Significance
Polyomavirus LT viral helicases recruit the host cell replisome machinery to replicate their viral DNA. We show that differences between viral and cellular replication helicase DNA melting mechanisms may explain how viral DNA can be rapidly and repeatedly amplified during a single cell cycle. We find that viral LTs melt origin DNA through their multimeric binding energy and do not require helicase activity for initial dsDNA melting and double-hexamer assembly.
Keywords: Merkel cell polyomavirus, large T antigen helicase, single-molecule fluorescence microscopy, SV40 large T antigen, unlicensed origin replication
Abstract
Cellular eukaryotic replication initiation helicases are first loaded as head-to-head double hexamers on double-stranded (ds) DNA origins and then initiate S-phase DNA melting during licensed (once per cell cycle) replication. Merkel cell polyomavirus (MCV) large T (LT) helicase oncoprotein similarly binds and melts its own 98-bp origin but replicates multiple times in a single cell cycle. To examine the actions of this unlicensed viral helicase, we quantitated multimerization of MCV LT molecules as they assembled on MCV DNA origins using real-time single-molecule microscopy. MCV LT formed highly stable double hexamers having 17-fold longer mean lifetime (τ, >1,500 s) on DNA than single hexamers. Unexpectedly, partial MCV LT assembly without double-hexamer formation was sufficient to melt origin dsDNA as measured by RAD51, RPA70, or S1 nuclease cobinding. DNA melting also occurred with truncated MCV LT proteins lacking the helicase domain, but was lost from a protein without the multimerization domain that could bind only as a monomer to DNA. SV40 polyomavirus LT also multimerized to the MCV origin without forming a functional hexamer but still melted origin DNA. MCV origin melting did not require ATP hydrolysis and occurred for both MCV and SV40 LT proteins using the nonhydrolyzable ATP analog, adenylyl-imidodiphosphate (AMP-PNP). LT double hexamers formed in AMP-PNP, and melted DNA, consistent with direct LT hexamer assembly around single-stranded (ss) DNA without the energy-dependent dsDNA-to-ssDNA melting and remodeling steps used by cellular helicases. These results indicate that LT multimerization rather than helicase activity is required for origin DNA melting during unlicensed virus replication.
The primary control step regulating eukaryotic DNA replication involves helicase-mediated unwinding and melting of double-stranded DNA (dsDNA) by the minichromosome maintenance (MCM) protein complex. During late mitosis and early G1 phases, MCM is loaded on the origin by origin recognition complex (ORC) proteins as a dodecameric head-to-head double hexamer (1). MCM then associates with licensing factors Cdc45 and GINS to form Cdc45-MCM-GINS (CMG) during the late G1/early S phase (2). Crystallographic and cryoelectron microscopic studies show that CMG assembles as a fully formed dodecameric complex composed of two oppositely positioned hexamers (a “double hexamer”) surrounding the origin dsDNA. Once licensed to replicate during the S phase, each hexamer in the CMG complex hydrolyzes ATP to ratchet together the intervening dsDNA to achieve DNA melting (3–5). The MCM hexamers then each remodel around a single-stranded (ss)DNA to generate a melted replication bubble that attracts assembly of the replisome machinery (6). Generation of a melted bubble in this model requires the full assembly of the double hexamer and ATP hydrolysis for DNA melting.
Merkel cell virus (MCV) encodes its own replication helicase, the multifunctional large tumor (LT) oncoprotein, which is both necessary and sufficient to initiate viral DNA replication (7). MCV is one of seven human cancer viruses and causes the clinically aggressive skin cancer, Merkel cell carcinoma (MCC) (8). Nearly 3,000 people in the United States develop this cancer each year (9), of which ~80% are MCV infected. The remaining 20% of MCC cases have tumors negative for the virus but phenocopy viral infection through UV-driven somatic mutations (10). MCV was identified by digital transcriptome subtraction and was the first human pathogen discovered by nondirected metagenomic cDNA sequencing (11).
Unlike the human CMG, MCV LT can initiate multiple rounds of viral genome replication within a single cell cycle (unlicensed replication) (7). MCC oncogenesis generally occurs after viral replication (12) when fragmented viral genomes become integrated into the host cell genome (7, 13). Because LT can reinitiate DNA replication off of the integrated viral origin (7), leading to replication fork collision and DNA fragmentation, the nascent cancer cell survives because another, independent mutation is present in the LT gene to truncate its C-terminal helicase domain preventing LT-dependent DNA replication (7, 14). It is unknown which mutation comes first, LT gene truncation (7) or virus integration (11), but both are required, together with loss of effective cytotoxic T lymphocyte responses against early viral antigens (15, 16), for emergence of this virus-driven cancer (8).
MCV LT binds to a 98 base pair (bp) viral origin (ori) located within the 464 bp noncoding control region (NCCR) (17). MCV is related to the rhesus macaque SV40 polyomavirus that has been an extensively studied model for eukaryotic DNA replication for over 50 y. The first in vitro eukaryotic DNA replication studies were performed using the LT protein and DNA origin of SV40 (18, 19), leading to the discovery of critical cellular factors in eukaryotic replication (20, 21). SV40 LT helicase assembles as a head-to-head, double-hexameric homopolymer that is reported to unwind less than a single turn of DNA as it assembles through a mechanism requiring ATP binding but not hydrolysis (22). Origin melting by SV40 LT, however, is also reported to occur through a dsDNA ratcheting mechanism similar to that of CMG helicase (3, 23, 24), while still other studies indicate that origin melting occurs in the absence of ATP hydrolysis and helicase activity (25, 26).
SV40 and MCV LT proteins are homologous, but not identical (Fig. 1A), and the extensive literature on SV40 LT can help guide experimentation on MCV LT. Both MCV and SV40 LT proteins have origin-binding domains that recognize canonical G(A/G)GGC pentanucleotide sequences (PS or pentads) in the origin (17). Although pentad nucleotide sequences are identical for both viruses, their numbers and spacing differ at their respective origins (SI Appendix, Fig. S1A) such that the two LT proteins cannot replicate each other’s viral genomes (7, 27). Ten pentads are present in the MCV ori, but only four (PS1,2,4 and 7) are required for replication (17). A single point mutation at one critical pentad (PS7) recovered from an MCC tumor genome (MCC350) (11) prevents LT-mediated DNA replication (here called Ori98.Rep-) (17, 28).
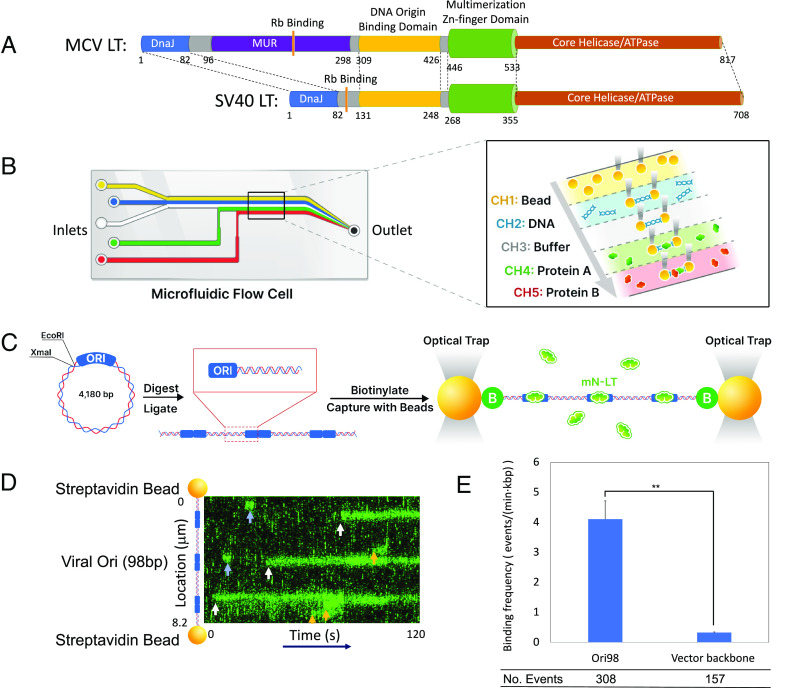
Fig. 1.
