Abstract
Background
Bovine mastitis, a condition with multifactorial etiology, imposes a significant economic burden on the dairy sector in Ethiopia, with Staphylococcus aureus (S. aureus) being one of the leading etiologic agents. The acquisition of a compiled source of information concerning S. aureus is imperative in order to enhance the control and prevention strategies, as well as to facilitate the successful implementation of the national action plan aimed at curbing antimicrobial resistance by the year 2025. Thus, the primary objective of this meta-analysis was to comprehensively summarize the estimates of the proportion and beta-lactam resistance profile of S. aureus in bovine mastitis in Ethiopia.
Methods
electronic bibliographic data such as PubMed, Web of Science, HINARI, Google Scholar, and other databases were used to search articles and quality assessment was performed using the AMSTAR-2. The pooled proportion, the rate of beta-lactam resistance, and a 95% confidence interval were calculated with a random effects model using STATA 17 statistical software. Funnel plots, and Eggers were used to assess publication bias.
Results
Twenty-six (26) cross-sectional studies were included in this meta-analysis. The overall pooled proportion of S.aureus was 35% (95% CI: 0.31 to 0.41). Considerable heterogeneity was observed in the included studies (I2 = 90.75%; P < 0.01). The subgroup analysis of the study region showed significant differences. The highest estimated regional pooled proportion of bovine mastitis-associated S.aureus was 40% in the Amhara and Tigray regions. Funnel plot and Eggers results showed no statistically significant publication bias (Eggers test: p = 0.5656) in estimating the proportion of S.aureus infections in association with bovine mastitis. A total of 14 articles were included to estimate beta-lactam antimicrobial resistance. The estimated pooled beta-lactam antimicrobial resistance rate of S.aureus was resistance to penicillin at 75%, followed by amoxicillin at 67%, ampicillin at 50% and cephalosporin at 57% were evaluated in the treatment of S. aureus. Therefore, the present meta-analysis has revealed that the prevalence of bovine-associated Staphylococcus aureus and its resistance to beta-lactam antibiotics are alarmingly high in the region of Ethiopia. This further emphasizes the vital necessity of implementing effective preventive measures to reduce the incidence and spread of this pathogen across the entire nation.
Keywords: Bovine mastitis, Beta-lactam, Pooled prevalence, Resistance, Staphylococcus aureus
1. Introduction
Bovine mastitis is one of the major and serious diseases that has a significant impact on dairy production [1,2]. The issue of bovine mastitis requires special attention for farmers residing in low-income countries, including Ethiopia. Losses due to mastitis include reduced milk production, condemnation of milk due to antibiotic residues, veterinary costs, and culling of cows infected over a long period of time [3]. Bovine mastitis is mainly categorized as subclinical and clinical based on the level of severity.
According to Ref. [4] production losses due to subclinical mastitis in Ethiopia accounts for over 90% of the total milk production loss [5] and are estimated at US$38 per lactation per cow from crossbred dairy cows. In addition, it has public health implications; with serious zoonotic potential due to the excretion of zoonotic pathogens and their toxins in dairy products [6]. Mastitis is caused by a wide range of pathogens including bacteria, fungi and viruses. At least 137 etiological agents that can cause mastitis in large domestic animals have been studied, with bacteria being the main culprits [7]. Staphylococcus aureus is one of the most important contagious causative agents of bovine mastitis and is responsible for milk spoilage [8].
Another serious public health impact of S. aureus is drug resistance. People with methicillin-resistant S.aureus (MRSA) infections have a 64% higher mortality rate than people with drug-sensitive infections. Methicillin resistance is caused by the acquisition of the mecA gene. This gene encodes an alternative penicillin-binding protein called PBP2A, which has a low affinity for beta-lactam antibiotics [9].
Numerous investigations have documented the frequencies of Staphylococcus. aureus resistance across diverse nations. In the United States, a cross-sectional investigation was carried out during the year 2005 at the annual American College of Veterinary Internal Medicine forum. The primary objective of this study was to ascertain the extent of methicillin-resistant S. aureus colonization among Veterinary personnel. Results indicated that 7% of veterinarians and 12% of technician attendees were colonized with MRSA [10]. Rates were above 70% in South Korea and Vietnam and below 50% in Portugal, Greece and Italy. In Egypt, a study found a prevalence rate of 17.2%. Another study showed that 70–73% of S. aureus strains isolated from various foods were resistant to -lactam antibiotics such as penicillin and ampicillin. A study conducted in South Africa found that the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) on commercial farms was 5.7–7%. In other African countries, MRSA prevalence rates were higher in Ethiopia (60.3%), Nigeria (28.57%) and Morocco (15%), while lower prevalence rates were recorded in Kenya (7.8%) [11].
Staphylococcus aureus is usually affects different body parts of dairy animals, including the head, skin, and nasal mucosa. However, an infected udder quarter remains the main reservoir of infection for non-infected animals during the milking time through contaminated milkers’ hands (developing countries), and milking machines in the case of developed countries [12].The identification and isolation methods, S.aureus could be differentiating from other species based on the formation of coagulase, the fermentation of mannitol, and trehalose [13]. Coagulase test may have the ability to identify S.aureus in more than 95% of all coagulase-positive staphylococci in bovine mastitis. However, it is not a 100% confirmatory diagnosis of S. aureus in mastitis infection. Staphylococcus.aureus is the most common contagious and zoonotic bacterial infection causing mastitis which results from high production loss in Ethiopia. Several investigations have been documented about the prevalence of S. aureus infection in bovine mastitis in different regions and different period of time, with prevalence at cow, herd, and quarter levels. According to most recent reports, the prevalence of S.aureus in clinical and sub-clinical bovine mastitis ranges from 10% to 66.07% [[14], [15], [16]] have been studied. Moreover, some investigations in the previous year reported that the average infected quarter suffers a 30% reduction in milk yield and at the cow level a 15% loss its production.
Milk is a comprehensive and highly nutritious food source for humans. Cattle are the main contributors to national milk production, accounting for 85–89% of total production [17,18]. Nonetheless, in several developing countries, including Ethiopia, the quality of dairy products has become a significant health concern for consumers, particularly infants and children. S. aureus is a major pathogen of the disease [19] and also the third most common foodborne pathogen worldwide, posing a significant threat to animal husbandry, and public health [20]. This results in a loss of up to €300 animals per cow per year, leading to an increase in clinical, subclinical and recurrent mastitis in cows. The situation is particularly alarming in underdeveloped countries such as Ethiopia, where there is a high prevalence of infectious diseases, limited surveillance networks, problems with laboratory capacity, and poor diagnostic practices [21]. The most worrying aspect of the situation is that contaminated milk may contain antimicrobial-resistant S. aureus, which poses a significant health risk to consumers and is recognized by international health organizations as one of the most critical health issues of the 21st century [22]. The most likely reason for the high prevalence of S. aureus is the lack of routine prevention and control measures for foodborne pathogens implemented by farms, milk collectors and dairy processors [23]. The findings of a survey conducted in central Ethiopia indicated that raw milk consumption was reported by 31.8% of individuals of all ages [24]. Given that milk is considered a complete meal for human consumption and also acts as a medium for microbial growth, it is crucial to ensure that proper hygiene standards are met for milk products. Beta-lactams serve as the first treatment option for bovine mastitis-associated S. aureus in Ethiopia [25]. However, improper and repeated use of these drugs has contributed to the emergence of resistant bacteria. Staphylococcus.aureus responds poorly to antibiotic treatments and can lead to prolonged infection and antimicrobial resistance [26].
Meta-analyses are conducted with the aim of summarizing existing evidence and informing specific decisions as well as it could be extended to incorporate economic considerations in a decision analysis framework [27]. Understanding the prevalence and antimicrobial resistance rate of S.aureus associated with bovine mastitis is crucial in enhancing therapeutic interventions and preventive measures. Therefore, this systematic review and meta-analysis aimed to provide an overall estimate of the proportion of bovine mastitis associated-S.aureu sand beta-lactam resistance rate in the available literature in Ethiopia.
2. Methods
The present review employed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis) 20220 checklist and PRISMA 2020 abstract checklist, as prescribed by the established guidelines [28]. The checklist served to ensure the comprehensive incorporation of pertinent data from the chosen articles, based on the underlying protocols (Supplementary file 1&2).
2.1. Search strategy
The literature search was conducted from September 10, 2021, to October 5, 2022. A comprehensive search strategy was made to identify included studies. Databases such as PubMed, Web of Science, Google Scholar, HINARI, retrieved articles and other manual methods were used for literature searches. The research question was “What is the proportion of S.aureus infection in clinical and subclinical mastitis among mastitis-causing pathogens in lactating dairy cows in Ethiopia? CoCoPop (Condition, Context, and Population) framework was undertaken to search for relevant articles. The condition was staphylococcus infection (Co), the context was Ethiopia (Co), and the Population was cows (Pop).
The search strategy included Medical Subject Heading (MeSH) terms and a range of important keywords. The MeSH terms incorporated in this step like staphylococcus aureus, staphylococcus infection, cross-sectional studies, prevalence, mastitis /mammary gland infection, bovine/lactating cows, and epidemiology. Then, the Boolean operator “AND /OR” were applied during an online search by combining related keywords/phrases. Searching protocols used were (Staphylococcus OR Staphylococcus infection OR Staphylococcus aureus) AND (occurrence OR prevalence OR infection rate) AND (cows OR dairy cows) AND (mastitis) AND (Ethiopia). All identified studies were imported to Endnote 20 software to remove duplicates.
2.2. Selection criteria
The establishment of inclusion and exclusion criteria based on quality was enacted before the commencement of the review processes. Subsequently, the two authors (F.A and N·B) independently conducted a sorting of all studies selected through the search strategy. The selection criteria utilized for research articles and published reports were centered on the primary objectives of the study, as outlined elsewhere. Consequently, any study to be included in the meta-analysis was required to satisfy the following eligibility criteria: (i) Studies considered only cross-sectional study designs, (ii) Studies provided only a clear quarter-level estimate of the proportion of S. aureus infection in both clinical and subclinical mastitis in cattle, (iii) selection was only made by the prevalence of S. aureus in cattle and lactating cows (iv) the methods used to detect and diagnose bacterial mastitis include the California Mastitis Test (CMT) and/or clinical examination method, (v) the isolation and identification of S.aureus was performed using standard bacteriological techniques (vi) articles written only in English (vii) the study period after 2008 was considered.
