This nonrandomized controlled trial assesses the long-term survival and prognosis in patients who undergo liver transplant for nonresectable liver metastases resulting from colorectal cancer.
Key Points
Question
Can liver transplant provide long-term survival and possible cure in patients with colorectal cancer with nonresectable liver metastases and extensive tumor load?
Finding
In this nonrandomized controlled trial of 61 patients who underwent liver transplant, based on various clinical predictive factors, a 10-year overall survival of 80% or more may be achieved in selected cases.
Meaning
Findings suggest that patients with nonresectable colorectal liver metastases have low probability of long-term survival when treated by conventional regimens; highly selected patients with colorectal cancer may obtain similar long-term survival after liver transplant as patients with other malignant and nonmalignant diagnoses who currently receive liver transplant as standard-of-care treatment.
Abstract
Importance
Liver transplant for colorectal cancer with liver metastases was abandoned in the 1990s due to poor overall survival. From 2006, liver transplant for in nonresectable colorectal liver metastases has been reexamined through different prospective trials.
Objective
To determine predictive factors for transplant long-term survival and cure after liver transplant.
Design, Setting, and Participants
This was a prospective, nonrandomized controlled cohort study derived from different clinical trials on liver transplant for colorectal liver metastases from 2006 to 2020 at Oslo University Hospital. The trials differed in prognostic inclusion criteria, but the design was otherwise identical regarding follow-up scheme to determine disease recurrence, overall survival, and survival after relapse. Final data analysis was performed on December 31, 2021. All patients with colorectal liver metastases from comparable prospective liver transplant studies were included.
Exposure
Liver transplant.
Main outcomes and measures
Disease-free survival, overall survival, and survival time after recurrence were determined in all participants.
Results
A total of 61 patients (median [range] age, 57.8 [28.7-71.1] years; 35 male [57.4%]) underwent liver transplant at Oslo University Hospital. Posttransplant observation time ranged from 16 to 165 months, and no patient was lost to follow-up. Median disease-free period, overall survival, and survival after relapse were 11.8 (95% CI, 9.3-14.2) months, 60.3 (95% CI, 44.3-76.4) months, and 37.1 (95% CI, 4.6-69.5) months, respectively. Negative predictive factors for overall survival included the following: largest tumor size greater than 5.5 cm (median OS, 25.3 months; 95% CI, 15.8-34.8 months; P <.001), progressive disease while receiving chemotherapy (median OS, 39.8 months; 95% CI, 28.8-50.7 months; P = .02), plasma carcinoembryonic antigen values greater than 80 μg/L (median OS, 26.6 months; 95% CI, 22.7-30.6 months; P <.001), liver metabolic tumor volume on positron emission tomography of greater than 70 cm3 (26.6 months; 95% CI, 11.8-41.5 months; P <.001), primary tumor in the ascending colon (17.9 months; 95% CI, 0-37.5 months; P <.001), tumor burden score of 9 or higher (23.3 months; 95% CI, 19.2-27.4 months; P = .02), and 9 or more liver lesions (42.5 months; 95% CI, 17.2-67.8 months; P = .02). An Oslo score of 0 or Fong Clinical Risk Score of 1 yielded 10-year survival of 88.9% and 80.0%, respectively.
Conclusions and relevance
Results of this nonrandomized controlled trial suggest that selected patients with liver-only metastases and favorable pretransplant prognostic scoring had long-term survival comparable with conventional indications for liver transplant, thus providing a potential curative treatment option in patients otherwise offered only palliative care.
Introduction
Colorectal cancer (CRC) is one of the most frequent malignancies in Western countries.1 Many patients have metastatic disease at time of diagnosis, and other patients develop metastatic disease after treatment of the primary tumor. The liver is the most common metastatic site.2 Liver resection is considered the only curative treatment for liver metastases. However, only approximately 20% of the patients with liver metastases are candidates for liver resection due to location, size or number of lesions, or extrahepatic disease.2 Five-year overall survival (OS) after liver resection is 30% to 50% in most studies.2,3 The treatment option for the majority of patients with CRC and liver metastases is palliative chemotherapy with a reported median overall survival (OS) of approximately 24 to 30 months and 5-year OS of 10%.4,5,6
Liver transplant (LT) is standard of care for patients with end-stage liver failure and for selected patients with hepatocellular carcinoma within the Milan criteria as well as selected patients with low-grade neuroendocrine tumors.7,8 The OS at 5 years for these patient groups often ranges from 63% to 75%.7,8,9,10 Similarly, LT for hilar cholangiocarcinoma according to the Mayo protocol is accepted as a valid indication in many countries.11,12 More recently, experimental LT for intrahepatic cholangiocarcinoma has been reported with a 5-year OS of approximately 50%.13
LT for patients with CRC and liver metastases was explored in the 1990s, but due to dismal long-term survival with 5-year OS of less than 20% and donor organ shortage, LT in these patients was abandoned.14 However, some long-term survivors were observed.15 In 2006, we started the pilot study Secondary Cancer I (SECA-I), where patients with CRC and metastases confined to the liver received LT (Supplement 1). The study examined LT in patients with CRC who had received modern chemotherapy before LT. We first reported results from the study when the first patient included in the SECA-I study had been observed for 5 years with an estimated Kaplan-Meier 5-year OS of 60%.16 The following factors were associated with inferior survival after LT in the SECA-I study: largest CRC liver metastasis greater than 5.5 cm, progressive disease while receiving chemotherapy at the time of LT, plasma carcinoembryonic antigen (CEA) levels greater than 80 μg/L (to convert CEA to nanograms per milliliter, divide by 1), and less than 2 years from resection of the primary tumor to LT.16
In the present study, we reported the OS, disease-free survival (DFS), and OS from time of relapse in patients with CRC who received liver transplants, and we reported prognostic factors in patients receiving a LT at Oslo University Hospital from November 2006 to November 2020. All patients listed for LT in different prospective studies are included.
