Abstract
Background
Five hundred milligrams of intravenous (IV) sotrovimab has been shown to be well tolerated and efficacious against pre-Omicron strains in treating patients with mild to moderate coronavirus disease 2019 (COVID-19) at high risk for disease progression.
Methods
This was an open-label, single-arm substudy of phase 3 COMET-TAIL (NCT04913675) assessing the safety and tolerability of a 2000 mg IV dose of sotrovimab. Symptomatic patients (aged ≥18 years) with COVID-19 at high risk for progression were enrolled from June 30 through July 11, 2022, when Omicron BA.5, BA.2.12.1, and BA.4 were the predominant circulating variants in the United States. The primary end point was the occurrence of adverse events (AEs), serious AEs (SAEs), AEs of special interest, and COVID-19 disease-related events (DREs) through day 8. Safety, pharmacokinetics, viral load, and hospitalization >24 hours for acute management of illness or death through day 29 were assessed.
Results
All participants (n = 81) were Hispanic, 58% were female, and 51% were aged ≥55 years. Through day 8, no AEs, including infusion-related reactions or hypersensitivity, were reported; 2 participants reported DREs (mild cough, n = 2). One SAE (acute myocardial infarction), which was considered unrelated to sotrovimab or COVID-19 by the investigator, occurred on day 27 and was the only hospitalization reported. Maximum serum concentration (geometric mean) was 745.9 µg/mL. Viral load decreased from baseline through day 29; only 2 (3%) participants had a persistently high viral load (≥4.1 log10 copies/mL) at day 8.
Conclusions
Two thousand milligrams of IV sotrovimab was well tolerated, with no safety signals observed.
Trial registration
ClinicalTrials.gov Identifier: NCT04913675.
Keywords: COVID-19, SARS-CoV-2, monoclonal antibody, Omicron, sotrovimab
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), remains a serious threat to public health, with continued strain on hospitals and deaths due to the disease [1, 2]. Older persons and those individuals with underlying health conditions (particularly immunocompromised patients) are at heightened risk of severe illness and death [3–6]. While oral agents are effective [7, 8] and may be convenient for administration, nirmatrelvir/ritonavir, the first-line oral agent, is contraindicated for many patients due to the risk of drug–drug interactions. Monoclonal antibodies represent a valuable early treatment option [9] for key patient groups, including those for whom oral agents are contraindicated and other antivirals (such as remdesivir) may not be feasible or appropriate. The COMET-ICE study showed the efficacy of sotrovimab, a monoclonal antibody, in the early treatment of COVID-19 [10]. This study was conducted from August 2020 through March 2021 in patients predominantly infected with wild-type Wuhan-Hu-1 virus [11]. In 1057 nonhospitalized participants with mild to moderate COVID-19, treatment with 500 mg of intravenous (IV) sotrovimab was associated with a 79% reduction in the risk of hospitalization >24 hours for acute management of any illness or death due to any cause through day 29 when compared with placebo. A second study, COMET-TAIL, conducted from June to August 2021, compared 500 mg IV sotrovimab with intramuscular (IM) administration of 250 mg or 500 mg sotrovimab in adults and adolescents with mild to moderate COVID-19 at high risk for progression to severe COVID-19 [12]. That enrollment period coincided with a surge of the Delta variant of SARS-CoV-2, and this was the predominant variant detected in COMET-TAIL participants [13]. Results showed that 500 mg IM administration was noninferior to 500 mg IV administration. In both large trials, sotrovimab demonstrated a favorable safety profile, with no unanticipated new safety signals detected, when given by the IV or IM route.
Notably, while the use of 500 mg IV sotrovimab was interrupted in the United States when Omicron BA.2 became the predominant variant, it continues to be used in patients at high risk of severe COVID-19 in other countries and remains recommended in national guidelines [14–19]. However, as new variants continue to emerge, the need has arisen to inform the safety and tolerability of higher doses of sotrovimab to accommodate further shifts in susceptibility.
Participants in the COMET-TAIL safety substudy were enrolled from June 30 through July 11, 2022, when Omicron BA.5, BA.2.12.1, and BA.4 were the predominant circulating variants in the United States. Understanding the safety and tolerability of higher doses of sotrovimab will provide useful information should higher doses be necessary to treat patients with COVID-19 caused by SARS-CoV-2 variants with reduced in vitro susceptibility to sotrovimab. The primary objective of this substudy of COMET-TAIL was to assess the safety and tolerability of 2000 mg sotrovimab when administered IV to nonhospitalized adults with mild to moderate COVID-19 at risk for progression to severe COVID-19. Exploratory objectives included assessment of clinical outcomes and antiviral activity.
METHODS
Participants
Participants were eligible for inclusion in the COMET-TAIL substudy (ClinicalTrials.gov Identifier: NCT04913675) if they were aged ≥18 years at the time of consent and at high risk for progression of COVID-19 based on the presence of 1 or more predefined risk factors. For participants aged 18 to 54 years, these included diabetes requiring medication, obesity (defined as body mass index ≥30 kg/m2), chronic kidney disease (estimated glomerular filtration rate <60 mL/min/1.73 m2 by Modification of Diet in Renal Disease criteria), congenital heart disease, congestive heart failure (New York Heart Association [NYHA] class II or higher), chronic lung diseases (ie, chronic obstructive pulmonary disease, moderate to severe asthma requiring corticosteroids, interstitial lung disease, cystic fibrosis, and pulmonary hypertension), sickle cell disease, neurodevelopmental disorders, immunosuppressive disease or receiving immunosuppressive medications, or chronic liver disease. Participants aged ≥55 years were eligible to participate in the study irrespective of any comorbidities.
