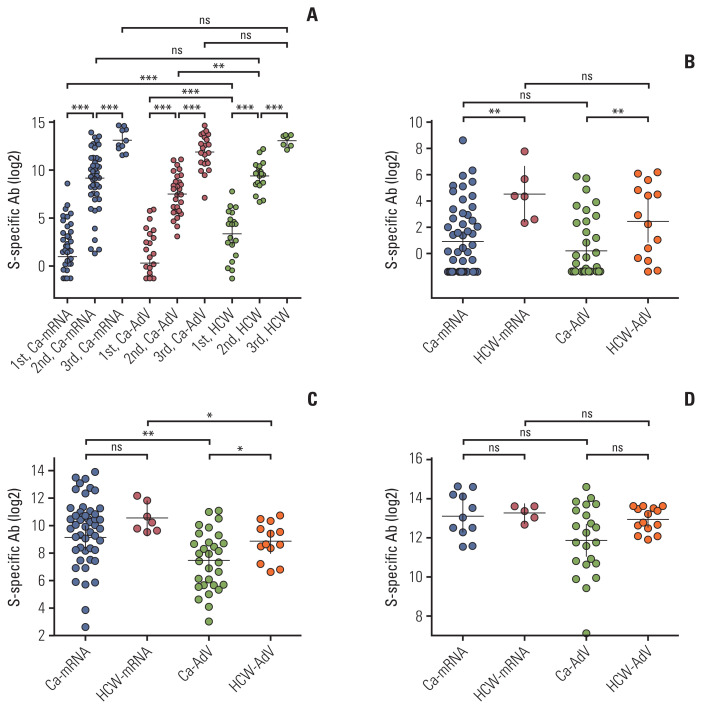
Spike-specific bAb titers after each dose of vaccination were compared between the CPs and HCWs. The GMTs of spike-specific antibody concentrations significantly increased following each dose of vaccination in all Ca-mRNA, Ca-AdV, and HCW groups (Fig. 1A, S3 Table) After the first vaccine dose, HCWs exhibited higher antibody concentrations than CPs, regardless of the type of vaccine received (23.1 vs. 1.9, p=0.004 in HCW-mRNA and CA-mRNA, 5.6 vs. 1.2, p=0.003 in HCW-AdV and Ca-AdV). Among the CPs, similar to HCWs, the vaccine type did not affect the concentration of spike-specific antibodies elicited by the first dose (Fig. 1B). After the second vaccine dose, participants receiving the mRNA vaccine showed higher antibody titers than those receiving the adenovirus vectored vaccine (566 vs. 177, p=0.001 in Ca-mRNA and Ca-AdV, 1,494 vs. 468, p=0.039 in HCW-mRNA and HCW-AdV). Ca-AdV showed significantly lower antibody concentrations than those observed in HCW-AdV (177 vs. 468, p=0.043), while GMTs in Ca-mRNA were insignificantly lower than those in HCW-mRNA (566.6 vs. 1,494, p=0.356) (Fig. 1C). After the booster dose, there were no statistical differences in the GMTs of bAbs between CPs and HCWs, regardless of the primary vaccine type (8,810 vs. 9,866, p=0.668 in Ca-mRNA and HCW-mRNA, 3,744 vs. 7,835, p=0.101 in Ca-AdV and HCW-AdV). After the booster dose of the mRNA vaccine, the primary series of vaccination with adenoviral vaccines exhibited comparable concentrations to those with mRNA vaccines in both CPs and HCWs (Fig. 1D). The details of antibody concentrations, intervals between two doses, and sampling for each vaccination are described in S3 Table.
Fig. 1.
Spike-specific binding antibody (S-specific Ab) concentrations following each vaccination. S-specific Ab concentrations following severe acute respiratory syndrome coronavirus 2 vaccine in the solid cancer patients (CPs) and healthcare workers (HCWs) (A) after vaccination in CPs and HCWs receiving either mRNA or adenovirus vector vaccine. Comparison of binding antibody concentrations between CPs and HCWs stratified by the type of primary vaccination set after the first vaccination (B), after the second vaccination (C), and after the third vaccination (D). Antibody levels are represented as U/mL, and described as geometric mean with 95% confidence interval. Statistical analyses within each group were performed using the 2-tailed Mann-Whitney test. The Ca-mRNA group received a primary set of mRNA vaccines (either BNT162b2 or mRNA-1273), and the Ca-AdV group received a primary set of adenovirus vector vaccines (AZD1222); HCW-mRNA and HCW-AdV are groups of age-matched HCWs who received a primary series of mRNA vaccines and adenovirus vector vaccines, respectively. Each circle indicates the reciprocal binding antibody titer. ns, no significance; *p < 0.05, **p < 0.01, ***p < 0.001.