Methods
All prospective studies had received approval from the regional ethics committee (South-Estern Health Region) and institutional review board of Oslo University Hospital, and all patients had signed an informed consent before inclusion. Patients with CRC who were on the waiting list for organ transplant underwent LT at Oslo University Hospital between November 2006 and November 2020. Patient follow-up ended on December 31, 2021. Data on race and ethnicity was not collected. It is not common to register such data on patients participating in studies in Norway. The reverse Kaplan-Meier method was used to estimate the median follow-up of patients included in the prospective studies and still alive. This study followed Transparent Reporting of Evaluations With Nonrandomized Designs (TREND) reporting guidelines.
The clinical trials from which this nonrandomized controlled trial is derived include SECA-I, SECA-II (arm A, B, C and D), and the Resection and Partial Liver Segment 2/3 Transplantation With Delayed Total Hepatectomy (RAPID) study. In the RAPID study, liver segments 1 to 3 were resected, and the patient received only donor liver segments 2 and 3. After 3 weeks, the donor segments had increased in volume, and patient liver segments 4 to 8 were resected. The inclusion and exclusion criteria and follow-up for the different LT trials as well as the specific immunosuppression used in the various studies have been previously reported.17 None of the patients received adjuvant chemotherapy after LT. Treatment and further follow-up at time of relapse were at the discretion of the responsible physician.
Fluorine-18 fluorodeoxyglucose positron emission tomography (PET) in combination with computed tomography (CT) was performed before the transplant procedure in all patients to exclude extrahepatic metastases and to determine the metabolic tumor volume (MTV) for all liver metastases as previously described.18
DFS was calculated as time from LT to suspected metastatic lesions or local relapse described by CT, magnetic resonance imaging, or PET-CT scans. OS was calculated from the date of LT to death or end of follow-up, which was December 31, 2021. Survival from time of relapse was calculated as OS minus DFS in patients with recurrent disease.
Risk stratification of the patients was performed using the Fong Clinical Risk Score (FCRS). Patients were given a score of 0 to 5 with 1 point for less than 12 months from diagnosis of the primary tumor to liver metastases, size of largest lesion greater than 5 cm, more than 1 lesion, plasma CEA level greater than 200 μg/L, and positive lymph node in the primary tumor.19 Risk stratification was also performed using the Oslo score, where the patients had a score of 0 to 4 based on the following factors: progressive disease while receiving chemotherapy at time of organ transplant, plasma CEA level greater than 80 μg/L, size of largest liver lesion greater than or equal to 5.5 cm, and time for resection of the primary tumor to LT less than 2 years.16 The Oslo score was originally based on the first 21 patients included in the SECA-I study and verified in the remaining patients. A cutoff value for PET MTV of 70 cm3 was established based on all patients in the SECA-I study and verified in the remaining patients. The prognostic significance of the Oslo score, PET-MTV value, and FCRS was determined in the total cohort.
Statistical Analyses
Survival analyses were estimated using the Kaplan-Meier method, and patient follow-up was calculated using the reverse Kaplan-Meier method. The log-rank test was used to compare outcomes between 2 groups. The difference between the median values of the groups was calculated by Mann-Whitney U test. A 2-sided P value < .05 was considered statistically significant for all tests. The analyses were performed using SPSS Statistics for Windows, version 28.0 (IBM Corp).
Results
A total of 72 patients with CRC were on the waiting list for LT, and 61 patients (median [range] age, 57.8 [28.7-71.1] years; 35 male [57.4%]; 26 female [42.6%]) underwent LT at Oslo University Hospital between November 2006 and November 2020 (eFigure 1 in Supplement 2). Of the initial 72 patients on the waiting list, 11 did not receive an LT for the following reasons: 7 due to metastatic disease determined by frozen section examination of hilar lymph nodes at the time of laparotomy; 3 due to the development of extrahepatic disease while on the waiting list, and 1 due to reduced performance status (Eastern Cooperative Oncology Group grade 3). Median OS and 5-year OS for all 72 patients accepted for the waiting list were 50.8 (95% CI, 32.2-69.4) months and 42.6%, respectively, when calculated from time of LT, laparotomy, and date of acceptance for the waiting list (eFigure 2 in Supplement 2).
Of the 61 patients who underwent LT, 60 patients had nonresectable metastases confined to the liver, and 1 patient was considered to have technically resectable disease; this patient was randomly assigned to the transplant group. DFS and OS were calculated by the Kaplan-Meier method for all 61 patients. One patient included in the prospective study, RAPID, died of complications 1.4 months after LT. This patient is excluded from analyses other than DFS and OS, thereby reducing the total number of patients for the various factors for long-term survival analyses to 60. The numbers of patients included in the different categories are given in eTable 1 in Supplement 2. Baseline characteristics are given in Table 1. Median follow-up of patients included in the prospective studies and still alive was 91.6 (95% CI, 84.9-98.4) months.
