Summary
Progestin-primed ovarian stimulation (PPOS) is a new ovulation stimulation protocol, and its role in ovulation and regulatory mechanism is unclear. The clinical PPOS protocol was simulated in mice. The ovulated oocytes, estradiol, progesterone, and luteinizing hormone (LH) levels were analyzed at different hours after trigger. mRNA extraction and real-time PCR, hematoxylin and eosin staining, and immunofluorescence of ovaries were used to explore the involved signaling pathways. The PPOS group had a delayed ovulation at 12.5 h after trigger. Its suppressed LH level reduced the expression of luteinizing hormone/choriogonadotropin receptor (LHCGR) on the preovulatory follicles before trigger and significantly decreased the following progesterone synthesis, blood progesterone level, and progesterone receptor (PGR) expression within 4–6 h after trigger. Furthermore, the important ovulatory genes regulated by PGR including ADAMTS-1, VEGF-A, and EDN2 were downregulated, ultimately delaying the ovulation. PPOS suppresses the LH level before trigger and decreases the synthesis of progesterone after trigger, thus delaying the ovulation by downregulating the LHCGR-PGR pathway.
Subject areas: Endocrine system physiology, Molecular biology
Graphical abstract
Highlights
-
•
PPOS inhibits LH level and LHCGR expression on preovulatory follicles before trigger
-
•
The synthesis of progesterone and PGR expression reduce within 4–6 h after hCG
-
•
The ovulatory genes regulated by PGR are downregulated, and this delays ovulation
Endocrine system physiology; Molecular biology
Introduction
For women undergoing controlled ovarian hyperstimulation (COH), progestin-primed ovarian stimulation (PPOS) could prevent the premature luteinizing hormone (LH) surge1,2 and has been an effective choice, by oral progestins which started simultaneously with the gonadotrophins and continued until the ovulation trigger. And available evidence3,4,5 shows that PPOS provides a similar number of retrieved oocytes and pregnancy rate per transfer as those of gonadotropin-releasing hormone (GnRH) analogues. PPOS shows its peculiarity and importance in the COH, which has been specially reported twice in the review articles by the “Human Reproduction Update” journal.6,7
From initial “Luteal-phase ovarian stimulation” in 2013,1 “double stimulations during the follicular and luteal phases (Shanghai protocol)” in 2014,8 “the prototype of PPOS” in 2015,9 and “the random start PPOS” in 201610 to “the flexible PPOS” in 2019,11 we find that all of them could effectively inhibit the premature LH surge and ovulation. Furthermore, the patients in the prolonged ovulation trigger-oocyte pickup (OPU) time interval group (36.4–37.1 h, 37.1–37.8 h) had significantly higher mature oocyte rate, implantation rate, and live birth rate per transfer than the earlier group (35.6–36.4 h).12 These results indicate that PPOS inhibits the LH level before trigger and might delay the process of ovulation, which is difficult to be verified in the clinic.
In natural menstrual cycle, the onset of LH surge would be triggered by estradiol (E2) originating from the dominant follicle, exceeding 200–300 pg/mL for a minimum of 50 h.13 Progesterone rise in circulation would be 12 h before LH surge, which also plays an important role in generating LH surge.14,15 Therefore, several medicines are used as a trigger for inducing an endogenous LH surge (GnRH agonist,16 progesterone,17,18 and kisspeptin19) or mimicking LH function (human chorionic gonadotropin, hCG20,21) and ovulation. After LH surge or hCG, a rapid and robust induction of progesterone receptor (PGR) expression is accompanied by expressions of progesterone biosynthetic enzymes in granulosa cells of preovulatory follicles.22 And several lines of evidence support that the steroid hormone progesterone and its receptor PGR are key regulators in the ovulation.23,24 Inhibition of progesterone synthesis by epostane blocks the ovulation in rats25; blocking PGR function with a selective antagonist mifepristone (RU486) or ulipristal acetate (CDB-2914) reduces the number of ovulated oocytes in mice26,27; and the mature preovulatory follicles fail to release the oocytes in the PGR-null mice.28,29
In view of the vital role of progesterone in ovulation, it seems contradictory that PPOS probably delay the process of ovulation. Because progesterone is injected along with human menopausal gonadotropin (hMG) in patients from menstruation cycle day 3 onward in the PPOS protocol, it might increase progesterone level and should accelerate ovulation. This drives us to uncover the role of PPOS in ovulation and its regulating mechanism in this research, which could provide the crucial reference of oocyte retrieval time for worldwide patients when choosing PPOS.
Results
The clinical PPOS and control protocols were simulated in mice
We simulated the clinical PPOS and set up its control protocol in mice (Figure 1A). Compared with the control group, PPOS group (5 mg/kg) reduced the oocyte yields significantly (Figure 1B), which is similar as the PPOS group (10 mg/kg) in our former research.30 While the PPOS group (2 mg/kg) had comparable oocyte yields with the control group (Figure 1B), the serum progesterone (P) level increased significantly every half an hour after progesterone intraperitoneal injection (p < 0.001 at 0.5 h and 24.5 h, two-way repeated ANOVA, Figure 1C). Meanwhile, the serum E2 increased gradually and reached the maximum concentration at 48 h after pregnant mare serum gonadotropin (PMSG) injection within two groups, but without difference (Figure 1D). Therefore, we chose 2 mg/kg progesterone to be used in the PPOS group for exploring its function in ovulation.
Figure 1.
The mice with simulated PPOS protocol have not ovulated at 12.5 h after hCG trigger
(A) Schematic of experimental design in mice: vehicle (control group) or two doses of progesterone (PPOS group) were administered.
(B) The number of retrieved oocytes in the control group and PPOS groups (2 mg/kg progesterone, 5 mg/kg progesterone at 16 h after triggering. (n = 10 mice in each group).
(C and D) Serum progesterone levels (C, n = 5–7 mice in each group) and estradiol levels (D, n = 5–8 mice in each group) of mice in the PPOS (red line) and control groups (black line) from 0 to 48 h after PMSG injection.
(E) The total number and percentage of oocytes in the ovaries between control and PPOS groups at 12 h after hCG injection. (n = 5–6 mice in each group).
(F) Micrographs of ovaries and oviducts at 12.5 h after hCG injection in control and PPOS groups. (n = 3–5 mice in each group). Scale bar, 100 μm.
(G) Comparisons of the number of total oocytes and ovulated oocytes at 12.5 h after hCG injection. (n = 8–11 mice in each group).
Statistical analysis was performed by Mann-Whitney test in (B, E-left, and G), chi-squared test in E-right and two-way repeated ANOVA with Holm-Sidak test in (C and D). Data are represented as mean ± SEM. ns, no significance (p ≥ 0.05). ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001.
