This genetic association study identifies genetic variants associated with hidradenitis suppurativa and sheds light on the underlying genes and genetic mechanisms.
Key Points
Question
What are the genetic variants associated with hidradenitis suppurativa (HS) and the genes that may affect disease pathogenesis?
Findings
A genome-wide association study (GWAS) of 753 patients from the University of North Carolina at Chapel Hill was performed and meta-analyzed with GWAS in 3 large biobanks (UK Biobank, FinnGen, and BioVU). Results identified 1 significant locus and 2 suggestive loci and replicated the HS associations in BioVU for 2 loci near the SOX9 and KLF5 genes.
Meaning
These findings implicated genetic variants in risk of HS and support that nearby genes SOX9 and KLF5, which play important roles in epidermal differentiation and follicular inflammation, may be associated with disease pathogenesis.
Abstract
Importance
Hidradenitis suppurativa (HS) is a common and severely morbid chronic inflammatory skin disease that is reported to be highly heritable. However, the genetic understanding of HS is insufficient, and limited genome-wide association studies (GWASs) have been performed for HS, which have not identified significant risk loci.
Objective
To identify genetic variants associated with HS and to shed light on the underlying genes and genetic mechanisms.
Design, Setting, and Participants
This genetic association study recruited 753 patients with HS in the HS Program for Research and Care Excellence (HS ProCARE) at the University of North Carolina Department of Dermatology from August 2018 to July 2021. A GWAS was performed for 720 patients (after quality control) with controls from the Add Health study and then meta-analyzed with 2 large biobanks, UK Biobank (247 cases) and FinnGen (673 cases). Variants at 3 loci were tested for replication in the BioVU biobank (290 cases). Data analysis was performed from September 2021 to December 2022.
Main Outcomes and Measures
Main outcome measures are loci identified, with association of P < 1 × 10−8 considered significant.
Results
A total of 753 patients were recruited, with 720 included in the analysis. Mean (SD) age at symptom onset was 20.3 (10.57) years and at enrollment was 35.3 (13.52) years; 360 (50.0%) patients were Black, and 575 (79.7%) were female. In a meta-analysis of the 4 studies, 2 HS-associated loci were identified and replicated, with lead variants rs10512572 (P = 2.3 × 10−11) and rs17090189 (P = 2.1 × 10−8) near the SOX9 and KLF5 genes, respectively. Variants at these loci are located in enhancer regulatory elements detected in skin tissue.
Conclusions and Relevance
In this genetic association study, common variants associated with HS located near the SOX9 and KLF5 genes were associated with risk of HS. These or other nearby genes may be associated with genetic risk of disease and the development of clinical features, such as cysts, comedones, and inflammatory tunnels, that are unique to HS. New insights into disease pathogenesis related to these genes may help predict disease progression and novel treatment approaches in the future.
Introduction
Hidradenitis suppurativa (HS) is a common chronic inflammatory disease characterized by painful nodules, abscesses, and tunnels with predilection for intertriginous sites.1 Strong evidence of heritability has been described in cohort and twin studies,2,3,4,5,6 and our analysis of 281 patient pedigrees in the US demonstrated that siblings of affected individuals have a nearly 20-fold risk of developing HS.7 While γ-secretase complex gene variants have been implicated in less than 5% of patients,8 most genetic risk factors are unknown. Genome-wide association studies (GWASs) provide an opportunity to identify common genetic variants that contribute to HS susceptibility.
Understanding of HS pathogenesis has shifted substantially in recent years. Hypotheses focused on apocrine gland inflammation and follicular occlusion have faded, with recent characterizations describing HS as a disease of chronic follicular inflammation and dysbiosis. Inflammatory cytokines, complement C5a, and several cell types have all been implicated in the inflammatory response in HS,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23 but the genetic and environmental factors predisposing to this response are unclear.
Pathogenesis of HS is characterized by unique clinical features, such as comedones, cysts, and chronically inflamed and/or epithelialized tunnels in areas of chronic involvement. Tunnels attract a chronic inflammatory response, and the epithelial lining has overlapping characteristics with nearby epidermis.24 The mechanisms leading to these unique structures are unclear and a notable gap in our understanding of disease progression.
To better understand genetic factors that may underlie pathogenesis, we performed a GWAS of 720 patients with HS in the Hidradenitis Suppurativa Program for Research and Care Excellence (HS ProCARE) at the University of North Carolina (UNC) Department of Dermatology with controls from the Add Health study.25,26 To our knowledge this is the first GWAS and meta-analysis for HS and uses HS ProCARE results along with association results from 2 biobanks,27,28 with replication in BioVU.29 We identified 2 loci associated with HS (P < 5 × 10−8). The location of HS-associated variants in skin regulatory elements and the functions of nearby genes in HS disease pathways suggest that the variants alter expression of nearby genes to affect disease risk.
Methods
HS ProCARE Patient Recruitment and DNA Isolation
Following UNC Chapel Hill Institutional Review Board approval, patients with HS presenting to UNC Dermatology outpatient clinics provided written informed consent and were enrolled in HS ProCARE from August 2018 to July 2021. Clinical and demographic data were collected by skilled interviewers using questionnaires and stored in a RedCAP database. This study followed the Strengthening the Reporting of Genetic Association Studies (STREGA) reporting guideline.
Samples were collected using Oragene_Dx saliva collection kits (DNA Genotek). A subset of patients provided peripheral blood mononuclear cells processed using lymphocyte separation media from blood collection in sodium citrate. Isolation of DNA was performed in the UNC BioSpecimen Processing facility using the PerkinElmer Chemagic Magnetic Separation Module I and magnetic bead separation kits.
UNC HS ProCARE Genetic Data
Quantity and quality of DNA were determined by NanoDrop OD and Quant-iT PicoGreen dsDNA Assay Kits (Invitrogen). The DNA was analyzed for human content with Femto Human DNA Quantification Kits (Zymo Research) and a QuantStudio 6 Real-time PCR system. Samples were genotyped in the UNC Mammalian Genotyping Core for approximately 654 000 variants using the Infinium Global Screening Array-24, version 3.0 (Illumina). We removed individuals with missing rate greater than 5% or sex mismatch and removed variants with missing rate greater than 5%. We confirmed genotype concordance for 7 pairs of duplicated samples. After quality control, we retained 720 of 753 samples and 644 188 variants. Genotypes were analyzed in build hg38.
Controls
The National Longitudinal Study of Adolescent to Adult Health (Add Health) is an ongoing longitudinal study of the health and developmental trajectories for US individuals from early adolescence into adulthood.25,26 Genotyping data collection was performed in 2008 with participant consent and has undergone extensive quality control and cleaning.25 Covariate data (age, sex, body mass index [BMI, calculated as weight in kilograms divided by height in meters squared], smoking status, and race and ethnicity) were also collected in 2008. Among 5801 Add Health participants with both genotypes and covariates, 3868 had European ancestry and 707 had African ancestry. We performed liftover from hg19 to hg38.