Table 1. Baseline Characteristics and Previous Treatments (n = 61).
| Characteristic | No. (%) |
|---|---|
| Age at LT, median (range), y | 57.8 (28.7-71.1) |
| Sex | |
| Female | 26 (42.6) |
| Male | 35 (57.4) |
| Treatment before resection of primary | |
| No treatment | 37 (60.7) |
| Chemotherapy | 14 (23.0) |
| Chemotherapy and radiation therapy | 8 (13.1) |
| Radiation therapy | 2 (3.3) |
| Primary | |
| (y)pT0 | 3 (4.9) |
| (y)pT1 | 1 (1.6) |
| (y)pT2 | 7 (11.5) |
| (y)pT3 | 46 (75.4) |
| (y)pT4 | 4 (6.6) |
| (y)pN0 | 24 (39.3) |
| (y)pN1 | 20 (32.8) |
| (y)pN2 | 17 (27.9) |
| Location of primary | |
| Ascending colon | 10 (16.4) |
| Colon transversum | 3 (4.9) |
| Left colon | 7 (11.5) |
| Sigmoideum | 20 (32.8) |
| Rectum | 21 (34.4) |
| Chemotherapy given before LT | |
| 5-FU | 61 (100) |
| Irinotecan | 51 (83.6) |
| Oxaliplatin | 47 (77.0) |
| EGFR-antibody | 26 (42.6) |
| Bevacizumab | 24 (39.3) |
| Tas-102 | 1 (1.6) |
| KRAS gene variant/wild type (n = 58) | 15 (25.9)/43 (74.1) |
| CEA at LT, median (range), μg/L | 6.0 (1-4346) |
| Other treatment before LT | |
| Liver resection (yes/no), No. of patients | 12 (19.7)/49 (80.3) |
| RFA (yes/no), No. of patients | 7 (11.5)/54 (88.5) |
| FCRS at LT, median (range) | 3 (1-5) |
| FCRS (1/2/3/4/5) | 5/16/25/10/5 |
| Oslo Score at LT, median (range) | 1 (0-4) |
| Oslo Score at LT (0/1/2/3/4) | 10/27/11/6/7 |
| MTV, median (range), cm3 | 21.3 (0-874) |
| Synchronous/metachronous CRC with mets | 56/5 |
| Max No. of lesions on CT /MRI scans at LT, median (range) | 8 (1-53) |
| Max size of lesions on CT/MRI scans at LT, median (range), mm | 35 (3-130) |
| Time from diagnosis to LT, median (range), mo | 20.9 (5.3-173.9) |
| Time from primary surgery to LT, median (range), mo | 16.9 (2.3-173.8) |
Abbreviations: 5-FU, fluorouracil; CEA, carcinoembryonic antigen; CRC, colorectal cancer; CT, computed tomography; EGFR, epidermal growth factor receptor; FCRS, Fong Clinical Risk Score; LT, liver transplant; max, maximum; met, metastasis; MRI, magnetic resonance imaging; MTV, metabolic tumor volume; RFA, radiofrequency ablation; Tas-102, trifluridine/tipiracil.
SI conversion factor: To convert CEA from micrograms per liter to nanograms per milliliter, divide by 1.
The median DFS for all 61 patients was 11.8 (95% CI, 9.3-14.2) months, and 5-year DFS was 18.3% (Figure 1A). Median OS in this cohort was 60.3 (95% CI, 44.3-76.4) months, and 5-year OS was 50.4%. (Figure 1B). One patient died of a surgical complication after 1.4 months, and 1 patient has been observed for 105 months without a relapse (Figure 1A). The patient with the longest survival is still alive 165 months after LT (Figure 1B).
Figure 1. Disease-Free Survival, Overall Survival, and Survival After Relapse After Liver Transplant.

A, Disease-free survival. B, Overall survival. C, Survival after relapse.
Forty-seven of 60 patients (78.3%) had a relapse after LT with median time to relapse of 9.0 (95% CI, 4.9-13.0) months. The majority of the relapses (94%) occurred within 2 years after LT, with last recurrence after 46.4 months. Median OS from time of relapse was 37.1 (95% CI, 4.6-69.5) months with 5-year survival after relapse of 34.8%, and 14 of 47 patients (29.8%) with recurrence were still alive more than 5 years after the diagnosis of recurrence. One patient is alive 156 months after relapse (Figure 1C).
Prognostic Factors for OS
Oslo Score
When survival outcomes were stratified by Oslo score 0 to 4, a total of 10 patients with an Oslo score of 0 had a median OS of 151.6 (0-328.1) months and both 5- and 10-year OS of 88.9%, whereas 27 patients with an Oslo score of 1 had a median OS of 60.3 (39.8-80.8) months and 5-year OS of 54.7%. None of the 6 patients with an Oslo score of 4 survived for 5 years after LT. The median OS for patients with an Oslo score of 0 to 2 was 92.0 (31.9-152.2) months, with a 5- and 10-year OS of 63.4% and 45.7%, respectively. The median OS for patients with an Oslo score of 3 to 4 was 24.8 (14.3-35.3) months, with a 5- and 10-year OS of 8.3% and 0%, respectively (P <.001) (Figure 2A).
Figure 2. Association of Overall Survival After Liver Transplant With Oslo Score, Lesion Size, Treatment Response, Carcinoembryonic Antigen (CEA) Levels, and Time From Diagnosis.

Association of overall survival after liver transplant with Oslo score (A), size of largest lesion (B), response to chemotherapy at time of transplant (C), CEA levels at time of transplant (D), and time from diagnosis to liver transplant (E).
There was a marked difference in OS between patients with largest tumor size greater than 5.5 cm (median OS, 25.3 months; 95% CI, 15.8-34.8 months; 5-year OS, 26.7%) or smaller than 5.5 cm (median OS, 92.0 months; 95% CI, 23.3-160.7 months; 5-year OS, 60.8%; P < .001) (Figure 2B). Patients with progressive disease at the time of LT had a median OS of 39.8 (95% CI, 28.8-50.7) months and 5-year OS of 35.1% compared with a median OS of 90.5 (95% CI, 22.8-158.2) months and 5-year OS of 60.9% in patients responsive to chemotherapy at the time of LT (P = .02) (Figure 2C). Patients with CEA levels above 80 μg/L had a reduced median OS after LT of 26.6 months (95% CI, 22.7-30.6 months; 5-year OS, 11.1%), and patients with CEA levels below 80 μg/L had a median OS after LT of 90.5 months (95% CI, 54.1-126.9 months; 5-year OS, 59.4%). OS at 10 years was 42.8% and 0% in patients with CEA levels below and above 80 μg/L, respectively (P < .001) (Figure 2D).
