Abstract
We have developed a catalytic asymmetric Nazarov cyclization that results in the formation of two contiguous all-carbon quaternary stereocenters in high yield with excellent levels of asymmetric induction. This method requires no catalyst recognition elements in the starting materials that are simple diketoesters. Geometrically pure E or Z isomers of the starting material lead to diastereomerically pure products with high enantioselectivity because the species that undergoes cyclization is a rhodium enolate that is configurationally stable.
Graphical Abstract
A catalytic asymmetric Nazarov cyclization that generates vicinal aliphatic all-carbon quaternary centers diastereoselectively and in high enantiomeric excess has been developed.

Introduction
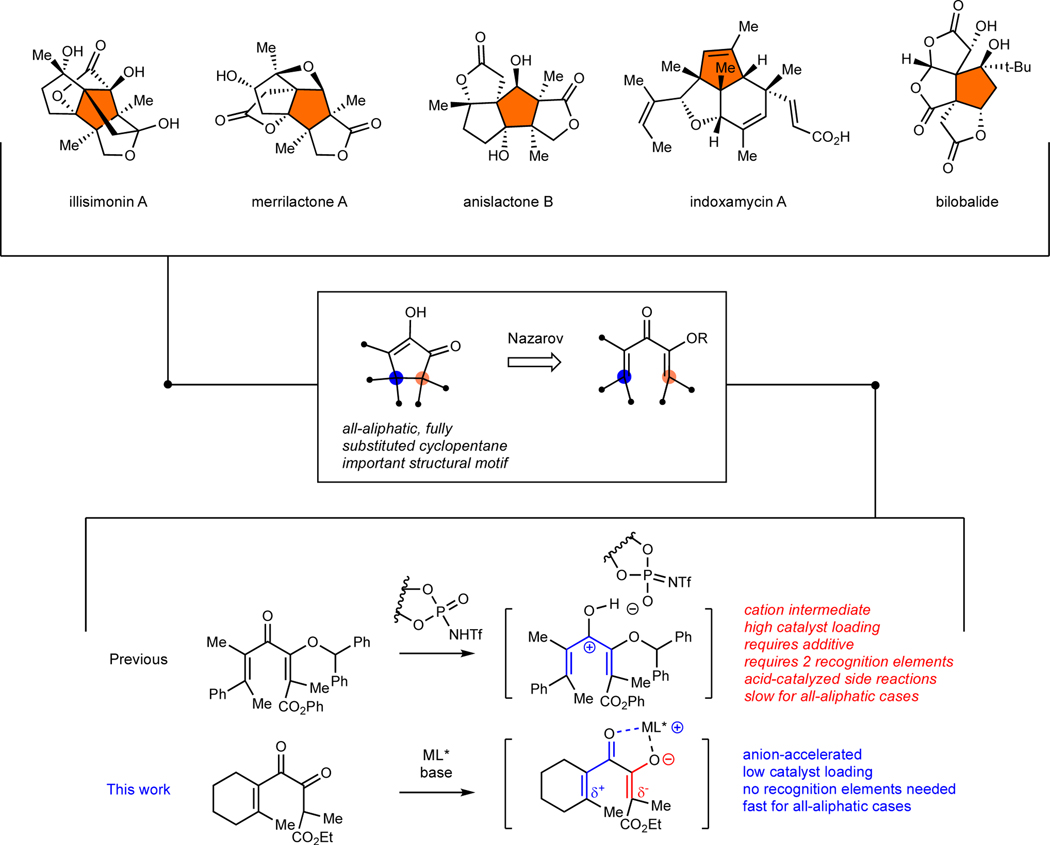
Natural products remain an important source of inspiration for the development of new drugs. A family of natural products whose syntheses have been especially challenging incorporate an embedded, highly substituted cyclopentane ring as seen in the examples of Figure 1.1 The presence of vicinal, all-carbon quaternary stereocenters in the ring presents a difficult problem that has generally been addressed through target-specific solutions.2,3 A general method to install this feature diastereo- and enantioselectively through a catalytic process would enable new strategies for total synthesis.
Figure 1.
Cyclopentane natural products with vicinal all-carbon quaternary stereocenters.
