Abstract
DDT (1,1,1-trichloro-2,2 bis(4-chlorophenyl) ethane) is a synthetic insecticide that has several negative effects on the environment and humans. Therefore, determining an effective method to reduce DDT may give a beneficial impact. Brown-rot fungus, Gloeophyllum trabeum, is well known to have the ability to degrade DDT, even though it might require long-term remediation. In this study, the effect of the addition of bacteria on the biodegradation of DDT by G. trabeum had been investigated. Bacillus subtilis, Pseudomonas aeruginosa, and Ralstonia pickettii were screened for the bacteria which the volume of bacteria at 1, 3, 5, and 10 mL and the time range of addition of bacteria on days 0, 1, 3, and 5. The addition of B. subtilis, P. aeruginosa, and R. pickettii bacteria into the G. trabeum culture increased DDT biodegradation to approximately 62.02; 74.66; and 75.72%, respectively, in which G. trabeum was only able to degrade DDT by 54.52% for 7 days of incubation. R. pickettii enhanced the degradation process, in which the addition of 10 mL of this bacterium at day 1 possessed the highest value of 92.41% within 7 days of incubation. DDD was detected to be a product metabolite through a dechlorination reaction. This study indicated that mixed cultures of G. trabeum and R. pickettii can be used to degrade DDT.
Keywords: Biodegradation, DDT, Bacillus subtilis, Pseudomonas aeruginosa, Ralstonia pickettii, Gloeophyllum trabeum
1. Introduction
Organochlorine pesticides are synthetic pesticides that are very effective against a wide variety of insects. One of them is the compound 1,1,1-trichloro-2,2-bis(4-chlorophenyl) ethane or dichloro diphenyl trichloroethane (DDT). DDT was the first synthetic insecticide to be widely used in the 1940s. DDT was first synthesized by the chemist Othmar Zeidler in 1874 but has yet to find its use. In 1939, Swiss chemist Paul Hermann Muller discovered that DDT could be used as an insecticide. During World War II, DDT was used to kill insects carrying malaria and typhoid. Between 1940 and 1970 the production and use of DDT increased from year to year, the maximum peak production in 1963 was approximately 600,000 tons and in 1959 users of DDT reached 1.35 billion people [1].
Due to the long-lasting and toxic nature of DDT, in 1972 the United States began to prohibit the use of DDT, and the United States Environmental Protection Agency classified DDT, and its metabolites such as 1,1-dichloro-2,2-bis(4-chlorophenyl) ethane (DDD) and 1,1-dichloro-2,2-bis(4-chlorophenyl)ethane (DDE) as pollutants [2]. Although its use has been banned, some developing countries still use DDT in health and agriculture [3]. DDT is highly hydrophobic and almost insoluble in water. The half-life of this compound in water is 30–60 days but can last 2–15 years in living organisms [4]. Direct exposure to low amounts of DDT results in symptoms such as headache, nausea, vomiting, confusion, and tremors. DDT and its metabolic products accumulate in food sources and adipose tissue in organisms that accumulate in the human body causing several problems including attacking the central tissue system, damaging DNA in blood cells decreasing male reproductive ability, interfering with the synthesis and metabolism of endogenous hormones, sex hormone disorders, and increase tumor production, pancreatic cancer, and breast cancer. In some animals, DDT accumulation can damage the endocrine system of birds and mammals [[5], [6], [7]]. Therefore, it is necessary to develop a remediation method to clean the residual DDT waste so that it is safe for the environment.
DDT remediation can be done physically and chemically, but it is more expensive and damaging to the environment, so there is a need for safe biological remediation at low cost by using the biodegradation method. Biodegradation is one of the bioremediation processes or reducing the level of contaminants into simple compounds that are less or non-toxic in soil, water, and the environment by using microorganisms (fungi or bacteria) as degrading agents. Microorganisms are known to have the ability to change or degrade xenobiotic compounds [8]. Some microorganisms already reported can degrade contaminants such as hydrocarbons and kerosene, polycyclic aromatic hydrocarbons (PAHs), pentachlorophenols (PCPs), polychlorinated biphenyls (PCBs), and pesticides [9].
Fungi are strong microorganisms and are more tolerant of chemical pollutants at high concentrations. One of the fungi that can degrade organic pollutants is the brown-rot Iungi (BRF). BRF are fungi that use hydroxyl radicals produced from the Fenton reaction in degrading cellulose and hemicellulose by modifying the lignin around them [10]. In addition to producing hydroxyl radicals, these fungi produce cellulase enzymes which are used to degrade cellulose as a source of carbon and energy. Some of the BRF that have been used for the degradation of organic pollutants include Gloeophyllum trabeum, Daedalea dickinsii, and Fomitopsis pinicola [11]. G. trabeum is a brown-rot fungus that uses the Fenton reaction in its degradation mechanism. Previous studies reported that the fungus G. trabeum was able to degrade 87% of DDT for 28 days in pure culture. This result was still relatively low and needed long incubation times. Thus, it is necessary to modify the degradation process [12].
On the other hand, bacteria also can live in various habitats and can degrade complex compounds into simpler compounds to meet their nutritional needs. Bacterial growth is quite fast and its high adaptability makes bacteria potential to be applied in degrading organic pollutants. Several types of bacteria have also been reported to be able to degrade organic pollutants. R. pickettii has been reported can degrade BTEX compounds such as benzene, ethylbenzene, toluene, and xylene isomers [13]. P. aeruginosa has been reported can degrade paracetamol up to 71,4% [14]. Another research reported that the P. aeruginosa 640x strain also can degrade DDT and its analogs [15]. The other bacteria, B. subtilis also reported can degrade Polyethylene [16]. This indicates that some bacteria have the ability and potential to be used as organic pollutant-degrading agents.