The mice of PPOS group had a postponed ovulation compared with control group
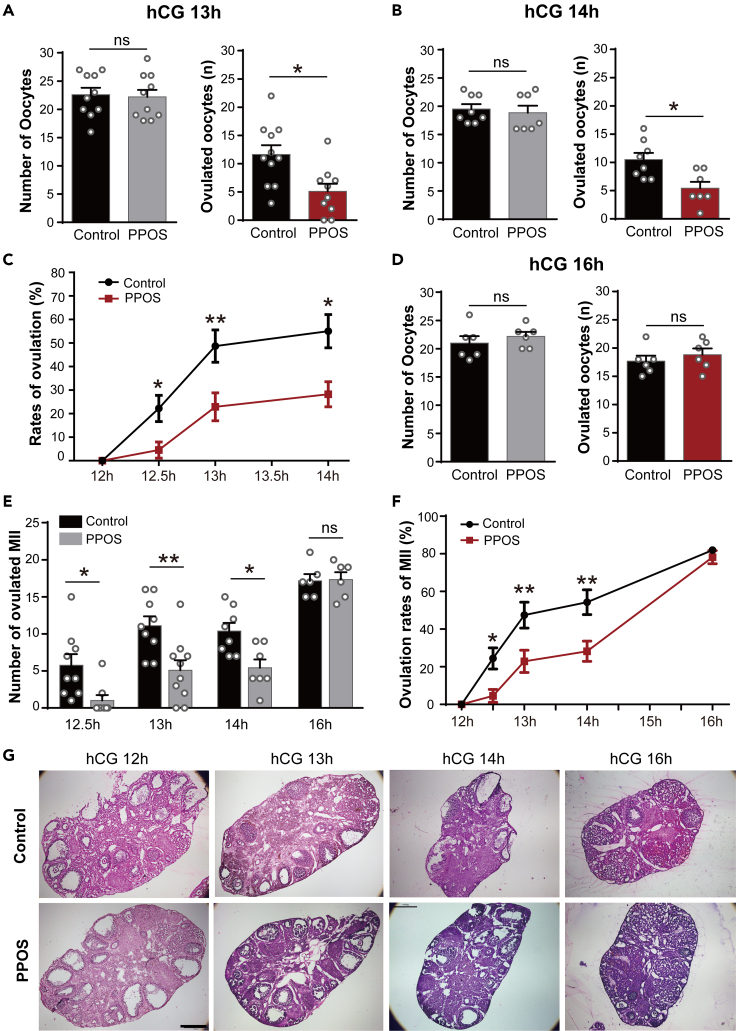
Both the PPOS and control groups were given exogenous gonadotropin (hCG) to trigger ovulation. After 12 h, two groups had a comparable number of oocytes in the ovaries before ovulation (Figure 1E, left), and the percentages of metaphase I (MI), metaphase II (MII), and death oocytes were similar in the ovaries (Figure 1E, right). However, the ovaries of control group had more large follicles and corpus luteum than the PPOS group at 12.5 h after hCG injection (Figure 1F, up). And the ampulla of oviduct was obviously enlarged and protruded in the control group at 12.5 h after hCG injection (Figure 1F, down). As seen in Figure 1G, when the total oocytes in the ovaries and oviduct were similar between the two groups, the control group had significantly more ovulated oocytes than the PPOS group at 12.5 h after hCG injection (4.8 ± 1.4 vs. 1.0 ± 0.8, p < 0.01).
The delayed ovulation of PPOS group still existed at the 13 h (11.6 ± 1.6 vs. 5.1 ± 1.3, p < 0.05, Figure 2A) and 14 h (10.5 ± 1.2 vs. 5.4 ± 1.1, p < 0.05, Figure 2B) after hCG trigger. As seen in Figure 2C, the rates of ovulation were significantly lower in the PPOS group from 12.5 to 14 h after hCG (p < 0.05 at 12.5 h and 14 h, p < 0.01 at 13 h, two-way ANOVA), while at 16 h after hCG, the PPOS and control groups had the similar oocytes in the oviduct (Figure 2D). Furthermore, the PPOS group had significantly less ovulated MII oocytes (Figure 2E) and ovulation rates (Figure 2F) than the control group at 12.5 h, 13 h, and 14 h after hCG, but without difference at 16 h after hCG (p < 0.05 at 12.5 h, p < 0.01 at 13 h, p < 0.01 or 0.05 at 14 h, two-way ANOVA). Ovaries were examined for histological change within 12–16 h after hCG administration (Figure 2G). At 12 h, 13 h, and 14 h after hCG, the ovaries of control group exhibited more corpora lutea (CL) and less preovulatory follicles than the PPOS group, while at 16 h after hCG, two groups had similar CL in the ovaries.
Figure 2.
The mice of PPOS group have a delayed process of ovulation than the control group
(A and B) At 13 h (A) and 14 h (B) after hCG injection, the total oocytes within the ovaries and oviducts and the ovulated oocytes in the oviducts between two groups (a: n = 9–11 mice in each group; b: n = 7–8 mice in each group).
(C) Comparison of ovulation rates between PPOS group (red line) and control group (black line) after hCG injection (n = 5–10 mice in each group).
(D) At 16 h after hCG injection, the total oocytes within the ovaries and oviducts and the ovulated oocytes in the oviducts between two groups (n = 6 mice in each group).
(E and F) Comparison of the number of ovulated MII oocytes (E) and ovulation rates of MII oocytes (F) from 12.5 to 16 h after hCG injection between two groups. (E and F: n = 6–10 mice in each group).
(G) HE-staining images of ovary sections in the PPOS and control groups at 12 h, 13 h, 14 h, and 16 h after hCG injection. (n = 3–5 mice in each group). Scale bar, 500 μm.
Statistical analysis was performed by Mann-Whitney test in A, B, and D and by two-way ANOVA in C, E, and F. Data are represented as mean ± SEM. ns, no significance (p ≥ 0.05). ∗p < 0.05, ∗∗p < 0.01.
The suppressed LH level of PPOS before trigger contributing to the reduced progesterone synthesis and PGR expression after hCG trigger
After hCG trigger, the E2 levels were gradually decreased till the ovulation, but without difference between two groups (Figure S1). The progesterone levels increased obviously within 4 h after hCG31 both in the control and PPOS groups (Figure 3A), but its level was significantly lower in the PPOS group at 4 h (49.65 ± 3.28 vs. 34.43 ± 7.28, p < 0.05, two-way repeated ANOVA). The endogenous LH levels of the two groups were without differences within 2–16 h after hCG (Figure 3B), while PPOS group had an obviously lower LH level than the control group before hCG trigger (1.96 ± 0.55 vs. 0.59 ± 0.17, p < 0.05, Figure 3D), although the two groups had the similar LH levels at the beginning of COH (Figure 3C). Furthermore, the expression of LH/choriogonadotropin receptor (LHCGR) on the theca cells of preovulatory follicles32,33,34 was significantly less in the PPOS group (Figures 3E and 3F).
Figure 3.
The serum progesterone and LH levels after hCG injection within the control and PPOS groups
(A and B) The serum progesterone (A) and endogenous LH levels (B) in the PPOS and control groups after hCG injection (A: n = 8 mice in each group; B: n = 5–6 mice in each group).
(C and D) The LH levels at 0 h before PMSG injection (C, n = 7–12 mice in each group) and 0 h before hCG trigger (D, n = 7–12 mice in each group) in the control and PPOS groups.
(E) Immunofluorescence images of LHCGR expression on the ovaries in the PPOS and control groups when giving hCG injection. (n = 3–5 mice in each group). The preovulatory follicles were indicated by asterisks and one of them indicated by dotted rectangle box was enlarged in the bottom (Scale bar: 500 μm in the upper panel, 100 μm in the middle panel, 50 μm in the bottom panel).
(F) The relative LHCGR fluorescence intensity within control and PPOS groups analyzed by ImageJ software (n = 5 preovulatory follicles within 3–5 mice in each group).
