Abstract
Background
Chemotherapy‐induced neutropenia is a common adverse effect in children with cancer. Due to the high relative risk of infections and infectious complications, standard care for children with cancer and febrile neutropenia consists of routine hospitalization and parenteral administration of broad‐spectrum antibiotics. However, there are less serious causes of febrile neutropenia; in a subgroup of these children, lengthy in‐hospital treatment might be unnecessary. Various research groups have studied the adjustment of standard care to shorten in‐hospital treatment for children with cancer and febrile neutropenia at low risk for bacterial infections. However, most of these studies were not done in a randomized matter.
Objectives
To evaluate whether early discharge (mean/median of less than five days) from in‐hospital treatment was not inferior to non‐early discharge (mean/median of five days or more) and whether very early discharge (mean/median of less than 24 hours) was not inferior to early discharge, non‐early discharge, or a combination of these, in children with cancer and febrile neutropenia.
Search methods
We searched the Cochrane Central Register of Controlled Trials (2015, issue 11), MEDLINE/PubMed (from 1945 to December 2015), EMBASE/Ovid (from 1980 to December 2015), the reference lists of relevant articles and review articles, and various conference proceedings (dependent on availability from 2005 to 2010 to 2013 to 2015). We scanned the International Standard Randomised Controlled Trials Number (ISRCTN) Register, the National Institute of Health Register for ongoing trials, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) on 9 January 2016.
Selection criteria
We included all randomized controlled trials and controlled clinical trials in which children with cancer and febrile neutropenia were divided in groups with different times of discharge.
Data collection and analysis
We used standard methods of Cochrane and its Childhood Cancer Group. Two independent review authors performed study selection, data extraction, and risk of bias assessment. We entered data extracted from the included studies into Review Manager 5 and undertook analyses according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
We included two randomized controlled trials assessing very early, early, non‐early (or a combination of these) discharge in children with cancer and febrile neutropenia. We graded the evidence as low quality; we downgraded for risk of bias and imprecision. One study, Santolaya 2004, consisted of 149 randomized low‐risk episodes and compared early discharge (mean/median of less than five days) to non‐early discharge (mean/median of five days or more). This study found no clear evidence of difference in treatment failure (risk ratio (RR) 0.91, 95% confidence interval (CI) 0.24 to 3.50, P value = 0.89 for rehospitalization or adjustment of antimicrobial treatment, or both; Fischer's exact P value = 0.477 for death) or duration of treatment (mean difference ‐0.3 days, 95% CI ‐1.22 to 0.62, P value = 0.52 for any antimicrobial treatment; mean difference ‐0.5 days, 95% CI ‐1.36 to 0.36, P value = 0.25 for intravenous antimicrobial treatment; mean difference 0.2 days, 95% CI ‐0.51 to 0.91, P value = 0.58 for oral antimicrobial treatment). Costs were lower in the early discharge group (mean difference USD ‐265, 95% CI USD ‐403.14 to USD ‐126.86, P value = 0.0002). The second included study, Brack 2012, consisted of 62 randomized low‐risk episodes and compared very early discharge (mean/median of less than 24 hours) to early discharge (mean/median of less than five days). This study also found no clear evidence of difference in treatment failure (RR 0.54, 95% CI 0.15 to 1.89, P value = 0.34 for rehospitalization or adjustment of antimicrobial treatment (or both); Fischer's exact P value = 0.557 for death). Regarding duration of treatment, median duration of intravenous antimicrobial treatment was shorter in the very early discharge group (Wilcoxon's P value ≤ 0.001, stated in the study) and median duration of oral antimicrobial treatment was shorter in the early discharge group (Wilcoxon's P ≤ 0.001, stated in the study) as compared to one another. However, there was no clear evidence of difference in median duration of any antimicrobial treatment (Wilcoxon's P value = 0.34, stated in the study). Costs were not assessed in this study. Neither of the included studies assessed quality of life. Meta‐analysis was not possible as the included studies assessed different discharge moments and used different risk stratification models.
Authors' conclusions
Very limited data were available regarding the safety of early discharge compared to non‐early discharge from in‐hospital treatment in children with cancer and febrile neutropenia and a low risk for invasive infection. The absence of clear evidence of differences in both studies could be due to lack of power.
Evidently, there are still profound gaps regarding very early and early discharge in children with cancer and febrile neutropenia. Future studies that assess this subject should have a large sample size and aim to establish uniform and objective criteria regarding the identification of a low‐risk febrile neutropenic episode.
Plain language summary
Very early discharge versus early discharge versus non‐early discharge in children with cancer and fever during neutropenia
Review question In this review of the literature we aimed to determine whether early discharge (less than five days, on average) from in‐hospital treatment, for a selected group of children, is not inferior to non‐early discharge (five days or more, on average) in children with cancer and fever during neutropenia. Furthermore, we wanted to evaluate whether very early discharge (less than 24 hours, on average) is not inferior to early discharge, and whether very early discharge is not inferior to non‐early discharge.
Background Treatment with chemotherapy can cause a low white blood cell count (neutropenia) in children with cancer. Due to the high risk of bacterial infections and of a sudden and severe course of infections, standard care for children with cancer and fever during neutropenia consists of routine hospitalization and intravenous administration of broad‐spectrum antibiotics (antibiotics that act against a wide range of disease‐causing bacteria). However, causes of fever during neutropenia can be less serious; in a subgroup of these children lengthy in‐hospital treatment might be unnecessary.
Study characteristics The evidence is current to December 2015. The current review identified one study, Santolaya 2004, in which early discharge was compared to non‐early discharge in this group of children, and one study, Brack 2012, in which very early discharge was compared to early discharge.
Key results Early discharge did not appear to be less safe than non‐early discharge in children with cancer and fever during neutropenia with a low risk for bacterial infections; there was no clear evidence of difference in treatment failure between the two groups. Moreover, the treatment costs in the early discharge group were lower than in the non‐early discharge group. Regarding very early discharge, this did not appear to be less safe than early discharge; there was no clear evidence of difference in treatment failure between the two groups. Duration of treatment differed between very early discharge and early discharge; duration of intravenous antibiotic treatment was shorter in the very early discharge group, and duration of oral antibiotic treatment was shorter in the early discharge group, as compared to one another. However, there was no clear evidence of difference in total treatment duration of any antibiotic treatment between these groups.
Quality of the evidence For both reported comparisons, the quality of the evidence was low. The included studies were relatively small with a low number of participants, thus it was possible that the absence of clear evidence of differences in the included studies could be due to, for example, the lack of power. Unfortunately, it was not possible to pool data in the two studies.
Conclusion In conclusion, regarding both rehospitalization or adjustment of antibiotics (or both) and death, evidence was fairly limited; however, there was no evidence that early discharge was less safe than non‐early discharge or very early discharge was less safe than early discharge of children with cancer and fever during neutropenia and a low risk for invasive bacterial infection. Future larger trials are needed to confirm or contradict these results.
Summary of findings
Summary of findings for the main comparison. Early discharge versus non‐early discharge for children with cancer and febrile neutropenia at low risk for invasive bacterial infection.
| Early discharge versus non‐early discharge for children with cancer and febrile neutropenia at low risk for invasive bacterial infection | ||||||
| Patient or population: children with cancer Settings: hospital/home Intervention: early discharge (mean/median of < 5 days) Comparison: non‐early discharge (mean/median of ≥ 5 days) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of episodes (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Non‐early discharge (mean/median ≥ 5 days) | Early discharge (mean/median < 5 days) | |||||
| Treatment failure; rehospitalization or adjustment of antimicrobial treatment, or both in number of episodes | 56 per 10001 | 51 per 1000 (14 to 197) | RR 0.91 (0.24 to 3.5) | 149 (1 study) | ⊕⊕⊝⊝ low2 | ‐ |
| Treatment failure; death | 14 per 10001 | 0 per 1000 (0 to 0) | RR not estimable1 | 149 (1 study) | ⊕⊕⊝⊝ low2 | ‐ |
| Costs in USD | The mean cost of treatment was USD903 | MD USD 265 lower (403.14 lower to 126.86 lower) | ‐ | 149 (1 study) | ⊕⊕⊝⊝ low2 | ‐ |
| Duration of total antimicrobial treatment in days | The mean duration of treatment was 6.4 days | MD 0.3 days fewer (1.22 fewer to 0.62 more) | ‐ | 149 (1 study) | ⊕⊕⊝⊝ low2 | ‐ |
| Duration of intravenous antimicrobial treatment in days | The mean duration of treatment was 4.8 days | MD 0.5 days fewer (1.36 fewer to 0.36 more) | ‐ | 149 (1 study) | ⊕⊕⊝⊝ low2 | ‐ |
| Duration of oral antimicrobial treatment in days | The mean duration of treatment was 1.6 days | MD 0.2 days fewer (0.51 fewer to 0.91 more) | ‐ | 149 (1 study) | ⊕⊕⊝⊝ low2 | ‐ |
| Quality of life | Not assessed | Not assessed | ‐ | ‐ | Not estimable | No information on quality of life was provided |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There were no deaths in the early discharge group and one death in the non‐early discharge group (Fischer's exact P value = 0.477).
2 We downgraded the level of evidence one level because of an unclear risk of bias that lowered our confidence in the estimate of effect (i.e. high risk of performance bias and unclear risk of selection bias, detection bias, attrition bias and reporting bias). In addition, we downgraded the level of evidence one level because of imprecision (dichotomous outcome did not meet their "rule of thumb" threshold, i.e. total number of events was fewer than 300, and continuous outcomes did also not meet their "rule of thumb" threshold, i.e. total number of participants was fewer than 400).
Summary of findings 2. Very early discharge versus early discharge for children with cancer and febrile neutropenia at low risk for invasive bacterial infection.
| Very early discharge versus early discharge for children with cancer and febrile neutropenia at low risk for invasive bacterial infection | ||||||
| Patient or population: children with cancer Settings: hospital/home Intervention: very early discharge (mean/median of < 24 hours) Comparison: early discharge (mean/median of < 5 days) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of episodes (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Early discharge (mean/median < 5 days) | Very early discharge (mean/median < 24 hours) | |||||
| Treatment failure; rehospitalization or adjustment of antimicrobial treatment (or both) in number of episodes | 206 per 10001 | 111 per 1000 (31 to 389) | RR 0.54 (0.15 to 1.89) | 61 (1 study) | ⊕⊕⊝⊝ low2 | ‐ |
| Treatment failure; rehospitalization or adjustment of antimicrobial treatment (or both), only first episode in number of participants | 259 per 10001 | 83 per 1000 (18 to 363) | RR 0.32 (0.07 to 1.4) | 51 (1 study) | ⊕⊕⊝⊝ low2 | ‐ |
| Treatment failure; death | 29 per 10001 | 0 per 1000 (0 to 0) | RR not estimable. Fischer's exact P value = 0.557 | 61 (1 study) | ⊕⊕⊝⊝ low2 | ‐ |
| Costs | Not assessed | Not assessed | ‐ | ‐ | Not estimable | No information on costs was provided |
| Duration of total antimicrobial treatment | Median duration of treatment: 5 days (range 2 to 19 days) | Median duration of treatment: 5 days (range 3 to 18 days) | RR not estimable. Wilcoxon's P value = 0.34 |
61 (1 study) | ⊕⊕⊝⊝ low2 | Data from study, Wilcoxon's P as stated in the study |
| Duration of intravenous antimicrobial treatment | Median duration of treatment: 4.5 days (range 1 to 18 days) | Median duration of treatment: 1 day (range 1 to 13 days) | RR not estimable. Wilcoxon's P value ≤ 0.001 |
61 (1 study) | ⊕⊕⊝⊝ low2 | Data from study, Wilcoxon's P as stated in the study |
| Duration of oral antimicrobial treatment | Median duration of treatment: 0 days (0 to 7 days) | Median duration of treatment: 4 days (0 to 14 days) | RR not estimable. Wilcoxon's P ≤ 0.001 |
61 (1 study) | ⊕⊕⊝⊝ low2 | Data from study, Wilcoxon's P as stated in the study |
| Quality of life | Not assessed | Not assessed | ‐ | ‐ | Not estimable | No information on quality of life was provided |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The assumed risk was based on the prevalence in the control group, i.e. early discharge, of the included study.
