Abstract
Non-invasive control of the drug molecules accessibility is a key issue in improving diagnostic and therapeutic procedures. Some studies have explored the spatiotemporal control by light as a peripheral stimulus. Phototriggered drug delivery systems (PTDDSs) have received interest in the past decade among biological researchers due to their capability the control drug release. To this end, a wide range of phototrigger molecular structures participated in the DDSs to serve additional efficiency and a high-conversion release of active fragments under light irradiation. Up to now, several categories of PTDDSs have been extended to upgrade the performance of controlled delivery of therapeutic agents based on well-known phototrigger molecular structures like o-nitrobenzyl, coumarinyl, anthracenyl, quinolinyl, o-hydroxycinnamate and hydroxyphenacyl, where either of one endows an exclusive feature and distinct mechanistic approach. This review conveys the design, photochemical properties and essential mechanism of the most important phototriggered structures for the release of single and dual (similar or different) active molecules that have the ability to quickly reason of the large variety of dynamic biological phenomena for biomedical applications like photo-regulated drug release, synergistic outcomes, real-time monitoring, and biocompatibility potential.
Key words: Phototrigger, Light irradiation, Excited state proton transfer, Drug delivery system, Dynamic process, Controlling chemistry
Graphical abstract
Here, the design, photochemical properties and essential mechanism of the most important phototriggered structures for the release of single and dual (similar or different) active molecules have been discussed.
1. Introduction
Over the past decade, special and great regard has been paid to the combination of the stimuli-triggered molecules with active biomaterial owing to multiple advantages in human healthcare such as controlled delivery of diagnostic, regulated drug delivery, therapeutic and pharmaceutical factors1, 2, 3, 4, 5, 6. The triggered molecules are defined as the units, which can change their structure due to isomerization, dimerization or bond cleavage in response to an external stimulus for the release of active molecules7, 8, 9, 10, 11, 12, 13. Living organisms in nature are rich in examples that can reversibly regulate their configuration and properties in response to environmental stimuli. Heat-shock transformation in bacteria14, camouflage in chameleons15 and color changes in echinoderms in response to light16 are wide ranging instances for this. Such triggered materials with the ability of responding to stimulus are considered to be an important class of advanced materials that can be utilized in biomedical applications and nanomedicine, particularly in the development of stimuli-triggered drug delivery systems (DDSs)17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28.
So far, several external stimuli have been developed, such as light29, 30, 31, 32, 33, 34, 35, temperature36, 37, 38, magnetism39, 40, 41, 42, ultrasound43,44 and electricity45. Among the mentioned external stimuli, light stimulation has attracted exceptional attention due to its ease of application without chemical contaminants, high spatial resolution, noninvasive nature and exact temporal and spatial control29,46, 47, 48, 49, 50, 51. Wider ranges of the light wavelengths from ultraviolet (UV, λmax = 200–400 nm) to visible (Vis, λmax = 400–750 nm) or near-infrared (NIR, λmax = 750–2000 nm) can be employed to trigger photo-sensitivity. Compared to UV and visible light, NIR light has less photo-toxicity, better tissues penetration depth and reduced background signal for biological applications. However, the application of NIR light is restricted due to its long wavelength, which has not enough energy to disrupt of chemical structures through bond-breaking or conformation-switching to triggered DDSs52, 53, 54, 55. Recently, this issue solved by two-photon actuation (Section 3.1.1) in the range of 650–900 nm or upconversion NPs (UCNPs) technologies56. In comparison, UV light is a somewhat inferior nominee due to its toxicity under prolonged treatment and poor tissue penetration capacities (around 10 mm) due to light scattering and absorbance by intrinsic biological chromophores57,58. However, attempts have been provided to address these restrictions by a micro-light (MLight) source that can be implanted locally inside the human body59,60. In contrast, visible light can lead minor damage than UV light in vivid systems that has been recommended as an alternative to phototriggered DDSs (PTDDSs)61.
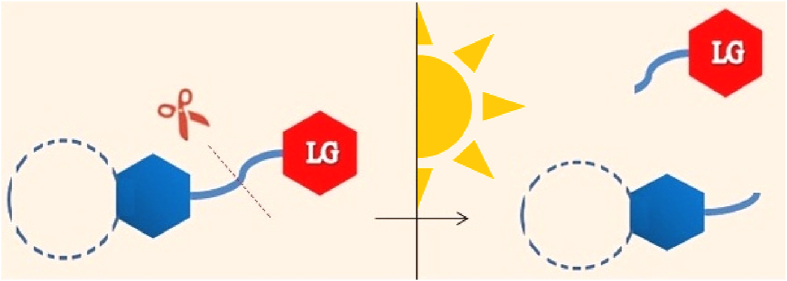
Phototriggers, also known as photo-removable protecting groups (PRPG), undergo an irreversible dissociation by selectively breaking a chemical bond can release leaving groups (LG) as bioactive molecules at specific time under light irradiation (Fig. 1)62. Therefore, a PTDDS can distribute a bioactive agent upon a specific wavelength light instantly at the preferred place in instant to attain a focused high value of drug while reducing generally injected dosage level and total poisonous effects as a result of their non-invasiveness and spatiotemporal accuracy63. This proves greatly assure for drugs with adverse toxicity and side-effects or for targeted therapy efficiency of them. With increasing applications of light-responsive DDSs particularly in biomedical applications, new improvements of phototriggers are needed to fulfill the requirements for better sensitivity, low toxicity, structural simplicity, desirable solubility in the targeted media, faster kinetics, effective fluorescence embodiment and an adjustable and strong absorption spectrum above 300 nm. So far, several phototriggers such as o-nitrobenzyl (ONB)64,65, coumarin66,67, p-hydroxylphenacyl (pHP)68,69, hydroxycinnmate70,71, benzoin (Bnz)72, 73, 74, nitroindoline (NI)75, 76, 77 and 8-bromo-7-hydroxylquinoline (BHQ)78,79 have been applied in DDS during the past decade.
Figure 1.
The general representative of phototriggered molecules.
To date, to further improvement the therapeutic results and reduce side effects, integration of nanotechnology with phototriggered molecules has opened new horizons for synergistic therapies25. Nano-platforms such as polymeric nanoparticles (NPs), liposomes, metal–organic cages and metallic NPs have provided a complementary means for delivery of therapeutic agents into diseased cells and tissues using safe and effective directions80, 81, 82, 83, 84, 85. In this review, we will focus on latest developments of conventional phototriggers to provide more effective therapies against serious illnesses with reduced the damage to healthy cells and open new perspective toward favorable bioavailability and drug delivery efficiency.
2. Phototriggered drug delivery systems (PTDDSs)
PTDDSs facilitate the release of LGs such as therapeutic agents or drug cargos through different mechanisms including bond cleavage86, 87, 88, 89, isomerization90, 91, 92, photo-oxidation93,94, photo-reduction95,96, cross-linking97,98 and photocaging/uncaging99,100, by light irradiation. These transformations can be caused disruption and dissociation of extant structures or even altering the lower critical solution temperature (LCST) transition8,101,102. Here, we discuss important latest advances on phototriggered molecules together with their essential mechanisms and their conditions for stimulation based on two types of light-induced drug release. One is the direct release of the drug as a LG by photochemical bond cleavage of p-hydroxyphenacyl (pHP), o-hydroxycinnamate, tetraphenylethylene (TPE), and coumarin substituted and the other is the photoinduced disruption of nanoscale structures such as micelles and MOCs having nitroaryl derivatives, thioketal, maleimide-anthracene, spiropyranes (SP) and azobenzenes (Azo) moiety to release the drug cargo. In addition, the dual-releasing phototriggers as a novel combination therapy with the ability to release two anticancer drugs are discussed at the end of review (Fig. 2).
Figure 2.
Schematic illustration of PTDDSs.
3. PTDDSs based on photochemical bond cleavage
The photochemical bond cleavage strategy assists intramolecular self-immolation to the release of the bioactive molecules such as enzymes, neurotransmitters, cell-signaling molecules, fluorophores, fragrances and drugs on a particular site with exact control of their dosages using light irradiation length86,103. The pHP, o-hydroxycinnamate, nitroaryl, TPE, and coumarin derivatives are good examples for these purposes, which noticed as a critical step for biomedical applications of phototriggers.
3.1. p-Hydroxyphenacyl
The pHP groups are good phototrigger example for the study of very fast biological procedures with high photochemical quantum yield104, 105, 106, however, due to non-fluorescent behavior and excitation wavelength below 400 nm, pHP group receives less attention as a delivery agent107. To overcome these issues, Barman et al.108 have incorporated 2-(2′-hydroxyphenyl)benzothiazole (HBT) moiety to pHP derivative toward the design a photo-induced DDS, entitled p-hydroxyphenacyl-benzothiazole-chlorambucil (pHP-Benz-Cbl), see Scheme 1.
Scheme 1.
Synthesis of the pHP-Benz-Cbl and its potential photorelease mechanism.
First, the salicylaldehyde 1 transformed to derivative 3 using Friedel–Crafts acylation and treated with the anticancer drug chlorambucil (Cbl) to afford 4. Afterward, the treatment of 4 with 2-aminothiophenol 5 yielded the pHP-Benz-Cbl as excited-state intramolecular proton transfer (ESIPT)-assisted phototrigger for the very fast photorelease of Cbl inside the cell (15 min) using the visible wavelength (≥410 nm). Herein, the pHP group integration on the HBT caused a distinct fluorescence discolor from green to blue after photorelease and assisted in the deprotonation of pHP segment to accelerate the release process via ESIPT. Upon visible light irradiation, pHP-Benz-Cbl (with an intense green-emission band) excites to the singlet state undertakes a fast ESIPT, where a proton translocation occurs from the pHP to the benzo-thiazole segment, producing 7 and subsequently zwitterionic 8. The intermediate 8 exceeds during proficient intersystem crossing (ISC) to triplet excited state that converts to a supposed spirodiketone 10 with the parallel release of the Cbl accompanied by a photo-Favorskii rearrangement. Finally, the hydrolytic ring opening of spirodiketone 10 yielded pHP-Benz-COOH 11 with an intense blue-emission band owing to conjugation disconnection from a phenolic hydroxy functional group to a carbonyl moiety. This phenomenon is obvious in emission spectrum (Fig. 3a) during 15 min. Fig. 3b‒d, show the real-time monitoring of Cbl release by pHP-Benz-Cbl within malignant neoplastic disease tissue (MDA-MB 231). At first, the tissues have green fluorescence because of pHP-Benz-Cbl sorption (Fig. 3b, 0 min). After 10 min visible light irradiation with λmax = 410 nm, two different green and blue fluorescence detected, signifying the Cbl incomplete release (Fig. 3c). In conclusion, after 15 min discolor from green to blue, suggesting a complete photorelease of Cbl drug and highest toxicity level (above 90%) toward cancer tissues (Fig. 3d).
Figure 3.
(a) Increase of emission spectra of pHP-Benz-Cbl during 0–15 min, and real-time monitoring of the Cbl liberation from pHP-Benz-Cbl during visible-light irradiation (b) 0 min, (c) 10 min and (d) 15 min by confocal microscopy. Reprinted with permission from Ref. 108. Copyright@2016 John Wiley & Sons, Inc.
Singh et al.109 recently developed pHP based DDS with an excellent uncaging capacity in the area of 700 nm. Aggregation-induced-emission (AIE) chromophores have gained immense interest in biomedical purposes due to their unique possessions including exceptional photostability, excellent luminescence and biocompatibility110, 111, 112. In this way, they incorporated the naphthalene group into the pHP moiety so that a strong internal charge transfer occurred, causing in a red-shift in absorption bond and improved two-photon uncaging cross-section (Scheme 2). The prepared photo-induced DDS, pHP-Naph-Cbl exhibits exceptional properties like two-photon absorption in the phototherapeutic window (700 nm), high real-time monitoring ability due to a specific fluorescence color change from greenish-yellow to blue through the Cbl release and demonstrates AIE behavior, therefore remarkably releases the Cbl in the aggregated state with distinct fluorescence discolor.
Scheme 2.
Two-photon uncaging of pHP-Naph-Cbl.
3.1.1. Two-photon excitation uncaging
The two-photon excitation (2 PE) uncaging is a growing alternative method to evade the photo-toxicity of UV light and enhance spatial resolution via three-dimensional (3D) imaging in various fields, especially in cell biology113, 114, 115, 116, 117, 118. This non-linear absorption occurrence can excite a molecule by simultaneous absorption of two photons with about half-energy instead of one, which doubles the corresponding irradiation wavelength compared with conventional one-photon excitation (1 PE). The phototrigger subsequently becomes red or NIR light, which their wavelengths can penetrate deeper into tumor cells and decrease the photodamage under treatment. Therefore, designing structures with two-photon photosensitivity is one of the most prevalent challenges in biomedical treatment. Recently, Klausen et al.119 provide a systematically review to the explanation and design of two-photon phototriggered structures in uncaging of bioactive molecules.
3.2. Tetraphenylethylene
Among the various AIE chromophores, tetraphenyletheylene (TPE) and its analogs have achieved considerable importance in the field of cancer therapy mostly through their potential to perform as photosensitizers for photodynamic therapy (PDT) and cellular imaging120,121. The TPE derivatives are one of the ideal units for assembling macrocycles and cages due to its simple C2 symmetry and as minimum tetratopic reaction situations122,123. To use the benefits of AIE and ESIPT combination, the Parthiban et al.124 group developed a PTDDS using connection of TPE with pHP-Cbl (TPE-pHP-Cbl) that, released the Cbl only in their aggregated state under visible light (λ ≥ 410 nm) irradiation with high real-time monitoring ability. This organized PTDDS due to its AIE phenomenon showed distinct fluorescence. Although, the visible light activated based on DDS by Barman et al.108 and Parthiban et al.124 did not display hopeful uncaging capacity in the area of 650–950 nm, which obstructs their sensible usage in the biomedical applications.
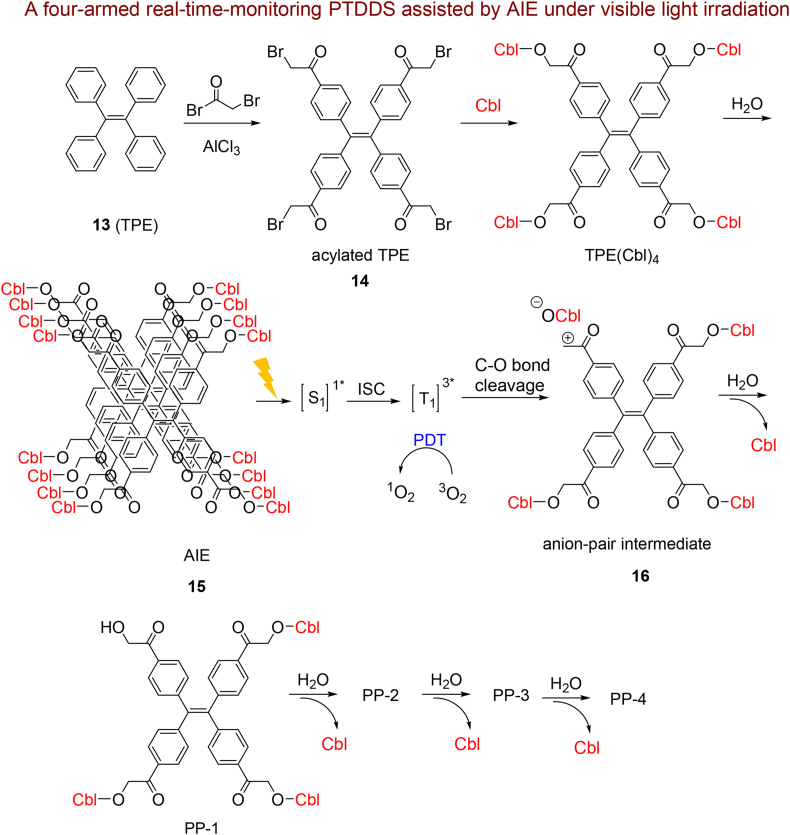
In the following, Parthiban et al.125 synthesized for the first time a photo-induced nano-DDS with strong fluorescence by a TPE 13 functionalized with 4 equivalent Cbl as anticancer agent (TPE(Cbl)4 NPs) (Scheme 3). The four-armed phototriggers TPE(Cbl)4 was initially synthesized by Friedel–Crafts acylation and then reacted with Cbl. Subsequently, the TPE(Cbl)4 NPs were synthesized by re-precipitation methods. They proved that 4 equiv of Cbl is librated in an ordinal procedure when the TPE(Cbl)4 NPs induced by visible light through a C–O bond cleavage. Furthermore, TPE(Cbl)4 and the released photoproducts exhibited a PDT property during drug release.
Scheme 3.
Synthetic route and potential mechanism for produced singlet oxygen and sequential Cbl release.
Upon photolysis, TPE(Cbl)4 NPs get excited to their singlet state and afterward to their triplet states through ISC. Then, TPE(Cbl)4 NPs as a photosensitizer produce singlet oxygen and undergo heterolytic fission of the carbonate ester C–O bond to cause anion-pair intermediate 16. After that, reaction of anion pair intermediate 16 with H2O generated the photoproducts PP-1, PP-2, PP-3 and PP-4 sequentially, together with the ordinal Cbl release in their aggregated state. During cellular uptake, the cells exhibited a deep-green color due to existence of TPE(Cbl)4 which has the AIE process capability (Fig. 4a). Upon 10 and 25 min visible irradiation, respectively (Fig. 4b and c), the amount of the fluorescence initially reduced and a weak green fluorescence observed that obviously signifies the breakup of TPE(Cbl)4 NPs and disseminated Cbl inside the tumor cell. Incubation of tumor tissue (HeLa cell) with non-fluorescent dichlorodihydrofluorescein diacetate (DCFDA) and TPE(Cbl)4 NPs displays weak green fluorescent (Fig. 4d) that after irradiation due to generation of a singlet oxygen through TPE(Cbl)4 NP a strong green fluorescence observed (Fig. 4e and f). In fact, non-fluorescent DCFDA convert to green fluorescent dichlorofluorescein owing to oxidation by produced singlet oxygen species. Prominently, they confirmed that TPE functionalized with 4 equivalents of Cbl are very efficient (lower 16% viability) compared to 1 equivalent of Cbl against cancer cells thanks to the synergistic effects of 4 equivalent of released Cbl and PDT activities.
Figure 4.
(a–c) Confocal microscopy representations of TPE(Cbl)4 NPs in HeLa cell; (a) 0, (b) 10 and (c) 25 min irradiation, and (d–f) generation of a singlet oxygen via TPE(Cbl)4 by DCFDA in HeLa cell; (d) 0, (e) 15 and (f) 25 min irradiation. Reprinted with permission from Ref. 125. Copyright@2019 American Chemical Society.
In the other research work by the same group, they synthesized tetraphenylethylene conjugated pHP NPs (TPE-pHP-H2S) for the controlled liberation of hydrogen sulfide (H2S) upon exposure to visible light without the assistance of any peripheral reagent126. This H2S donor triggered by light displays both AIE and ESIPT properties by TPE and pHP moieties, respectively. Furthermore, a real-time monitoring at the cellular level is possible with a simple fluorescence color change from yellow to green after photorelease.
3.3. o-Hydroxycinnamate
According to preceding considerations127,128, Paul et al.129 studied the application HBT as an ESIPT moiety in o-hydroxycinnamate fragment (HBT@o-hydroxycinnamate). As shown in Scheme 4, the authors designed the fluorescent phototrigger HBT@o-hydroxycinnamate by the attachment of HBT moiety to the o-hydroxycinnamate group for rapid and shortest release (60 min) of methyl salicylate 21 with distinct fluorescence color change from orange (because of the ESIPT occurrence) to blue (due to formed benzothiazole-coumarin 20) following photorelease. The light-induced conversion of the (E)-photoisomer to the (Z)-photoisomer causes the release of alcohol in company with a coumarin as by-product (with intense fluorescent property and emission wavelength). The potent fluorescent of coumarin byproduct can be useful in the release of alcohol derivatives from these systems. Although, the limitation of this work is the phototrigger activates in the UV region (≥365 nm) as well as the strong chromophore benzothiazole-coumarin byproduct 20 operates as an optical filter.
Scheme 4.
Photoinduced uncaging of methyl salicylate 21 from compound HBT@o-hydroxycinnamate.
3.4. Coumarin substituted
Among the synthetic phototriggers, coumarin-based derivatives as another important class of phototriggered molecules exhibit unique fluorescence visualization and liberate free drug molecules through a C–O bond breaking upon exposure light irradiation102,130, 131, 132, 133, 134. Accordingly, some researchers nominate a coumarin incorporated with heterocycle derivatives as the PTDD system for making the photo-induced release of antitumor agents56,135,136. In general, coumarins may be utilized both as a cross-linker and as a divisible moiety especially owing to their faster release rate compared to other phototriggered molecules such as ONB derivatives137. Coumarin functionalized at the 7-position with an electron-donating group (EDG) and at the 3-position with an electron-withdrawing group (EWG) display red-shifted absorption and emission in the blue-green light area (Scheme 5). The design of derivative 22 was a critical point burgeoning in the development of cages for the biological and biomedical studies138, 139, 140, 141, 142, 143. Wang et al.144 examined the synthesis of two carbazole-coumarin derivatives 23 (with –COOH group) and 24 (with triphenylphosphonium (TPP) group) for the photocontrolled release of Cbl, in an in vitro model of cancer cells. The amine group of the carbazole moiety supplies as an EDG and the lactone segment of the coumarin serves as an EWG and permits the visible light of 405 nm to trigger the photodecomposition of the carbazole-coumarin-Cbl connection. Carbazole-coumarins 23 and 24 showed emission bands at 360 and 450 nm, correspondingly.
Scheme 5.
Structures of (a) designed coumarin 22 and (b) a visible-light-triggered drug release of 23, 24.
The photochemical release of Cbl drug is similar to what will be expressed later in Scheme 24352. Carbazole-coumarin 24 with the lower IC50 due to synergistic effect of chemo-drug strength and photosensitization proposed the high efficiency of the TPP structure in raising the bioavailability carbazole-coumarin-drug derivatives.
Scheme 24.
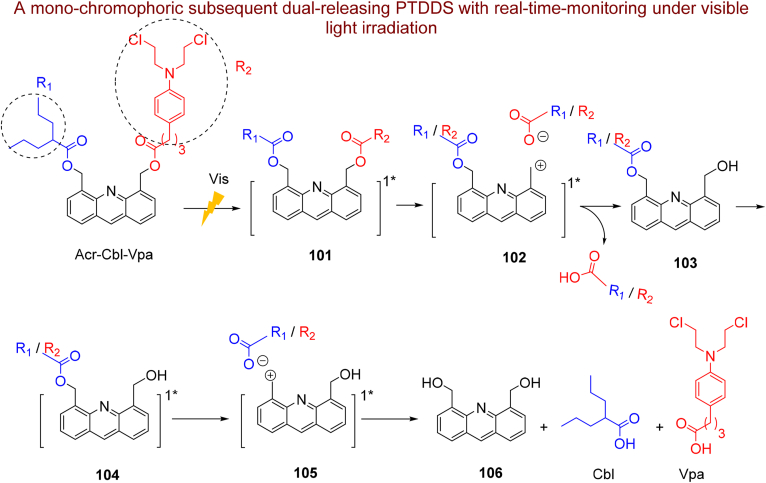
The Acr-Cbl-Vpa structure and subsequent release of Vpa and Cbl.
