Abstract
BACKGROUND:
Severe exacerbations requiring hospitalization contribute a substantial portion of the morbidity and costs of chronic obstructive pulmonary disease (COPD). Triple therapy (inhaled corticosteroid + long-acting β-agonist + long-acting muscarinic antagonist) is a recommended option for patients who experience recurrent COPD exacerbations or persistent symptoms. Few real-world studies have specifically examined the effect of prompt initiation of triple therapy, specifically among patients hospitalized for a COPD exacerbation.
OBJECTIVE:
To assess whether prompt initiation of triple therapy following a severe COPD exacerbation was associated with lower risk of subsequent exacerbations and lower health care use and costs and the effects of each 30-day delay of initiation.
METHODS:
Adults aged 40 years or older with COPD were identified in the Merative MarketScan Databases between January 1, 2010, and December 31, 2019, and were required to meet the following criteria: open or closed triple therapy (date of first closed prescription or last component of open=index treatment date), more than 1 inpatient admission with a primary COPD diagnosis (ie, severe exacerbation) in the prior 12 months (index exacerbation), 12 months of continuous enrollment before (baseline) and after (follow-up) index exacerbation, and absence of select respiratory diseases and cancer. Patients were stratified based on timing of open or closed triple therapy after the index exacerbation: prompt (≤30 days), delayed (31-180 days), or very delayed (181-365 days). Multivariable regression controlled for baseline characteristics (age, sex, insurance type, index year, comorbidities, prior treatment, and prior exacerbations) and estimated the odds of subsequent exacerbations, change in the number of exacerbations, and change in health care costs during 12-month follow-up associated with each 30-day delay of triple therapy initiation.
RESULTS:
A total of 6,772 patients met inclusion criteria (2,968 [43.8%] prompt, 1,998 [29.5%] delayed, and 1,806 [26.7%] very delayed). The adjusted odds of any exacerbation and a severe exacerbation during 12-month follow-up increased by 13% (odds ratio [95% CI]: 1.13 [1.11-1.15]) and 10% (1.10 [1.08-1.12]), respectively, for each 30-day delay in triple therapy initiation, and the mean number of exacerbations increased by 5.4% (95% CI = 4.7%-6.1%). There was a 3.0% increase (95% CI = 2.2%-3.8%) in mean all-cause costs and a 3.7% increase (95% CI = 2.9%-4.6%) in total COPD-related costs for each 30-day delay of triple therapy initiation.
CONCLUSIONS:
Longer delays in triple therapy initiation after a COPD hospitalization result in greater risk of subsequent exacerbations and higher health care resource use and costs. Adequate post-discharge follow-up care and earlier consideration of triple therapy may improve clinical and economic outcomes among patients with COPD.
Plain language summary
Patients with delayed triple therapy initiation after a hospitalization for chronic obstructive pulmonary disease (COPD) are more likely to have more exacerbations and higher health care costs in the following year. Patients who are hospitalized for COPD may benefit from starting triple therapy within 30 days after leaving the hospital.
Implications for managed care pharmacy
Triple therapy initiation within 30 days after a COPD hospitalization may improve patient outcomes in the following year. Benefits include lower risk of future exacerbations and avoiding thousands of dollars in health care costs. Triple therapy should be considered as an alternative to other treatments after a severe COPD exacerbation.
Chronic lower respiratory diseases, including chronic obstructive pulmonary disease (COPD), were the fourth leading cause of death in 2019,1 with approximately 135,000 American deaths attributed to COPD each year since 1999.2 COPD affects nearly 7% of American adults,3,4 who are at risk of experiencing severe disease exacerbations in which acute worsening of symptoms warrants hospitalization. Severe exacerbations may account for the majority of the estimated $40 billion annual COPD-related medical costs in the United States,5-8 and estimates of mortality following a COPD hospitalization range from 23% after 1 year9 to more than 50% after 5 years.10,11
The recent 2022 report of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) continues to recommend escalation from dual maintenance therapy (long-acting β-agonist [LABA] + inhaled corticosteroid [ICS], or LABA + long-acting muscarinic antagonist [LAMA]) to triple therapy (ICS + LABA + LAMA) in response to recurring COPD exacerbations or persistent symptoms.12 Randomized clinical trials have demonstrated the efficacy of triple therapy for improving lung function and reducing exacerbation frequency,13-16 and the recent real-world PRIMUS study17 showed a strong association between delaying triple therapy and adverse clinical and economic consequences following 1 severe or 2 moderate COPD exacerbations. There is also some support for a role of triple therapy in reducing mortality in both clinical trial and real-world analyses.18-20 Although prior investigations have examined the effect of initiating maintenance therapy in general among patients with a history of exacerbations,21-27 there has been little research focused specifically on patients with a recent history of hospitalization for COPD and the role of triple therapy in post-discharge outcomes.28 As of 2015, COPD is one of the conditions targeted by the Centers for Medicare and Medicaid Services (CMS) Hospital Readmissions Reduction Program (HRRP), which aims to reduce hospital readmissions through enhanced communication and care coordination29; however, only modest changes in COPD readmissions have been observed since program implementation.30-32 There remains a need for improvement and consideration of treatment strategies that could further reduce posthospitalization events.
Timely initiation of triple therapy following a COPD hospitalization could be an important treatment consideration to help avoid future COPD hospitalizations, outpatient exacerbations, and unnecessary costs. Therefore, the objectives of this study were to assess the risk of subsequent exacerbations and the impact on health care resource utilization and health care costs associated with delayed triple therapy initiation after a severe COPD exacerbation requiring hospitalization.
Methods
STUDY DESIGN AND DATA SOURCE
This retrospective observational study used health insurance claims from the Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Research Databases, January 1, 2009, to March 31, 2020. All patient records were deidentified and certified to be fully compliant with US patient confidentiality requirements set forth in the Health Insurance Portability and Accountability Act of 1996. Because this was a secondary database analysis, institutional review board approval was not required.
