Abstract
Background
Tegumentary leishmaniasis is often subject to limited funding, underpowered studies, and a paucity of high-quality interventional studies. Intravenous liposomal amphotericin B (L-AmB) has been increasingly used to treat cutaneous and mucosal leishmaniasis (CL and ML, respectively) despite the lack of well-conducted interventional studies. We conducted a systematic review to consolidate the descriptive evidence on the efficacy and safety of L-AmB in treating CL and ML.
Methods
Several online databases and the reference lists of included studies were searched to extract data from 132 studies comprising both case reports and case series. The population, intervention, comparison, outcome, and study design strategy and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines were used.
Results
Of 132 studies included, 92 were case reports and 40 were case series. Of the 92 cases, 65 (82.3%) were considered cured after receiving L-AmB as part of their treatment regimen. Twenty-one of the 92 (22.8%) cases reported adverse reactions to L-AmB. A pooled cure rate of 87.0% (95% CI, 79.0%–92.0%) was reported for the 38 case series that reported on treatment efficacy; 40.7% of the cases were associated with an adverse reaction.
Conclusions
Observational data on cure rates using L-AmB suggest efficacy between 80% and 90%, similar to rates reported for other antileishmanial drugs. The highest efficacy rates were observed when a single cycle of L-AmB was administered to patients with mild–moderate CL and ML. The limitations of this study include the heterogeneity observed among the included studies and the increased likelihood of publication bias associated with the inclusion of case reports and case series. This systematic review further illustrates the need for high-quality comparative trials of intravenous L-AmB for the treatment of tegumentary leishmaniasis.
Keywords: Leishmania spp, migrants, tegumentary leishmaniasis, travel medicine, travelers
Leishmaniasis is a neglected tropical disease caused by Leishmania parasites transmitted by the bite of infected female sandflies. More than 20 different species of Leishmania are involved in human leishmaniasis, with the geographic distribution of infection being divided between the Old World (Mediterranean basin, the Middle East, the Horn of Africa, and South and Central Asia) and the New World (Americas). There are 3 major presentations of leishmaniasis: cutaneous leishmaniasis (CL), mucosal leishmaniasis (ML), and visceral leishmaniasis (VL) [1]. An estimated 0.7 to 1.2 million new cases of CL occur annually worldwide, facilitated by factors such as climate change, increased migration, and political instability [2]. According to the World Health Organization (WHO), most cases of CL occur in the Americas, the Middle East, the Mediterranean basin, and Central Asia. Cases of mucocutaneous leishmaniasis mostly occur in South America (>90% of cases) [3].
Clinical manifestations of CL and ML are highly variable, depending on factors such as the Leishmania species, the strains and virulence dynamics of the infection-causing parasites, and host characteristics, such as age, gender, socioeconomic conditions, and immune status (especially in relation to HIV status) [2, 4]. Diagnosis of CL and ML can be achieved using several laboratory tests, which are not universally standardized. Identifying the species of Leishmania is recommended by the Infectious Diseases Society of America (IDSA) to improve case management [1].
Treatment of CL and ML remains challenging given that there is no ideal or universally applicable therapeutic approach. A lack of randomized controlled studies and sparse comparative literature complicate treatment decisions. Recommendations on optimal treatment depend on factors such as parasitic species, clinical form of the disease, region of acquisition, and immunologic status of the patient, but are based on limited evidence [5].
Systemic use of antimonial compounds has served as principal antileishmanial treatment for both Old and New World parasites for decades and has been considered the gold standard against which all other treatments are assessed [1, 6]. Limitations of antimonial therapy include major adverse reactions such as cardiotoxicity, hepatotoxicity, nephrotoxicity, and pancreatitis. Limited accessibility is a barrier in locations such as Canada and the United States.
Liposomal amphotericin B (L-AmB) is the treatment of choice for VL in many regions. While L-AmB had not initially been indicated as the standard treatment for tegumentary leishmaniasis, it has proven efficacy over time in the treatment of VL [7, 8]. Since then, L-AmB has increasingly been used with variable regimens to treat both CL and ML based on the extrapolation that the treatment is effective for VL in addition to other observational data. Most authors in the literature use L-AmB based on the VL regimen (total cumulative dose of 21 mg/kg) with heterogeneous results [9]. However, caution should be taken when extrapolating L-AmB efficacy from VL studies; skin penetration of L-AmB is not well studied, and species-related differences in response to L-AmB are important considerations.
Through this systematic review, we aim to consolidate the evidence on the efficacy and safety of intravenous (IV) L-AmB treatment for CL and ML acquired by travelers, migrants, and residents of Leishmania-endemic regions among the New and Old Worlds.
METHODS
This systematic review has been registered in the PROSPERO database (registration number CRD42020173440) and was prepared in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. The systematic review protocol was published in BMJ Open [11].