2 We downgraded the level of evidence one level because of an unclear risk of bias that lowered our confidence in the estimate of effect (i.e. high risk of performance bias and other bias and unclear risk of selection bias, detection bias and reporting bias). In addition, we downgraded the level of evidence another level because of imprecision (outcomes did not meet the "rule of thumb" threshold, i.e. total number of events was fewer than 300).
Background
Description of the condition
Survival rates for children with cancer have improved substantially in recent decades (O'Leary 2008). This has been attributed to better understanding of the disease, improvement of treatment protocols and optimalization of supportive care. However, cancer treatment also has unwanted adverse effects. One of the most important adverse effects in children with cancer is chemotherapy‐induced neutropenia, a haematological disorder characterized by an abnormally low number of neutrophils (type of granulocytes; category of white blood cells). In 1966, it was shown that low numbers of granulocytes were associated with an increased risk of severe infections (Bodey 1966). Due to the high relative risk of infections and infectious complications, standard care for children with cancer and febrile neutropenia (severe neutropenia with fever) consists of routine hospitalization and parenteral administration of broad‐spectrum antibiotics. Children are considered eligible for discharge from in‐hospital treatment when they are afebrile, have completed their antibiotic course, their absolute neutrophil count is recovering or has recovered, or a combination of these.
Description of the intervention
In 2002, the Infectious Diseases Society of America published a clear protocol for early discharge in adult with cancer and febrile neutropenia (Hughes 2002). A weighted scoring index for identification of adult low‐risk febrile neutropenia at time of presentation with fever comprised extent of illness, presence of hypotension, presence of chronic obstructive pulmonary disease, presence of solid tumor, presence of fungal infection, presence of dehydration, outpatient location of onset of fever and age. A recent review focussed on the effects and applicability of this weighted scoring index after 10 years of use (Klastersky 2013). This review concluded that the weighted scoring index has been validated in several studies as a reliable tool for identifying low‐risk febrile neutropenia episodes in adult cancer patients and is shown to be part of the selection process of patients who can safely be treated at home.
However, early discharge of pediatric patients was a more delicate topic; it was stated that after a minimum of 48 hours of in‐hospital treatment with parenteral antibiotics and observation, early discharge with oral antibiotics might be considered for selected children at low risk of bacterial infections (Hughes 2002). In this protocol, low risk for bacterial infection in children with cancer was defined as proposed by Paganini et al. and Shenep et al.; absence of severe co‐morbidity, good clinical condition, negative blood cultures, no Pseudomonas aeruginosa or methicillin‐resistant Staphylococcus aureus (MRSA) in cultures in the last 12 weeks, control of local infection and afebrile for the last 24 hours (Paganini 2000; Shenep 2001).
Why it is important to do this review
In 70% to 89% of febrile neutropenia episodes no causative organism is found (Ariffin 2006; Hodgson‐Viden 2005; Oude Nijhuis 2005; Petrilli 1993; Santolaya 1997). This implies that people with febrile neutropenia are a heterogeneous group, in which fever can be caused by a bacterial infection, but also, for example, by viruses, transfusion of blood products or chemotherapeutics. As a consequence, a large proportion of people are admitted to hospital and receive standard care unnecessarily.
Various research groups have studied the adjustment of standard care to shorten in‐hospital treatment for children with cancer with low‐risk febrile neutropenia, however, not in a randomized matter (Oude Nijhuis 2005; Paganini 2000; Shenep 2001; Wacker 1997). In this review, we have compared early discharge (mean/median of less than five days) from in‐hospital treatment to non‐early discharge (mean/median of five days or more) from in‐hospital treatment, very early discharge (mean/median of less than 24 hours) from in‐hospital treatment to early discharge (mean/median of less than five days) from in‐hospital treatment, and very early discharge (mean/median of less than 24 hours) from in‐hospital treatment to non‐early discharge (mean/median of five days or more) from in‐hospital treatment in children with cancer and febrile neutropenia, and evaluated the effects on treatment failure. The main importance was to gather and share the evidence in safety of non‐early discharge versus early and very early discharge and moreover, that unnecessary non‐early discharge might lead, for example, to unnecessary occupation of hospital beds, increased bacterial resistance, reduced quality of life and increased healthcare costs.
Objectives
To evaluate whether early discharge (mean/median of less than five days) from in‐hospital treatment was not inferior to non‐early discharge (mean/median of five days or more) and whether very early discharge (mean/median of less than 24 hours) was not inferior to early discharge, non‐early discharge, or a combination of these, in children with cancer and febrile neutropenia.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials and controlled clinical trials.
Types of participants
Children with cancer under 21 years of age, presenting with febrile neutropenia. We defined neutropenia as an absolute neutrophil count less than 0.5 x 109 cells/L, or a leukocyte count less than 1.0 x 109 cells/L (when an absolute neutrophil count was not available). We defined fever as a single oral reading of greater than 38.2 °C or two readings of a temperature greater than 37.9 °C within 24 hours.
Types of interventions
We defined:
non‐early discharge as discharge from in‐hospital treatment after (a mean or median of) at least five days;
early discharge as discharge from in‐hospital treatment (with a mean or median of) less than five days after presentation with fever and neutropenia;
very early discharge as discharge from in‐hospital treatment (with a mean or median of) less than 24 hours after presentation with fever and neutropenia.
We planned to include studies that compared the following in children with cancer and febrile neutropenia at low risk of invasive bacterial infection:
early discharge from in‐hospital treatment versus non‐early discharge from hospital treatment;
very early discharge from in‐hospital treatment versus non‐early discharge from hospital treatment;
very early discharge from in‐hospital treatment versus early discharge from hospital treatment.
Types of outcome measures
Primary outcomes
Treatment failure:
rehospitalization or adjustment of antimicrobial treatment (or both) related to febrile neutropenia (participant deterioration or other febrile neutropenia‐related causes), within one week or within the same neutropenic episode;
all death and death due to (complications of) febrile neutropenia within one week after hospital discharge or within the same neutropenic episode after hospital discharge.
Secondary outcomes
Quality of life.
Costs.
Duration of antimicrobial treatment:
duration of total antimicrobial treatment;
duration of intravenous antimicrobial treatment;
duration of oral antimicrobial treatment.
Search methods for identification of studies
See: Cochrane Childhood Cancer Group methods used in reviews (Module CCG). We imposed no language restrictions. We will update the searches every two years.
Electronic searches
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (2015; Issue 11), MEDLINE/PubMed (from 1945 to 9 December 2015) and EMBASE/Ovid (from 1980 to 9 December 2015). The appendices show the search strategies for the different electronic databases (using a combination of controlled vocabulary and text words) (Appendix 1; Appendix 2; Appendix 3).
Searching other resources
We located information about trials not registered in CENTRAL, MEDLINE or EMBASE, either published or unpublished, by searching the reference lists of relevant articles and review articles. We handsearched the conference proceedings of the International Society for Paediatric Oncology (SIOP) (2005 to 2015), the American Society of Clinical Oncology (ASCO) (2005 to 2015), the European Society for Paediatric Infectious Diseases (ESPID) (2005 to 2015), the Infectious Diseases Society of America (IDSA) (2005 to 2014), the European Hematology Association (EHA) (2006 to 2013), the American Society of Hematology (ASH) (2008 to 2015), the American Academy of Pediatrics (AAP) (2010 to 2015) and the Multinational Association for Supportive Care in Cancer (MASCC) (2005 to 2015), dependent on availability. We scanned the International Standard Randomised Controlled Trials Number (ISRCTN) Register, the National Institute of Health Register for ongoing trials and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) on 9 January 2016. Appendix 4 and Appendix 5 summarize the search strategies for the different conference proceedings and the ongoing trial registries.
Data collection and analysis
Selection of studies
After employing the search strategy described above, two review authors independently identified studies meeting the inclusion criteria. We resolved discrepancies between review authors by consensus. If this had been impossible, we planned to achieve final resolution by using a third party arbitrator. However, this was not necessary. We obtained the full‐text reports of any study that seemed to meet the inclusion criteria on the grounds of the title, abstract, or both, for closer inspection. Regarding the studies excluded after closer inspection, we stated the reasons for exclusion in the Characteristics of excluded studies table.
Data extraction and management
Two review authors independently performed data extraction using standardized forms. We extracted data on the following items.
Study design.
-
Participants, including:
age;
sex;
number of children entering the trial;
number of children randomized;
number of children excluded (with reasons);
number of children evaluable (for each outcome);
degree of neutropenia at the moment of presentation with febrile neutropenia.
Intervention: duration of admittance to the hospital in hours/days until discharge from in‐hospital treatment. In‐hospital treatment: no, oral or intramuscular/intravenous antibiotic treatment. Treatment after discharge: no, oral or intramuscular/intravenous antibiotic treatment.
Outcome measures.
Length of follow‐up.
When data were missing in a published report, we attempted to contact the authors for the missing information. This was the case in one of the included studies (Brack 2012), as is stated below (Dealing with missing data). In cases of disagreement regarding data extraction, we planned to re‐examined the abstracts and articles and undertake discussion until we achieved consensus. If this was impossible, we planned to achieve final resolution using a third party arbitrator. However, this was not necessary.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of the included randomized controlled trials, according to the following criteria:
random sequence generation;
concealment of allocation;
blinding of care provider/participants/outcome assessors;
incomplete outcome data;
selective reporting;
other bias, specifically baseline imbalance (e.g. due to selective randomization), differential diagnostic activity (e.g. due to other follow‐up programmes for different discharge moments) and selective reporting of subgroups.
For the quality items, we used the definitions as described in the module of the Childhood Cancer Group, based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Module CCG). We resolved discrepancies between review authors by consensus. If this was impossible, we planned to achieve final resolution using a third party arbitrator. However, this was not necessary. In the analyses, we took the quality of study into account in the interpretation of the review results.
