Abstract
Using protein AG in an enzyme-linked immunosorbent assay (ELISA), we tried to detect antibodies against parapoxvirus in 9 species of wild animals in Japan: the Japanese badger (Meles meles anakuma), Japanese black bear (Ursus thibetanus japonicus), Japanese deer (Cervus nippon centralis), Japanese monkey (Macaca fuscata), Japanese raccoon dog (Nyctereutes procyonoides viverrinus), Japanese serow (Capricornis crispus), Japanese wild boar (Sus scrofa leucomystax), masked palm civet (Paguma larvata), and nutria (Myocastor coypus). A total of 272 serum samples were collected over the period from 1984 to 1995 and were tested by the protein AG-ELISA, the agar gel immunodiffusion test, and an indirect immunofluorescence assay. The protein AG-ELISA was effective in a serological survey for parapoxvirus in wild animals, and antibodies were detected only in Japanese serows. A total of 24 of 66 (36.4%) Japanese serows reacted positively, and they were found in almost all prefectures in all years tested. These results suggest that epizootic cycles of parapoxvirus exist widely in Japanese serows and that they could be reservoirs for the virus in the field in Japan. Moreover, it is probable that they might carry the virus to domestic animals such as cattle, sheep, and goats.
The genus Parapoxvirus includes bovine papular stomatitis virus and pseudocowpox virus in cattle and orf virus in sheep and goats (15). The parapoxviruses cause a disease characterized by a contagious papular dermatitis around the mouth, teats, or skin of infected animals. The members of the Parapoxvirus genus are immunologically closely related and exhibit serological cross-reactivity (11, 16, 21, 22, 28). The viruses occasionally infect humans after close contact of humans with the skin lesions of infected animals or the handling of virus-contaminated materials, and the infections are therefore known as zoonoses (5, 12, 13, 19, 23, 25).
Parapoxvirus infections have also been described in other animals such as camels, seals, reindeer, musk ox, and squirrels (4, 19). Recently, a new parapoxvirus was isolated from red deer in New Zealand (6, 20). Thus, parapoxvirus infection may exist among wild animals in Japan, especially Japanese deer. However, data that support this speculation are available only for Japanese serows (17, 18, 26, 27). Since the foraging ranges of wild animals overlap those of domestic animals in pastures in certain areas, there is a possibility that wild animals infected with parapoxvirus are a factor in the spread of parapoxvirus infection among domestic animals.
In the study described here, we examined the utility of protein AG for use in a serological survey for evidence of parapoxvirus infection in nine species of wild animals in Japan and showed the prevalence of antibodies against parapoxvirus in the animals.
MATERIALS AND METHODS
Serum samples.
A total of 272 serum samples were collected over the period from 1984 to 1995 from nine species of wild animals in Japan, as shown in Table 1 and Fig. 1. They included the Japanese badger (Meles meles anakuma), Japanese black bear (Ursus thibetanus japonicus), Japanese deer (Cervus nippon centralis), Japanese monkey (Macaca fuscata), Japanese raccoon dog (Nyctereutes procyonoides viverrinus), Japanese serow (Capricornis crispus), Japanese wild boar (Sus scrofa leucomystax), masked palm civet (Paguma larvata), and nutria (Myocastor coypus). Nutria is a species that was introduced into Japan. Most samples were acquired for previous serological studies of infections caused by various bacterial and virological agents (7, 26, 30).
TABLE 1.