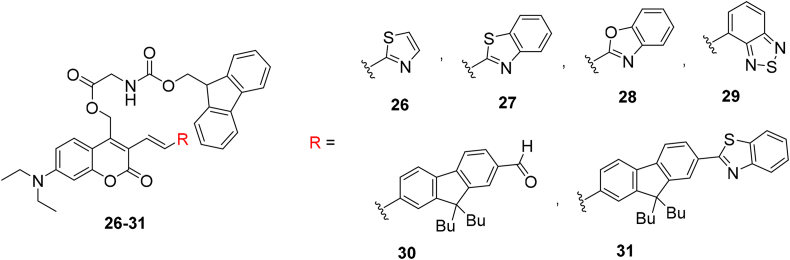
Klausen et al.145 in other attempt attached an EWG and a π-conjugated linker through a vinyl-acceptor function at the 3-position of the coumarin, the respective results conveyed Fmoc-protected glycine release both at 700 and 900 nm. As already mentioned (Section 3.1.1), contrary to UV–visible light, the 2 PE by NIR wavelengths affords different benefits including profounder penetration in tumor cells, abridged photo-damage and inherent 3D resolution. A tiny collection of advanced dipolar coumarinylmethyl structure 26–31 that demonstrate great two-photon sensibility at two supplementary wavelengths in the NIR spectral area is illustrated in Scheme 6.
Scheme 6.
Structures of π-extended coumarins 26–31.
Further investigation revealed that the existence of the fused-ring on the EWG and most powerful EWG in the categories can intensify the photorelease efficiency. Therefore, compounds 26–28 are not proper for uncaging applications during C–O bond cleavage upon excitation. Meanwhile, a distinct performance examined for developed analogs 30 and 31 with a fluorenyl component in the conjugated π-connector.
Bojtar et al.146 synthesized a click-and-release system based on coumarin with a supplementary degree of spatiotemporal manage for the liberation of the caged kinds (Scheme 7). They synthesized vinylene-linked coumarinyl-tetrazine 32 as a photocage that jointed with several amino acids including, Boc-phenylalanine, Fmoc-lysine and Boc-tyrosine-tBu-ester as the model caged substrates. The presence of vinylene tetrazine moiety in the photocages 33–35 quenches the fluorescence of the coumarin moiety and they were fairly photostable and no liberation of the amino acids was perceived following blue laser lighting (λmax = 488 nm).
Scheme 7.
Structure and conditional uncaging of the photocages.
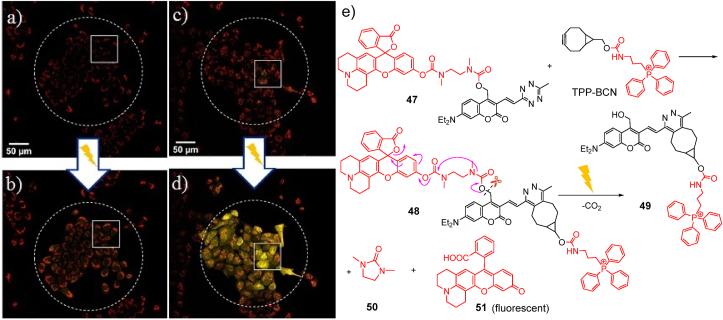
However, fluorescence capability was changed upon altering the tetrazine 33–35 in a bioorthogonal reaction with bicyclo[6.1.0]non-4-yn-9-ylmethanol (BCN) 36. Recently, bioorthogonal reactions allow researchers to label or manipulate biological systems in a single experiment147, 148, 149, 150, 151. The BCN-conjugated derivatives 37–39 demonstrated the most fluorescence (with a bright green-emission band) as well as the light-induced bond breaking to fast liberation of all three amino acids 44–46. Fig. 5a and b clearly demonstrate that the live cells remediated alone with tetrazine derivative 47 demonstrated a tiny fluorescence growth while cells treated with TPP-BCN and then with 47 displayed bright yellow emission of coumarin 51 after irradiation (Fig. 5c and d). This can be due to the formation of BCN-conjugated derivative 48 caused by the bioorthogonal reaction of 47 with TPP-BCN according to Fig. 5e. The tetrazine derivative 47 have an excellent ability for uncaging monitoring process. Further, BCN with triphenylphosphonium moiety (TPP-BCN) used as a tiny organelle marker, to focus the bioorthogonal reaction into the mitochondria (Fig. 5e)152.
Figure 5.
(a, b) Confocal images of tissues remediated alone with tetrazine derivative 47 (a) before and (b) after irradiation and (c, d) cells treated with TPP-BCN and then with 47 (c) before and (d) after irradiation and (e) reaction of tetrazine derivative 47 with TPP-BCN. Reprinted with permission from Ref. 146. Copyright@2020 American Chemical Society.
4. PTDDSs based on photoinduced disruption of nanoscale structures
4.1. Nitroaryl derivatives
Recently, nitroaryl derivatives including the ONB, o-nitro-2-phenethyl and o-nitro-anilide (ONA) groups used phototriggers to assemble photocages for the regulated liberation of important metallic ions, drugs or bioactive molecules153, 154, 155, 156, 157. In the ONB derivatives, an aci-nitro intermediate can form via an intramolecular hydrogen transfer to the nitro group (tautomerization) by irradiation. Despite their extensive applications, ONB photocages often show low quantum yield values (<0.05) owing to resonance stabilization of aci-nitro intermediates and their application is limited due to the highly absorbing and reactive side products72,158. A one photon blue visible light responsive polymeric carrier (up to 500 nm) based on ONB derivatives as a subcutaneously implanted depot developed by Carling et al.159 and it has been used for medical cargo release in the hydrophobic microenvironments including the internal space of a polymeric particle. The improvement of one-photon visible photo-responsive structures represents an attractive alternative for in vivo applications due to very shorter lighting time period with lower powers consumption and is significantly less dangerous to the tissues than UV and NIR laser lights. In this work, to facilitate polymerization and enhance the kinetics of photo-degradation, the butanediol derivative 2-(4′-N-dimethylamino-4-nitro-[1,1′-biphenyl]-3-yl)butane-1,4-diyl dicarbonyl (ANBB) was firstly synthesized160,161 and then basic tertiary amine groups introduced in the polymer backbone for promotion photo-cleavage in hydrophobic environments (Scheme 8).
Scheme 8.
(a) Schematic representative of Dex@PP52 and its swelling and Dex release under visible light irradiation and (b) in vivo photorelease of Dex@PP52 depot upon exposure to blue visible light. Reprinted with permission from Ref. 159. Copyright@2014 The Royal Society of Chemistry.
The tertiary amine functional group connected to photo-responsive polymer spine 46 (PP52) facilitate deprotonation of the aci-nitro intermediate directing to β-elimination and photo-cleavage according to Scheme 8. Therefore, the visible light irradiation of PP52 induces swelling and release of dexamethasone (Dex) as an anti-inflammatory agent in vivo. The therapeutic effects of Dex@PP52 were more effective than free Dex in local inflammation because of the release of Dex with greater efficiency in the target tissue, rapid diffusion and remaining as local depot on-demand at the injection site with minimizing adverse side effects. Dex@PP52 together with the NIR fluorescent probe IR780 permitted in vivo real-time monitoring of the depot release. Photograph Scheme 8b, demonstrates the in vivo photorelease of Dex@PP52 depot upon exposure to blue visible light. Advantages of this photo-responsive carrier were the strong visible light absorption above 500 nm and photo-reactivity in hydrophobic surroundings.
4.2. Thioketal linkers
4.2.1. Reactive oxygen species-responsive thioketal carriers
The irregular biochemical change of reactive oxygen species (ROS) level (lack/excess) in the disease sites can promote different diseases like autoimmune, cardiovascular, neurodegenerative and etc.162, 163, 164, 165, 166, 167, 168, 169. This issue has motivated investigators to utilize imparity ROS amounts for creating ROS-responsive drug carriers. Thioketal, thioacetal, thioether, vinyldithioether, aryloxalate, selenium, tellurium and arylboronic esters linkers are effective ROS-cleavable chemical groups undergoing bond breaking upon treatment with ROS, therefore, have already been utilized to synthesis ROS-responsive DDSs170, 171, 172, 173, 174, 175, 176. Chen et al.177 synthesized an amphiphilic and cationic ROS-responsive poly(β-amino ester) (PBAEROS) via insertion of a thioketal segment in its monomer unit to attain synergistic antitumor outcomes integrated with photothermal/photodynamic therapy (PTT/PDT) and chemotherapy treatment according to Scheme 9. Hereupon, PBAEROS was used for load of photosensitizer IR780 (a near NIR dye, which has both intense PTT and PDT efficacies under laser illumination) and chemotherapeutic drug doxorubicin (DOX). Because the poly(β-amino ester) chains of PBAEROS as hydrophobic moiety and side-chain hydroxyl groups as hydrophilic were conjugated, it was predictable that the poly(β-amino ester) chains and hydroxyl groups form a core/shell architecture in an aqueous medium, respectively. Nanomicelles of PBAEROS@IR780-DOX were prepared under stirring with dropwise addition of DOX and IR780 in DMSO and surface modification of polymer PBAEROS@IR780-DOX (PPID) was accomplished through propylene glycol alginate sodium sulfate (PSS) using an easy nanoprecipitation procedure (Scheme 9). The positive charges on the surface of the PPID nanosystem facilitate the rapid penetration into the tumor tissues because of interplay with the negatively charged cell membranes. Upon exposure 808 nm laser light, PPID NPs cause a quick rise temperature and generated a wide range of cytotoxic ROS. The ROS generated by the IR780 advanced further breakage of the thioketal bonds and release of DOX, thereby synergistic PTT/PDT and chemotherapy efficiencies was observed in vitro photorelease. Further examinations unraveled the formation of by-products acetone 53 and dithiol 54 upon the cleavage of the thioketal bond of PBAEROS.
Scheme 9.
Preparation of PPID NPs and their mechanisms via integrated with PTT/PDT and chemotherapy.
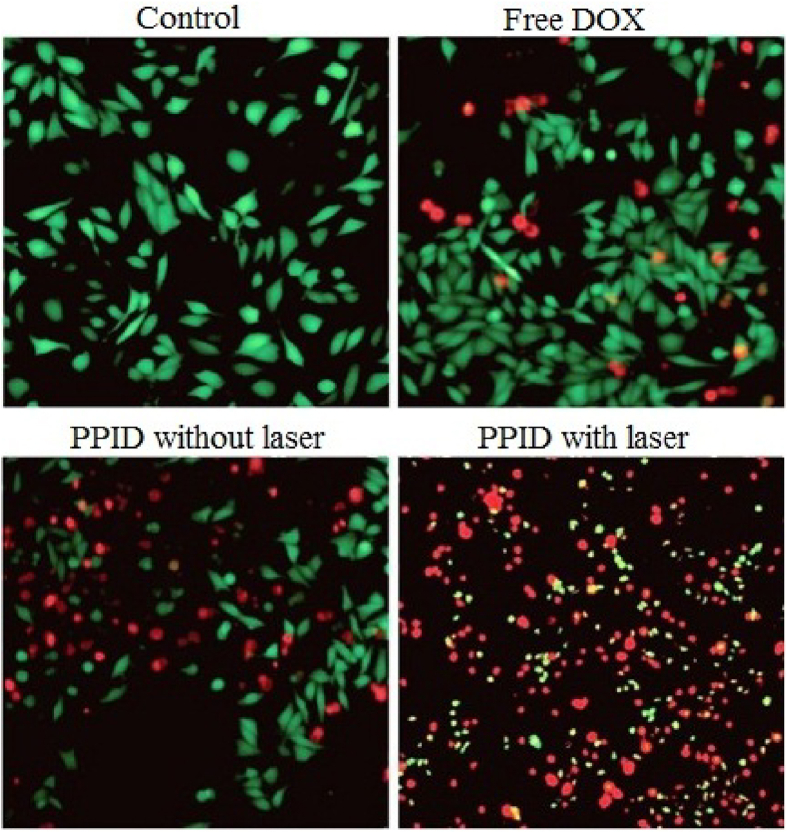
The fluorescence microscopic photographs of Hep1-6 tissues after various remediations with free DOX, PPID without laser and PPID with laser lighting are revealed in Fig. 6. Few tumor cells died after 24 h remediation with free DOX (IC50 = 1.3 μg/mL), a greater number of the cells significantly died with PPID NPs (IC50 = 0.72 μg/mL) and these cells were approximately completely died after laser illumination (IC50 = 0.22 μg/mL) meaning that PPID NPs possibly will merge PTT/PDT and chemotherapy to apply synergistic effects in cancer tissues.
Figure 6.
Fluorescence microscopic photographs of live and dead Hep1-6 tissues after various treatments. Reprinted with permission from Ref. 177. Copyright@2019 The Royal Society of Chemistry. Scale bar: 200 μm.
4.2.2. o-Nitroaryl thioketal carriers
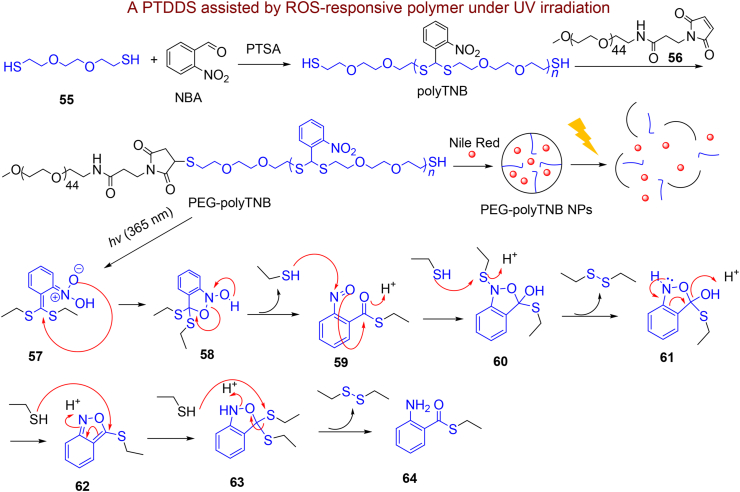
In an interesting study, Men et al.178 have presented a new cleavable polymer containing o-nitroaryl thioacetal structure with more stability than reported thioketal and thioacetal-based polymers to ROS. They first used o-nitrobenzaldehyde (o-NBA) and a dithiol derivative for the preparation of thioacetal polymer containing thiol terminated polyTNB. It can be easily conjugated to methoxypolyethylene glycol maleimide (mPEG-maleimide) through mPEG-maleimide click chemistry. The prepared amphiphilic polymers PEG-polyTNB capable of forming NPs in a combination of THF/dioxane with Nile Red as a hydrophobic drug pattern have been utilized as a light-cleavable nano-drug assembly (Scheme 10). The treatment of PEG-polyTNB with some ROS types specified a significant shift in the oxidation potential of these thioacetal compounds due to the presence of EWD group (NO2) of NBA, demonstrating improved stability of the polymer against ROS condition compared to the often-reported thioacetal polymers. In addition, irradiation of PEG-polyTNB with UV-A (365 nm) led to ONB product 59 that is subsequently reduced to benzisoxazole derivative 62 and lastly the thioester amine derivative 64 produced, see Scheme 10. Indeed, light leads to degradation of PEG-PolyTNB NPs surface and induced liberation of the Nile red as a hydrophobic drug pattern.
Scheme 10.
Synthesis of PEG-polyTNB NPs and its phototriggered release.
4.2.3. Metal–organic cage thioketal carriers
Recently, in a very attractive piece of work, the metal–organic cages (MOCs) were functionalized with ROS-cleavable thioketal linker by Shen et al.179 (Scheme 11). In this attempt, a ROS-responsive thioketal platform was developed to decrease some dilemmas restrictive biomedical application of MOCs. MOCs are a class of coordination-driven assembly of metallic ions and organic compounds with special hollows that are attractive for DDS180, 181, 182, 183, 184, 185, 186, 187, 188. The central cavity of MOCs provides new opportunities for controlled microenvironments with distinct shape and size that the cavity properties can be modified simply with infinite structural possibilities189. This allows that MOCs will be further extended for synergistic result in biomedical fields190, 191, 192, 193. The ultra-small size of MOCs makes possible tumor penetration; however, the fast release and negligible agglomeration at the cancer cells restrict their biomedical uses194, 195, 196, 197, 198, 199. As well as, the hydrophobicity of the MOC surfaces progresses internalization on the cancer sites leading to reduced blood circulation period and decreased biocompatibility.
Scheme 11.
Synthesis of ZnPC@polySCage and related photoinduced ROS-cleavable pathway.
To this end, a ROS-responsive thioketal platform is developed to decrease some dilemmas restrictive biomedical application of MOCs. As described, in this study 3,5-bis(pyridin-4-ylethynyl)aniline (BPA) and PEGylation dithioketal BPA (mPEG-SS-BPA) coordinate with Pd2+ to form MOCs and then it filled with photosensitizer Zinc Phthalocyanine (ZnPC) in the cavity called ZnPC@polySCage. Heteroleptic strategies were utilized to synthesis of ZnPC@polySCage and construct a cantellated tetrahedral cage using Pd(II) ions and two bent dipyridyl ligands200, 201, 202. The prepared ZnPC@polySCage can easily penetrate in the depth of cancer cell due to its nanoscale particle size (less than 10 nm). Then, the cavity-loaded ZnPC provided ROS for the degradation of PEG thioketal bond and PDT to destroy the cancer tissues. Interestingly, the PEG dissociation caused surface switching from hydrophilic to hydrophobic and automatically and simultaneous accumulation of MOCs (sizes as big as 671 nm). Therefore, the cellular uptake, tumor accumulation and the PDT efficacy were significantly enhanced under subsequent laser irradiation in the tumor cell. As illustrated in Fig. 7a. The mice mediated with ZnPC@polySCage upon exposure to light compared to only irradiation, ZnPC@polySCage without irradiation, ZnPC with irradiation, and ZnPC@polyCage with irradiation exhibited the significant anti-tumor effect that showed the phototriggered cellular sorption, drug aggregation and the PDT efficacy. Also by comparison, the in vivo safety evaluation results revealed that ZnPC@polySCage can be a prospective structure for tumor treatment with attractive biocompatibility and security. The main difference between ZnPC@polySCage and ZnPC@polyCage is that ZnPC@polyCage does not have thioketal linker in its structure that caused by the reaction of 1,3-bis(pyridin-4-ylethynyl)benzene (BPB), PEGylation BPA (mPEG-BPA) as well as Pd2+ and then filled with ZnPC according to Fig. 7b. The ZnPC@polyCage showed weaker tumor activity compared with ZnPC@polySCage due to diminished aggregation of ZnPC loaded in MOCs.
Figure 7.
(a) The photographs of the cancer tissues after the mice were sacrificed and (b) synthesis of ZnPC@polyCage. Reprinted with permission from Ref. 179. Copyright@2021 John Wiley & Sons, Inc.
4.3. Maleimide-anthracene linker
Maleimide-anthracene linkers with excellent thermal stability (up to 200 °C) have been utilized as mechanophores to explore the effects of macromolecular architecture or micellar aggregation on the duration of cycloreversion203, 204, 205, 206, 207, 208. Mechanophores, a class of stimuli-responsive compounds, have recently fascinated the interest of engineers due to their prospective applications as stress sensors209, 210, 211, 212, 213. These mechanically responsive molecules undergo fluorescent/color changes by the imposition of a mechanical stimulus such as stretch or rotation214, 215, 216, 217. More recently, Kabb et al.203 designed a crosslinker rely on the maleimide-anthracene linkage and employed in preparing exclusive networks that exhibit a fluorescence response when damaged by compressive forces. From a mechanistic point of view, the cycloreversion of adduct occurs through pressure and release of the fluorescent anthracene groups as shown in the top inset of Scheme 12. Accordingly, Cheng et al.218 recently synthesized ultra-stimuli responsive systems based on multiarmed poly(ethylene glycol)-block-poly(ε-caprolactone) (PEG-b-PCL) copolymer-linked by maleimide-anthracene as a photoresponsive segment to organize a globular phototriggered micellar NPs (maleimide-anthracene@PEG-b-PCL) in aqueous and phosphate buffered saline solution for the controlled drug release (Scheme 12).
Scheme 12.
Structure of maleimide-anthracene@PEG-b-PCL micelles, controlled drug loading and phototriggered drug release.
A part from various advantages like biodegradability and biocompatibility for clinical usages, the application of PEG-b-PCL polymeric micelles as DDS still is limited by the poor hydrolytic dissociation of the PCL segment in the aqueous surroundings. To overcome this obstacle a maleimide-anthracene linker as an ultra light-sensitive group was attached to the PEG-b-PCL polymers to generate micelles with unique responsiveness and amphiphilic characteristics. The including of the three PEG fragments in the copolymer structures significantly enhanced hydrophilicity and cause to the development of self-assembled hierarchical arrangements in water with DOX as a hydrophobic drug. As shown in Scheme 12, when DOX-loaded micelles irradiated with UV light (254 nm) for 10 s, the maleimide-anthracene linker disrupted, segments of 65, 66 produced and the drug rapidly and completely released from micellar NPs. These micellar NPs exhibited a small critical micellar concentration (below 10−5 mg/mL), potential micellar stability, improved-controlled photoresponsive property, appropriate drug-loading and ultra-sensitive light-responsive drug-release performance (for only 10 s) and could develop the protection and efficiency of chemotherapy.
4.4. Photochromic derivatives
In recent years, photochromic compounds integrated into the supramolecular systems have been extensively applied in phototriggers-based DDSs137,219, 220, 221, 222, 223, 224, 225, 226, 227. To date, a variety of photochromic derivatives such as stilbene228, 229, 230, spiropyranes231, 232, 233, 234, 235, spirooxazine236,237, dithienylethene238, 239, 240, 241, azobenzenes242, 243, 244, 245, 246 and 1,3-diazabicyclo[3.1.0]hex-3-ens, as a rather less renowned photochromic derivative247, 248, 249, 250, 251 have been developed that undergo structural changes through rotation or inversion on the original double bond, ring-opening process or the mixture of both processes in response to light irradiation. PTDDSs can be either reversible or irreversible depending on the type of the substrates that absorb the light. PTDDSs containing non-photochromic moiety such as ONB or coumarins mostly undergo an irreversible reaction, whereas PTDDSs based on photochromic molecules show reversible reactions and disordered substrate can be recovered again after removing the applied light. In addition, different light wavelengths can be used to induce light responsiveness. These items show the importance and benefits of utilizing photochromic derivatives in PTDDSs. Lately, almost all PTDDSs based on photochromic molecules are functionalized with photochromic spiropyranes (SP) or azobenzenes (Azo) that can isomerize under UV light irradiation and change their hydrophobic-hydrophilic balance.