Measures of treatment effect
We entered data into Review Manager 5 (RevMan 2012) and undertook analyses according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We analyzed dichotomous variables using risk ratios (RR). We analyzed continuous outcomes using the mean difference (MD). We presented results with the corresponding 95% confidence interval (CI). For outcomes where only one study was available, we were unable to calculate an RR if one of the treatment groups experienced no events and we used the Fischer’s exact test instead (using SPSS 20.0 (IBM Corp., Armonk, NY, USA)).
Dealing with missing data
When information relevant to study selection, data extraction, assessment of risk of bias, or a combination of these was missing, we attempted to contact the authors in order to obtain the missing data. We contacted the authors of one of the included studies because their definition of the outcome treatment failure was slightly different from ours (i.e. in addition to rehospitalization or adjustment of antimicrobial therapy (or both) and death (our definition of treatment failure), in the included study fever greater than 48 hours, bacteraemia, persistent symptoms and signs of local infection or addition of antifungal therapy (or both) were also considered as treatment failure) (Brack 2012). We asked the authors to specify the reasons for treatment failure of all episodes of which the reason was not stated specifically or clearly (or both) in the full‐text article. They responded swiftly and provided the requested data.
We planned to perform intention‐to‐treat analyses. In Brack 2012, it was clear that the intention‐to‐treat principle was used, therefore we performed intention‐to‐treat analyses of these data. However, in Santolaya 2004, it was not explicitly described whether or not the intention‐to‐treat principle was used (although it seems to be done), so we were unable to do this.
Assessment of heterogeneity
In the protocol, we stated that we would assess heterogeneity both by visual inspection of the forest plots and by a formal statistical test for heterogeneity, the I2 statistic. In the absence of substantial heterogeneity (I2 less than 50%) (Higgins 2011), we wanted to use a fixed‐effect model for the estimation of treatment effects. Otherwise, we wanted to explore possible reasons for the occurrence of heterogeneity and take appropriate measures by using the random‐effects model. However, since we could not pool the data from the included studies, this was not applicable.
Assessment of reporting biases
In the protocol, we stated that we would construct a funnel plot to ascertain the risk of publication bias graphically (Higgins 2011). However, since we could not pool the data from the included studies, this was not applicable.
Data synthesis
In the protocol, we stated that when possible we would analyze data for different types of malignancies. Due to lack of information, this was not possible. We included outcome measures in this systematic review only if it was the intention of the study to perform the necessary assessments in all participants (i.e. not optional or only performed in some centres). When less than 50% of the participants of a study had an acceptable follow‐up for a particular outcome measure, due to the associated high risk of attrition bias, we planned not to report the results of this outcome measure. However, since in Santolaya 2004 there were no episodes lost to follow‐up and in Brack 2012 there was one out of 62 episodes lost to follow‐up, this was not applicable. If pooling was not possible, we summarized the results qualitatively.
For each comparison. we prepared when possible a 'Summary of findings' table using the GRADEpro software in which we presented the following outcomes: treatment failure (i.e. rehospitalization or adjustment of antimicrobial treatment (or both), and death), quality of life, costs and duration of antimicrobial treatment (i.e. total, intravenous and oral). However, in Table 2 (very early discharge versus early discharge), we divided treatment failure into three outcomes (i.e. rehospitalization or adjustment of antimicrobial treatment in number of episodes (or both) and in number of participants, and death). Two review authors independently assessed the quality of the evidence using the five GRADE considerations (i.e. study limitations, inconsistency, indirectness, imprecision and publication bias).
Subgroup analysis and investigation of heterogeneity
In the protocol, we stated that when possible we would perform subgroup analyses. However due to lack of information or lack of stratified data subgroup analyses were not possible. We planned to analyze:
-
in‐hospital treatment:
in‐hospital treatment with intravenous or intramuscular antibiotics versus oral antibiotics;
in‐hospital treatment with intravenous or intramuscular antibiotics versus no antibiotics;
in‐hospital treatment with oral antibiotics versus no antibiotics;
-
treatment after discharge:
treatment after discharge with intravenous or intramuscular antibiotics versus oral antibiotics;
treatment after discharge with intravenous or intramuscular antibiotics versus no antibiotics;
treatment after discharge with oral antibiotics versus no antibiotics;
age: birth to under four years versus four to less than 21 years;
type of malignancy: haematological malignancies versus solid tumours;
degree of neutropenia at the moment of presentation with febrile neutropenia; absolute neutrophil count of 0.1 to 0.5 x 109 cells/L versus less than 0.1 x 109 cells/L.
Sensitivity analysis
In the protocol, we stated that we wanted to perform a sensitivity analysis; however, this was not possible or applicable (or both). Regarding the individual risk of bias criteria, it was not possible to perform sensitivity analyses considering the fact that pooling was not possible.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
See: flow diagram of selection of studies (Figure 1).
1.
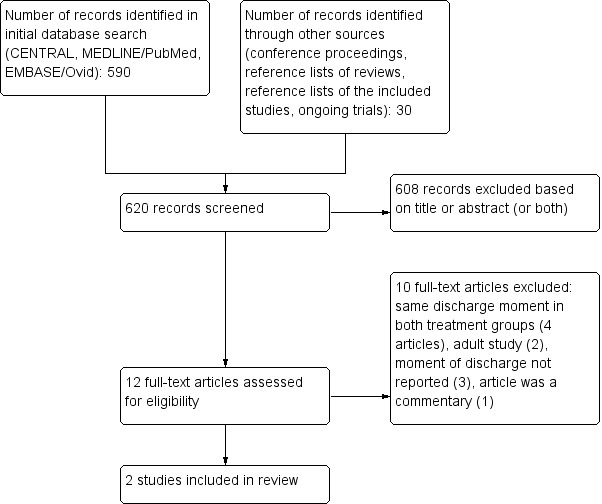
Flow diagram of selection of studies.
The search strategy identified 590 references in the three electronic databases, of which we evaluated 12 studies as full text as potential studies for inclusion. Two of these fulfilled all criteria for inclusion in this review and were thus included (Brack 2012; Santolaya 2004). We excluded the other 10 studies for reasons stated in the Characteristics of excluded studies table. We excluded all other studies based on title or abstract, since they were not randomized controlled trials or controlled clinical trials, did not include children, did not include children with cancer, defined fever otherwise than described in our inclusion criteria or defined neutropenia otherwise than described in our inclusion (or both).
The search performed on the conference proceedings, reference lists of reviews and the reference lists of the included studies identified 30 additional studies. We excluded all references based on title and abstract, for same reasons as described in the initial database search.
In the latest search for ongoing trials, we searched the registries of ISRCTN (www.ISRCTN.com), National Institutes of Health (NIH) (clinicaltrials.gov) and WHO ICTRP (apps.who.int/trialsearch/) in January 2016): we identified no relevant ongoing trials.
Included studies
For inclusion in this review, only children with fever during neutropenia at low risk for invasive bacterial infection were eligible.
Santolaya 2004 was a randomized controlled trial that compared outcome and costs of early discharge versus non‐early discharge among children with fever during neutropenia at low risk for invasive bacterial infection. A total of 390 episodes of febrile neutropenia occurred in 313 children with cancer; 168 episodes were classified as low‐risk at enrolment. After the second assessment of the low‐risk children, five children appeared to be high risk; they were excluded from the study. Of the 161 episodes at low‐risk for invasive bacterial infection, 12 (41%) could not be randomized due to various reasons. The remaining 149 episodes in 107 children were randomly assigned in parallel groups after 24 to 36 hours of hospitalization to receive ambulatory (78 children) or hospital‐based (71 children) treatment and they were monitored until episode resolution. Antibiotic treatment consisted of intravenous ceftriaxone and teicoplanin. Intravenous antibiotic treatment was switched to oral cefuroxime after a minimum of 72 hours when the clinical evolution was favourable. Outcome and costs were determined for each episode and compared between both groups using pre‐defined definitions and questionnaires.
Brack 2012 was a randomized controlled trial that investigated safety and efficacy of very early discharge versus early discharge among children with fever during neutropenia at low risk for invasive bacterial infection. All included children were re‐assessed after eight to 22 hours of inpatient therapy. Children then identified with low‐risk febrile neutropenia were randomized to either very early discharge (mean/median of less than 24 hours) or early discharge (continued inpatient therapy for more than 24 hours with a median of four days). A total of 355 potentially eligible episodes of febrile neutropenia occurred, of which 93 (26%) fulfilled low‐risk criteria at re‐assessment after eight to 22 hours of inpatient therapy. Of these, informed consent for randomization was declined in 25 (27%) episodes, and randomization was not performed for unknown reasons in six (6%) episodes. Thus, 62 (67%) low‐risk episodes in 52 children (eight children with two episodes, one child with three episodes) were randomized to very early discharge (28 children, one lost to follow‐up) or early discharge (34 children). All children but one (lost to follow‐up; centre stopped study participation) were monitored until antimicrobial therapy had been stopped for at least seven days and severe neutropenia had resolved. All children were initially treated with empirical intravenous broad‐spectrum antimicrobial therapy. In episodes randomized to early discharge, intravenous antimicrobial therapy was continued, in episodes randomized to very early discharge, intravenous antimicrobial therapy was replaced by a combination of oral ciprofloxacin and oral amoxicillin. Outcomes regarding safety and efficacy were determined for each episode and compared between both groups using pre‐defined definitions.
For more information, see the Characteristics of included studies table.
Excluded studies
After full‐text review, we excluded 10 studies that initially appeared to be potential for inclusion based on same discharge moment in both groups (four studies), adult study (two studies), moment of discharge not reported (three studies) and article was a commentary (one study).
Risk of bias in included studies
See 'Risk of bias' section of the Characteristics of included studies table and the 'Risk of bias' summary (Figure 2).
2.
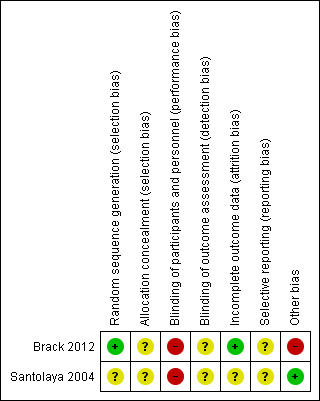
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
In Santolaya 2004, there was an unclear risk of selection bias (based on random sequence generation and allocation concealment), a high risk of performance bias (based on lack of blinding of participants and personnel), an unclear risk of detection bias (based on blinding of outcome assessment) for all reported outcomes, an unclear risk of attrition bias (based on incomplete outcome data), an unclear risk of reporting bias (based on selective reporting) and a low risk of other bias (no other risk of bias identified, clear explanation of not performed randomizations).
In Brack 2012, there was an unclear risk of selection bias (based on a low risk of random sequence generation, but an unclear risk of allocation concealment), a high risk of performance bias (based on lack of blinding of participants and personnel), an unclear risk of detection bias (based on blinding of outcome assessment) for all reported outcomes, a low risk of attrition bias (based on incomplete outcome data), an unclear risk of reporting bias (based on selective reporting) and a high risk of other bias (based on a high risk of baseline imbalance due to lack of explanation of declined and not performed randomizations in 25 (declined) and six (not performed) of 93 episodes).