One method to optimize the degradation of DDT by fungi is the addition of bacteria. Several studies have reported that the use of mixed cultures of fungi and bacteria can increase the ability of degradation. Mixed cultures of Coriolus versicolor and bacteria have been reported to be able to remove 47, 98, and 62% of aldicarb, atrazine, and alachlor in liquid culture for 14 days [17]. Other studies reported that the addition of bacteria (P. aeruginosa, R. pickettii, and B. subtilis) has an effect to increase DDT biodegradation by Daedalea dickinsii [18]. P. aeruginosa, R. pickettii, and B. subtilis are also reported to have the capability to increase methylene blue degradation by G. trabeum [19,20].
Based on this background, the objective of this research is to investigate the effect of adding bacteria on the DDT biodegradation ability by G. trabeum. The bacteria used were B. subtilis, P. aeruginosa, and R. pickettii. The increase in the amount of degradation occurs because of the synergy between fungi and bacteria. Besides being able to degrade pollutant compounds, these bacteria can also produce biosurfactants [13]. Biosurfactants are surface-active compounds that are able to reduce the surface tension or interfacial tension of two phases. In the presence of biosurfactants produced by bacteria, the dissolved DDT levels increased so that the G. trabeum fungus became easier to degrade DDT.
2. Materials and methods
2.1. Materials
The materials used in this study were the fungus Gloeophyllum trabeum (NBRC 6430), the bacterium Bacillus subtilis (NBRC 3009), Pseudomonas aeruginosa (NBRC 3080), and the Ralstonia pickettii (NBRC 102503) strain obtained from the collection of the Microbial Chemistry Laboratory, Department of Chemistry, Faculty of Science and Data Analytics ITS, DDT, DDD (Tokyo Chemical Industry Co., Japan), Potato Dextrose Agar (PDA, Merck, Darmstadt, Germany), Potato Dextrose Broth (PDB, Merck, Darmstadt, Germany), Nutrient Borth (NB, Merck, Darmstadt, Germany), Nutrient Agar (NA, Merck, Darmstadt, Germany), 70% ethanol, methanol, acetone, aqua DM, anhydrous Na2SO4 (Merck Millipore, Darmstadt, Germany), n-hexane (Anhui Fulltime, China), and dimethyl sulfoxide (DMSO).
2.2. Preparation of G. trabeum culture
The stock culture of G. trabeum was inoculated on the 90-mm diameter of PDA plates and incubated at 30 °C for 7 days. Then the incubated mycelia were transferred into an aseptic blender jar containing 25 mL of aseptic aqua distillation and homogenized for 3 min. A milliliter of this homogenate was added into 8 mL of PDB medium in 100-mL Erlenmeyer flasks. Then the cultures were incubated statically at 30 °C for 7 days [21].
2.3. Preparation of bacterium culture
The bacterium culture (single culture of B. subtilis, P. aeruginosa and R. pickettii) stock was inoculated on the 90-mm diameter of NA plates and incubated at 37 °C. Then the bacterium colony was inoculated in a 500 mL Erlenmeyer flask with 250 mL of sterile NB medium. Subsequently, the culture was pre-incubated on a shaker incubator at 180 rpm for 44 h [22,23].
2.4. Effect of bacteria addition on the growth of G. trabeum
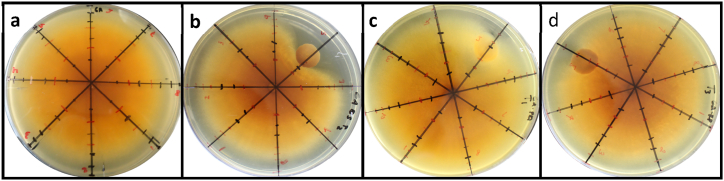
G. trabeum was inoculated into a Petri dish containing a sterile agar medium (PDA). B. subtilis, P. aeruginosa, and R. pickettii bacteria were inoculated into PDB media and incubated based on their stationary phase. The pre-incubated bacterial cells were taken as much as 20 mL and centrifuged for 10 min. The centrifuged biomass was added with 20 mL of water, then homogenized and centrifuged for 10 min at 3000 rpm. After that, the biomass and supernatant were separated, added 20 mL of distilled water was homogenized and centrifuged. The centrifuged biomass was added with 20 mL of water, then homogenized. G. trabeum fungus was inoculated using a 5 mm loop into a Petri dish containing sterile agar medium (PDA). Then added the bacteria at a distance of 4 cm from the G. Trabeum as shown in Fig. 1, followed by incubation at 28 °C for 2 days. After that, the mycelium growth was measured every day until the entire surface of the medium was covered with mycelium. As a control, the treatment was the same without the addition of bacteria [18].
Fig. 1.

The Position of The bacteria and the G. Trabeum in interaction assays.
2.5. DDT biodegradation by G. trabeum
G. trabeum cultures with a total volume of 20 mL were pre-incubated for 7 days, and each culture was added with 50 μL 5 mM DDT in DMSO (final concentration: 0.25 mol DDT/culture). Oxygen was added to each flask by flowing it through an oxygen tube inserted into the culture, when the culture medium was saturated as indicated by the coldness of the Erlenmeyer bottom surface, the addition of oxygen was stopped. Then the culture was covered with a glass stopper and tape to prevent the evaporation of DDT. The cultures were statically incubated for 7 days at 30 °C. As a negative control, the cultures were inactivated by autoclaving at 121 °C for 15 min before adding the DDT [18].
2.6. Effect of bacterial strains on DDT biodegradation by G. trabeum (bacteria screening)
Pre-incubated cultures of bacteria in NB medium with a volume of 5 mL was added to the pre-incubated G. trabeum culture in 10 mL PDB medium. Then each culture was added with 50 μL DDT 5 mM in DMSO (final concentration 0.25 mol DDT/culture). Then PDB was added to each culture up to a total volume of 20 mL. The cultures were statically incubated for 7 days at 30 °C. As a negative control, the cultures were sterilized by autoclaving at 121 °C for 15 min before adding DDT [18].