Statistical analysis was performed by two-way repeated ANOVA with Holm-Sidak test in A-B and Mann-Whitney test in C, D, and F. Data are represented as mean ± SEM. ns, no significance (p ≥ 0.05). ∗p < 0.05. ∗p < 0.01.
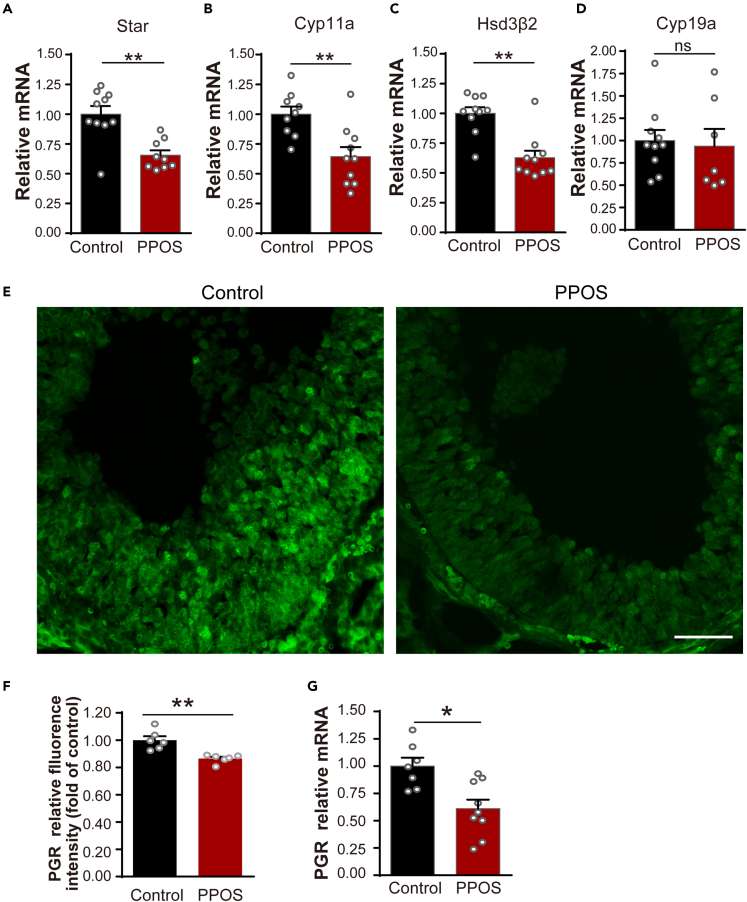
The mRNA levels of Star, Cyp11a, and Hsd3B2, which are key-point steroidogenic enzymes of progesterone synthesis,35,36 were significantly less in the PPOS group (Figures 4A–4C), while, CYP19a, which was mainly needed for E2, but not progesterone synthesis,36,37 was without difference within the two groups (Figure 4D). The PGR mRNA level of PPOS group was significantly reduced at 4 h after hCG (Figure 4G). Furthermore, PGR expression on the mural granular cells of preovulatory follicles23,38,39 also obviously decreased in the PPOS group at 6 h after hCG stimulation (Figures 4E and 4F).
Figure 4.
The decreased mRNA levels of progesterone synthesis enzymes and PGR expression in the mice of PPOS group
(A–D) Star (A, n = 9–10 mice in each group), Cyp11a (B, n = 9–10 mice in each group), Hsd3B2 (C, n = 10 mice in each group), and Cyp19a (D, n = 7–10 mice in each group) relative mRNA levels in the PPOS and control groups at 4 h after hCG injection.
(E) Immunofluorescence images of PGR expression on the preovulatory follicles in the PPOS and control groups at 6 h after hCG injection (n = 3–5 mice in each group). Scale bar, 50 μm.
(F) The relative PGR fluorescence intensity within control and PPOS groups analyzed by ImageJ software (n = 6 preovulatory follicles within 3–5 mice in each group).
(G) PGR relative mRNA expression levels at 4 h after hCG injection within two groups (n = 7–9 mice in each group).
Statistical analysis was performed by Mann-Whitney test in A–D, F-G. Data are represented as mean ± SEM. ns, no significance (p ≥ 0.05). ∗p < 0.05, ∗∗p < 0.01.
The PGR-regulated gene network which is essential for ovulation was downregulated in the PPOS group
ADAMTS-1 (a disintegrin and metalloproteinase with thrombospondin motifs) plays a key role in the remodeling of extensive extracellular matrix (ECM) to facilitate assembly of the viscoelastic cumulus-oocyte complex (COC) matrix before ovulation.40,41 Its mRNA levels at 4 h (Figure 5B) and protein expressions at 6 h (Figures 5F and 5J) after hCG were significantly reduced in the PPOS group. There was also a significant decrease of HIFs (hypoxia-inducible factors) in the PPOS group (Figures 5A, 5E, and 5I), which serves as critical regulators of the tissue’s response to changes in oxygen levels.42 EDN2 (Endothelin-2), a potent vasoactive hormone and contributing to the follicle-wall breakdown,43 was also significantly decreased in the PPOS group (Figures 5D, 5H, and 5L). Furthermore, VEGF-A (vascular endothelial growth factor A), which promotes vascular permeability during ovulation,23 was obviously reduced in the PPOS group (Figures 5C, 5G, and 5K). Images by confocal microscope also show that expressions of ADAMTS-1, HIF, VEGF-A, and EDN2 were significantly less in the granular cells of preovulatory follicles27,38,39 within the PPOS group at 6 h after hCG injection (Figures S2E–S2H).
Figure 5.
The mRNA levels and protein expressions of genes regulated by PGR were downregulated in the mice of PPOS group
(A–D) The relative mRNA levels of HIF (A), ADAMTS-1 (B), VEGF-A (C), and EDN2 (D) in the control group (n = 6, 8, 11, 10 mice in A–D) and PPOS groups (n = 9, 10, 10, 9 mice in A–D).
(E–H) The immunofluorescence images of HIF (E), ADAMTS-1 (F), VEGF-A (G), and EDN2 (H) expression in the ovaries (n = 3–5 mice in each group). The preovulatory follicles indicated by asterisks in the upper panel were enlarged in the lower panel. Scale bar in the upper panel, 500 μm. Scale bar in the lower panel, 200 μm.
(I–L) The relative fluorescence intensity of HIF (I), ADAMTS-1 (J), VEGF-A (K), and EDN2 (L) within control and PPOS groups analyzed by ImageJ software (n = 6–8 preovulatory follicles within 3–5 mice in each group).
Statistical analysis was performed by Mann-Whitney test in A–D and I–L. Data are represented as mean ± SEM. ns, no significance (p ≥ 0.05). ∗p < 0.05. ∗∗p < 0.01. ∗∗∗p < 0.001.
However, PGR-regulated genes mediating the inflammatory process in breaking down the wall of preovulatory follicles23 had not been influenced in the PPOS group. Neither mRNA levels of PPARr (peroxisome proliferator-activated receptors, Figure S2A) and IL6 (interleukin-6, Figure S2B) nor PTGS2 (prostaglandin endoperoxide synthase2, Figure S2C) had been obviously influenced in the PPOS group, although all of them had the same tendency of slight increase. SNAP25 (Synaptosomal-associated protein 25) is involved in the membrane fusion and may also participate in the ovulatory process;44 its mRNA level was without difference (Figure S2D).
