Abstract
Background
Asian Indians are at higher risk of cardiometabolic disease compared to other ethnic groups, and the age of onset is typically younger. Cardiac structure and function are poorly characterized in this ethnic group. In this study, we describe image-acquisition methods and the reproducibility of measurements and detailed echocardiography characteristics in two large Indian population-based cohorts (the New Delhi and Vellore Birth Cohorts) from India.
Methods
The IndEcho study captured transthoracic echocardiographic measurements of cardiac structure and function from 2,322 men and women aged 43–50 years. M-mode measurements in the parasternal long axis (PLAX) and 2-dimensional (2D) short axis recordings at the mitral valve, mid-papillary and apical level were recorded. Apical 2D recordings of two- three- and four-chamber (2C, 3C and 4C) views and Doppler images (colour, pulsed and continuous) were recorded in cine-loop format. Left ventricular (LV) mass, LV hypertrophy, and indices of LV systolic and diastolic function were derived.
Results
Echocardiographic measurements showed good/excellent technical reproducibility. Hetero-geneity across sites, sex and rural/urban differences in cardiac structure and function were observed. Overall, this cohort of South Asian Indians had smaller LV mass and normal systolic and diastolic function when compared with published data on other Asian Indians and the West, (LV mass indexed for body surface area: Delhi men: 68 g/m2, women 63.9; Vellore men: 65.8, women 61.6) but were within ethnic-specific reference ranges. The higher prevalence of obesity, diabetes and hypertension is reflected by the higher proportion of LV remodelling and lesser hypertrophy.
Conclusions
Our study adds to scarce population-based echocardiographic data for mid-life Asian Indians. Compared to published literature on other ethnic groups, the Asian Indian heart is characterised by smaller cardiac dimensions and normal range systolic and diastolic function on a background of a high prevalence of hypertension, diabetes and cardiac disease at a relatively young age. This data will form the basis for further analyses of lifecourse, metabolic and body composition predictors of cardiac structure and function, and echocardiographic predictors of future mortality.
ISRCTN registration number
13432279
Keywords: echocardiography, Asian Indian, cardiac size, systolic function, diastolic function
Introduction
Cardiovascular disease (CVD) is a major cause of premature mortality globally, accounting for about 52% in India and less than 20% in high income countries (1–3). Differences in CVD mortality and morbidity exist between various ethnic groups (4, 5). South Asian Indians develop CVD at a younger age and lower body mass index (BMI) compared to Europeans/white caucasians (1). These differences have been attributed to numerous factors, including the coexistence of and interaction with key drivers of CVD such as lifestyle factors, diabetes, hypertension and obesity (3). Left ventricular (LV) geometry and function are associated with CVD and mortality independent of conventional risk factors (6) but there is sparse data on LV structure and function, and their relationship to these risk factors, in Asian Indians.
The IndEcho study was designed to evaluate the associations of birth size, early childhood growth and adult CVD risk factors with mid-life cardiac structure and function in South Asian Indians, assessed using transthoracic echocardiography (7). In the current paper, we describe the image-acquisition methodology in over 2,000 participants aged 43–50 years with the aims of providing (1) descriptive data of cardiac structure and function in the cohort, (2) descriptive data of these measures in an apparently healthy sub-set free of CVD, diabetes and hypertension and (3) reproducibility data for the measurements.
Materials and methods
The IndEcho study used standardized protocols, in accordance with the 2015 Joint Update of American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) (8), to measure left ventricular (LV) and left atrial dimensions, systolic and diastolic function. Measurements were recorded by trained and experienced sonographers (two each in Delhi and Vellore) under the regular supervision of cardiologists, using transthoracic echocardiography on a Philips CX50 Compact Xtreme system equipped with an IPx-7, S5-1 (Cardiac Sector Probe) transducer. M-mode measurements in the parasternal long axis (PLAX) and short axis recordings at mitral valve, mid-papillary and apical levels were recorded. Apical 2D recordings of two- four- and three chamber (2C, 4C and 3C) views and Doppler images (colour, pulsed and continuous wave on three consecutive cardiac cycles) were recorded in cine-loop format. After joint training sessions before the study started, the measurements were recorded at the two centres independently, but all images were analysed using a Freeland digitizer and software packages (Alpharetta, USA) by a single echo-technician in Vellore (JS). A list of all cardiac parameters measured are provided in Table 1.
Table 1.
IndEcho echocardiographic measurements.
| Primary measures | Derived measures | |
|---|---|---|
| Cardiac size | LV wall thickness | Relative wall thickness (RWT) = [(2 X PWT)/LVIDD]) |
| LVIDD, LVISD | Fractional shortening = (LVIDd-LVIDs)/LVIDd | |
| Septal thickness | ||
| Relative wall thickness | ||
| LVEDV, LVESV | ||
| LV mass | ||
| Systolic function | Global longitudinal strain | LV-ejection fraction = (LVEDV-LVESV)/LVEDV |
| Diastolic function | E | E/A ratio = E wave/A wave |
| A | Average E/e’ = Average medial E/average lateral e' | |
| Medial e’ | LA volume - LA volume indexed to BSA | |
| Lateral e’ | ||
| TR max | ||
| Definition of categories | ||
| LV mass category | ||
| Concentric re-modelling | LV mass ≤102 g in men or ≤88 g in women AND RWT > 0.42 | |
| Concentric hypertrophy | LV mass >102 g in men or >88 g in women AND RWT > 0.42 | |
| Eccentric hypertrophy | LV mass >102 g in men or >88 g in women AND RWT ≤ 0.42 | |
| No remodeling or hypertrophy | LV mass ≤102 g in men or ≤88 g in women AND RWT ≤ 0.42 | |
| GLS categories | ||
| Normal GLS | <minus 17% | |
| Borderline GLS | minus 17–minus 15 | |
| Abnormal GLS | >minus 15% | |
| LV diastolic dysfunction | ||
| None | ||
| Indeterminate | if exactly two of the following abnormalities are present: 1) septal (medial) e' <7 cm/sec or lateral e' <10 cm/sec; 2) E/e' >14; 3) LA volume >34 ml/m2; and 4) TRmax >2.8 m/sec. | |
| Dysfunction | if more than two of the following four abnormalities were present: 1) septal (medial) e' <7 cm/sec or lateral e' <10 cm/sec; 2) E/e' >14; 3) LA volume >34 ml/m2; and 4) TRmax >2.8 m/sec. | |
LV, left ventricle; PWT, posterior wall thickness; LVIDd and LVIDs, left ventricular internal diameter in diastole and systole; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; LA, left atrial; BSA, body surface area; GLS, global longitudinal strain; E, passive ejection of left atrium; A, active atrial contraction; e', diastolic annular velocity.
The IndEcho cohorts
The IndEcho study is a longitudinal observational study that recalled participants from two birth cohorts in New Delhi (NDBC) and Vellore (VBC), India. These are prospective population-based cohorts originally set up in 1969–1972 to collect data on birth outcomes and infant mortality. The original cohorts included 18,700 singleton live births recruited from urban (the city of New Delhi and Vellore town) and rural (Vellore only) populations. Both studies collected longitudinal data on the growth of the children. In an adult follow-up at the age of 26–33 years, data was collected on a range of classical CVD risk factors. Details of both cohorts and previous rounds of follow-up are presented elsewhere (9–11). In the current study, men and women aged 43–46 years underwent echocardiography and cardiometabolic phenotyping. The sampling frame included all the men and women who took part in the 26–33 year follow-up in both cohorts (N = 1,526 in New Delhi and N = 2,218 in Vellore). The study was approved by the Health Ministry Steering, Government of India, institutional ethics committees of the participating centres and the University of Southampton, UK.