PATIENT SELECTION AND COHORT ASSIGNMENT
The patients included in this analysis were a subset of the patients in the recent PRIMUS study17 of triple therapy initiation among patients with 2 or more moderate or 1 or more severe COPD exacerbations in the prior year. In the PRIMUS study, patients were first identified who had 1 or more nondiagnostic inpatient or outpatient claims with a COPD diagnosis from January 1, 2010, to March 31, 2020, using International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification diagnosis codes (ICD-9-CM and ICD-10-CM) (Supplementary Table 1 (983.6KB, pdf) , available in online article). Evidence of open or closed triple therapy was required between January 1, 2011, and March 31, 2019; the date of the first closed triple therapy claim or the last component of open triple therapy was patients’ index treatment date. Open triple therapy was defined as claims for ICS, LABA, and LAMA with 14 or more consecutive and 30 or more total overlapping days of supply in a 90-day period. Closed triple therapy was defined as a claim for a single product containing ICS + LABA + LAMA.
Patients with COPD with triple therapy were required to have 1 or more severe or 2 or more moderate exacerbations during the 12 months before the index treatment date to qualify. Severe exacerbations were defined as inpatient claims with a primary diagnosis of COPD, and moderate exacerbations were defined as nondiagnostic outpatient claims with a COPD diagnosis in any position and either (1) a procedure code for a corticosteroid injection on the same claim (Supplementary Table 1 (983.6KB, pdf) ) or (2) a prescription claim for a short course of antibiotics or systemic corticosteroids within ±7 days. The earliest of the first severe or second moderate exacerbation was patient’s index exacerbation date in the PRIMUS study. Patients were required to be aged 40 years or older on the index exacerbation date and have 12 months or more of continuous enrollment with medical and pharmacy benefits before (baseline) and after (followup) the index exacerbation. Patients were excluded if they had evidence of interstitial fibrosis, sarcoidosis, pulmonary embolism, or malignancy (other than basal/squamous cell skin cancer) during baseline or follow-up (Supplementary Table 1 (983.6KB, pdf) ). Patients were also excluded if they showed any overlap in claims for ICS, LABA, and LAMA during the baseline period or between their index exacerbation and index treatment dates (ie, overlap not meeting the minimum requirement for open triple therapy).
This analysis is focused on patients meeting the above criteria for the PRIMUS study whose index exacerbation was severe. Qualifying patients with an index severe exacerbation were stratified based on the time between their index exacerbation and their index treatment date: prompt (≤ 30 days), delayed (31-180 days), and very delayed (181-365 days).
OUTCOMES ASSESSMENT
Primary outcomes were assessed during the 12-month follow-up period after the index exacerbation, including number and type of COPD exacerbations, health care resource utilization, and health care costs. Moderate and severe exacerbations were defined as described above; 14 days or more were required between events to be considered separate exacerbations. All-cause and COPD-related health care resource utilization included inpatient admissions, 90-day readmissions, emergency department (ED) visits, outpatient office visits (including primary care and pulmonologists), other outpatient visits, and outpatient prescriptions. COPD-related inpatient admissions included inpatient claims with a primary diagnosis of COPD (ie, a severe exacerbation), and COPD-related outpatient encounters included outpatient claims with a COPD diagnosis in any position. Ninety-day COPD-related readmissions were defined as COPD-related admissions within 90 days following discharge from a prior COPD-related admission. Readmissions were assessed for Commercial and Medicaid patients only. Because there is no patient responsibility for Medicare readmissions within 60 days, there are no health care claims generated for such readmissions, and they cannot be identified in the MarketScan database. COPD-related outpatient prescriptions included pharmacy claims for ICS, LABA, LAMA, LABA/LAMA, ICS/LABA, short-acting β-agonists (SABA), short-acting muscarinic antagonists (SAMA), SABA/SAMA, and oral corticosteroids (Supplementary Table 2 (983.6KB, pdf) ). All-cause and COPD-related health care costs were assessed overall and within each utilization category. Costs include payer plus patient payments, adjusted for inflation and standardized to 2020 US dollars. Health care costs were reported for the 90% of Commercial and Medicare patients who had full paid amounts on their claims during the study period; costs for all Medicaid patients were reported.
BASELINE ASSESSMENTS
Demographic characteristics were assessed on the index exacerbation date, including age, sex, payer, insurance plan type, race and ethnicity (Medicaid only), geographic region, (Commercial/Medicare only), urban/rural residence, and index year. Clinical characteristics were assessed during the 12-month baseline period, including the Elixhauser Comorbidity Index, other comorbid conditions, tobacco use, nebulizer use, oxygen therapy, and COPD medications. COPD-related office visits, ED visits, and inpatient admissions were also assessed between the index exacerbation and index treatment dates.
STATISTICAL ANALYSES
Descriptive statistics were reported for each triple therapy cohort (prompt [≤ 30 days to initiation], delayed [31-180 days],27,28 and very delayed [181-365 days]), including means, SDs, and medians for continuous variables and frequencies and proportions for categorical variables. Analyses of variance and Kruskal-Wallis tests compared continuous variables between groups and chi-square tests compared categorical variables. Multivariable logistic regression models estimated the effect of each 30-day delay of triple therapy initiation on the odds of another severe exacerbation, any exacerbation, any inpatient admission, any ED visit, a COPD-related ED visit, and a 90-day COPD readmission during follow-up. Multivariable negative binomial regression estimated the effect of each 30-day delay of triple therapy on the number of COPD exacerbations during follow-up. Generalized linear models with log-link and Ύ distribution estimated the change in all-cause and COPD-related health care costs per 30-day delay of triple therapy. All multivariable models controlled for demographics and baseline clinical characteristics, exacerbations, and health care utilization.