Discussion
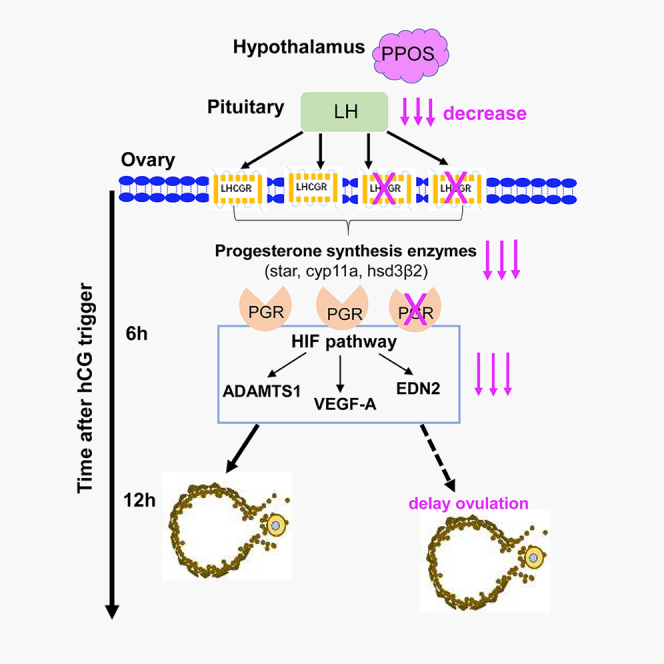
Our research firstly finds that the PPOS protocol in the clinic could suppress the endogenous LH level and LHCGR expression on the preovulatory follicles before trigger, which leads to the decreased biosynthesis of progesterone and PGR expression within 4–6 h after hCG trigger and thus postpones the process of ovulation.
These findings further verify our former clinical research12 in patients with PPOS protocol, which used the propensity score to auto-match patients with the homogeneous clinical characteristics among three trigger-OPU interval groups (group 1: 35.6–36.4 h; group 2: 36.4–37.1 h; group 3: 37.1–37.8 h) and found that the trigger-OPU interval within groups 2 and 3 had significantly higher mature oocyte rates (84.54% vs. 84.60% vs. 82.34%, p = 0.002) and implantation rates (34.17% vs. 34.37% vs. 29.61%, p < 0.05) than group 1. A relative prolonged ovulation trigger-OPU interval (36.4–37.8 h) is optimal for most patients using a PPOS protocol.
For triggering ovulation in the COH, the same dosage of exogenous gonadotropin (hCG) was given to simulate the endogenous LH surge in both PPOS and control groups. After hCG, the endogenous LH levels of the two groups were without differences within 2–16 h, while the progesterone level was significantly lower in the PPOS group at 4 h. It is confusing that the same dosage of exogenous hCG brought about the different progesterone levels after trigger within the two groups, especially that the PPOS group is the lower group. Progesterone is injected along with hMG in the PPOS protocol, which might increase the progesterone level. Actually, the serum progesterone level increased significantly every half an hour after progesterone intraperitoneal injection in the PPOS protocol, but this increasing was temporary and only before hCG trigger.
After trigger, activation of LHCGR on preovulatory follicles in response to the LH/hCG surge induces the expression of PGR specifically in granulosa cells, as well as luteinization-specific genes including steroidogenic enzymes that synthesize progesterone.38 Former1,2 and this research has found that PPOS could effectively suppress LH level and LHCGR expression on the preovulatory follicles before hCG trigger. Therefore, despite giving the same dosage of exogenous hCG to trigger, the granulosa cells of preovulatory follicles in the PPOS group could not synthesize the similar level of progesterone as the control group.
This speculation was further verified by the mRNA levels of Star, Cyp11a, and Hsd3B2, which are key-point steroidogenic enzymes of progesterone synthesis.35,36 They were obviously less in the PPOS group. CYP19a, which was mainly needed for E2, but not progesterone synthesis,36,37 was without difference within the two groups. As the key regulator of ovulation, progesterone plays its biological effects through the PGR. Therefore, a rapid and robust expression of PGR was induced in the preovulatory follicles after hCG injection; it peaks at 4–8 h and then declines to undetectable levels.29 Exactly, the PGR mRNA level and expression on the preovulatory follicles were significantly reduced in the PPOS group.
Research during the past years29,45,46,47,48 has identified that several genes, such as ADAMTS-1, HIFs, VEGF-A, and EDN2, are acting as downstreams of PGR and essential for ovulation in regulating follicle-wall degradation, vascular permeability, and vascular dynamics. These PGR-regulating genes which are essential for ovulation were obviously downregulated in the PPOS group, from the mRNA level to protein expression, leading to the delayed ovulation.
The possible molecular mechanism involved in the delayed ovulation of PPOS group might be the following: (1) the PGR, which was activated by locally secreted progesterone and expressed in mural granulosa cells of preovulatory follicles, transactivates intermediary genes including HIF, which acts in concert with PGR to induce ADAMTS-1 and VEGF-A.38 (2) ADAMTS-1, which plays a key role in the remodeling of extensive ECM to facilitate assembly of the viscoelastic COC matrix before ovulation,40,41 also probably plays an important role in the degradation of the collagen layer.23,47,48 (3) VEGF as a vascular permeability factor (VPF49) could alter local fluid fluxes and thus change hydrostatic pressure within the follicle and play critical roles during ovulation.50 4) EDN2 is produced downstream of PGR action in mural granulosa cells of preovulatory follicles immediately preceding ovulation.27 It regulates blood-vessel dynamics by controlling the constriction or dilation of the vessels and also plays a role in inflammation.51,52 The decreased EDN2 of PPOS group contributes to the delayed follicle-wall degradation by influencing the dynamic changes in the vasculature during ovulation. Through this sequential cascade of decreased PGR-initiated gene induction, integrated functional events including protease activation, cumulus expansion, smooth muscle contraction, vascular permeability, and angiogenesis all contribute to delayed follicle rupture and oocyte release in the PPOS group.
Our research firstly finds that the clinical PPOS protocol could inhibit the LH level and LHCGR expression on the preovulatory follicles before trigger. When given the same dosage of exogenous hCG, the progesterone synthesis, blood progesterone level, and PGR expression of PPOS group were obviously decreased, thus downregulating the PGR-modulatory genes and postponing the ovulation. The possible regulating mechanism of PPOS in delaying ovulation was summarized in the graphical abstract. Our research from the important clinical experience to the mechanism exploration provides the crucial evidence for the patients using PPOS when arranging the OPU time interval, according to its endocrine effects on the hypothalamic-pituitary-ovarian axis.