Clinical assessment
All participants, after consenting to participate, underwent clinical assessment using standardised protocols. Assessment included anthropometry (height, weight, waist and hip circumferences), biochemistry (fasting glucose and an oral glucose tolerance test, fasting plasma insulin and serum lipids, urinary albumin-creatinine ratio as a measure of microalbuminuria), assessments of life-style behaviour (tobacco and alcohol consumption, physical activity) and socio-economic status [using the standard of living index (SLI)], detailed echocardiographic assessment of LV dimensions and function and a 12-lead ECG with Minnesota coding. SLI was calculated as a composite score based on education, type of housing, household amenities, and material possessions (12).
Echocardiographic measurements
Cardiac size
LV internal dimensions in systole (LVIDs) and diastole (LVIDd), inter-ventricular septal thickness (IVS) and posterior wall thickness (PWT) were measured by M-mode using the leading-edge method. Relative wall thickness (RWT) was computed using the formula [(2 X PWT)/LVIDd]. Fractional shortening (FS) was calculated as the ratio of the difference between LVIDd and LVIDs to LVIDd. LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were measured from trans-thoracic 2D recordings using the modified Simpson's biplane method, indexed to body surface area (BSA). LV mass was derived using the area-length method by contouring the epicardial and endocardial cross-sectional areas at the papillary muscle level along with LV length measured from the from the mitral annulus to the apex.
Systolic function
From the 2D-recordings of LVEDV and LVESV, LV ejection fraction (LVEF) was calculated as [(LVEDV-LVESV)/LVEDV]. Global longitudinal strain (GLS) was computed by speckle tracking from the acquired 2-D; 2-, 3- and 4-chamber and long axis views using the in-built QLAB software in the CX50 echo machine. Measurements were acquired offline in standard grey-scale with a frame rate of at least 40 frames per minute.
Diastolic function
Myocardial velocities were measured using tissue-doppler imaging at septal and lateral mitral annulus. Trans-mitral inflow parameters were measured in early and late diastole in the apical 4C-view. Peak mitral flow velocities (early E and late A) of LV filling, the deceleration time (DT) of the E-wave, and the ratio of E/A was calculated. Peak mitral annular velocity (e') was measured in the apical 4C-view at the septal and lateral mitral annulus, and the average e' was calculated from two (septal and lateral) mitral annular segments. The E/e' ratio was derived subsequently. The LA volumes (indexed to BSA) were recorded in both 2C- and 4C-views and the average of these values was calculated. Right ventricular systolic pressure (RVSP) was determined from peak TR jet velocity, using the simplified Bernoulli equation, and combining this value with an estimate of the RA pressure: RVSP = 4(V)2 + RA pressure, where V is the peak velocity (in meters per second) of the tricuspid valve regurgitant jet. The RA pressure was estimated using the IVC diameter and collapsibility using criteria described by the ASE.
Outcome definitions
Type 2 diabetes (T2DM) was diagnosed from the fasting and 120 min glucose concentration after a 75 g oral glucose load using WHO criteria (13). Participants were also defined as having T2DM if they had a prior diagnosis of the condition managed by diet and/or medication. Hypertension was defined as a systolic blood pressure ≥140 mmHg or diastolic pressure ≥90 mmHg or being on prescribed treatment for hypertension. A past history of myocardial infarction was defined as a history of a heart attack diagnosed by their treating physician, and/or the presence of major Q waves on the ECG. Overweight was defined as BMI > 25–<30 kg/m2 and obesity as BMI > 30 kg/m2.
Cardiac chamber dimensions were calculated according to ASE/EACVI guidelines. LV remodelling and hypertrophy were categorised based on LV mass (BSA indexed) and RWT as concentric remodelling (LV mass ≤102 g in men/≤88 g in women and RWT >0.42), concentric hypertrophy (LV mass >102 g in men/>88 g in women and RWT > 0.42), and eccentric hypertrophy (LV mass >102 g in men/>88 g in women and RWT ≤ 0.42) (8).
Systolic dysfunction was based on EF and GLS values. Low EF was defined as <52% in men and <54% in women (12). GLS was categorised as normal, borderline or abnormal if strain measurements were <−17%, between −17 and −15%, or >−15% respectively (8).
Diastolic dysfunction was defined only if the EF was normal according to the criteria proposed by the joint ASE/European Association of Echocardiography guidelines (14). Diastolic dysfunction was defined if more than two of the following four abnormalities were present: (1) septal (medial) e' <7 cm/sec or lateral e' <10 cm/sec; (2) E/e' >14; (3) LA volume >34 ml/m2; and (4) TRmax >2.8 m/sec. If exactly two of these abnormalities were present, diastolic function was defined as indeterminate.
Quality control and reproducibility of measurements
The quality of images was assessed by cardiologists. Images deemed “good” or “fair” were included in the analysis. “Good” was defined as adequate images with clear endocardial and epicardial boundaries, allowing clear measurements of the IVS, PW, LVID in PLAX and for calculating LV volumes and LVEF from short axis and apical projections. “Fair” was defined as adequate apical views allowing the tracing of the endocardial boundary for apical 2- and 3-chamber views and adequate calculations of LVEF, but inadequate short axis views, precluding optimal wall motion and LV area estimates, or clear measurements for IVS, PW and LVID in only one view. Unsatisfactory images were defined as those in which it was not possible to trace endocardial boundaries and therefore to calculate reliable LVEF, LV areas or wall motion abnormalities, and/or M-mode projections inadequate to measure IVS, PW or LVID.
To quantify the reproducibility of measurements, the intra-observer variation of selected echocardiographic measurements (pre-specified indices of LV systolic function, LV diastolic function and LV mass) was analysed in two independent readings from the recordings of a subset of 20 randomly selected participants.
Statistical analysis
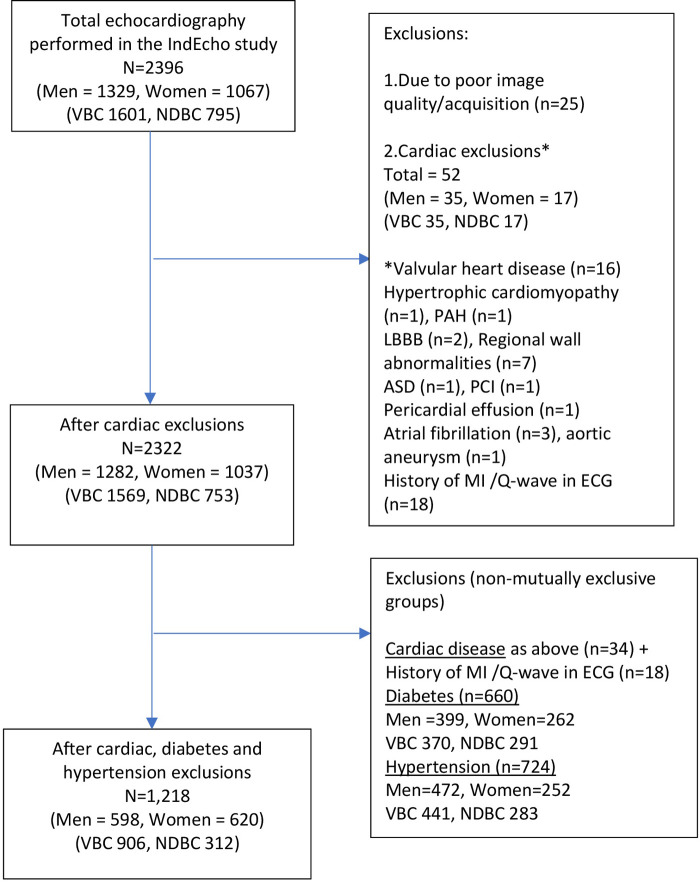
Intra-observer reproducibility was assessed by intraclass correlation coefficients (ICC) with 95% CI, interpreted as follows: ICC < 0.5 as poor reproducibility; ICC, 0.5–<0.75 as moderate; ICC, 0.75–0.9 as good; and >0.9 as excellent (15). Agreement was measured by Bland-Altman (BA) analysis and reported as the mean difference with 95% limits of agreement. Descriptive data are presented stratified by cohort and sex, because of differences in the frequencies of underlying co-morbidities such as diabetes, hypertension and heart disease. Continuous variables are presented as mean and standard deviation (SD) or median and interquartile range (IQR) depending on the skewness of the data. Frequency (percentages, %) were calculated for categorised variables. We present data for the whole sample, excluding individuals with known major cardiac disease likely to distort the echocardiographic measurements (valve disease, septal defects, hypertrophic cardiomyopathy, and pericardial effusion) (Table 2) and separately for a “healthy” sub-set free of hypertension, diabetes or a history of myocardial infarction (Table 3). A study flowchart and exclusions are presented in Figure 1. All analyses were performed using the STATA statistical package, version IC/16.0 (StataCorp LLC, United States). A p-value of <0.05 was considered statistically significant.