4. Neutralizing antibodies in CPs
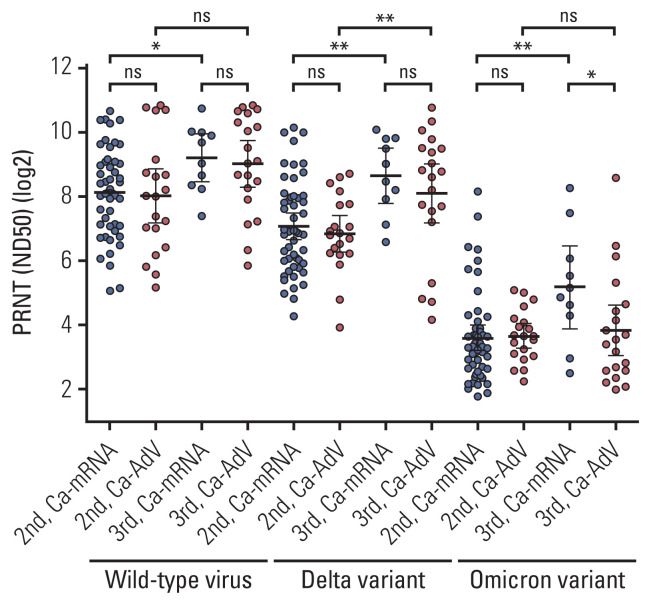
After the second vaccine dose, nAb titers against the wild-type virus, Delta, and Omicron variants in Ca-mRNA were comparable to those against Ca-AdV (278 vs. 256, p=0.706; 133 vs. 114, p=0.843; and 12 vs. 13, p=0.274, respectively). After the booster dose, nAb titers against the wild-type virus and the delta variant in Ca-mRNA were comparable to those with Ca-AdV (582 vs. 513, p=0.965 and 397 vs. 272, p=0.644, respectively), whereas nAbs against the Omicron variant were relatively lower in Ca-AdV than in Ca-mRNA (36 vs. 14, p=0.053). The booster dose significantly increased the neutralization titers against the wild-type virus (278 vs. 582, p=0.018 in Ca-mRNA; 256 vs. 513, p=0.088 in Ca-AdV), Delta variant (133 vs. 397, p=0.003 in Ca-mRNA; 114 vs. 272, p=0.008 in Ca-AdV), and Omicron variant in Ca-mRNA (12 vs. 36, p=0.008); however, the nAb titers against the Omicron variant in CPs with AdV were not quantitatively increased following the booster dose (13 vs. 14, p=0.695, Ca-AdV) (Fig. 2, S4 Table).
Fig. 2.
Neutralization titers against wild-type virus and the Delta and Omicron variants following the second and third vaccination in patients with solid cancers, in accordance with the type of primary series of vaccine. Antibody titers are described as geometric mean with 95% confidence interval. Significance was analyzed using the 2-tailed Mann-Whitney test between two groups. The Ca-mRNA group received a primary series of mRNA vaccines (either BNT162b2 or mRNA-1273) and the Ca-AdV group received a primary series of adenovirus vector vaccines (AZD1222). Each circle indicates the reciprocal binding antibody titer. ns, no significance; *p < 0.05, **p < 0.01.
5. Factors associated with a robust immune response (ad hoc analysis)
To identify the factors associated with a good humoral response among CPs, we analyzed CPs who presented robust and weak spike-specific bAb responses. CPs with spike-specific bAb values after the second vaccine dose, belonging to the highest quartiles in the distribution of anti-body concentrations within the age-matched HCW group stratified by 10 years, were defined as good responders, whereas those with the lowest quantiles were defined as poor responders.
In the age-matched analysis between good and poor responders among CPs, there was no significant difference in other factors, including the vaccine type, cancer type, duration between cancer treatment and vaccination, or the administered anticancer treatment. However, compared to CPs in the good responder group, those in the poor responder group showed lower leukocyte counts when treated with cytotoxic agents before vaccination. In addition, the proportion of patients with leukopenia (white blood cells < 4,500/μL) was also higher in the poor responder group than that in the good responder group (67.9% vs. 30%, p=0.018) (Table 2).
