Significance
Two-component systems are critically important to modulating bacterial gene expression in response to environmental signals. In Proteobacteria, the NifL-NifA system regulates the expression of genes related to biological nitrogen fixation in response to intracellular oxygen, cellular energy, and the carbon–nitrogen ratio. Modifications of nifL have been demonstrated to promote the excretion of ammonium in model diazotrophs. Resolving the structure of NifL in active and inactive conformations provides key insights into signal transduction in the noncanonical two-component regulatory system and lays the groundwork for synthetic biology approaches for engineering biofertilizers that are compatible with a wide variety of crop plants.
Keywords: biological nitrogen fixation regulation, small angle x-ray scattering, sensor histidine kinase, MS-coupled surface labeling, structural model
Abstract
NifL is a conformationally dynamic flavoprotein responsible for regulating the activity of the σ54-dependent activator NifA to control the transcription of nitrogen fixation (nif) genes in response to intracellular oxygen, cellular energy, or nitrogen availability. The NifL-NifA two-component system is the master regulatory system for nitrogen fixation. NifL serves as a sensory protein, undergoing signal-dependent conformational changes that modulate its interaction with NifA, forming the NifL–NifA complex, which inhibits NifA activity in conditions unsuitable for nitrogen fixation. While NifL-NifA regulation is well understood, these conformationally flexible proteins have eluded previous attempts at structure determination. In work described here, we advance a structural model of the NifL dimer supported by a combination of scattering techniques and mass spectrometry (MS)-coupled structural analyses that report on the average structure in solution. Using a combination of small angle X-ray scattering-derived electron density maps and MS-coupled surface labeling, we investigate the conformational dynamics responsible for NifL oxygen and energy responses. Our results reveal conformational differences in the structure of NifL under reduced and oxidized conditions that provide the basis for a model for modulating NifLA complex formation in the regulation of nitrogen fixation in response to oxygen in the model diazotroph, Azotobacter vinelandii.
In Proteobacteria, including the free-living associative diazotroph Azotobacter vinelandii, nitrogen fixation-related (nif) gene expression is controlled by the NifL-NifA two-component system (1–3). NifA is a σ54-dependent transcriptional activator that stimulates the expression of nif genes (4–8). While NifL is homologous to sensor histidine kinases (SHKs) in canonical two-component systems, NifL does not hydrolyze ATP or function as a kinase (6). Instead, NifL modulates NifA activity by binding or releasing NifA as it undergoes conformational changes in response to cellular signals of oxygen, energy, and fixed nitrogen status (6, 9–11). NifL senses these cellular cues and propagates the signal via its four discrete domains (Fig. 1A), similar to class I SHKs (12). The amino-terminal (N-terminal) portion of NifL contains tandem PAS domains, PAS1 and PAS2. The PAS1 domain includes a solvent-accessible FAD cofactor that is readily oxidized by intracellular oxygen (13–15) and is the only part of NifL with a known structure (16). Oxidation of the PAS1 FAD causes reorganization of hydrogen bonds within the FAD-binding pocket that leads to reorientation of the non-FAD containing PAS2 domain to stimulate NifL binding to NifA, inhibiting nitrogenase expression in oxidizing conditions (17–20). The carboxy-terminal (C-terminal) kinase-like DH and GHKL domains (21) perceive energy and fixed nitrogen signals. The NifL DH domain is homologous to the dimerization and histidine phosphotransfer (DHp) domain that contains the conserved His in SHKs, and the GHKL domain closely resembles the catalytic domain of canonical SHKs (21, 22). However, NifL does not function either as a kinase or a phosphatase. Rather than hydrolyzing ATP to catalyze phosphorylation, the GHKL domain binds adenosine nucleotides ADP and ATP to sense the cellular energy status via the ADP/ATP ratio, assuming a NifA-binding conformation when bound to ADP. NifL exhibits a 10-fold higher affinity for ADP than ATP, ensuring nitrogenase is only expressed in energy-rich conditions that can support the energetic demands of nitrogenase (11). The GHKL domain also indirectly perceives cellular, fixed nitrogen signals through interaction with GlnK, the PII nitrogen signal transduction protein in A. vinelandii (23–25). GlnK is uridylylated in response to the cellular carbon/nitrogen ratio (26, 27). Under nitrogen-replete conditions, GlnK is deuridylylated, allowing GlnK to interact with NIfL promoting a NifA-binding conformation and formation of the GlnK–NifL–NifA ternary complex (26, 27). While biochemical and genetic studies revealed the role of the essential proteins and domains involved in perceiving redox and fixed nitrogen status, the mechanism by which these signals are transduced through the different NifL domains to modulate the affinity for NifA remains unclear. Novel structural insights into the protein partners enrolled in this sophisticated regulatory network would enlighten our understanding of these signal transduction cascades.
Fig. 1.
NifL domain diagram and NifL dimer structure model. (A) Domain structure of A. vinelandii NifL. Each domain is colored and labeled, Per-ARNT-Sim1 (PAS1) is red, PAS2 is orange, the Q-linker (Q) is yellow, the dimerization helices (DH) domain is teal, and the Gyrase, Histidine Kinase-like (GHKL) domain is blue. The numbers above the diagram indicate each domain's first and last residues. (B) The NifL dimer model following the coloring scheme described in the domain diagram. The solvent-accessible FAD cofactor in PAS1 and ADP in the nucleotide-binding pocket of the GHKL domain are shown as stick models with all C atoms shown in green.
The conformational flexibility that facilitates NifL-NifA regulation makes them challenging targets for structure determination. Conformationally flexible proteins inherently have higher structural degrees of freedom, sometimes hampering crystallization and complicating particle picking in cryogenic electron microscopy (Cryo-EM) (28). In many cases, proteins can be poised in specific states by introducing ligands and controlling the redox conditions to reduce the degrees of freedom, increasing the chances of crystallization and structure determination (29). However, NifL and NifA have eluded previous attempts at structure determination even in conditions that promote a specific conformation. Therefore, there is considerable interest in strategies for the structural characterization of proteins that exhibit a high level of structural dynamics and conformational flexibility, such as NifL and NifA. In this work, we have employed a combination of next-generation structure prediction, small angle X-ray scattering (SAXS), and a suite of mass spectrometry (MS)-coupled techniques that report on the average conformation in solution to investigate the structure of NifL providing insights into molecular mechanisms of perception and propagation of NifL responses to oxygen and cellular energy status.
Results and Discussion
We first sought to generate a full-length structural model of NifL through protein structure prediction. While SHKs are well studied, initial attempts to model NifL were complicated by few available full-length template structures. Even with an experimentally determined structure for the N-terminal PAS1 domain and high confidence predictions for the C-terminal GHKL domain, poor template coverage in the central PAS2 and Q-linker domains left many questions concerning how the terminal domains are connected. Considering that close homologs of NifL likely suffer similar difficulties with structure determination (30, 31), this limitation is expected when relying primarily on template-based structure prediction methods. Taking advantage of the recent advances in protein structure prediction of AlphaFold (32), we generated high-quality full-length models of NifL using the RoseTTAfold method on the Robetta server (33). RoseTTAfold applies a combination of multiple sequence alignment, interresidue distance maps, and 3D coordinate representations to inform structure prediction. This strategy capitalized on all suitable domain-specific templates to generate a high-quality NifL peptide model (SI Appendix, Fig. S1).
Anticipating NifL to be tetrameric (11), we attempted to generate an oligomeric model by computationally docking four copies of the NifL peptide model together (34). However, the resulting docking models displayed minimal interactions between peptides, and none were consistent with the interactions reported in the PAS1 domain structure (16). Lacking any tetrameric templates or experimental restrictions to construct the full-length NifL tetramer, we leveraged the PAS1 structure as an N-terminal anchor to inform NifL oligomer construction. While full-length NifL and the isolated PAS1 domain were both reported to be tetrameric in solution (15), no tetramer could be identified in the crystal lattice of the isolated PAS1 domain (16). Instead, the PAS1 structure revealed a dimer that closely resembled other dimeric PAS domains of known structure, such as FixL from Sinorhizobium meliloti and DosH from Escherichia coli (35–37). Following this lead, we aligned copies of the full-length NifL peptide model to each chain of the PAS1 crystal structure to visualize how the other domains might oligomerize (SI Appendix, Fig. S2). Despite minimal interaction in the Q-linker and DH domains, the resulting PAS1-aligned NifL dimer model displayed greater buried surface area than our initial rigid-body docking models.