Participants were also required to have a positive SARS-CoV-2 test result within 7 days of randomization (by any validated diagnostic test, including reverse transcriptase–polymerase chain reaction [RT-PCR] or antigen-based testing on any specimen type), oxygen saturation ≥94% on room air, and symptoms of COVID-19 (≥1 of the following: fever, chills, cough, sore throat, malaise, headache, joint or muscle pain, change in smell or taste, vomiting, diarrhea, or shortness of breath on exertion) and to have started study treatment within ≤7 days of the onset of symptoms. Participants were not eligible for the study if they were currently hospitalized, were likely to require hospitalization in the next 24 hours, or had severe COVID-19 (respiratory distress or required hospitalization for oxygen supplementation). Receiving convalescent plasma from a patient who had recovered from COVID-19 or an anti-SARS-CoV-2 monoclonal antibody within the preceding 3 months was not permitted. Additionally, antiviral treatment with nirmatrelvir/ritonavir, remdesivir, molnupiravir, or passive antibody therapies was not allowed during the study unless given as local standard of care.
Study Design and Treatment
Details of the primary COMET-TAIL study have been reported previously [12]. The primary objective of this open-label, nonrandomized, single-arm substudy of COMET-TAIL was to describe the safety and tolerability of high-dose (2000 mg) IV sotrovimab, with an optional arm of up to 3000 mg IV. The substudy enrolled participants at 7 sites in the United States from June 30 through July 11, 2022. Given the positive efficacy data from previous studies of sotrovimab in preventing disease progression in high-risk participants [10, 12], it was considered unethical to include a placebo comparator group. The study is ongoing, with participants remaining under follow-up through week 36. An initial 32 enrolled participants comprised a safety lead-in group, who received sotrovimab per study protocol and for whom relevant safety data were reviewed. An additional 49 participants were then enrolled and treated. Enrollment was discontinued early in the context of evolving variants with increased fold changes in the in vitro half maximal inhibitory concentration (IC50) and uncertainty in the clinical relevance of these changes.
Following screening, all treated participants received open-label 2000 mg sotrovimab infused IV over 60 minutes on day 1 with follow-up through 36 weeks. A 2000 mg IV sotrovimab dose was evaluated after anticipating that it would maintain serum levels sufficiently above the 90% maximal effective concentration (EC90) to protect against some SARS-CoV-2 variants with reduced in vitro neutralization potency relative to wild type. Based on preliminary clinical pharmacology modeling at the time of protocol development, a 2000 mg IV dose of sotrovimab was predicted to maintain serum concentrations ≥120 μg/mL in 90% of participants for 28 days after dosing. These concentrations are ≥111-fold above wild-type tissue-adjusted EC90 (using a measured sotrovimab lung:serum ratio of 0.25 [20]) or ≥45-fold above tissue-adjusted EC90 (assuming a more conservative lung:serum ratio of 0.10).
Participants in the safety lead-in group were monitored for 2 hours following sotrovimab administration, with vital signs assessments predose and at 15 minutes, 30 minutes, 45 minutes, 1 hour, and 2 hours after infusion. Participants in the remainder of the cohort were monitored at similar intervals for up to 1 hour. Through day 29, further active monitoring was conducted on an outpatient basis, starting with weekly clinic visits, including physical examination, vital signs, laboratory assessments, and collection of nasopharyngeal swabs for virologic analyses. The study is ongoing and has additional clinic visits at weeks 12, 20, and 24. Sparse pharmacokinetic (PK) samples were collected on day 1 (predose and at the end of the infusion) and on days 3, 5, 8, 15, and 29. Sotrovimab serum concentrations were determined using a validated electrochemiluminescent method with a lower limit of quantification of 0.1 µg/mL. Additional samples for PK analyses are being collected through week 24. At weeks 8, 16, 28, 32, and 36, participants continue to be monitored via phone calls to assess for adverse events (AEs), disease-related events (DREs), serious AEs (SAEs), and the incidence and severity of any subsequent COVID-19 illness after sotrovimab dosing.
Patient Consent
The study was conducted according to the Declaration of Helsinki, Council for International Organizations of Medical Sciences international ethics guidelines, and Good Clinical Practice. The study protocol was approved by the institutional review boards/independent ethics committees at each participating study site. The authorizing body was Advarra Institutional Review Board (Columbia, MD, USA). All participants provided written informed consent at the screening visit.
Study Outcomes
The primary end point of the substudy was the occurrence of AEs, SAEs, AEs of special interest, and DREs through day 8. All AEs were classified according to Medical Dictionary for Regulatory Activities (MedDRA) terminology. Secondary end points included the occurrence of AEs, AEs of special interest, and DREs through week 12 and SAEs through week 36; the incidence and titers of serum antidrug antibody (ADA) and neutralizing antibody to sotrovimab through week 24; and serum sotrovimab PK through week 24. AEs of special interest included infusion-related reactions (IRRs; including hypersensitivity reactions occurring ≤24 hours after infusion), hypersensitivity reactions occurring at any time following dosing, immunogenicity-related AEs, and AEs potentially related to antibody-dependent enhancement of disease. This manuscript reports safety, viral load, and PK data collected through day 29.
