Abstract
BACKGROUND:
Cold agglutinin disease (CAD) is a rare autoimmune hemolytic anemia (AIHA). Information regarding the impact of CAD from the patient and health care system perspective is limited.
OBJECTIVE:
To understand longitudinal trends in outcomes in patients with CAD, including anemia severity, hemolytic status, administration of CAD-related therapies, and health care resource utilization (HCRU).
METHODS:
This retrospective, observational cohort study used data from the US Optum Electronic Health Record database. Included patients were aged 18 years and older at the index date (first CAD mention in physician"s notes), had 1 or more medical encounters with an AIHA-related diagnosis code during the study period, and had 3 or more CAD mentions during the patient identification period (January 2008 to March 2019). The baseline period was the 12 months preceding the index date. Anemia severity (severe, hemoglobin < 8.0 g/dL; moderate, 8.0-10.0 g/dL; mild, 10.1-11.9 g/dL; no anemia, ≥ 12.0 g/dL) and hemolytic status (elevated lactate dehydrogenase [LDH; > 250 μ/L] and/or elevated bilirubin [> 1.2 mg/dL]) were assessed at baseline and 6-monthly followup intervals. Use of CAD-related therapies, blood transfusions, and all-cause HCRU were analyzed every 6 months; results were stratified by anemia severity.
RESULTS:
The analysis included 610 adults with CAD (median [interquartile range; IQR] age 72.0 [61.0-78.0] years; 65.4% female). Median (IQR) duration of follow-up was 42.8 (22.8-68.4) months. The proportion of patients with moderate/severe anemia was 51.0% at baseline, 57.7% over 12 months’ follow-up, and 66.6% over full follow-up. During the full follow-up period, approximately 50% of patients had elevated bilirubin and LDH levels.
Corticosteroids were the most frequently used medication (65.6% of patients) over full follow-up. Mean (SD) number of blood transfusions per patient was 3.26 (9.21) over 12 months and 5.47 (17.11) over the full follow-up. At full follow-up, 68.7% of patients with severe anemia received a transfusion vs 12.6% and 0.0% with moderate or mild anemia, respectively. At 12 months, 34.1%, 97.7%, and 29.3% of patients had 1 or more hospitalizations, outpatient services, or emergency department visits (full follow-up: 52.5%, 99.0%, and 53.9%), respectively. Across all time periods, HCRU was greater in patients with severe anemia vs mild or moderate anemia.
CONCLUSIONS:
CAD imposed a substantial long-term burden on patients and health care systems, and despite the use of several therapies, hemolysis and anemia still occurred. The use of CAD-related therapies and HCRU was generally greater with greater anemia severity. These results suggest a lack of effective treatment options available for patients with CAD at the time of this analysis.
Plain language summary
Cold agglutinin disease (CAD) is a rare type of anemia. We do not know much about this illness or how it can affect peoples’ lives. This study showed that people with CAD who had severe anemia needed more blood transfusions, hospital admissions, and visits to the doctor than people with CAD with mild anemia. Overall, we found that the burden of disease was high at 6 months and remained high at 5 years.
Implications for managed care pharmacy
These results demonstrate that CAD imposes a substantial long-term burden on patients and health care systems. The use of CAD-related therapies and health care resource use was generally greater with greater anemia severity. These results provide evidence of the unmet medical need to treat and control the disease burden of CAD, particularly in patients with severe anemia, and may prompt further investigation into treatments and management of this rare disease.
Cold agglutinin disease (CAD) is a serious and rare autoimmune hemolytic anemia (AIHA) in which red blood cells are bound by cold agglutinin autoantibodies.1-3 The annual incidence of AIHA disorders in the United States is estimated to be 0.8 to 4 per 100,000 adults,4-6 and CAD affects approximately 15% to 25% of these patients.7,8 Little is known about the epidemiology of CAD; however, recent European studies estimate prevalence rates for CAD of 2.1, 1.0, and 0.5 per 100,000 in Norway (2017-2019), Denmark (2015), and the Lombardy region of Italy (2017-2019), respectively.9,10 The median age at diagnosis is approximately 68 to 72 years.9,11
The exact etiology of CAD is unknown but is believed to be caused by an underlying clonal lymphoproliferative disorder. Cold agglutinin autoantibodies bind to red blood cells in cold temperatures (optimal 3-4°C) while passing through the peripheral circulation and activate the classical complement system. Red blood cells are then phagocytized, causing hemolysis and anemia.1,2,12,13 Symptom severity may vary from mild to severe and include fatigue, weakness, dizziness, headaches, jaundice, cardiac problems, circulatory problems (livedo reticularis, Raynaud disease, acrocyanosis), and pain in the back, legs, or chest.11,14,15 Hemolytic anemia and the symptoms of CAD can be exacerbated by cold temperatures, viral infection, febrile illness, trauma, or surgery.11,14 Most patients with CAD experience anemia, which varies in severity over time, with many patients experiencing severe anemia during their disease course.9,16,17 Despite receiving transfusions and multiple lines of therapy for their anemia, many patients continue having hemolysis.16,18 There is limited evidence on the impact of this disease from both the patient and health care system perspective.