To refine the nascent PAS1-aligned NifL dimer model, we proceeded to refine the predicted oligomeric interfaces in the other domains of NifL. Moving sequentially from the N-terminal PAS1 domain, we found a set of residues in PAS2 (I153, V157, V166, L175, L177, R240, R262) that were identified as essential for dimerization in previous mutagenesis studies of the isolated PAS2 domain (13, 18). Mapping these residues onto the NifL peptide model revealed that most were localized to the β-sheet interface of PAS2, consistent with the conserved dimerization motif of PAS domains (35, 38, 39). Demonstrating support for our strategy, these residues were poised to form an oligomeric interface in the PAS1-aligned dimer model (SI Appendix, Fig. S2). However, the Q-linker helices protruded in opposite directions, suggesting that rigid body docking may not accurately reflect the oligomerization of the elongated NifL peptide model. With this in mind, we hypothesized that the extended helices in our NifL model might form a coiled-coil in the oligomer. To test this, we performed sequence analysis to identify any putative coiled-coils that could play a role in NifL oligomerization (40). Surpassing our expectations, we identified heptad repeat patterns consistent with parallel coiled-coils in the inter-PAS helix, Q-linker, and DH domains (SI Appendix, Fig. S2). Analysis of the heptad repeat in the Q-linker and DH domain revealed hydrophobic residues with a periodicity predicted to be buried in an assembled coiled-coil (41), and this pattern in the DH domain of NifL corresponds to the dimerization interface observed in DHp domains of other SHKs (42–46).
Mapping the predicted oligomeric interfaces described above onto the PAS1-aligned NifL dimer model, we made sequential, minor changes to bond angles in the flexible linkers connecting each domain to poise predicted oligomeric interfaces for interaction. Individual subunit models were energy minimized after each bond angle modification to prevent clashes and minimize quality loss (47). Once the oligomeric interfaces were oriented to interact, the NifL dimer model was submitted for water refinement to capture the flexibility expected in the interdomain helices and generate our final NifL dimer model (Fig. 1B) (34).
Initially, our goal in modeling the NifL dimer was to use it as an intermediate in rationalizing the NifL tetramer. However, no other oligomeric interfaces could be identified, and it remained unclear how the dimer model could assemble into a tetramer. Moreover, the PAS1, PAS2, and DH domains of NifL contain dimerization interfaces, and the NifL dimer is consistent with structures of other SHKs (12, 21, 30, 43, 44, 48). Since the results of the modeling were not consistent with previous work analyzing the quaternary structure of NifL in solutions, we decided to revisit the oligomeric state of NifL. Contrary to our previous analytical gel filtration results (11), native mass spectrometry returned a mass of 117 kDa for the complex with a charge state distribution centered on +24 (Fig. 2A), and no other oligomeric states were detected, supporting the oligomeric state of the NifL dimer model. As this result was unexpected, we sought independent confirmation using mass photometry. Molecular mass distributions calculated from Strep-tagged NifL samples at concentrations of 10 nM, 25 nM, and 100 nM display a single peak at 119 kDa, 119 kDa, and 114 kDa (±5%), respectively (Fig. 2B), within expected instrument error of the theoretical Strep-tagged NifL dimer mass of 119.6 kDa. Considering the elongated nature of the NifL dimer model, the previous determination of NifL as a tetramer was likely an artifact of native-PAGE and analytical gel filtration, both of which fractionate proteins based on Stokes radius, not molecular weight. Stokes radius is the radius of a perfect sphere that diffuses through solution at the same rate as the molecule of interest. Since elongated molecules are known to move more slowly through solution than spherical molecules of the same mass, these techniques would be expected to yield an artificially inflated molecular weight for elongated proteins like NifL (49).
Fig. 2.
Native mass spectrum and mass photometry of oxidized NifL. (A) Native mass spectrum of oxidized NifL. Blue circles indicate the charge state envelope centered around a charge of +24. The mass of NifL was determined to be 117,264 Da (the expected mass of the nucleotide-free NifL dimer (based on primary sequence) including two FAD cofactors is 117,046 Da). (B) Molecular mass distribution histogram of NifL (bin = 4.5 kDa). At concentrations of 10 nM (red), 25 nM (black), and 100 nM (blue) NifL, peaks are observed at 119 kDa, 119 kDa, and 114 kDa, respectively, within expected instrument error of the theoretical Strep-tagged NifL dimer mass of 119.6 kDa.
With experimental support for the quaternary structure of NifL, we went on to treat the NifL dimer model as the hypothesized structure of NifL. To independently validate the specific shape and particle dimensions of the NifL dimer model, we applied dynamic light scattering (DLS) and SAXS. While these methods are low resolution relative to Cryo-EM or X-ray diffraction, DLS and SAXS report directly on the dominant particles in solution, and recent advances in SAXS data processing can capture dynamic structural features unresolved by crystallographic techniques (50), making them well-suited to study conformationally dynamic proteins. To facilitate comparison to experimental results, theoretical particle diameter, the radius of gyration (Rg), and maximal diameter (Dmax) were calculated from the NifL dimer model using CRYSOL (51). DLS revealed a concentration-independent hydrodynamic diameter of 112 Å ± 6.4 when normalized by particle number, just above the 104 Å diameter expected for the NifL dimer model (SI Appendix, Fig. S3). However, aggregates were detected in NifL samples at all concentrations. To eliminate any interference from aggregation in the data quality, NifL samples were also analyzed by size exclusion chromatography coupled to SAXS (SEC-SAXS) to separate aggregates from native NifL immediately before data collection (52). Scattering curves were collected for NifL poised in oxidizing or reducing conditions in the presence of ADP or ATP, and each poised state displayed a different scattering profile (CorMap p-value = 0.645). For all poised states analyzed, the pair-wise distribution plot, p(r), is consistent with the ellipsoid shape of the NifL dimer model, and experimentally determined Rg, Dmax, and molecular weight calculations for all NifL conformers analyzed closely match predictions for the NifL dimer model [NifLox(ATP) Rsas = 0.00877, NifLox(ADP) Rsas = 0.0068, NifLred(ATP) Rsas = 0.00795, NifLred(ADP) Rsas = 0.00553] (SI Appendix, Table S1).
To probe nucleotide-dependent conformational changes, oxidized NifL samples were exposed to iodoacetamide (IAA) to label solvent-accessible Cys residues in nucleotide-free conditions or in the presence of ADP or ATP. Each NifL subunit contains four Cys residues, two in the PAS2 domain, and two in the nucleotide-binding pocket of the GHKL domain (Fig. 3C). Analysis of IAA incorporation revealed two labeling populations in each poised state, indicating asymmetry between peptide chains in the NifL dimer (Fig. 3A). The greatest extent of labeling was observed in the nucleotide-free state, in which IAA was incorporated at three or four sites. In the presence of ADP or ATP, IAA was incorporated in only two or three sites. The difference in IAA incorporation is consistent with an ADP- and ATP-induced conformational change in NifL. To confirm that the FAD cofactor remains attached during IAA labeling and in the presence of ADP/ATP, an extracted ion chromatogram was used to track FAD (Fig. 3B). Two models for NifL could arise from the labeling data, 1) NifL dimer has conformationally asymmetric subunits, 2) NifL is a symmetric dimer that has at least two conformations. Since the ratio of subunits labeled on 3 or 4 Cys is 1:1, this indicates that the equilibrium between the conformations is essentially 1:1. When bound with nucleotide (ATP or ADP) the labeling ratio remains (1:1), although only 2 or 3 Cys are labeled. This again indicates a 1:1 conformational equilibrium. While is it possible that the equilibrium between symmetrical NifL conformers is 50:50, the fact the equilibrium is the same without and with nucleotide would require that nucleotide binding does not alter the conformational equilibrium, whereas this labeling pattern is exactly what would be predicted by the asymmetric model in both cases. Nucleotide-dependent conformational differences in NifL are well supported in the literature and provide a straightforward mechanism for transduction of cellular energy signals in NifL (11, 53–55). Therefore, binding of ADP or ATP is expected to promote different conformers of NifL. For the ratio of labeling to remain 1:1 in model 2, both symmetric NifL conformers would need to change conformation, without altering their relative energies. This scenario would require very unusual circumstances, making it less favored. Therefore, our data and previously published work on histidine kinases (42, 43) support the asymmetric NifL dimer proposed above as model 1. Together, these data indicate that the presence of ADP/ATP limits the solvent accessibility of specific Cys residues in NifL. While the IAA labeling data presented above cannot distinguish between steric hindrance from nucleotides and conformational change upon binding, the peak splitting described above suggests that at least two distinct conformations of subunits remain after nucleotide binding.
