Abstract
Invasive fungal infections (IFIs) have been associated with high mortality, highlighting the urgent need for developing novel antifungal strategies. Herein the first light-responsive antifungal agents were designed by optical control of fungal ergosterol biosynthesis pathway with photocaged triazole lanosterol 14α-demethylase (CYP51) inhibitors. The photocaged triazoles completely shielded the CYP51 inhibition. The content of ergosterol in fungi before photoactivation and after photoactivation was 4.4% and 83.7%, respectively. Importantly, the shielded antifungal activity (MIC80 ≥ 64 μg/mL) could be efficiently recovered (MIC80 = 0.5–8 μg/mL) by light irradiation. The new chemical tools enable optical control of fungal growth arrest, morphological conversion and biofilm formation. The ability for high-precision antifungal treatment was validated by in vivo models. The light-activated compound A1 was comparable to fluconazole in prolonging survival in Galleria mellonella larvae with a median survival of 14 days and reducing fungal burden in the mouse skin infection model. Overall, this study paves the way for precise regulation of antifungal therapy with improved efficacy and safety.
KEY WORDS: Antifungal agents, Ergosterol biosynthesis, Triazoles, Photocaged, Optical control
Graphical abstract
Novel photo-responsive antifungal agents were designed by photocaging modification of fluconazole. Compound A1 precisely modulate fungal growth, virulence, ergosterol biosynthesis, and showed potent light-responsive in vivo therapeutic effect.

1. Introduction
In the past few decades, invasive fungal infections (IFIs) have accounted for a large proportion of infectious-related deaths. It is estimated that approximately a million people die from IFIs each year1. The increase in immunocompromised populations (e.g., AIDS patients) and the eukaryotic character of pathogenic fungi make fungal infections more difficult to eradicate2. Recently, IFIs have become an important complication in a large number of critically ill patients with coronavirus disease 2019 (COVID-19), leading to high mortality rates3. COVID-19-associated IFIs have gained worldwide attention, highlighting the need for developing novel antifungal agents and treatment strategies4. However, clinical availability of antifungal drugs (for IFIs: only triazoles, polyenes and echinocandins) remains rather limited compared to drug development for other infectious diseases5. Clinical use of these antifungal agents is often hampered by drug toxicity, drug–drug interactions and drug resistance6,7. Moreover, the increasing multidrug resistance of fungi has threatened the efficacy of current antifungal drugs in clinical treatment and the lag in the development of new antifungal agents has resulted in few drugs that effectively address this challenge. Only one antifungal drug (isavuconazole, a triazole derivative) has been approved in the past decade8. Acquired drug resistance is mostly attributed to the overuse of antifungal agents and the accumulation of antifungal agents in the environment9. For example, the accumulation of antifungal drugs in the environment caused by their widespread use in health care has exposed fungi to non-lethal concentrations of drugs for long periods of time, putting pressure on the fungi to evolve resistance. Fungi acquire resistance through mutation and alteration of gene expression10. Therefore, new strategies to control the activity of antifungal drugs for precision therapy is highly desirable.
Photopharmacology utilizes synthetic light-responsive ligands to achieve light-dependent pharmacological activity11,12. This new strategy has the advantage of reducing the systemic toxicity and emergency of resistance and achieving therapeutic precision. Light is used as an external controller because of its rapid and uninjurious character with spatiotemporal precision13. Spatiotemporal precision therapy that combining optical control with the therapeutic effect of drugs provides a promising concept for future clinical treatment. Tremendous progress on photopharmacological agents acting on a wide range of targets has been made12,14, 15, 16, 17, 18, 19. However, there is a lack of chemical strategies to light-dependent modulate antifungal drug targets in living fungal cells.
Triazoles (e.g., fluconazole and itraconazole) are the first-line antifungal agents in clinical treatment of IFIs5,20. They acted by inhibiting lanosterol 14α-demethylase (CYP51), an important enzyme in the fungal ergosterol biosynthesis pathway. CYP51 catalyzes the demethylation of the fungal lanosterol at the 14α position21. The catalytic product of CYP51 continues to participate in the biosynthesis of ergosterol on the fungal cell membrane22. Inhibition of CYP51 by triazoles causes the depletion of ergosterol and accumulation of lanosterol, leading to the impairment of fungal cell membrane and thus inhibition of the fungal cell growth. However, CYP51 belongs to the cytochrome P450 protein family, and triazole antifungal agents are generally suffered from hepatoxicity. Frequent use of triazoles also led to the emergence of resistant fungi, especially for Candida albicans23. Thus, the development of new chemical tools to control CYP51 with optical precision would enable improving the efficacy and safety of clinical antifungal therapy.
Inspired by our continuous efforts in the discovery novel antifungal agents24, 25, 26, 27, herein we developed the first-in-class light-responsive antifungal agents by designing photopharmacological triazole CYP51 inhibitors. Both irreversible (photocaging)28 and reversible (photoswitching)11 photo-activation strategies were applied to modify azoles. After biological screening of photocaged and photoswitchable triazoles, the photocaged agents turned out to completely shield the CYP51 inhibition and antifungal activity of fluconazole (FLC), which could be efficiently recovered by light irradiation. These light-responsive triazoles expanded the scope of the chemical toolbox available in the antifungal field, which achieved external control of CYP51 function by irradiation, thereby increased therapeutic index and reduced the risk of acquired drug resistance. This proof-of-concept study also extends the application of photopharmacology to treating infectious fungal diseases.
2. Results and discussion
2.1. Rational design of light-responsive triazole CYP51 inhibitors
Ideally, the activity of a photo-responsive antifungal agent is restored only after photo-activation29, 30, 31. The photochemistry of such modified molecules can be reversible or irreversible. Triazole CYP51 inhibitors FLC, itraconazole (ITC) and iodiconazole (Fig. 1A) were used as template molecules for the design of photo-responsive antifungal agents. In order to control the inhibition of fungal ergosterol biosynthesis by triazoles in a light-dependent manner and improve its therapeutic precision, the triazoles were modified in the form of photocages or photoswitches. Representative photoactive protecting groups (Fig. 1C)32, such as 4,5-dimethoxy-2-nitrobenzyl (DMNB), 4,5-dimethoxy-2-nitrobenzyloxycarbonyl (DMNBOC), 2-(3,4-methylenedioxy-6-nitrophenyl)-propyloxycarbonyl (MNPPOC), 2-(3,4-methylenedioxy-6-nitrophenyl)-ethyloxycarbonyl (MNPEOC) and diethylamino coumarin (DEACM), were used as photocages for the modification of triazoles. Next, an important concern is to select an appropriate site for photo-protection. The tertiary alcohol in FLC was an essential pharmacophore, which is involved in the water mediated hydrogen-bonding network with CYP51 (Fig. 1B)33. Thus, the photocaging groups were introduced on the hydroxyl group to design compounds A1–A4 (Table 1). Iodiconazole is an investigational antifungal agent developed by our group, whose phase III clinical trials have been finished34,35. Photocaging groups were also attached on the side chain amine group of iodiconazole analogues (compounds B1–B5, Table 2) in an attempt to block the interaction with the substrate entry channel in the active site of CYP51.
Figure 1.
Design of light-responsive antifungal compounds. (A) Chemical structures of FLC, ITC, and iodiconazole. (B) Binding models of FLC (PDB code: 4WMZ) and ITC (PDB code: 5V5Z) with CYP51. The figure was generated using PyMol (http://www.pymol.org/). (C) Chemical structures of cages commonly used for photocaging modification. (D) Design strategy of photocaging modification of FLC and iodiconazole. (E) Design strategy of photoswitching modification of ITC and FLC.