Wild animals tested for antibodies against parapoxvirus
| Animal | Genus and species | No. of serum samples tested | Period of serum collection | Prefectures where sera were collected |
|---|---|---|---|---|
| Japanese badger | Meles meles anakuma | 2 | 1992 | Gifu |
| Japanese black bear | Ursus thibetanus japonicus | 30 | 1991–1993 | Gifu, Shiga |
| Japanese deer | Cervus nippon centralis | 55 | 1991–1993 | Aomori, Iwate, Miyagi, Hyogo |
| Japanese monkey | Macaca fuscata | 30 | 1991–1992 | Gifu |
| Japanese raccoon dog | Nyctereutes procyonoides viverrinus | 24 | 1991–1992 | Gifu, Mie |
| Japanese serow | Capricornis crispus | 66 | 1984–1995 | Yamagata, Tochigi, Kanagawa, Gifu |
| Japanese wild boar | Sus scrofa leucomystax | 30 | 1991–1992 | Gifu, Shiga, Mie, Hyogo |
| Masked palm civet | Paguma larvata | 5 | 1991–1992 | Gifu |
| Nutria | Myocastor coypus | 30 | 1991–1992 | Gifu |
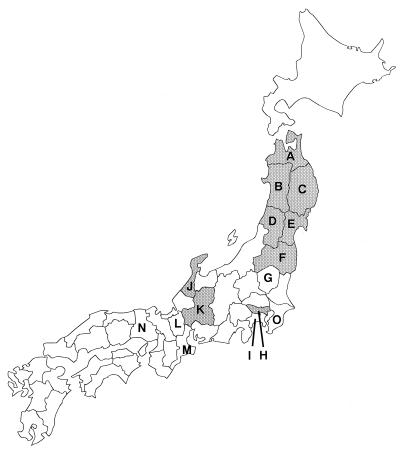
FIG. 1.
Map of Japan showing prefectures where serum samples from wild animals were collected and parapoxvirus infections of Japanese serows were observed. The prefectures are labeled as follows: A, Aomori; B, Akita; C, Iwate; D, Yamagata; E, Miyagi; F, Fukushima; G, Tochigi; H, Tokyo; I, Kanagawa; J, Ishikawa; K, Gifu; L, Shiga; M, Mie; N, Hyogo; and O, Chiba. Serum samples from wild animals were collected from prefectures labeled A, C to E, G, and I to N. In this study antibodies against parapoxvirus were detected from Japanese serows from prefectures labeled D, G, and K. Parapoxvirus infections of Japanese serows were clinically observed previously in some prefectures (shaded prefectures). A parapoxvirus strain Chiba used as an antigen in the protein AG-ELISA was isolated from a cow in the prefecture labeled O.
Virus and cells.
A parapoxvirus strain Chiba was isolated from a vesiculopapular lesion on the teat of a dairy cow (10). The virus was propagated in primary fetal bovine muscle (FBM) or Madin-Darby bovine kidney cells (MDBK). The cells were maintained in Eagle’s minimum essential medium (Nissui, Tokyo, Japan) supplemented with 0.3% tryptose phosphate broth (Difco, Detroit, Mich.), 5% fetal calf serum, 100 μg of streptomycin per ml, and 100 U of penicillin per ml.
Purification of virus.
The virus was purified in sodium diatrizoate gradients as follows. Culture fluids containing infected FBM cells were collected when a cytopathic effect appeared. They were frozen and thawed three times and were centrifuged at 1,500 × g for 5 min in an RS-720 rotor (Kubota, Tokyo, Japan). The resulting supernatant was further centrifuged at 90,000 × g for 1 h at 4°C in a Beckman SW28 rotor (Beckman, Fullerton, Calif.). Then the pellet was suspended in a small volume of TE (0.25 mM Tris-HCl [pH 7.5], 0.01 M EDTA) and disrupted by sonication (UR-200P; Tomy Seiko, Tokyo, Japan). The viral suspension was overlaid onto a discontinuous gradient consisting of 2 ml of 50% sodium diatrizoate, 2 ml of 25% sodium diatrizoate, and 1 ml of 10% dextran T-10 as described previously (3) and was centrifuged at 60,000 × g for 18 h at 4°C in a Beckman SW55Ti rotor. The visible virus band was withdrawn and was layered onto 1 ml of a 40% sucrose cushion and centrifuged at 60,000 × g for 30 min at 4°C in an SW55Ti rotor. The pellet of the purified virus was resuspended in a small volume of TE.
Protein AG-ELISA.
