Abstract
BACKGROUND:
Cystic fibrosis (CF) is a rare, life-threatening disease that results in severe respiratory, digestive, and metabolic problems. Elexacaftor/tezacaftor/ivacaftor is an oral drug that was approved by the US Food and Drug Administration (FDA) on October 21, 2019, after demonstrating clinical improvements compared with previous CF transmembrane conductance regulator modulators. Use of CF transmembrane conductance regulator modulators has improved CF care, but their high costs exceed commonly used cost-effectiveness thresholds. The Institute for Clinical and Economic Review issued an access and affordability alert warning that these high costs could threaten sustainable access to high-value care. There exists little real-world evidence on the uptake of elexacaftor/tezacaftor/ivacaftor and the impact on total cost of care and other health care resource utilization. This exploratory study analyzed the uptake and total cost-of-care impact of elexacaftor/tezacaftor/ivacaftor using pharmacy and medical claims data in a commercially insured patient population.
OBJECTIVE:
To analyze the uptake of elexacaftor/tezacaftor/ivacaftor by members who qualified for treatment and to evaluate the differences in total cost of care and health care resource utilization in members who started treatment with elexacaftor/tezacaftor/ivacaftor.
METHODS:
Uptake and per-member per-month information was obtained from Prime Therapeutics databases using cystic fibrosis transmembrane conductance regulator (CFTR) modulator claims. The total cost-of-care and resource utilization analysis used pharmacy and medical claims from Prime Therapeutics and Blue Cross NC across approximately 1.34 million commercially insured members over 20 months. Members with CF were identified by 2 or more International Classification of Diseases, Tenth Revision codes (E84.xx) in any field at least 30 days apart or by a CFTR modulator claim. Only continuously enrolled members with CF with an elexacaftor/tezacaftor/ivacaftor pharmacy claim were included. The date of the first claim served as the index date.
RESULTS:
At 12 months after FDA approval, 77 (68%) Blue Cross NC members with CF were using elexacaftor/tezacaftor/ivacaftor. Of these, 33 had switched from a different CFTR modulator and 44 were naive to CFTR modulator therapy. Pharmacy and medical claims for 51 continuously enrolled members that initiated elexacaftor/tezacaftor/ivacaftor were analyzed. The average total cost of care increased by 52% (P < 0.00001). Hospitalizations decreased from an average of 7.7 (± 7.2) to 3.9 (± 5.5) (P < 0.00001). The sum and average number of Pseudomonas aeruginosa infections were numerically lower, but the results did not meet statistical significance. Use of other supportive medications was numerically lower, but no statistically significant differences were observed.
CONCLUSIONS:
The uptake of elexacaftor/tezacaftor/ivacaftor was rapid, and the total cost of care increased despite reductions in hospitalizations and nonpharmacy costs. Differences in use of other CF-related medications appeared to be minimally affected.
Plain language summary
Elexacaftor/tezacaftor/ivacaftor is a therapy approved for the treatment of cystic fibrosis. It delivers many benefits to patients with cystic fibrosis but is very expensive. It is not known how it impacts total health care costs. The purpose of this study was to measure the change in total costs for patients taking elexacaftor/tezacaftor/ivacaftor.
Implications for managed care pharmacy
Elexacaftor/tezacaftor/ivacaftor uptake was rapid and resulted in a significant per-member per-month (PMPM) pharmacy cost increase. Total plan costs increased despite reductions in nonpharmacy costs for newly initiated members. We expect use to continue to increase given recent and future label expansions.
The implication for managed care pharmacy is that as the US Food and Drug Administration label is expanded to larger patient populations, PMPM costs are likely to increase quickly and significantly for plans and should be planned for accordingly.
Cystic fibrosis (CF) is an autosomal recessive disease that occurs because of mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene. It is a rare disease that significantly impacts the quality of life of those with the condition.1 The overall prevalence of CF in the United States was 30,755 in 2016.2 Over time, the incidence rates have decreased as diagnostic criteria and methods have changed, and data from registries indicates that prevalence is increasing because of improved survival rates with the development of better supportive care and new treatments.1,3,4
CF is managed through a combination of best supportive care (BSC) treatments that work by preventing chronic lung infections and pulmonary exacerbations while improving nutritional status. Treatment of pulmonary manifestations includes both nonpharmacologic and pharmacologic options.1-3 As reported by patients themselves, BSC is a substantial time burden to both patients and caregivers: airway clearance therapies and taking the pills and inhaled therapies can require daily treatment durations of 1-3 hours,5,6 sometimes even 6 hours a day.7 These complex regimens are usually coordinated through specialty clinics.
The standardization of BSC increased the median predicted survival for patients with CF. Between 2000 and 2010, the median survival age improved 1.8% each year (before the approval of CFTR modulators).1 The median age of individuals in the CF Foundation registry has increased from 11.5 in 1988 to 19.8 in 2018.8 Improvements in BSC have driven improved outcomes for patients with CF, but none of these treatment modalities addresses the underlying cause of CF.2,5
CFTR modulators have been developed over the past 2 decades and have transformed CF treatment and life expectancy. These medications directly target the underlying cause of the defects in the CFTR protein, improving lung function, pulmonary exacerbations, and nutritional status.9 There are 2 main classes of modulator medications with US Food and Drug Administration (FDA)–approved medications: potentiators and correctors. All the currently approved CFTR modulators are manufactured by the same company, Vertex Pharmaceuticals. All the CFTR modulators are oral medications that require daily dosing.