Table 2.
Demographics, antibody titers, and test results in poor and good responders
| Poor responders (n=41) | Good responders (n=31) | p-value | |
|---|---|---|---|
| Age (yr) | 57.9±10.9 | 58.3±11.9 | 0.927 |
| Male sex | 24 (58.5) | 24 (77.4) | 0.096 |
| BMI | 22.8±3.2 | 23.4±3.4 | 0.272 |
| Spike antibody titer after first vaccine dose, GMT | 0.9 | 4.7 | 0.003 |
| Spike antibody titer after second vaccine dose, GMT | 78.4 | 2,595.5 | < 0.001 |
| Spike antibody titer after second vaccine dose, GMT | 2,589 | 9,703 | 0.006 |
| Vaccine (1st and 2nd doses) | |||
| BNT162b2 | 23 (56.1) | 15 (48.4) | 0.176 |
| ChAdOx1 nCoV-19 | 15 (36.6) | 10 (32.3) | |
| mRNA-1273 | 3 (7.3) | 6 (19.4) | |
| Cancer type | |||
| Stomach | 26 (63.4) | 22 (71.0) | 0.473 |
| Pancreas | 4 (9.8) | 1 (3.2) | |
| Colorectum | 4 (9.8) | 1 (3.2) | |
| Melanoma | 2 (4.9) | 3 (9.7) | |
| Biliary | 1 (2.4) | 1 (3.2) | |
| Other | 4 (9.8) | 3 (9.7) | |
| Aim | |||
| Adjuvant | 8 (19.5) | 9 (29.0) | 0.523 |
| Palliative | 33 (80.5) | 22 (71.0) | |
| Chemotherapy regimen | |||
| Cytotoxic agents | 27 (65.9) | 23 (74.2) | 0.353 |
| Cytotoxic+immunotherapy | 4 (9.8) | 2 (6.5) | |
| Immunotherapy | 5 (12.2) | 4 (12.9) | |
| Others | 5 (12.2) | 2 (6.5) | |
| Days from the start of chemotherapy to vaccination | 14 (8–25) | 13 (5.5–20) | 0.463 |
| Laboratory results in patients with cytotoxic agents | 28 | 20 | |
| WBC, per microliter | 4,190 (3,520–5,240) | 5,125 (4,410–6,838) | 0.012 |
| Leukopenia (<4,500/μL) | 19 (67.9) | 6 (30.0) | 0.018 |
| Neutrophil, per microliter | 2,000 (1,580–2,840) | 2,795 (2,040–4,062) | 0.085 |
| Hemoglobin (g/dL) | 11.5 (10.6–12.7) | 12.0 (10.9–12.9) | 0.288 |
| Platelet, thousand per microliter | 182 (137–263) | 222 (137–263) | 0.315 |
| Reactogenicity of 2nd vaccine | |||
| Systemic symptoms | 25 (61.0) | 19 (61.3) | 0.840 |
| Localized symptoms | 30 (73.2) | 23 (74.2) | 0.922 |
Values are presented as mean±SD, number (%), or median (IQR). Good and poor responders were defined as those with higher and lower antibody titers than the mean of the age-stratified healthy cohort after the second vaccination, respectively. BMI, body mass index; GMT, geometric mean titer; IQR, interquartile range; SD, standard deviation; WBC, white blood cell.