Table 1.
In vitro antifungal activity of photocaged FLC derivatives under light or non-light conditions (MIC80a, μg/mL).
| Compd. | Structure (R) |
C. albicans SC5314 |
C. neoformans H99 |
||
|---|---|---|---|---|---|
| black | hvb | black | hvb | ||
| A1 | MNPPOC | >64 | 0.5 | 64 | 2 |
| A2 | DMNB | >64 | 1 | 64 | 4 |
| A3 | DMNBOC | 16 | 16 | 64 | 64 |
| A4 | MNPEOC | 64 | 2 | 32 | 8 |
| FLC | / | 0.25 | 0.25 | 1 | 1 |

The minimum concentration of the compound at which the inhibition rate is greater than or equal to 80%.
Light condition: 365 nm, 25 mW/cm2, 3 min.
Table 2.
In vitro antifungal activity photocaged iodiconazole analogues under light or non-light conditions (MIC80a, μg/mL).
| Compd. | Structure |
C. albicans SC5314 |
C. neoformas H99 |
||
|---|---|---|---|---|---|
| black | hvb | black | hvb | ||
| B1 | R1 = DMNB, R2 = I X = CH2 |
0.25 | <0.125 | 1 | 0.5 |
| B2 | R1 = DMNB, R2 = H X = (CH2)3 |
2 | <0.125 | 8 | 4 |
| B3 | R1 = DMNB, R2 = H X = (CH2)4 |
2 | <0.125 | 8 | 4 |
| B4 | R1 = DEACM, R2 = H X = (CH2)3 |
2 | 0.25 | >64 | 8 |
| B5 | R1 = DEACM, R2 = H X = (CH2)4 |
1 | 0.25 | 16 | 4 |
| b1 | R1 = H, R2 = I X = CH2 |
<0.125 | <0.125 | <0.125 | <0.125 |
| b2 | R1 = H, R2 = H X = (CH2)3 |
<0.125 | <0.125 | 2 | 2 |
| b3 | R1 = H, R2 = H X = (CH2)4 |
<0.125 | <0.125 | 1 | 1 |
| FLC | / | 0.25 | 0.25 | 1 | 1 |

The minimum concentration of the compound at which the inhibition rate is greater than or equal to 80%.
Light conditions: 365 nm, 25 mW/cm2, 3 min.
Azo-benzenes or azo-heterocycles have been widely used for photoswitchable modification36,37. ITC is a long-tailed triazole antifungal agent, whose long side chain was located in the substrate entry channel (Fig. 1B). To optically regulate the binding interactions, an azo photoswitching group was introduced into the side chain of ITC by replacing the piperazine group linking the two benzene rings (compound C1, Supporting Information Table S2). Moreover, another series of photoswitchable triazoles were also designed by linking azobenzene group to the triazole group of FLC (compounds C2–C5, Table S2).
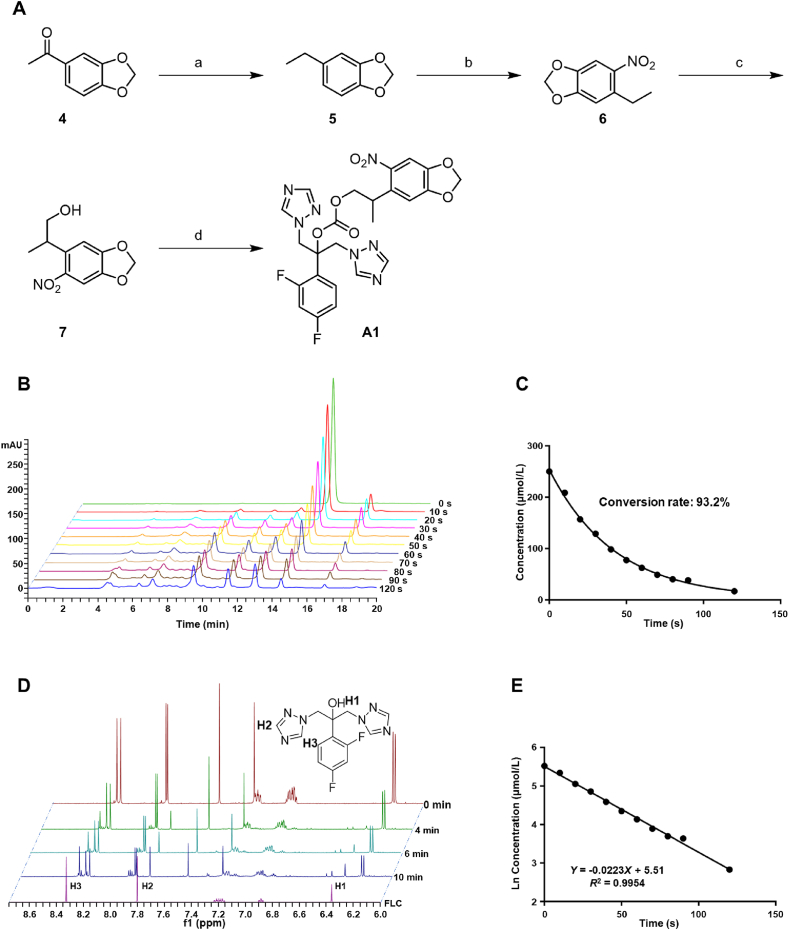
The synthetic scheme of photocaging compound A1 is shown in Fig. 2A. The carbonyl group of starting material 4 was reduced to methylene in the presence of hydrazine hydrate to give intermediate 5. Then, a nitro group was introduced by nitration reaction of compound 5, followed by the addition reaction with paraformaldehyde to afford intermediate 7. Finally, target compound A1 was obtained by the substitution reaction between intermediate 7 and FLC via two steps. Chemical synthesis of other target compounds is depicted in Supporting Information Schemes S1–S5.
Figure 2.
Compound A1 was photouncaged by the UV light. (A) Chemical synthesis of compound A1. Reagents and conditions: (a) N2H4/H2O, KOH, EtOH, glycol, reflux, yield 83.6%; (b) HNO3, DCM, 0 °C, yield 66.7%; (c) benzyltrimethylammonium hydroxide (Triton B), CH2O; MeOH, reflux, yield 33.0%; (d) i) triphosgene, Na2CO3, THF; ii) FLC, NaH, DMF, −20 °C, yield 10.0% two steps. (B) HPLC analysis of compound A1 irradiated with 365 nm light at 25 mW/cm2. (C) Photolysis kinetics of compound A1 upon 25 mW/cm2, 365 nm light irradiation. The concentration of compound A1 was monitored by HPLC at different time point after irradiation at 365 nm light. (D) Recording of 1H NMR changes in the photouncaging process of compound A1. (E) Linear fit of photolysis rate of compound A1. The data were derived from natural logarithm of the concentration of compound A1 in Fig. 2C.