Search Strategy
The search strategy was developed with the assistance of a medical librarian and consisted of text words and relevant indexing to identify studies treating CL and ML with IV L-AmB. The MEDLINE search strategy was applied to all databases with appropriate modifications [11].
Two independent reviewers (J.C. and K.N.) searched a variety of online databases: MEDLINE (via Ovid & via PubMed), The Cochrane CENTRAL Register of Controlled Trials & Cochrane Database of Systematic Reviews (via Wiley), Embase (via Ovid), Africa-Wide Information (via EBSCO), Global Health (via Ovid), Global Index Medicus (via WHO), and Scopus (via Elsevier). Additionally, 2 clinical trial registries were searched (clinicaltrials.gov, International Clinical Trials Registry Platform) along with citation searches of the reference lists of the included studies using Web of Science and Scopus. No restrictions on date and language of publication were applied in an effort to optimize the evidence to be captured. An initial search was conducted in April 2020, followed by an updated rerun of the search in June 2021. Some studies were added through references cited in screened publications.
Study Selection, Inclusion, and Exclusion Criteria
We used the Population, Intervention, Comparison, Outcome and Study Design (PICOS) strategy to formulate our research question, the inclusion and exclusion criteria, and to guide the overall review process [11].
The study populations included children and adults who are migrants, travelers, and residents of CL/ML-endemic areas. Studies that assessed patients with VL and post-kala-azar dermal leishmaniasis were excluded. We included studies that used IV L-AmB treatment (as monotherapy or as a drug combination) for CL and/or ML, while those focusing solely on any other formulations (intralesional, topical amphotericin B, or other forms of systemic treatment including other lipid formulations of amphotericin B) were excluded. Diagnosis of CL/ML had to be confirmed through visualization of amastigotes on a smear or on histopathology of a lesion biopsy, a positive culture, or nucleic acid amplification testing such as polymerase chain reaction. Studies that used only serology tests for diagnosis were excluded. Studies that failed to report the efficacy or response rate of IV L-AmB treatment for CL/ML and those that did not follow study participants for a minimum of 4 weeks were excluded from review. Additionally, authors of studies with missing information related to our study objectives (such as therapeutic regimen details, follow-up times, and adverse reactions) were contacted individually via email when possible.
Outcomes of Interest
The primary outcome of interest analyzed in this review was the efficacy (cure rate) of IV L-AmB in the healing of cutaneous and/or mucosal lesions. Cure outcomes were defined in multiple ways: (1) as reported by the respective study authors and (2) as defined by the World Health Organization: “initial response” lesion resolution 6–9 weeks after final treatment, “initial cure” lesion resolution after 90 days of follow-up, “definitive cure” lesion resolution after 180–360 days of follow-up. Cure rates have been categorized based on the sequence of L-AmB treatment, such as: used alone as monotherapy, used after a previous treatment, used before a second treatment (including >1 cycle of L-AmB), or concomitant use with another therapy.
A secondary outcome of interest assessed in this review was the toxicity profile of L-AmB. Adverse reactions were categorized in 1 of 8 categories for the purposes of this review: (1) renal insufficiency/toxicity, (2) electrolyte disorders, (3) infusion reactions, (4) rash/cutaneous reaction, (5) gastrointestinal symptoms, (6) cardiac toxicity, (7) anaphylaxis, and (8) other.
Screening and Data Extraction
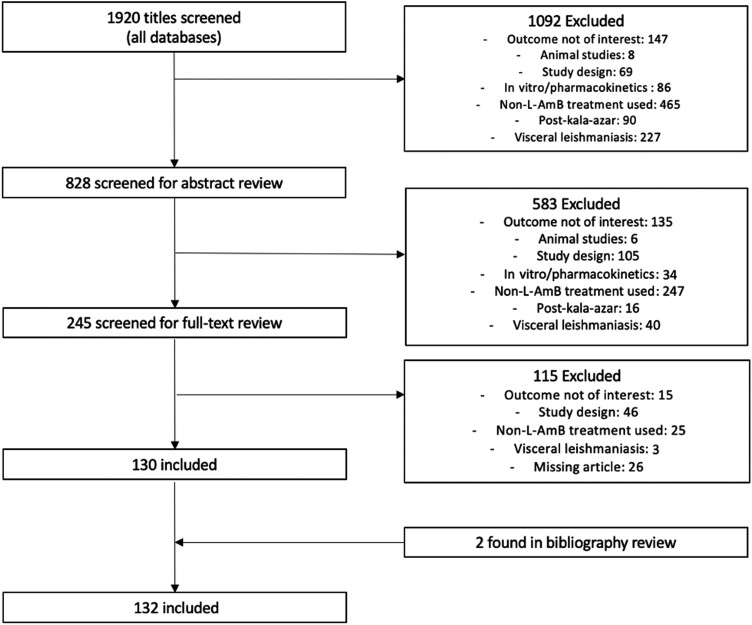
Titles and abstracts of studies retrieved using the predefined search strategies and those from additional sources were screened independently by the 2 review authors (J.C. and K.N.) to identify studies that met the inclusion criteria. Any discrepancies were resolved by a senior reviewer (S.B.). Figure 1 illustrates the flow diagram as per the PRISMA guidelines [10].