6. Reactogenicity
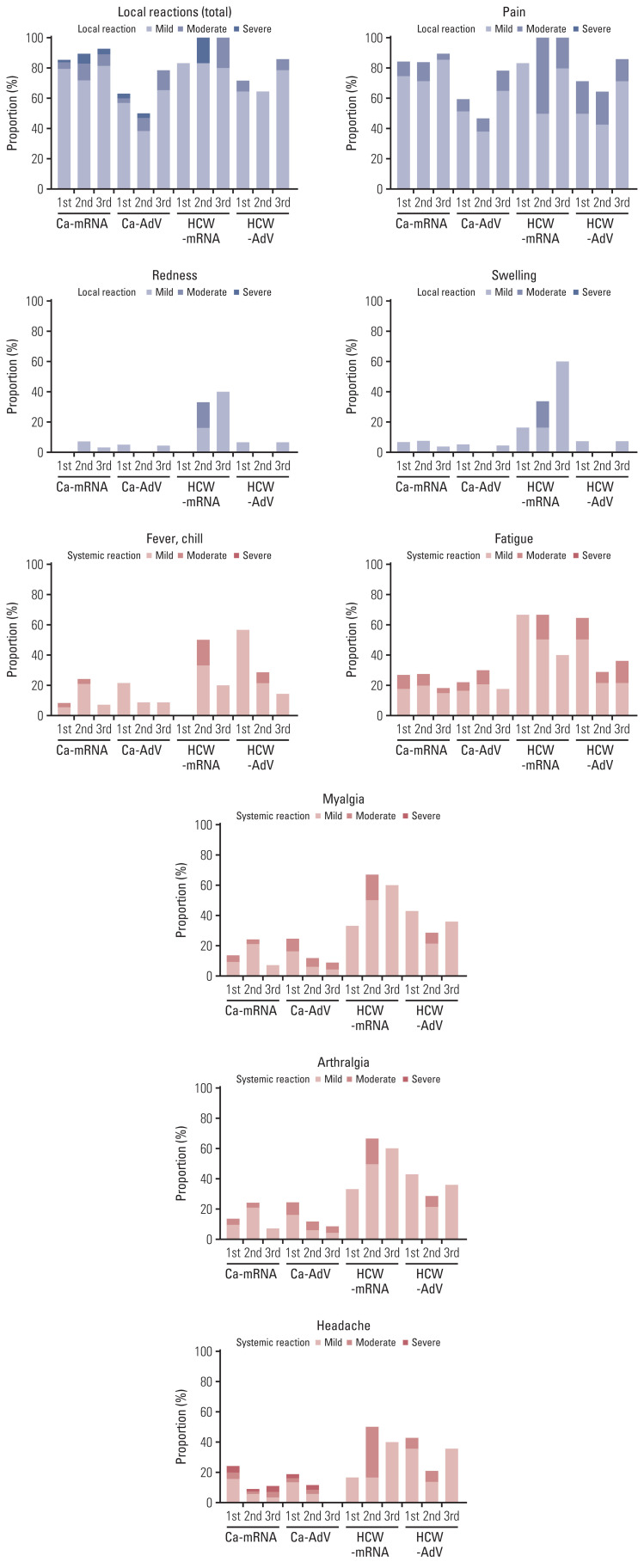
The most common solicited ARs following vaccination were injection site pain, followed by fatigue, myalgia, and headache (Fig. 3). Local reactions, including pain, swelling, and redness after the first vaccine dose were reported in 85% and 83% of Ca-mRNA and HCW-mRNA, respectively and 63% and 71% of Ca-AdV and HCW-AdV, respectively. The second and third doses caused lesser local reactions in CPs than those in the controls, regardless of vaccine type. The third mRNA vaccine dose caused more local reactions than those after the second adenoviral-vectored vaccine (78% and 50% in CP group and 86% and 64% in HCW group, respectively). The incidence and severity of the overall solicited ARs were similar or lower in CPs than in HCWs. Specifically, in the first vaccinated AdV group, the incidence of either fatigue or fever/chill was significantly higher in HCWs than in CPs; in the second vaccinated mRNA group, injection site redness, headache, myalgia, and arthralgia were significantly more common in HCWs than in CPs. However, pain at the injection site was more frequent and severe in CPs than in HCWs, regardless of the vaccine or dose (S5 Table). No life-threatening vaccine-related events or unsolicited reactions were observed in our study.
Fig. 3.
Reactogenicity after each vaccination with mRNA and adenoviral vector vaccine among cancer patients and healthcare workers (HCWs). Participants were questioned about the duration and severity of each symptom with grades, within 7 days following each dose of vaccine. To calculate the cumulative symptom scores, the scores were summed and adapted to severity: asymptomatic (grade 0), mild (below 4 scores, grade 1), moderate (below 8 scores, grade 2), and severe (> 8 scores, grade 3). Proportion of adverse reactions stratified by severity in each group was calculated.