Table 2.
Descriptive data stratified by cohort (NDBC and VBC) free of major structural cardiac disease.
| Variable units | DELHI | VELLORE | p-value between sexes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MALES | FEMALES | MALES | FEMALES | ||||||
| N | Mean SD/Median IQR†/N % | N | Mean SD/Median IQR†/N % | N | Mean SD/Median IQR†/N % | N | Mean SD/Median IQR†/N % | ||
| Clinical characteristics | |||||||||
| Age (years) | 460 | 46.1 (1.13) | 293 | 46.1 (1.06) | 824 | 45.9 (1.11) | 745 | 46.0 (1.14) | 0.034 |
| Weight (kg) | 457 | 80.0 (15.1) | 289 | 70.7 (13.0) | 824 | 68.5 (13.4) | 745 | 62.5 (13.1) | <0.001 |
| Height (cm) | 456 | 169.8 (6.6) | 290 | 154.9 (5.7) | 824 | 167.2 (6.4) | 745 | 154.6 (5.7) | <0.001 |
| BMI (kg/m2) | 456 | 27.7 (4.7) | 289 | 29.4 (5.1) | 824 | 24.4 (4.2) | 745 | 26.1 (5.1) | <0.001 |
| Body surface area (m2) | 456 | 1.91 (0.18) | 289 | 1.69 (0.15) | 824 | 1.76 (0.18) | 745 | 1.60 (0.16) | <0.001 |
| Systolic blood pressure (mmHg) | 456 | 129.4 (16.6) | 289 | 121.3 (17.6) | 824 | 130.5 (19.2) | 745 | 125.0 (18.5) | <0.001 |
| Diastolic blood pressure (mmHg) | 456 | 87.9 (10.9) | 289 | 82.5 (11.2) | 824 | 79.6 (13.9) | 745 | 75.7 (11.3) | <0.001 |
| Pulse (bpm) | 456 | 82.6 (10.6) | 289 | 85.5 (10.0) | 824 | 80.8 (12.0) | 745 | 85.4 (11.2) | <0.001 |
| SLI score | 460 | 41.6 (5.1) | 293 | 41.0 (5.6) | 824 | 29.5 (6.6) | 745 | 28.8 (6.7) | |
| Tobacco use | |||||||||
| Never | 297 (65.4) | 287 (99.0) | 499 (60.6) | 698 (93.7) | |||||
| Ex-user | 34 (7.5) | 0 (0.0) | 69 (8.4) | 5 (0.7) | <0.001 | ||||
| Current user | 123 (27.1) | 3 (1.0) | 256 (31.1) | 42 (5.6) | |||||
| Alcohol consumption | |||||||||
| None | 179 (38.9) | 281 (95.9) | 321 (39.0) | 745 (100.0) | |||||
| Mild | 207 (45.0) | 12 (4.1) | 327 (39.7) | 0 (0.0) | <0.001 | ||||
| Moderate | 49 (10.7) | 0 (0.0) | 104 (12.6) | 0 (0.0) | |||||
| Heavy | 25 (5.4) | 0 (0.0) | 72 (8.7) | 0 (0.0) | |||||
| CARDIAC SIZE | |||||||||
| LVIDd (mm) | 434 | 48.8 (6.1) | 272 | 45.4 (5.7) | 824 | 45.6 (4.6) | 745 | 43.0 (4.5) | <0.001 |
| LVIDs (mm) | 434 | 32.3 (4.2) | 272 | 29.8 (4.1) | 824 | 29.3 (3.2) | 745 | 27.5 (2.9) | <0.001 |
| Fractional shortening | 434 | 0.3 (0.05) | 272 | 0.3 (0.06) | 824 | 0.4 (0.04) | 745 | 0.4 (0.04) | 0.019 |
| PWT (mm)† | 434 | 9.0 (8.3, 10.0) | 272 | 8.7 (7.7, 9.0) | 824 | 9.0 (8.3, 9.3) | 745 | 8.7 (8.0, 9.0) | <0.001 |
| Septal thickness (mm)† | 434 | 10.0 (9.0, 10.7) | 272 | 9.0 (8.3, 10.0) | 824 | 10.0 (9.0, 10.3) | 745 | 9.3 (9.0, 10.0) | <0.001 |
| Relative wall thickness | 434 | 0.4 (0.06) | 272 | 0.4 (0.10) | 824 | 0.4 (0.06) | 745 | 0.4 (0.06) | 0.083 |
| LVEDV 4c (ml) | 383 | 59.3 (16.1) | 231 | 51.3 (13.3) | 824 | 68.2 (57.7, 82.6) | 745 | 58.0 (49.4, 70.0) | <0.001 |
| LVESV 4c (ml) | 383 | 21.4 (6.5) | 231 | 18.4 (5.1) | 824 | 23.5 (19.7, 28.4) | 745 | 20.1 (17.0, 23.7) | <0.001 |
| LV mass absolute (g)† | 348 | 128.5 (109.0, 152.0) | 212 | 109.0 (94.3, 130.0) | 824 | 117.0 (99.1, 135.0) | 745 | 99.3 (85.0, 114.0) | <0.001 |
| LV massi indexed for BSA (g/m2)† | 345 | 68.0 (58.8,79.4) | 208 | 63.9 (56.4, 75.3) | 824 | 65.8 (57.7, 75.9) | 745 | 61.6 (54.6, 70.3) | <0.001 |
| Categories of LV re-modelling/hypertrophy | |||||||||
| None (n, %) | 244 (73.9) | 139 (69.5) | 573 (69.5) | 471 (63.2) | 0.007 | ||||
| Concentric remodelling (n, %) | 70 (21.2) | 38 (19.0) | 231 (28.0) | 247 (33.2) | |||||
| Concentric hypertrophy (n, %) | 3 (0.9) | 6 (3.0) | 8 (1.0) | 10 (1.3) | |||||
| Eccentric hypertrophy (n, %) | 13 (3.9) | 17 (8.5) | 12 (1.5) | 17 (2.3) | |||||
| SYSTOLIC FUNCTION | |||||||||
| LV ejection fraction (%) † | 383 | 64.5 (61.6, 67.3) | 231 | 64.9 (61.7, 67.2) | 824 | 65.4 (63.2, 67.3) | 745 | 65.7 (63.6, 67.5) | 0.031 |
| Low LV ejection fraction (n, %) | 7 (1.8) | 4 (1.7) | 7 (0.9) | 2 (0.3) | 0.184 | ||||
| Global Longitudinal Strain (%) | 452 | −18.0 (3.4) | 289 | −18.3 (2.7) | 824 | −19.1 (2.2) | 744 | −19.7 (2.2) | <0.001 |
| Categories of GLS | |||||||||
| Normal (n, %) | 358 (79.2) | 238 (82.4) | 707 (85.8) | 672 (90.3) | 0.003 | ||||
| Borderline (n, %) | 75 (16.6) | 48 (16.6) | 83 (10.1) | 52 (7.0) | |||||
| Abnormal (n, %) | 19 (4.2) | 3 (1.0) | 34 (4.1) | 20 (2.7) | |||||
| DIASTOLIC FUNCTION additionally excluded people in atrial fibrillation | |||||||||