MCV LT specifically binds to the MCV replication origin. (A) MCV and SV40 LT helicase oncoprotein domains. MCV and SV40 LT are homologous helicases sharing DnaJ, retinoblastoma (Rb) protein-binding, DNA origin binding (OBD), multimerization Zn-finger and helicase domains. MCV LT contains an MCV unique region (MUR) that is not present in SV40 LT. (B) Microfluidic chip for DNA capture. (B, Left) Schematic flow cell. (B, Right) Details of the laminar flow channels. Initial DNA-polystyrene bead capture occurs in channel 1, followed by DNA tethering in channel 2, DNA quality check in PBS buffer in channel 3, and protein binding and imaging in channels 4 and 5. (C) Cloning of biotinylated Ori98 DNA. pMC.Ori98 Plasmids were digested at XmaI/EcoRI sites and self-ligated to form random origin multimers (1× to 7×), and CG ends were filled with biotinylated dCTP and dGTP using Klenow fragment. The number and location of Ori98 sequences were determined from DNA length in each assay. (D) Representative kymograph of mN-LT bound to multimeric pMC.Ori98 (3×) DNA showing both prolonged and transient binding events. Three origin (white arrows) and three non-origin-binding events (orange arrows) are shown. Transient binding events (< 5 s duration, blue arrows) were not included in subsequent analyses. (E) Ori98 sequence has high binding specificity compared to non-Ori98 pMC.BESPX backbone DNA sequence. Binding frequency for Ori98 and non-Ori98-binding events were collected from 30 DNAs, 5 min exposure each. Statistical analysis was performed using an unpaired t test, P = 0.0085.
In this study, we visualized real-time assembly of MCV LT on single-molecule MCV DNA replication origins with an optical tweezers/fluorescence microscope (Fig. 1B), using a Hidden Markov Model (HMM) simulation (29) to quantitate LT assembly. Our data help resolve how MCV and SV40 helicases initiate dsDNA melting. We find that the initial molecular steps in unlicensed MCV replication involve multimeric LT binding to the origin, which nonenzymatically pries apart the dsDNA. Unlike the reported DNA melting mechanism for cellular CMG, this initial viral DNA melting allows annular LT hexamers to directly form around single DNA strands to create a double-hexameric complex ready for subsequent helicase activation and DNA replication.
Results
Single-Molecule MCV LT Binding to Its Origin DNA.
To visualize real-time viral origin assembly and DNA melting by MCV LT molecules, we used a C-Trap microfluidics optical tweezers/fluorescence microscope (Fig. 1B). Ori98 was cloned into the pMC.BESPX vector, which was concatemerized (for observing multiple concurrent binding events in each experiment) and end biotinylated (Fig. 1C). A single DNA molecule was then captured between two streptavidin-coated beads and kept at 10 piconewton (pN) tension (SI Appendix, Fig. S1B). Nuclear extracts from 293 cells (30) expressing fluorescent N-terminally tagged mNeonGreen LT (mN-LT) were flowed over the DNA in 1mM ATP, 5 mM Mg2+ buffer at 25 °C [fluorescently tagged LT proteins were shown to retain replication competence in replicon assays (SI Appendix, Fig. S1C)]. A representative example for specific mN-LT binding to Ori98 DNA is shown in Fig. 1D and Movie S1. The mN-LT on-rate constant (kon) (31) and binding frequency were 47-fold and 15-fold higher, respectively, for Ori98 sites compared to the pMC.BESPX backbone sequence (Fig. 1E and SI Appendix, Fig. S2). Similarly, mN-LT localized to wild-type MCV Ori98 sequences with ~eightfold higher frequency per unit length of DNA than λ phage genome DNA, which has 140 G(A/G)GGC pentad sites scattered across its genome (SI Appendix, Fig. S1D). Capture of Ori98.Rep- DNA showed reduced mN-LT binding to levels not significantly greater than vector backbone sequence also confirming specificity of wild-type MCV origin recognition by LT in the C-Trap (Fig. 2A).
Fig. 2.
MCV LT multimerizes on the MCV origin. (A) LT specifically bound to wild-type origin but was reduced for tumor-derived mutant, MCV Ori98.Rep- origin DNA. Frequency plots from six DNAs each, with 61 and 22 binding events, respectively. Data collected from multimeric pMC-Ori98 and Ori98.Rep- (1× to 7×) were realigned as single copies. (B) LT protein multimerized on the wild-type Ori98. Representative kymograph for mN-LTK331A (Top) shows that K331A mutation in the LT origin binding domain (OBD) eliminated specific binding to Ori98. Binding was restored (Bottom) when mN-LTK331A was flowed together in the same channel with nonfluorescent wild-type LT. (C) Frequency plots for mN-LTK331A binding to Ori98 without and with nonfluorescent wild-type LT. Data collected from 6 DNA each with 2 and 17 binding events, respectively. (D) Coimmunoprecipitation of LT-FLAG and mN-LT expressed in 293 cells revealed LT multimerization in the absence of origin DNA. Retinoblastoma protein (Rb) detection was used as a positive control for LT pulldown. Representative blot of three repetitions.
To demonstrate single-molecule LT protein oligomerization, we introduced an alanine substitution mutation in the LT protein origin binding domain (OBD) at lysine 331 (mN-LTK331A) (32). The K331 residue has been previously mapped to be a critical DNA contact point (27). This mutation eliminated significant LT-DNA binding, irrespective of whether the DNA was Ori98 or pMC.BESPX (Fig. 2 B, Top). Origin-specific DNA binding fluorescence for mN-LTK331A was restored when flowed together with untagged (nonfluorescent) LT demonstrating molecular multimerization on the origin (Fig. 2 B, Bottom and Fig. 2C). Multimerization was confirmed by flowing mN-LT together with C-terminal mS-tagged LT (LT-mS), which colocalized on Ori98 (SI Appendix, Fig. S3). LT protein multimerization could occur in solution and did not require MCV DNA as determined by bulk immunoprecipitation and immunoblotting in the absence of viral origin DNA (Fig. 2D).
Origin DNA Melting by MCV LT at the Single Molecule Level.
To determine origin melting after LT binding, we used three independent approaches (Fig. 3). First, DNA cobinding by the ssDNA-binding protein RAD51 (33, 34) was examined in the presence or absence of mN-LT protein. To ensure that Cy5-RAD51 binding to ssDNA could be detected in the C-Trap, tethered dsDNA was stretched from 10 pN to 65 pN tension to generate local force-induced ssDNA regions (35), which then bound Cy5-RAD51 (SI Appendix, Fig. S4A). Cy5-labeled RAD51 did not significantly interact with tethered Ori98 dsDNA alone (Fig. 3 A, Top). When mN-LT was flowed in the same channel with Cy5-RAD51, Cy5-RAD51 bound to and colocalized with mN-LT (Fig. 3 A, Bottom). mN-LT temporally assembled on DNA first, followed by Cy5-RAD51, in 72% (n, 81) of 112 dual-binding events. All remaining dual-binding events (n, 31) were concurrent. Cy5-RAD51 binding prior to mN-LT binding was not observed, and only rarely did Cy5-RAD51 bind DNA alone without mN-LT cobinding (twice during 30 min of monitoring for dual binding events). DNA tension (10 pN) did not appreciably affect DNA melting and mN-LT and Cy5-RAD51 cobinding similarly occurred in the absence of DNA tension. In contrast to LT-LT interaction, no direct protein–protein interaction between RAD51 and MCV LT was found by bulk coimmunoprecipitation (SI Appendix, Fig. S4B).
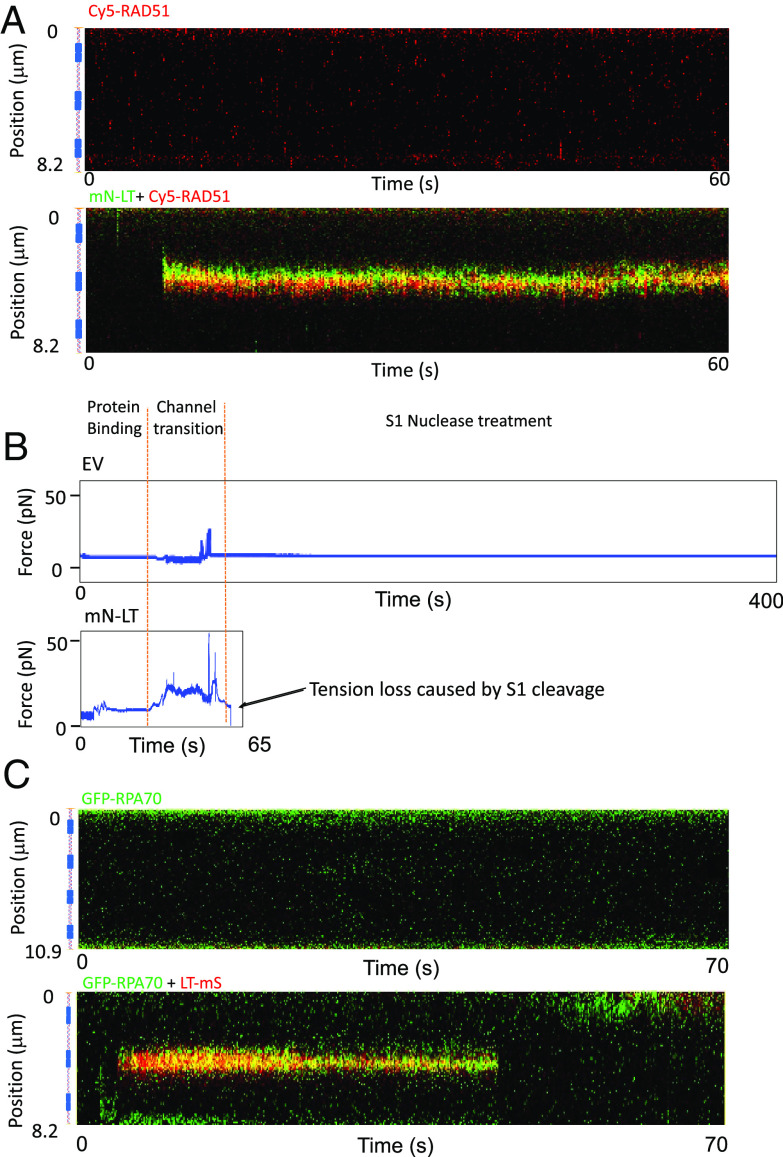
Fig. 3.
