Abstract
Background
Vascular closure devices (VCDs) are widely used to achieve haemostasis after procedures requiring percutaneous common femoral artery (CFA) puncture. There is no consensus regarding the benefits of VCDs, including potential reduction in procedure time, length of hospital stay or time to patient ambulation. No robust evidence exists that VCDs reduce the incidence of puncture site complications compared with haemostasis achieved through extrinsic (manual or mechanical) compression.
Objectives
To determine the efficacy and safety of VCDs versus traditional methods of extrinsic compression in achieving haemostasis after retrograde and antegrade percutaneous arterial puncture of the CFA.
Search methods
The Cochrane Vascular Trials Search Co‐ordinator searched the Specialised Register (April 2015) and the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 3). Clinical trials databases were searched for details of ongoing or unpublished studies. References of articles retrieved by electronic searches were searched for additional citations.
Selection criteria
We included randomised and quasi‐randomised controlled trials in which people undergoing a diagnostic or interventional procedure via percutaneous CFA puncture were randomised to one type of VCD versus extrinsic compression or another type of VCD.
Data collection and analysis
Two authors independently extracted data and assessed the methodological quality of trials. We resolved disagreements by discussion with the third author. We performed meta‐analyses when heterogeneity (I2) was < 90%. The primary efficacy outcomes were time to haemostasis and time to mobilisation (mean difference (MD) and 95% confidence interval (CI)). The primary safety outcome was a major adverse event (mortality and vascular injury requiring repair) (odds ratio (OR) and 95% CI). Secondary outcomes included adverse events.
Main results
We included 52 studies (19,192 participants) in the review. We found studies comparing VCDs with extrinsic compression (sheath size ≤ 9 Fr), different VCDs with each other after endovascular (EVAR) and percutaneous EVAR procedures and VCDs with surgical closure after open exposure of the artery (sheath size ≥ 10 Fr). For primary outcomes, we assigned the quality of evidence according to GRADE (Grades of Recommendation, Assessment, Development and Evaluation) criteria as low because of serious imprecision and for secondary outcomes as moderate for precision, consistency and directness.
For time to haemostasis, studies comparing collagen‐based VCDs and extrinsic compression were too heterogenous to be combined. However, both metal clip‐based (MD ‐14.81 minutes, 95% CI ‐16.98 to ‐12.63 minutes; five studies; 1665 participants) and suture‐based VCDs (MD ‐14.58 minutes, 95% CI ‐16.85 to ‐12.32 minutes; seven studies; 1664 participants) were associated with reduced time to haemostasis when compared with extrinsic compression.
For time to mobilisation, studies comparing collagen‐, metal clip‐ and suture‐based devices with extrinsic compression were too heterogeneous to be combined. No deaths were reported in the studies comparing collagen‐based, metal clip‐based or suture‐based VCDs with extrinsic compression. For vascular injury requiring repair, meta‐analyses demonstrated that neither collagen (OR 2.81, 95% CI 0.47 to 16.79; six studies; 5731 participants) nor metal clip‐based VCDs (OR 0.49, 95% CI 0.03 to 7.95; three studies; 783 participants) were more effective than extrinsic compression. No cases of vascular injury required repair in the study testing suture‐based VCD with extrinsic compression.
Investigators reported no differences in the incidence of infection between collagen‐based (OR 2.14, 95% CI 0.88 to 5.22; nine studies; 7616 participants) or suture‐based VCDs (OR 1.66, 95% CI 0.22 to 12.71; three studies; 750 participants) and extrinsic compression. No cases of infection were observed in studies testing suture‐based VCD versus extrinsic compression. The incidence of groin haematoma was lower with collagen‐based VCDs than with extrinsic compression (OR 0.46, 95% CI 0.40 to 0.54; 25 studies; 10,247 participants), but no difference was evident when metal clip‐based (OR 0.79, 95% CI 0.46 to 1.34; four studies; 1523 participants) or suture‐based VCDs (OR 0.65, 95% CI 0.41 to 1.02; six studies; 1350 participants) were compared with extrinsic compression. The incidence of pseudoaneurysm was lower with collagen‐based devices than with extrinsic compression (OR 0.74, 95% CI 0.55 to 0.99; 21 studies; 9342 participants), but no difference was noted when metal clip‐based (OR 0.76, 95% CI 0.20 to 2.89; six studies; 1966 participants) or suture‐based VCDs (OR 0.79, 95% CI 0.25 to 2.53; six studies; 1527 participants) were compared with extrinsic compression. For other adverse events, researchers reported no differences between collagen‐based, clip‐based or suture‐based VCDs and extrinsic compression.
Limited data were obtained when VCDs were compared with each other. Results of one study showed that metal clip‐based VCDs were associated with shorter time to haemostasis (MD ‐2.24 minutes, 95% CI ‐2.54 to ‐1.94 minutes; 469 participants) and shorter time to mobilisation (MD ‐0.30 hours, 95% CI ‐0.59 to ‐0.01 hours; 469 participants) than suture‐based devices. Few studies measured (major) adverse events, and those that did found no cases or no differences between VCDs.
Percutaneous EVAR procedures revealed no differences in time to haemostasis (MD ‐3.20 minutes, 95% CI ‐10.23 to 3.83 minutes; one study; 101 participants), time to mobilisation (MD 1.00 hours, 95% CI ‐2.20 to 4.20 hours; one study; 101 participants) or major adverse events between PerClose and ProGlide. When compared with sutures after open exposure, VCD was associated with shorter time to haemostasis (MD ‐11.58 minutes, 95% CI ‐18.85 to ‐4.31 minutes; one study; 151 participants) but no difference in time to mobilisation (MD ‐2.50 hours, 95% CI ‐7.21 to 2.21 hours; one study; 151 participants) or incidence of major adverse events.
Authors' conclusions
For time to haemostasis, studies comparing collagen‐based VCDs and extrinsic compression were too heterogeneous to be combined. However, both metal clip‐based and suture‐based VCDs were associated with reduced time to haemostasis when compared with extrinsic compression. For time to mobilisation, studies comparing VCDs with extrinsic compression were too heterogeneous to be combined. No difference was demonstrated in the incidence of vascular injury or mortality when VCDs were compared with extrinsic compression. No difference was demonstrated in the efficacy or safety of VCDs with different mechanisms of action. Further work is necessary to evaluate the efficacy of devices currently in use and to compare these with one other and extrinsic compression with respect to clearly defined outcome measures.
Keywords: Humans; Punctures; Vascular Closure Devices; Vascular Closure Devices/adverse effects; Collagen; Endovascular Procedures; Femoral Artery; Femoral Artery/surgery; Hemostasis, Surgical; Hemostasis, Surgical/instrumentation; Hemostasis, Surgical/methods; Length of Stay; Pressure; Randomized Controlled Trials as Topic; Surgical Instruments
Plain language summary
Effectiveness and safety of devices designed to close femoral artery puncture sites
Background
Endovascular procedures require access to the inside of an artery. A small hole is made in the artery at the groin, and a catheter is guided along to the site of interest. Once the procedure is complete, the hole in the artery must be closed and the bleeding stopped (haemostasis). Traditionally, the main method of closing the artery is compression, during which up to 30 minutes of manual pressure or mechanical clamps is applied directly to the patient's groin. This manual pressure can be painful and requires up to eight hours of bedrest. The process of closing the artery can lead to complications such as damage to the artery and bleeding, ranging from minor to life‐threatening. Pressure applied to the artery also affects the nearby vein and may cause blood clots (deep vein thrombosis). Vascular closure devices (VCDs) are designed to close the hole and stop bleeding. VCDs were developed in the 1990s in an attempt to reduce the time to stop bleeding, to enable earlier walking after a procedure and to improve patient comfort. Four main types of VCDs are based on the material used: collagen plugs, suture‐based, disc‐based and metal clips. No consensus has been reached on the effectiveness of VCDs in reducing procedure time, length of stay or time to mobilisation, and it is unknown whether they confer a cost benefit when compared with compression.
Study characteristics
This review measures the effectiveness and safety of these VCDs compared with one other and with manual or mechanical compression. After searching for relevant studies, we found 52 studies with a combined total of 19,192 participants (current until April 2015). Studies compared different VCDs with manual or mechanical compression and/or with one other. The main measures of effectiveness were time to haemostasis and time to mobilisation. The main safety outcomes included adverse events such as bleeding, arterial damage, infection and development of clots in the adjacent vein.
Key results
This review showed that for time to haemostasis and time to mobilisation, the studies were too different to be combined in a statistical analysis when VCDs are compared with compression. For safety outcomes, no robust evidence shows that VCDs reduce the number of serious puncture site complications, when compared with manual or mechanical compression. Furthermore, this review showed no difference in effectiveness or safety for one type of VCD versus another, but few studies made these comparisons. Further good quality studies are required before firm conclusions can be drawn.
Quality of the evidence
For time to haemostasis and time to mobilisation, the studies were too different to be combined and therefore were judged to provide low‐quality evidence. The quality of the evidence for the other outcomes was judged as moderate for precision, consistency and directness.
Background
Description of the condition
Percutaneous puncture of the common femoral artery is performed to enable sheath access to the arterial system for diagnostic catheter angiography and arterial intervention. Percutaneous arterial access carries risks of damage to the artery and adjacent vein, including haematoma and pseudoaneurysm formation and arterial dissection (Koreny 2004). If the adjacent vein is damaged at the time of the puncture, arteriovenous fistula formation is also possible (Merriweather 2012).
On completion of the procedure, haemostasis can be achieved by external compression of the artery against the underlying bone, either manually or with a mechanical compression device. After haemostasis has been achieved in this way, the patient is required to rest in bed, normally for four to six hours (Schwartz 2010). Successful and persistent haemostasis reduces the incidence of arterial bleeding and decreases the incidence of haematoma and pseudoaneurysm formation. Deep vein thrombosis has been reported after prolonged extrinsic compression of the adjacent artery (Zahn 1997).
Description of the intervention
Percutaneously deployed vascular closure devices (VCDs) are adjuncts to haemostasis that are deployed at the time of sheath removal. VCDs are suitable for use in many patients to provide instant haemostasis, obviating the need for extrinsic compression and prolonged bedrest. Over the past two decades, VCD use has been widely accepted by practitioners of endovascular medicine.
VCDs fall into four main categories: clip‐based (e.g. StarClose; Abbott), suture‐based (e.g. PerClose, ProStar; both Abbott), disc‐based (e.g. Cardiva Catalyst II; Cardiva Medical) and plug‐based (e.g. AngioSeal; St Jude Medical; ExoSeal; Cordis), in which the plugs are predominantly collagen in composition, except for ExoSeal, which is Polyglycolic Acidsee (Table 1). Indications for VCD use are device‐specific and depend on patient characteristics, calibre and quality of the arterial wall and arteriotomy size. Most devices are licenced to close 6 to 8 Fr puncture sites in non‐diseased arteries for patients without significant obesity. Recently, so‐called "pre‐closure" devices have become available (e.g. ProStar XL;, Abbott) that can close larger arteriotomies and can be used in large‐calibre arterial interventions such as percutaneous endovascular aortic aneurysm repair (EVAR) or transcatheter aortic valve implantation (TAVI). Device selection should be consistent with instructions for use. Operator and unit preference and device cost also play a significant role in device selection.
1. Types of vascular closure devices.
| Types of vascular closure devices (VCD) classified according to their mechanism to achieve haemostasis | Name | Recommended sheath size (Fr) | Extravascular haemostatic agent | Intravascular component |
| Balloon‐based device | Epiclose‐T | 6 | Temporary extravascular haemostatic balloon, which is withdrawn at the end of the procedure | Temporary anchor balloon, which is withdrawn at the end of the procedure |
| Disc‐based device | Cardiva Catalyst II | 4 to 10 | Temporary nitinol‐based wire with a nitinol braided mesh disc, which is removed at the end of the procedure | |
| Plug‐based device | AngioSeal VIP, AngioSeal STS‐Plus, AngioSeal Evolution | 6, 8 | Bovine collagen plug and an absorbable traction suture | Absorbable intra‐arterial anchor (co‐polymers of polylactic and polyglycolic acids, absorbed within 30 days) |
| VasoSeal VHD, ED, Elite | 5 to 8 | Purified bovine collagen‐based plug | ‐ | |
| VasoSeal Low Profile | 4, 5 | Purified bovine collagen‐based plug | ‐ | |
| Duett Pro, Duett | 5 to 9 | Thrombin with platelet activation of collagen | Temporary anchor balloon, which is withdrawn at the end of the procedure | |
| 6/7F Mynx, Mynx M5 | 5 to 7 | Water‐soluble, freeze‐dried polyethylene glycol (PEG) material | Temporary anchor balloon, which is withdrawn at the end of the procedure | |
| ExoSeal | PGA (polyglycolic acid), a trusted non‐collagen plug material | ‐ | ||
| Metal clip‐based device | StarClose | 5, 6 | Nitinol clip | ‐ |
| StarClose SE | 5, 6 | Nitinol clip | ‐ | |
| Angiolink EVS | 6 to 8 | Titanium staple | ‐ | |
| Suture‐based device | PerClose AT | 5 to 8 | Braided polyester suture | ‐ |
| PerClose ProGlide | 5 to 8 | Monofilament polypropylene suture | ‐ | |
| ProStar XL | 8.5 to 10 | Braided polyester suture | ‐ | |
| X‐Site | 5, 6 | Braided polyester suture | ‐ | |
| SuperStitch | 6 to 12 | Polypropylene suture | ‐ |
- Balloon‐based device (Epiclose‐T) (Kurşaklioğlu 2008): A temporary balloon‐positioning catheter is inflated inside the arterial puncture site, while a larger haemostasis balloon is inflated directly on the outer surface of the arteriotomy. The balloon applies direct pressure on the arteriotomy site, thus allowing natural coagulation to occur. After a few minutes of device deployment, the anchor balloon is pulled back into the distal end of the shaft, while the haemostasis balloon remains pressing against the arteriotomy site. At the end of the haemostasis waiting period, the haemostasis balloon is deflated and the device is removed, leaving no foreign body in the intraluminal nor the extraluminal space.
- Disc‐based closure device (Cardiva Catalyst II) (Schwartz 2010): conformable nitinol‐based wire with a temporary nitinol braided mesh disc on a tether, which is deployed inside the artery to achieve haemostasis. Temporary placement of a low‐profile, conformable disc against the intima creates site‐specific compression of both the arteriotomy and the tract. The haemostatic mechanism is based on the natural elastic recoil of the arteriotomy site back to its pre‐dilated state, around the wire. In addition, a biocompatible coating on the Catalyst II Wire assists the body's natural haemostatic process and promotes ease of removal. After a few minutes of device deployment, the nitinol mesh disc and wire are removed, thus leaving no foreign body in the intraluminal nor extraluminal space.
- Plug‐based device (predominantly of collagen in composition) consisting of an extraluminal sealant with or without an intraluminal anchor (VasoSeal VHD, ED, Elite, VasoSeal Low Profile) (Bechara 2010). The intra‐arterial anchor can be a temporary balloon‐positioning catheter that is removed at the end of the procedure (Duett Pro, Duett, 6/7F Mynx, Mynx M5) (Bechara 2010; Scheinert 2007) or an absorbable intra‐arterial anchor that is absorbed by the body in 30 days (AngioSeal VIP, AngioSeal STS Plus, AngioSeal Evolution). Collagen‐based devices without an intra‐arterial anchor can undergo repeated puncture for angiography. A commonly used extra‐arterial sealant is a bovine biodegradable product that triggers a haemostatic cascade and physical expansion to tamponade the puncture site and tissue tract.
- Metal clip‐based device (StarClose, StarClose SE, Angiolink EVS) (Bechara 2010): devices that utilise metal clip‐based technology and deploy metal staple or clip that penetrates the vessel wall to achieve haemostasis. Upon deployment, the metal clip or staple remains in situ over the vessel wall and forms a geometric configuration that approximates adventitial vessel layers to close the arterial hole. The metallic clips or staples do not undergo a bioresorption reaction, which therefore does not trigger significant soft tissue inflammatory response. Repeat puncture or surgical exploration of the artery can be done safely.
- Suture‐based device (PerClose AT, PerClose ProGlide, ProStar XL, X‐Site, SuperStitch) (Bechara 2010): Arterial haemostasis is achieved by deploying sutures to form a knot to close the arteriotomy. The knot is tied by a built‐in mechanism within the closure device or is tied manually. No proteinaceous biomaterial is left behind in the puncture tract; therefore, no inflammatory soft tissue reaction is associated with this closure technology. Consequently, repeat arterial access or surgical exploration of the same artery can be performed safely.
Why it is important to do this review
VCDs are thought to reduce time to haemostasis, but no consensus indicates whether they affect the incidence of complications at the arteriotomy site compared with haemostasis achieved through extrinsic compression (Smilowitz 2012). Furthermore, introduction of a delivery system and a foreign body into a patient could further damage the artery, and little is known about potentially increased incidence of complications arising directly from closure device use. This review compares the benefits and complications of different types of VCD with one other and with extrinsic compression.
Objectives
To determine the efficacy and safety of VCDs versus traditional methods of extrinsic compression in achieving haemostasis after retrograde and antegrade percutaneous arterial puncture of the common femoral artery (CFA).
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised and quasi‐randomised controlled clinical trials comparing vascular closure devices (VCDs) against manual compression (MC) or mechanical compression devices (MCDs), or both, for achieving common femoral artery (CFA) puncture site haemostasis. The review also encompasses comparisons between different vascular closure devices.
Types of participants
All studies involving people of both genders undergoing a diagnostic or interventional procedure in which vascular access was achieved through percutaneous puncture of the common femoral artery.
Types of interventions
-
Haemostasis after diagnostic or interventional endovascular procedures (sheath size ≤ 9 Fr).
Vascular closure device (VCD) versus manual compression (MC) or mechanical compression device (MCD), or both.
One type of VCD versus another.
-
Haemostasis after percutaneous EVAR (sheath size ≥ 10 Fr).
One type of VCD versus another.
-
Haemostasis after EVAR with open exposure of CFA (sheath size ≥ 10 Fr).
One type of VCD versus another.
Surgical suture‐based closure versus VCD.
Types of outcome measures
Primary outcomes
Primary end point: efficacy
Time to haemostasis: Haemostasis is defined as no or minimal subcutaneous bleeding and absence of expanding or developing haematoma.
Time to mobilisation: This was defined as the time between sheath removal and when the participant was able to mobilise without recurrence of bleeding.
-
Major adverse event (occurring at any time).
Mortality.
Vascular injury requiring vascular repair by surgical or non‐surgical techniques.
Secondary outcomes
-
Adverse events (occurring up to 30 days after arterial closure).
Infection.
Groin haematoma.
Retroperitoneal haemorrhage.
Pseudoaneurysm.
Arterial dissection.
Arteriovenous fistula.
Embolisation resulting in loss of distal pulse.
Deep vein thrombosis.
Limb ischaemia.
Femoral artery thrombosis.
Technical failure of VCDs.
Time spent in angiography suite.
Length of hospital stay.
Participant satisfaction.
Costs of VCD and extrinsic compression.
Search methods for identification of studies
Electronic searches
The Cochrane Vascular Trials Search Co‐ordinator (TSC) searched the Specialised Register (April 2015). In addition, the TSC searched the Cochrane Register of Studies (CRS) at http://www.metaxis.com/CRSWeb/Index.asp (the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 3)). See Appendix 1 for details of the search strategy used to search the CRS. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in The Cochrane Library (www.cochranelibrary.com).
The TSC searched the following trial databases in April 2015 for details of ongoing and unpublished studies using the terms 'vascular' and 'closure'.
World Health Organization International Clinical Trials Registry (http://apps.who.int/trialsearch/).
ClinicalTrials.gov (http://clinicaltrials.gov/).
International Standard Randomized Controlled Trial Number (ISRCTN) register (http://www.isrctn.com/).
Searching other resources
We searched citations within identified studies and contacted authors of identified studies to ask about unpublished studies. We applied no restrictions on language.
Data collection and analysis
All randomised and quasi‐randomised trials that compared the safety and efficacy of vascular closure devices with manual compression or mechanical compression methods, or both, were eligible for inclusion.
Selection of studies
Two review authors (LR and AA) independently assessed studies identified for inclusion in the review using the criteria stated above. They resolved disagreements by discussion or by consultation with a third review author (FC).
Data extraction and management
Two review authors (LR and AA) independently extracted data from the included studies using a standard data extraction form created for the review. Disagreements between the two review authors were resolved by discussion or by consultation with a third review author (FC).
Assessment of risk of bias in included studies
Two review authors (LR and AA) assessed the risk of bias for each study as described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011) for each of the following domains.
Randomisation sequence generation.
Allocation concealment.
Blinding (of participants, personnel and outcome assessors).
Completeness of data.
Selective outcome reporting.
Other sources of bias.
The review authors evaluated each criterion as 'Low risk' of bias or 'High risk' of bias according to Higgins 2011. If these criteria were not discussed in the publication, the review authors assessed risk of bias as 'Unclear'. Disagreements between the two review authors were resolved by discussion or by consultation with a third review author (FC).
Measures of treatment effect
When dealing with dichotomous outcome measures, we calculated a pooled estimate of the treatment effect for each outcome across trials using the odds ratio (OR) (the odds of an outcome among treatment‐allocated participants to the corresponding odds of the same outcome among participants in the control group) and estimated the 95% confidence interval (CI). For continuous outcomes, we recorded either the mean change from baseline for each group or mean post‐intervention values and standard deviation (SD) for each group. When appropriate, we calculated a pooled estimate of the treatment effect by calculating the mean difference (MD) and the SD.
Unit of analysis issues
The unit of analysis was the individual participant. We did not include cross‐over trials in the review because only a single treatment was designated to each group. In the case of cluster‐randomised trials, when the unit of randomisation was not the same as the unit of analysis, we performed appropriate adjustment for clustering, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
The review authors requested missing data from the original investigators, if appropriate. When these could not be obtained, an intention‐to‐treat (ITT) analysis was carried out. For the ITT analysis, we used data on the number of participants with each outcome event by allocated treatment group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow‐up.
Assessment of heterogeneity
If a meta‐analysis was possible, we assessed statistical heterogeneity by using the I2 statistic to quantify inconsistencies among included studies. A guide to interpretation of the I2 statistic is provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) as follows: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity. For the purposes of this review, 90% was the cutoff point for considerable heterogeneity. If considerable heterogeneity was observed (I2 ≥ 90%), the data were not pooled into a meta‐analysis. If heterogeneity was observed, we planned to conduct a subgroup analysis to explore possible causes.
Assessment of reporting biases
We investigated publication bias by using funnel plots if we were able to include a sufficient number of studies (≥ 10), as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; Sterne 2001). If we detected asymmetry, we explored causes other than publication bias. Asymmetrical funnel plots can indicate outcome reporting bias (ORB) or heterogeneity. If we suspected ORB, we contacted trialists. Outcome reporting bias can be assessed by comparing the Methods section of a published trial with the Results section when the original protocol is not available.
Data synthesis
We used a fixed‐effect model in our analysis (Higgins 2011). If we detected heterogeneity (I2 > 75%), we reassessed the significance of the treatment effect by using and reporting a random‐effects model.
Subgroup analysis and investigation of heterogeneity
The original protocol stipulated that the following analyses should be performed.
VCD for the conventional interventional vascular procedure using introducer sheaths up to 9 Fr versus VCD requiring larger introducer sheaths (e.g. for EVAR).
Comparison between antegrade and retrograde punctures.
However, data from the included studies did not permit these subgroup analyses.
In the presence of heterogeneity, we used a random‐effects model. To investigate heterogeneity further, we performed analyses comparing type of procedure (diagnostic or interventional) and brand of VCD when possible.
Sensitivity analysis
We planned to perform a sensitivity analysis to assess the impact of trials with high risk of bias on the overall outcome of pooling of data. However, most studies were classified as having low or unclear risk of bias; therefore, this was not possible.
Quality of evidence
We graded the quality of the evidence according to the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) principles described in Higgins 2011 and GRADE 2004.
Results
Description of studies
Results of the search
See Figure 1.
1.