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram.
A standardized data extraction table was developed to organize the extracted data from included studies. Before full extraction, 3 reviewers (J.C., K.N., and F.N.) performed a pilot data extraction using a small random sample of the included studies. One reviewer (S.B.) assessed the pilot data extraction for quality control and concordance purposes. Cohen's kappa (κ) statistic was calculated as 0.9 and suggested high inter-reviewer concordance.
Quality Assessment
A quality assessment tool for case series studies developed by the National Heart, Lung, and Blood Institute (NIH) was modified using items from the Care Report (CARE) guidelines in order to evaluate the quality of both case reports and case series [12, 13].
RESULTS
As illustrated in Figure 1, we reviewed a total of 1920 citation titles. After deduplication and a screening of study titles, we reviewed a total of 828 abstracts. Of 828 abstracts, 583 citations did not meet our eligibility criteria, such that 245 full-text articles were reviewed. Of 245 articles, 115 were excluded, while 2 additional studies were found from bibliography review, leading to a total of 132 articles (92 case reports and 40 case series) in our final set.
Description of Included Case Reports
Of the 92 case reports included in our review, 79 (85.9%) reported on the treatment efficacy of L-AmB, while 33 (35.9%) reported on the adverse reactions. Our review includes cases that reside in endemic areas (defined by “locals” in Table 1) and those who migrated from and traveled to endemic zones. Immunocompromised patients represented 34.8% of the case reports; however, several studies included in our review did not report on the immune status of their patients (20.7% of case reports). The majority of cases presented with CL (56.5%), while the remainder of cases were distributed among other clinical forms. The most common complexes identified were L. donovani (25.0%) and L. braziliensis (10.9%).
Table 1.
Demographic and Clinical Characteristics of L-AmB-Treated Patients in Case Reports
| Patient Demographics | No. (%) | |
|---|---|---|
| General characteristics | Total No. of patients included | 92 |
| Median age (IQR), y | 50 (32.5–61) | |
| Age group | … | |
| <18 y | 8 (8.70) | |
| 18–36 y | 18 (19.57) | |
| 36–50 y | 23 (25.00) | |
| 51–65 y | 26 (28.26) | |
| >65 y | 17 (18.48) | |
| Male | 71 (77.17) | |
| Type of population | … | |
| Locals (endemic zone) | 46 (50.00) | |
| Migrants | 11 (11.96) | |
| Travelers | 35 (38.04) | |
| Region of acquisition | … | |
| Americas | 35 (38.04) | |
| Europe | 29 (31.52) | |
| North Africa | 3 (3.26) | |
| Sub-Saharan Africa | 9 (9.78) | |
| Middle East/South Asia | 16 (17.39) | |
| Clinical form | Cutaneous leishmaniasis | 52 (56.52) |
| Mucosal leishmaniasis | 15 (16.30) | |
| Mucocutaneous leishmaniasis | 17 (18.48) | |
| Disseminated/diffuse leishmaniasis | 7 (7.61) | |
| Leishmaniasis recidivans | 1 (1.09) | |
| Old World/New World leishmaniasis | Old World | 57 (61.96) |
| New World | 35 (38.04) | |
| Leishmania complex | L. major | 5 (5.43) |
| L. tropica | 5 (5.43) | |
| L. donovani | 23 (25) | |
| L. mexicana | 4 (4.35) | |
| L. braziliensis | 10 (10.87) | |
| L. guyanensis | 7 (7.61) | |
| Mixed species | 2 (2.17) | |
| Not available | 36 (39.13) | |
Abbreviation: IQR, interquartile range.
As outlined in Table 2, cure rates are tabulated as per the definition of the study authors and as per the definitions of the WHO. We further categorized the cases based on their clinical form of leishmaniasis including CL, ML, mucocutaneous leishmaniasis (MCL), diffuse/disseminated leishmaniasis (DL), and leishmaniasis recidivans (LR). Of the 79 cases out of 92 that reported on L-AmB treatment efficacy, 65 (82.3%) were considered cured as per the respective study authors and had reported a minimum follow-up period of 4 weeks after the last dose of L-AmB. Additionally, we categorized these 65 cases as per the WHO's definition of cure (as defined above); the majority of cases, 39 (59.1%), resulted in definitive cure following treatment with L-AmB. Treatment with L-AmB was not consistent among the cases included in our review. Table 2 outlines the 4 possible ways L-AmB was administered to the cases. Among the 42 cases that were treated with a single cycle of L-AmB, 35 (83.3%) had reported on the efficacy of L-AmB treatment. Of the 35 cases, 32 (91.4%) cases were considered cured after a minimum of 4 weeks of follow-up time as per the respective study authors. Of these 32, as per the WHO definition, 20 (62.5%) demonstrated definitive cure.