Several exploratory end points were also included to evaluate the efficacy of 2000 mg IV sotrovimab. These were progression of COVID-19 through day 29 as defined by hospitalization >24 hours for acute management of illness due to any cause or death; visit to a hospital emergency room for management of illness or hospitalization for acute management of illness for any duration and for any cause or death; development of severe COVID-19 requiring respiratory support including oxygen at days 8, 15, 22, and 29; and the incidence of participants requiring intensive care unit (ICU) stay or mechanical ventilation through day 29. Viral load in nasal secretions was assessed using quantitative RT-PCR and evaluated as the change from baseline in viral load at days 3, 5, 8, 11, 15, 22, and 29; undetectable SARS-CoV-2 in nasal secretions at days 3, 5, 8, 11, 15, 22, and 29; and the proportion of participants with a persistently high SARS-CoV-2 viral load (PHVL; ≥4.1 log10 copies/mL) at day 8.
Sample Size and Statistical Analyses
A sample size of 200 participants was initially planned to assess the safety of 2000 mg IV sotrovimab. This sample size provided a 90% probability of observing ≥1 particular AE of interest if the true incidence of that AE was not below 1.14%, which was the frequency of IRRs observed in the COMET-ICE study.
The safety analysis set comprised all participants who were enrolled and exposed to study treatment. All analyses were conducted on this population except virologic analyses, which were conducted on all enrolled participants with a laboratory-confirmed quantifiable nasopharyngeal swab on day 1. All participants in the safety analysis set had ≥1 nonmissing PK assessment and were included in the PK analyses.
All statistical analyses were descriptive, and no formal statistical testing was conducted. Viral load measurements reported below the lower limits of detection or quantification were imputed to 1.78 log10 copies/mL. All statistical analyses were performed using SAS, version 9.4. (SAS Institute Inc., Cary, NC, USA). The study protocol and statistical analysis plan are included in the Supplementary Data.
RESULTS
Participants
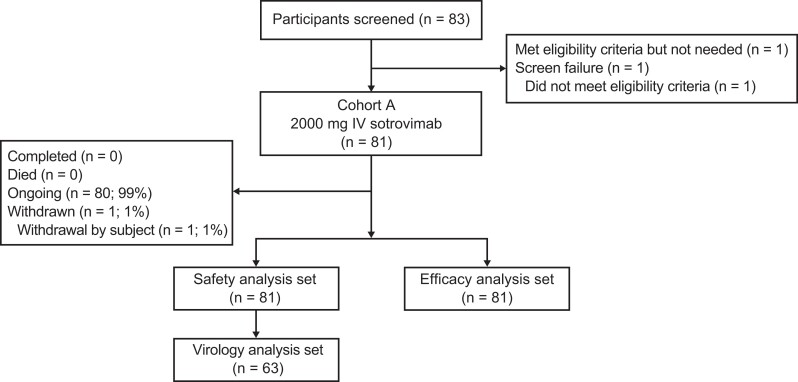
Due to early discontinuation of enrollment, only 81 participants received 2000 mg IV sotrovimab from the planned sample of 200 (Table 1, Figure 1). A total of 83 participants were screened; 1 failed screening, and another was not treated due to early study discontinuation. All participants were enrolled in the United States at 7 sites, with 6 in Florida. At the time of the data cutoff (October 4, 2022), 80 participants remained in the study and 1 participant had withdrawn.
Table 1.
Baseline Demographic and Clinical Characteristics
| Characteristic | 2000 mg IV Sotrovimab (n = 81) |
|---|---|
| Sex, No. (%) | |
| Male | 34 (42) |
| Female | 47 (58) |
| Age, mean (SD), y | 51.6 (14.6) |
| Age category, No. (%) | |
| <55 y | 40 (49) |
| 55–64 y | 26 (32) |
| 65–74 y | 12 (15) |
| 75–84 y | 2 (2) |
| ≥85 y | 1 (1) |
| Ethnicity, No. (%) | |
| Hispanic or Latino | 81 (100) |
| Not Hispanic or Latino | 0 |
| Race, No. (%) | |
| White | 77 (95) |
| Black/African American | 4 (5) |
| BMI, mean (SD), kg/m2 | 32.1 (5.2) |
| BMI category, No. (%) | |
| <30 kg/m2 | 27 (33) |
| 30–<35 kg/m2 | 24 (30) |
| ≥35 kg/m2 | 30 (37) |
| Conditions as a risk factor for progression to severe COVID-19, No. (%) | |
| Any condition | 81 (100) |
| Obesity | 54 (67) |
| Age ≥55 y | 41 (51) |
| Chronic lung diseases | 19 (23) |
| Diabetes | 13 (16) |
| No. of conditions met, No. (%) | |
| 1 | 43 (53) |
| 2 | 30 (37) |
| 3 | 8 (10) |
| >3 | 0 |
| Received any COVID-19 vaccination, No. (%) | 50 (62) |
| Fully vaccinated | 43 (53) |
| Symptom duration, No. (%) | |
| ≤3 d | 51 (63) |
| 4–5 d | 26 (32) |
| >5 d | 4 (5) |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; IV, intravenous.
Figure 1.
Patient enrollment and treatment assignment. Abbreviation: IV, intravenous.
All participants were Hispanic or Latino, and 77 (95%) were White; the mean age was 51.6 years, 41 (51%) participants were aged ≥55 years, and 47 (58%) participants were female. A total of 15 (19%) participants were aged ≥65 years, and 38 (47%) had ≥2 risk factors for progression to severe COVID-19. None had chronic kidney disease, chronic liver disease, congenital heart disease, congestive heart failure (NYHA class II or higher), immunosuppressive disease, neurodevelopmental disorder, or sickle cell disease. All participants were immunocompetent, and 50 (62%) had received ≥1 dose of a COVID-19 vaccine.