| Mitral - E (cm/sec)† | 459 | 71.8 (63.4, 81.2) | 293 | 75.8 (68.6, 85.0) | 824 | 74.4 (64.8, 84.0) | 741 | 78.8 (69.8, 89.5) | <0.001 |
| Mitral - A (cm/sec)† | 459 | 64.0 (57.2, 72.8) | 293 | 68.2 (61.8, 75.4) | 824 | 60.4 (53.1, 67.6) | 741 | 64.6 (57.0, 73.7) | <0.001 |
| Mitral E/A† | 459 | 1.1 (1.0, 1.3) | 293 | 1.1 (1.0, 1.3) | 824 | 1.2 (1.1, 1.4) | 741 | 1.2 (1.1, 1.4) | 0.983 |
| Deceleration time (ms) | 459 | 147.6 (28.4) | 293 | 147.1 (31.6) | 824 | 147.8 (31.1) | 741 | 143.1 (31.0) | 0.003 |
| Mitral E/average e’† | 457 | 7.4 (6.5, 8.4) | 293 | 7.8 (6.8, 9.2) | 824 | 7.9 (6.9, 9.2) | 741 | 8.6 (7.4, 9.7) | <0.001 |
| LA volumei (ml/m2) - 4c† | 380 | 10.0 (7.7, 11.8) | 227 | 10.9 (8.2, 12.6) | 824 | 11.6 (9.7, 13.6) | 744 | 11.9 (10.1, 14.1) | <0.001 |
| Average LA volumei (ml/m2)† | 352 | 9.8 (8.4, 11.6) | 206 | 10.5 (8.8, 12.6) | 824 | 11.4 (9.7, 13.1) | 744 | 11.9 (10.4, 13.8) | <0.001 |
| TR max m/sec | 298 | 154.6 (35.8) | 179 | 156.4 (36.7) | 822 | 174.0 (39.0) | 744 | 179.6 (37.9) | <0.001 |
| Categories of LV diastolic dysfunction participants with normal LV ejection fraction only | |||||||||
| None (n, %) | 295 (78.5) | 182 (80.2) | 809 (99.0) | 737 (99.2) | 0.038 | ||||
| Indeterminate (n, %) | 81 (21.5) | 45 (19.8) | 8 (1.0) | 6 (0.8) | |||||
| Dysfunction (n, %) | 0 | 0 | 0 | 0 | |||||
| Co-morbidities | |||||||||
| Diabetes (n, %) | 187 (40.7) | 104 (35.5) | 212 (25.7) | 158 (21.2) | 0.006 | ||||
| Hypertension (n, %) | 204 (44.4) | 79 (27.0) | 268 (32.5) | 173 (23.2) | <0.001 | ||||
| Myocardial infarction^ (n, %) | 4 (0.9) | 0 (0.0) | 5 (0.6) | 0 (0.0) | NA | ||||
| Atrial fibrillation (n, %) | – | – | 0 (0.0) | 1 (0.1) | NA | ||||
| Overweight (BMI ≥ 25 kg/m2), n (%) | 200 (43.9) | 112 (38.8) | 291 (35.3) | 256 (34.4) | <0.001 | ||||
| Obesity (BMI ≥ 30 kg/m2), n (%) | 126 (27.6) | 124 (42.9) | 77 (9.3) | 168 (22.5) | <0.001 | ||||
LV, left ventricle; LVIDD, LV internal diameter in diastole; LVISD, LV internal diameter in systole; PWT, posterior wall thickness; GLS, global longitudinal strain; E, passive ejection of left atrium; A, active atrial contraction; e’, diastolic annular velocity.
We excluded 52 participants (NDBC N = 17, VBC N = 35) due to major cardiac abnormalities likely to distort the echocardiographic measurements, including valvular heart disease, septal defects, hypertrophic cardiomyopathy and pleural effusion.
MI defined as history of MI in both cohorts.
denotes median (interquartile range).
Table 3.
Descriptive data stratified by cohort, further excluding participants with diabetes, hypertension or a history of MI.
| Variable units | DELHI | VELLORE | p-value between sexes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MALES | FEMALES | MALES | FEMALES | ||||||
| N | Mean SD/Median IQR†/N % | N | Mean SD/Median IQR†/N % | N | Mean SD/Median IQR†/N % | N | Mean SD/Median IQR†/N % | ||
| Clinical characteristics | |||||||||
| Age (years) | 164 | 46.2 (1.2) | 148 | 46.0 (1.0) | 434 | 45.8 (1.1) | 472 | 46.0 (1.2) | 0.349 |
| Height (cm) | 163 | 169.8 (5.9) | 148 | 154.1 (5.9) | 434 | 65.0 (12.8) | 472 | 59.9 (12.2) | <0.001 |
| Weight (kg) | 163 | 77.3 (14.6) | 148 | 69.7 (13.1) | 434 | 166.9 (6.4) | 472 | 154.7 (5.9) | <0.001 |
| BMI (kg/m2) | 163 | 26.8 (4.6) | 148 | 29.4 (5.4) | 434 | 23.3 (4.0) | 472 | 25.0 (4.8) | <0.001 |
| Body surface area (m2) | 163 | 1.88 (0.18) | 148 | 1.68 (0.15) | 434 | 1.72 (0.17) | 472 | 1.57 (0.16) | <0.001 |
| Systolic blood pressure (mmHg) | 163 | 118.9 (9.6) | 146 | 113.5 (11.0) | 434 | 120.2 (10.5) | 472 | 116.9 (12.1) | <0.001 |
| Diastolic blood pressure (mmHg) | 163 | 80.0 (6.2) | 146 | 77.4 (7.7) | 434 | 72.4 (9.5) | 472 | 71.2 (8.5) | <0.001 |
| Pulse (bpm) | 163 | 80.0 (9.6) | 146 | 84.8 (9.7) | 434 | 77.9 (11.0) | 472 | 83.6 (10.0) | <0.001 |
| SLI score | 164 | 41.7 (4.1) | 147 | 41.1 (4.6) | 434 | 28.7 (6.6) | 472 | 28.1 (6.9) | <0.001 |
| Tobacco use | |||||||||
| Never | 114 (69.5) | 144 (98.0) | 261 (60.1) | 436 (92.4) | |||||
| Ex-user | 7 (4.3) | 0 (0.0) | 25 (5.8) | 4 (0.9) | <0.001 | ||||
| Current user | 43 (26.2) | 3 (2.0) | 148 (34.1) | 32 (6.8) | |||||
| Alcohol consumption | |||||||||
| None | 72 (43.9) | 141 (95.9) | 192 (44.2) | 472 (100.0) | |||||
| Mild | 75 (45.7) | 6 (4.1) | 169 (38.9) | 0 (0.0) | <0.001 | ||||