Results
STUDY SAMPLE AND CHARACTERISTICS
Out of 24,770 patients who met all criteria for the PRIMUS study, 6,772 (27.3%) had a severe index exacerbation and were included in this analysis (Supplementary Figure 1 (983.6KB, pdf) ). Patients with a severe exacerbation initiated triple therapy an average (±SD) of 104 (±116) days after discharge from their exacerbation. About 44% of patients initiated triple therapy within 30 days after discharge (prompt), 29.5% initiated 31 to 180 days after discharge (delayed), and 26.7% initiated 181 to 365 days after discharge (very delayed). The mean (±SD) time from index exacerbation to triple therapy initiation was 5.3 (±8.5) days for prompt patients, 95.2 (±44.6) days for delayed patients, and 275.5 (±52.6) days for very delayed patients.
Prompt initiators were slightly older, less likely to have Medicaid, and more likely to be covered by an exclusive or preferred provider organization insurance plan, compared with delayed and very delayed initiators (Table 1). Overall comorbidity burden, summarized by the Elixhauser Comorbidity Index, was higher among delayed and very delayed patients, compared with prompt (Table 2). Cardiovascular disease (545%), uncomplicated hypertension (52.5%), asthma (24.6%), acute bronchitis/bronchiolitis (23.6%), chronic cough (22.8%), and pneumonia (20.6%) were the most common baseline comorbidities among all patients (Supplementary Table 3 (983.6KB, pdf) ), and more than one-third of patients (36.2%) had evidence of tobacco use during baseline (Table 2).
TABLE 1.
Demographic Characteristics on the Index Exacerbation Date
| All patients (N = 6,772) | Prompta (n = 2,968) | Delayedb (n = 1,998) | Very delayedc (n = 1,806) | P value | |
|---|---|---|---|---|---|
| Age, mean (SD) | 63.7 (11.3) | 64.1 (11.1) | 63.8 (11.6) | 62.7 (11.2) | < 0.001 |
| Sex, n (%) | |||||
| Female | 4,077 (60.2) | 1,745 (58.8) | 1,207 (60.4) | 1,125 (62.3) | 0.055 |
| Male | 2,695 (39.8) | 1,223 (41.2) | 791 (39.6) | 681 (37.7) | |
| Payer, n (%) | |||||
| Commercial | 2,161 (31.9) | 1,077 (36.3) | 625 (31.3) | 459 (25.4) | < 0.001 |
| Medicare Supplemental | 2,573 (38.0) | 1,191 (40.1) | 753 (37.7) | 629 (34.8) | |
| Medicaid | 2,038 (30.1) | 700 (23.6) | 620 (31.0) | 718 (39.8) | |
| Race and ethnicity—Medicaid patients, n (%) | |||||
| American Indian or Alaska Native | 11 (0.5) | 5 (0.7) | 4 (0.7) | 2 (0.3) | 0.081 |
| Asian or Pacific Islander | 15 (0.7) | 4 (0.6) | 7 (1.1) | 4 (0.6) | |
| Black | 543 (26.6) | 190 (27.1) | 161 (26.0) | 192 (26.7) | |
| Hispanic | 14 (0.7) | 4 (0.6) | 4 (0.7) | 6 (0.8) | |
| White | 1,146 (56.2) | 382 (54.6) | 351 (56.6) | 413 (57.5) | |
| Other/unknown | 309 (15.2) | 115 (16.4) | 93 (15.1) | 101 (14.1) | |
| US regiond—Commercial/Medicare patients, n (%) | |||||
| Midwest | 247 (5.2) | 134 (5.9) | 72 (5.2) | 41 (3.8) | < 0.001 |
| Northeast | 860 (18.2) | 436 (19.2) | 264 (19.2) | 160 (14.7) | |
| South Atlantic | 139 (2.9) | 62 (2.7) | 38 (2.8) | 39 (3.6) | |
| South Central | 954 (20.2) | 534 (23.5) | 232 (16.8) | 188 (17.3) | |
| West | 1,264 (26.7) | 605 (26.7) | 361 (26.2) | 298 (27.4) | |
| Unknown | 1,270 (26.8) | 497 (21.9) | 411 (29.8) | 362 (33.3) | |
| Urban residence, n (%) | 5,401 (79.8) | 2,415 (81.4) | 1,601 (80.1) | 1,385 (76.7) | 0.002 |
| Insurance plan type, n (%) | |||||
| Comprehensive/indemnity | 2,676 (39.5) | 1,046 (35.2) | 831 (41.6) | 799 (44.2) | < 0.001 |
| EPO/PPO | 2,133 (31.5) | 1,098 (37.0) | 574 (28.7) | 461 (25.5) | |
| HMO | 1,268 (18.7) | 511 (17.2) | 402 (20.1) | 355 (19.7) | |
| Other/unknown | 695 (10.3) | 313 (10.6) | 191 (9.6) | 191 (10.6) | |
| Year of index exacerbation, n (%) | |||||
| 2010-2014 | 4,281 (63.2) | 1,895 (63.8) | 1,195 (59.8) | 1,191 (65.9) | < 0.001 |
| 2015-2019 | 2,491 (36.8) | 1,073 (36.2) | 803 (40.2) | 615 (34.1) | |
a Triple therapy within 30 days after or on the index exacerbation date.
b Triple therapy between 31 and 180 days after index exacerbation.
c Triple therapy between 181 and 365 days after index exacerbation.
d See https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf for states included in each region. EPO=exclusive provider organization;
HMO = health maintenance organization; PPO = preferred provider organization.
TABLE 2.