Primary and Secondary End Points
For the primary end point, no AEs, SAEs, or AEs of special interest were observed through day 8 (Table 2). A total of 2 participants experienced DREs through day 8, including 2 cases of grade 1 cough (of 3 and 6 days’ duration, respectively). These events resolved during the study and were not associated with study discontinuation.
Table 2.
Primary, Secondary, and Key Exploratory End Points
| Day 8 (n = 81) | Day 29 (n = 81) | |
|---|---|---|
| Any AE, No. (%) | 0 | 1 (1.2) |
| AEs related to study treatment | 0 | 0 |
| AEs leading to study treatment discontinuation, dose interruption, or delay | 0 | 0 |
| Maximum grade 3/4 AEs | 0 | 1 (1.2) |
| Any SAEs | 0 | 1 (1.2) |
| SAEs related to study treatment | 0 | 0 |
| Fatal SAEs | 0 | 0 |
| DRE | 2 (2.5) | 4 (4.9) |
| Progression of COVID-19 through day 29, No. (%) | 0 | 0 |
| Hospitalization >24 h for acute management of illness due to any cause | 0 | 1 (1.2) |
| Death | 0 | 0 |
| Visit to a hospital emergency room for management of illness or hospitalization for acute management of illness for any duration and any cause | 0 | 1 (1.2) |
| Development of severe and/or critical respiratory COVID-19 as manifested by requirement for respiratory support (including oxygen) | 0 | 0 |
| ICU stay or mechanical ventilation | 0 | 0 |
| Missing | 0 | 1 (1.2) |
Abbreviations: AE, adverse event; COVID-19, coronavirus disease 2019; DRE, disease-related event; ICU, intensive care unit; SAE, serious adverse event.
For the secondary end point (reported through day 29), only 1 SAE occurred, which was a grade 3 acute myocardial infarction in a participant with multiple cardiac risk factors, including old age (50 years), male sex, type 2 diabetes mellitus, hypertension, and smoking. During the cardiac catheterization, a 95% stenosis of his mid-right coronary artery was found. The SAE occurred at 27 days after sotrovimab administration and was not considered related to sotrovimab or COVID-19. This SAE was reported as resolved within 3 days, and the participant continued in the study. Two participants experienced DREs through day 29, including 2 cases of grade 3 lipase elevation and 1 case of grade 2 insomnia. The lipase elevations resolved, the insomnia was resolving at the data cutoff, and none of these events led to study discontinuation. Assessments of AEs, AEs of special interest, and DREs through week 12 and SAEs through week 36 are ongoing.
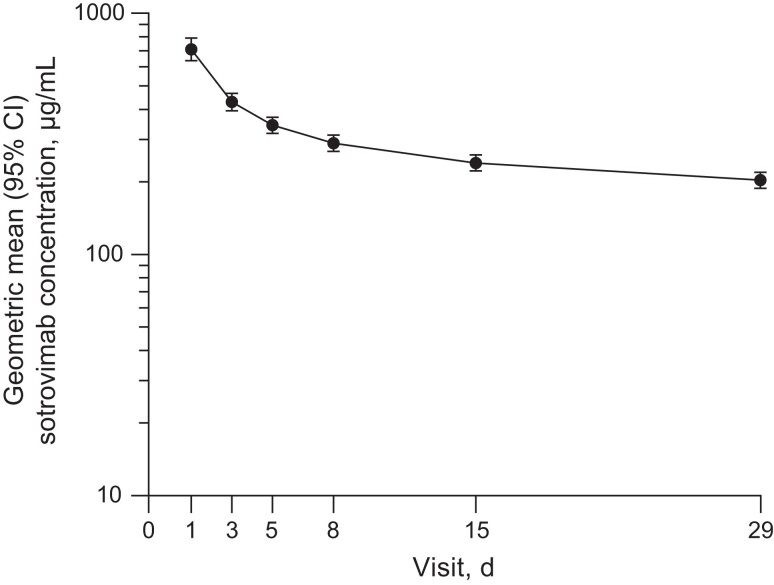
For the secondary end point assessing PK after a 60-minute 2000 mg IV infusion of sotrovimab, the geometric mean (% coefficient of variation [CV]) maximum serum concentration (Cmax) was 745.9 µg/mL (41.7) and the geometric mean (%CV) area under the plasma concentration-time curve from day 1 through day 29 was 7696 day·µg/mL (34.6). The geometric mean (%CV) of the serum concentration at day 29 was 203.4 µg/mL (35.5). The sotrovimab serum concentration-time plot through day 29 is displayed in Figure 2.
Figure 2.
Geometric mean (95% CI) sotrovimab serum concentration-time profile after administration of 2000 mg IV sotrovimab. Abbreviation: IV, intravenous.
Exploratory End Points
For the exploratory end points of progression of COVID-19 through day 29 as assessed by hospitalization >24 hours for acute management of illness due to any cause or death, or visit to a hospital emergency room for management of illness or hospitalization for acute management of illness for any duration and for any cause or death, only the participant with the unrelated SAE of acute myocardial infarction required hospitalization. No participants experienced progression to severe and/or critical respiratory COVID-19 or ICU stay or mechanical ventilation through day 29.