| Moderate | 14 (8.5) | 0 (0.0) | 51 (11.8) | 0 (0.0) | |||||
| Heavy | 3 (1.8) | 0 (0.0) | 22 (5.1) | 0 (0.0) | |||||
| CARDIAC SIZE | |||||||||
| LVIDd (mm) | 157 | 48.4 (5.8) | 139 | 45.1 (5.1) | 434 | 45.2 (4.4) | 472 | 42.8 (4.3) | <0.001 |
| LVIDs (mm) | 157 | 32.2 (4.1) | 139 | 29.6 (3.5) | 434 | 29.0 (2.9) | 472 | 27.4 (2.9) | <0.001 |
| Fractional shortening | 157 | 0.3 (0.04) | 139 | 0.3 (0.05) | 434 | 0.4 (0.04) | 472 | 0.4 (0.04) | 0.052 |
| PWT (mm)† | 157 | 9.0 (8.0, 9.3) | 139 | 8.0 (7.0, 9.0) | 434 | 9.0 (8.3, 9.0) | 472 | 8.7 (8.0, 9.0) | <0.001 |
| Septal thickness (mm)† | 157 | 9.5 (9.0, 10.2) | 139 | 9.0 (8.0, 10.0) | 434 | 10.0 (9.0, 10.3) | 472 | 9.0 (9.0, 10.0) | <0.001 |
| Relative wall thickness | 157 | 0.4 (0.06) | 139 | 0.4 (0.12) | 434 | 0.4 (0.05) | 472 | 0.4 (0.05) | 0.075 |
| LVEDV 4c (ml)† | 142 | 55.8 (45.8, 64.3) | 115 | 49.1 (41.8, 58.0) | 434 | 67.3 (57.0, 81.1) | 472 | 58.0 (50.6, 69.7) | <0.001 |
| LVESV 4c (ml)† | 142 | 19.1 (15.7, 21.8) | 115 | 17.4 (14.1, 21.5) | 434 | 23.1 (19.1, 27.9) | 472 | 20.1 (17.0, 23.8) | <0.001 |
| LV mass absolute (g)† | 127 | 123.0 (105.0, 145.0) | 108 | 104.0 (92.8, 124.5) | 434 | 112.0 (95.5, 127.0) | 472 | 95.3 (82.1, 111.0) | <0.001 |
| LV massi indexed for BSA (g/m2)† | 126 | 65.4 (57.3, 75.2) | 108 | 62.6 (56.5, 75.5) | 434 | 65.0 (57.2, 74.2) | 472 | 60.9 (54.2, 70.1) | <0.001 |
| Categories of LV re-modelling/hypertrophy | |||||||||
| None (n, %) | 97 (80.2) | 73 (70.9) | 319 (73.5) | 318 (67.4) | 0.023 | ||||
| Concentric remodelling (n, %) | 18 (14.9) | 17 (16.5) | 110 (25.4) | 142 (30.1) | |||||
| Concentric hypertrophy (n, %) | 2 (1.7) | 3 (2.9) | 0 (0.0) | 4 (0.9) | |||||
| Eccentric hypertrophy (n, %) | 4 (3.3) | 10 (9.7) | 5 (1.2) | 8 (1.7) | |||||
| SYSTOLIC FUNCTION | |||||||||
| LV ejection fraction (%)† | 142 | 65.2 (62.6, 67.8) | 115 | 64.9 (61.0, 67.6) | 434 | 65.7 (63.6, 67.5) | 472 | 65.9 (63.8, 67.7) | 0.633 |
| Low LV ejection fraction (n, %) | 2 (1.4) | 3 (2.6) | 434 | 0 (0.0) | 472 | 2 (0.4) | 0.425 | ||
| Average GLS | 162 | −17.9 (4.3) | 146 | −18.3 (3.4) | 434 | −19.6 (2.2) | 472 | −20.2 (2.1) | <0.001 |
| Categories of GLS | |||||||||
| Normal (n, %) | 130 (80.3) | 125 (85.6) | 393 (90.6) | 443 (93.9) | 0.045 | ||||
| Borderline (n, %) | 27 (16.7) | 20 (13.7) | 29 (6.7) | 21 (4.5) | |||||
| Abnormal (n, %) | 5 (3.1) | 1 (0.7) | 12 (2.8) | 8 (1.7) | |||||
| DIASTOLIC FUNCTION additionally excluded people in atrial fibrillation | |||||||||
| Mitral - E (cm/sec)† | 164 | 69.6 (62.6, 78.1) | 148 | 75.3 (67.0, 82.2) | 434 | 74.3 (65.0, 83.6) | 470 | 78.3 (69.4, 87.8) | <0.001 |
| Mitral - A (cm/sec)† | 164 | 60.7 (55.2, 67.6) | 148 | 66.5 (59.4, 73.7) | 434 | 56.7 (50.8, 64.2) | 470 | 62.4 (55.3, 70.0) | <0.001 |
| Mitral E/A† | 164 | 1.2 (1.1, 1.3) | 148 | 1.2 (1.0, 1.3) | 434 | 1.3 (1.2, 1.5) | 470 | 1.3 (1.2, 1.4) | 0.084 |
| Deceleration time (ms) | 164 | 149.4 (27.8) | 148 | 150.9 (30.2) | 434 | 150.1 (31.7) | 470 | 145.4 (28.9) | 0.045 |
| Mitral E/average e’† | 162 | 7.1 (6.3, 8.2) | 148 | 7.6 (6.7, 8.6) | 434 | 7.4 (6.5, 8.6) | 470 | 8.3 (7.1, 9.4) | 0.002 |
| LA volumei (ml/m2) - 4c† | 134 | 9.8 (8.1, 11.6) | 106 | 10.3 (8.6, 12.8) | 434 | 11.7 (9.6, 13.7) | 472 | 12.0 (10.2, 14.3) | 0.006 |
| Average LA volumei (ml/m2)† | 133 | 9.5 (8.1, 11.3) | 105 | 10.4 (8.7, 12.6) | 434 | 11.4 (9.8, 13.2) | 472 | 12.1 (10.5, 14.0) | <0.001 |
| TR max m/sec | 111 | 157.5 (34.0) | 84 | 159.9 (39.0) | 434 | 178.6 (37.5) | 472 | 182.6 (36.4) | 0.028 |
| Categories of LV diastolic dysfunction participants with normal LV ejection fraction only | |||||||||
| None (n, %) | 116 (82.9) | 86 (76.8) | 431 (99.3) | 468 (99.6) | 0.932 | ||||
| Indeterminate (n, %) | 24 (17.1) | 26 (23.2) | 3 (0.7) | 2 (0.4) | |||||
| Dysfunction (n, %) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||
| Co-morbidities | |||||||||
| Overweight (BMI ≥ 25 kg/m2), n (%) | 72 (42.9) | 57 (38.8) | 144 (30.9) | 144 (30.5) | <0.001 | ||||
| Obesity (BMI ≥ 30 kg/m2), n (%) | 35 (21.5) | 61 (41.5) | 20 (4.6) | 79 (16.7) | <0.001 | ||||
LV, left ventricle; LVIDD, LV internal diameter in diastole; LVISD, LV internal diameter in systole; PWT, posterior wall thickness; GLS, global longitudinal strain; E, passive ejection of left atrium; A, active atrial contraction; e’, diastolic annular velocity.