4.4.1. Spiropyranes (SP)
SP, as a family of photochromic molecular switches has so far gained particular attention in different DDSs due to inherent biocompatibility and their multi responsiveness to endo-/exogenous stimuli60,252, 253, 254, 255, 256, 257. SP undergoes a trigger-induced reversible conversion between the non-planer, hydrophobic and bleached spiro (SP) structure, likewise, the planer, hydrophilic, zwitterionic and colored merocyanine (MC) structure by the breaking of the Cspiro‒O bond258,259. These conversions are highly sensitive to environmental conditions including surface or solvent polarity, pH, ionic strength dependence, hydrogen bonding and polar–polar interfaces. Therefore, when this molecule was incorporated into the polymers after switching they are able to stimulate a considerable alteration in the polymer properties like polymer hydrophobicity219,260,261. The majority of phototriggered DDSs expressed here are founded on induced disruption and disorder of the drug carriers that experience an untimely release before arrival at the desired site. In addition, due to the non-reversibility and probable toxicity of the residual carrier pieces it can be appropriate to enable reversible photo-induced DDSs. They work through the intermolecular interplay (work of adhesion) among polymer, drug and solvent without the disruption of the carrier and according to desorption or release of the drugs262, 263, 264. In an exciting work, Ghani et al.265 established a photo-responsive interpenetrating polymer networks (IPN) as a new PTDDS, for the first time by the notion of work of adhesion. The work of adhesion declares when, the hydrophobic SP unit in the polymer converts to the hydrophilic MC, the phototriggered drug release can be done without decay and disconnection of the PTDDS. Therefore, the release (desorption) and adsorption of the drug can be frequently changed on and off, only by changing the light on and off. In this study, supercritical carbon dioxide (scCO2) tools were employed to produce IPNs using saturating silicone elastomers (as the host polymer) and copolymers of the photochromic spiropyran methacrylate (SPMA) as the guest polymer with varying hydrophilicity. Overall, the guest polymer mixture, particularly the hydrophilicity of the guest polymer, was the main point in the work of adhesion, untimely and triggered releases. The triggered-release of five different drugs doxycycline hyclate, dopamine, levodopa, prednisone and curcumin with varying hydrophobicity was evaluated, as illustrated in Scheme 13. After illumination of IPNs upon 365 nm UV light, conversion of the monoecious hydrophobic spiropyran to the hydrophilic zwitterionic merocyanine resulted due to a C–O bond division. As a consequence, the release of the drug was done as a result of the intermolecular interplays among the drug, the guest polymer and the solvent without the disruption of the system. Further, for the first time the release of drug can be increased or halted upon illumination in the form of reversible.
Scheme 13.
Desorption and liberation of bioactive components from the photoactive guest polymer in an IPN.
4.4.2. Azobenzenes (Azo)
Azo derivatives are excellent molecular switches that show great potential application in DDSs because they can be spontaneously, efficiently and reversibly switched between linear trans and bent cis forms under UV–visible light illumination266, 267, 268, 269, 270, 271, 272, 273. This transformation generates the supramolecular azo components reversible self-assembly and dissociation during light irradiation (UV and visible)274, 275, 276, 277, 278, 279, 280, 281. Also, using hydrophobic interplays and intermolecular van der Waals interactions, trans-Azo with an almost flat structure can spontaneously penetrate to the host molecules hollow and undertake complexation, while cis-Azo is unable to perform this due to the size difference between the host and guest structure282, 283, 284, 285, 286, 287, 288, 289, 290, 291, 292, 293, 294, 295. So, cis-Azo as a dynamic component and trans-Azo as a passive component are candidates for DDS and controlled arrangements268,296, 297, 298, 299. Wang et al.300 have prepared and studied a new in vivo DDS with hypoxic-responsive Azo bridge via efficient reduction of Azo segment to aniline groups using reductases and their surroundings oxygen deficiency, causing in the hypoxia-triggered drug release and giving a synergistic chemotherapy drug DOX with PDT on the inhibition of cancer progress. In other research, Bian et al.301 designed a photo-responsive silicone in accordance with host-guest interplays between thiolated Azo and β-cyclodextrin (β-CD) intended for controlled cell adhesion of particular cells (MCF-7). The selected tumor cell capture, particular aptamer (Section 2.2, a 25 mer DNA aptamer as GCA GTT GAT CCT TTG GAT ACC CTG G), was attached to the thiol-terminated-azobenzene by chemical coupling reactions for smart capture of MCF-7 when incubating a mixture of cells. Upon UV illumination, the Azo isomerized from trans to cis photoisomer and the cis-Azo cannot be known by β-CD thus releasing the captured MCF-7 cells. These innovative results give a novel avenue for the separation and assessment of tumor cell, mainly for regulated drug release. Therefore, Azo derivatives can be suitable for pharmaceutical and biomedical science due to its responsibility to light, hypoxia and enzymes, so showing gaining increasing attention in site-specific smart cargo delivery and prodrug302, 303, 304, 305, 306. The employment of Azo derivatives for triggered prodrugs and DDSs, and application of photoswitchable azo-based prodrugs, has been previously reviewed307.
4.4.3. Dual-stimuli-responsive phototriggers
4.4.3.1. Dual-stimuli-responsive SP-triggers
In recent years, the development of dual or multiple stimuli-responsive supramolecular DDSs has attracted increasing attention to suggest a safe and more efficient controlled drug release in cancer microenvironments and with better stability in normal cells using the synergistic retort to diverse triggers and thus reduce the hurt to normal tissues308, 309, 310, 311, 312, 313. Razavi et al.314 developed an interesting amphiphilic light/temperature sensitive block copolymers 67 using a hydrophobic poly(methyl methacrylate) (PMMA) segment with spiropyran unit as a light responsive group (UV and visible light irradiation) and poly (N-isopropylacrylamide) (PNIPAM) segment as a temperature-responsive moiety for controlled DOX release (SP-PNIPAM@DOX, Scheme 14). The amphiphilic copolymers can be self-assembled to nanomicelles in acetone and water solution with a hydrophobic core PMMA and the SP units and a hydrophilic PNIPAM shell. Subsequently, the anticancer drug DOX was loaded into the polymer assemblies 68. When SP→MC isomerization was performed upon exposure to UV light (365 nm), a movement of the polar MC units to the micelles surface, together with shrinkage of the micellar assemblies induced and DOX release triggered from micelles. This process was completely reversible under visible light irradiation. Besides, the shrinkage of the micellar assemblies occurred in response to temperature increase. PNIPAM has a LCST range of 31–33 °C and the PNIPAM's LCST demonstrated light-dependence. Under UV light irradiation, the LCST of PNIPAM enhanced to 37 °C due to the formation of the polar and water-soluble MC form after isomerization. Therefore, a considerable enhance in release of DOX was done under UV light irradiation and at temperatures over the PNIPAM's LCST (T = 40 °C) in acidic media. These polymer assemblies can be more efficient and applicable for multi-responsive DDSs by light, temperature and pH315,316.
Scheme 14.
Self-assembly of SP-PNIPAM of and controlled release of DOX in different conditions.
Further study by the same group, considered a multi-responsive polymer assembly based on SP and poly(dimethylaminoethyl methacrylate) (PDMAEMA) as a multi-responsive and hydrophobic polymer317. The PDMAEMA are generally used as a multi-responsive block toward different stimulants like temperature, pH and CO2 gas318, 319, 320, 321. In this study, dynamic light scattering (DLS) consequences indicated that the dimension of polymeric assemblies altered in response to light irradiation, temperature rising (above the LCST of PDMAEMA) and also pH variations (from 5 to 9) accordingly, DOX release was controlled by light irradiation, temperature changes and pH.
4.4.3.2. Dual-stimuli-responsive Azo-triggers
Cheng et al.322 developed pH/temperature responsive adenine poly(propylene) glycol-functionalized boron nitride nanospheres (BN-APPG) for regulated drug delivery and release in answer to internal pH and temperature stimuli in tumor tissues. While the cancer microenvironment has a somewhat higher temperature and a less pH than the surrounding healthy cells, these nanospheres showed excellent anticancer effect in vitro.
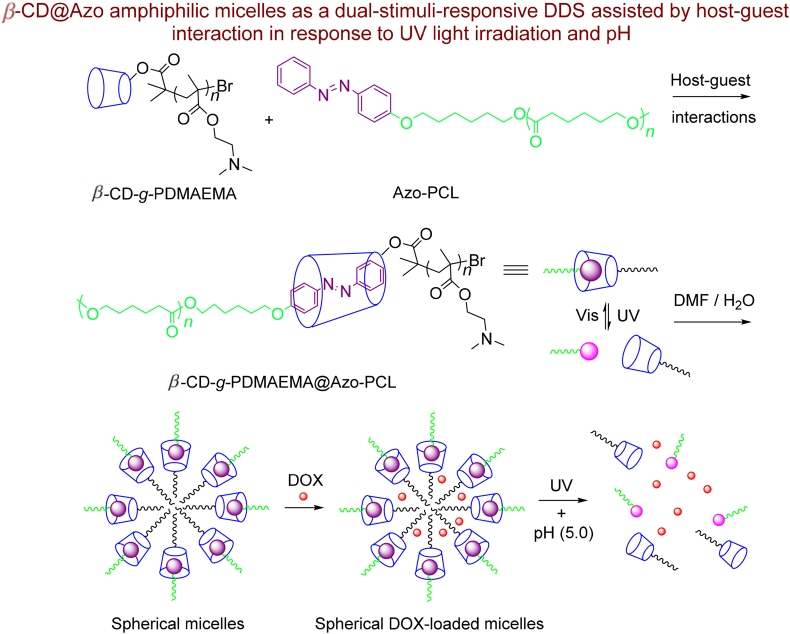
To further the development of multiple stimuli responsive DDSs with photo-response performance, Zhang et al.323 designed and synthesized an interesting dual light/pH-responsive supramolecular polymer via the host-guest interaction between β-CD-graft-poly(2-(dimethylamino)ethyl methacrylate) (β-CD-g-PDMAEMA) and Azo substituted poly(ε-caprolactone) (Azo-PCL) (Scheme 15). The supramolecular polymer β-CD-g-PDMAEMA@Azo-PCL can encapsulate DOX as spherical drug-loaded micelles and exhibits dual stimuli response to changes in light and pH. The hydrophobic PCL linkage as supramolecular micelles core is sensitive to light irradiation and the hydrophilic PDMAEMA moiety utilized as the micelle shell for response to pH changes. According to Scheme 15, Azo with trans-configuration can penetrate to the β-CD hallow during the host-guest complexation upon visible light irradiation. After UV illumination, the Azo configuration changes from the trans to cis, which cannot be recognized by the β-CD molecules. Therefore, the supramolecular micelles interrupt, causing the release of the DOX drug. Alternatively, in the acidic pH, the completely protonated tertiary amine groups in PDMAEMA moieties develop intense electrostatic repulsion interactions, so accelerating the drug release from the supramolecular micelles324.
Scheme 15.
Preparation, self-assembly and drug loaded of β-CD-g-PDMAEMA@Azo-PCL.
In addition, the electrostatic repulsion, accompanied by low protonation of Azo moieties triggers better inconstancy on the hydrophobic hallow and Azo-PCL core, therefore the host-guest complexation based on β-CD/Azo was weakened and DOX release from micelles was accelerated324. Also, under acidic medium, the re-protonation of the DOX amino functional groups enhances the DOX solubility in aqueous media and the micelle cores decay sooner, ensuing in the fast DOX release325. UV light irradiation in acidic surroundings more enhances the DOX release than a single stimulus due to synergistic effects of pH and UV light irradiation. These behaviors offer the possibility for the dual DOX-targeted and DOX-controlled release based on diverse physiological situations.
Gao et al.326 synthesized a dual light/temperature responsive supramolecular polymer brush through the host-guest complexation between β-CD structure on star-like side sequences and Azo moiety as a flexible spine, which supplies a novel platform for the preparation of self-assemblies with special sizes from unimolecular micelles, multi-molecular micelles and vesicles in water via alternating UV light irradiation and increasing temperature (Scheme 16). Polymer brushes are a unique kind of joined copolymers that are consists of a pliable unit and interlocked side chains327, 328, 329, 330, 331, 332, 333, 334, 335, 336. Upon UV light irradiation, the micelles or vesicles are disconnected due to the β-CD is divorced from the Azo backbone during photo-triggered isomerization from trans to cis conformer. Subsequently, the convened micelles or vesicles are able to accumulate into strawberry superframes via rising temperature. This report could be eventuality applied in DDSs.
Scheme 16.
Self-assemblies of unimolecular, multi-molecular micelles and vesicles through the host-guest interaction.
In the interesting work, Xiao et al.337 developed a dual stimuli-responsive azo-trigger to independent controlled release of multiple drugs. Since matrix metalloproteinases (MMPs) concentration is overexpressed in cancer tissues, they utilized it as interior stimulus for the controlled release of macromolecular drugs. UV light also used as secondary external stimulus for release of small molecule drugs. According to Scheme 17, the nanospheres were assembled by five bilayers of two particular polymer chains containing poly(acrylic acid-graft-azo-graft-proline-leucine-glycine-valine arginine-adamantane) (PAA-g-azo-g-PLGVR-AD) and poly(asparticacid-graft-β-CD) (PASP-g-β-CD) via layer-by-layer (LbL) method. The PASP-g-β-CD prepared by the ring-opening reaction of poly(l-succinimide) 81 with mono(6-(2-aminoethyl) amino-6-deoxy)-β-cyclodextrin 80 that functionalized firstly by p-toluenesulfonyl chloride and then ethylenediamine. The hydrophobic cavities of PASP-g-β-CD supplies possibilities to load guest molecules via non-covalent interactions. Also, the click reaction of alkynyl-terminated 76 with an adamantly azide derivative of 77 afford the PAA-g-azo-g-PLGVR-AD polymer. The azo-alkynyl-terminated 76 was prepared by cautiously controlling the reaction between conditions from both 69 to 71 and 73 to 76. This system can load two different drugs, macromolecular drugs were adsorbed in the hollow central cavity of nanospheres while, small molecule drugs attached by α-CD that aggregated on layers during the host–guest interaction between α-CD and Azo moiety. In this system, dextran5000-fluorescein isothiocyanate (Dex5000-FITC, with green fluorescence color) as a macromolecular model drug, α-CD-rhodamine B (α-CD-RhB, with red fluorescence color) as a small molecule model drug, and squamous cell carcinoma (SCC-7 cells) as high MMP activity were chosen. In the tumor tissues connection between AD and β-CD can be interrupt because PLGVR peptide hydrolyzed by MMPs and the macromolecular drugs regularly release from cavity. In addition, under UV irradiation, α-CD modified small molecule drugs that still bonded to PAA-g-azo polymers released from layers during photo-triggered Azo isomerization from trans to cis that this moment, the liberation of the small drugs occurs. The confocal microscopy images of SCC-7 cells confirm that the release of dual drugs can restricted by various stimulation of MMP or UV light irradiation, allowing cocktail treatment for tumor tissues.
Scheme 17.
Synthesis route of five bilayers nanospheres and schematic illustration of drugs release.
5. Dual-releasing phototriggers
Recently, dual-releasing PTDDSs have become a promising approach mainly in biomedical applications over single-releasing phototriggers due of their exceptional properties of quick and clean opening with spatio-temporal manage in the releasing two anticancer drugs and its application in combination treatment338, 339, 340, 341, 342, 343. Dual-releasing PTDDSs can be released two equivalent similar or different active molecules upon exposure to light as sequential (in order) or subsequent (irregular) according to Scheme 18. In the sequential release, the second active molecule is usually in the locked state when the first active molecule release simultaneously during light irradiation. Hence, this approach can exploit for the controlled release of two different active molecules selectively via stepwise pathways.
Scheme 18.
The schematic illustration of sequential and subsequent dual-releasing phototriggers.
5.1. Sequential dual-releasing phototriggers
5.1.1. Acetyl-nitrobenzyl (ANB) moiety
Firstly, Bochet et al.344 established a phototrigger for the sequential release of different LGs by orthogonal photolysis, although, this method requires two- or more-chromophoric structures. Since, the 4-acetyl-2-nitrobenzyl (ANB) segment is able to orthogonal photochemical release of distinct LGs, Kammari et al.345 synthesized a mono-chromophoric structure 76 during several steps345. The mono-chromophoric structure 82 design based on two well-known phototriggered molecules including ANB and phenacyl for the sequential release of two different LG in the presence of a chemical activator and UV light irradiation (<350 nm) (Scheme 19). Upon irradiation, initially, carboxylic acid or alcohol (R–OH) released essentially from ANB segment 82 which its photochemistry is firstly based on a conventional intramolecular 1,5-H shift by the nitro group 82 and formation of aci-nitro intermediate 83. Subsequently, formation of benzoxazolidines 84 and ring opening occur to release the first LG along with 2-nitrosobenzaldehyde derivative 86156,346. The second LG (R′CO2H) in the phenacyl position of 86 is photochemically released in the presence of a hydrogen atom donor like 2,2-propanol and light if required through photochemical electron transfer347. The most important restriction of this study is the necessity of using a chemical activator together with light for the second liberation phase and the sequential manage blocked if the 2,2-propanol (chemical activator) appended at first.
Scheme 19.
The structure and photochemistry of phototrigger substituted 82 in the benzylic and the phenacyl positions with LGs.
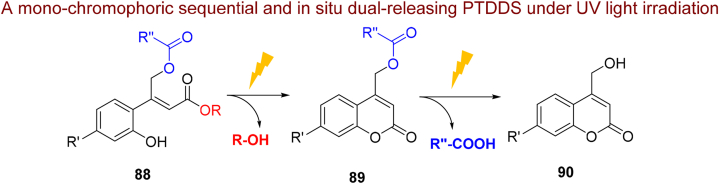
5.1.2. o-Hydroxycinnamate
In a valuable study, Paul et al.348 proposed a new approach for the sequential and in situ release of second active molecule from mono-chromophoric phototrigger 88 no need for an activator (Scheme 20). The first release (alcohol derivatives) occurs from o-hydroxycinnamate 88 as phototrigger I, due to the photoisomerisation of the double bond afterward lactonization during UV light irradiation. The second release (carboxylic acid derivatives) take places in situ simply from the generated coumarin 89 as second phototrigger, this step is located in an inactivated state throughout the first deprotecting step. The intended system also has real time monitoring capabilities owing to generation of fluorescence coumarin derivatives.
Scheme 20.
The structure and in situ liberation of phototrigger substituted 88 with different LGs.
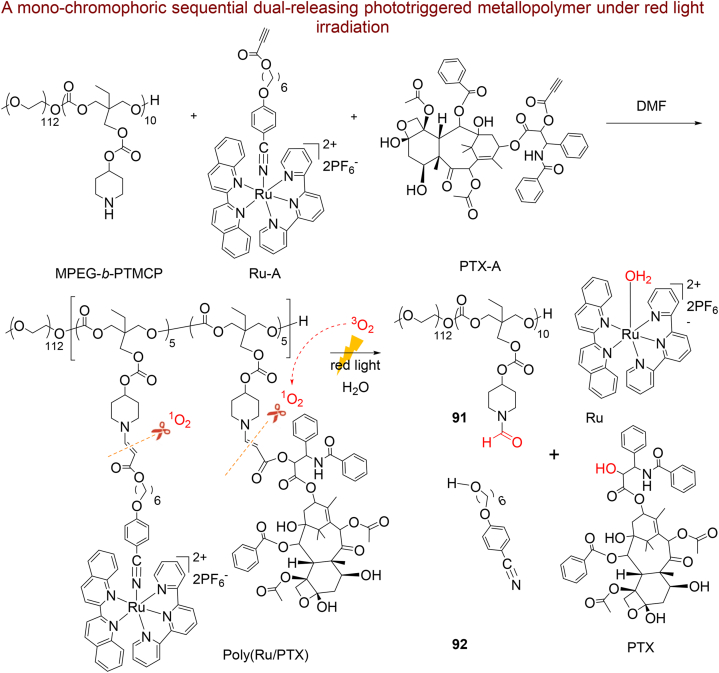
5.1.3. Metallopolymer
He et al.349 developed a sequential dual-releasing phototriggered metallopolymer (Poly@Ru/PTX) for release of photosensitizer ruthenium complex (Ru) and paclitaxel (PTX) as an anticancer drug for the first time (Scheme 21). The polymer filament includes methoxy polyethylene glycol (MPEG) and piperidine-functionalized polycarbonate (PTMCP) that Ru complexes and PTX covalently appended to the polymer backbone using a superficial amino-alkynoate click polymerization350. This type of innovative connection could let Ru and PTX to attain cancer spots concurrently via enhanced permeability and retention (EPR) effects and eschew unwanted drug release in the bloodstream. The red-light irradiation of the cancer cells released the anti-tumor Ru complexes straightforwardly and produced reactive singlet oxygen (1O2). The released 1O2 can cleave the ROS-sensitive β-aminoacrylate bond during oxidative degradation resulting to the liberation of PTX and other products 91, 92. The in vitro and in vivo investigations of poly(Ru/PTX) confirm a synergistic effects of chemotherapy PTX with PDT with exceptional cancer accumulation, cytotoxic activity (lower 32.4% viability) and high biosafety under red light irradiation.
Scheme 21.
The synthetic route of poly(Ru/PTX), cleavage of β-aminoacrylate bond and release of drugs and other products 91, 92 after oxidative degradation.
5.2. Subsequent dual-releasing phototriggers
5.2.1. Carbazole-combined to o-hydroxycinnamate
Carbazoles are of great importance among nitrogen containing heterocycles mostly due to efficient luminescence property351, various biological performances352 and easy modification and functionalization of core frame353. Accordingly, Venkatesh et al.354 designed a carbazole-combined to o-hydroxycinnamate derivative 93 for the subsequent dual release of similar and different alcohols upon one- and two-photon excitation (Scheme 22). The mechanism for the dual release upon irradiation progresses through excitation of 93 to its singlet state 94 and then a trans‒cis isomerization leading to the release of first alcohol and arrangement of the newly coumarin carbazole Cou-CBZ. The second LG also release by following a similar mechanism. The first and second release confirmed by an increase in fluorescence intensity and fluorescence color change, respectively. Since, in the Cou-CBZ internal charge transfer (ICT) take places between one carbazole moiety (as donor) and one ester-carbonyl moiety (as an acceptor) the fluorescence intensity is higher than Cou-CBZ-Cou. Furthermore, the fluorescence color change (from green to blue) is because, in Cou-CBZ-Cou, ICT does not occur. Although, the limitation of this work is the coumarin carbazole byproducts can operate as an inner filter.
Scheme 22.
The structure of dual-releasing phototrigger 93 and subsequent release of alcohols.
5.2.2. Acetyl carbazole
The same group355 synthesized a fluorescent dual-releasing phototrigger substrate (CBZ-CA-Cbl) based on acetyl carbazole chromophore with two arms to photocaging and subsequent release of both caffeic acid (CA) and Cbl simultaneously after UV light irradiation. Irradiation of CBZ-CA-Cbl causes to a singlet excited state 96 that undergoes ISC to their triplet state 97 and then cleavage of the C–O bond in carbazole continues 97 to form anion-pair intermediate 98. Solvation of the ion-pair intermediate 98, gives hydroxyacetyl 99 and released first drug. The second drug also release by related mechanism. By using the natural product CA, this dual-releasing phototrigger displayed improved anticancer effect (lower 45% viability, IC50 at 15 μmol/L) in comparison to single-releasing phototrigger CBZ-Cbl (60% viability) and even CBZ-CA (above 75% viability) (Scheme 23).