2.7. Effect of concentration of bacteria addition on the biodegradation of DDT by G. trabeum
The pre-incubated R. pickettii culture was added to the G. trabeum culture with variations in the number of bacteria 1, 3, 5, 7, and 10 mL. Then PDB medium was added to a total volume of 20 mL. Each culture was added to 50 μL of 5 mM DDT in DMSO (final concentration 0.25 mol DDT/culture). Oxygen was added to each flask by flowing it through an oxygen tube inserted into the culture, when the culture medium was saturated which was marked by the coldness of the bottom surface of the Erlenmeyer, the addition of oxygen was stopped. Then the culture is closed with a glass stopper and tape. The cultures were statically incubated for 7 days at 30 °C. As a negative control, the culture was deactivated by autoclaving at 121 °C for 15 min before adding DDT. The number of bacteria that produce the greatest DDT degradation ability was used in the next degradation process [18].
2.8. Effect of time of addition of bacteria to biodegradation of DDT by G. trabeum (variation of time of addition of bacteria)
A total of 50 L of 5 mM DDT in DMSO solvent (final concentration 0.25 mol DDT/pumpkin) was added to the pre-incubated G. trabeum culture in 10 mL PDB medium for 7 days at 30 °C. Furthermore, the pre-incubated culture of R. pickettii was added with variations in the added time of 0, 1, 3, and 5 days. Each flask is added oxygen by flowing it through an oxygen tube inserted into the culture, when the culture medium is saturated which is indicated by the coldness of the Erlenmeyer surface, the addition of oxygen is stopped. Then the culture is closed with a glass stopper and tape. The cultures were statically incubated for 7 days at 30 °C. As a negative control, the culture was deactivated by autoclaving at 121 °C for 15 min before adding DDT [18].
2.9. DDT recovery and product metabolite identification
A total of 20 mL of methanol and 50 L of 5 mM pyrene were added to the incubation culture. The sample was washed with 10 mL of acetone. The culture was filtered using Whatman paper diameter 9. The filtrate was evaporated at 40 °C until all the acetone and methanol had evaporated. The filtrate that did not evaporate was put into a separating funnel, then 50 mL of distilled water and 50 mL of n-hexane were added and shaken for 10 min. The organic phase was separated while the aqueous phase was re-extracted 2 times. The collected organic phase was put in Erlenmeyer which already contained anhydrous Na2SO4, then filtered. The sample was evaporated at a temperature of 65 °C until about 1 mL remained, then the sample was analyzed using GC/MS with an inlet temperature of 250 °C and a flow rate of 1 mL/min, the temperature of the first column was 80 °C held for 3 min, then increased for 12 min up to a temperature of 320 °C, when it reaches a temperature of 320 °C it is held for 2 min, then decreases to a temperature of 300 °C and held for 5 min. GC/MS was performed on an HP 6890 GC system (HP, USA) linked to an HP 5973 mass-selective detector (HP, USA) with a 30-m fused DB-5MS column (J&W Scientific, CA, USA). The remaining sample was re-evaporated and added 1 mL of methanol. The residue (sample) was homogenized with an ultrasonic cleaner, then analyzed using HPLC, the conditions used were isocratic with 80% acetonitrile and 20% water as the mobile phase, and the column temperature used was 45 °C [18]. The HPLC system consisted of a PU-1500 intelligent pump (Jasco, Japan) and an MD-1510 multiwavelength detector (Jasco, Japan) fitted with an Inertsil ODS-3 column (150 mm) with an inner diameter of 4.6 mm (GL Science, Japan).
2.10. Statistical analysis
The results were calculated as the average of three replicate determinations. Student’s t-test was used to detect any significant differences between or within groups during DDT transformation. Differences between mean values at a confidence level of 5% (P < 0.05) were considered to be statistically significant [18].
3. Results and discussion
3.1. Effect of bacteria addition on the growth of G. trabeum
The test of the effect of adding bacteria to the growth of G. trabeum was carried out by growing G. trabeum with bacteria in one culture. It aims to determine whether the added bacteria inhibit the growth of G. trabeum or accelerate its growth. The results of the interaction test are shown in Fig. 2. Fig. 2a shows the growth of G. trabeum without the addition of bacteria, Fig. 2b shows the growth of G. trabeum with B. subtilis addition, Fig. 2c shows the growth of G. trabeum with P. aeruginosa addition, and Fig. 2d shows the growth of G. trabeum with R. pickettii addition. Based on Fig. 2, shows that B. subtilis inhibited the growth of G. trabeum by comparing the growth of the control mycelium which showed no inhibition. B. subtilis is able to inhibit the growth of G. trabeum because B. subtilis is able to produce several peptides that act as antibiotics and antifungals, such as subtilin, aterimin, bacitracin, subtilisin, mycobacillin, subsporin, ituirin, sericin, serelaxin, surfactin, bafilomycin, cyanide acid, and basillocine [[24], [25], [26], [27]]. In addition, B. subtilis also produces macromolecular degradative enzymes that can destroy fungal cell walls, such as proteases (intracellular), and several extracellular enzymes secreted in the medium such as levansucrase, glucanase, amylase, xylanase, chitinase, and proteases [27].
Fig. 2.
Interaction Test between G. trabeum and bacteria after 10 days of incubation. a) control (G. trabeum without the addition of bacteria), b) G. trabeum with B. subtilis, c) G. trabeum with P. aeruginosa, d) G. trabeum with R. pickettii.
The growth of G. trabeum mycelium could not be inhibited by the presence of P. aeruginosa and R. pickettii bacteria, this was evidenced by the contact between the G. trabeum mycelium and bacterial colonies. Several studies have reported that P. aeruginosa and R. pickettii bacteria can promote fungal growth. Pseudomonas sp. P7014 has been reported can increase the growth rate of the mycelium of the fungus Pleurotus eryngii [28]. Pseudomonas sp. Also reported did not affect the mycelium growth of Suillus granulatus and R. pickettii to increase the mycelium growth of S. granulatus without cell contact between fungi and bacteria [29].