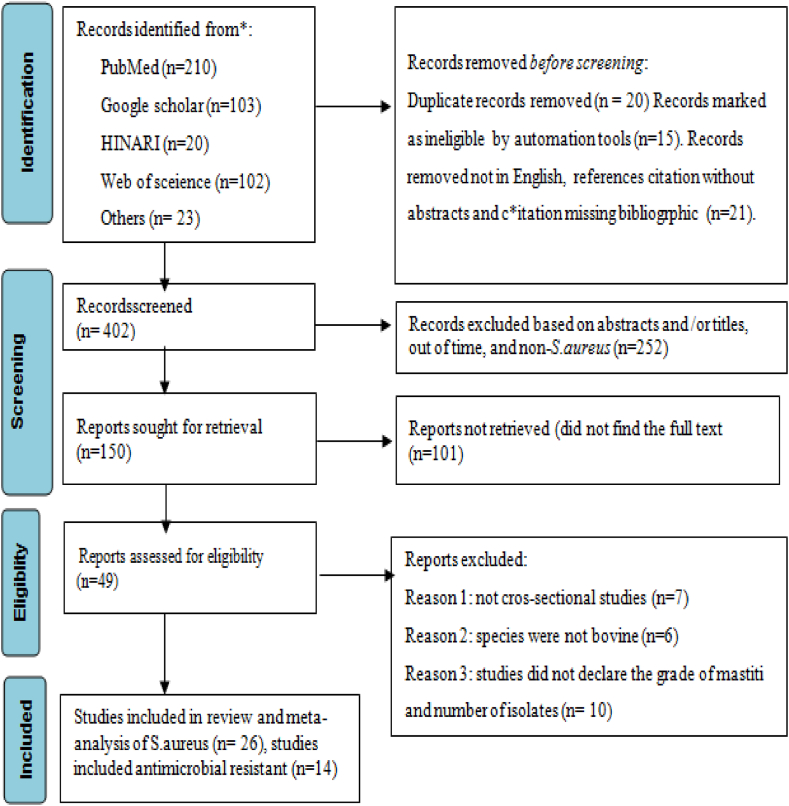
Outbreak reports, case series, traditional reviews, cohort and case-control studies, and experimental (clinical) studies, duplication records, the study samples which did not mastitis and the study not involves bacterial identification and isolation, ambiguous sample size, or bacterial isolate quantity not well determined, and the study was conducted out of the defined period (before 2008) were excluded. Finally, the identified inclusion and exclusion criteria were employed for data extraction and meta-analysis, thereby offering a comprehensive study screening strategy and reasons for exclusion (Fig. 1).
Fig. 1.
PRISMA flow chart for selection of included and excluded of studies.
2.3. Data extraction
From included studies, the following data were recorded by two authors (M.D and B.A) independently: the name of the first author, year of study, study design, study regions, geographical location, and number of cows, number of California mastitis positive quarter, and number of culture positive quarter, and true prevalence of bovine mastitis associated-S.aureus infection.
2.4. Study quality assessment
As meta-analysis considers the highest level of evidence, quality assessment is performed precisely by standard tools. Quality assessment was done by AMSTAR-2(Supplementary file 3) for this review to check the evidence and methodological quality of the research protocols. The results of meta-analysis rely on the assessment of quality of the included studies in this systematic review and met analysis. Quality assessment was done by two researchers (A.S and F.A) independently.
2.5. Data synthesis and statistical analysis
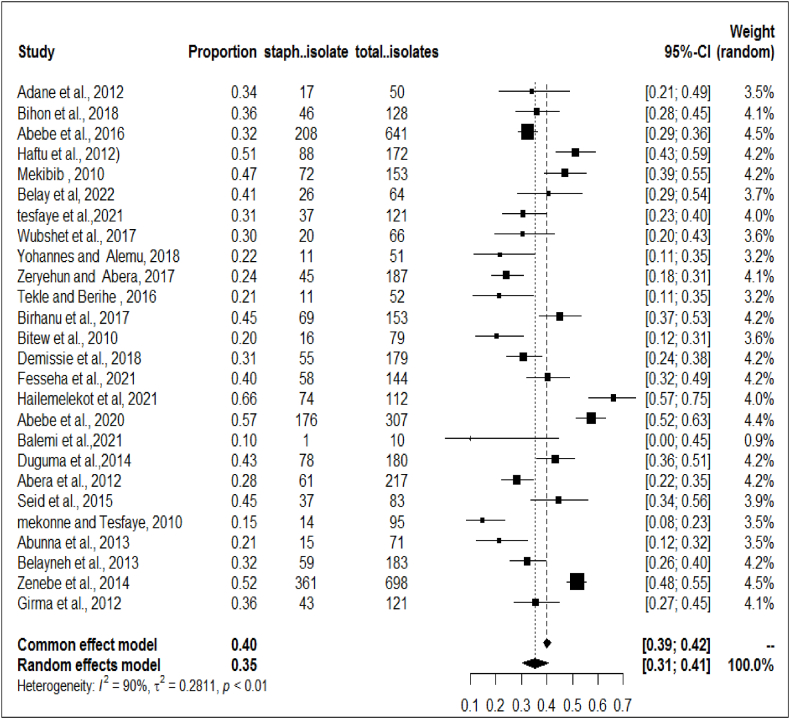
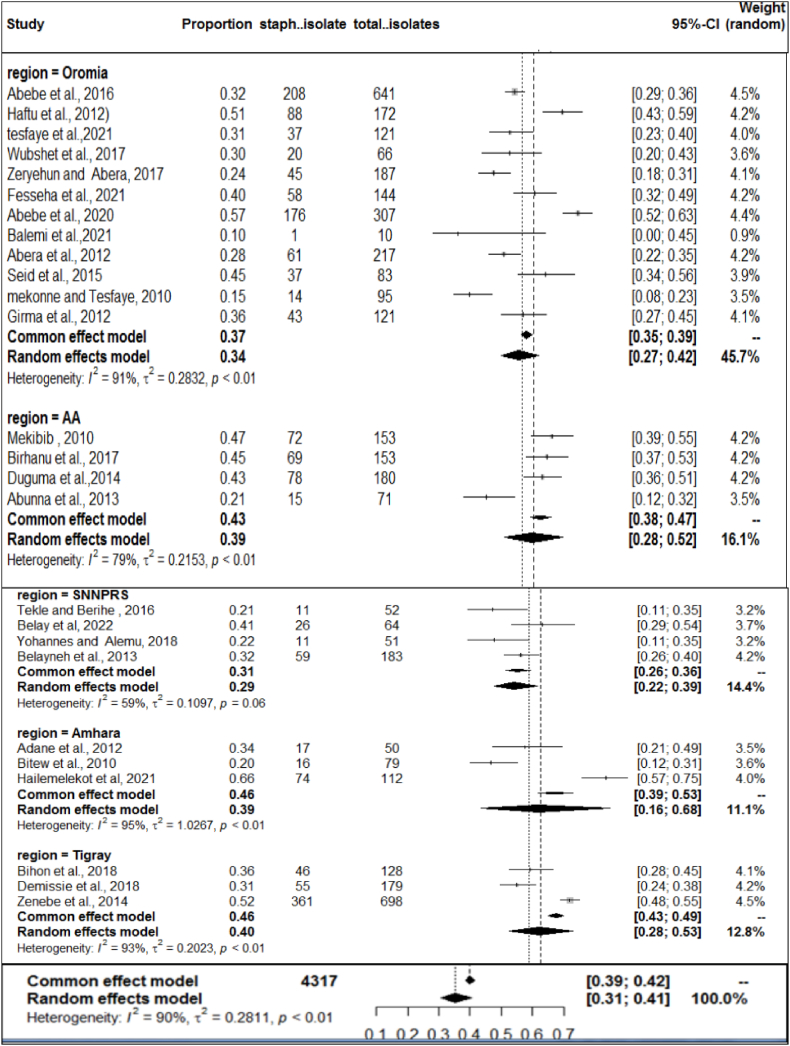
To determine the prevalence of S.aureus in clinical and subclinical bovine mastitis in the included studies, the proportion estimates for each study were pooled using a random effects model at a 95% confidence level. A meta-analysis, a subgroup analysis and a meta-regression analysis were performed using STATA-17 statistical software. Between-study heterogeneity was assessed using the Cochran's Q-test (expressed as a p-value) and the inverse index of variance (I2), which describes the percentage of the total observed variation between studies that is attributable to heterogeneity rather than chance. The I2 values of 25, 50, and 75% show low, medium, and high degrees of heterogeneity, respectively. The presence of heterogeneity between studies was assessed using a forest diagram. The forest plot diagram showed weights, the effect sizes of each study and their CI (Fig. 2). Similarly, subgroup analyzes for the proportion of S.aureus in both clinical and subclinical bovine mastitis with study year and study areas were performed to determine specific between-study variability. The academic year category such as (2012), (2013–2017) and (2018) was formed based on the number of articles included.
Fig. 2.
Forest plot for the proportion of bovine mastitis associated S.aureus in Ethiopia.
Furthermore, a meta-regression analysis was conducted to explore the potential factors that may contribute to the heterogeneity among studies, wherein study year, sample size, and regions were employed as covariates. Univariable meta-regression analysis was performed for each selected variable. The sample size was regarded as a continuous variable, whereas the study year and study regions were considered as categorical variables. Publication bias is an inevitable problem in the systematic review and meta-analysis; it is well known as one most important type of biases termed reporting bias, so it is also one of the main threats to the validity of meta-analysis [29]. Small study effects and publication bias were visualized using funnel plot diagrams, Egger's and Begg's test. Egger's regression test is used to test the funnel-plot symmetry, in which a regression model is built, using the standardized estimate of the effect size as a dependent variable and the inverse of the standard error (1/SE) as an independent variable. If the intercept is significantly different from zero, the estimate of the effect is considered biased [30].
3. Results
3.1. Search results
As viewed in Fig. 1, a total of 458 articles were browsed through different electronic databases and with other methods. A total of 56 articles were removed through duplicate, marked as ineligible and other reasons. A total of 252 articles were excluded through title and abstract screening. One hundred fifty (150) articles reports sought for retrieval and 49 evaluated for eligibility. Finally, only 26 full-text articles for qualitative and quantitative synthesis of bovine mastitis -associated S.aureus were included and 14 studies for antimicrobial test. Hence, it has come to our attention that within the selection process of articles, certain articles have been chosen for both the investigation of the prevalence of S. aureus and beta lactam antimicrobial resistance. Conversely, other articles were solely employed for either the assessment of S. aureus occurrence rate or its antimicrobial resistance.
3.2. Characteristics of included studies
This systematic review and meta-analysis was included published reports of staphylococcal infection in bovine mastitis among lactating cows in Ethiopia. It was comprises a total of twenty-six relevant studies for quantitative synthesis of S.aureus infection both clinical and subclinical bovine mastitis. All of the included studies were conducted by a cross-sectional study design. The included studies for this systematic review and meta-analysis were conducted between 2008 and 2022 in different parts of Ethiopia. The minimum sample size (quarter level) of individual studies included in this systematic review was 41 [14] while the maximum sample size for the was 1502 [16].