Although real-world studies have been conducted to examine health care resource utilization (HCRU) in CAD,16,19-21 most have included patients with secondary predisposing concomitant diseases, which recently have been distinguished from CAD and collectively termed cold agglutinin syndrome (CAS).3 There are limited population-based data available examining anemia severity, treatment patterns, and HCRU longitudinally in patients with CAD. Further information on the characteristics and burden of disease in patients with CAD is critical to understand the severity of this disease, understand the unmet health care needs in this population, and to optimize disease management. The objective of this descriptive analysis was to better understand long-term outcomes in patients with CAD, including anemia severity, hemolytic status, administration of CAD-related therapies (including the use of medications and blood transfusions), and all-cause HCRU.
Methods
STUDY DESIGN
This was a retrospective, observational cohort study examining laboratory parameters, CAD-related treatment choices, and HCRU data longitudinally using the US Optum Electronic Health Record (EHR) database in patients with CAD. Optum EHR is a longitudinal clinical repository derived from health care provider organizations in the United States and includes data from more than 700 hospitals and 7,000 clinics.
The study period ranged from January 1, 2007, to September 30, 2019. To allow for a 12-month baseline period and a minimum 6 months of follow-up for each patient, the patient identification period was set from January 1, 2008, until March 31, 2019. The index date was defined as date of first CAD mention in the physician’s notes section of the EHR, with the prior 12 months defined as the baseline period. The full follow-up period started at the index date and ended at death, the last recorded medical activity for the patient in the database, or September 30, 2019 (date of the last available data) (Supplementary Figure 1 (519.4KB, pdf) , available in online article). All patients were followed for 6 months or more post-index, and patients were assessed at 6-month cross-sectional intervals during the baseline and follow-up periods.
PATIENT SELECTION
Adult patients (aged ≥ 18 years) with CAD from the deidentified Optum EHR database were included in the analysis. Eligible patients had 1 or more medical encounters with an AIHA-related International Classification of Diseases Clinical Modification (ICD-CM) code (ICD-9-CM: 283.0 or ICD-10-CM: D59.1). Patients were included if the sentiment field in the physician notes was not negative (indicative of CAD not being ruled out as a diagnosis) and they had 3 or more documentations of the following CAD-related terms: cold agglutinin disease, cold autoimmune hemolytic anemia, or cold agglutinin hemoglobinuria. Patients were excluded if they were aged younger than 18 years on the index date, or if the period between the patient’s first and last activity recorded in the database (their “continuous medical activity”) was less than 365 days during the baseline period, or fewer than 180 days after the index date.
To select data from patients with CAD (primary causes only) and not CAS, patients with a coexisting diagnosis of lymphoma, mucosa-associated lymphoid-tissue lymphoma, chronic lymphoid leukemia, Waldenström macroglobulinemia, or multiple myeloma during the baseline period were excluded. Patients with infections at the index date that are known to be associated with CAS (mycoplasma, cytomegalovirus, and Epstein-Barr virus infection) were also excluded. ICD codes used for exclusion are shown in Supplementary Table 1 (519.4KB, pdf) .
STUDY ENDPOINTS
Anemia severity, bilirubin levels, and lactate dehydrogenase (LDH) levels were analyzed in two 6-month intervals during the 12-month baseline period and then at 6-month follow-up intervals. Patients were categorized for anemia severity by the lowest hemoglobin (Hb) value (severe, < 8.0 g/dL; moderate, 8.0-10.0 g/dL; mild, 10.1-11.9 g/dL; and no anemia, > 12.0 g/dL), for bilirubin levels by the highest value (normal, < 1.2 mg/dL; elevated, > 1.2 mg/dL), and for LDH levels by the highest value (normal, < 250 μ/L; elevated, > 250 μ/L) during each 6-month interval. Hemolysis was defined as elevated LDH and/or elevated bilirubin. Baseline results are reported for the 0- to 6-month interval only prior to the index date. During the post-index follow-up period, results are reported for the 0- to 6-month and 7- to 12-month intervals and for all available follow-up data.
The use of CAD-related therapies (including corticosteroids, danazol, ibrutinib, immunoglobulin, rituximab, immunosuppressants, antineoplastics, plasma exchange, splenectomy, and other biologics [eculizumab and lymphocyte immune globulin antithymocyte globulin]), blood transfusions, and all-cause HCRU (defined as inpatient hospitalizations, emergency department [ED] visits, and outpatient services) was analyzed every 6 months during follow-up and is reported for the 0- to 12-month and the full follow-up periods. Outpatient services included ambulatory patient services, day surgery patient visits, home visits, hospice, imaging, laboratory tests and diagnostics (nonimaging), long-term acute care, nursing home/assisted living, office or clinic patient visits, palliative care, swing bed, telephone/online consultations, and urgent care. ED visits and hospitalizations were captured separately even if a patient was admitted to hospital.
STATISTICAL ANALYSIS
Descriptive statistics were presented using patient counts with percentages for categorical variables and means with SDs or medians with interquartile ranges (IQRs) for continuous variables; formal sample size calculations and statistical comparisons were not conducted. Results were stratified by anemia severity category (severe, moderate, mild, and no anemia) during each follow-up interval. For all outcomes, the number and proportion of unique patients with missing data within each biomarker category were quantified but values were not imputed. Patients with missing baseline categorical data were assigned to unknown categories; means and medians only included patients with data for the characteristic or event.