3.2. Effect of bacteria addition on DDT biodegradation by G. trabeum
After the degradation process for 7 days was complete, the recovery process was carried out, where the recovery aimed to determine the amount of DDT that was degraded by comparing the peak area of DDT and pyrene (internal standard). The results of the analysis using HPLC showed that the DDT control recovery was 96.70%, while the recovery treatment was 42.17%. Based on these data, G. trabeum was able to degrade DDT by 54.52% for 7 days of incubation.
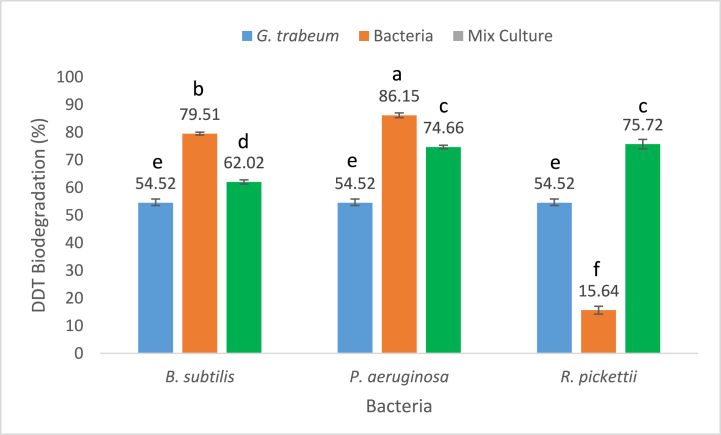
The effect of adding bacteria on the biodegradation of DDT by G. trabeum was also investigated. The results showed that the addition of bacteria can increase the performance of DDT biodegradation by G. trabeum. This was indicated by the addition of B. subtilis, P. aeruginosa, and R. pickettii bacteria into the G. trabeum culture increasing DDT biodegradation to approximately 62.02; 74.66; and 75.72%, respectively, which was higher compared to the degradation of G. trabeum without the addition of the bacteria itself, which was 54.52% (Fig. 3).
Fig. 3.
The Effect of different bacteria strains addition on DDT biodegradation by G. trabeum. The data is presented as the mean value with a sample size of 3. Bars with the same lowercase letter indicate no significant difference (P < 0.05) between them.
The degradation had increased because these three bacteria produce biosurfactants, where the biosurfactants produced by the bacteria include surfactin (B. subtillis), and rhamnolipids (P. aeruginosa and R. pickettii) as emulsifying agents that increase the solubility of DDT in culture media so that the availability of DDT for degradation is increasing. This assumption was reinforced by several studies that reported that biosurfactants, namely rhamnolipids produced by P. aeruginosa in pure culture, can increase the rate of hydrocarbon biodegradation [[30], [31], [32], [33], [34], [35]].
The addition of B. subtilis into G. trabeum culture showed the lowest degradation results among the others because B. subtilis inhibited the growth of the fungus G. trabeum as evidenced in Fig. 2(b) which showed that the mycelium growth of G. trabeum in PDA media was inhibited in the presence of B. subtilis. B. subtilis produces antifungal compounds that can inhibit the growth of fungi. Previous studies reported that B. subtilis produces volatile compounds, namely antifungals against the fungi Rhizoctonia solani and Pythium ultimum [25]. In addition, B. subtilis inhibits the growth of the fungus Phytophthora palmivora because this bacterium produces several peptides that act as antibiotics and antifungals, such as subtilin, aterimin, bacitracin, subtilisin, mycobacillin, subsporin, ituirin, surfactin, bafilomycin, basillisin, and cyanide acid. B. subtilis is a bacterial pathogen that produces macromolecular degradative enzymes that can destroy fungal cell walls, such as proteases (intracellular) and several extracellular enzymes secreted in the medium such as levansucrase, glucanase, amylase, xylanase, chitinase, and proteases [36].
The addition of P. aeruginosa and R. pickettii to the culture of G. trabeum showed the highest degradation with 74.66 and 75.72%, respectively. Judging from the results of bacterial performance, the degradation of the mixture of G. trabeum and P. aeruginosa decreased compared to the degradation performance of P. aeruginosa itself, but there was a significant increase in the mixture of G. trabeum and R. pickettii compared to the degradation performance of R. pickettii alone. This indicated that R. pickettii can synergize with G. trabeum. It was shown in Fig. 2(d) that the mycelium growth of G. trabeum was not inhibited in the presence of R. pickettii bacterium. Synergism is an interaction that occurs between fungi and bacteria which has a mutually beneficial effect on both parties [36]. Furthermore, studies on the biodegradation of organic pollutants using mixed cultures of fungi and bacteria were previously reported. The combination of the white-rot fungus (WRF) Coriolus versicolor and bacteria can remove aldicarb, atrazine, and alachlor to 47%, 98%, and 62%, respectively within 14 days of incubation period [37]. Several studies on DDT biodegradation were conducted using bacterium and fungus co-culture. The co-culture of WRF Ganoderma lingzhi and B. subtilis bacterium degraded DDT to about 82.30% [38]. Also, the co-culture of G. lingzhi and P. aeruginosa degraded DDT to 100%, while particular G. lingzhi degraded to 52.52% for 7 days incubation period in the PDB medium [39]. Other WRFs such as Pleurotus ostreatus and Pleurotus eryngii, which degraded DDT alongside bacterium, were mixed cultures of P. aeruginosa with P. ostreatus, R. pickettii with P. eryngii, and P. aeruginosa with P. eryngii [[40], [41], [42], [43], [44]]. P. ostreatus and P. eryngii with bacterium degraded 86 and 78% of DDT, respectively, while particular P. eryngii and P. ostreatus degraded 43% and 19.35% of DDT, respectively. Also, BRF mixed cultures with a bacterium or another species of fungus were reported to degrade some pollutants. The combination of 3 mL R. pickettii bacterium and BRF Fomitopsis pinicola showed enhanced DDT degradation up to 61%, while particular R. pickettii and F. pinicola degraded about 31% and 42%, respectively [41]. However, the addition of 10 mL P. aeruginosa and B. subtilis bacterium to F. pinicola culture degraded DDT to approximately 68% and 86%, respectively during the 7 days incubation period in the PDB medium. The result was higher than the biodegradation of DDT by F. pinicola, which was about 42% [45,46]. Another BRF, D. dickinsii, showed good synergistic ability with bacteria culture to degrade DDT. The addition of 10 mL of P. aeruginosa, B. subtilis, and R. pickettii on different flasks containing D. dickinsii culture degraded 96.70%, 67.70%, and 68.62% of DDT respectively for 7 days incubation period [18].