Table 2.
Treatment Efficacy (Cure Rate) Among Case Reports
| L-AmB Treatment Regimen |
No. of Cases (%) | No. of Immuno-compromised Patientsa (% Row) | Clinical Forms of Leishmaniasis (% row) |
No. of Cases for Which Primary Outcome Was Reported (%, Col) | No. of Curesb (%; 95% CI) n = 79 |
No. of Cures Using WHO Definitionc (% Row) n = 79 |
Follow-up Range, mo |
|---|---|---|---|---|---|---|---|
| L-AmB only (1 cycle) | 42 (45.65) | 13 (30.95) | CL = 27 (64.29) ML = 9 (21.43) MCL = 5 (11.90) DL = 1 (2.38) |
35 (44.30) | 32 (91.43; 76.94–98.20) | IR = 8 (25.00) IC = 3 (9.38) DC = 20 (62.50) |
1–60 |
| L-AmB with other concomitant treatment | 3 (3.26) | 2 (66.67) | CL = 0 (0) ML = 2 (66.67) MCL = 1 (33.33) DL = 0 (0) |
2 (2.53) | 2 (100; 15.81–100) | IR = 1 (50.00) IC = 1 (50.00) DC = 0 |
2–3 |
| Other treatment followed by L-AmB | 22 (23.91) | 4 (18.18) | CL = 13 (59.09) ML = 1 (4.55) MCL = 4 (18.18) DL = 3 (13.64 LR = 1 (4.55) |
19 (24.05) | 14 (73.68; 48.80–90.85) | IR = 1 (7.14) IC = 6 (42.86) DC = 7 (50.00) |
2–12 |
| L-AmB followed by another treatment (or >1 cycle of L-AmB) | 25 (27.17) | 13 (52.00) | CL = 12 (48.00) ML = 3 (12.00) MCL = 7 (28.00) DL = 3 (12.00) |
23 (29.11) | 17 (73.91; 51.59–89.77) | IR = 3 (16.67) IC = 3 (16.67) DC = 12 (66.67) |
1–36 |
| Overall results | 92 (100) | 32 (100) | CL = 52 (56.52) ML = 15 (16.30) MCL = 17 (18.48) DL = 7 (7.61) LR = 1 (1.09) |
79 (100) | 65 (82.28; 72.06–89.96) | IR = 13 (19.70) IC = 13 (19.70) DC = 39 (59.09) |
1–60 |
Abbreviations: CL, cutaneous leishmaniasis; DL, disseminated/diffuse leishmaniasis; LR, leishmaniasis recidivans; MCL, mucocutaneous leishmaniasis; ML, mucosal leishmaniasis; WHO, World Health Organization.
Immunosuppression status was available only for 73 of 92 (79.35%) patients.
Cure according to the respective study authors, with a minimal follow-up of 4 weeks. Including 79 studies that reported on this outcome.
WHO defines cure as follows: initial response, 28–83 days (IR); initial cure, 84–179 days (IC); definitive cure, ≥180 days (DC).
Adverse reactions due to L-AmB treatment among case reports are presented in Table 3. Of the 92 cases reports in our review, only 33 (35.9%) cases reported on the presence or absence of adverse reactions caused by L-AmB and were included for this outcome. Of the 33 cases, 21 (63.6%) experienced adverse reactions as a result of L-AmB treatment, such that 6 (28.6%) patients had their treatment modified and 8 (38.1%) had their treatment discontinued. A variety of adverse reactions were identified in our review, with the majority having renal toxicity (45.8%).
Table 3.
Adverse Reactions Among Case Reports
| Adverse Reactions | No. (%) | |
|---|---|---|
| Patients with adverse reactions | No. of patients who reported adverse reaction (%; 95% CI) | 21 (63.64; 45.12–79.60) |
| Impact on the treatment | Treatment unchanged | 7 (33.33) |
| Treatment modified (dose/length/etc.) | 6 (28.57) | |
| Treatment discontinued | 8 (38.10) | |
| Patients included (with or without adverse reactions) | Total No. of patients included | 33 (35.87) |
| Adverse reaction categories | Renal insufficiency/toxicity | 11 (45.83) |
| Electrolytes disorders | 1 (4.17) | |
| Infusion reaction | 3 (12.50) | |
| Rash/cutaneous reaction | 1 (4.17) | |
| GI symptoms | 1 (4.17) | |
| Cardiac toxicity | 2 (8.33) | |
| Anaphylaxis | 2 (8.33) | |
| Other | 3 (12.50) | |
| Total No. of events | Total No. of adverse reactions reported | 24 (100) |
Abbreviation: GI, gastrointestinal.