Discussion
In our study, the first dose of SARS-CoV-2 vaccination did not manifest sufficient seropositivity; however, high seropositive rates and increased humoral response were confirmed after the second vaccine dose among patients with solid cancers who were exposed to multiple chemotherapy sessions and actively undergoing anticancer treatment. One cohort study showed that patients with solid cancers had lower proportion of detectable nAb against wild-type virus than individuals without cancer, but median nAb titers showed no statistical difference [18]. Other cohort participants with solid organ or hematologic cancers receiving two doses of BNT162b2, mRNA-1273, or one dose of Ad26.COV2, bAb, and nAb titers are quantitatively lower in patients with solid cancers than in the healthy controls, regardless of the vaccine type and presence of previous infection [19]. In our study, CPs receiving two doses of mRNA vaccines elicited bAb concentrations comparable to those in age-matched healthy individuals. CPs who were administered two doses of AZD1222 exhibited relatively low bAb concentrations than those in age-matched healthy control. But after the third dose vaccination, the differential of bAb concentrations became insignificant.
Even though patients with solid tumors have shown the significant decrease of anti-spike IgG titers at 4–6 months of follow-up [20], higher concentrations of IgG titers and the stimulation of anti-SARS-CoV-2 T-cell activity have been observed after receiving BNT162b2 as a booster dose [20,21]. In addition, booster dose confers enhanced variant neutralization breadth, which is associated with an improved magnitude of wild-type neutralization [22]. In our study, booster vaccination increased neutralization titers against wild-type virus and delta variant in CPs regardless of vaccine type. Since the neutralizing antibodies are considered the most reliable predictor of protecting against symptomatic infection with SARS-CoV-2, the third booster vaccine induces enhanced immunogenicity in actively treated solid CPs.
Cross-neutralization of the Omicron variant has been reported in the sera from homologous BNT162b2 or heterologous vaccination, whereas immune escape has been observed in the sera from homologous AZD1222 vaccination in healthy participants [23]. The effect of mRNA booster vaccination in CPs with homologous AZD1222 vaccination was not evaluated. In the present study, after the second vaccination, the neutralization against the Omicron variant was relatively low in CPs. The booster dose did not elicit an expected increase in nAbs against the Omicron in AdV-cancer cohort who were older than mRNA cohort. Intriguingly, neutralizing titers against the Omicron variant following three doses of vaccination were not sufficient in these poor responders.
Low seroconversion rates have been observed in cytotoxic chemotherapy-treated patients compared to those treated with an immune checkpoint inhibitor (CPI) or hormonal therapy [24–26]. The receipt of chemotherapy in the previous year or current steroid treatment has been also associated with low bAb levels [19]. In contrast, some studies have not shown significant associations with reduced nAb in patients with systemic anticancer therapy [18] or a negative interaction between cytotoxic chemotherapy and antibody response [27]. In our study, leukopenia was associated with low immunogenicity among CPs treated with cytotoxic agents. Immunotherapy such as CPIs could stimulate the immunologic system, particularly T-cell response, and boost the immunogenicity after influenza vaccination [28]. The interaction between SARS-CoV-2 vaccines and immunotherapy has not been clearly demonstrated yet [29]. There were no immunologically related adverse events or superior humoral immune responses after the SARS-CoV-2 vaccination in patients with immunotherapy in our study.
This study also had several limitations. This was an observational cohort study, with no randomization of vaccine types, vaccination time points, or sampling among participants. Immunogenicity and reactogenicity may depend on sex and age, as CPs receiving primary series of adenoviral-vectored vaccines were approximately 10 years older than those who receiving the mRNA vaccine, and the cancer cohort had a higher proportion of male participants than those in the healthy controls. In addition, the sample size of HCW cohorts was small and due to insufficient sample volume, we did not evaluate neutralizing antibodies in HCWs; thus, the comparison of neutralization titers in the HCW and CP cohorts was not feasible. We could not evaluate the antibody titer before the third vaccination. However, our participants were regularly tested for SARS-CoV-2 during the study period, and there were no confirmed COVID-19. Even though the waning titers were not checked, we could evaluate the effect of the booster dose. Among the patients receiving mRNA vaccines as a primary series, 10 out of 80 patients participated in the third vaccine and were evaluated for immunogenicity, which could not represent the whole group. However, the third vaccine uptake was decided by patients themselves following the recommendation. Three and four patients participating in the third vaccination were good and poor responders to the second vaccination, respectively, indicating a similar distribution of CPs with mRNA. Lastly, we did not measure the T-cell response after SARS-CoV-2 vaccination, which is also important to prevent severe COVID-19 infection along with humoral immune response and neutralization antibodies against BA.5 predominantly circulating as of October 2022. However, this is a prospective study of patients with actively treated solid cancer who require more studies and real-world data. Participants in the cohort followed the recommendation from the time period, and are representative of the population. To our knowledge, this is the first study in Asia to report immunogenicity against SARS-CoV-2 following vaccination among actively treated CPs.