In this study, the addition of R. pickettii bacteria gave the highest effect of increasing the degradation ability compared to the others. For this reason, the culture optimization was also carried out by varying the number of additions of R. pickettii bacterium into the G. trabeum culture (Fig. 4). Based on Fig. 4, the variations in the amount of R. pickettii added to G. trabeum culture had an effect on DDT degradation. The increase in the amount of DDT degradation in the mixture of G. trabeum and R. pickettii reached 80.81% with the addition of 10 mL of bacteria, this indicated that the more bacteria added the greater the biosurfactant produced causing the amount of DDT solubility in G. trabeum culture to increase so that the amount of DDT the more degraded and the faster the degradation process. Synergy was seen in the mixture of G. trabeum and R. pickettii, where the degradation product of the mixture increased compared to the performance of each degrader. The addition of 10 mL of R. pickettii was used to test the effect of the time of adding R. pickettii on the biodegradation of DDT by G. trabeum.
Fig. 4.
The effect of bacteria concentration on DDT biodegradation by G. trabeum. The data is presented as the mean value with a sample size of 3. Bars with the same lowercase letter indicate no significant difference (P < 0.05) between them.
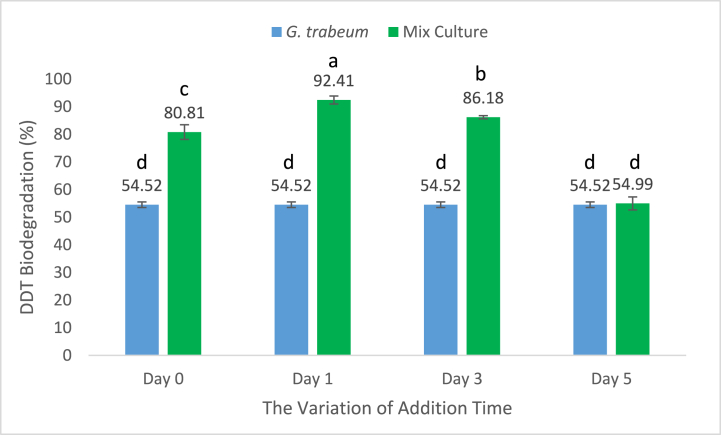
The optimal time for the addition of bacteria was also investigated. The results were shown in Fig. 5. Based on the figure, it showed that the time of adding R. pickettii to G. trabeum culture influenced DDT degradation. The ability of DDT degradation performance by G. trabeum increased when R. pickettii was added on the 1st and 3rd days. The maximum increase that occurred during the addition of the 1st day of 11.60%, while the decreased during the addition of the 3rd day with an increase of 5.37% compared to the addition on the 0th day. On day 5, the degradation ability of DDT by mixed cultures of G. trabeum and R. pickettii decreased by 25.82% compared to the addition on day 0, this result was close to the degradation ability of G. trabeum without the addition of bacteria by 54.52%. Giving an interval of 1 day and 3 days can provide an opportunity for G. trabeum to produce Fenton reactions first, so that when bacteria are added the solubility of DDT increases which causes the degradation of DDT with Fenton reactions produced by G. trabeum increases. However, when added on day 5, the degradation ability of the mixture of G. trabeum and R. pickettii decreased by 54.99%, in addition, when compared to the degradation ability of G. trabeum without the addition of R. pickettii, it did not show a significant difference, the difference was only 0.47%. This indicated that the biosurfactant produced by R. pickettii on the 5th-day addition was not maximal, so it did not affect the solubility of DDT in culture and the ability of DDT degradation performance by G. trabeum [40,41]. Previous studies reported that in a mixed culture between bacteria and fungi, fungi play a very large role in the degradation process compared to bacteria, because of the ability of fungi to degrade organic molecules, so that bacteria will continue the rest, the high number of enzymes evidences this [36].
Fig. 5.
The effect of addition time on DDT biodegradation by G. trabeum. The data is presented as the mean value with a sample size of 3. Bars with the same lowercase letter indicate no significant difference (P < 0.05) between them.
3.3. Product metabolites identification and proposed degradation pathway
In this study, the metabolites of DDT biodegradation products were analyzed using GCMS. Chromatogram results from GCMS analysis for metabolites of the degradation product of DDT by G. trabeum were shown in Fig. 6. The figure showed that there were three peaks with different retention times. Pyrene compound as an internal standard was detected at a retention time of 11.7 min, DDT at a retention time of 14.8 min, and DDD at a retention time of 13.4 min. DDD compound was a metabolite product of DDT degradation by G. trabeum during the 7-day incubation period. Identification of compounds was carried out based on MS spectra analysis results with the MS spectra database. Matching was based on the split pattern on the MS spectra database with the split pattern on the MS spectra as a result of the analysis.
Fig. 6.
GC chromatogram of DDT biodegradation by G. trabeum.