Melting of origin DNA by MCV LT. (A) mN-LT bound and melted dsDNA to ssDNA to allow Cy5-RAD51 cobinding. (A, Top) Cy5-RAD51 (red) did not bind pMC-Ori98 dsDNA in the absence of LT protein. No binding events were observed for 5 DNAs examined for 5 min each. (A, Bottom) Cy5-RAD51 (red) colocalized with mN-LT (green) bound to pMC.Ori98 DNA. Representative image from 12 DNAs, 5 min each, 122 events. (B) Single-strand S1 nuclease cleaved Ori98 DNA only after mN-LT binding. Top force diagram for Ori98 dsDNA without mN-LT (Top) exposed to S1 nuclease. The captured dsDNA was exposed to empty vector nuclear extract for 40 s and then moved into the S1 nuclease channel (200 units/mL). The DNA retained tension at 10 pN for 320 s. When mN-LT was captured on Ori98 (Bottom), and then moved into the S1 nuclease channel, tension was lost after 4 s, indicating DNA cleavage. (C) mN-LT bound and melted dsDNA as measured by GFP-RPA70 cobinding. (C, Top) representative GFP-RPA70 (green) flowed on multimeric Ori98 dsDNA alone. No binding events were observed for 5 DNAs, 5 min each. (C, Bottom) cobinding of LT-mS (red) and GFP-RPA70 present for 6 DNAs, 5 min each, 38 events.
We next assayed for molecular DNA melting by cleavage of tethered DNA using the single strand-specific S1 nuclease. S1 cleaved mN-LT-bound DNA within 4 s after introduction into the flow cell whereas in the absence of LT, tethered dsDNA was not cleaved during 320 s of S1 exposure (Fig. 3B). Finally, GFP-labeled RPA70 (36), one of the three replication protein A (RPA) subunits (37, 38), colocalized with LT-mS on DNA but did not bind captured dsDNA in the absence of LT-mS (Fig. 3C and SI Appendix, Fig. S4C). Taken together, these experiments show that Cy5-RAD51 cobinding with mN-LT to DNA was a reliable marker for single-molecule dsDNA melting.
mN-LT Assembles as a Dodecamer on Ori98 DNA.
To quantitate molecular assembly of LT on DNA, we developed a HMM simulation (29, 39). Based on the phenomenon that photobleaching causes equal, stepwise fluorescence decrements, fluorophore photo-oxidization was used to model the number of mN-LT molecules initially captured by DNA origins (Fig. 4 A and B and Materials and Methods). For technical reasons, the HHM could not reliably distinguish between monomer and dimer binding events. Therefore, these values were not included in the quantitative analysis.
Fig. 4.
Quantitation of assembly and mean lifetime of LT on the MCV origin. (A) Photobleaching of mN-LT. Representative mN-LT photobleaching (green) measured by photon counts per second. (B) Hidden Markov Model simulation (HMM) to estimate mN-LT molecule numbers for each initial binding event based on photobleaching. Photon counts from initial binding events were recorded using LUMICKS Pylake software and the best model for equal steps of photon loss was determined for each captured DNA. Estimated photon levels are displayed by red dashed lines. Monomer and dimer assemblies were not reliably discriminated and were removed from the analysis. (C) mN-LT assembled to a dodecamer on wild-type Ori98 but not on Ori98.Rep- DNA. Frequency of mN-LT molecules initially bound to Ori98 as determined by HMMs (blue bars, Top) vs. Ori98.Rep- (yellow bars, Bottom). mN-LT dodecamer assembly was observed in 22% of Ori98 binding events, whereas no assemblies greater than nonamer were observed for Ori98.Rep-. Approximately 30% of assemblies were trimers for both Ori98 and Ori98.Rep-. Rare Ori98 assemblies >12 molecules (3.6%) may represent binding to nonreplication pentads in the MCV origin in addition to origin assemblies. Error bars represent SEM among DNAs. A two-sample Kolmogorov–Smirnov test was significantly different for Ori98 and Ori98.Rep- distributions with D = 0.283, P < 0.05. (D) Mean binding lifetime for dodecamer, hexamer, and trimer mN-LT on Ori98 DNA as determined from koff rates corrected for photobleaching. The LT dodecamer has a 17-fold longer mean binding lifetime on origin DNA than the LT hexamer. The two-sample t test showed a significant difference of mean lifetime for 12-mers compared to 6-mers, with P < 0.0001.
LT molecular assembly on Ori98 for 308 protein binding events, obtained from 30 captured DNAs, ranged from 3 to 14 mN-LT molecules, with notable maxima at 3-mer (32%) and 12-mer (22%) LT complexes (blue bars, Fig. 4C). Some configurations, such as 10 and 11-mer assemblies, were exceedingly rare, which may reflect rapid allosteric promotion to 12-mer complexes from these lower ordered assemblies. The dodecameric assembly most likely represents two separate hexamers (a double hexamer), and the term double hexamer is used below, although we could not directly determine this assembly by C-Trap due to optical resolution limits. Other 12-mer assemblies remain formally possible. Rare complexes greater than 12-mer may represent double-hexamer formation plus additional LT-origin binding at nonreplication site pentads, (e.g., PS5 or PS6) (17). In contrast, when tumor-derived Ori98.Rep- DNA, having a single mutation in PS7, was substituted for Ori98, 12-mer assembly was not seen in 178 binding events (yellow bars, Fig. 4C). Maximum assembly on Ori98.Rep- reached only 6 to 8 mN-LT molecules, consistent with the origin having two separate hexamer nucleation sites, one at PS1, 2, and 4 and another at PS7. This is also supported by the reduced binding specificity seen in Fig. 2A, as well as bulk size-exclusion chromatography of nuclear lysates expressing untagged LT together with either 464 bp wild-type (WT) or Rep- NCCR DNA. Quantitative PCR revealed WT NCCR DNA eluted at higher molecular mass fractions than those eluting NCCR.Rep- DNA, consistent with higher order LT multimerization on the WT NCCR DNA (SI Appendix, Fig. S5A).
Double-Hexameric MCV LT Forms a Stable Complex on Origin DNA.
To determine the stability of mN-LT complexes on Ori98, we estimated the mean lifetime (τ = 1/koff) for mN-LT bound to DNA after correcting for photobleaching (tmN photobleaching = 33 s, SI Appendix, Fig. S5B). The mean LT-DNA binding lifetime increased from 36 s to 88 s for 3-mer and 6-mer assemblies, respectively (Fig. 4D). Since this was performed under active-flow channel conditions, transient disassembly–reassembly was unlikely. In contrast, mN-LT 12-mer assemblies on origin DNA had calculated mean binding lifetimes >1,500 s or greater than 17 times the mean binding lifetime for a single hexamer (Fig. 4D).
MCV and SV40 LT Origin Melting Does Not Require an LT Hexamer.
Ori98.Rep- did not form replication competent double-hexameric LT complexes, nevertheless, mN-LT recruited Cy5-RAD51 to Ori98.Rep- (Fig. 5A and Movie S1) as well as to Ori98 (Fig. 3A). Out of 34 mN-LT binding events on Ori98.Rep-, 26 (76%) were observed with Cy5-RAD51 cobinding. In the case of wild-type Ori98 DNA, Cy5-RAD51 binding and DNA melting was detected for subhexameric complexes (e.g., trimers). Bound Cy5-RAD51 fluorescence intensity increased linearly with the number of LT molecules coassembled on Ori98 (R2=0.86) with the shortest lag-time between initial mN-LT binding and subsequent Cy5-RAD51 binding (~1 s) occurring for an LT double hexamer (Fig. 5B). LT-Cy5/RAD51 binding lag time was inversely related to the number of assembled LT molecules (e.g., ~70 s for trimers; R2= 0.95, Fig. 5B). Since RAD51 forms polymeric fibrils on ssDNA, Cy5-RAD51 fluorescence intensity is not a reliable measure of single-strand bubble size, but these data taken together are consistent with extensive DNA melting upon subdodecameric LT assembly.