The concentrated purified viral material was diluted in an equal volume of TNE–NP-40 (0.01 M Tris-HCl [pH 8.0], 0.1 M NaCl, 0.001 M EDTA, 1% Nonidet P-40) and was used as the viral antigen for the protein AG–enzyme-linked immunosorbent assay (ELISA). The viral antigen was diluted with 0.015 M carbonate–0.035 M bicarbonate buffer (pH 9.6), and a suitable dilution for the assay was determined. Fifty microliters of the antigen was dispensed into the wells of ELISA microplates. After incubation at 4°C overnight, the wells were washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20 (PBS-T) with a Corty CW-40V microplate washer (Eisai, Tokyo, Japan). Two hundred microliters of blocking solution, which consisted of PBS containing 2% Block Ace (Dainippon Pharmaceutical, Osaka, Japan), 200 μg of ovalbumin (Sigma, St. Louis, Mo.) per ml, and 0.1% NaN3 was added, followed by incubation at 37°C for 1 h. The wells were washed with PBS-T as described above. The sera to be tested were diluted 1:100 with PBS containing 0.15% Tween 20, 2% Block Ace, 200 μg of ovalbumin per ml, and 0.1% NaN3, and 50 μl of diluted serum was placed in the wells. After incubation at 37°C for 1 h, the wells were washed with PBS-T and 50 μl of diluted peroxidase-conjugated protein A or G (1:4,000; Zymed, San Francisco, Calif.) or chimeric protein AG (1:2,000; ProZyme, San Leandro, Calif.) was added. After incubation at 37°C for 1 h, the wells were washed with PBS-T. Fifty microliters of substrate solution, which contained 0.05 M citric acid, 0.01% hydrogen peroxide, and 2,2′-azino-di-(3-ethylbenzthiazoline sulfonate [6]) (Boehringer Mannheim GmbH, Mannheim, Germany), was added, followed by incubation at 37°C for 1 h. The enzyme reaction was terminated by the addition of 50 μl of 5% sodium dodecyl sulfate. The optical density (OD) value at 414 nm was determined with an ImmunoMini NJ-2300 ELISA reader (InterMed, Tokyo, Japan). We used four positive serum samples and 1 negative serum sample from cattle as positive and negative controls, respectively.
AGID test.
The presence of a specific antibody was confirmed by the agar gel immunodiffusion (AGID) test and an indirect immunofluorescence assay (IFA). Infected MDBK cells were collected by trypsinization and were suspended in a small volume of PBS. The cell suspension was sonicated and was used as the viral antigen in the AGID test with 1% Noble agar (Difco) in buffer (0.05 M Tris-HCl [pH 7.2], 8.5% NaCl, 0.1% NaN3) as described previously (24). The wells were 5 mm in diameter, and six circumferential wells were placed at a distance of 3 mm from the central well. The central well was filled with the antigen, and the control antiserum was placed in alternate exterior wells. Serum samples were placed in the remaining three wells. The AGID plate was allowed to stand at room temperature for 3 days, and precipitation lines were observed.
IFA.
As an antigen in IFA, infected FBM cells were washed with PBS and were smeared onto the slides. After fixation with acetone, the antigen was incubated with serum samples at 37°C for 30 min and then incubated with fluorescein isothiocyanate-conjugated protein A or G (1:500; Zymed) at 37°C for 30 min.
RESULTS
Protein binding capacity.
Since no data on the binding capacities of proteins A and G to immunoglobulins of wild animals in Japan were available, serum samples from all species were tested with proteins A, G, and AG. Five serum samples selected from each animal at random were coated onto a microplate, and the binding capacity was tested by ELISA as described above. Sera from most species reacted with both proteins A and G, whereas those from the Japanese badger, Japanese black bear, and masked palm civet reacted only with protein A. Sera from the Japanese serow reacted strongly with protein G but weakly with protein A (Table 2). Protein AG had a broad binding ability and bound to sera from nine species, and it was therefore used for further serological experiments.
TABLE 2.
Protein binding capacities of sera from wild animals
| Animal | Mean (SD) ODa
|
||
|---|---|---|---|
| Protein AG | Protein A | Protein G | |
| Japanese monkey | 1.41 (0.03) | 1.68 (0.07) | 1.51 (0.05) |
| Japanese deer | 1.38 (0.05) | 1.28 (0.27) | 1.40 (0.03) |
| Japanese badger | 1.37 (0.00) | 1.50 (0.03) | 0.02 (0.01) |
| Japanese wild boar | 1.33 (0.11) | 1.41 (0.32) | 1.23 (0.18) |
| Nutria | 1.25 (0.15) | 1.55 (0.19) | 1.24 (0.13) |
| Japanese raccoon dog | 1.19 (0.23) | 1.04 (0.37) | 0.56 (0.27) |
| Masked palm civet | 1.11 (0.51) | 1.09 (0.55) | 0.02 (0.01) |
| Japanese black bear | 0.98 (0.51) | 1.09 (0.67) | 0.09 (0.08) |
| Japanese serow | 0.75 (0.45) | 0.15 (0.18) | 1.08 (0.33) |
Five serum samples from each animal except Japanese badgers were selected at random and were tested with proteins AG, A, and G. Two serum samples from Japanese badgers were tested.