Clinical Characteristics During the 12-Month Baseline Period
| All patients (N = 6,772) | Prompta (n = 2,968) | Delayedb (n = 1,998) | Very delayedc (n = 1,806) | P value | |
|---|---|---|---|---|---|
| Elixhauser comorbidity index, mean (SD) | 4.1 (8.2) | 3.5 (7.7) | 4.2 (8.4) | 5.0 (8.7) | < 0.001 |
| Tobacco use, n (%) | 2,451 (36.2) | 990 (33.4) | 712 (35.6) | 749 (41.5) | < 0.001 |
| Any long-acting maintenance medication, n (%) | 3,781 (55.8) | 1,422 (47.9) | 1,156 (57.9) | 1,203 (66.6) | < 0.001 |
| ICS | 593 (8.8) | 197 (6.6) | 190 (9.5) | 206 (11.4) | < 0.001 |
| LABA | 126 (1.9) | 37 (1.2) | 39 (2.0) | 50 (2.8) | 0.001 |
| LAMA | 1,324 (19.6) | 524 (17.7) | 412 (20.6) | 388 (21.5) | 0.002 |
| LAMA/LABA | 73 (1.1) | 31 (1.0) | 25 (1.3) | 17 (0.9) | 0.634 |
| ICS/LABA | 2,339 (34.5) | 877 (29.5) | 682 (34.1) | 780 (43.2) | < 0.001 |
| Number of long-acting medication claims, mean (SD) | 2.8 (4.0) | 2.4 (3.8) | 2.9 (3.9) | 3.4 (4.3) | < 0.001 |
| Any short-acting medication, n (%) | 5,168 (76.3) | 2,099 (70.7) | 1,542 (77.2) | 1,527 (84.6) | < 0.001 |
| SABA | 4,205 (62.1) | 1,696 (57.1) | 1,241 (62.1) | 1,268 (70.2) | < 0.001 |
| SAMA | 447 (6.6) | 133 (4.5) | 131 (6.6) | 183 (10.1) | < 0.001 |
| SABA/SAMA | 1,319 (19.5) | 427 (14.4) | 377 (18.9) | 515 (28.5) | < 0.001 |
| Oral corticosteroid | 3,486 (51.5) | 1,321 (44.5) | 1,037 (51.9) | 1,128 (62.5) | < 0.001 |
| Number of short-acting medication claims, mean (SD) | 5.7 (7.5) | 4.3 (5.8) | 5.6 (7.2) | 8.2 (9.6) | < 0.001 |
| No long- or short-acting medications, n (%) | 1,252 (18.5) | 710 (23.9) | 330 (16.5) | 212 (11.7) | < 0.001 |
| Nebulizer use, n (%) | 812 (12.0) | 257 (8.7) | 232 (11.6) | 323 (17.9) | < 0.001 |
| Oxygen therapy, n (%) | 1,317 (19.4) | 364 (12.3) | 401 (20.1) | 552 (30.6) | < 0.001 |
| Any COPD-related primary care visit, n (%) | |||||
| Commercial/Medicare | 1,227 (25.9) | 497 (21.9) | 381 (27.6) | 349 (32.1) | < 0.001 |
| Medicaid | 791 (38.8) | 238 (34.0) | 254 (41.0) | 299 (41.6) | 0.005 |
| Number of COPD-related primary care visits, mean (SD) | |||||
| Commercial/Medicare | 0.59 (1.35) | 0.42 (0.99) | 0.62 (1.32) | 0.91 (1.89) | < 0.001 |
| Medicaid | 1.03 (1.91) | 0.74 (1.46) | 1.04 (1.77) | 1.31 (2.32) | < 0.001 |
| Any COPD-related pulmonologist visit, n (%) | |||||
| Commercial/Medicare | 529 (11.2) | 171 (7.5) | 172 (12.5) | 186 (17.1) | < 0.001 |
| Medicaid | 54 (2.6) | 12 (1.7) | 16 (2.6) | 26 (3.6) | 0.082 |
| Number of COPD-related pulmonologist visits, mean (SD) | |||||
| Commercial/Medicare | 0.24 (0.84) | 0.13 (0.54) | 0.28 (0.90) | 0.41 (1.16) | < 0.001 |
| Medicaid | 0.05 (0.33) | 0.03 (0.22) | 0.04 (0.29) | 0.07 (0.42) | 0.030 |
a Triple therapy within 30 days after or on the index exacerbation date.
b Triple therapy between 31 and 180 days after index exacerbation.
c Triple therapy between 181 and 365 days after index exacerbation.
COPD = chronic obstructive pulmonary disease; ICS = inhaled corticosteroid; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist; SABA = short-acting β-agonist; SAMA = short-acting muscarinic antagonist.
Compared with the overall PRIMUS population, patients with a severe exacerbation were slightly older, less likely to have Medicaid, more likely to be Black (among Medicaid patients), and less likely to have evidence of tobacco use during the 12-month baseline period and had a higher comorbidity burden (Supplementary Table 4 (983.6KB, pdf) ).
Within the severe exacerbation population, the frequency and number of baseline prescription claims for any long- or short-acting maintenance medication was higher among delayed and very delayed patients, compared with prompt patients (Table 2). About 45% of all patients had no long-acting maintenance medication prescription fills during baseline, including more than 50% of patients with prompt triple therapy. Nearly one-quarter (23.9%) of prompt patients had no evidence of any long- or short-acting COPD medication during the baseline period. SABA, oral corticosteroids, and ICS/LABA were the most common baseline maintenance medications among all groups. Delayed and very delayed patients also had more baseline COPD-related primary care and pulmonologist visits. Most of the delayed (79.0%) and very delayed patients (92.4%) had at least 1 COPD-related office visit, ED visit, or inpatient admission between their index exacerbation and triple therapy initiation, and the majority (56.7% and 81.0%) had more than 1 COPD-related visit during that time (Supplementary Figure 2 (983.6KB, pdf) ).
FOLLOW-UP COPD EXACERBATIONS
About two-thirds (67.3%) of all study patients had at least 1 COPD exacerbation during the 12-month follow-up. Unadjusted results for the proportion of patients with 1 to 4 or more COPD exacerbations and the mean (±SD) number of exacerbations in each group are depicted in Figure 1. Delayed and very delayed patients were more likely to have any exacerbation and had more exacerbations, on average, compared with prompt patients. Delayed and very delayed patients were more than 2 times and 3 times as likely as prompt patients, respectively, to have a severe exacerbation during the 12 months after discharge from their index exacerbation.
FIGURE 1.