A sensitivity analysis was performed to assess the number of hemolysis events when stratified by anemia severity in the following subgroups: patients with 1 or more Hb values, patients with 5 or more Hb values, and patients with 10 or more Hb values less than 12 g/dL during the study period (including baseline and follow-up periods). The impact of missing laboratory values on hemolysis results was also assessed.
All data analyses were conducted using the Aetion Evidence Platform (AEP) (Version 4.3), a software developed by Aetion, Inc (http://www.aetion.com), for real-world data analysis. The Optum EHR data were minimally transformed at the point of connection to the AEP, thus preserving the original format of the raw data.
ETHICAL APPROVAL
The Optum EHR database is compliant with the Health Insurance Portability and Accountability Act of 1996. All data were deidentified; therefore, institutional review board approval was not required or sought.
This study was performed in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice, Good Pharmacoepidemiology Practice, and the applicable legislation on noninterventional studies and/or observational studies.
Results
STUDY POPULATION
A total of 1,135 patients with 3 or more non-negative mentions of CAD-related terms were identified from the database. Of these, 610 were aged 18 years or older at index, had an AIHA diagnosis, had no evidence of presenting with CAS (secondary causes of CAD), and had at least 12 and 6 months of continuous medical activity during the baseline and follow-up periods, respectively (Supplementary Figure 2 (519.4KB, pdf) ). Baseline characteristics are presented in Table 1. The majority of patients were female (65.4%), and the median age was 72.0 years, with 40.8% of patients aged 75 years or older. The median (IQR) duration of follow-up was 42.8 (22.8-68.4) months (mean [SD]: 48.1 [30.6] months), and 90% of patients had 12 or more months of follow-up. Overall, 536 patients had between 13 and 24 months of follow-up, 431 between 25 and 36 months, 349 between 37 and 48 months, and 261 between 49 and 60 months. Patients were based across the United States, with 50.0% in the Midwest, 23.3% in the South, 11.6% in the West, 11.1% in the Northeast, and 3.9% other/unknown. The median (IQR) distribution of time between the first and third documentation of a CAD-related term was 100.00 (21.00-328.00) days.
TABLE 1.
Baseline Characteristics of Patients With CAD
| Characteristic | All patients (N = 610) |
|---|---|
| Age, years | |
| Mean (SD) | 67.9 (14.5) |
| Median (IQR) | 72.00 (61.00-78.00) |
| Age groups, n (%), years | |
| 18-34 | 30 (4.9) |
| 35-44 | 22 (3.6) |
| 45-54 | 39 (6.4) |
| 55-64 | 105 (17.2) |
| 65-74 | 165 (27.0) |
| 75-84 | 216 (35.4) |
| 85+ | 33 (5.4) |
| Follow-up, months | |
| Mean (SD) | 48.1 (30.6) |
| Median (IQR) | 42.8 (22.8-68.4) |
| Sex, n (%) | |
| Male | 210 (34.4) |
| Female | 399 (65.4)a |
| Race/ethnicity, n (%) | |
| Caucasian | 529 (86.7) |
| African American | 39 (6.4) |
| Asian | 12 (2.0) |
| Other/Unknown | 30 (4.9) |
| CCI categories, n (%) | |
| 0 | 258 (42.3) |
| 1 | 120 (19.7) |
| 2 | 84 (13.8) |
| 3+ | 148 (24.3) |
| Anemia severityb, n (%) | n = 431 |
| No anemia (Hb ≥ 12.0 g/dL) | 116 (26.9) |
| Mild (Hb 10.1-11.9 g/dL) | 95 (22.0) |
| Moderate (Hb 8.0-10.0 g/dL) | 104 (24.1) |
| Severe (Hb < 8.0 g/dL) | 116 (26.9) |
| Bilirubin levelsb, n (%) | n = 389 |
| Normal (≤ 1.2 mg/dL) | 204 (52.4) |
| Elevated (> 1.2 mg/dL) | 185 (47.6) |
| LDH levelsb, n (%) | n = 214 |
| Normal (≤ 250 μ/L) | 79 (36.9) |
| Elevated (> 250 μ/L) | 135 (63.1) |
a 1 unknown.
b 0 to 6 months before index date.
CAD = cold agglutinin disease; CCI = Charlson comorbidity index;
Hb = hemoglobin; IQR = interquartile range; LDH = lactate dehydrogenase.
ANEMIA AND HEMOLYSIS STATUS AT BASELINE AND OVER TIME
At baseline (0-6 months prior to the first mention of CAD), 51.0% of patients had laboratory values indicating moderate/severe anemia, 47.6% had elevated bilirubin levels, and 63.1% had elevated LDH levels (Table 1). The proportion of patients with moderate/severe anemia was greater at 12 months follow-up (57.7%) and during the full followup (66.6%) than at baseline (Figure 1). The proportions of patients with elevated bilirubin and LDH levels were lower in the first year of follow-up than at baseline, and approximately half of patients had elevated levels of these markers during full follow-up (Supplementary Figure 3 (519.4KB, pdf) ).
FIGURE 1.