Limitations of the study
However, it is a pity and limitation that we could not set up a complete control for PPOS protocol in the clinic to compare their ovulation process. For the patients using PPOS, they need not use any other GnRH antagonist or agonist to suppress the pituitary gland and LH level within hyperstimulation process, while in the actual clinical ovary hyperstimulation process, a complete control without any inhibitory effect on LH level will induce premature LH surge and ovulation. We could only infer from the clinical research that the mature oocyte rate and implantation rate of patients using PPOS were significantly higher in the groups with prolonged trigger-OPU interval.12
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-LHCGR antibody | BOSTER | Cat# BA3590 |
| Rabbit anti- ET-2/Endothelin 2 antibody (FITC) | Biorbyt | Cat# orb466253 |
| Rabbit monoclonal anti- Progesterone Receptor antibody | Abcam | Cat# ab16661; RRID:AB_443421 |
| Mouse monoclonal anti- VEGF antibody (C-1) | Santa Cruz Biotechnology | Cat# sc-7269; RRID:AB_628430 |
| Mouse monoclonal anti- anti-HIF-1α antibody | Novus Biologicals | Cat# NB100-105; RRID:AB_10001154 |
| Sheep Polyclonal anti- ADAMTS-1 antibody | Thermo Fisher Scientific | Cat# PA5-47790; RRID:AB_2576699 |
| Alexa Fluor 488 Donkey anti-Mouse lgG (H + L) | Jackson Immuno Research | Cat# 715-546-150 |
| Alexa Fluor 488 Donkey anti-Sheep lgG (H + L) | Jackson Immuno Research | Cat# 713-545-147 |
| Alexa Fluor 488 Donkey anti-Rabbit gG (H + L) | Jackson Immuno Research | Cat# 711-546-152 |
| Chemicals, peptides, and recombinant proteins | ||
| Serum gonadotropin for injection (PMSG) | Ningbo Sansheng Biological Technology Co., Ltd. | B200920 |
| Progesterone | Sigma Aldrich | Cat# P0130 |
| Human chorionic gonadotropin (hCG) | Ningbo Sansheng Biological Technology Co., Ltd. | S200808 |
| PBS | Sangon Biotech (Shanghai) Co., Ltd. | Cat# E607008-0500 |
| Triton TM X-100 | Sigma | Cat# V900502-100 |
| Corn oil | Shanghai Aladdin Biochemical Technology Co., Ltd. | Cat# C116023-500 |
| 4% Paraformaldehyde/universal tissue fixative solution | Biosharp Life Sciences | Cat# BL539A |
| RNase-free Water | TAKARA | Cat# 9012 |
| Dimethyl sulfoxide (DMSO) | Diamond | Cat# A100231-0500 |
| Tween 20 | Diamond | Cat# A100777-0500 |
| Modified HTF medium | FUJIFILM Irvine Scientific | Cat# 90126 |
| Hematoxylin and Eosin Staining Kit | Beyotime Biotechnology | Cat# C0105S |
| 3-lsobutyl-1-methylxanthine (IBMX) | Sigma | Cat# I5879 |
| Total RNA isolation TRIzol Reagent | Shanghai Pufei Biotech Co., Ltd | Cat# 3101-100 |
| Critical commercial assays | ||
| PrimeScript™ RT reagent Kit | TAKARA | RR037A |
| TB Green Premix Ex Taq | TAKARA | RR420A |
| Experimental models: Organisms/strains | ||
| ICR mice | Beijing Vital River Laboratory Animal Technology Co., Ltd. | http://www2.vitalriver/order-terms |
| Oligonucleotides | ||
| Real-Time PCR primers | Sangon Biotech (Shanghai) Co., Ltd | This paper Table S1 |
| Software and algorithms | ||
| GraphPad Prism software | GraphPad Prism | RRID:SCR 002798 |
| QuantStudioTM Real-Time PCR Software v1.3 | Thermo Fisher Scientific | Cat# A31150 |
| ImageJ image analysis software | ImageJ (https://imagej.net/) | RRID:SCR_003070 |
| Other | ||
| Olympus inverted microscope | Olympus | IX71 |
| Olympus fluorescence microscope | Olympus | BX53 |
| Olympus confocal microscope | Olympus | FV3000 |
| Cryomicrotome | Leica | CM1860 |
| Maestro Nano pro | MAESTROGEN | N/A |
| Microscope cover glass | CITOTEST | Cat# 10212450C |
| Adhesion microscope slides | CITOTEST | Cat# 188105 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Li Wang (wanglishfd@126.com).
Materials availability
This study did not generate new unique reagents.
Experimental model and study participant details
Animals
Animal studies were approved by the Institutional Ethics Committees of Care and Use of Experimental Animals of Shanghai Ninth People’s Hospital. ICR female mice aged 3–5 weeks,44,53,54 were used for this experiment when monitoring two regular estrous cycles, which were purchased from the company (Beijing Vital River Laboratory Animal Technology Co., Ltd. China). littermates of the same sex were randomly assigned to experimental groups. All animals were maintained under regulated 12 h light on/12 h light off cycles and were fed with standard mice chow and water ad libitum.
The mouse model of Progestin-Primed Ovarian Stimulation Protocol
Mouse in diestrus of estrous cycle were chosen to induce COH. In order to simulate PPOS protocol, mice (30g ±2g) were intraperitoneally injected with 10 IU pregnant mare serum gonadotropin (PMSG) and progesterone (2 mg/kg, 5 mg/kg, dissolved in corn oil) at first day, while the mice of control group were only intraperitoneally injected with 10 IU PMSG and corn oil. After 24 h, the progesterone oil agent and oil were injected in PPOS group and control group respectively. After 48 h, the mice were given an intraperitoneal injection with 10 IU human chorionic gonadotropin (hCG) to induce the ovulation in the PPOS and control groups.
Method details
Oocyte isolation and detection of ovulation
Oocytes were collected in oviducts and ovaries at 12 h, 12.5 h, 13 h, 14 h and 16 h after hCG injection. Oocytes from oviducts were also collected by puncturing the ampulla of oviducts. Cumulus-cell-oocyte complexes (COCs) were isolated from large antral follicles (preovulatory follicles, containing an oocyte and the diameter of follicles >400 um55) in ovaries by needle puncture under a dissecting microscope. Removal of cumulus granulosa cells by repeated blowing and aspiration of COCs using glass pipette with adding traces of hyaluronidase enzyme into the medium. The medium used for COCs and oocytes was the modified HTF medium supplemented with 5% serum substitute supplement (FUJIFILM Irvine Scientific). Meanwhile, 5M IBMX (Sigma) was added for preventing oocytes from further mature. Oocytes isolated from ovaries may have four different developmental stages which include the germinal vesicle (GV), GV breakdown (GVBD), metaphase I (MI) and metaphase II (MII). Oocytes in different developmental stages were counted respectively, and their morphological appearance was photographed using an Olympus IX71 inverted microscope.
Hormone measurements
To determine estradiol (E2), progesterone (P) and LH levels, the whole blood of mice was collected at different hours after hCG injection. For LH testing, whole 6 μL blood was immediately diluted in 114 μL of 0.1M PBS with 0.05% Tween 20, vortexed, and snap frozen on dry ice. Samples were stored at −20°C for a subsequent LH ELISA.56,57 For estradiol (E2) and progesterone (P) testing, serum was isolated from whole blood by centrifuge at 3000 rpm for 10 min and cryopreserved at −80°C until test. Estradiol (E2) and progesterone (P) levels were examined by the laboratory of Shanghai Ninth People’s Hospital, using chemiluminescence (Abbott Biologicals B.V.).
Hematoxylin-eosin staining
Mice were injected anesthetic solution (ketamine: xylazine) with no more than 200 μL dose. Then mice died and perfused with saline followed by 4% paraformaldehyde in 0.1M PBS. The ovaries were quickly removed, post-fixed in 4% paraformaldehyde overnight, and subjected to dehydration in increasing saccharin solutions (20–30%) at 4°C. The frozen ovary slices were sectioned at 10 μm on a cryomicrotome (Leica) for hematoxylin and eosin (H&E) staining. Preovulatory follicle was defined as containing an oocyte and the diameter of follicles >400 um,55 and corpus luteum were defined as cellular and/or partially eosinophilic fluid-filled spaces lined by polygonal eosinophilic cells containing lipid droplets.54,55 Images were captured on an olympus fluorescence microscope (BX53).