In conclusion, the third vaccination elicited comparable spike-specific binding antibody concentrations between CPs and HCWs, regardless of the primary vaccine type. Neutralization against the Omicron variant was not robustly elicited in some solid CPs, implying the need for additional interventions to protect them from severe COVID-19.
Acknowledgments
We thank Dr. Kim HB, Dr. Kim ES, Dr. Song KH, Dr. Moon SM, Dr. Choi SJ (Seoul National University Bundang Hospital, Seoul National University College of Medicine), Dr. Kim SW (Kyungpook National University), Dr. Kwon KT (Chilgok Hospital, Kyungpook National University), Dr. Jeong HW (Chungbuk National University), Dr. Choi WS (Ansan Hospital, Korea University), and Dr. Ko JH (Samsung Medical Center, Sungkyunkwan University School of Medicine) for providing the results for binding antibodies in a healthcare worker cohort.
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A1A01055124) and a faculty research grant from the Yonsei University College of Medicine (6-2021-0066).
Footnotes
Ethical Statement
All studies were approved by the institutional review board of Severance Hospital (4-2020-0076) and written informed consent was obtained from all participants.
Author Contributions
Conceived and designed the analysis: Jung M, Ahn JY.
Collected the data: Kim KH, Beom SH, Lee CK, Shin SJ, Rha SY, Lee KH, Jung M.
Contributed data or analysis tools: Lee YJ, Park SR, Kim S.
Performed the analysis: Baek YJ, Kim S, Kim JH, Jeong SJ, Ku NS, Choi JY, Yeom JS, Ahn JY.
Wrote the paper: Baek YJ.
Supervision: Jung M, Ahn JY.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
References
- 1.Al-Quteimat OM, Amer AM. The impact of the COVID-19 pandemic on cancer patients. Am J Clin Oncol. 2020;43:452–5. doi: 10.1097/COC.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7:220–7. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian Y, Qiu X, Wang C, Zhao J, Jiang X, Niu W, et al. Cancer associates with risk and severe events of COVID-19: a systematic review and meta-analysis. Int J Cancer. 2021;148:363–74. doi: 10.1002/ijc.33213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, Chai P, Yu J, Fan X. Effects of cancer on patients with COVID-19: a systematic review and meta-analysis of 63,019 participants. Cancer Biol Med. 2021;18:298–307. doi: 10.20892/j.issn.2095-3941.2020.0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–15. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, et al. Phase 3 safety and efficacy of AZD1222 (ChAd-Ox1 nCoV-19) Covid-19 vaccine. N Engl J Med. 2021;385:2348–60. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becerril-Gaitan A, Vaca-Cartagena BF, Ferrigno AS, Mesa-Chavez F, Barrientos-Gutierrez T, Tagliamento M, et al. Immunogenicity and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after coronavirus disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2022;160:243–60. doi: 10.1016/j.ejca.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra SK, Pradhan SK, Pati S, Sahu S, Nanda RK. Waning of anti-spike antibodies in AZD1222 (ChAdOx1) vaccinated healthcare providers: a prospective longitudinal study. Cureus. 2021;13:e19879. doi: 10.7759/cureus.19879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreira ED, Jr, Kitchin N, Xu X, Dychter SS, Lockhart S, Gurtman A, et al. Safety and efficacy of a third dose of BNT162b2 Covid-19 vaccine. N Engl J Med. 2022;386:1910–21. doi: 10.1056/NEJMoa2200674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbel R, Hammerman A, Sergienko R, Friger M, Peretz A, Netzer D, et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413–20. doi: 10.1056/NEJMoa2115624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Information for healthcare practitioners [Internet] London: UK Health Security Agency; 2022. COVID-19 vaccination programme. [cited 2023 Feb 8]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1110045/COVID-19-vaccine-information-for-healthcare-practitioners-version-5.pdf. [Google Scholar]
- 12.Preliminary public health considerations for COVID-19 vaccination strategies in the second half of 2022 [Internet] Stockholm: European Centre for Disease Prevention and Control; 2022. [cited 2023 Feb 8]. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Preliminary-public-health-considerations-%20COVID-19-vaccination-2022.pdf. [Google Scholar]