Sixteen patients had an interval from resection of the primary tumor to LT of 2 years or more. These patients had a median OS of 151.6 (95% CI, 85.4-217.8) months and 5- and 10-year OS of 86.7% and 52.0%, respectively, compared with a median OS of 50.8 (95% CI, 28.0-73.6) months and 5- and 10-year OS of 38.4% and 28.9%, respectively, in patients with less than 2 years from resection of the primary tumor to LT. The difference was not statistically significant (eTable 1 in Supplement 2). Similarly, there was no significant difference in OS between patients with an interval from diagnosis to LT of more than 2 years or less than 2 years. However, patients with an interval of more than 3 years from diagnosis to LT had a statistically significant longer OS compared with patients with less than 3 years from diagnosis to LT, who had an estimated OS of 57.2 months (95% CI, 36.2-78.2; P = .02) (Figure 2E).
FCRS
FCRS of 1, 2, 3, 4, and 5 were determined in 5, 16, 25, 9, and 5 patients, respectively, with median OS of 164.9 (95% CI, 104.4-225.4) months, 90.5 (95% CI, 30.8-150.2) months, 59.9 (95% CI, 55.3-64.5) months, 32.8 (95% CI, 0-72.9) months, and 25.3 (95%, 9.4-41.2) months, respectively (P < .001). These patients had 5-year OS of 100%, 63.9%, 49.4, 33.3% and 0%, respectively. Patients with an FCRS of 1 had a 10-year OS of 80%. Nineteen patients had an FCRS of 0 to 2, and 41 patients had an FCRS of 3 to 5. Patients with an FCRS of 0 to 2 had a significantly better OS after LT compared with patients with an FCRS of 3 to 5 (FCRS 0-2: median OS, 151.6 months; 95% CI, 78.5-224.7 months; 5-year OS, 75.4%; FCRS 3-5: median OS, 50.8 months; 95% CI, 28.4-73.3 months; 5-year OS, 39.7%; P = .01) (Figure 3A).
Figure 3. Association of Overall Survival After Liver Transplant With Fong Clinical Risk Score (FCRS), Positron Emission Tomography (PET)–Metabolic Tumor Volume (MTV) Values, Tumor Location, and Liver Metastases.
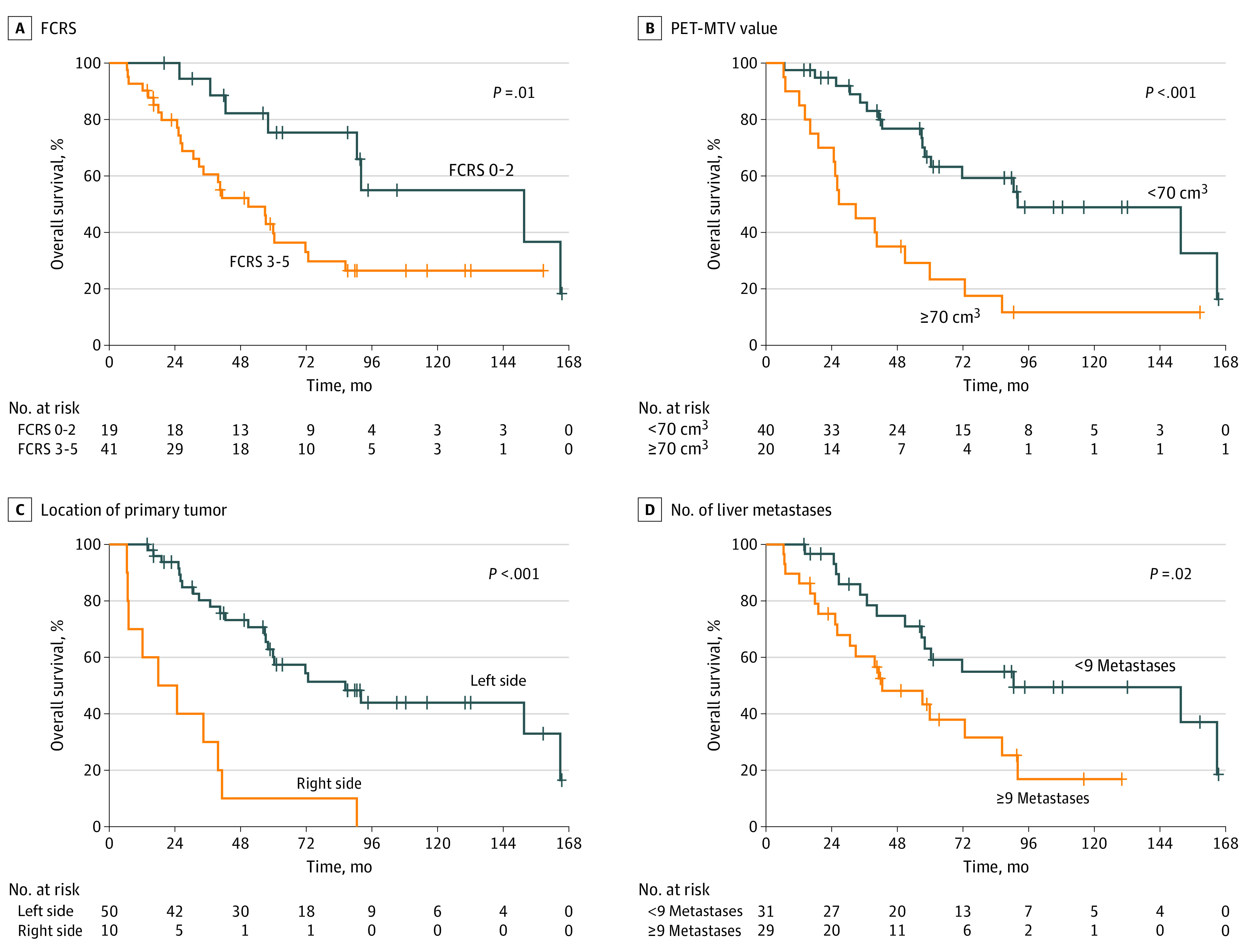
Overall survival associated with FCRS (A), PET-MTV values (B), location of primary tumor (C), and number of liver metastases (D).