2.2. Antifungal activity of light-responsive triazole CYP51 inhibitors
The antifungal activity of the designed compounds was tested to investigate the effectiveness of the two strategies. C. albicans and Cryptococcus neoformans, two clinically important fungal pathogens, were used in the assays. It was demonstrated that photoactive protecting groups could shield the antifungal activity of triazoles and restore their antifungal effects under 365 nm UV light conditions. As shown in Table 1, the antifungal activity of compounds installed with photocages at the hydroxyl group in FLC (A1, A2, A4) was significantly decreased. Among them, various cages played an important role in the shielding effects of antifungal activity. MNPPOC derivative A1 exhibited better shielding activity or more efficient photolysis efficiency than the other compounds (A2 or A4). Compound A1 was almost inactive in inhibiting the growth of C. albicans (MIC >64 μg/mL) and C. neoformans (MIC = 64 μg/mL). When it was irradiated by 365 nm UV light, the antifungal activity was restored to the similar level of FLC, with a MIC value against C. albicans and C. neoformans of 0.5 and 2 μg/mL, respectively. Although the major product of compound A1 after photolysis was FLC, we also determined the antifungal activity of other by-products, which were inactive in the assay (Supporting Information Fig. S1). However, compound A3 with a DMNBOC photocage was unable to be activated under 365 nm UV light. When the photocaging groups were linked to the side chain nitrogen atom of iodiconazole (B1–B5), weaker efficiency in masking the antifungal activity was observed (Table 2).
The photoisomerization properties of compounds C1–C5 were determined using UV–Vis absorption spectrum (Supporting Information Fig. S2E). The changes of relevant wavelengths were listed in Supporting Information Table S1. The absorption bands of compounds in the spectra with λmax of 348–393 nm for compound C1–C5 corresponded to the π–π∗ transition, and the moderate absorbance bands at 434–456 nm observed in the spectra of compounds C1–C5 illuminated with 365 nm UV light corresponded to the n–π∗ transition. Trans to cis isomerization can be induced by π–π∗ excitation of UV light38. After π–π∗ excitation at 365 nm UV light, the absorption properties of compounds C1–C5 were consistent with cis-isomer, including a significant decrease in the intensity of π–π∗ band and a enhance in the intensity of n–π∗ band39. On the other hand, of the two isomers, the trans-isomer is thermally stable and the cis-isomer is thermally unstable40. After illuminated with 365 nm UV light, compounds C1–C5 spontaneously changed to thermally stable state at 35 °C (HPLC spectra in Supporting Information). The change of absorption properties of compounds C1–C5 and the conversion of isomers to thermal stable state indicated that compounds C1–C5 were trans configuration before UV light. NOE effect between hydrogen atoms located in the azo-linked aromatic rings could also verified that compounds C1–C5 after 365 nm UV illumination were in cis configuration (1H–1H NOESY spectra of compounds C1 and C3 in Supporting Information). Compounds C1–C5 converted rapidly to the cis configuration with conversion rates in the range of 57.0%–98.2% after irradiation with light at 365 nm for 10 s (HPLC spectra in Supporting Information). Unfortunately, there was no significant difference in the activity of azobenzene (C1) or azoheterocycles (C2–C5) compounds under light or non-light conditions (Table S2). The results might be due to the fact that the substrate entry channel in CYP51 accommodates flexible side chains. As a result, the photoswitchable modification strategy failed to regulate the antifungal activity of triazole in a light-dependent manner. In addition, various clinical isolates or standard strains were used to test the broad-spectrum shielding effect of compound A1 (Supporting Information Table S3). Compound A1 was inactive against fungal strains including Candida glabrate, Candida lusitaniae, Candida parapsilosis, and Cryptococcus gattii before light exposure and this shielding effect could also be reversed by light. Taken together, the introduction of a photocaging group at the hydroxyl group of FLC was proven to be an effective strategy in shielding the antifungal activity and compound A1 was selected for further evaluations.
2.3. Photocaged triazoles release FLC effectively under 365 nm UV light
After demonstrating that the restoration of antifungal activity under the UV light was due to the photouncaging of compound A1, the photolysis process was investigated. UV-illuminated (365 nm) compound A1 could be converted into FLC in a short time as monitored by HPLC (about 93.2% conversion rate within 2 min) and 1H NMR spectroscopy (Fig. 2B and D). Furthermore, mass spectrometry analysis verified that the main photolysis product of compound A1 was FLC (Supporting Information Fig. S2A and S2C). The study on the photolysis kinetics of compound A1 showed an exponential trend in photouncaging (Fig. 2C). The natural logarithm of the concentration of compound A1 at each time point during the photolysis process was plotted with time. The results revealed that the photouncaging of compound A1 conformed to a first-order reaction rate equation (Fig. 2E). The photouncaging property of compound A2 was also monitored by HPLC (Fig. S2D). Similar to compound A1, compound A2 rapidly released FLC within 2 min. The photocaged compounds with similar ester structures have been reported41. The possible photouncaging process of compound A1 was shown in Supporting Information Fig. S3. Compound A1 was excited by light into a triplet state, and then gone through H-transfer, followed by β-elimination and fragmentation to release FLC.
2.4. Photocaged compounds regulate fungal growth and virulence factors via a light-dependent manner
Before photo-activation, compound A1 showed no significant difference in fungal proliferation compared with control group (Fig. 3A). Disk diffusion experiments confirmed that photouncaging of compound A1 led to effective inhibition of the proliferation of C. albicans SC5314 cells (Fig. 3A). Budded to hyphal transition (BHT) exists in the whole process of C. albicans invasion, plays an important role in diffusion and colonization42,43. In the spider medium that induced hyphal formation of C. albicans, morphological conversion was still observed in the group treated with photocaged compound A1, while FLC and photoactivated compound A1 successfully blocked the BHT process in C. albicans (Fig. 3B). Thus, the installation of photocage realized the precise external optical regulation of the morphological transformation of fungi. The photo-activation effects were also observed for compound A2, which was effective in optical modulation of fungal growth and morphological transition (Supporting Information Fig. S4A and S4B). Then, the optical regulation of photocaged compounds on biofilms, another important virulence factor associated with drug resistance44 was investigated. As shown in Fig. 3C and D, compounds A1 and A2 were unable to inhibit the biofilm formation of C. albicans under the non-light conditions. In contrast, photo-activated compounds A1 and A2 showed excellent inhibition on biofilm formation, and the inhibitory effect was comparable to that of FLC at the same dose. Therefore, the photoprotective groups of compounds A1 and A2 successfully masked the inhibition of FLC on C. albicans biofilm formation. With the release of FLC induced by illumination, its effect on biofilm was recovered. In addition, UV irradiation under the photouncaged conditions did not affect the growth and virulence levels of fungal cells, nor did it synergize with FLC.
Figure 3.
Optical control of the inhibitory activities against fungal cell growth and virulence factors caused by photouncaging of compound A1. (A) Growth of C. albicans on SDA (Sabouraud dextrose agar) medium containing drugs (16 μg/mL) or DMSO. About 1 × 104 C. albicans cells were plated in SDA medium and treated with UV irradiation or non-irradiation. UV light: 50 mW/cm2, 365 nm, 5 min. (B) Hyphal formation of C. albicans SC5314 in spider medium treated with compound A1 (16 μg/mL), FLC (16 μg/mL) or DMSO under black or hv conditions, scale: 100 μm. UV light: 50 mW/cm2, 365 nm, 2 min. (C) Compound A1 modulated biofilm formation of C. albicans under irradiated and non-irradiated conditions. UV light: 50 mW/cm2, 365 nm, 2 min. (D) Compound A2 modulated biofilm formation of C. albicans under irradiated and non-irradiated conditions. UV light: 50 mW/cm2, 365 nm, 2 min. (E) Disk diffusion of C. albicans on SDA medium containing compound A1 (16 μg/mL). About 1 × 104 cells were coated on the medium and irradiated with UV light only the right half. UV light: 50 mW/cm2, 365 nm, 5 min. (F) Time–growth curves of C. albicans with an initial fungal concentration of 1 × 105 cells/mL. The curves were obtained by counting the number of fungi treated with different concentrations of compound A1 with or without irradiation at different time points. UV light: 50 mW/cm2, 365 nm, 2 min. (G) Time–growth curves of C. albicans with an initial fungal concentration of 5 × 104 cells/mL. The fungal suspension was treated with irradiation at different time points after the addition of compound A1 (8 μg/mL) and the number of C. albicans cells at preset time points was recorded. UV light: 50 mW/cm2, 365 nm, 2 min.