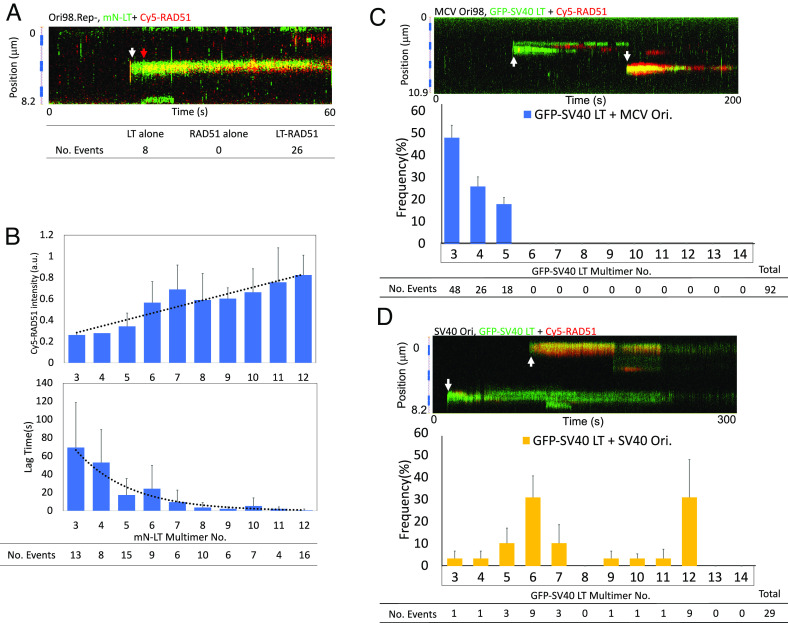
Fig. 5.
Partially assembled MCV or SV40 LT proteins melt MCV origin dsDNA. (A) ssDNA RAD51 binding occurs after LT assembly on Ori98.Rep-. A representative kymograph from 26 colocalization events for mN-LT (green) and Cy5-RAD51 (red) bound to Ori98.Rep- using six DNAs. The white arrow marks initial mN-LT DNA binding, and the red arrow marks subsequent Cy5-RAD51 assembly. (B) RAD51 cobinding was proportional to LT multimerization and lag time for RAD51 cobinding, after LT binding, decreased exponentially with size of the initially bound LT multimer. (Top) maximum Cy5-RAD51 fluorescence vs. mN-LT molecule assembly number on wild-type Ori98 for dual LT-RAD51 binding events (n, 94). Increased LT multimerization was associated with increased RAD51 ssDNA deposition, R2 = 0.8608 for a linear regression, and F = 49.48 for the F-test with P = 0.0001. six DNAs, 5 min exposure each. No RAD51 binding was seen for 52 origins that did not bind LT during the experiment. (Bottom) lag time between initial mN-LT and initial Cy5-RAD51 binding to the same origin for dual LT-RAD51 binding events (n, 94). Lag time was inversely related to initial LT multimerization. Dodecameric mN-LT recruited Cy5-RAD51 almost immediately, whereas trimeric mN-LT required 67 s (on average) to attract Cy5-RAD51 binding, R2 = 0.9509 for an exponential regression. (C) Nonreplicative SV40 LT melts MCV origin. (Top) GFP-SV40 LT (green) did not form hexamers on MCV Ori98 but was associated with DNA melting and subsequent Cy5-RAD51 (red) colocalization (white arrows). (Bottom) frequency of estimated SV40 LT-GFP multimers initially binding to MCV origin. Data collected from 12 DNAs, 5 min each. (D) SV40 LT melts the SV40 origin. GFP-SV40 LT (green) was associated with DNA melting and Cy5-RAD51 (red) colocalization (white arrows) on SV40 origin. (Bottom) frequency of estimated SV40 LT-GFP multimers initially binding to SV40 origin showed preferred hexamer and dodecamer assembly. Data were collected from six DNAs, 5 min each. Notably, sub-double-hexameric SV40 LT binding events were also observed to melt SV40 origin in a fashion similar to MCV LT on MCV origin.
When C-terminal GFP-tagged SV40 LT was flowed over MCV Ori98 DNA, only subhexameric SV40 LT binding was observed, and no SV40 LT hexamers or double hexamers were detected (Fig. 5C). All SV40 LT binding assemblies were trimeric (57%), tetrameric (27%), or pentameric (16%) in 92 binding events on 12 separate dsDNA molecules, consistent with the inability of SV40 LT to assemble a competent double-hexameric helicase on the MCV origin (7). When the MCV origin was replaced by the SV40 LT origin in the pMC plasmid, however, SV40 LT was able to readily assemble as a dodecamer on its own origin (Fig. 5D). Despite being unable to replicate MCV DNA or even form a single hexamer on MCV origin, SV40 LT recruited Cy5-RAD51 to MCV Ori98 DNA (Fig. 5C), demonstrating that SV40 LT melts the MCV origin when binding alone. As with MCV LT on MCV origin, Cy5-RAD51 cobinding was observed for subdodecameric as well as dodecameric SV40 LT assemblies on the SV40 origin (Fig. 5D).
MCV Origin DNA Melting Requires LT Multimerization but Not the Viral Helicase Domain.
Dispensability of the MCV LT helicase function for initial DNA melting was demonstrated with successive C-terminal truncations of the 817 aa LT protein (Fig. 6A). These truncation mutants all abrogated replication when used in replicon assays (SI Appendix, Fig. S6A). mN-LT700 lacks a critical cell growth-inhibitory domain (40) but retains canonical AAA+ Walker A and B sites required for ATP binding and hydrolysis (41, 42). mN-LT610 retains the Walker A site but is deleted for the Walker B site. Both mN-LT700 and mN-LT610 bound origin MCV DNA and induced Cy5-RAD51 colocalization (Fig. 6B). A point mutation in the Walker A site (mN-LTK599R, SI Appendix, Fig. S6B) (9, 41) also recruited Cy5-RAD51 (SI Appendix, Fig. S6C). Unexpectedly, this mutant showed enhanced movement along the DNA (y axis of SI Appendix, Fig. S6C), with the diffusion coefficient ranging from 0.05 to 0.4 μm2/s (30).
Fig. 6.

MCV LT melts MCV origin dsDNA in the absence of helicase activity. (A) MCV LT domains with truncation and site-directed mutation sites denoted. (B) Deletions of the MCV LT helicase domain (LT700 and LT610), but not the zinc-finger multimerization domain (LT455), retained capacity to melt MCV origin DNA. Representative kymographs for full-length LT, LT700, LT610, and LT455 binding are shown with 5 pMC-Ori98 DNAs for 5 min each. (C) MCV origin DNA melting by MCV LT required ATP binding but not hydrolysis. mN-LT (green) and Cy5-RAD51 colocalization (Top) was lost when nuclear extracts were treated with apyrase to eliminate ATP. Both mN-LT and Cy5-RAD51 binding to Ori98 were restored after apyrase treatment by exposure to 1 mM nonhydrolyzable AMP-PNP. Representative kymographs from 5 pMC-Ori98(4X) DNAs each, 5 min exposure. (D) MCV LT formed dodecameric assemblies on MCV origin DNA in the absence of hydrolyzable ATP. Frequency of estimated mN-LT multimers initially binding to MCV Ori with 1mM AMP-PNP. Data were collected from six DNAs, 5 min each. Error bars represent SEM among DNAs.
MCV LT C-terminally truncated at residue 455 (mN-LT455) corresponds to a tumor-derived (MCC339) mutant protein (11) that has an intact OBD but lacks the majority of the zinc-finger domain required for dimerization (43) as well as the helicase domain. This mutation only bound origin DNA as a monomer (Fig. 6B) and did not recruit Cy5-RAD51, consistent with LT multimerization being required for DNA melting and RAD51 recruitment.
MCV LT DNA Loading and Melting Requires ATP Binding but Not ATP Hydrolysis.
When mN-LT nuclear extracts were pretreated with apyrase, an ATP diphosphohydrolase, to deplete residual ATP from nuclear lysates, LT and Cy5-RAD51 binding to Ori98 DNA was eliminated (Fig. 6C). Binding and melting, however, was restored by addition of 1 mM nonhydrolyzable adenylyl-imidodiphosphate (AMP-PNP) (44). This is most consistent with ATP binding, but not enzymatic hydrolysis, being required for LT loading and initial origin melting. Notably, LT quantitation revealed that LT can assemble as a double hexamer (dodecamer) on origin DNA in the presence of AMP-PNP (Fig. 6D). Similar experiments using SV40 GFP-LT also revealed that SV40 LT/Cy5-RAD51 binding to the MCV origin was independent of ATP hydrolysis (SI Appendix, Fig. S6D).