†denotes median (interquartile range).
Figure 1.
Flow diagram of participants who underwent echocardiographic measurements in the IndEcho study.
Results
Echocardiographic assessment was performed on 1,329 men and 1,067 women. Of these, data from 25 participants in New Delhi were excluded, either because of a poor echo window (n = 11) or the scans were of unsatisfactory quality (n = 14). Fifty-two participants were excluded because of major pre-existing cardiac disease. The main analysis included a total of 2,322 individuals (753 in NDBC and 1,569 in VBC) (Figure 1). Their clinical characteristics and echocardiographic measurements are summarised in Table 2. Participants from both cohorts were of similar age. The BMI and BSA were higher in the NDBC compared with the VBC, as were the prevalence of other co-morbid conditions such as overweight, obesity, diabetes and hypertension.
The cardiac size parameters (LV mass, LV internal diameters, PWT and LV volumes) were generally larger in men compared with women in both cohorts, and higher in the NDBC compared with the VBC. Indexation for BSA did not alter the gender differences in LV mass. Based on current threshold definitions (8), about 30% of individuals met the diagnostic criteria for concentric remodelling and <3% had hypertrophy.
The average GLS was more negative among VBC participants (suggesting better LV systolic function) compared with NDBC participants. The mean LV ejection fraction of the IndEcho participants was well within the normal range for Indians reflecting very small variability in chamber dimensions. The proportion with low LV ejection fraction was <3% in the cohort. The trans-mitral flow velocities (E, A and E/A) and tissue doppler velocity (E/e') were comparable between both cohorts, and women generally had higher flow velocity values, but similar E/A ratio and mitral E-deceleration time compared with men.
To create the healthy sub-sample, we examined the distribution of echocardiographic outcomes in 1,215 participants (men 598, women 617) by excluding participants with diabetes, hypertension or a history of myocardial infarction (Table 3). This healthy group had smaller cardiac dimensions, more negative GLS values and similar LV filling pressures suggesting that underlying comorbid illnesses exert a pronounced effect on cardiac geometry and systolic function.
To further analyse the effect of lifestyle, we present data for the VBC participants stratified by area of residence (rural/urban) (Supplementary Table S1). The urban residents had higher SLI scores and higher tobacco and alcohol consumption. They also had higher BMI, higher BSA and a higher prevalence of diabetes, hypertension and MI. Cardiac size was similar between between rural and urban groups. A higher proportion of individuals from urban residence had LV remodelling and hypertrophy. A significant difference in GLS (p < 0.001) was observed between both groups and rural residents had higher proportion of abnormal GLS, particularly women (7.6%).
Intra-observer variability
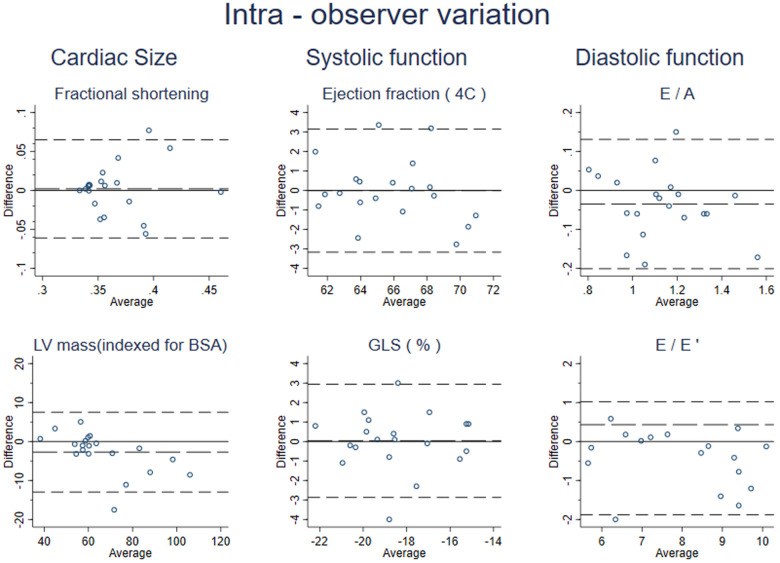
The Intra-observer repeatability of conventional echocardiographic measures was good or excellent in our study (Table 4). The mean difference [95% limit of agreement (LOA)] and ICC (95% CI) were −2.735 (−12.980, 7.510) and 0.971 (0.915, 0.989) for LV mass indexed for BSA. The corresponding values for other cardiac size, and systolic and diastolic function parameters are shown in Table 4. The LoA and the BA plots shown in Figure 2 demonstrate minimal bias in the data across the range of values.
Table 4.
Intra-observer variability of echocardiographic measurements.
| Variables | n | Correlation | Bias (SD) | LoA | CV | ICC (95% CI) |
|---|---|---|---|---|---|---|
| Cardiac size | ||||||
| LVIDD (mm) | 20 | 0.939 | −0.033 (1.841) | (−3.641, 3.574) | 2.3 | 0.967 (0.916, 0.987) |
| LVISD (mm) | 20 | 0.906 | −0.108 (1.524) | (−3.095, 2.879) | 3.1 | 0.950 (0.874, 0.980) |
| PWd (mm) | 20 | 0.701 | 0.442 (0.661) | (−0.853, 1.737) | 4.5 | 0.767 (0.320, 0.913) |
| LVEDV (4c) (ml) | 20 | 0.987 | −2.382 (2.618) | (−7.514, 2.750) | 2.7 | 0.988 (0.909, 0.996) |
| LVESV (4c) (ml) | 20 | 0.969 | −0.778 (1.468) | (−3.655, 2.098) | 4.1 | 0.980 (0.939, 0.993) |
| LV mass (2D) (g) | 20 | 0.982 | −4.675 (8.381) | (−21.102, 11.752) | 3.9 | 0.983 (0.947, 0.994) |
| LV massi (indexed for BSA, 2D) (g/m2) | 20 | 0.969 | −2.735 (5.227) | (−12.980, 7.510) | 3.9 | 0.971 (0.915, 0.989) |
| Systolic function | ||||||
| LV ejection fraction | 20 | 0.868 | −0.014 (1.611) | (−3.171, 3.143) | 1.3 | 0.929 (0.821, 0.972) |
| Average GLS (%) | 20 | 0.77 | 0.030 (1.482) | (−2.874, 2.934) | 4.1 | 0.876 (0.683, 0.951) |
| Diastolic function | ||||||
| E (cm/sec) | 20 | 0.98 | −2.372 (2.822) | (−7.904, 3.160) | 3.1 | 0.983 (0.899, 0.995) |
| A (cm/sec) | 20 | 0.893 | −0.250 (4.264) | (−8.608, 8.108) | 3.2 | 0.944 (0.857, 0.978) |
| E/A | 20 | 0.911 | −0.035 (0.085) | (−0.201, 0.131) | 4.4 | 0.946 (0.858, 0.979) |
| Deceleration time (ms) | 20 | 0.958 | 1.442 (9.260) | (−16.707, 19.590) | 3.5 | 0.976 (0.940, 0.990) |
| Lateral e’ (cm/sec) | 17 | 0.953 | 0.309 (0.654) | (−0.974, 1.591) | 3.9 | 0.966 (0.899, 0.988) |
| Medial e’ (cm/sec) | 16 | 0.916 | 0.152 (0.571) | (−0.966, 1.271) | 3.8 | 0.953 (0.869, 0.983) |
| LA volume (ml/m2) - 2c | 20 | 0.964 | 0.737 (1.051) | (−1.322, 2.796) | 3.8 | 0.974 (0.891, 0.991) |
| LA volume (ml/m2) - 4C | 20 | 0.978 | 0.138 (1.047) | (−1.913, 2.190) | 3.5 | 0.988 (0.969, 0.995) |
LoA, limits of agreement; CV, co-efficient of variation; ICC (95% CI), inter-class correlation (95% confidence interval).