Effects of interventions
Due to different study questions (i.e. very early discharge versus early discharge in Brack 2012 and early discharge versus non‐early discharge in Santolaya 2004), and the use of different low‐risk episode criteria, it was not possible to pool results. We entered the data from Santolaya 2004 and Brack 2012 into Review Manager 5 (RevMan 2012).
Early discharge versus non‐early discharge
One study compared early discharge versus non‐early discharge (Santolaya 2004).
Primary outcomes
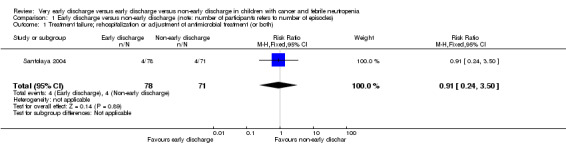
Rehospitalization or adjustment of antimicrobial treatment (or both)
The study stated that rehospitalization or adjustment of antimicrobial treatment, or both, within the same neutropenic episode, occurred in 4 out of the 78 episodes (5%) in the early discharge group and in 4 out of the 71 episodes (6%) in the non‐early discharge group (RR 0.91; 95% CI 0.24 to 3.50, P value = 0.89) (Figure 3).
3.
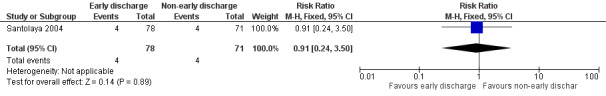
Forest plot of comparison: 1 Early discharge versus non‐early discharge, outcome: 1.1 Treatment failure; rehospitalization or adjustment of antimicrobial treatment (or both).
Death
The study stated that there were no deaths due to complications of febrile neutropenia within the same neutropenic episode in the early discharge group. There was one death in the non‐early discharge group (Fischer's exact P value = 0.477).
Secondary outcomes
Quality of life
This study did not assess the pre‐defined outcome measure quality of life.
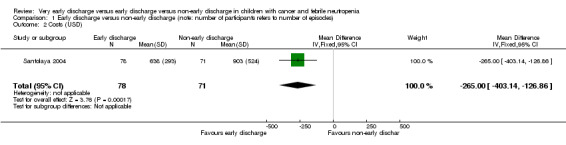
Costs
The study stated that the mean costs for early discharge treatment were significantly lower than the costs for the non‐early discharge treatment (USD 638; 95% CI 572 to 703 with early discharge versus USD 903; 95% CI USD 781 to USD 1025 with non‐early discharge, stated in the study). There was an MD of USD ‐265.00 (95% CI ‐403.14 to ‐126.86, P value = 0.0002) when early discharge was compared to non‐early discharge in favour of early discharge (Figure 4).
4.
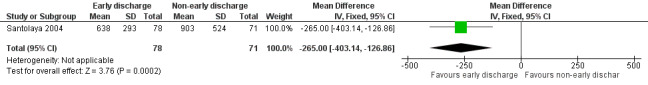
Forest plot of comparison: 1 Early discharge versus non‐early discharge, outcome: 1.2 Costs (USD).
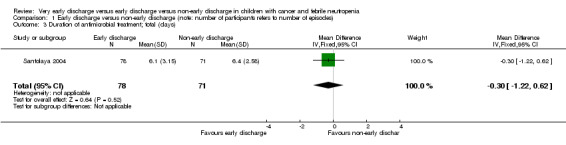
Duration of antimicrobial treatment
The study stated that the mean duration of antimicrobial treatment was 6.1 days (95% CI 5.4 to 6.8, stated in the study) in the early discharge group and 6.4 days (95% CI 5.9 to 7.0, stated in the study) in the non‐early discharge group. The MD of early discharge compared to non‐early discharge was ‐0.3 days (95% CI ‐1.22 to 0.62, P value = 0.52) (Figure 5).
The mean duration of intravenous antimicrobial treatment was 4.3 days (95% CI 3.7 to 5.0, stated in the study) in the early discharge group and 4.8 days (95% CI 4.4 to 5.3, stated in the study) in the non‐early discharge group. The MD of early discharge compared to non‐early discharge was ‐0.5 days (95% CI ‐1.36 to 0.36, P value = 0.25) (Figure 6).
The mean duration of oral antimicrobial treatment was 1.8 days (95% CI 1.2 to 2.3, stated in the study) in the early discharge group and 1.6 days (95% CI 1.1 to 2.1, stated in the study) in the non‐early discharge group. The MD of early discharge compared to non‐early discharge was 0.2 days (95% CI ‐0.51 to 0.91, P value = 0.58) (Figure 7).
5.
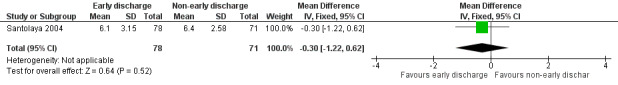
Forest plot of comparison: 1 Early discharge versus non‐early discharge, outcome: 1.3 Duration of antimicrobial treatment; total (days).
6.
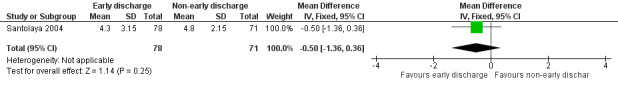
Forest plot of comparison: 1 Early discharge versus non‐early discharge, outcome: 1.4 Duration of antimicrobial treatment; intravenous (days).
7.
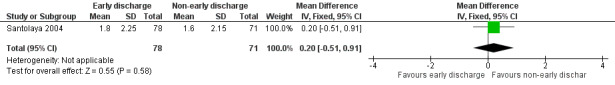
Forest plot of comparison: 1 Early discharge versus non‐early discharge, outcome: 1.5 Duration of antimicrobial treatment; oral (days).
Subgroup analyses and sensitivity analyses were not possible or applicable, or both.
Sensitivity analyses for risk of bias items were not possible.
We did not perform intention‐to‐treat analyses as it was not explicitly described whether or not the intention‐to‐treat principle was used.
Very early discharge versus early discharge
One study compared very early discharge versus early discharge (Brack 2012).
Primary outcomes
Rehospitalization or adjustment of antimicrobial treatment (or both)
The study stated that rehospitalization or adjustment of antimicrobial treatment (or both), within the same neutropenic episode, occurred in 3 out of 27 episodes (11%) in the very early discharge group and in 7 out of 34 episodes (21%) in the early discharge group (RR 0.54; 95% CI 0.15 to 1.89, P value = 0.34) (Figure 8).
8.
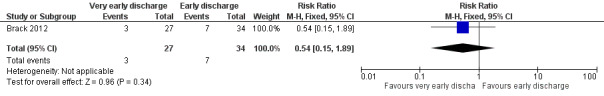
Forest plot of comparison: 2 Very early discharge versus early discharge, outcome: 2.1 Treatment failure; rehospitalization or adjustment of antimicrobial treatment (or both).
In this study, the authors also performed an analysis with only the first febrile neutropenic episode of all included children. In this study, there were 61 episodes of 51 children included, thus 10 episodes were excluded. Rehospitalization or adjustment of antimicrobial treatment (or both), within the same neutropenic episode, occurred in 2 out of 24 children (8%) in the very early discharge group and in 7 out of 27 children (26%) in the early discharge group (RR 0.32; 95% CI 0.07 to 1.40, P value = 0.13) (Figure 9).
9.
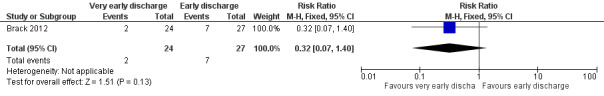
Forest plot of comparison: 2 Very early discharge versus early discharge (note: number of participants refers to number of episodes), outcome: 2.2 Treatment failure; rehospitalization or adjustment of antimicrobial treatment (or both), only first episode (note: number of participants stated instead of episodes).
Death
The study stated that there were no deaths due to complications of febrile neutropenia within the same neutropenic episode in the very early discharge group. There was one death in the early discharge group (Fischer's exact P value = 0.557).
Secondary outcomes
Quality of life
This study did not assess the pre‐defined outcome measure quality of life.
Costs
This study did not assess the pre‐defined outcome costs.
Duration of antimicrobial treatment
The study reported only the median and range duration of antimicrobial treatment. Therefore, we were unable to analyse results in Review Manager 5 (RevMan 2012).
The median duration of any antimicrobial treatment was 5 days (range 2 to 19 days, stated in the study) in the very early discharge group and 5 days (range 3 to 18 days, stated in the study) in the early discharge group (Wilcoxon's P value = 0.34, stated in the study).
The median duration of intravenous antimicrobial treatment was 1 day (range 1 to 13 days, stated in the study) in the very early discharge group and 4.5 days (range 1 to 18 days, stated in the study) in the early discharge group (Wilcoxon's P value ≤ 0.001, stated in the study).
The median duration of oral antimicrobial treatment was 4 days (range 0 to 14 days, stated in the study) in the very early discharge group and 0 days (range: 0 to 7 days, stated in the study) in the early discharge group (Wilcoxon's P value ≤ 0.001, stated in the study).
Subgroup analyses and sensitivity analyses were not possible or applicable, or both.
Sensitivity analyses for risk of bias items were not possible.
Discussion
Summary of main results
There were two studies that met the inclusion criteria for this review; Santolaya 2004 compared an early discharge (mean/median of less than five days) group with a non‐early discharge (mean/median of five days or more) group and Brack 2012 compared a very early discharge (mean/median of less than 24 hours) group with an early discharge (mean/median of less than five days) group. Both studies evaluated treatment failure and duration of treatment, only Santolaya 2004 evaluated costs of treatment and neither study evaluated quality of life. Regarding treatment failure, neither study identified significant differences between treatment groups in treatment failure (i.e. rehospitalization or adjustment of antimicrobial treatment (or both) and death). Regarding duration of antibiotic treatment (i.e. total, intravenous and oral antibiotics), Santolaya 2004 found no significant differences between treatment groups. Brack 2012 found significant shorter intravenous antimicrobial therapy in the very early discharge group and significantly shorter oral antimicrobial therapy in the early discharge group as compared to the other group; however, there was no significant difference in total duration of antibiotics between these groups. Santolaya 2004 found significantly lower costs in favour of the early discharge group as compared to the non‐early discharge group. Subgroup analyses were not possible.
Since both included studies had a relatively small number of randomized episodes (149 and 61), the non‐significant differences between treatment groups of the diverse results might be because the included studies were too small to detect a difference (i.e. low power). However, the included studies did not show that early discharge of children with cancer and febrile neutropenia at low risk for invasive bacterial infection was less safe than non‐early discharge, or that very early discharge of children with cancer and febrile neutropenia at low risk for invasive bacterial infection was less safe than early discharge; there were no significant differences in treatment failure between the two groups in both studies.