The Nazarov cyclization4 is an attractive choice for the synthesis of these complex cyclopentane rings because the stereochemical course of the reaction is orbital symmetry controlled and high diastereoselectivity can be achieved with geometrically pure starting materials. Nevertheless, inducing asymmetry at two fully substituted β-carbon atoms is very challenging. The only other report of a catalytic asymmetric Nazarov cyclization that achieves this is from our group using a chiral Brønsted acid catalyst (Fig 1).5 Although product yields and enantiomeric purities were excellent for substrates activated by an aryl group, they were lower for the all-aliphatic cases that were also slower to react. Two recognition elements were required in the starting material and acid-catalyzed side reactions were observed in some cases. In what follows, we report a conceptually different approach involving an anion-accelerated catalytic asymmetric Nazarov cyclization that produces fully substituted cyclopentenones under extraordinarily mild conditions.
Results and Discussion
Our starting point was a base-mediated Nazarov cyclization of α-ketoenones (eq 1).6 Treatment of diketoester 1 with substoichiometric strong base led to a fast reaction leading to a single diastereomer of (±)-3 in 60% yield. Catalytic activity was lost after 2–2.5 turnovers because of precipitation of MgCl2 with the formation of a lithium enolate that does not undergo cyclization at room temperature. For reasons discussed6 the cyclization leading to (±)-3 is unlikely to be an intramolecular Michael process. Instead, it is a conrotatory electrocyclization of enolate 2 that is greatly accelerated by the complementary polarization of the two reacting carbon atoms.7 The negative polarization of the β-ketoester is amplified in the magnesium enolate while chelation of magnesium to the ketone carbonyl group also polarizes the methylcyclohexenyl carbon atom positively. Since the reaction of eq 1 was rapid and highly diastereoselective, conditions were screened to develop an asymmetric process.8
 |
(1) |
The best result after an extensive screen of solvents, temperatures, asymmetric ligands and metals was a disappointing 65:35 e.r. (see page S-3 for greater detail). This may be an indication of two competing processes taking place through mono- and bidentate chelates (vide infra) or of a loose association of the substrate with the catalyst. To overcome this problem, two-point binding to the catalyst should be a requirement for cyclization to take place, thereby constraining the conformational freedom of the substrate. These considerations led us to consider Meggers’ excellent chiral-at-metal catalysts.9 We first developed a more convenient synthesis of the diketoester starting materials. Our original synthesis had made use of carboethoxy methylketene, however, the short solution lifetime of the ketene rendered the process challenging to perform on scale. Our improved method is illustrated in Scheme 1. Acid chloride 4 was converted to acetylenic ketoester 5 in 96% yield through reaction with benzyl propiolate in the presence of cuprous iodide and diisopropylethylamine. Regioselective conjugate addition of methylcopper followed by oxidation of the resulting vinyl copper intermediate with lithium tert-butylhydroperoxide led to diketoester 6 in 51–60% yield through a 1,2-metallate rearrangement.10 This practical method greatly simplified the preparation of the starting materials.
Scheme 1.
Preparation of the Starting Materials. (a) 1.5 equiv benzyl propiolate, 2.0 equiv iPr2NEt, 10 mol% CuI, CH2Cl2, rt, 2 h; 96%; (b) 1.5 equiv CuBr•Me2S, 1.5 equiv MeLi, Et2O, −78 °C, 15 min; 2.0 equiv tert-BuO2Li, −78 °C; 51–60%.
We optimized the cyclization in two iterations. The first iteration was carried out with reactive diketoester 7 and Meggers’ Λ-RhO catalyst9 to determine the effect of base and solvent on the enantiomeric excess of product (eq 2). Base was required for catalytic activity. All tertiary amines screened in acetonitrile led to a complete reaction to product in 86:14 e.r. (see page S-4) within 24 h at room temperature. The results were the same whether 1 equiv or 20 mol% of amine was used. Stoichiometric lithium tert-butoxide led to nearly racemic product (56:44 e.r.) whereas with 20 mol% a poor enantiomeric ratio was observed (ca. 78:22 e.r.) that varied from reaction to reaction. This is consistent with two competing processes taking place, one through a monometallic bidentate rhodium complex, the other through a bimetallic rhodium-lithium complex analogous to what is shown in eq 1. The base probably addresses the problem of product inhibition by shuttling a proton between the two oxygen atoms of the catalyst-bound product. There was a strong correlation between the dielectric constant of the solvent and the optical purity of product, with DMSO and acetonitrile leading to the best results and isooctane leading to nearly racemic product (see Table S2 on page S-5).