Fig. 3.
NifL iodoacetaide (IAA) labeling. (A) Total ion chromatograms (TIC) of oxidized NifL in nucleotide-free unlabeled (dashed line), as well as IAA-labeled NifL in the nucleotide-free (black), in the presence of ADP (blue), and in the presence of ATP (red) states. (B) Extracted ion chromatogram of FAD cofactor from NifL IAA labeling samples. (C) Location of Cys residues in the NifL dimer model structure (blue spheres). There are four Cys per subunit.
To improve the resolution of the labeling experiments of NifL poised in different states, we used glycine ethyl ester (GEE) labeling to identify changes in solvent accessibility of the carboxyl side chains of Asp and Glu residues. Each NifL subunit has 65 potential labeling sites (Fig. 4A). In order to leverage this coverage to probe the conformations that modulate NifL-NifA interactions, NifL was poised in either oxidizing or reducing conditions in the absence of nucleotide or presence of ADP or ATP for GEE labeling. To identify site-specific label incorporation, GEE-labeled samples were digested with trypsin, and analyzed by MS/MS to generate site-specific label incorporation maps for each conformation of NifL (SI Appendix, Fig. S5A).
Fig. 4.
Surface labeling and SAXS electron density maps fit to the NifL dimer. (A) GEE labeling sites on the NifL dimer model. Position of Asp and Glu residues that can be labeled by GEE (red spheres). All electron density maps were aligned to the NifL dimer model by DENSS followed by manual refinement in the orientation of each domain and localization of the GHKL domains. Electron density maps are shown as volumes colored according to density by sigma value (2.5 to 5 is blue, 5 to 7.5 is teal, 7.5 to 10 is green, 10 to 15 is yellow, and 15 to 200 is red). Electron density maps of (B) Oxidized NifL in the presence of ADP, (C) Oxidized NifL in the presence of ATP, (D) reduced NifL in the presence of ADP, and (E) reduced NifL in the presence of ATP, each with a 90° rotation in the plane of the page on the right to depict the 3D shape.
Multiple labeling distributions were apparent in all instances suggesting structural asymmetry, in agreement with Cys labeling results. While this technique is able to map asymmetry to the level of peptides, the nature of the conformational change and precise role in NifL signal transduction remains unclear. To simplify comparisons between the conformers of NifL, GEE label incorporation was reduced to a site-specific differential heatmap for each state (SI Appendix, Fig. S5B). Analysis of the heatmap results reveals differential labeling outside of PAS1 and GHKL in response to the redox state and nucleotide regime. Comparing label incorporation for each conformation of NifL reveals subtle conformational changes in response to the oxidation state and the presence of ADP or ATP, where most (41/65) of the label incorporation results are consistent for all conformations of NifL. However, consistent redox-dependent differences in GEE label incorporation were observed at NifL residues D327 and D500, indicating changes in the solvent accessibility of these residues at the C terminus of NifL in response to redox signals perceived by the N-terminal PAS1 domain. Similarly, GEE label incorporation at D267 exhibits differential labeling in residues distal from the GHKL domain, where D267 is labeled in the absence of nucleotide and unlabeled in the presence of ADP or ATP. Together, these results clearly demonstrate redox- and nucleotide-dependent conformational changes throughout the structure of NifL.
To ground the relative conformational differences identified by MS-coupled surface labeling in real space, electron density maps were calculated from the SAXS scattering profiles of NifL poised in oxidized or reduced states in the presence of ADP or ATP. Aligning the NifL dimer model to each electron density map shows reasonable agreement, with the highest correlation observed in the oxidized state in the presence of ADP (model correlation: 0.989, SI Appendix, Fig. S4 A–D). As all scattering curves were identified as highly ambiguous, 100 electron density maps were reconstructed from each scattering profile using DENSS. Preliminary reconstructions are aligned and evaluated to remove outliers or enantiomers, and the remaining electron density maps are averaged together to generate the final electron density map for each poised state (Fig. 4 B–E). The final maps reflect only the features common to all reconstructions. While preliminary electron density maps are different at high resolution, the final averaged electron density map captures common structural features of each conformation at low resolution, limiting potential ambiguity in the final reconstruction. Additionally, since the final map represents an averaged density, flexibility is captured by regions of low electron density, making this an ideal technique to evaluate conformationally dynamic proteins. Comparing these electron density maps to the NifL dimer model highlights consistent particle dimensions in all NifL-poised states (Fig. 4 A–E). Although, electron density maps calculated from oxidized samples depict a straighter structure with no apparent cleft (Fig. 4 B and C). In contrast, reduced NifL samples display a “bent” structure with an evident cleft in the center (Fig. 4 D and E). The differences in oxidized and reduced conformations of NifL were further modeled by fitting individual domains from the NifL dimer model to each electron density map manually (Fig. 4 B and E). A model of the NifL Q-linker coiled-coil was generated by submitting the NifL sequence from residue 267 to 299 to the CCFold server (56). The resulting model of the coiled NifL Q-linker predicted a section of random coil between residues 275 to 280, consistent with the location of the proposed pivot point in reduced NifL conformations (Fig. 4 D and E) with an angle between PAS1 and DH domains of approximately 45°. The resulting differences observed between the PAS and DH domains are similar to the conformational differences observed in the blue-light sensor histidine kinase from Brucella abortus (43). Comparing the NifL dimer model to the dark-adapted engineered blue-light photoreceptor SHK YF1 reveals a similar helical bending to that proposed in the reduced, “bent” conformation of NifL (44). In addition, the “linear” conformation proposed in oxidized NifL conformations is similar to what is observed for the light-adapted LOV-PAS-HK SHK construct (43). In addition to the differences in surface labeling throughout NifL, the data presented here clearly demonstrate that redox signals perceived in the PAS1 domain are transduced into conformational changes throughout the NifL dimer.
Concluding Remarks.