Scheme 23.
The CBZ-CA-Cbl structure and subsequent dual-release of CA and Cbl.
5.2.3. Functionalized acridines
Acridine derivatives with the planer structure are a DNA intercalator and topoisomerase II inhibitor356,357 that widely considered for their anticancer and antibacterial properties358, 359, 360, 361, 362. These results afforded the opportunity to develop acridine-based phototriggers for controlled release of drugs. Zhuang et al.363 first described C9-functionalized acridine phototrigger for the release of different alcohols under UV light irradiation. Furthermore, Jana et al.364 and Piloto et al.365 utilized the same C9-functionalized acridine phototrigger to the liberation of carboxylic acids and neurotransmitter amino acids. However, this phototrigger can liberate just one LG, limiting its application in combination treatment. Ray et al.366 designed a C4- and C5-functionalized acridine with dual arm to protection and then release of two anticancer drugs Cbl and valproic acid (Vpa) simultaneously under visible light irradiation (λ ≥ 410 nm) (Scheme 24). The photorelease pathway upon visible light irradiation is as follows: firstly, Acr-Cbl-Vpa excited to its singlet state and then undertakes a heterolytic C–O bond dissociation at the C4 or C5 benzyl substituent 101 to yield anion pair 102 that after solvation, one equivalent of Cbl or Vpa liberated. Subsequently, the attained acridine 103 once more excited to its singlet state and generates another equivalent of Cbl or Vpa. Irradiated Acr-Cbl-Vpa-incubated cells with visible light showed approximately double cytotoxicity (EC50 = 12.59 μmol/L) than free Cbl (EC50 = 20.23 μmol/L) with a synergistic efficacy of Vpa upon Cbl. This dual-releasing phototrigger also illustrated the real-time monitoring ability from green (uncleaved nano-drug) to blue (cleaved nano-drug) color change through photolysis.
5.2.4. Salicylaldazine
Biswas et al.367 have selected salicylaldazine (SDA) as the fundamental chromophore, which displays AIE and ESIPT process simultaneously. As previously mentioned (Section 3.2), the ESIPT incorporated with AIE provide further views to extend PTDDSs to drug photorelease with more efficiency in the aggregated state and dispelling the nonfluorescent character of the phototriggered molecules such as pHP groups. In this study, as displayed in Scheme 25, salicylaldazine moiety functionalized with two different drugs ferulic acid (FA) and Cbl on both sides (SDA-FA-Cbl) by simple merging of salicylaldazine to pHP. By virtue of which, it supplies the fast photorelease of two drugs sequentially inside the cell (5 min) with distinct fluorescent color change (from yellow to blue) using visible light irradiation (>410 nm) which confirmed the high efficiency of this phototriggered DDS.
Scheme 25.
The structure and photorelease of the dual PTDDS SDA-FA-Cbl.
The photochemical release of drugs from SDA-FA-Cbl initiates with AIE phenomena, ESIPT movement from pHP moiety to the imine group and then continues during photo-Favorskii rearrangement by forming the putative spirodiketone intermediate similar to what was expressed in Scheme 1. This PTDDS demonstrated a highest level of cytotoxicity towards HeLa cells (above 90%) upon irradiation compared with free Cbl due to effective biodistribution and the synergistic effect of FA and Cbl.
6. Hazard assessment and clinical studies of PTDDS methods
Despite the great attention on the design and effectiveness of PTDDS, it is important to pay attention to the side effects of this method due to the poor understanding of drug–tissue interactions and material properties368,369. In addition, it should be considered that the biomaterials and micro- or nanoparticulate formulations are not necessarily inert. Therefore, before use for clinical trials, several issues such as the assessment of formulation's biocompatibility via in vitro and in vivo sights, the safety of the all components of the PTDDS, including the drugs to be delivered locally and systemically, and mitigating tissue reaction directly via decoration and surface modification of particles with appropriate materials, should be addressed, accurately. In addition, it is still troubles to penetrate internal body parts even with IR. In current research, there are few in vivo phototargeted evaluations of PTDDSs, for example, Carling et al.159, Shen et al.179, and He et al.349 investigated the in vivo pharmacokinetic activity of prepared phototriggered structures in mice tissue. However, PDT clinically approved as an effective method to cancer treatment370, while PTT and PTDDS are still in clinical studies to treat cancer371.
7. Conclusions and future challenge
Various directions have been successfully extended for triggering the release of chemically or biologically moieties using peripheral stimuli, such as light that is a principally noteworthy stimulus. In this review, we have covered latest phototriggered molecules on DDSs together with their main mechanism for triggered drug release. The unique properties of all the mentioned PTDDSs in this review are given in Table 1. Phototrigered molecules are excellent and versatile tools for the release of various chemicals especially bioactive molecules in living tissue. Although various phototriggers have been developed, there are important challenges to be addressed for sensible demands. One of the major challenges to expand the biomedical applications of phototriggers is to increase the diversity of the phototriggers family. In continuation to our prior work248, 249, 250, 251 and despite this study, there has been an upsurge in interest in our group on design and exploitation of photochromic 1,3-diazabicyclo[3.1.0]hex-3-en derivatives to fulfill the demands for increase the variety of the phototriggers. Recently, we (Shamsipur et al.247) found that these photochromic structures are able to bind with charged molecules under sunlight. The capacity of these structures for photo-induced binding with active molecules might bring new opportunities to promote the development of innovative and new phototriggers. Furthermore, this family of photochromic derivatives is an excellent photo-responsive connection by short-term exposure to sunlight that may significantly influences their ability to form safe and ultrasensitive PTDDSs.
Table 1.
The unique properties of the foresaid PTDDSs in biomedical application.
| Entry | PTDDSs | Unique property/ies | Ref. | |
|---|---|---|---|---|
| 1 | pHP-Benz-Cbl | PTDDSs based on photochemical bond cleavage | 1. Very fast photorelease of Cbl (15 min) upon visible light with distinct fluorescence discolor | 108 |
| 2 | pHP-Naph-Cbl | 1. Two-photon absorption in the phototherapeutic window (700 nm) | 109 | |
| 2. Liberation of Cbl only in their aggregated state under visible light irradiation | ||||
| 3. High real-time monitoring ability (from greenish-yellow to blue) | ||||
| 3 | TPE-pHP-Cbl | 1. Liberation of Cbl only in their aggregated state under visible light irradiation | 124 | |
| 2. High real-time monitoring ability | ||||
| 3. PDT activity | ||||
| 4 | TPE(Cbl)4 NPs | 1. Release of 4 eq Cbl only in their aggregated state under visible light irradiation | 125 | |
| 2. High real-time monitoring ability, PDT activity | ||||
| 5 | HBT@o-hydroxycinnamate | 1. Rapid and shortest release (60 min) of methyl salicylate | 129 | |
| 2. Distinct fluorescence color change from orange to blue following photorelease | ||||
| 6 | Carbazole-coumarin derivatives 23,24 | 1. The phototrigger activates upon visible light irradiation | 144 | |
| 2. A synergic effect of phototriggered drug release and photosensitization of the carbazole-coumarin segment | ||||
| 7 | π-Extended coumarin 26-31 | 1. A two-photon uncaging sensitivity | 145 | |
| 8 | Click-and-release system based on coumarin | 1. The phototrigger activates upon the blue visible light | 146 | |
| 2. An extra level of spatial and temporal control for the release of the caged compounds | ||||
| 9 | Dex@PP52 | PTDDSs based on photoinduced disruption of nanoscale structures | 1. The phototrigger activates upon the blue visible light | 159 |
| 2. The strong visible light absorption above 500 nm | ||||
| 3. Photo-reactivity in hydrophobic surroundings | ||||
| 10 | PPID | 1. A ROS-responsive drug carrier | 177 | |
| 2. Synergistic effects of PTT/PDT and chemotherapy treatment | ||||
| 11 | PEG-polyTNB | 1. Resistant to high concentrations of ROS unlike reported thioacetal bonds and preventing non-triggered release | 178 | |
| 12 | ZnPC@polySCage | 1. Ultra-small size of ZnPC@polySCage | 179 | |
| 2. Hydrophobic ROS-responsive thioketal MOCs | ||||
| 3. PDT efficacy | ||||
| 13 | Maleimide-anthracene@PEG-b-PCL micelles | 1. An ultra light-sensitive characteristics (10 s) | 218 | |
| 2. A small critical micellar concentration (below 10−5 mg/mL) | ||||
| 3. Strong hydrolytic dissociation of the PCL segment in the aqueous surroundings | ||||
| 14 | IPN | 1. The drugs releasing without disruption of the system | 265 | |
| 2. Increasing or halting of drugs release upon illumination | ||||
| 15 | Hypoxic-responsive Azo bridge | 1. Synergistic effects of chemotherapy DOX with PDT | 300 | |
| 16 | Azo@β-CD | 1. Smart capture of MCF-7 cells in a mixture of cells under UV irradiation | 301 | |
| 17 | SP-PNIPAM@DOX | 1. A more enhance in release of DOX under UV light irradiation and at temperatures | 314 | |
| 18 | SP-PDMAEMA@DOX | 1. The DOX release by light irradiation, temperature changes and pH | 317 | |
| 19 | β-CD-g-PDMAEMA@Azo-PCL | 1. Dual stimuli response to changes in light and pH | 323 | |
| 2. The very fast release of DOX due to synergistic effects of pH and UV light irradiation | ||||
| 20 | Supramolecular polymer brush | 1. Dual stimuli response to changes in light and temperature | 326 | |
| 2. Self-assemblies of unimolecular, multi-molecular micelles and vesicles through the host-guest interaction with special sizes | ||||
| 21 | Five bilayers nanospheres | 1. Dual stimuli response to changes in light and MMP concentration | 337 | |
| 2. The ability to load two different drugs simultaneously | ||||
| 22 | Mono-chromophoric structure 82 | Dual-releasing phototriggers | 1. Sequential release of two different LG in the presence of a chemical activator and UV light irradiation | 345 |
| 23 | Phototrigger 88 | 1. The sequential release of two different LG under UV light irradiation no need for an activator | 348 | |
| 2. High real-time monitoring ability | ||||
| 24 | Poly@Ru/PTX | 1. The sequential release of two different LG under red light irradiation | 349 | |
| 2. Synergistic effects of chemotherapy PTX with PDT Ru | ||||
| 3. High biosafety under red light irradiation | ||||
| 25 | Carbazole-combined to o-hydroxycinnamate derivative 93 | 1. The subsequent dual release of similar and different LG upon one- and two-photon excitation | 354 | |
| 2. Confirmation of the first and second release by an increase in fluorescence intensity and fluorescence color change, respectively | ||||
| 26 | CBZ-CA-Cbl | 1. Fluorescent subsequent dual-releasing substrate | 355 | |
| 27 | Acr-Cbl-Vpa | 1. The sequential release of two different LG under visible light irradiation | 366 | |
| 2. High real-time monitoring ability | ||||
| 28 | SDA-FA-Cbl | 1. The sequential release of two different LG under visible light irradiation | 367 | |
| 2. Display AIE and ESIPT process simultaneously | ||||
| 3. High real-time monitoring ability | ||||
The type of light employed is another challenge to be improved for phototriggers. Especially NIR light is still worth of note due to deep penetration and safety. Although, sensitized methodology, multiphoton and UCNPs technologies are good examples of long wavelength with adequate energy to stimulate bond breaking, isomerization or rearrangement responses in triggered drug release, however these successes restricted for phototriggers due to their low efficiency values. Other criteria for the appropriate design of PTDDSs, include the following items:
-
•
Strong absorption of phototriggered molecules at wavelengths above 300 nm.
-
•
Fast responses of phototriggered molecules by a short-term light treatment.
-
•
Enhancement of their water solubility.
-
•
Biocompatibility of photo-produced byproducts.
-
•
The phototriggered intermediates or byproducts should not have absorption spectrum in the range of released bioactive components wavelengths to avoid competitive absorption.
In addition, combination of light into dual/multi stimuli-sensitive DDSs and development of single chromophoric PTDDS with simultaneous release of two drugs can lead to unprecedented and precision control over drug delivery, drug release and therapeutic efficacy. If all these requirements can be considered, the prospect of PTDDSs is very brilliant and we can hope that PTDDSs with avoiding unwanted side effects could be implemented in future studies.
Acknowledgments
The support of this work by the Razi University is gratefully acknowledged.
Author contributions
Mojtaba Shamsipur and Atefeh Ghavidast conceived the idea. Atefeh Ghavidast performed the literature search, wrote the original draft and revised the manuscript. Mojtaba Shamsipur and Afshin Pashabadi edited the manuscript. All authors read and approved the final manuscript.
Conflicts of interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Mojtaba Shamsipur, Email: mshamsipur@yahoo.com.
Atefeh Ghavidast, Email: at.ghavidast@yahoo.com.
References
- 1.Wang X., Chen X., Yang Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat Methods. 2012;9:266–269. doi: 10.1038/nmeth.1892. [DOI] [PubMed] [Google Scholar]
- 2.Fang L., Zhao Z., Wang J., Xiao P., Sun X., Ding Y., et al. Light-controllable charge-reversal nanoparticles with polyinosinic-polycytidylic acid for enhancing immunotherapy of triple negative breast cancer. Acta Pharm Sin B. 2022;12:353–363. doi: 10.1016/j.apsb.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi S., Chen Y., Nakajima S., Furuta T., Nagamune T. Light-activated gene expression from site-specific caged DNA with a biotinylated photolabile protection group. Chem Commun. 2010;46:2244–2246. doi: 10.1039/b922502a. [DOI] [PubMed] [Google Scholar]
- 4.Pinto M.N., Mascharak P.K. Light-assisted and remote delivery of carbon monoxide to malignant cells and tissues: photochemotherapy in the spotlight. J Photochem Photobiol C Photochem Rev. 2020;42:100341–100358. [Google Scholar]
- 5.Gupta S., Ahmad N., Mukhtar H. Involvement of nitric oxide during phthalocyanine (Pc4) photodynamic therapy-mediated apoptosis. Cancer Res. 1998;58:1785–1788. [PubMed] [Google Scholar]
- 6.Jori G., Spikes J.D. Photothermal sensitizers: possible use in tumor therapy. J Photochem Photobiol B Biol. 1990;6:93–101. doi: 10.1016/1011-1344(90)85078-b. [DOI] [PubMed] [Google Scholar]
- 7.Li M.H., Keller P. Stimuli-responsive polymer vesicles. Soft Matter. 2009;5:927–937. [Google Scholar]
- 8.Son S., Shin E., Kim B.S. Light-responsive micelles of spiropyran initiated hyperbranched polyglycerol for smart drug delivery. Biomacromolecules. 2014;15:628–634. doi: 10.1021/bm401670t. [DOI] [PubMed] [Google Scholar]
- 9.Jiang J., Qi B., Lepage M., Zhao Y. Polymer micelles stabilization on demand through reversible photo-cross-linking. Macromolecules. 2007;40:790–792. [Google Scholar]
- 10.Bao C., Zhu L., Lin Q., Tian H. Building biomedical materials using photochemical bond cleavage. Adv Mater. 2015;27:1647–1709. doi: 10.1002/adma.201403783. [DOI] [PubMed] [Google Scholar]
- 11.Lin F.C., Xie Y., Deng T., Zink J.I. Magnetism, ultrasound, and light-stimulated mesoporous silica nanocarriers for theranostics and beyond. J Am Chem Soc. 2021;143:6025–6036. doi: 10.1021/jacs.0c10098. [DOI] [PubMed] [Google Scholar]
- 12.Karimi M., Zangabad P.S., Baghaee-Ravari S., Ghazadeh M., Mirshekari H., Hamblin M.R. Smart nanostructures for cargo delivery: uncaging and activating by light. J Am Chem Soc. 2017;139 doi: 10.1021/jacs.6b08313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klajn R. Spiropyran-based dynamic materials. Chem Soc Rev. 2014;43:148–184. doi: 10.1039/c3cs60181a. [DOI] [PubMed] [Google Scholar]
- 14.Requena J.M. Calster academic press; Norfolk: 2012. Stress response in microbiology. [Google Scholar]
- 15.Stevens M., Merilaita S. Animal camouflage: current issues and new perspectives. Philos Trans R Soc London, Ser A B. 2009;364:423–427. doi: 10.1098/rstb.2008.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aizenberg J., Tkachenko A., Weiner S., Addadi L., Hendler G. Calcitic microlenses as part of the photoreceptor system in brittlestars. Nature. 2001;412:819–822. doi: 10.1038/35090573. [DOI] [PubMed] [Google Scholar]
- 17.Gu H., Mu S., Qiu G., Liu X., Zhang L., Yuan Y., et al. Redox-stimuli-responsive drug delivery systems with supramolecular ferrocenyl-containing polymers for controlled release. Coord Chem Rev. 2018;364:51–85. [Google Scholar]
- 18.Malachowski K., Breger J., Kwag H.R., Wang M.O., Fisher J.P., Selaru F.M., et al. Stimuli-responsive theragrippers for chemomechanical controlled release. Angew Chem Int Ed. 2014;53:8045–8049. doi: 10.1002/anie.201311047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rwei A.Y., Lee J.J., Zhan C., Liu Q., Ok M.T., Shankarappa S.A., et al. Repeatable and adjustable on-demand sciatic nerve block with phototriggerable liposomes. Proc Natl Acad Sci U S A. 2015;112:15719–15724. doi: 10.1073/pnas.1518791112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo D., Li N., Carter K.A., Lin C., Geng J., Shao S., et al. Rapid light-triggered drug release in liposomes containing small amounts of unsaturated and porphyrin-phospholipids. Small. 2016;12:3039–3047. doi: 10.1002/smll.201503966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bagheri A., Arandiyan H., Boyer C., Lim M. Lanthanide-doped upconversion nanoparticles: emerging intelligent light-activated drug delivery systems. Adv Sci. 2016;3:1500437–1500450. doi: 10.1002/advs.201500437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mura S., Nicolas J., Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 23.Allen T.M., Cullis P.R. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 24.Sun Q., Wang Z., Liu B., He F., Gai S., Yang P., et al. Recent advances on endogenous/exogenous stimuli-triggered nanoplatforms for enhanced chemodynamic therapy. Coord Chem Rev. 2022;451 [Google Scholar]
- 25.Karimi M., Ghasemi A., Sahandi Zangabad P., Rahighi R., Moosavi Basri S.M., Mirshekari H., et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev. 2016;45:1457–1501. doi: 10.1039/c5cs00798d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Lorenzo C., Concheiro A. Smart drug delivery systems: from fundamentals to the clinic. Chem Commun. 2014;50:7743–7765. doi: 10.1039/c4cc01429d. [DOI] [PubMed] [Google Scholar]
- 27.Timko B.P., Dvir T., Kohane D.S. Remotely triggerable drug delivery systems. Adv Mater. 2010;22:4925–4943. doi: 10.1002/adma.201002072. [DOI] [PubMed] [Google Scholar]
- 28.Abbas M., Zou Q., Li S., Yan X. Self-assembled peptide- and protein-based nanomaterials for antitumor photodynamic and photothermal therapy. Adv Mater. 2017;29:1605021–1605036. doi: 10.1002/adma.201605021. [DOI] [PubMed] [Google Scholar]
- 29.Bouchaala R., Anton N., Anton H., Vandamme T., Vermot J., Smail D., et al. Light-triggered release from dye-loaded fluorescent lipid nanocarriers in vitro and in vivo. Colloids Surf, B. 2017;156:414–421. doi: 10.1016/j.colsurfb.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 30.Lan G., Ni K., Lin W. Nanoscale metal-organic frameworks for phototherapy of cancer. Coord Chem Rev. 2019;379:65–81. doi: 10.1016/j.ccr.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Son J., Yi G., Yoo J., Park C., Koo H., Choi H.S. Light-responsive nanomedicine for biophotonic imaging and targeted therapy. Adv Drug Deliv Rev. 2019;138:133–147. doi: 10.1016/j.addr.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu B., Li C., Yang P., Hou Z., Lin J. 808-nm-Light-excited lanthanide-doped nanoparticles: rational design, luminescence control and theranostic applications. Adv Mater. 2017;29:1605434–1605457. doi: 10.1002/adma.201605434. [DOI] [PubMed] [Google Scholar]