For more information, see the Table 1 and Table 2. Due to insufficient data, we prepared no 'Summary of findings' table for the comparison of very early discharge (mean/median of less than 24 hours) with non‐early discharge (mean/median of five days or more).
Overall completeness and applicability of evidence
Currently there are no uniform criteria regarding identification of low‐risk febrile neutropenic episodes in children with cancer. The criteria used in the included studies are shown in the Characteristics of included studies table. The main difference between the included studies was that Brack 2012 mainly used clinical parameters(e.g. focal infection and temperature), where as Santolaya 2004 also incorporated laboratory values (e.g. C‐reactive protein and platelet count). With the studies using their own low‐risk criteria it makes it more difficult to interpret results correctly, therefore it is of the utmost importance to establish uniform low‐risk criteria. Regarding this matter, there is currently a Delphi survey being held among paediatric oncologists. Once uniform low‐risk criteria have been established, these should be prospectively validated in a large study among children with cancer, as has happened in adults with cancer.
At the moment, there is very little evidence available regarding early or very early discharge in children with cancer and febrile neutropenia at low risk for invasive bacterial infection. The current available evidence does not show that early discharge of children with cancer and febrile neutropenia and low risk of bacterial infection is less safe than non‐early discharge, or that very early discharge of children with cancer and febrile neutropenia and low risk of bacterial infection is less safe than early discharge. In addition, one study found a significant reduction of costs for the early discharge group in comparison to the non‐early discharge group. It is our opinion that, at this moment, both very early discharge and early discharge should only be practised in a trial setting in hospitals/oncology wards where close monitoring of very early and early discharged participants by well‐trained employees is guaranteed.
Quality of the evidence
Both included studies were randomized controlled trials and thus qualified as high quality evidence according to the GRADE approach. However, according to factor 1 in the GRADE assessment (limitations in the design and implementation of available studies suggesting high likelihood of bias), we downgraded the level of evidence one level because of an unclear risk of bias with potential limitations that are likely to lower confidence in the estimate of effect. Both included studies showed a high risk of performance bias (based on lack of blinding of participants and personnel), as it is obvious for participants and personnel whether participants are treated inside or outside the hospital. This can cause bias as participants can report their symptoms differently because they feel unsafe having fever and not being admitted to the hospital or this could lead to underreporting symptoms as participants or their parents want them to stay at home. However, due to the clear parameters for treatment failure, this risk seems to be small. In both studies, there was an unclear risk of detection bias (based on blinding of outcome assessment) for all reported outcomes; if there was no blinding of outcome measurement this could inflict bias in favour of both treatment arms depending on the beliefs of the person handling the outcome measures. Moreover, in both studies there was an unclear risk of reporting bias (based on selective reporting) and selection bias. In addition, in Santolaya 2004, there was an unclear risk of attrition bias and a low risk of other bias, and in Brack 2012, there was a high risk of other bias (based on a high risk of baseline imbalance) and a low risk of attrition bias.
Agreements and disagreements with other studies or reviews
Since the 1990s, there has been a tendency to earlier discharge or treatment with oral or no antibiotics (or both) in people with fever and neutropenia considered to be at low risk for serious bacterial infections or infectious complications. Parameters for risk stratification in the heterogeneous group of people with febrile neutropenia have changed over the years (te Poele 2009). The prediction model for episodes with low risk for invasive bacterial infection provided by Santolaya et al. consisted of objective items and was reliable in their study cohort (Santolaya 2002). It would be interesting to see results of this prediction model in studies performed in oncology units in other countries, to establish whether these results can be replicated in other populations with different genetic and environmental factors. The studies accomplished by Ammann et al. and Miedema et al. illustrated that there can be a different outcome in a different population (Ammann 2010; Miedema 2011). The study of Ammann et al. prospectively evaluated a risk assessment model for adverse events during febrile neutropenia, consisting of: preceding chemotherapy more intensive than acute lymphoblastic leukaemia maintenance (weight 4), haemoglobin 90 g/L or greater (weight 5), leukocyte count less than 0.3 g/L (weight 3) and platelet count less than 50 g/L (weight 3), where a score (sum of weights) of 9 or greater predicted future adverse events (Ammann 2010). One difference between the two risk assessment models was the moment of assessment; at presentation and after 24 to 36 hours in the study performed by Santolaya et al. and at presentation and within eight to 24 hours after admittance to the hospital in the study performed by Ammann et al. Another main difference was the parameters included in the risk assessment; the only corresponding item was platelet count. Both research groups had objective parameters. The risk assessment model used by Ammann et al. accurately predicted adverse events in their population of children with cancer. In the retrospective study performed by Miedema et al., the use of the identical risk assessment model had different sensitivity and specificity levels. This could be due to the retrospective nature of the study; however, the different treatment protocol, the different genetic background of the study population and environmental factors may also play a role. Both sets of authors stressed the necessity of prospective validation of the risk assessment score before broad clinical application and evaluation of the potential of markers of inflammation to increase its predictive performance (Ammann 2011; Miedema 2011).
Criteria for unfavourable outcome in Santolaya 2004 were haemodynamic instability (not attributed to volume loss), fever after day four, re‐appearance of fever after a 48‐hour afebrile period persisting for at least 24 hours, an ascending C‐reactive protein curve or a non‐descending curve over normal limits (defined as a value greater than 40 mg/L and less than 30% decrease from a previous recording), and isolation of a bacterial pathogen from a significant sample obtained on day three, and death occurring during the febrile episode attributable to infection. In Brack 2012, criteria for unfavourable outcome were occurrence of serious medical complication (i.e. death, intensive care unit treatment, potentially life‐threatening complications as judged by the treating physician), no resolution of infection (i.e. fever 38.0 °C or greater for 48 hours or longer, persistent symptoms and signs of local infection where applicable and positive control blood cultures where applicable), recurrent infection, modification of randomized antimicrobial therapy or addition of antifungal therapy, microbiologically defined infections and radiologically confirmed pneumonias. In both included studies, unfavourable outcome led to consideration of adjustment of antimicrobial treatment in both treatment groups and rehospitalization in the early (in Santolaya 2004) or very early (in Brack 2012) discharge group. The criteria used in both studies were relatively objective parameters, which seemed to be adequate. However, in our opinion, fever for more than two to four days is not adequate as a parameter for treatment failure as it can also be caused by a viral infection. Therefore, we did not mention it as an item in our outcome criteria. Finally, we valued rehospitalization or adjustment of antimicrobial treatment (or both) related to febrile neutropenia and death as adequate parameters of treatment failure, for both the in‐hospital and the outpatient treatment group.
Authors' conclusions
Implications for practice.
The current available evidence, however fairly limited, did not show that early discharge of children with cancer and febrile neutropenia and low risk of bacterial infection was less safe than non‐early discharge, or that very early discharge of children with cancer and febrile neutropenia and low risk of bacterial infection was less safe than early discharge in a carefully selected, carefully instructed and well‐monitored group. However, it should be taken into account that the included studies were relatively small, thus the non‐significant differences could be due to, for example, a lack of power. There was a significant reduction of costs for the early discharge group in comparison to the non‐early discharge group, which could be different in other countries and thus needs to be evaluated by other research groups.
The two available studies provided very limited evidence about the effects of very early discharge and early discharge. The lack of evidence to inform practice decisions justifies the evaluation of these discharge strategies in a trial setting in hospitals/oncology wards where close monitoring of very early and early discharged participants by well‐trained employees is guaranteed.
Implications for research.
Further research in large randomized controlled trials is required to confirm or contradict that early discharge is not less safe than non‐early discharge, and that very early discharge is not less safe than early discharge. In our opinion, it would also be valuable to have more information on quality of life, costs and duration of treatment. In addition, at this time, it is not clear which low‐risk criteria are superior, and future studies should address this matter with the objective of establishing uniform low‐risk criteria.
Acknowledgements
The authors thank Edith Leclercq, the Trials Search Co‐ordinator of Cochrane Childhood Cancer, for developing the search strategies and running the searches.
The authors thank Roland Ammann, the corresponding author of Brack 2012, for providing the missing data for this study.
The authors thank Roland Ammann, Pat Flynn and Joerg Meerpohl for peer reviewing the first version of this review.
Esther te Poele is supported by a research grant from the Foundation of Pediatric Oncology Groningen (SKOG 03‐001).
The editorial base of Cochrane Childhood Cancer is funded by Kinderen Kankervrij (KiKa).
Appendices
Appendix 1. Search strategy for Cochrane Central Register of Controlled Trials (CENTRAL)
1. ForEarly discharge the following text words were used:
discharge* OR patient discharge OR patient discharge* OR ambulatory care OR “outpatient management” OR home treatment OR out‐patient OR outpatient OR outpatients OR outpatient care OR outpatient health service* OR early discontinuation OR discontinue
2. ForNeutropenia the following text words were used:
febrile neutropenia OR fever OR febrile neutropenic OR neutropenia OR fevers OR hyperthermia* OR pyrexia* OR neutropenias OR febrile neutropenias
3. ForChildren the following text words were used:
infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR perinat* OR postnat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy
4. ForChildhood cancer the following text words were used:
leukemia OR leukemi* OR leukaemi* OR childhood ALL OR AML OR lymphoma OR lymphom* OR hodgkin OR hodgkin* OR T‐cell OR B‐cell OR non‐hodgkin OR sarcoma OR sarcom* OR Ewing* OR osteosarcoma OR osteosarcom* OR wilms tumor OR wilms* OR nephroblastom* OR neuroblastoma OR neuroblastom* OR rhabdomyosarcoma OR rhabdomyosarcom* OR teratoma OR teratom* OR hepatoma OR hepatom* OR hepatoblastoma OR hepatoblastom* OR PNET OR medulloblastoma OR medulloblastom* OR PNET* OR primitive neuroectodermal tumors OR retinoblastoma OR retinoblastom* OR meningioma OR meningiom* OR glioma OR gliom* OR pediatric oncology OR paediatric oncology OR childhood cancer OR childhood tumor OR childhood tumors OR brain tumor* OR brain tumour* OR brain neoplasms OR central nervous system neoplasm OR central nervous system neoplasms OR central nervous system tumor* OR central nervous system tumour* OR brain cancer* OR brain neoplasm* OR intracranial neoplasm* OR acute lymphocytic leukemia
5. 1 AND 2 AND 3 AND 4
The search was performed in title, abstract or keywords.