Detection of antibody.
A total of 272 serum samples were tested for the prevalence of antibody to parapoxvirus. In the protein AG-ELISA, the cutoff value was determined on the basis of the bimodal distribution of the antibody titer for each species. Most OD values for seronegative samples from wild animals were almost the same as those for the seronegative control samples from cattle. We designated as positive serum samples with OD values more than threefold that for the negative control.
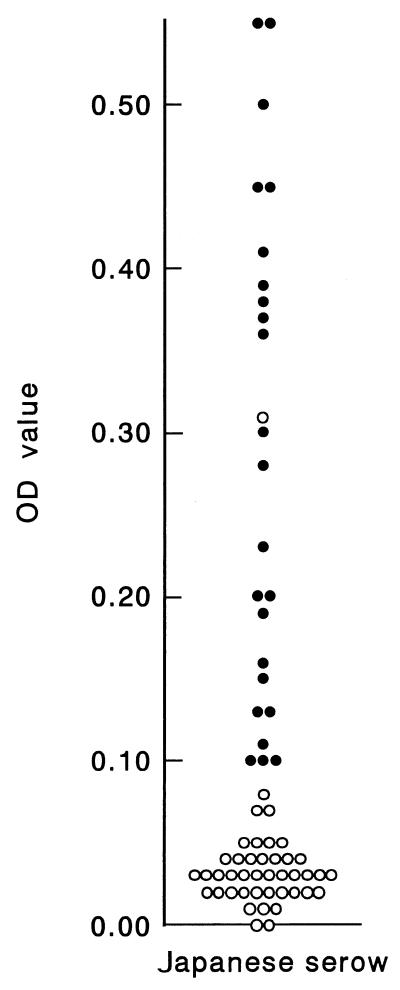
Antibodies against parapoxvirus were detected only in Japanese serows by the protein AG-ELISA (Fig. 2 and Table 3). The seroprevalence of antibodies against parapoxvirus among Japanese serows was 24 of 66 (36.4%), and seropositive serows were found in almost all prefectures in all years tested. The OD value for seropositivity ranged from 0.10 to 0.55 (Fig. 2). Seropositive samples were also determined to be positive by both the AGID test and IFA, in which a precipitation line and specific fluorescence were observed, respectively (data not shown). For some samples from other species and for one sample from a Japanese serow, the reactions were doubtful. The samples were positive by the protein AG-ELISA but had neither precipitation lines in the AGID test nor specific fluorescence in the IFA. We concluded that they were seronegative.
FIG. 2.
OD values for sera from Japanese serows in the protein AG-ELISA. ●, positive samples; ○, negative samples. One sample which was positive by the protein AG-ELISA (OD value = 0.31) but negative by the AGID test and IFA was determined to be negative.
TABLE 3.
Geographic and temporal distribution of seropositive Japanese serows
| Year | No. of seropositive serows/total no. of
serows tested (%) in the following prefecture:
|
|||
|---|---|---|---|---|
| Gifu | Kanagawa | Tochigi | Yamagata | |
| 1984-1985a | 14/30 (46.7) | |||
| 1989 | 0/1 (0.0) | |||
| 1991 | 1/2 (50.0) | 2/3 (66.7) | ||
| 1992 | 0/5 (0.0) | 1/6 (16.7) | ||
| 1993 | 0/3 (0.0) | 3/5 (60.0) | ||
| 1994 | 2/5 (40.0) | |||
| 1995 | 1/6 (16.7) | |||
Winter season.