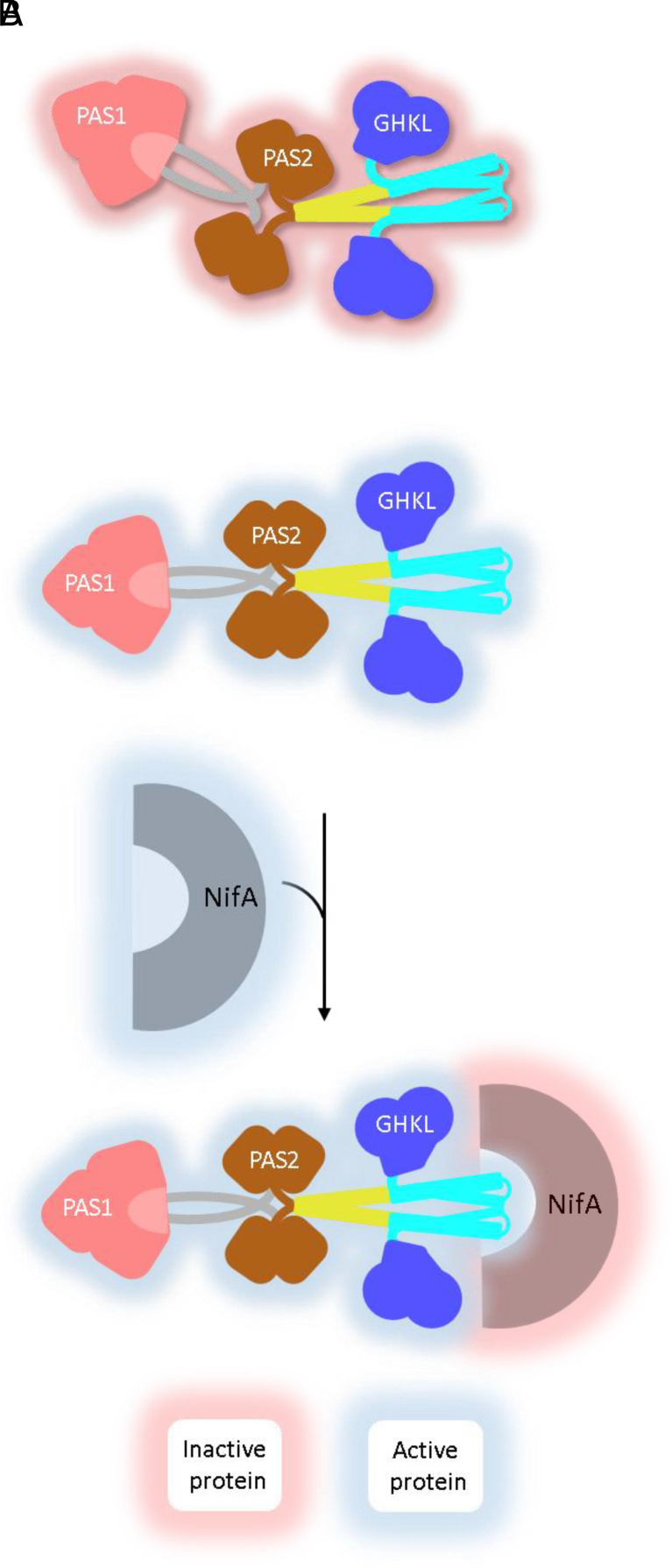
Previous studies hypothesized that the NifL oxygen signal is transduced via a signaling cascade where oxidation of the FAD cofactor in PAS1 causes separation of the PAS2 domains and reorientation of the GHKL domains relative to the DH helices to form the NifA-binding interface (13). In canonical SHKs, activity is modulated by controlling the proximity of the ATP-binding GHKL domain relative to the catalytic His residue in the DH domain in response to signals perceived by a sensory domain and propagated through subtle rearrangement of α helices in parallel coiled-coil linkers (22, 44). In light of the parallel coiled-coil predicted in the NifL Q-linker, we propose that the “bent” structure inferred in the SAXS reconstructions of the reduced, non-NifA-binding conformations (Fig. 5A) could be a result of twisting of the coiled-coil formed by the Q-linker (43, 44). Expanding on the previous hypothesis for NifL signal transduction, we propose that separation of the PAS2 domain is transduced to the Q-linker, potentially melting the coiled-coil observed in reduced conformations and straightening the structure of NifL to assume a NifA-binding conformation in the presence of intracellular oxygen (Fig. 5B). Similar to the mechanism of signal transduction observed in YF1 (44), this change in the coiled-coil could facilitate rearrangement of the GHKL domain relative to the DH helices, exposing the NifA-binding interface on the DH domain and orienting the GHKL domain to stabilize NifL–NifA interactions in the presence of intracellular oxygen (57). Nucleotide binding stabilizes the relative orientation of the DH domain and the GHKL domain in a similar manner to that observed for the SHK DesK in complex nucleotides (55).
Fig. 5.
Schematic representation of the proposed NifL conformational changes in the transition between noninhibitory and inhibitory states. (A) In conditions suitable for nitrogen fixation (high cellular energy and the absence of intracellular oxygen), NifL assumes a kinked, noninhibitory conformation (non-NifA binding) in which the proposed NifA-binding interface on the GHKL and DH domains are obscured. (B) In the presence of intracellular oxygen, NifL oxidation triggers a conformational change that straightens the structure of NifL, exposing the NifA-binding interface. NifL domains are drawn following the color scheme described in Fig. 1, while NifA is drawn as a single semicircle. Drawings are not to scale.
The mechanism of signal transduction implicated from this work on NifL is analogous to the bent and straight conformations that are modulated by the phosphorylation state in other SHKs (58). The MS-coupled surface labeling and SAXS electron density maps in this work indicate asymmetry in the NifL dimer and redox-dependent bending in NifL that may play a role in modulating NifL–NifA interactions. This work highlights the utility of SAXS and MS-coupled surface labeling to provide structural insights into signal transduction in full-length SHKs.
Materials and Methods
Cloning NifL.
For expression of A. vinelandii NifL protein with a cleavable N terminus His-tagged maltose-binding protein (MBP)-fusion in E. coli, the nifL (Avin_50990) gene sequence was amplified from A. vinelandii DJ genomic DNA and cloned into a duet expression system (Novagen). nifL was amplified by PCR with the Phusion™ High-Fidelity DNA Polymerase enzyme (ThermoFisher Scientific, Waltham, MA) using the following primers; nifL -F: 5′-ttggccggccttgaaaatctttattttcaaggtaccccggccaacccgaccctgagcaac-3′ and nifL -R: 5′-ttaccgcggtcaggtggaggccgagaagggcagctcgac-3′. FseI and SacII restriction sites (in italics above), respectively, were inserted upstream and downstream of the start and the stop codons in the primer sequences, and a tobacco etch virus (TEV) protease recognition sequence (underlined above) was inserted in place of the nifL start codon. The amplified fragment was digested by FseI and SacII and cloned inframe into MCS2 of the modified pRSFDuet-1 kanamycin-resistant vector containing the His-MBP sequence inserted between NdeI and AatII. The resulting plasmid His-MBP-NifL-pRSFDuet-1 was verified by sequencing (Functional Biosciences, Madison, WI).
Expression and Purification of NifL.
BL21(DE3)pLysS competent cells were transformed with the His-MBP-NifL-pRSFDuet-1 plasmid, and cells were grown on lysogeny broth (LB) agar plates supplemented with kanamycin (Kan) (0.05 mg/mL). A single colony was used to inoculate an overnight culture in liquid LB medium (200 mL), supplemented with Kan. Expression was initiated with 10% of the overnight culture and incubated at 37 °C with shaking at 250 rpm until the culture reached an OD600 of 0.4 to 0.6. Protein expression was induced with 10 µM IPTG, and cells were moved to a 20 °C shaking incubator at 180 rpm, proceeding for 16 h. Cells were harvested by centrifugation (5,000 rpm, 15 min), and cell pellets were flash-frozen in liquid nitrogen. For purification, cell pellets were resuspended in lysis buffer: 50 mM Bis-Tris, 100 mM ammonium sulfate, 5 mM imidazole, pH 7.0. Cell pellets from cultures were lysed using a 1-h lysozyme incubation in a 37 °C shaking incubator at 250 rpm, followed by sonication (Q700 Qsonica L.L.C, Newtown, CT). Lysates were cleared by centrifugation: 108,000 × g for 1 h prior to fast protein liquid chromatography (FPLC, Bio-Rad). Cleared lysate was loaded onto Ni-NTA columns equilibrated with 10 CV lysis buffer followed by washing with 50 mM Bis-Tris, 100 mM ammonium sulfate, 20 mM imidazole, 10% glycerol, pH 7. Elution was performed over a 30-min gradient from 0 to 50% elution buffer containing 50 mM Bis-Tris, 100 mM ammonium sulfate, 500 mMimidazole, 10% glycerol, pH 7. Protein concentration was evaluated using a Bradford assay and the NifL extinction coefficient ∈= at 445 nm. Purity was assessed using SDS-PAGE and western blot anti-His tag antibodies (alkaline phosphatase-conjugated monoclonal immunoglobulin, from hybridoma clone His-1 from SIGMA). To cleave the His-MBP affinity tag, fractions containing pure His-MBP-NifL were adjusted to 20% glycerol before concentration in 30-kDa centrifugal filters (Millipore Sigma) and buffer exchanged into 50 mM Bis-Tris, 100 mM ammonium sulfate, 20 mM imidazole, 5 mM DTT, 20% glycerol, pH 7. His-tagged TEV protease was added at a 1:10 ratio to His-MBP-NifL, and the cleavage reaction was incubated overnight at 4 °C. The TEV cleavage reaction was buffer exchanged on a PD-10 column equilibrated in the NifL recovery buffer containing 50 mM Bis-Tris, 100 mM ammonium sulfate, 20 mM imidazole, 20% glycerol, pH 7. Untagged NifL was recovered using FPLC Ni-NTA columns equilibrated in NifL recovery buffer, collecting NifL from the flowthrough. Untagged NifL was concentrated in 30-kDa centrifugal filters, and the buffer was exchanged into 50 mM Bis-Tris, 100 mM ammonium sulfate, and 50% glycerol. Finally, NifL protein samples were flash-frozen and stored in liquid nitrogen. Untagged NifL protein samples were used for DLS, SAXS, and MS-coupled surface labeling.