Potentiators restore function and increase the probability of the CFTR ion channel remaining open. Five percent of CF-causing mutations lead to impaired CFTR channel gating or conductance as the primary defect, representing mutation classes III and IV. The potentiator ivacaftor (Kalydeco) has shown clinical improvements in patient’s lives through reductions in sweat chloride levels, improved body mass index, increased exercise capacity, slower deterioration of lung function, reduction in pulmonary exacerbations, and less frequent lung infections.10 Further studies expanded its approval to include 38 different mutations. However, ivacaftor is only available to the limited number of patients with CF with class III and IV mutations.
Correctors address the folding of the CFTR protein and improve its transportation to the cell membrane. Most patients with CF carry at least one allele with this mutation, with the F508del allele being the most prevalent overall CF-causing mutation. According to 2019 data from the CF Foundation patient registry, 44.4% of patients are homozygous for the F508del mutation (F508del/F508del). Another 40.9% are heterozygous, possessing one F508del allele and either a residual function mutation (F508del/Res) or a minimal function mutation (F508del/MF). Correctors currently include lumacaftor, tezacaftor, and elexacaftor.
Lumacaftor is a first-generation corrector. When used in combination with ivacaftor, it demonstrated improvement in lung function in the F508del/F508del homozygous patient population. It was approved by the FDA/European Medicines Agency as the combination product lumacaftor/ivacaftor (Orkambi) in 2015 for patients that are F508del/F508del homozygous and aged 12 years and older; the label was expanded to patients aged 2 years and older in 2019.
Tezacaftor is a second-generation corrector. In clinical trials, treatment with tezacaftor/ivacaftor (Symdeko) demonstrated comparable therapeutic outcomes to lumacaftor/ivacaftor in F508del/F508del patients. When the combination was used in F508del/Res patients, it demonstrated better outcomes than ivacaftor alone, unlike the lumacaftor/ivacaftor combination. It was approved for use in patients aged 12 years and older with either the F508del/F508del or F508del/Res genotypes.
In F508del/minimal function (F508del/MF) genotype patients, neither dual-combination product restores CFTR function enough to treat this population. A minimal function mutation is one that does not produce protein or produces protein that is unresponsive to tezacaftor/ivacaftor.9 Approximately 30% of all patients with CF possess the F508del/MF genotype, meaning that these patients still had an unmet need.
The triple therapy combination product elexacaftor/tezacaftor/ivacaftor (Trikafta) was approved by the FDA in 2019 in patients aged 12 years and older with at least one F508del mutation.10,11 Its approval was based on two phase 3 trials in two different patient populations: those with the F508del/F508del12 genotype and those with the F508del/MF13 genotype. With its approval, the overall CFTR modulator eligible population has now risen to ~90%.14
For patients with the F508del/F508del genotype, treatment with elexacaftor/tezacaftor/ivacaftor resulted in improvements in lung function, sweat chloride concentration, respiratory-related quality of life, and nutritional parameters compared with tezacaftor/ivacaftor.12 Previously, patients with this genotype had an attenuated response to CFTR modulators compared with the recognized benchmark (ivacaftor treatment in G551 mutations). In this trial, patients were able to achieve improvements compared with tezacaftor/ivacaftor, including a 10-percentage-point improvement in lung function measured as absolute change in percent predicted forced expiratory volume in 1 second (ppFEV1) at 4 weeks (10.0; 7.4-12.6).12 Tezacaftor/ivacaftor was previously approved for treatment in younger age groups, but the label for elexacaftor/tezacaftor/ivacaftor was recently expanded to include patients aged 6-11 years.15 The costs are comparable between the 2 agents.
In patients with the F508del/MF genotype, elexacaftor/tezacaftor/ivacaftor demonstrated benefit.13 Compared with treatment with placebo and BSC, patients’ lung function improved with an absolute change in percentage of ppFEV1 from baseline at week 4 of 13.8%, which was sustained at 24 weeks at 14.3%. The trial was 24 weeks in duration, and the annualized rate ratio of pulmonary exacerbations leading to hospitalizations between the 2 groups was estimated at 0.29 (95% CI = 0.41-0.61).