- 13.Fendler A, de Vries EGE, GeurtsvanKessel CH, Haanen JB, Wormann B, Turajlic S, et al. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat Rev Clin Oncol. 2022;19:385–401. doi: 10.1038/s41571-022-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bae S, Ko JH, Choi JY, Park WJ, Lim SY, Ahn JY, et al. Heterologous ChAdOx1 and Bnt162b2 vaccination induces strong neutralizing antibody responses against SARS-CoV-2 including delta variant with tolerable reactogenicity. Clin Microbiol Infect. 2022;28:1390. doi: 10.1016/j.cmi.2022.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elecsys anti-SARS-CoV-2S instructions for use [Internet] Silver Spring MD: U.S. Food and Drug Administration; 2022. [cited 2023 Feb 8]. Available from: https://www.fda.gov/media/144037/download. [Google Scholar]
- 16.Elecsys-Anti-SARS-CoV-2-S-factsheet-SEPT-2020, 2 [Internet] Rotkreuz: Roche; 2020. [cited 2023 Feb 8]. Available from: https://diagnostics.roche.com/content/dam/diagnostics/Blueprint/en/pdf/cps/Elecsys-Anti-SARS-CoV-2-S-factsheet-SEPT-2020-2.pdf. [Google Scholar]
- 17.Center for Biologics Evaluation and Research . Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Rockville, MD: U.S. Food and Drug Administration; 2009. [Google Scholar]
- 18.Fendler A, Shepherd ST, Au L, Wilkinson KA, Wu M, Byrne F, et al. Adaptive immunity and neutralizing antibodies against SARS-CoV-2 variants of concern following vaccination in patients with cancer: the CAPTURE study. Nat Cancer. 2021;2:1305–20. doi: 10.1038/s43018-021-00274-w. [DOI] [PubMed] [Google Scholar]
- 19.Naranbhai V, Pernat CA, Gavralidis A, St Denis KJ, Lam EC, Spring LM, et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX cohort study. J Clin Oncol. 2022;40:12–23. doi: 10.1200/JCO.21.01891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shapiro LC, Thakkar A, Campbell ST, Forest SK, Pradhan K, Gonzalez-Lugo JD, et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell. 2022;40:3–5. doi: 10.1016/j.ccell.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasagna A, Bergami F, Lilleri D, Percivalle E, Quaccini M, Alessio N, et al. Immunogenicity and safety after the third dose of BNT162b2 anti-SARS-CoV-2 vaccine in patients with solid tumors on active treatment: a prospective cohort study. ESMO Open. 2022;7:100458. doi: 10.1016/j.esmoop.2022.100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naranbhai V, St Denis KJ, Lam EC, Ofoman O, Garcia-Beltran WF, Mairena CB, et al. Neutralization breadth of SARS-CoV-2 viral variants following primary series and booster SARS-CoV-2 vaccines in patients with cancer. Cancer Cell. 2022;40:103–8. doi: 10.1016/j.ccell.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med. 2022;386:698–700. doi: 10.1056/NEJMc2119236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agbarya A, Sarel I, Ziv-Baran T, Agranat S, Schwartz O, Shai A, et al. Efficacy of the mRNA-based BNT162b2 COVID-19 vaccine in patients with solid malignancies treated with anti-neoplastic drugs. Cancers (Basel) 2021;13:4191. doi: 10.3390/cancers13164191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thakkar A, Gonzalez-Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39:1081–90. doi: 10.1016/j.ccell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ligumsky H, Safadi E, Etan T, Vaknin N, Waller M, Croll A, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. J Natl Cancer Inst. 2022;114:203–9. doi: 10.1093/jnci/djab174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelli F, Fabbri A, Onorato A, Giannarelli D, Silvestri MA, Giron Berrios JR, et al. Effects of active cancer treatment on safety and immunogenicity of COVID-19 mRNA-BNT162b2 vaccine: preliminary results from the prospective observational Vax-On study. Ann Oncol. 2022;33:107–8. doi: 10.1016/j.annonc.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brest P, Mograbi B, Hofman P, Milano G. COVID-19 vaccination and cancer immunotherapy: should they stick together? Br J Cancer. 2022;126:1–3. doi: 10.1038/s41416-021-01618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piening A, Ebert E, Khojandi N, Alspach E, Teague RM. Immune responses to SARS-CoV-2 in vaccinated patients receiving checkpoint blockade immunotherapy for cancer. Front Immunol. 2022;13:1022732. doi: 10.3389/fimmu.2022.1022732. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.