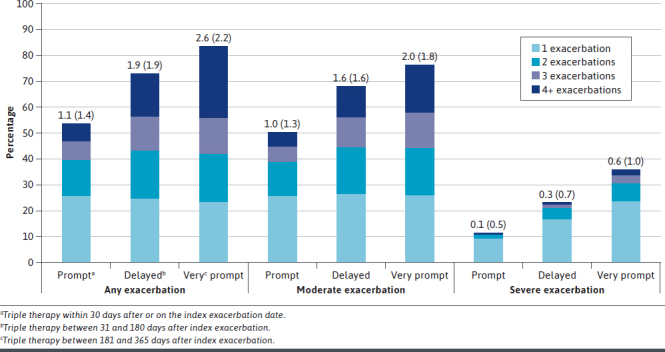
Proportion of Patients With 1, 2, 3, and 4+ Chronic Obstructive Pulmonary Disease Exacerbations, and Unadjusted Mean (SD) Number of Exacerbations per Group, During the 12-Month Follow-Up Period
After multivariable adjustment for baseline patient characteristics, each 30-day delay of triple therapy was associated with a 13% (95% CI = 1 1%-15%) increase in the odds of a moderate or severe exacerbation during 12-month follow-up (Figure 2). For the average patient in this study with 104 days to triple therapy, this equates to approximately 53% greater odds of another exacerbation within 12 months compared with a patient who received triple therapy within 30 days. The odds of experiencing another severe exacerbation in the next 12 months increased by 10% (95% CI = 8%-12%) for each 30-day delay of triple therapy so that a patient with the mean time to triple therapy would have approximately 40% greater odds of another severe exacerbation within a year compared with a patient who received prompt triple therapy. There was also a 5% increase in the mean number of exacerbations during follow-up for each 30-day delay of triple therapy. The adjusted mean estimate for the number of exacerbations during follow-up was 0.97 (95% CI = 0.91-1.03) among prompt patients and increased to 1.81 (95% CI = 1.57-2.08) exacerbations among patients who initiated triple therapy 12 months after their index exacerbation (Supplementary Figure 3 (983.6KB, pdf) ).
FIGURE 2.
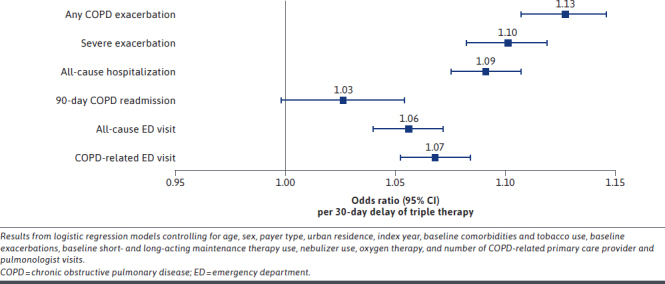
Adjusted Odds Ratios and 95% CIs for COPD Exacerbations and Health Care Resource Utilization During the 12-Month Follow-Up Period, per 30-Day Delay of Triple Therapy
FOLLOW-UP HEALTH CARE UTILIZATION AND COSTS
In unadjusted analyses, the frequency and number of allcause and COPD-related health care encounters were higher among delayed and very delayed patients, compared with prompt patients, in nearly all service categories during 12-month follow-up (Supplementary Figures 4 and 5 (983.6KB, pdf) ) (Supplementary Table 5 (983.6KB, pdf) ). Despite delayed and very delayed patients making up 56.2% of study patients, they contributed 73.5% of the all-cause hospitalizations and 79.9% of the COPD-related hospitalizations observed during 12-month follow-up.
After adjusting for baseline patient characteristics, each 30-day delay of triple therapy was associated with significant increases in the odds of an all-cause inpatient admission (9.1% [95% CI = 7.5%-10.7%]), an all-cause ED visit (5.6% [95% CI = 4.0%-7.2%]), and a COPD-related ED visit (6.8% [95% CI = 5.2%-8.4%]) (Figure 2). A patient with the average delay in triple therapy (104 days) would have approximately 36% greater odds of any inpatient admission and 21% and 26% greater odds of an all-cause or COPD-related ED visit, respectively, compared with a prompt patient. There was a 3% increase in the odds of a 90-day COPD readmission per 30-day delay of triple therapy, though the association was not statistically significant (P = 0.07).
In unadjusted analyses among Commercial/Medicare patients, mean total all-cause health care costs during 12-month follow-up were 33% and 57% greater among delayed and very delayed patients, respectively, compared with prompt patients (Supplementary Figure 6 (983.6KB, pdf) ). Unadjusted mean total all-cause costs were 23% and 49% greater among delayed and very delayed Medicaid patients, compared with prompt Medicaid patients (Supplementary Figure 7 (983.6KB, pdf) ).
After adjusting for baseline patient characteristics among all patients, each 30-day delay of triple therapy was associated with a 3% increase (95% CI = 2-4) in total all-cause health care costs, equating to an average increase of $1,266 per patient per month of delay. Each 30-day delay of triple therapy was associated with a 4% (95% CI = 3%-5%) increase in total COPD-related health care costs, equating to an average increase of $383 per patient per month of delay. The difference between the adjusted mean costs for patients with 1 to 12 months vs less than 1 month to triple therapy are depicted in Figure 3, illustrating excess all-cause costs exceeding $15,000 and excess COPD-related costs up to $4,600 associated with delayed triple therapy. Adjusted estimates of total costs during 12-month follow-up for delayed and very delayed patients with the average time to triple therapy within those groups (95.2 and 275.5 days, respectively) were 9% ($3,322) and 30% ($10,940) greater than prompt patients (5.3 days) for all-cause costs and 12% ($974) and 39% ($3,280) greater than prompt patients for COPD-related costs. After multivariable adjustment, the changes in allcause and COPD-related inpatient costs per 30-day delay of triple therapy were not statistically significant.
FIGURE 3.