Not much different from the results above, the results of the GC chromatogram analysis for the metabolites of the degradation products of DDT by the culture of G. trabeum with the addition of R. pickettii were shown in Fig. 7. The figure showed that there were two peaks with different retention times, namely 10.3 and 13.9 min. Identification of compounds was carried out based on the results of the MS spectra of each compound in the chromatogram with the spectra of the database. Furthermore, each compound was matched based on the fragments in the MS spectra of the database with the MS spectra from the analysis. Based on the results of the analysis, pyrene was released at a retention time of 10.367 min and DDD was released at a retention time of 13.917. minute. DDD compound is a product metabolite produced by a mixture of G. trabeum and R. pickettii. The disappearance of the DDT peak in the chromatogram indicated that the addition of R. pickettii bacteria increased the degradation ability of DDT by G. trabeum culture.
Fig. 7.
GC chromatogram of DDT biodegradation by G. trabeum with R. pickettii addition.
Determination of the degradation pathway was carried out by repeating the method of adding R. pickettii to the biodegradation of DDT by G. trabeum but the DDT substrate was replaced with a commercial product metabolite substrate, namely DDD according to the metabolite of the previous degradation product. Based on the results of the degradation of DDD by a mixture of G. trabeum and R. pickettii, the DDD compound did not degrade into other metabolites, so it was concluded that the DDD compound was a product metabolite produced by a mixture of G. trabeum and R. pickettii through a dechlorination reaction. The following is the proposed DDT degradation pathway by a mixture of G. trabeum and R. pickettii as shown in Fig. 8. The dotted arrows represent the DDT degradation pathway by G. trabeum.
Fig. 8.
The prediction of DDT Biodegradation Product.
4. Conclusion
The addition of B. subtilis, P. aeruginosa, and R. pickettii bacteria increased the degradation of DDT by G. trabeum. The addition of 10 mL of R. pickettii showed the highest DDT degradation of 80.81% for 7 days of incubation. The most appropriate time for adding R. pickettii to the degradation of DDT by G. trabeum was on day 1 with a yield of 92.41%. DDD was a metabolite product produced in the degradation of DDT by a mixture of R. pickettii and G. trabeum. This study showed that R. pickettii affected the biodegradation of DDT by G. trabeum and that there was a synergistic relationship between G. trabeum and R. pickettii.
Author contribution statement
Hamdan Dwi Rizqi: performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Adi Setyo Purnomo: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Aulia Ulfi: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This research was funded by the Indonesian Ministry of Education, Culture, Research and Technology, the Institur Teknologi Sepuluh Nopember (ITS), the Directorate of Research and Community Service, in accordance with the Scientific Research Grant with Number: 952/PKS/ITS/2022.
Data availability statement
Data included in article/supp. material/referenced in article.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
References
- 1.Padayachee K., Reynolds C., Mateo R., Amar A. A global review of the temporal and spatial patterns of DDT and dieldrin monitoring in raptors. Sci. Total Environ. 2022;858 doi: 10.1016/j.scitotenv.2022.159734. [DOI] [PubMed] [Google Scholar]
- 2.Yang X., Wang S., Bian Y., Chen F., Yu G., Gu C., Jiang X. Dicofol application resulted in high DDTs residue in cotton fields from northern Jiangsu province, China. J. Hazard Mater. 2007;150:92–98. doi: 10.1016/j.jhazmat.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 3.Foght J., April T., Biggar K.A.J. Bioremediation of DDT-contaminated soils: a review. Ann. Finance. 2001;5:225–246. doi: 10.1080/20018891079302. [DOI] [Google Scholar]
- 4.Vested A., Giwercman A., Bonde J.P., Toft G. Persistent organic pollutants and male reproductive health. Asian J. Androl. 2014;16(1):71–80. doi: 10.4103/1008-682X.122345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad R., Salem N.M., Estaitieh H. Occurrence of organochlorine pesticide residues in eggs, chicken and meat in Jordan. Chemosphere. 2009;78:667–671. doi: 10.1016/j.chemosphere.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Purnomo A.S. In: Microbe-Assisted Degradation of Aldrin and Dieldrin. Singh S., editor. Springer; Cham: 2017. (Microbe-Induced Degradation of Pesticides. Environmental Science and Engineering). [DOI] [Google Scholar]
- 7.Purnomo A.S., Mori T., Kamei I., Kondo R. Basic studies and applications on bioremediation of DDT: a review. Int. Biodeterior. Biodegrad. 2011;65:921–930. doi: 10.1016/j.ibiod.2011.07.011. [DOI] [Google Scholar]