Study flow diagram.
Included studies
See Characteristics of included studies.
We included in the review 52 studies involving a total of 19,192 participants (Amin 2000; Amine 1999; Ansel 2006; Behan 2007; Brachmann 1998; Camenzind 1994; Carere 2000; Castañeda 2003; Chen 2013; Deuling 2008; Diaz 2001; Doneaux 2001; Fargen 2011; Gerckens 1998; Gwechenberger 1997; Hattab 2012; Hermanides 2010; Hermiller 2005; Hermiller 2006; Holm 2014; Jensen 2008; Juergens 2004; Kalsch 2008; Kussmaul 1995; Legrand 2005; Machnik 2012; Magosaki 1999; Martin 2008; Michalis 2002; Nelson 2014; Noguchi 2000; Park 2005; Perlowski 2011; Rastan 2008; Reddy 2004; Rickli 2002; Sanborn 1993; Schräder 1992; Schulz‐Schüpke 2014; SEAL Trial Study Team; Seidelin 1997; Shammas 2002; Silber 1998; Sun 2009; Tron 2003; Upponi 2007; Veasey 2008; von Hoch 1995; Ward 1998; Wetter 2000; Wong 2009; Yadav 2003).
In all, 51 studies assessed the effectiveness of VCDs after diagnostic or interventional endovascular procedures (sheath size ≤ 9 Fr). One study compared the effectiveness of VCDs in people undergoing percutaneous EVAR and in those with open exposure of the common femoral artery (both sheath size ≥ 10 Fr) (Nelson 2014).
Eleven studies looked at the effectiveness of VCDs after diagnostic catheterisation procedures (Amine 1999; Behan 2007; Fargen 2011; Hermiller 2005; Holm 2014; Jensen 2008; Reddy 2004; Schulz‐Schüpke 2014; Seidelin 1997; Veasey 2008; Ward 1998), and 15 studies assessed interventional procedures (Amin 2000; Camenzind 1994; Chen 2013; Doneaux 2001; Hattab 2012; Hermiller 2006; Juergens 2004; Legrand 2005; Machnik 2012; Martin 2008; Rickli 2002; Silber 1998; Tron 2003; von Hoch 1995; Wetter 2000). Twenty‐four studies looked at both diagnostic and interventional procedures (Ansel 2006; Brachmann 1998; Carere 2000; Castañeda 2003; Deuling 2008; Diaz 2001; Gerckens 1998; Gwechenberger 1997; Kalsch 2008; Kussmaul 1995; Magosaki 1999; Michalis 2002; Noguchi 2000; Park 2005; Perlowski 2011; Rastan 2008; Sanborn 1993; Schräder 1992; SEAL Trial Study Team; Shammas 2002; Sun 2009; Upponi 2007; Wong 2009; Yadav 2003). One study (Nelson 2014) measured the effectiveness of two VCDs after percutaneous EVAR; the same study also compared devices with surgical suture‐based closure versus VCDs after EVAR with open exposure of the CFA.
Collagen‐based VCD versus manual or mechanical compression
Thirty studies measured the effectiveness of a collagen‐based vascular closing device versus manual or mechanical compression (Amin 2000; Behan 2007; Brachmann 1998; Camenzind 1994; Castañeda 2003; Deuling 2008; Diaz 2001; Doneaux 2001; Gwechenberger 1997; Hermanides 2010; Holm 2014; Jensen 2008; Juergens 2004; Kussmaul 1995; Legrand 2005; Machnik 2012; Magosaki 1999; Martin 2008; Reddy 2004; Sanborn 1993; Schräder 1992; Schulz‐Schüpke 2014; SEAL Trial Study Team; Seidelin 1997; Silber 1998; Upponi 2007; von Hoch 1995; Ward 1998; Wong 2009; Yadav 2003). Seventeen trials studied the AngioSeal device (Amin 2000; Behan 2007; Deuling 2008; Diaz 2001; Doneaux 2001; Hermanides 2010; Jensen 2008; Juergens 2004; Kussmaul 1995; Legrand 2005; Machnik 2012; Magosaki 1999; Martin 2008; Reddy 2004; Seidelin 1997; Upponi 2007; Ward 1998) ‐ seven in diagnostic procedures (Behan 2007; Deuling 2008; Doneaux 2001; Jensen 2008; Reddy 2004; Seidelin 1997; Ward 1998), six in interventional procedures (Amin 2000; Hermanides 2010; Juergens 2004; Legrand 2005; Machnik 2012; Martin 2008) and four (Diaz 2001; Kussmaul 1995; Magosaki 1999; Upponi 2007) in both diagnostic and interventional procedures. Seven studies tested the VasoSeal device (Brachmann 1998; Camenzind 1994; Gwechenberger 1997; Sanborn 1993; Schräder 1992; Silber 1998; von Hoch 1995) ‐ three (Camenzind 1994; Silber 1998; von Hoch 1995) in interventional procedures and four (Brachmann 1998; Gwechenberger 1997; Sanborn 1993; Schräder 1992) in both diagnostic and interventional procedures. One study (SEAL Trial Study Team) tested the Duett device, which is a liquid collagen and thrombin device, two studied QuickSeal (Castañeda 2003; Yadav 2003), two studied FemoSeal (Holm 2014; Schulz‐Schüpke 2014) and two studied ExoSeal (Schulz‐Schüpke 2014; Wong 2009), a device that uses a polyglycolic acid plug. Schulz‐Schüpke 2014 tested ExoSeal in interventional procedures, and Wong 2009 tested ExoSeal in both diagnostic and interventional procedures.
Metal clip‐based VCD versus manual or mechanical compression
Six studies measured the effectiveness of a metal clip‐based device versus manual compression (Ansel 2006; Deuling 2008; Hermiller 2005; Hermiller 2006; Perlowski 2011; Sun 2009). Four studied the StarClose device (Deuling 2008; Hermiller 2005; Hermiller 2006; Perlowski 2011) ‐ one (Hermiller 2005) in diagnostic procedures, one (Hermiller 2006) in interventional procedures and two (Deuling 2008; Perlowski 2011) in both diagnostic and interventional procedures. Sun 2009 was a three‐armed trial that compared StarClose, PerClose and manual compression in participants undergoing both diagnostic and interventional procedures. Finally, one study measured the effectiveness of the Angiolink EVS closure device (Ansel 2006) with both diagnostic and interventional procedures.
Suture‐based VCD versus manual or mechanical compression
Ten studies (Amine 1999; Carere 2000; Gerckens 1998; Jensen 2008; Martin 2008; Noguchi 2000; Rickli 2002; Sun 2009; Tron 2003; Wetter 2000) measured the effectiveness of a suture‐based device versus manual compression. Seven studies (Amine 1999; Jensen 2008; Martin 2008; Rickli 2002; Sun 2009; Tron 2003; Wetter 2000) looked at PerClose ‐ two (Amine 1999; Jensen 2008) in diagnostic participants, four (Martin 2008; Rickli 2002; Tron 2003; Wetter 2000) in interventional participants and one (Sun 2009) in both types of procedures. Three studies (Carere 2000; Gerckens 1998; Noguchi 2000) tested ProStar in participants undergoing diagnostic and interventional procedures.
Collagen‐based VCD versus metal clip‐based VCD: AngioSeal versus StarClose
Three studies compared AngioSeal versus StarClose (Deuling 2008; Rastan 2008; Veasey 2008). Veasey 2008 tested the device after diagnostic procedures, and Deuling 2008 and Rastan 2008 looked at both diagnostic and interventional procedures.
Collagen‐based VCD versus suture‐based VCD
Five studies (Hattab 2012; Jensen 2008; Kalsch 2008; Martin 2008; Park 2005) compared a collagen‐based VCD with a suture‐based VCD. Three studies (Jensen 2008; Kalsch 2008; Martin 2008) compared AngioSeal with PerClose ‐ one (Jensen 2008) in diagnostic participants, one (Martin 2008) in interventional participants and one (Kalsch 2008) in both diagnostic and interventional participants. One study (Park 2005) compared AngioSeal with Closure S in diagnostic and interventional participants, and Hattab 2012 compared ExoSeal with ProGlide in participants undergoing intervention. Park 2005 included participants with several femoral artery punctures. Outcomes are based on the number of punctures rather than on the number of individual participants. After personal communication with the study author, it was decided that although this study was relevant and met the inclusion criteria, data would not be included in the analyses, as they were not comparable with data based on individuals from the other included studies.
Metal clip‐based VCD versus suture‐based VCD: StarClose versus PerClose
One study (Sun 2009) compared the metal clip‐based StarClose with the suture‐based PerClose in participants undergoing diagnostic and interventional procedures.
Disc‐based VCD versus suture‐based VCD: Boomerang versus PerClose
One study (Chen 2013) compared a disc‐based device (Boomerang) with a suture‐based device (PerClose) in 60 participants undergoing coronary intervention.
Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus VasoSeal
Two studies (Michalis 2002; Shammas 2002) compared the collagen‐based devices AngioSeal and VasoSeal in both diagnostic and interventional procedures.
Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Mynx
One study (Fargen 2011) measured vascular injury requiring repair, infection, groin haematoma and patient satisfaction in diagnostic participants treated with the AngioSeal or another collagen device, Mynx.
Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Duett
Michalis 2002 was a three‐armed trial that tested the collagen devices AngioSeal and Duett in participants undergoing diagnostic and interventional procedures.
Collagen‐based VCD versus collagen‐based VCD: VasoSeal versus Duett
Michalis 2002 also tested the VasoSeal and Duett collagen devices.
Collagen‐based VCD versus collagen‐based VCD: FemoSeal versus ExoSeal
One study (Schulz‐Schüpke 2014) compared the collagen devices FemoSeal and ExoSeal in participants undergoing diagnostic procedures.
PerClose ProGlide versus ProStar XL after percutaneous EVAR (sheath size ≥ 10 Fr)
One study (Nelson 2014) compared PerClose ProGlide with ProStar XL in participants undergoing percutaneous EVAR.
PerClose ProGlide and ProStar XL versus suture‐based closure after EVAR with open exposure of CFA (sheath size ≥ 10 Fr)
Nelson 2014 also compared the PerClose ProGlide and ProStar XL devices with surgical suture‐based closure in participants undergoing open femoral exposure of the CFA.
Excluded studies
See Characteristics of excluded studies.
We excluded 14 studies (Baim 2000; Beyer‐Enke 1996; Chalmers 2007; Chevalier 2000; Jean‐Baptiste 2008; Kurşaklioĝlu 2008; Larzon 2015; Leinbudgut 2013; Lupi 2012; Neudecker 2003; Ratnam 2007; Slaughter 1995; Smilowitz 2012; Starnes 2003). Seven were not randomised controlled trials (Jean‐Baptiste 2008; Kurşaklioĝlu 2008; Lupi 2012; Ratnam 2007; Neudecker 2003; Ratnam 2007; Smilowitz 2012), two (Baim 2000; Starnes 2003) used 7 to 10 Fr sheath sizes and did not present data by sheath size and one (Chalmers 2007) used EVICEL and another (Larzon 2015) used the fascia suture technique (neither of which are VCDs); another study (Chevalier 2000) measured adverse events included in this review but did not present data, one (Leinbudgut 2013) randomised people by the drug they received to prevent bleeding rather than by VCD and another study (Beyer‐Enke 1996) was not clear on whether access for the procedure was attained through the femoral artery. For studies on which we had queries regarding data (Baim 2000; Beyer‐Enke 1996; Chevalier 2000; Starnes 2003), we wrote to the study authors but received no response and therefore had to exclude these studies from the review.
Risk of bias in included studies
2.
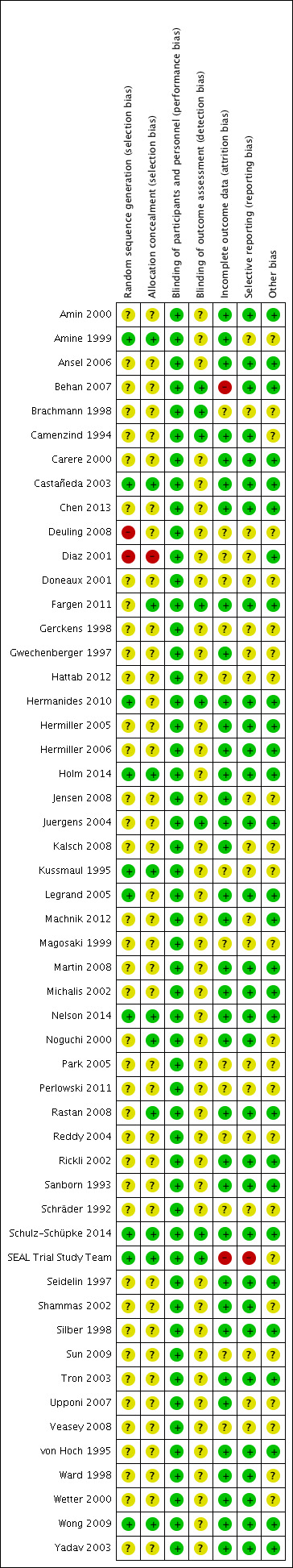
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation: Of the 52 studies included in this review, 10 were deemed to be at low risk of bias (Amine 1999; Castañeda 2003; Hermanides 2010; Holm 2014; Kussmaul 1995; Legrand 2005; Nelson 2014; Schulz‐Schüpke 2014; SEAL Trial Study Team; Wong 2009). Three studies (Legrand 2005; Schulz‐Schüpke 2014; Wong 2009) reported that randomisation was computer‐assisted, and another seven studies (Amine 1999; Castañeda 2003; Hermanides 2010; Holm 2014; Kussmaul 1995; Nelson 2014; SEAL Trial Study Team) reported using a block design to generate the random sequence. Two studies were judged to be at high risk of bias, as participants were assigned to treatment not randomly but rather on order of presentation (Deuling 2008) or by odd and even numbers (Diaz 2001). The remaining 40 studies did not provide enough information about the randomisation process to permit judgement on the risk of bias.
Allocation concealment: One study was deemed to be at high risk of bias as allocation was based on alternation (Diaz 2001). Eleven studies were at low risk of bias, as they reported using sealed envelopes (Amine 1999; Castañeda 2003; Fargen 2011; Kussmaul 1995; Nelson 2014; Noguchi 2000; Rastan 2008; SEAL Trial Study Team; Wong 2009) or a computer‐based system (Holm 2014; Schulz‐Schüpke 2014) to conceal allocation of treatment. The remaining 40 did not provide enough information about allocation concealment to permit judgement on the risk of selection bias.
Blinding
Blinding of study participants and personnel was not possible. However, we determined that outcomes of the review were not likely to be influenced by lack of blinding and therefore judged all studies to be at low risk of performance bias.
Blinding of outcome assessors was possible, and eight studies (Behan 2007; Brachmann 1998; Camenzind 1994; Fargen 2011; Hermanides 2010; Juergens 2004; Schulz‐Schüpke 2014; SEAL Trial Study Team) were judged to be at low risk of detection bias as study authors reported that outcome assessors were blinded to treatment assignment. No study was found to be at high risk of detection bias. In the remaining 44 studies included in this review, risk of detection bias was deemed to be unclear because reporting of blinding of outcome assessors was inadequate.
Incomplete outcome data
Thirty‐six of the 52 included studies were judged to be at low risk of attrition bias (Amin 2000; Amine 1999; Ansel 2006; Camenzind 1994; Carere 2000; Castañeda 2003; Chen 2013; Fargen 2011; Gwechenberger 1997; Hermanides 2010; Hermiller 2005; Hermiller 2006; Holm 2014; Jensen 2008; Juergens 2004; Kalsch 2008; Legrand 2005; Machnik 2012; Martin 2008; Michalis 2002; Nelson 2014; Noguchi 2000; Rastan 2008; Rickli 2002; Sanborn 1993; Schulz‐Schüpke 2014; Seidelin 1997; Shammas 2002; Silber 1998; Tron 2003; Upponi 2007; von Hoch 1995; Ward 1998; Wetter 2000; Wong 2009; Yadav 2003). Two studies were judged to be at high risk of attrition bias: SEAL Trial Study Team reported that only 227 of 392 participants treated with the Duett device completed the seven‐day and 30‐day quality of life study and did not explain the reason for this large loss to follow‐up and Behan 2007 reported that only 72% of AngioSeal and 71% of manual compression participants completed follow‐up at one week. The remaining 14 studies did not provide enough information about incomplete outcome data to permit judgement on the risk of attrition bias.
Selective reporting
One study (SEAL Trial Study Team) was judged to be at high risk of reporting bias, as study authors reported quality of life results at seven days and 30 days post procedure, but quality of life was not a clearly specified outcome of the study. Thirty‐one studies adequately reported data on all pre‐specified outcomes and therefore were judged to be at low risk of reporting bias (Amin 2000; Ansel 2006; Behan 2007; Camenzind 1994; Carere 2000; Castañeda 2003; Chen 2013; Fargen 2011; Hermanides 2010; Hermiller 2005; Hermiller 2006; Holm 2014; Juergens 2004; Legrand 2005; Martin 2008; Michalis 2002; Nelson 2014; Noguchi 2000; Rastan 2008; Rickli 2002; Sanborn 1993; Schulz‐Schüpke 2014; Seidelin 1997; Shammas 2002; Silber 1998; Tron 2003; von Hoch 1995; Ward 1998; Wetter 2000; Wong 2009; Yadav 2003). The remaining 20 studies did not provide enough information to permit judgement on low or high risk of reporting bias; therefore, the risk was deemed unclear.
Other potential sources of bias
Twenty‐eight studies appeared to be free from other sources of bias (Amin 2000; Ansel 2006; Behan 2007; Carere 2000; Castañeda 2003; Chen 2013; Diaz 2001; Fargen 2011; Hermanides 2010; Hermiller 2005; Hermiller 2006; Holm 2014; Juergens 2004; Legrand 2005; Machnik 2012; Martin 2008; Michalis 2002; Nelson 2014; Rastan 2008; Rickli 2002; Sanborn 1993; Schulz‐Schüpke 2014; Seidelin 1997; Silber 1998; Tron 2003; von Hoch 1995; Wong 2009; Yadav 2003). No study was deemed to be at high risk of bias. The remaining 24 studies included in the review did not provide enough information; therefore, risk of bias was unclear.
Effects of interventions
Haemostasis after diagnostic or interventional endovascular procedures (sheath size ≤ 9 Fr)
Collagen‐based VCD versus manual or mechanical compression
Thirty studies measured the effectiveness of a collagen‐based vascular closing device versus manual or mechanical compression (Amin 2000; Behan 2007; Brachmann 1998; Camenzind 1994; Castañeda 2003; Deuling 2008; Diaz 2001; Doneaux 2001; Gwechenberger 1997; Hermanides 2010; Holm 2014; Jensen 2008; Juergens 2004; Kussmaul 1995; Legrand 2005; Machnik 2012; Magosaki 1999; Martin 2008; Reddy 2004; Sanborn 1993; Schräder 1992; Schulz‐Schüpke 2014; SEAL Trial Study Team; Seidelin 1997; Silber 1998; Upponi 2007; von Hoch 1995; Ward 1998; Wong 2009; Yadav 2003).
Time to haemostasis
Nineteen studies that compared a collagen‐based VCD with manual compression measured time to haemostasis (Brachmann 1998; Castañeda 2003; Diaz 2001; Doneaux 2001; Gwechenberger 1997; Holm 2014; Juergens 2004; Kussmaul 1995; Magosaki 1999; Martin 2008; Reddy 2004; Sanborn 1993; Schulz‐Schüpke 2014; SEAL Trial Study Team; Seidelin 1997; Silber 1998; Ward 1998; Wong 2009; Yadav 2003). Data from 12 studies were entered into a meta‐analysis (Brachmann 1998; Castañeda 2003; Diaz 2001; Gwechenberger 1997; Juergens 2004; Kussmaul 1995; Magosaki 1999; Reddy 2004; Sanborn 1993; Seidelin 1997; Silber 1998; Wong 2009). Seven studies were not included in the meta‐analysis: Four studies (Doneaux 2001; Martin 2008; Ward 1998; Yadav 2003) did not report standard deviations for mean time to haemostasis, and three studies (Holm 2014; Schulz‐Schüpke 2014; SEAL Trial Study Team) presented time to haemostasis as a median and as an interquartile range.
When the 12 studies were combined in a meta‐analysis, considerable heterogeneity was evident (I2 = 98%) (Analysis 1.1). Subgroup analyses by type of procedure, brand of VCD and quality of the included studies revealed no differences between groups. Individually, 11 of the 12 studies showed that the collagen‐based VCD was associated with significantly shorter time to haemostasis when compared with manual compression (Brachmann 1998; Castañeda 2003; Diaz 2001; Gwechenberger 1997; Juergens 2004; Kussmaul 1995; Magosaki 1999; Sanborn 1993; Seidelin 1997; Silber 1998; Wong 2009). Only one study showed no significant improvement between the collagen‐based VCD and manual compression (Reddy 2004). Juergens 2004 reported a significantly longer time to haemostasis for both VCD and manual compression participants than was reported in other included studies. We contacted the study author, who did not reply to clarify whether results reported in the paper were correct. Exclusion of this study from the meta‐analysis had little impact on heterogeneity.
1.1. Analysis.
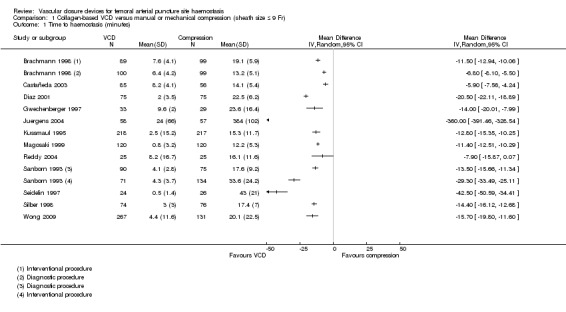
Comparison 1 Collagen‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 1 Time to haemostasis (minutes).
Time to mobilisation
Thirteen studies were included in a meta‐analysis (Behan 2007; Brachmann 1998; Castañeda 2003; Diaz 2001; Holm 2014; Juergens 2004; Legrand 2005; Machnik 2012; Magosaki 1999; Sanborn 1993; Schräder 1992; Seidelin 1997; Wong 2009). Doneaux 2001; Martin 2008; SEAL Trial Study Team; Ward 1998; and Yadav 2003 reported time to ambulation but did not provide standard deviations; the SEAL Trial Study Team reported time to ambulation as median and as interquartile range. We contacted the authors of these studies but did not obtain requested data.
Meta‐analysis of the 13 studies indicated heterogeneity (I2 = 100%) (Analysis 1.2). Subgroup analyses by type of procedure, brand of VCD and quality of included studies showed no differences between groups. All 13 studies individually showed that the collagen‐based VCD was associated with significantly shorter time to mobilisation than was seen with manual compression (Behan 2007; Brachmann 1998; Castañeda 2003; Diaz 2001; Holm 2014; Juergens 2004; Legrand 2005; Machnik 2012; Magosaki 1999; Sanborn 1993; Schräder 1992; Seidelin 1997; Wong 2009).
1.2. Analysis.

Comparison 1 Collagen‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 2 Time to mobilisation (hours).
Major adverse events
Mortality
Only one study (Castañeda 2003) presented data on mortality and reported no deaths in 141 participants (Analysis 1.3).
1.3. Analysis.
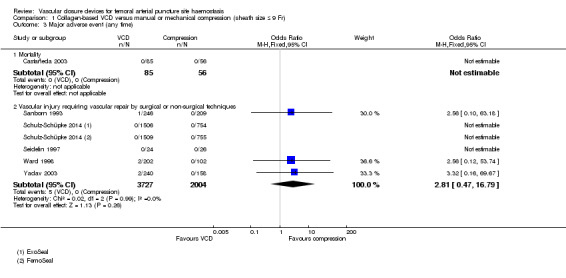
Comparison 1 Collagen‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 3 Major adverse event (any time).
Vascular injury requiring vascular repair by surgical or non‐surgical techniques
Five studies (Sanborn 1993; Schulz‐Schüpke 2014; Seidelin 1997; Ward 1998; Yadav 2003) reported on this outcome (Analysis 1.3). Of 3727 participants treated with a collagen‐based VCD, five (0.1%) had vascular injury requiring repair compared with none of 2004 manual compression participants (OR 2.81, 95% CI 0.47 to 16.79; P value = 0.26).
Adverse events
Infection
Nine studies (Behan 2007; Castañeda 2003; Deuling 2008; Holm 2014; Sanborn 1993; Schulz‐Schüpke 2014; SEAL Trial Study Team; Seidelin 1997; von Hoch 1995) recorded puncture site infection (Analysis 1.4). Of 4674 participants treated with a VCD, 15 (0.3%) experienced infection compared with six of 2942 (0.2%) participants treated with manual compression (OR 2.14, 95% CI 0.88 to 5.22; P value = 0.09). However, five of the nine included studies (Behan 2007; Castañeda 2003; Deuling 2008; Schulz‐Schüpke 2014; SEAL Trial Study Team) found no cases of infection, and another study (Seidelin 1997) included only 50 people.
1.4. Analysis.

Comparison 1 Collagen‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 4 Infection.
Groin haematoma
A total of 25 studies (Amin 2000; Camenzind 1994; Castañeda 2003; Deuling 2008; Diaz 2001; Doneaux 2001; Gwechenberger 1997; Hermanides 2010; Holm 2014; Jensen 2008; Juergens 2004; Kussmaul 1995; Legrand 2005; Machnik 2012; Magosaki 1999; Reddy 2004; Sanborn 1993; Schräder 1992; Schulz‐Schüpke 2014; Seidelin 1997; Silber 1998; Upponi 2007; Ward 1998; Wong 2009; Yadav 2003) measured groin haematoma (Analysis 1.5). Haematoma occurred in 327 of 6019 (5.4%) participants treated with a collagen‐based VCD compared with 456 of 4228 (10.8%) participants treated with manual compression, leading to an OR of 0.46 (95% CI 0.40 to 0.54; P value < 0.00001).
1.5. Analysis.

Comparison 1 Collagen‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 5 Groin haematoma.
Retroperitoneal haemorrhage
Three studies (Behan 2007; Martin 2008; Wong 2009), based on a total of 744 participants, found retroperitoneal haemorrhage in three of 444 (0.7%) VCD participants and one of 300 (0.3%) manual compression participants (OR 1.5, 95% CI 0.22 to 11.42; P value = 0.65) (Analysis 1.6).
1.6. Analysis.

Comparison 1 Collagen‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 6 Retroperitoneal haemorrhage.
Pseudoaneurysm
Twenty‐one studies (Amin 2000; Behan 2007; Camenzind 1994; Deuling 2008; Doneaux 2001; Gwechenberger 1997; Holm 2014; Juergens 2004; Legrand 2005; Machnik 2012; Magosaki 1999; Martin 2008; Reddy 2004; Sanborn 1993; Schulz‐Schüpke 2014; SEAL Trial Study Team; Silber 1998; Upponi 2007; von Hoch 1995; Ward 1998; Yadav 2003) reported pseudoaneurysm as an outcome (Analysis 1.7). Meta‐analysis showed that pseudoaneurysm occurred in 92 of 5573 (1.6%) VCD participants and in 83 of 3769 (2.2%) manual compression participants, leading to an OR of 0.74 (95% CI 0.55 to 0.99; P value = 0.04).
1.7. Analysis.
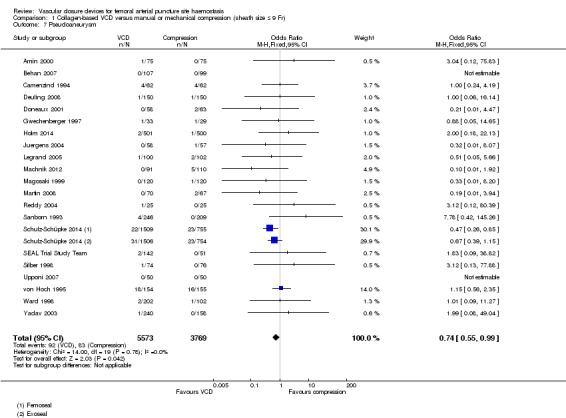
Comparison 1 Collagen‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 7 Pseudoaneurysm.
Arterial dissection
None of the included studies measured arterial dissection as an outcome.
Arteriovenous fistula
Meta‐analysis of eight studies (Gwechenberger 1997; Hermanides 2010; Machnik 2012; Martin 2008; Schulz‐Schüpke 2014; SEAL Trial Study Team; Upponi 2007; von Hoch 1995) showed that arteriovenous fistula occurred in 14 of 3868 (0.4%) VCD participants and in nine of 2285 (0.4%) manual compression participants (OR 0.98, 95% CI 0.43 to 2.21; P value = 0.96) (Analysis 1.8).
1.8. Analysis.

Comparison 1 Collagen‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 8 Arterio‐venous fistula.
Embolisation resulting in loss of distal pulse
None of the included studies measured this as an outcome.
Deep vein thrombosis
Among three studies (Camenzind 1994; Sanborn 1993; Seidelin 1997), deep vein thrombosis (DVT) occurred in four of 332 (1.2%) VCD participants and in one of 297 (0.3%) manual compression participants, leading to an OR of 2.41 (95% CI 0.46 to 12.50; P value = 0.30) (Analysis 1.9).
1.9. Analysis.
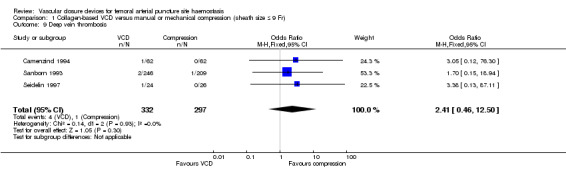
Comparison 1 Collagen‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 9 Deep vein thrombosis.
Limb ischaemia
Three studies (Behan 2007; Machnik 2012; Schulz‐Schüpke 2014) measured limb ischaemia as an outcome (Analysis 1.10). No cases occurred in the 3242 VCD participants nor in the 1728 participants treated with manual compression.
1.10. Analysis.

Comparison 1 Collagen‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 10 Limb ischaemia.
Femoral artery thrombosis
One study (Upponi 2007) measured femoral artery thrombosis but found no cases in VCD nor manual compression participants (Analysis 1.11).
1.11. Analysis.
Comparison 1 Collagen‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 11 Femoral artery thrombosis.
Technical failure of VCDs
In 24 studies (Amin 2000; Behan 2007; Castañeda 2003; Deuling 2008; Doneaux 2001; Gwechenberger 1997; Jensen 2008; Juergens 2004; Kussmaul 1995; Legrand 2005; Machnik 2012; Magosaki 1999; Martin 2008; Reddy 2004; Sanborn 1993; Schräder 1992; SEAL Trial Study Team; Seidelin 1997; Silber 1998; Upponi 2007; von Hoch 1995; Ward 1998; Wong 2009; Yadav 2003) with a combined total of 3033 participants treated with a collagen‐based VCD, 118 unsuccessful device deployments led to a technical failure rate of 3.9%.
Time spent in angiography suite
None of the included studies reported on this outcome.
Length of hospital stay
Eight studies (Castañeda 2003; Juergens 2004; Machnik 2012; Magosaki 1999; Silber 1998; Ward 1998; Wong 2009; Yadav 2003) measured length of hospital stay. However, Ward 1998 and Yadav 2003 did not report standard deviations for the mean stay and therefore could not be included in the meta‐analysis. Meta‐analysis of the six studies based on a random‐effects model showed considerable heterogeneity (I2 = 90%) (Analysis 1.12). Subgroup analyses that excluded two studies (Magosaki 1999; Silber 1998) with significantly longer hospital stay than the other studies showed no differences between groups.
1.12. Analysis.

Comparison 1 Collagen‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 12 Length of hospital stay (hours).
Patient satisfaction
Six studies (Amin 2000; Holm 2014; Juergens 2004; Legrand 2005; Martin 2008; Schräder 1992) reported on patient satisfaction. However, these studies used different measurement tools and scales; therefore, the results could not be meta‐analysed. Five studies reported that collagen‐based devices were associated with less pain and bedrest than were seen with manual compression (Amin 2000; Juergens 2004; Legrand 2005; Martin 2008; Schräder 1992). However, in one study (Holm 2014), participants in the VCD group reported greater pain and discomfort during the closure procedure when compared with participants in the manual compression group.
Cost of VCD and extrinsic compression
None of the included studies compared the cost of VCD versus manual compression.
Metal clip‐based VCD versus manual or mechanical compression
Six studies measured the effectiveness of a metal clip‐based device versus manual compression (Ansel 2006; Deuling 2008; Hermiller 2005; Hermiller 2006; Perlowski 2011; Sun 2009).
Time to haemostasis
Five studies (Ansel 2006; Hermiller 2005; Hermiller 2006; Perlowski 2011; Sun 2009) measured time to haemostasis, four using StarClose (Hermiller 2005; Hermiller 2006; Perlowski 2011; Sun 2009) and one using Angiolink (Ansel 2006). Ansel 2006 presented results according to type of procedure and therefore provided data on both diagnostic and interventional participants. Meta‐analysis using a random‐effects model indicated that the metal clip‐based VCD was associated with statistically significantly less time to haemostasis than manual compression (MD ‐14.81 minutes, 95% CI ‐16.98 to ‐12.63; participants = 1665; I2 = 84%; P value < 0.00001) (Analysis 2.1).
2.1. Analysis.

Comparison 2 Metal clip‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 1 Time to haemostasis (minutes).
Time to mobilisation
Three studies (Ansel 2006; Hermiller 2005; Sun 2009) including a total of 1303 participants measured time to haemostasis with Angiolink (Ansel 2006) or StarClose (Hermiller 2005; Sun 2009). Ansel 2006 presented results according to type of procedure, including data on both diagnostic and interventional participants. Meta‐analysis using a random‐effects model indicated substantial heterogeneity (I2 = 100%), and subgroup analysis performed by type of procedure and brand of VCD showed no differences between groups (Analysis 2.2). Individually, all three studies (Ansel 2006; Hermiller 2005; Sun 2009) showed that the metal clip‐based VCD was associated with significantly reduced time to mobilisation when compared with manual compression.
2.2. Analysis.
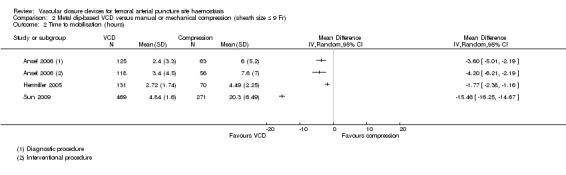
Comparison 2 Metal clip‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 2 Time to mobilisation (hours).
Major adverse event
Three studies (Hermiller 2005; Hermiller 2006; Perlowski 2011) with a combined total of 564 participants reported no deaths in either treatment group (Analysis 2.3). Three studies (Deuling 2008; Hermiller 2005; Hermiller 2006) with a combined total of 783 participants reported no differences in the incidence of vascular injury requiring repair (OR 0.49, 95% CI 0.03 to 7.95; P value = 0.62).
2.3. Analysis.

Comparison 2 Metal clip‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 3 Major adverse event (any time).
Adverse events
Infection
No cases of infection were reported in the 470 VCD and 313 manual compression participants among three studies reporting on infection (Deuling 2008; Hermiller 2005; Hermiller 2006) (Analysis 2.4).
2.4. Analysis.

Comparison 2 Metal clip‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 4 Infection.
Groin haematoma
Four studies (Deuling 2008; Hermiller 2005; Hermiller 2006; Sun 2009) determined that the incidence of groin haematoma was 30 of 939 (3.2%) and 28 of 584 (4.8%) VCD and manual compression participants, respectively (OR 0.79, 95% CI 0.46 to 1.34; P value = 0.38) (Analysis 2.5).
2.5. Analysis.

Comparison 2 Metal clip‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 5 Groin haematoma.
Retroperitoneal haemorrhage
None of the studies comparing metal clip‐based VCDs with manual compression measured retroperitoneal haemorrhage as an outcome.
Pseudoaneurysm
Pseudoaneurysm was reported in six of the included studies (Ansel 2006; Deuling 2008; Hermiller 2005; Hermiller 2006; Perlowski 2011; Sun 2009) (Analysis 2.6). The combined incidence was four of 1221 (0.3%) metal clip‐based VCD participants compared with three of 745 (0.4%) manual compression participants (OR 0.76, 95% CI 0.20 to 2.89; P value = 0.69).
2.6. Analysis.

Comparison 2 Metal clip‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 6 Pseudoaneurysm.
Arterial dissection
None of the studies comparing metal clip‐based VCDs with manual compression measured arterial dissection as an outcome.
Arteriovenous fistula
No cases of arteriovenous fistula were reported in 564 participants in three studies (Hermiller 2005; Hermiller 2006; Perlowski 2011) (Analysis 2.7).
2.7. Analysis.

Comparison 2 Metal clip‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 7 Arterio‐venous fistula.
Embolisation resulting in loss of distal pulse
None of the studies comparing metal clip‐based VCDs with manual compression measured embolisation with loss of distal pulse as an outcome.
Deep vein thrombosis
No cases of DVT were reported among 483 participants in two studies (Hermiller 2005; Hermiller 2006) (Analysis 2.8).
2.8. Analysis.

Comparison 2 Metal clip‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 8 Deep vein thrombosis.
Limb ischaemia
None of the 320 VCD participants nor 163 manual compression participants in two studies developed limb ischaemia (Hermiller 2005; Hermiller 2006) (Analysis 2.9).
2.9. Analysis.

Comparison 2 Metal clip‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 9 Limb ischaemia.
Femoral artery thrombosis
None of the studies comparing metal clip‐based VCDs with manual compression measured femoral artery thrombosis as an outcome.
Technical failure of VCDs
In six studies (Ansel 2006; Deuling 2008; Hermiller 2005; Hermiller 2006; Perlowski 2011; Sun 2009) on a combined total of 1039 participants treated with a metal clip‐based VCD, 71 unsuccessful device deployments occurred, leading to a technical failure rate of 6.8%.
Time spent in angiography suite
Time spent in the angiography suite was not measured in any of the studies comparing metal clip VCDs and manual compression.
Length of hospital stay
Length of hospital stay was not measured in any of the studies comparing metal clip VCDs and manual compression.
Patient satisfaction
One study (Hermiller 2006) measured pain on a scale of 0 to 10 and found StarClose to be non‐inferior to manual compression. A pain scale of 0 to 3 was reported by 87.3% of StarClose versus 93.3% of manual compression participants. Pain scales of 8 to 10 were seen in 2.2% of StarCose and 3.3% of compression participants.
Cost of VCD and extrinsic compression
No studies considered the costs of the different treatments.
Suture‐based VCD versus manual or mechanical compression
Ten studies (Amine 1999; Carere 2000; Gerckens 1998; Jensen 2008; Martin 2008; Noguchi 2000; Rickli 2002; Sun 2009; Tron 2003; Wetter 2000) measured the effectiveness of a suture‐based device versus manual compression.
Time to haemostasis
Eight studies (Amine 1999; Gerckens 1998; Martin 2008; Noguchi 2000; Rickli 2002; Sun 2009; Tron 2003; Wetter 2000) measured time to haemostasis. However, Martin 2008 did not report standard deviations and therefore was not included in the meta‐analysis. Pooled analysis of the seven studies using a random‐effects model showed that suture‐based VCDs were associated with a statistically significant reduction in time to haemostasis when compared with manual compression (MD ‐14.58 minutes; 95% CI ‐16.85 to ‐12.32; participants = 1664; I2 = 86%; P value < 0.0001) (Analysis 3.1).
3.1. Analysis.

Comparison 3 Suture‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 1 Time to haemostasis (minutes).
Time to mobilisation
Eight studies (Amine 1999; Carere 2000; Gerckens 1998; Martin 2008; Noguchi 2000; Rickli 2002; Sun 2009; Wetter 2000) measured time to mobilisation. Martin 2008 did not report standard deviations and therefore was not included in the meta‐analysis. Pooled analysis of the seven studies (Amine 1999; Carere 2000; Gerckens 1998; Noguchi 2000; Rickli 2002; Sun 2009; Wetter 2000) showed significant heterogeneity (I2 = 98%), and subgroup analysis performed by type of procedure and by brand of VCD showed no differences between groups (Analysis 3.2). Individually, all seven studies (Amine 1999; Carere 2000; Gerckens 1998; Noguchi 2000; Rickli 2002; Sun 2009; Wetter 2000) showed that the suture‐based VCD was associated with significantly reduced time to mobilisation when compared with manual compression.
3.2. Analysis.

Comparison 3 Suture‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 2 Time to mobilisation (hours).
Major adverse event
Only one study (Noguchi 2000) measured mortality and vascular injury requiring repair but reported no cases in either treatment group (Analysis 3.3).
3.3. Analysis.
Comparison 3 Suture‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 3 Major adverse event (any time).
Adverse events
Infection
Three studies (Amine 1999; Gerckens 1998; Noguchi 2000) with 750 participants reported the incidence of infection, describing two cases of infection in the VCD groups compared with one in the manual compression groups (OR 1.66, 95% CI 0.22 to 12.71; P value = 0.63) (Analysis 3.4).
3.4. Analysis.

Comparison 3 Suture‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 4 Infection.
Groin haematoma
Six studies (Amine 1999; Carere 2000; Gerckens 1998; Jensen 2008; Noguchi 2000; Sun 2009) including a total of 1350 participants measured the incidence of groin haematoma and found an incidence of 5.4% (34/633) among suture‐based VCD participants compared with 7.2% (52/717) among manual compression participants (OR 0.65, 95% CI 0.41 to 1.02; P value = 0.06) (Analysis 3.5).
3.5. Analysis.
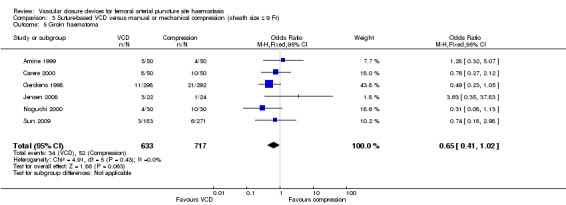
Comparison 3 Suture‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 5 Groin haematoma.
Retroperitoneal haemorrhage
One study measured the incidence of retroperitoneal haemorrhage in 63 suture‐based VCD (0/63) and 67 manual compression participants (1/67), reporting no association (OR 0.35, 95% CI 0.01 to 8.73) (Analysis 3.6).
3.6. Analysis.