Genotype Imputation and GWAS in HS ProCARE
We used variants shared by HS ProCARE and Add Health to perform genotype imputation with the TOPMed freeze 8 reference panel, following our publications,30,31,32 and using Eagle, version 2.433 for phasing and minimac434 for imputation. We reestimated imputation quality separately for European-ancestry HS cases, European-ancestry controls, African-ancestry HS cases, and African-ancestry controls and only retained variants with R2 of 0.8 or greater in all 4 groups.
We first performed logistic regression to correct for covariates, including age, age squared (age2), sex, BMI, smoking status (current smoker, former smoker, or nonsmoker), race and ethnicity indicators (Asian, Black, Hispanic/Latino, or White), and the first 20 genotype principal components (PCs), and inverse-normal transformed the residuals. Then we performed GWAS on the residuals with EPACTS, version 3.3.0 using the EMMAX test35 to control for sample relatedness,36,37 which was quantified by a kinship matrix constructed from genotyped variants with missing rate less than 1% and minor allele frequency greater than 1%. To evaluate potential confounding by race and ethnicity, we repeated the GWAS with European-ancestry–only and African-ancestry–only subsets of the data, conditioning on the same covariates except race and ethnicity indicators.
Biobank HS GWAS Analyses and Meta-Analyses
The UK Biobank (UKB)28 is a prospective biobank with approximately 500 000 individuals aged 40 to 69 years in 2006 to 2010. We identified 247 participants of primarily European ancestry with HS defined with either International Classification of Diseases, Ninth Revision (ICD-9) (705.83, Hidradenitis) or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) (L73.2, Hidradenitis suppurativa), with ancestry groups defined as in our previous work36 using k-means and self-reported race and ethnicity. All European-ancestry individuals without HS (n = 453 049) were included as controls. GWAS was performed using REGENIE38 with the same analysis strategy as HS ProCARE, accounting for the same covariates and 10 PCs. We included variants with minor allele frequency greater than 0.5% and imputation quality INFO score greater than 0.8 and performed liftover from hg19 to hg38.
FinnGen39 aims to collect and analyze genome and health data from 500 000 Finnish biobank participants. We analyzed version 7 data (June 2022) consisting of 309 154 individuals (173 746 females and 135 408 males), including 673 HS cases (defined by the same ICD-9 and ICD-10 codes as UKB) and 297 544 controls. The GWAS summary statistics from FinnGen were directly downloaded from https://www.finngen.fi/ in build hg38. FinnGen included age, sex, genotyping batch, and 10 PCs in models.
We performed meta-analyses using METAL40 with effective sample sizes calculated with the following equation:
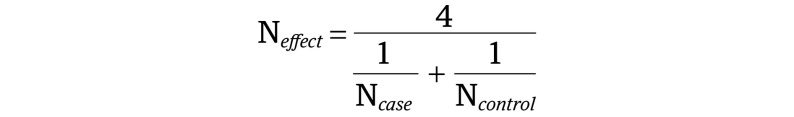 |
We first performed 3-way meta-analysis with UKB, FinnGen, and all HS ProCARE patients analyzed jointly with variants existing in 2 or more studies. After obtaining replication results from BioVU described below, we meta-analyzed the top variants in a 4-way meta-analysis (eTable 7 in Supplement 1). To further validate results and consider population stratification, we performed additional meta-analyses as sensitivity analyses: a 4-way meta-analysis with UKB, FinnGen, European-ancestry HS ProCARE, and European-ancestry BioVU individuals, and a 6-way meta-analysis also including African-ancestry HS ProCARE and African-ancestry BioVU individuals. We defined genome-wide significance as P < 5 × 10−8.
HS Association Analysis in BioVU
We tested lead variants from the GWAS meta-analyses for association with HS in the BioVU biobank based at Vanderbilt University Medical Center. BioVU DNA was extracted from blood samples, and genotypes were linked to deidentified medical records. Genotyping was performed on the Multi-Ethnic Global array, and genotype imputation was performed on Michigan Imputation Server using the TOPMed reference panel. Ancestry information was defined by PCs with 1000G as reference. Hidradenitis suppurativa disease status was determined with either ICD-9 or ICD-10 codes. We performed logistic regression in R (R Foundation for Statistical Computing) to test variant associations with HS. Covariates included age, age2, sex, smoking status, median BMI, self-reported race (Asian, Black, and White), ethnicity (Hispanic or not), genotype batch, median age, and the first 10 genotype PCs.
Annotations and Links to Genes
To identify potential candidate genes, we performed literature searches on the closest protein-coding genes. We identified candidate variants based on linkage disequilibrium (LD) (r2 > 0.8) using TOP-LD41 with European reference panels and used the University of California, Santa Cruz, genome browser (http://genome.ucsc.edu) to display annotation tracks for ENCODE candidate cis-regulatory elements, conservation across species, and epigenomic marks of accessible chromatin and histone chromatin immunoprecipitation corresponding to enhancers.42,43,44,45 We looked up lead variants in the GTEx eQTL database,46 but neither variant was in LD with the lead variant for an eQTL in any tissue (r2 < 0.5 in 1000 Genomes European participants).
Genetic Correlation Analysis
We performed genetic correlation analysis with LD score regression.47,48 We considered celiac disease,49 inflammatory bowel disease,50 polycystic ovary syndrome,51 psoriasis,52 rheumatoid arthritis,53 schizophrenia,54 and type 2 diabetes.55 GWAS summary statistics for these diseases were downloaded directly from the previous publications (eTable 6 in Supplement 1).
Results
Characteristics of Individuals Enrolled in HS ProCARE
We enrolled 720 patients with HS in an initial phase of an HS ProCARE registry and gathered detailed clinical characteristics (Table 1). Mean (SD) age at symptom onset was 20.3 (10.57) years and at enrollment was 35.3 (13.52) years; 360 (50.0%) patients were Black, and 575 (79.7%) female. More than half were obese (BMI >30), and fewer than 30% were smokers. Most patients had Hurley stage II (48%) or stage III (37%) disease. Almost half reported an affected first-degree relative. Phenotypes as described by van der Zee and Jemec56 were categorized at enrollment, with the majority having the regular phenotype.