The isolation of S.aureus in the included studies were carried out according to the standard microbiological procedures described by NMC(1). All included studies used CMT (sub-clinical mastitis), Bacteriological culture and standard bacterial isolation and identification technique. For this particular study, a comprehensive screening of a total of 8441 cattle was conducted and 10346 quarter-level milk samples analyzed to derive the overall proportion of S. aureus infection in Ethiopia. The observed prevalence of S. aureus in bovine mastitis ranged from 10% to 66.6%. The detailed characteristics of the studies are well documented in (Table 1).
Table 1.
Characteristic of the included studies.
| Study Name | Year | Region | Location | No. cows | TQE | NQP+ | TCP+ | Staph+ | OBP+ | PS* |
|---|---|---|---|---|---|---|---|---|---|---|
| Abebe et al. [1] | 2020 | Oromia | Southern | 529 | 2026 | 729 | 172 | 88 | 84 | 51 |
| Belay et al. [31] | 2020 | SNNPRS | Southern | 686 | 1662 | 132 | 64 | 26 | 38 | 40 |
| Mekibib [32], | 2008 | AA | Central | 107 | 428 | 192 | 153 | 72 | 81 | 47 |
| Tesfaye et al. [33] | 2015 | Oromia | Central | 384 | 1536 | 484 | 121 | 37 | 84 | 30 |
| Wubshet et al. [34] | 2013 | Oromia | Southern | 28 | 112 | 112 | 66 | 20 | 46 | 30 |
| Zeryehun and Abera [35], | 2015 | Oromia | Eastern | 384 | 1536 | 877 | 187 | 45 | 142 | 24 |
| Tekle and Berihe [36], | 2011 | SNNPRS | Southern | 384 | 384 | 70 | 52 | 11 | 41 | 21 |
| Birhanu et al. [37], | 2016 | AA | Central | 262 | 1048 | 170 | 153 | 69 | 84 | 45 |
| Bitew et al. [38] | 2010 | Amhara | Northern | 302 | 1208 | 134 | 79 | 16 | 63 | 20 |
| Demissie et al. [39] | 2015 | Tigray | Northern | 360 | 1440 | 229 | 179 | 55 | 124 | 30 |
| Fesseha et al. [40] | 2019 | Oromia | Southern | 384 | 1536 | 536 | 144 | 58 | 86 | 40 |
| Hailemelekot et al. [41], | 2018 | Amhara | Northern | 302 | 1208 | 126 | 112 | 74 | 38 | .66 |
| Abebe et al. [16] | 2018 | Oromia | Southern | 686 | 2633 | 773 | 307 | 176 | 131 | 57 |
| Balemi et al. [14] | 2018 | Oromia | Southern | 60 | 240 | 41 | 10 | 1 | 9 | 10 |
| Haftu et al. [42] | 2009 | Tigray | Northern | 305 | 1220 | 187 | 128 | 46 | 82 | 35 |
| Duguma et al. [43] | 2009 | AA | Central | 90 | 340 | 275 | 180 | 78 | 102 | 43 |
| Abera et al. [15] | 2008 | Oromia | Southern | 245 | 960 | 288 | 217 | 61 | 156 | 28 |
| Seid et al. [44] | 2014 | Oromia | Southern | 358 | 1422 | 490 | 83 | 37 | 46 | 44 |
| Mekonne and Tesfaye [45], | 2008 | Oromia | Sothern | 206 | 824 | 790 | 95 | 14 | 81 | 14 |
| Abunna et al. [46] | 2008 | AA | Central | 331 | 1324 | 146 | 71 | 15 | 56 | 21 |
| Belayneh et al. [47] | 2008 | SNNPRS | Northern | 303 | 1172 | 244 | 183 | 59 | 124 | 32 |
| Zenebe et al. [48] | 2011 | Tigray | Northern | 322 | 1288 | 696 | 698 | 361 | 337 | 52 |
| Girma et al. [49] | 2010 | Oromia | Eastern | 384 | 1536 | 1502 | 121 | 43 | 78 | 35 |
| Adane et al. [50] | 2010 | oromia | Southern | 460 | 1840 | 712 | 641 | 208 | 433 | 32 |
| Bihon et al. [51] | 2017 | Amhara | Northern | 334 | 1054 | 238 | 50 | 17 | 33 | 34 |
Note: G. Location = Geographical Location; St. Design = study design; TQE = Total quarter Examined; NQP+ = number of quarter positive: TCP+ = Total culture positive; Staph+ = staphylococcus aureus, Positive: OBP+ = other bacterial pathogen positive; PS* = prevalence of staphylococcus aureus.
3.3. Meta-analysis and bias assessment
A total of 26 studies were included in meta-analysis and estimated the prevalence of S.aureus infection in both clinical and subclinical mastitis. Substantial heterogeneity was observed across the included studies on bovine mastitis associated S.aureus infection among lactating cows (I2 = 90.75%; 2 = 0.2811, P < 0.01) and pooled effect size was 35% (95% CI: 0.31–0.41) as shown on the forest plot (Fig. 2). This observation signifies the presence of considerable variations across the investigations. Additionally, subsequent meta-regression and subgroup analysis were conducted to examine the potential factors that may account for the observed variance.
The result of the funnel plot (Fig. 3) showed that there was no asymmetrical distribution of studies and almost all studies are under 95% confidence interval which means smaller studies do not tend to be missed.
Fig. 3.
Funnel plot with pooled proportion of bovine mastitis associated S. auerus.
3.4. Subgroup analysis
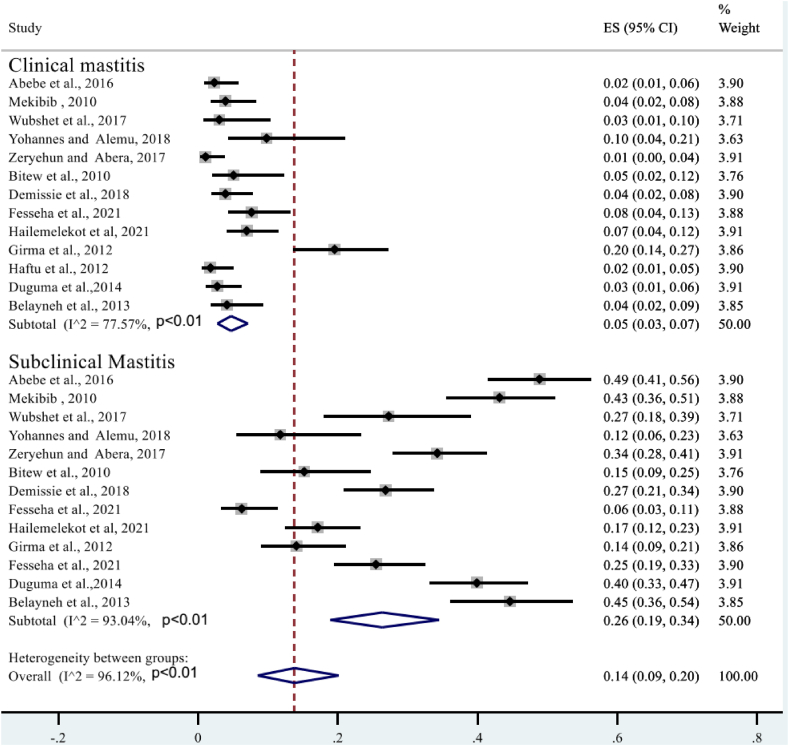
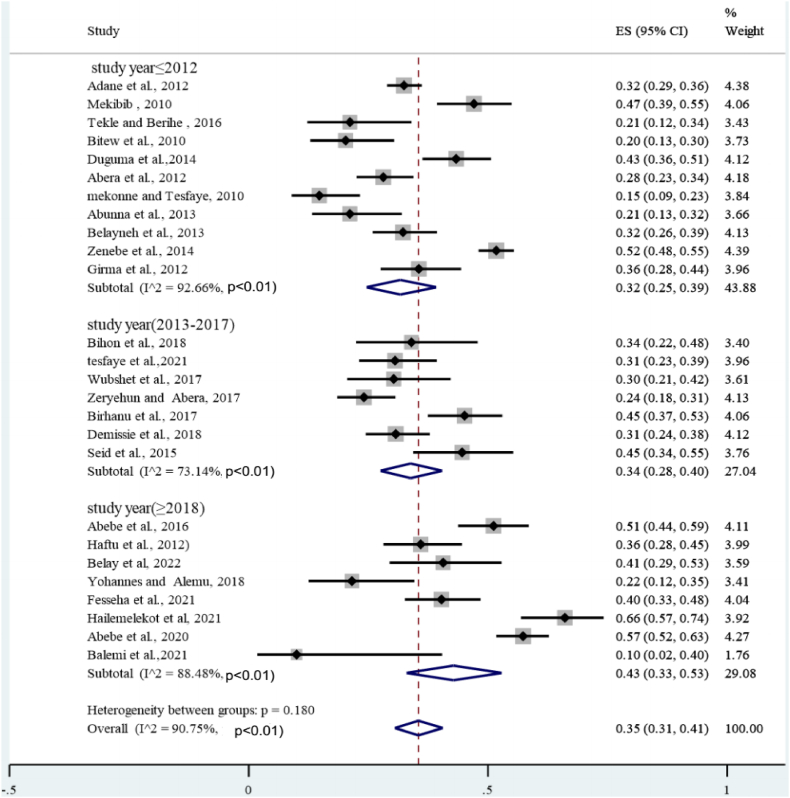
Given the high level of heterogeneity, a subgroup analysis was performed based on mastitis level (clinical and subclinical), year of study, study area (region), and sample size. A sub-analysis of subclinical mastitis studies revealed high heterogeneity (I2 = 96.12%, P < 0.01), whereas clinical mastitis studies showed moderate heterogeneity (I2 = 77.57%; P < 0.01) (Fig. 4). The subtotal percentage of staphylococcal infection was higher for subclinical mastitis (26%) than for clinical mastitis (5%). Sub-analysis also performed by year of study. First, all study years were divided into three groups: (2012), (2013–2017), and (2018), indicating statistically significant heterogeneity in each group. As shown in Fig. 5, the highest heterogeneity (92.66%: P < 0.01) was found in 2012. However, the sub-total proportion of S. aureus infection was the lowest at 32% (95% CI: 25–39%). A sub-analysis of the proportion of bovine mastitis associated with S. aureus infection by region had revealed the highest heterogeneity (I2 = 91.36%; P < 0.01: Fig. 6) in Oromia regional state. However, subtotal prevalence was the second lowest at 34% (95% CI: 27–42%), followed by SNNPR (29%). Also as exhibited in Fig. 7, subgroup analysis by sample size also showed significant heterogeneity between studies.