Comparison 3 Suture‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 6 Retroperitoneal haemorrhage.
Pseudoaneurysm
Six studies (Gerckens 1998; Martin 2008; Noguchi 2000; Rickli 2002; Sun 2009; Wetter 2000) measured this outcome. Pseudoaneurysm occurred in five of 720 (0.7%) suture‐based VCD and seven of 807 (0.9%) manual compression participants, leading to an OR of 0.79 (95% CI 0.25 to 2.53; P value = 0.70) (Analysis 3.7).
3.7. Analysis.
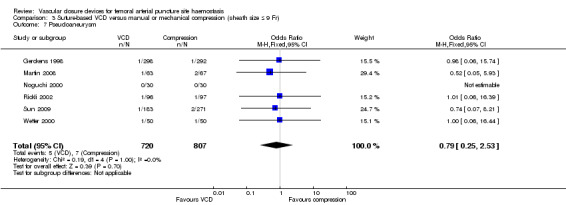
Comparison 3 Suture‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 7 Pseudoaneurysm.
Arterial dissection
Arterial dissection was not a reported outcome in any of the studies comparing suture‐based VCDs with manual compression.
Arteriovenous fistula
Four studies (Amine 1999; Gerckens 1998; Martin 2008; Noguchi 2000) reported on the incidence of arteriovenous fistula. Of the 441 VCD participants, none had an arteriovenous fistula, and one of the 439 (0.2%) manual compression participants experienced this outcome (OR 0.33, 95% CI 0.01 to 8.02; P value = 0.49) (Analysis 3.8).
3.8. Analysis.

Comparison 3 Suture‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 8 Arterio‐venous fistula.
Embolisation resulting in loss of distal pulse
One study (Noguchi 2000) measured distal embolisation but reported no cases in the VCD or manual compression group (Analysis 3.9).
3.9. Analysis.
Comparison 3 Suture‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 9 Embolisation.
Deep vein thrombosis
Deep vein thrombosis was not an outcome in any of the studies examining suture‐based VCDs and manual compression.
Limb ischaemia
Limb ischaemia was measured in two studies (Gerckens 1998; Martin 2008); the combined incidence was one of 361 (0.3%) VCD and one of 359 (0.3%) manual compression participants, respectively (OR 1.02, 95% CI 0.14 to 7.22; P value = 0.98) (Analysis 3.10).
3.10. Analysis.

Comparison 3 Suture‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 10 Limb ischaemia.
Femoral artery thrombosis
None of the studies comparing suture‐based VCDs with manual compression measured the incidence of femoral artery thrombosis.
Technical failure of VCDs
In 10 studies (Amine 1999; Carere 2000; Gerckens 1998; Jensen 2008; Martin 2008; Noguchi 2000; Rickli 2002; Sun 2009; Tron 2003; Wetter 2000) with a combined total of 843 participants who received a suture‐based VCD, 56 unsuccessful device deployments were reported, leading to a technical failure rate of 6.7%.
Time spent in angiography suite
This was not a reported outcome in any of the included studies.
Length of hospital stay
Three studies (Carere 2000; Noguchi 2000; Tron 2003) based on a total of 327 participants reported length of hospital stay. Meta‐analysis of the three studies using a random‐effects model showed that the suture‐based VCD was associated with shorter hospital stay when compared with manual compression (MD ‐11.66 hours, 95% CI ‐20.46 to ‐2.85; I2 = 85%; P value = 0.009) (Analysis 3.11).
3.11. Analysis.

Comparison 3 Suture‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr), Outcome 11 Length of hospital stay (hours).
Patient satisfaction
One study (Martin 2008) measured patient satisfaction in a questionnaire designed to address issues of discomfort and inconvenience at hospital discharge. On a 1 to 4 scale, mean groin discomfort was rated at 1.7 with the PerClose device compared with 2.0 among manual compression participants. Mean scores for the inconvenience of bedrest were 1.8 and 2.0 among VCD and manual compression participants, respectively. Discomfort at the time of sheath removal was 1.6 in both study arms.
Noguchi 2000 measured whether participants would be willing to undergo another procedure with the ProStar device if a repeat intervention was needed. Of 30 participants, 24 (80%) stated that ProStar would be their preferred choice.
Another study (Carere 2000) measured participant perception of the sheath removal procedure by questionnaire 24 hours after the procedure. Participants treated with the ProStar‐Plus device reported a more acceptable duration of bedrest when compared with those undergoing manual compression. Although discomfort during the procedure was similar between ProStar‐Plus and manual compression groups, discomfort after the procedure was greater among ProStar‐Plus participants. Overall, the 42 participants treated with ProStar reported that the procedure was very acceptable compared with 24 participants undergoing haemostasis by manual compression.
Participant comfort was reported on a visual analogue scale (VAS) (0 best to 10 worst) by Rickli 2002. Mean pain at sheath removal was 1.7 (SD 2.2) in PerClose and 2.9 (SD 2.7) in manual compression groups, with back pain reported as 2.8 (SD 2.7) and 4.5 (SD 2.9) and groin pain during follow‐up as 3.0 (SD 2.0) and 2.0 (SD 2.2), respectively.
Cost of VCD and extrinsic compression
Carere 2000 reported a total per‐patient cost (incremental savings) of $460.21 in the ProStar‐Plus group compared with $759.16 in the manual compression group but did not provide standard deviations for these means.
Noguchi 2000 reported that the hospital cost was $300 less in the ProStar group than in the manual compression group ($1310 (SD 248) vs $1613 (SD 460)).
Rickli 2002 measured treatment costs and reported that post‐percutaneous coronary intervention (PCI) costs were reduced in the PerClose group (€469 (SD 145) vs €539 (SD 57)) compared with the manual compression group. Additional costs of the PerClose device (€225) were exceeded by savings of ward costs due to earlier discharge (PerClose €178 (SD 132) vs manual compression €481 (SD 55)). PerClose was also associated with less cardiologist time (13.8 (SD 5.4) minutes vs 32.9 (SD 13.9) minutes) and less nursing time (6.9 (SD 3.5) vs 11.5 (SD 7.0) minutes).
Collagen‐based VCD versus metal clip‐based VCD: AngioSeal versus StarClose
Three studies compared AngioSeal versus StarClose (Deuling 2008; Rastan 2008; Veasey 2008).
Time to haemostasis
None of the studies comparing a collagen‐based VCD with a metal clip‐based VCD measured time to haemostasis.
Time to mobilisation
Time to mobilisation was not a reported outcome in any of the studies comparing collagen‐based and metal clip‐based VCDs.
Major adverse event
One study measured mortality and reported no deaths (Rastan 2008). Two studies (Deuling 2008; Rastan 2008) measured vascular injury requiring repair and found no differences between the two treatment groups (OR 0.33, 95% CI 0.01 to 8.22; P value = 0.50) (Analysis 4.1).
4.1. Analysis.

Comparison 4 Collagen‐based VCD versus metal clip‐based VCD: AngioSeal versus StarClose, Outcome 1 Major adverse event (any time).
Adverse events
Infection
No cases of infection occurred in two studies reporting this outcome (Deuling 2008; Veasey 2008) among a combined total of 701 participants (Analysis 4.2).
4.2. Analysis.

Comparison 4 Collagen‐based VCD versus metal clip‐based VCD: AngioSeal versus StarClose, Outcome 2 Infection.
Groin haematoma
Two studies (Deuling 2008; Rastan 2008) compared AngioSeal and StarClose devices among a combined total of 871 participants. Meta‐analysis using a random‐effects model showed no differences in the incidence of groin haematoma between the two devices (OR 0.84, 95% CI 0.43 to 1.65; P value = 0.61). The incidence was 3.9% (17/435) and 4.6% (20/436) among collagen‐based and metal clip‐based VCD participants, respectively (Analysis 4.3).
4.3. Analysis.

Comparison 4 Collagen‐based VCD versus metal clip‐based VCD: AngioSeal versus StarClose, Outcome 3 Groin haematoma.
Retroperitoneal haemorrhage
Only one study (Veasey 2008) measured the incidence of retroperitoneal haemorrhage and reported no cases in the collagen VCD (n = 208) nor metal clip VCD (n = 193) arms (Analysis 4.4).
4.4. Analysis.
Comparison 4 Collagen‐based VCD versus metal clip‐based VCD: AngioSeal versus StarClose, Outcome 4 Retroperitoneal haemorrhage.
Pseudoaneurysm
Pseudoaneurysm was measured in three studies (Deuling 2008; Rastan 2008; Veasey 2008). The incidence was four of 643 (0.6%) among collagen‐based VCD and eight of 629 (1.3%) among metal clip‐based VCD participants (OR 0.50, 95% CI 0.15 to 1.66; P value = 0.26) (Analysis 4.5).
4.5. Analysis.

Comparison 4 Collagen‐based VCD versus metal clip‐based VCD: AngioSeal versus StarClose, Outcome 5 Pseudoaneurysm.
Arterial dissection
Arterial dissection was not reported in any of the included studies.
Arteriovenous fistula
Only one study (Rastan 2008) measured arteriovenous fistula. Rastan 2008 reported one case in 285 collagen‐based VCD participants but no cases in 286 metal clip‐based VCD participants (OR 3.02, 95% CI 0.12 to 74.47) (Analysis 4.6).
4.6. Analysis.

Comparison 4 Collagen‐based VCD versus metal clip‐based VCD: AngioSeal versus StarClose, Outcome 6 Arterio‐venous fistula.
Embolisation resulting in loss of distal pulse
This was not measured in any of the included studies.
Deep vein thrombosis
Deep vein thrombosis was not reported in any of the studies comparing collagen‐based and clip‐based VCDs.
Limb ischaemia
One study (Veasey 2008) measured limb ischaemia but reported no cases in either treatment group (Analysis 4.7).
4.7. Analysis.

Comparison 4 Collagen‐based VCD versus metal clip‐based VCD: AngioSeal versus StarClose, Outcome 7 Limb ischaemia.
Femoral artery thrombosis
Femoral artery thrombosis was not reported in any of the studies comparing collagen‐based with clip‐based VCDs.
Technical failure of VCDs
Three studies (Deuling 2008; Rastan 2008; Veasey 2008) reported the technical failure of AngioSeal versus StarClose devices. The device failed in 22 of 643 (3.4%) AngioSeal participants compared with 53 of 629 (8.4%) StarClose participants, suggesting that the collagen‐based device has a significantly lower failure rate than the StarClose device (OR 0.38, 95% CI 0.23 to 0.64; P value = 0.0003) (Analysis 4.8).
4.8. Analysis.

Comparison 4 Collagen‐based VCD versus metal clip‐based VCD: AngioSeal versus StarClose, Outcome 8 Technical failure of VCD.
Time spent in angiography suite
Time spent in the angiography suite was not a reported outcome in any of the included studies.
Length of hospital stay
Length of hospital stay was not measured in any of the studies comparing collagen‐based and metal clip‐based VCDs.
Patient satisfaction
No study compared patient satisfaction with collagen‐based versus metal clip‐based VCDs.
Cost of VCD and extrinsic compression
Cost of the device was not reported in any of the included studies.
Collagen‐based VCD versus suture‐based VCD
Five studies (Hattab 2012; Jensen 2008; Kalsch 2008; Martin 2008; Park 2005) compared a collagen‐based VCD with a suture‐based VCD.
Time to haemostasis
Martin 2008 measured time to haemostasis among participants treated with AngioSeal and PerClose ProGlide. Although investigators reported the mean time to haemostasis, they did not provide standard deviations for the mean; therefore, it was not possible to perform statistical analysis on mean differences. We attempted to obtain these data, but study authors did not respond to our request. Martin 2008 did report that AngioSeal was associated with significantly reduced time to haemostasis compared with PerClose (P value < 0.01).
Time to mobilisation
Martin 2008 also reported mean time to mobilisation but did not present standard deviations; therefore, statistical tests on the mean difference could not be performed. However, study authors reported that AngioSeal was associated with reduced time to mobilisation when compared with the PerClose device (P value < 0.01).
Major adverse event
None of the five studies measured death or vascular injury requiring repair (Hattab 2012; Jensen 2008; Kalsch 2008; Martin 2008; Park 2005).
Adverse events
Infection
Kalsch 2008 measured the incidence of infection in 212 AngioSeal and 154 PerClose participants, reporting no cases of infection in either treatment group (Analysis 5.1).
5.1. Analysis.

Comparison 5 Collagen‐based VCD versus suture‐based VCD (sheath size ≤ 9 Fr), Outcome 1 Infection.
Groin haematoma
Three studies (Hattab 2012; Jensen 2008; Kalsch 2008) measured the incidence of groin haematoma in a combined total of 510 participants. . Haematoma occurred in 34 of 284 (12.0%) AngioSeal participants compared with 22 of 226 (9.7%) ExoSeal participants, resulting in an OR of 1.26 (95% CI 0.71 to 2.22; P value = 0.43); therefore, neither AngioSeal nor ExoSeal was superior in the prevention of haematoma (Analysis 5.2).
5.2. Analysis.

Comparison 5 Collagen‐based VCD versus suture‐based VCD (sheath size ≤ 9 Fr), Outcome 2 Groin haematoma.
Retroperitoneal haemorrhage
Martin 2008 reported one case of retroperitoneal haemorrhage in 70 AngioSeal participants but no cases in 63 PerClose ProGlide participants (OR 2.74, 95% CI 0.11 to 68.51; P value = 0.54) (Analysis 5.3).
5.3. Analysis.

Comparison 5 Collagen‐based VCD versus suture‐based VCD (sheath size ≤ 9 Fr), Outcome 3 Retroperitoneal haemorrhage.
Pseudoaneurysm
Martin 2008 reported no cases of pseudoaneurysm in 70 AngioSeal participants but one case in 63 PerClose ProGlide participants (OR 0.30, 95% CI 0.01 to 7.39; P value = 0.46) (Analysis 5.4).
5.4. Analysis.

Comparison 5 Collagen‐based VCD versus suture‐based VCD (sheath size ≤ 9 Fr), Outcome 4 Pseudoaneurysm.
Arterial dissection
This outcome was not measured in any of the studies included in this review.
Arteriovenous fistula
No cases of arteriovenous fistula were reported among participants treated with AngioSeal (n = 70) nor PerClose ProGlide (n = 63) in the study by Martin 2008 (Analysis 5.5).
5.5. Analysis.
Comparison 5 Collagen‐based VCD versus suture‐based VCD (sheath size ≤ 9 Fr), Outcome 5 Arterio‐venous fistula.
Embolisation resulting in loss of distal pulse
Embolisation was not reported as an outcome in any study.
Deep vein thrombosis
Deep vein thrombosis was not measured in any of the studies comparing collagen‐based and suture‐based VCDs.
Limb ischaemia
None of the studies comparing collagen‐based and suture‐based VCDs reported limb ischaemia.
Femoral artery thrombosis
Femoral artery thrombosis was not a reported outcome in any study.
Technical failure of VCDs
Three studies (Hattab 2012; Jensen 2008; Kalsch 2008) compared the technical failure of a collagen‐based VCD versus a suture‐based VCD. Two studies (Jensen 2008; Kalsch 2008) compared AngioSeal with PerClose, and Hattab 2012 compared ExoSeal with ProGlide. Meta‐analyses showed no differences in the technical failure of VCDs (OR 0.24, 95% CI 0.08 to 0.69; I2 = 74%; P value = 0.008) (Analysis 5.6).
5.6. Analysis.

Comparison 5 Collagen‐based VCD versus suture‐based VCD (sheath size ≤ 9 Fr), Outcome 6 Technical failure of VCD.
Time spent in angiography suite
Time spent in the angiography suite was not an outcome in any of the included studies.
Length of hospital stay
Length of hospital stay was not measured in any studies comparing collagen‐based and suture‐based VCDs.
Patient satisfaction
No study compared patient satisfaction with collagen‐based versus suture‐based VCDs.
Cost of VCD and extrinsic compression
Cost of treatment was not measured in any of the studies comparing collagen‐based and suture‐based VCDs.
Metal clip‐based VCD versus suture‐based VCD: StarClose versus PerClose
One study (Sun 2009) compared the metal clip‐based StarClose with the suture‐based PerClose.
Time to haemostasis
One study (Sun 2009) on 469 participants tested a metal clip‐based VCD (StarClose) against a suture‐based VCD (PerClose). Data on time to haemostasis were presented separately by type of procedure. For the purposes of the review, the mean and standard deviations for this outcome were combined according to the formula given in Table 7.7.a in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This study found that StarClose was associated with shorter time to haemostasis than PerClose (MD ‐2.24 minutes, 95% CI ‐2.54 to ‐1.94; P value < 0.00001) (Analysis 6.1).
6.1. Analysis.

Comparison 6 Metal clip‐based VCD versus suture‐based VCD: StarClose versus PerClose, Outcome 1 Time to haemostasis.
Time to mobilisation
Sun 2009 also presented data on time to mobilisation separately according to the type of procedure. As above, the mean and standard deviations for this outcome were combined according to the formula given in Table 7.7.a in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This study found that the StarClose device was associated with a reduction in the mean time to mobilisation (MD ‐0.30 hours, 95% CI ‐0.59 to ‐0.01; P value = 0.05) (Analysis 6.2).
6.2. Analysis.

Comparison 6 Metal clip‐based VCD versus suture‐based VCD: StarClose versus PerClose, Outcome 2 Time to mobilisation.
Major adverse event
Sun 2009 did not report mortality nor vascular injury requiring repair.
Adverse events
Infection
Sun 2009 did not report infection.
Groin haematoma
Sun 2009 measured the incidence of haematoma at 0.3% (1/286) in StarClose participants and 1.6% (3/183) in PerClose participants, leading to an OR of 0.21 (95% CI 0.02 to 2.04; P value = 0.18) (Analysis 6.3).
6.3. Analysis.

Comparison 6 Metal clip‐based VCD versus suture‐based VCD: StarClose versus PerClose, Outcome 3 Groin haematoma.
Retroperitoneal haemorrhage
Sun 2009 did not report retroperitoneal haemorrhage.
Pseudoaneurysm
In the Sun 2009 study, pseudoaneurysm did not occur in any of the StarClose participants, and one case was reported among the 183 (0.5%) PerClose participants (OR 0.21, 95% CI 0.01 to 5.24; P value = 0.34) (Analysis 6.4).
6.4. Analysis.
Comparison 6 Metal clip‐based VCD versus suture‐based VCD: StarClose versus PerClose, Outcome 4 Pseudoaneurysm.
Arterial dissection
This outcome was not measured in the Sun 2009 study.
Arteriovenous fistula
Sun 2009 did not report arteriovenous fistula.
Embolisation resulting in loss of distal pulse
Embolisation was not reported as an outcome in the Sun 2009 study.
Deep vein thrombosis
Sun 2009 did not report deep vein thrombosis.
Limb ischaemia
Sun 2009 did not report limb ischaemia.
Femoral artery thrombosis
Sun 2009 did not report femoral artery thrombosis.
Technical failure of VCDs
In the Sun 2009 study, the StarClose device demonstrated fewer incidences of technical failure compared with the PerClose device (OR 0.34, 95% CI 0.12 to 0.92; P value = 0.03) (Analysis 6.5)..
6.5. Analysis.

Comparison 6 Metal clip‐based VCD versus suture‐based VCD: StarClose versus PerClose, Outcome 5 Technical failure of VCD.
Time spent in angiography suite
Time spent in the angiography suite was not reported as an outcome in the Sun 2009 study.
Length of hospital stay
Sun 2009 did not measure length of hospital stay.
Patient satisfaction
Sun 2009 did not measure patient satisfaction.
Cost of VCD and extrinsic compression
Cost of treatment was not measured in the Sun 2009 study.
Disc‐based VCD versus suture‐based VCD: Boomerang versus PerClose
Chen 2013 compared a disc‐based device (Boomerang) with a suture‐based device (PerClose).
Time to haemostasis
Chen 2013 measured time to haemostasis in participants treated with the Boomerang and PerClose devices. The Boomerang device was associated with significantly longer time to haemostasis than the PerClose device (MD 32.05 minutes, 95% CI 29.09 to 35.01; P value < 0.00001) (Analysis 7.1).
7.1. Analysis.

Comparison 7 Disc‐based VCD versus suture‐based VCD: Boomerang versus PerClose, Outcome 1 Time to haemostasis (minutes).
Time to mobilisation
Chen 2013 also reported mean time to mobilisation but found no difference between the two devices (MD ‐0.04 hours, 95% CI ‐0.14 to 0.06 hours; P value = 0.41) (Analysis 7.2).
7.2. Analysis.

Comparison 7 Disc‐based VCD versus suture‐based VCD: Boomerang versus PerClose, Outcome 2 Time to mobilisation (hours).
Major adverse event
Chen 2013 did not report mortality nor vascular injury requiring repair.
Adverse events
Infection
Chen 2013 did not report infection.
Groin haematoma
Chen 2013 found no difference in the incidence of groin haematoma between the Boomerang and PerClose devices (OR 10.36, 95% CI 0.53 to 201.45; P value = 0.12) (Analysis 7.3).
7.3. Analysis.

Comparison 7 Disc‐based VCD versus suture‐based VCD: Boomerang versus PerClose, Outcome 3 Groin haematoma.
Retroperitoneal haemorrhage
Chen 2013 did not report retroperitoneal haemorrhage.
Pseudoaneurysm
Chen 2013 did not report pseudoaneurysm.
Arterial dissection
This outcome was not measured in the Chen 2013 study.
Arteriovenous fistula
Chen 2013 did not report arteriovenous fistula.
Embolisation resulting in loss of distal pulse
Embolisation was not reported as an outcome in the Chen 2013 study.
Deep vein thrombosis
Chen 2013 did not report deep vein thrombosis.
Limb ischaemia
Chen 2013 did not report limb ischaemia.
Femoral artery thrombosis
Chen 2013 did not report femoral artery thrombosis.
Technical failure of VCDs
Chen 2013 found no difference in the rate of technical failure between the Boomerang and PerClose devices (OR 2.07, 95% CI 0.18 to 24.15; P value = 0.56) (Analysis 7.4).
7.4. Analysis.
Comparison 7 Disc‐based VCD versus suture‐based VCD: Boomerang versus PerClose, Outcome 4 Technical failure of VCD.
Time spent in angiography suite
Time spent in the angiography suite was not reported as an outcome in the Chen 2013 study.
Length of hospital stay
Chen 2013 did not measure length of hospital stay.
Patient satisfaction
Chen 2013 measured pain on a scale of 0 to 10, with 0 representing no pain and 10 representing the worst possible pain. Results indicated that participants treated with the Boomerang device reported significantly lower pain levels (mean 1.10, SD 1.71) compared with participants treated with PerClose (mean 6.40, SD 2.92).
Cost of VCD and extrinsic compression
Cost of treatment was not measured in the Chen 2013 study.
Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus VasoSeal
Two studies (Michalis 2002; Shammas 2002) compared the collagen‐based devices AngioSeal and VasoSeal.
Time to haemostasis
Both studies (Michalis 2002; Shammas 2002) presented time to haemostasis separately for diagnostic and interventional procedures. When these studies were combined in a meta‐analysis, considerable heterogeneity was evident (I2 = 90%). When AngioSeal was compared with VasoSeal, VasoSeal was associated with shorter time to haemostasis for interventional procedures (MD 8.63, 95% CI 1.46 to 15.80; P value = 0.02; I2 = 86%) but not for diagnostic procedures (MD 6.68, 95% CI ‐2.31 to 15.67; P value = 0.15; I2 = 96%) (Analysis 8.1).
8.1. Analysis.

Comparison 8 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus VasoSeal, Outcome 1 Time to haemostasis (minutes).
Time to mobilisation
Both studies (Michalis 2002; Shammas 2002) compared the time to mobilisation between AngioSeal and VasoSeal devices. AngioSeal was not associated with a difference in time to mobilisation compared with VasoSeal, for both diagnostic (MD 0.01 hours, 95% CI ‐1.04 to 1.06; P value = 0.98; I2 = 90%) and interventional procedures (MD 0.45, 95% CI ‐1.91 to 2.82; P value = 0.71; I2 = 74%) (Analysis 8.2).
8.2. Analysis.

Comparison 8 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus VasoSeal, Outcome 2 Time to mobilisation (hours).
Major adverse event
Shammas 2002 found no cases of mortality nor vascular injury requiring repair (Analysis 8.3). Michalis 2002 did not measure mortality nor vascular injury requiring repair.
8.3. Analysis.
Comparison 8 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus VasoSeal, Outcome 3 Major adverse event (any time).
Adverse events
Infection
Shammas 2002 reported no cases of infection (Analysis 8.4). Michalis 2002 did not measure infection.
8.4. Analysis.
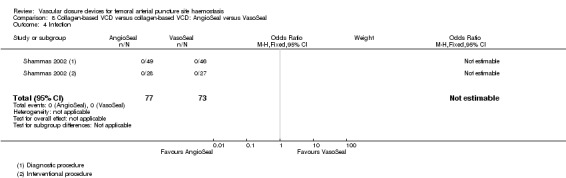
Comparison 8 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus VasoSeal, Outcome 4 Infection.
Groin haematoma
Both studies measured groin haematoma between collagen‐based devices (Michalis 2002; Shammas 2002). Shammas 2002 found no haematoma, but the incidence of haematoma in Michalis 2002led to an OR of 0.49 (95% CI 0.16 to 1.47; P = 0.20) (Analysis 8.5).
8.5. Analysis.

Comparison 8 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus VasoSeal, Outcome 5 Groin haematoma.
Retroperitoneal haemorrhage
Both studies (Michalis 2002; Shammas 2002) measured retroperitoneal haemorrhage as an outcome. They reported no cases in 367 AngioSeal‐treated participants and only one case in 353 VasoSeal‐treated participants, leading to an OR of 0.36 (95% CI 0.01 to 9.09; P value = 0.54) (Analysis 8.6).
8.6. Analysis.

Comparison 8 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus VasoSeal, Outcome 6 Retroperitoneal haemorrhage.
Pseudoaneurysm
Shammas 2002 reported just one case of pseudoaneurysm (OR 0.31, 95% CI 0.01 to 7.71; P value = 0.47) (Analysis 8.7). Michalis 2002 did not measure pseudoaneurysm.
8.7. Analysis.

Comparison 8 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus VasoSeal, Outcome 7 Pseudoaneurysm.
Arterial dissection
Arterial dissection was not reported in the Michalis 2002 and Shammas 2002 studies.
Arteriovenous fistula
Shammas 2002 reported no cases of arteriovenous fistula (Analysis 8.8). Michalis 2002 did not measure arteriovenous fistula.
8.8. Analysis.

Comparison 8 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus VasoSeal, Outcome 8 Arterio‐venous fistula.
Embolisation resulting in loss of distal pulse
The studies by Michalis 2002 and Shammas 2002 did not report on embolisation resulting in loss of distal pulse.
Deep vein thrombosis
Deep vein thrombosis was not an outcome of the Michalis 2002 and Shammas 2002 studies.
Limb ischaemia
Limb ischaemia was not an outcome of the Michalis 2002 and Shammas 2002 studies.
Femoral artery thrombosis
Femoral artery thrombosis was not an outcome of the Michalis 2002 and Shammas 2002 studies.
Technical failure of VCDs
Michalis 2002 showed that the device failed in 19 of 290 AngioSeal participants compared with 26 of 280 VasoSeal participants, leading to an OR of 0.68 (95% CI 0.37 to 1.26; P value = 0.22) (Analysis 8.9). Shammas 2002 did not report on the technical failure of the VCD.
8.9. Analysis.

Comparison 8 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus VasoSeal, Outcome 9 Technical failure of VCD.
Time spent in angiography suite
Neither Michalis 2002 nor Shammas 2002 reported on time spent in the angiography suite.
Length of hospital stay
Length of hospital stay was not an outcome of the Michalis 2002 and Shammas 2002 studies.
Patient satisfaction
Patient satisfaction was not an outcome of the Michalis 2002 and Shammas 2002 studies.
Cost of VCD and extrinsic compression
Device cost was not assessed in the two included studies (Michalis 2002; Shammas 2002).
Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Mynx
One study compared AngioSeal versus Mynx (Fargen 2011).
Time to haemostasis
Fargen 2011 did not measure time to haemostasis as an outcome.
Time to mobilisation
Fargen 2011 did not measure time to mobilisation as an outcome.
Major adverse event
Fargen 2011 did not measure mortality and found no cases of vascular injury requiring repair (Analysis 9.1).
9.1. Analysis.

Comparison 9 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Mynx, Outcome 1 Major adverse event (any time).
Adverse events
Infection
Fargen 2011 found no cases of infection (Analysis 9.2).
9.2. Analysis.
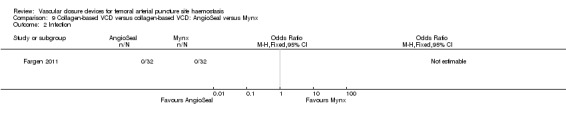
Comparison 9 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Mynx, Outcome 2 Infection.
Groin haematoma
Fargen 2011 found no cases of groin haematoma (Analysis 9.3).
9.3. Analysis.

Comparison 9 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Mynx, Outcome 3 Groin haematoma.
Retroperitoneal haemorrhage
Retroperitoneal haemorrhage was not reported as an outcome of the Fargen 2011 study.
Pseudoaneurysm
Pseudoaneurysm was not reported as an outcome of the Fargen 2011 study.
Arterial dissection
Arterial dissection was not reported as an outcome of the Fargen 2011 study.
Arteriovenous fistula
Arteriovenous fistula was not reported as an outcome of the Fargen 2011 study.
Embolisation resulting in loss of distal pulse
Fargen 2011 did not report on embolisation resulting in loss of distal pulse.
Deep vein thrombosis
Deep vein thrombosis was not reported as an outcome of the Fargen 2011 study.
Limb ischaemia
Limb ischaemia was not reported as an outcome of the Fargen 2011 study.
Femoral artery thrombosis
Femoral artery thrombosis was not reported as an outcome of the Fargen 2011 study.
Technical failure of VCDs
Fargen 2011 did not report technical failure of the VCDs.
Time spent in angiography suite
Fargen 2011 did not report on time spent in the angiography suite.
Length of hospital stay
Length of hospital stay was not reported as an outcome of the Fargen 2011 study.
Patient satisfaction
Fargen 2011 measured pain at closure and pain increase from baseline to closure, reporting that 88% of AngioSeal participants reported closure as the most painful part compared with 34% of Mynx participants.
Cost of VCD and extrinsic compression
Device cost was not assessed in the Fargen 2011 study.
Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Duett
Michalis 2002 compared AngioSeal with Duett.
Time to haemostasis
Data regarding time to haemostasis were presented separately for diagnostic and interventional procedures. The Duett device was associated with shorter time to haemostasis when compared with AngioSeal in both diagnostic (MD 10.60 minutes, 95% CI 9.74 to 11.46) and interventional procedures (MD 12.00 minutes, 95% CI 9.57 to 14.43) (Analysis 10.1).
10.1. Analysis.

Comparison 10 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Duett, Outcome 1 Time to haemostasis (minutes).
Time to mobilisation
Michalis 2002 showed that AngioSeal was associated with shorter time to mobilisation when compared with the Duett device in diagnostic procedures (MD ‐0.40 hours, 95% CI ‐0.51 to ‐0.29). However, no difference was noted when AngioSeal was compared with the Duett device in interventional procedures (MD ‐0.32 hours, 95% CI ‐0.71 to 0.07) (Analysis 10.2).
10.2. Analysis.

Comparison 10 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Duett, Outcome 2 Time to mobilisation (hours).
Major adverse event
Michalis 2002 did not measure mortality nor vascular injury requiring repair.
Adverse events
Infection
Michalis 2002 did not measure infection as an outcome.
Groin haematoma
Michalis 2002 reported the incidence of haematoma as 1.7% (5/290) in AngioSeal participants compared with 1.8% (5/281) in Duett participants (OR 0.97, 95% CI 0.28 to 3.38; P value = 0.97) (Analysis 10.3).
10.3. Analysis.

Comparison 10 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Duett, Outcome 3 Groin haematoma.
Retroperitoneal haemorrhage
Michalis 2002 measured retroperitoneal haemorrhage as an outcome, reporting no cases in AngioSeal nor Duett participants (Analysis 10.4).
10.4. Analysis.

Comparison 10 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Duett, Outcome 4 Retroperitoneal haemorrhage.
Pseudoaneurysm
Michalis 2002 did not measure pseudoaneurysm as an outcome.
Arterial dissection
Arterial dissection was not reported as an outcome in the Michalis 2002 study.
Arteriovenous fistula
Michalis 2002 did not measure arteriovenous fistula as an outcome.
Embolisation resulting in loss of distal pulse
Michalis 2002 did not report on embolisation resulting in loss of distal pulse.
Deep vein thrombosis
Deep vein thrombosis was not reported as an outcome of the Michalis 2002 study.
Limb ischaemia
Limb ischaemia was not reported as an outcome of the Michalis 2002 study.
Femoral artery thrombosis
Femoral artery thrombosis was not reported as an outcome of the Michalis 2002 study.
Technical failure of VCDs
Michalis 2002 showed that the device failed in 19 of 290 AngioSeal participants compared with 32 of 281 Duett participants, leading to an OR of 0.54 (95% CI 0.30 to 0.99; P value = 0.04) (Analysis 10.5).
10.5. Analysis.