Table 1. Characteristics of HS ProCARE Patients.
| Characteristic | No. (%) |
|---|---|
| Age at enrollment, y | |
| 0-18 | 81 (11.2) |
| 19-30 | 222 (30.8) |
| 31-50 | 318 (44.2) |
| >50 | 99 (13.8) |
| Body mass indexa | |
| <18.5 | 4 (0.6) |
| 18.5-24.9 | 93 (12.9) |
| 25.0-29.9 | 151 (21.0) |
| 30.0-39.9 | 307 (42.6) |
| 40-49.9 | 122 (16.9) |
| ≥50 | 43 (6.0) |
| Sex | |
| Female | 574 (79.7) |
| Male | 146 (20.3) |
| Race and ethnicity | |
| American Indian/Alaska Native | 5 (0.7) |
| Asian | 13 (1.8) |
| Black | 359 (49.9) |
| Black + Hispanic | 1 (0.1) |
| Hispanic | 21 (2.9) |
| Middle Eastern/Egyptian | 3 (0.4) |
| White | 308 (42.8) |
| White + Asian | 1 (0.1) |
| White + Black | 8 (1.1) |
| White + Hispanic | 1 (0.1) |
| Smoking status | |
| Current | 146 (20.3) |
| Former | 47 (6.5) |
| Never | 527 (73.2) |
| Hurley stage | |
| I | 106 (14.7) |
| II | 350 (48.6) |
| III | 264 (36.8) |
| Affected relatives | |
| 1st Degree | 351 (48.8) |
| 2nd Degree | 175 (24.3) |
| 1st and 2nd Degree | 115 (16.0) |
| 3rd Degree or no relatives | 309 (42.9) |
| Phenotype | |
| Regular | 502 (69.7) |
| Frictional furuncle | 42 (5.8) |
| Scarring folliculitis | 88 (12.2) |
| Conglobata | 44 (6.1) |
| Syndromic | 43 (6.0) |
| Ectopic | 1 (0.1) |
Abbreviation: HS ProCARE, Hidradenitis Suppurativa Program for Research and Care Excellence.
Calculated as weight in kilograms divided by height in meters squared.
HS ProCARE GWAS Results and Meta-Analysis With Biobanks
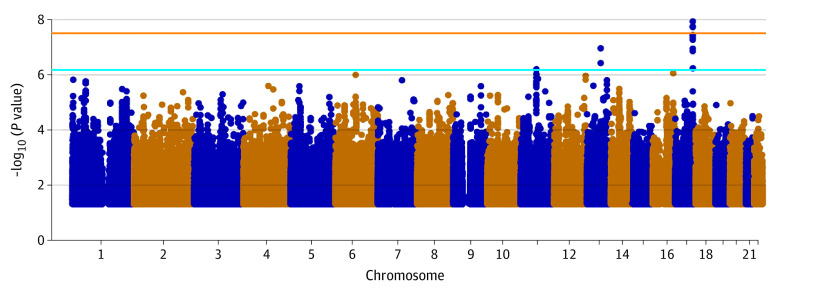
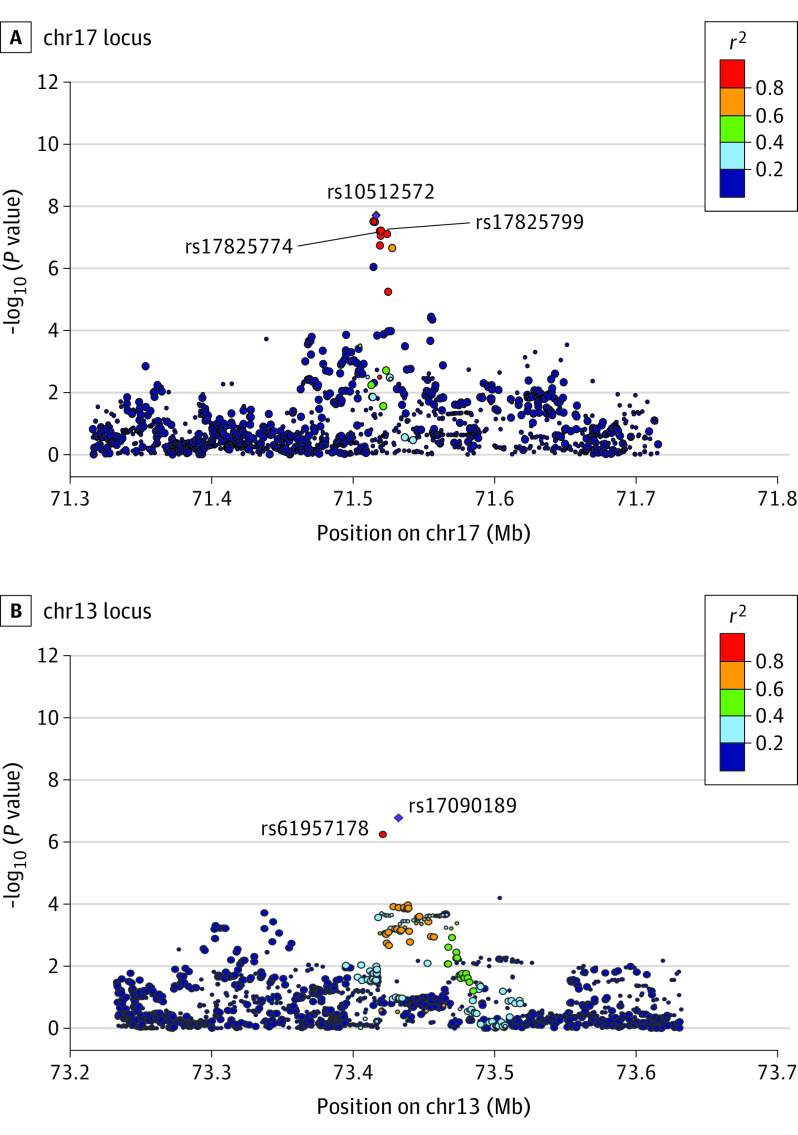
We performed initial GWAS analyses using HS ProCARE patients and Add Health controls (Methods) in all samples or separately for European- and African-ancestry participants. Eight loci showed suggestive evidence of association in the meta-analysis (P < 1 × 10−6) (eTables 1 and 2 in Supplement 1; eFigures 1-3 in Supplement 2). Despite use of external controls, we observed minimal inflation, with genomic control lambda ranging from 1.00 to 1.05 (eFigures 4-6 in Supplement 2). To increase power to detect associations, we performed GWAS in the United Kingdom Biobank (UKB; 247 cases, 453 048 controls) and meta-analyzed HS ProCARE GWAS results with UKB and the FinnGen biobank based in Finland (673 cases, 297 544 controls) (Methods). The 3-way meta-analysis revealed 1 genome-wide significant locus (P < 5 × 10−8), and 2 suggestive loci (Table 2, Figures 1 and 2; eTable 3 and eTable 10 in Supplement 1; eFigure 7 and eFigure 8 in Supplement 2). The most strongly associated variant, rs10512572 (meta-analysis P = 1.8 × 10−8) on chromosome (chr) 17, showed the strongest association in FinnGen (P = 1.9 × 10−7), with nominal support in HS ProCARE (P = 3.9 × 10−3) and a trend toward significance in UKB (P = .15), all with the same direction of effect. At the second locus, the most strongly associated variant, rs17090189 (meta-analysis P = 1.7 × 10−7) on chr13, also showed the strongest association in FinnGen (P = 3.7 × 10−7), with nominal support from HS ProCARE (P = .03) and UKB (P = .08), all with the same direction of effect. At a third locus, the most strongly associated variant, rs5792315 (meta-analysis P = 8.9 × 10−7) on chr11, showed the strongest association in HS ProCARE (P = 3.3 × 10−4), with support from FinnGen (P = 1.3 × 10−3) and a trend in UKB (P = .13), all in the same direction of effect. Notably, the human leukocyte antigen region on chr6 did not show even suggestive evidence of association with HS in any individual study or meta-analysis.