It is currently unknown what the impact of elexacaftor/tezacaftor/ivacaftor will be on long-term total cost of care. It is expected to lower the overall number of pulmonary exacerbations leading to hospitalizations, but the longest trial conducted was 24 weeks in duration. A 96-week open-label study is ongoing to evaluate the long-term safety and tolerability of elexacaftor/tezacaftor/ivacaftor (both genotypes, NCT03525574) and is also measuring relevant outcome measures such as pulmonary exacerbations.16 In addition, the SIMPLIFY trial (NCT04378153) is evaluating the impact of discontinuing chronic therapies in people with CF, specifically inhaled hypertonic saline or dornase alfa in patients taking elexacaftor/tezacaftor/ivacaftor.17
The overall CF population eligible for elexacaftor/tezacaftor/ivacaftor is likely to expand in the future with the ongoing trials. New users will result in a large and sustained increase in costs as CFTR modulators are chronic therapies that will presumably be taken for the lifetime of each patient. A phase 3 trial evaluating elexacaftor/tezacaftor/ivacaftor in patients with CF aged 6-11 years (NCT04353817)18 was recently completed and resulted in a label expansion to ~1,500 additional patients in this new age group.19,20 A future study is already planned to investigate the drug’s effectiveness in children aged 2-5 years.21 Recently the FDA expanded the labels for the 3 main CFTR modulators to include new responsive mutations based on in vitro data. This includes an additional 177 mutations for elexacaftor/tezacaftor/ivacaftor.22
It has been hypothesized that patients with a good response to CFTR modulator therapy will decrease use of other daily, chronic health care resources, such as hypertonic saline and dornase alfa, inhaled antibiotics, and nocturnal tube feeding, resulting in decreased overall time burden of these daily treatments. However, this has not been demonstrated in any clinical trial or in any published real-world evidence. To address this knowledge gap, we sought to conduct an analysis of pharmacy and medical claims data to provide evidence to complement what is already known about the use of elexacaftor/tezacaftor/ivacaftor. The aim of this study was to generate evidence that will provide insights for payers, providers, patients, and other stakeholders into what can be expected when the access to this medicine is expanded to younger age groups and larger numbers of patients with CF.
Methods
ANALYSIS OF ELEXACAFTOR/TEZACAFTOR/IVACAFTOR UPTAKE AND PHARMACY PMPM IMPACT
Data Source and Study Design. To analyze the uptake and per-member per-month (PMPM) impact of elexacaftor/tezacaftor/ivacaftor over time, databases of a commercially insured population were queried in the period of January 1, 2018, to December 31, 2020. This period was selected to allow for a holistic view of the changes in CFTR modulator use over time. All pharmacy claims for the 4 FDA-approved CFTR modulator medications were pulled for analysis (elexacaftor/tezacaftor/ivacaftor, tezacaftor/ivacaftor, lumacaftor/ivacaftor, and ivacaftor, summarized in Supplementary Table 1 (401.2KB, pdf) , available in online article). Information obtained from the data source included number of unique users, total plan pharmacy costs, and PMPM plan pharmacy costs. The pharmacy claims data were sourced from the Prime Therapeutics LLC pharmacy claims database. Prime Therapeutics is the Pharmacy Benefit Manager for Blue Cross Blue Shield of North Carolina. These results are summarized in Figure 1.
FIGURE 1.
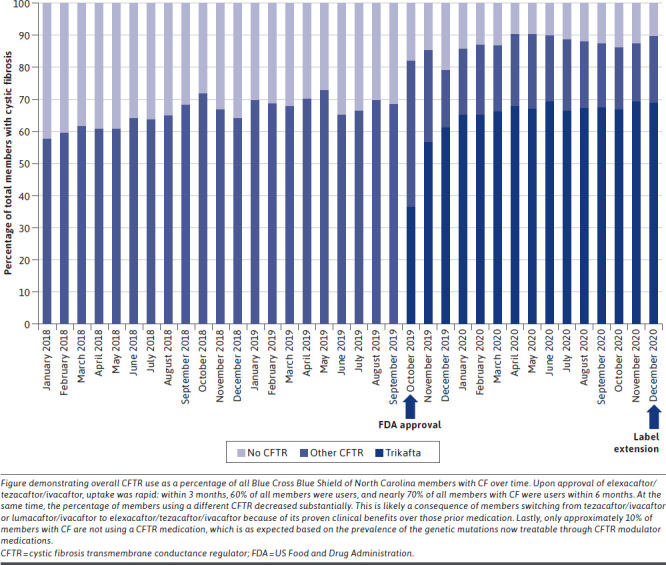
Elexacaftor/Ivacaftor/Tezacaftor Uptake by Members With Cystic Fibrosis Over Time
Study Population. For the analysis on the uptake of elexacaftor/tezacaftor/ivacaftor, members were identified by having any claim for a CFTR modulator medication. All users were included for the entire range of the data query (January 1, 2018, to December 31, 2021).
Elexacaftor/Tezacaftor/Ivacaftor Uptake Analysis. The analysis of elexacaftor/tezacaftor/ivacaftor uptake was completed by including all CFTR modulator claims and users during the data query period to capture the full impact of the medication. Use was reported as number of unique users of each CFTR modulator during each month of the query period.
PMPM Impact Analysis. Data on net costs for the PMPM impact analysis were supplied by Prime Therapeutics. The PMPM plan pharmacy costs were calculated using the total pharmacy costs divided by the total member months for each month. This allowed for an analysis of changes over time that accounts for changes in the number of members and is a standard normalization calculation used when looking at costs related to an insured population. A total PMPM pharmacy cost for members with CF was also calculated that included all plan medication costs for CF treatments. The month-over-month PMPM pharmacy cost changes are summarized in Figure 2.
FIGURE 2.