Comparison 10 Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Duett, Outcome 5 Technical failure of VCD.
Time spent in angiography suite
Michalis 2002 did not report on time spent in the angiography suite.
Length of hospital stay
Length of hospital stay was not reported as an outcome of the Michalis 2002 study.
Patient satisfaction
Patient satisfaction was not reported as an outcome of the Michalis 2002 study.
Costs of VCD and extrinsic compression
Device cost was not assessed in the included study (Michalis 2002).
Collagen‐based VCD versus collagen‐based VCD: VasoSeal versus Duett
One study compared VasoSeal and Duett (Michalis 2002).
Time to haemostasis
Michalis 2002 presented data regarding time to haemostasis separately for diagnostic and interventional procedures. No difference was found when VasoSeal was compared with the Duett device in diagnostic (MD ‐0.50 minutes, 95% CI ‐1.11 to 0.11) nor interventional (MD ‐1.00 minutes, 95% CI ‐3.15 to 1.15) procedures (Analysis 11.1).
11.1. Analysis.

Comparison 11 Collagen‐based VCD versus collagen‐based VCD: VasoSeal versus Duett, Outcome 1 Time to haemostasis (minutes).
Time to mobilisation
Michalis 2002 showed that when VasoSeal was compared with the Duett device, no differences in time to mobilisation were noted for diagnostic (MD 0.08 hours, 95% CI 0.05 to 0.21) nor interventional procedures (MD 0.16 hours, 95% CI ‐0.17 to 0.49) (Analysis 11.2).
11.2. Analysis.

Comparison 11 Collagen‐based VCD versus collagen‐based VCD: VasoSeal versus Duett, Outcome 2 Time to mobilisation (hours).
Major adverse event
Michalis 2002 did not measure mortality nor vascular injury requiring repair.
Adverse events
Infection
Michalis 2002 did not measure infection as an outcome.
Groin haematoma
Michalis 2002 found no difference in the incidence of haematoma when the VasoSeal was compared with the Duett device (OR 2.00, 95% CI 0.67 to 5.95; P value = 0.21) (Analysis 11.3).
11.3. Analysis.

Comparison 11 Collagen‐based VCD versus collagen‐based VCD: VasoSeal versus Duett, Outcome 3 Groin haematoma.
Retroperitoneal haemorrhage
Michalis 2002 reported only one case of retroperitoneal haemorrhage in 280 VasoSeal participants and no cases in Duett participants (OR 2.77, 95% CI 0.11 to 69.59; P value = 0.54) (Analysis 11.4).
11.4. Analysis.

Comparison 11 Collagen‐based VCD versus collagen‐based VCD: VasoSeal versus Duett, Outcome 4 Retroperitoneal haemorrhage.
Pseudoaneurysm
Michalis 2002 did not measure pseudoaneurysm as an outcome.
Arterial dissection
Arterial dissection was not reported in the Michalis 2002 study.
Arteriovenous fistula
Michalis 2002 did not measure arteriovenous fistula as an outcome.
Embolisation resulting in loss of distal pulse
Michalis 2002 did not report on embolisation resulting in loss of distal pulse.
Deep vein thrombosis
Deep vein thrombosis was not reported as an outcome of the Michalis 2002 study.
Limb ischaemia
Limb ischaemia was not reported as an outcome of the Michalis 2002 study.
Femoral artery thrombosis
Femoral artery thrombosis was not reported as an outcome of the Michalis 2002 study.
Technical failure of VCDs
Michalis 2002 showed that the Duett device failed in 32 of 281 participants, leading to an OR of 0.80 (95% CI 0.46 to 1.30; P value = 0.42) when compared with 26/280 failures with the VasoSeal device (Analysis 11.5).
11.5. Analysis.

Comparison 11 Collagen‐based VCD versus collagen‐based VCD: VasoSeal versus Duett, Outcome 5 Technical failure of VCD.
Time spent in angiography suite
Michalis 2002 did not report on time spent in the angiography suite.
Length of hospital stay
Length of hospital stay was not reported as an outcome of the Michalis 2002 study.
Patient satisfaction
Patient satisfaction was not reported as an outcome of the Michalis 2002 study.
Cost of VCD and extrinsic compression
Device cost was not assessed in the Michalis 2002 study.
Collagen‐based VCD versus collagen‐based VCD: FemoSeal versus ExoSeal
One study compared FemoSeal and ExoSeal (Schulz‐Schüpke 2014).
Time to haemostasis
Schulz‐Schüpke 2014 presented time to haemostasis as a median and as an interquartile range. Time to haemostasis was 0.5 minute (IQR 0.5 minute to 1 minute) in the group treated with FemoSeal and 2 minutes (IQR 1 minute to 2 minutes) in the group treated with ExoSeal.
Time to mobilisation
Schulz‐Schüpke 2014 did not measure time to mobilisation.
Major adverse event
Schulz‐Schüpke 2014 did not report on mortality and reported no cases of vascular injury requiring repair in either group (Analysis 12.1).
12.1. Analysis.

Comparison 12 Collagen‐based VCD versus collagen‐based VCD: FemoSeal versus ExoSeal, Outcome 1 Major adverse event (any time).
Adverse events
Infection
Schulz‐Schüpke 2014 found no cases of infection in the group treated with ExoSeal and only one case in the group treated with FemoSeal (OR 3.00, 95% CI 0.12 to 73.60; P value = 0.50) (Analysis 12.2).
12.2. Analysis.

Comparison 12 Collagen‐based VCD versus collagen‐based VCD: FemoSeal versus ExoSeal, Outcome 2 Infection.
Groin haematoma
Schulz‐Schüpke 2014 measured the incidence of groin haematoma as 4.3% (65/1509) and 5.3% (80/1506) in the two groups, respectively (OR 0.80, 95% CI 0.57 to 1.12; P value = 0.20) (Analysis 12.3).
12.3. Analysis.

Comparison 12 Collagen‐based VCD versus collagen‐based VCD: FemoSeal versus ExoSeal, Outcome 3 Groin haematoma.
Retroperitoneal haemorrhage
Schulz‐Schüpke 2014 did not report on retroperitoneal haemorrhage.
Pseudoaneurysm
Schulz‐Schüpke 2014 reported that the incidence of pseudoaneurysm was 1.5% (22/1509) in FemoSeal and 2.1% (31/1506) in ExoSeal participants (OR 0.70, 95% CI 0.41 to 1.22; P value = 0.21) (Analysis 12.4).
12.4. Analysis.

Comparison 12 Collagen‐based VCD versus collagen‐based VCD: FemoSeal versus ExoSeal, Outcome 4 Pseudoaneurysm.
Arterial dissection
Arterial dissection was not reported in the Schulz‐Schüpke 2014 study.
Arteriovenous fistula
Schulz‐Schüpke 2014 reported the incidence of arteriovenous fistula as 0.3% (4/1509) in FemoSeal and 0.5% (8/1506) in ExoSeal participants, respectively (OR 0.50, 95% CI 0.15 to 1.66; P value = 0.26) (Analysis 12.5).
12.5. Analysis.
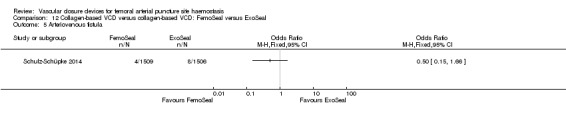
Comparison 12 Collagen‐based VCD versus collagen‐based VCD: FemoSeal versus ExoSeal, Outcome 5 Arteriovenous fistula.
Embolisation resulting in loss of distal pulse
Schulz‐Schüpke 2014 did not report on embolisation resulting in loss of distal pulse.
Deep vein thrombosis
Deep vein thrombosis was not reported as an outcome of the Schulz‐Schüpke 2014 study.
Limb ischaemia
Schulz‐Schüpke 2014 found no cases of limb ischaemia in the FemoSeal nor the ExoSeal group (Analysis 12.6).
12.6. Analysis.

Comparison 12 Collagen‐based VCD versus collagen‐based VCD: FemoSeal versus ExoSeal, Outcome 6 Limb ischaemia.
Femoral artery thrombosis
Femoral artery thrombosis was not reported as an outcome of the Schulz‐Schüpke 2014 study.
Technical failure of VCDs
Schulz‐Schüpke 2014 reported that the FemoSeal device failed to close in 5.3% (80/1509) of participants compared with 12.2% (184/1506) of ExoSeal participants, leading to an OR of 0.40 (95% CI 0.31 to 0.53; P value < 0.00001) (Analysis 12.7).
12.7. Analysis.

Comparison 12 Collagen‐based VCD versus collagen‐based VCD: FemoSeal versus ExoSeal, Outcome 7 Technical failure of VCD.
Time spent in angiography suite
Schulz‐Schüpke 2014 did not report on time spent in the angiography suite.
Length of hospital stay
Length of hospital stay was not reported as an outcome of the Schulz‐Schüpke 2014 study.
Patient satisfaction
Patient satisfaction was not an outcome of the Schulz‐Schüpke 2014 study.
Cost of VCD and extrinsic compression
Device cost was not assessed in the included study (Schulz‐Schüpke 2014).
Haemostasis after percutaneous EVAR (sheath size ≥ 10 Fr)
One study compared PerClose ProGlide with ProStar XL in participants undergoing percutaneous EVAR (Nelson 2014).
Time to haemostasis
Nelson 2014 measured time to haemostasis between the PerClose ProGlide and ProStar XL devices in participants undergoing percutaneous EVAR. They found no differences between the two devices (MD ‐3.20 minutes, 95% CI ‐10.23 to 3.83; P value = 0.37) (Analysis 13.1).
13.1. Analysis.
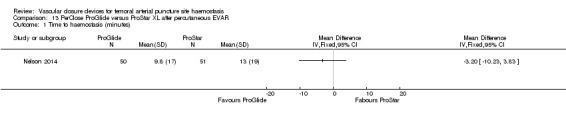
Comparison 13 PerClose ProGlide versus ProStar XL after percutaneous EVAR, Outcome 1 Time to haemostasis (minutes).
Time to mobilisation
Nelson 2014 also measured time to mobilisation between the PerClose ProGlide and ProStar XL devices in participants undergoing percutaneous EVAR. They found no differences between the two devices (MD 1.00 hour, 95% CI ‐2.20 to 4.20; P value = 0.54) (Analysis 13.2).
13.2. Analysis.

Comparison 13 PerClose ProGlide versus ProStar XL after percutaneous EVAR, Outcome 2 Time to mobilisation (hours).
Major adverse event
Nelson 2014 also measured major adverse events between the PerClose ProGlide and ProStar XL devices in participants undergoing percutaneous EVAR. They found no differences in the effectiveness of the devices in preventing death (OR 0.33, 95% CI 0.01 to 8.38; P value = 0.50) nor vascular injury requiring repair (OR 0.33, 95% CI 0.03 to 3.25; P value = 0.34) (Analysis 13.3).
13.3. Analysis.

Comparison 13 PerClose ProGlide versus ProStar XL after percutaneous EVAR, Outcome 3 Major adverse event (any time).
Adverse events
Infection
Nelson 2014 measured infection between the PerClose ProGlide and ProStar XL devices in participants undergoing percutaneous EVAR but found no cases in either group (Analysis 13.4).
13.4. Analysis.

Comparison 13 PerClose ProGlide versus ProStar XL after percutaneous EVAR, Outcome 4 Infection.
Groin haematoma
Nelson 2014 measured groin haematoma between the PerClose ProGlide and ProStar XL devices in participants undergoing percutaneous EVAR but found no cases in either group (Analysis 13.5).
13.5. Analysis.

Comparison 13 PerClose ProGlide versus ProStar XL after percutaneous EVAR, Outcome 5 Groin haematoma.
Retroperitoneal haemorrhage
Nelson 2014 did not measure retroperitoneal haemorrhage as an outcome.
Pseudoaneurysm
Nelson 2014 did not measure pseudoaneurysm as an outcome.
Arterial dissection
Arterial dissection was not reported in the Nelson 2014 study.
Arteriovenous fistula
Nelson 2014 measured arteriovenous fistula between the PerClose ProGlide and ProStar XL devices in participants undergoing percutaneous EVAR but found no cases in either group (Analysis 13.6).
13.6. Analysis.

Comparison 13 PerClose ProGlide versus ProStar XL after percutaneous EVAR, Outcome 6 Arterio‐venous fistula.
Embolisation resulting in loss of distal pulse
Nelson 2014 did not report on embolisation resulting in loss of distal pulse.
Deep vein thrombosis
Nelson 2014 measured deep vein thrombosis between the PerClose ProGlide and ProStar XL devices in participants undergoing percutaneous EVAR and found no differences between the two devices (OR 2.08, 95% CI 0.18 to 23.73; P value = 0.55) (Analysis 13.7).
13.7. Analysis.
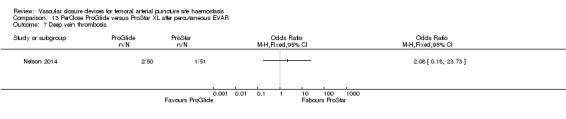
Comparison 13 PerClose ProGlide versus ProStar XL after percutaneous EVAR, Outcome 7 Deep vein thrombosis.
Limb ischaemia
Nelson 2014 measured limb ischaemia as an outcome but found no differences between the PerClose ProGlide and ProStar XL devices (OR 2.08, 95% CI 0.18 to 23.73; P value = 0.55) (Analysis 13.8).
13.8. Analysis.
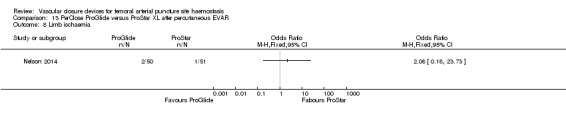
Comparison 13 PerClose ProGlide versus ProStar XL after percutaneous EVAR, Outcome 8 Limb ischaemia.
Femoral artery thrombosis
Nelson 2014 did not measure femoral artery thrombosis as an outcome.
Technical failure of VCDs
Nelson 2014 measured technical failure between the PerClose ProGlide and ProStar XL devices in participants undergoing percutaneous EVAR but found no cases in either group (Analysis 13.9).
13.9. Analysis.
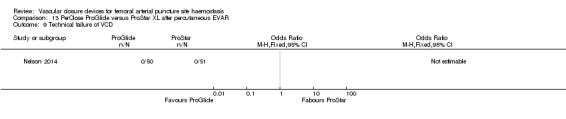
Comparison 13 PerClose ProGlide versus ProStar XL after percutaneous EVAR, Outcome 9 Technical failure of VCD.
Time spent in angiography suite
Nelson 2014 did not measure time spent in angiography suite as an outcome.
Length of hospital stay
Nelson 2014 measured length of hospital stay between the PerClose ProGlide and ProStar XL devices in participants undergoing percutaneous EVAR but found no differences (MD ‐0.10 hours, 95% CI ‐0.41 to 0.21; P value = 0.53) (Analysis 13.10).
13.10. Analysis.

Comparison 13 PerClose ProGlide versus ProStar XL after percutaneous EVAR, Outcome 10 Length of hospital stay (hours).
Patient satisfaction
Patient satisfaction was not reported as an outcome of the Nelson 2014 study.
Cost of VCD and extrinsic compression
Device cost was not assessed in the included study (Nelson 2014).
Haemostasis after EVAR with open exposure of CFA (sheath size ≥ 10 Fr)
One study compared the PerClose ProGlide and ProStar XL devices with surgical suture‐based closure in participants undergoing open femoral exposure of the CFA (Nelson 2014).
Time to haemostasis
Nelson 2014 measured time to haemostasis between suture‐based devices and surgically mediated closure in participants undergoing EVAR. The suture‐based device was associated with shorter time to haemostasis than surgical closure (MD ‐11.58 minutes, 95% CI ‐18.85 to ‐4.31; 101 participants; P value = 0.002) (Analysis 14.1).
14.1. Analysis.

Comparison 14 PerClose ProGlide ProStar XL versus suture‐based closure after EVAR with open exposure of CFA, Outcome 1 Time to haemostasis (minutes).
Time to mobilisation
Nelson 2014 measured time to mobilisation between suture‐based devices and surgically mediated closure in participants undergoing EVAR but found no differences between the two groups (MD: ‐2.50 hours, 95% CI ‐7.21 to 2.21) (Analysis 14.2).
14.2. Analysis.

Comparison 14 PerClose ProGlide ProStar XL versus suture‐based closure after EVAR with open exposure of CFA, Outcome 2 Time to mobilisation (hours).
Major adverse event
Nelson 2014 measured major adverse events between suture‐based devices and surgically mediated closure in participants undergoing EVAR and found no differences between the suture‐based device and surgical closure in preventing death (OR 1.51, 95% CI 0.66 to 37.67; P value = 0.80) nor in vascular injury requiring repair (OR 2.02, 95% CI 0.22 to 18.57; P value = 0.53) (Analysis 14.3).
14.3. Analysis.

Comparison 14 PerClose ProGlide ProStar XL versus suture‐based closure after EVAR with open exposure of CFA, Outcome 3 Major adverse event (any time).
Adverse events
Infection
Nelson 2014 measured infection between suture‐based devices and surgically mediated closure in participants undergoing EVAR but found no cases in either group (Analysis 14.4).
14.4. Analysis.

Comparison 14 PerClose ProGlide ProStar XL versus suture‐based closure after EVAR with open exposure of CFA, Outcome 4 Infection.
Groin haematoma
Nelson 2014 measured groin haematoma between suture‐based devices and surgically mediated closure in participants undergoing EVAR but found no cases in either group (Analysis 14.5).
14.5. Analysis.

Comparison 14 PerClose ProGlide ProStar XL versus suture‐based closure after EVAR with open exposure of CFA, Outcome 5 Groin haematoma.
Retroperitoneal haemorrhage
Nelson 2014 did not measure retroperitoneal haemorrhage as an outcome.
Pseudoaneurysm
Nelson 2014 did not measure pseudoaneurysm as an outcome.
Arterial dissection
Arterial dissection was not reported in the Nelson 2014 study.
Arteriovenous fistula
Nelson 2014 measured arteriovenous fistula between suture‐based devices and surgically mediated closure in participants undergoing EVAR but found no cases in either group (Analysis 14.6).
14.6. Analysis.

Comparison 14 PerClose ProGlide ProStar XL versus suture‐based closure after EVAR with open exposure of CFA, Outcome 6 Arterio‐venous fistula.
Embolisation resulting in loss of distal pulse
Nelson 2014 did not report on embolisation resulting in loss of distal pulse.
Deep vein thrombosis
Nelson 2014 measured deep vein thrombosis between suture‐based devices and surgically mediated closure in participants undergoing EVAR and found no differences between the two devices (OR 0.48, 95% CI 0.09 to 2.47; P value = 0.38) (Analysis 14.7).
14.7. Analysis.

Comparison 14 PerClose ProGlide ProStar XL versus suture‐based closure after EVAR with open exposure of CFA, Outcome 7 Deep vein thrombosis.
Limb ischaemia
Nelson 2014 measured limb ischaemia between suture‐based devices and surgically mediated closure in participants undergoing EVAR but found no differences between the two groups (OR 0.73, 95% CI 0.12 to 4.54; P value = 0.74) (Analysis 14.8).
14.8. Analysis.