- 33.Shabahang S., Kim S., Yun S.H. Light-guiding biomaterials for biomedical applications. Adv Funct Mater. 2018;28:1706635–1706651. doi: 10.1002/adfm.201706635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu L., Mou F., Gong H., Luo M., Guan J. Light-driven micro/nanomotors: from fundamentals to applications. Chem Soc Rev. 2017;46:6905–6926. doi: 10.1039/c7cs00516d. [DOI] [PubMed] [Google Scholar]
- 35.Tao Y., Chan H.F., Shi B., Li M., Leong K.W. Light: a magical tool for controlled drug delivery. Adv Funct Mater. 2020;30:2005029–2005066. doi: 10.1002/adfm.202005029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Croissant J., Zink J.I. Nanovalve-controlled cargo release activated by plasmonic heating. J Am Chem Soc. 2012;134:7628–7631. doi: 10.1021/ja301880x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S., Han M.Y. Silica-coated metal nanoparticles. Chem Asian J. 2010;5:36–45. doi: 10.1002/asia.200900228. [DOI] [PubMed] [Google Scholar]
- 38.Li H., Tan L.L., Jia P., Li Q.L., Sun Y.L., Zhang J., et al. Near-infrared light-responsive supramolecular nanovalve based on mesoporous silica-coated gold nanorods. Chem Sci. 2014;5:2804–2808. [Google Scholar]
- 39.Rosensweig R.E. Heating magnetic fluid with alternating magnetic field. J Magn Magn Mater. 2002;252:370–374. [Google Scholar]
- 40.Lin F., Zink I. Probing the local nanoscale heating mechanism of a magnetic core in mesoporous silica drug-delivery nanoparticles using fluorescence depolarization. J Am Chem Soc. 2020;142:5212–5220. doi: 10.1021/jacs.9b13082. [DOI] [PubMed] [Google Scholar]
- 41.Liu X., Zhang Y., Wang Y., Zhu W., Li G., Ma X., et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics. 2020;10:3793–3815. doi: 10.7150/thno.40805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas C.R., Ferris D.P., Lee J.H., Choi E., Cho M.H., Kim E.S., et al. Noninvasive remote-controlled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. J Am Chem Soc. 2010;132:10623–10625. doi: 10.1021/ja1022267. [DOI] [PubMed] [Google Scholar]
- 43.Boissenot T., Bordat A., Fattal E., Tsapis N. Ultrasound-triggered drug delivery for cancer treatment using drug delivery systems: from theoretical considerations to practical applications. J Contr Release. 2016;241:144–163. doi: 10.1016/j.jconrel.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 44.Lee S.F., Zhu X.M., Wang Y.X.J., Xuan S.H., You Q., Chan W.H., et al. Ultrasound, pH, and magnetically responsive crown-ether-coatedcore/shell nanoparticlesas drug encapsulation and release systems. ACS Appl Mater Interfaces. 2013;5:1566–1574. doi: 10.1021/am4004705. [DOI] [PubMed] [Google Scholar]
- 45.Santini J.T., Cima M.J., Langer R. A controlled-release microchip. Nature. 1999;397:335–338. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 46.Ni K., Luo T., Nash G.T., Lin W. Nanoscale metal-organic frameworks for cancer immunotherapy. Acc Chem Res. 2020;15:1739–1748. doi: 10.1021/acs.accounts.0c00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang G., Liu J., Wu Y., Feng L., Liu Z. Near-infrared-light responsive nanoscale drug delivery systems for cancer treatment. Coord Chem Rev. 2016;320–321:100–117. [Google Scholar]
- 48.Cheng H.B., Cui Y., Wang R., Kwon N., Yoon J. The development of light-responsive, organic dye based, supramolecular nanosystems for enhanced anticancer therapy. Coord Chem Rev. 2019;392:237–254. [Google Scholar]
- 49.Samadian H., Maleki H., Allahyari Z., Jaymand M. Natural polymers-based light-induced hydrogels: promising biomaterials for biomedical applications. Coord Chem Rev. 2020;420:213432–213461. [Google Scholar]
- 50.Marturano V., Kozlowska J., Bajek A., Giamberini M., Ambrogi V., Cerruti P., et al. Photo-triggered capsules based on lanthanide-doped upconverting nanoparticles for medical applications. Coord Chem Rev. 2019;398:213013–213016. [Google Scholar]
- 51.Thang D.C., Wang Z., Lu X., Xing B. Precise cell behaviors manipulation through light-responsive nano-regulators: recent advance and perspective. Theranostics. 2019;9:3308–3340. doi: 10.7150/thno.33888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ansari A.A., Parchur A.K., Chen G. Surface modified lanthanide upconversion nanoparticles for drug delivery, cellular uptake mechanism, and current challenges in NIR-driven therapies. Coord Chem Rev. 2022;457 [Google Scholar]
- 53.Yan C., Zhang Y., Guo Z. Recent progress on molecularly near-infrared fluorescent probes for chemotherapy and phototherapy. Coord Chem Rev. 2021;427:213556–213567. [Google Scholar]
- 54.Liu Q., Sun Y., Yang T., Feng W., Li C., Li F. Sub-10 nm hexagonal lanthanide-doped NaLuF4 upconversion nanocrystals for sensitive bioimaging in vivo. J Am Chem Soc. 2011;133:17122–17125. doi: 10.1021/ja207078s. [DOI] [PubMed] [Google Scholar]
- 55.Xie X., Gao N., Deng R., Sun Q., Xu Q.H., Liu X. Mechanistic investigation of photon upconversion in Nd3+-sensitized core-shell nanoparticles. J AmChem Soc. 2013;135:12608–12611. doi: 10.1021/ja4075002. [DOI] [PubMed] [Google Scholar]
- 56.Lin Q., Huang Q., Li C., Bao C., Liu Z., Li F., et al. Anticancer drug release from a mesoporous silica based nanophotocage regulated by either a one- or two-photon process. J Am Chem Soc. 2010;132:10645–10647. doi: 10.1021/ja103415t. [DOI] [PubMed] [Google Scholar]
- 57.Shen J., Chen G., Vu A.M., Fan W., Bilsel O.S., Chang C.C. Engineering the upconversion nanoparticle excitation wavelength: cascade sensitization of tri-doped upconversion colloidal nanoparticles at 800 nm. Adv Opt Mater. 2013;1:644–650. [Google Scholar]
- 58.Kobayashi H., Ogawa M., Alford R., Choyke P.L., Urano Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem Rev. 2009;110:2620–2640. doi: 10.1021/cr900263j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim A., Zhou J., Samaddar S., Song S.H., Elzey B.D., Thompson D.H., et al. An implantable ultrasonically-powered micro-light-source (MLight) for photodynamic therapy. Sci Rep. 2019;9:1395–1403. doi: 10.1038/s41598-019-38554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tong R., Hemmati H.D., Langer R., Kohane D.S. Photoswitchable nanoparticles for triggered tissue penetration and drug delivery. J Am Chem Soc. 2012;134:8848–8855. doi: 10.1021/ja211888a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ai1 X., Mu1 J., Xing B. Recent advances of light-mediated theranostics. Theranostics. 2016;6:2439–2457. doi: 10.7150/thno.16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Falvey D., Sundararajan S. Photoremovable protecting groups based on electron transfer chemistry. Photochem Photobiol Sci. 2004;3:831–838. doi: 10.1039/b406866a. [DOI] [PubMed] [Google Scholar]
- 63.Singh P.K., Majumdar P., Singh S.P. Advances in BODIPY photocleavable protecting groups. Coord Chem Rev. 2021;449 [Google Scholar]
- 64.Pal D.S., Kar H., Ghosh S. Phototriggered supramolecular polymerization. Chemistry. 2016;22:16872–16877. doi: 10.1002/chem.201603691. [DOI] [PubMed] [Google Scholar]
- 65.Li P., Song Y., Dong C.M. Hyperbranched polypeptides synthesized from phototriggered ROP of a photocaged Nε-[1-(2-nitrophenyl)ethoxycarbonyl]-l-lysine-N-carboxyanhydride: microstructures and effects of irradiation intensity and nitrogen flow rate. Polym Chem. 2018;9:3974–3986. [Google Scholar]
- 66.Schaal J., Dekowski B., Wiesner B., Eichhorst J., Marter K., Vargas C., et al. Coumarin-based octopamine phototriggers and their effects on an insect octopamine receptor. Chembiochem. 2012;13:1458–1464. doi: 10.1002/cbic.201200110. [DOI] [PubMed] [Google Scholar]
- 67.Bourbon P., Peng Q., Ferraudi G., Stauffacher C., Wiest O., Helquist P. Development of carbamate-tethered coumarins as phototriggers for caged nicotinamide. Bioorg Med Chem Lett. 2013;23:6321–6324. doi: 10.1016/j.bmcl.2013.09.067. [DOI] [PubMed] [Google Scholar]
- 68.Houk A.L., Givens R.S., Elles C.G. Two-photon activation of p-hydroxyphenacyl phototriggers: toward spatially controlled release of diethyl phosphate and ATP. J Phys Chem B. 2016;120:3178–3186. doi: 10.1021/acs.jpcb.5b12150. [DOI] [PubMed] [Google Scholar]
- 69.Ma C., Kwok W.M., Chan W.S., Du Y., Kan J.T.W., Toy P.H., et al. Ultrafast time-resolved transient absorption and resonance raman spectroscopy study of the photodeprotection and rearrangement reactions of p-hydroxyphenacyl caged phosphates. J Am Chem Soc. 2006;128:2558–2570. doi: 10.1021/ja0532032. [DOI] [PubMed] [Google Scholar]
- 70.Wijtmans M., Rosenthal S.J., Zwanenburg B., Porter N.A. Visible light excitation of CdSe nanocrystals triggers the release of coumarin from cinnamate surface ligands. J Am Chem Soc. 2006;128:11720–11726. doi: 10.1021/ja063562c. [DOI] [PubMed] [Google Scholar]
- 71.Duan X.Y., Zhai B.C., Song Q.H. Water-soluble o-hydroxycinnamate as an efficient photoremovable protecting group of alcohols with fluorescence reporting. Photochem Photobiol Sci. 2012;11:593–598. doi: 10.1039/c2pp05309h. [DOI] [PubMed] [Google Scholar]
- 72.Rajesh C.S., Givens R.S., Wirz J. Kinetics and mechanism of phosphate photorelease from benzoin diethyl phosphate: evidence for adiabatic fission to an α-keto cation in the triplet state. J Am Chem Soc. 2000;122:611–618. [Google Scholar]
- 73.Dai X.J., Yu Y.Q., Liu K.H., Su H.M. Photochemical reaction of benzoin caged compound: time-resolved fourier transform infrared spectroscopy study. Chin J Chem Phys. 2016;29:91–98. [Google Scholar]
- 74.McKay L.J., Carling C., Branda N.R. Improved polyaromatic benzoin photoremovable protecting groups. J Photochem Photobiol, A. 2021;421:113530–113535. [Google Scholar]
- 75.Corrie J.E.T., Furuta T., Givens R.S., Yousef A.L., Goeldner M. In: Dynamic studies in biology: phototriggers, photoswitches and caged biomolecules. Goeldner M., Givens R.S., editors. Wiley; 2005. Photoremovable protecting groups used for the caging of biomolecules; pp. 1–94. [Google Scholar]
- 76.Mayer G., Heckel A. Biologically active molecules with a “light switch”. Angew Chem Int Ed Engl. 2006;45:4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J., Zou Q., Tian H. Photochromic materials: more than meets the eye. Adv Mater. 2013;25:378–399. doi: 10.1002/adma.201201521. [DOI] [PubMed] [Google Scholar]
- 78.Zhu Y., Pavlos C.M., Toscano J.P., Dore T.M. 8-Bromo-7-hydroxyquinoline as a photoremovable protecting group for physiological use: mechanism and scope. J Am Chem Soc. 2006;128:4267–4276. doi: 10.1021/ja0555320. [DOI] [PubMed] [Google Scholar]
- 79.Huang J., Muliawan A.P., Ma J., Li M.D., Chiu H.K., Lan X., et al. A spectroscopic study of the excited state proton transfer processes of (8-bromo-7-hydroxyquinolin-2-yl)methyl-protected phenol in aqueous solutions. Photochem Photobiol Sci. 2017;16:575–584. doi: 10.1039/c6pp00377j. [DOI] [PubMed] [Google Scholar]
- 80.Wang D.D., Ge C.W., Wu G.A., Li Z.P., Wang J.Z., Zhang T.F., et al. A sensitive red light nano-photodetector propelled by plasmonic copper nanoparticles. J Mater Chem C. 2017;5:1328–1335. [Google Scholar]
- 81.Ge D., Issa A., Jradi S., Couteau C., Marguet S., Bachelot R. Advanced hybrid plasmonic nano-emitters using smart photopolymer. Photon Res. 2022;10:1552–1566. [Google Scholar]
- 82.Roco M.C., Bainbridge W.S. Kluwer Academic Publishers; 2003. Converging technologies for improving human performance: nanotechnology, biotechnology, information technology and cognitive science. [Google Scholar]
- 83.Shamsipur M., Barati A., Nematifar Z. Fluorescent pH nanosensors: design strategies and applications. J Photochem Photobiol C Photochem Rev. 2019;39:76–141. [Google Scholar]
- 84.Shamsipur M., Safavi A., Mohammadpour Z. Indirect colorimetric detection of glutathione based on its radical restoration ability using carbon nanodots as nanozymes. Sensor Actuator B Chem. 2014;199:463–469. [Google Scholar]
- 85.Rostami E., Kashanian S., Azandaryani A.H., Faramarzi H., Dolatabadi J.E.N., Omidfar K. Drug targeting using solid lipid nanoparticles. Chem Phys Lipids. 2014;181:56–61. doi: 10.1016/j.chemphyslip.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 86.Galangau O., Delbaere S., Ratel-Ramond N., Rapenne G., Li R., Calupitan J.P.D.C., et al. Dual photochemical bond cleavage for a diarylethene-based phototrigger containing both methanolic and acetic sources. J Org Chem. 2016;81:11282–11290. doi: 10.1021/acs.joc.6b02256. [DOI] [PubMed] [Google Scholar]
- 87.Li P., Liu K., Ye J., Xue F., Cheng Y., Lyu Z., et al. Facilitating the C–C bond cleavage on sub-10 nm concavity-tunable Rh@Pt core-shell nanocubes for efficient ethanol electrooxidation. J Mater Chem. 2019;7:17987–17994. [Google Scholar]
- 88.Kohman R.E., Cha S.S., Man H.Y., Han X. Light-triggered release of bioactive molecules from DNA nanostructures. Nano Lett. 2016;16:2781–2785. doi: 10.1021/acs.nanolett.6b00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan W., Bu W., Zhang Z., Shen B., Zhang H., He Q., et al. X-ray radiation-controlled NO-release for on-demand depth-independent hypoxic radiosensitization. Angew Chem Int Ed Engl. 2015;127:14232–14236. doi: 10.1002/anie.201504536. [DOI] [PubMed] [Google Scholar]
- 90.Butcher D.P., Rachford A.A., Petersen J.L., Rack J.J. Phototriggered S→O isomerization of a ruthenium-bound chelating sulfoxide. Inorg Chem. 2006;45:9178–9180. doi: 10.1021/ic061611g. [DOI] [PubMed] [Google Scholar]
- 91.Vittardi S.B., Magar R.T., Breen D.J., Rack J.J. A future perspective on phototriggered isomerizations of transition metal sulfoxides and related complexes. J Am Chem Soc. 2021;143:526–537. doi: 10.1021/jacs.0c08820. [DOI] [PubMed] [Google Scholar]
- 92.Olejniczak J., Carling C.J., Almutairi A. Photocontrolled release using one-photon absorption of visible or NIR light. J Contr Release. 2015;219:18–30. doi: 10.1016/j.jconrel.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 93.Biswas S., Rajesh Y., Barman S., Bera M., Paul A., Mandal M., et al. A dual-analyte probe: hypoxia activated nitric oxide detection with phototriggered drug release ability. Chem Commun. 2018;54:7940–7943. doi: 10.1039/c8cc01854e. [DOI] [PubMed] [Google Scholar]
- 94.Rogach A.L., Franzl T., Klar T.A., Feldmann J., Gaponik N., Lesnyak V., et al. Aqueous synthesis of thiol-capped CdTe nanocrystals: state-of-the-art. J Phys Chem C. 2007;111:14628–14637. [Google Scholar]
- 95.Xue Y., Liu D., Wang C., Bao C., Wang X., Zhu H., et al. Photo and reduction dual-responsive hydrogel for regulating cell adhesion and cell sheet harvest. ACS Appl Bio Mater. 2020;3:2410–2418. doi: 10.1021/acsabm.0c00139. [DOI] [PubMed] [Google Scholar]
- 96.Ruggiero E., Hernández-Gil J., Mareque-Rivas J.C., Salassa L. Near infrared activation of an anticancer PtIV complex by Tm-doped upconversion nanoparticles. Chem Commun. 2015;5:2091–2094. doi: 10.1039/c4cc07960d. [DOI] [PubMed] [Google Scholar]
- 97.Wang Z., Yu L., Lv C., Wang P., Chen Y., Tang X. Photoresponsive cross-linked polymeric particles for phototriggered burst release. Photochem Photobiol. 2013;89:552–559. doi: 10.1111/php.12038. [DOI] [PubMed] [Google Scholar]
- 98.Cheawchan S., Sogawa H., Takata T. Phototriggered crosslinking and surface modification via catalyst-free functionalization of a new orthogonal agent containing nitrile N-oxide and o-nitrobenzyl ether moieties. Macromol Chem Phys. 2021;222 [Google Scholar]
- 99.Tang S., Cannon J., Yang K., Krummel M.F., Choi S.K. Spacer-mediated control of coumarin uncaging for photocaged thymidine. J Org Chem. 2020;85:2945–2955. doi: 10.1021/acs.joc.9b02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mahmoodi M.M., Abate-Pella D., Pundsack T.J., Palsuledesai C.C., Goff P.C., Blank D.A., Distefano M.D. Nitrodibenzofuran: a one- and two-photon sensitive protecting group that is superior to brominated hydroxycoumarin for thiol caging in peptides. J Am Chem Soc. 2016;138:5848–5859. doi: 10.1021/jacs.5b11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jana A., Atta S., Sarkar S.K., Singh N.D.P. 1-Acetylpyrene with dual functions as an environment-sensitive fluorophore and fluorescent photoremovable protecting group. Tetrahedron. 2010;66:9798–9807. [Google Scholar]
- 102.Karthik S., Jana A., Selvakumar M., Venkatesh Y., Paul A., Shah S.S., et al. Coumarin polycaprolactone polymeric nanoparticles: light and tumor microenvironment activated cocktail drug delivery. J Mater Chem B. 2017;5:1734–1741. doi: 10.1039/c6tb02944b. [DOI] [PubMed] [Google Scholar]
- 103.Gallo R.D.C., Duarte M., da Silva A.F., Okada C.Y., Jr., Deflon V.M., Jurberg I.D. A selective C–C bond cleavage strategy promoted by visible light. Org Lett. 2021;23:8916–8920. doi: 10.1021/acs.orglett.1c03406. [DOI] [PubMed] [Google Scholar]
- 104.Givens R.S., Stensrud K., Conrad P.G., Yousef A.L., Perera C., Senadheera S.N., et al. p-Hydroxyphenacyl photoremovable protecting groups-robust photochemistry despite substituent diversity. Can J Chem. 2011;89:364–384. doi: 10.1139/V10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Givens R.S., Rubina M., Wirz J. Applications of p-hydroxyphenacyl (pHP) and coumarin-4-ylmethyl photoremovable protecting groups. Photochem Photobiol Sci. 2012;11:472–488. doi: 10.1039/c2pp05399c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Givens R.S., Weber J.F.W., Conrad P.G., Orosz G., Donahue S.L., Thayer S.A. New phototriggers 9: p-hydroxylphenacyl as a C-terminal photoremovable protecting group for oligopeptides. J Am Chem Soc. 2000;122:2687–2697. [Google Scholar]
- 107.Givens R.S., Park C.H. p-Hydroxyphenacyl ATP1: a new phototrigger. Tetrahedron Lett. 1996;37:6259–6262. [Google Scholar]
- 108.Barman S., Mukhopadhyay S.K., Biswas S., Nandi S., Gangopadhyay M., Dey S., et al. A p-hydroxyphenacyl-benzothiazole-chlorambucil conjugate as a real-time-monitoring drug-delivery system assisted by excited-state intramolecular proton transfer. Angew Chem Int Ed. 2016;55:4194–4198. doi: 10.1002/anie.201508901. [DOI] [PubMed] [Google Scholar]
- 109.Singh A.K., Kundu M., Roy S., Roy B., Shah S.S., Nair A.V., et al. Two-photon responsive napthyl tagged p-hydroxyphenacyl based drug delivery system: uncaging of anti-cancer drug in the phototherapeutic window with real-time monitoring. Chem Commun. 2020;56:9986–9989. doi: 10.1039/d0cc01903h. [DOI] [PubMed] [Google Scholar]
- 110.Wang H., Liu G. Advances in luminescent materials with aggregation-induced emission (AIE) properties for biomedical applications. J Mater Chem B. 2018;6:4029–4042. doi: 10.1039/c8tb00674a. [DOI] [PubMed] [Google Scholar]
- 111.Hu F., Xu S., Liu B. Photosensitizers with aggregation-induced emission: materials and biomedical applications. Adv Mater. 2018;30:1801350–1801378. doi: 10.1002/adma.201801350. [DOI] [PubMed] [Google Scholar]
- 112.Liang J., Tang B.Z., Liu B. Specific light-up bioprobes based on AIEgen conjugates. Chem Soc Rev. 2015;44:2798–2811. doi: 10.1039/c4cs00444b. [DOI] [PubMed] [Google Scholar]
- 113.Denk W., Strickler J.H., Webb W.W. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 114.Adams S.R., Tsien R.Y. Controlling cell chemistry with caged compounds. Annu Rev Physiol. 1993;55:755–784. doi: 10.1146/annurev.ph.55.030193.003543. [DOI] [PubMed] [Google Scholar]
- 115.Bort G., Gallavardin T., Ogden D., Dalko P.I. From one-photon to two-photon probes: “caged” compounds, actuators, and photoswitches. Angew Chem Int Ed. 2013;52:4526–4537. doi: 10.1002/anie.201204203. [DOI] [PubMed] [Google Scholar]
- 116.Abe M., Chitose Y., Jakkampudi S., Thuy P.T.T., Lin Q., Van B.T., et al. Design and synthesis of two-photon responsive chromophores for near-infrared light-induced uncaging reactions. Synthesis. 2017;49:3337–3346. [Google Scholar]
- 117.Chitose Y., Abe M. Design and synthesis of two-photon responsive chromophores for application to uncaging reactions. Photochemistry. 2018;46 [Google Scholar]
- 118.Jakkampudi S., Abe M. Caged compounds for two-photon uncaging. Module Chem Mol Sci Chem Eng. 2018;1:1–11. [Google Scholar]
- 119.Klausen M., Blanchard-Desce M. Two-photon uncaging of bioactive compounds: starter guide to an efficient IR light switch. J Photochem Photobiol C Photochem Rev. 2021;48 [Google Scholar]
- 120.Alifu N., Dong X., Li D., Sun X., Zebibula A., Zhang D., et al. Aggregation-induced emission nanoparticles as photosensitizer for two-photon photodynamic therapy. Mater Chem Front. 2017;1:1746–1753. [Google Scholar]