- 8.Al-Rashed S., Marraiki N., Syed A., Elgorban A.M., Prasad K.S., Shivamallu C., Bahkali A.H. Bioremediation characteristics, influencing factors of dichlorodiphenyltrichloroethane (DDT) removal by using non-indigenous Paracoccus sp. Chemosphere. 2020;270 doi: 10.1016/j.chemosphere.2020. [DOI] [PubMed] [Google Scholar]
- 9.Xu G., Zhao S., Liu J., He J. Bioremediation of organohalide pollutants: progress, microbial ecology, and emerging computational tools. Curr. Opin. Environ. Sci. Health. 2023;32 doi: 10.1016/j.coesh.2023.100452. [DOI] [Google Scholar]
- 10.Goodell B., Winandy J., Morrell J. Fungal degradation of wood: emerging data, new insights and changing perceptions. Coatings. 2020;10:1210. doi: 10.3390/coatings10121210. [DOI] [Google Scholar]
- 11.Setyo P.A., Dwi R.H., Sri F., Sulistyo P.H., Ichiro K. Effects of bacterium Ralstonia pickettii addition on DDT biodegradation by Daedalea dickinsii. Res. J. Chem. Environ. 2018;22:151–156. https://worldresearchersassociations.com/SpecialIssueAugust2018/26.pdf [Google Scholar]
- 12.Purnomo A.S., Koyama F., Mori T., Kondo R. DDT degradation potential of cattle manure compost. Chemosphere. 2010;80:619–624. doi: 10.1016/j.chemosphere.2010.04.059. [DOI] [PubMed] [Google Scholar]
- 13.Plaza G.A., Wypych J., Berry C., Brigmon R.L. Utilization of monocyclic aromatic hydrocarbons individually and in mixture by bacteria isolated from petroleum contaminated soil. World J. Microbiol. Biotechnol. 2007;23:533–542. doi: 10.1007/s11274-006-9256-8. [DOI] [Google Scholar]
- 14.Hu J., Zhang L.L., Chen J.M., Liu Y. Degradation of paracetamol by Pseudomonas aeruginosa strain HJ1012. J. Environ. Sci. Health: A Tox. Hazard. Subst. Environ. Eng. 2013;48(7):791–799. doi: 10.1080/10934529.2013.744650. [DOI] [PubMed] [Google Scholar]
- 15.Golovleva L.A., Zyakun A.M., Baskunov P.B., Pertsova R.N., Skryabin G.K. Degradation of DDT and its analogs by Pseudomonas aeruginosa 640X. Biol. Bull. Russ. Acad. Sci. 1980;7(2):143–151. PMID: 6784780. [PubMed] [Google Scholar]
- 16.Vimala P.P., Mathew L. Biodegradation of Polyethylene using Bacillus subtilis. Proc. Technol. 2016;24:232–239. doi: 10.1016/j.protcy.2016.05.031. [DOI] [Google Scholar]
- 17.Hai F.I., Modin O., Yamamoto K., Fukushi K., Nakajima F., Nghiem L. Pesticide removal by mixed culture of bacteria and white-rot fungi. J. Taiwan Inst. Chem. Eng. 2011;43:459–462. doi: 10.1016/j.jtice.2011.11.002. [DOI] [Google Scholar]
- 18.Rizqi H.D., Purnomo A.S., Kamei I. Interaction and effect of bacteria addition on dichlorophenyltrichloroethane biodegradation by Daedala dickinsii. Curr. Microbiol. 2021;78:668–678. doi: 10.1007/s00284-020-02305-8. [DOI] [PubMed] [Google Scholar]
- 19.Yuniarti E.P., Fuadah R.H., Rizqi H.D., Nawfa R., Putro H.S., Purnomo A.S. Effect of addition of bacterium Pseudomonas aeruginosa on biodecolorization of methylene blue by brown rot fungus Gloeophyllum trabeum. AIP Conf. Proc. 2022;2638(1) doi: 10.1063/5.0104088. [DOI] [Google Scholar]
- 20.Purnomo A.S., Rohmah A.A., Rizqi H.D., Putro H.S., Nawfa R. Biodecolorization of methylene blue by mixed cultures of brown rot fungus Gloeophylum trabeum and bacterium Bacillus subtilis. AIP Conf. Proc. 2021;2349(1) doi: 10.1063/5.0062270. [DOI] [Google Scholar]
- 21.Purnomo A.S., Ashari K.A., Hermansyah F.T. Evaluation of the synergistic effect of mixed cultures of white-rot fungus Pleurotus ostreatus and biosurfactant-producing bacteria on DDT biodegradation. J. Microbiol. Biotechnol. 2017;27(7):1306–1315. doi: 10.4014/jmb.1701.01073. [DOI] [PubMed] [Google Scholar]
- 22.Wahyuni S., Suhartono M.T., Khaeruni A., Purnomo A.S., Asranudin Holilah, Riupassa P.A. Purification and characterization of thermostable chitinase from Bacillus SW42 for chitin oligomer production. Asian J. Chem. 2016;28:2731–2736. doi: 10.1007/s12010-008-8328-7. [DOI] [Google Scholar]
- 23.Wahyuni S., Khaeruni A., Purnomo A.S., Asranudin Holilah, Fatahu Characterization of mannanase isolated from corncob waste bacteria. Asian J. Chem. 2017;29:1119–1120. doi: 10.14233/ajchem.2017.20437. [DOI] [Google Scholar]
- 24.Tuyen D., Trung N., Thao N., Nguyen T., Nguyen N., Tuyet N., Tien Cuong N., Chan S., Khoo K.S., Show P. Antifungal activity of secondary metabolites purified from Bacillus subtilis isolated in Vietnam and evaluated on in vitro and in vivo models. Int. Biodeterior. Biodegrad. 2022;179 doi: 10.1016/j.ibiod.2022.105558. [DOI] [Google Scholar]
- 25.Fiddaman P.J., Rossall S. The production of antifungal volatiles by Bacillus subtilis. J. Appl. Bacteriol. 1993;74:119–126. doi: 10.1111/j.1365-2672.1993.tb03004.x. [DOI] [PubMed] [Google Scholar]