Comparison 14 PerClose ProGlide ProStar XL versus suture‐based closure after EVAR with open exposure of CFA, Outcome 8 Limb ischaemia.
Femoral artery thrombosis
Nelson 2014 did not measure femoral artery thrombosis as an outcome.
Time spent in angiography suite
Nelson 2014 did not measure time spent in angiography suite as an outcome.
Length of hospital stay
Length of hospital stay was measured in the Nelson 2014 study, which reported no differences between participants treated with a suture‐based device and those who underwent surgical closure following EVAR (MD ‐10.80 hours, 95% CI ‐27.20 to 5.60; P value = 0.20).
Patient satisfaction
Patient satisfaction was not an outcome of the Nelson 2014 study.
Cost of VCD and extrinsic compression
Device cost was not assessed in the included study (Nelson 2014).
Subgroup analysis
The first planned subgroup analysis included looking at studies using smaller sheath sizes (5 to 6 Fr) compared with those with larger sheath sizes in participants undergoing EVAR procedures. However, all studies included in this review used sheath size 9 Fr or smaller. As only one study compared the effectiveness of VCDs in participants undergoing EVAR procedures, it was not possible to complete this subgroup analysis.
A second planned subgroup analysis was conducted to compare studies according to whether they used antegrade or retrograde puncture. However, very few studies reported on the direction of puncture used in the procedure; therefore, it was not feasible to conduct this subgroup analysis.
Discussion
Summary of main results
Time to haemostasis
Meta‐analysis indicated that collagen‐based or plug‐based, metal clip‐based and suture‐based vascular closure devices (VCDs) were all associated with reduced time to haemostasis when compared with manual or mechanical compression. However, considerable heterogeneity could not be explained; therefore, results of the meta‐analysis may not be meaningful. Every included study used a strict definition of time to haemostasis, defined as the time from sheath removal to complete cessation of bleeding and absence of palpable haematoma in minutes. Most studies (Ansel 2006; Brachmann 1998; Castañeda 2003; Gerckens 1998; Gwechenberger 1997; Hermiller 2005; Hermiller 2006; Kussmaul 1995; Magosaki 1999; Martin 2008; Perlowski 2011; Reddy 2004; Rickli 2002; Sanborn 1993; SEAL Trial Study Team; Seidelin 1997; Silber 1998; Sun 2009; Tron 2003; Ward 1998; Wetter 2000; Wong 2009; Yadav 2003) measured time to haemostasis in minutes. One study (Juergens 2004) measured time to haemostasis in hours, and another study (Diaz 2001) measured time to haemostasis in seconds. For the purposes of the review, data from Diaz 2001 and Juergens 2004 were converted to minutes. Seven studies (Gerckens 1998; Kussmaul 1995; Sanborn 1993; SEAL Trial Study Team; Sun 2009; Wong 2009; Yadav 2003) presented time to haemostasis for the entire study population and also separately for the subgroup of participants who underwent diagnostic and interventional procedures. Seven studies could not be used in the meta‐analysis because standard deviations were not reported (Doneaux 2001; Martin 2008; Ward 1998; Yadav 2003) or because median and interquartile range were presented (Holm 2014; Schulz‐Schüpke 2014; SEAL Trial Study Team).
Evidence on the effectiveness of one VCD compared with another VCD is lacking. No study compared time to haemostasis between collagen‐based and metal clip‐based VCDs. Only one study (Sun 2009) compared one metal clip‐based VCD (StarClose) with a suture‐based VCD (PerClose) and found that StarClose was associated with shorter time to haemostasis. However, this study was based on 469 participants, and the quality of the study could not be assessed because reporting of methods was insufficient. Two studies (Michalis 2002; Shammas 2002) compared the effectiveness of two collagen devices: AngioSeal and VasoSeal. In Michalis 2002, VasoSeal was associated with shorter time to haemostasis for both diagnostic and interventional procedures. However, when Shammas 2002 was added to the meta‐analysis, no difference in time to haemostasis was noted overall between the two devices. Michalis 2002 also provided a third treatment arm with the Duett device. In this study, VasoSeal was associated with shorter time to haemostasis than AngioSeal for both diagnostic and interventional procedures. Similarly the Duett device was associated with shorter time to haemostasis when compared with AngioSeal in both diagnostic and interventional procedures. However, no difference was found in either type of procedure when VasoSeal was compared with the Duett device. Martin 2008 reported that AngioSeal was associated with significantly reduced time to haemostasis compared with PerClose. Nelson 2014 compared ProStar and ProGlide devices in participants undergoing percutaneous EVAR but found no differences in time to haemostasis between the two devices. The same study (Nelson 2014) compared the ProStar and ProGlide devices with surgical suture‐based closure following EVAR and found reduced time to haemostasis for the VCDs.
Time to mobilisation
Meta‐analysis showed that collagen‐based, metal clip‐based and suture‐based VCDs were all associated with reduced time to mobilisation when compared with manual/mechanical compression. However, considerable heterogeneity could not be explained; therefore, results of the meta‐analysis may not be meaningful.
Some studies (Ansel 2006; Brachmann 1998; Carere 2000; Castañeda 2003; Gerckens 1998; Jensen 2008; Martin 2008; Rickli 2002; SEAL Trial Study Team; Seidelin 1997; Ward 1998; Wetter 2000; Wong 2009) encouraged VCD participants to ambulate sooner than manual compression participants. Other studies (Hermiller 2005; Hermiller 2006; Juergens 2004; Legrand 2005; Machnik 2012; Sanborn 1993; Yadav 2003) measuring time to mobilisation did so at the same time intervals regardless of the treatment. Some studies specified a particular distance for time to ambulation (Ansel 2006; Castañeda 2003; Gerckens 1998; Hermiller 2005; Hermiller 2006; Michalis 2002; SEAL Trial Study Team; Wong 2009; Yadav 2003), including 3 to 5 steps, 3 metres, 10 feet, 20 feet and 110 feet; other studies (Brachmann 1998; Carere 2000; Juergens 2004; Legrand 2005; Machnik 2012; Martin 2008; Rickli 2002; Sanborn 1993; Seidelin 1997; Ward 1998; Wetter 2000) simply specified out‐of‐bed moving or did not define time to mobilisation.
Although it was featured in the study protocol, time to ambulation is usually a clinician‐defined occurrence that varies according to local protocols and depends on the procedure undertaken as well as on the method of haemostasis. The authors of this review believe that it is not suitable for use as an outcome measure unless it is used in combination with the occurrence of vascular complications associated with different ambulation times.
Evidence on the effectiveness of one VCD compared with another VCD is lacking. Only one study (Sun 2009) compared time to ambulation between one metal clip‐based VCD (StarClose) and a suture‐based VCD (PerClose). This study found that StarClose was associated with shorter time to ambulation (0.30 hour) when compared with PerClose. However, the mean difference was very small and the confidence interval was close to zero, suggesting no differences between the two devices. Two studies (Michalis 2002; Shammas 2002) compared the effectiveness of two collagen devices: AngioSeal and VasoSeal. In the study by Michalis 2002, AngioSeal was associated with shorter time to mobilisation for both diagnostic and interventional procedures. However, when Shammas 2002 was added to the meta‐analysis, no differences in time to mobilisation were noted between the two devices. Michalis 2002 also included a third treatment arm using the Duett device. AngioSeal was associated with shorter time to mobilisation when compared with the Duett device in diagnostic but not in interventional procedures. Finally, no difference in time to haemostasis was noted for VasoSeal and Duett devices regardless of procedure type. No study compared time to ambulation between collagen‐based and metal clip‐based VCDs. Martin 2008 reported that AngioSeal was associated with less time to mobilisation when compared with PerClose. Nelson 2014 compared ProStar and ProGlide devices in participants undergoing percutaneous endovascular aortic aneurysm repair (EVAR) but found no differences in time to mobilisation between the two devices. The same study (Nelson 2014) also compared the VCDs with surgical closure following EVAR but reported no differences in time to mobilisation between the two treatment groups.
Major adverse events and adverse events
Very few studies eligible for inclusion in this review recorded the incidence of major adverse events including mortality and vascular injury requiring repair. When incidence of death was reported in the included studies,no cases of death were described, except when PerClose ProGlide was compared with ProStar XL for percutaneous EVAR (Nelson 2014). For vascular injury repair, meta‐analyses demonstrated that neither collagen‐based nor clip‐based VCDs were more effective than manual compression. Data on the effectiveness of one VCD versus another were lacking. Results showed no differences in effectiveness between ProGlide and ProStar after percutaneous EVAR nor when the same two devices were compared with surgical closure after EVAR.
Infection
Meta‐analyses of nine studies showed no difference in the incidence of this outcome between participants treated with a collagen‐based VCD and those treated with manual compression. In addition, four studies reported no cases of infection. No differences in the incidence of infection were noted between metal clip‐based VCDs nor suture‐based VCDs and manual compression. Furthermore, no difference was identified between different types of VCDs. Suture‐based devices were compared with each other following percutaneous EVAR, and also with surgical closure following EVAR, but no differences between treatment groups were reported.
Groin haematoma
Collagen VCDs were associated with a lower incidence of groin haematoma when compared with manual compression, but no differences were noted between metal clip‐based and suture‐based devices and manual compression. The clinical significance of the haematoma was not recorded in these studies, thus it was not possible to distinguish between the incidence of self‐limiting and clinically relevant haematoma. Furthermore, no differences between different types of VCDs were identified. Suture‐based devices were compared with each other following percutaneous EVAR and also with surgical closure following EVAR, but no differences in the incidence of groin haematoma were noted between treatment groups
The incidence of pseudoaneurysm was lower with collagen‐based devices than with manual or mechanical compression. The incidence of all other adverse events including retroperitoneal haemorrhage, arteriovenous fistula, deep vein thrombosis, limb ischaemia and femoral artery thrombosis did not differ by type of VCD nor when VCD was compared with manual or mechanical compression. Suture‐based devices were compared with one other following percutaneous EVAR and also with surgical closure following EVAR, but no differences in these outcomes were reported between treatment groups. No study measured arterial dissection nor time spent in angiography suite, and one study reported only distal embolisation and femoral artery thrombosis.
Length of hospital stay
Meta‐analysis showed that participants treated with a VCD were discharged from hospital earlier than those undergoing manual compression. Significant heterogeneity demonstrated in the reported data could not be controlled by the use of standard techniques of meta‐analysis. Owing to the short duration of stay often expected in patients undergoing percutaneous arterial procedures, the duration of stay could have been influenced mainly by procedural factors, not by the method of haemostasis. Furthermore, time to discharge after the procedure is often locally defined and controlled and may not be suitable as an outcome measure. Length of hospital stay did not differ significantly between suture‐based devices following percutaneous EVAR or between suture‐based devices and surgical closure following EVAR.
Patient satisfaction
Some studies have shown increased patient satisfaction when a closure device was used over extrinsic compression, but owing to differences in the tools used to assess this, we could not make a formal comparison between different mechanisms of action of VCD.
Cost of VCD and extrinsic compression
Only three studies reported on the cost of VCDs; all found that treatment with a suture‐based VCD cost less than manual compression. Study authors' calculations show that this was due to an associated time saving. These costs and calculations vary widely between different centres, and the review authors therefore believe that evidence is insufficient to allow firm conclusions.
Overall completeness and applicability of evidence
As detailed above, we observed heterogeneity in the studies identified including a wide variety of definitions for different outcome measures. The study protocol required that the closure device use must be assessed in diagnostic and interventional procedures. Practice varies between specialities, between centres and between countries, and possible differences between interventional and diagnostic procedures that could influence outcomes (e.g. anticoagulant use, time between procedure end and sheath removal) were not clearly defined in the included studies. Outcome measures such as local arterial damage could be caused by the arteriotomy or by the closure device used, and most of the included studies did not consider this. Arteriovenous fistula formation is a complication of arterial puncture, not of closure device use per se. Some studies did not specifically report whether the device was used in a manner consistent with the manufacturer's instructions for use or did not comment on operator training and experience with the device.
No study measured the pre‐defined outcomes of arterial dissection and time spent in the angiography suite, and only one study reported on distal embolisation and femoral artery thrombosis. Furthermore, some studies did not report data in useable format; therefore, we could not include those particular studies in the meta‐analysis. In such instances, we attempted to contact the study authors, but we did not receive the requested data.
Quality of the evidence
Very few studies reported on the method of randomisation used or the way in which treatment allocation was concealed. No study reported that participants were blinded. However, we judged that, as most reported outcomes required physical measurements, lack of blinding was unlikely to bias outcome measures. Only eight studies blinded outcome assessors. This is important to note as measures at the puncture site could be influenced by the assessor's knowledge of allocated treatment. We judged most studies as having low risk of attrition, reporting and other bias. Overall, we determined that the methodological quality of the studies included in this review was moderate to low.
The quality of evidence, defined according to GRADE (Grades of Recommendation, Assessment, Development and Evaluation) (GRADE 2004), was deemed to be low for the primary outcomes of this review: time to haemostasis, time to mobilisation and major adverse events. We downgraded the quality of the outcomes time to haemostasis and time to mobilisation for serious imprecision on the basis of substantial and unexplained heterogeneity between studies. We downgraded the outcome major adverse event to low in the domain of precision because we observed in the meta‐analysis a small sample size in relation to the expected effect size and wide confidence intervals. For secondary outcomes including adverse events, technical failure of the device and length of hospital stay, we judged the quality of the evidence as moderate for precision, consistency and directness.
A major limitation of this review is the inclusion of data from the past 20 years. Device and delivery system development throughout this period may have influenced success and complication rates, potentially allowing masking of significant differences. Furthermore, the remit of the review was to evaluate VCDs by mechanism of action (clip‐, collagen‐ or suture‐based) rather than by specific device, resulting in limited transferability to clinical practice. Differences between specific devices and their delivery systems may account, at least in part, for the substantial heterogeneity evident within the data set, and this could mask associated benefits or detrimental effects.
Many studies did not state the direction of puncture (as antegrade or retrograde), and many differentiated between diagnostic and interventional procedures without defining the relevant differences in procedure technique (e.g. sheath size, anticoagulant administration, differences in time between end of the procedure and sheath removal). Many studies showed wide variability in the stratification of complications into groups, meaning that a large quantity of the data was unfit for meta‐analysis.
It is important to note that other known techniques for closure of the puncture site have not been covered by this review but will be considered for inclusion in future versions of the review.
Potential biases in the review process
None of the authors of this review were involved in any of the included or excluded studies. Furthermore, none of the review authors have commercial or other conflicts of interest. The search was as comprehensive as possible, and two review authors independently assessed all studies for inclusion in the review. We are confident that we have included all relevant studies, and we have attempted to reduce bias in the review process by performing data extraction and assessing study quality independently. However, the possibility remains that studies may have been overlooked by the search methods.
For one study included in this review (Sun 2009), data on time to haemostasis and time to ambulation were presented separately by type of procedure. So these data could be included in a meta‐analysis, we combined the mean and standard deviations for the outcomes of time to haemostasis and time to ambulation according to the formula given in Table 7.7.a in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For a second study (Nelson 2014), which compared two suture‐based devices with surgical closure in participants undergoing EVAR, data on time to haemostasis, time to ambulation and length of hospital stay were presented separately for each device. So these data could be included in a meta‐analysis, we combined the mean and standard deviations for these outcomes according to the formula given in Table 7.7.a in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Agreements and disagreements with other studies or reviews
To date, four (Biancari 2010; Jiang 2015; Koreny 2004; Nikolsky 2004) systematic reviews and meta‐analyses have been conducted to investigate the efficacy and safety of VCDs.
Koreny 2004 included 30 randomised controlled trials (RCTs) of VCDs versus manual compression and found that participants treated with a VCD had shorter time to haemostasis (mean difference (MD) 17 minutes, range 14 to 19 minutes), shorter duration of bedrest (MD 10.8 hours, range 8.5 to 13.1 hours) and shorter duration of hospital stay (MD 0.6 days, range 0.1 to 1.1 days) than those treated with manual compression. However, considerable statistical heterogeneity was observed for these efficacy endpoints. With regard to safety outcomes, Koreny 2004 found no differences between VCDs and manual compression for groin haematoma (risk ratio (RR) 1.14, 95% confidence interval (CI) 0.86 to 1.51), bleeding (RR 1.48, 95% CI 0.88 to 2.48) and pseudoaneurysm (RR 1.19, 95% CI 0.75 to 1.88). The authors of the review raised concerns about the quality of the included studies, which they judged as poor to moderate.
Nikolsky 2004 included 30 studies on VCDs versus manual compression. However, only 18 of these were RCTs. Nikolsky 2004 performed device‐specific meta‐analysis and found no differences in complication rates between AngioSeal and mechanical compression (odds ratio (OR) 0.73, 95% CI 0.38 to 1.39) nor between PerClose ProGlide and mechanical compression (OR 1.00, 95% CI 0.53 to 1.88). The authors of the review did report that VasoSeal was associated with an increase in complications (OR 2.78, 95% CI 1.51 to 5.13); this consistent finding has led to removal of the device from clinical use.
Biancari 2010 included 31 RCTs with a total of 7528 participants randomised to VCD or manual/mechanical compression after diagnostic angiography or interventional procedures. Meta‐analysis showed no differences in the incidence of haematoma, bleeding and pseudoaneurysm between the two treatment groups. However, lower limb ischaemia (0.3% vs 0% P value = 0.02), the need for surgery for vascular complications (0.7% vs 0.4%; P value = 0.10) and groin infection (0.6% vs 0.2%; P value = 0.02) were more frequent in the VCD group. Meta‐analysis also showed that VCDs were associated with shorter time to haemostasis for participants undergoing both diagnostic (MD 16.64 minutes, range ‐21.96 to ‐11.32 minutes) and interventional procedures (MD ‐37.67 minutes, range ‐47.94 to ‐27.40 minutes). However, the authors of the review reported substantial heterogeneity for time to haemostasis outcomes.
Jiang 2015 included 40 RCTs with a total of 16,868 participants and found no differences in the rate of adverse vascular events between all VCDs combined and manual compression. However, subgroup analysis showed that both FemoSeal and AngioSeal devices were associated with fewer adverse events, in particular, haematoma.
Authors' conclusions
Implications for practice.
This review has included randomised controlled trials comparing extrinsic compression and VCDs in the closure of femoral arterial punctures and in surgical closure with percutaneous closure after large‐calibre arterial access. We have demonstrated no significant differences in the primary endpoints of time to haemostasis and time to mobilisation when VCD use is compared with extrinsic compression, owing to data heterogeneity in the included studies. Furthermore, we found no significant differences in the incidence of vascular injury or mortality when use of VCDs is compared with use of extrinsic compression. Collagen‐based VCDs are associated with a lesser incidence of groin haematoma when compared with extrinsic compression but no difference when metal clip‐based VCDs or suture‐based VCDs were compared with manual or mechanical compression. The incidence of pseudoaneurysm was lower among individuals treated with collagen‐based devices than in those treated with manual or mechanical compression, but no difference was evident when metal clip‐based VCDs or suture‐based VCDs were compared with manual or mechanical compression. For other adverse events, we observed no differences between collagen, clip‐based or suture‐based. and manual or mechanical compression. With larger‐calibre arterial access, percutaneous closure has been shown to be associated with shorter time to haemostasis without an increase in complication rates when compared with surgical closure.
A major limitation of this review is the inclusion of data spanning the past 20 years. Device and delivery system development throughout this period may have influenced success and complication rates, potentially allowing masking of significant differences.Differences between specific devices and their delivery systems may account, at least in part, for the substantial heterogeneity evident within the data set, and this could mask associated benefits or detrimental effects.
Many studies did not state the direction of puncture (as antegrade or retrograde), and many others differentiated between diagnostic and interventional procedures without defining the relevant differences in procedure technique (e.g. sheath size, anticoagulant administration, differences in time between end of the procedure and sheath removal). Many studies showed wide variety in the stratification of complications into groups, meaning that a large quantity of the data was unfit for analysis. This variability in study and reporting methodology means that the findings of thi review are of limited value in specific clinical situations.
Three studies describe cost‐savings associated with decreased time to haemostasis. Owing to wide variation in VCD cost and local hospital costs, formal economic analysis should be performed at a local level before VCDs are accepted on the basis of cost‐savings alone.
Implications for research.
Overall, successful VCD deployment is associated with a reduced incidence of groin hematoma and shorter length of hospital stay, with no increase in local arterial complications or major adverse events. Further work is necessary to evaluate the efficacy of specific devices currently used and to compare these with one other and with extrinsic compression with respect to clearly defined outcome measures. Researchers should evaluate primary and secondary outcomes of different devices in antegrade and retrograde puncture and should evaluate VCD outcome measures with respect to their on‐label and off‐label uses.
Some studies report increased patient satisfaction with successful VCD deployment. A formal assessment of this could be undertaken.
VCDs are widely used and are convenient for both patients and operators. Large longitudinal data demonstrate their safety and efficacy with low complication rates; thus investigators may resist development of randomised controlled trials of sufficiently robust methodology to truly evaluate their efficacy.
Acknowledgements
The review authors would like to thank the Cochrane Vascular Group for assistance provided in preparation of this review.
The review authors also would like to thank the authors of the protocol of this review: Charlie CT Hsu, Gigi NC Kwan, John A Rophael and Mieke L van Driel.
Appendices
Appendix 1. CRS search strategy
| Search run on Fri Apr 8 2015 | ||
| #1 | MESH DESCRIPTOR Endovascular Procedures EXPLODE ALL TREES | 5490 |
| #2 | MESH DESCRIPTOR Angiography EXPLODE ALL TREES | 5510 |
| #3 | MESH DESCRIPTOR Catheterization EXPLODE ALL TREES | 8520 |
| #4 | MESH DESCRIPTOR Femoral Artery | 723 |
| #5 | MESH DESCRIPTOR Groin | 88 |
| #6 | MESH DESCRIPTOR Punctures | 288 |
| #7 | vascular | 23798 |
| #8 | vessel | 5372 |
| #9 | catheter* :TI,AB,KY | 13346 |
| #10 | cannulat*:TI,AB,KY | 1002 |
| #11 | endovascular:TI,AB,KY | 920 |
| #12 | percutan*:TI,AB,KY | 7724 |
| #13 | PTA or PTCA | 1339 |
| #14 | angiograph*:TI,AB,KY | 9659 |
| #15 | angioplasty:TI,AB,KY | 5639 |
| #16 | arteriotom*:TI,AB,KY | 52 |
| #17 | femoral:TI,AB,KY | 5807 |
| #18 | groin:TI,AB,KY | 487 |
| #19 | punctur*:TI,AB,KY | 2580 |
| #20 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 | 57621 |
| #21 | MESH DESCRIPTOR Wound Closure Techniques EXPLODE ALL TREES | 1506 |
| #22 | MESH DESCRIPTOR Surgical Instruments EXPLODE ALL TREES | 537 |
| #23 | compres*:TI,AB,KY | 4491 |
| #24 | clamp*:TI,AB,KY | 3663 |
| #25 | press*:TI,AB,KY | 79983 |
| #26 | plug*:TI,AB,KY | 367 |
| #27 | closure:TI,AB,KY | 4668 |
| #28 | closing:TI,AB,KY | 511 |
| #29 | weight*:TI,AB,KY | 49193 |
| #30 | clip*:TI,AB,KY | 876 |
| #31 | seal*:TI,AB,KY | 2741 |
| #32 | mynx:TI,AB,KY | 1 |
| #33 | (starclose or star‐close or prostar or tecstar or perclose or boomerang or angioseal or proglide or vasoseal or duett):TI,AB,KY | 65 |
| #34 | MESH DESCRIPTOR Hemostasis | 697 |
| #35 | hemostasis:TI,AB,KY | 2687 |
| #36 | haemostasis:TI,AB,KY | 615 |
| #37 | #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 | 137390 |
| #38 | #20 AND #37 | 16375 |
| #39 | (arterial or artery):TI,AB,KY | 44222 |
| #40 | closure:TI,AB,KY | 4668 |
| #41 | #39 AND #40 | 553 |
| #42 | #38 OR #41 | 16635 |
| #43 | * NOT SR‐PVD:CC | 910030 |
| #44 | #42 AND #43 | 15550 |
Appendix 2. Glossary
Ambulation: mobilisation Arteriotomy: procedure in which a hole is made in an artery wall by a knife or a needleArteriovenous fistula: abnormal connection between an artery and a vein EVAR: endovascular aneurysm repair Haematoma: bruise Haemostasis: process that causes bleeding to stop Percutaneous: through the skin Pseudoaneurysm: haematoma that occurs as a result of a leaking hole in an artery 6 to 8 Fr: French catheter scale used to measure the size of the sheath or catheter
Data and analyses
Comparison 1. Collagen‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to haemostasis (minutes) | 12 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Time to mobilisation (hours) | 13 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Major adverse event (any time) | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Mortality | 1 | 141 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Vascular injury requiring vascular repair by surgical or non‐surgical techniques | 5 | 5731 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.47, 16.79] |
| 4 Infection | 9 | 7616 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.88, 5.22] |
| 5 Groin haematoma | 25 | 10247 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.40, 0.54] |
| 6 Retroperitoneal haemorrhage | 3 | 744 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.22, 11.42] |
| 7 Pseudoaneurysm | 21 | 9342 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.55, 0.99] |
| 8 Arterio‐venous fistula | 8 | 6153 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.43, 2.21] |
| 9 Deep vein thrombosis | 3 | 629 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.46, 12.50] |
| 10 Limb ischaemia | 3 | 4970 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Femoral artery thrombosis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Length of hospital stay (hours) | 6 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 2. Metal clip‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to haemostasis (minutes) | 5 | 1665 | Mean Difference (IV, Random, 95% CI) | ‐14.81 [‐16.98, ‐12.63] |
| 2 Time to mobilisation (hours) | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Major adverse event (any time) | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Mortality | 3 | 564 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Vascular injury requiring vascular repair by surgical or non‐surgical techniques | 3 | 783 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.03, 7.95] |
| 4 Infection | 3 | 783 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Groin haematoma | 4 | 1523 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.46, 1.34] |
| 6 Pseudoaneurysm | 6 | 1966 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.20, 2.89] |
| 7 Arterio‐venous fistula | 3 | 564 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Deep vein thrombosis | 2 | 483 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Limb ischaemia | 2 | 483 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Suture‐based VCD versus manual or mechanical compression (sheath size ≤ 9 Fr).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to haemostasis (minutes) | 7 | 1664 | Mean Difference (IV, Random, 95% CI) | ‐14.58 [‐16.85, ‐12.32] |
| 2 Time to mobilisation (hours) | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Major adverse event (any time) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Vascular injury requiring vascular repair | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Infection | 3 | 750 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.22, 12.71] |
| 5 Groin haematoma | 6 | 1350 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.41, 1.02] |
| 6 Retroperitoneal haemorrhage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Pseudoaneurysm | 6 | 1527 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.25, 2.53] |
| 8 Arterio‐venous fistula | 4 | 880 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
| 9 Embolisation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Limb ischaemia | 2 | 720 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.14, 7.22] |
| 11 Length of hospital stay (hours) | 3 | 327 | Mean Difference (IV, Random, 95% CI) | ‐11.66 [‐20.46, ‐2.85] |
Comparison 4. Collagen‐based VCD versus metal clip‐based VCD: AngioSeal versus StarClose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major adverse event (any time) | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Mortality | 1 | 571 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Vascular injury requiring vascular repair by surgical or non‐surgical techniques | 2 | 871 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.22] |
| 2 Infection | 2 | 701 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Groin haematoma | 2 | 871 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.43, 1.65] |
| 4 Retroperitoneal haemorrhage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Pseudoaneurysm | 3 | 1272 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.15, 1.66] |
| 6 Arterio‐venous fistula | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Limb ischaemia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Technical failure of VCD | 3 | 1272 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.23, 0.64] |
Comparison 5. Collagen‐based VCD versus suture‐based VCD (sheath size ≤ 9 Fr).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Groin haematoma | 3 | 510 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.71, 2.22] |
| 3 Retroperitoneal haemorrhage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Pseudoaneurysm | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Arterio‐venous fistula | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Technical failure of VCD | 3 | 510 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.08, 0.69] |
Comparison 6. Metal clip‐based VCD versus suture‐based VCD: StarClose versus PerClose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to haemostasis | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Time to mobilisation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Groin haematoma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Pseudoaneurysm | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Technical failure of VCD | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Disc‐based VCD versus suture‐based VCD: Boomerang versus PerClose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to haemostasis (minutes) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Time to mobilisation (hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Groin haematoma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Technical failure of VCD | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus VasoSeal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to haemostasis (minutes) | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Time to mobilisation (hours) | 2 | 720 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.63, 0.37] |
| 3 Major adverse event (any time) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Mortality | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Vascular injury requiring vascular repair by surgical or non‐surgical techniques | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Infection | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Groin haematoma | 2 | 720 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.16, 1.47] |
| 6 Retroperitoneal haemorrhage | 2 | 720 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 9.09] |
| 7 Pseudoaneurysm | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.71] |
| 8 Arterio‐venous fistula | 1 | 150 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Technical failure of VCD | 1 | 570 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.37, 1.26] |
Comparison 9. Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Mynx.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major adverse event (any time) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Vascular injury requiring vascular repair by surgical or non‐surgical techniques | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Groin haematoma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. Collagen‐based VCD versus collagen‐based VCD: AngioSeal versus Duett.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to haemostasis (minutes) | 1 | 571 | Mean Difference (IV, Fixed, 95% CI) | 10.75 [9.95, 11.56] |
| 2 Time to mobilisation (hours) | 1 | 571 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐0.50, ‐0.29] |
| 3 Groin haematoma | 1 | 571 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.28, 3.40] |
| 4 Retroperitoneal haemorrhage | 1 | 571 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Technical failure of VCD | 1 | 571 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.30, 0.99] |
Comparison 11. Collagen‐based VCD versus collagen‐based VCD: VasoSeal versus Duett.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to haemostasis (minutes) | 1 | 561 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.13, 0.05] |
| 2 Time to mobilisation (hours) | 1 | 561 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.03, 0.21] |
| 3 Groin haematoma | 1 | 561 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.67, 5.95] |
| 4 Retroperitoneal haemorrhage | 1 | 561 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.77 [0.11, 69.59] |
| 5 Technical failure of VCD | 1 | 561 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.46, 1.38] |
Comparison 12. Collagen‐based VCD versus collagen‐based VCD: FemoSeal versus ExoSeal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Major adverse event (any time) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Vascular injury requiring vascular repair by surgical or non‐surgical techniques | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Groin haematoma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Pseudoaneurysm | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Arteriovenous fistula | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Limb ischaemia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Technical failure of VCD | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 13. PerClose ProGlide versus ProStar XL after percutaneous EVAR.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to haemostasis (minutes) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Time to mobilisation (hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Major adverse event (any time) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Vascular injury requiring vascular repair by surgical or non‐surgical techniques | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Groin haematoma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Arterio‐venous fistula | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Deep vein thrombosis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Limb ischaemia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Technical failure of VCD | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Length of hospital stay (hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 14. PerClose ProGlide ProStar XL versus suture‐based closure after EVAR with open exposure of CFA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to haemostasis (minutes) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Time to mobilisation (hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Major adverse event (any time) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Vascular injury requiring vascular repair by surgical or non‐surgical techniques | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Groin haematoma | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Arterio‐venous fistula | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Deep vein thrombosis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Limb ischaemia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Length of hospital stay (hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
14.9. Analysis.