2.5. Photocaged compounds regulate fungal growth in a manner of optical precision
We next determined the optical precision of compound A1 in exerting antifungal effects by using light. The SDA medium containing compound A1 (16 μg/mL) coated with C. albicans was divided into light area (right side in Fig. 3E) and non-light area (left side in Fig. 3E). Disk diffusion experiments revealed that the combination of light could spatially and precisely modulate the antifungal effect of compound A1, because the growth of fungi in the right side was significantly inhibited after light activation (Fig. 3E). To demonstrate the precise temporal tunability of this photoprotective strategy, light irradiation was performed at different time points (0, 4, 6, 8, 10, 12 h) after administration and the number of fungi was counted at different time points (Fig. 3G). Before this, we measured the time growth curves of fungi treated with or without light at different drug concentrations (4, 8, and 16 μg/mL in Fig. 3F) to determine the appropriate drug concentration (8 μg/mL in Fig. 3G) and initial fungal concentration (5 × 104 cells/mL in Fig. 3G). With the release of FLC after light irradiation, the growth of fungi was inhibited and the growth curve showed a flat trend and gradually separated from the curve of the control group (Fig. 3F). The inhibition was similar to that of FLC at the same concentration (Fig. S4C). The growth of fungi slowed down at 4–6 h after light treatment, and the growth before light treatment was consistent with that of the control group (Fig. 3G). With the delay of light treatment, the growth inhibitory effect of light-activated compound A1 on fungi was gradually weakened, which was due to the increasing initial concentration of fungi resulted by fungal proliferation before light treatment (Fig. 3G). Compound A2 was activated by light with the same temporal precision (Fig. S4D and S4E). The introduction of photocage enabled the release of FLC in an optical precision manner.
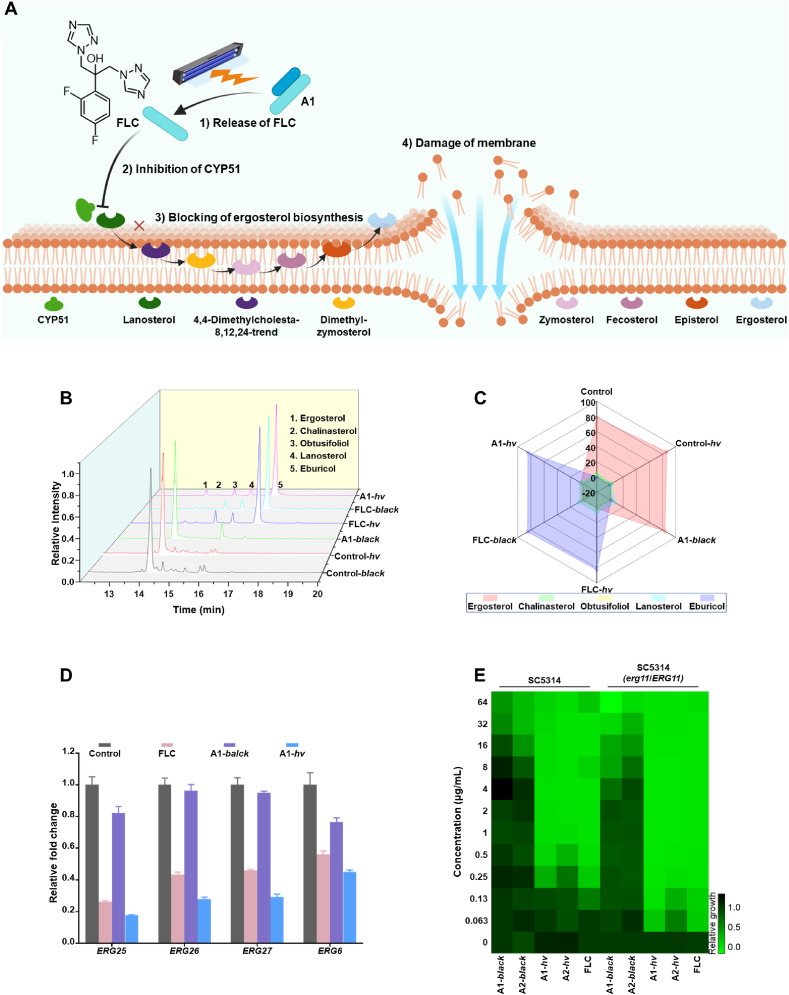
2.6. Photocaged compounds light-dependently regulate ergosterol biosynthesis of fungi (Fig. 4A)
Figure 4.
Photocaged compound A1 blocked the fungal ergosterol biosynthesis pathway via a photocontrol manner. (A) Schematic representation of compound A1 releasing FLC and blocking ergosterol biosynthesis in response to the UV light. Image element was created with BioRender.com. (B) Sterols detected by GC–MS on the cell membrane of C. albicans after treated with different compounds. UV light: 50 mW/cm2, 365 nm, 2 min. (C) Radar plot showing the relative content of each sterol in each treatment group. (D) Transcription of ERG11 downstream genes detected by real time qPCR. UV Light: 365 nm, 25 mW/cm2, 3 min. (E) Heat map showing the antifungal activity of compounds A1, A2 and FLC against C. albicans (SC5314) and its mutant (erg11Δ/ERG11) under different conditions. UV Light: 365 nm, 25 mW/cm2, 3 min.
The mechanism whether the installation of the photocaging group reduces the antifungal activity of FLC by shielding the inhibitory effect of CYP51 was further investigated. By measuring the changes of sterols in fungal cells (Fig. 4B), the radar chart clearly showed the relative contents of the five key sterols detected by GC–MS in each group (Fig. 4C). The accumulation of eburicol was concentrated in the FLC-black, FLC-hv, and A1-hv groups. The results indicated that the ergosterol synthesis was blocked in the FLC treated group, resulting in the accumulation of eburicol (a substrate of CYP51). In contrast, the accumulation of ergosterol was concentrated in the control, control-hv, and A1-black groups, confirming that the installation of photocaging group (compound A1) led to the loss of blocking effect of FLC on the ergosterol biosynthesis pathway. The combined treatment of compound A1 and light restored the inhibitory effect (Fig. 4B and C). Similar trend was also observed for compound A2 under light (Supporting Information Fig. S5A). Therefore, the photocaging modification successfully utilized light to achieve precise regulation of antifungal activity through optical control of the inhibition of CYP51.