Immunofluorescence
For immunofluorescence, the ovaries slices were washed three times with PBS, then separately incubated with the first antibody (anti-LHCGR receptor, BA3590, BOSTER; Rabbit anti- ET-2/Endothelin 2 (EDN2) antibody (FITC), orb466253, Biorbyt; anti-progesterone receptor, ab16661, Abcam; anti-VEGF, C-1-sc-7269, Santa Cruz Biotechnology; anti-HIF-1α, NB100-105, Novus Biologicals; anti-ADAMTS-1, PA5-47790, Thermo Fisher Scientific) with suggested dilution at 37°C 2 h. All primary antibodies were supplemented with 0.3% Triton X-100 and 3% equinum serum in PBS. After washing three times with PBS, ovary slices were stained with the Alexa Fluor 488 secondary antibodies respectively at 37°C 1 h. Then ovary slices were washed three times with PBS and stained with DAPI at 37°C for 10 min, and mounted after rinsing. Fluorescence images were captured on an olympus fluorescence microscope (BX53) and olympus FV3000 confocal microscope.
RNA extraction and real-time PCR
Mouse ovaries were isolated, frozen in TRIzol Reagent (Shanghai Pufei Biotech Co., Ltd) and stored at −70°C until extraction. Total RNA was extracted from ovaries using the TRIzol Reagent according to the manufacturer’s instructions, with the inclusion of a DNase digestion step. RNA concentrations were quantified using the Nano pro. The Superscript First-Strand Synthesis System for reverse transcription (RR037A, PrimeScript RT reagent Kit, TAKARA) was used with random primers. For amplification of the cDNA products, specific primers pairs were selected as indicated in Table S1. The target cDNA was amplified by gene-specific primers and SYBR (TB Green Premix Ex Taq, TAKARA), and carried out on an ABI-PRISM 5700 sequence detection system. The mRNA expression of target gene was normalized to the internal control ribosomal protein L-19 (L19).
Quantification and statistical analysis
The statistical analyses in mice were performed in GraphPad Prism (version 6.01). The number of oocytes, ovulated oocytes, LH level before PMSG and hCG, and the relative mRNA levels of specific gene within two groups were analyzed by Mann-Whitney test. When comparing the concentration of serum hormones at different timepoints within two groups, data were analyzed by two-way repeated ANOVA with Holm-Sidak test. The ovulated number of MII, rates of ovulation and the ovulation rates of MII, were analyzed by two-way ANOVA test. When comparing the percentage of oocytes in ovary, the chi-square test was used. The fluorescence intensities were calculated by ImageJ software. To normalize the protein expression levels, the average value of fluorescence intensity in control group was set as a normalized standard,58 then the relative fluorescence intensity of control and PPOS group was divided by this normalized standard. The relative fluorescence intensity within control and PPOS groups was analyzed by Mann-Whitney test. Differences were considered significant when p < 0.05. Values are given as mean ± SEM.
Acknowledgments
We gratefully acknowledge all the doctors, nurses, and laboratory staffs employed in the Department of Assisted Reproduction, Shanghai Ninth People’s Hospital Affiliated to Shanghai Jiao Tong University School of Medicine.
Funding: The study was financially supported by the following grants: National Natural Science Foundation of China (82071603 to LW), Natural Science Foundation of Shanghai (Grant number: 19ZR1429300 to HL, 18ZR1422600 to LW), National Natural Science Foundation of China (81871163 to QL), and Cross-disciplinary Research Fund of Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine (JYJC202014).
Author contributions
L.W., Q.F.L., and H.L. conceived and designed the experiments. X.S. did all the analysis with the assistance of L.W. Y.T.X., W.N.Y., and Y.P.K. performed the Progestin-Primed Ovarian Stimulation Protocol in mice. Y.T.X. did the oocyte isolation, detection of ovulation, estradiol (E2) and progesterone (P4) hormone test, hematoxylin-eosin staining, and immunofluorescence. Y.T.X., W.Y.G., and Q.J.C. performed the LH test, the RNA extraction, and real-time PCR amplification. L.W. analyzed all the data and prepared for all figures. L.W., Q.F.L., and H.L. wrote the manuscript with input from all authors. All the authors take full responsibility for the work.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: July 8, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107357.
Contributor Information
Hui Long, Email: longhuish@aliyun.com.
Qifeng Lyu, Email: lyuqifeng@126.com.
Li Wang, Email: wanglishfd@126.com.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Kuang Y., Hong Q., Chen Q., Lyu Q., Ai A., Fu Y., Shoham Z. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil. Steril. 2014;101:105–111. doi: 10.1016/j.fertnstert.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Yu S., Long H., Chang H.Y.N., Liu Y., Gao H., Zhu J., Quan X., Lyu Q., Kuang Y., Ai A. New application of dydrogesterone as a part of a progestin-primed ovarian stimulation protocol for IVF: a randomized controlled trial including 516 first IVF/ICSI cycles. Hum. Reprod. 2018;33:229–237. doi: 10.1093/humrep/dex367. [DOI] [PubMed] [Google Scholar]
- 3.Wang L., Yin M., Liu Y., Chen Q., Wang Y., Ai A., Fu Y., Yan Z., Jin W., Long H., et al. Effect of Frozen Embryo Transfer and Progestin-primed Ovary Stimulation on IVF outcomes in women with high body mass index. Sci. Rep. 2017;7:7447. doi: 10.1038/s41598-017-07773-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Chen Q., Wang N., Chen H., Lyu Q., Kuang Y. Controlled Ovarian Stimulation Using Medroxyprogesterone Acetate and hMG in Patients With Polycystic Ovary Syndrome Treated for IVF: A Double-Blind Randomized Crossover Clinical Trial. Medicine (Baltim.) 2016;95 doi: 10.1097/MD.0000000000002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Q., Wang Y., Sun L., Zhang S., Chai W., Hong Q., Long H., Wang L., Lyu Q., Kuang Y. Controlled ovulation of the dominant follicle using progestin in minimal stimulation in poor responders. Reprod. Biol. Endocrinol. 2017;15:71. doi: 10.1186/s12958-017-0291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ata B., Capuzzo M., Turkgeldi E., Yildiz S., La Marca A. Progestins for pituitary suppression during ovarian stimulation for ART: a comprehensive and systematic review including meta-analyses. Hum. Reprod. Update. 2021;27:48–66. doi: 10.1093/humupd/dmaa040. [DOI] [PubMed] [Google Scholar]
- 7.Massin N. New stimulation regimens: endogenous and exogenous progesterone use to block the LH surge during ovarian stimulation for IVF. Hum Reprod Update. 2017;23:211–220. doi: 10.1093/humupd/dmw047. [DOI] [PubMed] [Google Scholar]