Table 2. Summary Statistics of the Lead Variant at the 3 Suggestive Loci in 3-Way Meta-Analysis (MA).
| Variant | Chromosome:position (hg38) | Effect allelea | Effect allele frequencyb | OR 3-way MAc | P value, 3-way MA | OR BioVU replication | P value, BioVU replication | OR 4-way MA | P value, 4-way MA |
|---|---|---|---|---|---|---|---|---|---|
| rs10512572 | chr17:71515958 | A | 0.13 | 1.17 | 1.8 × 10−8 | 1.23 | 4.3 × 10−4 | 1.19 | 2.3 × 10−11 |
| rs17090189 | chr13:73432270 | A | 0.84 | 1.14 | 1.7 × 10−7 | 1.10 | .07 | 1.14 | 2.1 × 10−8 |
| rs5792315 | chr11:64223524 | A | 0.45 | 1.10 | 8.9 × 10−7 | 0.99 | .88 | 1.08 | 6.6 × 10−6 |
Abbreviations: HS ProCARE, Hidradenitis Suppurativa Program for Research and Care Excellence; OR, odds ratio; UKB, UK Biobank.
Represents 1 of the 4 alleles of human genetic variation (A, C, G, T).
Effect allele frequency is the weighted average across HS ProCARE, UKB, FinnGen, and BioVU.
Odds ratio is calculated based on the effective sample sizes (Methods).
Figure 1. Genetic Variants Associated With HS in a Meta-Analysis of GWAS Results From HS ProCARE, UKB, and FinnGen.
Each dot corresponds to 1 variant; the x-axis indicates genomic position, and the y-axis shows the −log10(P value) of variant association with hidradenitis suppurativa (HS). Dots above the orange and blue lines show variants that reached significant (P < 5 × 10−8) and suggestive (P < 1 × 10−6) thresholds, respectively, for genome-wide association studies (GWASs). HS ProCARE indicates HS Program for Research and Care Excellence; UKB, UK Biobank.
Figure 2. HS Association Plots for 2 Loci With European LD.
Variants associated with hidradenitis suppurativa (HS) in the 3-way meta-analysis located at the chromosome (chr) 17 locus (A) and the chr13 locus (B). Each point corresponds to 1 variant; the x-axis indicates genomic position, and the y-axis shows the −log10(P value) of variant association with HS, and the size of each point indicates relative sample size analyzed for that variant. Variants are colored according to their linkage disequilibrium (LD) r2 with the most significant variant in each region, which is shown as a purple diamond. The LD r2 values are based on TOPMed European-ancestry individuals. Lines point to variants located in skin enhancers (see Figure 3). The nearest protein-coding genes are not located within the 400-kilobase regions shown.
Replication of Variant Association With HS in BioVU
We tested the 10 most strongly associated variants at the 3 loci for association with HS in the BioVU biobank (Methods). BioVU contains 290 HS cases, including 189 European-ancestry and 101 African-ancestry individuals, with 64 234 and 12 105 controls for the 2 ancestry groups, respectively. The chr17 locus was replicated in BioVU (P = 1.9 × 10−4) in the same direction of effect (eTable 4 in Supplement 1). One variant at the chr13 locus showed nominal evidence of association (P = .05) in the same direction. In a 4-way meta-analysis of BioVU and the first 3 studies, the chr17 locus became more significant (P = 2.3 × 10−11), and the chr13 locus exceeded the genome-wide significance threshold (P = 2.1 × 10−8) (Table 2; eTable 5 in Supplement 1). The chr11 locus was not replicated in BioVU (P = .27).
Sensitivity Analyses
We performed 2 additional meta-analyses as sensitivity analyses to validate our findings. A 4-way meta-analysis of all European-ancestry individuals replicated the chr17 (P = 5.5 × 10−9) and chr13 (P = 1.6 × 10−8) loci (eTable 8 in Supplement 1). A 6-way meta-analysis adding African-ancestry individuals from HS ProCARE and BioVU showed that the 2 loci were more significant: chr17, P = 4.0 × 10−11; and chr13, P = 3.5 × 10−9 (eTable 9 in Supplement 1). These results suggest that the signals are robust to different analysis strategies.
Genes and Candidate Regulatory Variants at HS Loci
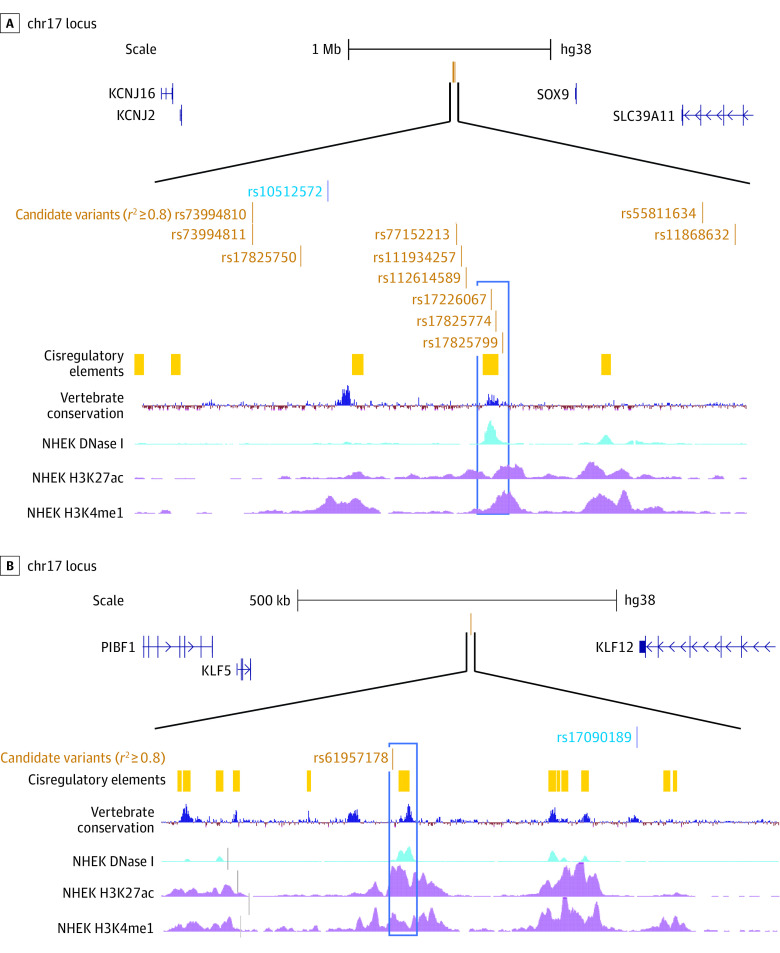
The HS-associated variants on chr17 and chr13 are located in intergenic regions (Figure 2, Figure 3). At the chr17 locus, the closest protein-coding gene, located more than 600 kb away, is SOX9, which encodes a transcription factor that regulates several developmental processes. SOX9 is expressed in the epidermal basal layer and outer root sheath (ORS) of hair follicles and is highly expressed in psoriasis and nonmelanoma skin cancers.57 Variants at this association signal, including rs17226067, rs17825774, and rs17825799, are located in a keratinocyte distal enhancer-like regulatory element (Figure 3) as defined by ENCODE SCREEN,45 suggesting that 1 or more variants may alter gene regulation.