PET-MTV Values
A total of 40 patients with MTV values less than 70 cm3 had a median OS of 92.0 (95% CI, 33.7-150.3) months and 5-year OS of 66.7% compared with patients with MTV values greater than 70 cm3 who had a median OS of 26.6 (95% CI, 11.8-41.5) months and 5-year OS of 23.3% (P < .001) (Figure 3B).
Site of Primary Tumor
Twenty-nine of 60 patients (48.3%) had a left-sided colon primary tumor, and 21 of 60 patients (35.0%) had a rectal primary tumor. Ten of 60 patients (16.7%) had their primary tumor in the ascending colon. Patients with an ascending colon primary tumor had a median OS of 17.9 (95% CI, 0-37.5) months and 5-year OS of only 10% compared with those with a more distal primary cancer with a median OS of 86.3 (95% CI, 48.9-123.7) months and 5-year OS of 60.1% (P < .001) (Figure 3C). When excluding patients with right-sided primary tumors, there was no significant difference in OS between patients with colon and rectal primary tumors.
Other Factors
The was no significant difference in OS associated with lymph node status of the primary resected tumor, although patients with pathologic stage (y)pN0 had numerically better OS (eTable 2 in Supplement 2). Furthermore, there was no significant difference in OS between patients with KRAS wild-type tumor compared with patients with KRAS variant primary tumor (eTable 1 in Supplement 2).
Only 5 patients had metachronous disease, defined as more than 12 months from diagnosis of CRC to appearance of liver metastasis. All these patients were alive at 5 years after LT, and 3 are alive more than 10 years after LT. One died of a sudden cardiac–related event 7.7 years after LT. However, the patient had a relapse of malignant disease at the time of death. The last patient with metachronous disease is alive without a relapse at 61.1 months.
Patients with CRC and 8 or fewer liver metastases at the time of LT had significantly longer OS (90.5 months; 95% CI, 7.8-173 months; P = .02) (Figure 3D) compared with patients with 9 of more liver metastases.
A tumor burden score (TBS) group of 2 was observed in 25 patients (median OS, 151.6 months; 95% CI, 25.4-277.8 months), and a TBS group of 3 was observed in 34 patients (median OS, 41.2 months; 95% CI, 28.7-53.8 months). Patients with a TBS group of 2 had 5- and 10-year OS of 72.3% and 56.1% compared with patients who had a TBS group of 3, with 5- and 10-year OS of 35.3% and 16.8% (P = .008). A summary of factors suggesting good prognosis after LT are given in Table 2.
Table 2. Patients With Colorectal Cancer Who Might Be Considered for Liver Transplant (LT).
| Very good prognosis after LT | No. of patients | Estimated 5-y survival |
|---|---|---|
| Metachronous disease (more than 12 mo from diagnosis of the primary tumor to detection of liver metastases) | 5 | 100% |
| Time from diagnosis to LT >3 y | 9 | 100% |
| Oslo score 0 | 10 | 88.9% |
| Fong Clinical Risk Score 1 | 5 | 100% |
| Good prognosis after liver transplant | ||
| PET-MTV value <70 cm3 | 40 | 66.7% |
| Oslo score 1 | 27 | 54.7% |
| Fong Clinical Risk Score 2 | 16 | 63.9% |
| Tumor Burden score, group 2 (score of 3-9) | 25 | 72.3% |
Abbreviations: MTV, metabolic tumor volume; PET, positron emission tomography.
Prognostic Factors for DFS
The factors predictive of OS were in general also significant for DFS. However, the difference in DFS was small compared with the difference in OS. DFS was often approximately 12 months or less (eTable 2 in Supplement 2). Median DFS of more than 20 months was only observed in the cohorts with FCRS of 0 to 2, pathologic stage pN0, and time from diagnosis to LT of more than 3 years (eTable 3 and eFigure 3 in Supplement 2).
Prognostic Factors for OS After Relapse
Many of the factors predictive of DFS and OS were also significant associated with survival time after relapse. There was a significant association between survival time after relapse and site of primary (ascending colon, 12.6 months; 95% CI, 0-27.4 months vs other localizations, 54.5 months; 95% CI, 43.5-65.6 months; P < .001), size of largest liver metastasis greater than 5.5 cm (17.5 months; 95% CI, 8.7-26.2 months vs size <5.5 cm, 54.5 months; 95% CI, 38.0-71.1 months; P < .001), Oslo score (0-2, 55.6 months; 95% CI, 48.0-63.1 vs 3-4, 17.4 months; 95% CI, 8.5-26.4; P < .001), CEA level greater than 80 μg/L (22.0 months; 95% CI, 17.9-26.1 vs <80 μg/L, 54.4 months; 95% CI, 30.0-79.8 months; P = .01), TBS (group 2, 55.6 months; 95% CI, 33.0-78.4 months vs TBS group 3, 23.3 months; 95% CI, 19.2-27.4 months; P = .02), PET-MTV (<70 cm3, 55.6 months; 95% CI, 37.2-73.9 months vs >70 cm3, 22.0 months; 95% CI, 15.9-28.0 months; P = .03), and more than 3 years from diagnosis to LT (141 months vs <3 years, 33.3 months; 95% CI, 16.5-50.1; P = .04) (eTable 2 and eFigure 3 in Supplement 2).