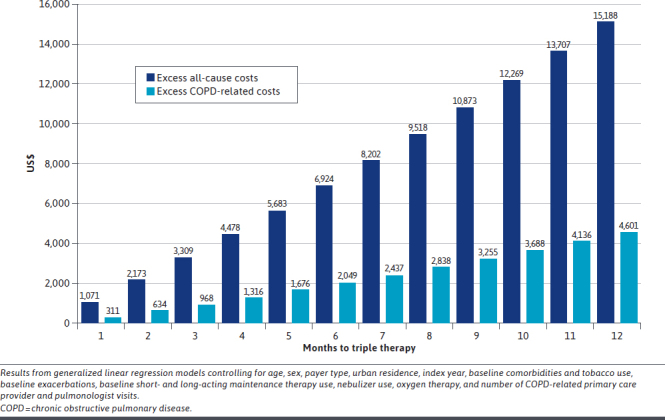
Adjusted Mean Excess All-Cause and COPD-Related Health Care Costs During 12-Month Follow-Up Associated With 1-Month to 12-Month Delays of Triple Therapy, Compared With <1 Month to Triple Therapy
Discussion
This is the first study to report a 6-month or greater delay in triple therapy initiation among more than half of patients experiencing a hospitalization for COPD and to describe patients who initiated triple therapy up to 1 year after hospitalization. Patients with delayed triple therapy, particularly those with very delayed initiation (> 6 months to 1 year), were more likely to have Medicaid insurance, had greater comorbidity burden, were more likely to be current or former tobacco users, and had indicators of more severe disease during baseline (eg, more maintenance medications and more pulmonologist visits). Notably, delayed and very delayed patients often had health care encounters after their COPD hospitalization in which they were still not escalated to triple therapy. Most had at least 1 COPD-related office, ED, or inpatient encounter between their index exacerbation and triple therapy initiation. The first of such encounters may have triggered treatment escalation for some, although nearly 60% of delayed and more than 80% of very delayed patients had at least 2 post-discharge COPD-related encounters before initiating triple therapy. These missed opportunities for treatment escalation may suggest therapeutic inertia among providers’33 and/or patients’ reluctance to change treatment. Even exacerbations experienced after delayed triple therapy initiation could be associated with the delay, because the index exacerbation may have accelerated lung function decline,34 which may have been slowed by triple therapy.35 It is also possible that multiple chronic comorbidities among delayed and very delayed patients may result in a lack of prioritization of COPD by health care providers or concern of interaction between triple therapy components and medications prescribed for other conditions. We observed significantly higher Elixhauser Comorbidity Index scores among delayed and very delayed patients, as well as greater prevalence of chronic conditions such as cardiovascular disease, asthma, and heart failure, compared with prompt patients. However, we did not assess the use of medications for other chronic conditions. In addition to timely initiation of appropriate COPD therapy, proper management of comorbidities as outlined by the GOLD report and others12 36 could improve COPD-related outcomes.
The pattern of these results regarding future exacerbations, health care resource use, and health care costs is consistent with the overall PRIMUS study. However, greater relative odds of some clinical outcomes and a greater increase in health care costs associated with delayed triple therapy was observed among this subset of patients hospitalized for a severe exacerbation. For example, the odds of a subsequent severe exacerbation increased by 6.5% per 30-day delay of triple therapy among all PRIMUS patients, compared with a 10% increase among the subset of patients with a severe index exacerbation. Additionally, the increase in all-cause and COPD-related health care costs was twice as high among patients with a severe exacerbation, compared with the overall PRIMUS cohort (all-cause: $1,266 vs $616 per 30-day delay; COPD-related: $383 vs $178 per 30-day delay). Although no statistical comparisons were made between this sample and PRIMUS patients with a moderate index exacerbation, these results suggest a greater clinical and economic impact of delayed triple therapy among patients who have been hospitalized for COPD. The increase in health care costs and the odds of a subsequent COPD hospitalization associated with delayed treatment are also in line with previous observations of higher costs and greater exacerbation risk among patients with delayed (31-180 days) vs prompt (≤ 30 days) triple therapy28,29 and other maintenance treatments25 after a COPD hospitalization or ED visit. Rather than only stratifying by time to triple therapy, the current analysis provides additional insight by demonstrating the incremental effect of each month of delay of triple therapy up to 1 year after discharge on costs and the risk of subsequent exacerbations.
Our results also suggest a potential role for prompt triple therapy in preventing readmissions after a COPD hospitalization. Although Medicare readmissions as defined by the CMS HRRP (readmission within 30 days) were not assessed, we did observe more frequent all-cause and COPD-related hospitalizations during the year after a COPD hospitalization among patients with delayed and very delayed triple therapy. Although they made up only 56% of the study population, delayed and very delayed patients accounted for 80% of the COPD-related hospitalizations during follow-up. GOLD advises that long-acting bronchodilators (LAMA, LABA, or both), with or without ICS, be either continued during a COPD hospitalization or initiated before discharge.12 We did not assess medications administered during index hospitalizations, but we did observe that 62% of delayed or very delayed patients had evidence of long-acting maintenance medication use before their index hospitalization. Although we cannot say whether those treatments were continued or changed during the hospitalization, and the GOLD advice is not specifically a recommendation for triple therapy, the current study does suggest that initiating triple therapy soon after discharge may produce more favorable outcomes than other treatment approaches. Clinical trials and real-world studies have demonstrated superiority of triple therapy (vs dual therapy) in reducing exacerbations among patients without prior maintenance treatment37,38 or with prior monotherapy37,38 or dual therapy,13-16,37,38 and there is growing consensus that prompt triple therapy initiation could improve outcomes over the step-up approach among patients who remain symptomatic or experience even a single severe exacerbation on other maintenance treatments.39
It should also be noted that prescription claims data reflect both prescriber and patient behavior; some patients may have been prescribed triple therapy at discharge but delayed filling their prescriptions. There is also some evidence that lack of early (within 1 month) post-discharge follow-up can substantially increase the risk of a COPD readmission.40 Consideration of triple therapy earlier, either in-hospital or during early post-discharge follow-up, in addition to sufficient patient education and coordination with outpatient care, may improve patient outcomes and avoid excess morbidity and costs.