Figure 3. Candidate Genes and Regulatory Variants at 2 Hidradenitis Suppurativa Loci.
Variants at the chromosome (chr) 17 locus (A) are located in an intergenic region more than 600 kb from SOX9. Among candidate variants, rs17226067, rs17825774, and rs17825799 (blue rectangle) are located in a regulatory region conserved across vertebrate species and characterized by DNase I hypersensitivity (accessible chromatin) and epigenomic marks of enhancers in NHEK epidermal keratinocytes (ENCODE consortium). Variants at the chr13 locus (B) are located in an intergenic region between KLF5 and KLF12, and candidate variant rs61957178 (blue rectangle) is located in a regulatory region characterized by epigenomic marks of enhancers in NHEK epidermal keratinocytes.
At the chr13 locus, the closest protein-coding genes are the transcriptional regulators KLF12 and KLF5, located approximately 260 kb and approximately 340 kb away, respectively. Deletion of KLF5 alters the skin barrier, and its expression is lower in human diseases with disrupted epidermal sphingolipid secretion.58 A variant at this locus, rs61957178, is located in a keratinocyte regulatory element (Figure 3) that may have a long-range effect on KLF5 and/or KLF12. This signal has also been associated with asthma and respiratory diseases.59,60
Genetic Correlation With Commonly Comorbid Conditions
To investigate evidence of shared genetic components between HS and autoimmune and inflammatory diseases, we performed genetic correlation analysis using LD score regression.47,48 Genetic correlation provides an estimate of the overlap in terms of evidence of associations for a pair of complex traits with existing genome-wide summary statistics, which could help understand the genetic components of HS comorbidities. We examined genetic correlation with celiac disease,49 inflammatory bowel disease,50 polycystic ovary syndrome,51 psoriasis,52 and rheumatoid arthritis,53 as well as schizophrenia54 and type 2 diabetes.55 We found nominally significant (P < .05) genetic correlation between HS and inflammatory bowel disease (rg = 0.39, P = .04), psoriasis (rg = 0.49, P = .03), and type 2 diabetes (rg = 0.18, P = .04) (eTable 6 in Supplement 1), although none of these results were significant at a stringent Bonferroni-corrected threshold (P < .05/7). These results show that inflammatory bowel disease, psoriasis, and type 2 diabetes may have correlated genetic segments with HS.
Discussion
Our GWAS meta-analysis of a cohort of patients with HS ascertained by the UNC Department of Dermatology, along with HS genetic data in large biobanks, identified variants associated with risk of HS. Variants at these loci are located in keratinocyte regulatory elements near the genes SOX9 and KLF5, suggesting that common variants may alter regulation of these or other nearby genes to influence HS risk. These genes have roles in skin and follicular inflammation, but have not previously been associated with HS pathogenesis.
The HS candidate gene SOX9, which encodes SRY-box transcription factor 9, is highly expressed in human epidermis and the ORS of hair follicles and is necessary for follicular differentiation and maintenance. The SOX9 gene upregulates MMP1, MMP2, and IL-8, which are linked to tumorigenesis and inflammation.61 Mouse models lacking SOX9 demonstrate follicular degeneration, epidermal thickening, and eventual dermal scarring. These mice also lost keratin 1 (K1) and keratin 10 (K10) expression in the basal layer of the follicular sheath,62 similar to loss of epidermal K1/K10 function in epidermolytic ichthyosis that leads to epidermal fragility, raising the possibility that follicular fragility and dissection play a role in HS pathogenesis. Blocking SOX9 expression in adult mice resulted in development of keratin pearls and loss of K1 expression at the upper ORS, leading to epidermal differentiation and loss of hair follicle stem cells.63 This differentiation toward epidermis may explain the development of characteristic cysts, comedones, and epithelialized tunnels.
Similarly, the candidate gene KLF5, which encodes Kruppel-like factor 5, may affect HS pathogenesis. Overexpression of mouse KLF5, a critical regulator of sphingolipid-metabolizing and secreting enzymes in the skin, led to hyperkeratosis, follicular occlusion, and skin erosions, which are characteristic of HS.64 Deletions of KLF5 disrupt barrier function and have been associated with atopic dermatitis, nonhealing wounds,58 and IL-17–driven colitis in mice.65 Colitis induced by KLF5 deletion was associated with altered gut microbiota and stimulation of the IL-22/STAT3/IL-17 pathway. This colitis was relieved by antibiotics and IL-17–neutralizing antibodies, similar to HS treatment responses.66,67,68 In human colitis, reduced intestinal KLF5 expression is linked to increased disease severity.65
Remarkably, genes at the 2 loci appear to have directly opposing and/or complementary roles in epithelial differentiation. The KLF5 gene is present primarily in epidermal keratinocytes that do not express SOX9, which is primarily expressed in the hair bulge.58 The KLF5 gene functions as a regulator of epidermal stem cells, while SOX9 is a primary regulator of hair follicle stem cells. More specifically, KLF5 and SOX9 expression demarcate epidermal and hair follicle differentiation, respectively. Coexpression is induced transiently during wound healing as stem cell populations are activated for repair, and induced expression of KLF5 downregulates SOX9 expression. Disruption of homeostasis in these expression patterns may explain the formation of chronic wounds such as inflammatory tunnels and ulcers in HS, as well as cysts and epithelialized tunnels that lack typical differentiation toward hair follicles or epidermis.
Genetic correlations of HS with common comorbidities such as psoriasis, inflammatory bowel disease, and type 2 diabetes suggest that shared genetic mechanisms may predispose to risk. Further investigations are needed with better-powered HS GWASs to evaluate other comorbidities and to uncover the mechanisms of shared risk.
Strengths and Limitations
An advantage of our work is the multiple large cohorts to validate association results, including ancestrally diverse patients with well-characterized disease and background. Careful selection of controls is vital for case-only participants. We used considerable effort to match the HS ProCARE cases with appropriate external controls, and Add Health controls resulted in negligible P value inflation. However, some Add Health participants may have HS, which would lead to misclassification bias toward the null. While HS ProCARE includes patients with ancestry from Europe and Africa, inadequate sample sizes limit our ability to detect significant loci within ancestry groups or assess generalizability in other ancestries.