DISCUSSION
We used chimeric protein AG in a serological survey of the prevalence of parapoxvirus among wild animals. Since chimeric protein AG can strongly bind to the immunoglobulins of many mammalian species but not to those of birds and reptiles (9), it is a powerful tool for use in immunological studies (1, 2). It is especially very useful for serological surveys of infectious agents in wild animals because antisera against immunoglobulins of wild animals are usually not commercially available. Among the sera from the nine species examined in this study, sera from six species reacted with both proteins A and G, whereas sera from three species reacted with only one protein. The protein AG-ELISA developed in this study could detect immunoglobulins against parapoxvirus in all species and might be applicable to serological surveys of the virus in other wild animals.
Antibodies against parapoxvirus were detected only in Japanese serows and were not detected in the other species tested. Parapoxvirus infections in Japanese serows were first reported in 1976 in Akita Prefecture (9a) and began to be observed in other areas, such as Aomori Prefecture in 1978 (27a) and Iwate, Yamagata, Fukushima, and Miyagi Prefectures from 1979 to 1982 (8, 18) but remained limited in the prefectures in the northern part of Japan (Fig. 1). In Gifu Prefecture, which is located in central part of Japan, a previous study demonstrated that although no antibodies were detected until the winter of 1982-1983, antibodies were detected from 1 of 189 serum samples in the winter of 1983-1984 and the disease occurred in the winter of 1984-1985 in Japanese serows (26). The disease is now spreading to other areas, such as Tokyo and Ishikawa (29, 31) (Fig. 1). These observations and our results suggest that the infections spread from northern Japan to central Japan and epizootic cycles of parapoxvirus infection are widely prevalent in Japanese serows.
A previous report showed that 37% of cattle in Aomori Prefecture in 1980 were seropositive for parapoxvirus (27a). However, our survey in 1998 revealed that 100% of cattle over 5 years old in Chiba Prefecture and the prefectures near Chiba Prefecture were positive for parapoxvirus (10). The increase in the rate of seropositivity among cattle may be associated with the spread of the disease in Japanese serows and with the increase in the rate of cattle transfer. The roles of some wild animals as reservoirs and/or amplifiers of viruses such as African swine fever and rabies viruses are reasonably well known. Japanese serows could be reservoirs for the virus in the field in Japan and might carry parapoxvirus to domestic animals such as cattle, sheep, and goats. It is also likely that there are virus cycles among Japanese serows and domestic animals.
Recently, a new parapoxvirus was isolated from red deer in New Zealand (6, 20). Clinical symptoms in red deer were observed at some geographically isolated farms. The isolated virus was genetically distinguishable from other parapoxviruses (6, 20). We first suspected the presence of antibodies in Japanese deer, but no antibodies were detected. However, there is a possibility that the virus could be introduced into Japanese deer by foreign deer that carry it. In 1997, contagious pustular dermatitis in captive Japanese livestock deer was added to the list of infectious diseases that we must monitor, according to the Animal Infectious Disease Control Law in Japan. Since there are no reports of clinical observations or infections at present, we can say that Japanese deer appear to have been free of this disease, at least until 1993.
Parapoxvirus infection was seen in many animals. The members of the genus Parapoxvirus are closely related, and there are no established serological distinctions (11, 16, 21, 22, 28). The relationship between the virus that infects wild animals and other parapoxviruses is still unclear. Restriction endonuclease analysis of viral DNA and DNA-DNA hybridization analyses are thought to be useful methods for the classification of parapoxviruses (14, 19). Thus, isolation of virus from Japanese serows and endonuclease analysis are required to clarify the relationship between parapoxvirus and other parapoxviruses in serows.
ACKNOWLEDGMENTS
We thank Yuichi Tagawa (Tohoku Branch, National Institute of Animal Health) and Shin-ichiro Hamasaki (Kansai Branch, Wildlife Management Office) for providing some serum samples from Japanese deer and serows, respectively. We are also thankful to Kim Barrymore for critical reading of the manuscript.
This study was supported in part by a grant from the Ministry of Agriculture, Forestry and Fisheries.