Description of Included Case Series
Among the 40 case series included in our review, 846 separate cases were identified, out of which 390 (46.1%) cases received L-AmB (Table 4). The majority, 205 (52.6%) of these cases, arose from leishmaniasis-endemic areas. The regions of acquisition were mostly the Americas (n = 271, 69.5%). Of the 390 cases, 30 (7.7%) cases were found to be immunocompromised. The most common clinical forms identified were CL (n = 248, 63.6%), followed by ML (n = 107, 7.4%).
Table 4.
Demographic and Clinical Characteristics of L-AmB-Treated Patients in Case Series
| Patient Demographics | No. (%) |
|---|---|
| Total No. of patients | 846 |
| No. of patients who received L-AmB | 390 |
| Weighted median age, y | 40.41 |
| Patients age <18 ya | 56 (16.77) |
| Male patienta | 282 (77.05) |
| Type of populationa | |
| Local (endemic area) | 205 (52.56) |
| Migrants | 19 (5.12) |
| Travelers | 147 (39.62) |
| Region of acquisition | |
| Americas | 271 (69.49) |
| Europe | 18 (4.62) |
| North Africa | 7 (1.79) |
| Sub-Saharan Africa | 0 (0) |
| Middle East/Asia | 60 (15.38) |
| Not available | 28 (7.18) |
| No. of immunocompromiseda | 30 (10.03) |
| Type of immunosuppression, No. (% of IC) | |
| HIV/AIDS | 11 (36.67) |
| RAID | 8 (26.67) |
| IBD | 4 (13.33) |
| AI | 3 (10) |
| OID | 2 (6.67) |
| SOT | 1 (3.33) |
| PULM | 1 (3.33) |
| Old World vs New World | |
| Old World | 113 (28.97) |
| New World | 271 (69.49) |
| Not available | 6 (1.54) |
| Clinical form | |
| CL | 248 (63.59) |
| ML | 107 (27.44) |
| MCL | 10 (2.56) |
| DL | 24 (6.15) |
| LR | 1 (0.26) |
| Leishmania complexes | |
| L. major | 13 (3.33) |
| L. tropica | 52 (13.33) |
| L. donovani | 29 (7.44) |
| L. mexicana | 3 (0.77) |
| L. braziliensis | 118 (30.26) |
| L. guyanensis | 38 (9.74) |
| Not available | 137 (35.13) |
Abbreviations: AI, autoimmune disease not otherwise specified; CL, cutaneous leishmaniasis; DL, disseminated/diffuse leishmaniasis; IBD, intestinal bowel disease; LR, leishmaniasis recidivans; MCL, mucocutaneous leishmaniasis; ML, mucosal leishmaniasis; OID, other immune disorders; PULM, pulmonary disease (asthma, chronic obstructive pulmonary disease); RAID, rheumatoid autoimmune disease; SOT, solid organ transplant.
The denominator used is the number of patients for which this variable was reported.
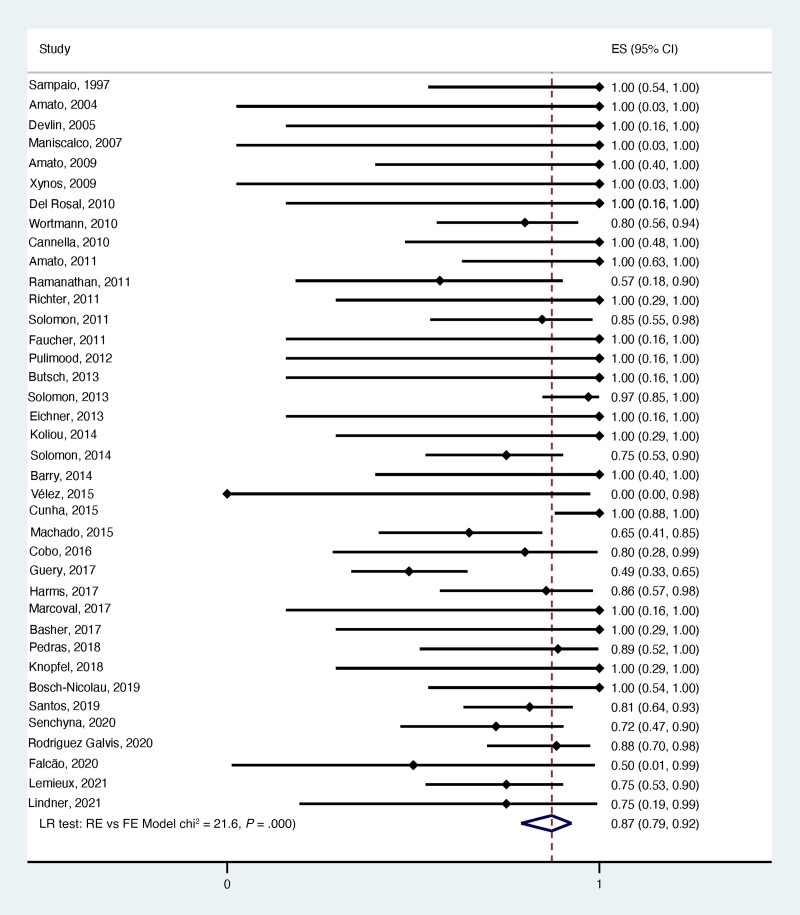
Table 5 summarizes the efficacy of L-AmB in treating leishmaniasis based on its placement in the overall treatment regimen as per the authors’ definition of cure. A cure rate of 95.0% was observed when cases were treated with a single cycle of L-AmB. Cure rates of 90%, 75%, and 69% were observed when L-AMB was used after, during, or before another treatment, respectively. Overall, 89.0% of the cases assessed among the 38 case series that reported on treatment efficacy were considered cured after receiving L-AmB as part of their treatment regimen (Figure 2). Supplementary Figures 1–4 illustrate the forest plots of the cure rates based on L-AmB treatments.