Comparison 14 PerClose ProGlide ProStar XL versus suture‐based closure after EVAR with open exposure of CFA, Outcome 9 Length of hospital stay (hours).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amin 2000.
| Methods | Study design : randomised prospective study | |
| Participants |
Country : UK Setting : hospital Number of centres : 1 Numbers : AngioSeal 75, FemoStop 75 Age (mean (SD)) : AngioSeal 58.0 (9.2) years, FemoStop 59.5 (9.6) years Sex : AngioSeal 48 M/17 F, FemoStop 49 M/16 F Inclusion criteria : patients undergoing intracoronary stent deployment Exclusion criteria : patients with a history of previous application of AngioSeal within 90 days, peripheral vascular disease, recent acute myocardial infarction, anticoagulation therapy, pre‐existing haematoma, formation of haematoma during the procedure or a concomitant femoral venous sheath. Patients in whom the puncture needle had penetrated the posterior wall of the artery during the procedure were also considered for exclusion |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : FemoStop groin compression device Sheath size : 8 Fr |
|
| Outcomes |
Primary : composite of bleeding, haematoma formation, bruise, requirement for blood transfusion, clinical indication for ultrasound examination and cross‐over to either method at 2 and 24 hours following the procedure; bleeding (defined as significant external blood loss after the application of either device, with significant bleeding defined as blood loss (Hb < 10.0 g/dL) requiring blood transfusion); haematoma (defined as development of a palpable mass over the access site classified as mild (< 5 cm), moderate (5 to 10 cm) or severe (> 10 cm)) Secondary : pseuodaneurysm (palpable expansible mass detected by clinical examination and confirmed by ultrasound imaging); groin discomfort (minimal, moderate or severe) Time of measurement : 2 and 24 hours after the procedure |
|
| Notes | All participants received a bolus dose of 10,000 units of heparin after diagnostic angiograms Antithrombotic agents following the procedure: aspirin 150 mg once daily long term and ticlopidine 250 mg twice daily for 3 weeks Outcomes measured at 2 and 24 hours post procedure, but 9 participants crossed over from AngioSeal to FemoStop because of persistent bleeding despite prolonged manual pressure |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Amine 1999.
| Methods | Study design : randomised study | |
| Participants |
Country : France Setting : hospital Number of centres : 1 Numbers: Techstar 50, manual compression 50 Age (mean (SD)) : Techstar 61 (10) years, manual compression 60 (8) years Sex : Techstar 72 M/28 F, manual compression 70 M/30 F Inclusion criteria : patients undergoing diagnostic coronography Exclusion criteria : patients in an acute phase of myocardial infarct, with or without thrombolysis treatment, with known anomalies of coagulation or plaque counting, with severe and uncontrollable arterial hypertension (systolic > 190 mmHg and diastolic >110 mmHg) and inflammation of arthritis of the inferior limbs |
|
| Interventions |
Intervention 1 : PerClose Intervention 2 : manual compression Sheath size : 6 Fr |
|
| Outcomes |
Primary : clinical and ultrasound complications during the first 15 days after treatment Secondary : time to haemostasis; time to ambulation (defined as time between removal of the closure device and the moment the participant could stand up and walk for 5 metres); success rate of the system Clinical exams at 1 hour and 24 hours looked for signs of ischaemia, haematoma, re‐bleeding, pseudo‐aneurysm and arteriovenous fistula. Doppler echographic examination at 24 hours looked for signs of haematoma, pseudo‐aneurysm, arteriovenous fistula, intra‐arterial thrombus or the presence of arterial narrowing at the puncture level |
|
| Notes | Coronography with 6 Fr sheath for all participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised in blocks of 4 for the manual compression group and by envelope opened at the end of the coronography procedure for the suture device group" Comment: Envelopes were used, so sequence generation was probably random. Study was judged to be at low risk of selection bias |
| Allocation concealment (selection bias) | Low risk | Quote: "closed envelope system" Comment: Envelopes were sealed, so the study was judged to be at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Ansel 2006.
| Methods | Study design : randomised multi‐centre study | |
| Participants |
Country : USA Setting : hospital Number of centres : 7 Numbers: Angiolink EVS 243, manual compression 119 Age (mean (SD)) : Angiolink EVS 61.2 (11.3) years, manual compression 62.9 (10.2) years Sex : Angiolink EVS 137 M/106 F, manual compression 75 M/44 F Inclusion criteria : patients 18 to 80 years of age undergoing percutaneous femoral access for elective or urgent transfemoral cardiac or peripheral vascular diagnostic or interventional procedures Exclusion criteria :
Type of procedure : 188 diagnostic (EVS n = 125, compression n = 63), 174 interventional (EVS n = 118, compression n = 56) |
|
| Interventions |
Intervention 1 : Angiolink Vascular Closure System (EVS) Intervention 2 : Manual compression Sheath size : > 6 Fr |
|
| Outcomes |
Primary : time to haemostasis (defined as time from sheath removal to complete cessation of bleeding); time to ambulation (defined as time from sheath removal to independent ambulation ≥ 20 feet without complication); time to device deployment (defined as the interval from sheath removal to device deployment); combined rate of major complications at 30‐day follow‐up Secondary :
Time of measurement : after sheath removal, before hospital discharge and 30 ± 7 days post procedure |
|
| Notes | EVS participants who did not receive IIb/IIIa inhibitors were ambulated 1 hour and those treated with IIb and IIIa were ambulated 2 hours post sheath removal. Manual compression participants were ambulated 4 (no IIb/IIIa inhibitors) and 6 hours (IIb/IIIa inhibitors) post sheath removal | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomised weighted 2:1 toward the device" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judge that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Behan 2007.
| Methods | Study design : prospective randomised controlled trial | |
| Participants |
Country : United Kingdom Setting : district general hospital Number of centres : 1 Numbers: AngioSeal 107, manual compression 99 Age (mean (SD)) : AngioSeal 66.3 (9.7) years, manual compression 65.4 (8.7) years Sex : AngioSeal 61 M/46 F, manual compression 57 M/42 F Inclusion criteria : patients undergoing day case cardiac catheterisation Exclusion criteria : unstable angina or acute myocardial infarction; significant peripheral vascular disease; previous peripheral vascular surgery or percutaneous intervention; inability to fully consent; pregnancy; age < 18 years; known ASD or VSD; previous femoral artery complication from angiography; patients whose vascular access site was obtained through a vascular graft; patients with uncontrolled hypertension (> 180 mmHg systolic); puncture site in superficial femoral artery, distal to or at the bifurcation of the superficial femoral and profunda femoris arteries, proximal to the inguinal ligament or multiple punctures |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : manual compression Sheath size : 6 Fr |
|
| Outcomes |
Primary : bruising; bleeding (defined as the requirement for transfusion); retroperitoneal bleed; pseudoaneurysm; leg ischaemia; vasovagal reaction; access site infection Secondary : participant satisfaction (comfort, experience, duration of hospital stay, immobilisation time, pain during and after the procedure and post‐procedure bruising) |
|
| Notes | Participants randomised to AngioSeal were mobilised within 30 minutes; manual compression participants were mobilised within 2 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Angiogram lists were randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Bruise size measurements were carried out for all the patients by the same trained nurse, who was separate from the group of operators. This person was not blinded to the method of haemostasis used" Comment: no blinding or incomplete blinding for bruising, but this was not an outcome of the review; therefore, it does not introduce detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Seventy‐six of 107 (71%) patients in the Angio‐Seal group attended for the 1 week follow up as did 71 of the 99 (72%) in the manual compression group. Furthermore the AngioSeal device was deployed in only 74 out of 107 patients randomised to the device. Reasons are stated for 23 patients but the reasons were not recorded in 10 cases" |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Brachmann 1998.
| Methods | Study design : prospective multi‐centre randomised trial | |
| Participants |
Country : Germany Setting : hospital Number of centres : not stated Numbers : VasoSeal 306, manual compression 204 Age (mean (SD)) : VasoSeal (angiography) 61 (12) years, VasoSeal (PTCA) 62 (11) years, manual compression (angiography) 61 (10) years, manual compression (PTCA) 61 (10) years Sex: VasoSeal 224 M/82 F, manual compression 153 M/51 F Inclusion criteria : patients 18 to 80 years of age who were acceptable candidates for diagnostic angiography and percutaneous transluminal coronary angioplasty (PTCA) procedures and manual compression after intervention Exclusion criteria: patients who were morbidly obese (body mass index > 40); with bleeding disorders; with a clinically significant haematoma (> 6 cm) present before sheath removal; admitted for emergency angioplasty; with known allergies to beef or collagen products; with elevated blood pressure (> 140/90 mmHg) that could not be controlled by medical therapy; pregnant women |
|
| Interventions |
Intervention 1 : VasoSeal Intervention 2 : manual compression Sheath size : 6 to 8 Fr |
|
| Outcomes |
Primary : time to haemostasis; time to mobilisation (defined as time from sheath pull to getting out of bed and moving about as necessary) Secondary : vascular repair; transfusion; infection prolonging hospital stay; haematoma > 6 cm; bleeding requiring > 30 minutes to re‐achieve haemostasis; pseudoaneurysm requiring mechanical compression; deep vein thrombosis; arteriovenous fistula; infection not prolonging hospital stay; retroperitoneal bleed; thromboembolism; failure to deploy collagen Time of measurement : immediately after sheath pull, 24 hours and 30 days post procedure |
|
| Notes | PTCA participants were randomised to VasoSeal normal (2 hours after sheath pull) and immediate (immediately after sheath pull) 21 participants removed from the analysis for time to mobilisation because of prior illness Initial mobilisation was attempted at 1 hour after sheath pull in VasoSeal participants and 6 hours post sheath pull in manual compression participants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information about the sequence generation process to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but review authors judge that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Camenzind 1994.
| Methods | Study design : randomised study | |
| Participants |
Country : The Netherlands Setting : hospital Number of centres : 1 Numbers : VasoSeal 62, manual compression 62 Age (mean (SD)) : VasoSeal 59 (12) years, manual compression 60 (11) years Sex : VasoSeal 50 M/12 F, manual compression 53 M/9 F Inclusion criteria : patients undergoing percutaneous transluminal coronary angioplasty performed with a 6F guiding catheter and full‐dose heparinisation for > 12 hours or coronary angioplasty with 7F or 8F guiding catheters and optional subsequent herparinisation Exclusion criteria : pre‐existing local haematoma and known allergy to collagen products |
|
| Interventions |
Intervention 1 : VasoSeal Intervention 2 : manual compression Sheath size : 8 Fr |
|
| Outcomes |
Primary : immediate haemostasis Secondary : haematoma; pseudoaneurysm; arteriovenous fistula; venous thrombosis; arterial occlusion |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information about the sequence generation process to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two physicians interpreted the ultrasound examinations without knowledge of treatment assignment" Comment: Blinding of outcome assessors was done. Study was judged to be at low risk of performance bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Study outcomes clearly reported |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Carere 2000.
| Methods | Study design : randomised controlled trial | |
| Participants |
Country : Canada Setting : hospital Number of centres : 1 Numbers : ProStar‐Plus 50, C‐Clamp 50 Age (mean (SD)) : ProStar‐Plus 62 (11) years, C‐Clamp 59 (12) years Sex : ProStar‐Plus 44 M/6 F, C‐Clamp: 39 M/11 F Inclusion criteria : patients with elective or urgent coronary angioplasty with or without stenting in whom same‐day discharge was reasonable Exclusion criteria : clinical evidence of peripheral arterial disease, pre‐existing femoral haematoma, serum creatinine > 150 mmol/L, blood pressure > 180/100 mmHg, participating in another research project |
|
| Interventions |
Intervention 1 : ProStar‐Plus Intervention 2 : C‐Clamp Sheath size : 8 Fr |
|
| Outcomes |
Primary : time to mobilisation; time to discharge Secondary : insertion failure; need for vascular surgery; external bleeding after initial haemostasis; an ooze of blood; haematomas (small < 5 cm, medium 5 to 10 cm, large > 10 cm); blood transfusion; participant satisfaction; cost per participant |
|
| Notes | Participants randomised to ProStar were mobilised after 4 hours; manual compression participants were mobilised 6 hours after sheath removal | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Castañeda 2003.
| Methods | Study design : randomised trial | |
| Participants |
Country : United States of America Setting : hospital Number of centres : 1 Numbers : QuickSeal 85, manual compression 56 Age (mean (SD)) : QuickSeal 62 (11.4) years, manual compression mean 65 (10.8) years Sex : QuickSeal 54 M/31 F, manual compression 31 M/25 F Inclusion criteria : patients 18 to 80 years of age undergoing percutaneous diagnostic or interventional procedure via the common femoral artery Exclusion criteria : pre‐existing autoimmune disease; punctures through a vascular graft; puncture depth < 3 cm or > 7.5 cm; haematoma present before sheath removal; significant bleeding or platelet disorders; uncontrolled hypertension; ipsilateral arterial site closure with QuickSeal within 6 weeks; closure with manual compression within 6 weeks, or closure with another device within 180 days; pregnant or breastfeeding women. Intraprocedural exclusion criteria included bleeding before sheath removal, suspected contamination of access site, multiple or double wall punctures, ipsilateral venous sheath, palpable haematoma before sheath removal and intraprocedural therapeutic thrombolysis |
|
| Interventions |
Intervention 1 : QuickSeal Intervention 2 : manual compression Sheath size : ≤ 8 Fr |
|
| Outcomes |
Primary : time to haemostasis (time between sheath pull and haemostasis); time to ambulation (defined as time between end of the procedure and the participant walking 10 feet); rate of major complications (need for medical intervention beyond that of standard procedure (haematoma requiring transfusion, severe vessel damage or infection) and that required extended hospitalisation, transfusion or unanticipated surgery); rate of minor complications (haematoma, ecchymosis, bleeding and minor pseudoaneurysm requiring no intervention) Secondary : time to hospital discharge |
|
| Notes | Ambulation was attempted at 1 hour, followed by subsequent checks at hourly intervals, in the QuickSeal group. The first check for ambulation in the manual compression group was attempted at 4 hours in participants undergoing diagnostic procedures and at 6 hours among participants undergoing interventional procedures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised according to a 3:2 ratio. Randomization was also stratified to type of procedure" Comment: random aspect of sequence generation. Study judged to be at low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque envelopes containing the random assignment that specified the method for achieving haemostasis were used to randomise patients" Comment: adequate concealment of allocation, so study judged to be at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Chen 2013.
| Methods | Study design : randomised study | |
| Participants |
Country : Taiwan Setting : hospital Number of centres : 1 Numbers : Boomerang 30, PerClose 30 Age (mean (SD)) : Boomerang 63.1 (9.9) years, PerClose mean 69.8 (10.6) years Sex : QuickSeal 21 M/9 F, manual compression 20 M/10 F Inclusion criteria : patients undergoing percutaneous interventional procedure via the common femoral artery Exclusion criteria : patients with “double wall” arterial punctures, intraluminal thrombi, pseudoaneurysms, haematomas, arteriovenous (AV) fistulas or infection in the target artery lesion; history of protamine allergy; previous injections of neutral protamine hagedorn (NPH). We also excluded patients who required a long sheath (> 23 cm) |
|
| Interventions |
Intervention 1 : Boomerang Intervention 2 : PerClose Sheath size : 7 Fr |
|
| Outcomes |
Primary : procedure success; device success; device deployment time; device dwell time; manual compression time; time to ambulation; major complications Secondary : pain score; minor complications |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Deuling 2008.
| Methods | Study design : prospective randomised trial | |
| Participants |
Country: The Netherlands Setting: hospital Number of centres : 1 Numbers : AngioSeal 150, StarClose 150, manual compression 150 Age (mean (SD)) : AngioSeal 62.7 (11.8) years, StarClose 64.1 (10.8) years, manual compression 62.9 (12.5) years Sex : AngioSeal 102 M/48 F, StarClose 109 M/41 F, manual compression 110 M/40 F Inclusion criteria : patients admitted for elective diagnostic or interventional cardiac catheterisation procedures who were eligible for femoral access Exclusion criteria : high or low arterial puncture |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : StarClose Intervention 3 : manual compression Sheath size : 6 Fr |
|
| Outcomes |
Primary: success of haemostasis; oozing; haemoglobin change during hospital admission; complications (haematoma, need for blood transfusion, surgical intervention at access site, infection) Secondary: participant comfort |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients received a device or manual compression based on order of presentation" Comment: non‐random sequence generation. Study judged to be at high risk of selection bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Diaz 2001.
| Methods | Study design : quasi‐randomised controlled trial | |
| Participants |
Country : Spain Setting : hospital Number of centres : 1 Numbers : AngioSeal 75, manual compression 75 Age (mean (SD)) : AngioSeal 59 (9.5) years, manual compression 60 (9) years Sex : AngioSeal 65 M/10 F, manual compression 64 M/11 F Inclusion criteria : patients aged over 18 years undergoing coronary angiography and/or percutaneous transluminal coronary angioplasty (PTCA) via the femoral artery with or without implantation of stent Exclusion criteria : the drilling of the posterior wall of the artery during puncture, presence of a femoral murmur, history of aortic vascular surgery or lower limb and the presence of haematoma before randomisation |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : manual compression Sheath size : 6 Fr |
|
| Outcomes |
Primary : time to haemostasis; time to ambulation Secondary : Hhaematoma |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated by odd and even numbers |
| Allocation concealment (selection bias) | High risk | Allocation based on alternation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Doneaux 2001.
| Methods | Study design : randomised comparison | |
| Participants |
Country : Belgium Setting : hospital Number of centres : not stated Numbers : AngioSeal 58, manual compression 63 Age : not stated Sex : not stated Inclusion criteria : patients undergoing percutaneous coronary intervention Exclusion criteria : not stated |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : manual compression Sheath size : not stated |
|
| Outcomes |
Primary : device success; time to compression; duration of compression; time to ambulation; participant satisfaction Secondary : large haematoma (> 5 cm); pseudoaneurysm; groin discomfort; subcutaneous bleeding |
|
| Notes | Study is published as an abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" Comment: insufficient information about the sequence generation process to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judge that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Fargen 2011.
| Methods | Study design : blinded randomised controlled trial | |
| Participants |
Country : United States of America Setting : hospital Number of centres : 1 Numbers : AngioSeal 32, Mynx 32 Age (mean (SD)) : Mynx 55.0 (1.9) years, AngioSeal 58.5 (2.6) years Sex : Mynx 11 M/21 F, AngioSeal 13 M/19 F Inclusion criteria : adult patients undergoing diagnostic cerebral angiography via femoral access Exclusion criteria : patients undergoing angiography through any non‐femoral artery percutaneous access site; those with documented psychiatric disorders, altered mental status or necessitating conscious sedation during their procedures; those reporting a baseline chronic pain rating ≥ 4 on the visual analogue scale (VAS) before closure device deployment; patients in which the puncture was distal to the bifurcation of the superficial femora and profunda femoris arteries; puncture site proximal to the inguinal ligament; puncture through a vascular graft; multiple punctures required to obtain arterial access; patients with clinically significant peripheral vascular disease; uncontrolled hypertension (systolic blood pressure > 180 mmHg); femoral artery size < 5 mm |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : Mynx M5 Sheath size : not stated |
|
| Outcomes |
Primary : change in pain from baseline (pre‐closure) to post closure, assessed by VAS Secondary : participant reporting of the most painful portion of the procedure from a multiple choice selection. Major complications (access site‐related surgical vascular repair; amputation related to access closure complication; permanent access site‐related nerve injury; access site‐related bleeding requiring transfusion; new ipsilateral lower extremity ischaemia by exam, Doppler or angiography requiring non‐surgical intervention; local access site‐related infection, inflammation or generalised infection due to the procedure requirement of intravenous antibiotics or prolonged hospitalisation); minor complications (arteriovenous fistula documented by ultrasound not requiring treatment; pseudoaneurysm not requiring treatment or treated with thrombin injection; access site haematoma ≥ 6 cm; access site bleeding requiring ≥ 30 minutes to achieve haemostasis; late (pre‐ or post‐discharge) access site‐related bleeding; ipsilateral deep vein thrombosis; transient access site‐related nerve injury; local access‐site related infection or inflammation requiring oral antibiotics) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed utilizing pre study‐created, randomly allotted, sealed envelopes containing the name of the VCD to be used" Comment: insufficient information regarding random sequence generation to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed utilizing pre study‐created, randomly allotted, sealed envelopes containing the name of the VCD to be used" Comment: adequate concealment of allocation. Study judged to be at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients, nurses administering the questionnaire and study coordinators were blinded to the VCD treatment used" Comment: Blinding of participants and personnel was done. Study was judged to be at low risk of performance bias |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients, nurses administering the questionnaire and study coordinators were blinded to the VCD treatment used" Comment: Blinding of outcome assessors was done. Study was judged to be at low risk of performance bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Gerckens 1998.
| Methods | Study design : single‐centre randomised trial | |
| Participants |
Country : Germany Setting : hospital Number of centres : 1 Numbers : suturing device 298, manual compression 292 Age (mean (SD)) : suturing device 60 (9) years, manual compression 62 (8) years Sex : suturing device 206 M/92 F, manual compression 207 M/85 F Inclusion criteria : patients who were possible candidates for same‐day ambulation and who had undergone a catheterisation procedure via the common femoral artery through a 5.5 Fr to 8 Fr introducer sheath Exclusion criteria : patients with suspected significant peripheral vascular disease; those in whom contralateral or bilateral arterial access site was punctured; previous vascular complication or repair; small common femoral artery; back wall of common femoral artery was punctured; multiple puncture attempts were made; patient's anatomy made successful device deployment unlikely |
|
| Interventions |
Intervention 1 : Techstar or ProStar Plus Intervention 2 : manual compression Sheath size : 6 Fr or 8 Fr |
|
| Outcomes |
Primary : incidence of major vascular complications; time to haemostasis; time to ambulation (elapsed time between randomisation and time the participant walked 3 meters) Secondary : surgery; untreated pseudoaneurysm; infection requiring oral antibiotics; arteriovenous fistula; peripheral ischaemia; haematoma > 4 cm |
|
| Notes | Guidelines for ambulation allowed the participant to walk within 1 hour after the suture‐mediated closure procedure in the diagnostic subset and within 4 hours in the interventional subset. Ambulation for compression participants was based on hospital standards (usually 4 hours after diagnotic procedures and 6 hours after achievement of haemostasis after interventional procedures) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation process" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Gwechenberger 1997.
| Methods | Study design : randomised study | |
| Participants |
Country : Austria Setting : hospital Number of centres : 1 Numbers: VasoSeal 33, manual compression 33 Age (mean (SD)) : VasoSeal 59.8 (8.1) years, manual compression 56.9 (10.8) years Sex : VasoSeal 31 M/2 F, manual compression 24 M/5 F Inclusion criteria : patients undergoing diagnostic or therapeutic percutaneous transluminal coronary angioplasty Exclusion criteria : not stated |
|
| Interventions |
Intervention 1 : VasoSeal Intervention 2 : manual compression Sheath size : not stated |
|
| Outcomes |
Primary : time to haemostasis Secondary : complications (arteriovenous fistula, pseudoaneurysm, bleeding, haematoma > 6 cm in diameter) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study outcomes are not clearly reported |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Hattab 2012.
| Methods | Study design : prospective randomised study | |
| Participants |
Country : France Setting : hospital Number of centres : 1 Numbers: ExoSeal 50, ProGlide 50 Age : not stated Sex : not stated Inclusion criteria : patients undergoing PCI and endovascular peripheral procedures via retrograde femoral artery access Exclusion criteria : not stated |
|
| Interventions |
Intervention 1 : ExoSeal Intervention 2 : PerClose ProGlide Sheath size : 6 Fr |
|
| Outcomes |
Primary : immediate total haemostasis Secondary : incidence of vascular complications (haematoma, blood transfusion) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Hermanides 2010.
| Methods | Study design : single‐centre retrospective randomised study | |
| Participants |
Country : The Netherlands Setting : hospital Number of centres : 1 Numbers: AngioSeal 313, manual compression 314 Age (mean (SD)) : AngioSeal 64.5 (11.3) years, manual compression 64.0 (11.0) years Sex : AngioSeal 238 M/75 F, manual compression 239 M/75 F Inclusion criteria : patients undergoing PCI via femoral artery access Exclusion criteria : age < 18 years; serious co‐morbidity such as cancer; advanced cerebrovascular disease; unwilling or unable to sign the consent form for participation; females of childbearing age not using medically prescribed contraceptives; unsuitable access site (severe peripheral vascular disease, poor location) |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : manual compression Sheath size : 6 Fr |
|
| Outcomes |
Primary : combined incidence of (1) severe haematoma > 5 cm at the puncture site or groin bleeding resulting in prolonged hospital stay, transfusion and/or surgical intervention at the puncture site; (2) arteriovenous fistula formation at the puncture site and/or surgical intervention at the puncture site Secondary : decrease in haemoglobin 1 day after inclusion; hospital admission duration |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by means of a computer program in blocks (randomly changing block size)" Comment: random sequence generation. Study was judged to be at low risk of selection bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A blinded independent clinical endpoint committee adjudicated all clinical endpoints" Comment: Blinding of outcome assessors was done. Study was judged to be at low risk of performance bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Hermiller 2005.
| Methods | Study design : prospective randomised multi‐centre trial (CLIP trial). Substudy of diagnostic arm | |
| Participants |
Country : United States of America Setting : hospital Number of centres : 17 Numbers : StarClose 136, manual compression 72 Age (mean (SD)) : StarClose 62.07 (12.12) years, manual compression 60.85 (11.11) years Sex : StarClose 93 M/43 F, manual compression 46 M/26 F Inclusion criteria : patients undergoing diagnostic angiography; arterial puncture site in an appropriate vessel; suitable as a candidate for vascular surgery; ability to complete required clinical follow‐up Exclusion criteria : patients with uncontrolled hypertension, clinically severe peripheral vascular disease (including calcification at the arteriotomy), obesity (BMI > 35) or a history of bleeding diathesis |
|
| Interventions |
Intervention 1 : StarClose Intervention 2 : manual compression Sheath size : 6 Fr |
|
| Outcomes |
Primary : major vascular complications (composite of vascular injury requiring repair, new ipsilateral distal ischaemia requiring revascularisation, access site nerve injury requiring intervention, access site bleeding requiring transfusion and access site infection requiring intravenous antibiotics or prolonged hospital stay); time to haemostasis (defined as time between sheath removal and first observed clinical haemostasis) Secondary : device success; procedure success; time to ambulation (defined as time from sheath pull to participant walking 20 feet without bleeding); time to discharge Follow‐up : 30 days post procedure |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information to permit judgement of low or high risk |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement of low or high risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement of low or high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Hermiller 2006.
| Methods | Study design : prospective randomised multi‐centre trial (CLIP trial). Substudy of interventional arm | |
| Participants |
Country : United States of America Setting : hospital Number of centres : 17 Numbers: StarClose 184, manual compression 91 Age : 62.8 (9.9) years Sex : 221 M/54 F Inclusion criteria : patients undergoing interventional catheterisation procedures; arterial puncture site in an appropriate vessel; suitability as a candidate for vascular surgery with ability to complete required clinical follow‐up Exclusion criteria : patients with uncontrolled hypertension; clinically severe peripheral vascular disease (including calcification at the arteriotomy); obesity (BMI > 35);history of bleeding diathesis |
|
| Interventions |
Intervention 1 : StarClose Intervention 2 : manual compression Sheath size : 6 Fr |
|
| Outcomes |
Primary : major vascular complications (composite of vascular injury requiring repair, new ipsilateral distal ischaemia requiring revascularisation, access site nerve injury requiring intervention, access site bleeding requiring transfusion and access site infection requiring intravenous antibiotics or prolonged hospital stay); time to haemostasis (defined as time between sheath removal and first observed clinical haemostasis) Secondary : device success; procedure success; time to ambulation (defined as time from sheath pull to participant walking 20 feet without bleeding); time to discharge |
|
| Notes | All participants received anticoagulation medication (heparin, aspirin, clopidogrel, glycoprotein IIb/IIIa inhibitors) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Holm 2014.
| Methods | Study design : prospective randomised non‐blinded single‐centre trial (CLOSE‐UP trial) | |
| Participants |
Country : Denmark Setting : hospital Number of centres : 1 Numbers: FemoSeal 500, manual compression 501 Age (mean (SD)) : FemoSeal 64.3 (11) years, manual compression 65.2 (11) years Sex : FemoSeal 310 M/190 F, manual compression 311 M/190 F Inclusion criteria : patients > 18 years of age eligible for femoral access and scheduled for elective diagnostic coronary angiography Exclusion criteria
|
|
| Interventions |
Intervention 1 : FemoSeal Intervention 2 : manual compression Sheath size : 6 Fr |
|
| Outcomes |
Primary
Secondary
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed 1:1 by telephone call to a voice prompt stand‐alone computer‐based system" Comment: random sequence generation. Study was judged to be at low risk of selection bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed 1:1 by telephone call to a voice prompt stand‐alone computer‐based system" Comment: adequate concealment of allocation. Study was judged to be at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Jensen 2008.
| Methods | Study design : prospective randomised single‐centre study | |
| Participants |
Country : Sweden Setting : hospital Number of centres : 1 Numbers : AngioSeal 22, PerClose 22, FemoStop 24 Age (mean (SD)) : AngioSeal 63 (11) years, PerClose 62 (9) years, FemoStop 61 (9) years, Sex : AngioSeal 15 M/7 F, PerClose 16 M/6 F, FemoStop 21 M/3 F Inclusion criteria : Patients undergoing planned coronary angiography because of stable angina pectoris were able to complete the required clinical follow‐up and provide informed consent Exclusion criteria : patients with unstable angina pectoris, ongoing infection, known inflammatory disease, previous PCI or coronary bypass grafting or other major surgery within 12 months and during follow‐up, and ongoing treatment with warfarin, steroids or non‐steroid anti‐inflammatory drugs |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : PerClose Intervention 3 : FemoStop Sheath size : 6 Fr |
|
| Outcomes |
Primary : inflammatory markers Secondary : immediate haemostasis; vascular injury; bleeding; haematoma |
|
| Notes | FemoStop participants were ambulated after 2 hours; those who received AngioSeal or PerClose were ambulated after 1 hour | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised to one of the following devices" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Number of side effects not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk. |
Juergens 2004.
| Methods | Study design : randomised trial | |
| Participants |
Country : Australia Setting : hospital Number of centres : 1 Numbers : 58 AngioSeal, 57 FemoStop Age (mean (SD)) : AngioSeal 59 (11) years, FemoStop 59 (10) years Sex : AngioSeal 44 M/14 F, FemoStop 46 M/11 F Inclusion criteria : patients undergoing percutaneous coronary intervention, clean (single puncture through the anterior wall only) arterial access with a 7 Fr sheath and guiding system, no development of haematoma during the procedure Exclusion criteria : patients in whom repeat femoral access through the same side was likely within the ensuing 90 days |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : FemoStop Sheath size : 7 Fr |
|
| Outcomes |
Primary : patient tolerance and resource utilisation (cost of disposals, amount of medical and nursing time spent attending to the femoral access site) Secondary : time to removal of participant from angiography suite; time to haemostasis; time to ambulation; time to hospital discharge; incidence of vascular complications |
|
| Notes | First outcome assessments were made at 4 hours, at 8 hours and on the morning after the procedure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised at the end of the procedure" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Patients underwent a duplex ultrasound reported by a vascular surgeon who was blinded to treatment assignment" Comment: outcome assessors blinded to treatment. Study was judged to be at low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Kalsch 2008.
| Methods | Study design : prospective single‐centre randomised study | |
| Participants |
Country : Germany Setting : hospital Number of centres : 1 Numbers : AngioSeal 214, PerClose 152 Age (mean (SD)) : AngioSeal 64 (11) years, PerClose 65 (10) years Sex : AngioSeal 151 M/61 F, PerClose 111 M/43 F Inclusion criteria : patients undergoing diagnostic cardiac catheterisation (n = 224) or interventional coronary procedures (n = 144) Exclusion criteria : patients < 18 years, pre‐existing large haematoma, known allergy to bovine products or reabsorbable suture material |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : PerClose Sheath size : 6 Fr or 8 Fr |
|
| Outcomes |
Primary : ankle‐brachial index; incidence of major complications during the in‐hospital period Secondary : successful technical deployment of the device; successful +haemostasis without additional treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised to..." Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Kussmaul 1995.
| Methods | Study design : randomised multi‐centre trial | |
| Participants |
Country : United States of America Setting : hospital Number of centres : 8 Numbers : AngioSeal 218, manual compression 217 Age (mean (SD)) : AngioSeal 61 (11) years, manual compression 62 (11) years Sex : AngioSeal 159 M/59 F, manual compression 156 M/61 F Inclusion criteria : patients undergoing cardiac catheterisation or angioplasty Exclusion criteria : patients < 18 or > 80 years; bleeding diathesis; warfarin therapy; thrombolytic therapy within 24 hours of or during catheterisation; acute myocardial infarction; marked obesity; uncontrolled hypertension and known allergy to bovine collagen or reabsorbable suture material; clinical or ultrasound evidence of significant peripheral vascular disease; history of claudication or vascular surgery; absent pedal pulses; femoral artery bruit; ankle/brachial systolic blood pressure index < 0.9; significant (> 20% occlusive) anterior atherosclerosis seen on ultrasound of the common femoral artery |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : manual compression Sheath size : 8 Fr |
|
| Outcomes |
Primary : time to haemostasis (defined as time between sheath removal and no bleeding) Secondary : complications (bleeding (any external blood loss after device deployment, ≥ 30 minutes of manual pressure required or any late bleeding, whether or not a measurable decrease in hematocrit occurred or transfusion was necessary); haematoma (any palpable mass); pseudoaneurysm (periarterial mass detected by physical examination or ultrasound‐containing Doppler‐detected flow); loss of pulse; infection; clinical evidence of leg ischaemia) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation procedure ensured that equal numbers of patients would be randomised to groups I and II by using a block scheme with six patients per block" Comment: random sequence generation. Study was judged to be at low risk of selection bias |
| Allocation concealment (selection bias) | Low risk | Quote: "The results of randomisation were place in sealed, sequential envelopes to be opened at each site as needed" Comment: adequate concealment of allocation. Study was judged to be at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 435 participants randomised, 115 underwent interventional procedures. However, data on only 109 interventional participants are reported. Therefore, 6 participants are missing from this analysis, and it is not stated why |
| Selective reporting (reporting bias) | Unclear risk | Study outcomes not clearly pre‐specified |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Legrand 2005.
| Methods | Study design : randomised trial | |
| Participants |
Country : Belgium Setting : hospital Number of centres : 1 Numbers : AngioSeal 100, manual compression 102 Age (mean (SD)) : AngioSeal 62.6 (10.3) years, manual compression 62.1 (13.0) years Sex : AngioSeal 79 M/21 F, manual compression 77 M/25 F Inclusion criteria : patients undergoing coronary intervention through a femoral 6 Fr access sheath Exclusion criteria : uncontrolled hypertension (> 200 mmHg); platelet count < 75,000; septicaemia; acute myocardial infarction; cardiogenic shock; severe acute non‐cardiac systemic disease or terminal illness; sheath in place longer than 24 hours; multiple femoral punctures; significant femoral disease and/or vascular tortuosity in the region of the puncture; vessel diameter < 5 mm; arterial puncture performed in the profunda femoris or close to the bifurcation; access through a femoral prosthesis; access sheath in the femoral vein; presence of a palpable haematoma at the end of the procedure |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : manual compression Sheath size : 6 Fr |
|
| Outcomes |
Primary : freedom from puncture site‐related complications (vasovagal response requiring atropine and fluid administration; large haematoma defined as any palpable mass > 5 cm diameter; pseudoaneurysm detected by Doppler ultrasound with significant bleeding after an initial period of haemostasis; loss of pulse; vessel occlusion; deep vein thrombosis; retroperitoneal haemorrhage; infection; arteriovenous fistula; crural nerve compression) Secondary : time to haemostasis; time to ambulation (defined as time between the end of PCI and cessation of bedrest); nursing time; participant satisfaction |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The computer assisted randomisation procedure ensured that approximately 100 patients would be included in each group" Comment: random sequence generation. Study was judged to be at low risk of selection bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Machnik 2012.