Cause of Death
Posttransplant mortality was caused by progressive malignant disease in all except 3 patients. These 3 patients died of a sudden cardiac event at 92 months after LT, traumatic cerebral hemorrhage at 59.9 months after LT, and postoperative hepatic artery thrombosis with biliary sepsis at 1.4 months after LT.
Discussion
This nonrandomized controlled trial describes the incremental development of LT in patients with CRC and metastases confined to the liver with the pilot trial as a proof of concept starting in 2006 followed by studies examining stricter selection criteria. The outcomes were superior to OS obtained in a similar cohort of patients receiving first-line chemotherapy.20 Other transplant centers have now started different LT studies in patients with CRC and nonresectable liver metastases with ongoing studies in Europe and North America. Several centers have performed organ transplant on selected patients outside of prospective study protocols,21 and the International Hepato-Pancreatico-Biliary Association has recently published consensus guidelines for LT in patients with CRC and nonresectable liver metastases.22
Most patients with CRC will have a disease relapse after LT23 and a short DFS with a median of approximately 12 months (Figure 1A). In contrast to patients with hepatocellular carcinoma who experience disease recurrence after organ transplant, patients with CRC and liver metastases had a long overall survival time after recurrence that in some was 10 years (Figure 1C). This is at least partly explained by the fact that the dominant recurrence pattern is small pulmonary metastases that grow slowly despite immunosuppression and where a majority can be offered pulmonary resection with curative intent.24 Patients receiving curatively intended treatment of pulmonary metastases had a 5-year OS of 70% from time of resection of pulmonary lesions.25 In particular, patients who had good prognostic factors including Oslo score of 0 to 2, PET-MTV values less than 70 cm3, or FCRS of 0 to 2 had long OS from time of resection of pulmonary metastases with 10-year OS of 76% to 100%.26 This long OS after resection of pulmonary relapses after transplant seems to be longer compared with patients who received a resection of their pulmonary metastases after prior liver resection.27,28There was no close correlation between DSF and OS as is conventionally seen in oncologic trials, and DFS is not a valid measure of treatment efficacy in LT for patients with CRC and liver metastases. This finding stands out compared with the experience in most other transplant oncology indications, where recurrence of disease usually is associated with limited treatment possibilities and short OS after posttransplant relapse.
Patients from different prospective studies with different inclusion and exclusion criteria were included in this trial, representing a very heterogeneous population. We have previously shown in a subset of these patients that FCRS, Oslo score, and PET-MTV values can stratify patients into groups with 5-year estimated OS of approximately 70% or higher and those with inferior posttransplant survival.17 These factors could be used as selection criteria to obtain 5-year OS of more than 70% and even a 10-year OS of more than 85% when only including patients with an Oslo score of 0. In the current cohort of patients, only 10 to 19 of the 60 included patients would have been eligible if the criteria of an Oslo score of 0 or FCRS of 0 to 2 had been applied. The results suggest that by applying stringent selection criteria, 5-year survival rates that are comparable with conventional indications for LT may be obtained.
In a retrospective analysis of 12 patients with CRC who received LT for liver metastasis, Toso et al21 found that 3 years or more from diagnosis to LT is important for OS after LT. The significance of a long interval from diagnosis to transplant is suggested by findings of the present study (Figure 2E). Many of these patients had metachronous disease and some had undergone liver resection and or radiofrequency ablation before organ transplant.
The hepatic tumor load of the included patients was substantial compared with most publications on liver resection in patients with CRC and metastases confined to the liver,2,3 and we are also not aware of any publication of patients with CRC and resected liver metastases with similar median tumor load. The 5-year OS in the present study is similar to that of patients included in the Perioperative FOLFOX-4 Chemotherapy and Surgery vs Surgery Alone for Resectable Liver Metastases From Colorectal Cancer (EORTC 40983) study, where patients with CRC and a median of 1 lesion and maximum of 4 lesions received liver resection.3 This may suggest that LT is the best medical option also in patients with CRC with extensive liver metastases even when all lesions are being considered to be resectable. The results of recently published comparative studies between liver resection and LT support this hypothesis.29,30
Limitations
We have previously shown that patients in the SECA-I trial who received LT had good quality of life up to 3 years after LT.31 Furthermore, LT is cost-effective compared with the cost of modern oncologic treatments.32 The major obstacles for being able to offer patients with CRC an LT internationally is the scarcity of donor grafts. This problem may be partly solved by use of extended-criteria donors. Only 1 of 11 extended-criteria donor grafts did not function well, and the patient was retransplanted with another extended-criteria donor graft.33 Furthermore, the shortage of donor grafts for transplant in CRC may be reduced also by use of the RAPID technique, where the patient during the first part of the hepatectomy has segments 1 to 3 resected and receives only the donor segments 2 and 3.34 Within 3 weeks, the donor segments have increased to sufficient size to avoid liver failure, and resection of segments 4 to 8 is performed.34 This opens the possibility for a donor graft offering a life extension for 2 patients or for use as a living donor with low risk for the donor.35,36
Conclusions
In conclusion, results of this nonrandomized controlled trial suggest that LT for patients with CRC and metastatic disease confined to the liver may yield survival rates comparable with conventional indications for LT when patients are selected according to an Oslo score of 0 to 1 and a PET-MTV value less than 70 cm3. The limitation represented by the shortage of available donor grafts may be reduced by using extended-criteria donor grafts and the RAPID technique with deceased or living donors.