In the biosynthetic pathway of ergosterol, the ERG11 gene is responsible for encoding CYP51. The transcription of genes encoding CYP51 downstream proteins could be reduced by the treatment of CYP51 inhibitors. Verified by the real time qPCR, the transcription levels of the downstream genes (ERG25, ERG26, ERG27, and ERG6) of the ERG11 gene were down-regulated after the treatment with FLC. The A1-treated group abolished this effect, which was recovered after photo-activation (Fig. 4D). In a heterozygous mutant strain of C. albicans SC5314 (erg11Δ/ERG11), FLC and light-activated compound A1 showed higher antifungal effect, whereas the MIC value of compound A1 without irradiation was decreased correspondingly (Fig. 4E and Supporting Information Table S4).
2.7. Photocaged compound A1 regulated in vivo antifungal effects in G. mellonella larvae and mice
In the field of photopharmacology, it is still highly challenging to translate molecular and cellular photocontrol into therapeutic effects. Herein the in vivo antifungal potency of photocaged compound A1 was evaluated in two models. Galleria mellonella larva is an emerging, facile and valuable model host in the studies of fungal pathogenesis and antifungal efficacy due to efficient inoculum delivery and handing45. Thus, invertebrate G. mellonella larvae model (Fig. 5A) was used to investigate the in vivo efficacy of the photocaged compound A1 under the optical control. The photo-activated compound A1 had a similar therapeutic effect as FLC in G. mellonella (Supporting Information Fig. S5B) when given prophylactic treatment (administrated 30 min earlier) and followed by inoculation (5 × 105 CFU/larva). Based on these results, we increased the inoculation amount of C. albicans (6 × 105 CFU/larva) to investigate the regulation of in vivo therapeutic effect of compound A1 using light in the prophylactic treatment and post-infection treatment, respectively (Fig. 5B and C). Both light-irradiated groups (A1-hv) and FLC-treated groups (FLC) significantly prolonged the survival time of G. mellonella larvae compared with control groups and non-light groups (A1-black).
Figure 5.
Photocontrol of the in vivo antifungal effects of photocaged compound A1 in G. mellonella larvae and mouse skin infection model. (A) Schematic diagram of in vivo efficacy evaluation of the G. mellonella larvae. Image element created with BioRender.com. (B) Survival of G. mellonella treated with FLC, compound A1, and compound A1 combined with light, respectively, 30 min before infection of C. albicans SC5314 (6 × 105 CFU/larva). (C) Survival of G. mellonella treated with FLC, compound A1, and compound A1 combined with light, respectively, 30 min after infection of C. albicans SC5314 (6 × 105 CFU/larva). (D) Schematic diagram of in vivo efficacy evaluation of mice model of cutaneous candidiasis. Image element created with BioRender.com. (E) Antifungal effects of light-controlled compound A1 in a mouse skin model of candidiasis, which was evaluated by local fungal burden on the skin. Mice were divided into four groups, and treated with solvent (control group), FLC (20 mg/mL, FLC group), compound A1 combined with UV light (20 mg/mL, A1-hv group), and compound A1 alone (20 mg/mL, A1-black group), respectively. The comparison between the two groups was completed by the One-way ANOVA. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001.
In a mouse skin model of candidiasis (Fig. 5D), drugs were administrated topically to the infected area using light or non-light treatments. The fungal burden of infected skin was investigated to evaluate the antifungal effect by compound A1 under light or without light (Fig. 5E). The activation of compound A1 via a photocontrol manner could significantly reduce the fungal burden in the mouse skin, whose therapeutic effect was comparable to that of FLC at the same dose. The results indicated that through light intervention, the photolysis of photocaged compound can be successfully regulated in an optical precision manner.
3. Conclusions
Light has emerged as a clinical tool with great potential, which controls the activation of light-responsive agents with high efficiency and high optical precision. We have developed novel chemical strategies to optical control of the fungal ergosterol synthesis pathway by introducing a photocaging group on the hydroxyl group of FLC. The photocaging group successfully blocked the binding of FLC to CYP51 and completely shielded the antifungal activity of FLC. Triggered by light, FLC was completely released, and exerted antifungal activities with precise optical control. The photocaged triazoles represent highly promising photopharmacological tools in antifungal therapy due to efficient and rapid photoconversion efficiency, optical regulation of fungal virulence factors and morphological transition. The potential clinical value of photoactivation of fungal CYP51 inhibition was demonstrated in the in vivo infectious models of G. mellonella larvae and mice, suggesting that light-controlled ergosterol biosynthesis is an efficient approach in antifungal therapy. In addition, the light-triggered activation allows for reduced exposure of antifungal agents, thereby decreasing the risk of acquired drug resistance. Although UV light might be harmful to the human body, the light is relatively controllable in this study. Photocaged compound A1 has a high photolysis rate under appropriate UV light intensity, which may effectively reduce the time required for light exposure and thus reduce the potential harm to human body. It should also be noted that, UV light activation has limitations in treat systemic infections, which might be overcome by using near-infrared photocaging groups or upconversion nanoparticles. Further optimizations of the wavelength of the photocaging groups are required. The direction of future efforts will be to explore how to effectively realize optical control within the biological tissue, and then expand the application of light-responsive antifungal agents to treat systemic infections.
4. Experimental
4.1. Chemistry
Reagents and solvents were analytically pure without further purification. Bruker AVANCE600 spectrometers were used to record the 1H NMR and 13C NMR spectra, using TMS as an internal standard and CDCl3 or DMSO-d6 as solvents. Chemical shifts in NMR spectra were given in ppm (δ). High-resolution mass spectrometry (HRMS) tests were performed on an Agilent UPLC–QTOF/MS mass spectrometer. Thin-layer chromatography (TLC, silica gel HSGF-254) was used to monitor the progress of reaction. The purities of the compounds were determined by HPLC (Agilent 1260), and all final compounds exhibited purities greater than 95.0%.
4.1.1. 2-(2,4-Difluorophenyl)-1,3-di (1H-1,2,4-triazol-1-yl)propan-2-yl (2-(6-nitrobenzo[d][1,3]dioxol-5-yl)propyl) carbonate (A1)
The synthesis of compounds 5–7 was according to the reported methods46. To a solution of 2-(6-nitrobenzo[d][1,3]dioxol-5-yl)propan-1-ol (7, 122 mg, 0.54 mmol) in THF (15 mL) was added triphosgene (158 mg, 0.54 mmol) and Na2CO3 (57 mg, 0.54 mmol) at 0 °C. The reaction mixture was warmed to room temperature and stirred for another 16 h. After the reaction mixture was filtered, the filtrate was concent rated under the reduced pressure at room temperature and dried in vacuo to give the crude product as an oil which was directly used in the next step without further purification.