Conclusions
In this genetic association study, as with all GWASs, identifying variant associations near candidate genes with plausible roles in disease biology does not prove a causal effect of these variants or genes on disease risk. Functional studies must assess whether the HS-associated variants and surrounding regulatory elements can alter SOX9 and KLF5 function or expression and, subsequently, affect HS pathology. These genes are strong candidates because they affect hair follicle and epidermal differentiation and inflammation. Other nearby genes such as KLF12 could also play a role. Larger sample sizes and sequencing are needed to detect rare gene variants, such as γ-secretase complex variants, that are implicated in small numbers of patients. Ultimately, understanding how genes lead to tunnel formation and an overactive inflammatory response may lead to therapeutic breakthroughs that target HS pathogenesis.
eTable 1. Variants most strongly associated with HS in ProCARE patients (n=720) and AddHealth controls (n=5801) passing suggestive threshold (p < 1e-6) in combined analysis with all the samples
eTable 2. Variants most strongly associated with HS in ProCARE patients and AddHealth controls passing a suggestive threshold (p < 1E-6) in ancestry-specific analyses
eTable 3. Most strongly associated variants at each of three suggestive or significant HS loci in the three-way meta-analysis of ProCARE, UKB and FinnGen
eTable 4. HS association results in BioVU for the most strongly-associated variants at three loci
eTable 5. HS association results from a meta-analysis of ProCARE, UKB, FinnGen and BioVU
eTable 6. Genetic correlation analyses
eTable 7. Detailed information for studies participating in HS meta-analysis
eTable 8. Sensitivity analyses 1: four-way meta-analysis of UKB, FinnGen, ProCARE-EUR and BioVU-EUR
eTable 9. Sensitivity analyses 2: six-way meta-analysis of UKB, FinnGen, ProCARE-EUR, BioVU-EUR, ProCARE-AFR and BioVU-AFR
eTable 10. Case- and control- specific effect allele frequencies for the top signals
eFigure 1. Genetic variants associated with HS in ProCARE patients and AddHealth controls
eFigure 2. Genetic variants associated with HS in ProCARE patients and AddHealth controls of primarily European ancestry
eFigure 3. Genetic variants associated with HS in ProCARE patients and AddHealth controls of primarily African ancestry
eFigure 4. QQ plot of GWAS for ProCARE patients and AddHealth controls
eFigure 5. QQ plot of GWAS for ProCARE patients and AddHealth controls of primarily European ancestry
eFigure 6. QQ plot of GWAS for ProCARE patients and AddHealth controls of primarily African ancestry
eFigure 7. HS association plots for two loci colored based on African LD
eFigure 8. HS association plots for two loci with recombination rates
Data Sharing Statement
References
- 1.Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81(1):76-90. doi: 10.1016/j.jaad.2019.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Straalen KR, Prens EP, Willemsen G, Boomsma DI, van der Zee HH. Contribution of genetics to the susceptibility to hidradenitis suppurativa in a large, cross-sectional Dutch twin cohort. JAMA Dermatol. 2020;156(12):1359-1362. doi: 10.1001/jamadermatol.2020.3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kjaersgaard Andersen R, Clemmensen SB, Larsen LA, et al. Evidence of gene-gene interaction in hidradenitis suppurativa: a nationwide registry study of Danish twins. Br J Dermatol. 2022;186(1):78-85. doi: 10.1111/bjd.20654 [DOI] [PubMed] [Google Scholar]
- 4.Fitzsimmons JS, Guilbert PR. A family study of hidradenitis suppurativa. J Med Genet. 1985;22(5):367-373. doi: 10.1136/jmg.22.5.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Von Der Werth JM, Williams HC, Raeburn JA. The clinical genetics of hidradenitis suppurativa revisited. Br J Dermatol. 2000;142(5):947-953. doi: 10.1046/j.1365-2133.2000.03476.x [DOI] [PubMed] [Google Scholar]
- 6.Pink AE, Simpson MA, Desai N, et al. Mutations in the γ-secretase genes NCSTN, PSENEN, and PSEN1 underlie rare forms of hidradenitis suppurativa (acne inversa). J Invest Dermatol. 2012;132(10):2459-2461. doi: 10.1038/jid.2012.162 [DOI] [PubMed] [Google Scholar]
- 7.Bruinsma RL, Fajgenbaum K, Yang Y, et al. Assessment of familial risk in patients with hidradenitis suppurativa. Br J Dermatol. 2021;184(4):753-754. doi: 10.1111/bjd.19664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frew JW, Vekic DA, Woods J, Cains GD. A systematic review and critical evaluation of reported pathogenic sequence variants in hidradenitis suppurativa. Br J Dermatol. 2017;177(4):987-998. doi: 10.1111/bjd.15441 [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira ASLE, Bloise G, Moltrasio C, et al. Transcriptome meta-analysis confirms the hidradenitis suppurativa pathogenic triad: upregulated inflammation, altered epithelial organization, and dysregulated metabolic signaling. Biomolecules. 2022;12(10):1371. doi: 10.3390/biom12101371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frings VG, Jopp L, Srivastava M, Presser D, Goebeler M, Schmidt M. Stress signalling and STAT1 activation characterize the keratinocytic gene expression pattern in Hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2022;36(12):2488-2498. doi: 10.1111/jdv.18465 [DOI] [PubMed] [Google Scholar]
- 11.Gudjonsson JE, Tsoi LC, Ma F, et al. Contribution of plasma cells and B cells to hidradenitis suppurativa pathogenesis. JCI Insight. 2020;5(19):e139930. doi: 10.1172/jci.insight.139930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rumberger BE, Boarder EL, Owens SL, Howell MD. Transcriptomic analysis of hidradenitis suppurativa skin suggests roles for multiple inflammatory pathways in disease pathogenesis. Inflamm Res. 2020;69(10):967-973. doi: 10.1007/s00011-020-01381-7 [DOI] [PubMed] [Google Scholar]
- 13.Frew JW, Grand D, Navrazhina K, Krueger JG. Beyond antibodies: B cells in hidradenitis suppurativa: bystanders, contributors or therapeutic targets? Exp Dermatol. 2020;29(5):509-515. doi: 10.1111/exd.14092 [DOI] [PubMed] [Google Scholar]
- 14.Zouboulis CC, Nogueira da Costa A, Makrantonaki E, et al. Alterations in innate immunity and epithelial cell differentiation are the molecular pillars of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2020;34(4):846-861. doi: 10.1111/jdv.16147 [DOI] [PubMed] [Google Scholar]
- 15.Hoffman LK, Tomalin LE, Schultz G, et al. Integrating the skin and blood transcriptomes and serum proteome in hidradenitis suppurativa reveals complement dysregulation and a plasma cell signature. PLoS One. 2018;13(9):e0203672. doi: 10.1371/journal.pone.0203672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hessam S, Sand M, Gambichler T, Skrygan M, Rüddel I, Bechara FG. Interleukin-36 in hidradenitis suppurativa: evidence for a distinctive proinflammatory role and a key factor in the development of an inflammatory loop. Br J Dermatol. 2018;178(3):761-767. doi: 10.1111/bjd.16019 [DOI] [PubMed] [Google Scholar]