 |
(2) |
In the second iteration thirteen chiral-at-rhodium catalysts were screened in the cyclization of 7 (see page S-7).11 The three best catalysts were subsequently evaluated in the reactions of the more challenging fully substituted diketoesters 9 so as to determine the effect of the ester group R1 (Table 1). The rhodium catalyst in which R = 1-adamantyl converted diketoester 9 (R1 = Et) to product 10 in 96:4 e.r. (entry 1). The same diketoester led to product of lower e.r. with Λ-RhO (R = tert-butyl) and when R = 3,5-dimethyladamantyl (entries 4 and 5, respectively). These results suggest that the 1-adamantyl substituent is optimal: dimethyladamantyl is too large and tert-butyl is too small. Comparing the results with ethyl, benzyl and tert-butyl esters (entries 1, 2 and 3) indicates a small advantage of benzyl over ethyl. The tert-butyl ester led to racemic product. The results from using the hexafluorophosphate salts of the catalyst were identical to those from the trifluoroacetate salts. Unless specified otherwise subsequent work was done with 1 mol% of catalyst Λ−11 in acetonitrile, 0.5 equiv of diisopropylethylamine and benzyl esters (Figure 2). An inert atmosphere and scrupulously dry reaction conditions were not necessary.
Table 1.
Catalyst and Ester Optimization

| |||
|---|---|---|---|
| entry | R1 | R | e.r. |
| 1 | Et | 1-adamantyl | 96:4 |
| 2 | Bn | 1-adamantyl | 97:3 |
| 3 | t-Bu | 1-adamantyl | 50:50 |
| 4 | Et | t-Bu (Λ-RhO) | 94:6 |
| 5 | Et | Δ−3,5-dimethyladamantyl | 12:88 |
Figure 2.
Reaction Scope. Typical scale was 150 to 200 mg of 12. aAbsolute stereochemistry determined by XRD of the (R)-camphanate derivative; see S.I. bA 3:1 mixture of 23 and 22 (97:3 e.r.) was isolated in 83% combined yield from an inseparable 3:1 Z/E mixture of starting material. All four stereoisomers were separable by hplc. cThe Δ-enantiomer of 11 was used (2 mol %). dDMSO used as solvent. eThe catalyst-product complex precipitated from MeCN during the reaction. fA mixed solvent of 3.6:1 MeCN/DMSO was used. g2.5 mol% of Λ−11 was used. hUse of the Δ-enantiomer of 11 led to ent-32 in 97:3 e.r. iReaction complete in 2 d.
Figure 2 summarizes our results. With the exception of 33, 35 and 36 all reactions led to single diastereomers (>100:1, as determined by hplc). Relative stereochemistry was assigned by nOe. Absolute stereochemistry was determined by XRD (x-ray diffraction) of the (R)-camphanate derivatives of 21 and 32 and the rest assigned by analogy.12 The enantiomeric ratios of products were determined by hplc on a chiral stationary phase with the racemate used as a standard. Unless noted otherwise, all racemates were prepared using stoichiometric scandium triflate in acetonitrile with excess diisopropylethylamine, and were isolated by preparative TLC. The scandium-catalyzed cyclizations were not highly diastereoselective.13
The tetrasubstituted cyclopentenones (13; R1, R2, R3, R4 ≠ H) were formed in slightly better enantiomeric purity than their trisubstituted counterparts, as revealed by comparison of 14 with 28 and of 20 with 31. Cyclopentenones 14 – 17 in which R2 is a primary aliphatic group were all formed in 97:3 e.r. whereas 19 and 20 in which R2 is branched were formed in 99:1 e.r. It is remarkable that diastereomers 20 and 21 were formed in comparable yields and enantiomeric purities and as single diastereomers. Diastereomer 21 is derived from the Z isomer of 12 (R3 = cyclohexyl; R1, R2, R4 = Me) in which the large “inside” cyclohexyl group impedes cyclization by disfavoring the reactive, U-shaped conformer of the diketoester. As expected, the scandium triflate-catalyzed cyclization that was used to prepare a sample of the racemate of 21 led to a very small amount of product and a large number of byproducts.14 Such diastereoselectivity is to the best of our knowledge unprecedented in any Nazarov cyclization and is revealing of the mechanism. Since the cyclization proceeds through the rhodium enolate of the diketoester (vide infra), competing isomerization of the double bond that would have led to diastereomeric products does not take place as it would have during a Lewis or Brønsted acid catalyzed Nazarov cyclization proceeding through a pentadienyl cation. Stereochemical scrambling does not compete with cyclization and the stereochemistry of the products is determined only by the sense of conrotation that is imposed by the catalyst. Catalyst 11 is also able to discriminate between groups R2 and R3 of similar size as demonstrated by the synthesis of 22 (97:3 e.r.). Curiously 23, the diastereomer of 22, was formed in only modest enantiomeric purity (83:17 e.r.). The diketoester starting material for 23 had undergone facile Z to E isomerization prior to cyclization so the inseparable 3:1 Z/E mixture of geometrical isomers was used which led to a 3:1 mixture of 23 and 22. The enantiomeric purity of 22 was the same (97:3) as from the cyclization that used geometrically pure E diketoester. We do not have an explanation for the difference between the reactions that lead to 21 and to 23. Hydroazulenone 24 that was formed in moderate 85:15 e.r. defines a limit of applicability of the catalyst.