A solution of FLC (110 mg, 0.36 mmol) in dry DMF (3 mL) was added dropwise into a solution of NaH (17 mg, 60% in oil, 0.43 mmol) in DMF (2 mL) at 0 °C. The reaction mixture was stirred at room temperature for 2 h and then kept at −20 °C. A solution of acyl chloride obtained from the previous step in DMF (5 mL) was added dropwise into the reaction mixture during a period of 30 min. The reaction mixture was stirred at room temperature for 24 h. Then, the residue was partitioned between EtOAc (30 mL) and aqueous NaHCO3 (15 mL). The separated aqueous layer was extracted with EtOAc (20 mL × 2). The combined organic layers were dried over Na2SO4, filtered and evaporated to dryness to give crude product. The residue was purified by silica gel chromatography (DCM/MeOH = 100/1–100/3) to give target compound A1 (20 mg, 10.0%) as light yellow solid. 1H NMR (600 MHz, CDCl3) δ 8.03 (s, 1H), 7.96 (s, 1H), 7.89 (s, 1H), 7.88 (s, 1H), 7.38 (s, 1H), 6.92–6.88 (m, 1H), 6.88 (s, 1H), 6.88–6.85 (m, 1H), 6.82–6.78 (m, 1H), 6.14 (d, J = 1.2 Hz, 1H), 6.11 (d, J = 1.2 Hz, 1H), 5.14 (d, J = 14.7 Hz, 1H), 5.09 (d, J = 14.3 Hz, 2H), 5.02 (d, J = 14.7 Hz, 1H), 4.36 (dd, J = 10.7, 6.9 Hz, 1H), 4.31 (dd, J = 10.8, 6.1 Hz, 1H), 3.89 (h, J = 6.9 Hz, 1H), 1.35 (d, J = 6.9 Hz, 3H). 13C NMR (151 MHz, CDCl3) δ 163.30 (dd, J = 253.2, 12.8 Hz), 159.25 (dd, J = 249.3, 11.6 Hz), 152.27 (s), 152.08 (s), 152.06 (s), 151.71 (s), 146.64 (s), 144.78 (s), 144.69 (s), 143.90 (s), 133.37 (s), 128.36 (dd, J = 9.4, 4.9 Hz), 119.29 (dd, J = 11.2, 3.6 Hz), 112.43 (dd, J = 21.1, 1.7 Hz), 106.85 (s), 105.61 (s), 105.18 (t, J = 26.5 Hz), 102.94 (s), 82.01 (d, J = 4.7 Hz), 72.13 (s), 51.38 (d, J = 5.1 Hz), 51.05 (d, J = 6.1 Hz), 33.42 (s), 17.72 (s). ESI-HRMS: Calcd. for C24H21F2N7O7 m/z [M+H]+ = 558.1544; found [M+H]+ = 558.1558.
The synthesis and structural characterization of compounds A2–A4, B1–B5, and C1–C5 were described in the Supporting Information.
4.2. Photolysis monitoring of compounds under 365 nm UV light
Aqueous solution of compounds was exposed to UV light (25 mW/cm2, 365 nm) and samples were taken at time points of 0, 10, 20, 30, 40, 50, 60, 70, 80, 90 and 120 s, respectively. The percentage of each component in the sample was determined by using HPLC (Eclipse Plus C18, 4.6 × 250 mm, 5 μm, MeOH/H2O = 60/40–80/20, 0–10 min, MeOH/H2O = 80/20, 10–18 min, 0.5 mL/min, 254 nm). LC–MS was used to determine the products of photolysis and the changes of each component in contents with time. Compound A1 dissolved in DMSO-d6 was placed in quartzose NMR tube and irradiated with UV light (25 mW/cm2, 365 nm). The 1H NMR of sample was recorded at different time points (0, 4, 6, and 10 min).
4.3. Determination of sterol content in fungal cell membrane
Exponentially growing terminal fungal cells (C. albicans SC5314) were centrifuged, washed with PBS (3 × 1 mL), and resuspended in 50 mL YEPD medium to a concentration of 1 × 106 cells/mL. The fungal suspension was divided into 8 groups, and respectively treated with DMSO (control), DMSO-hv (control-hv), FLC (16 μg/mL), FLC-hv (16 μg/mL), A1 (16 μg/mL), A1-hv (16 μg/mL), A2 (16 μg/mL), and A2-hv (16 μg/mL). Samples treated with UV light (50 mW/cm2, 365 nm, 2 min) or non-light were incubated at 30 °C for 24 h in shocking (200 rpm). After culture, the fungal suspension was centrifuged (4000 rpm, 5 min), washed with PBS (2 × 5 mL) and resuspended in PBS (1 mL). The fungal suspension was transferred to a 25 mL glass tube to which NaOH (15% in EtOH, 10 mL) was added and saponified at 80 °C for 3 h H2O (5 mL) was added into the glass tube once the reaction was allowed to room temperature and extracted with n-hexane (2 × 5 mL). The combined organic layers were washed with H2O (5 mL) and concentrated to dryness. The residue was redissolved in cyclohexane, centrifuged (10,000 rpm, 1 min) and supernatant (200 μL) was taken into a sample vial. The sterol content in fungal membrane was determined by a GC–MS.
4.4. Construction of a heterozygous mutant strain of C. albicans SC5314 (erg11Δ/ERG11)
E. coli containing plasmid vectors were cultured in 5 mL LB medium for 24 h for activation. The bacteria suspension was centrifuged at 9000×g for 1 min. The collected E. coli were used for plasmid extraction using a SanPrep Spin Column & Collection Tube (DNA Clean up, purchased from Sangon Biotech) according to the instruction manual. Approximately 100 μL of plasmid solution at a concentration of 150–300 ng/μL was obtained. ApaI endonuclease (1 μL), SacI endonuclease (1 μL), 10 × Smart buffer (15 μL), and double distilled H2O (ddH2O) were added into the solution to prepare an endonuclease system with total volume of 150 μL. The system was reacted at 37 °C for 6–8 h to obtain the solution of the tool fragment. Exponentially growing terminal fungal cells (C. albicans SC5314) were centrifuged, resuspended in 50 mL YEPD medium to obtain an OD600 approximately 0.2. The fungal suspension was cultured at 30 °C for another 6 h, centrifuged (5000 rpm, 7 min), and resuspended in TE buffer (500 μL) containing 0.1 mol/L LiOAc after washed with sterile water. The fungal suspension (100 μL), boiled ssDNA (100 μL), and tool fragment (100 μL) were mixed well and then cultured at 30 °C for 30 min 700 μL PLATE solution was added to the suspension described above and cultured for another 16 h at 30 °C. After centrifugation (13,000 rpm, 10 s) to remove the supernatant, gently washed the fungi with sterile water to remove the remaining liquid. The fungi were resuspended in YPD medium (1 mL) and cultured at 30 °C for 4 h with sharking. The fungal suspension was spread on SDA medium containing nourseothricin (200 μg/mL) and incubated at 30 °C for 48 h. The colonies on the plates were identified using PCR, and the correctly identified colonies were cultured in YPM medium for two generations and then coated on resistant plates containing nourseothricin (25 μg/mL) to culture at 30 °C for 24 h. The small colonies with limited growth on the plates were identified by PCR again, and the correct strains were kept for frozen storage.
4.5. Investigation on the survival of larval G. mellonella
G. mellonella larvae in good condition with a weight about 0.5 g and no obvious gray or black spots on the body surface were selected for the experiment. There were 15 larvae in each group, and each larva was inoculated with C. albicans SC5314 about 5 × 105 CFU or 6 × 105 CFU for infection. Drugs were administered before or after inoculation at a dose of 8 mg/kg. Briefly, exponentially growing terminal fungal cells (C. albicans SC5314) were centrifuged, washed with PBS (3 × 1 mL) and resuspended in PBS to a concentration of 6.25 × 107 or 7.5 × 107 cells/mL. The fungal suspension (8 μL) was injected into the larval from the site of last left proleg by a Hamilton syringe which cleaned thoroughly. Larvae infected with C. albicans were placed in 12-well plates (one per well) in the order of inoculation. 30 min after completion of inoculation, a solution of compound A1 in PBS (0.5 mg/kg, 8 μL) containing 25% DMSO was injected into the larvae from the site of last right proleg. The groups required illumination were treated with UV light (50 mW/cm2) at 365 nm for 10 min. The G. mellonella larvae were incubated at 37 °C. The survival of larvae was observed and recorded every 12 h. Pre-dosing groups were treated with UV light (as above) or non-light 30 min after administration followed by infected with C. albicans. The culture and survival records of G. mellonella larvae were consistent with the above operations.