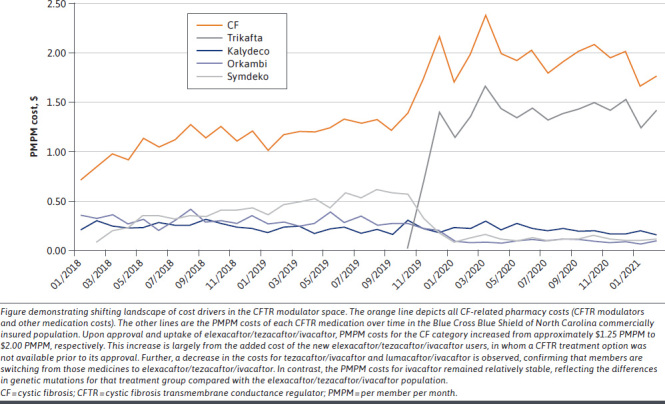
Elexacaftor/Tezacaftor/Ivacaftor Impact on Pharmacy PMPM Spending for Cystic Fibrosis
To better understand the specific impact of elexacaftor/tezacaftor/ivacaftor on the total plan CF pharmacy PMPM costs, an analysis was completed that measured the overall increase in PMPM after approval. To accomplish this, a total CF pharmacy PMPM pre-elexacaftor/tezacaftor/ivacaftor was calculated by taking an average of the 3 previous months of PMPM costs prior to the approval of elexacaftor/tezacaftor/ivacaftor. The total CF PMPM post-elexacaftor/tezacaftor/ivacaftor calculation was an average of the 10 months after elexacaftor/tezacaftor/ivacaftor approval. A longer duration in the post period was used to measure the full uptake of members starting elexacaftor/tezacaftor/ivacaftor. Averages were calculated to account for varying pharmacy fill dates each month and for members joining/leaving the plan. Although not as straightforward as a single calculation of the total pharmacy spend divided by the number of member months, this calculation presents the plan pharmacy PMPM impact over time after the approval and uptake of elexacaftor/tezacaftor/ivacaftor in a commercially insured population.
RETROSPECTIVE COHORT ANALYSIS: IMPACT OF ELEXACAFTOR/TEZACAFTOR/IVACAFTOR ON TOTAL COST OF CARE AND OTHER HEALTH CARE RESOURCE UTILIZATION
Data Source and Study Design. To analyze the changes in total cost of care and other health care resource utilization after elexacaftor/tezacaftor/ivacaftor initiation, a retrospective cohort study was conducted with pharmacy and medical claims data. Databases of a commercially insured population of approximately 1.34 million members were queried for the period of October 21, 2018, to December 31, 2020. Based on this query, it was determined at the time of writing that complete medical and pharmacy claims data existed fully through the date of August 31, 2020, meaning that a complete medical and pharmacy claims dataset only existed for ~10 months before and after the approval date of elexacaftor/tezacaftor/ivacaftor. To ensure equality of the data in both cohorts, only those members with continuous enrollment for the entire 20 months of duration were included in the analysis. The pharmacy claims data were sourced from the Prime Therapeutics database, and the medical claims from the Blue Cross Blue Shield of North Carolina (BCNC) database. Database information includes plan costs only for all pharmacy, facilities, and professional services claims for the queried members. The facilities category includes claims for inpatient and outpatient hospitals, emergency department visits, ambulatory care surgical centers, and independent clinics. Professional services include claims for ambulances, emergency departments, federally qualified health centers, independent clinics and laboratories, inpatient and outpatient hospitals, rehabilitation facilities, public health clinics, telehealth, and urgent care facilities.
Study Population. Members were identified as having CF with a claim containing an International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) code for the cystic fibrosis category (E84.xx). Only members aged older than 12 years were included. All pharmacy claims information was identified using Generic Product Identifier numbers.
For the analysis of the impact of elexacaftor/tezacaftor/ivacaftor on health care resource utilization, only members with a pharmacy claim for elexacaftor/tezacaftor/ivacaftor were included. The index date for each member was defined as the date of their first elexacaftor/tezacaftor/ivacaftor prescription pharmacy claim. The study period consisted of 10 months pre-index and 10 months post-index. Members were only included if they were continuously enrolled for the entire duration of the study period. The selection of a 10-month pre-/post-index period was made based on the completeness of the available medical claims data at the time and to include the largest possible population for this rare disease. A more traditional 12 months was desired, but the incompleteness of medical claims at the time of writing made this impossible. All members using elexacaftor/tezacaftor/ivacaftor were included regardless of individual adherence to their therapy, to better reflect real-world outcomes. The average elexacaftor/tezacaftor/ivacaftor prescription count was included to demonstrate the high adherence level of the cohort.
Member Characteristics. For the pre/post cohort section of the study, descriptive statistics were calculated on the included members using standard methods. Results were reported as averages, SDs, or percentages as appropriate. Characteristics of the members were summarized and are reported in Table 1.
TABLE 1.
Cohort Member Characteristics
| Characteristics | Value (N = 51) |
|---|---|
| Average age at Trikafta start date, years | 24 (± 11) |
| Sex | |
| Female | 29 (57) |
| Male | 21 (43) |
| Previously on a different CFTR modulator | 36 (71) |
| Members using dornase alfa at baseline | 32 (63) |
| Members receiving inhaled antibiotics | 24 (47) |
| Aztreonam | 8 (16) |
| Tobramycin | 11 (22) |
| Amikacin | 5 (10) |
| Members using diabetes medications | 8 (16) |
| Members using PERT | 43 (84) |
| Average Trikafta prescription count during study period | 11 (± 1.8) |
Characteristics of cohort members meeting inclusion criteria. Data are shown as n (%) or mean (± SD).