Fig. 4.
Forest plot and subgroup analysis by degree of mastitis due to S.aureus.
Fig. 5.
Subgroup analysis of the prevalence of S. aureus based on the study year.
Fig. 6.
Subgroup analysis of the prevalence of S.aureus based on the study region.
Fig. 7.
Subgroup analysis of the proportion of bovine mastitis associated S.aureus by sample size.
The present study outlines the outcomes of univariable meta-regression analyses in terms of coefficients, p values and Cochran's Q statistics. It is noteworthy that both region and study year exhibited a univariable p value < 0.1. Furthermore, a meta-regression analysis rate was conducted to determine the prevalence of bovine mastitis with respect to study year, which revealed a significant increase in the incidence of S.aureus infection. The univariable meta-regression analysis was followed by a multi-variable meta-regression analysis, wherein variables with a significance level of less than 0.1 were selected. The final results of this comprehensive analysis are presented in Table 2. Additionally, Fig. 8, Fig. 9 illustrate the event rate plot on the sample size and year of study, respectively.
Table 2.
Summary of statistics in multivariable meta-regression.
| Variables | category | Coefficient | P -value | 95%CI | Q |
|---|---|---|---|---|---|
| Study year | 2012 | Ref. | 247.33 | ||
| 2013–2017 | −0.27 | 0.00614 | −1.421–0.863 | ||
| 2018 | −0.118 | 0.0261 | −1.520–1.281 | ||
| Region | Oromia | Ref. | 42.33 | ||
| Amhara | 0.30 | 0.481 | −0.593–1.211 | ||
| Tigray | 0.62 | 0.211 | −0.386–1.636 | ||
| AA | 0.69 | 0.52 | −0.265–1.647 | ||
| SNNPR | 0.56 | 0.32 | −0.321–1.321 | ||
| Sample size | <100 | Ref. | 270.41 | ||
| 100–180 | 0.76 | 0.00 | 0.35–0.45 | ||
| >180 | 0.82 | 0.003 | 0.27–0.48 |
Fig. 8.
Regression of logit event rate by year of study.
Fig. 9.
Meta regression plot of sample size versus effect size of the rate of S.aureus.
4. Beta lactam antimicrobial resistance rate of S.aureus
Antimicrobials are the only option for treating bovine mastitis in Ethiopia. In particular, the main choices of antimicrobials are beta-lactams such as penicillin, ampicillin, amoxicillin, ceftriaxone and cefotaxime. It has been reported that the proportion of glands undergoing bacteriological healing after antimicrobial therapy for clinical mastitis varies widely [52].
Recovery rate is affected by factors related to the individual cow, factors related to management and bacterial associated factors (strain and presence of antimicrobial resistance; also determine the cure proportion [[53], [54], [55]]. The presence of differently sized plasmids was associated with the carriage of multiple antimicrobial resistances. In S. auerus, two main classes of plasmids were identified, which contribute to the resistance against antimicrobials and/or virulence. Antimicrobial resistance can be assessing in different methods including agar disc-diffusion assays broth dilution testing and the detection of genes encoding resistance [56].
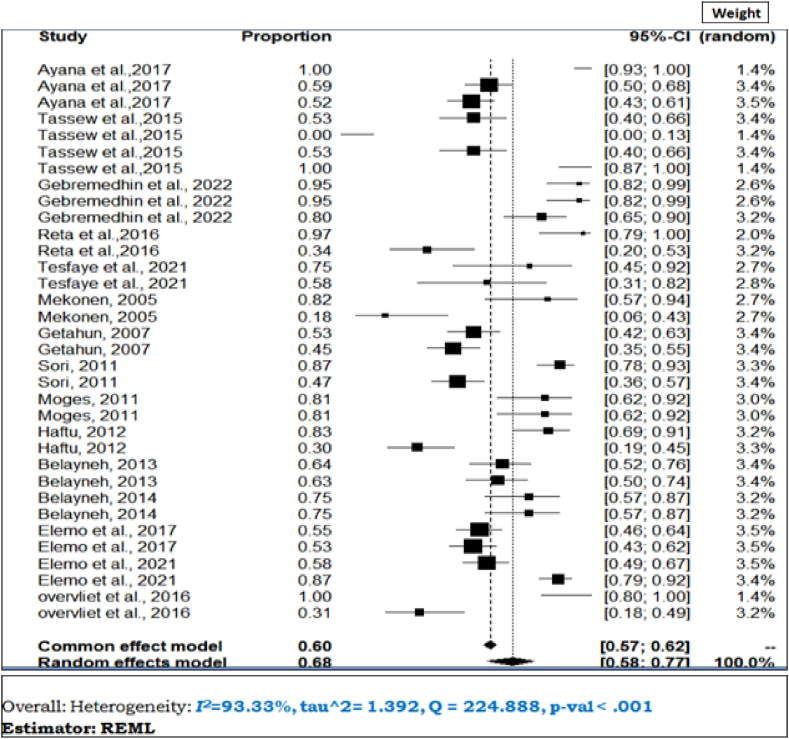
In the current review we have include a total of 14 studies (Table 3) for beta-lactam antimicrobial (Penicillin, amoxicillin, ampicillin and cephalosporin's resistance) resistance rate of bovine mastitis associated S.aureus.
Table 3.
The various beta-lactam resistance for bovine mastits associated-S.aureus (n = 14).
| Author | Antimicrobials | SA + isolated | Resistance | Susceptible | Proportion |
|---|---|---|---|---|---|
| Ayana et al. [21] | Penicillin | 110 | 110 | 0 | 1.000 |
| Amoxicillin | 110 | 65 | 45 | 0.590 | |
| Ampicillin | 110 | 57 | 53 | 0.518 | |
| Tassew et al. [57] | Penicillin | 53 | 0 | 53 | 0.000 |
| Cefoxitin | 53 | 28 | 25 | 0.472 | |
| Amoxicillin | 53 | 53 | 0 | 1.000 | |
| Gebremedhin et al. [23] | Ampicillin | 40 | 38 | 2 | 0.950 |
| Amoxicillin | 40 | 38 | 2 | 0.950 | |
| Cefotaxime | 40 | 32 | 8 | 0.800 | |
| Reta et al. [58] | Penicillin | 29 | 28 | 1 | 0.034 |
| Amoxicillin | 29 | 10 | 19 | 0.345 | |
| Tesfaye et al. [33] | Amoxicillin | 12 | 9 | 3 | 0.750 |
| Ceftriaxzone | 12 | 7 | 5 | 0.583 | |
| Mekonen and Tesfaye [45], | Penicillin | 17 | 14 | 3 | 0.824 |
| Ampicillin | 17 | 3 | 14 | 0.176 | |
| Getahun [59], | Pencillin | 85 | 45 | 40 | 0.530 |
| Ampicillin | 85 | 38 | 47 | 0.450 | |
| Sori [60], | Pencillin | 86 | 75 | 11 | 0.872 |
| Amoxillin | 86 | 40 | 46 | 0.460 | |
| Moges et al. [61] | Penicillin | 27 | 22 | 5 | 0.815 |
| Amoxicillin | 27 | 22 | 5 | 0.800 | |
| Haftu [40], | Penicillin | 46 | 38 | 8 | 0.830 |
| Ampicillin | 46 | 14 | 3 | 0.304 | |
| Belayneh (a) [47], | Penicillin | 59 | 38 | 21 | 0.650 |
| amoxicillin | 59 | 37 | 22 | 0.620 | |
| Belayneh(b) [62], | Penicillin | 32 | 24 | 8 | 0.750 |
| amoxicillin | 32 | 24 | 8 | 0.750 | |
| Elemo et al. [63], | Ampicillin | 112 | 62 | 50 | 0.550 |
| Amoxicillin | 112 | 59 | 53 | 0.530 | |
| Cefoxitin | 112 | 65 | 47 | 0.580 | |
| Penicillin | 112 | 97 | 15 | 0.870 | |
| overvliet et al. [53] | Penicillin | 32 | 32 | 0 | 1.000 |
| Cefoxitin | 32 | 10 | 22 | 0.300 |
4.1. Selection criteria
Out of the total 1907 isolates of S. auerus, 1234 were found to exhibit antimicrobial resistance. One article was used multiple times for quantitative data analysis. The selection criteria for the chosen studies were as follows, (1) almost all of the included studies provided a comprehensive overview of the pathogen isolation rates for both clinical and sub-clinical bovine mastitis, (2) each article had conducted multiple antimicrobial susceptibility tests. (3), the chosen articles had isolated S.aureus at least once, (4) in each study, at least one beta-lactam antimicrobial was present, (5) the chosen article may or may not be included in the previous section of our meta-analysis which focused on the prevalence of S. auerus, (6) we clearly estimated the proportion of antimicrobial resistance and susceptibility, (7) we only selected articles that exclusively used milk as the primary sample for dairy cattle in Ethiopia. Finally, we only included studies that conducted the antimicrobial susceptibility test according to the criteria of the clinical laboratory standards institute.
Articles were deemed ineligible if they met any of the following criteria: (1) duplication; (2) deviation from the topic at hand and a small sample size of less than 60; (3) lack of bacterial identification in the study; (4) presence of non-mastitis diseases in the study samples; (5) an unclear sample size or bacterial isolate quantity in the study; and (6) absence of beta lactam antimicrobials in the study.
4.2. Meta-analysis
Due to expectation of heterogeneity across studies, a random-effects meta-analysis was selected using the total number of S. auerus isolated as total sample size and number of resistance (as event rate). The result of the quantitative analysis (meta-analysis) of 14 studies showed that the individual study resistance rate of S.aureus ranged from 0% to 100%, with the overall pooled prevalence being 68% (95% CI; 58–77%). As shown in Fig. 10, the heterogeneity between and within studies was high (I2 = 93.33%; τ2 = 1.39, Q = 224.88), P < 0.001).
Fig. 10.
The forest plot, heterogeneity and resistance rate of beta lactamase of S. aureus.
4.3. Publication bias assessment
The funnel plot in Fig. 11 revealed that no asymmetrical distribution of studies and most of the studies are under a 95% confidence interval, the regression test was computed by using-mixed effect Meta regression model and standard error of the effect size as the predictor. Egger's regression test and rank correlation test of the funnel plot asymmetry were confirmed no significant publication bias (b = 39.32; P = 0.62) and rank correlation test (Kendall's tau = −0.1277; P = 0.2920), respectively.
Fig. 11.
Publication bias assessment of overall beta lactam antimicrobial resistance rate of mastitis associated S.aureus.
Penicillin resistance rate: of 14 studies, 12 studies were pooled to estimate the proportion of S. auerus resistance to penicillin. In Supplementary F. 1, the overall proportion of S. auerus resistance for penicillin was 75% (95% CL; 59–91%) in the treatment of bovine mastitis.