Figure 2.
Bland–Altman plots for selected echocardiographic variables.
Discussion
The current study presents comprehensive echocardiographic data in over 2,300 individuals aged 43–50 years and for a “healthy” sub-set apparently free from major cardiac disease, hypertension or diabetes, as well as presenting the reproducibility of echocardiographic measurements.
Our results confirm expected sex differences, heterogeneity across sites and rural/urban differences in cardiac dimensions and function. Overall, the cardiac dimensions and functional measurements align with other previously published literature among Indians (16–19). The NDBC participants generally had larger ventricular internal dimensions than VBC participants, including indexed LV mass, which could be due to the higher prevalence of underlying hypertension and higher BMI in this cohort. Systolic function was characterised by similar EF between both groups, while the NDBC participants demonstrated lower (less negative) GLS, possibly denoting a sizeable proportion with undetected subclinical heart disease that warrants further investigation. The heterogeneity between sites could possibly be driven by lifestyle differences and other risk factors. Overall, men had higher LV volume and lower systolic function compared with women. Consistent site-specific and sex differences were observed even after exclusion of individuals with diabetes and hypertension. Differences were also observed between urban and rural residents, with the former group having a higher LV mass, lower LV volumes and poor systolic function.
The Indian Normative Data of Echocardiography Analyzed (INDEA) (17) and World Alliance Societies of Echocardiography (WASE) (19) studies are the largest published studies that provide reference ranges for echo parameters in Asian Indians. The reference values of the WASE study that included 2008 individuals across 15 countries including India report variation in normative values across countries and between sexes. Our values fall within the most commonly used reference range provided by INDEA (17), WASE (Indian reference) (19) and ASE/EACVI (Western range) (8) (Supplementary Table S2). Similarly, cardiac dimensions (LV mass, LVEDV and RWT) in our study are comparable to other native Indian reports (16, 20–22), but smaller than the western population. A retrospective singe-centred study from central India by Sullere et al., reported that Indians have similar or even larger cardiac dimensions and volumes than those of Western origin (20). Ethnic based differences in LV size are well recognised (23, 24) and could be partly attributed to the differences in body composition between the ethnic groups.
Our data showed a higher prevalence of LV re-modelling and lower annular velocities than in US/European studies. The LV remodelling could reflect the high prevalence of underlying hypertension in this cohort and may contribute to increased CVD risk in this ethnic group, rather than hypertrophy. However, systolic (EF and GLS values) and diastolic function are comparable to other ethnic groups despite the smaller cardiac volume (18, 19, 25).
Comparative data on the parameters used to define diastolic function are less readily available; the INDEA study reported E, A, and E/e' values for a very small sub-set of the participants (N = 100) whose age was not defined (medial e' 10.2 cm/s in men, 10.1 cm/s in women; average E/e' 7.4 and 7.7; LA volume indexed 17.8 and 18.6 ml/m2; TR max 134 and 135 cm/s). The diastolic function in our study participants was well within the global (ASE/EACVI) and INDEA thresholds (8, 17).
The sex differences reported in our study have also been recognised previously (16, 17, 26). Consistent demonstration of sex differences in various ethnic groups may suggest that women generally have better LV adaptation to CVD compared with men (27, 28). The heterogeneity observed between cohorts within India as well as rural-urban differences could be attributed to burden of underlying co-morbid illnesses such as diabetes, obesity and hypertension in the community as well as differences in life-style factors within a country.
Strengths and limitations
Strengths were that the IndEcho study is one of the largest population-based echocardiographic studies from India and adds to the existing Echo-normative data in Indians. This resource is additionally valuable because of the past serially collected data on childhood growth and cardiometabolic risk markers from young adulthood, which will enable us to analyse these as longitudinal predictors of LV structure and function in future. The echocardiographic measurements demonstrated good to excellent repeatability. Due to the cohort design, our study sample covered a narrow age range, and therefore our findings in relation to cardiac structure and function may not be generalisable to other ages. The INDEA study, which included three age-groups (<35, 36–55 and ≥56 years) found little change in cardiac dimensions or systolic function with age (14). However, there are well-documented changes in LV diastolic function with age (9). Few participants (n = 25) had to be excluded completely due either to failure to obtain a suitable echo window, or poor image quality, most commonly associated with obesity, leading to some loss of study population. However, the proportion lost in IndEcho was similar to that in other studies (14% in the INDEA study). Since there are no widely accepted reference standards for LV remodelling/hypertrophy, systolic and diastolic function, among Asian Indians, our definitions were based on the US and European cut-offs which may not be ideal given the differences in cardiac size and could underestimate the prevalence of LV remodelling/hypertrophy in this cohort, a potential pitfall in applying western normal range values to Indian population.
Conclusions
In this large population-based analysis of cardiac structure and function in mid-life, our results confirm that healthy Asian Indians have smaller cardiac chamber volumes and dimension with similar systolic and diastolic function compared with published data from Europeans and white Caucasians. These reinforce the need for ethnic-specific and age-appropriate reference data to diagnose CVD as well as optimise clinical management. The high prevalence of overweight, obesity, diabetes, hypertension or a history of MI in these cohorts highlights the high prevalence of mid-life cardiometabolic disease currently in India. It is possible that inherent differences in cardiac geometry and function in Indians on a background of a high prevalence of cardiometabolic risk factors may in part contribute to premature CVD risk in this ethnic group. Future analysis will examine these risk factors, and early life growth patterns as predictors.
Acknowledgments
We thank the members of the NBDC and VBC and their families for their participation in research over more than four decades. We acknowledge the IndEcho field teams in Delhi and Vellore, which include administrative staff, field workers and supervisors, research officers, research nurses, phlebotomists, DXA and echo technicians, data management teams and data entry operators.
Funding Statement
The original cohort studies were supported by the National Center for Health Statistics, USA and the Indian Council of Medical Research. The two earlier follow-up studies in young adult life were supported by the British Heart Foundation. The IndEcho study was supported by British Heart Foundation Clinical Research (grant no. CRM: 0022324). Professor Fall's work on the study was supported by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the research committees of Sunder Lal Jain Hospital, New Delhi (13th August 2015; SLJ/IEC/1); Sitaram Bhartia Institute of Science and Research, New Delhi (23 October 2015; IEC/SBSR/2015/1); Centre for Chronic Disease Control, New Delhi (no.50/7/TF-CVD/15- NCD-II); All-India Institute of Medical Sciences, New Delhi (21 October 2015, IEC/ NP-410/09.10.2015); Christian Medical College, Vellore [22 July 2015; IRB 9548 (OBSERV)]; and the Faculty of Medicine, University of Southampton, UK (11 April 2016; RE 18694). The patients/participants provided their written informed consent to participate in this study.
Author contributions
The New Delhi Birth Cohort Study was directed by SB and HS, and the Vellore Birth Cohort Study by BA and MG. The IndEcho study was conceived and designed by SV, AA, AR, HS, FK, NT, FJ, SB, BA, DP, CF and VT. Data collection was co-ordinated by SV, BA, HS and SB. Echocardiography was carried out by JS, RP, JK and NTh under the supervision of AA, VT, AR and DP. Biochemistry was carried out by LR and GA. Data was analysed by MG, SS and CO. The initial manuscript was prepared by SV, CF, AB and MG and all authors made intellectual contributions to the final version. All members of the IndEcho study group contributed to data collection. All authors contributed to the article and approved the submitted version.