[*=zero or more characters]
Appendix 2. Search strategy for MEDLINE/PubMed
1. ForEarly discharge the following MeSH headings and text words were used:
discharge* OR patient discharge OR patient discharge* OR ambulatory care OR “outpatient management” OR home treatment OR out‐patient OR outpatient OR outpatients OR outpatient care OR outpatient health service* OR early discontinuation OR discontinue
2. ForNeutropenia the following MeSH headings and text words were used:
febrile neutropenia OR fever OR febrile neutropenic OR neutropenia OR fevers OR hyperthermia* OR pyrexia* OR neutropenias OR febrile neutropenias
3. ForChildren the following MeSH headings and text words were used:
infant OR infan* OR newborn OR newborn* OR new‐born* OR baby OR baby* OR babies OR neonat* OR perinat* OR postnat* OR child OR child* OR schoolchild* OR schoolchild OR school child OR school child* OR kid OR kids OR toddler* OR adolescent OR adoles* OR teen* OR boy* OR girl* OR minors OR minors* OR underag* OR under ag* OR juvenil* OR youth* OR kindergar* OR puberty OR puber* OR pubescen* OR prepubescen* OR prepuberty* OR pediatrics OR pediatric* OR paediatric* OR peadiatric* OR schools OR nursery school* OR preschool* OR pre school* OR primary school* OR secondary school* OR elementary school* OR elementary school OR high school* OR highschool* OR school age OR schoolage OR school age* OR schoolage* OR infancy OR schools, nursery OR infant, newborn
4. ForChildhood cancer the following MeSH headings and text words were used:
(((leukemia OR leukemi* OR leukaemi* OR (childhood ALL) OR AML OR lymphoma OR lymphom* OR hodgkin OR hodgkin* OR T‐cell OR B‐cell OR non‐hodgkin OR sarcoma OR sarcom* OR sarcoma, Ewing's OR Ewing* OR osteosarcoma OR osteosarcom* OR wilms tumor OR wilms* OR nephroblastom* OR neuroblastoma OR neuroblastom* OR rhabdomyosarcoma OR rhabdomyosarcom* OR teratoma OR teratom* OR hepatoma OR hepatom* OR hepatoblastoma OR hepatoblastom* OR PNET OR medulloblastoma OR medulloblastom* OR PNET* OR neuroectodermal tumors, primitive OR retinoblastoma OR retinoblastom* OR meningioma OR meningiom* OR glioma OR gliom*) OR (pediatric oncology OR paediatric oncology)) OR (childhood cancer OR childhood tumor OR childhood tumors)) OR (brain tumor* OR brain tumour* OR brain neoplasms OR central nervous system neoplasm OR central nervous system neoplasms OR central nervous system tumor* OR central nervous system tumour* OR brain cancer* OR brain neoplasm* OR intracranial neoplasm*) OR (leukemia lymphocytic acute) OR (leukemia, lymphocytic, acute[mh])
5. ForRCTs and CCTs the following MeSH headings and text words were used:
(randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) AND humans[mh]
6.1 AND 2 AND 3 AND 4 AND 5
[pt = publication type; tiab = title, abstract; sh = subject heading; mh = MeSH term; RCT = randomized controlled trial; CCT = controlled clinical trial]
Appendix 3. Search strategy for EMBASE/Ovid
1. ForEarly discharge the following Emtree terms and text words were used:
1. (discharge$ or patient discharge$).mp. 2. patient discharge.mp. or exp Hospital Discharge/ 3. ambulatory care.mp. or exp Ambulatory Care/ 4. outpatient management.mp. 5. home treatment.mp. or exp Home Care/ 6. out‐patient.mp. 7. exp OUTPATIENT CARE/ or outpatient.mp. or exp OUTPATIENT/ 8. outpatients.mp. 9. outpatient health service$.mp. 10. (early discontinuation or discontinue).mp. 11. or/1‐10
2. ForNeutropenia the following Emtree terms and text words were used:
1. febrile neutropenia.mp. 2. febrile neutropenias.mp. 3. febrile neutropenic.mp. 4. exp Febrile Neutropenia/ 5. exp NEUTROPENIA/ or neutropenia.mp. 6. fever.mp. or exp FEVER/ 7. fevers.mp. 8. Hyperthermia/ 9. hyperthermia$.mp. 10. hyperthermia.mp. 11. pyrexia.mp. 12. pyrexia$.mp. 13. (neutropenias or neutropaenia or neutropaenias).mp. 14. or/1‐13
3. ForChildhood cancer the following Emtree terms and text words were used:
1. (leukemia or leukemi$ or leukaemi$ or (childhood adj ALL) or acute lymphocytic leukemia).mp. 2. (AML or lymphoma or lymphom$ or hodgkin or hodgkin$ or T‐cell or B‐cell or non‐hodgkin).mp. 3. (sarcoma or sarcom$ or Ewing$ or osteosarcoma or osteosarcom$ or wilms tumor or wilms$).mp. 4. (nephroblastom$ or neuroblastoma or neuroblastom$ or rhabdomyosarcoma or rhabdomyosarcom$ or teratoma or teratom$ or hepatoma or hepatom$ or hepatoblastoma or hepatoblastom$).mp. 5. (PNET or medulloblastoma or medulloblastom$ or PNET$ or neuroectodermal tumors or primitive neuroectodermal tumor$ or retinoblastoma or retinoblastom$ or meningioma or meningiom$ or glioma or gliom$).mp. 6. (pediatric oncology or paediatric oncology).mp. 7. ((childhood adj cancer) or (childhood adj tumor) or (childhood adj tumors) or childhood malignancy or (childhood adj malignancies) or childhood neoplasm$).mp. 8. ((pediatric adj malignancy) or (pediatric adj malignancies) or (paediatric adj malignancy) or (paediatric adj malignancies)).mp. 9. ((brain adj tumor$) or (brain adj tumour$) or (brain adj neoplasms) or (brain adj cancer$) or brain neoplasm$).mp. 10. (central nervous system tumor$ or central nervous system neoplasm or central nervous system neoplasms or central nervous system tumour$).mp. 11. intracranial neoplasm$.mp. 12. LEUKEMIA/ or LYMPHOMA/ or brain tumor/ or central nervous system tumor/ or teratoma/ or sarcoma/ or osteosarcoma/ 13. nephroblastoma/ or neuroblastoma/ or rhabdomyosarcoma/ or hepatoblastoma/ or medulloblastoma/ or neuroectodermal tumor/ or retinoblastoma/ or meningioma/ or glioma/ or childhood cancer/ 14. or/1‐13
4. ForChildren the following Emtree terms and text words were used:
1. infant/ or infancy/ or newborn/ or baby/ or child/ or preschool child/ or school child/ 2. adolescent/ or juvenile/ or boy/ or girl/ or puberty/ or prepuberty/ or pediatrics/ 3. primary school/ or high school/ or kindergarten/ or nursery school/ or school/ 4. or/1‐3 5. (infant$ or newborn$ or (new adj born$) or baby or baby$ or babies or neonate$ or perinat$ or postnat$).mp. 6. (child$ or (school adj child$) or schoolchild$ or (school adj age$) or schoolage$ or (pre adj school$) or preschool$).mp. 7. (kid or kids or toddler$ or adoles$ or teen$ or boy$ or girl$).mp. 8. (minors$ or (under adj ag$) or underage$ or juvenil$ or youth$).mp. 9. (puber$ or pubescen$ or prepubescen$ or prepubert$).mp. 10. (pediatric$ or paediatric$ or peadiatric$).mp. 11. (school or schools or (high adj school$) or highschool$ or (primary adj school$) or (nursery adj school$) or (elementary adj school) or (secondary adj school$) or kindergar$).mp. 12. or/5‐11 13. 4 or 12
5. ForRCTs and CCTs the following Emtree terms and text words were used:
1. (randomized controlled trial or controlled clinical trial).mp. 2. (randomized or placebo or randomly or trial or groups).ti,ab. 3. drug therapy.sh. 4. 1 or 2 or 3 5. limit 4 to human
6. 1 and 2 and 3 and 4 and 5
[mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name; sh = subject heading; ti,ab = title, abstract; / = Emtree term; RCT = randomized controlled trial; CCT = controlled clinical trial]
Appendix 4. Search strategy for conference proceedings
We handsearched the conference proceedings of the following conferences:
The International Society of Paediatric Oncology (SIOP) annual meeting. Search in abstract books: all titles in “supportive care” section, full document search for individual terms "febrile", "fever", "neutropen" and "discharge".
The Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). Search in abstract books: all titles in "pediatrics" and "neutropenia‐infections" sections.
The European Society for Paediatric Infectious Diseases (ESPID). Search in abstract books: full document for "febrile", "cancer" and "oncol".
The European Hematology Association (EHA). Search in abstract books: all titles in “infectious diseases, supportive care” and “health economics” sections.
The Infectious Diseases Society of America (IDSA). Search in abstract books: full document search for "pediatric oncol", "cancer" and "febrile neutropen".
The American Society of Hematology (ASH). Search in online abstract books: individual searches for "febrile neutropenia", "pediatric oncology" and "children with cancer".
The American Society of Clinical Oncology (ASCO). Search in online annual meeting abstracts library: ("febrile neutropenia" OR "febrile neutropenic") AND ("pediatric" OR "child").
The American Academy of Pediatrics (AAP). Search in online abstract books: individual searches for "febrile", "cancer" and "oncology".
Appendix 5. Search strategy for trial registers
International Standard Randomised Controlled Trials Number (ISRCTN) bywww.isrctn.com
At the advanced search page, the following searches were performed:
Text search: febrile neutropenia, conditions: cancer, participant age range: child
Text search: fever, conditions: cancer, participant age range: child
Text search: febrile neutropenia
National Institute of Health (NIH) register, by clinicaltrials.gov
At the advances search page, the following search was performed:
Search terms: febrile neutropenia, conditions: cancer, age group: child
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) byapps.who.int/trialsearch/
The following searches were performed:
febrile AND neutropen* AND child* AND cancer
febrile AND neutropen* AND pediatr* AND oncol*
fever AND child* AND cancer
fever AND pediatr* AND oncol*
Data and analyses
Comparison 1. Early discharge versus non‐early discharge (note: number of participants refers to number of episodes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure; rehospitalization or adjustment of antimicrobial treatment (or both) | 1 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.24, 3.50] |
| 2 Costs (USD) | 1 | 149 | Mean Difference (IV, Fixed, 95% CI) | ‐265.0 [‐403.14, ‐126.86] |
| 3 Duration of antimicrobial treatment; total (days) | 1 | 149 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.22, 0.62] |
| 4 Duration of antimicrobial treatment; intravenous (days) | 1 | 149 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.36, 0.36] |
| 5 Duration of antimicrobial treatment; oral (days) | 1 | 149 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.51, 0.91] |
1.1. Analysis.
Comparison 1 Early discharge versus non‐early discharge (note: number of participants refers to number of episodes), Outcome 1 Treatment failure; rehospitalization or adjustment of antimicrobial treatment (or both).
1.2. Analysis.
Comparison 1 Early discharge versus non‐early discharge (note: number of participants refers to number of episodes), Outcome 2 Costs (USD).
1.3. Analysis.
Comparison 1 Early discharge versus non‐early discharge (note: number of participants refers to number of episodes), Outcome 3 Duration of antimicrobial treatment; total (days).
1.4. Analysis.
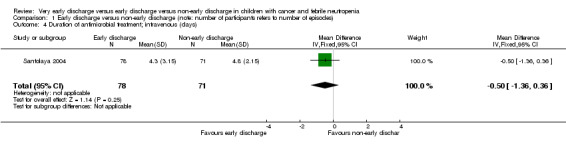
Comparison 1 Early discharge versus non‐early discharge (note: number of participants refers to number of episodes), Outcome 4 Duration of antimicrobial treatment; intravenous (days).