Table 5.
Treatment Efficacy (Cure Rate) Among Case Series
| L-AmB Treatment Regimen |
No. of Case Studies (%) | No. of Casesa | Cure Rate (95% CI), % |
|---|---|---|---|
| L-AmB only (1 cycle) | 22 (57.89) | 148 | 95.00 (82.00–99.00) |
| L-AmB with other concomitant treatment | 2 (5.26) | 4 | 75.00 (24.00–97.00) |
| Other treatment followed by L-AmB | 15 (39.47) | 112 | 90.00 (78.00–96.00) |
| L-AmB followed by another treatment (or >1 cycle of L-AmB) | 8 (21.05) | 18 | 69.00 (35.00–90.00) |
| Overall results | 38 (100) | 387 | 87.00 (79.00–92.00) |
Certain case series only reported the overall efficacy as opposed to the efficacy for each type of L-AmB treatment regimen. For this reason, the total number of cases do not add up to 387. Of note, no outcomes were reported for 3 out of 390 patients who received L-AmB.
Figure 2.
Forest plot of treatment efficacy (cure rate) among all case series. Abbreviations: FE, fixed-effects; LR, likelihood-ratio; RE, random-effects.
Adverse reactions because of L-AmB treatment throughout the case series were categorized in the same way as case reports (Table 6). Of the 327 cases that reported on the presence or absence of adverse reactions, 133 (40.7%) had at least 1 adverse reaction. Of these 133 cases, 112 (84.2%) cases did not change treatment despite the adverse reaction, while 10 (7.5%) had their treatment modified and 11 (8.3%) had their treatment discontinued. A variety of adverse reactions were identified among the case series included in our review, with the majority (43.9%) of cases suffering from renal toxicity.
Table 6.
Adverse Reactions Among Case Series
| Adverse Reactions | No. (%) | |
|---|---|---|
| Patients with adverse reactions | No. of patients who reported adverse reaction | 133 (40.67) |
| Impact on the treatment | Treatment unchanged | 112 (84.21) |
| Treatment modified (dose/length/etc.) | 10 (7.52) | |
| Treatment discontinued | 11 (8.27) | |
| Patients included (with or without adverse reactions) | Total No. of patients included | 327 (100) |
| Adverse reaction categories | Renal insufficiency/toxicity | 79 (43.89) |
| Electrolytes disorders | 12 (6.67) | |
| Infusion reaction | 50 (27.78) | |
| Rash/cutaneous reaction | 1 (0.56) | |
| GI symptoms | 18 (10.00) | |
| Cardiac toxicity | 2 (1.11) | |
| Anaphylaxis | 2 (1.11) | |
| Other | 31 (17.22) | |
| Total No. of events | Total No. of adverse reactions reported | 180 (100) |
Quality Assessment
The quality assessment tool by the NIH and the CARE guidelines were used to evaluate the quality of the case series and case reports, respectively. In general, the majority of case reports (n = 42, 51.1%) and case series (n = 25, 62.5%) had an adequate amount of follow-up to determine definitive cure. Supplementary Figures 5A and 5B provide a breakdown of the studies according to their respective assessment tools.
DISCUSSION
In our review, we identified 92 case reports and 390 patients from case series who received IV L-AmB as part of their treatment for CL/ML. Migrants and travelers together represented almost half of cases identified, highlighting the need for worldwide awareness of this disease in primary care settings. The majority of cases included in our review presented with cutaneous leishmaniasis. Our findings also suggest that mucocutaneous leishmaniasis mostly occurs in the Americas [3].