Heterogeneity: based on the twelve identified studies, a meta-analysis was conducted to investigate heterogeneity within and across studies using a random effect model. Studies reporting the resistance rate of S.aureus penicillin, there was evidence of between-study heterogeneity (I2 = 77.41%, P < 0.01).
Assessment of bias: The funnel plot revealed that there was no asymmetrical distribution of articles (Supplementary F. 2), and the majority of studies are within the Pseudo 95% confidence interval. The results of the egger's regression test for small-study effects showed no statistically significant publication bias (Egger's test: b = 0.63, P = 0.7653).
Amoxicillin resistance rate: of 14 studies, 10 studies were selected for estimation of the proportion of resistance of S.aureus to amoxicillin for the treatment bovine mastitis.
Heterogeneity: based on the ten identified studies, a meta-analysis was conducted to investigate heterogeneity within and across studies by using a random effect model. For studies reporting resistance of S.aureus to amoxicillin, there was evidence of between-study heterogeneity (I2 = 55.46%, p = 0.02; Supplementary F. 3); the pooled effect estimate was 67% (95%CI: 54–80%) in random effect meta-analysis.
Assessment of bias: the funnel plot was used to assess publication bias, which was confirmed by the Egger regression test (b = 1.37; P = 0.3364) (Supplementary F. 4).
Ampicillin resistance rate: six studies were selected for estimation of the proportion of S. auerus resistance to ampicillin in the treatment of bovine mastitis among dairy cows. The overall proportion of S. auerus resistance for ampicillin was 50% (95% CI; 29–71%).
Heterogeneity: according to six identified studies, a meta-analysis was carrying out to examine variability within and across studies using a random effect model. For studies reporting resistance of S. aureus in the treatment of ampicillin, there was high variability (I2 = 96.17%, Q = 153; P < 0.01; Supplementary F. 5).
Assessment of publication bias: the funnel plot was used to assess publication bias, which was confirmed by the Egger regression test. There was no publication biased because the regression-based Egger test for small-study effects was not statistically significant (b = 0.37; P = 0.2364) by using a random effect model with the method of restricted maximum likelihood (Supplementary F. 6).
Cephalosporin's resistance rate: five studies were selected for estimation of the proportion of resistance of S. auerus cephalosporin sin treatment of bovine mastitis among dairy cows. As supplementary F. 7 showed, the overall proportion of S. auerus resistance rate to cephalosporin was 57% (95% CI; 45–69%) in the treatment of bovine mastitis.
Heterogeneity: based on the five identified studies, a meta-analysis was conducted to investigate heterogeneity within and across studies using a random effect model. For studies reporting resistance of S. auerus to cephalosporin, there was no evidence of between-study heterogeneity (I2=0.00%, Q= 4.40; P = 0.35).
Publication bias: there was publication biased (Supplementary F. 8) because the funnel plot showed asymmetrical distribution of studies, as well as the regression-based egger test for small-study effects, was also statistically significant (b= -12.32; P=0.001). The findings of the various antimicrobial agents, the collective resistance, and the potential for publication bias across all Meta –analysis have been consolidated into Table 4.
Table 4.
The summary of the beta lactams with pooled estimates, heterogeneity and bias assent.
| Type of antimicrobial | Pooled resistance rate | Heterogeneity% (I2) | 95%CI | Bias(Egger's test) |
|---|---|---|---|---|
| Penicillin | 75 | 77.41 | 59–91 | b = 0.63, P = 0.7653 |
| Amoxicillin | 67 | 55.46 | 54–80 | b = 1.37; P = 0.3364 |
| Ampicillin | 50 | 96.17 | 29–71 | b = 0.37; P = 0.2364 |
| Cephalosporin | 57 | 0.00 | 45–69 | b = −12.32; P = 0.001 |
5. Discussion
A systematic review and meta-analysis was performed to estimate the pooled proportion of S.aureus infection in clinical and subclinical bovine mastitis in lactating dairy cows in Ethiopia. The current study, involving 26 studies, found that the pooled proportion of S. aureus-associated bovine mastitis in Ethiopia was 35% of the bacterial pathogens causing mastitis in lactating cows. Comparable results were reported by Girma et al. [49] reported in Tigray Regional State, Ethiopia.
In view of its global importance, S. aureus is widely recognized as a major cause of intramammary infections in dairy cows. This particular study found that the pooled prevalence of S. aureus-associated bovine mastitis (35%) found to be almost identical to that which was stated in a meta-analysis conducted in China (36.23%) by Wang et al. [64], figures to be higher in Canada at 20–22% [65] and in the United States at 20.8–23.3% [66] and at 15.7% in Kenya [67]. In contrast, the result was lower than the results reported by Elhaig and Selim [68] in Nigeria at 56.7% and in Egypt at 38.3%. These differences may be related to cow cleanliness, availability of vaccination, farm size, farm management systems, breed of cows and housing styles in different countries and regions are some of the factors contributing to the complexity of mammary health [69].
In terms of study year, the sub-total prevalence of S.aureus infection in clinical and subclinical mastitis in each study year category were 32% (>2012), 34% (2013–2017) and 43% (>2018). According to this systematic and meta-analysis the pattern of rate of S. auerus infection in bovine mastitis had increased in the subsequent stratification of included studies based on study year. This may due to increased antimicrobial resistance by various mechanisms and it is believed that about 50% of mastitis causing S.aureus produces β-lactamase [70].This may related with the transfer of resistant strain among environment, livestock and human. This may also due to with limited therapeutic treatment in Ethiopia and improper use of antimicrobials in veterinary practice. Another justification S. aureus biofilm formation is related with diminished antibiotic sensitivity in bovine mastitis, which is attributed to impaired antibiotic penetration through the biofilm matrix and decreased metabolic activity of bacteria within biofilms [71]. Furthermore, several circumstances, such as sub-inhibitory concentrations of various antibiotics [72].And milk components such as lactose and proteases, stimulate S. aureus biofilm formation inside the bovine mammary gland [73].
Regarding the study regions, the pooled prevalence of S. aureus infection was highest in Addis Ababa 40% whereas lowest in SNNPRS region 29%. The observed variation can be attributed to differences in management practices. Such discrepancies are particularly notable given the infectious nature of S.aureus, which is easily transmitted between cows. This is favored by unhygienic milking processes, such as the use of contaminated milking equipment and hands, and the involvement of flies and cross suckles [74].
Regarding level of mastitis, this systematic and meta-analysis indicated that the level of occurrence of S. aureus infection was higher (26%) in subclinical mastitis than clinical bovine mastitis 5%. Thus, this result confirmed that S. aureus infection has the great economic impact on dairy sector in Ethiopia because subclinical mastitis is the major bottle neck of dairy sector in developing countries [75] S.aureus is a very well opportunistic infection that is frequently related to subclinical mastitis and causes significant economic losses due to reduced milk quality and production [76]. Intrammary infection of lactating cows by CC97 strains has been shown to result in asymptomatic, sub-clinical, or persistent infections, increasing the difficulty of pathogen control in dairy herds [77].Although the overall rate of resistance varies widely among antimicrobial agents, resistance of S.aureus to antimicrobial agents is a serious problem. According to this meta-analysis, the overall rate of beta-lactam resistance in Ethiopia was very high (68%). This finding is corroborated by research carried out in Iran [78] and Brazil [79], which demonstrates that S.aureus displays a pronounced resistance to β-lactam antibiotics in comparison to alternative antibiotics. This may be because S.aureus produces beta-lactamase, which breaks down the drug's beta-lactam ring or it could be the synthesis of a redesigned PBP that has a decreased affinity for the majority of beta-lactam antimicrobials. Staphylococcus aureus may have acquired resistance to β-lactam antibiotics through a variety of mechanisms. These include modifications and expressions of penicillin-binding proteins (PBP), β-lactamase synthesis leading to drug inactivation, biofilm formation limiting drug uptake, and expression of efflux pumps reducing drug uptake. Specifically, the production of beta-lactamase by S.aureus can catalyze the breakdown of the drug's beta-lactam ring. Alternatively, a redesigned PBP with decreased affinity for beta-lactam antimicrobials may have been synthesized. In cases of bovine mastitis, biofilm formation by S. aureus has been linked to decreased susceptibility to antibiotics. This is likely due to reduced antibiotic diffusion through the biofilm matrix and decreased metabolic activity of bacteria within biofilms as suggested by Song et al. [80]. Furthermore, it has not been previously recognized that S.aureus could act as an intracellular pathogen. It was not previously known that S. aureus could act as an intracellular pathogen. However, there is now substantial evidence to suggest that the bacteria can survive and persist within a variety of mammalian cells, rendering antibiotic treatments ineffective [81]. This mechanism of survival confers a protective advantage to S. aureus as it enables the bacteria to evade both the host immune response and antibiotics, thereby rendering the bacteria inaccessible [82].
The prevalence of S.aureus resistance to penicillin is widespread in dairy herds across the globe. In the present review, it was observed that penicillin exhibited the highest resistance to S.aureus (75%) as compared to other beta lactam. This percentage value was higher than the values reported in Argentina (47.6%), Brazil (69.9%), Turkey (62%), Estonia (61%), France (30%), Hungary (30%), Germany (17%), the United States (10%), and Norway (6%) as reported by Gianneechini et al. [83], André et al. [84], Turutoglu et al. [85], Kalmus et al. [86], Sakwinska et al. [87], Peles et al. [88], Jørgensen et al. [89], Anderson et al. [90], and Tenhagen et al. [91], respectively. However, the resistance percentage was lower than the values reported in Mukaturi and Sululta (35%), Tigray (over 90%), Addis Ababa (95.3%), Hawassa (100%), Iran (100%), and China (26%). This variation may due the importation and distribution of poor quality drugs to the country [92]. This is also related to the fact that β-lactams rank first regarding the number of compounds commercially accessible and in terms of their use in the treatment of bovine mammary infections. In addition, penicillin belongs to the β-lactam category of drugs; the drug has been adopted for long-term and repeated administration in cattle, for example, for the treatment of diarrhea and other diseases, which may bring in increased resistance to its utilization of the treatment of mastitis [93].
Notably in Ethiopia, the occurrence of methicillin resistance ranges from 24 to 40.9% [94]. In this particular investigation, it was found that the S. aureus exhibited the lowest level of resistance towards ampicillin (50%). However, it has been observed that in Japan, the level of resistance towards ampicillin can escalate up to 76.1%∼89.7% [95]. In Nepal, all S. auerus isolates demonstrated complete resistance (100%) [96], towards ampicillin, while in Uganda it was recorded to be 73.2% [97] and in India it was found to be 74.42% [98], in India 20.5% [99].