Cloning of the Gene Encoding Av-Strep-NifL.
A codon-optimized version of the A. vinelandii nifL gene, with an added coding sequence for the Strep-Tag II affinity tag (before the original nifL starting codon) and the NcoI and PstI restriction sites at the 5′ and 3′ ends, respectively, was synthesized by GenScript (Piscataway, NJ). The resulting DNA fragment (SI Appendix, Supplementary File S1) was cloned into the pETDuet vector cut with the above-mentioned enzymes to generate the plasmid pMB2223.
Expression and Purification of Av-Strep-NifL.
To purify Av-Strep-NifL, 2 L of E. coli Nico21 (DE3) was grown at 30 °C, 250 rpm, to an OD600 nm of 0.5 to 0.6. Protein overexpression was induced for 3 h by the addition of 0.5 mM IPTG. The cells were harvested by centrifugation, and the cell paste was resuspended in Buffer A_ST (100 mM Tris-HCl pH 8.0 and 150 mM NaCl) and frozen at −20 °C. The next day, after thawing, the cells were disrupted by three to four passages through an Avestin EmulsiFlex-B15 cell homogenizer at an air pressure of 40 psi (12,000 psi homogenizing pressure) and immediately centrifuged at 32,000 g at 4 °C for 30 min. The supernatant was filtered through a 0.22 μM membrane and then loaded onto a 1 mL HiTrap StrepTrap XT column (Cytiva #29401320) previously equilibrated with Buffer A_ST, using an AKTA pure FPLC system (Cytiva). Av-Strep-NifL was eluted in a step gradient with 100% Buffer B_ST (100 mM Tris-HCl pH 8.0, 150 mM NaCl, and 15 mM biotin). The fractions containing Av-Strep-NifL were pooled and concentrated using a 50-kDa cutoff Amicon Ultra-15 centrifugal filter (Merck #UFC905024). To ensure maximum homogeneity, the concentrated fraction was purified by size exclusion chromatography using a Superdex 200 increase 10/300 GL (Cytiva #28990944) and eluted in NifL SEC Buffer (25 mM Tris-HCl pH 8.0, 150 mM NaCl, and 2.5% glycerol). The resulting peak aliquots with the protein of interest were collected and quantified using a Nanodrop nano spectrophotometer (molar extinction ε: 45,170 M−1 cm−1, MW: 59826.47 Da). Prior to storage, samples were flash-frozen in liquid nitrogen and kept at −80 °C. Av-Strep-NifL samples were used for mass photometry experiments.
Mass Photometry.
The oligomeric state of purified Av-Strep-NifL was assessed by mass photometry (59) using the OneMP mass photometer (Refeyn) instrument following the manufacturer’s instructions. Samples were diluted in PBS (8 mM Na2HPO4, 2 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4) to a concentration 10-fold higher than the final intended droplet dilution in the instrument objective slide. The instrument was calibrated by interpolating the ratiometric contrast signal against the molecular weight (kDa) of a set of calibrants, β-amylase (56, 112, and 224 kDa), thyroglobulin (670 kDa), and urease (90.7, 272, and 545 kDa) also diluted to the same molar rage in PBS.
DLS.
NifL samples were exchanged into 50 mM Tris, 200 mM NaCl, 10% glycerol, pH 8 for DLS data collection using the Zetasizer Pro (Malvern Instruments Ltd.). Samples were filtered using a 0.2-µm cellulose acetate syringe filter immediately prior to data collection in 40-µL plastic cuvettes (ZEN0040). Data collection was performed in triplicate for each sample, and analysis was conducted using the Malvern software package (60).
SEC-SAXS.
Datasets were collected at the Stanford Synchrotron Radiation Lightsource (SSRL) Small Angle X-ray Scattering (SAXS) beamline, BL4–2. NifL samples were exchanged into the SEC-SAXS buffer containing 50 mM Bis-Tris, 100 mM ammonium sulfate, and 10% glycerol pH 7 for SAXS measurement. Samples were prepared at a concentration of approximately 85 µM (10 mg/mL) NifL, in the presence of 5 mM ADP or ATP, respectively. Reduced samples were prepared by two sequential exchanges into degassed SEC-SAXS buffer supplemented with 1 mM 1,1′-bis(3-sulfonatopropyl)-4,4′-bipyridinium (S2V). Samples injections of 20 µL, 35 µL, and 50 µL were run on a Superdex 200 PC 3.2/30 column at a flow rate of 0.05 mL/min. Scattering data were collected from the eluent stream with a 1-s exposure every 5 s using a Dectris Pilatus 8 single photon counting device with a 2.5-m sample-to-detector distance and beam energy of 11 keV (wavelength, λ = 1.100 Å). Scattering profiles were generated, after buffer subtraction, using the beamline software SasTool (http://ssrl.slac.stanford.edu/~saxs/analysis/sastool.htm). SAXS analysis was performed using RAW 2.1.4 and the 2.8.0 ATSAS Package (61). The scattering curves corresponding to NifL and displaying a constant Rg from each injection volume of each sample were averaged, and GNOM from the ATSAS suite, was used to fit the data, calculate a P(r) curve, and estimate the Porod volume. Final experimental scattering curves for each sample were selected based on the best Guinier fit and GNOM quality estimates. The final averaged scattering profiles for each sample were then analyzed by dens.fit_data.py (62), which estimates uncertainty through error propagation using the Shannon information theory and taking into account variances and covariances in the coefficients. Fit between the NifL dimer model and structural parameters derived from the scattering data of each conformation was assessed by calculating Rsas (63). GNOM files were used as inputs to calculate electron density maps for each sample using DENSS in the RAW interface, using the slow mode to average 100 reconstructions together and the NifL dimer model as a reference structure. The proposed publication metrics for DENSS models are included in SI Appendix, Table S1F (64). Alignment of the NifL dimer model to electron density maps was performed internally by DENSS, and the correlation between the NifL dimer model and the final electron density map is reported for each conformation in SI Appendix, Table S1. To depict the proposed conformations of NifL, domains were extracted from the aligned NifL dimer model, and fit was manually refined into each electron density map in pymol. The angle formed between PAS1 and DH domains in each conformation was estimated by selecting the alpha carbon of residues 135, 277, and 327 and using the angle command in pymol. The Scattering data reported here are available on SASBDB (65) (accession codes: SASDRT5, SASDRU5, SASDRV5, SASDRW5 (66–69)).
Native Mass Spectrometry.
The oligomeric state of NifL was investigated using native mass spectrometry protocol. All experiments were conducted on a SYNAPT G2-Si instrument (Waters) as described previously (70, 71). Briefly, the NifL-oxidized samples were buffer exchanged into 250 mM ammonium acetate, pH 8 (Sigma) using 10-kDa molecular weight cutoff spin filters (Pall Corporation) or a 3.5-kDa cutoff slide-a-lyzer minidialysis device (Thermo-Fisher) and infused from in-house prepared gold-coated borosilicate glass capillaries to the electrospray source at a protein concentration of 1 to 5 μM and a flow rate around 90 nL/min. The instrument was tuned to enhance performance in the high mass-to-charge range with the following settings: source temperature 30 °C, capillary voltage 1.2 kV, trap bias voltage 16 V and argon flow in collision cell (trap) 7 mL/min. Transfer collision energy was held at 10 eV while trap energy was varied between 10 and 200 eV. Data analysis was performed in MassLynx software version 4.1 (Waters).