Although our primary interest were cyclopentenones bearing vicinal quaternary stereocenters we also examined the scope of the quaternary-tertiary case. Cyclopentenones 28 – 32 were formed in good enantiomeric excess. Diastereomeric cyclopentenones 31 and 32 that were derived from the E and Z isomers of 12 (for 32, R1 = Me; R2 = H; R3 = cyclohexyl) were both formed in excellent enantiomeric excess, although the reaction leading to 32 was slow.15 When the Δ-enantiomer of 11 was used as the catalyst ent-32 was formed in 97:3 e.r. In these two cases the catalyst bound to the product precipitated in acetonitrile after some time. In the case of 31, addition of DMSO (3.6:1 MeCN:DMSO) and an additional 1.5 mol% of 11 restored catalytic activity. In the case of 32 the reaction was carried out in neat DMSO with 1 mol% catalyst. It is unclear what factors contribute to the precipitation of the bound complex from acetonitrile in these two cases. For example, 20 differs from 31 only in having a methyl group in place of hydrogen (R3 in 13) yet gave no indication of product inhibition in acetonitrile. Regardless, the problem can be addressed effectively by adding DMSO to solubilize the precipitated complex.
A limited number of cyclopentenones substituted with aryl rings were prepared. A comparison of 31 (92:8 e.r.) and 33 (89:11 e.r.; 38:1 d.r.) shows that a phenyl ring in place of cyclohexyl erodes the enantioselectivity and also leads to formation of a small amount of a diastereomer. The same trend is seen by comparing 27 (87.5:12.5 e.r) and 21 (99:1 e.r.). These results are unsurprising since the catalyst was optimized using an all-aliphatic acyclic substrate. The preparation of 34 in good yield (83%, 86:14 e.r.) is noteworthy. Dienones 12 in which R1 = H generally undergo Nazarov cyclization in poor yield because the steric bias to favor the U-shaped conformer is lacking. The conformational constraints imposed on the substrate by Λ−11 appear to compensate for this.16
The cyclization that takes place through a metal enolate distinguishes this method from all other Nazarov cyclizations.
Because Brønsted acid is not used as a catalyst, acid-catalyzed Scheme 2 Transformations of the Products side reactions do not take place and the geometric purity of the starting material is not degraded. Consequently, the process is highly diastereoselective as illustrated by the examples of 21 and 32 in which a large “inside” cyclohexyl group is tolerated. This enables the synthesis of cyclopentenone diastereoisomers that are inaccessible using acid catalysis. The diketoester functionality is unique to our substrates and contributes to high reactivity as a result of the complementary polarization of the two reacting carbon atoms (Eq. 1). Because cyclization through diketoesters is terminated through proton transfer to the enolic oxygen atom of the product, both stereocenters are preserved. This is in contrast with the majority of Nazarov cyclizations which are terminated through loss of one of the β protons, thereby ablating one of the two stereocenters created during cyclization.
Scheme 2.
Transformations of the Products
The strong correlation between the solvent dielectric constant and the optical purity of product noted above supports the formation of a bidentate rhodium enolate, illustrated for 7 in Figure 3. In polar aprotic solvents the ammonium ion is dissociated from the diketoester that is bound to the rhodium through a bidentate chelate. As solvent polarity decreases, stronger ion-pairing between the enolate of the diketoester and the ammonium ion is favored, leading to a monodentate interaction with rhodium and poor asymmetry transfer. DFT studies also support the intermediacy of a bidentate rhodium enolate.17
Figure 3.