4.6. The fungal burden assay in skin model of candidiasis in mice
BALB/c mice (about 20 g each mouse) were used for the experiment with 3 mice each group. The animals were purchased from Huachaung Sino (Jiangsu, Certificate SCXK2020-0009). The conduct of animal experiments was approved by the Committee on Ethics of Medicine of Navy Medical University and the animal laboratory meets IACUC standards (Certificate SCXK-2022-0002, China). All mice were divided into 4 groups which respectively treated with normal saline, FLC, compound A1 (hv) and compound A1 (black). Briefly, exponentially growing terminal C. albicans SC5314 cells were harvested and resuspended in PBS to a concentration of 1 × 107 cells/mL. The mice were intradermally inoculated with fungal suspension at a dose of 1 × 106 CFU per mouse after shaving the back hair. Compounds (20 mg/mL, 100 μL) dissolved in a mixed solvent of 50% EtOH and 50% PEG-40 hydrogenated castor oil were smeared locally to the inoculation site of mice 24 h after inoculation. The group requiring light treatment was exposed to UV light (50 mW/cm2, 365 nm) for 2 min after administration. The mice were sacrificed after 4 days of continuous treatment. The skin affected with fungi was cut and weighted, and then ground with PBS (1 mL) to give a tissue homogenate. The tissue homogenate was diluted to an appropriate concentration and the diluent suspension (100 μL) was plated on SDA medium containing chloramphenicol and incubated 35 °C for 48 h. The number of colonies was counted, and the fungal burden in skin was calculated. GraphPad Prism was used to plot and calculate differences between groups.
Acknowledgments
This work was supported by the National Natural Science Foundation (81725020, 82003591 and 81973175, China), the Innovation Program of Shanghai Municipal Education Commission (2019-01-07-00-07-E00073, China), and Science and Technology Commission of Shanghai Municipality (20S11900400, China).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2023.02.008.
Contributor Information
Wei Wang, Email: wwang@pharmacy.arizona.edu.
Chunquan Sheng, Email: shengcq@smmu.edu.cn.
Author contributions
Na Liu, Jie Tu and Yahui Huang performed the literature search and data collection. Wei Wang and Chunquan Sheng proposed the project, Chunquan Sheng, Wanzhen Yang and Zhuang Li performed data analysis and contributed to the writing-review&editing of the manuscript. All authors have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Brown G.D., Denning D.W., Gow N.A., Levitz S.M., Netea M.G., White T.C. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadi A., Mohammadnejadi E., Karami P., Razzaghi-Asl N. Current status and structure activity relationship of privileged azoles as antifungal agents (2016–2020) Int J Antimicrob Agents. 2022;59 doi: 10.1016/j.ijantimicag.2022.106518. [DOI] [PubMed] [Google Scholar]
- 3.Hoenigl M., Seidel D., Sprute R., Cunha C., Oliverio M., Goldman G.H., et al. COVID-19-associated fungal infections. Nat Microbiol. 2022;7:1127–1140. doi: 10.1038/s41564-022-01172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doman M., Banyai K. COVID-19-associated fungal infections: an urgent need for alternative therapeutic approach? Front Microbiol. 2022;13 doi: 10.3389/fmicb.2022.919501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gintjee T.J., Donnelley M.A., Thompson G.R., 3rd Aspiring antifungals: review of current antifungal pipeline developments. J Fungi (Basel) 2020;6:28. doi: 10.3390/jof6010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu N., Tu J., Dong G., Wang Y., Sheng C. Emerging new targets for the treatment of resistant fungal infections. J Med Chem. 2018;61:5484–5511. doi: 10.1021/acs.jmedchem.7b01413. [DOI] [PubMed] [Google Scholar]
- 7.Liu N., Wang C., Su H., Zhang W., Sheng C. Strategies in the discovery of novel antifungal scaffolds. Future Med Chem. 2016;8:1435–1454. doi: 10.4155/fmc-2016-0020. [DOI] [PubMed] [Google Scholar]
- 8.Mota Fernandes C., Dasilva D., Haranahalli K., McCarthy J.B., Mallamo J., Ojima I., et al. The future of antifungal drug therapy: novel compounds and targets. Antimicrob Agents Chemother. 2021;65:e01719–e01720. doi: 10.1128/AAC.01719-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fisher M.C., Hawkins N.J., Sanglard D., Gurr S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science. 2018;360:739–742. doi: 10.1126/science.aap7999. [DOI] [PubMed] [Google Scholar]
- 10.Fisher M.C., Alastruey-Izquierdo A., Berman J., Bicanic T., Bignell E.M., Bowyer P., et al. Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol. 2022;20:557–571. doi: 10.1038/s41579-022-00720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchter M.J. On the promise of photopharmacology using photoswitches: a medicinal chemist's perspective. J Med Chem. 2020;63:11436–11447. doi: 10.1021/acs.jmedchem.0c00629. [DOI] [PubMed] [Google Scholar]
- 12.Impastato A.C., Shemet A., Vepřek N.A., Saper G., Hess H., Rao L., et al. Optical control of mitosis with a photoswitchable Eg5 inhibitor. Angew Chem Int Ed Engl. 2022;61 doi: 10.1002/anie.202115846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayer G., Heckel A. Biologically active molecules with a "light switch". Angew Chem Int Ed Engl. 2006;45:4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- 14.Parasar B., Chang P.V. Engineered Th17 cell differentiation using a photoactivatable immune modulator. J Am Chem Soc. 2020;142:18103–18108. doi: 10.1021/jacs.0c07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naro Y., Darrah K., Deiters A. Optical control of small molecule-induced protein degradation. J Am Chem Soc. 2020;142:2193–2197. doi: 10.1021/jacs.9b12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morstein J., Romano G., Hetzler B.E., Plante A., Haake C., Levitz J., et al. Photoswitchable serotonins for optical control of the 5-HT2A receptor. Angew Chem Int Ed Engl. 2022;61 doi: 10.1002/anie.202117094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Velema W.A., van der Berg J.P., Hansen M.J., Szymanski W., Driessen A.J., Feringa B.L. Optical control of antibacterial activity. Nat Chem. 2013;5:924–928. doi: 10.1038/nchem.1750. [DOI] [PubMed] [Google Scholar]
- 18.Lauxen A.I., Kobauri P., Wegener M., Hansen M.J., Galenkamp N.S., Maglia G., et al. Mechanism of resistance development in E. coli against TCAT, a trimethoprim-based photoswitchable antibiotic. Pharmaceuticals. 2021;14:392. doi: 10.3390/ph14050392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wegener M., Hansen M.J., Driessen A.J.M., Szymanski W., Feringa B.L. Photocontrol of antibacterial activity: shifting from UV to red light activation. J Am Chem Soc. 2017;139:17979–17986. doi: 10.1021/jacs.7b09281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S., Hou Y., Chen X., Gao Y., Li H., Sun S. Combination of fluconazole with non-antifungal agents: a promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery. Int J Antimicrob Agents. 2014;43:395–402. doi: 10.1016/j.ijantimicag.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Shafiei M., Peyton L., Hashemzadeh M., Foroumadi A. History of the development of antifungal azoles: a review on structures, SAR, and mechanism of action. Bioorg Chem. 2020;104 doi: 10.1016/j.bioorg.2020.104240. [DOI] [PubMed] [Google Scholar]