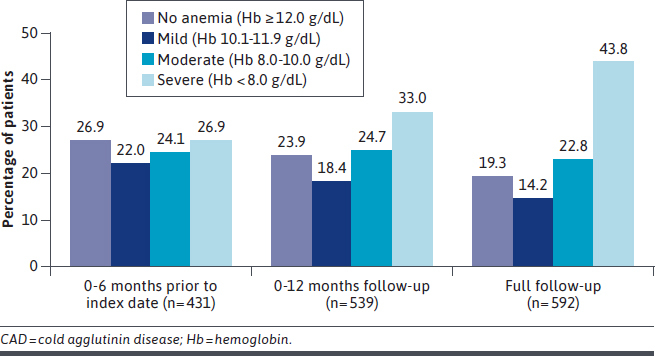
Anemia Severity of Patients With CAD Prior to Index Date, and at 12-Month Follow-Up and Full Follow-Up Periods
The mean (95% CI) number of severe anemia events and hemolysis events per patient-year was 0.72 (0.57-0.91) and 1.44 (1.25-1.66), respectively, during the full follow-up period. The greatest incidence of severe anemia events and hemolysis events occurred in the first 6 months of follow-up (mean [95% CI] 2.02 [1.55-2.63] and 3.42 [2.914.03] events per patient-year, respectively). Hemolysis or moderate/severe anemia events per patient-year remained high over the first year of follow-up, and although they were lower at later time periods, events were still observed at 5 years of follow-up (Figure 2).
FIGURE 2.
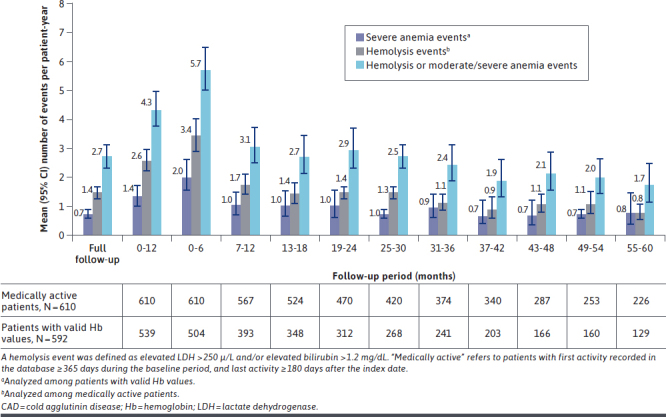
Anemia and Hemolysis Events of Patients With CAD Over Time
Results from the sensitivity analysis showed that the number of moderate/severe anemia and hemolysis events was similar among patients with at least 1, 5, or 10 Hb values during the study (data not presented).
CAD-RELATED THERAPIES (EXCLUDING BLOOD TRANSFUSIONS)
Most patients (74.6%) received at least 1 CAD-related therapy over the full follow-up period, and 52.1% during the first 12 months. The median (IQR) number of therapies per patient was 7.00 (2.00-19.00) during the full follow-up period and 5.00 (2.00-13.00) during the first 12 months. In patients with severe anemia, the median (IQR) number of therapies per patient was 11.0 (3.00-27.00) for the full followup period and 7.00 (2.00-22.00) during the first 12 months. At full follow-up, the median (IQR) number of CAD therapies in patients with severe anemia was more than 2-fold greater than that seen in patients with mild anemia (4.00 [1.00-14.00]).
Corticosteroids were the most frequently used CAD-related drug treatment during the full follow-up period (by 65.6% of patients), followed by antineoplastics (24.9%) and rituximab (19.8%) (Table 2). The proportion of patients receiving any CAD-related therapy generally increased with anemia severity, except for therapies given to very low proportions of patients (Table 2). The use of CAD-related therapies (excluding blood transfusions) was also 3-fold greater in patients with hemolysis compared with those with no reported hemolysis events (Supplementary Table 2 (519.4KB, pdf) ).
TABLE 2.
CAD-Related Therapies, Hospitalizations, Outpatient Services, and Emergency Department Visits in Patients With CAD by Severity of Anemia
| All patients | Severe anemia (Hb < 8.0 g/dL) | Moderate anemia (Hb 8.0-10.0 g/dL) | Mild anemia (Hb 10.1-11.9 g/dL) | No anemia (Hb ≥ 12.0 g/dL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Full follow-up (N = 610) | 0-12 months follow-up (N = 610) | Full follow-up (n = 259) | 0-12 months follow-up (n=178) | Full follow-up (n = 135) | 0-12 months follow-up (n = 133) | Full follow-up (n=84) | 0-12 months follow-up (n=99) | Full follow-up (n = 114) | 0-12 months follow-up (n = 129) | |
| Patients receiving CAD therapies, n (%) | ||||||||||
| Corticosteroids | 400 (65.6) | 260 (42.6) | 194 (74.9) | 118 (66.3) | 93 (68.9) | 60 (45.1) | 47 (56.0) | 33 (33.3) | 58 (50.9) | 34 (26.4) |
| Blood transfusions | 195 (32.0) | 127 (20.8) | 178 (68.7) | 114 (64.0) | 17 (12.6) | 10 (7.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| Antineoplastics | 152 (24.9) | 108 (17.7) | 98 (37.8) | 58 (32.6) | 31 (23.0) | 28 (21.1) | 11 (13.1) | 10 (10.1) | 10 (8.8) | 8 (6.2) |
| Biologies | 121 (19.8) | 88 (14.4) | 81 (31.3) | 49 (27.5) | 26 (19.3) | 23 (17.3) | 5 (6.0) | 7 (7.1) | 8 (7.0) | 6 (4.7) |
| Immunosuppressants | 42 (6.9) | 26 (4.3) | 21 (8.1) | 12 (6.7) | 8 (5.9) | 5 (3.8) | 5 (6.0) | 3 (3.0) | 7 (6.1) | 5 (3.9) |
| IV immunoglobulin | 21 (3.4) | 14 (2.3) | 17 (6.6) | 11 (6.2) | 2 (1.5) | 1 (0.8) | 1 (1.2) | 1 (1.0) | 1 (0.9) | 1 (0.8) |