- 8.Kuang Y., Chen Q., Hong Q., Lyu Q., Ai A., Fu Y., Shoham Z. Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol) Reprod. Biomed. Online. 2014;29:684–691. doi: 10.1016/j.rbmo.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Kuang Y., Chen Q., Fu Y., Wang Y., Hong Q., Lyu Q., Ai A., Shoham Z. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil. Steril. 2015;104:62–70.e3. doi: 10.1016/j.fertnstert.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Qin N., Chen Q., Hong Q., Cai R., Gao H., Wang Y., Sun L., Zhang S., Guo H., Fu Y., et al. Flexibility in starting ovarian stimulation at different phases of the menstrual cycle for treatment of infertile women with the use of in vitro fertilization or intracytoplasmic sperm injection. Fertil. Steril. 2016;106:334–341.e1. doi: 10.1016/j.fertnstert.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Yildiz S., Turkgeldi E., Angun B., Eraslan A., Urman B., Ata B. Comparison of a novel flexible progestin primed ovarian stimulation protocol and the flexible gonadotropin-releasing hormone antagonist protocol for assisted reproductive technology. Fertil. Steril. 2019;112:677–683. doi: 10.1016/j.fertnstert.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Shen X., Long H., Guo W., Gao H., Cai R., Jin W., Yan Z., Zhang S., Wang Y., Lyu Q., et al. Optimal Ovulation Trigger-Oocyte Pickup Interval in Progestin-Primed Ovarian Stimulation Protocol: A Retrospective Study Using Propensity Score Matching. Front. Endocrinol. 2019;10:694. doi: 10.3389/fendo.2019.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young J.R., Jaffe R.B. Strength-duration characteristics of estrogen effects on gonadotropin response to gonadotropin-releasing hormone in women. II. Effects of varying concentrations of estradiol. J. Clin. Endocrinol. Metab. 1976;42:432–442. doi: 10.1210/jcem-42-3-432. [DOI] [PubMed] [Google Scholar]
- 14.Hoff J.D., Quigley M.E., Yen S.S. Hormonal dynamics at midcycle: a reevaluation. J. Clin. Endocrinol. Metab. 1983;57:792–796. doi: 10.1210/jcem-57-4-792. [DOI] [PubMed] [Google Scholar]
- 15.De Geyter C., De Geyter M., Huber P.R., Nieschlag E., Holzgreve W. Progesterone serum levels during the follicular phase of the menstrual cycle originate from the crosstalk between the ovaries and the adrenal cortex. Hum. Reprod. 2002;17:933–939. doi: 10.1093/humrep/17.4.933. [DOI] [PubMed] [Google Scholar]
- 16.Casper R.F. Basic understanding of gonadotropin-releasing hormone-agonist triggering. Fertil. Steril. 2015;103:867–869. doi: 10.1016/j.fertnstert.2014.12.129. [DOI] [PubMed] [Google Scholar]
- 17.Villar L., Tralik B., Diamond M.P., Allon M., Maldonado I., Dozortsev D.I. Ovulation and birth after administration of progesterone trigger-two case reports. J. Assist. Reprod. Genet. 2023;40:1037–1044. doi: 10.1007/s10815-023-02750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dozortsev D.I., Diamond M.P. Luteinizing hormone-independent rise of progesterone as the physiological trigger of the ovulatory gonadotropins surge in the human. Fertil. Steril. 2020;114:191–199. doi: 10.1016/j.fertnstert.2020.06.016. [DOI] [PubMed] [Google Scholar]
- 19.Kasum M., Franulić D., Čehić E., Orešković S., Lila A., Ejubović E. Kisspeptin as a promising oocyte maturation trigger for in vitro fertilisation in humans. Gynecol. Endocrinol. 2017;33:583–587. doi: 10.1080/09513590.2017.1309019. [DOI] [PubMed] [Google Scholar]
- 20.Espey L.L. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol. Reprod. 1994;50:233–238. doi: 10.1095/biolreprod50.2.233. [DOI] [PubMed] [Google Scholar]
- 21.Espey L.L., Ujioka T., Russell D.L., Skelsey M., Vladu B., Robker R.L., Okamura H., Richards J.S. Induction of early growth response protein-1 gene expression in the rat ovary in response to an ovulatory dose of human chorionic gonadotropin. Endocrinology. 2000;141:2385–2391. doi: 10.1210/endo.141.7.7582. [DOI] [PubMed] [Google Scholar]
- 22.Ronen-Fuhrmann T., Timberg R., King S.R., Hales K.H., Hales D.B., Stocco D.M., Orly J. Spatio-temporal expression patterns of steroidogenic acute regulatory protein (StAR) during follicular development in the rat ovary. Endocrinology. 1998;139:303–315. doi: 10.1210/endo.139.1.5694. [DOI] [PubMed] [Google Scholar]
- 23.Kim J., Bagchi I.C., Bagchi M.K. Control of ovulation in mice by progesterone receptor-regulated gene networks. Mol. Hum. Reprod. 2009;15:821–828. doi: 10.1093/molehr/gap082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J., Li D., Liu X., Li Q., He X., Wei J., Li X., Li M., Rehman A.U., Xia Y., et al. IDDB: a comprehensive resource featuring genes, variants and characteristics associated with infertility. Nucleic Acids Res. 2021;49:D1218–D1224. doi: 10.1093/nar/gkaa753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka N., Espey L.L., Kawano T., Okamura H. Comparison of inhibitory actions of indomethacin and epostane on ovulation in rats. Am. J. Physiol. 1991;260:E170–E174. doi: 10.1152/ajpendo.1991.260.2.E170. [DOI] [PubMed] [Google Scholar]
- 26.Loutradis D., Bletsa R., Aravantinos L., Kallianidis K., Michalas S., Psychoyos A. Preovulatory effects of the progesterone antagonist mifepristone (RU486) in mice. Hum. Reprod. 1991;6:1238–1240. doi: 10.1093/oxfordjournals.humrep.a137519. [DOI] [PubMed] [Google Scholar]
- 27.Palanisamy G.S., Cheon Y.P., Kim J., Kannan A., Li Q., Sato M., Mantena S.R., Sitruk-Ware R.L., Bagchi M.K., Bagchi I.C. A novel pathway involving progesterone receptor, endothelin-2, and endothelin receptor B controls ovulation in mice. Mol. Endocrinol. 2006;20:2784–2795. doi: 10.1210/me.2006-0093. [DOI] [PubMed] [Google Scholar]
- 28.Lydon J.P., DeMayo F.J., Funk C.R., Mani S.K., Hughes A.R., Montgomery C.A., Jr., Shyamala G., Conneely O.M., O'Malley B.W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 29.Robker R.L., Russell D.L., Espey L.L., Lydon J.P., O'Malley B.W., Richards J.S. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc. Natl. Acad. Sci. USA. 2000;97:4689–4694. doi: 10.1073/pnas.080073497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long H., Yu W., Yu S., Yin M., Wu L., Chen Q., Cai R., Suo L., wang L., Lyu Q., Kuang Y. Progesterone affects clinic oocyte yields by coordinating with follicle stimulating hormone via PI3K/AKT and MAPK pathways. J. Adv. Res. 2021;33:189–199. doi: 10.1016/j.jare.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wade R.L., Van Andel R.A., Rice S.G., Banka C.L., Dyer C.A. Hepatic lipase deficiency attenuates mouse ovarian progesterone production leading to decreased ovulation and reduced litter size. Biol. Reprod. 2002;66:1076–1082. doi: 10.1095/biolreprod66.4.1076. [DOI] [PubMed] [Google Scholar]