- 26.Joshi S., Bharucha C., Desai A.J. Production of biosurfactant and antifungal compound by fermented food isolate Bacillus subtilis 20B. Bioresour. Technol. 2007;99:4603–4608. doi: 10.1016/j.biortech.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 27.Parthipan P., Preetham E., Machuca L.L., Rahman P.K.S.M., Murugan K., Rajasekar A. Biosurfactant and degradative enzymes mediated crude oil degradation by bacterium Bacillus subtilis A1. Front. Microbiol. 2017;8:193. doi: 10.3389/fmicb.2017.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim M.K., Math R.K., Cho K.M., Shin J.K., Kim J.O., Ryu J.S., Lee Y.H., Yun H.D. Effect of Pseudomonas sp. P7014 on the growth of edible mushroom Pleurotus eryngii in bottle culture for commercial production. Bioresour. Technol. 2007;99:3306–3308. doi: 10.1016/j.biortech.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 29.Kataoka R., Futai K. A new mycorrhizal helper bacterium, Ralstonia species, in the ectomycorrhizal symbiosis between Pinus thunbergii and Suillus granulatus. Biol. Fertil. Soils. 2009;45:315–320. doi: 10.1007/s00374-008-0340-0. [DOI] [Google Scholar]
- 30.Hisatsuka K., Nakahara T., Sano N., Yamada K. Formation of rhamnolipid by Pseudomonas aeruginosa and its function in hydrocarbon fermentation. Agric. Biol. Chem. 1971;35:686–692. doi: 10.1080/00021369.1971.10859987. [DOI] [Google Scholar]
- 31.Itoh S., Suzuki T. Effect of rhamnolipids on growth of Pseudomoonas aeruginosa mutant deficient in n-paraffin-utilizing ability. Agric. Biol. Chem. 1972;36:2233–2235. doi: 10.1080/00021369.1972.10860546. [DOI] [Google Scholar]
- 32.Koch A.K., Kappeli O., Fiechter A., Reiser J. Hydrocarbon assimilation and biosurfactant production in Pseudomonas aeruginosa mutants. J. Bacteriol. 1991;173:2233–4219. doi: 10.1128/jb.173.13.4212-4219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., Miller R.M. Enhanced octadecane dispersion and biodegradation by a Pseudomonas rhamnolipid surfactant (biosurfactant) Appl. Environ. Microbiol. 1992;58:3276–3282. doi: 10.1128/aem.58.10.3276-3282.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shreve G.S., Inguva S., Gunnam S. Rhamnolipid biosurfactant enhancement of hexadecane biodegradation by Pseudomonas aeruginosa. Mol. Mar. Biol. Biotechnol. 1995;4:331–337. PMID: 8541984. [PubMed] [Google Scholar]
- 35.Zhang Y., Maier W.J., Miller R.M. Effect of rhamnolipids on the dissolution, bioavailability and biodegradation of phenanthrene. Environ. Sci. Technol. 1997;31:2211–2217. doi: 10.1021/es960687g. [DOI] [Google Scholar]
- 36.Deveau A., Bonito G., Uehling J., Paoletti M., Becker M., Bindschedler S., Hacquard S., Hervé V., Labbé J., Lastovetsky O., Mieszkin S., Millet L., Vajna B., Junier P., Bonfante P., Krom B., Olsson S., Elsas J., Wick L. Bacterial - fungal Interactions: ecology, mechanisms and challenges. FEMS Microbiol. Rev. 2018;42 doi: 10.1093/femsre/fuy008. [DOI] [PubMed] [Google Scholar]
- 37.Hai F., Modin O., Yamamoto K., Fukushi K., Nakajima F., Nghiem L. Pesticide removal by a mixed culture of bacteria and white rot fungi. J. Taiwan Inst. Chem. Eng. 2011;43:459–462. doi: 10.1016/j.jtice.2011.11.002. [DOI] [Google Scholar]
- 38.Boelan E.G., Purnomo A.S. Abilities of Co-cultures of white-rot fungus Ganoderma lingzhi and bacteria Bacillus subtilis on biodegradation DDT. J. Phys.: Conf. Ser. 2018;1095 doi: 10.1088/1742-6596/1095/1/012015. [DOI] [Google Scholar]
- 39.Boelan E.G., Purnomo A.S. Biodegradation of 1,1,1-Trichloro-2,2-bis (4-chlorophenyl) ethane (DDT) by mixed cultures of white-rot fungus Ganoderma lingzhi and bacterium Pseudomonas aeruginosa. Hayati J. Biosci. 2019;26:90–95. doi: 10.4308/hjb.26.2.90. [DOI] [Google Scholar]
- 40.Purnomo A.S., Maulinawati D., Kamei I. Ralstonia pickettii enhances the DDT biodegradation by Pleurotus eryngii. J. Microbiol. Biotechnol. 2019;29(9):1424–1433. doi: 10.4014/jmb.1906.06030. [DOI] [PubMed] [Google Scholar]
- 41.Purnomo A.S., Sariwati A., Kamei I. Synergistic interaction of a consortium of the brown-rot fungus Fomitopsis pinicola and bacterium Ralstonia pickettii for DDT biodegradation. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purnomo A.S., Rahmadini F.D., Nawfa R., Putra S.R. The effect of addition of bacterium Pseudomonas aeruginosa on biodegradation of methyl orange dye by brown-rot fungus Gloeophyllum trabeum. IOP Conf. Ser. Mater. Sci. Eng. 2020;980 doi: 10.1088/1757-899X/980/1/012074. [DOI] [Google Scholar]
- 43.Maulianawati D., Purnomo A.S., Kamei I. Biodegradation of DDT by Co-cultures of Pleurotus eryngii and Pseudomonas aeruginosa. Hayati J. Biosci. 2021;28(3):240–248. doi: 10.4308/hjb.28.3.240. [DOI] [Google Scholar]
- 44.Purnomo A.S., Rahayu D.M., Nawfa R., Putra S.R. The addition effect of Pseudomonas aeruginosa on biodegradation of methyl orange dye by brown-rot fungus Fomitopsis pinicola. IOP Conf. Ser. Mater. Sci. Eng. 2020;959 doi: 10.1088/1757-899X/959/1/012008. [DOI] [Google Scholar]
- 45.Sariwati A., Purnomo A.S., Kamei I. Abilities of Co-cultures of Brown-rot fungus Fomitopsis pinicola and Bacillus subtilis on biodegradation of DDT. Curr. Microbiol. 2017;74:1068–1075. doi: 10.1007/s00284-017-1286-y. [DOI] [PubMed] [Google Scholar]
- 46.Sariwati A., Purnomo A.S. The effect of Pseudomonas aeruginosa addition on 1,1,1-Trichloro-2,2-bis(4-chlorophenyl)ethane (DDT) biodegradation by Brown-rot fungus Fomitopsis pinicola. Indones. J. Chem. 2018;18(1):75–81. doi: 10.22146/ijc.25158. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.