Bidentate vs. Monodentate Chelation
The structures shown in Figure 2 open new chemical space. For example, ent-18 (Scheme 2) is the core structure of merrilactone A, of illisimonin A and of anislactone B. The α-hydroxyenone can be transformed to other functionality in a number of ways5b some of which are shown in Scheme 3. Oxidative cleavage of the cyclopentenone ring in 20 by periodate in aqueous methanol18 leads to a single isomer of γ-butyrolactone 37 in which all three contiguous ring carbon atoms are fully substituted. This oxidation also takes place in air at room temperature.19 Conversion of 14 to the enol triflate followed by Negishi cross coupling5b led to fully substituted cyclopentenone 38. The oxidative Wagner-Meerwein rearrangement of 22 led to α-diketone 39 as a single geometrical isomer at the exocyclic double bond as determined by nOe. The exclusive migration of the methyl group may be a consequence of favorable overlap of the σ bond of the allylic methyl group with the π orbital in the preferred conformer of 22.20 All three reactions take place with no loss of optical purity and are unoptimized.
Conclusions
Trauner described the first enantioselective protonation in a catalytic asymmetric Nazarov cyclization.21 More recently Jacobsen and Rueping have applied a hydrogen bonding organocatalyst22 and a CBA,23 respectively, for this reaction. Torquoselective Nazarov cyclizations have been accomplished through bidentate chelation of α-carboalkoxy divinyl ketones to an asymmetrically ligated Cu(II),24 Ni(II),25 Fe(II)26 or Co(II)26 catalyst.27 Cyclization in these cases has been limited to substrates that are strongly activated by aryl substituents at both β carbon atoms of the divinyl ketone starting material. Meggers was the first to apply chiral-at-metal catalysts to torquoselective Nazarov cyclizations of highly activated substrates.9,28 Rawal’s Cr(II) salen29 and List’s IDPi catalysts30 provide the only examples to date of asymmetric Nazarov cyclizations of unactivated dienones leading to products bearing two contiguous tertiary stereocenters.31 The challenges of developing general methods to address this problem have been reviewed.2 This provides the context for our findings that represent only the second example of a catalytic asymmetric Nazarov cyclization that forms two contiguous all-carbon quaternary stereocenters with high levels of asymmetric induction. Key to the success of the method is the fact that the species that undergoes cyclization is a rhodium enolate that is configurationally stable, rather than a cation that is not. This mode of catalysis is likely to be applicable to reactions other than the Nazarov cyclization.
Supplementary Material
Acknowledgements
G.P.A.Y. (University of Delaware) thanks the National Institutes of Health for S10-OD026896A. The authors thank Mr. Wesley Yoshida (University of Hawaii) for performing all of the 2D NMR experiments and Dr. Thanh C. Ho (Northeastern University) for providing mass spectrometry data.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Conflicts of interest
There are no conflicts to declare.
Notes and references
- 1.For recent syntheses of these natural products see: (a) illisimonin A: Burns AS and Rychnovsky SD, S. D. J. Am. Chem. Soc, 2019, 141, 13295. [DOI] [PubMed] [Google Scholar]; Etling C, Tedesco G, Di Marco A, A. and Kalesse M, J. Am. Chem. Soc, 2023, 145, 7021. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) merrilactone A: Liu W and Wang B, Chem. Eur. J, 2018, 24, 16511; [Google Scholar]; Huffman BJ, Chu T, Hanaki Y, Wong JJ, Chen S, Houk KN and Shenvi RA, Angew. Chem. Int. Ed, 2022, 61, e202114514. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) anislactone B: Shen Y, Li L, Xiao X, Yang S, Hua Y, Wang Y, Zhang Y and Zhang Y, J. Am. Chem. Soc, 2021, 143, 3256. [DOI] [PubMed] [Google Scholar]; (d) indoxamycin A: Hu N, Dong C, Zhang C and Liang G, Angew. Chem. Int. Ed, 2019, 58, 6659. See also: [DOI] [PubMed] [Google Scholar]; Jeker OF and Carreira EM, Angew. Chem. Int. Ed, 2012, 51, 3474. [DOI] [PubMed] [Google Scholar]; (e) bilobalide: Baker MA, Demoret RM, Ohtawa M and Shenvi RA, Nature, 2019, 575(7784), 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For example see: (a) Chan W-L. Tang X, Zhang F, Quek G, Mei G-J and Lu Y, Angew. Chem. Int. Ed, 2019, 58, 6260. [DOI] [PubMed] [Google Scholar]; (b) Chen S-K, Ma W-Q, Yan Z-B, Zhang F-M, Wang S-H, Tu Y-Q, Zhang X-M and Tian J-M, J. Am. Chem. Soc, 2018, 140, 10099. [DOI] [PubMed] [Google Scholar]; (c) Liu X, Zhao C, Zhu R and Liu L, Angew. Chem. Int. Ed, 2021, 60, 18499. [DOI] [PubMed] [Google Scholar]; (d) Zhu Y, Wang H, Wang G, Wang Z, Liu Z and Liu L, Org. Lett, 2021, 23, 7248. [DOI] [PubMed] [Google Scholar]
- 3.Reviews: (a) Büschleb M, Dorich S, Hanessian S, Tao D, Schenthal KB and Overman LE, Angew. Chem. Int. Ed, 2016, 55, 4156. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Long R, Huang J, Gong J and Yang Z, Nat. Prod. Rep, 2015, 32, 1584. [DOI] [PubMed] [Google Scholar]
- 4.(a) Frontier AJ and Hernandez JJ, Acc. Chem. Res, 2020, 53, 1822. [DOI] [PubMed] [Google Scholar]; (b) Wenz DR and Read de Alaniz J, Eur. J. Org. Chem, 2015, 2015, 23. [Google Scholar]; (c) Di Grandi MJ, Org. Biomol. Chem, 2014, 12, 5331. [DOI] [PubMed] [Google Scholar]; (d) Tius MA, Chem. Soc. Rev, 2014, 43, 2979. [DOI] [PubMed] [Google Scholar]; (e) Vaidya T, Eisenberg R and Frontier AJ, ChemCatChem, 2011, 3, 1531. [Google Scholar]; (f) Shimada N, Stewart C and Tius MA, Tetrahedron, 2011, 67, 5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Jolit A, Walleser PM, Yap GPA and Tius MA, Angew. Chem. Int. Ed, 2014, 53, 6180. [DOI] [PubMed] [Google Scholar]; (b) Jolit A, Dickinson CF, Kitamura K, Walleser PM, Yap GPA and Tius MA, Eur. J. Org. Chem, 2017, 2017, 6067. [Google Scholar]
- 6.Batson WA, Sethumadhavan D and Tius MA, Org. Lett, 2005, 7, 2771. [DOI] [PubMed] [Google Scholar]
- 7.(a) Casson S and Kocienski P, J. Chem. Soc., Perkin Trans. 1, 1994, 1187. [Google Scholar]; (b) Tius MA, Kwok C-K, Gu X-q. and Zhao C, Synth. Commun, 1994, 24, 871. [Google Scholar]; (c) He W, Sun X and Frontier AJ, J. Am. Chem. Soc, 2003, 125, 14278. [DOI] [PubMed] [Google Scholar]
- 8.The origin of the diastereoselectivity of cyclization of the enol has been studied in detail computationally. It seems what is true for the enol is also true for the enolate: Asari A, Lam Y, Tius M and Houk K, J. Am. Chem. Soc, 2015, 137, 13191. [Google Scholar]
- 9.Mietke T, Cruchter T, Larionov VA, Faber T, Harms K and Meggers E, Adv. Synth. Catal, 2018, 360, 2093. [Google Scholar]
- 10.Minko Y, Pasco M, Lercher L, Botoshansky M and Marek I, Nature 2012, 490, 522. [DOI] [PubMed] [Google Scholar]
- 11.Catalyst Λ-RhO was prepared according to Meggers’ procedure in which ion exchange with ammonium hexafluorophosphate on silica converts an initially formed trifluoroacetate salt to the hexafluorophosphate. Catalyst Λ−11 and many of the trifluoroacetate salts of the other chiral-at-rhodium catalysts that we screened were mobile in dichloromethane/acetonitrile leading to poor recovery of the hexafluorophosphate after ion exchange. Because trifluoroacetate Λ−11 led to the same results as the corresponding hexafluoroacetate it was used instead. See: Brunen S, Grell Y, Steinlandt PS, Harms K and Meggers E, Molecules, 2021, 26, 1822.33804954 [Google Scholar]
- 12.See S.I. Camphanates have been used by us (reference 5a) and others for the assignment of absolute stereochemistry: Cane DE and Nachbar RB, Tetrahedron Lett., 1977, 49, 4277. [Google Scholar]
- 13.Any background reaction catalyzed by the tertiary amine is extremely slow. In the case of the tetrasubstituted compounds there was no product detectable by TLC (visualization by uv) from the control experiment after 24 h at room temperature. In the trisubstituted cases only a trace <2% of cyclic product could be detected after 24 h.