An overall treatment efficacy of 82.3% was determined for the 79 cases reports that reported on this primary outcome. This relatively high cure rate among the cases assessed in case reports is consistent with our own analysis of 38 case series demonstrating an overall cure rate of 87.0% (95% CI, 79%–92%). Among the case series included in our review, 390 patients received IV L-AmB as part of their regimen, with the majority of cases being from the New World (69.5%). Our findings seem to show that for New World tegumentary leishmaniasis, the overall efficacy of L-AmB exceeds 87%, which is comparable to other drugs. For example, pentavalent antimonials, which are historically known to be the gold standard of treatment, have an overall cure rate of 76.5% in New World CL [14]. Treatment with miltefosine for New World CL showed a cure rate ranging from 50% to 90% depending on the species and 70% independent of species [15–17]. A recent systematic review regarding pentamidine for tegumentary leishmaniasis reported a cure rate of 79.0%–92.0% [18]. Patients receiving 1 cycle of L-AmB treatment had high cure rates of 91.4% (95% CI, 76.9%–98.2%) among the 35 case reports and 95.0% (95% CI, 82.0%–99.0%) among the 22 case series. This group represents the majority (44.3% of case reports and 57.9% of case series) of the cases included in our analysis. However, these cases may have been milder or simpler than the ones that used different and multiple treatments.
It is noteworthy that most studies did not provide explicit definitions of cure, making the interpretation of results more difficult. In contrast to the WHO definition of cure mentioned previously, many studies did not specify that patients who were cured had also complete re-epithelialization of their lesions. In addition, long-term follow-up was missing in many studies. We decided to include studies with minimal follow-up of 1 month even though definitive cure or treatment failure of CL and ML cannot be determined before 180 days following treatment.
Case reports may form a seriously biased sample. Authors may have chosen to report cases that were more or less complex than average. There may be a publication bias in favor of or against successful results. Cases treated with a single course of L-AmB are likely less severe than those with more complex regimens. In our analysis of case reports, those treated with L-AmB followed by another treatment (19 cases) or another treatment followed by L-AmB (23 cases) had a relatively low combined cure rate of 73%.
Regarding adverse reactions, renal toxicity (45.9% in case reports and 43.9% in case series), mostly indicated by mild elevation of serum creatinine, was the most common adverse effect reported. In case reports, adverse effects were considered mild and temporary in most of the cases, with less than half (38.1%) having to definitively stop treatment. The occurrence of adverse effects is similar to or somewhat lower than those reported in cohorts receiving antimonial compounds, miltefosine, or pentamidine [18–21].
A major limitation of our systematic review is the lack of RCTs, comparative cohort studies, or even well-defined observational cohort studies published on IV L-AmB efficacy for tegumentary leishmaniasis [22]. This makes it impossible to compare cure rates between different drug regimens. During our search, we encountered 1 open-label trial including a total of 35 patients comparing L-AmB (1.5 mg/kg/d for 5 days) with pentavalent antimony (20 mgSbV/kg/d of N-methyl glucamine for 20 days), which showed a 50% clinical cure rate for L-AmB [23].
The articles included in this analysis exhibit heterogeneity regarding the population characteristics, clinical presentations, Leishmania species, L-AmB treatment regimen, and duration of follow-up. Included studies used different regimens, with a cumulative dose range between 6 mg/kg and 56 mg/kg and a duration range from 5 days to a few months. Some included studies did not specify how long patients were treated with L-AmB before starting another treatment. Several studies used the IDSA guidelines, which recommend a cumulative dose of 18–21 mg/kg. Furthermore, in most case series, it was not possible to know if the patients who responded well to the treatment were being treated for CL or ML. Therefore, our review does not allow us to compare treatment responses for CL vs ML. Lastly, the inclusion of case reports and case series introduces a high risk of publication bias.
CONCLUSIONS
With travel and migration from endemic zones fueling the increasing global burden of leishmaniasis, L-AmB offers potential for optimal case management given its presumed good treatment efficacy and moderate risk of adverse reactions. Our review consolidates data from several hundred cases of CL and ML worldwide and gives a rough portrait of the efficacy and safety profile of L-AmB. In particular, efficacy appeared to be high in cases reporting a single cycle of L-AmB administered to patients with mild to moderate CL and ML. This systematic review further illustrates the need for high-quality trials on IV L-Amb for the treatment of tegumentary leishmaniasis.
Supplementary Material
Acknowledgments
Financial support. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Patient consent. This study does not include factors necessitating patient consent.
Contributor Information
Jeffrey Chivinski, Division of Dermatology, Department of Medicine, Centre Hospitalier de l’Université de Montréal, Montreal, Quebec, Canada.
Keren Nathan, Division of Infectious Diseases, Department of Pediatrics, McGill University Health Centre, Montreal, Quebec, Canada.
Faheel Naeem, J.D. MacLean Centre for Tropical Diseases at McGill University, Montreal, Quebec, Canada; Research Institute of the McGill University Health Centre, Montreal, Quebec, Canada.
Taline Ekmekjian, Medical Libraries, McGill University Health Centre, Montreal, Quebec, Canada.
Michael D Libman, J.D. MacLean Centre for Tropical Diseases at McGill University, Montreal, Quebec, Canada; Research Institute of the McGill University Health Centre, Montreal, Quebec, Canada; Division of Infectious Diseases, Department of Medicine, McGill University Health Centre, Montreal, Quebec, Canada.