- 121.Zhuang W., Xu Y., Li G., Hu J., Ma B., Yu T., et al. Redox and pH dual-responsive polymeric micelles with aggregation-induced emission feature for cellular imaging and chemotherapy. ACS Appl Mater Interfaces. 2018;10:18489–18498. doi: 10.1021/acsami.8b02890. [DOI] [PubMed] [Google Scholar]
- 122.Feng H.T., Yuan Y.X., Xiong J.B., Zheng Y.S., Tang B.Z. Macrocycles and cages based on tetraphenylethylene with aggregation-induced emission effect. Chem Soc Rev. 2018;47:7452–7476. doi: 10.1039/c8cs00444g. [DOI] [PubMed] [Google Scholar]
- 123.Jana A., Nguyen K.T., Li X., Zhu P., Tan N.S., Agren H., et al. Perylene-derived single-component organic nanoparticles with tunable emission: efficient anticancer drug carriers with real-time monitoring of drug release. ACS Nano. 2014;8:5939–5952. doi: 10.1021/nn501073x. [DOI] [PubMed] [Google Scholar]
- 124.Parthiban C., Pavithra M., Reddy V.K.L., Sen D., Samuel M.S., Singh N.D.P. Visible light triggered fluorescent organic nanoparticles for chemo-photodynamic therapy with real time cellular imaging. ACS Appl Nano Mater. 2018;1:6281–6288. [Google Scholar]
- 125.Parthiban C., Pavithra M., Reddy L.V.K., Sen D., Singh N.D.P. Single-component fluorescent organic nanoparticles with four-armed phototriggers for chemo-photodynamic therapy and cellular imaging. ACS Appl Nano Mater. 2019;2:3728–3734. [Google Scholar]
- 126.Parthiban C., Pavithra M., Reddy L.V.K., Sen D., Samuel S.M., Singh N.D.P. Tetraphenylethylene conjugated p-hydroxyphenacyl: fluorescent organic nanoparticles for the release of hydrogen sulfide under visible light with real-time cellular imaging. Org Biomol Chem. 2018;16:7903–7909. doi: 10.1039/c8ob01629a. [DOI] [PubMed] [Google Scholar]
- 127.Turner A.D., Pizzo S.V., Rozakis G., Porter N.A. Photoreactivation of irreversibly inhibited serine proteinases. J Am Chem Soc. 1988;110:244–250. [Google Scholar]
- 128.Li H., Yang J., Porter N.A. Preparation and photochemistry of o-aminocinnamates. J Photochem Photobiol, A. 2005;169:289–297. [Google Scholar]
- 129.Paul A., Mengji R., Chandy O.A., Nandi S., Bera M., Jana A., et al. ESIPT-induced fluorescent o-hydroxycinnamate: a self-monitoring phototrigger for prompt image-guided uncaging of alcohols. Org Biomol Chem. 2017;17:8544–8855. doi: 10.1039/c7ob02280h. [DOI] [PubMed] [Google Scholar]
- 130.Abdallah M., Hijazi A., Dumur F., Lalevée J. Coumarins as powerful photosensitizers for the cationic polymerization of epoxy-silicones under near-UV and visible light and applications for 3D printing technology. Molecules. 2020;25:2063–2075. doi: 10.3390/molecules25092063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ji P.W., Li N., Chen D., Qi X., Sha W., Jiao Y., et al. Coumarin-containing photo-responsive nanocomposites for NIR light-triggered controlled drug release via a two-photon process. J Mater Chem B. 2013;1:5942–5949. doi: 10.1039/c3tb21206h. [DOI] [PubMed] [Google Scholar]
- 132.Huang Q., Bao C., Ji W., Wang Q., Zhu L. Photocleavable coumarin crosslinkers based polystyrene microgels: phototriggered swelling and release. J Mater Chem. 2012;22:18275–18282. [Google Scholar]
- 133.Trenor S.R., Shultz A.R., Love B.J., Long T.E. Coumarins in polymers: from light harvesting to photo-cross-linkable tissue scaffolds. Chem Rev. 2004;104:3059–3078. doi: 10.1021/cr030037c. [DOI] [PubMed] [Google Scholar]
- 134.Maddipatla M.V.S.N., Wehrung D., Tang C., Fan W., Oyewumi M.O., Miyoshi T., et al. Photoresponsive coumarin polyesters that exhibit cross-linking and chain scission properties. Macromolecules. 2013;46:5133–5140. [Google Scholar]
- 135.Lin Q., Bao C., Cheng S., Yang Y., Ji W., Zhu L. Target-activated coumarin phototriggers specifically switch on fluorescence and photocleavage upon bonding to thiol-bearing protein. J Am Chem Soc. 2012;134:5052–5055. doi: 10.1021/ja300475k. [DOI] [PubMed] [Google Scholar]
- 136.Lin Q., Bao C., Yang Y., Liang Q., Zhang D., Cheng S., et al. Highly discriminating photorelease of anticancer drugs based on hypoxia activatable phototrigger conjugated chitosan nanoparticles. Adv Mater. 2013;25:1981–1986. doi: 10.1002/adma.201204455. [DOI] [PubMed] [Google Scholar]
- 137.Beauté L., McClenaghan N., Lecommandoux S. Photo-triggered polymer nanomedicines: from molecular mechanisms to therapeutic applications. Adv Drug Deliv Rev. 2019;138:148–166. doi: 10.1016/j.addr.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 138.Olson J.P., Kwon H.B., Takasaki K.T., Chiu C.Q., Higley M.J., Sabatini B.L., et al. Optically selective two-photon uncaging of glutamate at 900 nm. J Am Chem Soc. 2013;135:5954–5957. doi: 10.1021/ja4019379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fournier L., Aujard I., LeSaux T., Maurin S., Beaupierre S., Baudin J.B., et al. Coumarinyl methyl caging groups with redshifted absorption. Chem Eur J. 2013;19:17494–17507. doi: 10.1002/chem.201302630. [DOI] [PubMed] [Google Scholar]
- 140.Chitose Y., Abe M., Furukawa K., Katan C. Design, synthesis, and reaction of -extended coumarin-based new caged compounds with two-photon absorption character in the near-IR region. Chem Lett. 2016;45:1186–1188. [Google Scholar]
- 141.Schiedel M.S., Briehn C.A., Bauerle P. Single-compound libraries of organic materials: parallel synthesis and screening of fluorescent dyes. Angew Chem Int Ed. 2001;40:4677–4680. doi: 10.1002/1521-3773(20011217)40:24<4677::aid-anie4677>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 142.Yu J., Shirota Y. A new class of high-performance red-fluorescent dyes for organic electroluminescent devices, [7-diethylamino-3-(2-thienyl)chromen-2-ylidene]-2,2-dicyanovinylamineand{10-(2-thienyl)-2,3,6,7-tetrahydro-1H,5H-chromeno[8,7,6-ij]quinolizin-11-ylidene}-2,2-dicyanovinylamine. Chem Lett. 2002;31:984–985. [Google Scholar]
- 143.Gandioso A., Contreras S., Melnyk I., Oliva J., Nonell S., Velasco D., et al. Development of green/red-absorbing chromophores based on a coumarin scaffold that are useful as caging groups. J Org Chem. 2017;82:5398–5408. doi: 10.1021/acs.joc.7b00788. [DOI] [PubMed] [Google Scholar]
- 144.Wang B.Y., Lin Y.C., Lai Y.T., Ou J.Y., Chang W.W., Chu C.C. Targeted photoresponsive carbazole-coumarin and drug conjugates for efficient combination therapy in leukemia cancer cells. Bioorg Chem. 2020;100:103904–103911. doi: 10.1016/j.bioorg.2020.103904. [DOI] [PubMed] [Google Scholar]
- 145.Klausen M., Dubois V., Clermont G., Tonnel´e C., Castet F., Blanchard-Desce M. Dual-wavelength efficient two-photon photorelease of glycine by p-extended dipolar coumarins. Chem Sci. 2019;10:4209–4219. doi: 10.1039/c9sc00148d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bojtar M., Nemeth K., Domahidy F., Knorr G., Verkman A., Kallay M., et al. Conditionally activatable visible-light photocages. J Am Chem Soc. 2020;142:15164–15171. doi: 10.1021/jacs.0c07508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim E., Koo H. Biomedical applications of copper-free click chemistry: in vitro, in vivo, and ex vivo. Chem Sci. 2019;10:7835–7851. doi: 10.1039/c9sc03368h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kozma E., Girona G.E., Paci G., Lemke E.A., Kele P. Bioorthogonal double-fluorogenic siliconrhodamine probes for intracellular superresolution microscopy. Chem Commun. 2017;53:6696–6699. doi: 10.1039/c7cc02212c. [DOI] [PubMed] [Google Scholar]
- 149.Kormos A., Kern D., Egyed A., Soveges B., Nemeth K., Kele P. Microscope laser assisted photooxidative activation of bioorthogonal clickox probes. Chem Commun. 2020;56:5425–5428. doi: 10.1039/d0cc01512a. [DOI] [PubMed] [Google Scholar]
- 150.Knorr G., Kozma E., Schaart J.M., Nemeth K., Torok G., Kele P. Bioorthogonally applicable fluorogenic cyanine-tetrazines for no-wash super-resolution imaging. Bioconjugate Chem. 2018;29:1312–1318. doi: 10.1021/acs.bioconjchem.8b00061. [DOI] [PubMed] [Google Scholar]
- 151.Versteegen R.M., tenHoeve W., Rossin R., deGeus M.A.R., Janssen H.M., Robillard M.S. Click-to-release from trans-cyclooctenes: mechanistic insights and expansion of scope from established carbamate to remarkable ether cleavage. Angew Chem Int Ed. 2018;57:10494–10499. doi: 10.1002/anie.201800402. [DOI] [PubMed] [Google Scholar]
- 152.Werther P., Yserentant K., Braun F., Kaltwasser N., Popp C., Baalmann M., et al. Live-cell localization microscopy with a fluorogenic and self-blinking tetrazine probe. Angew Chem Int Ed. 2020;59:804–810. doi: 10.1002/anie.201906806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wçll D., Smirnova J., Galetskaya M., Prykota T., Buhler J., Stengele K.P., et al. Intramolecular sensitization of photocleavage of the photolabile 2-(2-nitrophenyl)propoxycarbonyl (NPPOC) protecting group: photoproducts and photokinetics of the release of nucleosides. Chem Eur J. 2008;14:6490–6497. doi: 10.1002/chem.200800613. [DOI] [PubMed] [Google Scholar]
- 154.Mbatia H.W., Bandara H.M.D., Burdette S.C. Cupro Cleav-1, a first generation photocage for Cu+ Chem Commun. 2012;48:5331–5333. doi: 10.1039/c2cc31281f. [DOI] [PubMed] [Google Scholar]
- 155.Donato L., Mourot A., Davenport C.M., Herbivo C., Warther D., Leonard J., et al. Water-soluble, donor-acceptor biphenyl derivatives in the 2-(o-nitrophenyl)propyl series: highly efficient two-photon uncaging of the neurotransmitter γ-aminobutyric acid at λ = 800 nm. Angew Chem Int Ed. 2012;51:1840–1843. doi: 10.1002/anie.201106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Walbert S., Pfleiderer W., Steiner U.E. Photolabile protecting groups for nucleosides: mechanistic studies of the 2-(2-nitrophenyl)ethyl group. Helv Chim Acta. 2001;84:1601–1611. [Google Scholar]
- 157.Wang X., Yang Y., Liu C., Guo H., Chen Z., Xia J., et al. Photo- and pH-responsive drug delivery nanocomposite based on o-nitrobenzyl functionalized upconversion nanoparticles. Polymer. 2021;229:123961–123969. [Google Scholar]
- 158.Monteiro D.C.F., Amoah E., Rogers C., Pearson A.R. Using photocaging for fast time-resolved structural biology studies. Acta Crystallogr D Struct Biol. 2021;77:1218–1232. doi: 10.1107/S2059798321008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carling C.J., Viger M.L., Huu V.A.N., Garcia A.V., Almutairi A. In vivo visible light-triggered drug release from an implanted depot. Chem Sci. 2015;6:335–341. doi: 10.1039/c4sc02651a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Olejniczak J., Sankaranarayanan J., Viger M.L., Almutairi A. Highest efficiency two-photon degradable copolymer for remote controlled release. ACS Macro Lett. 2013;2:683–687. doi: 10.1021/mz400256x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sun S., Chamsaz E.A., Joy A. Photoinduced polymer chain scission of alkoxyphenacyl based polycarbonates. ACS Macro Lett. 2012;1:1184–1188. doi: 10.1021/mz3002947. [DOI] [PubMed] [Google Scholar]
- 162.Yuan X., Wang B., Yang L., Zhang Y. The role of ROS-induced autophagy in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2018;42:306–312. doi: 10.1016/j.clinre.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 163.Qi S., Guo L., Yan S., Lee R.J., Yu S., Chen S. Hypocrellin A-based photodynamic action induces apoptosis in A549 cells through ROS-mediated mitochondrial signaling pathway. Acta Pharm Sin B. 2019;9:279–293. doi: 10.1016/j.apsb.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liou G.Y., Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gong H., Chao Y., Xiang J., Han X., Song G., Feng L., et al. Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy. Nano Lett. 2016;16:2512–2521. doi: 10.1021/acs.nanolett.6b00068. [DOI] [PubMed] [Google Scholar]
- 166.Liu B., Li C., Cheng Z., Hou Z., Huang S., Lin J. Functional nanomaterials for near-infrared-triggered cancer therapy. Biomater Sci. 2016;4:890–909. doi: 10.1039/c6bm00076b. [DOI] [PubMed] [Google Scholar]
- 167.Yue C., Zhang C., Alfranca G., Yang Y., Jiang X., Yang Y., et al. Near-infrared light triggered ROS-activated theranostic platform based on Ce6-CPT-UCNPs for simultaneous fluorescence imaging and chemo-photodynamic combined therapy. Theranostics. 2016;6:456–469. doi: 10.7150/thno.14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tapeinos C., Pandit A. Physical, chemical, and biological structures based on ROS-sensitive moieties that are able to respond to oxidative microenvironments. Adv Mater. 2016;28:5553–5585. doi: 10.1002/adma.201505376. [DOI] [PubMed] [Google Scholar]
- 169.Nguyen V.N., Ha J., Cho M., Li H., Swamy K.M.K., Yoon J. Recent developments of BODIPY-based colorimetric and fluorescent probes for the detection of reactive oxygen/nitrogen species and cancer diagnosis. Coord Chem Rev. 2021;439:213936–213953. [Google Scholar]
- 170.Shi S., Zhang L., Zhu M., Wan G., Li C., Zhang J., et al. Reactive oxygen species-responsive nanoparticles based on peglated prodrug for targeted treatment of oral tongue squamous cell carcinoma by combining photodynamic therapy and chemotherapy. ACS Appl Mater Interfaces. 2018;10:29260–29272. doi: 10.1021/acsami.8b08269. [DOI] [PubMed] [Google Scholar]
- 171.Saravanakumar G., Kim J., Kim W.J. Reactive-oxygen-species-responsive drug delivery systems: promises and challenges. Adv Sci. 2017;4:1600124–1600143. doi: 10.1002/advs.201600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jin H., Zhu T., Huang X., Sun M., Li H., Zhu X., et al. ROS-responsive nanoparticles based on amphiphilic hyperbranched polyphosphoester for drug delivery: light-triggered size-reducing and enhanced tumor penetration. Biomaterials. 2019;211:68–80. doi: 10.1016/j.biomaterials.2019.04.029. [DOI] [PubMed] [Google Scholar]
- 173.Rapp T.L., DeForest C.A. Targeting drug delivery with light: a highly focused approach. Adv Drug Deliv Rev. 2021;171:94–107. doi: 10.1016/j.addr.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kuncewicz J., Dąbrowski J.M., Kyzioł A., Brindell M., Łabuz P., Mazuryk O., et al. Perspectives of molecular and nanostructured systems with d- and f-block metals in photogeneration of reactive oxygen species for medical strategies. Coord Chem Rev. 2019;398:113012–113043. [Google Scholar]
- 175.Detty M.R., Gibson S.L., Wagner S.J. Current clinical and preclinical photosensitizers for use in photodynamic therapy. J Med Chem. 2004;47:3897–3915. doi: 10.1021/jm040074b. [DOI] [PubMed] [Google Scholar]
- 176.Konan Y.N., Gurny R., Allémann E. State of the art in the delivery of photosensitizers for photodynamic therapy. J Photochem Photobiol B Biol. 2002;66:89–106. doi: 10.1016/s1011-1344(01)00267-6. [DOI] [PubMed] [Google Scholar]
- 177.Chen B., Zhang Y., Ran R., Wang B., Qin F., Zhang T., et al. Reactive oxygen species-responsive nanoparticles based on thioketal-containing poly(β-amino ester) for combining photothermal/photodynamic therapy and chemotherapy. Polym Chem. 2019;10:4746–4757. [Google Scholar]
- 178.Men Y., Brevé T.G., Liu H., Denkova A.G., Eelkema R. Photo cleavable thioacetal block copolymers for controlled release. Polym Chem. 2021;12:3612–3618. doi: 10.1039/d1py00514f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shen Y., Xu C., Chen J., Guan Z., Huang Y., Zeng Z., et al. Phototriggered self-adaptive functionalized moc-based drug delivery platform promises high antitumor efficacy. Adv Healthcare Mater. 2021;1:2100676–2100687. doi: 10.1002/adhm.202100676. [DOI] [PubMed] [Google Scholar]
- 180.Bloch W.M., Clever G.H. Integrative self-sorting of coordination cages based on ‘naked’ metal ions. Chem Commun. 2017;53:8506–8516. doi: 10.1039/c7cc03379f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Beuerle F., Gole B. Covalent organic frameworks and cage compounds: design and applications of polymeric and discrete organic scaffolds. Angew Chem Int Ed. 2018;57:4850–4878. doi: 10.1002/anie.201710190. [DOI] [PubMed] [Google Scholar]
- 182.Hiraoka S., Kubota Y., Fujita M. Self- and hetero-recognition in the guest-controlled assembly of Pd(II)-linked cages from two different ligands. Chem Commun. 2000;1:1509–1510. [Google Scholar]
- 183.Kumazawa K., Biradha K., Kusukawa T., Okano T., Fujita M. Multicomponent assembly of a pyrazine-pillared coordination cage that selectively binds planar guests by intercalation. Angew Chem Int Ed. 2003;42:3909–3913. doi: 10.1002/anie.200351797. [DOI] [PubMed] [Google Scholar]
- 184.Yamashina M., Yuki T., Sei Y., Akita M., Yoshizawa M. Anisotropic expansion of an M2L4 coordination capsule: host capability and frame rearrangement. Chem Eur J. 2015;21:4200–4204. doi: 10.1002/chem.201406445. [DOI] [PubMed] [Google Scholar]
- 185.Sun Q.F., Sato S., Fujita M. An M12(L1)12(L2)12 cantellated tetrahedron: a case study on mixed-ligand self-assembly. Angew Chem Int Ed. 2014;53:13510–13513. doi: 10.1002/anie.201408652. [DOI] [PubMed] [Google Scholar]
- 186.Bloch W.M., Abe Y., Holstein J.J., Wandtke C.M., Dittrich B., Clever G.H. Geometric complementarity in assembly and guest recognition of a bent heteroleptic cis-[Pd₂LA₂LB₂] coordination cage. J Am Chem Soc. 2016;138:13750–13755. doi: 10.1021/jacs.6b08694. [DOI] [PubMed] [Google Scholar]
- 187.Bloch W.M., Holstein J.J., Hiller W., Clever G.H. Morphological control of heteroleptic cis- and trans-Pd2L2L′2 cages. Angew Chem Int Ed. 2017;56:8285–8289. doi: 10.1002/anie.201702573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li J.R., Zhou H.C. Bridging-ligand-substitution strategy for the preparation of metal-organic polyhedral. Nat Chem. 2010;2:893–898. doi: 10.1038/nchem.803. [DOI] [PubMed] [Google Scholar]
- 189.Feng L., Wang K.Y., Day G.S., Zhou H.C. The chemistry of multi-component and hierarchical framework compounds. Chem Soc Rev. 2019;48:4823–4853. doi: 10.1039/c9cs00250b. [DOI] [PubMed] [Google Scholar]
- 190.Zhu W., Guo J., Ju Y., Serda R.E., Croissant J.G., Shang J., et al. Modular metal-organic polyhedra superassembly: from molecular-level design to targeted drug delivery. Adv Mater. 2019;31:1806774–1806783. doi: 10.1002/adma.201806774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang X., Dong X., Lu W., Luo D., Zhu X.W., Li X., et al. Fine-tuning apertures of metal-organic cages: encapsulation of carbon dioxide in solution and solid state. J Am Chem Soc. 2019;141:11621–11627. doi: 10.1021/jacs.9b04520. [DOI] [PubMed] [Google Scholar]
- 192.Nihei M., Ida H., Nibe T., Moeljadi A.M.P., Trinh Q.T., Hirao H., et al. Ferrihydrite particle encapsulated within a molecular organic cage. J Am Chem Soc. 2018;140:17753–17759. doi: 10.1021/jacs.8b10957. [DOI] [PubMed] [Google Scholar]
- 193.Gosselin E.J., Rowland C.A., Bloch E.D. Permanently microporous metal-organic polyhedra. Chem Rev. 2020;120:8987–9014. doi: 10.1021/acs.chemrev.9b00803. [DOI] [PubMed] [Google Scholar]
- 194.Chen Z., Chen B., He M., Wang H., Hu B. A porous organic polymer with magnetic nanoparticles on a chip array for preconcentration of platinum(IV), gold(III) and bismuth(III) prior to their on-line quantitation by ICP-MS. Mikrochim Acta. 2019;186:107–114. doi: 10.1007/s00604-018-3139-1. [DOI] [PubMed] [Google Scholar]
- 195.Das S., Heasman P., Ben T., Qiu S. Porous organic materials: strategic design and structure-function correlation. Chem Rev. 2017;117:1515–1563. doi: 10.1021/acs.chemrev.6b00439. [DOI] [PubMed] [Google Scholar]
- 196.Pan M., Wu K., Zhang J.H., Su C.Y. Chiral metal-organic cages/containers (MOCs): from structural and stereochemical design to applications. Coord Chem Rev. 2019;378:333–349. [Google Scholar]
- 197.Gao Y., Deng S.Q., Jin X., Cai S.L., Zheng S.R., Zhang W.G. The construction of amorphous metal-organic cage-based solid for rapid dye adsorption and time-dependent dye separation from water. Chem Eng J. 2019;357:129–139. [Google Scholar]
- 198.An Y., Zhu J., Liu F., Deng J., Meng X., Liu G., et al. Boosting the ferroptotic antitumor efficacy via site-specific amplification of tailored lipid peroxidation. ACS Appl Mater Interfaces. 2019;11:29655–29666. doi: 10.1021/acsami.9b10954. [DOI] [PubMed] [Google Scholar]
- 199.Li H.J., Du J.Z., Liu J., Du X.J., Shen S., Zhu Y.H., et al. Smart superstructures with ultrahigh pH-sensitivity for targeting acidic tumor microenvironment: instantaneous size switching and improved tumor penetration. ACS Nano. 2016;10:6753–6761. doi: 10.1021/acsnano.6b02326. [DOI] [PubMed] [Google Scholar]
- 200.Johnson A.M., Hooley R.J. Steric effects control self-sorting in self-assembled clusters. Inorg Chem. 2011;50:4671–4673. doi: 10.1021/ic2001688. [DOI] [PubMed] [Google Scholar]