- 14.We were unable to separate the enantiomers of 21 by hplc. Dr. Jay Ferraro at Daicel kindly separated the enantiomers on an IA-3 column in the highly enriched sample from the reaction catalyzed by Λ−11 as well as from the complex mixture derived from the reaction that was catalyzed by scandium triflate. Although the retention times of the enantiomers from the two hplc samples matched, we performed an additional experiment to confirm the result. We converted the mixture of enantiomers of 21 that was derived from the reaction catalyzed by Λ−11 to the p-bromobenzoate esters that we were able to separate by hplc. Our ratio of the p-bromobenzoates was 97.5:2.5, identical to Daicel’s ratio of the enantiomers of 21.
- 15.A nearly racemic sample of 32 was prepared from a mixture of approximately equal quantities of Λ−11 and its enantiomer.
- 16.Compounds bearing an aryl group underwent cyclization rapidly, typically within 12 h, whereas the time to completion for the all-alkyl compounds was much slower (ca. 5 d). It may be that with aryl substitution the cyclization takes place before the diketone-catalyst reaches equilibrium.
- 17.Dr. Rui Sun and Mr. Kazuumi Fujioka (University of Hawaii Chemistry Department) will publish their findings in a separate paper.
- 18.Wozniak B, Spannenberg A, Li Y, Hinze S and de Vries JG, ChemSusChem, 2018, 11, 356. [DOI] [PubMed] [Google Scholar]
- 19.This oxidative process may lead to a hydroperoxy α-diketone as a first intermediate that forms an endoperoxide following nucleophilic addition of the peroxidic hydroxyl to the distal ketone carbonyl group. Subsequent collapse through homolysis of the oxygen-oxygen bond followed by loss of carbon monoxide would lead to the observed product. Dr. Robert Hahn was the first to observe this reaction in a compound structurally related to 14.
- 20.Compound 22 was recrystallized once to 99:1 e.r. prior to the reaction with PIFA. When 15 and 24 were allowed to react with PIFA only the product of methyl migration was observed.
- 21.Liang G and Trauner D, J. Am. Chem. Soc, 2004, 126, 9544. [DOI] [PubMed] [Google Scholar]
- 22.Metternich JB, Reiterer M and Jacobsen EN, Adv. Synth. Catal, 2020, 362, 4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23 <j/>(a).Rueping M, Ieawsuwan W, Antonchick AP and Nachtsheim BJ, Angew. Chem. Int. Ed, 2007, 46, 2097. [DOI] [PubMed] [Google Scholar]; (b) Rueping M and Ieawsuwan W, Adv. Synth. Catal, 2009, 351, 78. [Google Scholar]
- 24.Cao P, Deng C, Zhou Y-Y, Sun X-L, Zheng J-C, Xie Z and Tang Y, Angew. Chem. Int. Ed, 2010, 49, 4463. [DOI] [PubMed] [Google Scholar]
- 25.Takeda T, Harada S and Nishida A, Org. Lett, 2015, 17, 5184. [DOI] [PubMed] [Google Scholar]
- 26.Kawatsura M, Kajita K, Hayase S and Itoh S, Synlett, 2010, 1243. [Google Scholar]
- 27.A recent paper describes a Au(I)-catalyzed asymmetric Nazarov cyclization: Solas M, Suárez-Pantiga S and Sanz R, Angew. Chem. Int. Ed, 2022, 61, e202207406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong Y, Jarrige L, Harms K and Meggers E, J. Am. Chem. Soc. 2019, 141, 4569. [DOI] [PubMed] [Google Scholar]
- 29.Hutson GE, Türkmen YE and Rawal VH, J. Am. Chem.Soc, 2013, 135, 4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ouyang J, Kennemur JL, Kanta De C, Farès C and List B, J. Am. Chem. Soc, 2019, 141, 3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawal’s reaction controls three tertiary stereocenters. The two at the β carbon atoms are controlled through the torquoselective cyclization whereas the α stereocenter is the result of proton transfer; see reference 29.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.