CFTR = cystic fibrosis transmembrane conductance regulator;
PERT = pancreatic enzyme replacement therapy.
Total Cost of Care and Other Health Care Resource Utilization. The total cost-of-care impact of elexacaftor/tezacaftor/ivacaftor was calculated in the cohort as both a total sum and a per-member average. Total plan costs were measured as a sum and broken down by claim site: facilities, professional services, and pharmacy. Other health care resource utilization was measured between the pre-/post-elexacaftor/tezacaftor/ivacaftor groups by calculating average hospitalizations, Pseudomonas infections, and other medication costs and prescription counts. Hospitalizations were defined as an inpatient hospital claim with a positive inpatient indicator and admission and discharge dates. Prescription counts were adjusted to 30-day supplies. The number of Pseudomonas infections was calculated by searching across all 51 members for the ICD-10 code B96.5, “Pseudomonas (aeruginosa) (mallei) (pseudomallei) as the cause of diseases classified elsewhere.” All calculations were completed using all 51 members (even if a specific variable did not occur for a member during a period, the data were still included as an average of all the 51 members). For all variables in which averages were calculated, statistical testing was done to measure for significant differences.
Statistical Analysis. Descriptive statistics, averages, and SDs were completed in a standard fashion. Owing to the small sample size, our data were found to be nonnormally distributed by examination as histograms and by using the Shapiro-Wilk test. To test for differences between averages, we used the Wilcoxon signed-rank test as an alternative to the paired Student’s t-test.
Results
ELEXACAFTOR/TEZACAFTOR/IVACAFTOR UPTAKE
The uptake of elexacaftor/tezacaftor/ivacaftor over time is illustrated in Figure 1. The percentage of members with CF at this health plan using elexacaftor/tezacaftor/ivacaftor increased to ~61% (68/111) within the first 3 months of its approval, reached ~68% (77/114) at 12 months, and reached a high of ~76% (77/101) at 16 months. Of the members with CF using any CFTR modulator therapy, the share of those using elexacaftor/tezacaftor/ivacaftor increased from 0% (0/56) at approval to 77% (77/100) 12 months after approval. Of the 77 new users of elexacaftor/tezacaftor/ivacaftor at 12 months after approval, 33 of the members had switched from a different CFTR modulator. The percentage of CF members not using any CFTR modulator decreased from 30% in October 2019 to 12% in October 2020.
PHARMACY PMPM COST IMPACT
The pharmacy PMPM costs over time are summarized in Figure 2. The plan pharmacy PMPM costs for all members with CF increased from $1.27 PMPM to $1.96 PMPM during the first 10 months after FDA approval of elexacaftor/tezacaftor/ivacaftor. The average total PMPM costs for elexacaftor/tezacaftor/ivacaftor were $1.31 PMPM during the first 10 months of its approval. The CF pharmacy PMPM costs increased by $0.69 during the first 10 months after FDA approval of elexacaftor/tezacaftor/ivacaftor.
In the same period, the combined PMPM costs of the other CFTRs (ivacaftor, lumacaftor/ivacaftor, and tezacaftor/ivacaftor) decreased from $1.05 PMPM to $0.47 PMPM. The PMPM costs for ivacaftor increased from $0.18 to $0.22. The PMPM costs for lumacaftor/ivacaftor decreased from $0.29 to $0.11, and the PMPM costs for tezacaftor/ivacaftor decreased from $0.57 to $0.14. Non-CFTR modulator pharmacy PMPM costs decreased overall from $0.22 to $0.16.
IMPACT OF ELEXACAFTOR/IVACAFTOR/TEZACAFTOR ON TOTAL COST OF CARE AND BY SITE OF CARE
We identified 51 members who started using elexacaftor/tezacaftor/ivacaftor and were continuously enrolled for the entire 20 months of the cohort study period. The full cohort member characteristics are reported in Table 1. The average age at initiation was 24 years, and 36 (71%) were previously on a different CFTR modulator. Eight (16%) of the members were using diabetes medications at study initiation, and 43 (84%) were on pancreatic enzyme replacement therapy.
The differences in total cost of care are illustrated in Figure 3. For the 51 continuously enrolled members who initiated elexacaftor/tezacaftor/ivacaftor, total costs increased by $5.8 million (a 52% increase). Pharmacy costs increased by $6.8 million (a 73% increase), facilities costs decreased by $0.8 million (a 73% decrease), and professional costs decreased by $0.3 million (a 50% decrease) during the study period.
FIGURE 3.