| Othera | 23 (3.8) | 15 (2.5) | 18 (6.9) | 12 (6.7) | 3 (2.2) | 1 (0.8) | 1 (1.2) | 1 (1.0) | 0 | 1 (0.8) |
| Health care resources used per patient-year, rate (95% CI) | ||||||||||
| Hospitalizations | 0.51 (0.44-0.60) | 0.79 (0.67-0.95) | 0.94 (0.78-1.13) | 1.94 (1.57-2.40) | 0.39 (0.28-0.55) | 0.70 (0.52-0.93) | 0.22 (0.15-0.33) | 0.41 (0.27-0.62) | 0.08 (0.04-0.14) | 0.15 (0.09-0.26) |
| Emergency department visits | 1.61 (1.31-1.98) | 2.08 (1.45-3.00) | 2.39 (1.79-3.19) | 4.43 (2.49-7.87) | 1.61 (1.17-2.22) | 2.13 (1.41-3.21) | 0.77 (0.45-1.30) | 1.01 (0.50-2.05) | 0.91 (0.44-1.89) | 0.87 (0.51-1.49) |
| Outpatient servicesb | 45.31 (40.95-50.13) | 52.61 (47.69-58.03) | 56.64 (48.72-65.84) | 76.73 (64.09-91.86) | 47.41 (38.64-58.17) | 67.00 (56.79-79.04) | 41.71 (32.26-53.92) | 39.31 (33.47-46.17) | 28.29 (22.88-34.99) | 37.84 (31.97-44.77) |
| Blood transfusions | 1.35 (1.00-1.81) | 3.48 (2.54-4.75) | 2.82 (2.09-3.78) | 7.38 (5.43-10.02) | 0.18 (0.10-0.32) | 0.50 (0.23-1.08) | 0 | 0 | 0 | 0.08 (0.01-0.53) |
| LOS per patient with ≥ 1 hospitalization days), mean (SD) | 6.23 (5.60) | 6.49 (6.36) | 6.76 (5.72) | 7.54 (7.09) | 6.04 (5.73) | 5.03 (4.38) | 5.55 (5.54) | 5.86 (5.30) | 2.81 (1.35) | 4.90 (6.76) |
a Includes danazol, ibrutinib, plasmapheresis, and splenectomy.
b Outpatient services included the following: ambulatory patient services, day surgery patient visits, home visits, hospice, imaging, laboratory tests and diagnostics (nonimaging), long-term acute care, nursing home/assisted living, office or clinic patient visits, palliative care, swing bed, telephone/online consultations, and urgent care.
CAD = cold agglutinin disease; Hb = hemoglobin; IV = intravenous; LOS = length of stay.
BLOOD TRANSFUSIONS
The mean [SD] number of blood transfusions received per patient was high during the 12-month baseline period (3.26 [9.21]) and the full follow-up period (5.47 [17.11]) (Supplementary Figure 4 (519.4KB, pdf) ). The mean (95% CI) number of blood transfusions per patient-year was 3.48 (2.54-4.75) over the first 12 months and 1.35 (1.00-1.81) during the full follow-up period (Supplementary Table 3 (519.4KB, pdf) ). Patients with severe anemia received the highest number of transfusions at all followup intervals compared with patients with mild, moderate, or no anemia (Table 2). At full follow-up, blood transfusions were received by 0.0%, 0.0%, 12.6%, and 68.7% of patients with no anemia, mild, moderate, and severe anemia, respectively. At full followup, 43.3% of patients with hemolysis events and 12.2% without hemolysis events received blood transfusions (Supplementary Table 2 (519.4KB, pdf) ).
HCRU
Over the full study period, 52.5%, 99.0%, and 53.9% of patients had 1 or more hospitalizations, outpatient services, and ED visits, respectively. Corresponding values at 12 months’ follow-up were 34.1%, 97.7%, and 29.3%, respectively; from 13 to 24 months were 22.0%, 93.8%, and 25.4%; 25 to 36 months were 18.6%, 92.1%, and 24.1%; 37 to 48 months were 18.1%, 92.8%, and 23.5%; and 49 to 60 months were 13.4%, 92.7%, and 24.9%. The mean (SD) number of hospitalizations per patient was 2.04 (3.98) during the full follow-up period, and total inpatient days per patient with 1 or more hospitalizations were 24.03 (36.82); corresponding median (IQR) values were 1.00 (0.00-3.00) and 13.00 (5.00-26.00), respectively. Mean (SD) number of outpatient services (a list of included outpatient services is in the Methods section) and ED visits per patient was 181.46 (250.59) and 6.46 (16.72), respectively, over the full follow-up period, and 50.56 (62.41) and 2.0 (9.22) during the first 12 months follow-up. The median (IQR) number of outpatient services and ED visits was 96.00 (44.00-220.25) and 1.00 (0.00-6.00), respectively, over the full follow-up period, and 31.00 (13.0065.25) and 0.00 (0.00-1.00) during the first 12 months.