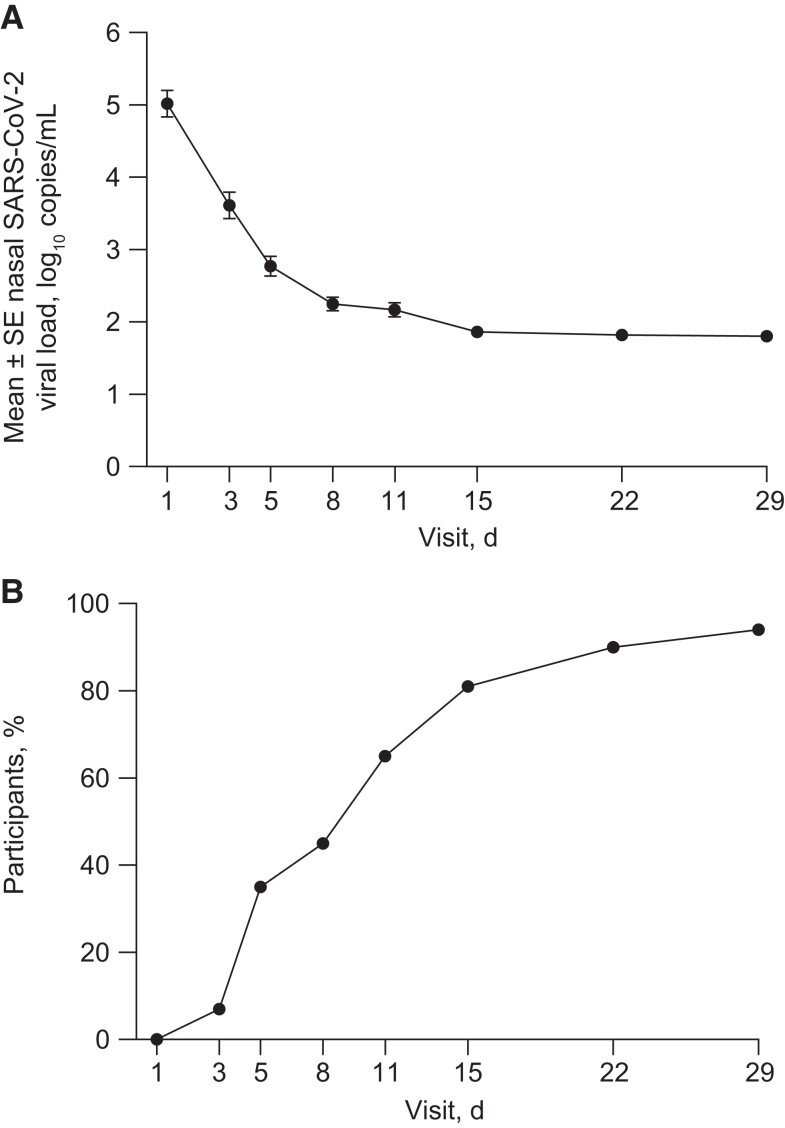
Among participants in the virology population (n = 63), the mean (SD) viral load at baseline was 5.02 (1.44) log10 copies/mL, had decreased to 2.25 (0.75) log10 copies/mL at day 8, and continued to decline through day 29 (Figure 3A). The proportion of participants with an undetectable SARS-CoV-2 viral load was 35% at day 5 and consistently increased over time, rising to 45% of participants at day 8, 81% of participants at day 15, and 94% of participants at day 29 (Figure 3B). Only 2 (3%) participants in the virology population demonstrated PHVL (≥4.1 log10 copies/mL) at day 8.
Figure 3.
Mean ± SE nasal SARS-CoV-2 viral load (A) and percentage of participants with undetectable SARS-CoV-2 in nasal secretions (B) through day 29. Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
DISCUSSION
This substudy of COMET-TAIL showed that a high dose (2000 mg) of sotrovimab administered IV over 60 minutes to participants with mild to moderate COVID-19 was well tolerated, with no new safety signals through day 29 relative to the studies using the 500 mg dose.
No AEs or AEs of special interest were reported through day 8; only 1 SAE occurred at day 27 after dosing, which was deemed by the investigator to be unrelated to sotrovimab or COVID-19. Safety data through weeks 12 and 36 (secondary end point) will be reported in the future.
There were no cases of progression, including COVID-19 hospitalization or death or development of severe COVID-19, reported during the study. Clearance of SARS-CoV-2 was observed in almost all participants by day 29 following sotrovimab administration, with most of the viral load decline (mean, 2.78) seen by day 8. This is of particular interest as enrollment was conducted during a period where the Omicron subvariants BA.2.12.1, BA.4, and BA.5 were the predominant circulating variants of SARS-CoV-2 (June 30 through July 11, 2022). Although in vitro studies show a moderate reduction in the neutralization of Omicron BA.2 and BA.5 by sotrovimab (15.7- and 21.6-fold change in IC50 relative to wild-type, respectively, using an authentic virus assay) [21, 22], recent observational studies conducted in Europe suggest continued clinical effectiveness of sotrovimab through the Omicron BA.2 and BA.5 surges [23–26]. In a study of >8000 patients in England who were infected with the Omicron BA.1 or BA.2 SARS-CoV-2 variants, there were no differences in the risk of hospitalization for patients with mild to moderate COVID-19 treated with sotrovimab [23]. Similarly, the risk of severe COVID-19 outcomes between nonhospitalized high-risk adult patients with COVID-19 was not substantially different between those who received nirmatrelvir/ritonavir or sotrovimab during a period of Omicron BA.2 and BA.5 dominance in England [26]. A separate UK-based study demonstrated greater effectiveness of sotrovimab than molnupiravir in preventing COVID-19 hospitalization and death in nonhospitalized patients at high risk for progression during the BA.1 and BA.2 variant waves [24]. In a French patient cohort, there was no difference in the percentage of hospitalizations or deaths in patients with COVID-19 caused by BA.1 or BA.2 variants following treatment with sotrovimab [25]. Similarly, in a US observational study, reduced risk of all-cause hospitalization and mortality was associated with sotrovimab vs no monoclonal antibody treatment during the Delta, BA.1, and BA.2 variant waves [27]. Compared with Omicron BA.2 and BA.5, sotrovimab demonstrates similar in vitro neutralization potency against the Omicron subvariants BA.2.12.1 and BA.4 (25.1- and 48.4-fold change in IC50, respectively, relative to wild-type using an authentic virus assay) [21]. In this study, treatment with sotrovimab 2000 mg IV was associated with a low rate of hospitalization due to any cause.