- 32.Baena V., Owen C.M., Uliasz T.F., Lowther K.M., Yee S.P., Terasaki M., Egbert J.R., Jaffe L.A. Cellular Heterogeneity of the Luteinizing Hormone Receptor and Its Significance for Cyclic GMP Signaling in Mouse Preovulatory Follicles. Endocrinology. 2020;161 doi: 10.1210/endocr/bqaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsuda M., Hirata M. Phospholipase C-related but catalytically inactive proteins regulate ovarian follicle development. J. Biol. Chem. 2017;292:8369–8380. doi: 10.1074/jbc.M116.759928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M., Shi H., Segaloff D.L., Van Voorhis B.J. Expression and localization of luteinizing hormone receptor in the female mouse reproductive tract. Biol. Reprod. 2001;64:179–187. doi: 10.1093/biolreprod/64.1.179. [DOI] [PubMed] [Google Scholar]
- 35.Dou Y.D., Zhao H., Huang T., Zhao S.G., Liu X.M., Yu X.C., Ma Z.X., Zhang Y.C., Liu T., Gao X., et al. STMN1 Promotes Progesterone Production Via StAR Up-regulation in Mouse Granulosa Cells. Sci. Rep. 2016;6 doi: 10.1038/srep26691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen X., Li D., Tozer A.J., Docherty S.M., Iles R.K. Estradiol, progesterone, testosterone profiles in human follicular fluid and cultured granulosa cells from luteinized pre-ovulatory follicles. Reprod. Biol. Endocrinol. 2010;8:117. doi: 10.1186/1477-7827-8-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hillier S.G. Sex steroid metabolism and follicular development in the ovary. Oxf. Rev. Reprod. Biol. 1985;7:168–222. [PubMed] [Google Scholar]
- 38.Robker R.L., Akison L.K., Russell D.L. Control of oocyte release by progesterone receptor-regulated gene expression. Nucl. Recept. Signal. 2009;7 doi: 10.1621/nrs.07012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ismail P.M., Li J., DeMayo F.J., O'Malley B.W., Lydon J.P. A novel LacZ reporter mouse reveals complex regulation of the progesterone receptor promoter during mammary gland development. Mol. Endocrinol. 2002;16:2475–2489. doi: 10.1210/me.2002-0169. [DOI] [PubMed] [Google Scholar]
- 40.Brown H.M., Dunning K.R., Robker R.L., Boerboom D., Pritchard M., Lane M., Russell D.L. ADAMTS1 cleavage of versican mediates essential structural remodeling of the ovarian follicle and cumulus-oocyte matrix during ovulation in mice. Biol. Reprod. 2010;83:549–557. doi: 10.1095/biolreprod.110.084434. [DOI] [PubMed] [Google Scholar]
- 41.Richards J.S., Hernandez-Gonzalez I., Gonzalez-Robayna I., Teuling E., Lo Y., Boerboom D., Falender A.E., Doyle K.H., LeBaron R.G., Thompson V., Sandy J.D. Regulated expression of ADAMTS family members in follicles and cumulus oocyte complexes: evidence for specific and redundant patterns during ovulation. Biol. Reprod. 2005;72:1241–1255. doi: 10.1095/biolreprod.104.038083. [DOI] [PubMed] [Google Scholar]
- 42.Semenza G.L. Targeting HIF-1 for cancer therapy. Nat. Rev. Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 43.Jamieson M.E., Fleming R., Kader S., Ross K.S., Yates R.W., Coutts J.R. In vivo and in vitro maturation of human oocytes: effects on embryo development and polyspermic fertilization. Fertil. Steril. 1991;56:93–97. doi: 10.1016/s0015-0282(16)54424-0. [DOI] [PubMed] [Google Scholar]
- 44.Shimada M., Yanai Y., Okazaki T., Yamashita Y., Sriraman V., Wilson M.C., Richards J.S. Synaptosomal-associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol. Endocrinol. 2007;21:2487–2502. doi: 10.1210/me.2007-0042. [DOI] [PubMed] [Google Scholar]
- 45.Mittaz L., Russell D.L., Wilson T., Brasted M., Tkalcevic J., Salamonsen L.A., Hertzog P.J., Pritchard M.A. Adamts-1 is essential for the development and function of the urogenital system. Biol. Reprod. 2004;70:1096–1105. doi: 10.1095/biolreprod.103.023911. [DOI] [PubMed] [Google Scholar]
- 46.Shozu M., Minami N., Yokoyama H., Inoue M., Kurihara H., Matsushima K., Kuno K. ADAMTS-1 is involved in normal follicular development, ovulatory process and organization of the medullary vascular network in the ovary. J. Mol. Endocrinol. 2005;35:343–355. doi: 10.1677/jme.1.01735. [DOI] [PubMed] [Google Scholar]
- 47.Andreini C., Banci L., Bertini I., Elmi S., Rosato A. Comparative analysis of the ADAM and ADAMTS families. J. Proteome Res. 2005;4:881–888. doi: 10.1021/pr0500096. [DOI] [PubMed] [Google Scholar]
- 48.Russell D.L., Doyle K.M.H., Ochsner S.A., Sandy J.D., Richards J.S. Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J. Biol. Chem. 2003;278:42330–42339. doi: 10.1074/jbc.M300519200. [DOI] [PubMed] [Google Scholar]
- 49.Ferrara N., Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 50.Hazzard T.M., Xu F., Stouffer R.L. Injection of soluble vascular endothelial growth factor receptor 1 into the preovulatory follicle disrupts ovulation and subsequent luteal function in rhesus monkeys. Biol. Reprod. 2002;67:1305–1312. doi: 10.1095/biolreprod67.4.1305. [DOI] [PubMed] [Google Scholar]
- 51.Meidan R., Levy N. The ovarian endothelin network: an evolving story. Trends Endocrinol. Metab. 2007;18:379–385. doi: 10.1016/j.tem.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Filipovich T., Fleisher-Berkovich S. Regulation of glial inflammatory mediators synthesis: possible role of endothelins. Peptides. 2008;29:2250–2256. doi: 10.1016/j.peptides.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Tam K.K.Y., Russell D.L., Peet D.J., Bracken C.P., Rodgers R.J., Thompson J.G., Kind K.L. Hormonally regulated follicle differentiation and luteinization in the mouse is associated with hypoxia inducible factor activity. Mol. Cell. Endocrinol. 2010;327:47–55. doi: 10.1016/j.mce.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Cacioppo J.A., Lin P.C.P., Hannon P.R., McDougle D.R., Gal A., Ko C. Granulosa cell endothelin-2 expression is fundamental for ovulatory follicle rupture. Sci. Rep. 2017;7:817. doi: 10.1038/s41598-017-00943-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jamnongjit M., Gill A., Hammes S.R. Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc. Natl. Acad. Sci. USA. 2005;102:16257–16262. doi: 10.1073/pnas.0508521102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steyn F.J., Wan Y., Clarkson J., Veldhuis J.D., Herbison A.E., Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154:4939–4945. doi: 10.1210/en.2013-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L., Guo W., Shen X., Yeo S., Long H., Wang Z., Lyu Q., Herbison A.E., Kuang Y. Different dendritic domains of the GnRH neuron underlie the pulse and surge modes of GnRH secretion in female mice. Elife. 2020;9 doi: 10.7554/eLife.53945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao F., Zhang X., Ni P., Yu H., Gao Q., Li M., Huo P., Wei Z., Wang S., Zhang Y., et al. Voltage-dependent potassium channel Kv4.2 alleviates the ischemic stroke impairments through activating neurogenesis. Neurochem. Int. 2021;150 doi: 10.1016/j.neuint.2021.105155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.