Staphylococcus auerus is recognized for its proficiency in acquiring antimicrobial resistance in both humans and animals. The utilization of beta lactams as the customary antibiotic therapy for S.aureus has led to numerous concerns regarding public health. such concerns include the potential for transmission of resistant microorganisms through both the food chain [100,101] and various ecosystems [102]. In addition to posing a threat to public health, the presence of antibiotic residues in milk can prove problematic for the dairy industry, adversely affecting the production technology of fermented milk products [103]. Thus, it is crucial that we increase awareness about the incorporation of a multifaceted One Health approach to address complex issues such as antimicrobial resistance (AMR) to ensure optimal health for humans, animals, and the environment.
This systematic review is subject to several limitations, which must be taken into consideration. Firstly, the review is exclusively focused on a single pathogen, namely S. aureus. This limited scope may not provide a comprehensive overview of the broader category of pathogens. Secondly, despite the vast quantity of literature that has been systematically reviewed, the Meta analysis depends on a relatively small sample of articles, with just 14 studies included for the analysis of antimicrobial resistance. This narrow scope may not be representative of the entire body of literature. Thirdly, the meta-analysis conducted was limited to studies in Ethiopia, and a few sample sizes or small numbers of studies were used to estimate the individual beta-lactam antimicrobial resistances. This may limit the generalizability of the findings. It is important to note that our eligibility criteria were limited to articles published in English, which creates potential language bias. Finally, these limitations must be taken into account when assessing the findings of this review.
6. Conclusion
This meta-analysis and systematic review estimated the pooled proportion of bovine mastitis associated-S. aureus among infection episode in Ethiopia. Hence, this figure revealed that bovine mastitis associated-S.aureus is a widely distributed and economically important disease of dairy cows throughout Ethiopia. Subgroup analysis showed that the pooled proportion of bovine associated S.aureus was greater in subclinical mastitis in Northern (Amhara and Tigray region) and central part of Ethiopia in between 2018 and 2022 study years. Furthermore, the overall beta lactamase resistance rate of the bovine associated S.aureus was high (68%). Among the beta lactam, the resistance rate of bovine mastitis associated-S.aureus was highest in Penicillin. This highest prevalence highest prevalence of S.aureus in bovine mastitis and highest resistance rate for the commonly use of beta-lactam antimicrobials should have given big attention. Therefore, this systematic review and meta-analysis was generated concrete and pooled evidence of the distribution of S. aureus in bovine mastitis. In general, the summarized findings presented here serve as a valuable tool for healthcare professionals, veterinarians and healthcare organizations alike; enabling them to design and implement appropriate interventions to control and mitigate adverse consequences of S.aureus infection in bovine mastitis and to monitor drug resistance S. aureus in dairy cows is an essential part of mastitis control.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author unreasonable request.
Authors’ contributions
Melkie Dagnaw Fenta: Data curation, Methodology, Supervision, Formal analysis, Conceptualization, Software, Validation.
Firdyawukal Abuhay Tafere: Conceptualization, writing the original draft and review and editing.
Atsede Solomon Mebratu: Conceptualization, Writing the Original Draft, Methodology.
Birhan Anagaw Malede: Visualization, Formal Analysis, Validation, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e18180.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Abebe R., Hatiya H., Abera M., Megersa B., Asmare K. Bovine mastitis : prevalence , risk factors and isolation of Staphylococcus aureus in dairy herds at Hawassa milk shed , South Ethiopia. BMC Vet. Res. 2016 doi: 10.1186/s12917-016-0905-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardhan I., Lin S. 2013. Effect of IT and R & D on Tobin ’ S Q Business Value of Information Technology : Testing the Interaction Effect of IT and R & D on Tobin ’ S Q; pp. 1147–1161. [Google Scholar]
- 3.Yusuf-Isleged M.A. Prevalence and associated risk factors of bovine mastitis on dairy cattle in Mogadishu Somalia. Anim. Vet. Sci. 2022;10 [Google Scholar]
- 4.Mungube E.O., Tenhagen B., Regassa F., Kyule M.N., Shiferaw Y. 2005. Reduced Milk Production in Udder Quarters with Subclinical Mastitis and Associated Economic Losses in Crossbred Dairy Cows in Ethiopia; pp. 503–512. [DOI] [PubMed] [Google Scholar]
- 5.Birhanu M., Leta S., Mamo G., Tesfaye S. Prevalence of bovine subclinical mastitis and isolation of its major causes in Bishoftu. BMC Res. Notes. 2017 doi: 10.1186/s13104-017-3100-0. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervinkova D., Vlkova H., Borodacova I., Makovcova J., Babak V., Lorencova A., et al. 2013. Prevalence of Mastitis Pathogens in Milk from Clinically Healthy Cows; pp. 567–575. [Google Scholar]
- 7.Bradley A.J. 116–28. 2002. (Bovine Mastitis : an Evolving Disease). [DOI] [PubMed] [Google Scholar]
- 8.Al-majali A.M., Al-qudah K.M. 193–200. 2008. (Risk Factors Associated with Camel Brucellosis in Jordan). [DOI] [PubMed] [Google Scholar]
- 9.Methicillin-resistant Staphylococcus aureus (MRSA) in Food Production Animals. 2010. pp. 606–625. [DOI] [PubMed] [Google Scholar]
- 10.Hanselman B.A., Kruth S.A., Rousseau J., Low D.E., Willey B.M., McGeer A., Weese J.S. Methicillin-resistant Staphylococcus aureus colonization in veterinary personnel. Emerg. Infect. Dis. 2006;12(12):1933. doi: 10.3201/eid1212.060231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lozano C., Gharsa H., Ben S.K., Zarazaga M., Torres C. Staphylococcusaureus in animals and food: methicillin resistance, prevalence and population structure. A review in the African continent. Microorganisms. 2016;4(12):1–19. doi: 10.3390/microorganisms4010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nitz J., Wente N., Zhang Y., Klocke D., Tho Seeth M., Krömker V. Dry Period or early lactation—time of onset and associated risk factors for intramammary infections in dairy cows. Pathogens. 2021 Feb 18;10(2):224. doi: 10.3390/pathogens10020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rusenova N.V., Rusenov A.G. Detection of Staphylococcus aureus among coagulase positive staphylococci from animal origin based on conventional and molecular methods. Maced. Vet. Rev. 2017 Mar 1;40(1):29–36. [Google Scholar]
- 14.Balemi A., Gumi B., Amenu K., Girma S., Gebru M., Tekle M. 2021. Prevalence of Mastitis and Antibiotic Resistance of Bacterial Isolates from CMT Positive Milk Samples Obtained from Dairy Cows , Camels , and Goats in Two Pastoral Districts in. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abera M., Elias B., Aragaw K., Denberga Y., Amenu K. Major causes of mastitis and associated risk factors in smallholder dairy cows in Shashemene. southern Ethiopia. 2012;7(24):3513–3518. doi: 10.1007/s11250-011-0055-3. [DOI] [PubMed] [Google Scholar]
- 16.Abebe R., Abera M., Denbarga Y., Suleyman M., Feka -A., Abunna F., et al. Prevalence , risk factors and bacterial causes of bo- vine mastitis in southern Ethiopia. 2020;24(1):52–68. [Google Scholar]
- 17.CSA Central statistical authority of Ethiopia: report on livestock and livestock characteristics (private peasant holdings) Stat. Bull. 2020;587 II [Google Scholar]
- 18.FAO . Animal Production and Health Working Paper. No. 13. 2014. Impact of mastitis in small scale dairy production systems. [Google Scholar]
- 19.Yilma Z., Loiseau G., Faye B. Manufacturing efficiencies and microbial properties of butter and Ayib—Ethiopian cottage cheese. Livest. Res. Rural Dev. 2007;19(7):2004. [Google Scholar]
- 20.Hachemi A., Zenia S., Denia M.F., Guessoum M., Hachemi M.M., Ait-Oudhia K. Epidemiological study of sausage in Algeria: prevalence, quality assessment, and antibiotic resistance of Staphylococcus aureusisolates and the risk factors associated with consumer habits affecting foodborne poisoning. Vet. World. 2019;12(8):1240–1250. doi: 10.14202/vetworld.2019.1240-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayana H.W., Mekonnen B.T., Bulle A.S., Berecha M.S. Isolation and identification of methicillin resistant Staphlococcus aureus from mastitic dairy cows in Bishoftu town, Ethiopia. Afr. J. Microbiol. Res. 2017;11:1606–1613. [Google Scholar]
- 22.WHO . World Health Organization; Geneva, Switzerland: 2001. World Health Organization Global Strategy for Containment of Antimicrobial Resistance. [Google Scholar]
- 23.Gebremedhin E.Z., Ararso A.B., Borana B.M., Kelbesa K.A., Tadese N.D., Marami L.M., Sarba E.J. Isolation and identification of Staphylococcus aureus from milk and milk products, associated factors for contamination, and their antibiogram in Holeta, Central Ethiopia. Vet. Med. Int. 2022 May 6:2022. doi: 10.1155/2022/6544705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makita K., Desissa F., Teklu A., Zewde G., Grace D. Risk assessment of staphylococcal poisoning due to consumption of informally-marketed milk and home-made yoghurt in Debre Zeit, Ethiopia. Int. J. Food Microbiol. 2012 Feb 1;153(1–2):135–141. doi: 10.1016/j.ijfoodmicro.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 25.Kemal K.E., Tesfaye S., Ashanafi S., Muhammadhussien A.F. Prevalence, risk factors and multidrug resistance profile of Staphylococcus aureus isolated from bovine mastitis in selected dairy farms in and around Asella town, Arsi Zone, South Eastern Ethiopia. Afr. J. Microbiol. Res. 2017 Dec 7;11(45):1632–1642. [Google Scholar]