1.5. Analysis.
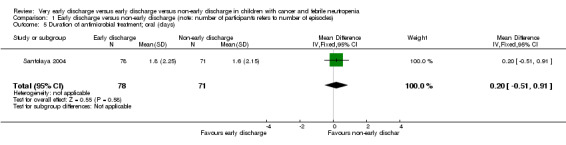
Comparison 1 Early discharge versus non‐early discharge (note: number of participants refers to number of episodes), Outcome 5 Duration of antimicrobial treatment; oral (days).
Comparison 2. Very early discharge versus early discharge (note: number of participants refers to number of episodes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure; rehospitalization or adjustment of antimicrobial treatment (or both) | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.15, 1.89] |
| 2 Treatment failure; rehospitalization or adjustment of antimicrobial treatment (or both), only first episode (note: number of participants stated instead of episodes) | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.40] |
2.1. Analysis.
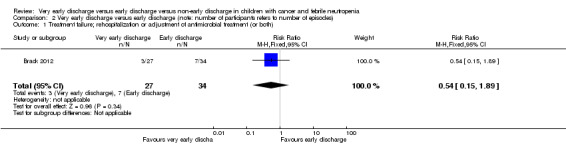
Comparison 2 Very early discharge versus early discharge (note: number of participants refers to number of episodes), Outcome 1 Treatment failure; rehospitalization or adjustment of antimicrobial treatment (or both).
2.2. Analysis.
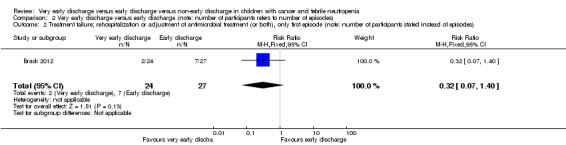
Comparison 2 Very early discharge versus early discharge (note: number of participants refers to number of episodes), Outcome 2 Treatment failure; rehospitalization or adjustment of antimicrobial treatment (or both), only first episode (note: number of participants stated instead of episodes).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brack 2012.
| Methods | Randomized controlled trial Study design: parallel group |
|
| Participants | Children with cancer presenting with febrile neutropenia after non‐myeloablative chemotherapy Age: 1‐18 years The study enrolled 52 children with a total of 62 episodes with low risk of adverse events Children at low risk fulfilled 10 pre‐defined low‐risk criteria, and 6 additional criteria regarding continuity of supportive therapy, and applicability of oral antimicrobial treatment Low‐risk criteria
Additional criteria: continuity of supportive therapy
Additional criteria: applicability of oral antimicrobial therapy
Definition of neutropenia: ANC ≤ 500/μL Definition of fever: axillary recording of ≥ 38.5 °C once or ≥ 38.0 °C during ≥ 2 hours Sex: very early discharge 41% boys; early discharge 44% boys ANC < 100/μL at presentation (%); very early discharge 72%; early discharge 47%. Type of malignancy (%): acute lymphoblastic leukaemia; very early discharge 48%; early discharge 53%. Tumour of the central nervous system; very early discharge 11%; early discharge 12%. Solid tumour outside the central nervous system; very early discharge 41%; early discharge 35% |
|
| Interventions | After 8‐22 hours of inpatient therapy, children who fulfilled low‐risk criteria were randomized to very early discharge or early discharge. Children randomized to very early discharge were given a combination of oral ciprofloxacin plus oral amoxicillin, and were discharged within 9‐24 hours from presentation with febrile neutropenia. These children were rehospitalized in case of fever ≥ 38.0 °C within ≥ 5 days from presentation with febrile neutropenia, and in case of shaking chills, toxic appearance, new signs of local infection or any other reason for inpatient management as determined by the treating physician. Discharge criteria for children randomized to early discharge were absence of fever ≥ 38.0 °C for ≥ 48 hours, of toxic appearance and of any other reason for inpatient management as determined by the treating physician. In both groups, modification of therapy was suggested in case of bacteraemia known after re‐assessment, signs and symptoms of a local infection, toxic appearance or adverse event/intolerance requiring its discontinuation. Criteria for ending antibiotics were no fever ≥ 38.0 °C for 48 hours, an ANC > 500/μL or rising for ≥ 48 hours, no bacteraemia, no local infection and no toxic appearance | |
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Follow‐up continued daily during antimicrobial therapy, and every second day until the resolution of severe neutropenia (ANC > 500/μL). Minimal required observations for follow‐up included history and physical examination at each visit, daily blood cultures if febrile ≥ 38.0 °C, and complete blood cell count every second day We contacted the authors of this study because their definition of outcome treatment failure was slightly different from ours, i.e. in the included study fever > 48 hours, bacteraemia, persistent symptoms and signs of local infection, and addition of antifungal therapy were also considered as treatment failures while, in our review, these were not. We asked the authors to specify the reasons for treatment failure of all episodes of which the reason was not stated specifically or clearly in the full‐text article, or both. They responded swiftly and provided the requested data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was block‐stratified per center and based on a list of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "At randomization, one of a set of numbered, sealed randomization envelopes containing the randomization allocation was opened by the treating oncologist" Comment: not mentioned if envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment of the various outcomes was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reason of the 1 episode lost to follow‐up stated (centre stopped study participation) and unlikely to be related to true outcome. Of the first episodes of all included children none were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We cannot ascertain that unexpected effects were not reported |
| Other bias | High risk | High risk of baseline imbalance. Quote: "informed consent for randomization was declined in 25 (27%), and randomization was not performed for other reasons in 6 (6%)" Comment: reasons not stated |
Santolaya 2004.
| Methods | Randomized controlled trial Study design: parallel group |
|
| Participants | Children with cancer, fever and neutropenia Age: ≤ 18 years; mean age 55 months for the early discharge group and 66 months for the non‐early discharge group Diagnosis: leukaemia, lymphoma and different types of solid tumours. No recipients of stem‐cell transplantation The study enrolled 107 children with a total of 149 episodes at low risk for invasive bacterial infection Children at low risk had 0 or only criteria 4 or 5 of the following (Santolaya 2002):
Definition of neutropenia: ANC < 500/μL Definition of fever: 1 axillary recording of ≥ 38.5 °C or 2 recordings of ≥ 38 °C separated by at least 1 hour Sex: early discharge 43% boys; non‐early discharge 49% boys Mean ANC at presentation (95% CI); early discharge 146 /μL (111‐182); non‐early discharge 137 /μL (98‐180) |
|
| Interventions | Children were randomised to ambulatory or hospital‐based treatment of febrile neutropenia after 24‐36 hours after presentation. Low‐risk children were discharged from the hospital after 24‐36 hours when assigned to the early discharge group and after a mean of 5.3 days (range 3‐9 days) when assigned to the non‐early discharge group. After a minimum of 3 days of intravenous antibiotics (ceftriaxone and teicoplanin); either given on the ward for the inpatients or during the daily visit to the oncology clinic for the ambulatory group, the decision to switch to oral antibiotics (cefuroxime axetil) was made on an individual basis, based on pre‐defined criteria. Criteria for ending antibiotics were 2 consecutive CRP values ≤ 40 mg/L and 1 full day without fever | |
| Outcomes |
Primary outcomes
Secondary outcomes
|
|
| Notes | Follow‐up continued daily until fever resolved and ANC ≥ 500/μL. Daily follow‐up consisted of physical examination, CRP measurement, monocyte until they reached 100 /μL and platelet counts until they reached 50,000/μL. For children with a positive culture on admission a repeat culture was obtained at day 3 It should be noted that in this study the used antimicrobial therapy did not meet the Infectious Diseases Society of America Clinical Practice Guidelines recommendations for empiric antimicrobial therapy for febrile neutropenia of the time the study was commenced. The used antimicrobial therapy did not cover for Pseudomonas bacteria, which was the identified micro‐organism in the only death in the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method was not described |
| Allocation concealment (selection bias) | Unclear risk | The method was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and personnel was not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment of the various outcomes was not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was not mentioned if there were participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | We could not ascertain that unexpected effects were not reported |
| Other bias | Low risk | We did not identify any other possible risk of bias |
ANC: absolute neutrophil count; CRP: C‐reactive protein; G‐CSF: granulocyte colony‐stimulating factor; PJP: Pneumocystis jirovecii pneumonia; SpO2: arterial oxygen saturation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmed 2007 | Both treatment groups were discharged ≥ 5 days after admission and thus fitted criteria for the same discharge group, i.e. non‐early discharge |
| Arora 2014 | Conference abstract without sufficient information to use for this review |
| Feng 2014 | Both treatment groups were discharged ≥ 5 days after admission and thus fitted criteria for the same discharge group, i.e. non‐early discharge |
| Georgala 2012 | Study in adults |
| Gupta 2009 | Both treatment groups were discharged immediately and thus fitted criteria for the same discharge group, i.e. very early discharge |
| Kern 2013 | Study in adults |
| Mathew 2014 | Commentary (to Orme 2014, also in excluded studies) |
| Orme 2014 | Study did not provide data to use in our comparisons. Potential early discharge was not a study question, moment of discharge for both treatment groups was not reported |
| Paganini 2000 | Both treatment groups were discharged after 72 hours and thus fitted criteria for the same discharge group, i.e. early discharge |
| Shenep 2001 | Participants were randomized to different antibiotic regimens, i.e. intravenous antibiotics vs. oral antibiotics. Potential early discharge was not a study question, moment of discharge for both treatment groups was not reported |
Differences between protocol and review
We changed the 'Primary outcomes' changed from ".... rehospitalization due to febrile neutropenia" to ".... rehospitalization or adjustment of antimicrobial treatment (or both) related to febrile neutropenia (participant deterioration or other febrile neutropenia‐related causes)", as rehospitalization is not a good parameter for treatment failure for people who receive in‐hospital treatment. Adjustment of antimicrobial treatment is a more accurate parameter. In addition, we added "patient deterioration or other febrile neutropenia‐related causes" between parentheses to emphasize that narrowing of antibiotic therapy when blood culture was known was not as an adverse outcome.
We changed the definition of non‐early discharge in the 'Methods' section from "Non‐early discharge was defined as discharge from in‐hospital treatment after at least five days" to "Non‐early discharge as discharge from in‐hospital treatment after (a mean or median of) at least five days", as otherwise important studies comparing of in‐hospital treatment might be excluded. For the same reason, we changed "Early discharge from hospital treatment was defined as discharge from the hospital before patients had received five days of in‐hospital treatment" was changed to "early discharge as discharge from in‐hospital treatment (with a mean or median of) less than five days after presentation with fever and neutropenia" and "Very early discharge was defined as discharge from in‐hospital treatment within 24 hours after presentation with fever and neutropenia" to "very early discharge as discharge from in‐hospital treatment (with a mean or median of) less than 24 hours after presentation with fever and neutropenia".
We added the durations of intravenous and oral antibiotics to the secondary outcomes as they provide information about whether the different treatment groups were comparable.