Mass Spectrometry-Coupled Surface Labeling.
Protein–protein interactions within the NifL homodimers were probed using the surface labeling protocol adopted from ref. 72 and further modified to be applicable in anaerobic conditions. Investigation was carried out on the intact protein level and peptide level (tryptic digest). Briefly, half of NifL samples were reduced with 5 mM 1,1′-bis(3-sulfonatopropyl)-4,4′-bipyridinium also known as S2V (73) and kept in anaerobic conditions. Redox status was evaluated by monitoring solution color, which remained blue for the entire duration of the labeling reaction. Both, reduced and nonreduced (“oxidized”) samples were divided into three aliquots and were incubated with 5 mM (final concentration) ATP (Sigma), ADP (Sigma), or buffer (nucleotide-free form). A few minutes before labeling reactions, NifL samples were diluted into water (first reaction) or 200 mM ammonium acetate, pH 7 (second reaction), which resulted in 2.5 mM Bis-Tris buffer pH 7, 5 mM ammonium sulfate, 1% glycerol final reaction conditions. In the first reaction, samples at 6 µM final concentration (based on NifL dimer) were surface labeled in the presence of 5.7 mM 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC, Thermo-Fisher) and 200 mM GEE (Sigma) at room temperature. After 5 min, the reaction was quenched by addition of 1 µL 1 M ammonium acetate, pH 7. Immediately before labeling, 50 mM EDC and 2 M GEE stock solutions were prepared in water. In the second reaction, the NifL samples (6 µM) were surface labeled with 55 mM (final) IAA (Sigma) for 30 min in a dark at room temperature. The labeling reaction was quenched with 100 mM (final) dithiothreitol (DTT, Sigma) and further incubated at room temperature for 15 min. 2.7 M IAA and 1 M DTT stock solutions were prepared immediately before labeling/quenching in 200 mM ammonium acetate, pH 7.
In the intact protein level protocol, the GEE- and IAA-labeled NifL samples were analyzed on a 1,290 ultrahigh pressure series chromatography stack (Agilent Technologies) coupled directly to electrospray-time of a flight mass spectrometer (Micro-TOF, Bruker Daltonics). Before infusion to the ionization source, samples were separated on a reverse phase column (Onyx monolithic C18, 100 mm × 2 mm; Phenomenex) at 50 °C using a flow rate of 600 µL/min under the following conditions: 1 min, 10% B; 1 to 7 min, 10 to 70% B; 7 to 8 min, 70 to 90% B; 8 to 9 min, 90 to 95% B; 9 to 10 min, 95-10% B; 10 to 12 min, 10% B. Mobile phase: solvent A and solvent B were 0.1% formic acid (FA, Sigma) in water (Thermo-Fisher) and 0.1% FA in acetonitrile (Thermo-Fisher), respectively. Electrospray conditions were as follows: nebulizer at 6.0 bar, drying gas at a flow rate of 6.0 L/min, drying temperature at 200 °C, capillary voltage at 4.5 kV, capillary exit voltage at 150 V, skimmer 1 set at 60 V, skimmer 2 set at 22 V, set at hexapole RF 350 V, and lens transfer at 87 µs. Data were collected in positive mode at 1-Hz rate over the 200 to 3,000 m/z scan range. Data processing and analysis were done in the Bruker Data Analysis package 4.2. Charge deconvolution was performed using a maximum entropy algorithm for H+ adducts only and 0.1 m/z data point spacing. The low mass end was defined by the theoretical mass of an NifL monomer (calculated based on NifL amino acid sequence) while the high mass end was defined as 3.3× of the NifL monomer. The number of incorporated labels was established based on additional mass of 85.05 m/z (GEE) or 57.02 m/z (hydrolyzed GEE what is glycine) or 57.02 m/z for carbamidomethyl (IAA). An extracted ion chromatogram for FAD was created by plotting the intensity of the signal observed at 786.2 mass-to-charge value as a function of retention time.
In the peptide level protocol, the GEE- and IAA-labeled NifL samples were allowed to separate on SDS-PAGE (4 to 20% linear gradient minigel, Bio-Rad) for about 20 min, and the gel was stained with Coomassie brilliant blue (Thermo-Fisher). The major protein bands (one per condition) were excised, briefly destained with 50% acetonitrile in 50 mM ammonium bicarbonate and subjected to in-gel trypsin digestion by addition of 100-ng porcine sequencing grade modified trypsin (Promega) in 50 mM ammonium bicarbonate (Sigma). After overnight incubation at 37 °C peptide products were acidified in 0.1% FA (Pierce). Tryptic peptides were separated by reverse phase XSelect CSH C18 2.5-µm resin (Waters) on an in-line 150 × 0.075 mm column using an UltiMate 3000 RSLCnano system (Thermo). Peptides were eluted using a 60-min gradient from 2% to 35% B (solution A: 0.1% FA, 0.5% acetonitrile; solution B: 0.1% FA, 99.9% acetonitrile). Eluted peptides were ionized by electrospray (2.4 kV) followed by analysis on an Orbitrap Eclipse Tribrid mass spectrometer (Thermo). MS data were acquired using the FTMS analyzer in profile mode at a resolution of 120,000 over a range of 375 to 1,400 m/z with advanced peak determination. Following HCD activation, MS/MS data were acquired using the ion trap analyzer in centroid mode and normal mass range with a normalized collision energy of 30%. Peptides were identified by database search (UniProt_2022_02_Azotobacter_vinelandii_UP000002424 database) using MaxQuant version 2.1.3.0 (74) with a parent ion tolerance of 5 ppm and a fragment ion tolerance of 0.5 Da assuming the digestion enzyme trypsin (max two missed cleavages). Oxidation of methionine, acetyl of the N terminus, carbamidomethyl of cysteine, aspartic acid and glutamic acid, carbamidomethyl (M) of methionine and GEE (DE) of aspartic acid and glutamic acid were specified in MaxQuant as variable modifications. Scaffold Q+S version 5.0.1 (Proteome Software Inc., Portland OR) was used to verify MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 5.0% probability to achieve an FDR less than 1.0% by the Peptide Prophet algorithm (75) with Scaffold delta-mass correction.