- 17.Witte K, Sabat R, Witte-Händel E, Ghoreschi K, Wolk K. Phytotherapeuthics affecting the IL-1/IL-17/G-CSF axis: a complementary treatment option for hidradenitis suppurativa? Int J Mol Sci. 2022;23(16):9057. doi: 10.3390/ijms23169057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenei A, Dajnoki Z, Medgyesi B, et al. Apocrine gland-rich skin has a non-inflammatory IL-17-related immune milieu, that turns to inflammatory IL-17-mediated disease in hidradenitis suppurativa. J Invest Dermatol. 2019;139(4):964-968. doi: 10.1016/j.jid.2018.10.020 [DOI] [PubMed] [Google Scholar]
- 19.Thomi R, Cazzaniga S, Seyed Jafari SM, Schlapbach C, Hunger RE. Association of hidradenitis suppurativa with T helper 1/T helper 17 phenotypes: a semantic map analysis. JAMA Dermatol. 2018;154(5):592-595. doi: 10.1001/jamadermatol.2018.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melnik BC, John SM, Chen W, Plewig G. T helper 17 cell/regulatory T-cell imbalance in hidradenitis suppurativa/acne inversa: the link to hair follicle dissection, obesity, smoking and autoimmune comorbidities. Br J Dermatol. 2018;179(2):260-272. doi: 10.1111/bjd.16561 [DOI] [PubMed] [Google Scholar]
- 21.Matusiak Ł, Szczęch J, Bieniek A, Nowicka-Suszko D, Szepietowski JC. Increased interleukin (IL)-17 serum levels in patients with hidradenitis suppurativa: implications for treatment with anti-IL-17 agents. J Am Acad Dermatol. 2017;76(4):670-675. doi: 10.1016/j.jaad.2016.10.042 [DOI] [PubMed] [Google Scholar]
- 22.Hotz C, Boniotto M, Guguin A, et al. Intrinsic defect in keratinocyte function leads to inflammation in hidradenitis suppurativa. J Invest Dermatol. 2016;136(9):1768-1780. doi: 10.1016/j.jid.2016.04.036 [DOI] [PubMed] [Google Scholar]
- 23.Lima AL, Karl I, Giner T, et al. Keratinocytes and neutrophils are important sources of proinflammatory molecules in hidradenitis suppurativa. Br J Dermatol. 2016;174(3):514-521. doi: 10.1111/bjd.14214 [DOI] [PubMed] [Google Scholar]
- 24.Navrazhina K, Frew JW, Gilleaudeau P, Sullivan-Whalen M, Garcet S, Krueger JG. Epithelialized tunnels are a source of inflammation in hidradenitis suppurativa. J Allergy Clin Immunol. 2021;147(6):2213-2224. doi: 10.1016/j.jaci.2020.12.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris KM, Halpern CT, Whitsel EA, et al. Cohort profile: the National Longitudinal Study of Adolescent to Adult Health (Add Health). Int J Epidemiol. 2019;48(5):1415-1415k. doi: 10.1093/ije/dyz115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris KM, Halpern CT, Smolen A, Haberstick BC. The National Longitudinal Study of Adolescent Health (Add Health) twin data. Twin Res Hum Genet. 2006;9(6):988-997. doi: 10.1375/twin.9.6.988 [DOI] [PubMed] [Google Scholar]
- 27.Kurki MI, Karjalainen J, Palta P, et al. FinnGen: unique genetic insights from combining isolated population and national health register data. medRxiv. Preprint posted online March 6, 2022. doi: 10.1101/2022.03.03.22271360 [DOI]
- 28.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203-209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roden DM, Pulley JM, Basford MA, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84(3):362-369. doi: 10.1038/clpt.2008.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Q, Yang Y, Rosen JD, et al. MagicalRsq: machine-learning-based genotype imputation quality calibration. Am J Hum Genet. 2022;109(11):1986-1997. doi: 10.1016/j.ajhg.2022.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Q, Liu W, Rosen JD, et al. ; Cystic Fibrosis Genome Project . Leveraging TOPMed imputation server and constructing a cohort-specific imputation reference panel to enhance genotype imputation among cystic fibrosis patients. HGG Adv. 2022;3(2):100090. doi: 10.1016/j.xhgg.2022.100090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen J, Xie M, Rowland B, et al. Transcriptome-wide association study of blood cell traits in African ancestry and Hispanic/Latino populations. Genes (Basel). 2021;12(7):1049. doi: 10.3390/genes12071049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loh PR, Danecek P, Palamara PF, et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet. 2016;48(11):1443-1448. doi: 10.1038/ng.3679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Das S, Forer L, Schönherr S, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48(10):1284-1287. doi: 10.1038/ng.3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang HM, Sul JH, Service SK, et al. Variance component model to account for sample structure in genome-wide association studies. Nat Genet. 2010;42(4):348-354. doi: 10.1038/ng.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Q, Graff M, Rowland B, et al. Analyses of biomarker traits in diverse UK biobank participants identify associations missed by European-centric analysis strategies. J Hum Genet. 2022;67(2):87-93. doi: 10.1038/s10038-021-00968-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W, Sun Q, Huang L, et al. Innovative computational approaches shed light on genetic mechanisms underlying cognitive impairment among children born extremely preterm. J Neurodev Disord. 2022;14(1):16. doi: 10.1186/s11689-022-09429-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mbatchou J, Barnard L, Backman J, et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat Genet. 2021;53(7):1097-1103. doi: 10.1038/s41588-021-00870-7 [DOI] [PubMed] [Google Scholar]
- 39.Kurki MI, Karjalainen J, Palta P, et al. ; FinnGen . FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613(7944):508-518. doi: 10.1038/s41586-022-05473-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190-2191. doi: 10.1093/bioinformatics/btq340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang L, Rosen JD, Sun Q, et al. ; NHLBI Trans-Omics for Precision Medicine (TOPMed) Consortium . TOP-LD: a tool to explore linkage disequilibrium with TOPMed whole-genome sequence data. Am J Hum Genet. 2022;109(6):1175-1181. doi: 10.1016/j.ajhg.2022.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kent WJ, Sugnet CW, Furey TS, et al. The human genome browser at UCSC. Genome Res. 2002;12(6):996-1006. doi: 10.1101/gr.229102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Consortium EP; ENCODE Project Consortium . An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57-74. doi: 10.1038/nature11247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo Y, Hitz BC, Gabdank I, et al. New developments on the Encyclopedia of DNA Elements (ENCODE) data portal. Nucleic Acids Res. 2020;48(D1):D882-D889. doi: 10.1093/nar/gkz1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore JE, Purcaro MJ, Pratt HE, et al. ; ENCODE Project Consortium . Expanded encyclopaedias of DNA elements in the human and mouse genomes. Nature. 2020;583(7818):699-710. doi: 10.1038/s41586-020-2493-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Consortium GT; GTEx Consortium . The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369(6509):1318-1330. doi: 10.1126/science.aaz1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bulik-Sullivan BK, Loh PR, Finucane HK, et al. ; Schizophrenia Working Group of the Psychiatric Genomics Consortium . LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47(3):291-295. doi: 10.1038/ng.3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bulik-Sullivan B, Finucane HK, Anttila V, et al. ; ReproGen Consortium; Psychiatric Genomics Consortium; Genetic Consortium for Anorexia Nervosa of the Wellcome Trust Case Control Consortium 3 . An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47(11):1236-1241. doi: 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang L, Zheng Z, Fang H, Yang J. A generalized linear mixed model association tool for biobank-scale data. Nat Genet. 2021;53(11):1616-1621. doi: 10.1038/s41588-021-00954-4 [DOI] [PubMed] [Google Scholar]