After publication of the protocol, the Childhood Cancer Group adjusted the risk of bias criteria and their definitions based on a new version of the Cochrane Handbook for Systematic Reviews of Interventions; we used the updated version of their criteria (Higgins 2011).
We handsearched more conference proceedings than stated in the protocol to yield additional studies. In the protocol, we stated that we would handsearch the conference proceedings of the International Society for Paediatric Oncology (SIOP), the American Society of Clinical Oncology (ASCO) and the Multinational Association for Supportive Care in Cancer (MASCC). We have added the conference proceedings of the European Society for Paediatric Infectious Diseases (ESPID), the Infectious Diseases Society of America (IDSA), the European Hematology Association (EHA), the American Society of Hematology (ASH) and the American Academy of Pediatrics (AAP) to our search.
We have searched more trial registers than stated in the protocol to yield additional ongoing trials. In the protocol, we stated that we would search the ISRCTN Register and the National Institute of Health (NIH) Register. We have added the World Health Organization (WHO) ICTRP Register to our search.
In the protocol, we did not state that we would include 'Summary of findings' tables. As this is strongly recommended in the most recent version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we included 'Summary of findings' tables.
Contributions of authors
EMP and ESJMB designed the study.
EMP wrote the protocol.
WJET and ESJMB revised the protocol.
EAHL, EMP, WJET and ESJMB performed the searches, data extraction and risk of bias assessment.
HMB, EAHL, EMPand WJET performed the statistical analysis.
EAHL and EMP wrote the review.
WJET, ESJMB and HMB revised the review.
All authors approved the final version of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
The Foundation of Pediatric Oncology Groningen (SKOG 03‐001), Netherlands.
Declarations of interest
None known.
Joint first authorship with Erik AH Loeffen
New
References
References to studies included in this review
Brack 2012 {published data only}
- Brack E, Bodmer N, Simon A, Leibundgut K, Kühne T, Niggli FK, et al. First‐day step‐down to oral outpatient treatment versus continued standard treatment in children with cancer and low‐risk fever in neutropenia. A randomized controlled trial within the multicenter SPOG 2003 FN study. Pediatric Blood & Cancer 2012;59:423‐30. [DOI] [PubMed] [Google Scholar]
Santolaya 2004 {published data only}
- Santolaya ME, Alvarez AM, Avilés CL, Becker A, Cofré J, Cumsille MA, et al. Early hospital discharge followed by outpatient management versus continued hospitalization of children with cancer, fever, and neutropenia at low risk for invasive bacterial infection. Journal of Clinical Oncology 2004;22(18):3784‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahmed 2007 {published data only}
- Ahmed N, El‐Mahallawy HA, Ahmed IA, Nassif S, El‐Beshlawy A, El‐Haddad A. Early hospital discharge versus continued hospitalization in febrile pediatric cancer patients with prolonged neutropenia: a randomized, prospective study. Pediatric Blood & Cancer 2007;49:786‐92. [DOI] [PubMed] [Google Scholar]
Arora 2014 {published data only}
- Arora B, Banavali S, Vora T, Chinnaswamy G, Prasad M, Paradkar A, et al. A randomized open labeled parallel group phase III study of antibiotics plus G‐CSF in pediatric cancer patients with febrile neutropenia in a low‐income setting. Pediatric Blood & Cancer 2014;61 Suppl 2:S105‐433. [Google Scholar]
Feng 2014 {published data only}
- Feng X, Ruan Y, He Y, Zhang Y, Wu X, Liu H, et al. Prophylactic first‐line antibiotics reduce infectious fever and shorten hospital stay during chemotherapy‐induced agranulocytosis in childhood acute myeloid leukemia. Acta Haematologica 2014;132(1):112‐7. [DOI] [PubMed] [Google Scholar]
Georgala 2012 {published data only}
- Georgala A, Loizidou A, Berghmans T, Aoun M, Laethem V, Dubreucq L, et al. Feasibility of very early discharge of febrile neutropenia (FN) patients (pts) selected using the Mascc score: a Belgian study. 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy. 2012; Vol. T1028:110.
Gupta 2009 {published data only}
- Gupta A, Swaroop C, Agarwala S, Pandey RM, Bakhshi S. Randomized control trial comparing oral amoxicillin‐clavulanate and ofloxacin with intravenous ceftriaxone and amikacin as outpatient therapy in pediatric low‐risk febrile neutropenia. Journal of Pediatric Hematology‐Oncology 2009;31:635‐41. [DOI] [PubMed] [Google Scholar]
Kern 2013 {published data only}
- Kern WV, Marchetti O, Drgona L, Akan H, Aoun M, Akova M, et al. Oral antibiotics for fever in low‐risk neutropenic patients with cancer: a double‐blind, randomized, multicenter trial comparing single daily moxifloxacin with twice daily ciprofloxacin plus amoxicillin/clavulanic acid combination therapy ‐ EORTC infectious diseases group trial XV. Journal of Clinical Oncology 2013;31(9):1149‐56. [DOI] [PubMed] [Google Scholar]
Mathew 2014 {published data only}
- Mathew JL, Arora RS, Sankar J. Outpatient versus inpatient IV antibiotic management for pediatric oncology patients with low risk febrile neutropenia: a randomised trial. Indian Pediatrics 2014;51:659‐61. [DOI] [PubMed] [Google Scholar]
Orme 2014 {published data only}
- Orme LM, Babl FE, Barnes C, Barnett P, Donath S, Ashley DM. Outpatient versus inpatient IV antibiotic management for pediatric oncology patients with low risk febrile neutropenia: a randomised trial. Pediatric Blood & Cancer 2014;61(8):1427‐33. [DOI] [PubMed] [Google Scholar]
Paganini 2000 {published data only}
- Paganini HR, Sarkis CM, Martino MG, Zubizarreta PA, Casimir L, Fernandez C, et al. Oral administration of cefixime to lower risk febrile neutropenic children with cancer. Cancer 2000;88(12):2848‐52. [DOI] [PubMed] [Google Scholar]
Shenep 2001 {published data only}
- Shenep JL, Flynn PM, Baker DK, Hetherington SV, Hudson MM, Hughes WT, et al. Oral cefixime is similar to continued intravenous antibiotics in the empirical treatment of febrile neutropenic children with cancer. Clinical Infectious Diseases 2001;32(1):36‐43. [DOI] [PubMed] [Google Scholar]
Additional references
Ammann 2010
- Ammann RA, Bodmer N, Hirt A, Niggli FK, Nadal D, Simon A, et al. Predicting adverse events in children with fever and chemotherapy‐induced neutropenia: the prospective multicenter SPOG 2003 FN study. Journal of Clinical Oncology 2010;28(12):2008‐14. [DOI] [PubMed] [Google Scholar]
Ammann 2011
- Ammann RA, Hirt A, Nadal D, Simon A, Kühne T, Aebi C. Reply to K.G.E. Miedema et al. Journal of Clinical Oncology 2011;29(7):e185. [Google Scholar]
Ariffin 2006
- Ariffin H, Ai CL, Lee CL, Abdullah WA. Cefepime monotherapy for treatment of febrile neutropenia in children. Journal of Paediatrics and Child Health 2006;42(12):781‐4. [DOI] [PubMed] [Google Scholar]
Bodey 1966
- Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Annals of Internal Medicine 1966;64(2):328‐40. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodgson‐Viden 2005
- Hodgson‐Viden H, Grundy PE, Robinson JL. Early discontinuation of intravenous antimicrobial therapy in pediatric oncology patients with febrile neutropenia. BioMed Central Pediatrics 2005;5(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2002
- Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clinical Infectious Diseases 2002;34(6):730‐51. [DOI] [PubMed] [Google Scholar]
Klastersky 2013
- Klastersky J, Paesmans M. The Multinational Association for Supportive Care in Cancer (MASCC) risk index score: 10 years of use for identifying low‐risk febrile neutropenic cancer patients. Supportive Care in Cancer 2013;21:1487‐95. [DOI] [PubMed] [Google Scholar]
Miedema 2011
- Miedema KG, Bont ES, Oude Nijhuis CS, Vliet D, Kamps WA, Tissing WJ. Validation of a new risk assessment model for predicting adverse events in children with fever and chemotherapy‐induced neutropenia. Journal of Clinical Oncology 2011;29(7):e182‐4. [DOI] [PubMed] [Google Scholar]
Module CCG
- Kremer LCM, Dalen EC, Moher D, Caron HN. Childhood Cancer Group. In: The Cochrane Library, 2010, Issue 2 . Chichester: Wiley‐Blackwell. Updated quarterly.
O'Leary 2008
- O'Leary M, Krailo M, Anderson JR, Reaman GH, Children's Oncology Group. Progress in childhood cancer: 50 years of research collaboration, a report from the Children's Oncology Group. Seminars in Oncology 2008;35(5):484‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oude Nijhuis 2005
- Oude Nijhuis C, Kamps WA, Daenen SM, Gietema JA, Graaf WT, Groen HJ, Vellenga E, Vergert EM, Vermeulen KM, Vries‐Hospers HG, Bont ES. Feasibility of withholding antibiotics in selected febrile neutropenic cancer patients.. Journal of Clinical Oncology 2005 Oct 20;23(30):7437‐44. [DOI] [PubMed] [Google Scholar]
Petrilli 1993
- Petrilli AS, Melaragno R, Barros KV, Silva AA, Kusano E, Ribeiro RC, et al. Fever and neutropenia in children with cancer: a therapeutic approach related to the underlying disease. Pediatric Infectious Disease Journal 1993;12(11):916‐21. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2012.
Santolaya 1997
- Santolaya ME, Villarroel M, Avendano LF, Cofré J. Discontinuation of antimicrobial therapy for febrile, neutropenic children with cancer: a prospective study. Clinical Infectious Diseases 1997;25(1):92‐7. [DOI] [PubMed] [Google Scholar]
Santolaya 2002
- Santolaya ME, Alvarez AM, Avilés CL, Becker A, Cofré J, Enríquez N, et al. Prospective evaluation of a model of prediction of invasive bacterial infection risk among children with cancer, fever, and neutropenia. Clinical Infectious Diseases 2002;35(6):678‐83. [DOI] [PubMed] [Google Scholar]
te Poele 2009
- Poele EM, Tissing WJ, Kamps WA, Bont ES. Risk assessment in fever and neutropenia in children with cancer: what did we learn?. Critical Reviews in Oncology/Hematology 2009;72(1):45‐55. [DOI] [PubMed] [Google Scholar]
Wacker 1997
- Wacker P, Halperin DS, Wyss M, Humbert J. Early hospital discharge of children with fever and neutropenia: a prospective study . Journal of Pediatric Hematology/Oncology 1997;19(3):208‐11. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
te Poele 2010
- Poele EM, Tissing WJE, Bont ESJM. Very early discharge versus early discharge versus non‐early discharge in pediatric cancer patients with febrile neutropenia. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD008382] [DOI] [PMC free article] [PubMed] [Google Scholar]