Discussion
Our results are most consistent with multimer MCV and SV40 LT, as small as a trimer, being able to nonenzymatically bind and pry open the dsDNA origin so that LT can directly form two hexamers (a double hexamer) around the ssDNA strands (Fig. 7A). This “strand invasion model” can occur if multimeric LT has a higher affinity for origin ssDNA than for dsDNA, as has been described for SV40 LT (45), and LT’s ssDNA affinity exceeds the local corresponding binding affinity of the complementary ssDNA strand. After the double hexamer is assembled onto complementary ssDNA strands, DNA unwinding and unzipping through helicase activity and ATP hydrolysis would be able to allow DNA polymerase processivity in replication. If MCV LT followed the same steps as the CMG origin melting model instead (1), MCV LT hexamers would have to first form as annuli around dsDNA, initiate helicase shearing even without two complete hexamers, and then remodel onto ssDNA without use of ATP hydrolysis, which is energetically unlikely to happen.
Fig. 7.
Models for CMG and MCV LT helicase initiation of dsDNA melting for recruitment of replication machinery. (A) Model for eukaryotic CMG helicase initiation of DNA replication (4, 6). CMG double hexamer first assembles around dsDNA during the late M/G1 phase. On S phase entry, CMG melts origin DNA by ATP-driven DNA distortion and then hexamers remodel around ssDNA. The two hexamers bypass each other to initiate dsDNA unzipping and recruitment of the replisome. (B) Model for MCV and SV40 LT origin melting. After initial LT binding to viral DNA pentads using LT origin binding domains, LT multimerizes to pry apart the MCV origin sequence and melt dsDNA in the absence of ATP hydrolysis. Hexamers then directly assemble around ssDNA. Once assembled, the MCV LT initiates ATP-driven helicase processivity similarly to cellular CMG.
It is not surprising for viral helicases to have a molecular mechanism for origin melting that differs from cellular CMG since viruses initiate multiple rounds of replication during each cell cycle. CMG is preloaded by ORC onto dsDNA eukaryotic origins to assure complete replication of the genome, and thus, CMG double hexamers must wait until they are fully loaded and licensed before initiating origin melting. The LT strand invasion model may explain how these viruses can rapidly reinitiate origin melting on newly synthesized dsDNA strands to iteratively amplify viral genomes in a single cell cycle. While MCV and SV40 LT proteins have similarities, caution is needed to assume that both viral proteins have identical replication mechanisms. For example, initial SV40 LT origin melting is reported to occur at an early palindrome region that is not present in the MCV origin (46). Instead, MCV origin has an AT-rich tract (SI Appendix, Fig. S1A) between PS6 and PS7 that may allow melting during LT assembly on the origin sequence. Despite having different origin sequences, these two viral LT proteins are similar enough to each other to initiate melting, but not replication, of the MCV origin (Fig. 5C).
There are several key pieces of data in our single-molecule experiments that support the MCV LT direct strand invasion mechanism rather than helicase-dependent compression of dsDNA between the two hexamers to initially melt DNA. Measurements of kon and koff rates allowed stability to be determined for different configurations of LT-DNA (SI Appendix, Fig. S2 and Fig. 4D) and support the hypothesis that LT hexamers melt and directly surround ssDNA rather than first assembling around dsDNA. A free complementary ssDNA strand would compete to eject a single hexamer in our flow experiments making it more unstable than a double hexamer in which both strands are occupied. Further, the kinetics for initial RAD51 cobinding with partially multimerized (6-mer through 10-mer) MCV LT approached dodecameric LT rates, consistent with partial multimers and dodecamers of LT opening up similar-sized ssDNA bubbles (Fig. 5 B, Top). Additionally, double-hexamer loading and melting occurred in the absence of hydrolyzable ATP (Fig. 6 C and D), which is inconsistent with helicase activity being responsible for shearing dsDNA. Finally, MCV LT mutations eliminated functional helicase activity but retained dsDNA origin melting. The results for SV40 LT shown here (Fig. 5C and SI Appendix, Fig. S6), in which no SV40 hexamers are formed on MCV origin, and studies using bulk KMnO4 oxidation assays on SV40 origin (25, 26), suggest viral LT multimers pry apart origin dsDNA rather than using a helicase mediated shearing mechanism.
Single-molecule microscopy complements X-ray crystallography and cryo-EM studies in determining the functions for LT structural features. The requirement for ATP binding in LT assembly on dsDNA (Fig. 6C), for example, may be due to anchoring interactions of the AAA+ domain on the dsDNA minor groove (47). A recent single molecule study for activated yeast CMG revealed that nucleotide binding anchors CMG to DNA to prevent bidirectional diffusion of the helicase along dsDNA (48). This may explain the diffusion along DNA we saw for the MCV LT Walker A box mutant (mN-LTK599R, SI Appendix, Fig. S6C). This mutant still generated Cy5-RAD51 cobinding, which tracked with the LT complex. The movement is most likely a result of physical flow conditions in our experiment, wherein LT hexamers are nudged along the DNA by the channel flow to unzip dsDNA.
The minimum number of assembled LT subunits needed for MCV melting is not addressed by our study. Monomeric MCV mN-LT455 was incapable of melting origin DNA, in agreement with structure studies showing that monomer MCV OBD binding to the origin major groove at PS1 and PS2 causes a 5º bend in the DNA but not strand separation (27). For SV40, multimeric LT binding causes local distortion and melting of origin DNA (25, 26), but dimeric LT alone is not capable of melting origin DNA (49). Structural studies of bovine papillomavirus E1 (a distantly related virus) suggest that E1 trimerization is sufficient to initiate viral strand separation (50) and is consistent with trimeric MCV LT (Fig. 5B) being able to initiate detectable melting in at least a fraction of bound DNAs.
In addition to binding to origin sequences, we find that MCV LT and RAD51 can also bind to nonorigin DNA sequences, most likely at single G(A/G)GGC pentad sequences. This binding is not expected to allow adventitious replication but could promote single strand breaks if the bound LT persistently melts dsDNA. DNA damage responses due to LT expression—as well as expression of the replication accessory MCV small T protein inhibitor of anaphase-promoting complex/cyclosome (51)—might halt host cell DNA replication (6), but not viral replication, thereby shifting cellular replication resources to the virus (46). It is not known whether MCV LT is inherently mutagenic, but both SV40 and MCV LT have been reported to induce cellular DNA damage responses independent of oncoprotein domains (52, 53). Whether cellular DNA damage from MCV LT expression might contribute to clonal viral integration is unknown.
This study focused only on the initial steps in origin melting since directed LT movement in the DNA axis (expected for in situ DNA helicase processivity) was rarely seen on the kymographs in our experiments performed at 25 °C. Our dynamic study complements static atomic resolution X-ray crystallography (54) and cryoelectron microscopy structural studies (23, 27), yet generates an unexpected model for viral DNA replication initiation. Use of nuclear extracts was particularly critical to these experiments, but we recognize that unmeasured, nonfluorescent cellular replication/repair proteins may also affect MCV DNA melting and should be considered. Extension of these single-molecule experiments to chromatinized DNA or by achieving in situ helicase activity will provide important additional information on events controlling replication of this human tumor virus.
Materials and Methods
Cell Lines.
293 cells (ATCC) were maintained in Dulbecco’s modified Eagle medium (ThermoFisher) supplemented with 10% fetal bovine serum (FBS), in a 37 °C and 5% CO2 incubator.
Plasmid Mutagenesis.
mN-LT plasmid was constructed by inserting codon optimized MCPyV LT sequence to the C terminus of pmNeongreen-C1, using XhoI and BamHI cutting sites (a 6 a.a. GSTGSR nonspecific protein tag was appended to the C terminus of LT due to cloning strategy). To generate the pMC-Ori98 plasmid, a fragment of Ori98 sequence was produced through PCR from pMC-MCV and then inserted into the pMC.BESPX backbone using EcoRI and BamHI sites. All point mutations (mN-LTK331A, mN-LTK599R etc.) were produced using QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent) following the manufacturer’s protocol. All Chang-Moore (CM) laboratory plasmid numbers are listed in the Key Resources table.
Origin Replication Assay.
293 cells were seeded in 6-well plates and transfected with appropriate sample plasmid combinations to equal 1 µg total plasmid using Lipofectamine 2000 (ThermoFisher). At 48 h posttransfection, cells were collected for DNA and protein extraction. Total genomic DNA was purified from cells using DNeasy Blood and Tissue Kit (Qiagen). To linearize the replicated Ori98 DNA and remove transfected bacterial DNA, 1.25 µg of total genomic DNA was digested overnight using BamHI and DpnI.