Sapha Barkati, J.D. MacLean Centre for Tropical Diseases at McGill University, Montreal, Quebec, Canada; Research Institute of the McGill University Health Centre, Montreal, Quebec, Canada; Division of Infectious Diseases, Department of Medicine, McGill University Health Centre, Montreal, Quebec, Canada.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis 2016; 63:1539–57. [DOI] [PubMed] [Google Scholar]
- 2. Barkati S, Ndao M, Libman M. Cutaneous leishmaniasis in the 21st century: from the laboratory to the bedside. Curr Opin Infect Dis 2019; 32:419–25. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO). Leishmaniasis. Available at: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis#:∼:text=Cutaneous%20leishmaniasis%20(CL)%20is%20the, Middle%20East%20and%20Central%20Asia. Accessed February 9,2022.
- 4. Aronson NE, Joya CA. Cutaneous leishmaniasis: updates in diagnosis and management. Infect Dis Clin North Am 2019; 33:101–17. [DOI] [PubMed] [Google Scholar]
- 5. Blum J, Buffet P, Visser L, et al. Leishman recommendations for treatment of cutaneous and mucosal leishmaniasis in travelers, 2014. J Travel Med 2014; 21:116–29. [DOI] [PubMed] [Google Scholar]
- 6. Palumbo E. Treatment strategies for mucocutaneous leishmaniasis. J Glob Infect Dis 2010; 2:147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berman JDU. US Food and Drug Administration approval of AmBisome (liposomal amphotericin B) for treatment of visceral leishmaniasis. Clin Infect Dis 1999; 28:49–51. [DOI] [PubMed] [Google Scholar]
- 8. Davidson RN, Di Martino L, Gradoni L, et al. Liposomal amphotericin B (AmBisome) in Mediterranean visceral leishmaniasis: a multi-centre trial. Q J Med 1994; 87:75–81. [PubMed] [Google Scholar]
- 9. Sundar S, Chakravarty J. Liposomal amphotericin B and leishmaniasis: dose and response. J Glob Infect Dis 2010; 2:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moher D, Shamseer L, Clarke M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naeem F, Nathan K, Chivinski J, Ekmekjian T, Libman M, Barkati S. Intravenous liposomal amphotericin B efficacy and safety for cutaneous and mucosal leishmaniasis: a systematic review and meta-analysis protocol. BMJ Open 2021; 11:e045707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Heart, lung and blood institute (NIH) . Study quality assessment tools. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed February 9,2022.
- 13. Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med 2013; 2:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tuon FF, Amato VS, Graf ME, Siqueira AM, Nicodemo AC, Amato Neto V. Treatment of new world cutaneous leishmaniasis—a systematic review with a meta-analysis. Int J Dermatol 2008; 47:109–24. [DOI] [PubMed] [Google Scholar]
- 15. Soto J, Arana BA, Toledo J, et al. Miltefosine for new world cutaneous leishmaniasis. Clin Infect Dis 2004; 38:1266–72. [DOI] [PubMed] [Google Scholar]
- 16. Chrusciak-Talhari A, Dietze R, Chrusciak Talhari C, et al. Randomized controlled clinical trial to access efficacy and safety of miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania (Viannia) guyanensis in Manaus, Brazil. Am J Trop Med Hyg 2011; 84:255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vélez I, López L, Sánchez X, Mestra L, Rojas C, Rodríguez E. Efficacy of miltefosine for the treatment of American cutaneous leishmaniasis. Am J Trop Med Hyg 2010; 83:351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piccica M, Lagi F, Bartoloni A, Zammarchi L. Efficacy and safety of pentamidine isethionate for tegumentary and visceral human leishmaniasis: a systematic review. J Travel Med 2021; 28:taab065. [DOI] [PubMed] [Google Scholar]
- 19. Wise ES, Armstrong MS, Watson J, Lockwood DN. Monitoring toxicity associated with parenteral sodium stibogluconate in the day-case management of returned travellers with New World cutaneous leishmaniasis [corrected]. PLoS Negl Trop Dis 2012; 6:e1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ware JM, O'Connell EM, Brown T, et al. Efficacy and tolerability of miltefosine in the treatment of cutaneous leishmaniasis. Clin Infect Dis 2021; 73:e2457–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Machado PR, Ampuero J, Guimarães LH, et al. Miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis in Brazil: a randomized and controlled trial. PLoS Negl Trop Dis 2010; 4:e912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pinart M, Rueda JR, Romero GA, et al. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst Rev 2020; 8:CD004834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Motta JO, Sampaio RN. A pilot study comparing low-dose liposomal amphotericin B with N-methyl glucamine for the treatment of American cutaneous leishmaniasis. J Eur Acad Dermatol Venereol 2012; 26:331–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.