REFERENCES
- 1.Eliasson M, Andersson R, Olsson A, Wigzell H, Uhlén M. Differential IgG-binding characteristics of staphylococcal protein A, streptococcal protein G, and a chimeric protein AG. J Immunol. 1989;142:575–581. [PubMed] [Google Scholar]
- 2.Eliasson M, Olsson A, Palmcrantz E, Wiberg K, Inganäs M, Guss B, Lindberg M, Uhlén M. Chimeric IgG-binding receptors engineered from staphylococcal protein A and streptococcal protein G. J Biol Chem. 1988;263:4323–4327. [PubMed] [Google Scholar]
- 3.Esposito J J, Obijeski J F, Nakano J H. Orthopoxvirus DNA: strain differentiation by electrophoresis of restriction endonuclease fragmented virion DNA. Virology. 1978;89:53–66. doi: 10.1016/0042-6822(78)90039-9. [DOI] [PubMed] [Google Scholar]
- 4.Falk E S. Parapoxvirus infections of reindeer and musk ox associated with unusual human infections. Br J Dermatol. 1978;99:647–654. doi: 10.1111/j.1365-2133.1978.tb07059.x. [DOI] [PubMed] [Google Scholar]
- 5.Fenner F. Poxviruses. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2673–2702. [Google Scholar]
- 6.Horner G W, Robinson A J, Hunter R, Cox B T, Smith R. Parapoxvirus infections in New Zealand farmed red deer (Cervus elaphus) N Z Vet J. 1987;35:41–45. doi: 10.1080/00480169.1987.35376. [DOI] [PubMed] [Google Scholar]
- 7.Imada T, Tsuboi T, Takahashi N, Hamaoka T, Haritani M, Miyamoto T, Murata H. Serological survey of 8 bovine viral pathogens in sika deer (Cervus nippon) of northern Japan. Jpn J Zoo Wildl Med. 1996;1:42–44. [Google Scholar]
- 8.Kato H, Sato K, Ishikawa Y, Takahashi S, Gonai Y, Yatsu K. Papular stomatitis with dictyocaulosis in Japanese serows, Capricornis crispus, in captivity. J Jpn Assoc Zool Gard Aqua. 1980;22:46–50. . (In Japanese.) [Google Scholar]
- 9.Kelly P J, Tagwira M, Matthewman L, Mason P R, Wright E P. Reactions of sera from laboratory, domestic and wild animals in Africa with protein A and a recombinant chimeric protein AG. Comp Immunol Microbiol Infect Dis. 1993;16:299–305. doi: 10.1016/0147-9571(93)90159-3. [DOI] [PubMed] [Google Scholar]
- 9a.Kumagai T, et al. Abstracts of the 87th Meeting of the Japanese Society of Veterinary Science. 1979. [Google Scholar]
- 10.Kuroda, Y., M. Yoshida, T. Shibahara, T. Matsui, T. Nakane, H. Hara, Y. Inoshima, and H. Sentsui. An epidemic of parapoxvirus infection among cattle: isolation and antibody survey. J. Vet. Med. Sci., in press. [DOI] [PubMed]
- 11.Lard S L, Roehring J T, Pearson L D. Differentiation of parapoxviruses by application of orf virus-specific monoclonal antibodies against cell surface proteins. Vet Immunol Immunopathol. 1991;28:247–258. doi: 10.1016/0165-2427(91)90118-v. [DOI] [PubMed] [Google Scholar]
- 12.Mayr A, Büttner M. Milker’s node virus. In: Dinter Z, Morein B, editors. Virus infections of ruminants. Amsterdam, The Netherlands: Elsevier Science Publishers; 1990. pp. 29–32. [Google Scholar]
- 13.Memar O, Tyring S K. Cutaneous viral infections. J Am Acad Dermatol. 1995;33:279–287. doi: 10.1016/0190-9622(95)90262-7. [DOI] [PubMed] [Google Scholar]
- 14.Mercer A, Fleming S, Robinson A, Nettleton P, Reid H. Molecular genetic analyses of parapoxviruses pathogenic for humans. Arch Virol. 1997;13(Suppl.):25–34. doi: 10.1007/978-3-7091-6534-8_3. [DOI] [PubMed] [Google Scholar]
- 15.Moss B. Poxviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2637–2671. [Google Scholar]
- 16.Nagington J, Lauder I M, Smith J S. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;81:306–313. doi: 10.1136/vr.81.13.306. [DOI] [PubMed] [Google Scholar]