Quantitation of Replication by Quantitative Real-Time PCR.
After overnight digestion of DNA from harvested cells, qPCR was performed using PowerUp™ SYBR™ Green Master Mix (ThermoFisher) with 5 ng DNA and Ori98 primers Fw: 5′-GCCGCCAAGGATCTGATG-3′ and Rev: 5′-CTGCGCAAGGAACGCCCGTCG-3′, with GAPDH primers: Fw: 5′-TGTGTCCCTCAATATGGTCCTGT-C-3′ and Rev: 5′-ATGGTGGTGAAGACGCCAGT-3′ as the endogenous control. Using a QuantStudio™ three Real-Time PCR Machine (ThermoFisher) and the ΔΔCT comparative method, threshold cycle (CT) values were used to calculate relative DNA replication levels, normalized to GAPDH levels.
Immunoblotting.
Total protein was extracted from transfected cells using RIPA Lysis Buffer (150 mM NaCl, 1% NP-40 ,0.5% DOX, 0.1% SDS, and 50 mM Tris–HCl, pH 7.4) and protease inhibitors (0.2 mM Vanadate, 0.3 mM PMSF, 1 mg/mL Leupeptin, 1 mg/mL Pepstatin A, and 1 mg/mL Aprotinin). Samples were then sonicated with Fisherbrand™ Model 505 Sonic Dismembrator (ThermoFisher) at 20% Amp 4× for 5 s each on ice. 2× Laemmli loading buffer (65.8 mM Tris–HCl pH 6.8, 26.3% glycerol, 2.1% SDS, and 0.01% bromophenol blue, 10% 2-mercaptoethanol were added to samples which were then separated by SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were incubated with primary mouse monoclonal antibody to MCV LT (CM2B4) overnight at 4 °C, followed by incubation with IRD800 conjugated goat anti-mouse secondary antibody (LI-COR Biotechnology) diluted 1:10,000 and Rhodamine conjugated anti-tubulin antibody (Bio-Rad) diluted 1:10,000 for 1 h at room temperature. A ChemiDoc™ MP Imaging system (Bio-Rad) was used to detect signals.
Coimmunoprecipitation.
293 cells were cotransfected with 1ug each plasmid (LT-FLAG and mN-LT; mN-LT and T7-RAD51) with Lipofectamine 2000 (ThermoFisher) for 48 h. Lysates were precleared with Protein A/G PLUS-agarose beads (Santa Cruz) and incubated with antibody overnight at 4 °C, then with protein A/G PLUS-agarose beads for 3 h at 4 °C. The beads were then washed twice with IP buffer (50 mM Tris pH7.4, 150 mM NaCl) and twice with LiCl buffer (500 mM LiCl 50 mM Tris pH7.4). Beads were boiled in 50 µL SDS loading dye. 15 µL of sample was run on 10% acrylamide gel, transferred to nitrocellulose, blocked in 5% milk, incubated with antibody at 4 °C overnight, washed, and incubated with secondary antibody at room temperature for 1 h. Blots were imaged on a ChemiDoc™ MP Imaging system (Bio-Rad). Antibodies: for LT-FLAG and mN-LT, IP: Mouse anti-FLAG (Sigma) 1µg; IB: Rabbit anti-FLAG (Sigma) 1:1,000, Mouse anti-mNeon (Chromotek) 1:1,000, Mouse anti-Rb (Cell Signaling) 1:1,000; for mN-LT and T7-RAD51, IP: CM2B4 anti-LT 1 µg; IB: Mouse anti-mNeon (Chromotek) 1:1,000, Mouse anti-T7 (Novagen) 1:3,000, Mouse anti-Rb (Cell Signaling) 1:1,000.
Size-Exclusion Chromatography.
293 cells were transfected with pcDNA6-LT, and nuclear extracts were prepared 48 h after transfection as described in SMADNE method below. 150 µL of nuclear extracts were added to an equal volume of 2× reaction buffer (50 mM Tris–acetate, 20 mM magnesium acetate, 100 mM potassium acetate, 0.2 mM EDTA, 4 mM TCEP, 2 mM ATP, and 6 mM DTT) and incubated at 37 °C for 1 h. Diluted nuclear extracts were loaded onto a Superose 6 10/300 GL column and eluted with BC150 buffer (20 mM HEPES pH 7.9, 150 mM KCl, 0.2 mM EDTA, 10% glycerol, 1 mM DTT, and 0.5 mM PMSF), and 250 µL fractions were collected. 100 µL of each fraction was trichloroacetic acid (TCA)-precipitated and boiled in 25 µL of 2× Laemmli loading buffer. 20 µL of each sample was loaded on a 10% SDS-gel and transferred to a nitrocellulose membrane at 30 V overnight at 4 °C. Membranes were treated with SuperSignal western blot enhancer (Thermo) according to the manufacturer’s protocol and then incubated with primary antibody (CM2B4, 1:1,000 dilution) overnight followed by incubation with secondary antibody (goat anti-mouse-IR800, 1:10,000 dilution) for 1 h at room temperature. Images were taken with ChemiDoc™ MP Imaging system (Bio-Rad). Quantitative PCR was applied using SYBR Green Master buffer (ThermoFisher) and Primers: FW: 5′-ATCGGGATCCGGTGACTTTTTTTTTTCAAGTTG-3′ and Rev: 5′-ATCGGAATTCTAAGCCTCTTAAGCCTCAGAG-3′ to quantify NCCR oligo DNA copies in each sample. Thermal cycling was performed on a QuantStudio™ three Real-Time PCR machine. Threshold cycle (CT) values were used to calculate relative NCCR oligo DNA abundance.
SMADNE.
Following the SMADNE protocol(30), 293 cells at 70% confluency were transfected with 2 µg of plasmid (e.g. mN-LT, LT-mS, or sT-GFP) and 2 µL of Lipofectamine 2,000 (Thermo Fisher) in six-well plates. Cells were collected for nuclear extract preparation 48 h after transfection using the NE-PER™ Nuclear and Cytoplasmic Extraction Reagents kit (ThermoFisher) to prepare 50 µL of nuclear extract per well. Immediately prior to single-molecular experiments, nuclear extracts were diluted in reaction buffer (25 mN Tris–acetate, 10 mM magnesium acetate, 50 mM potassium acetate, 0.1 mM EDTA, 2 mM TCEP, 1 mM ATP, and 3 mM DTT) at 1:100 ratio (denoted as 1×).
Linear Biotinylated DNA Substrate Preparation.
Fig. 1B and SI Appendix, Fig. S1B, show the schematic for multimeric Ori98 biotinylated DNA preparation. First, 2 µg of pMC.Ori98 plasmid was digested with XmaI and EcoRI-HF (NEB) overnight and then column purified using the NucleoSpin Gel and PCR Clean-up Mini Kit (Macherey-Nagel). The resulting linear DNA was self-ligated using T4 ligase (NEB) for 48 h and column purified again, creating randomly multimerized pMC-Ori98 with 5′-GGCC and/or 5′-AATT overhangs. Then, the 5′-GGCC overhangs were filled-in with 10 mM biotin-14-dCTP (and 10 mM biotin-11-dGTP (AAT Bioquest) using 10U DNA Polymerase I Klenow Fragment (NEB) for 1 h at 37 °C. After a final column purification, DNA was stored in 0.1× TE buffer and diluted 1:250 in 1× phosphate buffered saline (PBS) for use.
Optical Tweezer–Fluorescence Microscope.
Optical Tweezer–Fluorescence Microscope (C-Trap, LUMICKS) with triple-color confocal fluorescence microscope and dual-trap laser optical tweezers was used in single-molecule experiments and has successfully been used to characterize nuclear extracts (30). The instrument contains five microfluidic channels combined into one chamber (Fig. 1A). Polystyrene beads (Spherotech, IL) coated with streptavidin at a diameter of 4.5 to 4.9 µm were flowed into channel 1 and captured by two optical tweezers with a stiffness of 0.3 pN/nm. The beads were then moved by optical traps to channel 2 to capture biotin-conjugated linear dsDNA. The length of tethered DNAs was quantified by the force–distance curve and fit into a worm-like chain (WLC) model to verify presence of a single DNA tether in channel 3. All channels were flowed at 0.2 bar to maintain laminar flow. Channels 4 and 5 were loaded with nuclear extracts diluted in reaction buffers. For the mN-LT binding assay, channel 4 was loaded with nuclear extract of mN-LT transfected 293 cells. Cy5-RAD51/GFP-RPA70 was mixed with mN-LT immediately before loading into channel 4. For S1 nuclease assays (see details below), mN-LT was loaded to channel 4, and S1 nuclease was loaded to channel 5. 2D scanning images and kymographs were taken in these two channels. When DNA-tethered beads were moved to these channels, protein–DNA binding events were recorded at a DNA tension of 10 pN, unless otherwise specified For high protein binding efficiency, the flow pressure was adjusted to 0.03 bar in channels 3 and 4 while images were taken. mNeongreen and GFP fluorophores were excited by laser at 488 nm and emission was collected in a 500-550-nm band-pass filter. mScarlet fluorophore was excited at 532 nm, and emission was collected in a 575 to 625 nm band-pass filter. All data were collected with a 1.2 numerical aperture 60X water immersion. Kymographs were generated via a 1D scan through the center of the two beads, at pixel size = 50 nm, pixel scanning time = 0.1 ms, and line scanning time = 0.1 s. 2D scanning was performed at a focal plane that passes the center of the two beads, with frame rate = 2.0 s/frame.