| Methods | Study design : randomised study | |
| Participants |
Country : Poland Setting : hospital Number of centres : 1 Numbers : AngioSeal 91, manual compression 110 Age (mean (SD)) : AngioSeal 66.9 (8) years, manual compression 66.7 (8) years Sex : AngioSeal 53 M/38 F, manual compression 66 M/44 F Inclusion criteria : patients undergoing percutaneous interventions on carotid, vertebral or peripheral arteries Exclusion criteria : not stated |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : manual compression Sheath size : 6 Fr and 8 Fr |
|
| Outcomes |
Primary : complications (large haematomas (> 10 cm), acute lower limb ischaemia requiring surgical intervention, pseudoaneurysm, arteriovenous fistula) Secondary : time to mobilisation; duration of post‐procedural hospitalisation |
|
| Notes | All participants were receiving dual antiplatelet therapy (aspirin 75 mg + clopidogrel 75 mg). Unfractionated heparin was used during the procedure to achieve activated coagulation time > 250 seconds. Oral anticoagulant therapy was stopped before the procedure in all participants receiving long‐term treatment with acenocoumarol/warfarin to obtain INR < 1.4, allowing an elective percutaneous intervention. Subcutaneous injections of low molecular weight heparin were stopped for ≥ 12 hours before the procedure and after the procedure for all participants receiving this type of therapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Study outcomes are not clearly pre‐specified |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Magosaki 1999.
| Methods | Study design : propsective randomised trial | |
| Participants |
Country : Japan Setting : hospital Number of centres : 4 Numbers : AngioSeal 120, manual compression 120 Age (mean (SD)) : AngioSeal 61.1 (10.9) years, manual compression 60.9 (9.9) years Sex : AngioSeal 93 M/27 F, manual compression 90 M/30 F Inclusion criteria : patients undergoing diagnostic angiography or coronary angioplasty Exclusion criteria : not stated |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : manual compression Sheath size : 5 to 8 Fr |
|
| Outcomes |
Primary : time to haemostasis; time to ambulation; complications Secondary : activated clotting time; successful placement of device |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Martin 2008.
| Methods | Study design : prospective randomised trial | |
| Participants |
Country : United States of America Setting : hospital Number of centres : 1 Numbers : AngioSeal 70, PerClose ProGlide 63, manual compression 67 Age (mean (SD)) : AngioSeal 63 (12) years, PerClose 66 (12) years, manual compression 68 (11) years Sex : AngioSeal 50 M/20 F, PerClose 40 M/23 F, manual compression 50 M/17 F Inclusion criteria : patients undergoing PCI using a 6 Fr femoral sheath Exclusion criteria : arterial insertion site not in the common femoral artery; more than minimal arterial calcification or common femoral artery < 6 mm in diameter; patients with INR > 1.4 or who had prior arterial access at the same femoral site within 30 days |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : PerClose Intervention 3 : manual compression Sheath size : not stated |
|
| Outcomes |
Primary : major and minor bleeding; time to haemostasis (defined as time at which no compression was required to control bleeding at the arteriotomy site) Secondary : time to ambulation; vascular complications (retroperitoneal haemorrhage, pseudoaneurysm, thrombosis, arteriovenous fistula); participant satisfaction |
|
| Notes | Ambulation was allowed 3 hours after PerClose or AngioSeal and 6 hours after compression | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "A patient questionnaire was administered at hospital discharge by study personnel blinded to treatment assignment" Comment: Study personnel assessing participant satisfaction were blinded to treatment allocation. However, it is not clear whether assessors measuring other study outcomes such as time to haemostasis, time to mobilisation and complications were blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Michalis 2002.
| Methods | Study design : prospective randomised single‐centre trial | |
| Participants |
Country : Greece Setting : hospital Number of centres : 1 Numbers : AngioSeal 290, VasoSeal 280, Duett 281 Age (mean (SD)) : AngioSeal 62.7 (14.3) years, VasoSeal 64.1 (10.2) years, Duett 62.4 (12.6) years Sex : AngioSeal 200 M/90 F, VasoSeal 196 M/84 F, Duett 205 M/76 F Inclusion criteria : patients undergoing coronary angiography and/or angioplasty who were deemed appropriate for immediate sheath removal Exclusion criteria : patients in whom the sheath was retained for prolonged access |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : VasoSeal Intervention 3 : Duett Sheath size : 6 Fr |
|
| Outcomes |
Primary : successful deployment of the device; time of device deployment; time to haemostasis (defined as time between completion of the device insertion procedure to achievement of haemostasis by compression); time to ambulation (measured from end of the catheterisation procedure until participant was able to stand and walk 3 to 5 steps unaided) Secondary : major complications (haematoma > 5 cm in diameter; haematoma or bleeding requiring transfusion; pseudoaneurysm; arteriovenous fistula; retroperitoneal haemorrhage; plug embolism; groin infection; death); minor complications (bleeding from the puncture site that did not require transfusion and/or vascular surgery; haematoma < 5 cm in diameter; pain at the puncture site; skin allergic reaction related to the device) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were prospectively randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Nelson 2014.
| Methods | Study design : prospective multi‐centre randomised controlled trial | |
| Participants |
Country : America Setting : hospital Number of centres : 20 Numbers : ProGlide 50, ProStar XL 51 Age (mean (SD)) : ProGlide (70 (6.6)) years, ProStar XL (74 (11)) years Sex (M/F) : ProGlide 47 M/3 F, ProStar XL 44 M/7 F Inclusion criteria : patients ≥ 18 years old with an abdominal aortic aneurysm (AAA) of maximum diameter ≥ 5 cm, or in the range of 4 to 5 cm, which has increased by ≥ 0.5 cm in the past 6 months; suitable ipsilateral common femoral artery for percutaneous access using a 'PreClose' technique as detailed in the protocol Exclusion criteria
|
|
| Interventions |
Intervention 1 : ProGlide Intervention 2 : ProStar XL Sheath size : ProGlide 8 Fr, ProStar XL 10 Fr |
|
| Outcomes |
Primary : treatment success defined as the composite of procedural technical success, absence of vascular complications and absence of major adverse events Secondary : all serious and non‐serious adverse events; stent graft patency and integrity; health‐related quality of life survey; clinical utility measures |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted by study site, using two block sizes (3 or 6) with random choice of block size order" Comment: random sequence generation. Study was judged to be at low risk of selection bias |
| Allocation concealment (selection bias) | Low risk | Quote: "One set of sealed randomization envelopes was provided to each site after completion of roll‐in cases and on sponsor approval to initiate the randomized trial. On screening eligibility confirmation, the next sequential randomization envelope was opened and the assignment was immediately allocated" Comment: adequate concealment of allocation. Study was judged to be at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Noguchi 2000.
| Methods | Study design : prospective randomised controlled trial | |
| Participants |
Country : Japan Setting : hospital Number of centres : 1 Numbers : ProStar 30, manual compression 30 Age (mean (SD)) : ProStar 63 (10) years, manual compression 61 (12) years Sex : ProStar 27 M/3 F, manual compression 25 M/5 F Inclusion criteria : patients undergoing angioplasty or stenting Exclusion criteria : arterial access at a site other than the right or left femoral artery; emergency procedure; continued use of warfarin before the procedure; haematoma formation during the procedure; history of claudication due to arteriosclerosis obliterans or vascular surgery that involved the groin area such as aortofemoral bypass surgery; unwillingness or inability to provide written informed consent |
|
| Interventions |
Intervention 1 : ProStar Intervention 2 : manual compression Sheath size : 8 Fr |
|
| Outcomes |
Primary : time to haemostasis; time to ambulation; time to discharge Secondary : surgical repair; infection; arteriovenous fistula; pseudoaneurysm; distal embolisation; haematoma; participant comfort; hospital costs |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information about the sequence generation process to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was carried out using consecutive sealed envelopes" Comment: Envelopes were sealed, so the study was judged to be at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Study outcomes are clearly reported |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Park 2005.
| Methods | Study design : prospective randomised study | |
| Participants |
Country : Korea Setting : hospital Number of centres : 1 Numbers : AngioSeal 961, Closure S 715 Age (mean) : AngioSeal 56.8 years, Closure S 51.1 years Sex : AngioSeal 727 M/234 F, Closure S 435 M/280 F Inclusion criteria : patients undergoing diagnostic angiography or endovascular interventional treatment Exclusion criteria : difficulty in puncturing the artery; severe peripheral vascular disease; marked obesity; age < 15 years; arterial sheath size < 4 Fr or > 8 Fr; patient’s refusal to provide written informed consent |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : Closure S Sheath size : 6 Fr or 8 Fr |
|
| Outcomes |
Primary : immediate haemostasis; successful vascular device closure (defined as immediate haemostasis without complications) Secondary : major complications (need for vascular surgery; haemorrhage requiring transfusion; pseudoaneurysm; arteriovenous fistula; arterial occlusion or distal arterial embolism; infection necessitating treatment with intravenous antibiotics or surgical debridement); minor complications (haemorrhage from the puncture site that was controlled via conservative management without transfusion (i.e. additional manual compression, sandbag placement or prolonged bedrest); infection that could be treated with oral antibiotics). All complications were categorised as early (< 24 hours of the procedure) and late (≥ 24 hours after the procedure) |
|
| Notes | This study included participants with several femoral artery punctures. Outcomes are based on the number of punctures rather than on the number of individual participants. After personal communication with the study author, it was decided that although this study was relevant and met the inclusion criteria, we would not include data in the analyses, as they were not comparable with data based on individuals from the other included studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Perlowski 2011.
| Methods | Study design : single‐centre prospective randomised trial | |
| Participants |
Country : United States of America Setting : hospital Number of centres : 1 Numbers : StarClose 39, manual compression 42 Age : not stated Sex : not stated Inclusion criteria : patients with confirmed peripheral arterial disease undergoing percutaneous endovascular and coronary procedures Exclusion criteria : not stated |
|
| Interventions |
Intervention 1 : StarClose Intervention 2 : manual compression Sheath size : not stated |
|
| Outcomes |
Primary : vascular complications (pseudoaneurysm, arteriovenous fistula) Secondary : time to haemostasis; procedural success; device success; death |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised to" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Rastan 2008.
| Methods | Study design : prospective single‐centre randomised controlled trial | |
| Participants |
Country : Switzerland Setting : hospital Number of centres : 1 Numbers : AngioSeal 285, StarClose 286 Age (mean (SD)) : AngioSeal 67.6 (9.3) years, StarClose 66.1 (10.3) years Sex : AngioSeal 201 M/84 F, StarClose 204 M/82 F Inclusion criteria : patients undergoing a percutaneous transluminal procedure through a 5 Fr or 6 Fr femoral sheath Exclusion criteria : patients with a history of vascular surgery at the intended access site; treatment with glycoprotein IIb/IIIa inhibitors or fibrinolytic therapy; history of bleeding diathesis or documented puncture of the superficial or deep femoral artery near the femoral artery bifurcation |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : StarClose Sheath size : 5 Fr or 6 Fr |
|
| Outcomes |
Primary : composite incidence of access site pseudoaneurysm, major bleeding requiring transfusion, in‐hospital access site vascular surgery or catheter intervention or in‐hospital death from all causes Secondary : incidence of arteriovenous fistula; device failure (defined as access site bleeding requiring adjunctive therapy such as prolonged manual compression and/or pressure bandage); groin haematoma ≥ 5 cm in diameter |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was by envelope" Comment: insufficient information regarding random sequence generation to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was by sealed envelope" Comment: adequate concealment of allocation. Study was judged to be at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Reddy 2004.
| Methods | Study design : prospective randomised study | |
| Participants |
Country : United States of America Setting : hospital Number of centres : 1 Numbers : AngioSeal 25, manual compression 25 Age (mean (SD)) : AngioSeal 64 (3) years, manual compression 61 (3) years Sex : AngioSeal 15 M/10 F, manual compression 14 M/11 F Inclusion criteria : patients undergoing diagnostic cardiac catheterisation; ≥ 18 years of age; easily palpable femoral and radial pulses; a normal Allen's test Exclusion criteria : vascular disease of the upper or lower extremities precluding access at the femoral or radial artery; prior femoral artery graft surgery; unstable coronary syndromes; unstable patients with myocardial infarction who require an intervention within 7 days; patients for whom additional procedures were planned at the same setting or during the same hospital stay; those who were unable or unwilling to provide informed consent |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : manual compression Sheath size : 6 Fr |
|
| Outcomes |
Primary : quality of life Secondary : cardiovascular and major vascular complications |
|
| Notes | All participants were ambulated within 1 hour | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were prospectively randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Rickli 2002.
| Methods | Study design : single‐centre multiple‐operator prospective study | |
| Participants |
Country : Switzerland Setting : hospital Number of centres : 1 Numbers : PerClose 96, manual compression 97 Age (mean (SD)) : PerClose 62 (11) years, manual compression 59 (10) years Sex : PerClose 71 M/25 F, manual compression 81 M/16 F Inclusion criteria : patients undergoing percutaneous coronary intervention by 6 Fr or 7 Fr femoral access Exclusion criteria : not stated |
|
| Interventions |
Intervention 1 : PerClose Intervention 2 : manual compression Sheath size : 6 Fr and 7 Fr |
|
| Outcomes |
Primary : major (need for surgical intervention; local infection; need for blood transfusion); minor complications (pseudoaneurysm; local haematoma > 1 mL assessed by ultrasound); time to haemostasis; time to ambulation; time to discharge Secondary : participant discomfort; costs |
|
| Notes | Participants treated with PerClose were allowed to ambulate 4 hours after the procedure, but manual compression participants were ambulated the morning after the procedure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Sanborn 1993.
| Methods | Study design : prospective multi‐centre randomised trial | |
| Participants |
Country : United States of America Setting : hospital Number of centres : not stated Numbers : VasoSeal 246 (90 diagnostic catheterisation, 156 coronary angioplasty), manual compression 209 (75 diagnostic catheterisation, 134 coronary angioplasty) Age (mean (SD)) : VasoSeal diagnostic catheterisation 62.4 (10.8) years, VasoSeal coronary angioplasty without heparin 58.6 (9.8) years, VasoSeal coronary angioplasty with heparin 60.8 (9.8) years, manual compression diagnostic catheterisation 62.8 (10.7) years, manual compression coronary angioplasty 61.3 (11.0) years Sex : VasoSeal 181 M/65 F, manual compression 141 M/68 F Inclusion criteria : patients undergoing diagnostic cardiac catheterisation or balloon angioplasty; > 20 years of age; ability to give written consent and understand the obligation for a follow‐up study Exclusion criteria : patients who were markedly obese; known platelet dysfunction; uncontrolled hypertension; haematoma during catheterisation or angioplasty procedure. Patients undergoing atherectomy or intracoronary stent placement were not enrolled because the interventions required larger sheaths or greater anticoagulant regimens |
|
| Interventions |
Intervention 1 : VasoSeal Intervention 2 : manual compression Sheath size : 6 to 9 Fr |
|
| Outcomes |
Primary : time to haemostasis (defined as time elapsed from initial compression and removal of pre‐existing sheath until completion of compression); time to ambulation (calculated as total time from start of the procedure until ambulation) Secondary : peripheral vascular complications requiring vascular surgical repair for bleeding; large pseudoaneurysm; arteriovenous fistula; thrombosis or loss of distal pulse; transfusion due to bleeding at puncture site; deep vein thrombosis; infection at the puncture site requiring intravenous antibiotics; bleeding from the puncture site; small pseudoaneurysm treated medically; haematomas 2 to 6 cm and > 6 cm Follow‐up : in hospital, 3 days and 30 days after sheath removal |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information to provide judgement on low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to provide judgement on low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to provide judgement on low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Schräder 1992.
| Methods | Study design : randomised single‐centre study | |
| Participants |
Country : Germany Setting : hospital Number of centres : 1 Numbers: VasoSeal 50, manual compression 50 Age (mean (SD)) : VasoSeal 58.5 (10.2) years, manual compression 58.5 (9.2) years Sex : VasoSeal 43 M/7 F, manual compression 45 M/5 F Inclusion criteria : patients undergoing femoral artery catheterisation for coronary angiography or coronary artery dilation Exclusion criteria : patients taking vitamin K antagonists; thrombyte disorders; allergy to collagen products |
|
| Interventions |
Intervention 1 : VasoSeal Intervention 2 : pressure dressing |
|
| Outcomes |
Primary : compression time; time to ambulation Secondary : bleeding; haematoma Follow‐up : not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information to provide judgement on low or high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to provide judgement on low or high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to provide judgement on low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to provide judgement on low or high risk of bias |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to provide judgement on low or high risk of bias |
| Other bias | Unclear risk | Insufficient information to provide judgement on low or high risk of bias |
Schulz‐Schüpke 2014.
| Methods | Study design : randomised large‐scale multi‐centre open‐label clinical trial | |
| Participants |
Country : Germany Setting : hospital Number of centres : 4 Numbers: FemoSeal 1509, ExoSeal 1506, manual compression 1509 Age (mean (range)) : FemoSeal 66.6 (57.8 to 74.3) years, ExoSeal 68.1 (59.3 to 74.9) years, manual compression 68.4 (59.5 to 74.8 years) Sex : FemoSeal 1040 M/469 F, ExoSeal 1058 M/448 F, manual compression 1031 M/478 F Inclusion criteria : Patients were eligible for enrolment if they provided written informed consent and were undergoing diagnostic coronary angiography (without subsequent percutaneous coronary intervention) with a 6 Fr sheath through the common femoral artery, which had to have a diameter > 5 mm (proven by angiography) Exclusion criteria : Major exclusion criteria were implantation of a VCD within the last 30 days, symptomatic leg ischaemia, prior thromboendarteriectomy (TEA) or patch plastic of the common femoral artery, planned invasive diagnostic or interventional procedure in the following 90 days, a heavily calcified vessel, active bleeding or bleeding diathesis, severe arterial hypertension (> 220/110 mmHg), local infection, autoimmune disease, allergy to resorbable suture and pregnancy |
|
| Interventions |
Intervention 1 : FemoSeal Intervention 2 : ExoSeal Intervention 3 : manual compression Sheath size : 6 Fr |
|
| Outcomes |
Primary : incidence of vascular access site complications (i.e. the composite of haematoma measuring ≥ 5 cm, pseudoaneurysm, arteriovenous fistula, access site–related major bleeding, acute ipsilateral leg ischaemia, need for vascular surgical or interventional treatment or local infection at 30 days after randomisation Secondary : time to haemostasis; repeat manual compression; VCD failure Follow‐up : 30 days |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Sealed opaque envelopes containing a computer‐generated sequence were used" Comment: random sequence generation. Study was judged to be at low risk of selection bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed opaque envelopes containing a computer‐generated sequence were used" Comment: adequate concealment of allocation. Study was judged to be at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All events were adjudicated and classified by an event adjudication committee in which members were unaware of the assigned treatment" Comment: Outcome assessors were blinded to treatment allocation. Study was judged to be at low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
SEAL Trial Study Team.
| Methods | Study design : multi‐centre randomised study | |
| Participants |
Country : United States of America Setting : hospital Number of centres : 16 Numbers : Duett 392, manual compression 238 Age (mean (SD)) : Duett 62 (11) years, manual compression 63 (12) years Sex : Duett 309 M/83 F, manual compression 165 M/73 F Inclusion criteria : patients >18 years undergoing a diagnostic (n = 209) or interventional (n = 421) cardiac procedure with femoral arterial access who could provide written informed consent Exclusion criteria : arterial sheath < 5 Fr or > 9 Fr or longer than 10 cm; presence of a ≥ 6 cm diameter haematoma before initial sheath removal; presence of clinically severe peripheral vascular disease manifested by claudication at < 100 feet; weak or absent pulses in the affected limb; ankle brachial index < 0.5 at rest; known stenosis ≥ 50% in the iliac or femoral artery on the affected side; prior vascular bypass surgery or stent placement involving the affected femoral artery; suspected posterior femoral artery puncture or puncture distal to the common femoral artery bifurcation; known bleeding disorder including platelet count < 100,000 or receipt of thrombolytic therapy within the previous 24 hours; haemoglobin < 10 g/dL; international normalised ratio > 1.5; activated clotting time > 400 seconds at the conclusion of the catheterisation procedure; suspected pregnancy; life expectancy < 1 year; Q wave myocardial infarction within 72 hours; uncontrolled severe hypertension (systolic blood pressure > 180 mmHg or diastolic blood pressure > 110 mmHg); medical indication for continued intravenous heparin therapy after the procedure; known allergy to bovine‐derived products; estimated femoral artery diameter < 6 mm on the basis of femoral angiography results |
|
| Interventions |
Intervention 1 : Duett Intervention 2 : manual compression + FemoStop or C‐Clamp Sheath size : 5 to 9 Fr |
|
| Outcomes |
Primary : time to haemostasis; time to ambulation (defined as time from the end of sheath removal to time when the participant had gotten out of bed and walked 110 feet without loss of haemostasis); incidence of major complications (vascular surgery; ultrasound scan‐guided compression to treat a pseudoaneurysm; bleeding requiring transfusion; infection of the puncture site requiring extended hospitalisation; antibiotic administration within 30 days) Secondary : device success rate; time to discharge; composite of the primary endpoints divided by diagnostic and interventional procedures Follow‐up : 30 days |
|
| Notes | Participants randomised to the Duett device were ambulated 2 to 4 hours after the procedure according to manufacturer guidelines. Participants randomised to standard compression were ambulated according to the institution's practice | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated to 1 of the 2 treatment groups with a closed envelope system and permuted block design supplied by the coordinating centre" Comment: random sequence generation. Study was judged to be at low risk of selection bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Closed envelope system" Comment: adequate concealment of allocation. Study was judged to be at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Treatment masked analysis of ultrasound examination tape" Comment: Outcome assessors were blinded to treatment allocation. Study was judged to be at low risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 227 of 392 Duett participants completed 7‐day and 30‐day follow‐up examinations in the quality of life substudy. No explanation for incomplete data was provided by the study authors |
| Selective reporting (reporting bias) | High risk | Under Results, study authors reported 7‐day and 30‐day follow‐up examinations in the quality of life substudy participant group to measure participant discomfort, but this is not specified as an outcome of the study |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Seidelin 1997.
| Methods | Study design : randomised pilot trial | |
| Participants |
Country : Canada Setting : hospital Number of centres : 1 Numbers: AngioSeal 24, manual compression 26 Age (mean (SD)) : AngioSeal 55 (10) years, manual compression 56 (9) years Sex : AngioSeal 18 M/6 F, manual compression 19 M/7 F Inclusion criteria : patients < 75 years of age referred for elective coronary angiography Exclusion criteria : patients with a femoral bruit; reduced femoral pulses; previous vascular surgery of the aorta or lower limb arteries; history or evidence of peripheral vascular disease; history of puncture site complications from prior percutaneous procedures; known allergy to materials in the device; current anticoagulant therapy; known hypercoagulation or hypocoagulation conditions; presence of severe acute non‐cardiac systemic disease or terminal illness; female of child‐bearing potential; evidence of systemic bacterial or cutaneous infection |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : manual compression Sheath size : 7 Fr |
|
| Outcomes |
Primary : time to haemostasis (defined as time elapsed from sheath removal to time haemostasis was first confirmed); time to mobilisation (defined as time elapsed from sheath removal to time the participant was mobilised) Secondary : groin complications (re‐bleeding after initial haemostasis (3 categories): insignificant oozing requiring further compression or requiring transfusion); swelling; haematoma (minor ≤ 3 cm, moderate 3 to 6 cm, large > 6 cm); pseudoaneurysm; need for blood transfusion; need for vascular surgery; other groin complications not pre‐specified Follow‐up : at 30 minutes, at 2 hours, at 4 hours, at discharge, at 7 days after the procedure |
|
| Notes | Participants treated with the AngioSeal device were mobilised within 5 minutes of tamper removal, but manual compression participants were placed on bedrest for 4 to 6 hours before mobilisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Shammas 2002.
| Methods | Study design : single‐centre randomised study | |
| Participants |
Country : United States of America Setting : hospital Number of centres : 1 Numbers : AngioSeal 79, VasoSeal 78 Age (mean (SD)) : AngioSeal 60 (11.4) years, VasoSeal 60 (10.9) years Sex : AngioSeal 37 M/42 F, VasoSeal 42 M/36 F Inclusion criteria : patients undergoing cardiac catheterisation or percutaneous interventional procedures Exclusion criteria : arteriotomy larger than 8 Fr; any suspicion that the introducer has been placed through the superficial femoral artery and the profunda femoris, or at the bifurcation of these 2 vessels; the presence of significant vascular disease as judged by the cardiologist; uncontrolled hypertension at the time of deployment of the device; allergy to beef product, collagen or polyglycolic or polylactic acid polymers; emergency cases; therapeutic thrombolysis; vascular graft puncture; bleeding disorder; pregnant or lactating females; previous device placed within 6 weeks in the same common femoral artery; pre‐existing autoimmune disease; morbid obesity; hematoma before the procedure; < 18 or > 80 years of age |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : VasoSeal Sheath size : 8 Fr |
|
| Outcomes |
Primary : time to haemostasis; time to ambulation Secondary : major complications (pseudoaneurysm; arteriovenous fistula; thrombosis of common femoral artery;retroperitoneal bleed; infection; bleeding from the puncture site requiring transfusion; death) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Silber 1998.
| Methods | Study design : prospective randomised study | |
| Participants |
Country : Germany Setting : hospital Number of centres : 1 Numbers : VasoSeal 74, manual compression 76 Age (mean (SD)) : VasoSeal 59.8 (9.0) years, manual compression 58.0 (9.2) years Sex : VasoSeal 58 M/16 F, manual compression 58 M/18 F Inclusion criteria : patients undergoing PTCA Exclusion criteria : prolonged duration PTCA patients requiring an additional bolus of heparin; patients with need for overnight heparin infusion or coumadin; inadvertent penetration of the dorsal arterial wall within the puncture needle; previous application of collagen sealing of the femoral access site; known allergy to collagen; peripheral artery disease; patients with acute myocardial infarction; status post thrombolytic therapy; known coagulation defects or known platelet dysfunction; severe uncontrolled arterial hypertension (systolic > 220 mmHg or diastolic > 120 mmHg); pre‐existing haematoma; haematoma developed during the procedure; patients with a venous femoral sheath |
|
| Interventions |
Intervention 1 : VasoSeal Intervention 2 : manual compression Sheath size : 8 Fr |
|
| Outcomes |
Primary : time to haemostasis; groin discomfort; haematoma (small < 7 cm, medium 7 to 15 cm, large > 15 cm) Secondary : major complications (thrombosis; loss of distal pulses; large pseudoaneurysm; arteriovenous fistula; bleeding with need for transfusion or any vascular surgery); minor complications (bleeding from the puncture site not requiring transfusion; vascular surgery and small pseudoaneurysm treated medically) Follow‐up : 24 hours after sheath pull |
|
| Notes | As time to ambulation was not an endpoint of the study, participants had to stay in bed until the morning after the procedure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Sun 2009.
| Methods | Study design : randomised comparative study | |
| Participants |
Country : China Setting : hospital Number of centres : 1 Numbers : StarClose 286, PerClose 183, manual compression 271 Age (mean (SD)) : StarClose CAG 62.0 (10.5) years, StarClose PCI 63.9 (12.1) years, PerClose CAG 61.9 (11.1) years, StarClose PCI 64.0 (10.1) years, manual compression CAG 61.7 (10.5) years, manual compression PCI 64.1 (12.3) years Sex : StarClose 179 M/107 F, PerClose 115 M/68 F, manual compression 158 M/113 F Inclusion criteria : patients undergoing coronary angiography (CAG) and percutaneous coronary intervention (PCI) Exclusion criteria : not reported |
|
| Interventions |
Intervention 1 : StarClose Intervention 2 : PerClose Intervention 3 : Boomerang Sheath size : not reported |
|
| Outcomes |
Primary : haemostasis operation time; immobilisation time; incidence of vascular complications Secondary : device failures Follow‐up : not stated |
|
| Notes | Data presented by diagnosis/intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized" Insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Tron 2003.
| Methods | Study design : randomised study | |
| Participants |
Country : France Setting : hospital Number of centres : 1 Numbers : PerClose 91, manual compression 76 Age (mean (SD)) : PerClose 59 (10) years, manual compression 59 (11) years Sex : PerClose 85 M/6 F, manual compression 76 M/0 F Inclusion criteria : patients undergoing successful PTCA through the femoral artery with a 6 Fr or 8 Fr sheath Exclusion criteria : difficulty in puncturing the artery; peripheral vascular disease; marked obesity; age > 80 years; acute myocardial infarction; ilio‐femoral tortuosities; presence of a venous sheath; arterial sheath already inserted the day before PTCA; > 3 previous punctures in the same artery; refusal to give written informed consent |
|
| Interventions |
Intervention 1 : PerClose Intervention 2 : manual compression Sheath size : 6 Fr or 8 Fr |
|
| Outcomes |
Primary : success rate; time to haemostasis; haematoma (defined as a palpable mass at the puncture site); blood oozing; length of hospital stay Secondary : participant pain Follow‐up : 2 days post procedure |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Upponi 2007.
| Methods | Study design : prospective randomised study | |
| Participants |
Country : United Kingdom Setting : hospital Number of centres : 1 Numbers: AngioSeal 50, manual compression 50 Age (mean (SD)) : AngioSeal 68.9 years, manual compression 70.1 years Sex : AngioSeal 33 M/17 F, manual compression 35 M/15 F Inclusion criteria : patients undergoing retrograde femoral arterial puncture Exclusion criteria : not stated |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : manual compression Sheath size : 5 Fr to 7 Fr |
|
| Outcomes |
Primary : minor complications (haematoma < 6 cm); major complications (false aneurysm; arterio‐venous fistula; vessel occlusion; those requiring further percutaneous or surgical intervention) Secondary : none Follow‐up : up to 6 weeks post procedure |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "50 patients were randomised to each group" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are not clearly stated |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Veasey 2008.
| Methods | Study design : randomised single‐blind prospective trial | |
| Participants |
Country : United Kingdom Setting : district general hospital Number of centres : 1 Numbers: AngioSeal 208, StarClose 193 Age (mean (SD)) : AngioSeal 65.3 (10.6) years, StarClose 66.5 (10.8) years Sex : AngioSeal 115 M/93 F, StarClose 107 M/86 F Inclusion criteria : patients undergoing elective day‐case diagnostic coronary angiography with immediate post‐procedure mobilisation Exclusion criteria : patients with a diagnosis of unstable angina or acute myocardial infarction; significant peripheral vascular disease; previous peripheral vascular surgery; pregnant; unable to give written informed consent; younger than 18 years; previous femoral artery complication from coronary angiography |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : StarClose Sheath size : 5 Fr |
|
| Outcomes |
Primary : complications (induration; haematoma; bleeding; retroperitoneal bleed; pseudoaneurysm; leg ischaemia; access site infection) Secondary : participant satisfaction; pain scores Follow‐up : 1 week post procedure |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised to" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Outcomes are not clearly stated |
| Selective reporting (reporting bias) | Unclear risk | Outcomes are not clearly stated |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
von Hoch 1995.
| Methods | Study design : prospective randomised study | |
| Participants |
Country : Germany Setting : hospital Number of centres : 1 Numbers: VasoSeal 154, manual compression 155 Age (median (IQR)) : VasoSeal 63 (54 to 70) years, manual compression 60 (53 to 70) years Sex : VasoSeal 125 M/29 F, 118 M/37 F Inclusion criteria : patients requiring emergency coronary stenting or elective percutaneous coronary angioplasty Exclusion criteria : patients with known allergies to collagen or other animal products; any haemostatic disorder; previous catheterisation from the right femoral artery within 1 week before the qualifying procedure; acute myocardial infarction |
|
| Interventions |
Intervention 1 : VasoSeal Intervention 2 : manual compression Sheath size : not stated |
|
| Outcomes |
Primary : complications at the vascular access site (pseudoaneurysm; arteriovenous fistula; local bleeding requiring blood transfusion or surgical treatment; groin infection; occlusion of the femoral artery) Secondary : time to haemostasis |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to" Insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Ward 1998.
| Methods | Study design : randomised multi‐centre study | |
| Participants |
Country : United States of America Setting : hospital Number of centres : 8 Numbers: AngioSeal 202, manual compression 102 Age (mean (SD)) : AngioSeal 61.7 (12) years, manual compression 64.7 (10) years Sex : AngioSeal 140 M/62 F, manual compression 72 M/30 F Inclusion criteria : patients undergoing diagnostic catheterisation via the femoral approach with an 8 Fr sheath or smaller Exclusion criteria : patients with severe systemic or terminal illness, systemic or cutaneous infection, platelet count < 75,000 cells/µL, use of an intra‐aortic balloon pump, thrombolytic therapy within previous 24 hours, fibrinogen count < 100 mg/dL, sheath in place > 36 hours, presence of haematoma before sheath removal, suspected profunda femoris puncture, younger than 18 years or unable to give informed consent |
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : manual compression Sheath size : 5 Fr to 8 Fr |
|
| Outcomes |
Primary : time to haemostasis (defined as elapsed time between sheath removal and first observed haemostasis); time to ambulation; time to discharge Secondary : vascular injury (need for vascular repair or pseudoaneurysm); bleeding complications (bleeding requiring transfusion; haematoma < 6 cm; haematoma ≥ 6 cm; late bleeding) Follow‐up : 14 days and 30 days after hospital discharge |
|
| Notes | Participants randomised to AngioSeal were ambulated at 1 hour; manual compression participants were ambulated 4 to 6 hours after sheath removal | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Wetter 2000.
| Methods | Study design : prospective randomised study | |
| Participants |
Country : Switzerland Setting : hospital Number of centres : 1 Numbers: PerClose 50, manual compression 50 Age (mean (SD)) : PerClose 58.5 (10.5) years, manual compression 59.9 (9.7) years Sex : not stated Inclusion criteria : patients who had undergone elective percutaneous transluminal coronary angioplasty Exclusion criteria : patients not ambulatory on the day of the intervention |
|
| Interventions |
Intervention 1 : PerClose Intervention 2 : manual compression Sheath size : 6 Fr or 7 Fr |
|
| Outcomes |
Primary : time to haemostasis (measured from insertion of device until application of bandage); time to mobilisation Secondary : haematoma; pseudoaneurysm; arteriovenous fistula Follow‐up : 4 hours post procedure |
|
| Notes | PerClose participants had 4 hours of bedrest; manual compression participants were kept in bed until the morning after the procedure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Unclear risk | Insufficient information to permit judgement of high or low risk |
Wong 2009.
| Methods | Study design : randomised non‐blinded trial | |
| Participants |
Country : United States of America Setting : hospital Number of centres : 17 Numbers: ExoSeal 267, manual compression 134 Age (mean (SD)) : ExoSeal 63.3 (11.1) years, manual compression 61.4 (10.5) years Sex : ExoSeal 182 M/85 F, manual compression 83 M/51 F Inclusion criteria : patients 18 to 84 years of age scheduled to undergo a diagnostic or interventional coronary or peripheral procedure via arterial puncture of a > 5 mm lumen diameter common femoral artery Exclusion criteria : patients who had sustained a myocardial infarction with ST‐segment elevation < 48 hours before the catheterisation procedure; uncontrolled hypertension at time of closure; symptomatic leg ischaemia in the target vessel limb or prior femoral vascular surgery or placement of a vascular graft at the target site; history of bleeding or platelet disorder or previous treatment with a thrombin‐specific anticoagulant or low molecular wegiht heparin < 24 hours before the catheterisation procedure; required puncture of both femoral arteries; prior closure of the target artery with any vascular closure device; pre‐existing systemic or cutaneous infection |
|
| Interventions |
Intervention 1 : ExoSeal Intervention 2 : manual compression Sheath size : 6 Fr |
|
| Outcomes |
Primary : time to haemostasis (defined as time from sheath removal to time haemostasis was achieved); time to ambulation (defined as time from sheath removal to time the participant was able to walk > 20 feet without recurrence of bleeding) Secondary : time to eligibility for hospital discharge; time to hospital discharge; procedure success; major adverse event (need for vascular repair by surgical or non‐surgical techniques; bleeding requiring a blood transfusion; infection requiring antibiotics; new‐onset ischaemia of the ipsilateral lower extremity; permanent access site‐related nerve injury); device success; post‐procedural complications (recurrent local bleeding requiring a haemostatic intervention or a > 6 cm haematoma or ecchymosis; pseudoaneurysm; arterio‐venous fistula; vascular laceration or retroperitoneal bleeding; ipsilateral manifestations of vascular insufficiency or embolisation including loss of distal pulse, total arterial occlusion or deep vein thrombosis, infection and nerve injury) Follow‐up : 30 days post procedure |