LIMITATIONS
These results should be interpreted with consideration of some limitations. First, no direct measures of disease severity are available in the MarketScan Databases, so the definition of a severe exacerbation was limited to data available from health care claims (ie, diagnosis and setting). Claims data are also subject to coding limitations, thereby introducing the potential for misclassification of outcomes and other variables. However, any misclassification is not expected to differ among study groups. There is also no standard definition of open triple therapy, so our requirement of a minimum number and duration of overlapping days of supply was developed to minimize misclassification of patients who were switching between treatments rather than initiating triple therapy. The initiation of triple therapy was based on filled prescriptions; it was assumed that patients used their medications as prescribed, although adherence, administration technique, and persistence were not assessed. Because this study was limited to patients with employer-sponsored commercial or Medicare Supplemental insurance, or those with Medicaid coverage, the results may not be generalizable to patients without insurance or who purchased plans directly from the Health Insurance Marketplace. We also required at least 24 months of continuous enrollment in the MarketScan Databases, thus potentially introducing bias wherein patients without the requisite amount of enrollment, including those who died within 12 months after a COPD hospitalization, may be systematically different from patients who met enrollment criteria.
Residual confounding from unobservable patient characteristics may be present. Confounding by indication may exist in comparisons between study groups, because it is possible that physicians provide different levels of counseling to patients based on the perceived severity of their disease. Different patients may therefore be more or less likely to fill prescribed medications, fill them in a timely manner, and use them regularly and correctly. We addressed this bias to some extent by including proxies for baseline disease severity (eg, medication use and pulmonologist visits) in multivariable modeling. Some patients with a severe exacerbation may have been prescribed triple therapy but did not fill their prescriptions during the study period; outcomes were not assessed among those patients because triple therapy initiation was a requirement for inclusion. Similarly, we did not assess outcomes among patients with a severe exacerbation who were never prescribed triple therapy. Healthy adherer bias may also be present, because patients who promptly filled triple therapy prescriptions may be more likely to adhere to COPD treatment and other recommended behaviors (eg, quit smoking). Multivariable models also attempted to reduce this bias by controlling for behaviors that can be assessed in claims, such as baseline tobacco use and use of COPD maintenance therapies. There may also be unmeasured provider or health plan characteristics (eg, level of provider knowledge of guidelines; formulary step therapy requirements) that contribute to the timing of triple therapy and postdischarge outcomes after a severe exacerbation.
Conclusions
Delaying triple therapy after a severe COPD exacerbation requiring hospitalization can increase risk for future hospitalizations and subsequent outpatient exacerbations, leading to higher health care resource utilization and health care costs. Ensuring patients hospitalized for COPD are discharged with proper therapy and follow-up care, including early consideration of triple therapy, could reduce the clinical and economic impacts of COPD.
ACKNOWLEDGMENTS
Analytic support was provided by Helen Varker, Diana Stetsovsky, and David Smith of Merative.
REFERENCES
- 1.Heron M. Deaths: Leading causes for 2019. Nat Vital Stat Rep. 2021;70(9). [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying cause of death 1999-2020 on CDC WONDER online database, 2021. Accessed February 11, 2022. http://wonder.cdc.gov/ucd-icd10.html
- 3.National Center for Health Statistics. Percentage of COPD, emphysema, or chronic bronchitis for adults aged 18 and over, United States, 2019. National Health Interview Survey. Accessed February 14, 2022. https://wwwn.cdc.gov/NHISDataQueryTool/SHS_adult/index.html
- 4.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System: Web Enabled Analysis Tool. 2019. Accessed February 14, 2022. https://nccd.cdc.gov/weat/#/analysis
- 5.Foster TS, Miller JD, Marton JP, et al. Assessment of the economic burden of COPD in the U.S.: A review and synthesis of the literature. COPD. 2006;3(4):211-8. doi: 10.1080/15412550601009396 [DOI] [PubMed] [Google Scholar]
- 6.Dalal AA, Christensen L, Liu F, et al. Direct costs of chronic obstructive pulmonary disease among managed care patients. Int J Chron Obstruct Pulmon Dis. 2010;5:341-9. doi: 10.2147/COPD.S13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwab P, Dhamane AD, Hopson SD, et al. Impact of comorbid conditions in COPD patients on health care resource utilization and costs in a predominantly Medicare population. Int J Chron Obstruct Pulmon Dis. 2017;12:735-44. doi: 10.2147/COPD.S112256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zafari Z, Li S, Eakin MN, et al. Projecting long-term health and economic burden of COPD in the United States. Chest. 2021;159(4):1400-10. doi: 10.1016/j.chest.2020.09.255 [DOI] [PubMed] [Google Scholar]
- 9.Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003;124(2):459-67. doi: 10.1378/chest.124.2.459 [DOI] [PubMed] [Google Scholar]
- 10.Hoogendoorn M, Hoogenveen RT, Rutten-van Molken MP, et al. Case fatality of COPD exacerbations: A meta-analysis and statistical modelling approach. Eur Respir J. 2011;37;508-15. doi: 10.1183/09031936.00043710 [DOI] [PubMed] [Google Scholar]
- 11.Gudmundsson G, Ulrik CS, Gislason T, et al. Long-term survival in patients hospitalized for chronic obstructive pulmonary disease: A prospective observational study in the Nordic countries. Int J Chron Obstruct Pulmon Dis. 2012;7:571-6. doi: 10.2147/COPD.S34466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2022. [Google Scholar]
- 13.Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): A doubleblind, parallel-group multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747-58. doi: 10.1016/S2213-2600(18)30327-8 [DOI] [PubMed] [Google Scholar]