Member of the IndEcho study group
Bhaskar Singh, Siva Dora, Sukanya Sarma, Gupta KD (D-7 Gulmohar Park, New Delhi), Srinath Reddy (Public Health Foundation of India, New Delhi, India), Thomas V Paul (Endocrinology, Christian Medical College, Vellore), Prasanna Samuel (Biostatistics, Christian Medical College, Vellore)
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1055454/full#supplementary-material
References
- 1.Prabhakaran D, Jeemon P, Roy A. Cardiovascular diseases in India: Current epidemiology and future directions. Circulation. (2016) 133(16):1605–20. 10.1161/CIRCULATIONAHA.114.008729 [DOI] [PubMed] [Google Scholar]
- 2.Prabhakaran D, Anand S, Watkins DA, Gaziano TA, Wu Y, Mbanya JC, et al. Cardiovascular, respiratory, and related disorders: Key messages and essential interventions to address their burden in low-and middle-income countries. In: Prabhakaran D, Anand S, Gaziano TA, Mbanya JC, Wu Y, Nugent R, editors. Cardiovascular, respiratory, and related disorders. 3rd ed. Washington, DC: The International Bank for Reconstruction and Development/The World Bank; (2017). Chapter 1. PMID: 30212087. [PubMed] [Google Scholar]
- 3.India State-Level Disease Burden Initiative CVDC. The changing patterns of cardiovascular diseases and their risk factors in the states of India: The global burden of disease study 1990–2016. Lancet Glob Health. (2018) 6(12):e1339–51. 10.1016/S2214-109X(18)30407-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. (2015) 132(17):1667–78. 10.1161/CIRCULATIONAHA.114.008720 [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the interheart study): case-control study. Lancet. (2004) 364(9438):937–52. 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 6.Vakili BA, Okin PM, Devereux RB. Prognostic implications of left ventricular hypertrophy. Am Heart J. (2001) 141(3):334–41. 10.1067/mhj.2001.113218 [DOI] [PubMed] [Google Scholar]
- 7.Vasan SK, Roy A, Samuel VT, Antonisamy B, Bhargava SK, Alex AG, et al. Indecho study: Cohort study investigating birth size, childhood growth and young adult cardiovascular risk factors as predictors of midlife myocardial structure and function in South Asians. BMJ Open. (2018) 8(4):e019675. 10.1136/bmjopen-2017-019675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28(1):1–39 e14. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 9.Antonisamy B, Raghupathy P, Christopher S, Richard J, Rao PS, Barker DJ, et al. Cohort profile: The 1969–73 vellore birth cohort study in South India. Int J Epidemiol. (2009) 38(3):663–9. 10.1093/ije/dyn159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huffman MD, Prabhakaran D, Osmond C, Fall CH, Tandon N, Lakshmy R, et al. Incidence of cardiovascular risk factors in an Indian urban cohort results from the New Delhi birth cohort. J Am Coll Cardiol. (2011) 57(17):1765–74. 10.1016/j.jacc.2010.09.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. (2004) 350(9):865–75. 10.1056/NEJMoa035698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassani DG, Corsi DJ, Gaffey MF, Barros AJ. Local distributions of wealth to describe health inequalities in India: a new approach for analyzing nationally representative household survey data, 1992–2008. PLoS One. (2014) 9(10):e110694. 10.1371/journal.pone.0110694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes Mellitus. Diabetes Care. (2003) 26(11):3160–7. 10.2337/diacare.26.11.3160 [DOI] [PubMed] [Google Scholar]
- 14.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2016) 29(4):277–314. 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 15.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. (2016) 15(2):155–63. 10.1016/j.jcm.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bansal M, Mohan JC, Sengupta SP. Normal echocardiographic measurements in Indian adults: how different are we from the western populations? A pilot study. Indian Heart J. (2016) 68(6):772–5. 10.1016/j.ihj.2016.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sengupta SP, Burkule N, Bansal M, Mohan JC, Karande A, Chatterjee D, et al. Normative values of cardiac chamber dimensions and global longitudinal strain in Indians: the Indian normative data of echocardiography analyzed (indea) study. Int J Cardiovasc Imaging. (2021) 37(3):871–80. 10.1007/s10554-020-02060-8 [DOI] [PubMed] [Google Scholar]
- 18.Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. Ethnicity-related differences in left ventricular function, structure and geometry: a population study of UK Indian Asian and European white subjects. Heart. (2010) 96(6):466–71. 10.1136/hrt.2009.173153 [DOI] [PubMed] [Google Scholar]
- 19.Asch FM, Miyoshi T, Addetia K, Citro R, Daimon M, Desale S, et al. Similarities and differences in left ventricular size and function among races and nationalities: results of the world alliance societies of echocardiography normal values study. J Am Soc Echocardiogr. (2019) 32(11):1396–406 e2. 10.1016/j.echo.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 20.Sullere V, Jain D, Sullere S, Anthony C. Global longitudinal strain, ejection fraction, effort tolerance and normal echocardiography measurements in healthy Indians. Indian Heart J. (2018) 70(5):637–41. 10.1016/j.ihj.2018.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhambhani A, John N, Mathew A. Real-time three-dimensional echocardiographic left heart parameters in healthy Indian adults. Indian Heart J. (2018) 70(5):642–8. 10.1016/j.ihj.2017.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherjee A, Halder SK, Nandi S, Mandal M, Khanra D, Biswas K. A study on normal reference values of echocardiographic chamber dimensions in young eastern Indian adults. Indian Heart J. (2021) 73(1):77–84. 10.1016/j.ihj.2020.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park CM, March K, Ghosh AK, Jones S, Coady E, Tuson C, et al. Left-ventricular structure in the southall and brent revisited (sabre) study: explaining ethnic differences. Hypertension. (2013) 61(5):1014–20. 10.1161/HYPERTENSIONAHA.111.00610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Echocardiographic Normal Ranges Meta-Analysis of the Left Heart C. Ethnic-specific normative reference values for echocardiographic La and Lv size, Lv mass, and systolic function: The echonormal study. JACC Cardiovasc Imaging (2015) 8(6):656–65. 10.1016/j.jcmg.2015.02.014 [DOI] [PubMed] [Google Scholar]
- 25.Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. Population-based reference values for 3d echocardiographic Lv volumes and ejection fraction. JACC Cardiovasc Imaging. (2012) 5(12):1191–7. 10.1016/j.jcmg.2012.07.014 [DOI] [PubMed] [Google Scholar]
- 26.Gardin JM, Siscovick D, Anton-Culver H, Lynch JC, Smith VE, Klopfenstein HS, et al. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The cardiovascular health study. Circulation. (1995) 91(6):1739–48. 10.1161/01.cir.91.6.1739 [DOI] [PubMed] [Google Scholar]
- 27.Beale AL, Nanayakkara S, Segan L, Mariani JA, Maeder MT, van Empel V, et al. Sex differences in heart failure with preserved ejection fraction pathophysiology: A detailed invasive hemodynamic and echocardiographic analysis. JACC Heart Fail. (2019) 7(3):239–49. 10.1016/j.jchf.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 28.Kuch B, Muscholl M, Luchner A, Doring A, Riegger GA, Schunkert H, et al. Gender specific differences in left ventricular adaptation to obesity and hypertension. J Hum Hypertens. (1998) 12(10):685–91. 10.1038/sj.jhh.1000689 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.