NifL surface labeling maps with GEE were generated based on the presence/absence of the GEE (or Gly) label incorporated into the side chain of aspartic and/or glutamic acid. GEE/Gly modifications were called “present” only in the case of peptide identification probability equal 100%. Molecular graphics were created using the UCSF Chimera package 1.13.1 (76).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
J.W.P. and B.B. were supported by the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences under Award DE-SC001814. N.R.B. was supported by a fellowship from the Agricultural and Food Research Initiative grant no. 2021-67034-35148 from the USDA National Institute of Food and Agriculture. M.B.B. and R.D. were supported by the UKRI Biotechnology and Biological Sciences Research Council, grants BB/W009986/1 to MBB, BB/N013476/1 and BBS/E/J/000PR9797 to R.D. M.B.B. and R.D. were also supported by the Royal Society grant ICA\R1\180088. Funding for the Proteomics, Metabolomics, and Mass Spectrometry Facility used in this publication was made possible in part by the MJ Murdock Charitable Trust, the MSU Office of Research, Economic Development and Graduate Education, and the National Institute of General Medical Sciences of the NIH under Award Number P20GM103474 and S10OD28650. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the US DOE, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-76SF00515. The Stanford Synchrotron Radiation Lightsource Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the NIH, National Institute of General Medical Sciences (P30GM133894). Julia Mundy and Abbas Maqbool are acknowledged for assistance with the mass photometry analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Author contributions
N.R.B., M.T.-L., M.B.B., F.M., R.D., B.B., and J.W.P. designed research; N.R.B., M.T.-L., M.B.B., and F.M. performed research; N.R.B., M.T.-L., and M.B.B. analyzed data; and N.R.B., M.T.-L., M.B.B., F.M., R.D., B.B., and J.W.P. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
SAXS data have been deposited in SASBDB (https://www.sasbdb.org/data/SASDRT5/, https://www.sasbdb.org/data/SASDRU5/, https://www.sasbdb.org/data/SASDRV5/, https://www.sasbdb.org/data/SASDRW5/) (66–69). All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Martinez-argudo I., Little R., Shearer N., Johnson P., Dixon R., The NifL-NifA system: A multidomain transcriptional regulatory complex that integrates environmental signals. J. Bacteriol. 186, 601–610 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bueno Batista M., Dixon R., Manipulating nitrogen regulation in diazotrophic bacteria for agronomic benefit. Biochem. Soc. Trans. 47, 603–614 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon R., The oxygen-responsive NIFL-NIFA complex: A novel two-component regulatory system controlling nitrogenase synthesis in γ-Proteobacteria. Arch. Microbiol. 169, 371–380 (1998), 10.1007/s002030050585. [DOI] [PubMed] [Google Scholar]
- 4.Poza-Carrión C., Jiménez-Vicente E., Navarro-Rodríguez M., Echavarri-Erasun C., Rubio L. M., Kinetics of nif gene expression in a nitrogen-fixing bacterium. J. Bacteriol. 196, 595–603 (2014), 10.1128/JB.00942-13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei S. H. I., Pulakat L., Gavini N., Genetic analysis of nif regulatory genes by utilizing the yeast two-hybrid system detected formation of a NifL-NifA complex that is implicated in regulated expression of nif genes. J. Bacteriol. 181, 6535–6539 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin S., Buck M., Cannon W., Eydmann T., Dixon R., Purification and in vitro activities of NifL and NifA. J. Bacteriol. 176, 3460–3465 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Little R., Dixon R., The amino-terminal GAF domain of Azotobacter vinelandii NifA binds 2-oxoglutarate to resist inhibition by NifL under nitrogen-limiting conditions. J. Biol. Chem. 278, 28711–28718 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Argudo I., Little R., Dixon R., Role of the amino-terminal GAF domain of the NifA activator in controlling the response to the antiactivator protein NifL. Mol. Microbiol. 52, 1731–1744 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Argudo I., Little R., Dixon R., A crucial arginine residue is required for a conformational switch in NifL to regulate nitrogen fixation in Azotobacter vinelandii. Proc. Natl. Acad. Sci. U.S.A. 101, 16316–16321 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Little R., Signal transduction to the Azotobacter vinelandii NIFL-NIFA regulatory system is influenced directly by interaction with 2-oxoglutarate and the PII regulatory protein. EMBO J. 19, 6041–6050 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin S., Hill S., Dixon R., The redox- and fixed nitrogen-responsive regulatory protein NIFL from Azotobacter vinelandii comprises discrete flavin and nucleotide-binding domains. Mol. Microbiol. 28, 179–192 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Dutta R., Qin L., Inouye M., Histidine kinases: Diversity of domain organization. Mol. Microbiol. 34, 633–640 (1999). [DOI] [PubMed] [Google Scholar]
- 13.Slavny P., Little R., Salinas P., Clarke T. A., Dixon R., Quaternary structure changes in a second Per-Arnt-Sim domain mediate intramolecular redox signal relay in the NifL regulatory protein. Mol. Microbiol. 75, 61–75 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Little R., Salinas P., Slavny P., Clarke T. A., Dixon R., Substitutions in the redox-sensing PAS domain of the NifL regulatory protein define an inter-subunit pathway for redox signal transmission. Mol. Microbiol. 82, 222–235 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Little R., Slavny P., Dixon R., Influence of PAS domain flanking regions on oligomerisation and redox signalling by NifL. PLoS One 7, e46651 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Key J., Hefti M., Purcell E. B., Moffat K., Structure of the redox sensor domain of Azotobacter vinelandii NifL at atomic resolution: Signaling, dimerization, and mechanism. Biochemistry 46, 3614–3623 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Little R., Dixon R., Role of the central region of NifL in conformational switches that regulate nitrogen fixation. Biochem. Soc. Trans. 34, 162–164 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Slavny P. A., Role of the PAS2 domain of the NifL regulatory protein in redox signal transduction, Doctoral thesis, University of East Anglia, Norwich, UK. (2010). [Google Scholar]
- 19.Macheroux P., et al. , Electron donation to the flavoprotein NifL, a redox-sensing transcriptional regulator. Biochem. J. 332, 413–419 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill S., Austint S., Eydmannt T., Jonest T., Dixontt R. A. Y., Azotobacter vinelandii NIFL is a flavoprotein that modulates transcriptional activation of nitrogen-fixation genes via a redox-sensitive switch. Biochemistry 93, 2143–2148 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhate M. P., Molnar K. S., Goulian M., Degrado W. F., Francisco S., Signal transduction in histidine kinases: Insights from new structures. Structure 23, 981–994 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Möglich A., Signal transduction in photoreceptor histidine kinases. Protein Sci. 28, 1923–1946 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Little R., Colombo V., Leech A., Dixon R., Direct interaction of the NifL regulatory protein with the GlnK signal transducer enables the Azotobacter vinelandii NifL-NifA regulatory system to respond to conditions replete for nitrogen*. J. Biol. Chem. 277, 15472–15481 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Arcondéguy T., Lawson D., Merrick M., Two residues in the T-loop of GlnK determine NifL-dependent nitrogen control of nif gene expression. J. Biol. Chem. 275, 38452–38456 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Rudnick P., Kunz C., Gunatilaka M. K., Hines E. R., Kennedy C., Role of GlnK in NifL-mediated regulation of NifA activity in Azotobacter vinelandii. J. Bacteriol. 184, 812–820 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little R., Colombo V., Leech A., Dixon R., Direct interaction of the NifL regulatory protein with the GlnK signal transducer enables the Azotobacter vinelandii NifL-NifA regulatory system to respond to conditions replete for nitrogen. J. Biol. Chem. 277, 15472–15481 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Rudnick P., Kunz C., Gunatilaka M. K., Hines E. R., Kennedy C., Role of GlnK in NifL-mediated regulation of NifA activity in Azotobacter vinelandii. J. Bacteriol. 184, 812–20 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu L., Reutzel-Edens S. M., Mitchell C. A., Crystallization and polymorphism of conformationally flexible molecules: Problems, patterns, and strategies. Org. Process Res. Dev. 4, 396–402 (2000). [Google Scholar]
- 29.Artz J. H., Zadvornyy O. A., Mulder D. W., King P. W., Peters J. W., Structural Characterization of Poised States in the Oxygen Sensitive Hydrogenases and Nitrogenases (Elsevier Inc., ed. 1, 2017). [DOI] [PubMed] [Google Scholar]