Data Extraction.
Kymographs of protein–DNA binding were taken and then analyzed by LUMICKS custom codes, and the line tracking of each fluorophore over time was performed based on a Gaussian fit over the signal intensity and connected over time. Visual aids were performed to ensure that each tracking result was continuous and clear. Instantaneous events (<5 s) were discarded since they might represent unstable protein attaching temporarily to DNA. The graphical user interface (GUI) allowed for quantitation and extraction of each event start/end time, event location tracking, photon count of the event over time, and tension applied to the DNA. Kymographs were generated from LUMICKS Lakeview software and exported as PNG files. Since the software showed the 500 to 550-nm channel in blue, all kymographs containing this channel were further imported to ImageJ to pseudocolor the 500-550-nm channel to green.
Simulation for Fluorophore Levels with HMM Simulations.
The LUMICKS C-Trap optical tweezer–fluorescence microscope records raw data of binding events including original photon counts over time. By defining each protein binding events with pylake, photon count distribution of each event was extracted (Fig. 4 A and B). Then a HMM was applied using Matlab to analyze the dataset to estimate each fluorophore level. The code was adapted from Sgouralis et al (29). Original code is available at https://github.com/JamesLiWan/MultimerizationCode. After each dataset was analyzed and the maximum multimer number was obtained, the fluorophore level of each binding event was recognized and recorded. A complete statistical analysis to count the frequency of each multimer was then applied across different DNA datasets. Monomers and dimers were excluded because the photon count distribution dataset does not clearly distinguish between adjacent monomer/dimer events, causing potential inaccuracy.
Localization Analysis.
Colocalization analysis was performed using the “Colocalization Analyzer” script available at harbor.lumicks.com. This script functions by performing a Gaussian fit to determine the positions of each event and then comparing each time and position of the binding events in one color with the times and positions of all binding events in a second color to determine the frequency and nature of interactions.
Photobleaching Analysis.
Photobleaching decay constants for each fluorophore was experimentally determined by testing the fluorescently labeled proteins immobilized at the bottom of the flow cell on the glass slides. The objective of the confocal microscope in C-Trap was lowered to the glass surface with identical laser power settings. At least 5 kymographs were obtained using the same data collection setup to observe photobleaching decay of these fluorophores. The images were processed through event data extraction and the photon counts of all events were fit into a single-exponential decay function to determine photobleaching lifetimes. Then, the binding mean lifetime of all events on DNA was corrected for photobleaching effect with the following equation:
S1 Nuclease Cleavage Experiment.
Channels 1, 2, and 3 were flowed with polystyrene beads, biotinylated Ori98 DNAs, and 1XPBS, respectively. Channel 4 was flowed with nuclear extracts of mN-LT or pcDNA6 empty vector (EV) diluted 1:100 in reaction buffer. Channel 5 was flowed with S1 nuclease (NEB) at 1 µL (100 Units) in 500 µL of reaction buffer (40 mM sodium acetate pH 4.5, 0.3 M NaCl, and 2 mM ZnSO4.) DNA tension was monitored until breakage (0 pN) or for > 300 s.
Protein Purification and Native Page Complex Formation for Cy5-RAD51.
Human RAD51 was purified from Escherichia coli (AB1157ΔRecA) as described (55). To label RAD51 N-terminally with Cy5, recombinant RAD51 was dialyzed in buffer containing 250 mM NaPi (pH 7.0), 150 mM NaCl, 1 mM DTT, and 10% glycerol and labeled with Cy5-Mono-Reactive Dye (VWR). Cy5-RAD51 was further purified as described (55). Labeling efficiency was determined by measuring the absorbance of RAD51 at 280 nm and of Cy5 at 650 nm using their extinction coefficients (ε280 = 14,900 M−1cm−1 for RAD51 and ε650 = 250,000 M−1cm−1 for Cy5). Labeling efficiency was determined to be 39.7% for Cy5-RAD51.
ATP Hydrolysis Assay.
5 µL of 293 cell nuclear extract transfected with mN-LT was mixed with 2 µL apyrase (NEB) and 1× apyrase reaction buffer in a total reaction volume of 20 µL for 20 min at 30 °C for ATP hydrolysis. The reaction mixture was immediately diluted in 500 µL of reaction buffer (25 mM Tris–acetate pH 7.5, 10 mM magnesium acetate, 50 mM potassium acetate, 0.1 mM EDTA, 2 mM TCEP, 1 mM ATP, and 3 mM DTT) for single-molecule DNA binding experiments. For recovery with nonhydrolyzable ATP, adenylyl-imidodiphosphate (AMP-PNP) (Sigma) was added to the solution after ATP hydrolysis to a 1 mM final concentration.
Supplementary Material
Appendix 01 (PDF)
mN-LT (green) and Cy5-RAD51 (red) binding to pMC.Ori98 tethered to polystyrene beads (shown by the two auto-fluorescent spheres) in the C-Trap system. Recording speed = 2.0 s/frame. Total Recording Time = 200s.
Acknowledgments
We thank Maria Spies for providing the Cy5-RAD51 labeling protocols and the pCH1-RAD51 plasmid, Yufei Wang for drawing schematics, and Marco Pereira for help with the manuscript. This study was supported by NIH Grants R35CA197463 (to P.S.M.), R01CA232604 (to Y.C.), R35ES031638 (to B.V.H), K99ES033738 (to S.R.H.), R01ES031796 and R01ES030335 (to K.A.B), 2P30CA047904 (to the University of Pittsburgh Medical Center, Hillman Cancer Center), and a major equipment grant S10OD032158-01A1 (to B.V.H). This study was also supported by Hillman Postdoctoral Fellowship for Innovative Cancer Research (to M.A.S and S.R.H.) and American Cancer Society Postdoctoral Fellowship (133947-PF-19-132-01-DMC) (to S.R.H.).
Author contributions
L.W., Y.C., and P.S.M. designed research; L.W., S.T., L.R.R.-M., N.L., and X.L. performed research; M.A.S., S.R.H., K.A.B., and B.V.H. contributed new reagents/analytic tools; L.W., Y.C., P.S.M, M.A.S., and B.V.H. analyzed data; and L.W., Y.C., and P.S.M. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: J.A.D., Dana-Farber Cancer Institute; and R.D.W., University of Texas MD Anderson Cancer Center.
Contributor Information
Yuan Chang, Email: yc70@pitt.edu.
Patrick S. Moore, Email: psm9@pitt.edu.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information. Software: Code used for the simulation of fluorophore levels has been deposited in GitHub at https://github.com/JamesLiWan/MultimerizationCode (56).