HCRU BY SEVERITY OF ANEMIA
Across the full follow-up period, the percentage of patients with any hospitalization, the number of hospitalizations and ED visits per patient-year, and the length of stay per hospitalization were all highest in patients with severe anemia compared with mild, moderate, or no anemia (Supplementary Table 3 (519.4KB, pdf) ). A high proportion of patients with CAD were hospitalized during the first year of disease (34.1%), and the burden of hospitalization remained high over the study period (Figure 3). The rate (95% CI) of outpatient services per patient-year was high across all anemia severity categories and remained high over the full study period; 56.64 (48.7265.84) for patients with severe anemia, compared with 47.41 (38.64-58.17) for moderate, 41.71 (32.26-53.92) for mild, and 28.29 (22.88-34.99) with no anemia (Supplementary Table 3 (519.4KB, pdf) ).
FIGURE 3.
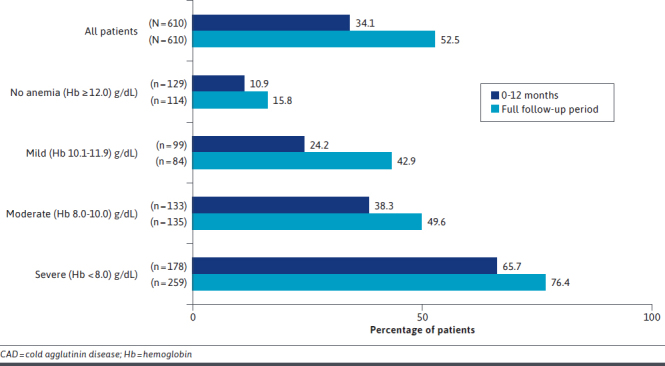
Patients With CAD With Any Hospitalization (All Patients and by Anemia Severity) at 12 Months Follow-Up and Full Follow-Up Periods
HCRU BY HEMOLYTIC STATUS
Patients with hemolysis had a larger number of hospitalizations, outpatient services, and ED visits during the first year of disease and the full follow-up period compared with patients with no hemolysis events (Supplementary Figure 5 (519.4KB, pdf) ). At full follow-up, the rate of hospitalizations per patient-year was approximately 2 times greater in patients with hemolysis events than in those without (accounting for differences in follow-up time). The rates of hospitalizations and ED visits per patient-year over the full follow-up period was greater in patients with elevated bilirubin and LDH than in those with normal levels (Supplementary Table 3 (519.4KB, pdf) ). The number of outpatient services per patient-year over the full follow-up period was greater in patients with elevated bilirubin levels than in those with normal levels, but similar in patients with and without elevated LDH levels.
Discussion
This retrospective, real-world data analysis demonstrates that primary CAD has a substantial, long-term burden on both patients and health care systems. In our study, moderate to severe anemia and hemolysis events were frequently observed during the first 12 months of follow-up and remained high up to 4 to 5 years after diagnosis despite many patients receiving treatment to manage their disease. Over the full follow-up period (median duration 42.8 months), two-thirds of all patients had moderate to severe anemia and approximately half of all patients had elevated markers of hemolysis. The burden on health care systems was especially pronounced in patients with moderate or severe anemia or hemolysis events, who required high numbers of blood transfusions and larger numbers of CAD-related therapies, hospitalizations, ED visits, and outpatient services. This level of HCRU may be indicative of the lack of effective treatment options available for patients with CAD at the time of the analysis. Patients’ use of health care resources was greatest during the first year after diagnosis, as might be expected. However, results over the full follow-up period showed a continued high demand. This was particularly evident in the blood transfusions received among patients with moderate or severe anemia, among whom 12.6% and 68.7% of patients, respectively, continued to require transfusions during the full follow-up period.
This is, to our knowledge, the first study to evaluate trends in treatment and HCRU among patients diagnosed with CAD using a large real-world dataset. However, some smaller or more limited real-world studies have been published.9,16,20-22 Mullins et al conducted a retrospective, single-center study in the United States, which analyzed 29 patients with CAD (including patients with CAS), based on the Stanford Translational Research Integrated Database Environment (STRIDE).16 In total, 45% of patients had severe anemia at disease onset (vs 27% in the present study), all patients used outpatient services in the first year after diagnosis (median of 42.0 visits), and 67% of patients used hospital inpatient services.16 Comparative values at 1-year follow-up in our study were 97.7% of patients using outpatient services (median of 31.0 visits) and 34.1% of patients using inpatient services. Our study also builds on work by Su et al, who undertook a 12-month postdiagnosis comparison of the HCRU of 410 patients with CAD from the Optum-Humedica database with a matched non-CAD cohort of 3,390 patients in the United States.19 These results expand on Su et al by providing a longitudinal assessment of HCRU according to anemia severity and hemolytic status, as well as the use of CAD-related therapies, in a larger CAD cohort. Several European studies have also been published. Barcellini et al, for example, reported a median of 20 outpatient visits in a subcohort of 63 Italian patients with CAD over 4 years’ follow-up,20 compared with 96 services in this study. This may reflect differences in the reimbursement of visits between the United States and Italy with a higher number of visits in the United States likely due to opportunistic behavior. Additionally, Vágó et al recently showed that 104 patients with CAD or CAS in the Danish National Patient Registry used health care resources significantly more than demographically and disease-matched patients without CAD, with 5-fold more inpatient hospitalization admissions and outpatient visits reported, and greater transfusion use.21
The results reported in these studies vary because of differences in the disease management and health care systems across countries, sample sizes, and data sources used. For example, Optum, used in the present study, collected data from more than 700 hospitals and 7,000 clinics in the United States, included more than 103 million patients, and enabled distinction between patients with CAD and CAS, whereas STRIDE included data from 2.1 million patients based in 2 hospitals in California and did not offer this distinction. The data in STRIDE also included a large number of referrals, which may have occurred more frequently for severe cases of CAD.