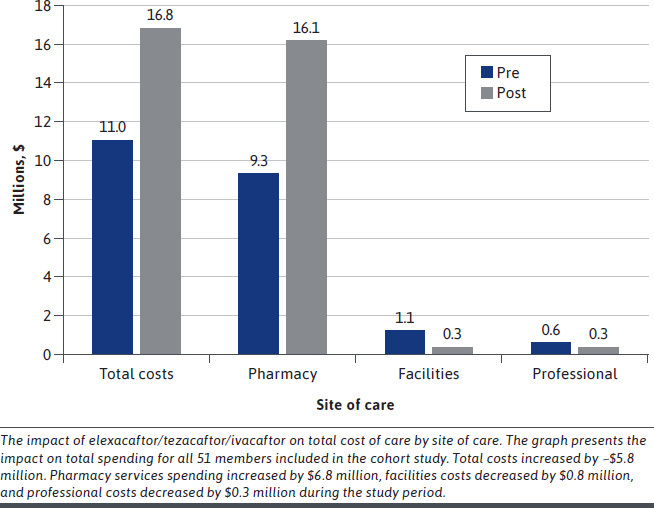
Impact of Elexacaftor/Ivacaftor/Tezacaftor on Total Cost of Care
The average impact on total costs per patient is summarized in Table 2. There was an average total increase of $114,000 (P < 0.00001) during the 10-month post-period. Average pharmacy costs increased by $134,000 (P < 0.00001), average facilities costs decreased by $15,000 (P < 0.00001), and average professional costs decreased by $6,000 (P < 0.00001).
TABLE 2.
Mean Changes in Total Costs, Hospitalizations, Pseudomonas Infections, and Other Health Care Resource Utilization
| Variable | Pre | Post | P value |
|---|---|---|---|
| Total costs, $ | 216,381 (124,066) | 329,583 (86,843) | < 0.00001 |
| Facilities costs, $ | 21,995 (36,454) | 7,245.75 (17,984) | < 0.00001 |
| Professional costs, $ | 11,603 (12,931) | 6,129 (6,984) | < 0.00001 |
| Pharmacy costs, $ | 182,783 (117,629) | 316,635 (76,714) | < 0.00001 |
| Hospitalizations | 7.7 (7.2) | 3.9 (5.5) | < 0.00001 |
| Pseudomonas infections, n | 0.27 (1.002) | 0.1 (0.361) | 0.833 |
| Dornase alfa prescriptions, n | 4.2 (4.1) | 3.8 (4.2) | 0.276 |
| Dornase alfa cost, $ | 17,707 (18,929) | 15,721 (19,045) | 0.263 |
| Inhaled aztreonam prescriptions, n | 0.61 (1.47) | 0.41 (1.2) | 0.826 |
| Inhaled aztreonam costs, $ | 5,155 (12,657) | 3,756 (11,023) | 0.857 |
| Inhaled tobramycin prescriptions, n | 2.36 (1.69) | 2.29 (1.86) | 0.726 |
| Inhaled tobramycin costs, $ | 1,382 (3,503) | 1,572 (3,808) | 0.976 |
| Inhaled amikacin prescriptions, n | 0.51 (1.92) | 0.29 (1.35) | 0.857 |
| Inhaled amikacin costs, $ | 5,345 (20,092) | 3,382 (15,579) | 0.857 |
| Insulin prescriptions, n | 1 (3.16) | 0.84 (2.53) | 0.897 |
| Insulin costs, $ | 361 (1,239) | 306 (969) | 0.889 |
| Oral diabetes prescriptions, n | 0.43 (2.94) | 0.22 (1.27) | 0.873 |
| Oral diabetes costs, $ | 214 (1,450) | 106 (611) | 0.873 |
| PERT prescriptions, n | 5.5 (3.4) | 4.9 (3.4) | 0.153 |
| PERT costs, $ | 26,188 (21,446) | 26,173 (19,774) | 0.741 |
Table of results from 51 continuously enrolled members who started therapy with elexacaftor/tezacaftor/ivacaftor. Total study duration was 20 months. Pre- and post-periods were 10 months before and after elexacaftor/tezacaftor/ivacaftor use, respectively. All data are presented as mean change per patient. Total costs were broken down by specific site. Facilities costs include hospital-related services. Professional costs include all nonpharmacy and physician services. All P values were generated using Wilcoxon signed-rank tests. Data are shown as mean (SD).
PERT = pancreatic enzyme replacement therapy.
IMPACT ON OTHER HEALTH CARE RESOURCE UTILIZATION
All other changes in other health care resource utilization were analyzed and reported in Table 2. The changes in average number of hospitalizations and Pseudomonas infections were calculated. The differences in both average cost and use of various CF comorbidity–related medications were also measured and analyzed. Hospitalizations decreased from an average of 7.7 (± 7.2) to 3.9 (± 5.5) (P < 0.00001). No average differences between the other variables were found to be statistically different between the 2 groups.
Discussion
This study analyzed the uptake, use, and impact of the new CFTR modulator elexacaftor/tezacaftor/ivacaftor among commercially insured members. It provides insights into future trends as its use expands to younger age groups.
UPTAKE AND PHARMACY PMPM IMPACT
For this part of the analysis, all the elexacaftor/tezacaftor/ivacaftor users and claims were included to present the most complete picture of its impact. This not only provides a holistic picture of the true initial impact elexacaftor/tezacaftor/ivacaftor had on the commercial population at this plan but also is informative on the impact that future indications for elexacaftor/tezacaftor/ivacaftor may have in similar populations. The largest driver of this adoption was from CFTR-naive members, but adoption was also observed from members that were previously on tezacaftor/ivacaftor or lumacaftor/ivacaftor. This is likely because of the additional clinical benefit of elexacaftor/tezacaftor/ivacaftor over those 2 medications. Ivacaftor use remained relatively unaffected because members who qualify for ivacaftor have different genetic mutations that do not meet the criteria for elexacaftor/tezacaftor/ivacaftor use. A total of 14.7% of patients with CF have no copies of the F508del allele according to the most recent CF Patient Registry report,23 which corroborates the observation here that only 12% of members with CF are not using a CFTR modulator and is consistent with the overall prevalence of treatable CFTR mutations in the CF population.