Moving forward, it is anticipated that other emerging variants [28] and treatments providing adequate activity against current and future variants will be increasingly important. Sotrovimab has demonstrated neutralization activity in vitro against the recent Omicron XBB.1.5, BQ.1, and BQ.1.1 variants with a range in potency that is reduced relative to Wuhan-Hu-1 (11.3-, 28.5-, and 94-fold change in IC50, respectively, using a pseudotyped virus assay) [29]. This clinical experience with sotrovimab 2000 mg and the virology data reported, together with real-world evidence data on 500 mg IV in variants with modest reductions in in vitro neutralization, suggest that at higher doses, sotrovimab may be useful in treating patients infected with variants for which sotrovimab has reduced neutralization activity relative to Wuhan-Hu-1. Additionally, the preliminary PK results from our study are consistent with the expectations for a 2000 mg IV dose under assumptions of dose proportionality.
There are a number of limitations to the current study. Specifically, this was a single arm without a placebo control design with a small sample size, and almost all the participants were enrolled at sites in Florida (USA) over a period of 2 weeks, potentially leading to less heterogeneity in the circulating SARS-CoV-2 variants than the variants that may have been observed with a broader geographic sample. Furthermore, there was limited racial and ethnic diversity among the study population, and no immunocompromised participants were enrolled. The early closure of enrollment and the resultant small sample size decreased the probability of observing an AE of interest with a true incidence of 1.14% from 90% to 60%. The frequency of IRRs in the COMET-TAIL main and COMET-ICE studies was ∼1.0%. Even with 81 participants receiving 2000 mg IV sotrovimab there was a 90% chance of observing at least 1 IRR if the true incidence rate of IRRs is not below 2.85%. Thus, the safety results suggest that AEs of interest did not increase in a dose-proportional manner.
The observed mean viral load change from baseline on day 8 in the COMET-TAIL substudy was −2.78 log10 copies/mL, which was similar in magnitude to the viral load changes observed on day 8 postdosing with 500 mg IV sotrovimab in the COMET-TAIL main and COMET-ICE studies (−2.99 and −2.63 log10 copies/mL, respectively) [10, 12]. Additionally, the percentage of participants with PHVL was 3% on day 8 in the COMET-TAIL substudy, which was lower than the percentage of PHVL observed in the 500 mg IV sotrovimab arm of the COMET-TAIL main study (13%) [30]. Although the virologic analyses provide useful information with respect to the antiviral effects of high-dose sotrovimab, no firm conclusions regarding efficacy can be drawn as these outcomes were exploratory in nature and there was no control comparator.
Further findings from this study will be reported as the data become available, including safety through weeks 12 and 36, incidence and titers of ADAs, full viral sequencing data including identification of SARS-CoV-2 variants and treatment-emergent mutations, and PK of sotrovimab after the 2000 mg IV dose through week 24. A 3000 mg IV dose of sotrovimab is being evaluated in healthy volunteers (COSMIC; ClinicalTrials.gov Identifier: NCT05280717) to support clinical doses and alternative infusion rates for potential future clinical studies [31]. Ongoing real-world studies outside of the United States will continue to inform a broader picture of sotrovimab treatment effectiveness against emerging SARS-CoV-2 variants.
Although the need for IV infusion may present as a challenge to treatment with monoclonal antibodies in the community setting during the COVID-19 pandemic [32, 33], some hospitals have created protocols to bring patients with COVID-19 into infusion centers [34, 35]. Infusion centers may also be positioned in various locations, including ambulatory centers, emergency departments, skilled nursing facilities, and residential facilities [36, 37]. Ultimately, however, the length of infusion dictates the throughput of patients requiring monoclonal antibody treatment for COVID-19 [35]. Thus, agents with shorter infusion times or agents that may be administered non-IV (ie, IM or subcutaneous) are needed to increase access to care in patients with COVID-19. Unfortunately, the non-IV route is not feasible even with the concentrated formulation utilized in the COSMIC study [31]. To deliver a 2000 mg dose of sotrovimab, IV administration is necessary due to the substantial medication volume and the requirement for multiple injections.
In conclusion, sotrovimab, when administered at a dose of 2000 mg IV infused over 60 minutes, was safe and well tolerated, with a low frequency of AEs (none of which were considered related to sotrovimab) and no hypersensitivity, IRRs, or deaths in participants with mild to moderate COVID-19.
Supplementary Material
Acknowledgments
The authors thank Jeanne McKeon, PhD, of Lumanity Scientific Inc. for medical writing support (ensured proper formatting, added references, helped with the electronic submission, etc.), which was funded by Vir Biotechnology, Inc., and GSK. The authors also thank Joseph Hogan, MS, Mimi Chae, MS, and Emma Gierman, MPH, of Vir Biotechnology, Inc., for clinical operations support.