- 201.Preston D., Barnsley J.E., Gordon K.C., Crowley J.D. Controlled formation of heteroleptic [Pd2(La)2(Lb)2](4+) cages. J Am Chem Soc. 2016;138:10578–10585. doi: 10.1021/jacs.6b05629. [DOI] [PubMed] [Google Scholar]
- 202.Zheng Y.R., Zhao Z.G., Wang M., Ghosh K., Pollock J.B., Cook T.R., et al. A facile approach toward multicomponent supramolecular structures: selective self-assembly via charge separation. J Am Chem Soc. 2010;132:16873–16882. doi: 10.1021/ja106251f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kabb C.P., O'Bryan C.S., Morley C.D., Angelini T.E., Sumerlin B.S. Anthracene-based mechanophores for compression-activated fluorescence in polymeric networks. Chem Sci. 2019;10:7702–7708. doi: 10.1039/c9sc02487e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Syrett J.A., Mantovani G., Barton W.R.S., Price D., Haddleton D.M. Self-healing polymers prepared via living radical polymerization. Polym Chem. 2010;1:102–106. [Google Scholar]
- 205.Li J., Shiraki T., Hu B., Wright R.A.E., Zhao B., Moore J.S. Mechanophore activation at heterointerfaces. J Am Chem Soc. 2014;136:15925–15928. doi: 10.1021/ja509949d. [DOI] [PubMed] [Google Scholar]
- 206.Church D.C., Peterson G.I., Boydston A.J. Comparison of mechanochemical chain scission rates for linear versus three-arm star polymers in strong acoustic fields. ACS Macro Lett. 2014;3:648–651. doi: 10.1021/mz5003068. [DOI] [PubMed] [Google Scholar]
- 207.Li H., Gostl R., Delgove M., Sweeck J., Zhang Q., Sijbesma R.P., et al. Promoting mechanochemistry of covalent bonds by noncovalent micellar aggregation. ACS Macro Lett. 2016;5:995–998. doi: 10.1021/acsmacrolett.6b00579. [DOI] [PubMed] [Google Scholar]
- 208.Sun H., Kabb C.P., Dai Y., Hill M.R., Ghiviriga I., Bapat A.P., et al. Macromolecular metamorphosis via stimulus-induced transformations of polymer architecture. Nat Chem. 2017;9:817–823. doi: 10.1038/nchem.2730. [DOI] [PubMed] [Google Scholar]
- 209.Wang J., Piskun I., Craig S.L. Mechanochemical strengthening of a multi-mechanophore benzocyclobutene polymer. ACS Macro Lett. 2015;4:834–837. doi: 10.1021/acsmacrolett.5b00440. [DOI] [PubMed] [Google Scholar]
- 210.Gordon M.B., Wang S., Knappe G.A., Wagner N.J., Epps T.H., Kloxin C.J. Force-induced cleavage of a labile bond for enhanced mechanochemical crosslinking. Polym Chem. 2017;8:6485–6489. [Google Scholar]
- 211.Chen Z., Mercer J.A.M., Zhu X., Romaniuk J.A.H., Pfattner R., Cegelski L., et al. Mechanochemical unzipping of insulating polyladderene to semiconducting polyacetylene. Science. 2017;357:475–479. doi: 10.1126/science.aan2797. [DOI] [PubMed] [Google Scholar]
- 212.Larsen M.B., Boydston A.J. “Flex-activated” mechanophores: using polymer mechanochemistry to direct bond bending activation. J Am Chem Soc. 2013;135:8189–8192. doi: 10.1021/ja403757p. [DOI] [PubMed] [Google Scholar]
- 213.Cao B., Boechler N., Boydston A.J. Additive manufacturing with a flex activated mechanophore for nondestructive assessment of mechanochemical reactivity in complex object geometries. Polymer. 2018;152:4–8. [Google Scholar]
- 214.Gossweiler G.R., Hewage G.B., Soriano G., Wang Q., Welshofer G.W., Zhao X., et al. Mechanochemical activation of covalent bonds in polymers with full and repeatable macroscopic shape recovery. ACS Macro Lett. 2014;3:216–219. doi: 10.1021/mz500031q. [DOI] [PubMed] [Google Scholar]
- 215.Peterson G.I., Larsen M.B., Ganter M.A., Storti D.W., Boydston A.J. 3D-Printed mechanochromic materials. ACS Appl Mater Interfaces. 2015;7:577–583. doi: 10.1021/am506745m. [DOI] [PubMed] [Google Scholar]
- 216.Song X., Song Y., Cui X., Wang J.P., Luo Y., Qi T., et al. Intrinsic healable mechanochromic materials via incorporation of spiropyran mechanophore into polymer main chain. Polymer. 2022;250 [Google Scholar]
- 217.Larsen M.B., Boydston A.J. Successive mechanochemical activation and small molecule release in an elastomeric material. J Am Chem Soc. 2014;136:1276–1279. doi: 10.1021/ja411891x. [DOI] [PubMed] [Google Scholar]
- 218.Cheng C.C., Huang J.J., Lee A.W., Huang S.Y., Huang C.Y., Lai J.Y. Highly effective photocontrollable drug delivery systems based on ultrasensitive light-responsive self-assembled polymeric micelles: an in vitro therapeutic evaluation. ACS Appl Bio Mater. 2019;2:2162–2170. doi: 10.1021/acsabm.9b00146. [DOI] [PubMed] [Google Scholar]
- 219.Cardano F., Canto E.D., Giordani S. Spiropyran for light-controlled drug delivery. Dalton Trans. 2019;48:15537–15544. doi: 10.1039/c9dt02092f. [DOI] [PubMed] [Google Scholar]
- 220.Cheng H., Yoon J., Tian H. Recent advances in the use of photochromic dyes for photocontrol in biomedicine. Coord Chem Rev. 2018;372:66–84. [Google Scholar]
- 221.Velema W.A., Szymanski W., Feringa B.L. Photopharmacology: beyond proof of principle. J Am Chem Soc. 2014;136:2178–2191. doi: 10.1021/ja413063e. [DOI] [PubMed] [Google Scholar]
- 222.Cheng H.B., Zhang S., Qi J., Liang X.J., Yoon J. Advances in application of azobenzene as a trigger in biomedicine: molecular design and spontaneous assembly. Adv Mater. 2021;1:2007290–2007331. doi: 10.1002/adma.202007290. [DOI] [PubMed] [Google Scholar]
- 223.Liu J., Bu W., Pan L., Shi P.J. NIR-triggered anticancer drug delivery by upconverting nanoparticles with integrated azobenzene-modified mesoporous silica. Angew Chem Int Ed. 2013;52:4375–4379. doi: 10.1002/anie.201300183. [DOI] [PubMed] [Google Scholar]
- 224.Peddie V., Abell A.D. Photocontrol of peptide secondary structure through non-azobenzene photoswitches. J Photochem Photobiol C Photochem Rev. 2019;40:1–20. [Google Scholar]
- 225.Wang X., Hu J., Liu G., Tian J., Wang H., Gong M., et al. Reversibly switching bilayer permeability and release modules of photochromic polymersomes stabilized by cooperative noncovalent interactions. J Am Chem Soc. 2015;137:15262–15275. doi: 10.1021/jacs.5b10127. [DOI] [PubMed] [Google Scholar]
- 226.Pavlukhina S., Sukhishvili S. Polymer assemblies for controlled delivery of bioactive molecules from surfaces. Adv Drug Deliv Rev. 2011;63:822–836. doi: 10.1016/j.addr.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 227.Volodkin D.V., Madaboosi N., Blacklock J., Skirtach A.G. Surface-supported multilayers decorated with bio-active material aimedat light-triggered drug delivery. Langmuir. 2009;25:14037–14043. doi: 10.1021/la9015433. [DOI] [PubMed] [Google Scholar]
- 228.Marturano V., Cerruti P., Cerruti P., Giamberini M., Tylkowski B., Ambrogi V. Light-responsive polymer micro- and nano-capsules. Polymers. 2017;9:8–26. doi: 10.3390/polym9010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Görner H., Kuhn H.J. In: Advance in photochemistry. Neckers D.C., Volman D.H., von Bünau G., editors. John Wiley & Sons, Inc.; Hoboken: 1994. Cis-trans photoisomerization of stilbenes and stilbene-like molecules. [Google Scholar]
- 230.Granados A., Vallribera A. Fluorous hydrophobic fluorescent (E)-stilbene derivatives for application on security paper. Dyes Pigments. 2019;170:107597–107608. [Google Scholar]
- 231.Abdollahi A., Sahandi-Zangabad K., Roghani-Mamaqani H. Rewritable anticounterfeiting polymer inks based on functionalized stimuli-responsive latex particles containing spiropyran photoswitches: reversible photopatterning and security marking. ACS Appl Mater Interfaces. 2018;10:39279–39292. doi: 10.1021/acsami.8b14865. [DOI] [PubMed] [Google Scholar]
- 232.Abdollahi A., Mouraki A., Sharifian M.H., Mahdavian A.R. Photochromic properties of stimuli-responsive cellulosic papers modified by spiropyran-acrylic copolymer in reusable pH-sensors. Carbohydr Polym. 2018;200:583–594. doi: 10.1016/j.carbpol.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 233.Li E., Kang J., Ye P., Zhang W., Cheng F., Yin C. A prospective material for the highly selective extraction of lithium ions based on a photochromic crowned spirobenzopyran. J Mater Chem B. 2019;7:903–907. doi: 10.1039/c8tb02906g. [DOI] [PubMed] [Google Scholar]
- 234.Sakai H., Ebana H., Sakai K., Tsuchiya K., Ohkubo T., Abe M. Photo-isomerization of spiropyran-modified cationic surfactants. J Colloid Interface Sci. 2007;316:1027–1030. doi: 10.1016/j.jcis.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 235.Sahoo P.R., Prakash K., Kumar S. Light controlled receptors for heavy metal ions. Coord Chem Rev. 2018;357:18–49. [Google Scholar]
- 236.Ranjan P., Kumar S.S. Photochromic spirooxazine as highly sensitive and selective probe for optical detection of Fe3+ in aqueous solution. Sensor Actuator B Chem. 2016;226:548–552. [Google Scholar]
- 237.Sun B., He Z., Hou Q., Liu Z., Cha R., Ni Y. Interaction of a spirooxazine dye with latex and its photochromic efficiency on cellulosic paper. Carbohydr Polym. 2013;95:598–605. doi: 10.1016/j.carbpol.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 238.Guerchais V., Ordronneau L., Bozec H.L. Recent developments in the field of metal complexes containing photochromic ligands: modulation of linear and nonlinear optical properties. Coord Chem Rev. 2010;254:2533–2545. [Google Scholar]
- 239.Li Z., Liu Y., Yang X.G., Gao X., Zhang Y., Zhang H., et al. Cyanostilbene-functionalized dithienylethenes with aggregation -induced emission for photoswitching behavior in multi-media. J Lumin. 2022;250 [Google Scholar]
- 240.Yan Q., Qiao Z., Xu J., Ren J., Wang S. All-visible-light triggered photochromic fluorescent dithienylethene-phenanthroimidazole dyads: synthesis, crystal structure, multiple switching behavior and information storage. Dyes Pigments. 2022;202 [Google Scholar]
- 241.Zhang H., Qi Y., Zhao X., Li M., Wang R., Cheng H., et al. Dithienylethene-bridged fluoroquinolone derivatives for imaging-guided reversible control of antibacterial activity. J Org Chem. 2022;87:7446–7455. doi: 10.1021/acs.joc.2c00797. [DOI] [PubMed] [Google Scholar]
- 242.Huang Y., Dong R., Zhu X., Yan D. Photo-responsive polymeric micelles. Soft Matter. 2014;10:6121–6138. doi: 10.1039/c4sm00871e. [DOI] [PubMed] [Google Scholar]
- 243.Yuan Q., Zhang Y., Chen T., Lu D., Zhao Z., Zhang X., et al. Photon-manipulated drug release from a mesoporous nanocontainer controlled by azobenzene-modified nucleic acid. ACS Nano. 2012;7:6337–6344. doi: 10.1021/nn3018365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tong X., Wang G., Soldera A., Zhao Y. How can azobenzene block copolymer vesicles be dissociated and reformed by light? J Phys Chem B. 2005;109:20281–20287. doi: 10.1021/jp0524274. [DOI] [PubMed] [Google Scholar]
- 245.Jiang J., Tong X., Morris D., Zhao Y. Toward photocontrolled release using light-dissociable block copolymer micelles. Macromolecules. 2006;39:4633–4640. [Google Scholar]
- 246.Liu X., He J., Niu Y., Li Y., Hu D., Xia X., et al. Photo-responsive amphiphilic poly(α-hydroxy acids) with pendent o-nitrobenzyl ester constructed via copper-catalyzed azide-alkyne cycloaddition reaction. Polym Adv Technol. 2015;26:449–456. [Google Scholar]
- 247.Shamsipur M., Ghavidast A. Facile synthesis of magnetic photo-responsive nanoparticles based on 1,3-diazabicyclo[3.1.0]hex-3-en: an enhanced adsorption of toxic dyes from aqueous solution under sunlight. J Mol Struct. 2022;263:133130–133143. [Google Scholar]
- 248.Mahmoodi N.O., Ahmadi N.K., Ghavidast A. Light-induced switching of 1,3-di-azabicyclo-[3.1.0]hex-3-enes on gold nanoparticles. J Mol Struct. 2018;1160:463–470. [Google Scholar]
- 249.Mahmoodi N.O., Ghavidast A., Mirkhaef S., Zanjanchi M.A. Photochromism of azobenzene-thiol-1,3-diazabicyclo-[3.1.0]hex-3-ene on silver nanoparticles. Dyes Pigments. 2015;118:110–117. [Google Scholar]
- 250.Fasihi-Ramandi M., Mahmoodi N.O., Ghavidast A., Shirini F., Nahzomi H.T. Synthesis and exploring the excited-state PES of photochromic hydrogen bond-assembled [2]rotaxane based on 1,3-diazabicyclo-[3.1.0]hex-3-enes. Res Chem Intermed. 2021;47:1–16. [Google Scholar]
- 251.Ghavidast A., Mahmoodi N.O., Zanjanchi M.A. Synthesis and photochromicproperties of a novel thiol-terminated 1,3-diazabicyclo[3.1.0]hex-3-ene on silver nanoparticles. J Mol Struct. 2013;1048:166–171. [Google Scholar]
- 252.Rad J.K., Balzade Z., Mahdavian A.R. Spiropyran-based advanced photoswitchable materials: a fascinating pathway to the future stimuli-responsive devices. J Photochem Photobiol C Photochem Rev. 2022;51 [Google Scholar]
- 253.ter Schiphorst J., Coleman S., Stumpel J.E., Azouz A.B., Diamond D., Schenning A.P.H.J. Molecular design of light-responsive hydrogels, for in situ generation of fast and reversible valves for microfluidic applications. Chem Mater. 2015;27:5925–5931. [Google Scholar]
- 254.Zhang Q.M., Wang W., Su Y.Q., Hensen E.J.M., Serpe M.J. Biological imaging and sensing with multiresponsive microgels. Chem Mater. 2016;28:259–265. [Google Scholar]
- 255.Zhu M.Q., Zhu L., Han J.J., Wu W., Hurst J.K., Li A.D.Q. Spiropyran-based photochromic polymer nanoparticles with optically switchable luminescence. J Am Chem Soc. 2006;128:4303–4309. doi: 10.1021/ja0567642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Li M., Zhang Q., Zhou Y.N., Zhu S. Let spiropyran help polymers feel force. Prog Polym Sci. 2018;79:26–39. [Google Scholar]
- 257.Lee H.I., Wu W., Oh J.K., Mueller L., Sherwood G., Peteanu L., et al. Light-induced reversible formation of polymeric micelles. Angew Chem Int Ed. 2007;46:2453–2457. doi: 10.1002/anie.200604278. [DOI] [PubMed] [Google Scholar]
- 258.Jochum F.D., Theato P. Temperature- and light-responsive smart polymer materials. Chem Soc Rev. 2013;42:7468–7483. doi: 10.1039/c2cs35191a. [DOI] [PubMed] [Google Scholar]
- 259.Jochum F.D., Theato P. Temperature- and light-responsive polyacrylamides prepared by a double polymer analogous reaction of activated ester polymers. Macromolecules. 2009;42:5941–5945. [Google Scholar]
- 260.Minkin V.I. Photo-, thermo-, solvato-, and electrochromic spiroheterocyclic compounds. Chem Rev. 2004;104:2751–2776. doi: 10.1021/cr020088u. [DOI] [PubMed] [Google Scholar]
- 261.Berkovic G., Krongauz V., Weiss V. Spiropyrans and spirooxazines for memories and switches. Chem Rev. 2000;100:1741–1754. doi: 10.1021/cr9800715. [DOI] [PubMed] [Google Scholar]
- 262.Weissleder R. A clearer vision for in vivo imaging: progress continues in the development of smaller, more penetrable probes for biological imaging. Nat Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 263.Cho H.J., Chung M., Shim M.S. Engineered photo-responsive materials for near-infrared-triggered drug delivery. J Ind Eng Chem. 2015;31:15–25. [Google Scholar]
- 264.Linsley C.S., Wu B.M. Recent advances in light-responsive on-demand drug-delivery systems. Ther Deliv. 2017;8:89–107. doi: 10.4155/tde-2016-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Ghani M., Heiskanen A., Kajtez J., Rezaei B., Larsen N.B., Thomsen P., et al. On-demand reversible UV-triggered interpenetrating polymer network-based drug delivery system using the spiropyran-merocyanine hydrophobicity switch. ACS Appl Mater Interfaces. 2021;13:3591–3604. doi: 10.1021/acsami.0c19081. [DOI] [PubMed] [Google Scholar]
- 266.Tang Y., Wang G. NIR light-responsive nanocarriers for controlled release. J Photochem Photobiol C Photochem Rev. 2021;47:100420–100435. [Google Scholar]
- 267.Tylkowski B., Trojanowska A., Marturano V., Nowak M., Marciniak L., Giamberini M., et al. Power of light-functional complexes based on azobenzene molecules. Coord Chem Rev. 2017;351:205–217. [Google Scholar]
- 268.Wang D., Wu S. Red-light-responsive supramolecular valves for photocontrolled drug release from mesoporous nanoparticles. Langmuir. 2016;32:632–636. doi: 10.1021/acs.langmuir.5b04399. [DOI] [PubMed] [Google Scholar]
- 269.Hartley G.S. The cis-form of azobenzene. Nature. 1937;140:281–282. [Google Scholar]
- 270.Yang H., Yuan B., Zhang X. Supramolecular chemistry at interfaces: host-guest interactions for fabricating multifunctional biointerfaces. Acc Chem Res. 2014;47:2106–2115. doi: 10.1021/ar500105t. [DOI] [PubMed] [Google Scholar]
- 271.Deng J., Liu X., Shi W., Cheng C., He C., Zhao C. Light-triggered switching of reversible and alterable biofunctionality via β-cyclodextrin/azobenzene-based host-guest interaction. ACS Macro Lett. 2014;3:1130–1133. doi: 10.1021/mz500568k. [DOI] [PubMed] [Google Scholar]
- 272.Shen Q., Liu L., Zhang W. Fabrication of a photocontrolled surface with switchable wettability based on host-guest inclusion complexation and protein resistance. Langmuir. 2014;30:9361–9369. doi: 10.1021/la500792v. [DOI] [PubMed] [Google Scholar]
- 273.Wan P., Chen Y., Xing Y., Chi L., Zhang X. Combining host-guest systems with nonfouling material for the fabrication of a biosurface: toward nearly complete and reversible resistance of cytochrome c. Langmuir. 2010;26:12515–12517. doi: 10.1021/la102336a. [DOI] [PubMed] [Google Scholar]
- 274.Becker D., Konnertz N., Böhning M., Schmidt J., Thomas A. Light-switchable polymers of intrinsic microporosity. Chem Mater. 2016;28:8523–8529. [Google Scholar]
- 275.Robertus J., Browne W.R., Feringa B.L. Dynamic control over cell adhesive properties using molecular-based surface engineering strategies. Chem Soc Rev. 2010;39:354–378. doi: 10.1039/b906608j. [DOI] [PubMed] [Google Scholar]
- 276.Szymanski W., Beierle J.M., Kistemaker H.A.V., Velema W.A., Feringa B.L. Reversible photocontrol of biological systems by the incorporation of molecular photoswitches. Chem Rev. 2013;113:6114–6178. doi: 10.1021/cr300179f. [DOI] [PubMed] [Google Scholar]
- 277.Beharry A.A., Woolley G.A. Azobenzene photoswitches for biomolecules. Chem Soc Rev. 2011;40:4422–4437. doi: 10.1039/c1cs15023e. [DOI] [PubMed] [Google Scholar]
- 278.Merino E., Ribagorda M. Control over molecular motion using the cis-trans photoisomerization of the azo group. Beilstein J Org Chem. 2012;8:1071–1090. doi: 10.3762/bjoc.8.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Bandara H.M., Burdette S.C. Photoisomerization in different classes of azobenzene. Chem Soc Rev. 2012;41:1809–1825. doi: 10.1039/c1cs15179g. [DOI] [PubMed] [Google Scholar]
- 280.Weis P., Wu S. Light-switchable azobenzene-containing macromolecules: from UV to near infrared. Macromol Rapid Commun. 2018;39:1700220–1700231. doi: 10.1002/marc.201700220. [DOI] [PubMed] [Google Scholar]
- 281.Broichhagen J., Frank J.A., Trauner D. A roadmap to success in photopharmacology. Acc Chem Res. 2015;48:1947–1960. doi: 10.1021/acs.accounts.5b00129. [DOI] [PubMed] [Google Scholar]
- 282.Qu D.H., Wang Q.C., Zhang Q.W., Ma X., Tian H. Photoresponsive host-guest functional Systems. Chem Rev. 2015;115:7543–7588. doi: 10.1021/cr5006342. [DOI] [PubMed] [Google Scholar]
- 283.Han Y., Meng Z., Ma Y.X., Chen C.F. Iptycene-derived crown ether hosts for molecular recognition and self-assembly. Acc Chem Res. 2014;47:2026–2040. doi: 10.1021/ar5000677. [DOI] [PubMed] [Google Scholar]
- 284.Mathapa B.G., Paunov V.N. Self-assembly of cyclodextrin-oil inclusion complexes at the oil-water interface: a route to surfactant-free emulsions. J Mater Chem. 2013;1:10836–10846. [Google Scholar]
- 285.Zhao Y., Huang Y., Zhu H., Zhu Q., Xia Y. Three-in-one: sensing, self-assembly, and cascade catalysis of cyclodextrin modified gold nanoparticles. J Am Chem Soc. 2016;138:16645–16654. doi: 10.1021/jacs.6b07590. [DOI] [PubMed] [Google Scholar]
- 286.Yang S., Yan Y., Huang J., Petukhov A.V., Kroon-Batenburg L.M.J., Drechsler M., et al. Giant capsids from lattice self-assembly of cyclodextrin complexes. Nat Commun. 2017;8:15856–15862. doi: 10.1038/ncomms15856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Yan H., Qiu Y., Wang J., Jiang Q., Wang H., Liao Y., et al. Wholly, visible-light-responsive host-guest supramolecular gels based on methoxy azobenzene and β-cyclodextrin dimmers. Langmuir. 2020;36:7408–7417. doi: 10.1021/acs.langmuir.0c00964. [DOI] [PubMed] [Google Scholar]
- 288.Tanaka Y., Miyachi M., Kobuke Y. Selective vesicle formation from calixarenes by self-assembly. Angew Chem Int Ed. 1999;38:504–506. doi: 10.1002/(SICI)1521-3773(19990215)38:4<504::AID-ANIE504>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 289.Zorzi R.D., Guidolin N., Randaccio L., Purrello R., Geremia S. Nanoporous crystals of calixarene/porphyrin supramolecular complex functionalized by diffusion and coordination of metal ions. J Am Chem Soc. 2009;131:2487–2489. doi: 10.1021/ja808850d. [DOI] [PubMed] [Google Scholar]