As elexacaftor/tezacaftor/ivacaftor was rapidly adopted, it became the main driver of PMPM pharmacy costs for members with CF and drove a $0.69 PMPM increase in overall pharmacy costs for the CF category. With elexacaftor/tezacaftor/ivacaftor itself constituting ~$1.50 PMPM of the CF pharmacy costs, it now makes up ~75% of the pharmacy costs for this group of commercially insured members with CF.
IMPACT ON TOTAL COST OF CARE AND OTHER HEALTH CARE RESOURCE UTILIZATION
To measure the specific impact on costs and other resource utilization, a retrospective claims analysis included 51 continuously enrolled members from the commercial population that began using the medication elexacaftor/tezacaftor/ivacaftor and measured its impact over a 10-month period on several variables. This study provides unique data because of its length (10 months pre/post) and completeness (contains both medical and pharmacy claims). Further, this study provides real-world evidence, complementing the randomized control trial data. Finally, it allows for the examination of changes in several variables after using the novel medicine elexacaftor/tezacaftor/ivacaftor for nearly a year.
A major aim of this study was to determine the impact of elexacaftor/tezacaftor/ivacaftor use on total cost of care. The total cost of care increased significantly, both in terms of absolute total for the 51 included members and as a per-member average. This was observed despite reductions in both facilities and professional costs by 50% or greater during the same period.
A second major aim was to measure differences in other health care resource utilization, including the number of hospitalizations, Pseudomonas infections, and the use and costs of other medications. Although the SDs were large, there was a statistically significant reduction in the average number of hospitalizations between the 2 groups. This is consistent with results from a clinical trial that showed a reduction in pulmonary exacerbations that lead to hospitalizations in patients with a single F508del allele.14 The analysis of all the other variables showed that the absolute numbers and group averages decreased after starting elexacaftor/tezacaftor/ivacaftor. However, no statistically significant differences were observed in any of these variables. This question specifically warrants future study, as larger datasets may improve the ability to detect differences in use.
The approval of elexacaftor/tezacaftor/ivacaftor has expanded coverage of this medication class to address an important unmet need in this patient population, but these results highlight the importance of the access and affordability alert warning issued by the Institute for Clinical and Economic Review that these high costs could threaten sustainable access to high-value care for all patients.
LIMITATIONS
There were several limitations to this study. First, our study was conducted entirely using retrospective claims data. Although useful in adding to the body of evidence, it is important to avoid the inherent biases that can happen with this type of data. Another weakness of claims data is their incompleteness for a variety of reasons. Because we only included continuously enrolled members in this study for the cohort differences analysis, the discontinuity that occurs when members switch plans reduced our sample size substantially. Second, the small sample size is a weakness, and it likely increased the chances of committing type 2 errors when completing the statistical analyses. For instance, the differences in total cost of care were large enough to detect statistically significant differences, but we were unable to detect differences in the other variables with smaller overall changes in magnitude, and a larger sample size would have perhaps made this possible if a difference exists. In CF or any rare disease, this is a typical problem, owing to the rarity of the disease. Third, the fact that this was an observational study limits the conclusions to those that can be reasonably made for all observational studies. Lastly, the study period included a large portion of the COVID-19 pandemic. We attempted to account for this by selecting study variables that should be minimally affected, but there was no precise way to fully adjust for the confounding that this event would bring to the data.
Conclusions
The uptake of elexacaftor/tezacaftor/ivacaftor reached 68% of all eligible members with CF within the first 12 months of FDA approval. There were decreases in nonpharmacy costs (facilities and professional services), but total costs increased by ~$5.8 million (52%) over the 10-month observation period. The average number of hospitalizations decreased by 3.8 after initiating elexacaftor/tezacaftor/ivacaftor during the 10-month period. Statistically significant reductions in Pseudomonas infections or CF comorbidity–related medication use were not observed despite numerical decreases in the averages. Although the small sample size limits conclusions, these are some of the first real-world data on the impact of elexacaftor/tezacaftor/ivacaftor in a commercially insured group of patients with CF.
ACKNOWLEDGMENTS
We would like to thank Kathy Roberts, Senior Data Scientist, and Kimberly Gray, MPH, Senior Analytics Consultant (no longer with BCBSNC), for assistance in querying and indexing the pharmacy and medical claims data.
REFERENCES
- 1.Ratjen F, Bell SC, Rowe SM, Goss CH, Quittner AL, Bush A. Cystic fibrosis. Nat Rev Dis Primer. 2015;1(1):15010. doi: 10.1038/nrdp.2015.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ICER. Modulator Treatments for Cystic Fibrosis: Effectiveness and Value Final Evidence Report and Meeting Summary, September 23, 2020.