Supporting Information
References
- 1.Costa A., Diffley J. F. X., The initiation of eukaryotic DNA replication. Annu. Rev. Biochem. 91, 107–131 (2022). [DOI] [PubMed] [Google Scholar]
- 2.Todorov I. T., et al. , A human nuclear protein with sequence homology to a family of early S phase proteins is required for entry into S phase and for cell division. J. Cell Sci. 107, 253–265 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Langston L. D., O’Donnell M. E., An explanation for origin unwinding in eukaryotes. Elife 8, e46515 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis J. S., et al. , Mechanism of replication origin melting nucleated by CMG helicase assembly. Nature 606, 1007–1014 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abid Ali F., et al. , Cryo-EM structure of a licensed DNA replication origin. Nat. Commun. 8, 1–10 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas M. E., Ali F. A., Costa A., Diffley J. F., The mechanism of eukaryotic CMG helicase activation. Nature 555, 265–268 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuda M., et al. , T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc. Natl. Acad. Sci. U.S.A. 105, 16272–16277 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore P. S., Chang Y., Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat. Rev. Cancer 10, 878–889 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulson K. G., et al. , Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad Dermatol. 78, 457–463.e452 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed M. M., Cushman C. H., DeCaprio J. A., Merkel cell polyomavirus: Oncogenesis in a stable genome. Viruses 14, 58 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng H., Shuda M., Chang Y., Moore P. S., Clonal integration of a polyomavirus in human merkel cell carcinoma. Science 319, 1096–1100 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastrana D. V., et al. , Quantitation of human seroresponsiveness to merkel cell polyomavirus. PLoS Pathog. 5, e1000578 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang Y., Moore P. S., Merkel cell carcinoma: A virus-induced human cancer. Annu. Rev. Pathol. 7, 123–144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spurgeon M. E., et al. , Merkel cell polyomavirus large T antigen binding to pRb promotes skin hyperplasia and tumor development. PLoS Pathog. 18, e1010551 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowlatshahi M., et al. , Tumor-specific T cells in human Merkel cell carcinomas: A possible role for Tregs and T-cell exhaustion in reducing T-cell responses. J. Invest. Dermatol. 133, 1879–1889 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afanasiev O. K., et al. , Merkel polyomavirus-specific T cells fluctuate with merkel cell carcinoma burden and express therapeutically targetable PD-1 and Tim-3 exhaustion markers. Clin. Cancer Res. 19, 5351–5360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwun H. J., et al. , The minimum replication origin of merkel cell polyomavirus has a unique large T-antigen loading architecture and requires small T-antigen expression for optimal replication. J. Virol. 83, 12118–12128 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waga S., Bauer G., Stillman B., Reconstitution of complete SV40 DNA replication with purified replication factors. J. Biol. Chem. 269, 10923–10934 (1994). [PubMed] [Google Scholar]
- 19.Kelly T. J., et al. , Replication of adenovirus and SV40 chromosomes in vitro. Philos. Trans. R Soc. Lond. B Biol. Sci. 317, 429–438 (1987). [DOI] [PubMed] [Google Scholar]
- 20.Melendy T., Stillman B., An interaction between replication protein A and SV40 T antigen appears essential for primosome assembly during SV40 DNA replication. J. Biol. Chem. 268, 3389–3395 (1993). [PubMed] [Google Scholar]
- 21.Tsurimoto T., Melendy T., Stillman B., Sequential initiation of lagging and leading strand synthesis by two different polymerase complexes at the SV40 DNA replication origin. Nature 346, 534–539 (1990). [DOI] [PubMed] [Google Scholar]
- 22.Dean F. B., Hurwitz J., Simian virus 40 large T antigen untwists DNA at the origin of DNA replication. J. Biol. Chem. 266, 5062–5071 (1991). [PubMed] [Google Scholar]
- 23.Langston L. D., Yuan Z., Georgescu R., Li H., O’Donnell M. E., SV40 T-antigen uses a DNA shearing mechanism to initiate origin unwinding. Proc. Natl. Acad. Sci. U.S.A. 119, e2216240119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li D., et al. , Structure of the replicative helicase of the oncoprotein SV40 large tumour antigen. Nature 423, 512–518 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Borowiec J. A., Hurwitz J., ATP stimulates the binding of simian virus 40 (SV40) large tumor antigen to the SV40 origin of replication. Proc. Natl. Acad. Sci. U.S.A. 85, 64–68 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A., et al. , Model for T-antigen-dependent melting of the simian virus 40 core origin based on studies of the interaction of the beta-hairpin with DNA. J. Virol. 81, 4808–4818 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison C. J., et al. , Asymmetric assembly of merkel cell polyomavirus large T-antigen origin binding domains at the viral origin. J. Mol. Biol. 409, 529–542 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abere B., et al. , Replication kinetics for a reporter merkel cell polyomavirus. Viruses 14, 473 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sgouralis I., Pressé S., Icon: An adaptation of infinite hmms for time traces with drift. Biophys. J. 112, 2117–2126 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaich M. A., et al. , Single-molecule analysis of DNA-binding proteins from nuclear extracts (SMADNE). Nucleic Acids Res. 51, e39 (2023), 10.1093/nar/gkad095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vauquelin G., Effects of target binding kinetics on in vivo drug efficacy: Koff, kon and rebinding. Br. J. Pharmacol. 173, 2319–2334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siebels S., et al. , Merkel cell polyomavirus DNA replication induces senescence in human dermal fibroblasts in a Kap1/Trim28-dependent manner. mBio 11, e00142–00120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benson F. E., Stasiak A., West S. C., Purification and characterization of the human Rad51 protein, an analogue of E. coli RecA. EMBO J. 13, 5764–5771 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Heijden T., et al. , Real-time assembly and disassembly of human RAD51 filaments on individual DNA molecules. Nucleic Acids Res. 35, 5646–5657 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wasserman M. R., Schauer G. D., O’Donnell M. E., Liu S., Replication fork activation is enabled by a single-stranded DNA gate in CMG helicase. Cell 178, 600–611.e616 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohr L., et al. , ER-directed TREX1 limits cGAS activation at micronuclei. Mol. Cell 81, 724–738.e729 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wold M. S., Weinberg D. H., Virshup D. M., Li J. J., Kelly T. J., Identification of cellular proteins required for simian virus 40 DNA replication. J. Biol. Chem. 264, 2801–2809 (1989). [PubMed] [Google Scholar]
- 38.Coverley D., et al. , Requirement for the replication protein SSB in human DNA excision repair. Nature 349, 538–541 (1991). [DOI] [PubMed] [Google Scholar]
- 39.Messina T. C., Kim H., Giurleo J. T., Talaga D. S., Hidden Markov model analysis of multichromophore photobleaching. J. Phys. Chem. B 110, 16366–16376 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng J., Rozenblatt-Rosen O., Paulson K. G., Nghiem P., DeCaprio J. A., Merkel cell polyomavirus large T antigen has growth-promoting and inhibitory activities. J. Virol. 87, 6118–6126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanson P. I., Whiteheart S. W., AAA+ proteins: Have engine, will work. Nat. Rev. Mol. Cell Biol. 6, 519–529 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Koonin E. V., A common set of conserved motifs in a vast variety of putative nucleic acid-dependent ATPases including MCM proteins involved in the initiation of eukaryotic DNA replication. Nucleic Acids Res. 21, 2541–2547 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wendzicki J. A., Moore P. S., Chang Y., Large T and small T antigens of merkel cell polyomavirus. Curr. Opin. Virol. 11, 38–43 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao N. Y., Zhang D., Yurieva O., O’Donnell M. E., CMG helicase can use ATPγS to unwind DNA: Implications for the rate-limiting step in the reaction mechanism. Proc. Natl. Acad. Sci. U.S.A. 119, e2119580119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onwubiko N. O., et al. , SV40 T antigen interactions with ssDNA and replication protein A: A regulatory role of T antigen monomers in lagging strand DNA replication. Nucleic Acids Res. 48, 3657–3677 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fanning E., Zhao K., SV40 DNA replication: From the A gene to a nanomachine. Virology 384, 352–359 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gai D., Wang D., Li S.-X., Chen X. S., The structure of SV40 large T hexameric helicase in complex with AT-rich origin DNA. ELife 5, e18129 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Ramírez Montero D., Nucleotide binding halts diffusion of the eukaryotic replicative helicase during activation. Nat. Commun. 14, 2082 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang Y. P., et al. , Mechanism of origin DNA recognition and assembly of an initiator-helicase complex by SV40 large tumor antigen. Cell Rep. 3, 1117–1127 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu X., Schuck S., Stenlund A., Adjacent residues in the E1 initiator β-hairpin define different roles of the β-hairpin in Ori melting, helicase loading, and helicase activity. Mol. Cell 25, 825–837 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Shuda M., et al. , Merkel cell polyomavirus small T antigen induces cancer and embryonic merkel cell proliferation in a transgenic mouse model. PLoS One 10, e0142329 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boichuk S., Hu L., Hein J., Gjoerup O. V., Multiple DNA damage signaling and repair pathways deregulated by simian virus 40 large T antigen. J. Virol. 84, 8007–8020 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J., et al. , Merkel cell polyomavirus large T antigen disrupts host genomic integrity and inhibits cellular proliferation. J. Virol. 87, 9173–9188 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meinke G., et al. , The crystal structure of the SV40 T-antigen origin binding domain in complex with DNA. PLoS Biol. 5, e23 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subramanyam S., Kinz-Thompson C. D., Gonzalez R. L. Jr., Spies M., Observation and analysis of RAD51 nucleation dynamics at single-monomer resolution. Methods Enzymol. 600, 201–232 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wan L., MultimerizationCode. Github. https://github.com/JamesLiWan/MultimerizationCode. Deposited 22 February 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
mN-LT (green) and Cy5-RAD51 (red) binding to pMC.Ori98 tethered to polystyrene beads (shown by the two auto-fluorescent spheres) in the C-Trap system. Recording speed = 2.0 s/frame. Total Recording Time = 200s.
Data Availability Statement
All study data are included in the article and/or supporting information. Software: Code used for the simulation of fluorophore levels has been deposited in GitHub at https://github.com/JamesLiWan/MultimerizationCode (56).