In the present study, 74.6% of patients received at least 1 CAD-related therapy during the full follow-up period. This is in line with results reported by Barcellini et al and Berentsen et al, who observed, respectively, that among 107 patients with CAD treated at 8 Italian reference centers, 84% received at least 1 line of therapy (median follow-up of 4.3 years),20 and in a multicenter study of 232 patients with CAD in Europe, 76% received at least 1 line of therapy (median follow-up of 6 years).9 Corticosteroids were the most frequently reported CAD-related drug therapy in our study, used by 65.6% of patients, based on data from 2007 to 2019. This was lower than the 90% of patients reported from STRIDE, which covered patients treated from 2000 to 2016.16 This difference may reflect a change in treatment approaches, as corticosteroids are no longer recommended for the treatment of CAD because of poor response rates and the high doses required.3,23 Despite rituximab monotherapy being recommended as first-line therapy for CAD,3 only 20% of patients in our study received such treatment. In the current analysis, a blood transfusion rate of 1.35 per patient-year was observed, with a mean number of transfusions per patient of 5.47 during the full follow-up period. This was lower than reported during the 12-month follow-up, which likely reflects the reduction in hospitalizations over time. This transfusion rate was also lower than the 11.00 per patient-year reported by Mullins et al over a median of 4.9 years of follow-up.16 This may be because more patients had severe disease in the Mullins study, as previously discussed, with different median follow-up durations, and that blood transfusions are indicated as rescue therapy for emergency events.3 Although several therapies were used to treat CAD, hemolysis and anemia still occurred, indicating a need for new CAD-specific therapeutic strategies that can provide long-term disease control and avoid rescue therapies.
LIMITATIONS
Although the data presented here provide information on trends over time, this analysis did not give continuous follow-up of patients, but rather a series of cross-sectional analyses in which individual patients entered and exited at different times. This allowed analyses of a larger number of patients than would be possible if the analysis had included only a single group identified at a specific timepoint and followed longitudinally. Patients were excluded from the analysis if their continuous medical activity was fewer than 180 days after the index date (to provide ≥ 6 months followup); however, it is possible that this may have excluded patients with the most severe disease.
The provider’s notes from different health care centers could have used different CAD diagnosis terms. Our inclusion and exclusion criteria were carefully selected to include all patients with CAD and exclude those without CAD or with CAS. Nevertheless, patients could have been incorrectly included or excluded. Additionally, although indirect bilirubin and/or haptoglobin are generally accepted as hemolysis markers, in the database the results of these biomarkers were limited, and they were not included in this study.
Real-world studies using EHR databases are subject to limitations such as incomplete or inaccurate coding of diagnoses, leading to potential misclassification or underreporting of baseline characteristics or outcomes of interest. It cannot be known whether all prescriptions for study-relevant medications were recorded accurately or if patients used the medication. However, given the large sample size in our study, the impact of minor inaccuracies for medication use is likely negligible. Additionally, as with all real-world data sources, there may be missing data that impacted the study outcomes; however, we report all sample sizes and numbers available for analysis at each time point to inform the interpretation of the results.
We included all-cause hospitalizations and ED and outpatient services recorded for patients with CAD in our analysis and did not attempt to exclude medical visits unrelated to CAD. The results thus cannot be used to compare outcomes between patients with and without CAD; however, they did enable examination of outcomes according to severity of anemia and hemolysis markers. Furthermore, the results of this study should be interpreted with caution as only a subset of patients have data at the longer follow-up times. Finally, as results are based on US data, they cannot be extrapolated beyond the population studied.
Conclusions
The results of this observational cohort study confirmed that high levels of HCRU are observed in CAD, which generally increased with higher severity of anemia and hemolysis, highlighting the substantial long-term burden of CAD on patients and health care systems. Despite high proportions of patients receiving CAD-related therapies during the first year following diagnosis, the number of moderate/severe anemia or hemolysis events remained high in patients with CAD at 4 to 5 years after diagnosis, and the need for blood transfusions remained substantial. These results provide evidence of the lack of effective CAD-specific treatments available and therefore suggest there is an unmet medical need in CAD.
ACKNOWLEDGMENTS
The authors thank Melitza Iglesias-Rodriguez of Sanofi, Cambridge, MA, for her invaluable contribution to the study. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Amy Watkins, PhD, of Ashfield Health Ltd and was funded by Sanofi in accordance with Good Publication Practice guidelines.