- 14.Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): A double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076-84. doi: 10.1016/S0140-6736(18)30206-X [DOI] [PubMed] [Google Scholar]
- 15.Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671-80. doi: 10.1056/NEJMoa1713901 [DOI] [PubMed] [Google Scholar]
- 16.Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35-48. doi: 10.1056/NEJMoa1916046 [DOI] [PubMed] [Google Scholar]
- 17.Tkacz J, Evans KA, Touchette DR, et al. PRIMUS - Prompt Initiation of Maintenance Therapy in the US: A real-world analysis of clinical and economic outcomes among patients initiating triple therapy following a COPD exacerbation. Int J Chron Obstruct Pulmon Dis. 2022;17:329-42. doi: 10.2147/COPD.S347735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am J Respir Crit Care Med. 2021;203(5):553-64. doi: 10.1164/rccm.202006-2618OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Short PM, Williamson PA, Elder DH, et al. The impact of tiotropium on mortality and exacerbations when added to inhaled corticosteroids and long-acting β2-agonist therapy in COPD. Chest. 2012;141(1):81-6. doi: 10.1378/chest.11-0038 [DOI] [PubMed] [Google Scholar]
- 20.Lipson DA, Crim C, Criner GJ, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201(12):1508-16. doi: 10.1164/rccm.201911-2207OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalal AA, Shah M, D’Souza AO, et al. Rehospitalization risks and outcomes in COPD patients receiving maintenance pharmacotherapy. Respir Med. 2012;106(6):829-37. doi: 10.1016/j.rmed.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 22.Dalal AA, Shah MB, D’Souza AO, et al. Outcomes associated with timing of maintenance treatment for COPD exacerbation. Am J Manag Care. 2012;18(9):e338-45. [PubMed] [Google Scholar]
- 23.Dalal AA, Shah MB, D’Souza AO, et al. Observational study of the outcomes and costs of initiating maintenance therapies in patients with moderate exacerbations of COPD. Respir Res. 2012;13(1):41. doi: 10.1186/1465-9921-13-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker CL, Zou KH, Su J. Long-acting bronchodilator use after hospitalization for COPD: An observational study of health insurance claims data. Int J Chron Obstruct Pulmon Dis. 2014;9:431-9. doi: 10.2147/COPD.S59322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coutinho AD, Lokhandwala T, Boggs RL, et al. Prompt initiation of maintenance treatment following a COPD exacerbation: Outcomes in a large insured population. Int J Chron Obstruct Pulmon Dis. 2016;11:1223-31. doi: 10.2147/COPD.S102570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buikema AR, Brekke L, Anderson A, et al. The effect of delaying initiation of umeclidinium/vilanterol in patients with COPD: An observational administrative claims database analysis using marginal structural models. Multidiscip Respir Med. 2018;13:38. doi: 10.1186/s40248-018-0151-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mainar AS, Huerta A, Artieda RN, et al. Economic impact of delaying initiation with multiple-inhaler maintenance triple therapy in Spanish patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulm Dis. 2019;14:2121-9. doi: 10.2147/COPD.S211854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogart M, Glassberg MB, Reinsch T, et al. Impact of prompt versus delayed initiation of triple therapy post COPD exacerbation in a US-managed care setting. Respir Med. 2018;145:138-44. doi: 10.1016/j.rmed.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 29.Centers for Medicare and Medicaid Services. Hospital Readmissions Reduction Program (HRRP). Accessed March 7, 2022. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program
- 30.Tadahiro G, Faridi MK, Gibo K, et al. Trends in 30-day readmission rates after COPD hospitalization, 2006-2012. Respir Med. 2017;130:92-7. doi: 10.1016/j.rmed.2017.07.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buhr RG, Jackson NJ, Kominski GF, et al. Readmission rates for chronic obstructive pulmonary disease under the hospital readmissions reduction program: An interrupted time series analysis. J Gen Intern Med. 2020;35(12):3581-90. doi: 10.1007/s11606-020-05958-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puebla Neira DA, Hsu ES, Kuo YF, et al. Readmissions reduction program: Mortality and readmissions for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(4):437-46. doi: 10.1164/rccm.202002-0310OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh D, Holmes S, Adams C, et al. Overcoming therapeutic inertia to reduce risk of COPD exacerbations: Four action points for healthcare professionals. Int J Chron Obstruct Pulmon Dis. 2021;16:3009-16. doi: 10.2147/COPD.S329316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halpin DM, Decramer M, Celli BR, et al. Effect of a single exacerbation on decline in lung function in COPD. Respir Med. 2017;128:85-91. doi: 10.1016/j.rmed.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 35.Singh D, Fabbri LM, Vezzoli S, et al. Extrafine triple therapy delays COPD clinically important deterioration vs ICS/LABA, LAMA, or LABA/LAMA. Int J Chron Obstruct Pulm Dis. 2019;14:531-46. doi: 10.2147/COPD.S196383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Recio Iglesias J, Diez-Manglano J, Lopez Garcia F, et al. Management of the COPD patient with comorbidities: An experts recommendation document. Int J Chron Obstruct Pulmon Dis. 2020;15:1015-37. doi: 10.2147/COPD.S242009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voorham J, Corradi M, Papi A, et al. Comparative effectiveness of triple therapy versus dual bronchodilation in COPD. ERJ Open Res. 2019;5(3);00106-2019. doi: 10.1183/23120541.00106-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng W, Duan J, Zhou A, et al. Real-world effectiveness of inhalation therapy among patients with symptomatic COPD in China: A multicenter prospective study. Front Pharmacol. 2021;12:753653. doi: 10.3389/fphar.2021.753653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanfleteren L, Ullman A, Nordenson A, et al. Triple therapy (ICS/LABA/LAMA) in COPD: Thinking out of the box. ERJ Open Res. 2019;5(1):00185-2018. doi: 10.1183/23120541.00185-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gavish R, Levy A, Dekey OK, et al. The association between hospital readmission and pulmonologist follow-up visits in patients with COPD. Chest. 2015;148(2);375-81. doi: 10.1378/chest.14-1453 [DOI] [PubMed] [Google Scholar]