Trial Protocol
eTable 1. Colorectal Cancer Patients Receiving Liver Transplantation in Different Studies
eTable 2. Median Overall Survival and 5-Year Overall Survival Related to Baseline Factors
eTable 3. Median Disease-Free Survival and 3-Year Disease-Free Survival Related to Baseline Factors
eFigure 1. Flowchart for all Patients Listed for Liver Transplantation in the Different Prospective Studies
eFigure 2. Overall Survival for all 72 Patients Accepted for the Liver Transplantation Waiting List Calculated From Date of Transplantation, Date of Laparoscopy, and Date of Being Listed for Transplantation
eFigure 3. Disease-Free Survival After Liver Transplantation and Survival After Relapse
Data Sharing Statement
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Quan D, Gallinger S, Nhan C, et al. ; Surgical Oncology Program at Cancer Care Ontario . The role of liver resection for colorectal cancer metastases in an era of multimodality treatment: a systematic review. Surgery. 2012;151(6):860-870. doi: 10.1016/j.surg.2011.12.018 [DOI] [PubMed] [Google Scholar]
- 3.Nordlinger B, Sorbye H, Glimelius B, et al. ; EORTC Gastro-Intestinal Tract Cancer Group; Cancer Research UK; Arbeitsgruppe Lebermetastasen und–tumoren in der Chirurgischen Arbeitsgemeinschaft Onkologie (ALM-CAO); Australasian Gastro-Intestinal Trials Group (AGITG); Fédération Francophone de Cancérologie Digestive (FFCD) . Perioperative FOLFOX4 chemotherapy and surgery vs surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomized, controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1208-1215. doi: 10.1016/S1470-2045(13)70447-9 [DOI] [PubMed] [Google Scholar]
- 4.Masi G, Vasile E, Loupakis F, et al. Randomized trial of 2 induction chemotherapy regimens in metastatic colorectal cancer: an updated analysis. J Natl Cancer Inst. 2011;103(1):21-30. doi: 10.1093/jnci/djq456 [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29(15):2011-2019. doi: 10.1200/JCO.2010.33.5091 [DOI] [PubMed] [Google Scholar]
- 6.Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab vs FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306-1315. doi: 10.1016/S1470-2045(15)00122-9 [DOI] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Llovet JM, Miceli R, et al. ; Metroticket Investigator Study Group . Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35-43. doi: 10.1016/S1470-2045(08)70284-5 [DOI] [PubMed] [Google Scholar]
- 8.Le Treut YP, Grégoire E, Klempnauer J, et al. ; For ELITA . Liver transplantation for neuroendocrine tumors in Europe-results and trends in patient selection: a 213-case European liver transplant registry study. Ann Surg. 2013;257(5):807-815. doi: 10.1097/SLA.0b013e31828ee17c [DOI] [PubMed] [Google Scholar]
- 9.Kwong AJ, Ebel NH, Kim WR, et al. OPTN/SRTR 2020 annual data report: liver. Am J Transplant. 2022;22(suppl 2):204-309. doi: 10.1111/ajt.16978 [DOI] [PubMed] [Google Scholar]
- 10.Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I, et al. Liver transplantation in patients with liver metastases from neuroendocrine tumors: a systematic review. Surgery. 2017;162(3):525-536. doi: 10.1016/j.surg.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 11.Gores GJ, Darwish Murad S, Heimbach JK, Rosen CB. Liver transplantation for perihilar cholangiocarcinoma. Dig Dis. 2013;31(1):126-129. doi: 10.1159/000347207 [DOI] [PubMed] [Google Scholar]
- 12.Ziogas IA, Rauf MA, Matsuoka LK, et al. Liver transplantation for cholangiocarcinoma: charting a path with lessons learned from center experience. Transplant Direct. 2021;7(4):e686. doi: 10.1097/TXD.0000000000001133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lunsford KE, Javle M, Heyne K, et al. ; Methodist–MD Anderson Joint Cholangiocarcinoma Collaborative Committee (MMAJCCC) . Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3(5):337-348. doi: 10.1016/S2468-1253(18)30045-1 [DOI] [PubMed] [Google Scholar]
- 14.Mühlbacher F, Huk I, Steininger R, et al. Is orthotopic liver transplantation a feasible treatment for secondary cancer of the liver? Transplant Proc. 1991;23(1 Pt 2):1567-1568. [PubMed] [Google Scholar]
- 15.Hoti E, Adam R. Liver transplantation for primary and metastatic liver cancers. Transpl Int. 2008;21(12):1107-1117. doi: 10.1111/j.1432-2277.2008.00735.x [DOI] [PubMed] [Google Scholar]
- 16.Hagness M, Foss A, Line PD, et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg. 2013;257(5):800-806. doi: 10.1097/SLA.0b013e3182823957 [DOI] [PubMed] [Google Scholar]
- 17.Dueland S, Grut H, Syversveen T, Hagness M, Line PD. Selection criteria related to long-term survival following liver transplantation for colorectal liver metastasis. Am J Transplant. 2020;20(2):530-537. doi: 10.1111/ajt.15682 [DOI] [PubMed] [Google Scholar]
- 18.Grut H, Dueland S, Line PD, Revheim ME. The prognostic value of 18F-FDG PET/CT prior to liver transplantation for nonresectable colorectal liver metastases. Eur J Nucl Med Mol Imaging. 2018;45(2):218-225. doi: 10.1007/s00259-017-3843-9 [DOI] [PubMed] [Google Scholar]
- 19.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230(3):309-318. doi: 10.1097/00000658-199909000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dueland S, Guren TK, Hagness M, et al. Chemotherapy or liver transplantation for nonresectable liver metastases from colorectal cancer? Ann Surg. 2015;261(5):956-960. doi: 10.1097/SLA.0000000000000786 [DOI] [PubMed] [Google Scholar]