- 3.Elborn JS. Cystic fibrosis. Lancet. 2016;388(10059):2519-31. doi: 10.1016/S0140-6736(16)00576-6 [DOI] [PubMed] [Google Scholar]
- 4.De Boeck K. Cystic fibrosis in the year 2020: A disease with a new face. Acta Paediatr Oslo Nor 1992. 2020;109(5): 893-99. doi: 10.1111/apa.15155 [DOI] [PubMed] [Google Scholar]
- 5.Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: Challenges to disease self-management. J Cyst Fibros. 2009;8(2):91-96. doi: 10.1016/j.jcf.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tice JA, Kuntz KM, Wherry K, Seidner M, Rind DM, Pearson SD. The effectiveness and value of novel treatments for cystic fibrosis: A summary from the Institute for Clinical and Economic Review’s California Technology Assessment Forum. J Manag Care Spec Pharm. 2021;27(2):276-80. doi: 10.18553/jmcp.2021.27.2.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cystic Fibrosis Research Inc. Voice of the Cystic Fibrosis Patient: A Summary Report of the Externally-Led Patient-Focused Drug Development Meeting on Cystic Fibrosis. Published online 2018. Accessed March 16, 2021. http://cfri.org/wp-content/uploads/2019/03/EL-PFDD.CFRI_.VoiceofCFPatientReport.pdf
- 8.Cystic Fibrosis Foundation. Patient Registry 2018 Annual Data Report. Published online 2018.
- 9.Taylor-Cousar JL, Mall MA, Ramsey BW, et al. Clinical development of triplecombination CFTR modulators for cystic fibrosis patients with one or two F508del alleles. ERJ Open Res. 2019;5(2):00082-02019. doi: 10.1183/23120541.00082-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopes-Pacheco M. CFTR modulators: The changing face of cystic fibrosis in the era of precision medicine. Front Pharmacol. 2019;10:1662. doi: 10.3389/fphar.2019.01662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridley K, Condren M. Elexacaftortezacaftor-ivacaftor: The first triple-combination cystic fibrosis transmembrane conductance regulator modulating therapy. J Pediatr Pharmacol Ther. 2020;25(3):192-97. doi: 10.5863/1551-6776-25.3.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heijerman HGM, McKone EF, Downey DG, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet. 2019;394(10212):1940-48. doi: 10.1016/S0140-6736(19)32597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809-19. doi: 10.1056/NEJMoa1908639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FDA approves new breakthrough therapy for cystic fibrosis. Published March 24, 2020. Accessed October 27, 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-new-breakthrough-therapy-cystic-fibrosis
- 15.FDA Accepts Vertex Application for Expansion of Trikafta to Include Children ages 6-11. Cystic Fibrosis Foundation. Accessed June 16, 2021. https://www.cff.org/News/News-Archive/2021/FDA-Accepts-Vertex-Application-for-Expansion-of-Trikafta-to-Include-Children-ages-6-11/ [Google Scholar]
- 16.A Study Evaluating the Longterm Safety and Efficacy of VX-445 Combination Therapy. Accessed December 7th, 2020. https://clinicaltrials.gov/ct2/show/NCT03525574
- 17.Impact of Discontinuing Chronic Therapies in People With Cystic Fibrosis on Highly Effective CFTR Modulator Therapy (SIMPLIFY). Accessed December 2, 2020. https://clinicaltrials.gov/ct2/show/NCT04378153
- 18.A Phase 3b, Randomized, Placebo-Controlled Study Evaluating the Efficacy and Safety of Elexacaftor/Tezacaftor/Ivacaftor in Cystic Fibrosis Subjects 6 Through 11 Years of Age Who Are Heterozygous for the F508del Mutation and a Minimal Function Mutation (F/MF). Accessed October 27, 2020. https://clinicaltrials.gov/ct2/show/NCT04353817
- 19.Cystic Fibrosis Foundation. Vertex Announces Positive Study Results for elexacaftor/tezacaftor/ivacaftor in Children Ages 6-11. Accessed October 28, 2020. https://www.cff.org/node/671
- 20.Vertex Announces U.S. FDA Acceptance of Supplemental New Drug Application for ELEXACAFTOR/TEZACAFTOR/IVACAFTOR® (elexacaftor/tezacaftor/ivacaftor and ivacaftor) in Children With Cystic Fibrosis Ages 6 through 11 With Certain Mutations. Accessed March 20, 2021. https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-us-fda-acceptance-supplemental-new-drug
- 21.Study to Evaluate Biological & Clinical Effects of Significantly Corrected CFTR Function in Infants & Young Children. Accessed October 28, 2020. https://clinicaltrials.gov/ct2/show/NCT04509050
- 22.Vertex Announces FDA Approvals of TRIKAFTA® (elexacaftor/tezacaftor/ivacaftor and ivacaftor), SYMDEKO ® (tezacaftor/ivacaftor and ivacaftor) and KALYDECO® (ivacaftor) for Use in People With CF With Certain Rare Mutations. Accessed January 10, 2021. https://news.vrtx.com/press-release/vertex-announces-fda-approvals-trikaftar-elexacaftortezacaftorivacaftor-and-ivacaftor
- 23.Cystic Fibrosis Foundation Patient Registry 2019 Annual Data Report Bethesda, Maryland: ©2020 Cystic Fibrosis Foundation. [Google Scholar]