- 26.Santajit S., Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. BioMed Res. Int. 2016 doi: 10.1155/2016/2475067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. 2002;1558:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 28.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borenstein M. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods. 2010 Apr;1(2):97–111. doi: 10.1002/jrsm.12. http://www.prisma-statement.org/ [DOI] [PubMed] [Google Scholar]
- 30.van Enst W.A., Ochodo E., Scholten R.J., Hooft L., Leeflang M.M. Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study. BMC Med. Res. Methodol. 2014 Dec;14(1):1–10. doi: 10.1186/1471-2288-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belay R., Belihu K., Wubete A. Dairy cow's mastitis survey in Adama town, Ethiopia. Journal of Veterinary Medicine and Animal Health. 2013 Oct 31;5(10):281–287. [Google Scholar]
- 32.Mekibib B., Furgasa M., Abunna F., Megersa B., Regassa A. Bovine mastitis: prevalence, risk factors and major pathogens in dairy farms of Holeta Town, Central Ethiopia. Vet. World. 2010;3(9):397 403. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=4ae481fcf67c9c8d74d4cd888b3e692f2683406b [Google Scholar]
- 33.Tesfaye K., Gizaw Z. 2021. Prevalence of Mastitis and Phenotypic Characterization of Methicillin-Resistant Staphylococcus aureus in Lactating Dairy Cows of Selected Dairy Farms in and Around Adama Town , Central Ethiopia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wubshet A.K., Tesema T.S., Gebru M., Derib B.T., Haile A.F., Wedeabyezgi H.A. 2017. Incidence of Heifer Mastitis and Identification of Major Associated Pathogens in Dairy Farms at Wolaita Soddo Town , Southern Ethiopia; pp. 169–176. [Google Scholar]
- 35.Zeryehun T., Abera G. Vol. 2017. 2017. Prevalence and Bacterial Isolates of Mastitis in Dairy Farms in Selected Districts of Eastern Harrarghe Zone , Eastern Ethiopia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tekle Y., Berihe T. Bovine mastitis: prevalence, risk factors and major pathogens in the Sidamo Zone SNNPRS, Ethiopia. European journal of biology and medical science research. 2016 Nov;4(5):27–43. [Google Scholar]
- 37.Birhanu M., Leta S., Mamo G., Tesfaye S. Prevalence of bovine subclinical mastitis and isolation of its major causes in Bishoftu. BMC Res. Notes. 2017 doi: 10.1186/s13104-017-3100-0. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bitew M., Tafere A., Tolosa T. Study on bovine mastitis in dairy farms of Bahir Dar and its environs. J. Anim. Vet. Adv. 2010;9(23):2912–2917. [Google Scholar]
- 39.Demissie T.F., Menghistu H.T., Mitiku M.A. Prevalence of mastitis and identification of its bacterial causative agents in small holder dairy farms in and around Wukro of Tigray region, Ethiopia. Int. J. Adv. Res. Biol. Sci. 2018;5(11):10–22. [Google Scholar]
- 40.Fesseha H., Mathewos M., Aliye S., Wolde A. Study on prevalence of bovine mastitis and associated risk factors in dairy farms of Modjo town and suburbs, central Oromia, Ethiopia. Vet. Med. Res. Rep. 2021 Oct 8:271–283. doi: 10.2147/VMRR.S323460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hailemelekot M. Clinical and subclinical bovine mastitis and associated risk factors in small-scale dairy farms in bahir dar and its envirion. Amhara region. 2021;1–16 [Google Scholar]
- 42.Haftu R., Taddele H., Gugsa G., Kalayou S. Prevalence, bacterial causes, and antimicrobial susceptibility profile of mastitis isolates from cows in large-scale dairy farms of Northern Ethiopia. Trop. Anim. Health Prod. 2012 Oct;44:1765–1771. doi: 10.1007/s11250-012-0135-z. [DOI] [PubMed] [Google Scholar]
- 43.Duguma A., Tolosa T., Yohannes A. Prevalence of clinical and sub-clinical mastitis on cross bred dairy cows at Holleta Agricultural Research Center, Central Ethiopia. Journal of Veterinary Medicine and Animal Health. 2014 Jan 31;6(1):13–17. [Google Scholar]
- 44.Seid U., Zenebe T., Almaw G., Edao A., Disassa H., Kabeta T., Gerbi F., Kebede G. Prevalence, risk factors and major bacterial causes of bovine mastitis in west arsi zone of Oromia region, southern Ethiopia. Nat. Sci. 2015;13(8):19–27. [Google Scholar]
- 45.Mekonnen H., Tesfaye A. 2010. Prevalence and Etiology of Mastitis and Related Management Factors in Market Oriented Smallholder Dairy Farms in Adama; pp. 574–579. [Google Scholar]
- 46.Abunna F., Fufa G., Megersa B., Regassa A. Bovine mastitis: prevalence, risk factors and bacterial isolation in small-holder dairy farms in Addis Ababa City, Ethiopia. Global Vet. 2013;10(6):647–652. https://www.academia.edu/download/54694664/mastitis.pdf [Google Scholar]
- 47.Belayneh R., Belihu K., Tesfaye A(a) Microbiological study on bacterial causes of bovine mastitis and its antibiotics susceptibility patterns in East Showa Zone, Akaki District, Ethiopia. J Vet Med Anim Health. 2014 Apr 30;6(4):116–122. [Google Scholar]
- 48.Zenebe N., Habtamu T., Endale B. Study on bovine mastitis and associated risk factors in Adigrat, Northern Ethiopia. Afr. J. Microbiol. Res. 2014 Jan 22;8(4):327–331. [Google Scholar]
- 49.Girma S., Teshale S., Tadesse F., Beyene T.J. 2012. Study on Prevalence of Bovine Mastitis and its Major Causative Agents in West Harerghe Zone , Doba District , Ethiopia. [Google Scholar]
- 50.Adane B., Bayissa B., Tuffa S., Tola T., Mekonnen S. Participatory impact assessment of ticks on cattle milk production in pastoral and agro-pastoral production systems of Borana Zone, Oromia Regional State, Southern Ethiopia. Ethiopian Veterinary Journal. 2012;16(1):1–3. [Google Scholar]
- 51.Bihon A., Syoum A., Assefa A. Assessment of risk factors and isolation of Staphylococcus aureus and Escherichia coli from bovine subclinical mastitic milk in and around Gondar, Northwest Ethiopia. Trop. Anim. Health Prod. 2019 May 1;51:939–948. doi: 10.1007/s11250-018-1777-2. [DOI] [PubMed] [Google Scholar]
- 52.Roberson J.R., Warnick L.D., Moore G. Mild to moderate clinical mastitis: efficacy of intramammary amoxicillin, frequent milk-out, a combined intramammary amoxicillin, and frequent milk-out treatment versus no treatment. J. Dairy Sci. 2004 Apr 1;87(3):583–592. doi: 10.3168/jds.S0022-0302(04)73200-2. [DOI] [PubMed] [Google Scholar]
- 53.Mcdougall S., Agnew K.E., Cursons R., Hou X.X., Compton C.R.W. Parenteral treatment of clinical mastitis with tylosin base or penethamate hydriodide in dairy cattle. J Dairy Sci [Internet] 2007;90(2):779–789. doi: 10.3168/jds.S0022-0302(07)71562-X. [DOI] [PubMed] [Google Scholar]
- 54.Schukken Y.H. Factors associated with cure after therapy of clinical mastitis caused by Staphylococcus aureus. J Dairy Sci [Internet] 2000;83(2):278–284. doi: 10.3168/jds.S0022-0302(00)74875-2. [DOI] [PubMed] [Google Scholar]
- 55.Taponen B.S., Jantunen A., Pyörälä E., Pyörälä S. 2003. Efficacy of Targeted 5-day Combined Parenteral and Intramammary Treatment of Clinical Mastitis Caused by Penicillin-Susceptible or Penicillin- Resistant Staphylococcus aureus; pp. 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bradley A.J., Green M.J. Factors affecting cure when treating bovine clinical mastitis with cephalosporin-based intramammary preparations. J Dairy Sci [Internet] 2009;92(5):1941. doi: 10.3168/jds.2008-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tassew A., Negash M., Demeke A., Feleke A., Tesfaye B., Sisay T. Isolation, identification and drug resistance patterns of methicillin resistant Staphylococcus aureus from mastitic cows milk from selected dairy farms in and around Kombolcha, Ethiopia. Journal of Veterinary Medicine and Animal Health. 2016 Jan 31;8(1):1–10. [Google Scholar]
- 58.Reta M.A., Bereda T.W., Alemu A.N. Bacterial contaminations of raw cow's milk consumed at Jigjiga City of Somali Regional State, Eastern Ethiopia. Int. J. Flow Control. 2016 Dec;3(1):1–9. [Google Scholar]
- 59.Getahun K., Kelay B., Bekana M., Lobago F. Bovine mastitis and antibiotic resistance patterns in Selalle smallholder dairy farms, central Ethiopia. Trop. Anim. Health Prod. 2008 May;40:261–268. doi: 10.1007/s11250-007-9090-5. [DOI] [PubMed] [Google Scholar]
- 60.Sori T., Hussien J., Bitew M. Prevalence and susceptibility assay of Staphylococcus aureus isolated from bovine mastitis in dairy farms of Jimma town, South West Ethiopia. J. Anim. Vet. Adv. 2011 Jan 1;10(6):745–749. [Google Scholar]
- 61.Moges N., Asfaw Y., Belihu K., Tadesse A. Aantimicrobial susceptibility of mastitis pathogens from smallholder dairy herds in and around Gondar, Ethiopia. J. Anim. Vet. Adv. 2011 Jan 1;10(12):1616–1622. [Google Scholar]
- 62.Belayneh R., Belihu K., Tesfaye A(b) Microbiological study on bacterial causes of bovine mastitis and its antibiotics susceptibility patterns in East Showa Zone, Akaki District, Ethiopia. J Vet Med Anim Health. 2014 Apr 30;6(4):116–122. [Google Scholar]
- 63.Elemo K.K., Sisay T., Shiferaw A., Fato M.A. Prevalence, risk factors and multidrug resistance profile of Staphylococcus aureus isolated from bovine mastitis in selected dairy farms in and around Asella town, Arsi Zone, South Eastern Ethiopia. Afr. J. Microbiol. Res. 2017 Dec 7;11(45):1632–1642. [Google Scholar]
- 64.Wang K., Cha J., Liu K., Deng J., Yang B., Xu H., Wang J., Zhang L., Gu X., Huang C., Qu W. The prevalence of bovine mastitis-associated Staphylococcus aureus in China and its antimicrobial resistance rate: a meta-analysis. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.1006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levison L.J., Miller-Cushon E.K., Tucker A.L., Bergeron R., Leslie K.E., Barkema H.W., et al. Incidence rate of pathogen-specific clinical mastitis on conventional and organic Canadian dairy farms. J. Dairy Sci. 2016;99:1341–1350. doi: 10.3168/jds.2015-9809. [DOI] [PubMed] [Google Scholar]
- 66.Mullen K.A., Sparks L.G., Lyman R.L., Washburn S.P., Anderson K.L. Comparisons of milk quality on North Carolina organic and conventional dairies. J. Dairy Sci. 2013;96:6753–6762. doi: 10.3168/jds.2012-651. [DOI] [PubMed] [Google Scholar]