- 50.Liu JZ, van Sommeren S, Huang H, et al. ; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium . Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979-986. doi: 10.1038/ng.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Day F, Karaderi T, Jones MR, et al. ; 23andMe Research Team . Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 2018;14(12):e1007813. doi: 10.1371/journal.pgen.1007813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sakaue S, Kanai M, Tanigawa Y, et al. ; FinnGen . A cross-population atlas of genetic associations for 220 human phenotypes. Nat Genet. 2021;53(10):1415-1424. doi: 10.1038/s41588-021-00931-x [DOI] [PubMed] [Google Scholar]
- 53.Saevarsdottir S, Stefansdottir L, Sulem P, et al. ; Members of the DBDS Genomic Consortium; Danish RA Genetics Working Group; Swedish Rheumatology Quality Register Biobank Study Group (SRQb) . Multiomics analysis of rheumatoid arthritis yields sequence variants that have large effects on risk of the seropositive subset. Ann Rheum Dis. 2022;81(8):1085-1095. doi: 10.1136/annrheumdis-2021-221754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trubetskoy V, Pardiñas AF, Qi T, et al. ; Indonesia Schizophrenia Consortium; PsychENCODE; Psychosis Endophenotypes International Consortium; SynGO Consortium; Schizophrenia Working Group of the Psychiatric Genomics Consortium . Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604(7906):502-508. doi: 10.1038/s41586-022-04434-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahajan A, Spracklen CN, Zhang W, et al. ; FinnGen; eMERGE Consortium . Multi-ancestry genetic study of type 2 diabetes highlights the power of diverse populations for discovery and translation. Nat Genet. 2022;54(5):560-572. doi: 10.1038/s41588-022-01058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van der Zee HH, Jemec GB. New insights into the diagnosis of hidradenitis suppurativa: clinical presentations and phenotypes. J Am Acad Dermatol. 2015;73(5)(suppl 1):S23-S26. doi: 10.1016/j.jaad.2015.07.047 [DOI] [PubMed] [Google Scholar]
- 57.Shi G, Sohn KC, Li Z, et al. Expression and functional role of Sox9 in human epidermal keratinocytes. PLoS One. 2013;8(1):e54355. doi: 10.1371/journal.pone.0054355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyu Y, Guan Y, Deliu L, et al. KLF5 governs sphingolipid metabolism and barrier function of the skin. Genes Dev. 2022;36(13-14):822-842. doi: 10.1101/gad.349662.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han Y, Jia Q, Jahani PS, et al. Genome-wide analysis highlights contribution of immune system pathways to the genetic architecture of asthma. Nat Commun. 2020;11(1):1776. doi: 10.1038/s41467-020-15649-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kichaev G, Bhatia G, Loh PR, et al. Leveraging polygenic functional enrichment to improve GWAS power. Am J Hum Genet. 2019;104(1):65-75. doi: 10.1016/j.ajhg.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo H, Wang C, Liu M, et al. Inhibition of SOX9 promotes inflammatory and immune responses of dental pulp. J Endod. 2018;44(5):792-799. doi: 10.1016/j.joen.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 62.Vidal VP, Chaboissier MC, Lützkendorf S, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15(15):1340-1351. doi: 10.1016/j.cub.2005.06.064 [DOI] [PubMed] [Google Scholar]
- 63.Kadaja M, Keyes BE, Lin M, et al. SOX9: a stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev. 2014;28(4):328-341. doi: 10.1101/gad.233247.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sur I, Rozell B, Jaks V, Bergström A, Toftgård R. Epidermal and craniofacial defects in mice overexpressing Klf5 in the basal layer of the epidermis. J Cell Sci. 2006;119(pt 17):3593-3601. doi: 10.1242/jcs.03070 [DOI] [PubMed] [Google Scholar]
- 65.Shieh J, Chu TH, Liu Y, et al. KLF5 protects the intestinal epithelium against Th17 immune response in a murine colitis model. JCI Insight. 2022;7(7):e153488. doi: 10.1172/jci.insight.153488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81(1):91-101. doi: 10.1016/j.jaad.2019.02.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Navrazhina K, Frew JW, Grand D, et al. Interleukin-17RA blockade by brodalumab decreases inflammatory pathways in hidradenitis suppurativa skin and serum. Br J Dermatol. 2022;187(2):223-233. doi: 10.1111/bjd.21060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.is Kashetsky N, Mufti A, Alabdulrazzaq S, et al. Treatment outcomes of IL-17 inhibitors in hidradenitis suppurativa: a systematic review. J Cutan Med Surg. 2022;26(1):79-86. doi: 10.1177/12034754211035667 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Variants most strongly associated with HS in ProCARE patients (n=720) and AddHealth controls (n=5801) passing suggestive threshold (p < 1e-6) in combined analysis with all the samples
eTable 2. Variants most strongly associated with HS in ProCARE patients and AddHealth controls passing a suggestive threshold (p < 1E-6) in ancestry-specific analyses
eTable 3. Most strongly associated variants at each of three suggestive or significant HS loci in the three-way meta-analysis of ProCARE, UKB and FinnGen
eTable 4. HS association results in BioVU for the most strongly-associated variants at three loci
eTable 5. HS association results from a meta-analysis of ProCARE, UKB, FinnGen and BioVU
eTable 6. Genetic correlation analyses
eTable 7. Detailed information for studies participating in HS meta-analysis
eTable 8. Sensitivity analyses 1: four-way meta-analysis of UKB, FinnGen, ProCARE-EUR and BioVU-EUR
eTable 9. Sensitivity analyses 2: six-way meta-analysis of UKB, FinnGen, ProCARE-EUR, BioVU-EUR, ProCARE-AFR and BioVU-AFR
eTable 10. Case- and control- specific effect allele frequencies for the top signals
eFigure 1. Genetic variants associated with HS in ProCARE patients and AddHealth controls
eFigure 2. Genetic variants associated with HS in ProCARE patients and AddHealth controls of primarily European ancestry
eFigure 3. Genetic variants associated with HS in ProCARE patients and AddHealth controls of primarily African ancestry
eFigure 4. QQ plot of GWAS for ProCARE patients and AddHealth controls
eFigure 5. QQ plot of GWAS for ProCARE patients and AddHealth controls of primarily European ancestry
eFigure 6. QQ plot of GWAS for ProCARE patients and AddHealth controls of primarily African ancestry
eFigure 7. HS association plots for two loci colored based on African LD
eFigure 8. HS association plots for two loci with recombination rates
Data Sharing Statement