- 30.Diensthuber R. P., Bommer M., Gleichmann T., Mo A., Full-length structure of a sensor histidine kinase pinpoints coaxial coiled coils as signal transducers and modulators. Structure 21, 1127–1136 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Wang C., et al. , Mechanistic insights revealed by the crystal structure of a histidine kinase with signal transducer and sensor domains. PLoS Biol. 11, e1001493 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J., Anishchenko I., Park H., Peng Z., Ovchinnikov S., Improved protein structure prediction using predicted interresidue orientations. Proc. Natl. Acad. Sci. U.S.A. 117, 1496– 1503 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baek M., Accurate prediction of protein structures and interactions using a three-track neural network. Science 373, 871–876 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Zundert G. C. P., et al. , The HADDOCK2.2 web server: User-friendly integrative modeling of biomolecular complexes. J. Mol. Biol. 428, 720–725 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Möglich A., Ayers R. A., Moffat K., Addition at the molecular level: Signal integration in designed Per-ARNT-Sim receptor proteins. J. Mol. Biol. 400, 477–486 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Miyatake H., et al. , Sensory mechanism of oxygen sensor FixL from Rhizobium meliloti: Crystallographic, mutagenesis and resonance Raman spectroscopic studies. J. Mol. Biol. 301, 415–431 (2000). [DOI] [PubMed] [Google Scholar]
- 37.Park H. J., Suquet C., Satterlee J. D., Kang C. H., Insights into signal transduction involving PAS domain oxygen-sensing heme proteins from the X-ray crystal structure of Escherichia Coli dos heme domain (Ec DosH). Biochemistry 43, 2738–2746 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Ayers R. A., Moffat K., Changes in quaternary structure in the signaling mechanisms of PAS domains. Biochemistry 47, 12078–12086 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stuffle E. C., Johnson M. S., Watts K. J., PAS domains in bacterial signal transduction. Curr. Opin. Microbiol. 61, 8–15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ludwiczak J., Winski A., Szczepaniak K., Alva V., Dunin-Horkawicz S., DeepCoil–A fast and accurate prediction of coiled-coil domains in protein sequences. Bioinformatics 35, 2790–2795 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Truebestein L., Leonard T. A., Coiled-coils: The long and short of it. BioEssays 38, 903–916 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mechaly A. E., Sassoon N., Betton J. M., Alzari P. M., Segmental helical motions and dynamical asymmetry modulate histidine kinase autophosphorylation. PLoS Biol. 12, e1001776 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rinaldi J., et al. , Dimer asymmetry and light activation mechanism in brucella blue-light sensor histidine kinase. MBio 12, e00264-21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berntsson O., et al. , Sequential conformational transitions and α-helical supercoiling regulate a sensor histidine kinase. Nat. Commun. 8, 1–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dago A. E., et al. , Structural basis of histidine kinase autophosphorylation deduced by integrating genomics, molecular dynamics, and mutagenesis. Proc. Natl. Acad. Sci. U.S.A. 109, E1733–E1742 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Little R., Martinez-argudo I., Perry S., Dixon R., Role of the H domain of the histidine kinase-like protein NifL in signal transmission*. J. Biol. Chem. 282, 13429–13437 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Feig M., Local protein structure refinement via molecular dynamics simulations with locPREFMD. J. Chem. Inf. Model. 56, 1304–1312 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dikiy I., et al. , Insights into histidine kinase activation mechanisms from the monomeric blue light sensor EL346 CA. Proc. Natl. Acad. Sci. U.S.A. 116, 4963–4972 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erickson H. P., Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 11, 32–51 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grant T. D., Ab initio electron density determination directly from solution scattering data. Nat. Methods 15, 191–193 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Franke D., et al. , ATSAS 2.8: A comprehensive data analysis suite for small-angle scattering from macromolecular solutions. J. Appl. Crystallogr. 50, 1212–1225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bucciarelli S., et al. , Size-exclusion chromatography small-angle X-ray scattering of water soluble proteins on a laboratory instrument. J. Appl. Crystallogr. 51, 1623–1632 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones T., et al. , Transcriptional activation of the nitrogenase promoter in vitro: Adenosine nucleotides are required for inhibition of NIFA activity by NIFL. J. Bacteriol. 177, 1186–1195 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perry S., Shearer N., Little R., Dixon R., Mutational analysis of the nucleotide-binding domain of the Anti-activator NifL. J. Mol. Biol. 346, 935–949 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Albanesi D., et al. , Structural plasticity and catalysis regulation of a thermosensor histidine kinase. Proc. Natl. Acad. Sci. U.S.A. 106, 16185–16190 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guzenko D., Strelkov S. V., CCFold: Rapid and accurate prediction of coiled-coil structures and application to modelling intermediate filaments. Bioinformatics 34, 215–222 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Little R., Martinez-Argudo I., Perry S., Dixon R., Role of the H domain of the histidine kinase-like protein NifL in signal transmission. J. Biol. Chem. 282, 13429–13437 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Jacob-Dubuisson F., Mechaly A., Betton J. M., Antoine R., Structural insights into the signalling mechanisms of two-component systems. Nat. Rev. Microbiol. 16, 585–593 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Young G., Quantitative mass imaging of single molecules HHS Public Access. Science 360, 423–427 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stetefeld J., McKenna S. A., Patel T. R., Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 8, 409–427 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hopkins J. B., Gillilan R. E., Skou S., BioXTAS RAW: Improvements to a free open-source program for small-angle X-ray scattering data reduction and analysis. J. Appl. Crystallogr. 50, 1545–1553 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grant T. D., Describing small-angle scattering profiles by a limited set of intensities. J. Appl. Crystallogr. 55, 1116–1124 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rambo R. P., Tainer J. A., Accurate assessment of mass, models and resolution by small-angle scattering. Nature 496, 477–481 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grant T. D., Reconstruction of 3D Density from Solution Scattering (Elsevier Inc., ed. 1, 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kikhney A. G., Borges C. R., Molodenskiy D. S., Jeffries C. M., Svergun D. I., SASBDB: Towards an automatically curated and validated repository for biological scattering data. Protein Science, 29, 66–75 (2019). Portico. 10.1002/pro.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyer N., Structural insights into redox signal transduction mechanisms in the control of nitrogen fixation by the NifLA system (SASDRT5). Small Angle Scattering Biological Data Bank. https://www.sasbdb.org/data/SASDRT5/. Deposited 21 February 2023. [DOI] [PMC free article] [PubMed]
- 67.Boyer N., Structural insights into redox signal transduction mechanisms in the control of nitrogen fixation by the NifLA system (SASDRU5). Small Angle Scattering Biological Data Bank. https://www.sasbdb.org/data/SASDRU5/. Deposited 21 February 2023. [DOI] [PMC free article] [PubMed]
- 68.Boyer N., Structural insights into redox signal transduction mechanisms in the control of nitrogen fixation by the NifLA system (SASDRV5). Small Angle Scattering Biological Data Bank. https://www.sasbdb.org/data/SASDRV5/. Deposited 21 February 2023 [DOI] [PMC free article] [PubMed]
- 69.Boyer N., Structural insights into redox signal transduction mechanisms in the control of nitrogen fixation by the NifLA system (SASDRW5). Small Angle Scattering Biological Data Bank. https://www.sasbdb.org/data/SASDRW5/. Deposited 21 February 2023 [DOI] [PMC free article] [PubMed]
- 70.Luo M. L., et al. , The CRISPR RNA-guided surveillance complex in Escherichia coli accommodates extended RNA spacers. Nucleic Acids Res. 44, 7385–7394 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Patterson A., Tokmina-Lukaszewska M., Bothner B., Probing Cascade Complex Composition and Stability Using Native Mass Spectrometry Techniques (Elsevier Inc., ed. 1, 2019). [DOI] [PubMed] [Google Scholar]
- 72.Brown K. A., Correction: The oxygen reduction reaction catalyzed by: Synechocystis sp. PCC 6803 flavodiiron proteins (Sustainable Energy and Fuels (2019) DOI: 10.1039/c9se00523d. Sustain. Energy Fuels 4, 417 (2019). [DOI] [Google Scholar]
- 73.Debruler C., Hu B., Moss J., Luo J., Liu T. L., A sulfonate-functionalized viologen enabling neutral cation exchange, aqueous organic redox flow batteries toward renewable energy storage. ACS Energy Lett. 3, 663–668 (2018). [Google Scholar]
- 74.Cox J., Mann M., MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008). [DOI] [PubMed] [Google Scholar]
- 75.Keller A. D., Nesvizhskii A. I., Kolker E., Aebersold R., Empirical statistical model to estimate the accuracy of protein identifications made by MS/MS and database search. Proc. 50th ASMS Conf. Mass Spectrom. Allied Top. 74, 37–38 (2002). [DOI] [PubMed] [Google Scholar]
- 76.Pettersen E. F., et al. , UCSF Chimera–A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
SAXS data have been deposited in SASBDB (https://www.sasbdb.org/data/SASDRT5/, https://www.sasbdb.org/data/SASDRU5/, https://www.sasbdb.org/data/SASDRV5/, https://www.sasbdb.org/data/SASDRW5/) (66–69). All study data are included in the article and/or SI Appendix.