- 67.Mbindyo C.M., Gitao G.C., Mulei C.M. Prevalence, etiology, and risk factors of mastitis in dairy cattle in Embu and Kajiado Counties, Kenya. Vet. Med. Int. 2020 Aug 4:2020. doi: 10.1155/2020/8831172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elhaig M.M., Selim A. Molecular and bacteriological investigation of subclinical mastitis caused by Staphylococcus aureus and Streptococcus agalactiae in domestic bovids from Ismailia, Egypt. Trop. Anim. Health Prod. 2015 Feb;47:271–276. doi: 10.1007/s11250-014-0715-1. [DOI] [PubMed] [Google Scholar]
- 69.Hill A.E., Green A.L., Wagner B.A., Dargatz D.A. Relationship between herd size and annual prevalence of and primary antimicrobial treatments for common diseases on dairy operations in the United States. Prev. Vet. Med. 2009;88:264–277. doi: 10.1016/j.prevetmed.2008.12.00164. [DOI] [PubMed] [Google Scholar]
- 70.Bradley A.J., Green M.J. Factors affecting cure when treating bovine clinical mastitis with cephalosporin-based intramammary preparations. J. Dairy Sci. 2009;92(5):1941–1953. doi: 10.3168/jds.2008-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Song M., Li Q., Zhang Y., Song J., Shi X., Shi C. 2017. Bio Fi Lm Formation and Antibiotic Resistance Pattern of Dominant Staphylococcus aureus Clonal Lineages in China; pp. 1–7. [Google Scholar]
- 72.Ster C., Lebeau V., Leclerc J., Fugère A., Veh K.A., Roy J.P. In vitro antibiotic susceptibility and biofilm production of Staphylococcus aureus isolates recovered from bovine intramammary infections that persisted or not following extended therapies with cephapirin , pirlimycin or ceftiofur. Vet. Res. 2017:1–10. doi: 10.1186/s13567-017-0463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Melchior M.B., Osch M.H.J., Graat R.M., Duijkeren E Van, Mevius D.J. Vol. 137. 2009. Biofilm Formation and Genotyping of Staphylococcus aureus Bovine Mastitis Isolates : Evidence for Lack of Penicillin-Resistance in Agr -type II Strains; pp. 83–89. [DOI] [PubMed] [Google Scholar]
- 74.Radostits O.M., Gay C.C., Blood D.C., Hinchlif K.W., Mastitis . Harcourt Ltd; London: 2007. Veterinary Medicine 9 Thed; pp. 174–758. [Google Scholar]
- 75.Fabres-klein M.H., Junior M., Santos C., Klein R.C., Souza GN De, Oliveira A De, et al. 2015. An Association between Milk and Slime Increases Biofilm Production by Bovine Staphylococcus aureus; pp. 1–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonçalves J.L., Kamphuis C., Martins C.M.M.R., Barreiro J.R., Tomazi T., Gameiro A.H. Bovine subclinical mastitis reduces milk yield and economic return. Livest Sci [Internet] 2018;210(January):25–32. doi: 10.1016/j.livsci.2018.01.016. [DOI] [Google Scholar]
- 77.Hata E., Kobayashi H., Nakajima H., Shimizu Y., Eguchi M. 2010. Epidemiological Analysis of Staphylococcus aureus Isolated from Cows and the Environment of a Dairy Farm in Japan. [DOI] [PubMed] [Google Scholar]
- 78.Hassani S., Moosavy M.H., Gharajalar S.N., Khatibi S.A., Hajibemani A., Barabadi Z. High prevalence of antibiotic resistance in pathogenic foodborne bacteria isolated from bovine milk. Sci. Rep. 2022;12:3878. doi: 10.1038/s41598-022-07845-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Freu G., Tomazi T., Filho A.F., Heinemann M.B., Dos Santos M.V. Antimicrobial resistance and molecular characterization of Staphylococcus aureus recovered from cows with clinical mastitis in dairy herds from southeastern Brazil. Antibiotics. 2022;11:424. doi: 10.3390/antibiotics11040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song T., Duperthuy M., Wai S.N. Sub-optimal treatment of bacterial biofilms. Antibiotics. 2016 Jun 22;5(2):23. doi: 10.3390/antibiotics5020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zaatout N., Hezil D. A meta-analysis of the global prevalence of methicillin-resistant Staphylococcus aureus (MRSA) isolated from clinical and subclinical bovine mastitis. J. Appl. Microbiol. 2022;132:140–154. doi: 10.1111/jam.15192. [DOI] [PubMed] [Google Scholar]
- 82.Garcia-Migura L., Hendriksen R.S., Fraile L., Aarestrup F.M. Antimicrobial resistance of zoonotic and commensal bacteria in Europe: the missing link between consumption and resistance in veterinary medicine. Vet. Microbiol. 2014;170:1–9. doi: 10.1016/j.vetmic.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 83.Gianneechini R.E., Concha C., Franklin A. Antimicrobial susceptibility of udder pathogens isolated from dairy herds in the west littoral region of Uruguay. Acta Vet. Scand. 2002 Mar;43(1):1–10. doi: 10.1186/1751-0147-43-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.André M.C., Campos M.R., Borges L.J., Kipnis A., Pimenta F.C., Serafini A.B. Comparison of Staphylococcus aureus isolates from food handlers, raw bovine milk and Minas Frescal cheese by antibiogram and pulsed-field gel electrophoresis following SmaI digestion. Food Control. 2008 Feb 1;19(2):200–7007. [Google Scholar]
- 85.Turutoglu H.U., Ercelik S., Ozturk D. Antibiotic resistance of Staphylococcus aureus and coagulase-negative staphylococci isolated from bovine mastitis. Bull. Vet. Inst. Pulawy. 2006 Jan 1;50(1):41. [Google Scholar]
- 86.Kalmus P., Aasmäe B., Kärssin A., Orro T., Kask K. Udder pathogens and their resistance to antimicrobial agents in dairy cows in Estonia. Acta Vet. Scand. 2011 Dec;53:1–7. doi: 10.1186/1751-0147-53-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sakwinska O., Morisset D., Madec J.Y., Waldvogel A., Moreillon P., Haenni M. Link between genotype and antimicrobial resistance in bovine mastitis-related Staphylococcus aureus strains, determined by comparing Swiss and French isolates from the Rhone Valley. Appl. Environ. Microbiol. 2011 May 15;77(10):3428–3432. doi: 10.1128/AEM.02468-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peles F., Wagner M., Varga L., Hein I., Rieck P., Gutser K., Keresztúri P., Kardos G., Turcsányi I., Béri B., Szabó A. Characterization of Staphylococcus aureus strains isolated from bovine milk in Hungary. Int. J. Food Microbiol. 2007 Sep 15;118(2):186–193. doi: 10.1016/j.ijfoodmicro.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 89.Jørgensen H.J., Mørk T., Caugant D.A., Kearns A., Rørvik L.M. Genetic variation among Staphylococcus aureus strains from Norwegian bulk milk. Appl. Environ. Microbiol. 2005 Dec;71(12):8352–8361. doi: 10.1128/AEM.71.12.8352-8361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Anderson K.L., Lyman R.L., Bodeis-Jones S.M., White D.G. Genetic diversity and antimicrobial susceptibility profiles among mastitis-causing Staphylococcus aureus isolated from bovine milk samples. Am. J. Vet. Res. 2006 Jul 1;67(7):1185–1191. doi: 10.2460/ajvr.67.7.1185. [DOI] [PubMed] [Google Scholar]
- 91.Tenhagen B.A., Hansen I., Reinecke A., Heuwieser W. Prevalence of pathogens in milk samples of dairy cows with clinical mastitis and in heifers at first parturition. J. Dairy Res. 2009 May;76(2):179–187. doi: 10.1017/S0022029908003786. [DOI] [PubMed] [Google Scholar]
- 92.Ismael A. Journal of Veterinary Medicine Epidemiology of Bovine Mastitis in Ethiopia. 2018;2(1):1–7. [Google Scholar]
- 93.Gao J., Barkema H.W., Zhang L., Liu G., Deng Z., Cai L., et al. Incidence of clinical mastitis and distribution of pathogens on large Chinese dairy farms. J. Dairy Sci. 2017;100:4797–4806. doi: 10.3168/jds.2016-12334. [DOI] [PubMed] [Google Scholar]
- 94.Eshetie S., Tarekegn F., Moges F., Amsalu A., Birhan W., Huruy K. Methicillin resistant Staphylococcus aureus in Ethiopia: a meta-analysis. BMC Infect. Dis. 2016;16:1–9. doi: 10.1186/s12879-016-2014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thongratsakul S., Usui M., Higuchi H., Takahashi T., Sato T., Poolkhet C., et al. Prevalence and characterization of Staphylococcus aureus isolated in raw milk from cows in Hokkaido, Japan. Trop. Anim. Health Prod. 2020;52:1631–1637. doi: 10.1007/s11250-019-02169-6. [DOI] [PubMed] [Google Scholar]
- 96.Shrestha A., Bhattarai R.K., Luitel H., Karki S., Basnet H.B. Prevalence of methicillin-resistant Staphylococcus aureus and pattern of antimicrobial resistance in mastitis milk of cattle in Chitwan, Nepal. BMC Vet. Res. 2021;17:239. doi: 10.1186/s12917-021-02942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Asiimwe B.B., Baldan R., Trovato A., Cirillo D.M. Prevalence and molecular characteristics of Staphylococcus aureus, including methicillin resistant strains, isolated from bulk can milk and raw milk products in pastoral communities of South-West Uganda. BMC Infect. Dis. 2017;17(1):422. doi: 10.1186/s12879-017-2524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sudhanthiramani S., Swetha C.S., Bharathy S., Veterinary S.V. Prevalence of antibiotic resistant Staphylococcus aureus from raw milk samples collected from the local vendors in the region of Tirupathi, India. Vet. World. 2015;8(4):478–481. doi: 10.14202/vetworld.2015.478-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Annamanedi M., Sheela P., Sundareshan S., Isloor S., Gupta P., Jasmeen P., et al. Molecular fingerprinting of bovine mastitis-associated Staphylococcus aureus isolates from India. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-94760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abdi R.D., Gillespie B.E., Ivey S., Pighetti G.M., Almeida R.A., Kerro Dego O. Antimicrobial resistance of major bacterial pathogens from dairy cows with high somatic cell count and clinical mastitis. Animals. 2021;11:131. doi: 10.3390/ani11010131. ([CrossRef]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kabelitz T., Aubry E., van Vorst K., Amon T., Fulde M. The role of Streptococcus spp. in bovine mastitis. Microorganisms. 2021;9:1497. doi: 10.3390/microorganisms9071497. ([CrossRef]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cagnardi P., Grilli G., Villa R., Di Cesare F., Piccirillo A. Antimicrobials in farm animals: impact on the environment and consequent antimicrobial resistance dissemination. Int. J. Health Anim. Sci. Food Saf. 2018;5:22. [Google Scholar]
- 103.Andelkovi′c J., Radonji′c V. Usage of intramammary antimicrobial veterinary medicinal products in the republic of Serbia from ‾ 2011 to 2014. Serbian J. Exp. Clin. Res. 2017;18:27–31. ([CrossRef]) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author unreasonable request.