- 21.Toso C, Pinto Marques H, Andres A, et al. ; Compagnons Hépato-Biliaires Group . Liver transplantation for colorectal liver metastasis: survival without recurrence can be achieved. Liver Transpl. 2017;23(8):1073-1076. doi: 10.1002/lt.24791 [DOI] [PubMed] [Google Scholar]
- 22.Bonney GK, Chew CA, Lodge P, et al. Liver transplantation for nonresectable colorectal liver metastases: the International Hepato-Pancreato-Biliary Association consensus guidelines. Lancet Gastroenterol Hepatol. 2021;6(11):933-946. doi: 10.1016/S2468-1253(21)00219-3 [DOI] [PubMed] [Google Scholar]
- 23.Dueland S, Foss A, Solheim JM, Hagness M, Line PD. Survival following liver transplantation for liver-only colorectal metastases compared with hepatocellular carcinoma. Br J Surg. 2018;105(6):736-742. doi: 10.1002/bjs.10769 [DOI] [PubMed] [Google Scholar]
- 24.Grut H, Solberg S, Seierstad T, et al. Growth rates of pulmonary metastases after liver transplantation for unresectable colorectal liver metastases. Br J Surg. 2018;105(3):295-301. doi: 10.1002/bjs.10651 [DOI] [PubMed] [Google Scholar]
- 25.Dueland S, Smedman TM, Røsok B, et al. Treatment of relapse and survival outcomes after liver transplantation in patients with colorectal liver metastases. Transpl Int. 2021;34(11):2205-2213. doi: 10.1111/tri.13995 [DOI] [PubMed] [Google Scholar]
- 26.Dueland S, Smedman TM, Grut H, Syversveen T, Jørgensen LH, Line PD. PET-uptake in liver metastases as method to predict tumor biological behavior in patients transplanted for colorectal liver metastases developing lung recurrence. Cancers (Basel). 2022;14(20):5042. doi: 10.3390/cancers14205042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Angelica M, Kornprat P, Gonen M, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18(4):1096-1103. doi: 10.1245/s10434-010-1409-1 [DOI] [PubMed] [Google Scholar]
- 28.Salah S, Ardissone F, Gonzalez M, et al. Pulmonary metastasectomy in colorectal cancer patients with previously resected liver metastasis: pooled analysis. Ann Surg Oncol. 2015;22(6):1844-1850. doi: 10.1245/s10434-014-4173-9 [DOI] [PubMed] [Google Scholar]
- 29.Lanari J, Hagness M, Sartori A, et al. Liver transplantation versus liver resection for colorectal liver metastasis: a survival benefit analysis in patients stratified according to tumor burden score. Transpl Int. 2021;34(9):1722-1732. doi: 10.1111/tri.13981 [DOI] [PubMed] [Google Scholar]
- 30.Dueland S, Yaqub S, Syversveen T, et al. Survival outcomes after portal vein embolization and liver resection compared with liver transplant for patients with extensive colorectal cancer liver metastases. JAMA Surg. 2021;156(6):550-557. doi: 10.1001/jamasurg.2021.0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dueland S, Line PD, Hagness M, Foss A, Andersen MH. Long-term quality of life after liver transplantation for nonresectable colorectal metastases confined to the liver. BJS Open. 2018;3(2):180-185. doi: 10.1002/bjs5.50116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjørnelv GMW, Dueland S, Line PD, et al. Cost-effectiveness of liver transplantation in patients with colorectal metastases confined to the liver. Br J Surg. 2019;106(1):132-141. doi: 10.1002/bjs.10962 [DOI] [PubMed] [Google Scholar]
- 33.Smedman TM, Line PD, Hagness M, Syversveen T, Grut H, Dueland S. Liver transplantation for unresectable colorectal liver metastases in patients and donors with extended criteria (SECA-II arm D study). BJS Open. 2020;4(3):467-477. doi: 10.1002/bjs5.50278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Line PD, Hagness M, Berstad AE, Foss A, Dueland S. A novel concept for partial liver transplantation in nonresectable colorectal liver metastases: the RAPID concept. Ann Surg. 2015;262(1):e5-e9. doi: 10.1097/SLA.0000000000001165 [DOI] [PubMed] [Google Scholar]
- 35.Königsrainer A, Templin S, Capobianco I, et al. Paradigm shift in the management of irresectable colorectal liver metastases: Living Donor Auxiliary Partial Orthotopic Liver Transplantation in Combination With 2-stage Hepatectomy (LD-RAPID). Ann Surg. 2019;270(2):327-332. doi: 10.1097/SLA.0000000000002861 [DOI] [PubMed] [Google Scholar]
- 36.Settmacher U, Ali-Deeb A, Coubeau L, et al. Auxilliary liver transplantation according to the RAPID procedure in noncirrhotic patients: technical aspects and early outcomes. Ann Surg. 2023;277(2):305-312. doi: 10.1097/SLA.0000000000005726 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Colorectal Cancer Patients Receiving Liver Transplantation in Different Studies
eTable 2. Median Overall Survival and 5-Year Overall Survival Related to Baseline Factors
eTable 3. Median Disease-Free Survival and 3-Year Disease-Free Survival Related to Baseline Factors
eFigure 1. Flowchart for all Patients Listed for Liver Transplantation in the Different Prospective Studies
eFigure 2. Overall Survival for all 72 Patients Accepted for the Liver Transplantation Waiting List Calculated From Date of Transplantation, Date of Laparoscopy, and Date of Being Listed for Transplantation
eFigure 3. Disease-Free Survival After Liver Transplantation and Survival After Relapse
Data Sharing Statement