Author contributions. M.T., M.A., E.L.A., A.S., G.S., A.E.-Z., J.E.S., and S.P. contributed to study conception and design. G.A., J.M., E.J., J.C.M.G., R.H.-L., J.D., R.H., M.T., and S.P. contributed to data analysis. M.T., E.L.A., A.S., G.S., A.E.-Z., J.E.S., S.S., C.X., and S.P. made substantial contributions to analysis and interpretation of data. All authors were involved in drafting the article and/or revising it critically for important intellectual content, and all authors approved of its final version.
Data availability. The data collected for this study will not be made available to others.
Financial support. This work was supported by Vir Biotechnology, Inc., and GSK.
Contributor Information
Jaynier Moya, Pines Care Research Center, Pembroke Pines, Florida, USA.
Marisol Temech, Vir Biotechnology, Inc., San Francisco, California, USA.
Sergio Parra, Vir Biotechnology, Inc., San Francisco, California, USA.
Erick Juarez, Florida International Medical Research, Miami, Florida, USA.
Reinaldo Hernandez-Loy, Dynamic Medical Research, LLC, Miami, Florida, USA.
Juan C Moises Gutierrez, Continental Clinical Research, Miami, Florida, USA.
Jorge Diaz, Doral Medical Research, Doral, Florida, USA.
Rubaba Hussain, RH Medical Urgent Care, New York, New York, USA.
Scott Segal, GSK, Collegeville, Pennsylvania, USA.
Claire Xu, GSK, Collegeville, Pennsylvania, USA.
Andrew Skingsley, GSK, Brentford, UK.
Gretja Schnell, Vir Biotechnology, Inc., San Francisco, California, USA.
Asma El-Zailik, Vir Biotechnology, Inc., San Francisco, California, USA.
Jennifer E Sager, Vir Biotechnology, Inc., San Francisco, California, USA.
Melissa Aldinger, Vir Biotechnology, Inc., San Francisco, California, USA.
Elizabeth L Alexander, Vir Biotechnology, Inc., San Francisco, California, USA.
Gerard Acloque, Universal Medical and Research Center, Miami, Florida, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Our World in Data . Coronavirus (COVID-19) hospitalizations. Available at: https://ourworldindata.org/covid-hospitalizations. Accessed January 19, 2023.
- 2. World Health Organization . Weekly epidemiological update on COVID-19 - 11 January 2023. Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---11-january-2023. Accessed January 19, 2023.
- 3. Reyna-Villasmil E, Caponcello MG, Maldonado N, et al. . Association of patients’ epidemiological characteristics and comorbidities with severity and related mortality risk of SARS-CoV-2 infection: results of an umbrella systematic review and meta-analysis. Biomedicines 2022; 10:2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Demombynes G, de Walque D, Gubbins P, Urdinola P, Veillard J. Are COVID-19 age-mortality curves for 2020 flatter in developing countries? Evidence from a cross-sectional observational study of population-level official death counts and excess deaths estimates. BMJ Open 2022; 12:e061589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vardavas CI, Mathioudakis AG, Nikitara K, et al. . Prognostic factors for mortality, intensive care unit and hospital admission due to SARS-CoV-2: a systematic review and meta-analysis of cohort studies in Europe. Eur Respir Rev 2022; 31:220098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nguyen JL, Alfred T, Reimbaeva M, et al. . Population attributable fractions of underlying medical conditions for coronavirus disease 2019 (COVID-19) diagnosis and COVID-19 hospitalizations, ventilations, and deaths among adults in the United States. Open Forum Infect Dis 2022; 9:ofac099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. . Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients. N Engl J Med 2022; 386:509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hammond J, Leister-Tebbe H, Gardner A, et al. . Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med 2022; 386:1397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miguez-Rey E, Choi D, Kim S, Yoon S, Sandulescu O. Monoclonal antibody therapies in the management of SARS-CoV-2 infection. Expert Opin Investig Drugs 2022; 31:41–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gupta A, Gonzalez-Rojas Y, Juarez E, et al. . Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA 2022; 327:1236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GlaxoSmithKline Manufacturing S.p.A. Xevudy summary of product characteristics. 2023. Available at: https://www.ema.europa.eu/en/documents/product-information/xevudy-epar-product-information_en.pdf. Accessed January 17, 2023.
- 12. Shapiro AE, Sarkis E, Acloque J, et al. . Intramuscular versus intravenous SARS-CoV-2 neutralizing antibody sotrovimab for treatment of COVID-19 (COMET-TAIL): a randomized non-inferiority clinical trial. Open Forum Infect Dis 2023:ofad354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agostini ML, Schnell G, di Iulio J, et al. . Resistance analysis in the COMET-TAIL study: participants with mild-to-moderate COVID-19 treated with intramuscular or intravenous sotrovimab. Open Forum Infect Dis 2022; 9:ofac492.988. [Google Scholar]
- 14. Italian Medicines Agency . Medicines usable for treatment of COVID-19 disease. Available at: https://www.aifa.gov.it/en/web/guest/aggiornamento-sui-farmaci-utilizzabili-per-il-trattamento-della-malattia-covid19. Accessed March 10, 2023.
- 15. National Institute for Health and Care Excellence . Casirivimab plus imdevimab, nirmatrelvir plus ritonavir, sotrovimab and tocilizumab for treating COVID-19. Available at: https://www.nice.org.uk/guidance/TA878/history. Accessed March 10, 2023.