- 17.Okada H M, Okada K, Numakunai S, Ohshima K. Electron microscopy on mucosal and cutaneous lesions in contagious papular dermatitis of Japanese serow (Capricornis crispus) Jpn J Vet Sci. 1984;46:297–302. doi: 10.1292/jvms1939.46.297. [DOI] [PubMed] [Google Scholar]
- 18.Okada H M, Okada K, Numakunai S, Ohshima K. Histopathologic studies on mucosal and cutaneous lesions in contagious papular dermatitis of Japanese serow (Capricornis crispus) Jpn J Vet Sci. 1984;46:257–264. doi: 10.1292/jvms1939.46.257. [DOI] [PubMed] [Google Scholar]
- 19.Robinson A J, Lyttle D J. Parapoxviruses: their biology and potential as recombinant vaccines. In: Binns M, Smith G L, editors. Recombinant poxviruses. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 285–327. [Google Scholar]
- 20.Robinson A J, Mercer A A. Parapoxvirus of red deer: evidence for its inclusion as a new member in the genus parapoxvirus. Virology. 1995;208:812–815. doi: 10.1006/viro.1995.1217. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbusch R F, Reed D E. Reaction of convalescent bovine antisera with strain-specific antigens of parapoxviruses. Am J Vet Res. 1983;44:875–878. [PubMed] [Google Scholar]
- 22.Rossi C R, Kiesel G K, Jong M-H. A paravaccinia virus isolated from cattle. Cornell Vet. 1977;67:72–80. [PubMed] [Google Scholar]
- 23.Schuler G, Hönigsmann H, Wolff K. The syndrome of milker’s nodules in burn injury. J Am Acad Dermatol. 1982;6:334–339. doi: 10.1016/s0190-9622(82)70025-8. [DOI] [PubMed] [Google Scholar]
- 24.Sentsui H, Nishimori T, Nagai I, Nishioka N. Detection of sheep-associated malignant catarrhal fever virus antibodies by complement fixation tests. J Vet Med Sci. 1996;58:1–5. doi: 10.1292/jvms.58.1. [DOI] [PubMed] [Google Scholar]
- 25.Smith K J, Skelton III H G, James W D, Lupton G P. Parapoxvirus infections acquired after exposure to wildlife. Arch Dermatol. 1991;127:79–82. [PubMed] [Google Scholar]
- 26.Suzuki T, Minamoto N, Sugiyama M, Kinjo T, Suzuki Y, Sugimura M, Atoji Y. Isolation and antibody prevalence of a parapoxvirus in wild Japanese serows (Capricornis crispus) J Wildl Dis. 1993;29:384–389. doi: 10.7589/0090-3558-29.3.384. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki Y, Sugimura M, Atoji Y, Minamoto N, Kinjo T. Widespread of parapox infection in wild Japanese serows, Capricornis crispus. Jpn J Vet Sci. 1986;48:1279–1282. doi: 10.1292/jvms1939.48.1279. [DOI] [PubMed] [Google Scholar]
- 27a.Takatori I, et al. Abstracts of the 89th Meeting of the Japanese Society of Veterinary Science. 1980. [Google Scholar]
- 28.Wittek R, Herlyn M, Schümperli D, Bachmann P A, Mayr A, Wyler R. Genetic and antigenic heterogeneity of different parapoxvirus strains. Intervirology. 1980;13:33–41. doi: 10.1159/000149104. [DOI] [PubMed] [Google Scholar]
- 29.Yamagami T, Takahashi K, Sugiyama M, Uematsu K, Noguchi Y, Haritani M, Sudo Y. Parapox virus infection of wild Japanese serows (Capricornis crispus) in Tokyo. J Jpn Vet Med Assoc. 1996;49:257–259. . (In Japanese with English summary.) [Google Scholar]
- 30.Yamaguchi T, Shirota K, Fukushi H, Minamoto N, Kinjo T, Hirai K. Prevalence of infectious agents, drug-resistant Escherichia coli and residual organochlorine in wild animals inhabiting the mountainous areas of central Japan. Jpn J Zoo Wildl Med. 1998;3:1–7. . (In Japanese with English summary.) [Google Scholar]
- 31.Yata S, Murakami T, Ozawa T, Kitano H. A case of parapoxvirus infection in wild Japanese serow (Capricornis crispus) in Ishikawa prefecture. Jpn J Zoo Wildl Med. 1996;1:93–97. . (In Japanese with English summary.) [Google Scholar]