|
| Notes | ExoSeal participants were ambulated at 1 hour (received no glycoprotein IIb/IIIa inhibitors), 2 hours (received glycoprotein IIb inhibitor) and 6 hours (received glycoprotein IIIa inhibitors); manual compression participants were ambulated no later than 4 hours post haemostasis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Assignment was based on a computer‐generated treatment list, which balanced the randomisation by centre and type of procedure performed" Comment: random sequence generation. Study was judged to be at low risk of selection bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly assigned using sealed envelopes" Comment: allocation of treatment was concealed. Study was judged to be at low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Nonblinded" Comment: no blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
Yadav 2003.
| Methods | Study design : randomised multi‐centre controlled clinical investigation | |
| Participants |
Country : United States of America Setting : hospital Number of centres : 9 Numbers: QuickSeal 240, manual compression 158 Age (mean (SD)) : QuickSeal 62 (10.4) years, manual compression 61 (11.2) years Sex : QuickSeal 156 M/84 F, manual compression 102 M/56 F Inclusion criteria : patients 18 to 80 years of age who provided written informed consent and underwent a percutaneous diagnostic or interventional procedure by way of the common femoral artery Exclusion criteria : patients with pre‐existing autoimmune disease; ipsilateral arterial site closure with the QuickSeal device or manual compression within previous 6 weeks; closure utilising another device within 180 days; pregnant or lactating women; significant bleeding or platelet disorders; platelet count < 100,000; haemoglobin < 10 mg/dL; hematocrit < 30%; blood pressure > 170/100 mmHg |
|
| Interventions |
Intervention 1 : QuickSeal Intervention 2 : manual compression Sheath size : 5 to 8 Fr |
|
| Outcomes |
Primary : time to haemostasis (defined as time between end of the procedure and haemostasis); time to ambulation (defined as time between end of the procedure and participant ambulation > 10 feet); incidence of major complications (vascular repair; bleeding requiring transfusion and/or intervention; infection requiring intravenous antibiotics and/or extended hospitalisation) Secondary : time to discharge; minor complications (haematoma; ecchymosis; bleeding and pseudoaneurysm) Follow‐up : 30 days after discharge |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised" Comment: insufficient information to permit judgement of high or low risk |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but review authors judged that outcomes and outcome measurements are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of high or low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Data on pre‐specified outcomes reported |
| Other bias | Low risk | Study appears to be free from other sources of bias |
ASD: atrial septal defect BMI: body mass index CAG: coronary angiography CFA: common femoral artery INR: international normalised ratio PCI: percutaneous coronary intervention PTCA: percutaneous transluminal coronary angioplasty SD: standard deviation VAS: visual analogue scale VCD: vascular closure device VSD: ventricular septal defect
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baim 2000 | ProStar‐Plus device was used with an 8 Fr or 10 Fr sheath size, but data are not presented separately. We contacted study authors twice but received no response |
| Beyer‐Enke 1996 | Could not confirm with study authors that access for the procedure was attained through the femoral artery |
| Chalmers 2007 | EVICEL is not a vascular closure device |
| Chevalier 2000 | Data on review outcomes were not presented in the study report (abstract) |
| Jean‐Baptiste 2008 | Not a randomised controlled trial |
| Kurşaklioĝlu 2008 | Cannot confirm that this is a randomised controlled trial and that access was attained via the femoral artery |
| Larzon 2015 | Did not use a vascular closure device |
| Leinbudgut 2013 | Participants were randomised by drug received to prevent bleeding rather than by vascular closure device (VCD) |
| Lupi 2012 | Not a randomised controlled trial |
| Neudecker 2003 | Not a randomised controlled trial |
| Ratnam 2007 | Not a randomised controlled trial |
| Slaughter 1995 | Cost‐effectiveness analysis. No relevant data |
| Smilowitz 2012 | Not a randomised controlled trial |
| Starnes 2003 | Arteriography participants who were treated with 7 Fr to 10 Fr sheaths. Data are not presented according to sheath size. Attempts to contact the study author for specific data were unsuccessful |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT01297322.
| Methods |
Study design : randomised open‐label trial Country : United States of America Setting : hospital Number of centres : 21 |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 : VASCADE Vascular Closure System Intervention 2 : manual compression |
| Outcomes |
Primary
Secondary
|
| Notes | This study was published just after the searches were run and will be included in a future update |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12611001248954.
| Trial name or title | ACTRN12611001248954 |
| Methods |
Study design : open‐label randomised multi‐centre controlled trial Country : United States of America Setting : hospital Number of centres : multi‐centre |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 : VASCADE Vascular Closure System Intervention 2 : manual compression |
| Outcomes |
Primary
Secondary
|
| Starting date | December 2011 |
| Contact information | charles_maroney@cardivamedical.com |
| Notes |
CTRI/2014/09/004946.
| Trial name or title | Closure of puncture site in the groin after coronary stenting |
| Methods |
Study design randomised parallel‐group active controlled trial Country : India Setting : hospital Number of centres : 1 |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 : PerClose ProGlide Intervention 2 : manual compression |
| Outcomes |
Primary
Secondary
|
| Starting date | 01/09/2014 |
| Contact information | drssandeep@hotmail.com |
| Notes |
DRKS00000802.
| Trial name or title | VCD trial |
| Methods |
Study design : prospective, randomised clinical trial Country : Germany Setting : hospital Number of centres : multi‐centre |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 : ExoSeal Intervention 2 : Safeguard Intervention 3: manual compression |
| Outcomes |
Primary
Secondary
|
| Starting date | May 2011 |
| Contact information | stefan.köberich at uniklinik‐freiburg.de marc.kollum at hbh‐kliniken.de |
| Notes |
NCT00264264.
| Trial name or title | ACDC trial |
| Methods |
Study design : randomised parallel‐assignment double‐blind trial Country : Canada Setting : hospital Number of centres : 1 |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 : vascular closure device Intervention 2 : direct compression |
| Outcomes |
Primary
Secondary
|
| Starting date | July 2006 |
| Contact information | Asim Cheema, St Michael's Hospital, Toronto |
| Notes |
NCT00428155.
| Trial name or title | ACDC trial II |
| Methods |
Study design : randomised single‐group assignment single‐blind trial Country : Canada Setting : hospital Number of centres : 1 |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : StarClose |
| Outcomes |
Primary
Secondary
|
| Starting date | January 2007 |
| Contact information | Asim Cheema, St Michael's Hospital, Toronto; cheemaa@smh.toronto.on.ca |
| Notes |
NCT01389375.
| Trial name or title | Instrumental sealing of arterial puncture site closure device versus manual compression trial |
| Methods |
Study design : prospective randomised open‐label trial Country : Germany Setting : hospital Number of centres : not stated |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 : ExoSeal Intervention 2 : FemoSeal Intervention 3 : manual compression |
| Outcomes |
Primary
Secondary
|
| Starting date | July 2011 |
| Contact information | Maryam Linhardt, Deutsches Herzzentrum München, Klinik für Herz‐und Kreislauferkrankungen |
| Notes |
NCT01600482.
| Trial name or title | Clinical investigation for safety and efficacy study of CELT ACD arterial closure device |
| Methods |
Study design : randomised multi‐centre open‐label study Countries : United States of America, Germany and Ireland Setting : hospital Number of centres : multi‐centre |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 : CELT ACD Intervention 2 : manual compression |
| Outcomes |
Primary
Secondary
|
| Starting date | May 2012 |
| Contact information | Turi‐Zoltan@CooperHealth.edu scwong@med.cornell.edu michael.laule@charite.de |
| Notes |
NCT01669382.
| Trial name or title | ACCESS |
| Methods |
Study design : randomised multi‐centre single‐blinded trial Country : Germany Setting : hospital Number of centres : multi‐centre |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 : ExoSeal Intervention 2 : AngioSeal |
| Outcomes |
Primary
Secondary
|
| Starting date | January 2012 |
| Contact information | johannes.brachmann@klinikum‐coburg.de harald.rittger@uk‐erlangen.de holger.nef@innere.med.uni‐giessen.de |
| Notes |
NCT02061696.
| Trial name or title | A randomised controlled trial to assess safety and efficacy of AXERA (device name) 2 Access System compared with manual compression |
| Methods |
Study design : randomised parallel‐assignment open‐label Country : United States of America Setting : hospital Number of centres : multi‐centre |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 : AXERA 2 Access System Intervention 2 : manual compression |
| Outcomes |
Primary
Secondary
|
| Starting date | 30 January 2014 |
| Contact information | frank.saltiel@borgess.com vishal.gupta@borgess.com |
| Notes |
NCT02234830.
| Trial name or title | CLOSE‐UP II trial |
| Methods |
Study design : randomised parallel‐assignment open‐label Country : Denmark Setting : hospital Number of centres : multi‐centre |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 : AngioSeal Intervention 2 : ExoSeal |
| Outcomes |
Primary
Secondary
|
| Starting date | December 2012 |
| Contact information | hellbarg@rm.dk niels.holm@clin.au.dk |
| Notes |
NCT02237430.
| Trial name or title | CLOSE‐UP III trial |
| Methods |
Study design : randomised parallel‐assignment open‐label Country : Denmark Setting : hospital Number of centres : multi‐centre |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention 1 : Mynx Intervention 2 : manual compression |
| Outcomes |
Primary
Secondary
|
| Starting date | June 2014 |
| Contact information | niels.holm@clin.au.dk evald.christiansen@dadlnet.dk |
| Notes |
AV: arterio‐venous BARC: Bleeding Academic Research Consortium BMI: body mass index BP: blood pressure CABG: coronary artery bypass graft CAG: coronary angiography CFA: common femoral artery cm: centimetres FFR: fractional flow reserve Hct: haematocrit Hgb: haemoglobin INR: International normalised ratio ITP: idiopathic thrombocytopenic purpura IV: intravenous IVUS: intravascular ultrasound MI: myocardial infarction mm: millimetres NIRS: near infrared spectroscopy NSTEMI: non‐ST elevation myocardial infarction OCT: optical coherence tomography PCI: percutaneous coronary intervention PEG: polyethylene glycol STEMI: ST elevation myocardial infarction VCD: vascular closure device
Contributions of authors
Lindsay Robertson, Alina Andras, Frances Colgan, Ralph Jackson
LR selected studies for inclusion in the review, extracted data, assessed risk of bias for included studies, inputted studies into Review Manager, performed analysis and wrote the review. AA selected studies for inclusion, extracted data, assessed risk of bias, checked data entry and commented on the review. FC provided clinical advice and wrote the review. RJ provided advice on clinical aspects of the review and reviewed the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Programme Grant funding (10/4001/14) to Cochrane Vascular. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
LR: none known. AA: none known. FC: travel/accommodation/meeting expenses: Gore Medical training (product training/aortic intervention, October 2014), Cook Medical training (Vista Education programme) (product training in embolisation, September 2014), Medtronic Aortic University (aortic stent grafting training, 2015), Vascutek (Terumo) (sponsorship for BSIR meeting, November 2014; sponsorship for LINC meeting, January 2015). RJ: none known.
New
References
References to studies included in this review
Amin 2000 {published data only}
- Amin FR, Yousufuddin M, Stbales R, Shamim Q, Al‐Nasser FM, Coats AJS, et al. Femoral haemostasis after transcatheter therapeutic intervention: a prospective randomised study of the AngioSeal device vs. the FemoStop device. International Journal of Cardiology 2000;76:235‐40. [DOI] [PubMed] [Google Scholar]
Amine 1999 {published data only}
- Amine el S, Teiger E, Aptecar E, Dubois‐Randé J‐L, Pernès J‐M, Dupouy P. Suture percutanée du point de ponction artériel fémoral après coronarographie diagnostique. Archives des Malaides du Coeur et des Vaisseaux 1999;92(11):1447‐53. [PubMed] [Google Scholar]
Ansel 2006 {published data only}
- Ansel G, Yakubov S, Neilson C, Allie D, Stoler R, Hall P, et al. Safety and efficacy of staple‐mediated femoral arteriotomy closure: results from a randomised multicentre study. Catheterization and Cardiovascular Interventions 2006;67:546‐53. [DOI] [PubMed] [Google Scholar]
Behan 2007 {published data only}
- Behan MWH, Large JK, Patel NR, Lloyd GW, Sulke AN. A randomised controlled trial comparing the routine use of an AngioSeal STS device strategy with conventional femoral haemostasis methods in a district general hospital. International Journal of Clinical Practice 2007;61(3):367‐72. [DOI] [PubMed] [Google Scholar]
Brachmann 1998 {published data only}
- Brachmann J, Asnsah M, Kosinksi EJ, Schuler GC. Imprved clinical effectiveness with a collagen vascular hemostasis device for shortened immobilization time following diagnostic angiography and percutaneous transluminal coronary angioplasty. The American Journal of Cardiology 1998;81(12):1502‐5. [DOI] [PubMed] [Google Scholar]
Camenzind 1994 {published data only}
- Camenzind E, Grossholz M, Urban P, Dorsaz PA, Didier D, Meier B. Collagen application versus manual compression: a prospective randomized trial for arterial puncture site closure after coronary angioplasty. Journal of the American College of Cardiology 1994;24(3):655‐62. [DOI] [PubMed] [Google Scholar]
Carere 2000 {published data only}
- Carere RG, Webb JG, Buller CEH, Wilson M, Rahman T, Spinelli J, et al. Suture closure of femoral arterial puncture sites after coronary angioplasty followed by same‐day discharge. American Heart Journal 2000;139:52‐8. [DOI] [PubMed] [Google Scholar]
Castañeda 2003 {published data only}
- Castañeda F, Swischuk JL, Smouse B, Brady T. Gelatin sponge closure device versus manual compression after peripheral arterial catheterization procedures. Journal of Vascular and Interventional Radiology 2003;14(12):1517‐23. [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen CP, Huang HP, Hsia CH, Chang YM, Lin LS, Lee CL. Short‐term safety and efficacy of femoral vascular closure after percutaneous coronary intervention with combination of the BoomerangTM device and intravenous Protamine Sulfate. Acta Cardiologica Sinica 2013;29(6):531‐8. [PMC free article] [PubMed] [Google Scholar]
Deuling 2008 {published data only}
- Deuling JHH, Jaarsma T, Vermeulen RP, Heuvel AFM. A randomized controlled trial comparing StarClose and AngioSeal vascular closure devices in a district general hospital. International Journal of Clinical Practice 2008;62(11):1801. [DOI: 10.1111/j.1742-1241.2008.01865.x] [DOI] [PubMed] [Google Scholar]
- Deuling JHH, Vermeulen RP, Anthonio RA, Heuvel AFM, Jaarsma T, Jessurun G, et al. Closure of the femoral artery after cardiac catheterization: a comparison of AngioSeal, StarClose and manual compression. Catheterization and Cardiovascular Interventions 2008;71(4):518‐23. [DOI] [PubMed] [Google Scholar]
Diaz 2001 {published data only}
- Diaz De La Llera LS, Fournier Andray JA. Early deambulation following cardiac catheterization by the use of 6 Fr Angio‐Seal, a new hemostatic percutaneous puncture closure device. Revista Espanola de Cardiologia 2001;54(12):1406‐10. [DOI] [PubMed] [Google Scholar]
Doneaux 2001 {published data only}
- Doneaux P, Gach O, Martinez CH, Legrand V. Femoral puncture closure devices versus manual compression after percutaneous coronary interventions. European Heart Journal 2001;22(Suppl):216. [Google Scholar]
Fargen 2011 {published data only}
- Fargen KM, Hoh BL, Mocco J. A prospective randomized single‐blind trial of patient comfort following vessel closure: extravascular synthetic sealant closure provides less pain than a self‐tightening suture vascular compression device. Journal of Neurointerventional Surgery 2011;3:219‐33. [DOI] [PubMed] [Google Scholar]
- NCT00998023. Patient comfort with vascular closure. http://clinicaltrials gov/show/NCT00998023 (accessed 26 October 2013).
Gerckens 1998 {published data only}
- Gerckens U, Cattelaens N, Lampe EG, Grube E. Management of arterial puncture site after catheterization procedures: evaluating a suture‐mediated closure device. American Journal of Cardiology 1999;83(12):1658‐63. [DOI] [PubMed] [Google Scholar]
- Gerckens U, Cattelaens N, Müller R, Lampe E‐G, Grube E. Perkutaner nahtverschluß von femoralarteriezugängen nach diagnostischen herzkatheterisierungen oder koronarinterventionen. Sicherheit and effekitivtät einer neuen verschlußtechnik arterieller punktionsstellen. Herz 1998;23(1):27‐34. [DOI] [PubMed] [Google Scholar]
Gwechenberger 1997 {published data only}
- Gwechenberger M, Katzenschlager R, Heinz G, Gottsauner‐Wolf M, Probst P. Use of a collagen plug versus manual compression for sealing arterial puncture site after cardiac catheterization. Angiology 1997;48(2):121‐6. [DOI] [PubMed] [Google Scholar]
Hattab 2012 {published data only}
- Hattab M, Hokin M, Carreira VB, Elhadad S. A randomized trial comparing two vascular closure devices: PROGLIDE and the novel EXOSEAL after percutaneous femoral procedures. Journal of the American College of Cardiology 2012;60(17 Suppl B):B112. [Google Scholar]
Hermanides 2010 {published data only}
- Hermanides RS, Ottervanger JP, Dambrink JH, Boer MJ, Hoorntje JC, Gosselink AT, et al. Closure device or manual compression in patients undergoing percutaneous coronary intervention: a randomized comparison. Journal of Invasive Cardiology 2010;22(12):562‐6. [PubMed] [Google Scholar]
Hermiller 2005 {published data only}
- Hermiller J, Simonton C, Hinohara T, Lee D, Cannon L, Mooney M, et al. Clinical experience with a circumferential clip‐based vascular closure device in diagnostic catheterization. Journal of Invasive Cardiology 2005;17(10):504‐10. [PubMed] [Google Scholar]
Hermiller 2006 {published data only}
- DiDonato K, Jaff M, Hermiller J, Simonton C, Hinohara T, Mooney M. 6 month results for the CLIP trial. Transcatheter Cardiovascular Therapeutics. 2006; Vol. Abstract 479.
- Hermiller JB, Simonton C, Hinohara T, Cannon L, Mooney M, O'Shaughnessy C, et al. The StarClose® vascular closure system: interventional results from the CLIP study. Catheterization and Cardiovascular Interventions 2006;68(5):677‐83. [DOI] [PubMed] [Google Scholar]
- Jaff MR, Hadley G, Hermiller JB, Simonton C, Hinohara T, Cannon L, et al. The safety and efficacy of the StarClose Vascular CLosure system: the ultrasound substudy of the CLIP study. Catheterization and Cardiovascular Interventions 2006;68(5):684‐9. [DOI] [PubMed] [Google Scholar]
Holm 2014 {published data only}
- Holm NR, Sindberg B, Schou M, Maeng M, Kaltoft A, Bottcher M, et al. Randomised comparison of manual compression and FemoSeal vascular closure device for closure after femoral artery access coronary angiography: the CLOSure dEvices Used in everyday Practice (CLOSE‐UP) study. Eurointervention 2014;10(2):183‐90. [DOI] [PubMed] [Google Scholar]
- Sindberg B, Schou M, Hansen L, Christiansen KJ, Jørgensen KS, Søloft M, et al. Pain and discomfort in closure of femoral access coronary angiography. The CLOSuredEvices Used in everyday Practice (CLOSE‐UP) pain substudy. European Journal of Cardiovascular Nursing 2014;13(3):221‐6. [DOI] [PubMed] [Google Scholar]
Jensen 2008 {published data only}
- Jensen J, Saleh N, Jensen U, Svane B, Jönsson A, Tornvall P. The inflammatory response to femoral arterial closure devices: a randomized comparison among FemoStop, AngioSeal, and PerClose. Cardiovascular and Interventional Radiology 2008;31(4):751‐5. [DOI] [PubMed] [Google Scholar]
Juergens 2004 {published data only}
- Juergens CP, Leung DYC, Crozier JA, Wong AM, Robinson JTC, Lo S, et al. Patient tolerance and resource utilisation associated with an arterial closure versus an external compression device after percutaneous coronary intervention. Catheterization and Cardiovascular Interventions 2004;63:166‐70. [DOI] [PubMed] [Google Scholar]
Kalsch 2008 {published data only}
- Kalsch HIM, Eggebrecht H, Mayringer S, Konorza T, Sievers B, Sack S, et al. Randomized comparison of effects of suture‐based and collagen‐based vascular closure devices on post‐procedural leg perfusion. Clinical Research in Cardiology 2008;97(1):43‐8. [DOI] [PubMed] [Google Scholar]
Kussmaul 1995 {published data only}
- Kussmaul WG, Buchbinder M, Whitlow PL, Heuser RR, King SB, Kent KM, et al. Rapid arterial hemostasis and decreased access site complications after cardiac catheterization and angioplasty: results of a randomized trial of a novel hemostatic device. Journal of the American College of Cardiology 1995;25(7):1685‐92. [DOI] [PubMed] [Google Scholar]
Legrand 2005 {published data only}
- Legrand V, Doneux P, Martinez C, Gach O, Bellekens M. Femoral access management: comparison between two different vascular closure devices after percutaneous coronary intervention. Acta Cardiologica 2005;60(5):482‐8. [DOI] [PubMed] [Google Scholar]
Machnik 2012 {published data only}
- Machnik R, Pleniążek, Musiałek P, Przewłocki T, Tekieli Ł, Trystuła M, et al. Control of local haemostasis with the AngioSeal® vascular closure device in peripheral endovascular interventions via 6‐9 F femoral artery access. Postępy w Kardiologii Interwencyjnej 2012;8(1):1‐7. [Google Scholar]
Magosaki 1999 {published data only}
- Magosaki N, Hosoda S, Kawagishi N, Yamaguchi T, Sakatani H, Haze K, et al. Efficacy of hemostatic puncture closing device for hemostasis and early ambulation after coronary angiography and angioplasty: results of a multicentre trial. Journal of Cardiology 1999;34:131‐8. [PubMed] [Google Scholar]
Martin 2008 {published data only}
- Martin JL, Pratsos A, Magargee E, Mayhew K, Pensyl C, Nunn M, et al. A randomized trial comparing compression, PerClose ProGlideTM and AngioSeal VIPTM for arterial closure following percutaneous coronary intervention: the CAP trial. Catheterization and Cardiovascular Interventions 2008;71(1):1‐5. [DOI] [PubMed] [Google Scholar]
Michalis 2002 {published data only}
- Michalis LK, Rees MR, Patsouras D, Katsouras CS, Goudevenos J, Pappas S, et al. A prospective randomized trial comparing the safety and efficacy of three commercially available closure devices (AngioSeal, VasoSeal and Duett). Cardiovascular and Interventional Radiology 2002;25:423‐9. [DOI] [PubMed] [Google Scholar]
Nelson 2014 {published data only}
- Nelson PR, Kracjer MSZ, Kansal N, Rao V. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). Journal of Vascular Surgery 2014;59:1181‐94. [DOI] [PubMed] [Google Scholar]
Noguchi 2000 {published data only}
- Noguchi T, Miyazaki S, Yasuda S, Baba T, Sumida H, Morii I, et al. A randomised controlled trial of ProStar‐Plus for haemostasis in patients after coronary angioplasty. European Journal of Vascular and Endovascular Surgery 2000;19(5):451‐5. [DOI] [PubMed] [Google Scholar]
Park 2005 {published data only}
- Park Y, Roh HG, Choo SW, Lee SH, Shin SW, Do YS, et al. Prospective comparison of collagen plug (AngioSealTM) and suture‐mediated (the Closure STM) closure devices at femoral access sites. Korean Journal of Radiology 2005;6:248‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Perlowski 2011 {published data only}
- Perlowski A, Jaff M, O'Shaughnessy D, Brueggeman C, Rosenfield K. StarClose device closure of femoral arteriotomy site versus manual compression in patients with peripheral arterial disease: the CLIPIT trial. Journal of the American College of Cardiology 2011;57(14(Suppl 1)):E1982. [Google Scholar]
Rastan 2008 {published data only}
- Rastan A, Sixt S, Schwarzwälder U, Schwarz T, Frank U, Bürgelin K, et al. VIPER‐2: a prospective, randomized single‐centre comparison of 2 different closure devices with a hemostatic wound dressing for closure of femoral artery access sites. Journal of Endovascular Therapy 2008;15:83‐90. [DOI] [PubMed] [Google Scholar]
Reddy 2004 {published data only}
- Reddy BK, Brewster PS, Walsh T, Burket MW, Thomas WJ, Cooper CJ. Randomized comparison of rapid ambulation using radial, 4 French femoral access, or femoral access with AngioSeal closure. Catheterization and Cardiovascular Interventions 2004;62:143‐9. [DOI] [PubMed] [Google Scholar]
Rickli 2002 {published data only}
- Rickli H, Unterweger M, Sütsch G, Brunner‐La Rocca HP, Sagmeister M, Ammann P, et al. Comparison of costs and safety of a suture‐mediated closure device with conventional manual compression after coronary artery interventions. Catheterization and Cardiovascular Interventions 2002;57(3):297‐302. [DOI] [PubMed] [Google Scholar]
Sanborn 1993 {published data only}
- Sanborn TA, Gibbs HH, Brinker JA, Knopf WD, Kosinski EK, Roubin GS. A multicentre randomised trial comparing a percutaneous collagen haemostasis device with conventional manual compression after diagnostic angiography and angioplasty. Journal of the American College of Cardiology 1993;22(5):1273‐9. [DOI] [PubMed] [Google Scholar]
Schräder 1992 {published data only}
- Schräder R, Steinbacher ST, Kaltenbach M. Randomisierter vergleich zwischen kollagenpplikation und druckverband zum verschluß der arteriellen punktionsstelle nach koronarangiographie und koronardilatation. Zeitschrift fűr Kardiologie 1992;81:507‐11. [PubMed] [Google Scholar]
Schulz‐Schüpke 2014 {published data only}
- Schulz‐Schüpke S, Helde S, Gewalt S, Ibrahim T, LinhardtM, Haas K, et al. Comparison of vascular closure devices vs manual compression after femoral artery puncture. The ISAR‐CLOSURE randomized clinical trial. Journal of the American Medical Association 2014;312(19):1981‐7. [DOI] [PubMed] [Google Scholar]
SEAL Trial Study Team {published data only}
- The SEAL Trial Study Team. Assessment of the safety and efficacy of the DUETT vascular hemostasis device: final results of the Safe and Effective Vascular Hemostasis (SEAL) trial. American Heart Journal 2002;143(4):612‐9. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Mahoney EM, Gershony G, Ellis S, Saucedo JF, Talley JD, et al. Impact of the Duett sealing device on quality of life and hospitalization costs for coronary diagnostic and interventional procedures: results from the Study of Economic and Quality of Life substudy of the SEAL trial. American Heart Journal 2001;142:982‐8. [DOI] [PubMed] [Google Scholar]
Seidelin 1997 {published data only}
- Seidelin PH, Adelman AG. Mobilization within thirty minutes of elective diagnostic coronary angiography: a feasibility study using a hemostatic femoral puncture closure device. Journal of Interventional Cardiology 1997;10:409‐15. [Google Scholar]
Shammas 2002 {published data only}
- Shammas NW, Rajendran VR, Alldredge SG, Witcik WJ, Robken JA, Lewis JR, et al. Randomized comparison of VasoSeal and AngioSeal closure devices in patients undergoing coronary angiography and angioplasty. Catheterization and Cardiovascular Interventions 2002;55:421‐5. [DOI] [PubMed] [Google Scholar]
Silber 1998 {published data only}
- Silber S, Björvik A, Mühling H, Rösch A. Usefulness of collagen plugging with VasoSeal® after PTCA as compared to manual compression with identical sheath dwell times. Catheterization and Cardiovascular Diagnosis 1998;43:421‐7. [DOI] [PubMed] [Google Scholar]
Sun 2009 {published data only}
- Sun JJ, Zhang HT, Huang CC, Luo HL, Wang JH, Tan WJ, et al. Three kinds of vascular closure devices StarClose, PerClose and Boomerang in the femoral artery hemostasis applications. Journal of Clinical Rehabilitative Tissue Engineering Research 2009;13(13):2485‐90. [Google Scholar]
Tron 2003 {published data only}
- Tron C, Koning R, Eltchaninoff H, Douillet R, Chassaing S, Sanchez‐Giron C, et al. A randomized comparison of a percutaneous suture device versus manual compression for femoral artery hemostasis after PTCA. Journal of Interventional Cardiology 2003;16(3):217‐21. [DOI] [PubMed] [Google Scholar]
Upponi 2007 {published data only}
- Upponi SS, Ganeshan AG, Warakaulle DR, Phillips‐Hughes J, Boardman P, Uberoi R. AngioSeal versus manual compression for haemostasis following peripheral vascular diagnostic and interventional procedures ‐ A randomized controlled trial. European Journal of Radiology 2007;61(2):332‐4. [DOI] [PubMed] [Google Scholar]
Veasey 2008 {published data only}
- Veasey RA, Large JK, Silberbauer J, Paul G, Taggu Wm Ellery S, Rathore VS, et al. A randomised controlled trial comparing StarClose and AngioSeal vascular closure devices in a district general hospital. International Journal of Clinical Practice 2008;62(6):912‐8. [DOI] [PubMed] [Google Scholar]
von Hoch 1995 {published data only}
- Hoch F, Neumann F‐J, Theiss W, Kastrati A, Schömig A. Efficacy and safety of collagen implants for haemostasis of the vascular access site after coronary balloon angioplasty and coronary stent implantation: a randomized study. European Heart Journal 1995;16(5):640‐6. [DOI] [PubMed] [Google Scholar]
Ward 1998 {published data only}
- Ward SR, Casale P, Raymond R, Kussmaul WG, Simpfendorfer C for the Angio‐Seal Investigators. Efficacy and safety of a hemostatic puncture closure device with early ambulation after coronary angiography. American Journal of Cardiology 1998;81:569‐72. [DOI] [PubMed] [Google Scholar]
Wetter 2000 {published data only}
- Wetter DR, Rickli H, Smekal A, Amann FW. Early sheath removal after coronary artery interventions with use of a suture‐mediated closure device: clinical outcome and results of Doppler US evaluation. Journal of Vascular and Interventional Radiology 2000;11:1033‐7. [DOI] [PubMed] [Google Scholar]
Wong 2009 {published data only}
- Wong SC, Bachinsky W, Cambier P, Stoler R, Aji J, Rogers JH, et al. A randomized comparison of a novel bioabsorbable vascular closure device versus manual compression in the achievement of hemostasis after percutaneous femoral procedures. Journal of the American College of Cardiology: Cardiovascular Interventions 2009;2(8):785‐93. [DOI] [PubMed] [Google Scholar]
Yadav 2003 {published data only}
- Yadav JS, Ziada KM, Almany S, Davis TP, Castaneda F. Comparisons of the QuickSeal femoral arterial closure system with manual compression following diagnostic and interventional catheterization procedures. American Journal of Cardiology 2003;91:1463‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baim 2000 {published data only}
- Baim DS, Knopf WD, Hinohara T, Schwarten DE, Schatz RA, Pinkerton CA, et al. Suture‐mediated closure of the femoral access site after cardiac catheterization: results of the Suture To Ambulate aNd Discharge (STAND I and STAND II) trials. American Journal of Cardiology 2000;85(7):864‐9. [DOI] [PubMed] [Google Scholar]
Beyer‐Enke 1996 {published data only}
- Beyer‐Enke SA, Soldner J, Zeitler E. Immediate sealing of arterial puncture site following femoropopliteal angioplasty: a prospective randomized trial. Cardiovascular and Interventional Radiology 1996;19:406‐10. [DOI] [PubMed] [Google Scholar]
Chalmers 2007 {published data only}
- Chalmers R, Clement Darling R, Wingard JT, Chetter I, Cutler B, Kern JA, FIBRIN SEALANT PV Investigator Group. A prospective, randomized controlled trial comparing the effects of a fibrin sealant (EVICEL) versus manual compression on hemostatic effectiveness during vascular surgical procedures utilizing polytetrafluorethylene graft material. Transfusion 2007;47(11(Suppl)):22A. [Google Scholar]
Chevalier 2000 {published data only}
- Chevalier B, Henry M, Pillière R, Koning R, Lancelin B, Puel J, et al. Duplex scan assessment of femoral access for coronary stenting: compression versus AngioSeal comparison in the HEMOSTASE trial. European Heart Journal 2000;21(Abstract Supplement 642):3522. [Google Scholar]
- Chevalier B, Lancelin B, Koning R, Henry M, Gommeaux A, Pilliere R, et al. Effect of a closure device on complication rates in high‐local‐risk patients: results of a randomized multicenter trial. Catheterization and Cardiovascular Interventions 2003;58(3):285‐91. [DOI] [PubMed] [Google Scholar]
Jean‐Baptiste 2008 {published data only}
- Jean‐Baptiste E, Hassen‐Khodja R, Haudebourg P, Bouillanne PJ, Declemy S, Batt M. Percutaneous closure devices for endovascular repair of infrarenal abdominal aortic aneurysms: a prospective, non‐randomized comparative study. European Journal of Vascular and Endovascular Surgery 2008;35(4):422‐8. [DOI] [PubMed] [Google Scholar]
Kurşaklioĝlu 2008 {published data only}
- Kurşaklioĝlu H, Iyisoy A, Barҫin C, Celik T, Nitzan R, Köse S, et al. The experience with the Epiclose‐T vascular access closure device: a human study. Anadolu Kardiyoloju Dergisi 2008;8(1):38‐42. [PubMed] [Google Scholar]
Larzon 2015 {published data only}
- Larzon T, Roos H, Gruber G, Henrikson O, Magnuson A, Falkenberg M. A randomised controlled trial of the fascia suture technique compared with a suture‐mediated closure device for femoral arterial closure after endovascular aortic repair. European Jouranl of Vascular and Endovascular Surgery 2015;49(2):166‐73. [DOI] [PubMed] [Google Scholar]
Leinbudgut 2013 {published data only}
- Leibundgut G, Pache J, Schulz S, Berger PB, Ferenc M, Gick M, et al. Collagen plug vascular closure devices and reduced risk of bleeding with bivalirudin versus heparin plus abciximab in patients undergoing percutaneous coronary intervention for non ST‐segment elevation myocardial infarction. Journal of Interventional Cardiology 2013;26(6):623‐9. [DOI] [PubMed] [Google Scholar]
Lupi 2012 {published data only}
- Lupi A, Rognoni A, Secco CG, Lazzero M, Plebani L, Cossa G. Different spectrum of vascular complications after AngioSeal deployment of manual compression. Journal of Invasive Cardiology 2012;24(3):90‐6. [PubMed] [Google Scholar]
Neudecker 2003 {published data only}
- Neudecker A, Manke C, Lenhart M, Zorger N, Paetzel C, Feurbach S, et al. Evaluation of a haemostatic device with percutaneous collagen application (VasoSeal) compared to a mechanical compression system (Compressar) after transfemoral catheterization of patients suffering from arterial occlusive disease [Evaluation eines Verschlusssystems mit perkutaner Kollageneinbringung (VasoSeal) im Vergleich zu einem mechanischen Kompressionssystem (Compressar) nach Femoralispunktion bei Patienten mit AVK]. Rofo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2003;175(5):676‐81. [DOI] [PubMed] [Google Scholar]
Ratnam 2007 {published data only}
- Ratnam LA, Raja J, Munneke GJ, Morgan RA, Belli AM. Prospective non‐randomized trial of manual compression and AngioSeal and StarClose arterial closure devices in common femoral punctures. Cardiovascular and Interventional Radiology 2007;30(2):182‐8. [DOI] [PubMed] [Google Scholar]
Slaughter 1995 {published data only}
- Slaughter PM, Chetty R, Flintoft VF, Lewis S, Sykora K, Beattie DM, et al. A single center randomized trial assessing use of a vascular hemostasis device vs. conventional manual compression following PTCA: what are the potential resource savings?. Catheterization and Cardiovascular Diagnosis 1995;34(3):210‐4. [DOI] [PubMed] [Google Scholar]
Smilowitz 2012 {published data only}
- Smilowitz NR, Kirtane AJ, Guiry M, Gray WA, Dolcimascolo P, Querijero M, et al. Practices and complications of vascular closure devices and manual compression in patients undergoing elective transfemoral coronary procedures. American Journal of Cardiology 2012;110(2):177‐82. [DOI] [PubMed] [Google Scholar]
Starnes 2003 {published data only}
- Starnes BW, O'Donnell SD, Gillespie DL, Goff JM, Rosa P, Parker MV, et al. Percutaneous arterial closure in peripheral vascular disease: a prospective randomized evaluation of the PerClose device. Journal of Vascular Surgery 2003;38(2):263‐71. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01297322 {published data only}
- NCT01297322. RESPECT Trial ‐ (Rapid Extravascular Sealing Via PercutanEous Collagen ImplanT). http://clinicaltrials.gov/showNCT01297322 (accessed 26 October 2013).
References to ongoing studies
ACTRN12611001248954 {published data only}
- ACTRN12611001248954. Femoral artery closure using the Cardiva VASCADE Vascular Closure System (VCS) to reduce time to hemostasis and reduce time to ambulation and hospital discharge versus manual compression in patients who have undergone diagnostic or interventional endovascular catheterization procedures utilizing 6 Fr or 7 Fr procedural sheaths. http://www anzctr org au/ACTRN12611001248954 (accessed 26 October 2013).
CTRI/2014/09/004946 {published data only}
- CTRI/2014/09/004946. Impact of femoral access site closure after percutaneous coronary interventions. A comparison between manual compression and the use of vascular closure device (PerClose ProGlide). http://apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2014/09/004946 (accessed 27 May 2015).
DRKS00000802 {published data only}
- DRKS00000802. A prospective randomized trial to evaluate the complication rate and patient comfort using three different vascular closure strategies following coronary angiography. http://drks‐neu.uniklinik‐freiburg.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00000802 (accessed 26 October 2013).
NCT00264264 {published data only}
- NCT00264264. Arterial closure vs direct compression for hemostasis after PCI ‐ ACDC Trial. http://clinicaltrials.gov/show/NCT00264264 (accessed 25 October 2013).
NCT00428155 {published data only}
- NCT00428155. Arterial Closure Device Comparison Trial II ‐ ACDC Trial II. http://clinicaltrials.gov/show/NCT00428155 (accessed 25 October 2103).
NCT01389375 {published data only}
- NCT01389375. Instrumental sealing of arterial puncture site closure device versus manual compression trial (ISAR‐CLOSURE). http://clinicaltrials.gov/show/NCT01389375 (accessed 26 October 2013).
NCT01600482 {published data only}
- NCT01600482. Clinical investigation for safety and efficacy study of CELT ACD arterial closure device. http://clinicaltrials.gov/show/NCT01600482 (accessed 26 October 2013).
NCT01669382 {published data only}
- NCT01669382. AngioSeal® vs. ExoSeal® for closure of arterial puncture sites. http://clinicaltrials.gov/show/NCT01669382 (accessed 26 October 2013).
NCT02061696 {published data only}
- NCT02061696. A randomized controlled trial to assess safety and efficacy of AXERA (device name) 2 Access System compared to manual compression. https://clinicaltrials.gov/ct2/show/NCT02061696 (accessed 27 May 2015).
NCT02234830 {published data only}
- NCT02234830. Randomized comparison of ExoSeal® and AngioSeal vascular closure devices: the CLOSE‐UP II trial. https://clinicaltrials.gov/ct2/show/NCT02234830 (accessed 27 May 2015).
NCT02237430 {published data only}
- NCT02237430. Randomized comparison of MynxGrip vascular closure device and manual compression for closure after femoral access angiography. The Closure Devices Used in Every Day Practice Study, CLOSE‐UP III Trial. https://clinicaltrials.gov/ct2/show/NCT02237430 (accessed 27 May 2015). [DOI] [PMC free article] [PubMed]
Additional references
Bechara 2010
- Bechara CF, Annambhotla S, Lin PH. Access site management with vascular closure devices for percutaneous transarterial procedures. Journal of Vascular Surgery 2010;52(6):1682‐96. [DOI] [PubMed] [Google Scholar]
Biancari 2010
- Biancari F, D'Andrea V, Marco C, Savino G, Tiozzo V, Catania A. Meta‐analysis of randomized trials on the efficacy of vascular closure devices after diagnostic angiography and angioplasty. American Heart Journal 2010;159:518‐31. [DOI] [PubMed] [Google Scholar]
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jiang 2015
- Jiang J, Zou J, Ma H, Jiao Y, Yang H, Zhang X, et al. Network meta‐analysis of randomized trials on the safety of vascular closure devices for femoral arterial puncture site haemostasis. Scientific Reports 2015;5:13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koreny 2004
- Koreny M, Riedmüller E, Nikfardjam M, Siostrzonek P, Müllener M. Arterial puncture closing devices compared with standard manual compression after cardiac catheterization. Systematic review and meta‐analysis. Journal of American Medicine Association 2004;291(3):350‐7. [DOI] [PubMed] [Google Scholar]
Kurşaklioğlu 2008
- Kurşaklioğlu H, Iyisoy A, Barçin C, Celik T, Nitzan R, Köse S, et al. The experience with the Epiclose‐T vascular access closure device: a human study. Anadolu Kardiyoloji Dergisi ‐ The Anatolian Journal of Cardiology 2008;8(1):38‐42. [PubMed] [Google Scholar]
Merriweather 2012
- Merriweather N, Sulzbach‐Hoke LM. Managing risk of complications at femoral vascular access sites in percutaneous coronary intervention. Critical Care Nurse 2012;32(5):16‐30. [DOI] [PubMed] [Google Scholar]
Nikolsky 2004
- Nikolsky E, Mehran R, Halkin A, Aymong ED, Mintz GS, Lasic Z, et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures: a meta‐analysis. Journal of the American College of Cardiology 2004;44(6):1200‐9. [DOI] [PubMed] [Google Scholar]
Scheinert 2007
- Scheinert D, Sievert H, Turco MA, Schmidt A, Hauptmann KE, Mueller R, et al. The safety and efficacy of an extravascular, water‐soluble sealant for vascular closure: initial clinical results for Mynx. Catheterization and Cardiovascular Interventions 2007;70(5):627‐33. [DOI] [PubMed] [Google Scholar]
Schwartz 2010
- Schwartz BG, Burstein S, Economides C, Kloner RA, Shavelle DM, Mayeda GS. Review of vascular closure devices. Journal of Invasive Cardiology 2010;22(12):599‐607. [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54(10):1046‐55. [DOI] [PubMed] [Google Scholar]
Zahn 1997
- Zahn R, Fromm E, Thoma S, Lotter R, Zander M, Wagner S. Local venous thrombosis after cardiac catheterization. Angiology 1997;48:1‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hsu 2011
- Hsu CCT, Kwan GNC, Rophael JA, Anthony C, Driel ML. Vascular closure devices for femoral arterial puncture site haemostasis. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009541] [DOI] [PMC free article] [PubMed] [Google Scholar]