- 22.Desmond E., Gribaldo S. Phylogenomics of sterol synthesis: insights into the origin, evolution, and diversity of a key eukaryotic feature. Genome Biol Evol. 2009;1:364–381. doi: 10.1093/gbe/evp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arendrup M.C., Dzajic E., Jensen R.H., Johansen H.K., Kjaeldgaard P., Knudsen J.D., et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect. 2013;19:E343–E353. doi: 10.1111/1469-0691.12212. [DOI] [PubMed] [Google Scholar]
- 24.Tu J., Liu N., Huang Y., Yang W., Sheng C. Small molecules for combating multidrug-resistant superbug Candida auris infections. Acta Pharm Sin B. 2022;12:4056–4074. doi: 10.1016/j.apsb.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W., Yun Z., Ji C., Tu J., Yang W., Li J., et al. Discovery of novel sertraline derivatives as potent anti-cryptococcus agents. J Med Chem. 2022;65:6541–6554. doi: 10.1021/acs.jmedchem.1c01845. [DOI] [PubMed] [Google Scholar]
- 26.Li Z., Tu J., Han G., Liu N., Sheng C. Novel carboline fungal histone deacetylase (HDAC) inhibitors for combinational treatment of azole-resistant candidiasis. J Med Chem. 2021;64:1116–1126. doi: 10.1021/acs.jmedchem.0c01763. [DOI] [PubMed] [Google Scholar]
- 27.Han G., Liu N., Li C., Tu J., Li Z., Sheng C. Discovery of novel fungal lanosterol 14α-demethylase (CYP51)/histone deacetylase dual inhibitors to treat azole-resistant candidiasis. J Med Chem. 2020;63:5341–5359. doi: 10.1021/acs.jmedchem.0c00102. [DOI] [PubMed] [Google Scholar]
- 28.Brieke C., Rohrbach F., Gottschalk A., Mayer G., Heckel A. Light-controlled tools. Angew Chem Int Ed Engl. 2012;51:8446–8476. doi: 10.1002/anie.201202134. [DOI] [PubMed] [Google Scholar]
- 29.Hansen M.J., Feringa F.M., Kobauri P., Szymanski W., Medema R.H., Feringa B.L. Photoactivation of MDM2 inhibitors: controlling protein–protein interaction with light. J Am Chem Soc. 2018;140:13136–13141. doi: 10.1021/jacs.8b04870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klán P., Šolomek T., Bochet C.G., Blanc A., Givens R., Rubina M., et al. Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem Rev. 2013;113:119–191. doi: 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen M.J., Velema W.A., Lerch M.M., Szymanski W., Feringa B.L. Wavelength-selective cleavage of photoprotecting groups: strategies and applications in dynamic systems. Chem Soc Rev. 2015;44:3358–3377. doi: 10.1039/c5cs00118h. [DOI] [PubMed] [Google Scholar]
- 32.Anhäuser L., Klöcker N., Muttach F., Mäsing F., Špaček P., Studer A., et al. A benzophenone-based photocaging strategy for the N7 position of guanosine. Angew Chem Int Ed Engl. 2020;59:3161–3165. doi: 10.1002/anie.201914573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagatova A.A., Keniya M.V., Wilson R.K., Monk B.C., Tyndall J.D. Structural insights into binding of the antifungal drug fluconazole to Saccharomyces cerevisiae lanosterol 14α-demethylase. Antimicrob Agents Chemother. 2015;59:4982–4989. doi: 10.1128/AAC.00925-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu L., Zhou K., Zong W., Chen Y., Sheng C. Single dose pharmacokinetics of topical iodiconazole creams in healthy Chinese volunteers. Xenobiotica. 2021;51:427–433. doi: 10.1080/00498254.2021.1876962. [DOI] [PubMed] [Google Scholar]
- 35.Sheng C., Zhang W., Ji H., Zhang M., Song Y., Xu H., et al. Structure-based optimization of azole antifungal agents by CoMFA, CoMSIA, and molecular docking. J Med Chem. 2006;49:2512–2525. doi: 10.1021/jm051211n. [DOI] [PubMed] [Google Scholar]
- 36.Mafy N.N., Matsuo K., Hiruma S., Uehara R., Tamaoki N. Photoswitchable CENP-E inhibitor enabling the dynamic control of chromosome movement and mitotic progression. J Am Chem Soc. 2020;142:1763–1767. doi: 10.1021/jacs.9b12782. [DOI] [PubMed] [Google Scholar]
- 37.Albert L., Xu J., Wan R., Srinivasan V., Dou Y., Vázquez O. Controlled inhibition of methyltransferases using photoswitchable peptidomimetics: towards an epigenetic regulation of leukemia. Chem Sci. 2017;8:4612–4618. doi: 10.1039/c7sc00137a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bléger D., Schwarz J., Brouwer A.M., Hecht S. o-Fluoroazobenzenes as readily synthesized photoswitches offering nearly quantitative two-way isomerization with visible light. J Am Chem Soc. 2012;134:20597–20600. doi: 10.1021/ja310323y. [DOI] [PubMed] [Google Scholar]
- 39.Aggarwal K., Kuka T.P., Banik M., Medellin B.P., Ngo C.Q., Xie D., et al. Visible light mediated bidirectional control over carbonic anhydrase activity in cells and in vivo using azobenzenesulfonamides. J Am Chem Soc. 2020;142:14522–14531. doi: 10.1021/jacs.0c05383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kolarski D., Miller S., Oshima T., Nagai Y., Aoki Y., Kobauri P., et al. Photopharmacological manipulation of mammalian CRY1 for regulation of the circadian clock. J Am Chem Soc. 2021;143:2078–2087. doi: 10.1021/jacs.0c12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X., Xi W., Gao G., Wang X., Stansbury J.W., Bowman C.N. o-Nitrobenzyl-based photobase generators: efficient photoinitiators for visible-light induced thiol-Michael addition photopolymerization. ACS Macro Lett. 2018;7:852–857. doi: 10.1021/acsmacrolett.8b00435. [DOI] [PubMed] [Google Scholar]
- 42.Gow N.A., van de Veerdonk F.L., Brown A.J., Netea M.G. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat Rev Microbiol. 2011;10:112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grald A., Yargosz P., Case S., Shea K., Johnson D.I. Small-molecule inhibitors of biofilm formation in laboratory and clinical isolates of Candida albicans. J Med Microbiol. 2012;61:109–114. doi: 10.1099/jmm.0.034124-0. [DOI] [PubMed] [Google Scholar]
- 44.Wu S., Wang Y., Liu N., Dong G., Sheng C. Tackling fungal resistance by biofilm inhibitors. J Med Chem. 2017;60:2193–2211. doi: 10.1021/acs.jmedchem.6b01203. [DOI] [PubMed] [Google Scholar]
- 45.Fuchs B.B., O'Brien E., Khoury J.B., Mylonakis E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence. 2010;1:475–482. doi: 10.4161/viru.1.6.12985. [DOI] [PubMed] [Google Scholar]
- 46.Bhushan K.R. Light-directed maskless synthesis of peptide arrays using photolabile amino acid monomers. Org Biomol Chem. 2006;4:1857–1859. doi: 10.1039/b601390b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.