- 16. STAKOB (Robert Koch-Institut) . Hinweise zu erkennung, diagnostik und therapie von patienten mit COVID-19. Available at: https://www.rki.de/DE/Content/Kommissionen/Stakob/Stellungnahmen/Stellungnahme-Covid-19_Therapie_Diagnose.pdf?__blob=publicationFile. Accessed March 10, 2023.
- 17. Spanish Agency of Medicines and Medical Products . Criteria for assessing the administration of new antiviral therapeutic alternatives against SARS-CoV-2 infection. Available at: https://www.aemps.gob.es/medicamentos-de-uso-humano/acceso-a-medicamentos-en-situaciones-especiales/criterios-para-valorar-la-administracion-de-las-nuevas-alternativas-terapeuticas-antivirales-frente-a-la-infeccion-por-sars-cov-2/?lang=en. Accessed March 10, 2023.
- 18.United Arab Emirates Ministry of Health and Prevention. National Guidelines for Clinical Management and Treatment of COVID-19. Version 9.0. United Arab Emirates Ministry of Health and Prevention;2023.
- 19. Japanese Association for Infectious Diseases . Approach to drug treatment for COVID-19 Version 15.1 (14 February 2023). Available at: https://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_drug_230217.pdf. Accessed June 13, 2023.
- 20. Aweda TA, Cheng SH, Lenhard SC, et al. . In vivo biodistribution and pharmacokinetics of sotrovimab, a SARS-CoV-2 monoclonal antibody, in healthy cynomolgus monkeys. Eur J Nucl Med Mol Imaging 2023; 50:667–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park YJ, Pinto D, Walls AC, et al. . Imprinted antibody responses against SARS-CoV-2 Omicron sublineages. Science 2022; 378:619–27. [DOI] [PubMed] [Google Scholar]
- 22. Cathcart AL, Havenar-Daughton C, Lempp FA, et al. . The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv 434607 [Preprint]. April 1, 2022. Available at: 10.1101/2021.03.09.434607. Accessed June 12, 2023. [DOI]
- 23. Harman K, Nash SG, Webster HH, et al. . Comparison of the risk of hospitalisation among BA.1 and BA.2 COVID-19 cases treated with sotrovimab in the community in England. Influenza Other Respir Viruses 2023; 17:e13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng B, Green ACA, Tazare J, et al. . Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe COVID-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform. BMJ 2022; 379:e071932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin-Blondel G, Marcelin AG, Soulie C, et al. . Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2. J Infect 2022; 85:e104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng B, Tazare J, Nab L, et al. . Comparative effectiveness of Paxlovid versus sotrovimab and molnupiravir for preventing severe COVID-19 outcomes in non-hospitalised patients: observational cohort study using the OpenSAFELY platform. medRxiv 23284849 [Preprint]. January 22, 2023. Available at: 10.1101/2023.01.20.23284849. Accessed June 12, 2023 [DOI] [PMC free article] [PubMed]
- 27. Cheng MM, Reyes C, Satram S, et al. . Real-world effectiveness of sotrovimab for the early treatment of COVID-19 during SARS-CoV-2 Delta and Omicron waves in the USA. Infect Dis Ther 2023; 12:607–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention . COVID Data Tracker, Nowcast. Available at: https://covid.cdc.gov/covid-data-tracker/#variant-proportions. Accessed January 19, 2023.
- 29. Addetia A, Piccoli L, Case JB, et al. . Therapeutic and vaccine-induced cross-reactive antibodies with effector function against emerging Omicron variants. bioRxiv 523798 [Preprint]. January 17, 2023. Available at: 10.1101/2023.01.17.523798. [DOI]
- 30. ClinicalTrials.gov. NCT04913675: Intramuscular and intravenous VIR-7831 (sotrovimab) for mild/moderate COVID-19. Available at: https://www.clinicaltrials.gov/ct2/show/NCT04913675. Accessed June 15, 2023.
- 31. ClinicalTrials.gov. NCT05280717: Relative bioavailability, safety, and tolerability of single-dose sotrovimab injection in adults (COSMIC). Available at: https://clinicaltrials.gov/ct2/show/NCT05280717. Accessed January 17, 2023.
- 32. Dotan I, Panaccione R, Kaplan GG, O'Morain C, Lindsay JO, Abreu MT. Best practice guidance for adult infusion centres during the COVID-19 pandemic: report from the COVID-19 International Organization for the Study of IBD [IOIBD] task force. J Crohns Colitis 2020; 14:S785–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kwan BM, Sobczak C, Gorman C, et al. “All of the things to everyone everywhere”: a mixed methods analysis of community perspectives on equitable access to monoclonal antibody treatment for COVID-19. PLoS One 2022; 17:e0274043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Berry JR, Liebl MG, Todd PH, Brownewell V. Rapid operationalization of COVID-19 monoclonal antibody infusion clinics. NEJM Catal Innov Care Deliv 2021; 2:1–10. [Google Scholar]
- 35. Razonable RR, Aloia NCE, Anderson RJ, et al. . A framework for outpatient infusion of antispike monoclonal antibodies to high-risk patients with mild-to-moderate coronavirus disease-19: the Mayo Clinic model. Mayo Clin Proc 2021; 96:1250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Minnich A, Rocha D, Hu Y, et al. . Delivering monoclonal antibody infusions to novel outpatient settings. NEJM Catal Innov Care Deliv 2022; 3:1-10. [Google Scholar]
- 37. Pham DH, Wong S, Nguyen CT, Lee SC, Won KJ. Development of a bamlanivimab infusion process in the emergency department for outpatient COVID-19 patients. Healthcare (Basel) 2021; 10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.