REFERENCES
- 1.Berentsen S. Role of complement in autoimmune hemolytic anemia. Transfus Med Hemother. 2015;42(5):303-10. doi: 10.1159/000438964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berentsen S. Cold agglutinin disease. Hematology Am Soc Hematol Educ Program. 2016;2016(1):226-31. doi: 10.1182/asheducation-2016.1.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. 2020;41:100648. doi: 10.1016/j.blre.2019.100648 [DOI] [PubMed] [Google Scholar]
- 4.Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69(4):258-71. doi: 10.1002/ajh.10062 [DOI] [PubMed] [Google Scholar]
- 5.Klein NP, Ray P, Carpenter D, et al. Rates of autoimmune diseases in Kaiser Permanente for use in vaccine adverse event safety studies. Vaccine. 2010;28(4):1062-8. doi: 10.1016/j.vaccine.2009.10.115 [DOI] [PubMed] [Google Scholar]
- 6.Silberstein P. Autoimmune hemolytic anemia. In: Enna SJ, Bylund DB, eds. xPharm: The Comprehensive Pharmacology Reference. New York: Elsevier; 2007:1-6. doi: 10.1016/B978-008055232-3.60624-5 [DOI] [Google Scholar]
- 7.Barcellini W, Fattizzo B. The changing landscape of autoimmune hemolytic anemia. Front Immunol. 2020;11:946. doi: 10.3389/fimmu.2020.00946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berentsen S, Röth A, Randen U, Jilma B, Tjønnfjord GE. Cold agglutinin disease: Current challenges and future prospects. J Blood Med. 2019;10:93-103. doi: 10.2147/JBM.S177621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berentsen S, Barcellini W, D’Sa S, et al. Cold agglutinin disease revisited: A multinational, observational study of 232 patients. Blood. 2020;136(4):480-8. doi: 10.1182/blood.2020005674 [DOI] [PubMed] [Google Scholar]
- 10.Hansen DL, Möller S, Andersen K, Gaist D, Frederiksen H. Increasing incidence and prevalence of acquired hemolytic anemias in Denmark, 1980-2016. Clinical Epidemiol. 2020;12:497-508. doi: 10.2147/CLEP.S250250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013;122(7):1114-21. doi: 10.1182/blood-2013-02-474437 [DOI] [PubMed] [Google Scholar]
- 12.Baines AC, Brodsky RA. Complementopathies. Blood Rev. 2017;31(4):213-23. doi: 10.1016/j.blre.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barcellini W, Zaninoni A, Giannotta JA, Fattizzo B. New insights in autoimmune hemolytic anemia: From pathogenesis to therapy stage 1. J Clin Med. 2020;9(12):3859. doi: 10.3390/jcm9123859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berentsen S, Randen U, Tjønnfjord GE. Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol Oncol Clin North Am. 2015;29(3):455-71. doi: 10.1016/j.hoc.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 15.Genetic and Rare Diseases Information Center. Cold agglutinin disease. Accessed May 7, 2021. https://rarediseases.info.nih.gov/diseases/6130/cold-agglutinin-disease#ref_8843
- 16.Mullins M, Jiang X, Bylsma LC, et al. Cold agglutinin disease burden: A longitudinal analysis of anemia, medications, transfusions, and health care utilization. Blood Adv. 2017;1(13):839-48. doi: 10.1182/bloodadvances.2017004390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berentsen S. How I treat cold agglutinin disease. Blood. 2021;137(10):1295-303. doi: 10.1182/blood.2019003809 [DOI] [PubMed] [Google Scholar]
- 18.Berentsen S, Ulvestad E, Langholm R, et al. Primary chronic cold agglutinin disease: A population based clinical study of 86 patients. Haematologica. 2006;91(4):460-6. [PubMed] [Google Scholar]
- 19.Su J, Bylsma LC, Jiang X, et al. Healthcare resource utilization among commercially insured patients with cold agglutinin disease in the United States. J Med Econ. 2020;23(8):902-7. doi: 10.1080/13696998.2020.1764006 [DOI] [PubMed] [Google Scholar]
- 20.Barcellini W, Zaninoni A, Fattizzo B, et al. Predictors of refractoriness to therapy and healthcare resource utilization in 378 patients with primary autoimmune hemolytic anemia from eight Italian reference centers. Am J Hemtaol. 2018;93(9):E243-6. doi: 10.1002/ajh.25212 [DOI] [PubMed] [Google Scholar]
- 21.Vágó EK, Nicholson G, Horváth-Puhó E, Hooda N, Fryzek JP, Su J. Healthcare resource utilization among patients with cold agglutinin disease in Denmark. Curr Med Res Opin. 2021;37(10):1829-35. doi: 10.1080/03007995.2021.1960494 [DOI] [PubMed] [Google Scholar]
- 22.Lamarque M, Michel M. La maladie des agglutinines froides: Spectre clinicobiologique et données d’une étude rétrospective multicentrique française portant sur soixante-neuf patients. Hématologie. 2017;23(6):366-78. [Google Scholar]
- 23.Berentsen S. How I manage patients with cold agglutinin disease. B J Haematol. 2018;181(3):320-30. doi: 10.1111/bjh.15109 [DOI] [PubMed] [Google Scholar]