- 290.Lee J.W., Samal S., Selvapalam N., Kim H.J., Kim K. Cucurbituril homologues and derivatives: new opportunities in supramolecular chemistry. Acc Chem Res. 2003;36:621–630. doi: 10.1021/ar020254k. [DOI] [PubMed] [Google Scholar]
- 291.Reany O., Li A., Yefet M., Gilson M.K., Keinan E. Attractive interactions between heteroallenes and the cucurbituril portal. J Am Chem Soc. 2017;139:8138–8145. doi: 10.1021/jacs.6b13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Yang X., Wang R., Kermagoret A., Bardelang D. Oligomeric cucurbituril complexes: from peculiar assemblies to emerging applications. Angew Chem Int Ed. 2020;59:21280–21292. doi: 10.1002/anie.202004622. [DOI] [PubMed] [Google Scholar]
- 293.Xue M., Yang Y., Chi X., Zhang Z., Huang F. Pillararenes, a new class of macrocycles for supramolecular chemistry. Acc Chem Res. 2012;45:1294–1308. doi: 10.1021/ar2003418. [DOI] [PubMed] [Google Scholar]
- 294.Kaizerman-Kane D., Hadar M., Tal N., Dobrovetsky R., Zafrani Y., Cohen Y. pH-Responsive pillar[6]arene-based water-soluble supramolecular hexagonal boxes. Angew Chem Int Ed. 2019;58:5302–5306. doi: 10.1002/anie.201900217. [DOI] [PubMed] [Google Scholar]
- 295.Zhu H., Li Q., Gao Z., Wang H., Shi B., Wu Y., et al. Pillararene host-guest complexation induced chirality amplification: a new way to detect cryptochiral compounds. Angew Chem Int Ed. 2020;59:10868–10872. doi: 10.1002/anie.202001680. [DOI] [PubMed] [Google Scholar]
- 296.Zhao Y.L., Stoddart J.F. Azobenzene-based light-responsive hydrogel system. Langmuir. 2009;25:8442–8446. doi: 10.1021/la804316u. [DOI] [PubMed] [Google Scholar]
- 297.Zhang X., Lei B., Wang Y., Xu S., Liu H. Dual-sensitive on-off switch in liposome bilayer for controllable drug release. Langmuir. 2019;35:5213–5220. doi: 10.1021/acs.langmuir.8b04094. [DOI] [PubMed] [Google Scholar]
- 298.Liu H., Fu Y., Li Y., Ren Z., Li X., Han G., t al e. A fibrous localized drug delivery platform with NIR-triggered and optically monitored drug release. Langmuir. 2016;32:9083–9090. doi: 10.1021/acs.langmuir.6b02227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Sarkar D., Chowdhury M., Das P.K. Naphthalimide-based azo-functionalized supramolecular vesicle in hypoxia-responsive drug delivery. Langmuir. 2022;38:3480–3492. doi: 10.1021/acs.langmuir.1c03334. [DOI] [PubMed] [Google Scholar]
- 300.Wang W., Lin L., Ma X., Wang B., Liu S., Yan X., et al. Light-induced hypoxia-triggered living nanocarriers for synergistic cancer therapy. ACS Appl Mater Interfaces. 2018;10:19398–19407. doi: 10.1021/acsami.8b03506. [DOI] [PubMed] [Google Scholar]
- 301.Bian Q., Wang W., Wang S., Wang G. Light-triggered specific cancer cell release from cyclodextrin/azobenzene and aptamer-modified substrate. ACS Appl Mater Interfaces. 2016;8:27360–27367. doi: 10.1021/acsami.6b09734. [DOI] [PubMed] [Google Scholar]
- 302.Long M., Liu X., Huang X., Lu M., Wu X., Weng L., et al. Alendronate-functionalized hypoxia-responsive polymeric micelles for targeted therapy of bone metastatic prostate cancer. J Contr Release. 2021;334:303–317. doi: 10.1016/j.jconrel.2021.04.035. [DOI] [PubMed] [Google Scholar]
- 303.Joshi U., Filipczak N., Khan M.M., Attia S.A., Torchilin V. Hypoxia-sensitive micellar nanoparticles for co-delivery of siRNA and chemotherapeutics to overcome multi-drug resistance in tumor cells. Int J Pharm. 2020;590:119915–119929. doi: 10.1016/j.ijpharm.2020.119915. [DOI] [PubMed] [Google Scholar]
- 304.Zhang Y., Chan H.F., Leong K.W. Advanced materials and processing for drug delivery: the past and the future. Adv. Drug Deliv Rev. 2013;65:104–120. doi: 10.1016/j.addr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Zhou Y., Ye H., Chen Y., Zhu R., Yin L. Photoresponsive drug/gene delivery systems. Biomacromolecules. 2018;19:1840–1857. doi: 10.1021/acs.biomac.8b00422. [DOI] [PubMed] [Google Scholar]
- 306.Wang X., Sun B., Ye Z., Zhang W., Xu W., Gao S., et al. Enzyme-responsive COF-based thiol-targeting nanoinhibitor for curing bacterial infections. ACS Appl Mater Interfaces. 2022;34:38483–38496. doi: 10.1021/acsami.2c08845. [DOI] [PubMed] [Google Scholar]
- 307.Zhu J., Guo T., Wang Z., Zhao Y. Triggered azobenzene-based prodrugs and drug delivery systems. J Contr Release. 2022;345:475–493. doi: 10.1016/j.jconrel.2022.03.041. [DOI] [PubMed] [Google Scholar]
- 308.Ma X., Zhou N., Zhang T., Guo Z., Hu W., Zhu C., et al. In situ formation of multiple stimuli-responsive poly[(methyl vinyl ether)-alt-(maleic acid)] based supramolecular hydrogels by inclusion complexation between cyclodextrin and azobenzene. RSC Adv. 2016;6:13129–13136. [Google Scholar]
- 309.McConnell A.J., Wood C.S., Neelakandan P.P., Nitschke J.R. Stimuli-responsive metal-ligand assemblies. Chem Rev. 2015;115:7729–7793. doi: 10.1021/cr500632f. [DOI] [PubMed] [Google Scholar]
- 310.Ma X., Tian H. Stimuli-responsive supramolecular polymers in aqueous solution. Acc Chem Res. 2014;47:1971–1981. doi: 10.1021/ar500033n. [DOI] [PubMed] [Google Scholar]
- 311.Kelley E.G., Albert J.N.L., Sullivan, Epps T.H. Stimuli-responsive copolymer solution and surface assemblies for biomedical applications. Chem Soc Rev. 2013;42:7057–7071. doi: 10.1039/c3cs35512h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Zhang K., Liu J., Guo Y., Li Y., Ma X., Lei Z. Synthesis of temperature, pH, light and dual-redox quintuple-stimuli-responsive shell-crosslinked polymeric nanoparticles for controlled release. Mater Sci Eng C Mater Biol Appl. 2018;87:1–9. doi: 10.1016/j.msec.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 313.Cheng W., Gu L., Ren W., Liu Y. Stimuli-responsive polymers for anti-cancer drug delivery. Mater Sci Eng C Mater Biol Appl. 2014;45:600–608. doi: 10.1016/j.msec.2014.05.050. [DOI] [PubMed] [Google Scholar]
- 314.Razavi B., Abdollahi A., Roghani-Mamaqani H., Salami-Kalajahi M. Light- and temperature-responsive micellar carriers prepared by spiropyran-initiated atom transfer polymerization: investigation of photochromism kinetics, responsivities, and controlled release of doxorubicin. Polymer. 2019;187 [Google Scholar]
- 315.Chen S., Gao Y., Cao Z., Wu B., Wang L., Wang H., et al. Nanocomposites of spiropyran-functionalized polymers and upconversion nanoparticles for controlled release stimulated by near-infrared light and pH. Macromolecules. 2016;49:7490–7496. [Google Scholar]
- 316.Wang X., Liu X., Wang L., Tang C.Y., Law W.C., Zhang G., et al. Synthesis of yolk-shell polymeric nanocapsules encapsulated with monodispersed upconversion nanoparticle for dual-responsive controlled drug release. Macromolecules. 2018;51:10074–10082. [Google Scholar]
- 317.Razavi B., Abdollahi A., Roghani-Mamaqani H., Salami-Kalajahi M. Light-, temperature-, and pH-responsive micellar assemblies of spiropyran-initiated amphiphilic block copolymers: kinetics of photochromism, responsiveness, and smart drug delivery. Mater Sci Eng C. 2019;109 doi: 10.1016/j.msec.2019.110524. [DOI] [PubMed] [Google Scholar]
- 318.Lee H., Pietrasik J., Matyjaszewski K. Phototunable temperature-responsive molecular brushes prepared by ATRP. Macromolecules. 2006;39:3914–3920. [Google Scholar]
- 319.Zeinali E., Haddadi-Asl V., Roghani-Mamaqani H. Nanocrystalline cellulose grafted random copolymers of N-isopropylacrylamide and acrylic acid synthesized by RAFT polymerization: effect of different acrylic acid contents on LCST behavior. RSC Adv. 2014;4:31428–31442. [Google Scholar]
- 320.Haqani M., Roghani-Mamaqani H., Salami-Kalajahi M. Synthesis of dual-sensitive nanocrystalline cellulose-grafted block copolymers of N-isopropylacrylamide and acrylic acid by reversible addition-fragmentation chain transfer polymerization. Cellulose. 2017;24:2241–2254. [Google Scholar]
- 321.Hajebi S., Rabiee N., Bagherzadeh M., Ahmadi S., Rabiee M., Roghani-Mamaqani H., et al. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019;92:1–18. doi: 10.1016/j.actbio.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Cheng C.C., Muhabie A.A., Huang S.Y., Wu C.Y., Gebeyehu B.T., Lee A.W., et al. Dual stimuli-responsive supramolecular boron nitride with tunable physical properties for controlled drug delivery. Nanoscale. 2019;11:10393–10401. doi: 10.1039/c8nr09537j. [DOI] [PubMed] [Google Scholar]
- 323.Zhang J.G., Zhou Z.H., Li L., Luo Y.L., Xu F., Chen Y. Dual-stimuli responsive supramolecular self-assemblies based on the host-guest interaction between β-cyclodextrin and azobenzene for cellular drug release. Mol Pharm. 2020;17:1100–1113. doi: 10.1021/acs.molpharmaceut.9b01142. [DOI] [PubMed] [Google Scholar]
- 324.Stubbs E., Laskowski E., Conor P., Heinze D.A., Karis D., Glogowski E.M. Control of pH- and temperature-responsive behavior of mPEG-b-PDMAEMA copolymers through polymer composition. J Macromol Sci. 2017;54:228–235. [Google Scholar]
- 325.Shuai X., Ai H., Nasongkla N., Kim S., Gao J. Micellar carriers based on block copolymers of poly(q-caprolactone) and poly(ethylene glycol) for doxorubicin delivery. J Contr Release. 2004;98:415–426. doi: 10.1016/j.jconrel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 326.Gao Z., Chen M., Hu Y., Dong S., Cui J., Hao J. Tunable assembly and disassembly of responsive supramolecular polymer brushes. Polym Chem. 2017;8:2764–2772. [Google Scholar]
- 327.Wataoka I., Urakawa H., Kajiwara K., Tsukahara Y. Molecular bottlebrushes. Macromolecules. 1996;29:978–983. [Google Scholar]
- 328.Bhattacharya A., Misrab B.N. Grafting: a versatile means to modify polymers–techniques, factors and applications. Prog Polym Sci. 2004;29:767–814. [Google Scholar]
- 329.Müllner M., Dodds S.J., Nguyen T.H., Senyschyn D., Porter C.H., Boyd B.J., et al. Size and rigidity of cylindrical polymer brushes dictate long circulating properties in vivo. ACS Nano. 2015;9:1294–1304. doi: 10.1021/nn505125f. [DOI] [PubMed] [Google Scholar]
- 330.Blum A.P., Kammeyer J.K., Gianneschi N.C. Activating peptides for cellular uptake via polymerization into high density brushes. Chem Sci. 2016;7:989–994. doi: 10.1039/c5sc03417e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Liu G.Q., Cai M.R., Zhou F., Liu W.M. Charged polymer brushes-grafted hollow silica nanoparticles as a novel promising material for simultaneous joint lubrication and treatment. J Phys Chem B. 2014;118:4920–4931. doi: 10.1021/jp500074g. [DOI] [PubMed] [Google Scholar]
- 332.Yu G.C., Zhao R., Wu D., Zhang F.W., Shao L., Zhou J., et al. Pillar[5]arene-based amphiphilic supramolecular brush copolymers: fabrication, controllable self-assembly and application in self-imaging targeted drug delivery. Polym Chem. 2016;7:6178–6188. doi: 10.1039/C6PY01402J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Miyake G.M., Weitekamp R.A., Piunova V.A., Grubbs R.H. Synthesis of isocyanate-based brush block copolymers and their rapid self-assembly to infrared-reflecting photonic crystals. J Am Chem Soc. 2012;134:14249–14254. doi: 10.1021/ja306430k. [DOI] [PubMed] [Google Scholar]
- 334.Song D.P., Li C., Colella N.S., Lu X.M., Lee J.H., Watkins J.J. Thermally tunable metallodielectric photonic crystals from the self-assembly of brush block copolymers and gold nanoparticles. Adv Opt Mater. 2015;3:1169–1175. [Google Scholar]
- 335.Macfarlane R.J., Kim B., Lee B., Weitekamp R.A., Bates C.M., Lee S.F., et al. Improving brush polymer infrared one-dimensional photonic crystals via linear polymer additives. J Am Chem Soc. 2014;136:17374–17377. doi: 10.1021/ja5093562. [DOI] [PubMed] [Google Scholar]
- 336.Ballauff M., Borisov O.V. Phase transitions in brushes of homopolymers. Polymer. 2016;98:402–408. [Google Scholar]
- 337.Xiao W., Zeng X., Lin H., Han K., Jia H.Z., Zhang X.Z. Dual stimuli-responsive multi-drug delivery system for individual controlled release of anti-cancer drugs. Chem Commun. 2015;51:1475–1478. doi: 10.1039/c4cc08831j. [DOI] [PubMed] [Google Scholar]
- 338.San Miguel V., Bochet C.G., Del Campo A. Wavelength-selective caged surfaces: how many functional levels are possible? J Am Chem Soc. 2011;133:5380–5388. doi: 10.1021/ja110572j. [DOI] [PubMed] [Google Scholar]
- 339.Priestman M.A., Sun L., Lawrence D.S. Dual wavelength photoactivation of cAMP-and cGMP-dependent protein kinase signaling pathways. ACS Chem Biol. 2011;6:377–384. doi: 10.1021/cb100398e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Rodrigues-Correia A., Weyel X.M.M., Heckel A. Four levels of wavelength-selective uncaging for oligonucleotides. Org Lett. 2013;15:5500–5503. doi: 10.1021/ol402657j. [DOI] [PubMed] [Google Scholar]
- 341.Scott T.F., Kowalski B.A., Sullivan A.C., Bowman C.N., McLeod R.R. Two-color single-photon photoinitiation and photoinhibition for subdiffraction photolithography. Science. 2009;324:913–917. doi: 10.1126/science.1167610. [DOI] [PubMed] [Google Scholar]
- 342.Wong P.T., Tang S., Cannon J., Mukherjee J., Isham D., Gam K., et al. A thioacetal photocage designed for dual release: application in the quantitation of therapeutic release by synchronous reporter decaging. Chembiochem. 2017;18:126–135. doi: 10.1002/cbic.201600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wong P.T., Tang S., Cannon J., Chen D., Sun R., Lee J., et al. Photocontrolled release of doxorubicin conjugated through a thioacetal photocage in folate-targeted nanodelivery systems. Bioconjugate Chem. 2017;28:3016–3028. doi: 10.1021/acs.bioconjchem.7b00614. [DOI] [PubMed] [Google Scholar]
- 344.Bochet C.G. Orthogonal photolysis of protecting groups. Angew Chem Int Ed. 2001;40:2071–2073. doi: 10.1002/1521-3773(20010601)40:11<2071::AID-ANIE2071>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 345.Kammari L., Solomek T., Ngoy B.P., Heger D., Klan P. Orthogonal photocleavage of a monochromophoric linker. J Am Chem Soc. 2010;132:11431–11433. doi: 10.1021/ja1047736. [DOI] [PubMed] [Google Scholar]
- 346.Pelliccioli A.P., Wirz J. Photoremovable protecting groups: reaction mechanisms and applications. Photochem Photobiol Sci. 2002;1:441–458. doi: 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- 347.Banerjee A., Falvey D.E. Protecting groups that can be removed through photochemical electron transfer: mechanistic and product studies on photosensitized release of carboxylates from phenacyl esters. J Org Chem. 1997;62:6245–6251. [Google Scholar]
- 348.Paul A., Bera M., Gupta P., Singh N.D.P. o-Hydroxycinnamate for sequential photouncaging of two different functional groups and its application in releasing cosmeceuticals. Org Biomol Chem. 2019;17:7689–7693. doi: 10.1039/c9ob01148j. [DOI] [PubMed] [Google Scholar]
- 349.He M., He G., Wang P., Jiang S., Jiao Z., Xi D., et al. A sequential dual-model strategy based on photoactivatable metallopolymer for on-demand release of photosensitizers and anticancer drugs. Adv Sci. 2021;8:2103334–2103344. doi: 10.1002/advs.202103334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Bio M., Nkepang G., You Y. Click and photo-unclick chemistry of aminoacrylate for visible light-triggered drug release. Chem Commun. 2012;48:6517–6519. doi: 10.1039/c2cc32373g. [DOI] [PubMed] [Google Scholar]
- 351.Van Dijken A., Bastiaansen J.J.A.M., Kiggen N.M.M., Langeveld B.M.W., Rothe C., Monkman A., et al. Carbazole compounds as host materials for triplet emitters in organic light-emitting diodes: polymer hosts for high-efficiency light-emitting diodes. J Am Chem Soc. 2004;126:7718–7727. doi: 10.1021/ja049771j. [DOI] [PubMed] [Google Scholar]
- 352.Bashir M., Bano A., Ijaz A.S., Chaudhary B.A. Recent developments and biological activities of N-substituted carbazole derivatives: a review. Molecules. 2015;20:13496–13517. doi: 10.3390/molecules200813496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Ameen S., Lee S.B., Yoon S.C., Lee J., Lee C. Diphenylaminocarbazoles by 1,8-functionalization of carbazole: materials and application to phosphorescent organic light-emitting diodes. Dyes Pigments. 2016;124:35–44. [Google Scholar]
- 354.Venkatesh Y., Srivastava H.K., Bhattacharya S., Mehra M., Datta P.K., Bandyopadhyay S., et al. One- and two-photon uncaging: carbazole fused o-hydroxycinnamate platform for dual release of alcohols (same or different) with real-time monitoring. Org Lett. 2018;20:2241–2244. doi: 10.1021/acs.orglett.8b00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Venkatesh Y., Rajesh Y., Karthik S., Chetan A.C., Mandal M., Jana A., et al. Photocaging of single and dual (similar or different) carboxylic and amino acids by acetyl carbazole and its application as dual drug delivery in cancer therapy. J Org Chem. 2016;81:11168–11175. doi: 10.1021/acs.joc.6b02152. [DOI] [PubMed] [Google Scholar]
- 356.Ferguson L.R., Denny W.A. The genetic toxicology of acridines. Mutat Res. 1991;258:123–160. doi: 10.1016/0165-1110(91)90006-h. [DOI] [PubMed] [Google Scholar]
- 357.Chen Y.Y., Lukka P.B., Joseph W.R., Finlay G.J., Paxton J.W., McKeage M.J., et al. Selective cellular uptake and retention of SN28049, a new DNA-binding topoisomerase II-directed antitumor agent. Cancer Chemother Pharmacol. 2014;74:25–35. doi: 10.1007/s00280-014-2469-x. [DOI] [PubMed] [Google Scholar]
- 358.Mitra P., Chakraborty P.K., Saha P., Ray P., Basu S. Antibacterial efficacy of acridine derivatives conjugated with gold nanoparticles. Int J Pharm. 2014;473:636–643. doi: 10.1016/j.ijpharm.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 359.Zawada Z., Safarík M., Dvoráková E., Janousková O., Brezinová A., Stibor I., et al. Quinacrine reactivity with prion proteins and prion-derived peptides. Amino Acids. 2013;44:1279–1292. doi: 10.1007/s00726-013-1460-x. [DOI] [PubMed] [Google Scholar]
- 360.Belmont P., Bosson J., Godet T., Tiano M. Acridine and acridone derivatives, anticancer properties and synthetic methods: where are we now? Anti Cancer Agents Med Chem. 2007;7:139–169. doi: 10.2174/187152007780058669. [DOI] [PubMed] [Google Scholar]
- 361.Denny W.A. Acridine derivatives as chemotherapeutic agents. Curr Med Chem. 2002;9:1655–1665. doi: 10.2174/0929867023369277. [DOI] [PubMed] [Google Scholar]
- 362.Cholewinski G., Dzierzbicka K., Kołodziejczyk A.M. Natural and synthetic acridines/acridones as antitumor agents: their biological activities and methods of synthesis. Pharmacol Rep. 2011;63:305–336. doi: 10.1016/s1734-1140(11)70499-6. [DOI] [PubMed] [Google Scholar]
- 363.Zhuang H.B., Tang W.J., Yu J.Y., Song Q.H. Acridin-9-ylmethoxycarbonyl (Amoc): a new photochemically removable protecting group for alcohols. Chin J Chem. 2006;24:1465–1468. [Google Scholar]
- 364.Jana A., Saha B., Karthik S., Barman S., Ikbal M., Ghosh S.K., et al. Fluorescent Photoremovable precursors (acridin-9-ylmethyl)ester: synthesis, photophysical, photochemical and biological applications. Photochem Photobiol Sci. 2013;12:1041–1052. doi: 10.1039/c3pp25362g. [DOI] [PubMed] [Google Scholar]
- 365.Piloto A.M., Hungerford G., Costa S.P.G., Goncalves M.S.T. Acridinyl methyl esters as photoactive precursors in the release of neurotransmitter amino acids. Photochem Photobiol Sci. 2013;12:339–347. doi: 10.1039/c2pp25261a. [DOI] [PubMed] [Google Scholar]
- 366.Ray S., Banerjee S., Singh A.K., Ojha M., Mondal A., Singh N.D.P. Visible light-responsive delivery of two anticancer drugs using single-component fluorescent organic nanoparticles. ACS Appl Nano Mater. 2022;5:7512–7520. [Google Scholar]
- 367.Biswas S., Mengji R., Barman S., Vangala V., Jana A., Singh N.D.P. ‘AIE + ESIPT’ Assisted photorelease: fluorescent organic nanoparticles for dual anticancer drug delivery with real-time monitoring ability. Chem Commun. 2018;54:168–171. doi: 10.1039/c7cc07692d. [DOI] [PubMed] [Google Scholar]
- 368.Jesus S., Schmutz M., Som C., Borchard G., Wick P., Borges O. Hazard assessment of polymeric nanobiomaterials for drug delivery: what can we learn from literature so far. Front Bioeng Biotechnol. 2019;7:261–297. doi: 10.3389/fbioe.2019.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Kohane D.S., Langer R. Biocompatibility and drug delivery systems. Chem Sci. 2010;1:441–446. [Google Scholar]
- 370.Ratjen L., Arrue L. Internal targeting and external control: phototriggered targeting in nanomedicine. ChemMedChem. 2017;12:1908–1916. doi: 10.1002/cmdc.201700621. [DOI] [PubMed] [Google Scholar]
- 371.Abueva C. Photo-triggered theranostic nanoparticles in cancer therapy. Med Lasers. 2021;10:7–14. [Google Scholar]